Abstract
A major challenge for advancing our understanding of the functional role of soil microbial communities is to link changes in their structure and function under climate change. To address this challenge requires new understanding of the mechanisms that underlie the capacity of soil microbial communities to resist and recover from climate extremes. Here, we synthesize emerging understanding of the intrinsic and extrinsic factors that influence the resistance and resilience of soil microbial communities to climate extremes, with a focus on drought, and identify drivers that might trigger abrupt changes to alternative states. We highlight research challenges and propose a path for advancing our understanding of the resistance and resilience of soil microbial communities to climate extremes, and of their vulnerability to transitions to alternative states, including the use of trait-based approaches. We identify a need for new approaches to quantify resistance and resilience of soil microbial communities, and to identify thresholds for transitions to alternative states. We show how high-resolution time series coupled with gradient designs will enable detecting response patterns to interacting drivers. Finally, to account for extrinsic factors, we suggest that future studies should use environmental gradients to track soil microbial community responses to climate extremes in space and time.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions’.
Keywords: soil microbial communities, microbial traits, resistance, resilience, alternative states, ecosystem function
1. Introduction
Climate extremes, including droughts, heat waves and floods, cause major fluctuations in the structure and functioning of ecosystems [1,2], and, in some cases, pave the way for abrupt changes (i.e. rapid transition, box 1) from one ecosystem state to another [6–8]. Further, the potential for such abrupt changes in ecosystem states is rising due to anthropogenic climate change, and expected increases in the severity and frequency of climate extremes [9]. In this light, the concepts of resistance and resilience, or the ability of an ecological system to resist (i.e. resistance) and recover (i.e. resilience) from a perturbation and hence maintain its structure and function, have fast become a major focus of ecological research [10–12]. Yet, while this area of research has boomed, it has focused mainly on aboveground and aquatic ecosystems [7,13–15]. As such, our understanding of the mechanisms that confer resistance and resilience of belowground microbial communities to climate extremes remains limited.
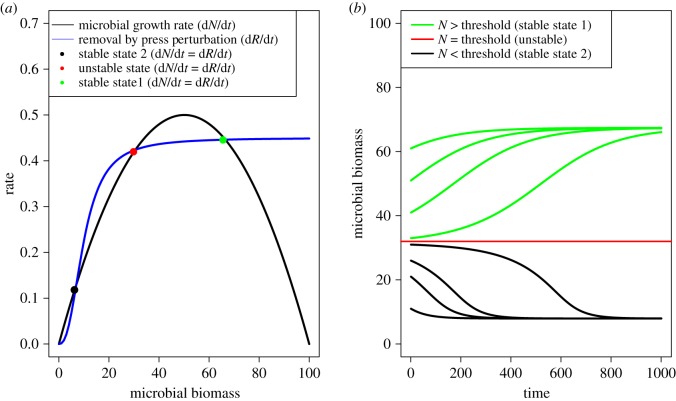
Box 1. Multiple stable states and perturbation.
Different types of perturbations interact to drive soil microbial communities to alternative states. Here, we show how pulse and press perturbations, and different intrinsic and extrinsic factors [3,4] typically applied to soil microbial systems, can drive such transitions using a modified version of Noy-Meyr's model [5]. We adapted the original model to a hypothetical soil microbial model where the growth rate of the state variable (originally plant density but microbial biomass in our example) is modelled with a logistic function (figure 1a, black hump-shaped curve). The model assumes that a press perturbation is any abiotic or biotic factor that negatively affects microbial biomass (the state variable of our model). The perturbing factor is a constant parameter of the model, and so a press perturbation [3,4]. The net effect of this perturbation depends on the nonlinear shape of the function that describes the variation of perturbation as a function of the state variable. In the original model, this shape was modelled as a type III functional response, which is sigmoidal (figure 1a, blue curve) and leads to multiple stable states if parametrized as in our example of figure 1 (for model equations and parameter values, see the R_code_Figure1.R in the electronic supplementary material). The net removal of microbial biomass described by the blue curve in figure 1a equals microbial growth rate only at three points (black, red and green dots), which correspond to three equilibrium states. One of these equilibria (red dot, figure 1a) is unstable and represents a critical threshold (red horizontal line, figure 1b). If microbial biomass moves above the threshold value, it will eventually settle on stable state 1 (green dot, figure 1a, green curves, figure 1b). Otherwise, microbial biomass will settle on stable state 2 (black dot, figure 1a and black curves, figure 1b). The shape of the rate curves and the specific parameter values (figure 1a) are intrinsic to the model system. Different realizations of microbial biomass (i.e. different starting points and trajectories of black and green curves in figure 1b) reflect the initial conditions of the system and are due to the extrinsic factors, such as climate history and soil abiotic properties, that determine initial conditions.
An enormous diversity of microbial life is found in soil, forming one of the most complex and biologically diverse communities on Earth [16,17]. These soil microbial communities, including fungi, protists, viruses, bacteria and archaea, have major roles in shaping the structure and function of terrestrial ecosystems through their involvement in biogeochemical cycles and by forming intimate relationships with plant roots [16]. The soil environment is highly dynamic: soil microbial communities are constantly exposed to natural fluctuations in environmental conditions, including changes in moisture, resource availability and temperature. Under such conditions, microbial communities are relatively stable in terms of fluctuations in the abundance and composition of major functional groups [16]. Nevertheless, and despite a large literature demonstrating the vulnerability of soil microbial communities to environmental change [16], major uncertainties exist regarding the mechanisms that underlie their ability to resist and recover from abrupt and intense ‘pulse’ perturbations (sensu Bender et al. [3]) such as those associated with climate extremes. Little is also known about what makes soil microbial communities vulnerable to abrupt changes (box 1) from one taxonomic and functional state to another, and what the consequences of transitions to alternative microbial states are for ecosystem functioning (figure 2). These represent important gaps in knowledge given the sensitivity of soil microbial communities to environmental change and their importance for ecosystem functioning [16,17].
Figure 1.
A theoretical model of soil microbial growth under press perturbation based on the classic grazing model of Noy-Meyr [5]: (a) growth and removal rates as a function of microbial biomass; (b) different trajectories of microbial biomass over time (the red, green and black solid lines corresponds to the black, red and green dots of panel (a). The dynamics of microbial biomass (state variable) are governed by two factors: the positive contribution of growth (a, black line), which follows a logistic function, and the negative contribution of biomass removal (a, blue line) caused by an abiotic (e.g. drought) or biotic (e.g. grazers) factor held constant (press). We assumed a sigmoidal shape of removal rate (blue curve in a) and searched for parameters that generated two stable states (black and green dots in a and black and green lines in b) and one unstable state (red dot in a, and red horizontal line in b). The unstable state is a critical threshold (red horizontal line): above it, the system settles on stable state 1; below it, the system settles on stable state 2.
Figure 2.
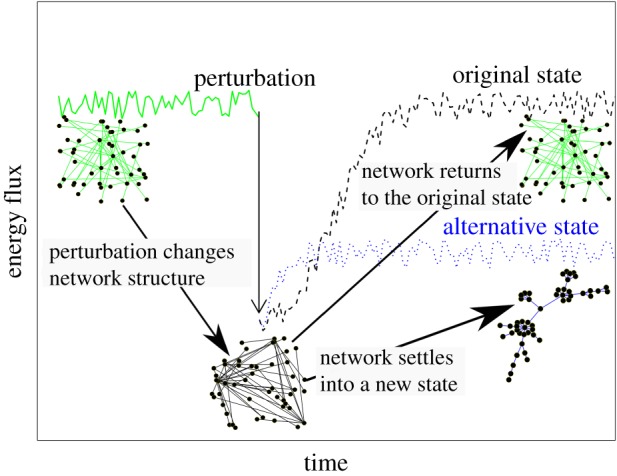
Aggregated soil microbial community properties (e.g. the total flux of energy through the community, see y-axis) emerge from the interactions that link different species in an ecological network. In the networks of this figure, solid dots represent different species and the lines connecting them the flux of energy between the connected species. The sum of energy fluxes across all possible species linkages is the total flux of energy through the network (y-axis and solid green, black and blue lines that show fluctuation of energy over time). Energy fluxes between species, and hence the total flux of energy, change over time (fluctuating green, solid line, that is that phase before any perturbation is applied), for example, in response to seasonal variation in soil abiotic properties, such as water availability. Perturbations (arrow) such as droughts alter these natural dynamics and change the structure of the network (compare the network with green linkages, before the perturbation, with the network with black linkages, after the perturbation), and thus total energy flux. Here, we assume that a sharp decline in microbial diversity and function decreases total energy flux, at least in the short to medium term. Over time, ecological networks fluctuate and reassemble to either recover to their original state (dotted black lines, network with green linkages) or settle in a new state (dotted blue line, network with blue linkages). A mechanistic understanding of the resilience of aggregated properties such as total energy flux requires a quantification of the natural variability of soil microbial networks over space and time and an understanding of how these networks reassemble in response to perturbations.
Here, we synthesize emerging understanding of the intrinsic (i.e. taxonomic and functional diversity) and extrinsic (i.e. climate, vegetation and soil abiotic properties) attributes that confer resistance and resilience of soil microbial communities to climate extremes. We focus on drought because it is globally prevalent and expected to increase in frequency and intensity in most regions of the world [9]. Further, changes in water availability are of primary importance for microbial life and biogeochemical processes [18]. Although a topic of much debate [19,20], we define resistance as the degree to which soil microbial communities do not change in the face of a pulse perturbation, whereas resilience is the rate at which they recover after that perturbation, both normalized to an undisturbed state [12]. We also consider the external drivers, or forcing factors, that might trigger abrupt changes in soil microbial communities to alternative states (boxes 1 and 2), with potentially deleterious consequences for ecosystem functioning. Finally, we highlight research challenges and propose a path for advancing understanding of the mechanisms that underpin the resilience of belowground communities to climate extremes.
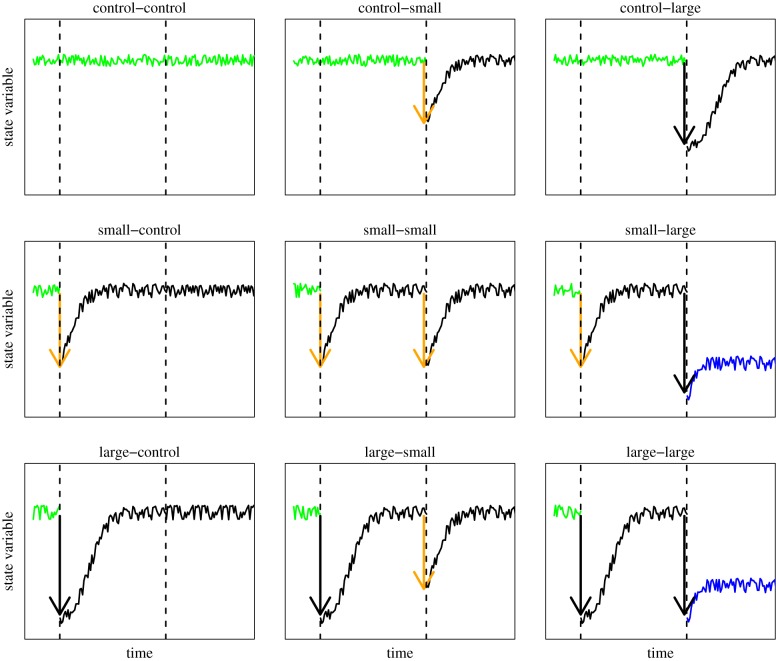
Box 2. Experimental measurement of resilience and detection of multiple stable states.
The collection of time series from perturbation experiments is central to the understanding of the dynamics that govern communities and their resistance and resilience. An important aspect of climate extremes is variability and unpredictability [21,22] in the intensity (perturbation magnitude) and frequency (the timing and sequence of perturbations) of these events. Also, perturbations that follow each other can be of a different type, for instance, drought and flood, and winter frost followed by summer drought. Studies of these types of perturbation regime require the application of pulse perturbations of different intensity and nature in sequence over time. Before–after control–impact (BACI) design, and their revised version accounting for the limitations of earlier formulations of this design [23], provides a starting point for this type of experiments. Ideally, there should be a baseline of the temporal variability of the state variables before any pulse perturbation is applied (figure 3, control–control, top left corner). A small (figure 3, orange arrows) or large (figure 3, black arrows) pulse is applied that negatively affects that state variable. The state variable can recover from the perturbation over time. A second perturbation of the same or different magnitude can then be applied sometime after the first perturbation. Assuming the system had recovered to its initial state after an initial small or large pulse, following the second pulse, the system could now recover towards a new stable state (i.e. small–large and large–large scenarios, figure 3) or return to its original state (i.e. small–small and large–large scenarios, figure 3). The full design to discriminate among all the possible trajectories of the state variable requires at least nine treatments [4], as illustrated in figure 3. These are: (i) a full control–control, with no perturbation, monitored over all duration of the experiment; (ii) a small–control, with one pulse at the start; (iii) a control–small, with one later pulse but not the early pulse; (iv) a small–small, with two small pulse perturbations in a row; (v) a small–large with one small perturbation followed by a large one; (vi) a large–small; (vii) a control–large, with just a later large pulse; (viii) a large–control, with just a large pulse at the start; and (ix) a large–large, with two large pulses in a row. The small versus large comparison could be substituted by two different types of perturbation, such as flood versus drought. A more complex design could incorporate both two perturbations of a different nature and two magnitudes (small versus large drought, and small versus large flood, with all possible combinations, including the controls). These designs are particularly useful to detect the onset of multiple stable states and test whether the system has memory, flips to alternative states or can return to its original state after perturbations, and how the intensity and nature of perturbations affect resistance and resilience. In a fully replicated factorial design, this experiment could be analysed with mixed-effect models [24] based on multifactorial design for fixed terms but also including random terms for locations and identity (repeated measure) of experimental units, and autocorrelation functions. The models should also explicitly model heterogeneity of variance, which is likely to occur given the nature of these experiments (e.g. variation in temporal variance caused by perturbations). The general design of these experiments is also amenable to time-series analysis. In the case of multivariate responses such as those of belowground ecological networks and food webs, multivariate autoRegressive or MAR [25–28] are a powerful approach to unravel the forces that structure communities over time. MAR models explicitly quantify the relative effects of biotic interactions, environmental and stochastic drivers of community dynamics, and deterministic environmental forcing such as those imposed by press perturbations. A relatively simple equation for MAR [25] is
which models the abundances x of each species or functional group on a logarithm scale. The key terms of this equation are the matrix B, which models species interactions, matrix C, which quantifies the effect of the environment on species, and the stochastic term wt, decomposable in demographic and environmental components [27]. The estimate of B is useful to parametrize networks of interactions. The parameters can then be used to create energetic versions of these interactions such as those typical of food webs [29]. The matrix B can also be used to calculate classic metrics of stability and resilience [28,30], while B, C and wt can be used to calculate metrics of stability in relation to synchrony of populations [31].
2. Intrinsic attributes associated with microbial resistance and resilience
(a). Overview of intrinsic attributes that determine resistance and resilience
Species richness is frequently proposed as a key intrinsic attribute associated with increased resistance and resilience of ecosystem functions (e.g. primary production) to pulse perturbations, which is largely attributed to the insurance hypothesis, i.e. that high diversity insures against declines in ecosystem function because many species provide greater guarantees that some will maintain their function if others do not [32]. The mechanistic basis for such relationships remains unclear, and multiple taxonomic and functional facets of diversity could determine the resistance and resilience of ecosystem functions in response to pulse perturbations such as climate extremes [33–37]. For instance, differences in species preferences for environmental conditions, which generate asynchronous species responses to perturbations, and the speed at which they recover, are key traits that determine positive diversity effects on the stability of ecosystem functions [31]. Functional diversity (i.e. the range and variance of functional traits) is also a key driver of ecosystem stability. Communities containing a wide range of traits linked to resistance (i.e. those related to stress tolerance, physiological plasticity or the capacity to enter dormancy) and resilience (e.g. those related to the capacity to grow rapidly, such as high reproduction and growth rates) are more able to buffer pulse perturbations associated with climate extremes [10]. Further, the traits of dominant species are likely to be a key driver of the resistance and resilience of ecosystem functions to climate extremes [38,39].
Figure 3.
A theoretical example of the nine possible treatment combinations of a manipulative experiment that applies multiple perturbations (two in this case) of different amplitude and monitors the response of state variables (e.g. biomass) over time. The green line represents natural fluctuations before any perturbation as in the control–control scenario (top left corner). Perturbations (arrows) can be applied at one of two points in time (vertical horizontal lines) and with small (orange arrow) or large (black arrow) amplitude. Here, we assume that the state variable drops after being perturbed and then starts to recover. Depending on the sequence and amplitude of perturbations, the state variable may recover to its original state or settle onto a new state (blue solid line). Here, we assume that a late and large perturbation makes the system shift to a new alternative stable state (see small–large and large–large scenarios) while one large perturbation in isolation does not necessarily shift the system to a new stable state. This is a specific assumption to illustrate just one of the possible outcomes. We also assume that the new state has an equilibrium at a lower level than the original one. The full experimental design would consist of nine treatments (all possible alternations of control, small and large) and multiple spatial replicates for each combination of treatments. The temporal resolution of measurements (frequency of data collection and time between the two pulses) will depend on the nature of the state variable and its natural temporal variability. Perturbations can also be of a different nature (e.g. drought versus flood), which combined with the size of the perturbation would further increase the complexity of the experimental design.
Many studies have documented how various climate extremes, such as drought, freezing events and floods, impact soil microbial communities and their functioning [40–42], but few have identified the intrinsic attributes that confer their resistance and resilience. Further, studies that have experimentally tested how changes in soil microbial diversity affect the resistance and resilience of ecosystem functions give mixed results [36,37], which is likely due to a variety of factors. These include differences in the way soil microbial diversity is experimentally manipulated, which can create confounding factors, and varying levels of functional redundancy within different microbial groups [36,37]. Here, we consider two broad mechanisms by which the diversity and composition of soil microbial communities can regulate their capacity to resist and recover from climate extremes, namely: (i) asynchrony of species responses, which is based on microbial life-history strategies related to the tolerance and speed of recovery; and (ii) changes in the strength of food web interactions and the asymmetry of food web energy channels.
(b). Asynchrony of species responses, microbial traits and life-history strategies
Numerous studies report divergent responses in population fluctuations and relative abundance of individual soil microbial taxa to climate extremes, especially drought [41,43,44], which points to asynchrony of species responses playing a role in determining the resistance and resilience of microbial-mediated functions. However, only recently have attempts been made to explain such asynchronous microbial responses to climate extremes on the basis of ecological life-history strategies, such as those related to tolerance to desiccation and rapid growth rate [43,45]. A study of soil microbial responses to extreme desiccation and rewetting, for instance, revealed contrasting phylum-level response patterns in soil bacterial communities expressed through different bacterial life-history strategies: members of the Actinobateria phylum, which are prevalent in soils of dry environments [46], were found to be highly resistant to drought due to their high tolerance to desiccation, whereas by contrast, members of the Acidobacteria phylum were less resistant to drying but more resilient, showing remarkable recovery due to their fast growth strategy [43]. Likewise, by exploring responses of individual soil bacterial taxa to moisture pulses, Evans & Wallenstein [45] identified three distinct life-history strategies, with bacterial species being classified as tolerant, opportunistic or sensitive to moisture pulses. Further, different precipitation histories were accompanied by shifts in the relative abundance of bacterial species employing these strategies: soil bacterial communities with a history of moisture pulses had a greater proportion of taxa exhibiting a stress-tolerant strategy, whereas those subject to ambient conditions contained a greater abundance of drought-sensitive taxa [45]. Finally, it has been proposed that certain bacterial phyla can be differentiated into broad copiotrophic and oligotrophic strategies: oligotrophs characterized by low growth rates and high resource use efficiency are more resistant to climate extremes than copiotrophs, which have high growth rates and low resource use efficiency, and are more resilient [47,48].
While the identification of broad life-history strategies associated with resistance and resilience has advanced our understanding of microbial responses to climate extremes, the specific microbial traits involved remain unclear. However, the use of trait-based approaches in microbial ecology is developing fast [17,49–51], and offers potential to identify subsets of microbial traits that are of importance for microbial life-history strategies related to resistance and resilience to climate extremes. For instance, microbial traits such as heterotrophic growth strategy and dormancy potential, measured, respectively, as community-weighted ribosomal operon count and dormancy genes (e.g. sporulation, toxin/antitoxin systems and resuscitation promoting factors), have been linked to an enhanced ability of microbial-mediated functions to buffer perturbations [52,53]. Further, the use of genomic data in association with existing and/adapted trait-based frameworks from plant ecology, such as Grime's competitor–stress tolerator–ruderal (CSR) framework [54], could inform on the adaptive strategies of dominant microbial taxa and their capacity to resist and recover from climate extremes [17,50,51]. For instance, it has been suggested that genomic data could be used to identify subsets of microbial traits of importance for microbial life-history strategies related to resource acquisition (C) (e.g. genes encoding enzymes activities and major metabolic pathways of nutrient cycling), stress tolerance (S) (e.g. genes associated with dormancy, desiccation, bio-molecular damage repair and osmoregulation) and rapid growth (R) (e.g. investment in assimilatory metabolic pathways, such as carbohydrate, amino acid, nucleotide and fatty acid synthesis) [17,51]. Such approaches are especially relevant given that a relatively small subset of bacterial and fungal phylotypes dominate soils globally [55,56], and that dominant bacterial taxa are often the strongest responders to drought [57]. This points to the importance of the traits of dominant species for predicting how soil microbial communities respond to climate extremes.
(c). Food web interactions and asymmetry of energy channels
Belowground communities are extremely complex and include a myriad of biotic interactions within the soil food web, with microbes representing the basal trophic levels of this food web [16]. A useful approach to tackling the problem of how soil food webs are structured to confer stability is to consider food webs as coupled fast and slow energy channels that broadly relate to rates of energy and nutrient flux in the soil system [29,58,59]. This idea, which is supported by theoretical analysis, is based on food webs being broadly structured into the ‘fast’ energy channel, which is formed of bacteria and their consumers, cycles nutrients rapidly and recovers quickly from disturbances, thereby increasing resilience, and the ‘slow’ energy channel that comprises fungi and their consumers, cycles nutrients more slowly and dampens responses to perturbations, thereby increasing resistance [58,59]. This way of compartmentalizing food webs is ubiquitous across aquatic and terrestrial ecosystems, including soil, and the complementary functions of these energy channels, which process organic matter at different rates, provides a mechanism for responding to perturbations such as climate extremes [29]. In particular, asymmetry in energy flux in coupled fast and slow channels provides top predators with complementary dynamics that stabilize their populations, and the productivity and structure of the system. In other words, a rapid response of the fast channel to a perturbation provides resources (i.e. prey) for top predators, allowing recovery of predator populations, and these oscillations are compensated for by the recovery of the slow channel that is freed of predation. Rooney et al. [29] argued that these complementary functions of coupled energy channels produce a rapid, yet stable recovery of the food web from a perturbation. Further, any factor that destroys this complementarity or changes the asymmetry of the two channels, such as increased resource supply or removal of organisms, will destabilize the food web and its functioning relative to an unperturbed state [29].
These theoretical ideas on soil food webs are still to be fully tested and have been questioned on the basis that, in reality, belowground feeding interactions are not restricted to these energy channels; rather, soil organisms, especially bacteria, fungi and the protists that consume both bacteria and fungi, display enormous versatility in their processing of soil organic matter [60–62]. Nevertheless, studies support the notion that broad shifts in the asymmetry of the two coupled energy channels regulate the resistance and resilience of soil functions to climate extremes. First, perturbations, especially those associated with intensive agriculture (e.g. nutrient enrichment), can shift the distribution of biomass and energy flow to the bacterial rather than the fungal channel [63,64]. Further, such increases in the bacterial over the fungal energy channel resulting from intensive land management are associated with faster rates of soil carbon and nitrogen cycling and reduced microbial retention of nutrients following dry/wet cycles, which leads to increased loss of nutrients from soil [65,66]. Such findings are broadly in line with theory, which purports that press perturbations (i.e. those that maintain a constant level over a prolonged period of time, such as nutrient enrichment) bias the asymmetry of food webs towards the fast energy pathway, leading to the loss of stability and oscillations in function [29]. Second, experimental studies support the notion that differences in the biomass of the two energy channels alter the ability of the soil system to resist and recover, and retain nutrients, following extreme drought: soil food webs with a greater biomass in the slow fungal energy channel, which are promoted by extensive, low input agricultural management, were more resistant and retained nutrients more effectively following drought than those dominated by the fast bacterial energy channel, which is promoted by intensive agriculture [30]. Although the bacterial–fungal energy channel concept is relatively simplistic, it provides a useful framework for predicting how broad shifts in soil food web structure resulting from sustained press perturbations impact the resistance and resilience of soil food webs and their functions to climate extremes.
Another approach that is gaining popularity for capturing the response of belowground communities of coexisting organisms to perturbations is the analysis of ecological networks [67]. Soil microbial communities form highly complex ecological networks that include multiple interactions between coexisting taxa, and evidence is emerging that properties of these networks can influence their response to climate extremes. A recent study, for instance, revealed that drought had a much stronger impact on bacterial than fungal co-occurrence networks [57], which is consistent with the expectation that soil bacterial communities are less resistant to drought than fungal communities [30,43,68]. However, it was also found that bacterial co-occurrence networks had properties associated in theory with low stability under perturbations, such as high connectivity and centrality, whereas fungal networks had properties associated with higher stability, such as fewer negative correlations, which stabilizes co-oscillation in communities [29,69–71]. Another important finding was that dominant bacterial taxa, which were the most responsive to drought, were highly central and connected within networks, suggesting that they are the major drivers of changes in bacterial network structure [57]. Although caution is needed in interpreting co-occurrence networks [72,73], it can yield important information on co-oscillation of microbial taxa and the stability of microbial communities in the face of pulse perturbations such as climate extremes [57,74].
3. Extrinsic attributes and microbial resistance and resilience
(a). Historical climate and soil properties
Extrinsic attributes or characteristics of the environment in which soil microbes live, such as the historical climate and soil physico-chemical conditions, have potential to modify intrinsic attributes of soil microbial communities that influence their capacity to resist and recover from climate extremes. Climate history can favour taxa with traits that enable them to buffer droughts, or cause shifts in the physiology of individual species due to adaptation or phenotypic plasticity [45,75]. Further, such shifts will affect the functioning of microbial communities in response to pulse perturbations such as dry–wet cycles [45,75]. For example, modelling studies indicate that soil microbial assemblages shaped by a more constant historical environment have intrinsic attributes that render them more sensitive to change, leading to poorer functional acclimatization than those from more fluctuating environments [75]. Further, soil microbial activity, measured as enzyme production, becomes increasingly sensitive to changes in soil moisture as the historical moisture regime becomes drier [76], and functional resistance of soil microbial communities to drought is greater in drier than in mesic sites due to a greater abundance of drought-tolerant phyla [41]. Recurring droughts can also increase the tolerance of microbial communities to subsequent dry–wet cycles, which is largely due to community reorganization in favour of drought-tolerant microbial taxa [45,77]. Similarly, a history of soil freezing has been linked to greater resistance of microbial community abundance and function to freeze–thaw cycles due to community reorganization and/or selection of frost-tolerant microbial taxa [78,79].
Soil abiotic properties, such as soil organic matter content and texture, can also modify the capacity of soil microbial communities to resist and recover from climate extremes, either by modifying the scale of environmental impact, for instance, by operating as a determinant of soil water holding capacity and hence moisture availability under drought, or by shaping the intrinsic attributes of soil microbial communities. Few studies have explored how variation in soil properties influences the resistance and resilience of microbial communities to climate extremes, but soil resource availability could play an important role. For instance, a global meta-analysis revealed that temporal variability or a lack of stability of soil microbial biomass was lower in soils of high organic carbon and pH, which suggests that these factors contribute to alleviation of stress and increase the stability of soil microbial communities [80]. Microbial resilience to perturbations also seems greater in soils of high resource availability, which fosters fast-growing microbial taxa with high rates of recovery from perturbations [81]. Experimental studies, however, paint a more complex picture. Orwin et al. [82], for example, studied how the resistance and resilience of microbial functional parameters varied with soil resources quantity and quality (based on total pools and ratios of C, N and P) during ecosystem development, using soils taken from three geographically distinct chronosequences. They found a consistent trade-off between microbial resistance and resilience in response to experimental drought across all three chronsequences, which suggests that subsets of microbial communities have different strategies associated with resistance and resilience [45]. However, the direction and strength of correlations between microbial resistance and resilience and soil resources depended on the identity of the soil microbial response variable analysed and the chronosequence studied. Nevertheless, these studies indicate that spatial variation in soil abiotic properties plays an important role in modifying microbial responses to climate extremes, either by moderating their impact on microbial communities or by shaping the intrinsic attributes of soil microbial communities that confer resistance and resilience.
(b). Plant community composition
Few studies have explored how plants and changes in plant community composition influence the resistance and resilience of soil microbial communities to climate extremes; rather, most have focused on responses of aboveground plant communities, with little consideration of interactions with soil microbes that could modify plant responses or have knock-on consequences for belowground communities. The primary mechanisms by which plants modify microbial community responses to climate extremes are largely indirect [83] and, as with soil abiotic factors, operate by changing the intrinsic attributes of microbial communities that confer resistance and resilience. One such indirect mechanism is a change in plant allocation and transfer of root-derived carbon to the soil microbial community in response to an extreme climatic event. Extreme drought, for example, reduces plant allocation of recent photosynthate to roots, which in turn reduces the transfer of plant-derived carbon to soil microbes, including root-associated mycorrhizal fungi [84–89]. This response is associated with differential uptake and turnover of plant-derived carbon by different groups of soil microbes, which modify intrinsic microbial community attributes that confer resistance and resilience [89,90]. For instance, a proportionally greater transfer of recently assimilated plant carbon to mycorrhizal fungi (measured using 13C pulse labelling) seems the basis for high resilience to drought [90], given the role of mycorrhizal fungi in increasing drought tolerance in plants via improved access to water and nutrients [91]. By contrast, increased uptake of root-derived carbon by bacteria following rewetting is a key mechanism underlying resilience or recovery of the plant–soil system from drought [90]. Drought-induced changes in root exudation, including qualitative changes, also have implications for ecosystem function, for instance, by increasing microbial activity and respiration [92] and/or by shifting the soil microbial community towards increased decomposition of soil organic carbon [89].
Over longer timescales, drought-induced shifts in vegetation phenology and composition [93,94], and vegetation mortality [95] can also alter plant carbon transfer to soil microbes via changes in root turnover and plant litter. However, the implications of this for intrinsic attributes that confer microbial community resistance and resilience to climate extremes are poorly understood [96], and are likely also moderated by other extrinsic factors, such as climate history and soil abiotic properties that vary across ecosystems [97,98]. Understanding the underpinning mechanisms that explain such variability and consequences for microbial resistance and resilience to climate extremes therefore represents a major challenge because the timescale involved is multiyear if not decadal.
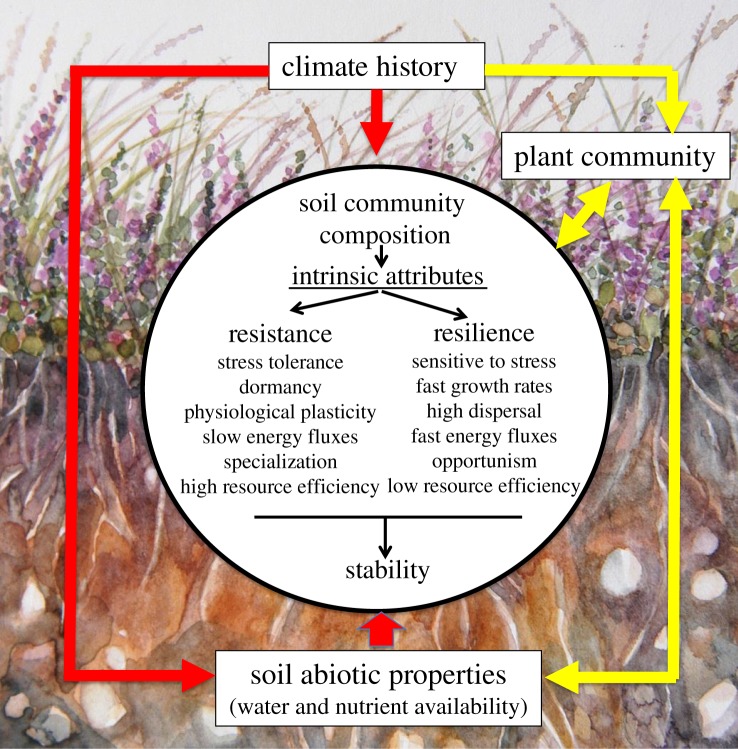
Another mechanism by which plants can modify the response of soil organisms to climate extremes is via changing other extrinsic factors, especially soil moisture and nutrient availability. Few studies have explored this issue, but vegetation change resulting from an extreme drought event, and concomitant increases in plant water uptake, can lead to reorganization of the soil microbial community by changing soil moisture content [57]. Drought can also favour plant associations with mycorrhizal fungi and mutualistic soil bacteria, which enhance plant drought tolerance via improved access to water and nutrients [91,99]. Plant species identity and composition can also alter the resilience of microbial activity to drought due to changes in soil nutrient availability, for instance, by changing plant-microbial competition for nitrogen [100,101]. Furthermore, plant community attributes that enhance the stability of primary production, such as high plant functional diversity and dominance of plant species with traits related to stress tolerance [102–104], are also likely to promote belowground community resilience to climate extremes such as drought. In support of this, high variation in rooting depth in restored grasslands increased the resistance to drought of a range of microbial-mediated processes key to soil functioning [105], which underlies the importance of coupling plant and belowground measurements for a mechanistic understanding of ecosystem stability. A major challenge is therefore to quantify the relative roles of the various indirect and direct ways through which plants operate as an extrinsic factor controlling intrinsic attributes of microbial communities that confer resistance and resilience (figure 4), and to identify the consequences for ecosystem function and feedbacks to plant community dynamics.
Figure 4.
Schematic of the pathways by which extrinsic factors can change the intrinsic attributes of soil microbial communities that confer resistance and resilience both directly (red pathways), via climate history and inherent variation in soil properties, and indirectly (yellow pathways) via changes in the plant communities which alter soil resource availability. Background image by Jill Colquhoun Bardgett.
4. Abrupt changes in soil microbial communities to alternative states
It is well established that ecosystems have critical thresholds, or tipping points, beyond which they shift abruptly from one state to another [106–109] (box 1). A key mechanism contributing to such abrupt transitions is a shift in community composition, for instance caused by sustained ‘press’ perturbations [3], that erodes the resilience of a community and increases its vulnerability to being tipped to an alternative state by a pulse perturbation (box 2). Many examples of such abrupt transitions exist, largely from aquatic and terrestrial plant communities where they are often triggered by pulse perturbations, such as climate extremes [110–114]. Studies of the human gut microbiome also reveal its vulnerability to abrupt transitions [115], showing that persistent pulse perturbations (e.g. poor diet) can degrade microbiome resilience, causing transitions to a species-poor state of simple metabolism following interventions [116].
Comparative knowledge of abrupt changes in soil microbial communities to alternative states resulting from climate extremes or other ‘pulse’ perturbations is lacking. Nevertheless, sustained ‘press’ perturbations, such as nutrient enrichment, elevated atmospheric CO2 and warming, cause progressive shifts in the composition and function of soil microbial communities from their original state [117–119], which, although not tested, could potentially render them more vulnerable to abrupt transitions to alternative taxonomic and functional states in response to climate extremes. For instance, sustained nutrient enrichment causes reductions in the abundance of fungi relative to bacteria [64–66], which reduces the resistance of microbial communities to drought [30], and potentially their vulnerability to abrupt transitions to alternative taxonomic and functional states. Also, elevated atmospheric CO2 can modify the structural properties of microbial networks [120], which although not tested, could alter the vulnerability of microbial communities to transitions to alternative states in response to climate extremes. Given that sustained press perturbations (e.g. nutrient enrichment and gradual warming) and pulse perturbations (e.g. such as drought and heat waves) commonly co-occur, we argue that their combined effects need to be considered to gain a more complete understanding of soil microbial community resistance and resilience to global change. Likewise, there is a need for improved understanding of how extrinsic factors, such as historical climate, soil abiotic properties and vegetation composition, modify the intrinsic attributes of soil microbial communities that render them more vulnerable to abrupt changes to alternative states triggered by climate extremes.
Despite a general lack of studies exploring abrupt transitions in soil microbial communities, evidence is beginning to emerge that these transitions can be triggered by climate extremes. Repeated summer droughts, for example, reduced soil moisture retention and increased carbon mineralization in heathland soil, and then a severe drought led to an abrupt change to an alternative state, characterized by impaired soil moisture retention and rewetting [121]. Repeated dry–wet cycles can also induce persistent shifts in the functional state of soils, measured as soil respiration [121], and this response is affected by land use history: soils previously under arable agriculture were more vulnerable to an abrupt transition in functional state than those with a history of grassland [122]. Finally, a severe drought, equivalent to a 100-year drought event, caused a strong and long-lasting shift in soil microbial community composition and diversity, and the structure of bacterial networks, which was also associated with changes in microbial function in terms of nitrogen cycling genes [57]. These studies demonstrate that climate extremes can trigger significant and long-lasting shifts in soil abiotic properties and the composition and functioning of soil microbial communities. Climate extremes can also impose a regime of repeated pulse perturbations that at the same time are profoundly affected by regimes of press perturbations such as nutrient enrichment and climate warming.
5. Future research needs
There is much literature exploring how the structure and function of soil microbial communities respond to climate extremes, especially drought, and it is clear that they often do not recover to their original state. But our understanding of how soil microbial communities and their collective metabolic activities resist and recover from climate extremes, and the consequences of abrupt transitions in soil microbial communities for ecosystem function, remain poor. This represents an important knowledge gap, given the known sensitivity of soil microbial communities to climate extremes and their central role in regulating ecosystem functioning [16,17].
Frameworks for quantifying resistance and resilience, and for diagnosing abrupt ecological change, already exist [8,12] and are increasingly being applied to soil microbial communities in the context of climate extremes, especially drought [30,43,45,57,82,117]. Nevertheless, this is not straightforward given the overwhelming complexity and diversity of soil microbial communities, which display enormous idiosyncrasies in their response to climate extremes, and the relative roles of the numerous direct and indirect factors that control this response (figure 4). Promising ways of addressing this complexity of responses of soil microbial communities to climate extremes are the use of frameworks based on species and community-level microbial traits, and/or biomass distribution and energy flux. In particular, the use of trait-based approaches in microbial ecology is developing fast in parallel to an increasing number of molecular tools to identify and quantify traits [17,49–51], and offers potential to identify microbial traits of importance for microbial life-history strategies related to resistance and resilience to climate extremes. Further, as we highlight in this paper, recent studies indicate that soil bacterial and fungal communities are dominated by relatively few taxa with strong environmental preferences [55,56]. Moreover, the majority of dominant fungi are characterized by high genomic potential for stress tolerance, which is a key trait associated with resistance to perturbations [56]. The mass-ratio hypothesis (i.e. ecosystem properties are driven by the characteristics of dominant species within a community [39]) and the fact that plant communities are highly resilient if dominated by species that rapidly recover following drought [38] indicates that a focus on the functional traits of dominant taxa will improve our understanding of intrinsic factors that regulate soil microbial community resistance and resilience to climate extremes.
There is also a need to improve understanding of the effects of type, timing and severity of climate extremes [37], and of interacting effects of different co-occurring perturbations, including sustained ‘pulse’ perturbations and legacies of seasonal climate extremes. Related to this is the need for studies targeted at better understating of the factors that drive transitions of soil microbial communities to alternative states. The thresholds leading to alternative states need also to be identified. Central to meeting these challenges are time series generated through manipulative experiments (box 2), which are currently lacking because of the technical and economic challenges they pose. However, experimental time series are the main tool to quantify resistance and resilience of soil microbial communities and functions, and to identify potential thresholds for abrupt changes to alternative states. As such, there is a need for studies that carry out repeated observations of soil microbial communities and their functions before, during and after different types, intensities and frequencies of climate extremes (box 2). Such experiments are by necessity large, but the use of gradient designs provides an opportunity to quantify response patterns to interacting drivers in a practical and statistically powerful way [123]. Resulting data will allow quantification of resistance and resilience, including trajectories of recovery and the existence of alternative states.
Finally, time series of soil microbial communities and their function need to be repeated in space, given strong evidence of the importance of historical contingencies, both biotic and abiotic. The use of such time series adds further logistic, technical and economic challenges to the study of the resilience of soil microbial communities. Molecular methods to profile the entire soil microbial communities are, however, becoming more affordable and accessible. In recent years, there has also been much progresses in the development and use of wireless sensors, which are becoming cheaper, easier to use and capable of measuring soil abiotic and biotic properties in multiple locations over time. The development of this technology will boost the temporal and spatial replication required to validate experimental time series and to track the dynamics of soil microbial communities and their functioning in response to climate extremes in both space and time.
6. Conclusion
The study of the resistance and resilience of soil microbial communities to climate extremes is not straightforward, given their incredible complexity and diversity and the heterogeneity of the soil environment within which they live. We identify approaches to overcome this complexity and gain an improved mechanistic understanding of the resistance and resilience of soil microbial communities to climate extremes, and of potential abrupt transitions into alternative microbial states. Central to this is a focus on key intrinsic attributes of soil microbial communities, such as the functional traits of dominant microbial taxa, coupled with measures of ecosystem functions related to biogeochemical cycles and plant production. Further, it would be fruitful to focus future research on disentangling the relative importance of different extrinsic factors (e.g. climate history, soil resource availability and indirect interactions with plants) in modifying the intrinsic attributes of microbial communities that confer resistance and resilience to climate extremes. The study of the relative importance of the multiple factors that modify soil microbial communities should be integrated with the role of interactions between sustained ‘press’ perturbations (e.g. nutrient enrichment and climate extremes) and ‘pulse’ perturbations associated with climate extremes in triggering abrupt shifts to alternative microbial states with potentially impaired functioning. New approaches to quantifying resistance and resilience, and abrupt changes to alternative states are needed. Promising approaches include the use of time series or repeated observations of soil microbial communities and their functioning, and gradient designs that enable response patterns to interacting drivers to be detected in a practical and statistically powerful way. Finally, given the importance of historical contingencies, we suggest that future studies should be carried out along environmental gradients to track soil microbial community dynamics in response to climate extremes in both space and time. We hope that the ideas presented here will serve as a basis on which understanding of the mechanisms that underpin the resistance and resilience of soil microbial communities to climate extremes and other perturbations can be improved.
Supplementary Material
Data accessibility
This article has no additional data.
Authors' contributions
The paper was conceived by R.D.B. and jointly written by R.D.B. and T.C.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by NERC Soil Security programme grant nos NE/M017028/1 and NE/P013708/1 to R.D.B. and NE/M017036/1 to T.C.
References
- 1.Smith M. 2011. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663. ( 10.1111/j.1365-2745.2011.01798.x) [DOI] [Google Scholar]
- 2.Reichstein M, et al. 2013. Climate extremes and the carbon cycle. Nature 500, 287–295. ( 10.1038/nature12350) [DOI] [PubMed] [Google Scholar]
- 3.Bender EA, Case TJ, Gilpin ME. 1984. Perturbation experiments in community ecology: theory and practice. Ecology 65, 1–13. ( 10.2307/1939452) [DOI] [Google Scholar]
- 4.Petraitis P. 2013. Multiple stable states in natural ecosystems. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Noy-Meir I. 1975. Stability of grazing systems: an application of predator-prey graphs. J. Ecol. 63, 459–481. ( 10.2307/2258730) [DOI] [Google Scholar]
- 6.Scheffer M, Carpenter S, Foley JA, Walker B. 2001. Catastrophic shifts in ecosystems. Nature 413, 591–596. ( 10.1038/35098000) [DOI] [PubMed] [Google Scholar]
- 7.Reyer CP, Rammig A, Brouwers N, Langerwisch F. 2015. Forest resilience, tipping points and global change processes. J. Ecol. 103, 1–4. ( 10.1111/1365-2745.12342) [DOI] [Google Scholar]
- 8.Ratajczak Z, Carpenter SR, Ives AR, Kucharik CJ, Ramiadantsoa T, Stegner MA, Williams JW, Zhang J, Turner MG. 2018. Abrupt change in ecological systems: inference and diagnosis. Trends Ecol. Evol. 33, 513–526. ( 10.1016/j.tree.2018.04.013) [DOI] [PubMed] [Google Scholar]
- 9.IPCC. 2007. Climate change 2007 working group I: the physical sciences basis. In Contribution of working group 1 to the fourth assessment report of the intergovernmental panel on climate change (eds Solomon S, Qin D, Manning M, Chen MZ, Marquis M, Averyt KB, Tignor M, Miller HL). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Nimmo DG, Mac Nally R, Cunningham SC, Haslem A, Bennett AF. 2015. Vive la résistance: reviving resistance for 21st century conservation. Trends Ecol. Evol. 30, 516–523. ( 10.1016/j.tree.2015.07.008) [DOI] [PubMed] [Google Scholar]
- 11.Oliver TH. 2015. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 30, 673–684. ( 10.1016/j.tree.2015.08.009) [DOI] [PubMed] [Google Scholar]
- 12.Ingrisch J, Bahn M. 2018. Towards a comparable quantification of resilience. Trends Ecol. Evol. 33, 251–259. ( 10.1016/j.tree.2018.01.013) [DOI] [PubMed] [Google Scholar]
- 13.Kimbro DL, Cheng BS, Grosholz ED. 2013. Biotic resistance in marine environments. Ecol. Lett. 16, 821–833. ( 10.1111/ele.12106) [DOI] [PubMed] [Google Scholar]
- 14.Alofs KM, Jackson DA, Lester NP. 2014. Ontario freshwater fishes demonstrate differing range-boundary shifts in a warming climate. Divers. Distrib. 20, 123–136. ( 10.1111/ddi.12130) [DOI] [Google Scholar]
- 15.Isbell F. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577. ( 10.1038/nature15374) [DOI] [PubMed] [Google Scholar]
- 16.Bardgett RD, van der Putten WH. 2014. Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. ( 10.1038/nature13855) [DOI] [PubMed] [Google Scholar]
- 17.Fierer N. 2017. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15, 579–590. ( 10.1038/nrmicro.2017.87) [DOI] [PubMed] [Google Scholar]
- 18.Schimel JP. 2018. Life in dry soils: effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 49, 409–432. ( 10.1146/annurev-ecolsys-110617-062614) [DOI] [Google Scholar]
- 19.Hodgson D, McDonald JL, Hosken DJ. 2015. What do you mean, ‘resilient’? Trends Ecol. Evol. 30, 503–506. ( 10.1016/j.tree.2015.06.010) [DOI] [PubMed] [Google Scholar]
- 20.Yeung ACY, Richardson JS. 2016. Some conceptual and operational considerations when measuring ‘resilience’: a response to Hodgson et al. Trends Ecol. Evol. 31, 2–3. ( 10.1016/j.tree.2015.10.005) [DOI] [PubMed] [Google Scholar]
- 21.Bellprat O, Guemas V, Doblas-Reyes F, Donat MG. 2019. Towards reliable extreme weather and climate event attribution. Nat. Commun. 10, 1732 ( 10.1038/s41467-019-09729-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 23.Underwood AJ. 1994. On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecol. Appl. 4, 3–15. ( 10.2307/1942110) [DOI] [Google Scholar]
- 24.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer Science & Business Media. [Google Scholar]
- 25.Hampton SE, Holmes EE, Scheef LP, Scheuerell MD, Katz SL, Pendleton DE, Ward EJ. 2013. Quantifying effects of abiotic and biotic drivers on community dynamics with multivariate autoregressive (MAR) models. Ecology 94, 2663–2669. ( 10.1890/13-0996.1) [DOI] [PubMed] [Google Scholar]
- 26.Mutshinda CM, O'Hara RB, Woiwod IP. 2009. What drives community dynamics? Proc. R. Soc. B 276, 2923–2929. ( 10.1098/rspb.2009.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutshinda CM, O'Hara RB, Woiwod IP. 2011. A multispecies perspective on ecological impacts of climatic forcing. J. Anim. Ecol. 80, 101–107. ( 10.1111/j.1365-2656.2010.01743.x) [DOI] [PubMed] [Google Scholar]
- 28.Ives AR, Dennis K, Cottingham KL, Carpenter SR. 2003. Estimating community stability and ecological interactions from time-series data. Ecol. Monogr. 73, 301–330. ( 10.1890/0012-9615(2003)073[0301:ECSAEI]2.0.CO;2) [DOI] [Google Scholar]
- 29.Rooney N, McCann K, Gellner G, Moore JC. 2006. Structural asymmetry and the stability of diverse food webs. Nature 442, 265–269. ( 10.1038/nature04887) [DOI] [PubMed] [Google Scholar]
- 30.De Vries FT, Liiri ME, Bjørnlund L, Bowker MA, Christensen S, Setälä HM, Bardgett RD. 2012. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Change 2, 276–280. ( 10.1038/nclimate1368) [DOI] [Google Scholar]
- 31.Loreau M, de Mazancourt C. 2013. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 16, 106–115. ( 10.1111/ele.12073) [DOI] [PubMed] [Google Scholar]
- 32.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brotherton SJ, Joyce CB. 2015. Extreme climate events and wet grasslands: plant traits for ecological resilience. Hydrobiologia 750, 229–243. ( 10.1007/s10750-014-2129-5) [DOI] [Google Scholar]
- 34.De Boeck HJ, Bloor JM, Kreyling J, Ransijn JC, Nijs I, Jentsch A, Zeiter M. 2018. Patterns and drivers of biodiversity–stability relationships under climate extremes. J. Ecol. 106, 890–902. ( 10.1111/1365-2745.12897) [DOI] [Google Scholar]
- 35.Griffin-Nolan RJ, et al. 2018. Trait selection and community weighting are key to understanding ecosystem responses to changing precipitation regimes. Funct. Ecol. 32, 1746–1756. ( 10.1111/1365-2435.13135) [DOI] [Google Scholar]
- 36.Griffiths BS, Philippot L. 2013. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 37, 112–129. ( 10.1111/j.1574-6976.2012.00343.x) [DOI] [PubMed] [Google Scholar]
- 37.Shade A, et al. 2012. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 3, 417 ( 10.3389/fmicb.2012.00417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoover DL, Knapp AK, Smith MD. 2014. Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95, 2646–2656. ( 10.1890/13-2186.1) [DOI] [Google Scholar]
- 39.Grime JP. 1998. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 8, 902–910. ( 10.1046/j.1365-2745.1998.00306.x) [DOI] [Google Scholar]
- 40.Barnes CJ, van der Gast CJ, McNamara NP, Rowe R, Bending GD. 2018. Extreme rainfall affects assembly of the root-associated fungal community. New Phytol. 220, 1172–1184. ( 10.1111/nph.14990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochoa-Hueso R, Collins SL, Delgado-Baquerizo M, Hamonts K, Pockman WT, Sinsabaugh RL, Smith MD, Knapp AK, Power SA. 2018. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob. Change Biol. 24, 2818–2827. ( 10.1111/gcb.14113) [DOI] [PubMed] [Google Scholar]
- 42.Sharma S, Szele Z, Schilling R, Munch JC, Schloter M. 2006. Influence of freeze-thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Appl. Environ. Microbiol. 72, 2148–2154. ( 10.1128/AEM.72.3.2148-2154.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnard RL, Osborne CA, Firestone MK. 2013. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 7, 2229–2241. ( 10.1038/ismej.2013.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preece C, Verbruggen E, Liu L, Weedon JT, Peñuelas J. 2019. Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biol. Biochem. 131, 28–39. ( 10.1016/j.soilbio.2018.12.022) [DOI] [Google Scholar]
- 45.Evans SE, Wallenstein MD. 2014. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 17, 155–164. ( 10.1111/ele.12206) [DOI] [PubMed] [Google Scholar]
- 46.Maestre FT, et al. 2015. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl Acad. Sci. USA 112, 15 684–15 689. ( 10.1073/pnas.1516684112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fierer N, Bradford MA, Jackson RB. 2007. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364. ( 10.1890/05-1839) [DOI] [PubMed] [Google Scholar]
- 48.De Vries FT, Shade A. 2013. Controls on soil microbial community stability under climate change. Front. Microbiol. 4, 265 ( 10.3389/fmicb.2013.00265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krause S, Le Roux X, Niklaus PA, Van Bodegom PM, Lennon JT, Bertilsson S, Grossart HP, Philippot L, Bodelier PL. 2014. Trait-based approaches for understanding microbial biodiversity and ecosystem functioning. Front. Microbiol. 27, 251 ( 10.3389/fmicb.2014.00251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood JL, Tang C, Franks AE. 2018. Competitive traits are more important than stress-tolerance traits in a cadmium-contaminated rhizosphere: a role for trait theory in microbial ecology. Front. Microbiol. 9, 121 ( 10.3389/fmicb.2018.00121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malik AA, Martiny JB, Brodie EL, Martiny AC, Treseder KK, Allison SD. 2020. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 14, 1–9. ( 10.1038/s41396-019-0510-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemergut DR, et al. 2016. Decreases in average bacterial community rRNA operon copy number during succession. ISME J. 10, 1147–1156. ( 10.1038/ismej.2015.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kearns PJ, Shade A. 2018. Trait-based patterns of microbial dynamics in dormancy potential and heterotrophic strategy: case studies of resource-based and post-press succession. ISME J. 12, 2575–2581. ( 10.1038/s41396-018-0194-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194. ( 10.1086/283244) [DOI] [Google Scholar]
- 55.Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK, Fierer N. 2018. A global atlas of the dominant bacteria found in soil. Science 359, 320–325. ( 10.1126/science.aap9516) [DOI] [PubMed] [Google Scholar]
- 56.Egidi E, Delgado-Baquerizo M, Plett JM, Wang J, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK. 2019. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 10, 2369 ( 10.1038/s41467-019-10373-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Vries FT, et al. 2018. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9, 3033 ( 10.1038/s41467-018-05516-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore JC, Hunt HW. 1988. Resource compartmentation and the stability of real ecosystems. Nature 333, 261–263. ( 10.1038/333261a0) [DOI] [Google Scholar]
- 59.De Ruiter PC, Neutel AM, Moore JC. 1995. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269, 1257–1260. ( 10.1126/science.269.5228.1257) [DOI] [PubMed] [Google Scholar]
- 60.Geisen S. 2016. The bacterial-fungal energy channel concept challenged by enormous functional versatility of soil protists. Soil Biol. Biochem. 102, 22–25. ( 10.1016/j.soilbio.2016.06.013) [DOI] [Google Scholar]
- 61.Bradford MA. 2016. Re-visioning soil food webs. Soil Biol. Biochem. 100, 1–3. ( 10.1016/j.soilbio.2016.08.010) [DOI] [Google Scholar]
- 62.De Vries FT, Caruso T. 2016. Eating from the same plate? Revisiting the role of labile carbon inputs in the soil food web. Soil Biol. Biochem. 102, 4–9. ( 10.1016/j.soilbio.2016.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bardgett RD, Wardle DA. 2010. Aboveground–belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford, UK: Oxford University Press. [Google Scholar]
- 64.De Vries FT, et al. 2013. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl Acad. Sci. USA 110, 14 296–14 301. ( 10.1073/pnas.1305198110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon H, Haygarth PM, Bardgett RD. 2008. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem. 40, 302–311. ( 10.1016/j.soilbio.2007.08.008) [DOI] [Google Scholar]
- 66.De Vries FT, Bloem J, Quirk H, Stevens CJ, Bol R, Bardgett RD. 2012. Extensive management promotes plant and microbial nitrogen retention in temperate grassland. PLoS ONE 7, e51201 ( 10.1371/journal.pone.0051201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramirez KS, Geisen S, Morriën E, Snoek BL, van der Putten WH. 2018. Network analyses can advance above-belowground ecology. Trends Plant Sci. 23, 759–768. ( 10.1016/j.tplants.2018.06.009) [DOI] [PubMed] [Google Scholar]
- 68.Bapiri A, Bååth E, Rousk J. 2010. Drying–rewetting cycles affect fungal and bacterial growth differently in an arable soil. Microb. Ecol. 60, 419–428. ( 10.1007/s00248-010-9723-5) [DOI] [PubMed] [Google Scholar]
- 69.Neutel AM, Heesterbeek JAP, de Ruiter PC. 2002. Stability in real food webs: weak links in long loops. Science 296, 1120–1123. ( 10.1126/science.1068326) [DOI] [PubMed] [Google Scholar]
- 70.Coyte KZ, Schluter J, Foster KR. 2015. The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666. ( 10.1126/science.aad2602) [DOI] [PubMed] [Google Scholar]
- 71.Stouffer DB, Bascompte J. 2011. Compartmentalization increases food-web persistence. Proc. Natl Acad. Sci. USA 108, 3648–3652. ( 10.1073/pnas.1014353108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freilich MA, Wieters E, Broitman BR, Marquet PA, Navarrete SA. 2018. Species co-occurrence networks: can they reveal trophic and non-trophic interactions in ecological communities? Ecology 99, 690–699. ( 10.1002/ecy.2142) [DOI] [PubMed] [Google Scholar]
- 73.Berry D, Widder S. 2014. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 5, 219 ( 10.3389/fmicb.2014.00219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barberan A, Bates ST, Casamayor EO, Fierer N. 2012. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 6, 343–351. ( 10.1038/ismej.2011.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawkes CV, Keitt TH. 2015. Resilience vs. historical contingency in microbial responses to environmental change. Ecol. Lett. 18, 612–625. ( 10.1111/ele.12451) [DOI] [PubMed] [Google Scholar]
- 76.Averill C, Waring BG, Hawkes CV. 2016. Historical precipitation predictably alters the shape and magnitude of microbial functional response to soil moisture. Glob. Change Biol. 22, 957–1964. ( 10.1111/gcb.13219) [DOI] [PubMed] [Google Scholar]
- 77.Evans SE, Wallenstein MD. 2012. Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 109, 101–116. ( 10.1007/s10533-011-9638-3) [DOI] [Google Scholar]
- 78.Stres B, Philippot L, Faganeli J, Tiedje JM. 2010. Frequent freeze–thaw cycles yield diminished yet resistant and responsive microbial communities in two temperate soils: a laboratory experiment. FEMS Microbiol. Ecol. 74, 323–335. ( 10.1111/j.1574-6941.2010.00951.x) [DOI] [PubMed] [Google Scholar]
- 79.Walker VK, Palmer GR, Voordouw G. 2006. Freeze-thaw tolerance and clues to the winter survival of a soil community. Appl. Environ. Microbiol. 72, 1784–1792. ( 10.1128/AEM.72.3.1784-1792.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wardle DA. 1998. Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol. Biochem. 30, 1627–1637. ( 10.1016/S0038-0717(97)00201-0) [DOI] [Google Scholar]
- 81.Wallenstein MD, Hall EK. 2012. A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry 109, 35–47. ( 10.1007/s10533-011-9641-8) [DOI] [Google Scholar]
- 82.Orwin KH, Wardle DA, Greenfield LG. 2006. Context-dependent changes in the resistance and resilience of soil microbes to an experimental disturbance for three primary plant chronosequences. Oikos 112, 196–208. ( 10.1111/j.0030-1299.2006.13813.x) [DOI] [Google Scholar]
- 83.Bardgett RD, Freeman C, Ostle NJ. 2008. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2, 805–814. ( 10.1038/ismej.2008.58) [DOI] [PubMed] [Google Scholar]
- 84.Hasibeder R, Fuchslueger L, Richter A, Bahn M. 2015. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 205, 1117–1127. ( 10.1111/nph.13146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Canarini A, Dijkstra FA. 2015. Dry-rewetting cycles regulate wheat carbon rhizodeposition, stabilization and nitrogen cycling. Soil Biol. Biochem. 81, 195–203. ( 10.1016/j.soilbio.2014.11.014) [DOI] [Google Scholar]
- 86.Bakhshandeh S, Corneo PE, Yin L, Dijkstra FA. 2019. Drought and heat stress reduce yield and alter carbon rhizodeposition of different wheat genotypes. J. Agron. Crop Sci. 205, 157–167. [Google Scholar]
- 87.Karlowsky S, Augusti A, Ingrisch J, Uddin Akanda MK, Bahn M, Gleixner G. 2018. Drought-induced accumulation of root exudates supports post-drought recovery of microbes in mountain grassland. Front. Plant Sci. 9, 1593 ( 10.3389/fpls.2018.01593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuchslueger L, Bahn M, Hasibeder R, Kienzl S, Fritz K, Schmitt M, Watzka M, Richter A. 2016. Drought history affects grassland plant and microbial carbon turnover during and after a subsequent drought event. J. Ecol. 104, 1453–1465. ( 10.1111/1365-2745.12593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chomel M, et al. 2019. Drought decreases incorporation of recent plant photosynthate into soil food webs regardless of their trophic complexity. Glob. Change Biol. 25, 3549–3561. ( 10.1111/gcb.14754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karlowsky S, Augusti A, Ingrisch J, Hasibeder R, Lange M, Lavorel S, Bahn M, Gleixner G. 2018. Land use in mountain grasslands alters drought response and recovery of carbon allocation and plant-microbial interactions. J. Ecol. 106, 1230–1243. ( 10.1111/1365-2745.12910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mariotte P, Canarini A, Dijkstra FA. 2017. Stoichiometric N:P flexibility and mycorrhizal symbiosis favour plant resistance against drought. J. Ecol. 105, 958–967. ( 10.1111/1365-2745.12731) [DOI] [Google Scholar]
- 92.De Vries FT, Williams A, Stringer F, Willcocks R, McEwing R, Langridge H, Straathof AL. 2019. Changes in root exudate induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 224, 132–145. ( 10.1111/nph.16001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bokhorst S, Bjerke JW, Street LE, Callaghan TV, Phoenix GK. 2011. Impacts of multiple extreme winter warming events on sub-Arctic heathland: phenology, reproduction, growth, and CO2 flux responses. Glob. Change Biol. 17, 2817–2830. ( 10.1111/j.1365-2486.2011.02424.x) [DOI] [Google Scholar]
- 94.Pardee GL, Jensen IO, Inouye DW, Irwin RE. 2019. The individual and combined effects of snowmelt timing and frost exposure on the reproductive success of montane forbs. J. Ecol. 107, 1970–1981. ( 10.1111/1365-2745.13152) [DOI] [Google Scholar]
- 95.McDowell NG, et al. 2013. Evaluating theories of drought-induced vegetation mortality using a multimodel–experiment framework. New Phytol. 200, 304–321. ( 10.1111/nph.12465) [DOI] [PubMed] [Google Scholar]
- 96.Bardgett RD, Manning P, Morrien E, De Vries FT. 2013. Hierarchical responses of plant–soil interactions to climate change: consequences for the global carbon cycle. J. Ecol. 101, 334–343. ( 10.1111/1365-2745.12043) [DOI] [Google Scholar]
- 97.Wilcox KR, von Fischer JC, Muscha JM, Petersen MK, Knapp AK. 2015. Contrasting above- and belowground sensitivity of three Great Plains grasslands to altered rainfall regimes. Glob. Change Biol. 21, 335–344. ( 10.1111/gcb.12673) [DOI] [PubMed] [Google Scholar]
- 98.Knapp AK, Carroll CJ, Denton EM, La Pierre KJ, Collins SL, Smith MD. 2015. Differential sensitivity to regional-scale drought in six central US grasslands. Oecologia 177, 949–957. ( 10.1007/s00442-015-3233-6) [DOI] [PubMed] [Google Scholar]
- 99.Rubin RL, van Groenigen KJ, Hungate BA. 2017. Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant Soil 416, 309–323. ( 10.1007/s11104-017-3199-8) [DOI] [Google Scholar]
- 100.Orwin KH, Wardle DA. 2005. Plant species composition effects on belowground properties and the resistance and resilience of the soil microflora to a drying disturbance. Plant Soil 278, 205–221. ( 10.1007/s11104-005-8424-1) [DOI] [Google Scholar]
- 101.Bloor JM, Bardgett RD. 2012. Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: interactions with plant species diversity and soil nitrogen availability. Perspect. Plant Ecol. Evol. Syst. 14, 193–204. ( 10.1016/j.ppees.2011.12.001) [DOI] [Google Scholar]
- 102.Craine JM, Ocheltree TW, Nippert JB, Towne EG, Skibbe AM, Kembel SW, Fargione JE. 2013. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Change 3, 63–67. ( 10.1038/nclimate1634) [DOI] [Google Scholar]
- 103.Polley HW, Isbell FI, Wilsey BJ. 2013. Plant functional traits improve diversity-based predictions of temporal stability of grassland productivity. Oikos 121, 1275–1282. ( 10.1111/j.1600-0706.2013.00338.x) [DOI] [Google Scholar]
- 104.Jung V, Albert CH, Violle C, Kunstler G, Loucougaray G, Spiegelberger T. 2014. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 102, 45–53. ( 10.1111/1365-2745.12177) [DOI] [Google Scholar]
- 105.Fry EL, Savage J, Hall AL, Oakley S, Pritchard WJ, Ostle NJ, Pywell RF, Bullock JM, Bardgett RD. 2018. Soil multifunctionality and drought resistance are determined by plant structural traits in restoring grassland. Ecology 99, 2260–2271. ( 10.1002/ecy.2437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dent CL, Cumming GS, Carpenter SR. 2002. Multiple states in river and lake ecosystems. Phil. Trans. R. Soc. Lond. B 357, 635–645. ( 10.1098/rstb.2001.0991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scheffer M, et al. 2009. Early-warning signals for critical transitions. Nature 461, 53–59. ( 10.1038/nature08227) [DOI] [PubMed] [Google Scholar]
- 108.Barnosky AD, et al. 2012. Approaching a state shift in Earth's biosphere. Nature 486, 52–58. ( 10.1038/nature11018) [DOI] [PubMed] [Google Scholar]
- 109.Dakos V, Carpenter SR, van Nes EH, Scheffer M. 2015. Resilience indicators: prospects and limitations for early warnings of regime shifts. Phil. Trans. R. Soc. B 370, 20130263 ( 10.1098/rstb.2013.0263) [DOI] [Google Scholar]
- 110.Wernberg T, et al. 2016. Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172. ( 10.1126/science.aad8745) [DOI] [PubMed] [Google Scholar]
- 111.Koerner SE, Avolio ML, La Pierre KJ, Wilcox KR, Smith MD, Collins SL. 2016. Nutrient additions cause divergence of tallgrass prairie plant communities resulting in loss of ecosystem stability. J. Ecol. 104, 1478–1487. ( 10.1111/1365-2745.12610) [DOI] [Google Scholar]
- 112.Hautier Y, et al. 2014. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 508, 521–525. ( 10.1038/nature13014) [DOI] [PubMed] [Google Scholar]
- 113.Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S. 2005. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl Acad. Sci. USA 102, 4387–4392. ( 10.1073/pnas.0408648102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chisholm RA, Menge DN, Fung T, Williams NS, Levin SA. 2015. The potential for alternative stable states in nutrient-enriched invaded grasslands. Theor. Ecol. 8, 399–417. ( 10.1007/s12080-015-0258-8) [DOI] [Google Scholar]
- 115.Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M, Eisen JA. 2009. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl Acad. Sci. USA 106, 17 187–17 192. ( 10.1073/pnas.0904847106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. ( 10.1038/nature11550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allison SD, Martiny JB. 2008. Resistance, resilience, and redundancy in microbial communities. Proc. Natl Acad. Sci. USA 105, 11 512–11 519. ( 10.1073/pnas.0801925105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leff JW, et al. 2015. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl Acad. Sci. USA 112, 10 967–10 972. ( 10.1073/pnas.1508382112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou J, et al. 2012. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Change 2, 106–110. ( 10.1038/nclimate1331) [DOI] [Google Scholar]
- 120.Zhou J, Deng Y, Luo F, He Z, Yang Y. 2011. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. MBio 2, e00122-11 ( 10.1128/mBio.00122-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robinson DA, Jones SB, Lebron I, Reinsch S, Domínguez MT, Smith AR, Jones DL, Marshall MR, Emmett BA. 2016. Experimental evidence for drought induced alternative stable states of soil moisture. Sci. Rep. 6, 20018 ( 10.1038/srep20018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Todmam LC, Fraser FC, Corstanje R, Harris JA, Pawlett M, Ritz K, Whitmore AP. 2018. Evidence for functional state transitions in intensively-managed soil ecosystems. Sci. Rep. 8, 11522 ( 10.1038/s41598-018-29925-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kreyling J, Schweiger AH, Bahn M, Ineson P, Migliavacca M, Morel-Journel T, Christiansen JR, Schtickzelle N, Larsen KS. 2018. To replicate, or not to replicate—that is the question: how to tackle nonlinear responses in ecological experiments. Ecol. Lett. 21, 1629–1638. ( 10.1111/ele.13134) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.