Abstract
Natural climate solutions (NCS) in the Arctic hold the potential to be implemented at a scale able to substantially affect the global climate. The strong feedbacks between carbon-rich permafrost, climate and herbivory suggest an NCS consisting of reverting the current wet/moist moss and shrub-dominated tundra and the sparse forest–tundra ecotone to grassland through a guild of large herbivores. Grassland-dominated systems might delay permafrost thaw and reduce carbon emissions—especially in Yedoma regions, while increasing carbon capture through increased productivity and grass and forb deep root systems. Here we review the environmental context of megafaunal ecological engineering in the Arctic; explore the mechanisms through which it can help mitigate climate change; and estimate its potential—based on bison and horse, with the aim of evaluating the feasibility of generating an ecosystem shift that is economically viable in terms of carbon benefits and of sufficient scale to play a significant role in global climate change mitigation. Assuming a megafaunal-driven ecosystem shift we find support for a megafauna-based arctic NCS yielding substantial income in carbon markets. However, scaling up such projects to have a significant effect on the global climate is challenging given the large number of animals required over a short period of time. A first-cut business plan is presented based on practical information—costs and infrastructure—from Pleistocene Park (northeastern Yakutia, Russia). A 10 yr experimental phase incorporating three separate introductions of herds of approximately 1000 individuals each is costed at US$114 million, with potential returns of approximately 0.3–0.4% yr−1 towards the end of the period, and greater than 1% yr−1 after it. Institutional friction and the potential role of new technologies in the reintroductions are discussed.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions'.
Keywords: Arctic, megafauna, herbivory, geoengineering, natural climate solutions, permafrost
1. Introduction
Rapid climate change [1] and biodiversity decline [2] pose huge challenges to humankind and call for new approaches involving active nature management strategies that are able to secure the resilience of ecosystems. In this context, there is a growing interest in the potential of nature-based solutions, defined by the International Union for Conservation of Nature as ‘actions to protect, sustainably manage, and restore natural or modified ecosystems, that address societal challenges effectively and adaptively, simultaneously providing human well-being and biodiversity benefits’. The 2015 Paris Agreement on Climate Change signified a high-level recognition of the potential for natural ecosystems to play a role in tackling climate change: 66% of signatories committed to nature-based solutions in their climate pledges. Natural climate solutions (NCS) involve improved land management and ecological restoration practices that avoid emissions and/or increase carbon sequestration. Griscom et al. [3] estimated that in the next 20 years, NCS could contribute over a third of the CO2 mitigation to keep the planet on a 2°C pathway. The emerging NCS narrative particularly emphasizes forests as carbon sinks, but there is increasing interest to expand the focus to climate solutions in non-tree ecosystems including peatlands, grasslands and agriculture [4].
Concurrent with these developments in climate policy is the rise of a new paradigm of recovery-based conservation and environmental management under the label of rewilding. Rewilding [5] is an approach to ecological restoration that aims to restore trophic complexity, stochastic disturbance events and the ability of organisms to disperse over time and space. The degree to which each can be achieved is dependent on context, vision and ambition [6]. On the spectrum of rewilding initiatives, one of the boldest is the restoration of ecosystems and ecosystem functions which were at least partly or fully lost owing to the megafaunal Late Pleistocene/early-Holocene (LP/EH) extinctions [7]. The science and practice of rewilding has emerged in different contexts, but can be understood as a response to new insights on (i) the coevolution of grassland systems with grazing and browsing herbivores and the role of megafauna in creating and modifying both biotic and abiotic habitat components [8–10]; (ii) the large-scale modification of Earth System processes brought about by the megafaunal extinctions in LP/EH, encompassing fire regimes, community composition [11], biogeochemical cycling [9,12,13], hydrology [14] and the global climate [15]; (iii) a new emphasis on restoring ecosystem process and dynamics and the role of these in generating diversity, abundance and resilience [16]; and (iv) a desire for wilder natures and a more hopeful and empowering environmental narrative [17]. In Europe, rewilding practice is inspiring the creation of new natural assets that ‘take inspiration from the past’ but are designed to generate nature-based solutions to a range of social and environmental challenges, including climate change [18].
Rewilding initiatives have generally targeted areas and ecosystems with missing functional species and where wildlife populations and trophic complexity have been downgraded by human practices. A distinctive feature of rewilding is that it responds to new knowledge on the ecosystem impacts of past large-bodied faunal extinctions [19,20]. The potential for trophic rewilding in the Arctic has become an increasing focus of discussion [21]. Because rewilding is usually associated with restoring degraded ecosystems, Arctic rewilding (and more particularly Pleistocene Arctic rewilding) requires some explanation and assumptions, given that this biome is often seen as free of high-intensity human disturbance and hence natural or wild. Ample evidence exists on (i) the current effects of large herbivores on the resilience of treeless tundra against the encroachment of erect woody plants [22] and on the resilience of grasslands against moss- and shrub-dominated tundra [23]; and (ii) the role of human expansion across the high latitudes in the LP/EH extinction of key megafauna species [24]. Whereas (i) links large animals to key properties such as carbon sequestration, productivity, albedo, the hydrological cycle and the energy budget of the Earth surface (including the dynamics of the upper layers of the permafrost), (ii) questions the degree of natural intactness of tundra ecosystems and justifies considering rewilding strategies in the terrestrial Arctic. In this study, we will refer to Pleistocene Arctic rewilding as a form of megafaunal ecological engineering (MEE), because the inherent restoration component embedded in the term rewilding would require considering the Arctic tundra a degraded ecosystem.
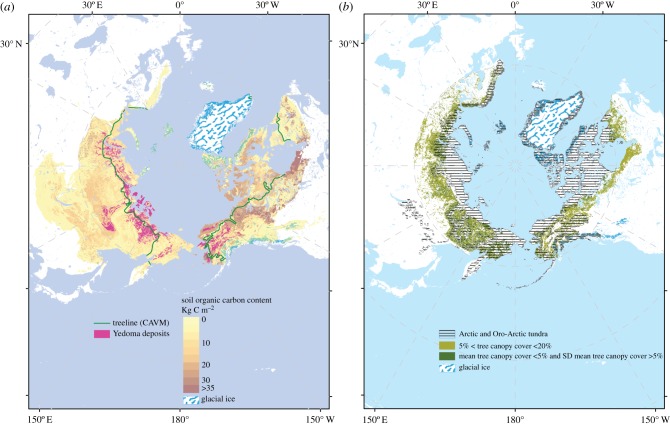
The characteristics of the terrestrial Arctic—loosely defined in here as the high latitude regions covered by tundra and forest–tundra and underlain by permafrost, figure 1—make it singular in terms of opportunities and challenges for MEE. Low human population densities (but by no means lack of people or of millennia-long land use) and remoteness from large urbanized and industrial centres; extreme abiotic conditions (notably the high seasonality in light and temperature and the pervasive role of ice and snow) that have prevented the development of agriculture; fast rates of warming (0.76°C decade−1 over 1998–2012; greater than 6× Earth's average [30]); provide an opportunity, and perhaps an imperative, to test whether MEE can create more resilient/adaptable ecosystems. Further, in the Arctic, such actions can be conceived at a scale that can influence the functioning of the Earth System globally on its own through carbon cycle and energy balance modification, most notably—but not only—if the large amounts of carbon stored in its deep permafrost soils are accounted for. It is thus timely to assess the feasibility of one of the most audacious hypotheses proposed in this region [14,31], namely that reconstituting an arctic large-herbivore guild at sufficient density will stabilize the permafrost–climate warming positive feedback [32].
Figure 1.
(a) Shades of brown: estimated soil organic carbon storage (kg C m−2) in the 0–300 cm depth range of the northern circumpolar permafrost region. Data normalized for total polygon area (including non-soil areas) [25]; pink regions: areas of deep, organic-rich Yedoma deposits [26]; Green line: tundra/boreal forest treeline [27]. (b) Dashed area: areas of treeless Arctic tundra—any land north of the Arctic treeline [27]—and ‘Oro-Arctic’ areas [28]; shades of green: map of selected areas with defined tree canopy cover over the circumpolar taiga–tundra ecotone. Derived from the 500-m MODIS Vegetation Continuous Fields product as averaged over 6 years from 2000 to 2005 and processed as described in [29]. It depicts patches of low tree canopy cover indicative of the forest–tundra ecotone. Map covers 60° N–70° N (Eurasia) and 50° N–70° N (North America). Map projection: Azimuthal Equidistant, geodetic datum: WGS84.
In this paper, we present an initial assessment of whether MEE at a large scale (i.e. that able to modify the global carbon and climate systems) is within the bounds of the possible. Such an action would require megafaunal engineering at a scale not previously considered. We do so by (i) reviewing the environmental context of MEE in the Arctic; (ii) exploring the mechanisms through which MEE can help fight climate change; and (iii) analysing the potential and practicalities of such initiatives at scales where they could make a significant contribution to climate mitigation and adaptation. As an example, we draw on Pleistocene Park in northeastern Siberia. If successful, such an initiative may constitute a first-order NCS form of geoengineering.
2. Arctic terrestrial ecosystems: effects of defaunation and trophic downgrading
Pollen, plant macrofossils and ancient DNA (aDNA) suggest that during the Quaternary, ice-free Northern Hemisphere terrestrial environments north of 40° N were largely dominated by open landscapes that formed a grassland biome, referred to as the mammoth steppe, that sustained a guild of large herbivores including mammoth, woolly rhino, bison, horse, elk and reindeer [31]. Such a biome was not a uniform grass carpet, but instead a savannah-like mosaic, with trees and shrubs on poor soils and productive grasslands on loess and loamy soils [31]. Heterogeneity of plant functional types was probably topography-mediated [33], for example, through soil moisture [34]. The abundance of predators (wolf, cave lion) [31] also contributed to the heterogeneity of vegetation cover through the generation of landscapes of fear [35]. But how stable was this system, and how long did it last under different climatic conditions?
The identification of this biome in the fossil record is not straightforward. Fossil pollen-based reconstructions struggle to identify steppe and dry tundra because it is partly defined by the presence of grass species [36] that cannot be identified below the family level (Poaceae; [37]). Further, pollen is thought to be rare in heavily grazed grassland since there is much vegetative reproduction [31]. Macrofossil findings add clearer taxonomical information but are rarer [34]. A precise quantification of the dominance of steppe versus tundra in the Arctic in the late Quaternary is thus challenging. Notable insights have come from environmental aDNA [38], despite the still incomplete reference libraries of mammoth steppe species upon which these studies depend. Willerslev et al. [38] targeted the mammoth steppe in their pan-Arctic aDNA study of the last 50 ka, using a limited library of mammoth steppe plant taxa, plus nematode indicator species. Their study clearly shows that mammoth steppe dominated the Arctic landscape for much of this period. The suggestion that the mammoth steppe was dominated by forbs—with graminoids amounting to less than 20%—in contrast to pollen-based reconstructions [38] requires further research. The technique used in [38] favours the amplification of forb DNA [39] and the likely over-representation of forbs in soil DNA [40], although special attention was paid to include as many graminoids as possible in [38]. Beyond 50 ka, previous interglacials were probably characterized by more open vegetation than the Holocene [35,41].
A major ecological shift occurred in the LP/EH. The vast herds of large herbivores collapsed as humans moved into the area [24,31,42,43]. Wet and shrubby present tundra established in much of the terrestrial vegetated Arctic, with larch forest dominating the permafrost regions of northeastern-Eurasia [31]. Wetter soil conditions are inferred from increases in peatland, lakes and Sphagnum fossil pollen after 14 ka cal BP [36], as well as in sedimentary aDNA of aquatic taxa [38]. Nematode species employed as indicators of steppe versus tundra show a dominance of steppe indicators before and during the last glacial maximum (LGM), with tundra nematodes dominating after 10 ka cal BP [38]. Evidence thus suggests that the Holocene in the Arctic and high latitudes of the Northern Hemisphere is non-analogous to the Late Pleistocene, even when compared with previous interglacials [41]: its flora is different [38], as are its soils, and the guild of large megaherbivores is gone.
An important question with regard to Arctic MEE is to which extent the mammoth steppe was generated and controlled by large-herbivore herds. Zimov et al. [14,31] maintain that the mammoth steppe was ‘created and maintained’ by these animals. Several mechanisms have been identified to drive this top-down control, such as increased nutrient cycling, selection in favour of more palatable species, increased evapotranspiration associated with the dominance of grasses and forbs, together with the bark-stripping behaviour of many megaherbivores such as bison [14,35]. Current experimental evidence from herbivore enclosures confirms these mechanisms [21,35], and it is likely that such effects would be even more remarkable than current observations with extant fauna in the presence of the extinct megafauna [35]. Further evidence comes from the fossil record: herbivory represented a significant vegetation driver on glacial–interglacial cycles in northeastern Siberia during the Pliocene and early Pleistocene, potentially decoupling it from climate forcing [44]. This does not mean that the mammoth steppe was static: it was probably a highly dynamic biological system that offered higher resilience to external forcing given its very intense biotic component. Finally, (i) the facts that the inferred mammoth steppe climatic niche is within the current climatic envelope of northern Siberia, Alaska and Yukon, and (ii) the state of the Arctic Holocene as a moist/wet tundra-dominated interglacial [31] further agree with the hypothesis that the megafaunal extinctions of the LP/EH radically modified land cover and soil conditions in these regions, and that current terrestrial Arctic ecosystems might indeed be heavily affected by the ‘ghosts of nature's past’ [45].
Despite not all Arctic megafauna going extinct at the LP/EH, the mammoth steppe has not developed during the Holocene (except for small refugia; e.g. [46]), even in North America where the bison, but not the horse, survived. This would give weight to the climate hypothesis for ecosystem change following the LP/EH (e.g. [47]). However, Holocene diversity of herbivores (and thus dietary diversity) was much lower than during the Pleistocene, and most importantly, human hunter–gatherer societies have been present in the high latitudes of Eurasia and America throughout, adding pressure on species which have long generation times and slow growth rates, and thus probably keeping them at low densities [48]. No estimates of megaherbivore density in the Holocene come close to those estimated for the Pleistocene mammoth steppe [31].
Independently of what caused the Arctic megafaunal extinctions, the likely large consequences of the megaherbivore extinctions for terrestrial Arctic ecosystem dynamics set the stage for Arctic MEE. van der Wal [23] argues that large herbivores are able to change the state of vegetated Arctic terrestrial ecosystems by increasing their carrying capacity through favouring more productive grasslands through the mechanisms discussed above. Hence, the reintroduction of large herbivores in large enough densities might favour a phase transition in regions where climatic conditions could allow alternative vegetation states [35]: such regions have been estimated to cover approximately 42% of the vegetated Arctic [23]. The role of herbivory in decoupling vegetation from climatic conditions—both seen in present experiments [21,49] and in the palaeoecological record [44]—strongly suggests that it could confer increased resilience to Arctic terrestrial ecosystems to changes in climate.
3. Arctic lands and the global carbon budget
The climatic oscillations of the Quaternary implied large changes in ice sheet, sea ice and ice shelf extents in the Arctic [50]. Although their spatio-temporal dynamics are not yet fully resolved, especially for periods prior to the LGM, large parts of the high latitudes in the Northern Hemisphere have lacked land ice over several glacial/interglacial cycles, despite very low temperatures that promoted the existence and expansion of permafrost. In particular, low moisture during the late Pliocene and Pleistocene meant that the region stretching from eastern Siberia to Alaska (broadly corresponding to the area known as Beringia) remained largely free of glacial ice and its scarifying effects [51]. A thick (up to 50 m) sedimentary layer of fine-grained, ice-rich, aeolian-deposited sediment accumulated since Marine Isotope Stage 4 (71–60 thousand years ago) in the oldest places and ended abruptly at the Pleistocene/Holocene transition [26]. This layer includes large amounts of fossil material and organic matter, preserved in deep permafrost and known as Yedoma in Russia [52]. The Holocene development of peatlands has given rise to another, younger large high latitude carbon reservoir [53] (figure 1). The possibility that such large quantities of permafrost-stored carbon become vulnerable owing to rising temperatures and permafrost thaw—especially abrupt thaw in ice-rich regions [54]—is one of the main positive climate feedbacks identified in the large latitudes [55]. Lenton et al. [56] suggest that such an Arctic permafrost tipping point is already ‘active’.
The terrestrial permafrost regions of the Arctic are estimated to host around 1500 Pg C, about 40% of total terrestrial soil carbon [57]. Carbon stocks are particularly concentrated in the 1.2 million km2 of deep soils of the Yedoma regions of Siberia and Alaska (210–460 Pg C), and in Arctic river deltas (91 ± 39 Pg C), with the remaining stocks (1035 ± 150 Pg C) being spread across the broad surface permafrost pool (0–3 m, figure 1). There are likely to be additional poorly described deep carbon stocks (approx. 400 Pg C). In summary, the total known pool of terrestrial permafrost carbon in the northern permafrost zone is 1330–1580 Pg C [57]. These stocks are similar in magnitude to the total soil carbon stocks in all other (excluding Arctic and boreal zones) biomes (2050 Pg C), and greater than the total estimated carbon stock in live vegetation (approx. 1000 Pg C).
Incubation experiments and associated modelling efforts [57] suggest that 5–15% of permafrost carbon reserves would be emitted over the twenty-first century under a representative concentration pathway (RCP) 8.5 scenario (the pathway that best captures ‘business-as-usual’ greenhouse emissions pathways; [58]). Assuming 10% loss and a permafrost carbon stock of 1450 Pg C, this is equivalent to 145 Pg C, or an annual rate of 1.45 Pg C yr−1 if spread evenly over the twenty-first century. Local emission rates vary depending on many factors, including in situ carbon stocks, local warming and thawing rates, degree of waterlogging (which influences the relative importance of faster aerobic versus slower anaerobic processes) and soil carbon–nitrogen ratios. Averaged over the permafrost zone (excluding Antarctica, about 13–16 million km2, we take 14.5 million km2) and assumed a constant rate of release over the century, annual emission rates would be 100 gC m−2 yr−1. In high carbon stock zones such as the Yedoma region, these rates may be substantially higher. These rates are based on gradual warming and decomposition scenarios—there is increasing awareness and concern that nonlinear abrupt thaw events may approximately double the rate of release of CO2 to approximately 200 g C m−2 yr−1 [54]. Indeed, abrupt permafrost thaw in ice-rich cold permafrost regions has already been observed [59]. Moreover, it is estimated that associated emissions of CH4 will add an additional 25% (in mineral soil) to 45% (in organic soil) of radiative forcing effect over the next century [57]. On the other hand, increased woody vegetation growth in warming Arctic zones may act as a carbon sink, but also as an agent of radiative warming because of reduced surface albedo [60]. A number of studies suggest the net effect of increased woody vegetation in arctic and boreal latitudes is one of warming [61]. Estimating the opposing effects carbon and radiative forcing effects of woody vegetation increase is beyond the goals of this initial scoping, and for the present purpose, we neglect the effects of woody vegetation, while noting that the net effect of preventing woody vegetation spread (e.g. through megafauna) is likely to be one of cooling [61] (see the electronic supplementary material).
Allowing for scaling factors for abrupt thaw events and the CO2 equivalent effects of CH4 (taken to be uniform across all zones), we estimate total annual permafrost carbon emissions of 300 g C m−2 yr−1, or 4.35 Pg C yr−1 over the permafrost zone over the twenty-first century, increasing to 600 g C yr−1 over the Yedoma zones. While these calculations are crude and hence represent an initial approximation, they do suggest that the potential emissions from permafrost warming are larger than annual emissions estimated from current or projected land-use change (approx. 1–2 Pg C yr−1) but smaller than those from fossil fuel emissions (currently 9–10 Pg C yr−1) [62]. These numbers suggest that Arctic carbon emissions and associated land-use options to deal with them warrant at least as much attention as land use considerations in other biomes that currently receive much greater attention. Moreover, the Arctic is warming very rapidly, and Arctic ecosystems are already changing at a fast pace in response. A ‘do nothing’ or ‘leave as is’ approach to managing these ecosystems does not imply that they would remain unchanged in their previous late Holocene state.
4. Arctic megafaunal ecological engineering as a nature climate solution
(a). The mammoth steppe and climate change
The Pleistocene mammoth steppe interacted with the Earth System differently to the current wet tundra/forest–tundra. Key differences relevant to the thermal regime and carbon budget, and hence climate change mitigation, are:
-
(i)
grassland-dominated ecosystems have more reflective surfaces than shrub-dominated tundra and forest-tundra, both because of vegetation type and exposed snow cover, and thus enhanced albedo [31];
-
(ii)
snow trampling in winter by large herbivores as they move and forage for food implies a more compact snow layer and reduced surface insulation from very low winter air temperatures, enabling colder and deeper winter soil freezing [31];
-
(iii)
(ii) is enhanced by the lack of snow-trapping by shrubs and trees [63,64];
-
(iv)
increased evapotranspiration of graminoids promotes lower soil moisture and decreases waterlogging [23,31];
-
(v)
large-herbivore densities increase nutrient cycling and productivity by orders of magnitude, as the death-and-slow-composition nutrient pathway is overwhelmed by the herbivory-and-egestion pathway [23]; and
-
(vi)
the root structure of grasses and forbs—they both have deep, diffused roots, contrary to the shallow root systems of tundra shrubs and larch—increase soil carbon storage in the first 1 m [21].
Together, these mechanisms imply that mammoth steppe systems resulted in (i) colder annual soil temperature driven by enhanced winter freezing, (ii) enhanced albedo, (iii) enhanced carbon capture and storage given increased productivity and deeper roots, and (iv) reduced waterlogging. This implies an enhanced protection of the carbon-rich permafrost, with consequently reduced carbon emissions from permafrost thaw, increased carbon capture and an overall negative feedback to global warming. Indeed, modelling studies suggest that the LP/EH transition to tundra and forest-tundra altered albedo and may have contributed to regional and global warming at that time [15]. Considering that the shift from grasslands to moss-tundra was probably a consequence of reduced herbivore densities (see §2) that can be reversed [21,65], restoring a proxy of the mammoth steppe ecosystems could stabilize—or at least delay—Arctic permafrost carbon release [32]. Thus, an ecosystem-based solution based on the introduction of megafaunal herbivores as ecosystem engineers is worthy of evaluation.
(b). Megafaunal engineering guild
In order to consider the feasibility of enabling a phase shift from wet/moist tundra to a mammoth steppe-like ecosystem through enhanced herbivory, it is necessary to estimate a density and diversity of large herbivores able to enact and maintain it. In the following quantitative thought exercise, we will assume that a target state for each km2 of converted/reverted Arctic mammoth steppe is comparable to the population density inferred from Pleistocene Arctic sites: we term this a megafaunal engineering guild (MEG).
(i). Target megafaunal engineering guild density
Assuming that animal density in the mammoth steppe can be estimated from the number of bones found in the permafrost, an estimated average of 1 mammoth, 5 bison, 7.5 horses, 15 reindeer, 0.25 cave lions and 1 wolf, 1 km−2 roamed the area surrounding the site of Duvanniy Yar, an eroding bank on the lower reaches of the Kolyma River in northeastern Siberia that has been exhaustively studied (figure 2a). These estimates are based on length of the soil exposure, thickness of Yedoma, rate of erosion of the soil exposure, density of bones m−3 of soil column, the time period over which the soil was accumulated (42 000 ka to 13 000 ka in Duvanniy Yar), and the average age of the animals at the time of death. If we account for the average weight of adults for each species, the system sustained approximately 10.5 Mg of herbivores km−2 [31]. We can expect heterogeneity in the above density values across the vast expanses of the Pleistocene mammoth steppe. However, commercial tusk collection data from Yedoma in different regions suggest similar numbers for mammoth [31], and multi-taxon herbivory values in Mamontovy Khayata—another intensively exposure east of the Lena River Delta, as well as in the New Siberian Islands, are roughly similar to those in Duvanniy Yar [66]. In fact, data from multiple sources suggest herbivore biomass values ranging between 8.8 and 10.5 Mg of herbivores km−2, with even greater values in Great Britain in last interglacial [35], suggesting that herbivore density values might have been even higher in past interglacials, because improved climatic conditions would translate in higher productivity. These values are within the range of present-day African savannah game reserves, 0.9–19.1 Mg of herbivores km−2 [35].
Figure 2.
(a) Ice-rich Yedoma permafrost exposure in Duvanniy Yar, an eroding bank on the lower reaches of the Kolyma River in northeastern Siberia. Note the small Dahurian larch—Larix gmelinii (Ruprecht) Kuzeneva 1920—trees on the top of the ridge, heavily disturbed by permafrost dynamics, the rich syngenetic ice-wedges and the lush grassy vegetation on top of nutrient-rich detached soil fragments; (b) aerial view of a section of Pleistocene Park. The thick black line marks the outer fence of the area; (c) Yakutian horses in Pleistocene Park; (d) European bison browsing on Alnus viridis (Chaix.) D.C. shrubs; (e) herd of yaks grazing on a drained lake in Pleistocene Park.
(ii). Megafaunal engineering guild composition
Because mammoths and cave lions are not available in the near future, and reindeer and wolf are currently present in the Arctic, we focus our thought experiment on the potential introduction of bison and horses as the likely essential megafaunal engineers: bison to open up woody vegetation and horses to maintain grasslands (figure 2c,d). Apart from being present in the tundra already, reindeer/caribou are known to favour land cover transitions to grasslands [21]; however, these shifts occur only at very high densities where these animals have been managed with fences in small areas (e.g. in reindeer pens) or across migration routes along linear features in the landscape [67]. In this respect, they are not considered an ecosystem engineer of the same calibre as bison or horse. Both horse and bison were abundant in the high latitudes during the Pleistocene and currently exist at high latitudes (e.g. bison in Alaska, horse in Yakutia and Alaska). American bison diet consists mostly of sedges and grasses, with a seasonal contribution of woody plants—mainly deciduous shrubs such as willow in summer [68]. This alone would favour the conversion to a savannah-type system of many deciduous-shrub-dominated tundra regions. The closely related European bison is known to strip bark and open up closed forest [3,35], showing an equal preference for oak (deciduous broadleaf) and spruce (conifer) [69,70]. Metabarcoding studies highlight the varied nature of the bison diet, highly rich in woody species (in some cases 48.6 to 51.9% of their summer diet are trees and shrubs; [71]), suggesting that the traditional understanding of bison diet might be limited by the current limited distribution of habitats it currently inhabits. This is in agreement with palaeoecological evidence, which gives an important role to bison (both American and Eurasian) as a first-order ecological engineer opening the landscape through its effects on woody vegetation [35]. Indeed, an examination of American bison palaeo-diets across its former continental-wide distribution through dental wear patterns showed a much wider dietary range and evidence of more activity on woody material than at present [72]. Horses are known to be strict grazers able to maintain grazing lawns but requiring other guilds to convert a woody landcover into a grass-dominated one (e.g. [73]). We did not include musk oxen in the MEG scoping experiment because—although they are known to be important extant megafaunal ecosystem engineers—they were not found to be abundant across the Yedoma region during the Late Pleistocene [31], and they exist in low numbers at present and thus are not readily available for an Arctic MEE initiative.
It is important to consider whether mammoths are necessary for creating and maintaining the mammoth steppe. Mammoth has been estimated to directly consume a minor portion of the grassland productivity (<20% in Duvanniy Yar and Wrangel Island; [31]). Owing to this, it is hypothesized that although it most likely acted as a keystone species limiting the expansion of trees, this function was most important in the southern parts of the mammoth steppe, whereas in Arctic regions ungulates were probably able to maintain the ‘mammoth’ steppe even in the absence of mammoths [31].
(iii). Megafaunal engineering guild growth rate
Little information is available on growth rates of translocated herbivores. The European Wildlife Bank (https://rewildingeurope.com/european-wildlife-bank/) uses a general growth rate for all large herbivores (irrespective of type) of 25% a year. However, this figure is an upper estimate based on ideal release conditions. Following discussions with Rewilding Europe experts and review of efforts to re-establish bison in Romania, an annual growth rate 10% per year is probably more realistic. This ‘first estimate’ growth rate could be refined in a hypothetical experimental phase and finessed to account for the effects of acclimatization and herd size.
(iv). Megafaunal engineering guild growth model
We next estimate the potential dynamics of rate of megafaunal introduction and expansion, with the aim of evaluating how feasible it is to generate an ecosystem shift that is (i) potentially economically viable in terms of carbon benefits and (ii) of sufficient scale to play a significant role in climate change mitigation.
First, we assume that the required animal density to engender and maintain Arctic grassland is 1 MEG km−2 (i.e. estimated Late Pleistocene animal densities: 5 bison and 7.5 horses km−2). For the purposes of the model exercise, we assume that the herds are in place and the transition has occurred at t = 0, or occurs fast, and hence the effect on permafrost starts immediately (see §4e and the electronic supplementary material for more on this assumption):
where N is the number of MEG units at time t, I(t) is the annual rate of new animal introduction (in MEG units) and b is the annual intrinsic population growth rate (10% yr−1; see above).
If new mammoth steppe successfully prevents warming-related carbon loss from tundra, it generates an annual carbon benefit of c Mg C km−2 yr−1. Converted mammoth steppe keeps yielding carbon benefits (avoided annual C emissions) every year after conversion, so the carbon benefit needs to be integrated over time. Hence, the carbon benefit of megafaunal introduction is C. Assuming the carbon benefit c = 300 Mg km−2 yr−1 (see §3), the net carbon benefit is therefore
We explore numerical solutions of this equation for two scenarios: (i) a constant animal introduction rate of 10 MEG yr−1; (ii) a ramping up introduction rate starting at 5 MEG yr−1 in the first year and increasing by 5 MEG yr−1 each subsequent year. Under the first scenario, after 30 years, an area of 3100 km2 would be converted to grassland, resulting in 1.8 Mt of avoided carbon emissions from avoided/delayed thawing permafrost. Assuming a carbon price of $5 MgC−1 (see §4e for details on the carbon price estimate), this results in US$9 million of carbon income. Under the second scenario, the area converted to grassland after 30 years would be 8300 km2, resulting in 4.7 Mt of avoided carbon emissions, and US$23.5 million in carbon income. Hence, it is apparent that megafauna-based conversion to mammoth steppe may yield substantial income in a carbon market. However, implementing such projects to a scale that is significant for the global climate would be a challenge. Conversion of 1.5 million km2 of Arctic tundra (10% of the Arctic permafrost zone) in 30 years would result in 850 Mt C of avoided carbon emissions, which would be significant in terms of global climate, but would require an introduction rate of 10 000 MEGs yr−1, which is unrealistic. Hence the challenge of scalability is a major one.
(c). The Pleistocene Park experiment
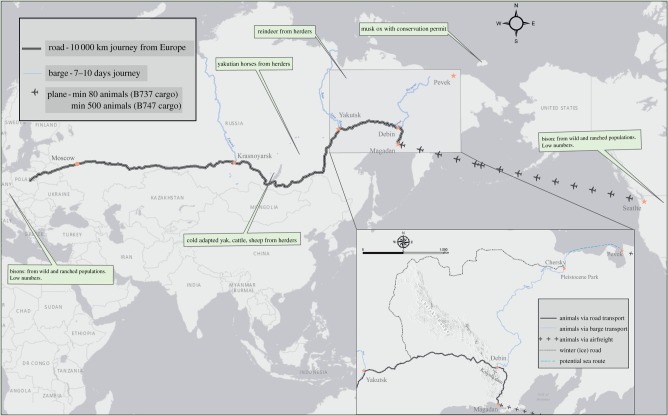
The Pleistocene Park experiment in northeast Siberia (figure 2b) offers information on the practical feasibility of large-scale MEE in the Arctic. Sergey Zimov [14] first proposed the idea that reconstructed grassland ecosystems could prevent permafrost from thawing and thereby mitigate climate warming. Pleistocene Park (68°30′48″ N 161°31′32″ E) was first established in 1996 with an initial 40 ha fenced area, now nested within a new 100 ha fenced area established in 2018, and surrounded by a still larger 20 km2 fenced area established in 2005/2006—the total area owned being 160 km2. The park offers a model to design experiments to test hypotheses along with practical information that can be used to outline the costs and logistics of large-scale arctic MEE.
The mammoth steppe biome was the product of interactions between vegetation and an assembly of large-bodied herbivores. The broad-range of body sizes and diet preferences had a strong effect on woody and other vegetation [35]. Different large-herbivore species have different feeding strategies that generate interspecific benefits through differential impacts on vegetation. Some permafrost dynamics are seen as a temporary practical advantage for the intended ecosystem phase shift: ice-wedge degradation leads to surface subsidence, disturbing extant ecosystems and exposing bare soils that are rapidly colonized by grasses. This already ongoing process emits large quantities of carbon but facilitates the introduction a guild of large herbivores which may maintain the new open landscape, prevent the regrowth of woody vegetation and stabilize permafrost, hence delaying or stopping further emissions. Likewise, drainage of standing water bodies owing to permafrost degradation [74] allows for new sites where grass initially grows and hence where large herbivores can be easily introduced. Indeed, a lake was artificially drained in Pleistocene Park to this effect (figure 2e). Mammoths apart, the complementary interaction between equids and bovids is considered a key driver of shifts to more open grassland landscapes. The ability of bovids (ruminants) to digest cellulose present in leaves, bark and fibrous grasses opens and suppresses woody vegetation, whereas equids (non-ruminants) nibble less fibrous vegetation and promote short grass growth. Together, bovids and equids create and maintain ‘grazing lawns’ that are expected to maximize the albedo effect (see §4b). Experience in the Pleistocene Park also suggests that horses are better able than bovids to plough and trample through the snow to expose winter forage.
The primary focus of Pleistocene Park is to establish an ecosystem where a variety of herbivore species (including the horse–bison grazing interaction in association with feeding strategies of extant reindeer and elk) drive vegetation dynamics and foster a diverse and productive grassland system, with the eventual incorporation of predators and the generation of heterogeneity through landscapes of fear. Bison are preferred over cold-adapted cattle (e.g. Kalmyk cattle) because, as intermediate grazers [75], they switch between a woody plant and fibrous grass diet and have the build, power and behavioural traits to create clearings. Horse and bison populations disappeared from northern Siberia during the mid-Holocene. However, between the thirteenth and seventeenth centuries, horse-riding Yakut people moved into the region and their horses rapidly evolved anatomical, morphological and physical adaptations to thrive in the open year-round and grazing on the vegetation below deep snow for seven to eight months [76].
The park has struggled to establish significant numbers of animals owing to the cost and logistical challenges associated with sourcing and translocation, and with keeping newly arrived animals alive over winter as a sufficiently productive grassland ecosystem has taken time to generate. As of October 2018, Pleistocene Park had 27 Yakutian horses, 1 bison, 4 musk ox (all male), 10 elk, 25 reindeer, 20 sheep, 9 yak and 20 Kalmyk cattle. A further 12 American bison were translocated from Denmark in June 2019 at a cost of $110 000. The difficulty and cost of sourcing bison prompted the Park to experiment with cold-adapted domestic species from the Lake Baikal region (4000 km to the southwest, figure 3), but initial trials have proved disappointing as the animals appear to avoid woody vegetation. To speed up the transition to grassland, serious consideration is being given to the option of seeding and fertilizing areas to create ‘founder grasslands’ that would act as ‘soft release’ areas for newly translocated animals. The grass seed pool is poor in present and undisturbed soils, where establishment may involve ‘pairing’ a MEG with a grasslands seed mix, which is important when considering expansion and scalability.
Figure 3.
Location of Pleistocene Park (inset) and the routes for transporting large herbivores from potential sources areas. See text for details. (Online version in colour.)
Owing to its remoteness from large-herbivore populations, Pleistocene Park has yet to establish herds of sufficient density to rigorously test the megafaunal phase-shift hypothesis. Nonetheless, progress to date has generated important knowledge, insight and evidence relevant to an assessment of the feasibility of establishing grasslands at scale. Initial monitoring data with belowground temperature sensors have shown a maximum difference of 14°C in a heavily grazed fenced area versus a control area (−24°C versus −10°C at 25 cm, −20°C versus −9°C at 50 cm, −16°C versus −8°C at 90 cm; March 2018), but more importantly, mean soil annual temperatures 2.2°C cooler in grazed areas, as well as increased carbon sequestration in the first 1 m of soil in grazed areas [77]. Furthermore, Pleistocene Park has developed practical knowledge on the logistics of translocating animals, and generated creative and grounded insight on herbivore ecology and future strategies for scaling up. Lastly, the fact that one family in a remote location has successfully overcome major translocation challenges with their own resources only suggests that an organized and properly financed effort could achieve major impact.
(d). Strategy for creating a rolling frontier
Large-scale MEE would require an experimental, design and socialization phase followed by an implementation phase. We envisage that the first phase would comprise three components that could be completed within 10 years: (i) establishment of a trans-Arctic network of experimental reserves. Based on Pleistocene Park experience, we estimate that rigorous hypothesis-testing would require enclosure experiments involving around 1000 bison and horses; (ii) modelling and planning a ‘rolling frontier’ that integrates biophysical, ecological, cultural, administrative and logistical considerations in its design and generates authoritative carbon budget predictions; and (iii) policy and public dialogues to generate interest, acceptability, buy-in and the new policy mechanisms that an initiative of this scale and novelty would require (see §4f).
The design of a rolling frontier could take inspiration from the migration and settlement strategy of the early Sakha Turkic people to northern Siberia since at least the last 500 years: they exploited the natural thermokarst dynamics, settling in thermokarst depressions or drained thaw lake basins—known as alas—often draining the lakes themselves, where their horses and cattle maintained grass growth to form pastures [78]. Ongoing permafrost thawing accelerates thermokarst processes: as mentioned before, disturbed soils owing to ice wedge degradation and drained water bodies offer prime opportunity areas to target within this MEE, because they facilitate the rapid establishment and expansion of grasslands. Moreover, integrating the affordance of technology into the design could further accelerate the expansion of the megafauna frontier and also make it a more attractive investment. For instance, integrated information system (ISS) designs involving remote sensing, satellite tracking of animals, carbon flux sensor networks and climate modes could enable regional-scale environmental monitoring. In addition, ISS-generated change-of-state metrics could interface with blockchain platforms to generate verified carbon credits and/or action smart contracts for payments associated with proof of action/impact [79]. This would dramatically reduce operational transaction costs thereby increasing the prospect of investment returns: a bison blockchain pilot is already underway in Romania and planetary satellite analytics are undergoing a step-change in terms of resolution, power and cost (https://rewildingeurope.com/).
(e). First-cut business plan for Arctic megafaunal ecological engineering
We have calculated an approximate cost of the bison, horse and reindeer component of an MEG. We have included reindeer in this costing exercise because they are available for purchase, they have a distinct ecological niche and function, and their inclusion would increase the speed of the experimental and establishment phases, although because they are present in most Arctic lands, their introduction might not be required in some cases. We estimate an experimental 10-yr implementation cost of 1 MEG to be US$383 000. This figure includes costs on animal purchase (5 bison, 7.5 Yakutian horses, 15 reindeer), translocation (transport, permit fees, food), introduction (fencing and other infrastructure, winter fodder, husbandry for 10 years). The figure is derived from a combination of local price knowledge of horse and reindeer and experience of translocating large herbivores to the Pleistocene Park, 2018 US bison auction prices and a rough quote for airfreight bison from Chicago to Magadan from a leading livestock transport firm. The cost assumes that bison will be sourced from North American ranches in shipments of 120 (= sole freight charter) which brings down costs, but would require minimum investments of 24 MEGs (i.e. US$9.2 million). It does not take into account the likely increases in prices that a high demand for these animals might create. We assume that over time (10–25 years), and as herds build up, prices will drop and compensate for higher initial purchasing costs. This is a rough estimate figure with scope for refinement and financial modelling.
The cost of establishing an experimental Arctic MEE area (comprised of approximately 1000 bison + horses, approximately 80 MEG—see §4d) would be US$30.5 million. Scientific infrastructure, research personnel and area management would cost in the order US$0.75 million per year, such that each 10 yr experimental area would cost US$38 million. An experimental MEE area would generate key empirical knowledge on the effects of MEE on the ecological and Earth system processes discussed above, but it would also be designed to produce practical knowledge on logistics, and on how to create an efficient rolling frontier strategy appealing to investors.
These areas might generate cost recovery in terms of carbon sequestration. In the following calculation, an estimate of the market price of US$5 MgC−1 is used. This figure is a compromise between the 2018 US$10 MgC−1 midpoint value of carbon pricing in the compliance market (World Bank [80]) and the 2017 average of US$3 MgC−1 in voluntary market initiatives [81]. Carbon prices are likely to rise in the near future as more countries, sub-national governments and corporations commit to ambitious net carbon emissions goals as part of their Paris climate goals, and NCS are incorporated within those targets. If an Arctic MEE was established in Russia, generated carbon credits would probably to be traded on the voluntary market because Russia lacks a carbon tax and hence a pricing mechanism. It is currently a buyers' market, where the value of credits depends on factors beyond carbon, such as country of origin and, importantly, the story of their generation. Using the estimated carbon benefit of 300 Mg C km−2 yr−1 MEG−1, once an experimental area reaches full impact, it could generate up to 24 000 Mg C yr−1 and US$120 000 yr−1 in carbon revenues. This would represent a 0.32% (0.4% without research cost) annual return—i.e. financial benefits expressed as a proportion of the invested capital—on the investment after 8–10 years (the implementation phase over which the herd would establish and change vegetation cover substantially to affect permafrost).
It is perhaps unfair to judge the appeal for the investment of Pleistocene Arctic MEE in the light of the experimental phase annual returns. Once this phase is completed, and assuming that (i) MEGs establish and maintain a phase shift to a grassland-herbivore system, (ii) source herds develop, and (iii) an efficient roll-out strategy is designed, a business case for Arctic MEE could look promising. Annual revenues for Arctic MEE initiatives post-experimental phase would increase in proportion to their cost-efficiency in relation to the experimental MEE areas (i.e. one-third of the initial experimental cost would translate in revenues greater than 1% yr−1 after 8–10 years). Returns could be significantly increased if/when Arctic countries introduce a carbon tax and pricing mechanism. While we recognize that these figures are approximate and contain many assumptions (see the electronic supplementary material for further discussion on this), they do enable us to generate an order of magnitude figure for MEE in the Arctic under the framework of the global carbon budget. This is US$114 million for feasibility testing if we consider three experimental Arctic MEE areas over a period of 10 years.
(f). Institutional friction
Even with available funds to test, design and develop Arctic MEE, there would still be significant institutional challenges to overcome. Institutions are assemblies of rules, norms, worldviews, discourses and practice structures that shape (constrain or enable) the possible in the social and political realm [82]. Institutions order society and are embedded in the political economy. This creates powerful forces to retain the status quo: deviance from the established way of doing things is often perceived as unsettling and undesirable by influential publics, both political and social. From an institutional perspective, the concept of large-scale megafaunal engineering outlined here is radical in almost every dimension. As a result, its development and implementation are likely to generate considerable institutional ‘friction’ at all levels. In the geo-political realm, Arctic regions such as Russia, Alaska and the Canadian Yukon would be providing a global public good (avoiding large carbon emissions), which would add a major new dimension to international relations. In the bureaucratic realm, the bison and horses would become a new category of wildlife that is neither fully domestic nor wild. Veterinary and livestock institutions generate significant power and rents from managing disease in domestic herds through animal movement controls. Negotiation of a transnational ‘deal’ with these institutions would be necessary to support the movement of animals at the scale required. In the realm of intellectual and popular discourse, the idea of transforming large portions of the Arctic with livestock is likely to prompt comparisons with the Lysenkoism agriculture of Soviet era Stalinism that produced famines killing millions [83] and claims that it is a neo-colonial project that frames the Arctic as a free un-populated resource for others to appropriate. Nevertheless, we anticipate that societies at large will probably be more open to direct interventions in nature (e.g. geoengineering) in order to adapt to or mitigate climate change in the near future as climate change impacts increase.
5. Discussion and conclusion
In this study, we explored if Pleistocene-inspired Arctic MEE is viable and could be scaled up to play a significant role in global climate change mitigation. Whereas we found it to be reasonably viable economically, our results suggest that ramping it up to a scale likely to be a significant contribution to global climate change mitigation on its own would be a challenge, because the numbers of large herbivores required for such an undertaking do not exist. Nevertheless, such initiatives might avoid high carbon emissions where and when implemented, especially over Yedoma permafrost soils.
Our quantitative scoping exercise aimed at providing, to our knowledge for the first time, a rough estimate of the feasibility of such an approach. It is based on a number of assumptions, which are discussed at length in the electronic supplementary material, and which comprise (i) determining an effective megafaunal density and guild; (ii) the immediate effect of a MEG on the landscape; (iii) effectiveness of the mammoth steppe on thermal, nutrient, and carbon budget; (iv) the MEG growth rates; (v) the size of the experimental units; (vi) the role of predators; and (vii) the price of carbon. Together, they make a compelling case for a systematic monitoring and the implementation of a plan as described in §§4d–4f. Our essential assumption is that Arctic MEE is effective to protect permafrost and delay its thaw. There is ample palaeoecological and empirical evidence to assume that the sequence of processes and the net effect of Arctic MEE discussed in this study are plausible, although we did not aim at testing them but at analysing the feasibility of such an action assuming that they work. All other assumptions follow from this and have been conservatively applied according to values obtained from the literature.
Ongoing climate change-caused rapid permafrost degradation is creating thermokarst lakes that enhance carbon emissions, but is also enhancing the drainage of water bodies through catastrophic drainage (e.g. [74]) and land subsidence that causes disturbance. This results in the exposure of fertile soils and the creation of grazing landscapes over large areas that could be readily used in Pleistocene-inspired MEE actions taking past Sakha horse and cattle herders' land use techniques as an inspiration [78]. Our approach did not incorporate the increase in carbon capture and storage by the more productive, deeper-rooted grassland community [77] and thus underestimated the carbon balance by neglecting increased soil carbon capture.
Our first-cut business case estimate assumed that governments and groups with title or claims over land would choose to sponsor such large-scale landscape changes (i.e. it did not cost land): many ongoing MEE initiatives work this way (https://rewildingeurope.com/). Because our economic feasibility plan only focused on the carbon market, it did not include other co-benefits that might arise such as employment, new tourism economies, carbon-negative wild meat and other carbon-negative products. This could potentially enhance substitution effects—e.g. reduced demand for beef and thus reduced pressure on forested areas in tropical regions, although this later point would require a thorough consideration of the socio-economic consequences of geographically shifting economic activities. In any case, the finding that this initiative is viable offers an opportunity but also potential friction that might need being negotiated at the local scale.
Considering safeguards to avoid perverse outcomes for existing Arctic biodiversity is pertinent given the scale at which such an initiative could take place. In this respect, approximately 42% of the pan-Arctic tundra region has been deemed susceptible to such changes [23], and a much smaller extent than this is estimated to be within reach of such ecological engineering within the next decades given scalability issues (this study). Even the regions where such transition would occur would be heterogeneous, with a diversity of vegetation cover according to the topography as it probably was in the Pleistocene [31]. Moreover, no Arctic or alpine plant species with a fossil record has become extinct in the Quaternary [84], the only well-documented Pleistocene extinctions in the Arctic being those of the megafauna. Hence, even though the shift would represent a large re-balance of species abundances regionally, there are no signs that it could carry over increased extinction risks for current Arctic biota.
In summary, our analysis suggests that land-use change in the Arctic has similar or greater implications for climate change and the carbon cycle than other regions at lower latitudes where land use issues receive much more attention. Irrespective of what action we take, terrestrial systems in high latitudes will influence the character of global climate change and, given current rates of warming, will not remain unchanged under a ‘do nothing’ approach. Although there is a debate on the role of human agency in LP/EH extinctions (e.g. [85]), and thus in the consideration of current Arctic terrestrial systems as defaunated or trophically downgraded, the weight of evidence is in favour of a decisive role of human over-hunting in the demise of many megafaunal species. Regardless of the cause, there is ample evidence of the role of large herbivores on the region's ecological and biogeochemical processes. If this initiative is to work, a large experimental phase would need to be implemented for the generation of empirical data, and logistics and practical knowledge. We invite expertise from all disciplines—e.g. livestock breeding, financial modelling, ecology, social sciences—to collaborate in determining the feasibility of such a mammoth task.
Supplementary Material
Data accessibility
All data used in this study are presented in the main manuscript.
Authors' contributions
M.M.-F., P.J., N.Z. and Y.M. designed the research presented and provided the data required. M.M.-F., P.J., Y.M. designed and wrote the manuscript. M.M.-F. led the manuscript writing and editing.
Competing interests
N.Z. received funds for implementing and managing the Pleistocene Park.
Funding
This work stemmed from a University of Oxford John Fell Fund Award (reference: 171/307) which enabled a visit by M.M.-F., P.J. and Y.M. to Pleistocene Park. A natural Environment Research Council Independant Research Fellowship (NE/L011859/1) funded M.M.-F.'s contribution. Y.M. is supported by the Jackson Foundation.
References
- 1.IPCC. 2013. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, 1535 p Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO. 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 ( 10.1126/science.1246752) [DOI] [PubMed] [Google Scholar]
- 3.Griscom BW, et al. 2017. Natural climate solutions. Proc. Natl Acad. Sci. USA 114, 11 645–11 650. ( 10.1073/pnas.1710465114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seddon N, Turner B, Berry P, Chausson A, Girardin CAJ. 2019. Grounding nature-based climate solutions in sound biodiversity science. Nat. Clim. Change 9, 84–87. ( 10.1038/s41558-019-0405-0) [DOI] [Google Scholar]
- 5.Donlan CJ, et al. 2006. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. Am. Nat. 168, 660–681. ( 10.1086/508027) [DOI] [PubMed] [Google Scholar]
- 6.Torres A, et al. 2018. Measuring rewilding progress. Phil. Trans. R. Soc. B 373, 20170433 ( 10.1098/rstb.2017.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svenning JC, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhi Y, Doughty CE, Galetti M, Smith FA, Svenning J-C, Terborgh JW. 2016. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl Acad. Sci. USA 113, 838–846. ( 10.1073/pnas.1502540113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith FA, Doughty CE, Malhi Y, Svenning J-C, Terborgh J. 2016. Megafauna in the Earth system. Ecography 39, 99–108. ( 10.1111/ecog.02156) [DOI] [Google Scholar]
- 10.Berzaghi F, Longo M, Ciais P, Blake S, Bretagnolle F, Vieira S, Scaranello M, Scarascia-Mugnozza G, Doughty CE. 2019. Carbon stocks in central African forests enhanced by elephant disturbance. Nat. Geosci. 12, 725–729. ( 10.1038/s41561-019-0395-6) [DOI] [Google Scholar]
- 11.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. 2009. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 326, 1100–1103. ( 10.1126/science.1179504) [DOI] [PubMed] [Google Scholar]
- 12.Doughty CE, Wolf A, Malhi Y. 2013. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 6, 761–764. ( 10.1038/ngeo1895) [DOI] [Google Scholar]
- 13.Pires MM, Guimarães PR, Galetti M, Jordano P. 2017. Pleistocene megafaunal extinctions and the functional loss of long-distance seed-dispersal services. Ecography 41, 153–163. ( 10.1111/ecog.03163) [DOI] [Google Scholar]
- 14.Zimov SA, Chuprynin VI, Oreshko AP, Chapin Iii FS, Reynolds JF, Chapin MC. 1995. Steppe-tundra transition: a herbivore-driven biome shift at the end of the Pleistocene. Am. Nat. 146, 765–794. ( 10.1086/285824) [DOI] [Google Scholar]
- 15.Doughty CE, Wolf A, Field CB. 2010. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: the first human-induced global warming? Geophys. Res. Lett. 37, L15703 ( 10.1029/2010gl043985) [DOI] [Google Scholar]
- 16.Perino A, et al. 2019. Rewilding complex ecosystems. Science 364, eaav5570 ( 10.1126/science.aav5570) [DOI] [PubMed] [Google Scholar]
- 17.Jepson P. 2018. Recoverable Earth: a twenty-first century environmental narrative. Ambio 48, 123–130. ( 10.1007/s13280-018-1065-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jepson P, Schepars F, Helmer W. 2018. Governing with nature: a European perspective on putting rewilding principles into practice. Phil. Trans. R. Soc. B 373, 20170434 ( 10.1098/rstb.2017.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorimer J, Sandom C, Jepson P, Doughty C, Barua M, Kirby KJ. 2015. Rewilding: science, practice, and politics. Ann. Rev. Environ. Resour. 40, 39–62. ( 10.1146/annurev-environ-102014-021406) [DOI] [Google Scholar]
- 20.Root-Bernstein M, Gooden J, Boyes A. 2018. Rewilding in practice: projects and policy. Geoforum 97, 292–304. ( 10.1016/j.geoforum.2018.09.017) [DOI] [Google Scholar]
- 21.Olofsson J, Post E. 2018. Effects of large herbivores on tundra vegetation in a changing climate, and implications for rewilding. Phil. Trans. R. Soc. B 373, 20170437 ( 10.1098/rstb.2017.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bråthen KA, Ravolainen VT, Stien A, Tveraa T, Ims RA. 2017. Rangifer management controls a climate-sensitive tundra state transition. Ecol. Appl. 27, 2416–2427. ( 10.1002/eap.1618) [DOI] [PubMed] [Google Scholar]
- 23.van der Wal R. 2006. Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos 114, 177–186. ( 10.1111/j.2006.0030-1299.14264.x) [DOI] [Google Scholar]
- 24.Sandom C, Faurby S, Sandel B, Svenning J-C. 2014. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hugelius G, Tarnocai C, Broll G, Canadell JG, Kuhry P, Swanson DK. 2013. The Northern Circumpolar Soil Carbon Database: spatially distributed datasets of soil coverage and soil carbon storage in the northern permafrost regions. Earth Syst. Sci. Data 5, 3–13. ( 10.5194/essd-5-3-2013). [DOI] [Google Scholar]
- 26.Strauss J, et al. 2017. Deep Yedoma permafrost: a synthesis of depositional characteristics and carbon vulnerability. Earth Sci. Rev. 172, 75–86. ( 10.1016/j.earscirev.2017.07.007) [DOI] [Google Scholar]
- 27.Walker DA, et al. 2005. The circumpolar Arctic vegetation map. J. Veg. Sci. 16, 267–282. ( 10.1111/j.1654-1103.2005.tb02365.x) [DOI] [Google Scholar]
- 28.Virtanen R, et al. 2016. Where do the treeless tundra areas of northern highlands fit in the global biome system: toward an ecologically natural subdivision of the tundra biome. Ecol. Evol. 6, 143–158. ( 10.1002/ece3.1837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranson KJ, Montesan PM, Nelson R. 2014 doi: 10.3334/ORNLDAAC/1218. Tree canopy cover for the circumpolar taiga-tundra ecotone: 2000–2005. Data set. Available from: http://daac.ornl.gov , from ORNL DAAC, Oak Ridge, TN. ( ) [DOI] [Google Scholar]
- 30.Huang J, et al. 2017. Recently amplified arctic warming has contributed to a continual global warming trend. Nat. Clim. Change 7, 875–879. ( 10.1038/s41558-017-0009-5) [DOI] [Google Scholar]
- 31.Zimov SA, Zimov NS, Tikhonov AN, Chapin FS. 2012. Mammoth steppe: a high-productivity phenomenon. Quat. Sci. Rev. 57, 26–45. ( 10.1016/j.quascirev.2012.10.005) [DOI] [Google Scholar]
- 32.Wolf A. 2008. The big thaw. Stanford Magazine, pp. 63–69 Stanford, CA: Stanford University. [Google Scholar]
- 33.Edwards ME, et al. 2000. Pollen-based biomes for Beringia 18,000, 6000 and 0 14C yr bp. J. Biogeogr. 27, 521–554. ( 10.1046/j.1365-2699.2000.00426.x) [DOI] [Google Scholar]
- 34.Goetcheus VG, Birks HH. 2001. Full-glacial upland tundra vegetation preserved under tephra in the Beringia National Park, Seward Peninsula, Alaska. Quat. Sci. Rev. 20, 135–147. ( 10.1016/S0277-3791(00)00127-X) [DOI] [Google Scholar]
- 35.Bakker ES, Gill JL, Johnson CN, Vera FWM, Sandom CJ, Asner GP, Svenning J-C. 2016. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl Acad. Sci. USA 113, 847–855. ( 10.1073/pnas.1502545112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binney H, et al. 2017. Vegetation of Eurasia from the last glacial maximum to present: key biogeographic patterns. Quat. Sci. Rev. 157, 80–97. ( 10.1016/j.quascirev.2016.11.022) [DOI] [Google Scholar]
- 37.Swanson DK. 2006. Biogeographical evidence for the grass (Poaceae) species of Pleistocene Beringian lowlands. Arctic 59, 191–200. [Google Scholar]
- 38.Willerslev E, et al. 2014. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506, 47–51. ( 10.1038/nature12921) [DOI] [PubMed] [Google Scholar]
- 39.Nichols RV, Vollmers C, Newsom LA, Wang Y, Heintzman PD, Leighton M, Green RE, Shapiro B. 2018. Minimizing polymerase biases in metabarcoding. Mol. Ecol. Resour. 18, 927–939. ( 10.1111/1755-0998.12895) [DOI] [PubMed] [Google Scholar]
- 40.Yoccoz NG, et al. 2012. DNA from soil mirrors plant taxonomic and growth form diversity. Mol. Ecol. 21, 3647–3655. ( 10.1111/j.1365-294X.2012.05545.x) [DOI] [PubMed] [Google Scholar]
- 41.Sandom CJ, Ejrnæs R, Hansen MDD, Svenning J-C. 2014. High herbivore density associated with vegetation diversity in interglacial ecosystems. Proc. Natl Acad. Sci. USA 111, 4162–4167. ( 10.1073/pnas.1311014111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin PS. 1973. The discovery of America. Science 179, 969–974. ( 10.1126/science.179.4077.969) [DOI] [PubMed] [Google Scholar]
- 43.Guthrie RD. 2006. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209. ( 10.1038/nature04604) [DOI] [PubMed] [Google Scholar]
- 44.Herzschuh U, Birks HJB, Laepple T, Andreev A, Melles M, Brigham-Grette J. 2016. Glacial legacies on interglacial vegetation at the Pliocene-Pleistocene transition in NE Asia. Nat. Commun. 7, 11967 ( 10.1038/ncomms11967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silliman BR, Hughes BB, Gaskins LC, He Q, Tinker MT, Read A, Nifong J, Stepp R. 2018. Are the ghosts of nature's past haunting ecology today? Curr. Biol. 28, R532–R537. ( 10.1016/j.cub.2018.04.002) [DOI] [PubMed] [Google Scholar]
- 46.Pavelková Řičánková V, Robovský J, Riegert J. 2014. Ecological structure of recent and last glacial mammalian faunas in Northern Eurasia: the case of Altai-Sayan refugium. PLoS ONE 9, e85056 ( 10.1371/journal.pone.0085056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guthrie RD. 2001. Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammal tooth pits, buckles, and inside-out Beringia. Quat. Sci. Rev. 20, 549–574. ( 10.1016/s0277-3791(00)00099-8) [DOI] [Google Scholar]
- 48.Surovell TA, Pelton SR, Anderson-Sprecher R, Myers AD. 2016. Test of Martin's overkill hypothesis using radiocarbon dates on extinct megafauna. Proc. Natl Acad. Sci. USA 113, 886–891. ( 10.1073/pnas.1504020112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693. ( 10.1111/j.1365-2486.2009.01935.x) [DOI] [Google Scholar]
- 50.Jakobsson M, Long A, Ingólfsson Ó, Kjær KH, Spielhagen RF. 2010. New insights on Arctic Quaternary climate variability from palaeo-records and numerical modelling. Quat. Sci. Rev. 29, 3349–3358. ( 10.1016/j.quascirev.2010.08.016) [DOI] [Google Scholar]
- 51.Schirrmeister L, Froese D, Tumskoy V, Grosse G, Wetterich S. 2013. Yedoma: late Pleistocene ice-rich syngenetic permafrost of Beringia. In The encyclopedia of Quaternary science (ed. SA Elias), pp. 542–552. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 52.Zimov SA, Schuur EAG, Chapin FS. 2006. Permafrost and the global carbon budget. Science 312, 1612 ( 10.1126/science.1128908) [DOI] [PubMed] [Google Scholar]
- 53.Gajewski K, Viau A, Sawada M, Atkinson D, Wilson S. 2001. Sphagnum peatland distribution in North America and Eurasia during the past 21,000 years. Global Biogeochem. Cycles 15, 297–310. ( 10.1029/2000GB001286) [DOI] [Google Scholar]
- 54.Turetsky MR, et al. 2019. Permafrost collapse is accelerating carbon release. Nature 569, 32–34. ( 10.1038/d41586-019-01313-4) [DOI] [PubMed] [Google Scholar]
- 55.Duarte CM, Lenton TM, Wadhams P, Wassmann P. 2012. COMMENTARY: abrupt climate change in the Arctic. Nat. Clim. Change 2, 60–62. ( 10.1038/nclimate1386) [DOI] [Google Scholar]
- 56.Lenton TM, Rockström J, Gaffney O, Rahmstorf S, Richardson K, Steffen W, Schellnhuber HJ. 2019. Climate tipping points – too risky to bet against. Nature 575, 592–595. ( 10.1038/d41586-019-03595-0) [DOI] [PubMed] [Google Scholar]
- 57.Schuur EAG, et al. 2015. Climate change and the permafrost carbon feedback. Nature 520, 171–179. ( 10.1038/nature14338) [DOI] [PubMed] [Google Scholar]
- 58.Riahi K, Rao S, Krey V, Cho C, Chirkov V, Fischer G, Kindermann G, Nakicenovic N, Rafaj P. 2011. RCP 8.5 – a scenario of comparatively high greenhouse gas emissions. Clim. Change 109, 33–57. ( 10.1007/s10584-011-0149-y) [DOI] [Google Scholar]
- 59.Farquharson LM, Romanovsky VE, Cable WL, Walker DA, Kokelj SV, Nicolsky D. 2019. Climate change drives widespread and rapid thermokarst development in very cold permafrost in the Canadian High Arctic. Geophys. Res. Lett. 46, 6681–6689. ( 10.1029/2019GL082187) [DOI] [Google Scholar]
- 60.Loranty MM, et al. 2018. Reviews and syntheses: changing ecosystem influences on soil thermal regimes in northern high-latitude permafrost regions. Biogeosciences 15, 5287–5313. ( 10.5194/bg-15-5287-2018) [DOI] [Google Scholar]
- 61.Bonan GB. 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449. ( 10.1126/science.1155121) [DOI] [PubMed] [Google Scholar]
- 62.Le Quéré C, et al. 2018. Global carbon budget 2018. Earth Syst. Sci. Data 10, 2141–2194. ( 10.5194/essd-10-2141-2018). [DOI] [Google Scholar]
- 63.Sturm M, Holmgren J, McFadden JP, Liston GE, Chapin FS, Racine CH. 2001. Snow–shrub interactions in Arctic tundra: a hypothesis with climatic implications. J. Clim. 14, 336–344. (). [DOI] [Google Scholar]
- 64.Sturm M, Racine C, Tape K. 2001. Climate change – increasing shrub abundance in the Arctic. Nature 411, 546–547. ( 10.1038/35079180) [DOI] [PubMed] [Google Scholar]
- 65.Cromsigt JPGM, te Beest M, Kerley GIH, Landman M, le Roux E, Smith FA. 2018. Trophic rewilding as a climate change mitigation strategy? Phil. Trans. R. Soc. B 373, 20170440 ( 10.1098/rstb.2017.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sher AV, Kuzmina SA, Kuznetsova TV, Sulerzhitsky LD. 2005. New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals. Quat. Sci. Rev. 24, 533–569. ( 10.1016/j.quascirev.2004.09.007) [DOI] [Google Scholar]
- 67.Forbes BC, Stammler F, Kumpula T, Meschtyb N, Pajunen A, Kaarlejarvi E. 2009. High resilience in the Yamal-Nenets social-ecological system, West Siberian Arctic, Russia. Proc. Natl Acad. Sci. USA 106, 22 041–22 048. ( 10.1073/pnas.0908286106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larter NC, Gates CC. 1991. Diet and habitat selection of wood bison in relation to seasonal changes in forage quantity and quality. Can. J. Zool. 69, 2677–2685. ( 10.1139/z91-376) [DOI] [Google Scholar]
- 69.Brender B. 2012. The European bison's (Bison bonasus): impact on pedunculate oak and Norway spruce in Almindingen on Bornholm. Odense, Denmark: University of Southern Denmark. [Google Scholar]
- 70.Gebczynska Z, Gebczynski M, Martynowicz E. 1991. Food eaten by the free-living European bison in Bialowieza Forest. Acta Theriol. 36, 307–313. ( 10.4098/AT.arch.91-32) [DOI] [Google Scholar]
- 71.Schmidt ENB. 2016. Meta-barcoding reveals high contribution of shrubs and trees in the diet of the European bison (Bison bonasus) on Bornholm, Denmark – local adaptation or global misconception? Copenhagen, Denmark: University of Copenhagen. [Google Scholar]
- 72.Rivals F, Solounias N, Mihlbachler MC. 2007. Evidence for geographic variation in the diets of Late Pleistocene and early Holocene Bison in North America, and differences from the diets of recent bison. Quat. Res. 68, 338–346. ( 10.1016/j.yqres.2007.07.012) [DOI] [Google Scholar]
- 73.Cromsigt JPGM, Kemp YJM, Rodriguez E, Kivit H. 2018. Rewilding Europe's large grazer community: how functionally diverse are the diets of European bison, cattle, and horses? Restor. Ecol. 26, 891–899. ( 10.1111/rec.12661) [DOI] [Google Scholar]
- 74.Jones BM, Arp CD. 2015. Observing a catastrophic thermokarst lake drainage in Northern Alaska. Permafrost Periglacial Process. 26, 119–128. ( 10.1002/ppp.1842) [DOI] [Google Scholar]
- 75.Hoffman RR. 1989. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457. ( 10.1007/BF00378733) [DOI] [PubMed] [Google Scholar]
- 76.Librado P, et al. 2015. Tracking the origins of Yakutian horses and the genetic basis for their fast adaptation to subarctic environments. Proc. Natl Acad. Sci. USA 112, E6889–E6897. ( 10.1073/pnas.1513696112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimov N, Zimova G, Gabaidulin A, Shipilov K, Sleptsov V, Zimov S. 2018. Pleistocene Park experiment: effect of grazing on the accumulation of soil carbon in the Arctic. Paper presented at the AGU Fall Meeting, 2018, Washington, DC. See https://agu.confex.com/agu/fm18/meetingapp.cgi/Paper/430445.
- 78.Crate S, et al. 2017. Permafrost livelihoods: a transdisciplinary review and analysis of thermokarst-based systems of indigenous land use. Anthropocene 18, 89–104. ( 10.1016/j.ancene.2017.06.001) [DOI] [Google Scholar]
- 79.Fang S, Xu LD, Zhu Y, Ahati J, Pei H, Yan J, Liu Z. 2014. An integrated system for regional environmental monitoring and management based on Internet of things. IEEE Trans. Ind. Inf. 10, 1596–1605. ( 10.1109/TII.2014.2302638) [DOI] [Google Scholar]
- 80.World Bank and Ecofys. 2018. State and trends of carbon pricing 2018 (May). Washington, DC: World Bank; ( 10.1596/978-1-4648-1292-7) [DOI] [Google Scholar]
- 81.Hamrick K, Gallant M. 2017. Unlocking potential: state of the voluntary carbon markets 2017, p. 42 Washington, DC: Forest Trends' Ecosystem Marketplace. [Google Scholar]
- 82.Hodgson GM. 2006. What are institutions? J. Econ. Issues 40, 1–25. ( 10.1080/00213624.2006.11506879) [DOI] [Google Scholar]
- 83.Kolchinsky EI, Kutschera U, Hossfeld U, Levit GS. 2017. Russia's new Lysenkoism. Curr. Biol. 27, R1042–R1047. ( 10.1016/j.cub.2017.07.045) [DOI] [PubMed] [Google Scholar]
- 84.Birks HH. 2008. The Late-Quaternary history of arctic and alpine plants. Plant Ecol. Divers. 1, 135–146. ( 10.1080/17550870802328652) [DOI] [Google Scholar]
- 85.Lorenzen ED, et al. 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364. ( 10.1038/nature10574) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are presented in the main manuscript.