Abstract
Integrated high-resolution maps of carbon stocks and biodiversity that identify areas of potential co-benefits for climate change mitigation and biodiversity conservation can help facilitate the implementation of global climate and biodiversity commitments at local levels. However, the multi-dimensional nature of biodiversity presents a major challenge for understanding, mapping and communicating where and how biodiversity benefits coincide with climate benefits. A new integrated approach to biodiversity is therefore needed. Here, we (a) present a new high-resolution map of global above- and below-ground carbon stored in biomass and soil, (b) quantify biodiversity values using two complementary indices (BIp and BIr) representing proactive and reactive approaches to conservation, and (c) examine patterns of carbon–biodiversity overlap by identifying 'hotspots' (20% highest values for both aspects). Our indices integrate local diversity and ecosystem intactness, as well as regional ecosystem intactness across the broader area supporting a similar natural assemblage of species to the location of interest. The western Amazon Basin, Central Africa and Southeast Asia capture the last strongholds of highest local biodiversity and ecosystem intactness worldwide, while the last refuges for unique biological communities whose habitats have been greatly reduced are mostly found in the tropical Andes and central Sundaland. There is 38 and 5% overlap in carbon and biodiversity hotspots, for proactive and reactive conservation, respectively. Alarmingly, only around 12 and 21% of these proactive and reactive hotspot areas, respectively, are formally protected. This highlights that a coupled approach is urgently needed to help achieve both climate and biodiversity global targets. This would involve (1) restoring and conserving unprotected, degraded ecosystems, particularly in the Neotropics and Indomalaya, and (2) retaining the remaining strongholds of intactness.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions’.
Keywords: carbon density, multi-dimensional biodiversity, climate change, spatial-planning, global environmental policy agenda, post-2020 framework
1. Introduction
Worldwide trends in biodiversity continue to be negative [1] and anthropogenic carbon emissions are changing the Earth's climate in ways that threaten human wellbeing [2]. Climate change is also a major and growing driver of biodiversity loss in its own right, amplifying the effects of existing threats [2–6]. Conversely, biodiversity and ecosystem functions and services can significantly contribute to climate change adaptation and mitigation [7,8]. Hence, there is a growing recognition of the necessity to integrate climate and biodiversity policy agendas at global and national scales, mainstreaming climate change issues into national biodiversity strategies and action plans and vice versa [9]. Moreover, there is a potential conflict between climate and biodiversity objectives, since many pathways towards decarbonizing energy systems in line with the Paris Agreement require large increases in biomass use and biofuel production [2], which appears inconsistent with biodiversity conservation [10].
Meeting the objectives of both the United Nations Framework Convention on Climate Change (UNFCCC) and the Convention on Biological Diversity (CBD) requires clear targets as well as guidance on how these carbon and biodiversity targets can be embedded into national policies and operationalized. In the case of climate change, governments have adopted the long-term target of keeping the rise of average global temperatures to well below 2°C above pre-industrial levels. This implies a carbon emissions budget of 590–1240 GtCO2 in total from 2015 onwards [11], which will be rapidly reached if emissions continue at the current annual rate of 40 GtCO2. In principle, governments and other stakeholders can translate the global climate target into specific actions. By contrast, for biodiversity, we currently lack an analogous overall ‘currency’ and the analyses needed to translate politically agreed levels of ambition into operational science-based targets.
Effective climate change mitigation policies are often focused on protecting or restoring high-carbon forests [12], which normally means that for biodiversity conservation success, the degree of spatial congruence between carbon storage and biodiversity is relevant [13,14]. Reducing conversion of natural ecosystems for agriculture, as under REDD+ (Reducing Emissions from Deforestation and forest Degradation ‘plus’ the conservation, sustainable management and enhancement of forest carbon stocks), is often one of the most cost-effective options for emissions reduction [15,16]. However, relying heavily on the assumption that carbon is positively related to biodiversity and so focusing on carbon also protects biodiversity can lead to undesired outcomes, such as replacing high-biodiversity, low-carbon ecosystems with low-biodiversity, high-carbon plantations (e.g. [17–23]) or optimizing carbon conservation at the expense of protecting other diverse ecosystems in the landscape [13,24].
There has been much debate on how to maximize co-benefits for carbon storage and biodiversity, and contrasting evidence exists on the correlation between these two attributes at different scales (e.g. [19,25–30]). We still need a much better understanding of the spatial relationship between carbon storage and biodiversity in order to maximize conservation co-benefits. This implies detailed mapping and understanding of the spatial distribution of both aspects.
One long-standing challenge is that the concept of biodiversity defies easy definition, has different meanings for different sectors, has multiple facets and scales to consider and is inherently multi-dimensional (e.g. [31–33]). The CBD defines biodiversity as ‘the variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part’ [34]. This definition includes three inherent dimensions—diversity within species, across species and of ecosystems. This is quite different from carbon storage, which is a single feature that is readily measured in biomass and in soils.
Biodiversity data are also sparse for some of the biodiversity's dimensions and in some parts of the world [35] and it has proven complex to integrate different dimensions into a single measure that it is easy to communicate, which means there is no agreed way to measure the overall condition of biodiversity. Fortunately, the quality and availability of data for many different dimensions of biodiversity have improved in recent years, which now makes it possible to develop high-quality and relatively high-resolution maps of terrestrial biodiversity and carbon stocks at global scale.
The focus of most previous research on patterns of carbon-biodiversity co-occurrence has been on considering a few species-based metrics of biodiversity and above-ground carbon (e.g. [19,36] but see [26,37]). Species richness is one of the most used measures of biodiversity [38] as it is relatively easy to understand and measure. However, there is evidence that the association between carbon storage and species richness varies with scale (e.g. [20,22,39–41]). Furthermore, local species richness alone is not sufficient for highlighting areas of biodiversity importance, as it would overlook individual species of high conservation concern in species-poor ecosystems.
Given the urgent need for detailed spatial information on carbon-biodiversity co-benefits, in this paper, we (a) present an upgraded global map of carbon stocks incorporating soil organic carbon, (b) provide a proof-of-concept for two integrated biodiversity indicators representing two different approaches to conservation, reactive (prioritizing areas of high threat and high irreplaceability) and proactive (prioritizing areas of low threat but high irreplaceability), and (c) assess global overlap of areas of high importance for biodiversity and carbon sequestration. Our biodiversity indicators incorporate local species diversity and ecosystem intactness, as well as regional habitat condition across a broader area containing a similar natural assemblage of species. We demonstrate how combining detailed spatial data can help to identify areas where the opportunities for carbon and biodiversity benefits coincide. This synthesis of information can help inform reactive or proactive conservation action, operationalize spatial targets and guide spatially explicit national assessments of potential co-benefits.
2. Material and methods
(a). Carbon data
We created a harmonized global map of above- and below-ground terrestrial carbon storage (tonnes (t) of C per hectare (ha)) in biomass and soil for the reference year 2010 by combining the most reliable publicly available datasets. We undertook a literature review to search for and review existing datasets on biomass and soil organic carbon in terrestrial ecosystems. To determine which datasets to combine to produce the global carbon density map, we evaluated the datasets identified against our criteria based on resolution, accuracy, biomass definition and reference date (see table 1 for further information on datasets selected).
Table 1.
Summary of datasets combined for the global carbon map following a comparative analysis.
| dataset | scope | year | spatial resolution | definition |
|---|---|---|---|---|
| Santoro et al. [42] | global | 2010 | 100 m | above-ground woody biomass (including all woody parts) for trees that are >10 cm diameter-at-breast-height, masked to Landsat-derived canopy cover for 2010 [43]; biomass is expressed as oven-dry weight of the woody parts (stem, bark, branches and twigs) of all living trees excluding stump and roots |
| Xia et al. [44] | global | 2010 | 8 km | above-ground grassland biomass |
| Bouvet et al. [45] | Africa | 2010 | 25 m | above-ground woodland and savannah biomass; low woody biomass areas, which therefore exclude dense forests and deserts |
| Spawn et al. [46] | global | 2010 | 300 m | synthetic, global above- and below-ground biomass maps that combine recently released satellite-based data of standing forest biomass with novel estimates for non-forest biomass stocks |
| Hengl et al. [47] | global | 2010 | 250 m | soil organic carbon content at seven standard depths (0, 5, 15, 30, 60, 100 and 200 cm) |
We overlaid the selected biomass carbon datasets with the ESA CCI landcover map for the year 2010 [48], assigning to each grid cell the corresponding above-ground biomass value from the biomass map that was most appropriate for the grid cell's landcover type. We resampled each dataset to a nominal scale of 300 m resolution. We added below-ground biomass using root-to-shoot ratios from the IPCC guidelines [49]. The values of the resulting map (in tonnes of dry organic matter per hectare) were multiplied by 0.5 to convert to carbon, following IPCC guidelines [49] (figure 1a). Finally, we added the soil organic carbon dataset [50] (to 1 m depth, also resampled to 300 m) to generate the combined ‘total’ carbon density map (figure 1b). We resampled the resulting map to a resolution of 1 km using average pixel values for analyses with biodiversity data. See electronic supplementary material for further description on methodology.
Figure 1.
Global carbon maps representing (a) terrestrial above- and below-ground vegetation biomass carbon density (CD) (range 0–415 t ha−1) and (b) terrestrial above- and below-ground vegetation biomass carbon and soil organic carbon density to 1 m depth [47] (range 0–4011 t ha−1) per 1 km grid cell for the reference year 2010. See Material and methods for a description of biomass carbon datasets used and aggregation methods.
(b). Biodiversity data
We used five metrics as indicators of biodiversity status (table 2; electronic supplementary material, figure S1). As local species-level metrics (S), we used Species Richness–Area of Habitat (SR-AOH) and Rarity-weighted Richness–Area of Habitat (RWR-AOH). As metrics of local ecosystem intactness (E) we used the GLOBIO Mean Species Abundance (MSA) [53,62] and the PREDICTS Biodiversity Intactness Index (BII) [55,56,58,63]. Finally, as a measure of regional habitat condition (c), we used the CSIRO Biodiversity Habitat Index (BHI) [59], which is a measure of average habitat condition across the broader area supporting, or previously supporting, a similar natural assemblage of species to the location of interest.
Table 2.
Datasets used to calculate the BIp and BIr indicators.
| dataset | reference | spatial resolution | dimension of biodiversity | definition |
|---|---|---|---|---|
| species richness—AOH | IUCN [51] | 1 km | species (community composition) | provides an indication of how many species are potentially present in a given pixel. It is based on aggregating maps of the extent of suitable habitat (ESH) for all mammals, amphibians and birds. |
| rarity-weighted richness—AOH | IUCN [51] | 1 km | species (community composition) | provides an indication of how ‘important’ a given area is for biodiversity. It is based on aggregating range-size rarity scores (i.e. a measure of endemism) from maps of the extent of suitable habitat (ESH) for all mammals, amphibians and birds. This results in a continuous layer of biodiversity importance. |
| GLOBIO 4—mean species abundance (MSA) | Alkemade et al. [52], Schipper et al. [53], Kim et al. [54] | 300 m | ecosystems (intactness) | provides a measure on the compositional integrity or intactness of local communities within a pixel. It represents the mean abundance of original species in relation to a particular pressure as compared with the mean abundance in an undisturbed reference situation. Pressures include climate change, atmospheric nitrogen deposition, land use, infrastructure, habitat fragmentation and hunting (in tropical regions). |
| PREDICTS—Biodiversity Intactness Index (BII—abundance based) | Newbold et al. [55], Purvis et al. [56], de Palma et al. [57], Hill et al. [58] | 1 km | ecosystems (intactness) | BII provides a measure of the intactness of ecological assemblages and it represents the average community abundance of the originally present species, as affected by four pressure variables: land use, land-use intensity, human population density and proximity to the nearest road, in the pixel (relative to the original state assuming a pristine cover). Raster dataset was downloaded from https://data.nhm.ac.uk/dataset/global-map-of-the-biodiversity-intactness-index-from-newbold-et-al-2016-science on September 2018. |
| CSIRO—Biodiversity Habitat Index (BHI) | Ferrier et al. [59], Allnutt et al. [60], Hoskins et al. [61] | 1 km | ecosystems (intactness) | BHI estimates the impacts of habitat transformation on the retention of terrestrial biodiversity. It integrates: (1) habitat condition, on a scale of 0–1, derived from statistical downscaling of coarse-resolution land-use data using 1 km resolution environmental and remotely sensed land-cover covariates; and (2) spatial turnover in species composition (beta diversity) of vascular plant communities modelled as a function of species occurrence records and 1 km resolution climate, terrain and soil surfaces. The BHI score assigned to a given 1 km cell is calculated as the average condition of all cells predicted to have supported a similar composition of species to the cell of interest (prior to habitat transformation). This score can therefore be interpreted as the effective proportion of habitat remaining across these compositionally similar cells. |
SR-AOH, RWR-AOH, MSA and BII are measures of local biodiversity in grid cell i and BHI is a measure of habitat condition within the ranges of species occurring in grid cell i. We used RWR-AOH as a proxy for uniqueness to complement SR-AOH, which is a count of the potential number of species remaining in a grid cell i. MSA and BII represent compositional turnover (temporal β-diversity) in biological communities as compared with an undisturbed reference state, providing a measure of the local intactness of ecological assemblages within a grid cell i. BHI is a measure of the average habitat condition of all grid cells predicted to have supported a similar composition of species to the grid cell i (prior to habitat transformation). This score can therefore be interpreted as the average intactness of habitat remaining across those cells that are compositionally similar to grid cell i. It provides context about the ecological habitat neighbourhood within which each grid cell i is found.
We compiled species richness and rarity-weighted richness maps using the geographical ranges of terrestrial mammals (n = 5530), birds (n = 10579) and amphibians (n = 6492) [51]. Species ranges were rasterized at a resolution of 1 km. Employing resolutions higher than 2° (ca 200 km) when using IUCN extent-of-occurrence maps can lead to overestimation of species richness as a result of commission errors (false indication of presence) [64,65]. To tackle this issue, we refined each species' range to obtain the area of habitat (AOH) in which the species could potentially persist [66], using information on altitudinal limits and habitat preferences from the IUCN Red List data [51] combined with ESA CCI land cover [48] and GMTED2010 [67]. Thus, we excluded areas of unsuitable habitat from each species’ range, which reduces commission errors and more closely approximates the actual occurrence of the species [68,69]. We retrieved layers of BII, MSA and BHI from the global biodiversity models GLOBIO [53], PREDICTS [56] and BILBI [61], respectively. Since we were specifically interested in the potential of relatively high-resolution maps to inform spatially explicit prioritization analyses at all levels (including CBD/UNFCCC, national and sub-national), we employed a fixed grain size in our analysis. See the electronic supplementary material for further information on all the biodiversity layers used for analyses.
(c). Composite biodiversity indicators (BIp and BIr)
We followed two different approaches to calculate a Biodiversity Index (BI) and incorporate vulnerability into our global biodiversity assessment following Brooks et al. [70]: a proactive biodiversity index (BIp) and a reactive biodiversity index (BIr). BIp prioritizes cells with high local biodiversity and intactness b, for which the average habitat condition (c) or intactness of cells supporting, or previously supporting, a similar natural assemblage of species to the cell of interest is also high:
where ci is the BHI value in grid cell i, and where local diversity bi is expressed as
BIr also prioritizes areas of high local biodiversity and intactness b, but for which the condition of compositionally similar cells c, is low, expressed as
We normalized all individual datasets to an index ranging between 0 and 1. We then calculated Si (species component) as the geometric mean of SR-AOH and RWR-AOH in grid cell i, and Ei (ecosystems component) as the arithmetic mean of MSA and BII in grid cell i. We used the geometric mean in calculating Si because the two variables have qualitatively different scales.
We used the summation of the two components Si and Ei to calculate bi so that the index would exhibit high values where either the species component was high or the ecosystem component was high, but that the index would peak in areas where both components were high. In this way, bi reflects the fact that both components are important, but that co-occurrence of high local diversity and uniqueness and high local intactness is even more valuable.
Grid cells with high values for BIp represent areas of high irreplaceability (high bi value) and low threat (high ci value), while grid cells with high BIr values represent areas of high irreplaceability (high bi value) but high threat (low ci value) as the habitat types of species occurring in those grid cells are largely deteriorated across their ranges.
(d). Spatial analyses
We used bivariate maps to illustrate the relationship between carbon and either BIp or BIr at the global level. We also defined hotspot locations as those pixels for which values of carbon and either BIp or BIr lay in the highest 20% of respective values globally. We then calculated what proportion of the terrestrial land surface these hotspots covered and, additionally, the proportion of the hotspot areas currently under protection according to the World Database on Protected Areas (WDPA) [71].
Spatial data preparation was done in ArcGIS Pro 2.1.0 and Google Earth Engine [72]. All statistical analyses were performed in R 3.5.2 [73] using the package ‘raster’ [74] and ‘RStoolbox’ [75].
3. Results
(a). Carbon and biodiversity maps
Our global map of terrestrial biomass carbon highlights, as expected, temperate/subtropical moist forests as peak areas (figure 1a). When soil organic carbon to 1 m depth is added (figure 1b), the quantities of carbon in the soil dominate the spatial patterns; this is particularly notable for the organic soils at high latitudes where biomass is low.
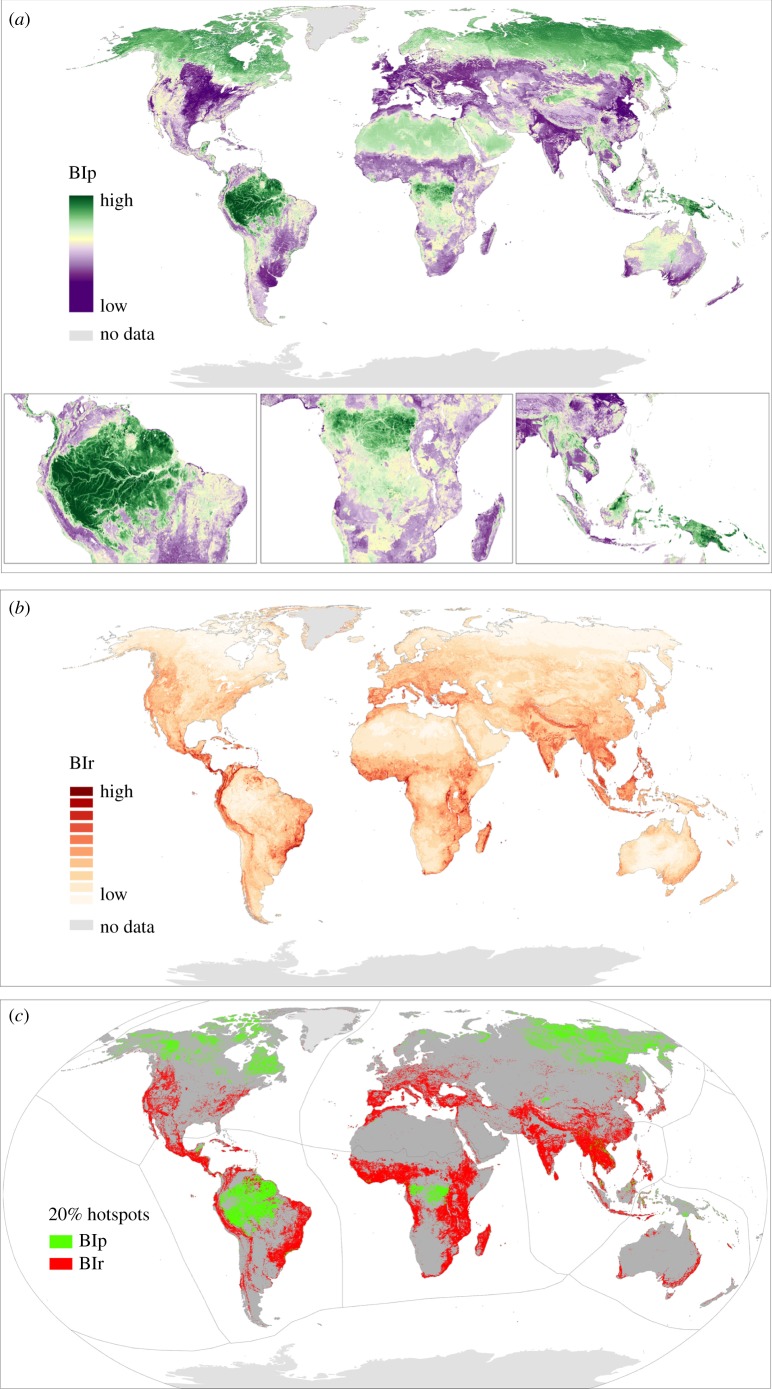
The BIp global map highlights three main regions with the highest biodiversity value (top 20% quantile) located in the Neotropics, Afrotropics and Indomalaya (figure 2a). In the Neotropics, these areas include the tropical/subtropical moist broadleaf forests of southwestern and central Amazonia, the northern moist broadleaf forests of Madeira-Tapajós and the Guiana Shield, as well as fragments of the Yucatán and Andean moist forests in Central and South America. Areas with the highest values for BIp in the Afrotropics are mostly found in the Congolian lowland forests of Central Africa, while the Indomalayan realm exhibits high BIp values in the montane rainforests of the Western Ghats, northern and southern Annamites, Cardamom Mountains, and northern Borneo. In the Australasian, the highest values for BIp are found in the montane rainforests of Sulawesi and New Guinea. The tundra and boreal forest of the Nearctic and Palaearctic also exhibit large areas with high BIp (figure 2c).
Figure 2.
Global biodiversity maps showing priority areas for proactive (BIp) and reactive (BIr) conservation actions. (a) BIp represents areas of high local biodiversity (high richness and range-size rarity of the species remaining in an area, high local intactness and high average habitat condition across the broader area supporting, or previously supporting, a similar natural assemblage of species to the location of interest); (b) BIr represents areas of high local biodiversity but low average habitat condition across the broader area supporting a similar assemblage of species; (c) 20% top quantile for both biodiversity indices (BIp and BIr). Maps are presented for the year reference 2015 and scaled to 0–1. See Material and methods for a description on datasets used and methodology.
The BIr global map shows the highest values (top 20%) in the tropical/subtropical grasslands, moist broadleaf forests, savannahs/shrublands, flooded grasslands and mangroves of Madagascar, Mozambique, Tanzania, southeastern Congo, eastern South Africa, Kenya and Ethiopia, as well as in the coastal areas of Angola, Gabon, Guinea, Cameroon and Nigeria in the Afrotropical realm (figure 2c). The highest BIr values within the Neotropics are found in the Atlantic forest (Serra do Mar and Bahia coastal forests), tropical Andes and the tropical/subtropical moist broadleaf and coniferous forests of Central America and the Caribbean. In the Afrotropical realm, the highest BIr values are found in the eastern Madagascar humid forests, Albertine Rift and East African montane forests of Congo, Kenya and Tanzania, Cross-Sanaga-Bioko, coastal and highland forests of Cameroon, Central African mangroves of Nigeria, eastern Guinean forests of Ghana and Côte d'Ivoire, Fynbos scrubland and eastern tropical/subtropical grasslands, savannahs and shrublands of South Africa. In the Indomalayan realm, BIr peaks in areas such as the montane and deciduous forests of the northwestern Ghats, the eastern Himalayas and Teri Arc, Peninsular Malaysia, the Sumatran and Philippines rainforests and the Bornean lowland rainforests.
(b). Overlap between carbon and biodiversity hotspots
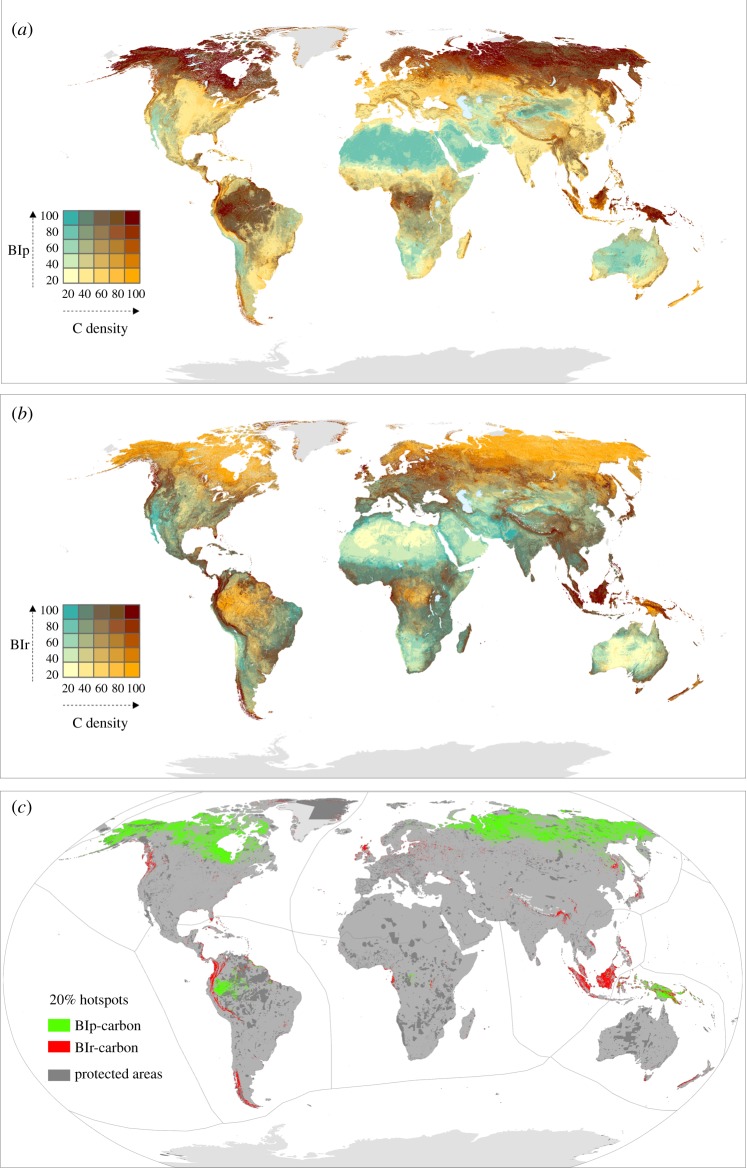
Our bivariate maps illustrate the spatial relationship between carbon and either BIp or BIr at the global level (figure 3a,b). Hotspots for carbon and either BIp or BIr show broad areas of the world with the highest potential for carbon and biodiversity co-benefits in terms of conservation and potential for recovery of degraded ecosystems, respectively (figure 3c).
Figure 3.
Global distribution of the spatial congruence between carbon density (carbon in biomass and soil organic carbon to 1 m depth) and (a) BIp and (b) BIr per 1 km grid cell. Areas with high biodiversity and carbon are represented in dark brown. (c) Map reporting 20% hotspots for both BIp carbon and BIr carbon and their overlap at the global level. Colour scales are based on quantile intervals (in 20% increments) with each class containing an equal number of values.
There is a moderate overlap between the hotspots (top 20% quantile) for carbon and BIp (38% overlap), of which 12% fall within protected areas (figure 3c). There is only a 5% overlap for carbon and BIr hotspots; 21% of that area is currently protected (figure 3c).
The areas in the world with the highest values for carbon and BIp (top 20% quantile) are found in the montane rainforests of Peninsular Malaysia, northern Borneo and West Sumatra and in the montane alpine meadows of Kinabalu in the Indomalayan realm. In the Australasian realm, these areas are found in the southeastern montane rainforests and freshwater swamp forests of New Guinea and Sulawesi and the Halmahera rainforests of southeastern Asia. In the Neotropics, the areas with highest values for both carbon and BIp are found in the Solimões-Japurá, Napo and Chocó-Darién moist forests, the Iquitos and Purus várzea, the Guianan freshwater and Orinoco Delta swamp forests of the Amazon Basin. The southern Hudson Bay, Alaska–Yukon and Siberian taiga, as well as the Canadian Arctic or central Siberian tundra in the Palaearctic and Nearctic realms, are also areas with high values for both carbon and BIp.
Finally, regions with hotspots for both carbon and BIr in the Neotropics realm are found in the Cordillera de Mérida páramo in the Venezuelan Andes, the Chocó-Darién moist forests and the Brazilian Atlantic forest in South America, the northern Andean páramo of Colombia and Ecuador and the Talamancan montane forests of Central America. In the Afrotropics, hotspots are found in the Rwenzori-Virunga montane moorlands of Rwanda, Uganda and Congo and in the Mount Cameroon and Bioko montane forests along the border between Cameroon and Nigeria. In Australasia, hotspots of both carbon and BIr are found in the sub-alpine grasslands of central Papua and the New Guinea mangroves. The most important areas for carbon and BIr in the Indomalayan realm are found in the peat swamp forests of Peninsular Malaysia, Sumatra and Borneo, the Kinabalu montane alpine meadows, Bornean lowland rainforests, Sundaland heath forests, Sumatran tropical pine forests and the Sunda Shelf mangroves.
4. Discussion
There is a growing need for urgent and coordinated global responses to mitigate climate change and reverse biodiversity loss. We argue that a clear spatially explicit understanding of the status, trends and priority regions for carbon storage and sequestration and biodiversity conservation is needed to inform such an integrated response, including the setting of post-2020 agendas under the CBD and UNFCCC. Without such information, countries and the international community cannot specify clear area-based conservation and restoration objectives that would allow them to meet the politically agreed targets to halt climate change and biodiversity loss.
We have used the most reliable and recent global datasets on carbon storage and biodiversity in terrestrial ecosystems to illustrate the potential of 1 km resolution maps to help decision-makers and local managers in identifying areas where conservation action can make substantial contributions to both climate stabilization and biodiversity conservation goals. Our results may help in setting priorities for land-use planning and in further analyses on degradation and restoration potential at international to national scales, aiming to achieve dual goals of climate stabilization under UNFCCC and biodiversity conservation under CBD.
(a). Global maps of carbon and biodiversity (BIp and BIr)
The updated global map of terrestrial carbon stocks we present here provides a globally consistent picture of how carbon stocks vary across the world. It also provides a helpful illustration as a frame of reference for national scale analyses to support decision-making on land use, including REDD+, where such data (ideally from national sources) are used along with information on changing the land cover and on other ecosystem services to identify areas for priority interventions [76].
The global BIp and BIr maps represent a proof-of-concept for integrating available data on different facets of biodiversity to develop transparent, scalable and easy-to-communicate measures that can be used by scientists, managers, policy-makers and the public to better understand, track and communicate the status of biodiversity and inform spatial-planning. BIp represents areas of high importance for biodiversity that are largely intact. It captures remaining wilderness areas which are the last stronghold of intact ecosystems across Earth. Wilderness areas are increasingly important buffers against the effects of climate change and other human impacts [77]. Also, their role in mitigating the global biodiversity crisis is crucial as they act as a buffer against species loss and they urgently require targeted protection to ensure the long-term persistence of biodiversity [78]. The complement to this estimate (BIr) represents areas that are the last refuges for a large number of species whose habitats have been greatly depleted across their range. Therefore, these areas can help inform further analyses on potential priorities for the protection and restoration of degraded environments. Unless conservation action is taken within these areas and habitat condition is improved, unique species communities are committed to disappear from their entire distribution. Our global BIp and BIr metrics offer a similar picture to that of Brooks et al. [70] on global patterns of priority regions for conservation approaches, while providing an integrated approach to understanding global patterns of biodiversity and at a much finer resolution. These metrics bring together latitudinal biodiversity gradients on species richness, a proxy for uniqueness or irreplaceability, patterns of intactness and wilderness more linked to remoteness and average condition of habitats.
The species component (S) of the global BIp and BIr metrics provides information about the remaining richness and rarity of the species occurring in a particular area after including the effects of habitat loss, whereas the ecosystem intactness component (E) summarizes the local condition of that area, and therefore the likelihood that the biodiversity originally associated with that location remains there (intactness). By combining and summing both components and multiplying by the habitat condition c, the BIp and BIr maps highlight areas where either one of the local biodiversity components of the equation is high and associates these with the average condition of habitats for the species naturally occurring in those areas (from intact to highly disturbed). Our BIp and BIr measures are distinct from previous approaches which have focused on identifying critical, irreplaceable and discrete areas for biodiversity conservation (Conservation International Hotspots, Key Biodiversity Areas, WWF Global 200 Priority Ecoregions) in providing a continuous surface of biodiversity values across different facets of biodiversity.
Nevertheless, both the carbon and biodiversity maps are associated with caveats that need to be addressed. Firstly, the global map of terrestrial carbon represents carbon stocks rather than emissions potential. While the relation between stock and emissions potential can approach one-to-one for biomass carbon stocks where land-use changes result in the loss of nearly all carbon stocks, the relationship is more nuanced for soil organic carbon stocks. The response of soil carbon stocks to land-use change varies with soil type, climate, depth and subsequent land use among other factors [79–82] and cannot accurately be inferred simply from the size of the existing stock. Given the uncertain future of a given piece of land, our map is agnostic to the vulnerability of soil organic carbon and thus represents a grid cell's absolute maximum potential emission. Further work is still needed to map the vulnerability of soil organic carbon stocks to land-use change at the global scale. There is also scope to improve this global map of carbon in terrestrial ecosystems by (a) further validation of areas with high uncertainty associated with the carbon datasets used (e.g. the AGB dataset by Santoro et al. [42] likely saturates at very high AGB values, greater than 300 Mg ha−1), (b) more formal comparative analysis between the new biomass carbon datasets to identify parts of the world where particular datasets have greater accuracy than others, and (c) improving the soil carbon layer to reflect the full peatland depth vulnerable to land-use change and the different carbon densities of peat in different parts of the world (see ‘Next steps for mapping global carbon and biodiversity’, §4c).
Secondly, despite being a useful framework for synthesizing data on biodiversity patterns, the BIp and BIr maps do not offer a complete picture of the status of biodiversity on Earth because of (1) constraints in our understanding of patterns of global biodiversity caused by substantial data gaps, and (2) limits to the representativeness of variables used here. Two main types of data gaps exist. Some dimensions of biodiversity are not represented at all or at sufficiently high resolution. For example, the scientific community is still far away from having comprehensive intraspecific data to represent the genetic dimension of biodiversity (population-level genetic diversity), although the Global Genome Initiative may accelerate progress. Also, existing phylogenetic trees can provide information on genetic diversity across species, for only a small number of species groups (e.g. through TimeTree, http://www.timetree.org/). At the species level, for many vertebrates, we have comprehensive spatially explicit data on distribution, together with information on extinction risk, threats and habitats, sourced from the IUCN Red List of Threatened Species. However, this list only includes a fraction of the planet's species diversity. In general, outside of vertebrate groups and plants, we are also lacking information on functional traits with which to construct patterns of functional diversity. The IUCN Red List is biased taxonomically towards higher vertebrates, and the majority of plants, invertebrates and fungi are broadly underrepresented [83]. Therefore, the BIp and BIr metrics we calculated from these data have a similar taxonomic bias. This is particularly relevant for vascular plants, which are included in the ecosystem intactness component but not in the species' component of the metrics. Finally, we note that our biodiversity patterns are based on modelling (either quantitatively or using expert-based approaches) and therefore the predicted patterns have associated uncertainties arising from multiple sources, including the covariates used in the modelling. Better understanding and narrowing of these uncertainties, thereby increasing confidence, will be critical for increasing the utility of the BIp and BIr maps for decision-makers.
(b). Spatial congruence between carbon and biodiversity
Our results demonstrate that there are areas where conservation actions can make substantial contributions to meeting both biodiversity and climate mitigation objectives. The greatest potential for co-benefits for carbon and biodiversity conservation of areas with relatively intact communities (high BIp) are found in the Napo and Solimōes-Japurá moist forests and Iquitos várzea in the southwestern Amazon Basin of the Neotropics realm. These areas exhibit high local biodiversity and largely remain intact but are at potential risk of conversion to oil palm plantations [84]. Further expansion of protected areas, as well as the strengthening of existing protection, will be a major component of conservation in the southwestern Amazon as the survival of species in these areas is largely dependent on habitat in good condition [78,84].
The Afrotropical and Indomalayan realms exhibited the lowest coverage of hotspots for both carbon and intact communities (BIp) of all realms. The last strongholds of intact biodiversity and high carbon in the Afrotropics, Indomalayan and Australasia are broadly found in the Western Congolian swamp forests and Albertine Rift montane forests of Central Africa, the montane rainforests of Peninsular Malaysia, northern Borneo and West Sumatra, the Halmahera rainforests of Southeast Asia and the freshwater swamp forests of New Guinea and Sulawesi. Our analyses also show relatively strong spatial congruence between carbon and BIp in boreal and tundra ecosystems (figure 3c) reflecting high soil carbon and high compositional intactness (E component of the BIp) (figure 1b; electronic supplementary material, figure S1). These hotspots of both total carbon and BIp in boreal and tundra biomes largely correspond with the Yukon–Kuskokwim delta in Alaska, the Hudson Bay lowlands and the Mackenzie River catchment in Canada, the western Siberian peatlands and the Kolyma lowlands in eastern Siberia. These areas characterized by larger extents of wilderness have been neglected in conservation efforts owing to a belief that they have lower conservation value as they are less vulnerable to threatening processes and less rich in threatened biodiversity [85]. Nevertheless, all these areas that are rich in soil carbon stocks, and that are often frozen (permafrost), are vulnerable to thaw due to anthropogenic climate change rather than to the direct impacts of human land use. They also host species communities with a high dependence on wilderness habitat [78].
The Neotropical areas which, if protected and restored, will generate the greatest carbon co-benefits are found in the tropical Andes, Talamancan montane forest of Central America and the Atlantic forest.
We also found high potential co-benefits for carbon and BIr in Madagascar, the Guinean lowland western forests and the eastern Afromontane region in the Afrotropical realm, as well as the lowland and peat swamp forests of central Sundaland. Our results reflect the extensive conversion to agriculture, such as oil palm, of deep peat soils that harbour high-carbon reserves in Borneo, Peninsular Malaysia and Sumatra [86,87]. These areas in the Afrotropical and Indomalayan realms that have already lost the largest part of their wilderness require conservation strategies more focused on the restoration of highly degraded ecosystems. These areas are critical for conservation as they host much of the remaining habitat of unique and irreplaceable species assemblages.
Restoration opportunities have been identified throughout the tropics, particularly in lowland tropical rainforest landscapes, where implementation is likely to provide the greatest potential benefits and cost-effective outcomes [88]. Also, the Indomalayan realm showed the highest overall hotspots coverage for both carbon and BIr and had the lowest hotspots coverage for both carbon and BIp of all realms, providing confirmation of the urgency for conservation actions in the region [89]. In these areas with a long history of human land use, both the protection of remaining natural habitats and restoration of degraded land may serve both biodiversity and carbon conservation purposes.
Alarmingly, our results show that these invaluable areas for both potential proactive and reactive conservation actions are globally under-protected. Of the 5% of the terrestrial surface where the highest values for both carbon and unique biodiversity under threat overlap, only 21% is currently protected. Protected areas can be seen as existing ‘wins’ but future protection needs to focus on unprotected and threatened or degraded ecosystems coupled with a targeted retention of the remaining areas of wilderness to make the best use of limited conservation resources [78].
(c). Next steps for mapping global carbon and biodiversity
To support governments in translating politically agreed levels of ambition into clear policy targets and inform decision-making at all levels (including CBD/UNFCCC, national and sub-national) on what post-2020 biodiversity targets would imply spatially, we need spatially detailed maps synthesizing data not only on carbon and biodiversity but also on other ecosystem services. Linking this to spatially explicit estimates of restoration potential across ecosystems would help to ensure that efforts to meet the objectives of the UN Decade on Ecosystem Restoration (2021–2030) provide the greatest possible benefits for climate change mitigation, food and water security and biodiversity.
Key next steps for such analyses include: incorporating data on other taxa (e.g. terrestrial plant species, reptiles, invertebrates) for the metrics based upon IUCN Red List data; accounting for plantation forests when estimating area of suitable habitat within species ranges, and in MSA, BII and BHI; improving the representation of peatlands in soil carbon data; including other ecosystem services (e.g. hydrological services from the Waterworld model [90]); and including updated information on human activities that threaten biodiversity and ecosystem functions. Such integrated information could then be used to identify globally which areas could be protected and/or restored to meet different quantitative biodiversity targets under consideration by the CBD. Recent restoration prioritization approaches incorporate ecological and economic efficiencies of scale and model-specific policy options [91]. The data could also be combined with information on land-use change to assess changes in biodiversity, carbon storage and other ecosystem services over time, compared with investment in conservation, and to track progress towards meeting the objectives of the CBD and the UNFCCC.
Similarly, we need further efforts to improve maps of coastal and marine biodiversity (building on Jones et al. [32]) and other ecosystem services that, once combined with the terrestrial data described above, will enable preparation of an integrated map for terrestrial and marine biodiversity, carbon storage and other critical ecosystems that can guide the management of oceans, forests and other biodiversity worldwide.
Integrated, globally consistent maps of biodiversity, organic carbon and key ecosystem services will enable governments and other stakeholders to express their conservation and restoration objectives for carbon, biodiversity and ecosystem services more tangibly in geospatial terms and to understand how national actions contribute to global goals. This will help countries to operationalize targets, agree on bold 2030 targets under the CBD (including a long-term 2050 vision) and implement nature-based solutions under the UNFCCC that increase the level of ambition to retain and restore nature worldwide.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to BirdLife International and the IUCN Red List team for providing data and advice for the AOH rarity-weighted richness and richness datasets.
Data accessibility
All input data used in these analyses and datasets generated in the current study are available from the corresponding author upon reasonable request and subject to agreement with authors and data providers.
Authors' contributions
C.S.-N. was the lead author of the article, performed the analysis, coordinated the writing of the preliminary draft and wrote the later drafts. C.R. and X.d.L. developed the carbon maps; A.A. and C.R. developed the SR-AOH and RWR-AOH layers; M.H., S.L.L.H., O.R.W., A.M.S., S.F., C.R. and A.A. contributed to the analyses; S.F. and T.H. provided the BHI layer for analyses; R.A. and A.M.S. provided the MSA layer for analyses; M.S., A.B., S.M., T.L.T., J.X., W.Y., S.A.S. and H.K.G. contributed with carbon datasets, and all authors reviewed and edited the first draft and contributed to the discussion.
Competing interests
We declare we have no competing interests.
Funding
Financial support for the collation of carbon data and initial carbon–biodiversity analysis came from the UN Sustainable Development Solutions Network. C.S-N. is supported by the Luc Hoffmann Institute.
References
- 1.IPBES. 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (eds S Díaz et al.). Bonn, Germany: IPBES Secretariat.
- 2.Masson-Delmotte V, et al. (eds). In press. Summary for policymakers. In Global warming of 1.5°C. Geneva, Switzerland: IPCC. [Google Scholar]
- 3.Millennium Ecosystem Assessment. 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute. [Google Scholar]
- 4.Lister AM, Stuart AJ. 2008. The impact of climate change on large mammal distribution and extinction: evidence from the last glacial/interglacial transition. C. R. Geosci. 340, 615–620. ( 10.1016/j.crte.2008.04.001) [DOI] [Google Scholar]
- 5.Pereira HM, et al. 2010. Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501. ( 10.1126/science.1196624) [DOI] [PubMed] [Google Scholar]
- 6.Barros VR, et al. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Geneva, Switzerland: IPCC. [Google Scholar]
- 7.Munang R, Thiaw I, Alverson K, Mumba M, Liu J, Rivington M. 2013. Climate change and ecosystem-based adaptation: a new pragmatic approach to buffering climate change impacts. Curr. Opin. Environ. Sustain. 5, 67–71. ( 10.1016/j.cosust.2012.12.001) [DOI] [Google Scholar]
- 8.Pereira HM, et al. 2013. Essential biodiversity variables. Science 339, 277–278. ( 10.1126/science.1229931) [DOI] [PubMed] [Google Scholar]
- 9.Biodiversity and Climate Change Working Goup II. 2018. CBD/COP/14 /L.23. Conference on Biological Diversity, Sharm El-Sheikh, Egypt, 17–29 November 2018, CBD/COP/14/L.23. See https://www.cbd.int/doc/c/9860/44b3/042fbf32838cf31a771bb145/cop-14-l-23-en.pdf.
- 10.Obersteiner M, et al. 2018. How to spend a dwindling greenhouse gas budget. Nat. Clim. Chang. 8, 7–10. ( 10.1038/s41558-017-0045-1) [DOI] [Google Scholar]
- 11.Rogelj J, Schaeffer M, Friedlingstein P, Gillett NP, van Vuuren DP, Riahi K, Allen M, Knutti R. 2016. Differences between carbon budget estimates unravelled. Nat. Clim. Chang. 6, 245 ( 10.1038/nclimate2868) [DOI] [Google Scholar]
- 12.Venter O, Koh LP. 2012. Reducing emissions from deforestation and forest degradation (REDD+): game changer or just another quick fix?. Ann. N. Y. Acad. Sci. 1249, 137–150. ( 10.1111/j.1749-6632.2011.06306.x) [DOI] [PubMed] [Google Scholar]
- 13.Miles L, Kapos V. 2008. Reducing greenhouse gas emissions from deforestation and forest degradation: global land-use implications. Science 320, 1454–1455. ( 10.1126/science.1155358) [DOI] [PubMed] [Google Scholar]
- 14.Larsen FW, Londoño-Murcia MC, Turner WR. 2011. Global priorities for conservation of threatened species, carbon storage, and freshwater services: scope for synergy?. Conserv. Lett. 4, 355–363. ( 10.1111/j.1755-263X.2011.00183.x) [DOI] [Google Scholar]
- 15.Griscom BW, et al. 2017. Natural climate solutions. Proc. Natl. Acad. Sci. USA 114, 11 645–11 650. ( 10.1073/pnas.1710465114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fargione JE, et al. 2018. Natural climate solutions for the United States. Sci. Adv. 4, eaat1869 ( 10.1126/sciadv.aat1869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armenteras D, Rodríguez N, Retana J. 2015. National and regional relationships of carbon storage and tropical biodiversity. Biol. Conserv. 192, 378–386. ( 10.1016/j.biocon.2015.10.014) [DOI] [Google Scholar]
- 18.Beaudrot L, et al. 2016. Limited carbon and biodiversity co-benefits for tropical forest mammals and birds. Ecol. Appl. 26, 1098–1111. ( 10.1890/15-0935) [DOI] [PubMed] [Google Scholar]
- 19.Di Marco M, Watson JEM, Currie DJ, Possingham HP, Venter O. 2018. The extent and predictability of the biodiversity–carbon correlation. Ecol. Lett. 21, 365–375. ( 10.1111/ele.12903) [DOI] [PubMed] [Google Scholar]
- 20.Gan J, McCarl BA. 2007. Measuring transnational leakage of forest conservation. Ecol. Econ. 64, 423–432. ( 10.1016/j.ecolecon.2007.02.032) [DOI] [Google Scholar]
- 21.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145 ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 22.Labrière N, Locatelli B, Vieilledent G, Kharisma S, Basuki I, Gond V, Laumonier Y. 2016. Spatial congruence between carbon and biodiversity across forest landscapes of northern Borneo. Glob. Ecol. Conserv. 6, 105–120. ( 10.1016/j.gecco.2016.01.005) [DOI] [Google Scholar]
- 23.Sullivan MJP, et al. 2017. Diversity and carbon storage across the tropical forest biome. Scient. Rep. 7, 39102 ( 10.1038/srep39102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson-Teixeira KJ. 2018. Prioritizing biodiversity and carbon. Nat. Clim. Change 8, 667–668. ( 10.1038/s41558-018-0242-6) [DOI] [Google Scholar]
- 25.Naidoo R, Balmford A, Costanza R, Fisher B, Green RE, Lehner B, Malcolm TR, Ricketts TH. 2008. Global mapping of ecosystem services and conservation priorities. Proc. Natl Acad. Sci. USA 105, 9495–9500. ( 10.1073/pnas.0707823105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strassburg BBN, et al. 2010. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv. Lett. 3, 98–105. ( 10.1111/j.1755-263X.2009.00092.x) [DOI] [Google Scholar]
- 27.Egoh B, Reyers B, Rouget M, Bode M, Richardson DM. 2009. Spatial congruence between biodiversity and ecosystem services in South Africa. Biol. Conserv. 142, 553–562. ( 10.1016/j.biocon.2008.11.009) [DOI] [PubMed] [Google Scholar]
- 28.Murray JP, Grenyer R, Wunder S, Raes N, Jones JPG. 2015. Spatial patterns of carbon, biodiversity, deforestation threat, and REDD+ projects in Indonesia. Conserv. Biol. 29, 1434–1445. ( 10.1111/cobi.12500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Jaen MC, Potvin C. 2010. Tree diversity explains variation in ecosystem function in a neotropical forest in Panama. Biotropica 42, 638–646. ( 10.1111/j.1744-7429.2010.00631.x) [DOI] [Google Scholar]
- 30.Kessler M, et al. 2012. Can joint carbon and biodiversity management in tropical agroforestry landscapes be optimized? PLoS ONE 7, e47192 ( 10.1371/journal.pone.0047192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Don C, DeLong J. 1996. Defining biodiversity. Wildl. Soc. Bull. 24, 738–749. [Google Scholar]
- 32.Jones KR, et al. 2018. The location and protection status of Earth's diminishing marine wilderness. Curr. Biol. 28, 2506–2512. ( 10.1016/j.cub.2018.06.010) [DOI] [PubMed] [Google Scholar]
- 33.Kaennel M. 1998. Biodiversity: a diversity in definition BT – assessment of biodiversity for improved forest planning. In Proc. Conf. Assessment of Biodiversity for Improved Planning, 7–11 October 1996, Monte Verità, Switzerland (eds P Bachmann, M Köhl, R Päivinen), pp. 71–81. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 34.Convention on Biological Diversity. 1992. Convention on Biological Diversity. United Nations Treaty Ser. 1760, p. 79. [Google Scholar]
- 35.Meyer C, Kreft H, Guralnick R, Jetz W. 2015. Global priorities for an effective information basis of biodiversity distributions. Nat. Commun. 6, 8221 ( 10.1038/ncomms9221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavanaugh KC, et al. 2014. Carbon storage in tropical forests correlates with taxonomic diversity and functional dominance on a global scale. Glob. Ecol. Biogeogr. 23, 563–573. ( 10.1111/geb.12143) [DOI] [Google Scholar]
- 37.Lecina-Diaz J, Alvarez A, Regos A, Drapeau P, Paquette A, Messier C, Retana J. 2018. The positive carbon stocks–biodiversity relationship in forests: co-occurrence and drivers across five subclimates. Ecol. Appl. 28, 1481–1493. ( 10.1002/eap.1749) [DOI] [PubMed] [Google Scholar]
- 38.Field R, et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147. ( 10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 39.Kapos V, et al. 2008. Carbon and biodiversity: a demonstration atlas. UNEP-WCMC. See https://www.unep-wcmc.org/resources-and-data/carbon-and-biodiversity–a-demonstration-atlas.
- 40.Ruesch A, Gibbs HK. 2008. New IPCC tier-1 global biomass carbon map for the year 2000. Oak Ridge National Laboratory, Oak Ridge, TN. See http://cdiac.ess-dive.lbl.gov.
- 41.Hiederer R, Köchy M. 2011. Global soil organic carbon estimates and the Harmonized World Soil Database. JRC Scient. Tech. Rep. no. 68528/EUR 25225 EN. Ispra, Italy: Joint Research Centre.
- 42.Santoro M, et al. 2018. A detailed portrait of the forest aboveground biomass pool for the year 2010 obtained from multiple remote sensing observations. Geophys. Res. Abstr. 20, EGU2018–EG18932. [Google Scholar]
- 43.Hansen MC, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. (doi:0.1126/science.1244693) [DOI] [PubMed] [Google Scholar]
- 44.Xia J, Liu S, Liang S, Chen Y, Xu W, Yuan W. 2014. Spatio-temporal patterns and climate variables controlling of biomass carbon stock of global grassland ecosystems from 1982 to 2006. Remote Sens. 6, 1783–1802. ( 10.3390/rs6031783) [DOI] [Google Scholar]
- 45.Bouvet A, Mermoz S, Le Toan T, Villard L, Mathieu R, Naidoo L, Asner GP. 2018. An above-ground biomass map of African savannahs and woodlands at 25 m resolution derived from ALOS PALSAR. Remote Sens. Environ. 206, 156–173. ( 10.1016/j.rse.2017.12.030) [DOI] [Google Scholar]
- 46.Spawn SA, Lark T, Gibbs H. 2017. New global biomass map for the year 2010. New Orleans, LA: American Geophysical Union. [Google Scholar]
- 47.Hengl T, et al. 2017. SoilGrids250 m: Global gridded soil information based on machine learning. PLoS ONE 12, e0169748 ( 10.1371/journal.pone.0169748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bontemps S, et al. 2013. Consistent global land cover maps for climate modelling communities: current achievements of the ESA's land cover CCI. In Proc. ESA Living Planet Symposium, 9–13 September 2013, Edinburgh, UK See http://due.esrin.esa.int/page_gcvRef.php. [Google Scholar]
- 49.IPCC. 2006. 2006 IPCC guidelines for national greenhouse gas inventories (eds HS Eggleston, L Buendia, K Miwa, T Ngara, K Tanabe. Kanagawa, Japan: IGES. [Google Scholar]
- 50.Penman J, et al. 2003. Good practice guidance for land use, land-use change and forestry. Kanagawa Prefecture, Japan: Institute for Global Environmental Strategies. [Google Scholar]
- 51.IUCN. 2017. IUCN Red List of Threatened Species, version 2017.3. See http://www.iucnredlist.org.
- 52.Alkemade R, van Oorschot M, Miles L, Nellemann C, Bakkenes M, ten Brink B. 2009. GLOBIO3: a framework to investigate options for reducing global terrestrial biodiversity loss. Ecosystems 12, 374–390. [Google Scholar]
- 53.Schipper A, Bakkenes M, Meijer J, Alkemade R, Huijbregts M. 2016. The GLOBIO model. A technical description of version 3.5, p. 34. The Hague, The Netherlands: PBL Netherlands Environmental Assessment Agency.
- 54.Kim H, et al. 2018. A protocol for an intercomparison of biodiversity and ecosystem services models using harmonized land-use and climate scenarios. Geosci. Model Dev. 11, 4537–4562. ( 10.5194/gmd-11-4537-2018) [DOI] [Google Scholar]
- 55.Newbold T, et al. 2016. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291. ( 10.1126/science.aaf2201) [DOI] [PubMed] [Google Scholar]
- 56.Purvis A, et al. 2018. Modelling and projecting the response of local terrestrial biodiversity worldwide to land use and related pressures: the PREDICTS project. In Next generation biomonitoring: part 1 (eds Bohan DA, Dumbrell AJ, Woodward G, Jackson M), pp. 201–241. New York, NY: Academic Press. [Google Scholar]
- 57.de Palma A, et al. 2018. Challenges with inferring how land-use affects terrestrial biodiversity: study design, time, space and synthesis. In Next generation biomonitoring: part 1 (eds Bohan DA, Dumbrell AJ, Woodward G, Jackson M), pp. 163–199. New York, NY: Academic Press. [Google Scholar]
- 58.Hill SLL, et al. 2018. Worldwide impacts of past and projected future land-use change on local species richness and the Biodiversity Intactness Index. bioRχiv, 311787 ( 10.1101/311787) [DOI]
- 59.Ferrier S, et al. 2004. Mapping more of terrestrial biodiversity for global conservation assessment. Bioscience 54, 1101–1109. ( 10.1641/0006-3568(2004)054[1101:MMOTBF]2.0.CO;2) [DOI] [Google Scholar]
- 60.Allnutt TF, et al. 2008. A method for quantifying biodiversity loss and its application to a 50-year record of deforestation across Madagascar. Conserv. Lett. 1, 173–181. ( 10.1111/j.1755-263X.2008.00027.x) [DOI] [Google Scholar]
- 61.Hoskins AJ, et al. 2018. Supporting global biodiversity assessment through high-resolution macroecological modelling: methodological underpinnings of the BILBI framework. bioRχiv, 309377 ( 10.1101/309377309377) [DOI]
- 62.Schipper AM, et al. In press. Projecting terrestrial biodiversity intactness with GLOBIO 4. Glob. Change Biol. ( 10.1111/gcb.14848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Palma A, Hoskins A, Gonzalez RE, Newbold T, Sanchez-Ortiz K, Ferrier S, Purvis A. 2018. Changes in the Biodiversity Intactness Index in tropical and subtropical forest biomes, 2001–2012. bioRχiv, 311688 ( 10.1101/311688) [DOI] [PMC free article] [PubMed]
- 64.Hurlbert AH, Jetz W. 2007. Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl Acad. Sci. 104, 13 384–13 389. ( 10.1073/pnas.0704469104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jetz W, Sekercioglu CH, Watson JEM. 2008. Ecological correlates and conservation implications of overestimating species geographic ranges. Conserv. Biol. 22, 110–119. ( 10.1111/j.1523-1739.2007.00847.x) [DOI] [PubMed] [Google Scholar]
- 66.Brooks TM, et al. 2019. Measuring terrestrial area of habitat (AOH) and its utility for the IUCN Red List. Trends Ecol. Evol. 34, 977–986. ( 10.1016/j.tree.2019.06.009) [DOI] [PubMed] [Google Scholar]
- 67.Danielson JJ, Gesch DB.2011. Global multiresolution terrain elevation data 2010 (GMTED2010). Open-File Report no. 2011–1073, p. 26. Reston, Virginia: U.S. Geological Survey.
- 68.Rondinini C, et al. 2011. Global habitat suitability models of terrestrial mammals. Phil. Trans. R. Soc. B 366, 2633–2641. ( 10.1098/rstb.2011.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santini L, Butchart SHM, Rondinini C, Benítez-López A, Hilbers JP, Schipper AM, Cengic M, Tobias JA, Huijbregts MAJ. 2019. Applying habitat and population-density models to land-cover time series to inform IUCN Red List assessments. Conserv. Biol. 33, 1084–1093. ( 10.1111/cobi.13279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL. 2006. Global biodiversity conservation priorities. Science 313, 58–61. ( 10.1126/science.1127609) [DOI] [PubMed] [Google Scholar]
- 71.UNEP-WCMC, IUCN. 2019. Protected planet: the world database on protected areas (WDPA). Cambridge, UK: UNEP-WCMC and IUCN; See www.protectedplanet.net. [Google Scholar]
- 72.Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R. 2017. Google Earth Engine: PLANETARY-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27. ( 10.1016/j.rse.2017.06.031) [DOI] [Google Scholar]
- 73.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See: http://www.R-project.org/.
- 74.Hijmans RJ. 2015. raster: Geographic data analysis and modeling.R Package version 2.4–15. See http://CRAN.R-project.org/package=raster.
- 75.Leutner B, Horning N. 2015. RStoolbox: tools for remote sensing data analysis. R Package version 0.2.6. See https://www.researchgate.net/publication/312456069_RStoolbox_Tools_for_Remote_Sensing_Data_Analysis.
- 76.Pollini B, Nimir R, Miles L. 2019. Spatial analysis: a tool for integrated land use planning for REDD+. Cambridge, UK: UNEP-WCMC. [Google Scholar]
- 77.Watson JEM, et al. 2018. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610. ( 10.1038/s41559-018-0490-x) [DOI] [PubMed] [Google Scholar]
- 78.Di Marco M, Ferrier S, Harwood TD, Hoskins AJ, Watson JEM. 2019. Wilderness areas halve the extinction risk of terrestrial biodiversity. Nature 573, 582–585. ( 10.1038/s41586-019-1567-7) [DOI] [PubMed] [Google Scholar]
- 79.Rasmussen C, et al. 2018. Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137, 297–306. ( 10.1007/s10533-018-0424-3) [DOI] [Google Scholar]
- 80.Poeplau C, Don A, Vesterdal L, Leifeld J, Van Wesemael BAS, Schumacher J, Gensior A. 2011. Temporal dynamics of soil organic carbon after land-use change in the temperate zone – carbon response functions as a model approach. Glob. Change Biol. 17, 2415–2427. ( 10.1111/j.1365-2486.2011.02408.x) [DOI] [Google Scholar]
- 81.Powers JS, Corre MD, Twine TE, Veldkamp E. 2011. Geographic bias of field observations of soil carbon stocks with tropical land-use changes precludes spatial extrapolation. Proc. Natl Acad. Sci. USA 108, 6318–6322. ( 10.1073/pnas.1016774108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spawn SA, Lark TJ, Gibbs HK. 2019. Carbon emissions from cropland expansion in the United States. Environ. Res. Lett. 14, 045009 ( 10.1088/1748-9326/ab0399) [DOI] [Google Scholar]
- 83.Stuart SN, Wilson EO, McNeely JA, Mittermeier RA, Rodríguez JP. 2010. The barometer of life. Science 328, 177 ( 10.1126/science.1188606) [DOI] [PubMed] [Google Scholar]
- 84.Vijay V, Reid CD, Finer M, Jenkins CN, Pimm SL. 2018. Deforestation risks posed by oil palm expansion in the Peruvian Amazon. Environ. Res. Lett. 13, 114010 ( 10.1088/1748-9326/aae540) [DOI] [Google Scholar]
- 85.Watson J, Venter O, Lee J, Jones K, Robinson J, Possingham H, Allan J. 2018. Protect the last of the wild. Nature 563, 27–30. ( 10.1038/d41586-018-07183-6) [DOI] [PubMed] [Google Scholar]
- 86.Miettinen J, Shi C, Liew SC. 2012. Two decades of destruction in Southeast Asia's peat swamp forests. Front. Ecol. Environ. 10, 124–128. ( 10.1890/100236) [DOI] [Google Scholar]
- 87.Gaveau DLA, et al. 2014. Four decades of forest persistence, clearance and logging on Borneo. PLoS ONE 9, e101654 ( 10.1371/journal.pone.0101654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brancalion PHS, et al. 2019. Global restoration opportunities in tropical rainforest landscapes. Sci. Adv. 5, eaav3223 (doi:0.1126/sciadv.aav3223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sodhi NS, Koh LP, Brook BW, Ng PKL. 2004. Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660. ( 10.1016/j.tree.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 90.Mulligan M. 2012. WaterWorld: a self-parameterising, physically based model for application in data-poor but problem-rich environments globally. Hydrol. Res. 44, 748–769. ( 10.2166/nh.2012.217) [DOI] [Google Scholar]
- 91.Strassburg BBN, et al. 2019. Strategic approaches to restoring ecosystems can triple conservation gains and halve costs. Nat. Ecol. Evol. 3, 62–70. ( 10.1038/s41559-018-0743-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All input data used in these analyses and datasets generated in the current study are available from the corresponding author upon reasonable request and subject to agreement with authors and data providers.