Abstract
Background:
Heart rate variability (HRV) is of interest to researchers due to its potential utility as a marker for both physiological and psychological stress. Sympatholytics are used to treat opioid withdrawal, but little information about the parasympathetic system’s role in mediating withdrawal symptoms exists. The goal of the current study was to evaluate changes in HRV during opioid withdrawal to provide a better understanding of the autonomic effects of opioid withdrawal.
Methods:
Ten male participants (mean age = 46.4 years) received intramuscular naloxone (mean dose =0.26 mg) to confirm opioid dependence. The presence and severity of withdrawal symptoms were assessed using subjective and objective measures (Wang et al., 1974). Electrocardiography (ECG) was measured continuously, and HRV was analyzed in 2-minute segments before naloxone injection (at baseline) and after participants were in moderate withdrawal (Wang Test score ≥10). Heart rate, blood pressure, pupil diameter, and respiratory rate were also examined.
Results:
Pupil diameter significantly increased after naloxone administration relative to baseline (t(9) = 5.562, p = 0.000). Both high frequency (HF) HRV (Z = −2.803, p = 0.005) and root mean square of successive differences (RMSSD) HRV (Z = −2.090, p = 0.037) were significantly lower during withdrawal relative to baseline. Increases in heart rate (Z = −2.090, p = 0.032) and systolic pressure (t(9) = 8.099, p = 0.0000) from baseline to withdrawal also were significant.
Conclusions:
These preliminary data indicate that a large reduction in cardiac vagal tone occurs during naloxone-induced withdrawal. This finding underscores the need for further research into the role of the parasympathetic nervous system in opioid withdrawal.
Keywords: Opioid withdrawal, Heart rate variability, Naloxone, Opioid dependence, Opioid use disorder, Autonomic nervous system
1. Introduction
Opioid use is responsible for a staggering proportion of drug-related morbidity and mortality. Opioid use disorder (OUD) is one of the most common substance use disorders, with ~26.8 million cases worldwide as of 2016 (Degenhardt et al., 2018). Treatment of OUD can be challenging, and one hurdle is the emergence of opioid withdrawal during various phases of the treatment process. It is characterized by a constellation of symptoms that include gastrointestinal distress, autonomic hyperactivity, anxiety, dysphoria, and pain (Koob et al., 1992; Ries et al., 2014; Sigmon et al., 2012). Although opioid withdrawal is typically not fatal, the experience is uncomfortable, and it remains a significant motivator for continued non-medical opioid use, and a barrier to the initiation of some treatment medications, such as oral or extended-release naltrexone (XR-NTX), an opioid antagonist, and buprenorphine, a partial agonist at mu opioid receptors and antagonist at kappa opioid receptors. Inducting patients onto XR-NTX requires 7–10 days of abstinence from opioids, and even starting buprenorphine typically involves a brief period of opioid withdrawal (Jarvis et al., 2018; Sigmon et al., 2012). Opioid withdrawal also often occurs after administration of naloxone, which is used to reverse opioid overdose (Neale and Strang, 2015).
Increased sympathetic nervous system (SNS) activity is likely involved in many of the symptoms of withdrawal. Increases in blood pressure, pupil size, and noradrenergic activity suggest that the SNS becomes hyperactive during withdrawal (Koob et al., 1992; Maldonado, 1997). Medications commonly used to treat opioid withdrawal (e.g., clonidine and lofexidine) are sympatholytic, inhibiting the SNS by acting as agonists at α2 adrenergic receptors. However, medications acting solely on the SNS may not be addressing the full scope of the autonomic disturbances that occur during opioid withdrawal. Very little research has evaluated changes in the parasympathetic nervous system (PNS) during opioid withdrawal in humans. Newlin et al. (1992) administered 0.4 mg intramuscular naloxone to 19 opioid-dependent individuals and found a non-statistically significant reduction in cardiac vagal tone during withdrawal. However, this study’s methodology differed from ours by utilizing a different assessment for withdrawal, and a wider frequency range for the frequency domain measures of heart rate variability (HRV).
Thus, the literature assessing the role of the PNS in opioid withdrawal is limited and further research is needed to assess the potential of the PNS as a therapeutic target in opioid withdrawal. The purpose of this pilot study was to assess the changes in cardiac vagal tone measured through high frequency (HF) HRV and the root mean square of successive differences (RMSSD) HRV in a sample of opioid-dependent participants undergoing a naloxone challenge procedure to assess for opioid physical dependence. HF and RMSSD are HRV indices that are pure measures of cardiac vagal tone (Task Force, 1997). We hypothesized that we would observe a significant decrease in cardiac vagal tone post-naloxone challenge, compared to baseline.
2. Methods
2.1. Participants
Data were collected from participants screening for various clinical research studies. In order to qualify for these trials, participants were required to have moderate-severe OUD and be physiologically dependent on opioids. These criteria were assessed using urine drug toxicologies at each screening visit, along with clinical interviews with nurses, psychologists, and physicians. The final assessment was a naloxone challenge procedure, which was used to verify physiological dependence on opioids by administering the opioid antagonist naloxone and assessing objective and subjective signs of withdrawal. Participants with cardiovascular disease (e.g. arrhythmias, unstable hypertension, past history of myocardial infarction, coronary artery disease) were excluded prior to completing this screening assessment. For the current study, pre- and post-naloxone assessments of HRV were added to the naloxone challenge procedure. The procedures of this study were approved by the New York State Psychiatric Institute Institutional Review Board.
2.2. Naloxone challenge procedure (modified Wang test)
All equipment needed to perform the challenge, including naloxone, rescue medication, and physiological equipment, was prepared prior to participant arrival. Participants were seated upright in an examination room and acclimated to orthostatic changes (i.e., changes in heart rate (HR) and blood pressure due to shifting from a supine position to sitting or standing and vice versa) for at least 5 min. During this time, a nurse explained the procedure to the subject. Following at least 1 min of rest and a 2-minute baseline HRV assessment period, participants received an intramuscular dose of 0.2 mg naloxone, with a second dose (0.2–0.4 mg) given 10 min after the first dose, if necessary, to induce withdrawal.
The Wang Test measures the severity of withdrawal, with scores determined by the presence of several signs and symptoms including: gooseflesh, vomiting, sweating, stomach pain, and restlessness. Directly observable signs, such as vomiting, are weighted more heavily than symptoms, such as muscle pain (Wang et al., 1974). In the original test, assessments are made every 10 min for 30 min and a sum score is calculated. For our modified test, repeated withdrawal assessments were made by a trained nurse every 10 min for up to 50 min after administration of naloxone and the average peak withdrawal score at any time point was calculated. Subjects also were assessed using the modified Wang Test after morphine rescue in order to ensure they were no longer experiencing significant withdrawal symptoms.
2.3. Physiological measures
ECG was recorded continuously throughout the naloxone challenges, and HRV was analyzed in 2-minute epochs. Throughout the procedure, subjects breathed at their natural rate. We compared a 2-minute baseline epoch to the two minutes immediately following the start of withdrawal (score ≥ 10). HF and RMSSD HRV, two commonly utilized measures of cardiac vagal tone, were calculated from the ECG recordings. The standard deviation of interbeat intervals (SDNN) and Low Frequency (LF) HRV were calculated from the ECG recordings. Heart rate (HR) was also calculated from the ECG. ECGs were recorded using the PowerLab model 8/35 with ECG BioAmp and respiration band accessories with a sampling rate of 1 KHz (AD Instruments, Sydney, Australia). Blood pressure (BP; systolic and diastolic) and pupil size were also measured due to the sympathetic input to these two measures.
2.4. Analyses
Data were analyzed in LabChart using AD Instrument’s proprietary HRV analysis module, with manual cleaning of artifacts and identification of R-Waves being performed as necessary. Both the absolute HF (frequency = 0.15–0.40) and RMSSD HRV were examined, as well as the natural log transformed HF (ln-HF) and RMSSD (ln-RMSSD). Due to the skewed distribution of the cardiac measures, a Wilcoxon signed-rank test was used to compare HF, LF, RMSSD, and HR, at baseline and during withdrawal. Paired samples T-tests on ln-HF and ln-RMSSD were used to compare the differences between baseline and withdrawal. Paired samples T-tests on BP, respiratory rate, pupil diameter, and SDNN were performed to compare differences between baseline and withdrawal.
3. Results
3.1. Participants
Data were collected from a total of 10 naloxone challenge procedures. Demographic and self-reported substance use is shown in Table 1. All participants showed signs of opioid withdrawal within 21 minutes of naloxone administration (Mean =11.3 min, SD =5.3 min, Min =5 min) though 30% of participants required an additional 0.2 mg dose of naloxone to exhibit notable withdrawal. The mean peak withdrawal score at any single time point during the assessment period was 15.2 (SD = 3.2), while the average score using the original Wang Test protocol (summative measurements taken every 10 min for 30 min) was 98.2 (SD = 51.1) indicating that every subject experienced moderate to severe withdrawal.
Table 1.
Participant demographics and substance use. Numbers represent means or number of participants with corresponding standard deviations or percentages, as indicated.
| Measurement/Parameters | Mean or # of participants |
|---|---|
| Age (yrs) ± SE | 46.4 ± 9.3 |
| Sex (M/F) | 10/0 |
| Race/Ethnicity (Caucasian/Black/Hispanic) | 1/7/2 |
| Height (cm) ± SE | 175.4 ± 6.0 |
| Weight (kg) ± SE | 74.1 ± 21.3 |
| Current Heroin Use (bags/day) ± SE | 6.1 ± 5.8 |
| Alcohol Use (y/n) | 6/4 |
| Daily Nicotine Use (y/n) | 10/0 |
| Positive Urine Drug Toxicologies On Day of Challenge (y/n) | |
| Amphetamine (y/n) | 0/10 |
| Barbiturates (y/n) | 0/10 |
| Benzodiazepines (y/n) | 1/9 |
| Buprenorphine (y/n) | 0/10 |
| Cocaine (y/n) | 4/6 |
| Fentanyl (y/n) | 9/1 |
| Heroin (y/n) | 10/0 |
| MDMA (y/n) | 0/10 |
| Methadone (y/n) | 3/7 |
| Methamphetamine (y/n) | 0/10 |
| Oxycodone (y/n) | 1/9 |
| PCP (y/n) | 0/10 |
| THC (y/n) | 3/7 |
SE = standard error.
Y = yes.
N = no.
3.2. Physiological measures
The mean values of recorded physiological measures for baseline and withdrawal are shown in Table 2. Pupil diameter increased by ~0.8 mm during withdrawal (t(9) = 5.562, p = 0.000) and systolic blood pressure increased by 12 mmHg (t(9) = 8.099, p = 0.000). HR also was elevated during withdrawal relative to baseline, (Z = −2.090, p = 0.032). The difference in average HR between conditions was ~5 bpm, which represents a ~6% increase from baseline.
Table 2.
Mean values for physiological parameters at baseline and during withdrawal.
| Physiological Measures | Baseline (n = 10) | Withdrawal (n = 10) |
|---|---|---|
| Heart Rate (beats per min) ± SE | 79.6 ± 3.7 | 84.5 ± 4.4* |
| Respiratory Rate (breaths per min) ± SE | 15.1 ± 0.9 | 16.0 ± 0.8 |
| SDNN (ms) ± SE | 41.2 ± 4.4 | 41.5 ± 4.9 |
| RMSSD (ms) ± SE | 28.5 ± 5.0 | 20.2 ± 3.1* |
| In RMSSD ± SE | 3.2 ± 0.2 | 2.9 ± 0.2* |
| HF (ms2) ± SE | 245.6 ± 57.9 | 126.7 ± 25.5** |
| In HF ± SE | 5.3 ± 0.2 | 4.4 ± 0.4** |
| LF (ms2) ± SE | 563.6 ± 195.9 | 615.4 ± 150.0 |
| ln LF ± SE | 5.8 ± 0.4 | 5.9 ± 0.4 |
| Pupil Diameter (mm) ± SE | 3.2 ± 0.2 | 4.0 ± 0.3** |
| Systolic BP (mmHg) ± SE | 121.6 ± 3.3 | 133.7 ± 3.4** |
| Diastolic BP (mmHg) ± SE | 76.7 ± 2.9 | 80.4 ± 3.5 |
SE = standard error.
p < 0.05.
p < 0.01.
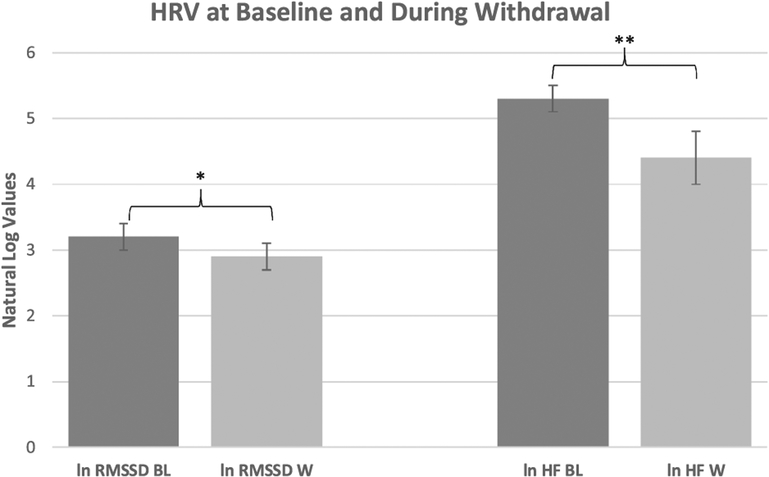
With regard to HRV, both HF (Z = −2.803, p = 0.005) and RMSSD (Z = −2.090, p = 0.037) decreased during withdrawal relative to baseline (Table 2). HF decreased by 49.4% and RMSSD decreased by 29.0%. These percentages correspond to a 119 ms2 difference in HF, and an 8.3 ms difference in RMSSD across conditions. Correspondingly, both ln-HF (t(9) = 3.434, p = 0.007) and ln-RMSSD (t(9) = 2.382, p = 0.041) were significantly lower during withdrawal compared to baseline (Fig. 1). Neither SDNN nor LF were significantly altered during withdrawal relative to baseline.
Fig. 1.
Comparison of ln RMSSD and ln HF at baseline versus during withdrawal.
BL=baseline HRV assessment.
W=withdrawal HRV assessment.
*p < 0.05.
**p < 0.01.
4. Discussion
The relationship between effects of naloxone on the autonomic nervous system has gone largely unstudied, in both opioid dependent and healthy populations. In this study, a significant decrease in cardiac vagal tone was observed following naloxone administration to opioid-dependent participants, which suggests that a decrease in PNS activity is involved in opioid withdrawal. The only other clinical study that the investigators could find on the effect of opioid withdrawal on vagal tone was performed by Newlin et al. (1992). In this study by Newlin and colleagues (1992), non-significant changes occurred in cardiac vagal tone, but the authors measured an older frequency domain measure of vagal tone (the vagal tone index), which spans a frequency currently thought to have substantial sympathetic contributions (Task Force, 1996). Newlin et al. (1992) also utilized a log transformation of HF as opposed to a natural log transformation when analyzing HF, as is the current common practice (Shaffer and Ginsberg, 2017). These differences may explain why our study produced a significant result in terms of cardiac vagal tone, while Newlin et al. (1992) did not.
The subjective symptoms of withdrawal, as opposed to the measurable physiologic signs, are a significant cause of treatment dropout and improve little from sympatholytic agents despite improvement of physiologic symptoms. One could argue that inhibiting the SNS should produce similar effects as increasing PNS activity, and that there are currently interventions available to achieve this. Inhibiting the SNS with agents such as clonidine and lofexidine has been shown to ameliorate the severity of withdrawal (Gold et al., 1978; Gorodetzky et al., 2017). However, previous work has found that clonidine and lofexidine do not effectively treat the anxiety, insomnia, and myalgias that accompany withdrawal (Charney et al., 1981). These medications also fail to impact measures of cardiac vagal tone such as HF and RMSSD (Fagermoen et al., 2015; Michaloudis et al., 1998).
Given the limitations of these treatments, it may be worthwhile to target the PNS for the treatment of opioid withdrawal. Currently, lofexidine is the only FDA-approved non-opioid medication available for treating opioid withdrawal. Patients and clinicians often report that the efficacy of this medication is inferior to opioid-based interventions such as buprenorphine or methadone (Ries et al., 2014). However, medically supervised withdrawal with opioid agonists limits its use to tightly controlled settings, and does not allow for the initiation of an opioid antagonist until after a long washout period. Therefore, investigating new physiological targets, such as the PNS, to assist in the treatment of opioid use disorder remains an important endeavor.
Our study is limited in several ways. Because the naloxone challenge test is part of standard screening procedures in our laboratory, we did not have a control group of individuals who were not physically dependent on opioids. However, the limited research on the effects of naloxone in healthy controls suggests that the effects on physiological parameters such as heart rate, blood pressure, and skin conductance are negligible (Jones and Herning, 2016). Jones and Herning (2016) did observe a statistically significant, although clinically insignificant elevation in HR of 4 bpm. However, it should be noted that the naloxone doses used by Jones and Herning (2016) were on the order of 10–100x greater than what was delivered to our participants. Therefore, these findings are not readily comparable to our own results. In addition, a placebo condition was not included, and we could not control for the recent use of other drugs of abuse on the day of the challenge. Finally, it is not clear whether these findings with naloxone-precipitated opioid withdrawal are generalizable to abstinence-induced opioid withdrawal. Future research should seek to replicate these findings with the aforementioned controls and assess for sex differences.
4.1. Conclusions
These preliminary data indicate that changes in cardiac vagal tone occur during naloxone-precipitated opioid withdrawal. Further research is needed to understand the contribution of the PNS to this effect, potentially to improve treatment of opioid withdrawal. The field might also benefit from future research to determine if there is a predictive relationship between withdrawal severity and the magnitude of change in cardiac vagal tone. Importantly, the data suggest that interventions targeting the parasympathetic nervous system may provide an alternative and novel treatment approach.
Acknowledgements
We would like to acknowledge the following medical and support staff for their assistance in the completion of this project: Claudia Tindall, NP; Janet Murray, RN; Ida Holt, RN; Ben Foote, BA; Annalise Perricone, BA; Johnathan Kaicher, BA; and Lindy Chiu, BA.
Role of funding source:
Financial support for the naloxone challenge tests was provided by the NIH and FDA grants: R01DA039169 (SDC), FDA-BAA-HHSF223201710119C (SDC), U54DA037842 (SDC), and NIDA T32-DA007294 (JMW).
Disclosures:
Dr. Comer has received compensation in the form of partial salary support from studies supported by Alkermes, Braeburn Pharmaceuticals, Cerecor Inc., Corbus, Endo Pharmaceuticals, Go Medical, Indivior PLC/Reckitt-Benckiser Pharmaceuticals, Intracellular. Therapies, Johnson & Johnson Pharmaceutical Research & Development, Lyndra, and MediciNova. In addition, Dr. Comer has consulted for: Alkermes, Charleston Labs, Clinilabs, Collegium, Daiichi Sankyo, Depomed, Egalet, Endo, Epiodyne, Inspirion Delivery Sciences, Janssen, KemPharm, Mallinckrodt, Nektar, Neurolixis, Newron, Opiant, Otsuka, Pfizer, and Sun Pharma. She also has received honoraria from the World Health Organization (WHO). Drs. Wai and Jones and Mr. Levin have no disclosures to report.
Footnotes
Declaration of Competing Interest:
The authors of this paper have no conflicts of interest to report.
References
- Charney DS, Sternberg DE, Kleber HD, Heninger GR, Redmond DE Jr, 1981. The clinical use of clonidine in abrupt withdrawal from methadone: effects on blood pressure and specific signs and symptoms. JAMA Psychiatry 38, 1273–1277. 10.1001/archpsyc.1981.01780360089010. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, Whiteford H, Leung J, Naghavi M, Griswold M, Rehm J, Hall W, Sartorius B, Scott J, Vollset SE, Knudsen AK, Haro JM, Patton G, Kopec J, Carvalho Malta D, Topor-Madry R, McGrath J, Haagsma J, Allebeck P, Phillips M, Salomon J, Hay S, Foreman K, Lim S, Mokdad A, Smith M, Gakidou E, Murray C, Vos T, 2018. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Psychiatry 5, 987–1012. 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagermoen E, Sulheim D, Winger A, Andersen AM, Gjerstad J, Godang K, Rowe PC, Saul JP, Skovlund E, Wyller VB, 2015. Effects of low-dose clonidine on cardiovascular and autonomic variables in adolescents with chronic fatigue: a randomized controlled trial. BMC Pediatr. 15 10.1186/s12887-015-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Kleber HD, 1978. Clonidine blocks acute opiate-withdrawal symptoms. Lancet 312, 599–602. 10.1016/S0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Gorodetzky CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, Biswas K, 2017. A phase III, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of Lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend. 10.1016/j.drugalcdep.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Berry MS, Subramaniam S, Umbricht A, Fingerhood M, Bigelow GE, Silverman K, 2018. Predictors of induction onto extended-release naltrexone among unemployed heroin-dependent adults. J. Subst. Abuse Treat. 10.1016/j.jsat.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, Herning RI, 2016. Naloxone-induced mood and physiologic changes in normal volunteers. Endorphins in Mental Health Research. 10.1007/978-1-349-04015-5_44. [DOI] [Google Scholar]
- Koob GF, Maldonado R, Stinus L, 1992. Neural substrates of opiate withdrawal.Trends Neurosci. 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Maldonado R, 1997. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci. Biobehav. Rev 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Michaloudis D, Kochiadakis G, Georgopoulou G, Fraidakis O, Chlouverakis G, Petrou A, Pollard BJ, 1998. The influence of premedication on heart rate variability. Anaesthesia. 10.1046/j.1365-2044.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- Neale J, Strang J, 2015. Naloxone: does over-antagonism matter? Evidence of iatrogenic harm after emergency treatment of heroin/opioid overdose. Addiction 110, 1644–1652. 10.1111/add.13027. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Wong CJ, Cheskin LJ, 1992. Cardiovascular responses to naloxone challenge in opiate-dependent individuals. Pharmacol. Biochem. Behav 10.1016/0091-3057(92)90162-9. [DOI] [PubMed] [Google Scholar]
- Ries R, Miller SC, Saitz R, Fiellin DA, Medicine AS, 2014. The ASAM principles of addiction medicine. of A.. The ASAM Principles of Addiction Medicine. [Google Scholar]
- Shaffer F, Ginsberg JP, 2017. An overview of heart rate variability metrics and norms.Front. Public Heal 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G, 2012. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am. J. Drug Alcohol Abuse 10.3109/00952990.2011.653426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force, 1996. Guidelines heart rate variability. Eur. Heart J 10.1161/01.CIR.93.5.1043. [DOI] [Google Scholar]
- Wang RIH, Wiesen RL, Lamid S, Roh BL, 1974. Rating the presence and severity of opiate dependence. Clin. Pharmacol. Ther 10.1002/cpt1974164653. [DOI] [PubMed] [Google Scholar]