Abstract
Regions and localities may lose many species to extinction under rapid climate change and may gain other species that colonize from nearby warmer environments. Here, it is argued that warming-induced species losses will generally exceed gains and there will be more net declines than net increases in plant community richness. Declines in richness are especially likely in water-limited climates where intensifying aridity will increasingly exceed plant tolerances, but also in colder temperature-limited climates where steep climatic gradients are lacking, and therefore, large pools of appropriate species are not immediately adjacent. The selectivity of warming-induced losses may lead to declines in functional and phylogenetic diversity as well as in species richness, especially in water-limited climates. Our current understanding of climate-caused diversity trends may be overly influenced by numerous studies coming from north-temperate alpine mountaintops, where conditions are unusually favourable for increases—possibly temporary—in local species richness.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions’.
Keywords: climate change, species richness, functional diversity, phylogenetic diversity
1. Introduction
Human-caused loss of biodiversity has long been a paradigm in conservation ecology, but recently two prominent meta-analyses concluded that there have been no consistent time trends in species richness at the local community scale over the past several decades [1,2]. Considerable controversy ensued because this finding undermined the rationale for the large subfield of experimental ecology examining the potential effects of diversity loss on ecosystem function [3,4]. Climate change was the principal driver of diversity change in only a handful of studies examined, and it showed the same mix of positive, negative and neutral effects as the other drivers [2]. Likewise, in the ever-expanding literature on the ecological effects of climate change [5,6], loss of community diversity has not been a prominent theme.
In the face of this seeming scarcity of evidence, here I argue that as climate change intensifies, its likely effects will include widespread losses of terrestrial plant community diversity across multiple spatial scales and organizational levels. This assertion is based on empirical and modelling evidence that, first, many future climates will be conducive to lower plant diversity than present ones, even if dispersal were unlimited; second, dispersal by most plants is in fact extremely limited relative to climate change velocity; and third, climatic tolerances are linked to particular functional strategies and lineages, implying potential losses of functional and phylogenetic diversity as well as taxonomic diversity. Present evidence on climatic warming and plant community diversity may be skewed by coming disproportionately from north-temperate alpine mountaintops, where conditions are unusually favourable for increases in species richness, at least at local scales and in the near term.
2. Warming affects plant diversity differently in water- and temperature-limited climates
Climatic warming and altered precipitation in the next century will affect the Earth's surface unequally. Just as important for forecasting the ecological future is that these changes will fall on environments with different climatic factors limiting plant growth and dominant sets of plant functional strategies. Over large areas of the terrestrial Earth, warming will make the climate effectively more arid, decreasing plant productivity and exacerbating the role of water as the critical limiting factor [7–9]. At high latitudes and elevations where the length and warmth of the growing season are more strongly limiting than water, and in regions where large increases in precipitation are also expected, the effects of warming on potential plant productivity will tend to be positive. However, realization of these potential increases in productivity, unlike the potential decreases in productivity in water-limited climates, will depend on either the presence or the immigration of species capable of thriving under the altered conditions.
While ecosystem productivity is a frequent subject for climate change forecasts, analogous predictions for species richness in ecological communities are less often examined. Globally, terrestrial plant and animal species richness follow similar patterns to productivity, in that they are highest in climates where mild temperatures and abundant water are jointly available [10]. Two main ecological (i.e. not involving speciation) hypotheses have been put forward to explain the positive relationship of climatic productivity to species richness [11]. The energetic or more-individuals hypothesis proposes that climatic productivity sets a carrying capacity in terms of how many species can attain minimum viable population sizes; evidence cited in its support includes the relatively high predictive power of the energy-richness relationship across continents [12]. The tolerance or climatic niche conservatism hypothesis proposes that the ability to live in mild and wet climates is ancestral in many terrestrial lineages, and requires fewer specialized adaptations than the ability to survive in cold or arid conditions. This idea is supported by, among other things, the existence of strong functional and phylogenetic structure in climate-richness relationships [13,14].
Whatever its causes, the ubiquitous climate-richness relationship offers a way to project the future of species richness under the assumption of unlimited dispersal. Potential species richness, or the ‘climatic capacity for species richness', is predicted to increase within colder and wetter (temperature-limited) regions of 101–105 km2, where current temperature-richness relationships are positive, specifically regions with precipitation minus potential evaporation greater than −500 mm [15]. Potential richness is expected to decline in warmer and drier (water-limited) regions where current temperature-richness relationships are negative, i.e. precipitation minus potential evaporation less than −500 mm [15]. Because water-limited regions are both extensive and species-rich, the expected net change in potential richness at the global scale is negative [15]. Similar forecasts have been made at subregional scales, using either models of the climate-richness relationship [16] or models of individual species distributions [17]; under either approach, net potential species richness decreases within warmer regions and increases within cooler ones. Where gradients cross the transition from cool temperature-limited to warm water-limited climates, models and observations show that the effects of warming will switch from net increases to net decreases in plant productivity and species richness (e.g. [18–20]).
Both gains and losses of species will occur in response to changing climates, and actual net magnitudes of change in richness (‘biodiversity balance’, [21]) will depend in part on the proximity and dispersal abilities of species adapted to the novel climate. Even before considering propagule supply and dispersal limitation, however, a critical assertion being made here is that species richness will generally decline in water-limited climates that are becoming drier as they become warmer—even in locations where there are warmer climates nearby to supply new species, although more so where there are not. This assertion is consistent with the energetic hypothesis for the climate-richness relationship, which proposes that drier and less productive climates have the capacity for fewer individuals and therefore fewer species. It is also consistent with the tolerance hypothesis, which proposes that the ability to survive under increasing levels of water stress requires increasingly specialized adaptations and thus selects for an increasingly narrow subset of species. Where the two hypotheses diverge is on what attributes of species will most directly predict the order of their loss in warming and drying climates; under the energetic hypothesis, the first species to disappear ought to be the rarest ones, whereas under the tolerance hypothesis, the order of species disappearance should be best predicted by traits related to drought resistance.
The kinds of community changes that may eventually lead to the predicted richness declines in drying climates are already observable, and seem consistent with the tolerance hypothesis. Widespread drought has led to extensive tree mortality from carbon starvation, xylem embolism and loss of antiherbivore defences [22–25]. Shrubs and trees have sometimes failed to regenerate after increasingly frequent and severe fires, because of either inadequate carbon reserves for resprouting or the inability of seedlings to establish in warmer postfire climates [26,27]. Drought stress may kill herbaceous plants before they reproduce, depleting soil seed banks [28–30]. As discussed below, growing evidence suggests that functional traits related to drought stress tolerance may predict the relative vulnerabilities of species to these changes.
In cold climates that are becoming more productive as they warm, potential species richness is expected to increase, either because there will be a higher carrying capacity for individuals and species (energetic hypothesis) or because more sets of functional strategies can tolerate a benign than a harsh environment (tolerance hypothesis). Unlike diversity loss in drying climates, however, this potential for diversity gain can only be realized if there are nearby sources of new species, dispersal rates are adequate and other barriers to establishment are surmounted. Otherwise, diversity will remain below its new potential value (a ‘biodiversity deficit’, [21]). Also, even where warming in colder climates combined with immigration leads to increased net species richness, some resident species will be lost that are unable to tolerate aspects of the new climate such as greater unpredictability of moisture [31,32], less protection from early spring frost by snowpack [33] or increased competition from faster-growing generalists [34]. Finally, since any climate-driven gains in species richness necessarily occur at less-than-global scales, they imply that β-diversity is being lost at some scale. This contrasts with climate-driven losses of species richness, which may occur at all scales from local to global.
In summary, global patterns indicate that within regions of the world where plant growth is primarily water-limited, net plant species richness will generally decline under warming as the climate becomes effectively drier, even without taking dispersal limitation into account. Within cold temperature-limited regions, potential species richness will increase, but this potential may be smaller in magnitude and extent, more scale-dependent and less certain to be fully realized.
3. Plants are highly dispersal-limited relative to the speed of change
It is commonly said that species must disperse, evolve or become extinct under climate change, but quantifying the required changes leads to sobering perspectives [35]. Models with no evolution or dispersal indicate that species-rich biomes could lose large fractions of their species in the coming century [36–38]. The speed at which a species must disperse to track its shifting climate is a geographically varying quantity termed ‘climate change velocity’ that can be estimated from rates of change in climate in space and time at a given location [39]. Climate change velocities are greater where climates change more rapidly in time, such as at high latitudes and in continental interiors, and where climates change less abruptly in space, such as in low-relief landscapes. For the twenty-first century under a moderate emissions scenario, mean velocities of change in temperature and precipitation for different biomes have been estimated at 0.08–1.26 km yr−1 and 0.08–1.9 km yr−1, respectively [39]. These projected future mean velocities far exceed the mean velocities of post-Pleistocene change, with the caveat that spatial and temporal variation in both past and future estimates span several orders of magnitude [40].
Whether plant populations can spread at rates comparable to climate change velocity is difficult to measure directly but has been examined through indirect means, including population modelling and analyses of palaeoecological change. Modelled rates of population spread depend on maximal seed dispersal distances, which are typically small (much less than 0.1 km) in most species, and also on the determinants of seed abundance including fecundity, generation time and source population size [41–43]. A host of external factors generally tend to reduce rates of spread below the maximum of which a species is capable, including habitat heterogeneity, fragmentation, competition and mutualisms. Modifying models to represent species endemic to patchily distributed soils, for example, can lead to a requirement for these often rare species to make improbable repeated ‘jumps’ of many kilometres at a time [44]. Considering all these factors, it has often been concluded that few plant species are likely to keep up with twenty-first-century climate change through dispersal (e.g. [43,45,46]), except for abundant, warm-adapted, well-dispersing ecological generalists and human commensals.
Present-day species distributions in relation to present and past climates provide indirect evidence about time lags in the responses of plant diversity to natural climate change. Geographical concentrations of endemic species tend to be found where estimated post-Pleistocene climate velocities were lowest, suggesting a link between past climatic instability and the extinction of small-ranged species [47]. Species distributions (or species richness) in north-temperate forests may be more accurately predicted by Pleistocene climates or the locations of Holocene climate refugia than by contemporary climate, suggesting that because of slow dispersal, low population growth rates, and biotic and abiotic obstacles to range shifts, climatic changes can leave their imprint on communities for centuries to millennia (e.g. [46,48–51]). Palaeoecological evidence on changes in species richness during climatic transitions is limited, and suggests both transient decreases due to dispersal lags [52] and transient increases due to extinction lags [53].
Modern upward shifts of species ranges in elevation and latitude, one of the most commonly studied fingerprints of climate change [5,6,54], appear considerably more common and rapid in animals than plants, and within plants, to be recorded more often along short and steep elevational gradients than long and shallow latitudinal extents. Even in the Arctic, where rapid warming has led to increases in tree recruitment and growth, there has been little long-distance range expansion by trees [55]. ‘Biodiversity deficits' [21], or richness below its new potential level owing to dispersal limitation, appear common in the climate change literature. Apparent upward elevational range shifts in plants are often caused by trailing-edge extirpations [56] or changes in abundance within the existing elevational range [57,58], rather than by dispersal and colonization beyond the former climatic range limits. Extinctions at trailing edges may be slowed by multiple factors, including microrefugia [59,60], longevity [46], overstory shading [61] and small-scale soil variation [62], leading to transient periods where richness is above its new potential level (‘biodiversity surpluses’, [21]). However, there is an asymmetry between leading-edge diversity deficits and trailing-edge diversity surpluses; maladapted species at the trailing edge are fated to die, but if the velocity of climate change considerably exceeds that of dispersal, better-adapted species may never catch up to the leading edge.
4. North-temperate alpine zones as exceptions
If climate change drives an increasing number of local extinctions, as it appears to be doing [56], and if colonizations are less frequent than extinctions, both because many climates are less conducive to species richness and because many barriers exist to successful dispersal, then declines in species richness and perhaps other forms of community diversity will be inevitable. Why, then, is the evidence for climate-driven declines in species richness so equivocal? One obvious answer is that so many other influences on diversity are more severe at least in the short term. Exotic species introductions frequently enhance species richness [63], while many canopy-opening disturbances (logging, grazing, fire) tend to cause local species richness to increase. As climate change continues and intensifies, it may come to play a more dominant role.
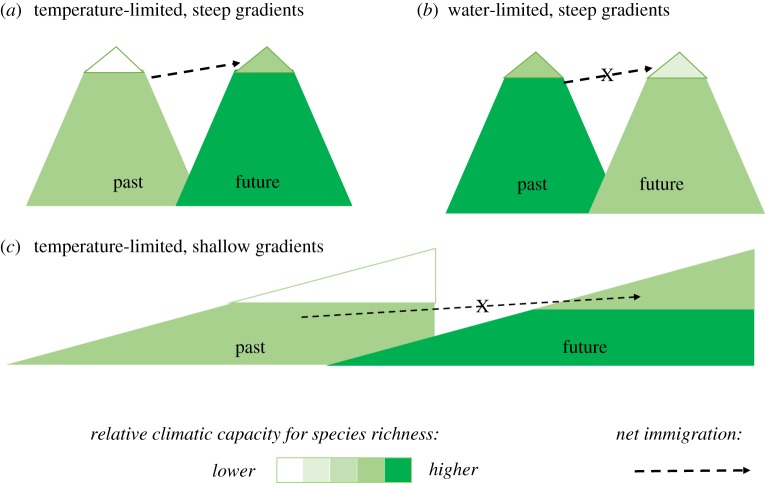
Another possible factor is that a disproportionate number of studies reporting climate-driven changes in species richness have come from alpine mountaintops in northern Europe (e.g. [20,64–67]). In this ecological setting, plant community richness has increased as upper montane species have moved into alpine zones, and there have been few extinctions of the resident alpine species so far. Alpine mountaintops may represent a best-case scenario for such an increase in local community diversity, as warmer and longer growing seasons have increased the climatic capacity for species richness, and the adjacent upper montane zones provide large numbers of warmer-adapted species to take advantage of this potential with minimal barriers to dispersal ([20,68]; figure 1a). These increases in local richness in the alpine zone come at the expense of β-diversity along the elevational gradient, and they may diminish over time as competition from faster-growing species drives alpine residents to extinction, or even cease if the available species pool is exhausted ([69,70]; but see [21]).
Figure 1.
Species richness changes in a warmer future: interactive effects of climate and topography on dispersal in space and time. Richness increases in (a), alpine mountaintops (white triangle) in cold climates where warming increases the capacity for species richness, and rugged topography provides nearby pools of species suited to the new conditions. Richness does not increase in either (b) mountaintops in warmer and drier climates where warming decreases the capacity for species richness or (c) less rugged settings in which there are no nearby sources of species suited to the new conditions. (Online version in colour.)
On mountaintops in Mediterranean Europe, plant species richness decreased during the same time period when it increased on mountaintops in northern Europe [20], a result that illustrates the key importance of climatic water balance and the climatic capacity for species richness (compare figure 1a,b). Warming in water-limited climates affects potential species richness in the opposite way as warming in temperature-limited climates, because it intensifies rather than relaxes the predominant limit to species distributions. Treating temperature and water as separate controls over plant distributions, rather than considering the effect of temperature on water balance, would produce the misleading conclusion that changes in plant species richness under warming should be independent of the macroclimatic context.
Increases in potential plant species richness in cold climates are unlikely to be realized along latitudinal gradients or elsewhere where steep topography is lacking, because of the absence of a nearby pool of warmer-adapted species (compare figure 1a,c). By contrast, in temperate marine fish faunas, regional species richness appears to be increasing as tropical species expand their latitudinal distributions towards the poles [71]. Widespread limitation of plant species distributions by water balance and seasonality rather than temperature per se, as well as the lower dispersal capacities of plants and the existence of more dispersal barriers on land, combine to make such an expansion of tropical diversity into temperate latitudes unlikely for terrestrial plants.
Observational studies that have tracked plant community richness in settings that are neither alpine mountaintops, nor strongly affected by more immediate impacts such as logging or fire, have found evidence for climate-induced diversity declines [20,26,72–75], as well as increases [76]. In the absence of seed addition, warming experiments in both temperature-limited and water-limited environments appear to more often show declines than increases in richness (e.g. [34,77–79]). Even with seed addition, warming may not always cause richness to increase in temperature-limited environments because of additional constraints such as intense herbivory, missing mutualists or inappropriate soils [80–83].
5. Interactions are unlikely to reverse diversity declines
As temperature-limited climates become warmer and more productive, species interactions may strongly modify and even reverse the direct effects of the new climate on the local richness and composition of plant communities. One general effect of warming in cold environments is to increase dominance by particular functional groups with the potential for rapid growth and tall stature, which outcompete shorter residents and erode plant community diversity at local scales [34,70,84,85]. However, susceptibility to herbivores may be promoted by some of the same traits that predispose plants to grow rapidly and become dominant, such as high foliar nitrogen; grazing may then serve to reduce dominance by the novel species and help maintain resident species diversity [83,86]. For similar reasons, grazing may also inhibit the establishment of lowland species in warming alpine and arctic environments, thus slowing the anticipated increase in species richness [82]. Plant–plant facilitation is considered to be especially prominent in harsh environments such as alpine zones and deserts. In alpine zones, where richness might otherwise be increasing under a warming climate, any disappearances of key facilitators such as cushion plants has the potential to cause losses of facilitated species [32].
In water-limited climates that are becoming less productive under climatic warming, there is less evidence for indirect effects, especially positive ones. In Californian grasslands, the severe drought of 2012–2014 disproportionately harmed the growth of competitively dominant exotic annual grasses, leading to a temporary increase in abundances although not diversity of native species [87,88]. The loss of tree cover and the conversion of forest understories into open grasslands could potentially increase local species richness in water-limited climates, but such an effect has been little documented. A key issue is whether, in water-limited climates, the direct negative effects of a warmer climate will be stronger or weaker than any positive effects caused by reduced competition [89].
In summary, species interactions can strongly affect the magnitude of change in diversity caused by warming, especially in cold climates. However, there seems little reason to expect that species interactions will transform diversity declines to diversity increases in many settings.
6. Declining functional and phylogenetic diversity
Climate change will clearly be highly selective in which species it affects the most within any given community. Current climatic distributions of species may serve as coarse integrated measures of their climatic tolerances that can be used to predict changes to local communities. The loss of species with high-latitude and high-elevation distributions, combined in some cases with gains of species with low-latitude or low-elevation distributions [61,90,91], is a widely documented form of change termed thermophilization. Similarly, membership in lineages considered to be of mesic versus arid biogeographic origin have been shown to predict climate-caused losses and gains of species [72,79,90–94].
Functional traits are another way to predict the selective losses and gains of species in changing climates. In environments where water stress is intensifying, species vulnerability may be predicted by specific leaf area [72,74,75,95], allocation to roots [96,97] and related traits associated with water use efficiency. In cold environments where productivity is increasing, short stature, shade intolerance and slow-growth-related traits may predict vulnerability [70]. Overall functional diversity will decline to the extent that novel climates select for particular functional subsets of existing plant communities. Under the assumption of unlimited dispersal, functional diversity might be expected to decline in warmer climates as these become more demanding of specialized traits, but to increase in cold climates as these become less demanding and more amenable to generalist species [98]. However, traits that govern dispersal capacity, including dispersal mode itself (e.g. [66]) and non-climatic niche requirements (e.g. [80]), may act as additional filters tending to reduce functional diversity under any scenario of rapid environmental change. Functional diversity in modern European plant communities is highest where the estimated velocity of past climate change is lowest [99,100].
Phylogenetic diversity tends to be highest in ancient mesic lineages that depend on mild temperatures and abundant water [13,101]. Accordingly, in European plant and animal communities, climate-driven extinctions are predicted to increase phylogenetic diversity in high latitudes and elevations but to decrease it in water-limited regions, with the net overall result being both decrease and homogenization of phylogenetic diversity [102].
7. Implications
At the local community scale, where species interact and compete for resources, it has been theorized and experimentally demonstrated that the loss of plant diversity threatens ecosystem functions including mean biomass, temporal stability, invasion resistance, nutrient and carbon retention, and diverse microbial composition and function [103–105]. The loss of diversity may also decrease the resilience of communities to climate change itself [106]. However, because existing evidence does not support consistent decline in species richness at local community scales [1,2], the relevance of the biodiversity and ecosystem function paradigm has recently come into question [4].
The arguments presented here suggest that declining plant community diversity at the species, functional and phylogenetic levels may become an increasingly visible aspect of human-induced change in the future. Importantly, though, these changes will not be well represented by randomly altering the numbers of species in experimental communities. Under climatic warming, future communities may be increasingly dominated by drought-tolerant species in water-limited climates and by weedy, fast-growing, fast-dispersing species in temperature-limited climates. A potentially valuable direction for experimental studies would be to examine the ecosystem consequences of such anticipated community changes.
Extrapolating from present-day ecological patterns to the future world is undeniably risky in the face of the high variability of these patterns and the novel climatic combinations, interactive CO2-climate effects, and prolonged periods of transient dynamics and disequilibrium expected in the future. Also, while this review focuses solely on climate, many drivers of diversity change will act both faster and more predictably than climate. For all of these reasons, the arguments presented here should be taken in the spirit of an attempt to stimulate further thought and research.
Acknowledgements
Howard Cornell, Dov Sax, Mark Vellend and three anonymous reviewers made many valuable comments on earlier versions of the manuscript.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
The work was supported by an NSF OPUS award no. (1748610) to S.H.
Disclaimer
The author is solely responsible for the rampant speculation expressed here.
References
- 1.Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE. 2014. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299. ( 10.1126/science.1248484) [DOI] [PubMed] [Google Scholar]
- 2.Vellend M, et al. 2013. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl Acad. Sci. USA 110, 19 456–19 459. ( 10.1073/pnas.1312779110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez A, et al. 2016. Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology 97, 1949–1960. ( 10.1890/15-1759.1) [DOI] [PubMed] [Google Scholar]
- 4.Vellend M, Baeten L, Becker-Scarpitta A, Boucher-Lalonde V, McCune JL, Messier J, Myers-Smith IH, Sax D. 2017. Plant biodiversity change across scales during the Anthropocene. Annu. Rev. Plant Biol. 68, 563–586. ( 10.1146/annurev-arplant-042916-040949) [DOI] [PubMed] [Google Scholar]
- 5.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 6.Walther G-R. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B. 365, 2019–2024. ( 10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins M, et al. 2013. Long-term climate change: projections, commitments and irreversibility. In Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (eds Stocker TF, et al.), pp. 1029–1136. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Cook BI, Ault TR, Smerdon JE. 2015. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 1, e1400082 ( 10.1126/sciadv.1400082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Yu H, Guan X, Wang G, Guo R. 2016. Accelerated dryland expansion under climate change. Nat. Clim. Change 6, 166–172. ( 10.1038/nclimate2837) [DOI] [Google Scholar]
- 10.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 11.Currie DJ, et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134. ( 10.1111/j.1461-0248.2004.00671.x) [DOI] [Google Scholar]
- 12.Rabosky DL, Hurlbert AH. 2015. Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583. ( 10.1086/680850) [DOI] [PubMed] [Google Scholar]
- 13.Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644. ( 10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 14.Harrison S, Grace JB. 2007. Biogeographic affinity helps explain the productivity-richness relationship at regional and local scales. Am. Nat. 170, S5–S15. ( 10.1086/519010) [DOI] [PubMed] [Google Scholar]
- 15.Sommer JH, Kreft H, Kier G, Jetz W, Mutke J, Barthlott W. 2010. Projected impacts of climate change on regional capacity for global plant species richness. Proc. R. Soc. B 277, 2271–2280. ( 10.1098/rspb.2010.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie DJ. 2001. Projected effects of climate change on patterns of vertebrate and tree species richness in the conterminous United States. Ecosystems 4, 216–225. ( 10.1007/s10021-001-0005-4) [DOI] [Google Scholar]
- 17.Iverson LR, Prasad AM. 2001. Potential change in tree species richness and forest community types following climate change. Ecosystems 4, 186–199. ( 10.1007/s10021-001-0003-6) [DOI] [Google Scholar]
- 18.Jolly WM, Dobbertin M, Zimmerman NE, Reichstein M. 2005. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys. Res. Lett. 32, L18409 ( 10.1029/2005GL023252) [DOI] [Google Scholar]
- 19.Schlaepfer DR, Lauenroth WK, Bradford JB. 2012. Consequences of declining snow accumulation for water balance of mid-latitude dry regions. Glob. Change Biol. 18, 1988–1997. ( 10.1111/j.1365-2486.2012.02642.x) [DOI] [Google Scholar]
- 20.Pauli H, et al. 2012. Recent plant diversity changes in Europe's mountain summits. Science 336, 353–355. ( 10.1126/science.1219033) [DOI] [PubMed] [Google Scholar]
- 21.Jackson S, Sax D. 2010. Balancing biodiversity in a changing environment: extinction debt, immigration credit, and species turnover. Trends Evol. Ecol. 25, 153–160. ( 10.1016/j.tree.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 22.Breshears DD, et al. 2005. Regional vegetation die-off in response to global change-type drought. Proc. Natl Acad. Sci. USA 102, 15 144–15 148. ( 10.1073/pnas.0505734102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Mantgem PJ, Stephenson NL. 2007. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Lett. 10, 909–916. ( 10.1111/j.1461-0248.2007.01080.x) [DOI] [PubMed] [Google Scholar]
- 24.Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Camilo Villegas J, Breshears DD, Zou CC, Troch PA, Huxman TE. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global change-type drought. Proc. Natl Acad. Sci. USA 106, 7063–7066. ( 10.1073/pnas.0901438106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderegg WRL, Plavcová L, Anderegg L, Hacke UG, Berry JA, Field CB. 2013. Drought's legacy: multiyear hydraulic deterioration underlies widespread aspen forest die-off and portends increased future risk. Glob. Change Biol. 19, 1188–1196. ( 10.1111/gcb.12100) [DOI] [PubMed] [Google Scholar]
- 26.Slingsby JA, Merow C, Aiello-Lammens M, Allsopp N, Hall S, Kilroy Mollman H, Turner R, Wilson AM, Silander JA. 2017. Intensifying postfire weather and biological invasion drive species loss in a Mediterranean-type biodiversity hotspot. Proc. Natl Acad. Sci. USA 114, 4697–4702. ( 10.1073/pnas.1619014114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guiterman C, Margolis EQ, Allen CD, Falk DA, Swetnam TW. 2018. Long-term persistence and fire resilience of oak shrubfields in dry conifer forests of northern New Mexico. Ecosystems 21, 943–959. ( 10.1007/s10021-017-0192-2) [DOI] [Google Scholar]
- 28.Tilman D, El Haddi A. 1992. Drought and biodiversity in grasslands. Oecologia 89, 257–264. ( 10.1007/BF00317226) [DOI] [PubMed] [Google Scholar]
- 29.Spasojevic M, Harrison S, Day HW, Southard RJ. 2014. Above- and belowground biotic interactions facilitate relocation of plants into cooler environments. Ecol. Lett. 17, 700–709. ( 10.1111/ele.12272) [DOI] [PubMed] [Google Scholar]
- 30.Hovenden MJ, Newton PCD, Porter M. 2017. Elevated CO2 and warming effects on grassland plant mortality are determined by the timing of rainfall. Ann. Bot. 119, 1225–1233. ( 10.1093/aob/mcx006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimenez-Benavides L, Escudero A, Iriondo JM. 2007. Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevational climate gradient. New Phytol. 173, 367–382. ( 10.1111/j.1469-8137.2006.01932.x) [DOI] [PubMed] [Google Scholar]
- 32.Bergstrom DM, et al. 2015. Rapid collapse of a sub-Antarctic alpine ecosystem: the role of climate and pathogens. J. Appl. Ecol. 52, 774–783. ( 10.1111/1365-2664.12436) [DOI] [Google Scholar]
- 33.Inouye DW. 2000. The ecological and evolutionary significance of frost in the context of climate change. Ecol. Lett. 3, 457–463. ( 10.1046/j.1461-0248.2000.00165.x) [DOI] [Google Scholar]
- 34.Walker MD, et al. 2006. Plant community responses to experimental warming across the tundra biome. Proc. Natl Acad. Sci. USA 103, 1342–1346. ( 10.1073/pnas.0503198103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis MB, Shaw D. 2001. Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679. ( 10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- 36.Midgley GF, Hannah L, Millar D, Rutherford MC, Powrie LW. 2002. Assessing the vulnerability of species richness to anthropogenic climate change in a biodiversity hotspot. Glob. Ecol. Biogeogr. 11, 445–451. ( 10.1046/j.1466-822X.2002.00307.x) [DOI] [Google Scholar]
- 37.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 38.Malcolm JR, Liu C, Neilson RP, Hansen L, Hannah L. 2006. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 20, 538–548. ( 10.1111/j.1523-1739.2006.00364.x) [DOI] [PubMed] [Google Scholar]
- 39.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052–1055. ( 10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 40.Sandel BL, Monnet A-C, Govaerts R, Vorontsova M. 2017. Late Quaternary climate stability and the origins and future of global grass endemism. Ann. Bot. 119, 279–288. ( 10.1093/aob/mcw178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark JS, Lewis M, Horvath L. 2001. Invasion by extremes: variation in dispersal and reproduction retards population spread. Am. Nat. 157, 537–554. ( 10.1086/319934) [DOI] [PubMed] [Google Scholar]
- 42.Clark JS, Lewis M, McLachlan JS, Hille Ris Lambers J. 2003. Estimating population spread: what can we forecast and how well? Ecology 84, 1979–1988. ( 10.1890/01-0618) [DOI] [Google Scholar]
- 43.Neilson RP, Pitelka LF, Solomon AM, Nathan R, Midgley GF, Fragoso JMV, Lischke H, Thompson K. 2005. Forecasting regional to global plant migration in response to climate change. Bioscience 55, 749–759. ( 10.1641/0006-3568(2005)055[0749:FRTGPM]2.0.CO;2) [DOI] [Google Scholar]
- 44.Damschen EI, Harrison S, Ackerly DD, Fernandez-Going BM, Anacker BL. 2012. Endemic plant communities on special soils: early victims or hardy survivors of climate change? J. Ecol. 100, 1122–1130. ( 10.1111/j.1365-2745.2012.01986.x) [DOI] [Google Scholar]
- 45.Corlett RT, Westcott DA. 2013. Will plant movements keep up with climate change? Trends Ecol. Evol. 28, 482–488. ( 10.1016/j.tree.2013.04.003) [DOI] [PubMed] [Google Scholar]
- 46.Svenning JC, Sandel B. 2013. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 100, 1266–1286. ( 10.3732/ajb.1200469) [DOI] [PubMed] [Google Scholar]
- 47.Sandel B, Arge L, Dalsgaard B, Davies RG, Gaston KJ, Sutherland WJ, Svenning J-C. 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664. ( 10.1126/science.1210173) [DOI] [PubMed] [Google Scholar]
- 48.Svenning JC, Skov F. 2004. Limited filling of the potential range in European tree species. Ecol. Lett. 7, 565–573. ( 10.1111/j.1461-0248.2004.00614.x) [DOI] [Google Scholar]
- 49.Svenning JC, Skov F. 2007. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol. Lett. 10, 453–460. ( 10.1111/j.1461-0248.2007.01038.x) [DOI] [PubMed] [Google Scholar]
- 50.Svenning JC, Skov F. 2007. Ice age legacies in the geographical distribution of tree species richness in Europe. Glob. Ecol. Biogeogr. 16, 234–245. ( 10.1111/j.1466-8238.2006.00280.x) [DOI] [Google Scholar]
- 51.Svenning JC, Normand S, Skov F. 2008. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography 31, 316–326. ( 10.1111/j.0906-7590.2008.05206.x) [DOI] [Google Scholar]
- 52.Cole K. 1985. Past rates of change, species richness, and a model of vegetational inertia in the Grand Canyon, Arizona. Am. Nat. 125, 289–303. ( 10.1086/284341) [DOI] [Google Scholar]
- 53.Jackson S, Betancourt JL, Lyford ME, Gray ST, Rylander KA. 2005. A 40,000-year woodrat-midden record of vegetational and biogeographical dynamics in north-eastern Utah, USA. J. Biogeography 32, 1085–1106. ( 10.1111/j.1365-2699.2005.01251.x) [DOI] [Google Scholar]
- 54.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 55.MacDonald GM, Kremenetski KV, Beilman DW. 2008. Climate change and the northern Russian tree zone. Phil. Trans. R. Soc. B 363, 2285–2299. ( 10.1098/rstb.2007.2200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiens JJ. 2016. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104 ( 10.1371/journal.pbio.2001104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenoir J, Gegout JC, Marquet PA, de Ruffray P, Brisse H. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771. ( 10.1126/science.1156831) [DOI] [PubMed] [Google Scholar]
- 58.Kelly AE, Goulden ML. 2008. Rapid shifts in plant distribution with recent climate change. Proc. Natl Acad. Sci. USA 105, 11 823–11 826. ( 10.1073/pnas.0802891105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashcroft MB, Gollan JR, Warton DI, Ramp D. 2012. A novel approach to quantify and locate potential microrefugia using topoclimate, climate stability, and isolation from the matrix. Glob. Change Biol. 18, 1866–1879. ( 10.1111/j.1365-2486.2012.02661.x) [DOI] [Google Scholar]
- 60.Keppel G, Van Niel KP, Wardell-Johnson GW, Yates CJ, Byrne M, Mucina L, Schut AGT, Hopper SD. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 21, 393–404. ( 10.1111/j.1466-8238.2011.00686.x) [DOI] [Google Scholar]
- 61.De Frenne P, et al. 2013. Microclimate moderates plant responses to macroclimate warming. Proc. Natl Acad. Sci. USA 110, 18 561–18 565. ( 10.1073/pnas.1311190110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fridley JD, Grime JP, Askew AP, Moser B, Stevens CJ. 2011. Soil heterogeneity buffers community response to climate change in a species-rich grassland. Glob. Change Biol. 17, 2002–2011. ( 10.1111/j.1365-2486.2010.02347.x) [DOI] [Google Scholar]
- 63.Sax DF, et al. 2007. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 22, 465–471. ( 10.1016/j.tree.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 64.Klanderud K, Birks HJB. 2003. Recent increases in species richness and shifts in altitudinal distributions of Norwegian mountain plants. Holocene 13, 1–6. ( 10.1191/0959683603hl589ft) [DOI] [Google Scholar]
- 65.Walther G-R, Beissner S, Burga CA. 2005. Trends in the upward shift of alpine plants. J. Veg. Sci. 16, 541–548. ( 10.1111/j.1654-1103.2005.tb02394.x) [DOI] [Google Scholar]
- 66.Holzinger B, Hulber K, Kamenisch M, Grabherr G. 2008. Changes in plant species richness over the last century in the eastern Swiss Alps: elevational gradient, bedrock effects, and migration rates. Plant Ecol. 195, 179–196. ( 10.1007/s11258-007-9314-9) [DOI] [Google Scholar]
- 67.Steinbauer M, et al. 2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234. ( 10.1038/s41586-018-0005-6) [DOI] [PubMed] [Google Scholar]
- 68.Engler R, Randin CF, Vittoz P, Czaka T, Beniston M, Zimmerman NE, Guisan A. 2009. Predicting future distributions of mountain plants under climate change: does dispersal capacity matter? Ecography 32, 34–45. ( 10.1111/j.1600-0587.2009.05789.x) [DOI] [Google Scholar]
- 69.Dullinger S, et al. 2012. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Change 2, 619–622. ( 10.1038/nclimate1514) [DOI] [Google Scholar]
- 70.Greenwood S, Jump A. 2014. Consequences of treeline shifts for the diversity and function of high altitude ecosystems. Arctic Antarctic Alpine Res. 46, 829–840. ( 10.1657/1938-4246-46.4.829) [DOI] [Google Scholar]
- 71.Batt RD, Morley JW, Selden RL, Tingley MW, Pinsky ML. 2016. Gradual changes in range size accompany long-term trends in species richness. Ecol. Lett. 20, 1148–1157. ( 10.1111/ele.12812) [DOI] [PubMed] [Google Scholar]
- 72.Damschen EI, Harrison S, Grace JB. 2010. Climate change effects on an endemic-rich edaphic flora: resurveying Robert H. Whittaker's Siskiyou sites (Oregon, USA). Ecology 91, 3609–3619. ( 10.1890/09-1057.1) [DOI] [PubMed] [Google Scholar]
- 73.Harrison S, Damschen EI, Grace JB. 2010. Ecological contingency in the effects of climate change on forest herbs. Proc. Natl Acad. Sci. USA 107, 19 362–19 367. ( 10.1073/pnas.1006823107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison S, Gornish E, Copeland S. 2015. Climate-driven diversity loss in a grassland community. Proc. Natl Acad. Sci. USA 112, 8672–8677. ( 10.1073/pnas.1502074112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrison S, LaForgia ML, Latimer AM. 2017. Climate-driven diversity change in annual grasslands: drought plus deluge does not equal normal. Glob. Change Biol. 24, 1782–1792. ( 10.1111/gcb.14018) [DOI] [PubMed] [Google Scholar]
- 76.Becker-Scarpitta A, Vissault S, Vellend M. 2019. Four decades of plant community change along a continental gradient of warming. Glob. Change Biol. 25, 1629–1641. ( 10.1111/gcb.14568) [DOI] [PubMed] [Google Scholar]
- 77.Peñuelas J, et al. 2007. Response of plant species richness and primary productivity in shrublands along a north-south gradient in Europe to seven years of experimental warming and drought: reductions in primary productivity in the heat and drought year of 2003. Glob. Change Biol. 13, 2563–2581. ( 10.1111/j.1365-2486.2007.01464.x) [DOI] [Google Scholar]
- 78.Prieto P, Peñuelas J, Llort F, Llorens L, Estiarte M. 2009. Experimental drought and warming decrease diversity and slow down post-fire succession in a Mediterranean shrubland. Ecography 32, 623–636. ( 10.1111/j.1600-0587.2009.05738.x) [DOI] [Google Scholar]
- 79.Pfeifer-Meister L, Bridgham SD, Reynolds LL, Goklany MEE, Wilson HE, Little CJ, Ferguson A, Johnson BR. 2015. Climate change alters plant biogeography in Mediterranean prairies along the West Coast, USA. Glob. Change Biol. 22, 845–855. ( 10.1111/gcb.13052) [DOI] [PubMed] [Google Scholar]
- 80.Le Roux PC, McGeoch MA. 2008. Rapid range expansion and community reorganization in response to warming. Glob. Change Biol. 14, 2950–2962. ( 10.1111/j.1365-2486.2008.01687.x) [DOI] [Google Scholar]
- 81.Wilson SD, Nilsson C. 2009. Arctic alpine vegetation change over 20 years. Glob. Change Biol. 15, 1676–1684. ( 10.1111/j.1365-2486.2009.01896.x) [DOI] [Google Scholar]
- 82.Kaarlejarvi E, Eskelinen A, Olofsson J. 2013. Herbivory prevents positive responses of lowland plants to warmer and more fertile conditions at high altitudes. Funct. Ecol. 27, 1244–1253. ( 10.1111/1365-2435.12113) [DOI] [Google Scholar]
- 83.Kaarlejarvi E, Eskelinen A, Olofsson J. 2017. Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nat. Commun. 8, 419 ( 10.1038/s41467-017-00554-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wookey PA, Aerts R, Bardgett RD, Baptist F, Brathen KA, Cornelissen JHC, Gough L, Hartley IP. 2009. Ecosystem feedbacks and cascade processes: understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob. Change Biol. 15, 1153–1172. ( 10.1111/j.1365-2486.2008.01801.x) [DOI] [Google Scholar]
- 85.Olsen S, Topper J, Skarpaas O, Vandvik V, Klanderud K. 2016. From facilitation to competition: temperature-driven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Glob. Change Biol. 22, 1915–1926. ( 10.1111/gcb.13241) [DOI] [PubMed] [Google Scholar]
- 86.Post E, Pedersen C. 2008. Opposing plant community responses to warming with and without herbivores. Proc. Natl Acad. Sci. USA 105, 12 353–12 358. ( 10.1073/pnas.0802421105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Copeland SM, Harrison SP, Latimer AM, Damschen EI, Eskelinen AM, Fernandez-Going B, Spasojevic MJ, Anacker BL, Thorne JH. 2016. Ecological effects of an extreme drought on Californian herbaceous plant communities. Ecol. Monogr. 86, 295–311. ( 10.1002/ecm.1218) [DOI] [Google Scholar]
- 88.LaForgia ML, Spasojevic MJ, Case EJ, Latimer AM, Harrison S. 2018. Seed banks of native forbs, but not exotic grasses, increase during extreme drought. Ecology 99, 896–903. ( 10.1002/ecy.2160) [DOI] [PubMed] [Google Scholar]
- 89.Levine JM, McEachern AK, Cowan C. 2010. Do competitors modulate rare plant response to climate change? Ecology 91, 130–140. ( 10.1890/08-2039.1) [DOI] [PubMed] [Google Scholar]
- 90.Gottfried M, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115. ( 10.1038/nclimate1329) [DOI] [Google Scholar]
- 91.Moradi H, Fakheran S, Peintinger M, Bergamini A, Schmid B, Joshi J. 2012. Profiteers of climate change: increase of thermophilous and generalist plants in wetland ecosystems within the last 10 years. Alpine Bot. 122, 45–56. ( 10.1007/s00035-012-0102-3) [DOI] [Google Scholar]
- 92.Peñuelas J, Boada M. 2003. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Change Biol. 9, 131–140. ( 10.1046/j.1365-2486.2003.00566.x) [DOI] [Google Scholar]
- 93.Lavergne S, Molina J, Debussche M. 2006. Fingerprints of environmental change on the rare Mediterranean flora: a 115-year study. Glob. Change Biol. 12, 1466–1478. ( 10.1111/j.1365-2486.2006.01183.x) [DOI] [Google Scholar]
- 94.Beckage B, Osborne B, Gavin DG, Pucko C, Siccama T, Perkins T. 2008. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl Acad. Sci. USA 109, 4197–4202. ( 10.1073/pnas.0708921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soudzilovskaia N, Elumeeva TG, Onipchenko VG, Shidakov II, Salpagarova FS, Khubiev AB, Tekeev DK, Cornelissen JHC. 2013. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl Acad. Sci. USA 110, 18 180–18 184. ( 10.1073/pnas.1310700110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang H, Li Y, Wu M, Zhang ZHE, Li L, Wan S. 2011. Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Glob. Change Biol. 17, 2936–2944. ( 10.1111/j.1365-2486.2011.02423.x) [DOI] [Google Scholar]
- 97.Dorji T, Totland O, Moe SR, Hopping KA, Pan J, Klein JA. 2013. Plant traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob. Change Biol. 19, 459–472. ( 10.1111/gcb.12059) [DOI] [PubMed] [Google Scholar]
- 98.Thuiller W, Lavorel S, Araujo MB, Sykes MT, Prentice IC. 2005. Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. USA 102, 8245–8250. ( 10.1073/pnas.0409902102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ordoñez A, Svenning JC. 2017. Consistent role of Quaternary climate change in shaping current plant functional diversity patterns. Nat. Sci. Rep. 7, 42988 ( 10.1038/srep42988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keppel G, Ottaviani G, Harrison S, Wardell-Johnson GW, Marcantonio M, Mucina L. 2018. Towards an eco-evolutionary understanding of endemism hotspots and refugia. Ann. Bot. 122, 927–934. ( 10.1093/aob/mcy173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anacker BL, Harrison S. 2012. Historical and ecological controls on phylogenetic diversity in Californian plant communities. Am. Nat. 180, 257–269. ( 10.1086/666650) [DOI] [PubMed] [Google Scholar]
- 102.Thuiller W, Lavergne S, Roquet C, Boulangeat I, Lafourcade R, Araujo M. 2011. Consequences of climate change on the tree of life in Europe. Nature 470, 531–534. ( 10.1038/nature09705) [DOI] [PubMed] [Google Scholar]
- 103.Chapin FS, III, et al. 2000. Consequences of changing biodiversity. Nature 405, 234–242. ( 10.1038/35012241) [DOI] [PubMed] [Google Scholar]
- 104.Isbell F, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202. ( 10.1038/nature10282) [DOI] [PubMed] [Google Scholar]
- 105.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 106.Isbell F, et al. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577. ( 10.1038/nature15374) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.