Abstract
Biodiversity has always responded dynamically to environmental perturbations in the geological past, through changes to the abundances and distributions of genes and species, to the composition of biological communities, and to the cover and locations of different ecosystem types. This is how the ‘nature’ that exists today has survived. The same is true in the Anthropocene. The entire planet surface has been altered by humans, ranging from direct vegetation transformation and removal of most of the world's megafauna, through to atmospheric changes in greenhouse gasses and consequent climatic changes and ocean acidification. These anthropogenic perturbations have led to the establishment of genes and species in new locations, thus generating novel communities and ecosystems. In this historical context, recent biological changes should be seen as responses to multiple drivers of change, rather than being a problem per se. These changes are the means by which the biosphere is adjusting to and will ultimately survive the Anthropocene. Thus, management and conservation of the biological world, and our place in it, requires a transition from trying to minimize biological change to one in which we facilitate dynamism that accelerates the rates at which species and ecosystems adjust to human-associated drivers of change.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions’.
Keywords: Anthropocene, biodiversity, biological invasions, climate change, conservation, land-use change
1. Introduction
Humans are generating novel combinations of physical and biological conditions across the world [1,2]. These perturbations include the removal of most terrestrial megafaunal diversity and over-exploitation of fisheries; the conversion of more than a third of the world's terrestrial ecosystems for agriculture, livestock (replacement megafauna) grazing and human dwellings; doubling global nitrogen fixation and the release of a wide array of novel chemicals into the environment; and the transfer of species around the planet at an unprecedented rate. Other rapid perturbations also include the release of greenhouse gasses, especially CO2, resulting in altered photosynthetic rates for plants, climate change and ocean acidification. Both the high speed of change (e.g. rate of atmospheric CO2 increase) and the destination (e.g. potential future CO2 concentrations not seen for 20 million years) are important to biotic responses, as well as the fact that multiple pressures are acting together [3–6]. The global extent of these changes means that essentially every location on the Earth has already been influenced by human activity, save for an unknown proportion of microbial communities within geological substrates. The result has been the decline and extermination of some genes, populations and species, a loss which has been characterized as ‘the biodiversity crisis'. However, the new anthropogenic conditions simultaneously favour other genes, populations and species, increases of which can be thought of as biological gains. These gains, as well as losses, are so extensive that it is no longer possible to disentangle human and non-human contributions to gene frequencies, geographical distributions, the composition of biological communities or ecosystem functions [7,8]. Humans and human-related activities are integral to the biological processes of the Earth system, arising from the unlikely evolution and then social and technological development of the human ape, ushering in the Anthropocene [2].
Despite the prevalence of human influences, the fundamental processes that underpin biological change remain qualitatively unaltered across the world's surface—at least, so far. Changes to the abundances and distributions of genes, populations and species are still achieved by the birth, death and movement of individuals, and their interactions determine community composition and ecosystem processes. They are simply doing so in the context of human modification of the physical and biotic environment (including humans both influencing the variation that comes into existence and acting as selective agents), reshuffling the Earth's biotic ‘building blocks’ as a faster rate than usual.
From an evolutionary perspective, individuals of each organism (bearing different combinations of genes and capacity for plasticity) still vary in their survival and reproduction in different environments, albeit in human-modified ones. Mutation, recombination, gene flow, hybridization (i.e. gene flow between less closely related entities) and horizontal gene transfer determine the variation available for evolution to act upon in new anthropogenic environments [9]. This includes any genetic variation accidentally or deliberately generated by humans and recognizes that the human animal is an important selective force. Evolutionary change can also be thought of as operating at a range of micro- to macroevolutionary levels: from mutations and changes in gene frequencies within populations and metapopulations to the relative success or failure of evolutionarily distinct populations and races within species, the success or failure of different species, through to differences in the origination and extinction rates of different clades or functional types. Thus, declines and extinctions, and also the success of alleles, populations, races, species and higher taxa—all of which have been observed in the Anthropocene—represent evolutionary changes taking place at multiple taxonomic, spatial and temporal scales (e.g. [10–15]). These processes (generation of variation and selection acting upon it) are fundamentally the same as they have been in previous epochs while accepting that the rates of change in the Anthropocene are exceptionally high.
In what follows, I am attempting to extract broad biological generalizations, rather than to describe the situation in every location or region. Regions differ in their biological histories, climates, geologies and human social and economic situations, resulting in a wide variety of specific changes. I am also not passing any value judgements, at least not until §6 of the paper! Our human opinions about the relative worth of different species, about the ecosystem services we derive from historic versus novel ecosystems, and about the importance of diversity levels on different spatial and temporal scales are societal issues for which there cannot be universal ‘correct’ answers. Whatever our individual perspective, biological diversity (with both inputs of new species arriving and the disappearance of species that were present previously) is changing at all spatial scales, and dynamic change is going to continue. It is our attitudes and responses to these changes, and how we might attempt to influence future trajectories of change, that are a matter for debate. Anthropocene change is commonly perceived as being characterized by biological collapse, with an emphasis on the need for humanity to ‘save biodiversity’ and the ecosystem services that it provides (e.g. [16–20]). I agree that we should. However, we have to work with biological, human and physical planetary systems as they are, rather than as we might wish them to be. My focus here, therefore, is on the dynamism of biological systems, and hence that maintaining biodiversity in the long term requires us to embrace rather than reject or repel many of the processes of biological change [7]. To this end, §§2–5 represent my interpretation of biological changes that have taken place in response to environmental perturbation in the recent and more distant past, while §6 discusses the implications of these changes for the conservation of biodiversity.
2. Biological communities are especially dynamic during periods of environmental change
The Pleistocene climatic switchback between frigid glacial conditions (such as the last glacial maximum, approximately 26 500 years ago) and relatively warm interglacials (as for the last approximately 11 700 years of the Holocene) has generated drastic and repeated changes to the composition of local biological communities and regional biotas, during which some species have been extirpated (extinction at the local or regional scale) while others have arrived and their populations grown [21–23]. There are potential lags both in times to extirpation (extinction debt) and the arrival and increases of species that will subsequently thrive under the new conditions (colonization deficits) [24,25]. There are also slow community and ecosystem development processes (e.g. population dynamics of long-lived trees, soil development), such that we observe trajectories of community change over decades to millennia [26–28]. Lags are difficult to determine precisely, partly because of the temporal resolution and spatial incompleteness of palaeo-records, and partly because it is hard to distinguish between delayed responses to an earlier perturbation and immediate responses to a later stimulus. Nonetheless, delays can be substantial. For example, the distributions of some European tree species may not have fully ‘caught up’ with climatic changes that took place over 10 000 years ago [29]. At a global scale, the relatively cold 2.6 Myr of the Pleistocene has seen both species-level extinctions of warm-adapted species, such as frost-sensitive trees that lived in Europe during the warmer Pliocene [30], and diversification of cold-adapted and colonizing lineages, including lupins in the Andes [31]. Changes in precipitation played an equally important role, especially in the tropics. Thus, the Quaternary (Pleistocene plus Holocene) and earlier episodes of climate instability (including the Palaeocene-Eocene Thermal Maximum approximately 55 Ma; [32]) have consistently been associated with high rates of distribution and community composition changes at local, regional and larger scales. Today, rates of warming are even faster, at a global scale.
More generally, biological changes within any given region—be that an island, landscape, seascape or present-day human administrative unit such as a county or country—are generated by changes to the physical environment, the arrival of new species from outside and the disappearance of species that are no longer able to survive under the new physical and biological conditions. Within each region, the populations of species present develop altered abundances and local distributions (as a result of birth, death and movement), evolve in relation to the new physical environment and co-evolve in relation to one another. Delayed arrivals, delayed extirpations and feedback loops involving community composition, ecosystem processes and evolutionary adjustments generate lags in the system [33].
It is helpful to consider the parallels between evolutionary and ecological change [34–36]. The rate of evolutionary change in a population commonly increases with the strength of selection, with the level of relevant genetic variation within that population, and with gene flow that increases the variation that is available for selection to act on. This is uncontroversial. The same is inevitably true at the community level: changes to the identities and relative abundances of species within communities (equivalent to changes in allele frequencies, or rate of evolution) accelerate with the rate of environmental change (equivalent to the strength of selection) and also increase with the diversity of species within the initial community (equivalent to the standing genetic diversity within a population) and with the immigration of new taxa (equivalent to gene flow).
Note that compositional (i.e. community) change is not equivalent to ecosystem change, and the functional attributes of an ecosystem may either be stabilized (e.g. a forest remains a forest through increases in drought-resistant species) or destabilized by community changes (e.g. a forest becomes a savannah through increases in drought-resistant species that are flammable). This is again equivalent to evolutionary change in a population, where selection, gene flow and evolution at particular loci may provide resilience and stability to the rest of that population's genome (adaptive introgression), or where gene flow may effectively replace (swamp) the previously resident genome [37]. Thus, an ecosystem type may be stabilized (e.g. maintained as forest) or disrupted (e.g. becomes another ecosystem) by changes to the species composition of a biological community, and a ‘locally adapted’ population type may be stabilized or disrupted (replaced) by the arrival of new alleles, depending on circumstances. However, in each case, the biotic change is normally generating populations, communities and ecosystems that are now ‘better adjusted’ to the new physical and biological reality of the location that is under consideration. I am using the term ‘adjusted’ as a very broad term to refer to any alteration, be that evolutionary or ecological, which increases some definable metric of individual, population or ecosystem performance, such as individual fitness, population growth rate or primary productivity.
While mutation is evidently central to the evolution and ecology of life on Earth in the long run, the immediate response to rapid environmental change is predominantly achieved by re-arrangements of those elements (genes and species—the latter can be thought of as bundles of genes) that existed previously. Changes to the abundances of alleles and species in space and time are what permit the global biotic system to adjust to rapid environmental change, generating successions of new populations, communities and functioning ecosystems. This means that certain genes and species have been rare or localized under some environmental circumstances but relatively common and widespread at other times, generating overall flexibility and resilience of biological systems at regional and global scales. For example, European warm-adapted tree species become confined to climatically suitable refugia in southern Europe during glacial maximum conditions but expand during interglacials [38,39], whereas cold-adapted bird species do the reverse, becoming confined to relatively cold refugia at high elevations and in northern Europe during warmer interglacial conditions [40].
In conclusion, all past biological communities and ecosystems have been constructed dynamically from combinations of genes and species. This remains the case today, and all future biological systems will be constructed from the descendants of today's species unless humans generate entirely new life forms.
3. Plus ça change in the Anthropocene
The new biological and physical reality of the Earth surface includes humans, who have collectively exterminated megafauna, increased atmospheric CO2 concentrations, changed the climate and increased the acidity of the oceans even in the remotest locations. The existence of areas without human influence passed into history long ago. All notions of wilderness, protected areas, saving a ‘half Earth’ for nature, or rewilding must be seen in this context. Our human abundance, consumption and functional capabilities (including cultural development and desires) have triggered environmental change everywhere.
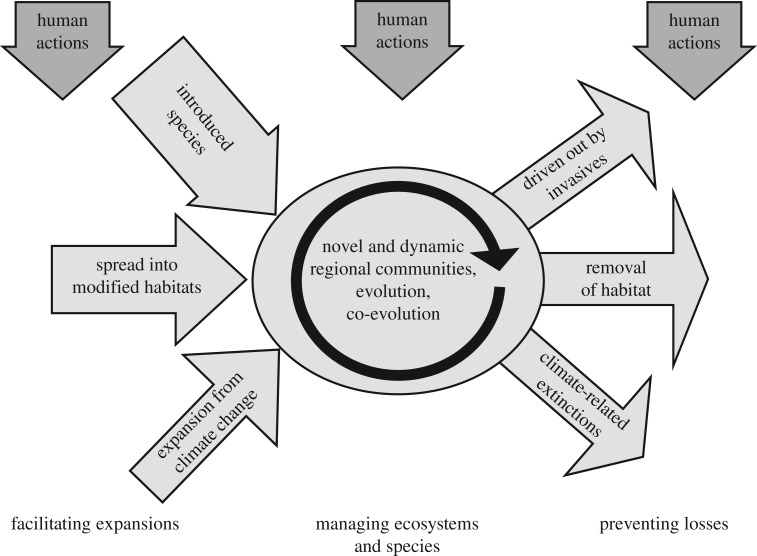
The basic processes by which biotic systems have responded to these changes remain broadly similar, however, with inputs and departures of genes and species at any given location, and new interactions then taking place among the novel set of organisms that establish in that location (figure 1). Some genotypes, populations, entire species (in endemic-containing regions) and ecosystem types are extirpated as a result. Humans are also instrumental in biological gains. Species have colonized new areas because the human-altered environment is more suitable for them. For example, the climate has become more suitable in new locations for many warmth-associated species, as evidenced by polewards range expansions of a majority of temperate zone animal species [6,41,42], and new human-derived habitats have become available for grassland and disturbance-dependent species in regions that were previously forested. Species have also spread because humans actively accelerate the rates at which species immigrate into new areas through accidental and deliberate introductions. A wide range of human-associated and non-human changes to the environment can trigger new arrivals and disappearances, and figure 1 simply illustrates three major anthropogenic impacts. As with Pleistocene perturbations of the climate, there are time lags in the delayed arrival and extirpation of genes and species, as well as ecological and evolutionary feedbacks [33]. Even if all humans were to disappear tomorrow, the past human-mediated transfer of species between continents, for example, will still affect the biological future of every region on Earth for an indefinite period. The Anthropocene represents multiple trajectories of change, not a new categorical state.
Figure 1.
Inputs and losses of biological diversity in the Anthropocene, from a regional perspective (the region can be small or large). The schema is illustrative rather than exhaustive. The larger ‘arrival’ than ‘departure’ arrows reflect that regional diversity (number of species per country or island) has increased for most parts of the world, over the last 300 years. Humans affect changes taking place within each region, the arrival of new species and the losses of ones that die out. The questions (bottom of figure) relate to what our targets should be for any regions when systems are dynamic (centre); who, why and how we should decide to import additional species deliberately (left); and why and how we might try to retain species for which the region is no longer suitable for their survival (right).
The re-mixed sets of species interact ecologically and evolve in relation to the new physical environment, and co-evolve in relation to one another. Local evolution, population declines, population increases, distribution changes, changes to communities and regional biotas, and changes to ecosystems are all being observed within novel anthropogenic environments (e.g. [7,43]). As during previous epoch-changing events, the net result is generating populations, communities and ecosystems that are more resilient under the new, albeit transient, conditions than were the biological communities that preceded them (the previous sets of organisms were in most cases better adjusted to the previous non-anthropogenic set of conditions). Some of these changes are regarded as beneficial by humanity, and some negative. For example, the development of pollinator communities associated with urban gardens and ruderal communities is typically seen as beneficial, as are increases in specific insects that pollinate widespread crop plants, whereas declines in other pollinators because of land-use changes and pesticides are seen as negative [44–46]. But this is a human perspective. The net result of both the gains and losses is an improved match between the distributions and abundances of pollinators and the current human-altered environment (including nectar and pollen resource availability, places to nest or otherwise reproduce, and insecticides as one aspect of the chemistry of the modern environment), not a failure of basic biological processes.
4. ‘Local diversity stays about the same, regional diversity increases and global diversity declines' [47]
The net balance of the human-driven gains and losses depicted in figure 1 depends on the temporal and spatial scale considered. These differences emerge because patterns of colonization and extirpation themselves vary in space and time. There are different spatial and temporal signatures of key processes, such as human-caused habitat change, over-exploitation (including megafaunal extinctions), humans acting as a vector for the transport of species around the world and, increasingly, climate change. Scaling differences are also linked to the relative importance of different population dynamic processes at different spatial scales [48]. Changes to the number of species at a very local scale primarily depend on the birth, death and movement of individuals, while (meta-) population-level colonization and extirpation are also important at regional scales, as are extinction and speciation at the global scale. Similarly, patterns of change may vary through time, depending on the durations over which extinction debts, colonization lags and community reassembly take place. These differences make it possible for diversity to increase at some spatial scales and decline at others [49] and to increase on some time scales and decline at others, depending on the temporal schedules of arrivals and departures. Diversity can, therefore, decline in response to some human-mediated perturbations at first and then increase later as species gradually colonize novel environments (transient diversity troughs), or vice versa if initial arrival rates exceed subsequent extirpation rates [50].
Consider the evidence, first in relation to spatial scale. An increasingly large literature suggests that the average number of species in local biological communities (i.e. for relatively small sample areas) has been fairly stable or has even increased slightly in recent decades [51–53]. In other words, the rate of arrival of new species matches the rate of disappearance of others when averaged across many sites, although some individual sites exhibit net increases while others show declines. The exception is when there is a ‘one-off’ reduction associated with major land-use change or intensification [54]. These observations (little or no net change despite one-off diversity reductions) are compatible because only a modest percentage of the world's land surface experiences fundamental diversity-reducing land-use change each year, and the transformed lands may then gradually accumulate species that are suited to the new environments. Inputs to these novel habitats include, for example, the colonization of urban areas by former cliff-dwelling birds, the establishment of disturbance-dependent communities of plants and insects, and the range expansions of introduced species. Given time, derived environments can potentially support substantial numbers of species, such as ‘semi-natural’ calcareous grasslands (livestock pastures) in parts of Europe, domestic parks and gardens in many parts of the world, and the development of reefs on human structures in marine systems. Anthropogenic climate change can also contribute to the growth of local species richness whenever range shifts take place along species richness gradients, for example, when the expansions of warm-associated species outnumber the retreats of cold-associated species (e.g. [6,7,25,50,55]). The trickle of species accumulating in novel environments and under altered climates counteract (but do not necessarily match) the more easily attributable losses taking place in the subset of localized areas where land-use transformation or intensification is taking place.
By contrast, if we increase the sample area considered, the number of species has substantially increased in recent centuries for most regions of the world (e.g. per country- or state-sized area of land, or per island group [49,55–58]). At this larger spatial scale, the number of species establishing has exceeded the number of extirpations and extinctions, and hence the ‘input’ arrows are shown as wider than the departure arrows in figure 1. These increases have predominantly been associated with the formation of novel anthropogenic habitats within each region, which provide new opportunities for colonization, and with the deliberate and accidental transport of immigrant species [59].
However, humans have been responsible for reducing the total number of species (and presumably unique alleles) on Earth. At this very large spatial scale, the rate of extinction has exceeded the rate of speciation in recent centuries, for animals at least [13,17,60,61], despite a human-associated acceleration in the speciation rate [62,63].
A consistent pattern is emerging. Measured species richness change shows a hump-shaped relationship with increasing ‘plot’ size. Local diversity has remained relatively stable (increasing in some locations, declining in others), regional diversity has generally increased, and global diversity has declined [47,49,55,57]. These conclusions are based on observed changes to the numbers of species, given that this is the information that is most readily available. Nonetheless, the same principles of the arrival and disappearance of genes will apply and, because species bear genes, we might generally expect similar results (considering total genetic variation at the assemblage level). There are inputs of new genetic variation to biological assemblages through gene flow, as well as through the establishment of colonizing species. Losses are associated with selection and the extirpation of individual alleles, populations and species. Newly arriving species might initially be expected to hold relatively low within- and among-population levels of variation arising from founder bottlenecks, but the addition of new species (especially unrelated ones from distant continents) also introduces genetic variation and functions not present in the original species within a particular site or broader region. At a global scale, allele extinction (associated with population, race and species-level extinctions) almost certainly exceeds the establishment of novel mutations. Even so, the many examples of populations undertaking evolutionary adaptation to anthropogenic environments imply that novel mutations are also establishing [7,9]. Thus, I hypothesize that there is also likely to be a humped-shaped relationship for total genetic diversity, with a net loss at a global scale, net increase at a regional level (mainly associated with the net increase in species richness and the taxonomic diversity of colonizing and imported species) and intermediate change at local scales. Net genetic diversity will undoubtedly have increased in some locations and declined in others, at a local scale, but insufficient data are available to deduce the overall average direction of change.
Contrary to the common perception of biodiversity decline, the positive trend in the number of species per region is very likely to continue as the processes that have driven the growth of regional richness are still operating (international transport more so than ever), notwithstanding that there will be exceptions in some regions. The postulated slight upwards trend in species richness at a local scale can also be expected to continue. There is generally a positive correlation between the size of the regional pool of species and the species richness of local communities [64,65], such that recent increases in regional diversity place ‘upwards pressure’ on the average species richness of local communities. However, fresh land conversion and intensification will generate ‘downwards pressure’ on those locations directly affected [54], and hence any possible growth in local richness is expected to be much slower than that of regional richness. At a global scale, species extinctions are expected to continue to exceed speciation [4,17], but not necessarily for higher plants [61,62,66].
5. The composition of communities
Differences in the identities of species and genetic variation at different locations (beta diversity) are equally important, and the scaling effects are reminiscent of those for species richness. The transport of species has reduced beta diversity at global and continental scales: biotas more than, say, 2000 km apart are usually more similar to one another than they used to be [49,67,68], and the same must be true for the unique genetic variation associated with these species. For example, Great Britain and New Zealand share many more species than they did 1000 years ago, and the extinction of New Zealand endemics has also eroded the difference.
By contrast, beta diversity has increased within each region for two reasons. First, ‘naturally colonizing’ and introduced species have disproportionately established populations in human-transformed ecosystems [69–72] over the course of recent centuries and millennia, leaving higher proportions of ‘native’ species in less disturbed locations, thereby contributing to increased beta diversity between ecosystem types ([73,74]; although this can reverse with extreme intensification, [7,75], cf. [49]). Second, despite the linguistic implications of terms such as ‘invasive species’, immigrant ‘non-native’ species tend to have smaller geographical range sizes within regions than do the longer-established ‘native’ species, at least for arrivals within the last 500 years [76,77]. This again increases compositional differences between locations. Within-region beta diversity of genetic variation is also likely to have increased, linked to the species-level differences and to different selection pressures operating in anthropogenic versus less disturbed ecosystems. Quite how beta diversity is changing at very local scales is less clear, but there is some indication that it could be declining [49]. If so, we also see a hump-shaped relationship for beta diversity change, with the greatest increases taking place at intermediate (within-region) spatial scales, and declines at very local (potentially) and global scales.
Turnover in the composition of biological communities through time is also highly relevant and is known to have accelerated during periods of rapid environmental change, in the Pleistocene and before (see above). It is no surprise, therefore, that the rate of turnover in species composition in any given location or region has increased in recent centuries [51–53,78]. This is likely to be true at all spatial scales [49], even at the global scale, where both extinction and speciation rates have apparently accelerated [13,17,47,60–63,66,79]. Genetic turnover is also likely to have increased at all spatial scales, with increased selection in favour of adaptations to novel environments, allele extinction (associated with population, race and species-level extinctions), gene flow (including establishment of species in new locations) and the establishment of novel mutations which provide increased fitness in anthropogenic environments. We can confidently predict that species and gene turnover will continue apace in coming decades, as they respond to multiple human-associated drivers of change—and show lagged responses to events that have already taken place.
6. Managing dynamic systems
Human actions will continue to affect the establishment as well as the loss of biological diversity at all spatial scales in the future, and it is inevitable that we will wish to manage biodiversity at local, regional and global scales to minimize risks of harm and maximize potential benefits, including the benefit of simply enjoying nature. However, this is challenging when nature is dynamic [80], and when human-caused changes to the atmosphere, land, freshwaters and seas are in the process of accelerating change. It is not straightforward to determine priorities in a dynamic system (figure 1), given that different individuals and interest groups will take different perspectives and imagine different futures.
If nature was ‘nearly static’ relative to human lifetimes and cultures, it might seem appropriate to define environmental and biodiversity ‘baselines’, representing either the current condition of ecosystems or some historical state that could in principle be maintained. However, this approach eventually fails in a dynamic system. Baseline thinking implicitly holds that all ecological and evolutionary change up to the baseline date or condition is ‘good’ (including the immigration of species and genes up to that time), whereas all subsequent deviations (including the immigration of additional species and in some instances genes) are deemed to be undesirable, in the sense that they represent departures from that ‘preferred’ state. This is philosophically and practically flawed. It is inevitable that every location will gradually depart from its baseline, without necessarily degrading the local, regional or global system, especially during episodes of rapid climate change. Such is the history of life on Earth.
To illustrate how these social attitudes can play out, England has adopted several biodiversity indicators representing changes to the status of priority ‘native’ species and one representing the spread of selected ‘non-native’ species [81]. Both the decreasing trend for ‘native’ species (most of which have colonized Britain in the last 12 000 years) and increasing trend for ‘non-native’ species (which have arrived in the last 500 years) are deemed to be negative. The net result is a near-universal perception that biodiversity is declining in Britain (it is by some metrics), even though it is unequivocally true that the total number of species in Britain is increasing, if we add all losses and all gains together ([82]; see above). The rate of arrival and establishment of species exceeds the rate of extirpation of others. This is not just a UK-centric perspective. For example, Sala et al.'s [3] renowned global scenarios for future biodiversity explicitly exclude gains of ‘exotic’ species and omit biological communities that are ‘maintained by regular human intervention’ [3, p. 1770], thereby implicitly assuming both that there is a correct distribution of species and that contributions of anthropogenic ecosystems to biodiversity are negligible (despite the fact that all ecosystems are somewhat modified). Neither assumption is consistent with the past, recent and future dynamics of species and ecosystems during a period of environmental change and human influence.
This apparent preference for what we already have, or used to have, is reflected in the United Nations Sustainable Development Goals, the Convention on Biological Diversity Aichi targets, the legislation of individual countries, and the mission statements of numerous global and national non-governmental organizations and corporations. By contrast, the same conventions, legislatures and organizations typically refer to recently successful species in the condemnatory language (for example, as weeds, pests, adventives, non-natives or invasive aliens) and urge individuals, institutions and nations to take action against them. The establishment of successful species in new locations is commonly interpreted as further evidence that the Earth system is departing from a more desirable state, located somewhere in the historical past. However, the reality is that we always have been, are and will continue to live with dynamic systems, in which gains are as much a reality as losses.
Aligned with this policy emphasis, most ongoing conservation effort focuses on retaining existing ecosystems (retarding change in the central part of figure 1) and reducing extirpations (right-hand side of figure 1), with an element of the reintroduction of previously extirpated species. By contrast, the inputs of new genes and species associated with novel environments are largely left to happenstance, with inputs from far afield (introductions of non-native species) commonly regarded or defined as negative (left-hand side of figure 1). Instead, actions and expenditure concentrate on repelling new arrivals. This is puzzling, given that inputs of new functional elements (genes, species) facilitate adjustments of ecosystems, and potentially generate ecosystem transformations that increase longer-term resilience.
Facilitating the arrival of species and genes that provide benefits (ecosystem goods and services, including pleasure from the presence of new species) is just as legitimate—no more, no less—an intervention in a dynamic system as managing existing biodiversity or attempting to avoid extirpations. Indeed, it is already widely applied in agriculture, forestry and, on occasion, in conservation (e.g. [83]). Facilitating arrivals may also be easier. When large-scale external forces cannot be counteracted locally or regionally, it could be less costly and more practical to introduce new elements that thrive under the new conditions than attempt to save the last few individuals of species that will inevitably die out from that location (provided that they are can survive elsewhere). Yet, the inputs of new genes and species commonly remain off the radar—at least as a specific approach to conservation management. Arrivals are still going to take place as conditions change, but this generates angst as often as it is perceived as an opportunity. By contrast, environmental mangers and conservationists could intervene to increase the rate of arrival of desirable organisms, as we already have in the contexts of agriculture, horticulture and forestry.
Given that climatic and other ongoing environmental changes are inevitable, conservation bodies are re-adjusting to the new reality (table 1). For example, ‘connectivity conservation’ is perceived as increasingly important, in the contexts of both minimizing negative impacts of habitat fragmentation and facilitating distributional responses to climate change [84–86]. However, setting goals for dynamic systems is genuinely challenging. Priority setting requires a shift away from ‘how do I save my country's species and ecosystems?’ (which will eventually fail because of ongoing environmental and distribution changes) to a more global perspective of ‘how can my site, county, or country contribute most effectively to global conservation in the long run?’. With the latter perspective, citizens who live in countries that contain many narrowly distributed endemic species are likely to maintain and develop existing priorities, but with increased flexibility, because the national ‘good’ (saving species x in country y) is similar to the global ‘good’ (saving entire species from extinction). Elsewhere—over most of the land and oceans because areas of high endemism are localized—it is possible to take a more relaxed attitude to the precise identities and abundances of the species present in each location because a recently arrived species can have as much global significance (in terms of rarity, functions and contributions to ecosystem services) as one that has been present in the region for longer. A far greater emphasis can be placed on facilitating adaptive change, including trans situ conservation (table 1), the notion of enabling genes and species to reach locations where they might survive, thrive and contribute to ecosystem processes. Trans situ conservation can be enhanced by the redesign of landscapes and by the designation of protected areas to maintain existing habitat continuity along environmental gradients, so as to facilitate large-scale distribution changes. It can also be enhanced by the deliberate transport of genes and species. The logic is to save rare and endangered genes and species somewhere within the global system so that they have the potential to contribute to additional ecosystem functions, resilience and services in the recipient regions either in the near future (benefiting current and near-future generations) or in the longer term (the equity of generations).
Table 1.
Conservation strategies. Caricatures of ‘recent’ and ‘emerging’ conservation strategies, in the context of climatic and other drivers of environmental change. (In dynamic systems, increasing emphasis needs to be on balancing the global and regional importance of any actions, and on trans situ conservation, enabling genes and species to survive somewhere, even if that is not within their historical distributions.)
| broad strategy | recent emphasis | emerging emphasis |
|---|---|---|
| planning | mainly static, with priority areas, holding the line and some attempts to reverse past changes | dynamic, with the mental shift to accept and encourage dynamic ranges and novel ecosystems, co-benefits |
|
in situ
reserves, ecosystem protection and management |
primary approach, often local, protecting species in existing ranges and ecosystems, restoration and reintroduction | primary approach, regional and global perspectives, refugia, heterogeneous environments, engineered ecosystems |
|
ex situ
zoos, botanic gardens, gene/seed banks |
modest contribution, largely back-up collections, also for reintroductions (mainly of vertebrates and plants) | increased contribution, but still modest, for trans situ conservation, with gene banks (DNA code) for lost causes |
|
trans situ
facilitating movement to new locations |
trivial contribution, mainly associated with landscape-scale conservation and ecological corridors | major role, connectivity (stepping-stones, corridors), translocation, managing ecosystem transitions |
At present, there is a lack of accepted national and international guidelines and legal frameworks to facilitate prioritization and decision-making to bring about trans situ conservation (although a number of approaches and methodologies have been proposed: e.g. [87–90]). Achieving an increased consensus on assessing risks and opportunities (from actions and inactions) of trans situ conservation is a growing priority. Otherwise, the default position will be to leave the inputs of biodiversity to chance. Or rather, conservationists will simply be leaving the identities of new arrivals to be influenced by the actions of other sectors of society.
7. Concluding remarks
The gains in biological diversity that have been taking place at local and regional scales generally illustrate the resilience of the global biological system to human perturbation. Change per se should not be defined as negative. Biological change is what maintains biodiversity and functioning ecosystems during periods of perturbation to the Earth system. Thus, we should think twice before embarking on the forever treatment of the biological symptoms of change, many of which are indications of biological resilience, rather than of forthcoming collapse. Trying to stop biological change is often ineffective, and sometimes counter-productive. We require a paradigm shift from any concepts that are still inspired by a notional ‘balance of nature’ to a dynamic perspective of nature at multiple temporal and spatial scales [7,91]. If we wish to slow change, we should concentrate more on its causes than on the consequences—this should be the focus of human choices. In the meantime, biological communities will continue to change, and they are likely to do so at an accelerating rate until at least the end of this century. By that time, we will have stored up a millennium or more's worth of lagged changes, in extinction debt, colonization lags and community reassembly. Given that change is inevitable, we need to contemplate the benefits and opportunities that might be associated with the arrival of new species in changing ecosystems, as well as the harms and risks, and then decide whether, when and how to intervene so as to accelerate new arrivals and rates of biological change.
Data accessibility
This article does not contain any additional data.
Competing interests
I declare I have no competing interests.
Funding
I received funding support from the Leverhulme Trust.
References
- 1.Steffen W, Grinevald J, Crutzen P, McNeill J. 2011. The Anthropocene: conceptual and historical perspectives. Phil. Trans. R. Soc. A 369, 842–867. ( 10.1098/rsta.2010.0327) [DOI] [PubMed] [Google Scholar]
- 2.Ellis EC. 2018. Anthropocene: a very short introduction. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 4.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 5.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 6.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 7.Thomas CD. 2017. Inheritors of the earth: how nature is thriving in an age of extinction. New York, NY: Allen Lane, London & Public Affairs Books. [Google Scholar]
- 8.Bliege Bird R, Nimmo D. 2018. Restore the lost ecological functions of people. Nat. Ecol. Evol. 2, 1050–1052. ( 10.1038/s41559-018-0576-5) [DOI] [PubMed] [Google Scholar]
- 9.Carroll SP, Hendry AP, Reznick DN, Fox CW. 2007. Evolution on ecological time-scales. Funct. Ecol. 21, 387–393. ( 10.1111/j.1365-2435.2007.01289.x) [DOI] [Google Scholar]
- 10.Jablonski D. 2001. Lessons from the past: evolutionary impacts of mass extinctions. Proc. Natl Acad. Sci. USA 98, 5393–5398. ( 10.1073/pnas.101092598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas CD, Bodsworth EJ, Wilson RJ, Simmons AD, Davies ZG, Musche M, Conradt L. 2001. Ecological and evolutionary processes at expanding range margins. Nature 411, 577–581. ( 10.1038/35079066) [DOI] [PubMed] [Google Scholar]
- 12.Isaac NJ, Turvey ST, Collen B, Waterman C, Baillie JE. 2007. Mammals on the EDGE: conservation priorities based on threat and phylogeny. PLoS ONE 2, e296 ( 10.1371/journal.pone.0000296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 14.Hill JK, Griffiths HM, Thomas CD. 2011. Climate change and evolutionary adaptations at species' range margins. Ann. Rev. Ent. 56, 143–159. ( 10.1146/annurev-ento-120709-144746) [DOI] [PubMed] [Google Scholar]
- 15.Bridle JR, Buckley J, Bodsworth EJ, Thomas CD. 2014. Evolution on the move: specialization on widespread resources associated with rapid range expansion in response to climate change. Proc. R. Soc. B 281, 20131800 ( 10.1098/rspb.2013.1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnosky AD, et al. 2012. Approaching a state-shift in the biosphere. Nature 486, 52–56. ( 10.1038/nature11018) [DOI] [PubMed] [Google Scholar]
- 17.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO. 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 ( 10.1126/science.1246752) [DOI] [PubMed] [Google Scholar]
- 18.Cavicchioli R, et al. 2019. Scientists’ warning to humanity: microorganisms and climate change. Nat. Rev. Microbiol. 17, 569–586. ( 10.1038/s41579-019-0222-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinerstein E, et al. 2019. A global deal for nature: guiding principles, milestones and targets. Sci. Adv. 5, eaaw2869 ( 10.1126/sciadv.aaw2869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IPBES (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2019. Summary for policymakers. See http://bit.ly/IPBESReport.
- 21.Atkinson TC, Briffa KR, Coope GR. 1987. Seasonal temperatures in Britain during the past 22,000 years, reconstructed using beetle remains. Nature 325, 587–592. ( 10.1038/325587a0) [DOI] [Google Scholar]
- 22.Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913. ( 10.1038/35016000) [DOI] [PubMed] [Google Scholar]
- 23.Davis MB, Shaw RG. 2001. Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679. ( 10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- 24.Davis MB. 1989. Lags in vegetation response to greenhouse warming. Clim. Change 15, 75–82. ( 10.1007/BF00138846) [DOI] [Google Scholar]
- 25.Menéndez R, Megías AG, Hill JK, Braschler B, Willis SG, Collingham Y, Fox R, Roy DB, Thomas CD. 2006. Species richness changes lag behind climate change. Proc. R. Soc. B 273, 1465–1470. ( 10.1098/rspb.2006.3484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen J, Hooijer A, Vernimmen R, Liew SC, Page SE. 2017. From carbon sink to carbon source: extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett. 12, 024014 ( 10.1088/1748-9326/aa5b6f) [DOI] [Google Scholar]
- 27.Talluto MV, Boulangeat I, Vissault S, Thuiller W, Gravel D. 2017. Extinction debt and colonization credit delay range shifts of eastern North American trees. Nature Ecol. Evol. 1, 0182 ( 10.1038/s41559-017-0182) [DOI] [Google Scholar]
- 28.Esquivel-Muelbert A. 2019. Compositional response of Amazon forests to climate change. Glob. Change Biol. 25, 39–56. ( 10.1111/gcb.14413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svenning JC, Skov F. 2004. Limited filling of the potential range in European tree species. Ecol. Lett. 7, 565–573. ( 10.1111/j.1461-0248.2004.00614.x) [DOI] [Google Scholar]
- 30.Svenning JC. 2003. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecol. Lett. 6, 646–653. ( 10.1046/j.1461-0248.2003.00477.x) [DOI] [Google Scholar]
- 31.Hughes C, Eastwood R. 2006. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA 103, 10 334–10 339. ( 10.1073/pnas.0601928103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing SL, Harrington GJ, Smith FA, Bloch JI, Boyer DM, Freeman KH. 2005. Transient floral change and rapid global warming at the Paleocene-Eocene boundary. Science 310, 993–996. ( 10.1126/science.1116913) [DOI] [PubMed] [Google Scholar]
- 33.Jackson ST, Sax DF. 2010. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol. Evol. 25, 153–160. ( 10.1016/j.tree.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 34.Vellend M, Geber MA. 2005. Connections between species diversity and genetic diversity. Ecol. Lett. 8, 767–781. ( 10.1111/j.1461-0248.2005.00775.x) [DOI] [Google Scholar]
- 35.Rosindell J, Harmon LJ, Etienne RS. 2015. Unifying ecology and macroevolution with individual-based theory. Ecol. Lett. 18, 472–482. ( 10.1111/ele.12430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vellend M. 2016. The theory of ecological communities. Monographs in population biology – 57. Princeton, NJ: Princeton University Press. [Google Scholar]
- 37.Ellstrand NC, Rieseberg LH. 2016. When gene flow really matters: gene flow in applied evolutionary biology. Evol. Appl. 9, 833–836. ( 10.1111/eva.12402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huntley B, Birks HJB. 1983. An atlas of past and present pollen maps for Europe, 0–13,000 years ago. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 39.Bennett KD, Tzedakis PC, Willis KJ. 1991. Quaternary refugia of north European trees. J. Biogeogr. 18, 103–115. ( 10.2307/2845248) [DOI] [Google Scholar]
- 40.Smith SE, Gregory RD, Anderson BJ, Thomas CD. 2013. The past, present and potential future distributions of cold-adapted bird species. Divers. Distr. 19, 352–362. ( 10.1111/ddi.12025) [DOI] [Google Scholar]
- 41.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 42.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 12, 450–455. ( 10.1111/j.1365-2486.2006.01116.x) [DOI] [Google Scholar]
- 43.IPCC. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. In Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change (eds Field CB. et al). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Hanson T. 2018. Buzz: the nature and necessity of bees. New York, NY: Basic Books. [Google Scholar]
- 45.Baldock KC, et al. 2019. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 3, 363–373. ( 10.1038/s41559-018-0769-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powney GD, Carvell C, Edwards M, Morris RK, Roy HE, Woodcock BA, Isaac NJ. 2019. Widespread losses of pollinating insects in Britain. Nat. Commun. 10, 1018 ( 10.1038/s41467-019-08974-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas CD. 2013. Local diversity stays about the same, regional diversity increases, and global diversity declines. Proc. Natl Acad. Sci. USA 110, 19 187–19 188. ( 10.1073/pnas.1319304110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas CD, Kunin WE. 1999. The spatial structure of populations. J. Anim. Ecol. 68, 647–657. ( 10.1046/j.1365-2656.1999.00330.x) [DOI] [Google Scholar]
- 49.McGill BJ, Dornelas M, Gotelli NJ, Magurran AE. 2015. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 30, 104–113. ( 10.1016/j.tree.2014.11.006) [DOI] [PubMed] [Google Scholar]
- 50.Suggitt AJ, Lister DG, Thomas CD. 2019. Widespread effects of climate change on local plant diversity. Curr. Biol. 29, 2905–2911. ( 10.1016/j.cub.2019.06.079) [DOI] [PubMed] [Google Scholar]
- 51.Vellend M, Baeten L, Myers-Smith IH, Elmendorf SC, Beauséjour R, Brown CD, De Frenne P, Verheyen K, Wipf S. 2013. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl Acad. Sci. USA 110, 19 456–19 459. ( 10.1073/pnas.1312779110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vellend M, et al. 2017. Estimates of local biodiversity change over time stand up to scrutiny. Ecology 98, 583–590. ( 10.1002/ecy.1660) [DOI] [PubMed] [Google Scholar]
- 53.Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE. 2014. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299. ( 10.1126/science.1248484) [DOI] [PubMed] [Google Scholar]
- 54.Newbold T, et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. ( 10.1038/nature14324) [DOI] [PubMed] [Google Scholar]
- 55.Vellend M, Baeten L, Becker-Scarpitta A, Boucher-Lalonde V, McCune JL, Messier J, Myers-Smith IH, Sax DF. 2017. Plant biodiversity change across scales during the Anthropocene. Ann. Rev. Plant Biol. 68, 563–586. ( 10.1146/annurev-arplant-042916-040949) [DOI] [PubMed] [Google Scholar]
- 56.Sax DF, Gaines SD, Brown JH. 2002. Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. Am. Nat. 160, 766–783. ( 10.1086/343877) [DOI] [PubMed] [Google Scholar]
- 57.Sax DF, Gaines SD. 2003. Species diversity: from global decreases to local increases. Trends Ecol. Evol. 18, 561–566. ( 10.1016/S0169-5347(03)00224-6) [DOI] [Google Scholar]
- 58.Ellis EC, Antill EC, Kreft H. 2012. All is not loss: plant biodiversity in the Anthropocene. PloS ONE 7, e30535 ( 10.1371/journal.pone.0030535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seebens H, et al. 2017. No saturation in the accumulation of alien species worldwide. Nature Commun. 8, 14435 ( 10.1038/ncomms14435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Vos JM, Joppa LN, Gittleman JL, Stephens PR, Pimm SL. 2015. Estimating the normal background rate of species extinction. Conserv. Biol. 29, 452–462. ( 10.1111/cobi.12380) [DOI] [PubMed] [Google Scholar]
- 61.Humphreys AE, Govaerts R, Ficinski SZ, Lughadha EN, Vorontsova MS. 2019. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nature Ecol. Evol. 3, 1043–1047. ( 10.1038/s41559-019-0906-2) [DOI] [PubMed] [Google Scholar]
- 62.Thomas CD. 2015. Rapid acceleration of plant speciation during the Anthropocene. Trends Ecol. Evol. 30, 448–455. ( 10.1016/j.tree.2015.05.009) [DOI] [PubMed] [Google Scholar]
- 63.Bull JW, Maron M. 2016. How humans drive speciation as well as extinction. Proc. R. Soc. B 283, 20160600 ( 10.1098/rspb.2016.0600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cornell HV, Lawton JH. 1992. Species interactions, local and regional processes, and limits to the richness of ecological communities: a theoretical perspective. J. Anim. Ecol. 61, 1–12. ( 10.2307/5503) [DOI] [Google Scholar]
- 65.Ricklefs RE, He F. 2016. Region effects influence local tree species diversity. Proc. Natl Acad. Sci. USA 113, 674–679. ( 10.1073/pnas.1523683113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas CD. 2013. The Anthropocene could raise biological diversity. Nature 502, 7 ( 10.1038/502007a) [DOI] [PubMed] [Google Scholar]
- 67.Winter M, et al. 2009. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl Acad. Sci. USA 106, 21 721–21 725. ( 10.1073/pnas.0907088106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capinha C, Essl F, Seebens H, Moser D, Pereira HM. 2015. The dispersal of alien species redefines biogeography in the Anthropocene. Science 348, 1248–1251. ( 10.1126/science.aaa8913) [DOI] [PubMed] [Google Scholar]
- 69.Hobbs RJ, et al. 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecol. Biogeogr. 15, 1–7. ( 10.1111/j.1466-822X.2006.00212.x) [DOI] [Google Scholar]
- 70.Maskell LC, Firbank LG, Thompson K, Bullock JM, Smart SM. 2006. Interactions between non-native plant species and the floristic composition of common habitats. J. Ecol. 94, 1052–1060. ( 10.1111/j.1365-2745.2006.01172.x) [DOI] [Google Scholar]
- 71.Chytrý M, Maskell LC, Pino J, Pyšek P, Vilà M, Font X, Smart SM. 2008. Habitat invasions by alien plants: a quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J. Appl. Ecol. 45, 448–458. ( 10.1111/j.1365-2664.2007.01398.x) [DOI] [Google Scholar]
- 72.Beniak M, Pauková Ž, Fehér A. 2015. Altitudinal occurrence of non-native plant species (neophytes) and their habitat affinity to anthropogenic biotopes in conditions of south-western Slovakia. Ekológia 34, 163–175. ( 10.1515/eko-2015-0017) [DOI] [Google Scholar]
- 73.Hiley JR, Bradbury RB, Thomas CD. 2016. Impacts of habitat change and protected areas on alpha and beta diversity of Mexican birds. Divers. Distr. 22, 1245–1254. ( 10.1111/ddi.12483) [DOI] [Google Scholar]
- 74.Newbold T, et al. 2016. Global patterns of terrestrial assemblage turnover within and among land uses. Ecography 39, 1151–1163. ( 10.1111/ecog.01932) [DOI] [Google Scholar]
- 75.Birks HJB, Felde VA, Seddon AW. 2016. Biodiversity trends within the Holocene. The Holocene 26, 994–1001. ( 10.1177/0959683615622568) [DOI] [Google Scholar]
- 76.Williamson M, Dehnen-Schmutz K, Kühn I, Hill M, Klotz S, Milbau A, Stout J, Pyšek P. 2009. The distribution of range sizes of native and alien plants in four European countries and the effects of residence time. Divers. Distr. 15, 158–166. ( 10.1111/j.1472-4642.2008.00528.x) [DOI] [Google Scholar]
- 77.Thomas CD, Palmer G. 2015. Non-native plants add to the British flora without negative consequences for native diversity. Proc. Natl Acad. Sci. USA 112, 4387–4392. ( 10.1073/pnas.1423995112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dornelas M, Gotelli NJ, Shimadzu H, Moyes F, Magurran AE, McGill BJ. 2019. A balance of winners and losers in the Anthropocene. Ecol. Lett. 22, 847–854. ( 10.1111/ele.13242) [DOI] [PubMed] [Google Scholar]
- 79.Mallet J. 2007. Hybrid speciation. Nature 446, 279–283. ( 10.1038/nature05706) [DOI] [PubMed] [Google Scholar]
- 80.Jackson ST. 2016. Reinventing conservation – again. Front. Ecol. Environ. 14, 519 ( 10.1002/fee.1439) [DOI] [Google Scholar]
- 81.Defra. 2018. Biodiversity 2020: a strategy for England's wildlife and ecosystem services (indicators). London, UK: Defra. [Google Scholar]
- 82.Roy HE, et al. 2014. GB Non-native species information portal: documenting the arrival of non-native species in Britain. Biol. Invasions 16, 2495–2505. ( 10.1007/s10530-014-0687-0) [DOI] [Google Scholar]
- 83.Hamilton JA, Miller JM. 2016. Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conserv. Biol. 30, 33–41. ( 10.1111/cobi.12574) [DOI] [PubMed] [Google Scholar]
- 84.Crooks KR, Sanjayan M. 2006. Connectivity conservation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 85.Hodgson JA, Thomas CD, Wintle BA, Moilanen A. 2009. Climate change, connectivity and conservation decision making: back to basics. J. Appl. Ecol. 46, 964–969. ( 10.1111/j.1365-2664.2009.01695.x) [DOI] [Google Scholar]
- 86.Isaac NJ, et al. 2018. Defining and delivering resilient ecological networks: nature conservation in England. J. Appl. Ecol. 55, 2537–2543. ( 10.1111/1365-2664.13196) [DOI] [Google Scholar]
- 87.Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Thomas CD. 2008. Assisted colonization and rapid climate change. Science 321, 345–346. ( 10.1126/science.1157897) [DOI] [PubMed] [Google Scholar]
- 88.IUCN SSC. 2013. Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission. [Google Scholar]
- 89.Nuñez TA, Lawler JJ, McRae BH, Pierce DJ, Krosby MB, Kavanagh DM, Singleton PH, Tewksbury JJ. 2013. Connectivity planning to address climate change. Conserv. Biol. 27, 407–416. ( 10.1111/cobi.12014) [DOI] [PubMed] [Google Scholar]
- 90.Foden WB, et al. 2019. Climate change vulnerability assessment of species. Wiley Interdiscip. Rev. Clim. Change 10, e551 ( 10.1002/wcc.551) [DOI] [Google Scholar]
- 91.Pickett SA. 2013. The flux of nature: changing worldviews and inclusive concepts. In Linking ecology and ethics for a changing world (eds Rozzi R, Pickett STA, Palmer C, Armesto JJ, Callicott JB), pp. 265–279. Dordrecht, The Netherlands: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.