Abstract
Tropical forests and coral reefs host a disproportionately large share of global biodiversity and provide ecosystem functions and services used by millions of people. Yet, ongoing climate change is leading to an increase in frequency and magnitude of extreme climatic events in the tropics, which, in combination with other local human disturbances, is leading to unprecedented negative ecological consequences for tropical forests and coral reefs. Here, we provide an overview of how and where climate extremes are affecting the most biodiverse ecosystems on Earth and summarize how interactions between global, regional and local stressors are affecting tropical forest and coral reef systems through impacts on biodiversity and ecosystem resilience. We also discuss some key challenges and opportunities to promote mitigation and adaptation to a changing climate at local and global scales.
This article is part of the theme issue ‘Climate change and ecosystems: threats, opportunities and solutions'.
Keywords: biodiversity, climate change, climate extremes, coral reefs, ecosystem functioning and resilience, tropical forests
1. Introduction
The tropics contain the overwhelming majority of Earth's biological diversity [1] disproportionately distributed in two key ecosystems: tropical forests and coral reefs. Tropical forests cover less than 12% of the planet's ice-free surface but host more than two-thirds of all terrestrial species [1]. They provide the largest contribution to Earth's productivity from any biome [2] and play a critical role in overall climate regulation by storing 25% of the carbon in the terrestrial biosphere [3]. Equally important are tropical coral reefs (hereafter ‘coral reefs’), covering just 0.1% of the ocean surface yet holding the highest species diversity of any marine ecosystem [4]. They also sustain crucial ecosystem processes for more than 500 million people who use coral reefs and reef products for food provisioning, fisheries and tourism [5,6], and through providing coastal protection against natural hazards [7].
Despite their global importance, tropical forests and coral reefs are subject to a complex mixture of more localized pressures such as overexploitation, habitat loss and degradation, pollution and global climate change [1,8]. Growing evidence also suggests that anthropogenic climate change is increasing the periodicity and intensity of some climate extremes (e.g. [9–11]), which can be defined as abrupt climatic events, such as abnormally intense storms, hurricanes, floods, heatwaves, droughts and associated large-scale wildfires [12]. The ecological impacts of these extreme climate events can be exacerbated by ongoing gradual changes in temperature and precipitation, as well as local anthropogenic pressures, such as land-use change [13,14]. Understanding how tropical rainforests and coral reefs respond to climate extremes—and their interactions with other stressors—is therefore essential to achieve global conservation targets [15] and sustainable development goals [16].
Evidence of the influence of gradual climate changes and extreme climatic events is growing, and many studies explore their interactions with other more localized human pressures that threaten tropical forests and reefs (e.g. [1,13]). Yet, the existing literature is patchy and our ability to protect and manage these ecosystems is limited by two important knowledge gaps. First, no study to our knowledge has summarized where climate extremes are known to already affect both tropical forests and coral reefs worldwide, or which extreme events drive ecological changes in these two ecosystems. Second, despite a growing literature on the subject, it is not clear how interactions between gradual climate change, extreme climatic events and local disturbance are influencing tropical forests and reefs. These two knowledge gaps motivate the first and second part of our review. The final part explores how our current understanding of ecosystem responses to multiple pervasive pressures could be applied to inform management and conservation strategies. Although we primarily focus on tropical forests and coral reefs, the interactions among climate-related and local human-driven stressors are also major threats to other global ecosystems both in tropical and extratropical regions [17–19].
2. Where and how are climate extremes affecting tropical forests and reefs?
(a). Storms and floods
Climate change is causing more intense and frequent cyclonic storm systems (i.e. hurricanes, cyclones and typhoons) [10], with more extreme events expected in regions already affected by tropical cyclones, including Central America and the Caribbean, East Africa, most of Asia, as well as in Australia and the Pacific islands [20]. Although their impacts on coral reefs are primarily physical, for example, through reef structural damage [21], storms and hurricanes can strongly influence marine ecosystems [22,23]. On the Great Barrier Reef (GBR), for example, heavy rainfall was associated with negative trends in live coral cover, and storms emerged as the major driver of changes in inshore reef dynamics [24]. Not surprisingly, cyclonic storms have been shown to trigger regime transitions, from coral to macroalgal dominance, through interactions with local stressors (e.g. overfishing and diseases) that drive coral cover declines [25].
Tropical forests are also being affected—hurricanes frequently affect tropical forests in the Caribbean and Central America [26–28], and heavy storms have caused severe landslides in Venezuela [29] and floods in the Amazon basin (e.g. in Brazil and Peru [30–32]; figure 1). Some of the most extreme hydrological events have been associated with La Niña-induced changes in precipitation and river flow (e.g. 1989, 1999, 2009 and 2012) [32,35,36]. The 1998/1999 La Niña, in particular, brought one of the strongest hurricane seasons ever recorded in the North Atlantic, while in the Indian Ocean over 50% of Bangladesh was flooded [37]. Consequently, a range of post-hurricane ecological consequences has been recorded in tropical forests, such as reductions in non-tree resources for nectarivorous and frugivorous fauna [38]; changes in plant-herbivore networks (e.g. negative effects on network size and specificity, but increased network connectance and robustness) [39]; and greater than 50% declines in rates of occupancy, and even local and global extinctions of forest birds on Caribbean islands [26,40].
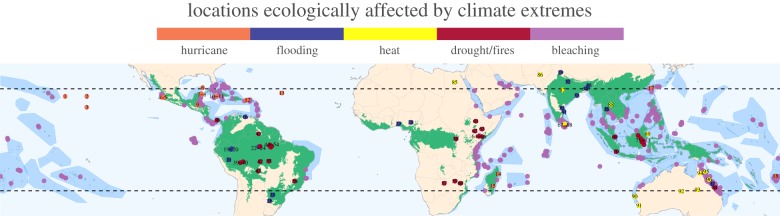
Figure 1.
Tropical forest and coral reef locations ecologically affected by climate extremes. Tropical forest biome (green) was defined following the ecoregions ‘Tropical & Subtropical Dry Broadleaf Forests' and ‘Tropical & Subtropical Moist Broadleaf Forests’ [33]. The tropical marine biome (darker blue polygons) was defined as the extent of shallow-water coral-forming ecoregions [34] on the basis of sea-surface temperature (mean minimum monthly 18°C sea-surface isotherm between 1988 and 2018; [1]). Colour-coding of the dots on the map indicates different extreme climatic events: drought/fires (red), flooding (blue), heatwaves (yellow) and hurricane/cyclones (orange). Purple-coloured dots show high-intensity bleaching reports from ReefBase (www.reefbase.org) between 1990 and 2010. Data sources and references for each number are presented in the electronic supplementary material, tables S1 and S2, respectively.
(b). Heatwaves and droughts
Extreme temperatures and droughts have been recently recorded across much of southern Africa, Southeast Asia and South America [41]. In recent decades, marine heatwaves have provoked widespread coral bleaching [42] (figure 1), leading to fundamental changes in coral reef ecosystems (e.g. [43–45]). In particular, the extremely high sea-surface temperatures across most of the tropical and extratropical oceans during the 2015/2016 record-breaking anomaly [46] caused one of the strongest mass bleaching events on a worldwide scale [47], resulted in unprecedented levels of coral mortality [48] and altered community composition of both corals and fish on the GBR [49]. Other heatwave-induced ecological impacts include flattening of reef structure [50] and loss of carbonate production [51], formation of persistent novel fish communities [43], shifts to macroalgal regimes [44] and synchronous multi-trophic ecological disruptions in marine, but also in terrestrial, ecosystems (e.g. coral bleaching and tree die-off) [52].
The combination of extreme high temperatures with longer and more severe dry seasons has also led to the spread of unprecedented and large-scale wildfires in tropical forests [53,54] (figure 1). For example, forests in the Amazon basin and Indonesia have witnessed at least four ‘mega-droughts’ in the last three decades [55,56]. Some of these heat and drought events were aggravated by the El Niño Southern Oscillations (ENSO), such as in 2015/2016 when fires devastated around 1 Mha of Amazonian forests [57,58] and greater than 4.6 Mha across Sumatra, Kalimantan and West Papua [54]. As a result of more frequent, extensive and intense drought and fire events, tropical forests have been affected through elevated tree mortality [59–61], impoverishment of biological communities [59,62–64] and loss of specific functional groups (e.g. evergreens and softwoods [65]). For instance, in Amazonia, hotter and drier seasons impose additional water-stress for trees even in the wetter environments [66], and tree recruitment has shifted species composition towards more dry-affiliated species, accompanied by increased mortality of wet-affiliated species [67]. These drought-related impacts can go beyond taxonomic and functional changes to effects on ecosystem resilience and stability (box 1), and in combination with wildfires, have led to reduced plant growth (e.g. [82] but see [83]) and ecosystem primary production [82,84]—all of which negatively affect the forest carbon cycling [85,86].
Box 1. Empirical examples of how climate extremes impact taxonomic and functional diversity, affecting the resilience and stability of tropical forests and coral reefs.
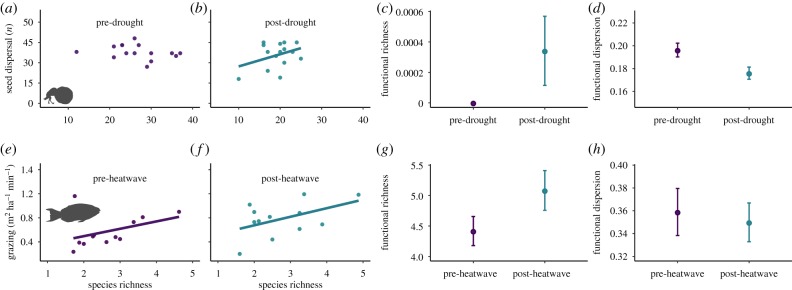
Securing functionally stable and resilient ecosystems is a pressing issue under ongoing global change. It is assumed that biodiversity increases ecosystem functioning and climate-resistance [68], and that functional trait-based approaches can better quantify disturbance consequences on ecological function and ecosystem stability [69]. However, the literature lacks evidence from the tropics [70,71]. To explore how an El Niño-related extreme drought and marine heatwave can affect the functional stability and ecosystem functioning of tropical forests and coral reefs, we used empirical data from dung beetles—which are important insects for secondary seed dispersal and seedling establishment processes in tropical forests [72,73]—within primary Amazonian forests and herbivore parrotfish within reefs throughout the inner Seychelles. We measured functional traits of dung beetles and parrotfish, along with two key ecosystem functions: secondary seed dispersal rates by dung beetles in forests and grazing rates by herbivorous parrotfishes on reefs. All datasets were sampled before and after the onset of the 2015–2016 El Niño (forest: 2010 and 2016; reef: 2014 and 2017; for further details see supplementary material and [44,74]). We, hence, compared post-El Niño functional diversity metrics and biodiversity-ecosystem function (BEF) relationships with those from pre-El Niño surveys.
Our findings suggest that climate extremes could reveal the importance of tropical biodiversity for ecosystem functioning, increasing the range of ecological niches occupied by functional groups (functional richness), and reducing the trait dissimilarity among communities (functional dispersion)—but these impacts are ecosystem-dependent [75] (figure 2). Specifically, lower seed dispersal rates occurred in forests with reduced beetle richness after the 2015–2016 El Niño drought (figure 2a,b), whereas positive BEF relationships were found in both pre- and post-El Niño surveys on Seychelles reefs (figure 2e,f). Although these findings focus only on the short-term responses, they suggest that disturbances could make tropical forests more dependent on biodiversity for their functioning [76]; while demonstrating that not only climate change, but also climatic extremes, may have filtering effects for terrestrial biological communities [17]. In addition, the maintenance of high post-disturbance grazing rates—under some specific ecological contexts [77]—may promote long-term coral recovery and stability by controlling competitive algae and reducing the likelihood of ecosystem transitions to algal-dominated states [44].
After the El Niño event in the Amazon, dung beetle functional richness was higher (figure 2c) and functional dispersion was lower (figure 2d). Similar results were found for ground beetle functional responses to flood disturbance in German grasslands [78]. These patterns could be explained by the loss of species with very distinctive traits and an increased dominance of functionally similar species such as generalists (often found in more disturbed environments [39,79,80]). By contrast, the lack of changes in functional richness and dispersion in the marine example (figure 2g,h) indicates no overall variation in the number of different functional traits and groups in parrotfish communities. Thus, the high taxonomic richness on coral reefs may support high functional redundancy, enabling functional groups to persist despite the El Niño event. Previous studies have similarly found no change in functional indices, including richness and dispersion, of coral reef fishes following habitat degradation due to storms or bleaching [49,81]. However, functional originality of coral reef fishes often decreases following climate extremes [49,81], which could make them more susceptible to future disturbances and to the interacting effects of climate change, climate extremes and local stressors (figure 3).
3. How do interactions among climate change, extreme climatic events and human-driven local stressors affect the resilience of studied ecosystems?
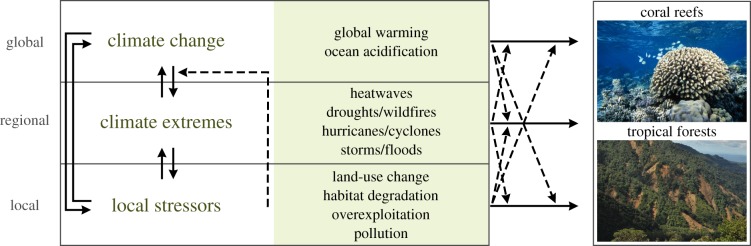
Following the framework proposed by Didham et al. [18], the interactions between climate-related stressors and local disturbances can result in ‘chain’ and ‘modification’ effects (figure 3). The interaction chain effects occur when multiple stressors have direct ecological impacts, with one driver amplifying the magnitude of another (a direct and synergistic interaction; e.g. land-use change increases climate warming via albedo effects or carbon release [87]). By contrast, interaction modification effects occur when the per unit or per capita influence of one stressor is modified by another (an indirect interaction), such as when habitat fragmentation prevents species from migrating to track their preferred climate niche [88]. These modification effects can occur through additive, antagonistic or synergistic interactions between stressors (reviewed by Côté et al. [89]). Regardless of how they interact and the scale on which they operate (figure 3), climate change, extreme climatic events and local stressors are likely to act as strong and interacting environmental filters [69,90]. As only a small subset of the original species pool is likely to respond positively to multiple stressors [1,91], this potential filtering of biological communities can result in subsequent effects on ecosystem functioning and functional stability of tropical coral reef and forest systems. These impacts, however, are likely to be ecosystem-dependent, as demonstrated by the empirical evidence from Brazilian Amazon forests and Seychelles coral reefs (box 1).
Figure 3.
Framework of interactive effects between climatic and anthropogenic stressors on tropical forests and reefs. Interactions may occur through modification effects, whereby the impacts per capita/per unit of one stressor is influenced by another pressure (dashed arrows), or through chain effects that may occur when both stressors have a direct influence, with one amplifying the severity of the other (adapted from the framework proposed by Didham et al. [18]). Photos represent a coral bleaching event in Moorea and landslides after massive thunderstorms in Peruvian cloud forests, by K. Chong-Seng and M. Dehling, respectively. (Online version in colour.)
(a). Climate and deforestation interactions threaten tropical forests and coral reefs
Climate stressors and land-use change, principally deforestation for food production and human settlement provision, have been exerting multi-taxa and -trophic effects on terrestrial and marine systems [1,92–95], and causing disproportionate biodiversity loss—particularly in the tropics [13]. Although climate change is considered the most important threat to coral reefs [77], deforestation impacts are also projected to outweigh future climate-change-driven declines in river flow and sediment load to reef systems in some regions [95]. However, the complex interactions between these stressors can make it challenging to tease apart their independent effects [89,96].
Deforestation has two effects on climate. First, it favours climate change through effects on greenhouse gas emissions and surface fluxes of radiation, moisture and heat [87]. Second, it increases the likelihood, intensity and extent of regional climatic extremes [97–99]. Consequently, many ecological responses to deforestation and fragmentation likely result from interaction modifications with climate. For instance, a global terrestrial analysis of 1319 papers found that habitat loss impacts on biodiversity were greatest in regions experiencing higher temperatures and lower rainfall [100]. Interaction modification effects would also imply that climate extremes occur under conditions of altered resilience generated by previous forest conversion. For example, deforestation can indirectly reduce the ability of tropical forest and reef biota to resist further climate disturbances by creating hostile landscapes and ocean conditions that hinder species capacity to track and achieve climate envelopes with more suitable conditions [88,101,102]. Moreover, habitat area, quality, heterogeneity and configuration can also affect the biota sensitivity and recovery after climatic disturbances [96,103,104].
(b). Enhanced heat and drought vulnerability within human-modified tropical forests
Most remaining tropical forests are currently subject to some form of anthropogenic disturbance [105]. Many of these alter forest microclimates—selective logging and wildfires, for example, increase tree mortality, which results in greater canopy openness [106,107] and drier understoreys [108]. These processes, combined with increasingly hotter and longer dry seasons, enhance forest flammability [109] and the likelihood of escaped fires ignited on agricultural lands [110] to burn neighbouring forests [111,112]. Although many tree species have molecular and physiological mechanisms that help them resist short-lived heat and drought [84], tropical rainforests are fire-sensitive and have few fire-resistant species [113]. Post-disturbance changes in carbon cycles [105] and evapotranspiration rates—a key source of aerial moisture—are also likely to affect atmospheric circulation patterns through biogeochemical feedbacks mediated by pollution through the release of CO2 and other aerosols [114,115], which have been shown to suppress cloud formation and regional precipitation [116,117]. Another example of an interaction modification effect occurs when climate change exacerbates the many negative impacts of ongoing forest degradation through declines in rainfall [59,118] that can enhance tree mortality through physiological mechanisms related to carbon starvation and hydraulic failure [84,119]. As rising global temperatures promote the occurrence and severity of extreme droughts [120] and wildfires [121], their interaction chain effects are also likely to be common in tropical forests (figure 3). Climate changes can also indirectly modify the susceptibility of tropical forests to climate extremes. For example, if cloud cover is declining over mid-latitudes [122] and elevated CO2 levels are enhancing liana biomass [123], then this could increase the mortality rates of drought-stressed trees even in otherwise undisturbed tropical forests [124].
(c). Climate-induced disturbances exacerbate impacts of local stressors on coral reefs
The current coral crisis is the result of a combination of large-scale climatic stressors and localized non-climatic disturbances [125]. Coral reef ecosystems are already widely threatened by local stressors such as overharvesting, land-based pollution, diseases, sedimentation and nutrient loading [125]. At a global scale, climate change is increasing the frequency, duration and intensity of marine heatwaves [46], resulting in interaction chain effects (figure 3) that are pushing coral communities towards their physiological stress limits [126] and causing widespread coral bleaching (figure 1). For example, the 1997/1998 and 2015/2016 bleaching events affected approximately 75% of well-studied coral reefs across the globe [47] and, in some regions, led to greater than 90% declines in live coral cover [127]. The individual effects of local and global stressors on coral reefs are relatively well-understood, but recent insights suggest that the impacts of climate extremes can also be exacerbated by local stressors. Corals on the GBR, for example, contend with multiple disturbances including sedimentation, nutrient run-off and crown-of-thorns starfish outbreaks [22]—and interactions between these disturbances determine coral resilience to bleaching (figure 3). For instance, coral declines are greatest and coral recovery is slowest on reefs where overfishing has compromised ecosystem processes such as predation and herbivory [128]. Furthermore, reefs adjacent to turbid river outflows have a lower probability of bleaching mortality due to lower light stress [23], providing an example of an antagonistic interaction. By contrast, reefs with elevated nutrient levels have reduced coral recovery rates by 12–27% [23], which signals an additive or synergistic interaction.
Although the magnitude of impacts of climate extremes will depend on the direct and indirect interactions with local and global pressures (figure 3), even isolated and relatively pristine reefs are vulnerable to both climate change and extremes [47,129]. Thus, local management alone is not expected to promote coral reef resilience in the face of climate stressors [130,131], although limited evidence shows that local stressor alleviation favoured post-bleaching recruitment and coral recovery in the GBR [128], Caribbean [132], Mesoamerican [133] and Kenyan reef systems [134]. In other regions, ecosystem protection of coral reefs can fail to mitigate bleaching impacts when compliance is weak and protected areas are small [135,136].
4. The way forward
We have herein outlined various examples of how climate extremes pose a broad range of challenges to tropical forests and coral reefs (figure 1 and box 1), particularly when combined with ongoing climate change and more localized human pressures. Guarding against negative impacts on the world's most biodiverse ecosystems will be challenging and dependent on local and global actions for climate adaptation and impact mitigation, while more traditional conservation strategies will need to be renewed to ameliorate the impacts of multiple interacting threats (figure 3).
(a). Climate-smart protected areas
Networks of connected protected areas have been the cornerstone of efforts to conserve biodiversity; however, interactions between local and climatic stressors (figure 3) require a new focus on functional and climate connectivity, with the particular aim of allowing species range shifts along climate gradients [88]. The global extent of marine protected areas protects just 7.66% of the ocean, and the size of the tropical network is far smaller than in the rest of the world [137]. Although the largest percentage of forest area under protected status (greater than 26%) is found in the tropics [138], most tropical reserves are smaller than 100 km2 [139]. The coverage of tropical forest and marine protected areas is therefore too small to allow long-distance range shifts by species, and over 62% of the tropical forests have been shown to be likely to fail in facilitating species movements to analogous future climates [88].
To enhance climate connectivity and hence resilience, decision-makers should also focus on viable patch-linkages and habitat corridors among protected areas preferably distributed along climate gradients and where connectivity loss and species vulnerability to climate are high [88]. Achieving successful reserves will also require the protection of habitat in the wider landscape—such as private lands—to ensure reserves remain functionally connected if climate change and extreme events result in enhanced environmental stochasticity [140], and species need to travel longer to find suitable bioclimatic conditions [88,141]. In addition, protected areas may also play a key role for both climatic mitigation and adaptation through reducing emissions from tropical deforestation [142], alleviating regional flood (drought) occurrence during extremely rainy (dry and hot) seasons [143–145], and avoiding overexploitation and loss of organisms and processes important for post-disturbance ecosystem recovery (e.g. [128,146]). However, to fulfil their role as an insurance policy for biodiversity and climate-mitigation, current protected area networks need to be well enforced and funded [147], while new marine and forest reserves should be strategically placed where they increase climate connectivity [88] and/or are predicted to escape the burden of climate-associated stressors [130]. This is important because even regions under low direct anthropogenic stress may be subject to impacts from regional and global stressors [77].
(b). We are all in the same boat: multi-level actions to tackle different stressors
As human populations and per capita consumption continue to grow [148], the fate and future benefits provided by tropical forest and reef systems will also depend greatly on how well these ecosystems are managed. Their long-term resilience to climate change and extremes will require the collective effort of a broad range of stakeholders at distinct levels. Acting locally is important, and there are different approaches to avoid further on-the-ground disturbance. For instance, the post-disturbance resilience of tropical ecosystems and biota may be enhanced through approaches for climatic adaptation such as the implementation of well-planned landscapes, reinstatement of connectivity and energy flows among ecosystems [149] and improvements in habitat quality through ecological restoration (e.g. green firebreaks in China [150]). Addressing the many distal drivers of degradation in tropical ecosystems is essential to foster the effectiveness of these approaches [1,125]. Research and climate-mitigation strategies are also more likely to have an effect if engaging with local actors, such as tropical scientists, managers, citizens and institutions [151–153], and encouraging land- and marine-use practices that respect local needs and diverse socio-ecological conditions (e.g. fire-safe agriculture in tropical forests [154] and community-based management programmes for coastal populations that depend on corals and small-scale fisheries [155]).
Managing locally may not be enough if we do not tackle global climate change issues [77]. Redoubling efforts to limit anthropogenic climate changes remains critical and is the most important mitigation option we have where climate stressors cause widespread damage independent of other local non-climatic disturbances. This issue needs to be addressed by local, national and international stakeholders, while balancing the needs for economic growth and environmental sustainability, a particular challenge for tropical countries [156]. For this, both tropical and extratropical nations will need to develop strategies such as low-carbon technologies to reduce the emissions of greenhouse gases while avoiding forest destruction to increase carbon intake [105]. Controlling climate change may also reduce the risks of more severe and frequent weather extremes [46,157], and, consequently, the need for a considerable amount of investments to prepare regions that are more vulnerable to them.
5. Conclusion
Our review shows that climate extremes are impacting forests and coral reefs throughout the tropics (figure 1), but their ecological consequences for ecosystem resilience and stability are likely to differ across realms (box 1). The fate of these ecosystems will be determined by a complex interplay between the impacts of local and climate-associated stressors [1,17] (figure 3). Ecological studies on species-specific physiological tolerance [158], changing species composition [60,159] and ecosystem recovery trajectories [27,48] may help us to inform management decisions where climatic stressors are the main drivers of disturbance. However, where local and climate-related stressors are jeopardizing ecosystems services, we need to develop better predictive models to understand how chain and modification interactions with local stressors can mediate the ecological consequences of climate change and climatic extremes. Such integrated approaches can better inform policy and climate-adjusted management solutions to ameliorate further disturbance impacts, helping to promote ecosystem adaptation and resilience. We urge the creation of conservation initiatives to develop interventions that effectively curb local disturbances, but these will be of limited success if they are not accompanied by international actions to decrease CO2 emissions and therefore slow global climate changes. Conserving the hyperdiverse biota of tropical forests and coral reefs for future generations will require much greater cooperation between nations and the involvement of a broader range of stakeholders in the development of solutions.
Supplementary Material
Acknowledgements
We thank Andrew Hoey for providing the parrotfish feeding data; and Simon Jennings, Laís F. Maia, Fernando Z. Vaz-de-Mello, Victor Hugo F. Oliveira, Rodrigo F. Braga and the numerous field and laboratory assistants that supported us with data collection, trait measures and fauna identification. Institutional support was provided by LBA Program (INPA) and ICMBio in Santarém.
Ethics
Surveys in Brazilian protected areas occurred with appropriate state and federal permits (Brazil: SISBIO no. 24164 in 2009, and 53271 in 2016–2017).
Data accessibility
The data used in this paper are available as part of the electronic supplementary material (figure 1) and/or at https://doi.org/10.5285/799db965-3ce7-4e9b-8590-de6a8624d652 (figure 2).
Figure 2.
Drought and bleaching impacts on tropical biodiversity-ecosystem functioning links, functional richness and functional dispersion in tropical forests and coral reefs, respectively. Dung beetle (a–d) and herbivore parrotfish communities (e–h) were surveyed before (purple) and after (blue) the 2015/2016 El Niño drought within Brazilian Amazonian forests and heatwave in Seychelles reefs, respectively. The x-axis shows dung beetle (a,b) and parrotfish (e–f) species richness, and pre- and post-drought/heatwave surveys (c,d/g,h). The y-axis represents rates of dung beetle-mediated secondary seed dispersal (a,b), parrotfish grazing rates (e,f), functional richness (c,g) and functional dispersion (d,h). Further details on functional traits, analyses and results are described in the electronic supplementary material. (Online version in colour.)
Authors' contributions
F.M.F. and J.B. conceived, designed and structured the review idea with essential support from G.P., C.E.B, J.P.W.R. and N.A.J.G. Data were provided by F.M.F., N.A.J.G. and J.L., while F.M.F., C.E.B. and J.P.W.R. carried out the analyses with critical inputs from G.P., J.M.T. and N.A.J.G. The manuscript was drafted by F.M.F., J.B., G.P., C.E.B. and J.P.W.R.; and reviewed by A.C.L, N.A.J.G., J.F., E.B. and J.M.T. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from CNPq (grant nos. 574008/2008-0, 458022/2013-6, 400640/2012-0), CNPq-CAPES-PELD (project 88887.136261/2017-00; grant no. 441659/2016-0; scholarships 88887.186650/2018-00 and 88887.358233/2019-00 for F.M.F., and 307788/2017-2 for J.F.), FAPESP (grant no. 2012/51872-5) and EMBRAPA (grant no. SEG:02.08.06.005.00), and The Nature Conservancy, in Brazil. Funding was also provided by the Darwin Initiative (grant no. 17-023) and NERC (grant nos. NE/F01614X/1, NE/G000816/1, NE/K016431/1, NE/F015356/2, NE/l018123/1 and NE/P004512/1), in UK; and the Swedish Formas (2013-1571). G.P. was supported by the Marsden Fund (grant no. UOC1705). C.E.B. was supported by the Bertarelli Foundation as part of the Bertarelli Programme in Marine Science.
References
- 1.Barlow J, et al. 2018. The future of hyperdiverse tropical ecosystems. Nature 559, 517–526. ( 10.1038/s41586-018-0301-1) [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Running SW. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943. ( 10.1126/science.1192666) [DOI] [PubMed] [Google Scholar]
- 3.Bonan GB. 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449. ( 10.1126/science.1155121) [DOI] [PubMed] [Google Scholar]
- 4.Roberts CM. 2002. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295, 1280–1284. ( 10.1126/science.1067728) [DOI] [PubMed] [Google Scholar]
- 5.Burke L, Reytar K, Spalding M, Perry A. 2011. Reefs at risk revisited. Washington, DC: World Resources Institute. [Google Scholar]
- 6.Buddemeier RW, Kleypas JA, Aronson RB. 2004. Coral reefs and climate change: potential contributions of climate change to stresses on coral reef ecosystems. Washington, DC: Pew Center on Global Climate Change. [Google Scholar]
- 7.Beck MW, Losada IJ, Menéndez P, Reguero BG, Díaz-Simal P, Fernández F. 2018. The global flood protection savings provided by coral reefs. Nat. Commun. 9, 2186 ( 10.1038/s41467-018-04568-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter KE, et al. 2008. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. ( 10.1126/science.1159196) [DOI] [PubMed] [Google Scholar]
- 9.Fischer EM, Knutti R. 2015. Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nat. Clim. Change 5, 560–564. ( 10.1038/nclimate2617) [DOI] [Google Scholar]
- 10.Patricola CM, Wehner MF. 2018. Anthropogenic influences on major tropical cyclone events. Nature 563, 339–346. ( 10.1038/s41586-018-0673-2) [DOI] [PubMed] [Google Scholar]
- 11.Sobel AH, Camargo SJ, Hall TM, Lee C, Tippett MK, Wing AA. 2016. Human influence on tropical cyclone intensity. Science 353, 242–246. ( 10.1126/science.aaf6574) [DOI] [PubMed] [Google Scholar]
- 12.IPCC. 2019. Glossary of acronyms and specialised terms on the Intergovernmental Panel on Climate Change (IPCC) and Data Distribution Centre (DDC) website. Definition of terms used within DDC page. http://www.ipcc-data.org/guidelines/pages/glossary/glossary_e.html (accessed on 1 February 2019).
- 13.Newbold T, et al. 2019. Climate and land-use change homogenise terrestrial biodiversity, with consequences for ecosystem functioning and human well-being. Emerg. Top. Life Sci. 3, 207–219. ( 10.1042/ETLS20180135) [DOI] [PubMed] [Google Scholar]
- 14.Ghedini G, Russell BD, Falkenberg LJ, Connell SD. 2015. Beyond spatial and temporal averages: ecological responses to extreme events may be exacerbated by local disturbances. Clim. Change Responses 2, 6 ( 10.1186/s40665-015-0014-8) [DOI] [Google Scholar]
- 15.Convention on Biological Diversity. 2014. Aichi biodiversity targets. Strategic Plan 2011-2020. https://www.cbd.int/sp/targets/#GoalB (accessed on 19 August 2015).
- 16.SDG. 2018. Sustainable development goals. https://sustainabledevelopment.un.org/sdgs (accessed on 20 January 2018).
- 17.Gibb H, et al. 2015. Climate mediates the effects of disturbance on ant assemblage structure. Proc. R. Soc. B 282, 20150418 ( 10.1098/rspb.2015.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Didham RK, Tylianakis JM, Gemmell N, Rand T, Ewers R. 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 22, 489–496. ( 10.1016/j.tree.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 19.Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. ( 10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 20.IPCC. 2013. Climate change 2013: the physical science basis. In Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (eds Stocker TF, et al.), p. 1535 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 21.Madin JS, Connolly SR. 2006. Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 444, 477–480. ( 10.1038/nature05328) [DOI] [PubMed] [Google Scholar]
- 22.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. ( 10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacNeil MA, Mellin C, Matthews S, Wolff NH, McClanahan TR, Devlin M, Drovandi C, Mengersen K, Graham NAJ. 2019. Water quality mediates resilience on the Great Barrier Reef. Nat. Ecol. Evol. 3, 620–627. ( 10.1038/s41559-019-0832-3) [DOI] [PubMed] [Google Scholar]
- 24.Lam VYY, Chaloupka M, Thompson A, Doropoulos C, Mumby PJ. 2018. Acute drivers influence recent inshore Great Barrier Reef dynamics. Proc. R. Soc. B 285, 20182063 ( 10.1098/rspb.2018.2063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes TP. 1994. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551. ( 10.1126/science.265.5178.1547) [DOI] [PubMed] [Google Scholar]
- 26.Wiley JW, Wunderle JM. 1993. The effects of hurricanes on birds, with special reference to Caribbean islands. Bird Conserv. Int. 3, 319–349. ( 10.1017/S0959270900002598) [DOI] [Google Scholar]
- 27.Schowalter TD, Willig MR, Presley SJ. 2017. Post-hurricane successional dynamics in abundance and diversity of canopy arthropods in a tropical rainforest. Environ. Entomol. 46, nvw155 ( 10.1093/ee/nvw155) [DOI] [PubMed] [Google Scholar]
- 28.Dunham AE, Erhart EM, Wright PC. 2011. Global climate cycles and cyclones: consequences for rainfall patterns and lemur reproduction in southeastern Madagascar. Glob. Change Biol. 17, 219–227. ( 10.1111/j.1365-2486.2010.02205.x) [DOI] [Google Scholar]
- 29.Takahashi T, Nakagawa H, Satofuka Y, Kawaike K. 2001. Flood and sediment disasters triggered by 1999 rainfall in Venezuela: a river restoration plan for an alluvial fan. J. Nat. Disaster Sci. 23, 65–82. [Google Scholar]
- 30.Espinoza JC, et al. 2012. From drought to flooding: understanding the abrupt 2010–11 hydrological annual cycle in the Amazonas River and tributaries. Environ. Res. Lett. 7, 024008 ( 10.1088/1748-9326/7/2/024008) [DOI] [Google Scholar]
- 31.Espinoza JC, Ronchail J, Frappart F, Lavado W, Santini W, Guyot JL. 2013. The major floods in the Amazonas river and tributaries (Western Amazon Basin) during the 1970–2012 period: a focus on the 2012 flood. J. Hydrometeorol. 14, 1000–1008. ( 10.1175/JHM-D-12-0100.1) [DOI] [Google Scholar]
- 32.Marengo JA, Espinoza JC. 2016. Extreme seasonal droughts and floods in Amazonia: causes, trends and impacts. Int. J. Climatol. 36, 1033–1050. ( 10.1002/joc.4420) [DOI] [Google Scholar]
- 33.Dinerstein E, et al. 2017. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545. ( 10.1093/biosci/bix014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleypas JA, McManus JW, Meñez LAB. 1999. Environmental limits to coral reef development: where do we draw the line? Am. Zool. 39, 146–159. ( 10.1093/icb/39.1.146) [DOI] [Google Scholar]
- 35.Satyamurty P, da Costa CPW, Manzi AO, Candido LA. 2013. A quick look at the 2012 record flood in the Amazon Basin. Geophys. Res. Lett. 40, 1396–1401. ( 10.1002/grl.50245) [DOI] [Google Scholar]
- 36.Chen JL, Wilson CR, Tapley BD. 2010. The 2009 exceptional Amazon flood and interannual terrestrial water storage change observed by GRACE. Water Resour. Res. 46, 1–10. ( 10.1029/2010WR009383) [DOI] [Google Scholar]
- 37.Kunii O, Nakamura S, Abdur R, Wakai S. 2002. The impact on health and risk factors of the diarrhoea epidemics in the 1998 Bangladesh floods. Public Health 116, 68–74. ( 10.1038/sj.ph.1900828) [DOI] [PubMed] [Google Scholar]
- 38.Scanlon AT, Petit S, Tuiwawa M, Naikatini A. 2018. Response of primary and secondary rainforest flowers and fruits to a cyclone, and implications for plant-servicing bats. Glob. Change Biol. 24, 3820–3836. ( 10.1111/gcb.14103) [DOI] [PubMed] [Google Scholar]
- 39.Luviano N, Villa-Galaviz E, Boege K, Zaldívar-Riverón A, Del-Val E. 2018. Hurricane impacts on plant-herbivore networks along a successional chronosequence in a tropical dry forest. For. Ecol. Manage. 426, 158–163. ( 10.1016/j.foreco.2017.09.011) [DOI] [Google Scholar]
- 40.Lloyd JD, Rimmer CC, Salguero-Faría JA. 2019. Short-term effects of hurricanes Maria and Irma on forest birds of Puerto Rico. PLoS ONE 14, e0214432 ( 10.1371/journal.pone.0214432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen JH, et al. 2013. Climate phenomena and their relevance for future regional climate change. In Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (eds Stocker TF. et al), pp. 1217–1308. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Baker AC, Glynn PW, Riegl B. 2008. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471. ( 10.1016/j.ecss.2008.09.003) [DOI] [Google Scholar]
- 43.Robinson JPW, Wilson SK, Jennings S, Graham NAJ. 2019. Thermal stress induces persistently altered coral reef fish assemblages. Glob. Change Biol. 25, 2739–2750. ( 10.1111/gcb.14704) [DOI] [PubMed] [Google Scholar]
- 44.Graham NAJJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK. 2015. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97. ( 10.1038/nature14140) [DOI] [PubMed] [Google Scholar]
- 45.Wernberg T, Smale DA, Tuya F, Thomsen MS, Langlois TJ, de Bettignies T, Bennett S, Rousseaux CS. 2013. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 3, 78–82. ( 10.1038/nclimate1627) [DOI] [Google Scholar]
- 46.Frölicher TL, Fischer EM, Gruber N. 2018. Marine heatwaves under global warming. Nature 560, 360–364. ( 10.1038/s41586-018-0383-9) [DOI] [PubMed] [Google Scholar]
- 47.Hughes TP, et al. 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. ( 10.1126/science.aan8048) [DOI] [PubMed] [Google Scholar]
- 48.Hughes TP, et al. 2018. Global warming transforms coral reef assemblages. Nature 556, 492–496. ( 10.1038/s41586-018-0041-2) [DOI] [PubMed] [Google Scholar]
- 49.Richardson LE, Graham NAJ, Pratchett MS, Eurich JG, Hoey AS. 2018. Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob. Change Biol. 24, 3117–3129. ( 10.1111/gcb.14119) [DOI] [PubMed] [Google Scholar]
- 50.Couch CS, Burns JHR, Liu G, Steward K, Gutlay TN, Kenyon J, Eakin CM, Kosaki RK. 2017. Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLoS ONE 12, e0185121 ( 10.1371/journal.pone.0185121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lange ID, Perry CT. 2019. Bleaching impacts on carbonate production in the Chagos Archipelago: influence of functional coral groups on carbonate budget trajectories. Coral Reefs 38, 619–624. ( 10.1007/s00338-019-01784-x) [DOI] [Google Scholar]
- 52.Ruthrof KX, et al. 2018. Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Sci. Rep. 8, 13094 ( 10.1038/s41598-018-31236-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nobre CA, Borma LDS. 2009. ‘Tipping points’ for the Amazon forest. Curr. Opin. Environ. Sustain. 1, 28–36. ( 10.1016/j.cosust.2009.07.003) [DOI] [Google Scholar]
- 54.Lohberger S, Stängel M, Atwood EC, Siegert F. 2018. Spatial evaluation of Indonesia's 2015 fire-affected area and estimated carbon emissions using Sentinel-1. Glob. Change Biol. 24, 644–654. ( 10.1111/gcb.13841) [DOI] [PubMed] [Google Scholar]
- 55.Marengo JA, Souza CM, Thonicke K, Burton C, Halladay K, Betts RA, Alves LM, Soares WR. 2018. Changes in climate and land use over the Amazon region: current and future variability and trends. Front. Earth Sci. 6, 228 ( 10.3389/feart.2018.00228) [DOI] [Google Scholar]
- 56.ESCAP, RIMES, UNDP. 2016. Assessment of El Niño-associated risks: the step-wise process. https://www.unescap.org/resources/assessment-el-ni%C3%B1o-associated-risks-step-wise-process.
- 57.Withey K, et al. 2018. Quantifying immediate carbon emissions from El Niño-mediated wildfires in humid tropical forests. Phil. Trans. R. Soc. B 373, 20170312 ( 10.1098/rstb.2017.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiménez-Muñoz JC, Mattar C, Barichivich J, Santamaría-Artigas A, Takahashi K, Malhi Y, Sobrino JA, van der Schrier G. 2016. Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep. 6, 33130 ( 10.1038/srep33130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen CD, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259, 660–684. ( 10.1016/j.foreco.2009.09.001) [DOI] [Google Scholar]
- 60.Barlow J, Peres CA. 2008. Fire-mediated dieback and compositional cascade in an Amazonian forest. Phil. Trans. R. Soc. B 363, 1787–1794. ( 10.1098/rstb.2007.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lwanga JS. 2003. Localized tree mortality following the drought of 1999 at Ngogo, Kibale National Park, Uganda. Afr. J. Ecol. 41, 194–196. ( 10.1046/j.1365-2028.2003.00428.x) [DOI] [Google Scholar]
- 62.Peres CA, Barlow J, Haugaasen T. 2003. Vertebrate responses to surface wildfires in a central Amazonian forest. Oryx 37, 97–109. ( 10.1017/S0030605303000188) [DOI] [Google Scholar]
- 63.Cleary DFR, Mooers AØ. 2006. Burning and logging differentially affect endemic vs. widely distributed butterfly species in Borneo. Divers. Distrib. 12, 409–416. ( 10.1111/j.1366-9516.2006.00256.x) [DOI] [Google Scholar]
- 64.Barlow J, Haugaasen T, Peres CA. 2002. Effects of ground fires on understorey bird assemblages in Amazonian forests. Biol. Conserv. 105, 157–169. ( 10.1016/S0006-3207(01)00177-X) [DOI] [Google Scholar]
- 65.Aleixo I, Norris D, Hemerik L, Barbosa A, Prata E, Costa F, Poorter L. 2019. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Change 9, 384–388. ( 10.1038/s41558-019-0458-0) [DOI] [Google Scholar]
- 66.Esquivel-Muelbert A, et al. 2017. Seasonal drought limits tree species across the Neotropics. Ecography (Cop.). 40, 618–629. ( 10.1111/ecog.01904) [DOI] [Google Scholar]
- 67.Esquivel-Muelbert A, et al. 2019. Compositional response of Amazon forests to climate change. Glob. Change Biol. 25, 39–56. ( 10.1111/gcb.14413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 69.Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. 2013. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. ( 10.1016/j.tree.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 70.Clarke DA, York PH, Rasheed MA, Northfield TD. 2017. Does biodiversity–ecosystem function literature neglect tropical ecosystems? Trends Ecol. Evol. 32, 320–323. ( 10.1016/j.tree.2017.02.012) [DOI] [PubMed] [Google Scholar]
- 71.Stroud JT, Feeley KJ. 2017. Neglect of the tropics is widespread in ecology and evolution: a comment on Clarke et al. Trends Ecol. Evol. 32, 626–628. ( 10.1016/j.tree.2017.06.006) [DOI] [PubMed] [Google Scholar]
- 72.Griffiths HM, Bardgett RD, Louzada J, Barlow J. 2016. The value of trophic interactions for ecosystem function: dung beetle communities influence seed burial and seedling recruitment in tropical forests. Proc. R. Soc. B 283, 20161634 ( 10.1098/rspb.2016.1634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andresen E. 2002. Dung beetles in a Central Amazonian rainforest and their ecological role as secondary seed dispersers. Ecol. Entomol. 27, 257–270. ( 10.1046/j.1365-2311.2002.00408.x) [DOI] [Google Scholar]
- 74.França FM, et al. In press El Niño impacts on human-modified tropical forests: consequences for dung beetle diversity and associated ecological processes. Biotropica. ( 10.1111/btp.12756) [DOI] [Google Scholar]
- 75.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 76.Manning P, Slade EM, Beynon SA, Lewis OT. 2017. Effect of dung beetle species richness and chemical perturbation on multiple ecosystem functions. Ecol. Entomol. 42, 577–586. ( 10.1111/een.12421) [DOI] [Google Scholar]
- 77.Bruno JF, Côté IM, Toth LT. 2019. Climate change, coral loss, and the curious case of the parrotfish paradigm: why don't marine protected areas improve reef resilience? Ann. Rev. Mar. Sci. 11, 307–334. ( 10.1146/annurev-marine-010318-095300) [DOI] [PubMed] [Google Scholar]
- 78.Gerisch M, Agostinelli V, Henle K, Dziock F. 2012. More species, but all do the same: contrasting effects of flood disturbance on ground beetle functional and species diversity. Oikos 121, 508–515. ( 10.1111/j.1600-0706.2011.19749.x) [DOI] [Google Scholar]
- 79.Tylianakis JM, Morris RJ. 2017. Ecological networks across environmental gradients. Annu. Rev. Ecol. Evol. Syst. 48, 25–48. ( 10.1146/annurev-ecolsys-110316-022821) [DOI] [Google Scholar]
- 80.Torres JA. 1992. Lepidoptera outbreaks in response to successional changes after the passage of Hurricane Hugo in Puerto Rico. J. Trop. Ecol. 8, 285–298. ( 10.1017/S0266467400006544) [DOI] [Google Scholar]
- 81.Brandl SJ, Emslie MJ, Ceccarelli DM, Richards ZT. 2016. Habitat degradation increases functional originality in highly diverse coral reef fish assemblages. Ecosphere 7, e01557 ( 10.1002/ecs2.1557) [DOI] [Google Scholar]
- 82.Reichstein M, et al. 2013. Climate extremes and the carbon cycle. Nature 500, 287–295. ( 10.1038/nature12350) [DOI] [PubMed] [Google Scholar]
- 83.Berenguer E, et al. 2018. Tree growth and stem carbon accumulation in human-modified Amazonian forests. Phil. Trans. R. Soc. B 373, 20170308 ( 10.1098/rstb.2017.0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niu S, Luo Y, Li D, Cao S, Xia J, Li J, Smith MD. 2014. Plant growth and mortality under climatic extremes: an overview. Environ. Exp. Bot. 98, 13–19. ( 10.1016/j.envexpbot.2013.10.004) [DOI] [Google Scholar]
- 85.Frank DD, et al. 2015. Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob. Change Biol. 21, 2861–2880. ( 10.1111/gcb.12916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silva CVJ, et al. 2018. Drought-induced Amazonian wildfires instigate a decadal-scale disruption of forest carbon dynamics. Phil. Trans. R. Soc. B 373, 20180043 ( 10.1098/rstb.2018.0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betts RA. 2005. Integrated approaches to climate–crop modelling: needs and challenges. Phil. Trans. R. Soc. B 360, 2049–2065. ( 10.1098/rstb.2005.1739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Senior RA, Hill JK, Edwards DP. 2019. Global loss of climate connectivity in tropical forests. Nat. Clim. Change 4, 164–166. ( 10.1038/s41558-019-0529-2) [DOI] [Google Scholar]
- 89.Côté IM, Darling ES, Brown CJ. 2016. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 283, 20152592 ( 10.1098/rspb.2015.2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balmford A. 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends Ecol. Evol. 11, 193–196. ( 10.1016/0169-5347(96)10026-4) [DOI] [PubMed] [Google Scholar]
- 91.Vinebrooke RD, Cottingham KL, Norberg J, Scheffer M, Dodson SI, Maberly SC, Sommer U. 2004. Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104, 451–457. ( 10.1111/j.0030-1299.2004.13255.x) [DOI] [Google Scholar]
- 92.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 93.Kroon FJ, Thorburn P, Schaffelke B, Whitten S. 2016. Towards protecting the Great Barrier Reef from land-based pollution. Glob. Change Biol. 22, 1985–2002. ( 10.1111/gcb.13262) [DOI] [PubMed] [Google Scholar]
- 94.Voigt W, et al. 2003. Trophic levels are differentially sensitive to climate. Ecology 84, 2444–2453. ( 10.1890/02-0266) [DOI] [Google Scholar]
- 95.Maina J, de Moel H, Zinke J, Madin J, McClanahan T, Vermaat JE. 2013. Human deforestation outweighs future climate change impacts of sedimentation on coral reefs. Nat. Commun. 4, 1986 ( 10.1038/ncomms2986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliver TH, Morecroft MD. 2014. Interactions between climate change and land use change on biodiversity: attribution problems, risks, and opportunities. Wiley Interdiscip. Rev. Clim. Change 5, 317–335. ( 10.1002/wcc.271) [DOI] [Google Scholar]
- 97.Bradshaw CJA, Sodhi NS, Peh KSH, Brook BW. 2007. Global evidence that deforestation amplifies flood risk and severity in the developing world. Glob. Change Biol. 13, 2379–2395. ( 10.1111/j.1365-2486.2007.01446.x) [DOI] [Google Scholar]
- 98.Findell KL, Berg A, Gentine P, Krasting JP, Lintner BR, Malyshev S, Santanello JA, Shevliakova E. 2017. The impact of anthropogenic land use and land cover change on regional climate extremes. Nat. Commun. 8, 1–9. ( 10.1038/s41467-017-01038-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baker JCA, Spracklen DV. 2019. Climate benefits of intact Amazon forests and the biophysical consequences of disturbance. Front. For. Glob. Change 2, 47 ( 10.3389/ffgc.2019.00047) [DOI] [Google Scholar]
- 100.Mantyka-pringle CS, Martin TG, Rhodes JR. 2012. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob. Change Biol. 18, 1239–1252. ( 10.1111/j.1365-2486.2011.02593.x) [DOI] [Google Scholar]
- 101.Eigenbrod F, Gonzalez P, Dash J, Steyl I. 2015. Vulnerability of ecosystems to climate change moderated by habitat intactness. Glob. Change Biol. 21, 275–286. ( 10.1111/gcb.12669) [DOI] [PubMed] [Google Scholar]
- 102.Oliver TH, Gillings S, Pearce-Higgins JW, Brereton T, Crick HQP, Duffield SJ, Morecroft MD, Roy DB. 2017. Large extents of intensive land use limit community reorganization during climate warming. Glob. Change Biol. 23, 2272–2283. ( 10.1111/gcb.13587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliver TH, Brereton T, Roy DB. 2013. Population resilience to an extreme drought is influenced by habitat area and fragmentation in the local landscape. Ecography (Cop.). 36, 579–586. ( 10.1111/j.1600-0587.2012.07665.x) [DOI] [Google Scholar]
- 104.Piessens K, Adriaens D, Jacquemyn H, Honnay O. 2009. Synergistic effects of an extreme weather event and habitat fragmentation on a specialised insect herbivore. Oecologia 159, 117–126. ( 10.1007/s00442-008-1204-x) [DOI] [PubMed] [Google Scholar]
- 105.Baccini A, Walker W, Carvalho L, Farina M, Sulla-Menashe D, Houghton RA. 2017. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 358, 230–234. ( 10.1126/science.aam5962) [DOI] [PubMed] [Google Scholar]
- 106.Asner GP, Keller M, Pereira R Jr, Zweede JC, Silva JNM. 2004. Canopy damage and recovery after selective logging in Amazonia: field and satellite studies. Ecol. Appl. 14, 280–298. ( 10.1890/01-6019) [DOI] [Google Scholar]
- 107.Slik JWF. 2004. El Niño droughts and their effects on tree species composition and diversity in tropical rain forests. Oecologia 141, 114–120. ( 10.1007/s00442-004-1635-y) [DOI] [PubMed] [Google Scholar]
- 108.Lindenmayer DB, Hunter ML, Burton PJ, Gibbons P. 2009. Effects of logging on fire regimes in moist forests. Conserv. Lett. 2, 271–277. ( 10.1111/j.1755-263X.2009.00080.x) [DOI] [Google Scholar]
- 109.Brando PM, et al. 2014. Abrupt increases in Amazonian tree mortality due to drought-fire interactions. Proc. Natl Acad. Sci. USA 111, 6347–6352. ( 10.1073/pnas.1305499111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uhl C, Buschbacher R. 1985. A disturbing synergism between cattle ranch burning practices and selective tree harvesting in the Eastern Amazon. Biotropica 17, 265 ( 10.2307/2388588) [DOI] [Google Scholar]
- 111.Nepstad D, Lefebvre P, da Silva U Lopes, Tomasella J, Schlesinger P, Solorzano L, Moutinho P, Ray D, Guerreira Benito J. 2004. Amazon drought and its implications for forest flammability and tree growth: a basin-wide analysis. Glob. Change Biol. 10, 704–717. ( 10.1111/j.1529-8817.2003.00772.x) [DOI] [Google Scholar]
- 112.Nepstad DC, Stickler CM, Filho BS, Merry F. 2008. Interactions among Amazon land use, forests and climate: prospects for a near-term forest tipping point. Phil. Trans. R. Soc. B 363, 1737–1746. ( 10.1098/rstb.2007.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cochrane MA. 2003. Fire science for rainforests. Nature 421, 913–919. ( 10.1038/nature01437) [DOI] [PubMed] [Google Scholar]
- 114.Betts RA, Cox PM, Collins M, Harris PP, Huntingford C, Jones CD. 2004. The role of ecosystem-atmosphere interactions in simulated Amazonian precipitation decrease and forest dieback under global climate warming. Theor. Appl. Climatol. 78, 157–175. ( 10.1007/s00704-004-0050-y) [DOI] [Google Scholar]
- 115.Bony S, Bellon G, Klocke D, Sherwood S, Fermepin S, Denvil S. 2013. Robust direct effect of carbon dioxide on tropical circulation and regional precipitation. Nat. Geosci. 6, 447–451. ( 10.1038/ngeo1799) [DOI] [Google Scholar]
- 116.Staal A, Tuinenburg OA, Bosmans JHC, Holmgren M, Van Nes EH, Scheffer M, Zemp DC, Dekker SC. 2018. Forest-rainfall cascades buffer against drought across the Amazon. Nat. Clim. Change 8, 539–543. ( 10.1038/s41558-018-0177-y) [DOI] [Google Scholar]
- 117.Andreae MO. 2004. Smoking rain clouds over the Amazon. Science 303, 1337–1342. ( 10.1126/science.1092779) [DOI] [PubMed] [Google Scholar]
- 118.Spracklen DV, Garcia-Carreras L. 2015. The impact of Amazonian deforestation on Amazon basin rainfall. Geophys. Res. Lett. 42, 9546–9552. ( 10.1002/2015GL066063) [DOI] [Google Scholar]
- 119.Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, art129 ( 10.1890/ES15-00203.1) [DOI] [Google Scholar]
- 120.Yeh S-W, et al. 2018. ENSO atmospheric teleconnections and their response to greenhouse gas forcing. Rev. Geophys. 56, 185–206. ( 10.1002/2017RG000568) [DOI] [Google Scholar]
- 121.Fernandes K, Verchot L, Baethgen W, Gutierrez-Velez V, Pinedo-Vasquez M, Martius C. 2017. Heightened fire probability in Indonesia in non-drought conditions: the effect of increasing temperatures. Environ. Res. Lett. 12, 054002 ( 10.1088/1748-9326/aa6884) [DOI] [Google Scholar]
- 122.Norris JR, Allen RJ, Evan AT, Zelinka MD, O'Dell CW, Klein SA. 2016. Evidence for climate change in the satellite cloud record. Nature 536, 72–75. ( 10.1038/nature18273) [DOI] [PubMed] [Google Scholar]
- 123.Phillips OL, et al. 2002. Increasing dominance of large lianas in Amazonian forests. Nature 418, 770–774. ( 10.1038/nature00926) [DOI] [PubMed] [Google Scholar]
- 124.McDowell N, et al. 2018. Drivers and mechanisms of tree mortality in moist tropical forests. New Phytol. 219, 851–869. ( 10.1111/nph.15027) [DOI] [PubMed] [Google Scholar]
- 125.Hughes TP, et al. 2017. Coral reefs in the Anthropocene. Nature 546, 82–90. ( 10.1038/nature22901) [DOI] [PubMed] [Google Scholar]
- 126.Pratchett MS, McCowan D, Maynard JA, Heron SF. 2013. Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS ONE 8, 1–10. ( 10.1371/journal.pone.0070443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goreau T, McClanahan T, Hayes R, Strong A. 2000. Conservation of coral reefs after the 1998 Global Bleaching Event. Conserv. Biol. 14, 5–15. ( 10.1046/j.1523-1739.2000.00011.x) [DOI] [Google Scholar]
- 128.Mellin C, MacNeil MA, Cheal AJ, Emslie MJ, Caley MJ. 2016. Marine protected areas increase resilience among coral reef communities. Ecol. Lett. 19, 629–637. ( 10.1111/ele.12598) [DOI] [PubMed] [Google Scholar]
- 129.Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS. 2013. Recovery of an isolated coral reef system following severe disturbance. Science 340, 69–71. ( 10.1126/science.1232310) [DOI] [PubMed] [Google Scholar]
- 130.Côté IM, Darling ES. 2010. Rethinking ecosystem resilience in the face of climate change. PLoS Biol. 8, e1000438 ( 10.1371/journal.pbio.1000438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 132.Mumby PJ, Harborne AR. 2010. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS ONE 5, e8657 ( 10.1371/journal.pone.0008657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carilli JE, Norris RD, Black BA, Walsh SM, McField M. 2009. Local stressors reduce coral resilience to bleaching. PLoS ONE 4, e6324 ( 10.1371/journal.pone.0006324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McClanahan TR. 2008. Response of the coral reef benthos and herbivory to fishery closure management and the 1998 ENSO disturbance. Oecologia 155, 169–177. ( 10.1007/s00442-007-0890-0) [DOI] [PubMed] [Google Scholar]
- 135.Graham NAJ, et al. 2008. Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE 3, e0003039 ( 10.1371/journal.pone.0003039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Selig ER, Casey KS, Bruno JF. 2012. Temperature-driven coral decline: the role of marine protected areas. Glob. Change Biol. 18, 1561–1570. ( 10.1111/j.1365-2486.2012.02658.x) [DOI] [Google Scholar]
- 137.UNEP-WCMC, IUCN. 2019. Marine protected planet. https://www.protectedplanet.net/marine (accessed on 16 July 2019).
- 138.Morales-Hidalgo D, Oswalt SN, Somanathan E. 2015. Status and trends in global primary forest, protected areas, and areas designated for conservation of biodiversity from the Global Forest Resources Assessment 2015. For. Ecol. Manage. 352, 68–77. ( 10.1016/j.foreco.2015.06.011) [DOI] [Google Scholar]
- 139.UNEP-WCMC, IUCN. 2019. Protected planet: the world database on protected areas (WDPA). www.protectedplanet.net (accessed on 16 July 2019).
- 140.Cormont A, Malinowska AH, Kostenko O, Radchuk V, Hemerik L, WallisDeVries MF, Verboom J. 2011. Effect of local weather on butterfly flight behaviour, movement, and colonization: significance for dispersal under climate change. Biodivers. Conserv. 20, 483–503. ( 10.1007/s10531-010-9960-4) [DOI] [Google Scholar]
- 141.Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742. ( 10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soares-Filho B, et al. 2010. Role of Brazilian Amazon protected areas in climate change mitigation. Proc. Natl Acad. Sci. USA 107, 10 821–10 826. ( 10.1073/pnas.0913048107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brauman KA, Daily GC, Duarte TK, Mooney HA. 2007. The nature and value of ecosystem services: an overview highlighting hydrologic services. Annu. Rev. Environ. Resour. 32, 67–98. ( 10.1146/annurev.energy.32.031306.102758) [DOI] [Google Scholar]
- 144.Weng W, Luedeke MKB, Zemp DC, Lakes T, Kropp JP. 2017. Aerial and surface rivers: downwind impacts on water availability from land use changes in Amazonia. Hydrol. Earth Syst. Sci. Discuss. 22, 911–927. ( 10.5194/hess-2017-526) [DOI] [Google Scholar]
- 145.Bhattacharjee K, Behera B. 2018. Does forest cover help prevent flood damage? Empirical evidence from India. Glob. Environ. Change 53, 78–89. ( 10.1016/j.gloenvcha.2018.09.004) [DOI] [Google Scholar]
- 146.Ashton LA, et al. 2019. No termites mitigate the ecosystem-wide effects of drought in tropical rainforest. Science 177, 174–177. ( 10.1126/SCIENCE.AAU9565) [DOI] [PubMed] [Google Scholar]
- 147.Kuempel CD, Adams VM, Possingham HP, Bode M. 2018. Bigger or better: the relative benefits of protected area network expansion and enforcement for the conservation of an exploited species. Conserv. Lett. 11, e12433 ( 10.1111/conl.12433) [DOI] [Google Scholar]
- 148.IPCC. 2019. IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. In Climate change and land (eds Arneth A, et al.), p. 1542 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 149.Graham NAJ, Wilson SK, Carr P, Hoey AS, Jennings S, MacNeil MA. 2018. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559, 250–253. ( 10.1038/s41586-018-0202-3) [DOI] [PubMed] [Google Scholar]
- 150.Cui X, Alam MA, Perry GL, Paterson AM, Wyse SV, Curran TJ. 2019. Green firebreaks as a management tool for wildfires: lessons from China. J. Environ. Manage. 233, 329–336. ( 10.1016/j.jenvman.2018.12.043) [DOI] [PubMed] [Google Scholar]
- 151.Baker K, Eichhorn MP, Griffiths M. 2019. Decolonizing field ecology. Biotropica 51, 288–292. ( 10.1111/btp.12663) [DOI] [Google Scholar]
- 152.Balvanera P, et al. 2017. Key features for more successful place-based sustainability research on social-ecological systems: a Programme on Ecosystem Change and Society (PECS) perspective. Ecol. Soc. 22, 45 ( 10.5751/ES-08826-220114) [DOI] [Google Scholar]
- 153.Waylen KA, Fischer A, McGowan PJK, Thirgood SJ, Milner-Gulland EJ. 2010. Effect of local cultural context on the success of community-based conservation interventions. Conserv. Biol. 24, 1119–1129. ( 10.1111/j.1523-1739.2010.01446.x) [DOI] [PubMed] [Google Scholar]
- 154.Carmenta R, Coudel E, Steward AM. 2018. Forbidden fire: does criminalising fire hinder conservation efforts in swidden landscapes of the Brazilian Amazon? Geogr. J. 185, 23–37. ( 10.1111/geoj.12255) [DOI] [Google Scholar]
- 155.Cinner JE, et al. 2012. Comanagement of coral reef social-ecological systems. Proc. Natl Acad. Sci. USA 109, 5219–5222. ( 10.1073/pnas.1121215109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Edelman A, et al. 2014. State of the tropics – 2014 report. https://researchonline.jcu.edu.au/35471/.
- 157.Cai W, et al. 2015. Increased frequency of extreme La Niña events under greenhouse warming. Nat. Clim. Change 5, 132–137. ( 10.1038/nclimate2492) [DOI] [Google Scholar]
- 158.Santiago LS, De Guzman ME, Baraloto C, Vogenberg JE, Brodie M, Hérault B, Fortunel C, Bonal D. 2018. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 218, 1015–1024. ( 10.1111/nph.15058) [DOI] [PubMed] [Google Scholar]
- 159.Darling ES, McClanahan TR, Côté IM. 2013. Life histories predict coral community disassembly under multiple stressors. Glob. Change Biol. 19, 1930–1940. ( 10.1111/gcb.12191) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this paper are available as part of the electronic supplementary material (figure 1) and/or at https://doi.org/10.5285/799db965-3ce7-4e9b-8590-de6a8624d652 (figure 2).
Figure 2.
Drought and bleaching impacts on tropical biodiversity-ecosystem functioning links, functional richness and functional dispersion in tropical forests and coral reefs, respectively. Dung beetle (a–d) and herbivore parrotfish communities (e–h) were surveyed before (purple) and after (blue) the 2015/2016 El Niño drought within Brazilian Amazonian forests and heatwave in Seychelles reefs, respectively. The x-axis shows dung beetle (a,b) and parrotfish (e–f) species richness, and pre- and post-drought/heatwave surveys (c,d/g,h). The y-axis represents rates of dung beetle-mediated secondary seed dispersal (a,b), parrotfish grazing rates (e,f), functional richness (c,g) and functional dispersion (d,h). Further details on functional traits, analyses and results are described in the electronic supplementary material. (Online version in colour.)