Abstract
The incidence of chronic disease is elevated in women after menopause. Increased expression of ESR1 (the gene that encodes the estrogen receptor alpha, ERα) in muscle is highly associated with metabolic health and insulin sensitivity. Moreover, reduced muscle expression levels of ESR1 are observed in women, men, and animals presenting clinical features of the metabolic syndrome (MetSyn). Considering that metabolic dysfunction elevates chronic disease risk, including type 2 diabetes, heart disease, and certain cancers, treatment strategies to combat metabolic dysfunction and associated pathologies are desperately needed.
This review will provide published work supporting a critical and protective role for skeletal muscle ERα in the regulation of mitochondrial function, metabolic homeostasis, and insulin action. We will provide evidence that muscle-selective targeting of ERα may be effective for the preservation of mitochondrial and metabolic health. Collectively published findings support a compelling role for ERα in the control of muscle metabolism via its regulation of mitochondrial function and quality control. Studies identifying ERα-regulated pathways essential for disease prevention will lay the important foundation for the design of novel therapeutics to improve metabolic health of women while limiting secondary complications that have historically plagued traditional hormone replacement interventions.
Keywords: estradiol action, estrogen receptor alpha, mitochondrial function, skeletal muscle metabolism, metabolic health
For over 2 decades researchers have shown strong relationships between estrogen action and metabolic health in women. Moreover, epidemiological reports indicate that chronic disease incidence increases in women following menopause. Considering that menopause occurs on average at age 51 (www.nia.nih.gov), and that life expectancy has increased for white females to ~81.1 years (National Vital Statistics Reports, 2019) (1), women in the modern era are challenged with heightened disease risk associated with increasing adiposity and metabolic dysfunction for up to 3 decades of life. Although many researchers and clinicians have focused on the impact of replacement estrogens to ameliorate clinical symptoms and provide protective health benefit, an incomplete understanding of hormone action as well as estrogen receptor distribution and function has contributed to our continued confusion and failure to advance therapeutic strategies to combat chronic disease-associated pathologies for women.
Regarding the benefits of exogenous hormone replacement therapy (HRT) on diabetes risk after menopause, large randomized clinical trials of postmenopausal estrogen-based HRT compared with placebo and prospective cohort studies have shown reductions in fasting glucose, insulin, and incidence of new-onset type 2 diabetes (T2D) (2–7). Meta-analyses indicate a 30% lower relative risk (RR 0.7 [CI, 0.6–0.9]) of new-onset T2D in postmenopausal women following HRT compared with placebo (8). The mechanism by which HRT reduces T2D incidence in postmenopausal women is not yet known; however, molecular studies in rodents indicate that this protective effect may be achieved in part as a consequence of estrogen-induced insulin-sensitization. Considering that 75% to 85% of insulin-stimulated glucose disposal is into skeletal muscle and since skeletal muscle typically represents 30% to 40% of total body mass, we have focused our efforts in understanding the effects of estradiol/estrogen receptor (ER) α action in this tissue.
Since insulin resistance and metabolic dysfunction are identified as major underpinnings in the pathobiology of chronic diseases that plague our society, in this review we will present studies related to the biological actions of estradiol and estrogen receptors on mitochondrial function in skeletal muscle, and the impact of these biological actions exert on glucose homeostasis and insulin sensitivity. We will present basic research suggesting that the ERα form, ERα (encoded by the gene ESR1), is an important target to combat metabolic dysfunction by enhancing mitochondrial metabolism.
Molecular Mechanisms of Estrogen Receptor Action
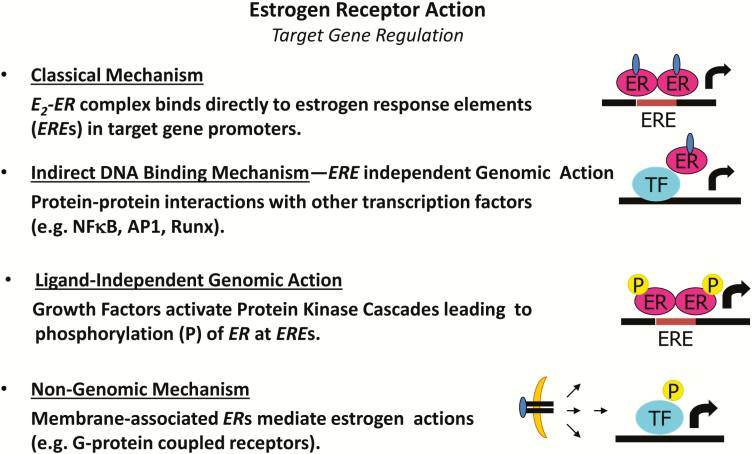
Phylogenetic analysis of steroid receptors in basal vertebrates and reconstruction of the sequences and functional attributes of ancestral proteins led to the conclusion that the primordial steroid receptor was an ER (9). Early studies in reproductive tissues investigating the actions of estradiol led to the paradigm of classical nuclear ERs as ligand-activated transcription factors (10). ERs exist in two main forms, α and β, with multiple splice variants of unknown function. ERs exhibit tissue specificity in expression and function, and determination of receptor specificity is an area of intense investigation (11). The classical, or genomic mechanism of ER action, describes a scenario whereby the ligand-activated ER dissociates from its chaperone and binds as a dimer either directly to estrogen response elements (EREs) in target genes promoters or indirectly to AP-1 or SP-1 response elements through protein tethering association with other transcription factors to DNA (12) (Fig. 1). Overlap in binding sites for E2-liganded ERα and ERβ is observed when receptors are expressed individually; however, when both ERs are present, few sites are shared. Each ER restricts the binding site occupancy of the other, with ERα typically dominating (13). Moreover, ligand-activated ERs promote transcription in a cyclic fashion. The repeated cycling of the receptor complex on and off target promoters in the presence of continuous E2 stimulation may represent a mechanism of continuous sensing and adaptation to the external hormonal milieu to yield the appropriate transcriptional response (14).
Figure 1.
Molecular actions of ERα to activate or repress target genes by classical DNA binding, non-ERE genomic action, or non-genomic actions. ERE, estrogen response element in target gene promoters; P, phosphorylation; TF, transcription factor.
In addition to classical signaling, E2-ERα can act within seconds to minutes via extranuclear and membrane-associated forms of the receptor (15) (Fig. 1). Membrane-associated receptors localize to caveolae where they congregate with other signaling molecules, including G proteins, growth factor receptors, tyrosine kinases (Src), linker proteins (MNAR), and orphan G-protein coupled receptors (GPCRs) (16). In a variety of cell types, membrane and extranuclear pools of ERs activate protein kinases that phosphorylate transcription factors to promote their nuclear translocation and transcriptional action (15,17). The G protein-coupled estrogen receptor (GPER), or GPR30, has been reported to respond to E2; however, its role as an ER is still controversial (18) (Fig. 1).
Currently the role of nuclear versus extranuclear actions of ERα in the regulation of metabolism and insulin action remains inadequately understood (19), so the prevailing theme in the field is that for many targets, nuclear and non-nuclear signaling must collaborate to achieve the full biological action of estradiol (20). Although nongenomic signaling is supported for specific cell types under defined conditions, scientific dissection of these pathways has remained challenging, thus the tissue-specific sites of action and the molecular mechanisms by which ERα selectively activates or represses target genes remains an open topic of active investigation.
ERα Genomic Actions
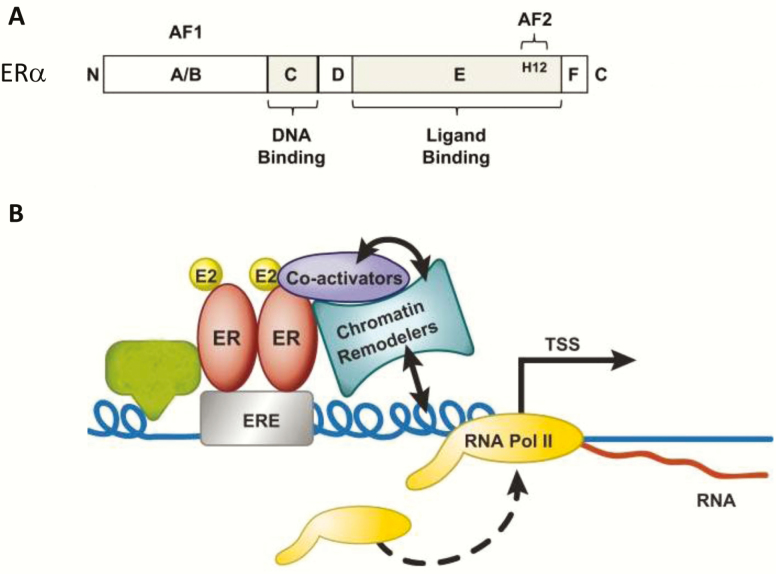
Because ERα is a ligand-dependent transcription factor that regulates a large number of genes in diverse target tissues to achieve selective action, the question arises as to how ERα exerts such specific and exacting control over so many different processes. The interplay between ligand, receptor, DNA sequence, cofactors, chromatin context, and post-translational modifications collectively governs transcriptional regulation by ERα. As stated above, ERα can bind directly to DNA (classical pathway), or can impact gene transcription indirectly via protein–protein tethering. In the classical sense ERα homodimers are thought to bind specific sequence motifs called estrogen response elements (EREs) (Fig. 2). ERs recognize DNA sequences, EREs, which have a 13-base pair consensus sequence (GGTCAnnnTGACC) separated by a 3-bp spacer (21–23). ERα-DNA binding was first identified by promoter analysis of the Xenopus vitellogenin gene (24). The binding of an ER dimer to an inverted palindrome indicates that the 2 monomers are arranged in symmetrical face-to-face configuration. Following DNA binding, ER dimers interact with basal transcription factors leading to activation or repression of target gene expression. Interestingly, there are over 70 000 estrogen responsive elements present in the human genome and >65 000 in the mouse genome, with one or more elements evolutionarily conserved in ~660 orthologous genes (26). However, in silico studies confirm that ERα action is dependent upon more factors than just DNA sequence.
Figure 2.
ERα structure (A) and DNA binding at estrogen response elements (B). From (25).
The factors governing ERα target site accessibility, including chromatin structure, is relatively unknown but is currently under intense investigation. It is estimated that only 23% of E2-responsive genes are direct targets (23). Lin et al. identified 1234 high confidence binding clusters of which 94% are projected to be bona fide ERα binding regions (23). Of importance, only 5% of the mapped estrogen receptor binding sites are located within 5 kb upstream of the transcriptional start sites of adjacent genes (regions containing the proximal promoters); therefore, the vast majority of ERα binding sites mapped to intronic or distal locations (> 5 kb from 5′ and 3′ ends of adjacent transcript), suggesting transcriptional regulatory mechanisms act over significant physical distances (27,28). Of the total ERα binding sites identified, 71% harbored putative full EREs, 25% ERE half sites, and 4% had no recognizable ERE sequences (27,28).
Classical genetics approaches provide evidence of redundant, additive, and synergistic enhancer relationships over a variety of loci. More recent studies using a multiplex interference approach reveal (in Isikawa and T-47D cells) that there is a strong collaboration between predominant and supportive ERα binding sites exposing a complex functional hierarchy of enhancers that regulate the expression of ERα target genes (28). Current thinking is that chromosomal looping allows for the collaborative action of these distal sites and that distance to the target gene and strength of the ERE motif predicts the ERα binding site necessity/importance (28). At least in liver, ERE sites, ERE half sites, AP1, bHLH, ETS, and forkhead-binding motifs were enriched for DNA sequences in ERα binding regions (29). Considering that most of what we know about ERα action is gleaned from MCF7 breast cancer cells, an important question is whether the genomics of ERα can be translated to muscle and other metabolic tissues (30). Now that we are moving beyond whole genome binding site cartography, putative ERα binding sites will require validation by functional interrogation using chromatin immunoprecipitation and mutagenesis approaches in a cell-specific context (27,28,31–34).
Estrogen Action, Metabolic Function, and Insulin Sensitivity
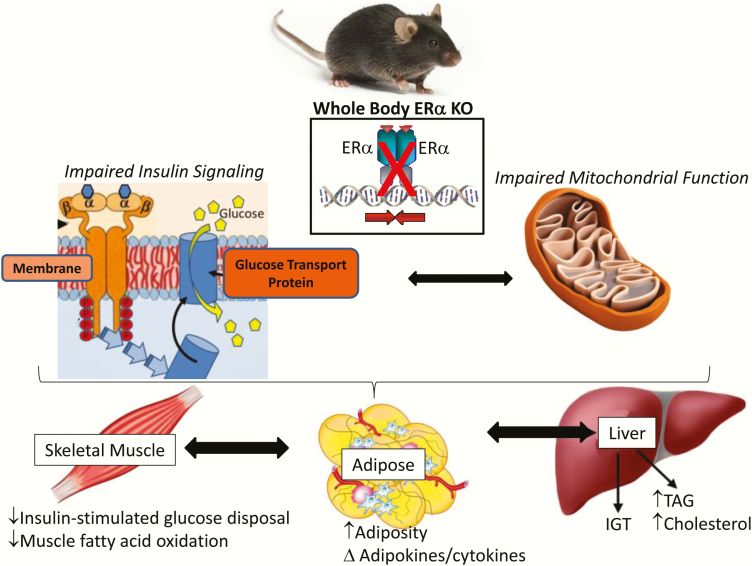
Reduced whole body ERα expression or impaired ERα function due to genetic alteration (including genetic variants) has been linked with increased prevalence of specific features of the metabolic syndrome including insulin resistance and obesity in both male and female human subjects and rodents (35–42). Since obesity is a prominent phenotype observed in estrogen- or ERα-deficient rodent models (Fig. 3), the specific role of ERα in adipocytes and the phenotypic outcomes of obesity as a consequence of adipose-specific ERα deletion in mice is currently under investigation by several laboratories around the world. Whether the obesity phenotype observed in whole body Esr1–/– mice or women harboring an ESR1 genetic variant is explained by impaired ERα action in adipose tissue specifically, or as a secondary phenotype of ERα impairment in other metabolic tissues, for example, skeletal muscle, requires resolution. Of interest, the metabolic phenotypes observed in estrogen or ERα-deficient women and female animals are consistently observed in men and male animals as well. These observations suggest that despite lower circulating estradiol and lower tissue expression levels of Esr1 in males compared with females, ERα regulatory nodes controlling specific metabolic traits are conserved between the sexes. The specific mechanisms underlying these sex-conserved regulatory nodes and trait outcomes require further delineation.
Figure 3.
The impact of whole body ERα deletion on metabolic phenotypes. From (43–45,46–48).
Insulin resistance is a central disorder in the pathogenesis of obesity and type 2 diabetes, and is a defining feature of the metabolic syndrome, a clustering of metabolic abnormalities including obesity, hypertension, glucose intolerance, and dyslipidemia (49,50). Metabolic dysfunction is worrisome as this clinical distinction is now thought to impact nearly a quarter of the US population, and drives a marked increase in the risk of numerous chronic disease states including type 2 diabetes, cardiovascular disease, neurodegeneration, and certain forms of cancer (51,52). Normally cycling premenopausal women show enhanced insulin sensitivity compared with men when sensitivity is normalized to lean mass (women have a reduced lean body mass compared to men) (53). The sex dimorphism in insulin sensitivity and an intrinsic protection against factors promoting insulin resistance in females are likely underpinnings of reduced type 2 diabetes incidence observed for premenopausal women compared with men (53,54). Although a 40% to 50% reduction in insulin-mediated glucose disposal is consistently observed in males following high-fat feeding (55,56), estradiol-replete females, humans and rodents are typically protected against a high-fat diet and acute fatty acid-induced insulin resistance (57,58).
In contrast to the metabolic protection seen in normally cycling premenopausal women, following menopause (biological or surgically induced) a precipitous decline in insulin sensitivity coincides with a dramatic increase in fat mass, and elevated circulating inflammatory markers, low-density lipoprotein, triglycerides, and fatty acids. Similar to humans, OVX mice and rats become insulin resistant, show impaired exercise-stimulated glucose disposal into muscle (59), and are more susceptible to the deleterious effects of high-fat diet or lipid oversupply. These physiological consequences of OVX are prevented by restoration of circulating estradiol or ERα-specific agonist within a physiological concentration (60–62).
Although chronic administration of E2 is shown to improve insulin sensitivity in rodents of both sexes, the acute action of estradiol to promote insulin-stimulated glucose uptake into muscle remains disputed; this despite consistent observations of E2-induced activation of Akt and AMP-activated protein kinase (AMPK) (62,63). Furthermore, although administration of intravenous conjugated estrogens and E2 to postmenopausal women or OVX rats elicited a significant increase in glucose disposal during hyperinsulinemic–euglycemic clamp studies (64,65), ex vivo treatment of skeletal muscle with E2 failed to recapitulate the same increase in insulin-stimulated glucose disposal in rodent muscle (63). It could be that the supraphysiological insulin concentrations tested thus far have masked the effects of estradiol on insulin action seen at physiological insulin doses. This ex vivo observation by Rogers et al. (63). is also in contrast to the short-term estradiol effects on insulin action in myotubes from postmenopausal women and age-matched men studied in culture (66).
Additionally, recent research by Park et al. (67) shows that the timing of E2 administration following menopause may also be of importance. This team found reduced nuclear expression of ERα in muscle from women > 10 years from final menstrual period (late postmenopause), versus early postmenopausal women < 6 years from final menstrual period (early postmenopause). Moreover, they described a lack of estradiol effect to improve insulin sensitivity and increase Pcg1a expression and AMPK phosphorylation in late postmenopause compared with the positive effects of estradiol on these endpoints in ERα-replete EPM (64,67). Collectively these data support the notion that the expression and functionality of ERα may be a key determinant of estradiol therapeutic efficacy on metabolic health (64,67).
Similar to findings for ovarian failure in women and rodents, a reduction in circulating estrogens resulting from rare inactivating mutations or experimental deletion of Cyp19 (gene that encodes aromatase cytochrome P450) confers an obesity–insulin resistance phenotype in mice of both sexes (35,68–75). The physiological and genetic evidence argues that E2 and ER favor insulin sensitivity in rodents and humans of both sexes when E2 is maintained within a tight physiological concentration. Indeed, replacement or augmentation of E2 to supraphysiological levels is thought to induce insulin resistance secondary to hyperinsulinemia and or a reduction in total GLUT4 expression in muscle (76,77). Two studies reported that higher plasma levels of E2 were prospectively associated with increased risk of developing T2D in postmenopausal women (78,79). Clearly, additional studies in rodents and humans using a dose–response strategy are necessary to better understand the interplay of steroid hormones including E2, testosterone and progesterone on the regulation of metabolism and insulin action in glucoregulatory tissues.
The Role of ESR1/ERα in Whole Body Metabolism
ESR1 is broadly expressed in the central nervous system and in peripheral tissues including adipose, skeletal muscle, liver, and immune cells (80). Women and men as well as male and female mice carrying specific ESR1 variants develop features of the metabolic syndrome including obesity, glucose intolerance, and insulin resistance. Clinical evidence shows that the clustering of these metabolic abnormalities increases disease risk (heart disease, type 2 diabetes, and certain forms of cancer) (35,36,43,44). Of translational relevance, whole body ERα knockout mice (ERαKO) recapitulate the metabolic dysfunction observed in a male human subject with a rare inactivating receptor mutation, as well as aspects of the phenotypes observed in subjects with genetic polymorphisms in the receptor (Fig. 3) (35,36,43). Not only do ERαKO mice have increased adiposity caused by reductions in energy expenditure, but they also exhibit glucose intolerance and insulin resistance, thus demonstrating a critical role for ESR1 in regulating energy and metabolic homeostasis (43–45). The integration of central and peripheral ESR1 action as well as the interaction of ERα and sex chromosome action remains to be defined; however, the tissue dissection approach to studying ERα using mice with conditional deletion alleles has allowed the research community the opportunity to delineate unique aspects of ERα biology in a tissue and sex-specific context.
Observational findings indicate that ESR1 expression levels are reduced in muscle from women with the metabolic syndrome, and studying the natural variation in muscle ESR1 expression in women revealed an inverse relationship between muscle ESR1 expression and adiposity, fasting insulin, and markers of metabolic health (ie, low muscle ESR1 expression levels are associated with metabolic dysfunction and increased adiposity) (81). Remarkably similar findings were observed across numerous strains of inbred female mice as well as in genetically obese animals illustrating the strong relationship between muscle ERα expression and metabolic health that is conserved in mouse and (wo)man. Collectively these data suggest that maintenance of ERα expression or activation of muscle ESR1 could serve as an effective means to combat diseases associated with metabolic dysfunction (81). Although these strong correlative findings suggest a relationship between muscle ERα expression levels and metabolic health, few studies have directly tested a causal relationship. Does a loss of ERα specifically from myocytes drive skeletal muscle insulin resistance, or does the insulin resistance phenotype observed in the ERαKO model arise from increased adiposity/altered adipokine/cytokine secretion and impaired central drive of feeding and ambulatory movement?
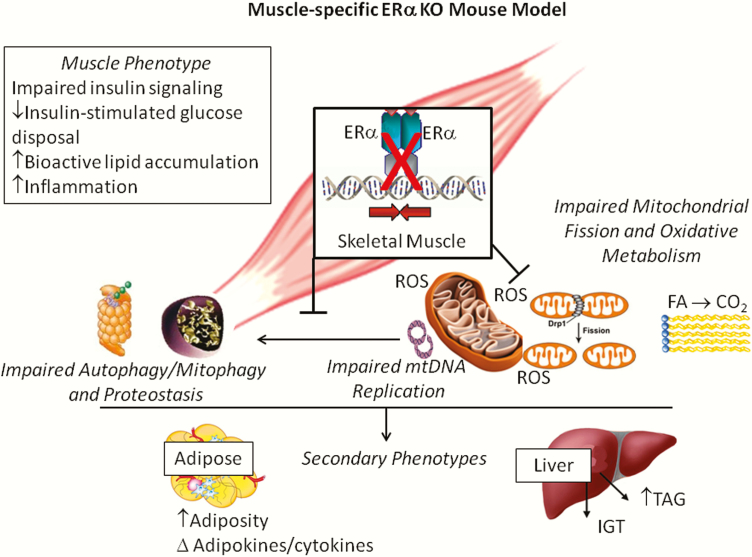
Although 2 forms of the receptor are expressed in many of the glucoregulatory tissues, ERα is expressed at much higher abundance than ERβ or GPR30, as these transcripts are nearly undetectable in muscle from human and rodents (66,81–83). Consistent with these observations, homozygous deletion of ERβ failed to produce insulin resistance (84) in contrast to the marked skeletal muscle insulin resistance observed in ERαKO animals (Fig. 3) (44,85). The underlying mechanism contributing to impaired insulin action in muscle of ERαKO animals remains disputed. Findings reported by Bryzgalova et al. (45). suggest reduced total GLUT4 levels in muscle as an underlying cause for the ERαKO insulin resistance phenotype; however, these findings have not consistently been supported (45,81). Furthermore, despite maintenance of GLUT4 mRNA and protein, Ribas et al. reported more dramatic skeletal muscle insulin resistance in ERαKO mice than Bryzgalova et al. Research by the Hevener laboratory suggests that the skeletal muscle insulin resistance observed in ERαKO mice is predominantly a consequence of direct ERα deletion effects on insulin action and secondary effects of inflammation or other factors on proximal insulin signaling. These hypotheses were subsequently tested in muscle-conditional deletion allele mice, where muscle insulin sensitivity was studied in vivo, ex vivo, and in vitro (Fig. 4).
Figure 4.
The impact of skeletal muscle-specific ERα deletion on metabolism and insulin sensitivity. Skeletal muscle-specific ERα knockout (MERKO) reduced mitochondrial DNA replication and impaired muscle oxidative metabolism, despite maintenance of mtDNA copy number. Increased PKA and reduced calcineurin activity promoted elongated, hyperfused mitochondria in MERKO muscle. The morphological changes coupled with an imbalanced PKA-calcineurin axis blunted mitochondrial fission signaling through DRP1 and impaired macroautophagy, both processes critical for mitochondrial turnover by mitophagy. Collectively, the retention of damaged mitochondria to the network was paralleled by increased ROS production, inflammation, and insulin resistance in skeletal muscle of MERKO mice. Findings implicate a critical role for ERα in the maintenance of muscle mitochondrial and metabolic health. From (81,113).
Indeed, in female muscle-specific ERα knockout mice, and myotubes with ERα knockdown, no alteration in GLUT4 mRNA or protein in skeletal muscle was observed despite reduced insulin-stimulated glucose disposal into muscle during clamp studies. Findings in the muscle-specific ERα mouse are consistent with those of whole body ERα mice (86). Furthermore, additional studies by Barros et al. (77,87). assessing GLUT4 expression in response to ovariectomy with/without E2 supplementation are in conflict with other studies of similar design (66,73,88–90). Given the lack of consensus ERE in the GLUT4 promoter (91) and absence of confirmatory findings in cellular reporter and chromatin immunoprecipitation assays, the regulation of GLUT4 expression by ERα requires further investigation. GLUT4 is regulated by several redundant transcriptional pathways (92,93). Considering that total GLUT 4 transcript and protein are not reduced in humans or rodents in the context of insulin resistance, obesity and type 2 diabetes, or between men and women (94,95), it is likely that in the absence of ERα, other transcription factors compensate to maintain GLUT4 levels (96–101). This is not to say that ERα is not involved in the exercise-stimulated increase in GLUT4 observed following training (94,102,103), as there is a concomitant increase in ERα and GLUT4 expression levels observed in muscle of exercise-trained humans and mice (82,104,105).
Myocyte enhancer factor 2 (MEF2) expression and a functional MEF2 element in the GLUT4 promoter are critical for GLUT4 gene expression (106). Furthermore, reciprocal regulation between ERα and MEF2 is observed in cardiomyocytes via ERα interaction with class II HDAC in female mice only (107). Despite complex transcriptional signal integration in the regulation of GLUT4 expression (92,93,108–111), it is conceivable that elevated ERα action could promote increased GLUT4 transcription via heightened protein tethering with MEF2 on the GLUT4 promoter or by indirect action via AMPK (63,112). It is important to note that transcriptional activity of the GLUT4 promoter is quite low under basal conditions and other ovarian hormones (eg, progesterone) are shown to play an antagonistic role in the regulation of GLUT4 expression (59). It could be that ERα acts at a distal enhancer to regulate GLUT4 transcription in muscle under specific conditions, but that ERα is not necessarily obligatory in the direct regulation of GLUT4 expression under basal conditions. In addition to MEFs role in the regulation of glucose uptake in muscle, MEF binding sites have been identified in the Pgc1a promoter (114). The role of ERα in the control of these transcription factors is of interest considering the powerful impact of ERα in regulating oxidative metabolism and mitochondrial function. The intersection of oxidative metabolism and insulin action in muscle remains incompletely understood despite decades of intense investigation.
Collectively, work by Ribas et al. suggests that the skeletal muscle insulin resistance observed in whole body ERαKO mice and animals with a muscle-specific deletion of ERα is predominantly the result of impaired insulin signal transduction (Fig. 4) (86). A role for ERα in the regulation of proximal insulin signal transduction has been suggested previously as E2 administration to insulin-resistant rodents increases insulin receptor substrate-1 abundance and insulin-stimulated tyrosine phosphorylation and as well as phosphorylation of Akt at activation site Ser473 (85,115). Akt serves many functions in myocytes including ERα-induced regulation of myogenic differentiation (116), suppression of muscle atrophy ubiquitin ligases via FOXO1 inhibition (117), and induction of genes associated with myocellular proliferation (116,118–121).
In breast cancer cell lines, endothelial cells and cortical neurons, ERα-specific binding and activation of PI3kinase as well as suppression of the tumor suppressor and PI3kinase inhibitory protein, PTEN, is well-established (122–126); however, studies on this direct interaction are limited in skeletal muscle. Additionally, E2 acting via ERα is also shown to promote phosphorylation of p38 MAPK (127,128), and transduction of a signaling cascade shown to enhance GLUT4 intrinsic activity and glucose uptake (129–131). Furthermore, ERα activation of Akt and MAPK pathways is thought to underlie E2-mediated protection of muscle against age-induced sarcopenia (132–138), exercise-induced muscle damage (120,134,139,140), and myocyte apoptosis in the face of a variety of cellular perturbations (141–144). Thus, ERα stimulation of muscle growth and insulin sensitivity via these pathways is reasonable to posit, but how these pathways converge with oxidative metabolism have remained less clear.
ERα and Skeletal Muscle Fatty Acid Metabolism
Normally cycling premenopausal women are protected against acute lipid-induced insulin resistance compared with estrogen-deficient women and men (58,145). Furthermore, muscle from premenopausal women shows enhanced insulin sensitivity despite a 47% higher triglyceride content than age-matched men (95). This observation in women is consistent with a reduced respiratory quotient and greater reliance on fatty acid oxidation as a fuel source (146). These data indicate interesting similarities between E2 replete women and exercise-trained subjects including elevated muscle ERα expression (82,104,105), heightened insulin sensitivity (99), elevated muscle lipid tolerance (147), and enhanced oxidative capacity (148,149). Consistent with the reported effects of E2 on metabolism, estrogen supplementation is shown to enhance lipid oxidation in vivo in men during acute endurance exercise (150), and stimulate palmitate oxidation in myotubes from male subjects ex vivo (66).
The effect of E2 to increase the expression of fatty acid transport protein FAT/CD36 and FABP as well as transcription factors and key enzymes that regulate oxidative metabolism (88,94,151) likely underlies these observations in human subjects. Moreover, E2 treatment reduced HFD-induced insulin resistance in skeletal muscle by 50% (assessed by hyperinsulinemic–euglycemic clamp) in an ERα-dependent manner (85). In addition, similar to exercise, E2 is shown to rapidly stimulate AMPK phosphorylation in both muscle and myotubes (63,152). AMPK is considered a central regulator of many cellular processes including growth, mitochondrial biogenesis, and oxidative metabolism (153,154). Similarly to the effects of E2 the ERα-selective agonist PPT stimulates AMPK phosphorylation in muscle of ovariectomized female rats (62) while OVX or whole body ERα deletion is associated with reduced skeletal muscle levels of phosphorylated AMPK (44,155). Recent evidence from Lipovka et al. shows that ERα but not β directly binds the βγ-subunit domain of AMPK α (156). Muscle PPARα, PPARδ, and UCP2 expression are also reduced in whole body ERαKO mice, and these factors are essential for this coordination of oxidative metabolism (Fig. 3). Interestingly, although the phenotype of impaired muscle fatty oxidation was recapitulated in the muscle-specific ERαKO mice (MERKO), no alteration in basal p-AMPK, PPARα, PPARδ, or UCP2 was observed in this mouse model (Fig. 4) (86), suggesting that these specific alterations in muscle gene expression are secondary to the loss of ERα in other metabolic tissues (eg, adipose tissue, liver, and CNS).
Despite model differences in gene and protein expression, skeletal muscle insulin resistance and bioactive lipid accumulation was surprisingly similar between ERαKO and MERKO animals (Figs. 3 and 4) (44,81). Triacylglycerol, diacylglycerol, and ceramides were all elevated significantly in muscle from female mice lacking ERα globally or specifically in muscle (44,81). Consistent with these observations, oxygen consumption rates in C2C12 myotubes with ERα knockdown were reduced significantly (81). In addition, mitochondria from muscle cells depleted of ERα or from muscle of animals lacking estradiol produced high levels of reactive oxygen species (ROS) indicative of oxidative stress (157). Analysis of mitochondrial function confirmed a defect in respiratory complex 1 activity in MERKO muscle (81,158). Moreover, these mitochondria produced increased levels of H2O2 and superoxide. Collectively these data point to a role for ERα in the direct regulation of mitochondrial function (possibly complex I); however, the precise mechanism(s) underlying these phenotypes in mice with hormone deficiency or ERα gene deletion require further investigation. Studies aimed at identifying ERα responsive genes that encode mitochondria-specific proteins are needed so that we can understand how estradiol precisely governs oxidative metabolism.
The Role of Estradiol and Muscle ERα in the Regulation of Mitochondrial Function
Mitochondria are dynamic organelles critical for the production of ATP by oxidative phosphorylation, as well as a central hub for β-oxidation, heme biosynthesis, calcium buffering, steroidogenesis, and apoptosis signaling (159). In the past decade considerable effort has focused on the role of estrogen action in disease prevention since estradiol has been linked with changes in mitochondrial function leading to clinical outcomes, including improved metabolism in type 2 diabetes, neuroprotection, and diminution of damage as a consequence of ischemia–reperfusion in cardiac tissue. Interestingly, skeletal muscle from estrogenized female rats shows increased mitochondrial mass, antioxidant protection, and a higher capacity for oxidative phosphorylation than males (160–162). Moreover, ovariectomy reduces oxygen consumption, markers of mitochondrial biogenesis, and protein abundance of key regulators of mitochondrial remodeling associated with increased hydrogen peroxide production (163,164). Estradiol replacement in OVX rats reverses the defect in oxygen consumption and increases citrate synthase and COX activity, and these changes were shown to parallel a restoration or increase in expression of Pgc1a, Tfam1, and Nrf1 (a master regulator of mitochondrial DNA replication and transcription). Similar findings by Torres et al. show that OVX reduced muscle electron transport chain complex I activity and that this defect in electron transport chain function was ameliorated by estradiol replacement (157). Although there is strong evidence for estradiol action on muscle metabolism, much of this research was performed in animals or human subjects receiving the hormone systemically, thus the findings are confounded by tissue crosstalk.
Because it is well established that estradiol promotes enhanced oxidative metabolism in muscle, and since estradiol is linked with changes in mitochondrial function contributing to disease protection in mice, several laboratories have begun to more precisely interrogate the sites of estradiol-induced action on the mitochondria (the central cellular organelle responsible for controlling oxidative metabolism). Mitochondria possess their own DNA that is maternally inherited, and exist as a circular, double-stranded genome organized into 16 569 base pairs. The mitochondrial genome encodes 37 mitochondrial genes: 22 transfer RNAs, 2 mitochondrial ribosomal RNAs, and 13 protein subunits of the electron transport chain complexes, with the exception of complex II which is entirely nuclear encoded. There are several mtDNA copies per mitochondrion and hundreds of mitochondria per cell. Importantly, although the mitochondria are home to ~20 000 polypeptides, the mitochondrial genome encodes very few of these, thus a precise communication between the mitochondria and nucleus must occur so that these organelles can maintain metabolic homeostasis. A major question puzzling the field relates to the mechanisms by which the mitochondria and nucleus communicate their requirements and coordinate activities. Since there is some evidence for the presence of ERs in both the mitochondria and the nucleus, it is provocative to posit that this female leaning transcription factor was evolutionarily conserved to preserve balance between these “symbiotes.” It is our view that additional studies to confirm the import and action of ERα within the mitochondria, especially in skeletal muscle, are required. Moreover, the signals emanating from the mitochondria to modulate nuclear gene expression, retrograde signaling, remain relatively understudied.
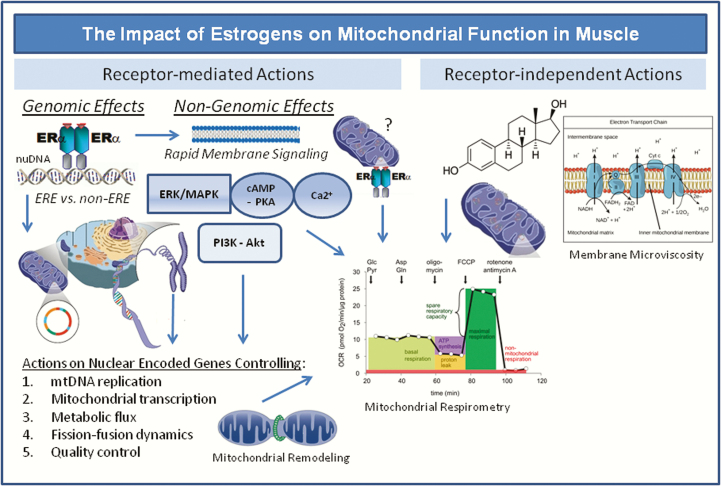
Because ovariectomy and ERα deletion are consistently shown to diminish mitochondrial function, many labs have searched for the direct targets of estradiol action that control aspects of mitochondrial biology. The field is divided into 2 major lines of scientific investigation, laboratories studying rapid nongenomic signaling of the ligand and its receptors, and those studying the DNA binding or transcription factor tethering actions of the estrogen receptor in the nucleus (Figs. 1 and 5). Although these nongenomic and genomic actions of estradiol/ERα would appear mutually exclusive, there is some consensus regarding interdependence of these pathways to achieve the full biological effectiveness of the hormone. Below we provide the most recent evidence supporting these research efforts.
Figure 5.
Genomic and non-genomic targets of estradiol/ERα action on mitochondrial function and metabolism. ERα is shown to bind estrogen response elements (EREs) or tether other transcription factors in promoters of genes controlling mitochondrial function. Nongenomic ERα membrane signaling activates kinases that alter mitochondrial remodeling and activate oxidative metabolism. Whether ERα directly tethers to the outer mitochondrial membrane has yet to be shown. Recently, estradiol was found to localize to mitochondrial membranes (independently of receptor) altering membrane microviscosity and bioenergetic function (158).
Since E2 treatment has been linked with direct actions on mitochondrial function including ATP production (165), membrane potential (166), ROS production in nonmuscle cell types, Torres et al. explored the direct effects of estradiol on mitochondrial function in isolated mitochondria from skeletal muscle (Fig. 5) (158). Using liquid chromatography mass spectrometry they detected estradiol in the membranes of the isolated mitochondria and these levels were elevated in OVX animals receiving estradiol compared with OVX. Estradiol was shown to change the mitochondrial membrane microviscosity and this was associated with enhanced complex I and I + III activities and OXPHOS responsiveness, and reduced H2O2 emission potential (158). This was the first study to show that E2 modulates muscle mitochondrial function directly, independent of receptor action. However, since these studies were conducted in culture it remains unclear whether estradiol alone, independent of receptor, is enough to ameliorate metabolic dysfunction in vivo. Evidence in women and female mice consistently points to the requirement of a functional receptor in mediating the health benefits of the hormone. This research question requires more rigorous investigation.
Since ERα has yet to be convincingly shown to reside in the mitochondria, greater efforts have focused on identifying ERα-controlled mitochondrial genes encoded in the nucleus. Studies in muscle-specific ERα knockout mice and C2C12 cells with Esr1-knockdown have shown that defects in mitochondrial function are a likely consequence of reduced expression in the only mammalian mtDNA polymerase, Polg1 (encodes the catalytic subunit of heterodimeric Polymerase γ, PolG) (81) (Figs. 4 and 5). Additionally, heavy water labeling of newly synthesized mtDNA showed a reduction in the rate of mtDNA replication, functionally supporting an impact of the reduction in Polg1 expression in MERKO mouse muscle (81). Further mechanistic studies showed that estradiol and ERα-selective ligand treatment induced Polg1 expression in muscle cells; however, ligand was ineffective to induce gene expression when the estrogen receptor was absent. Considering the presence of a consensus ERE in the Polg1 promoter, ongoing studies should delineate the mechanism(s) by which ERα regulates mtDNA replication via PolG. These observations appear internally consistent with the observed defects in mitochondrial complex I previously observed by Torres et al. (157,158) and Ribas et al. (81) considering that Complex I contains the largest number of subunits transcribed by the mitochondrial genome. Although traditional immunoblotting failed to show a difference in electron transport chain subunit abundance, it could be that the turnover of select proteins of the complexes is altered and that these proteins are less effective over time to maintain complex structure–function in the absence of estradiol/ERα action. This research question has yet to be directly tested.
Importantly, mitochondrial DNA replication is intimately linked with mitochondrial fission remodeling (severing of a mitochondrion into 2 daughter organelles), and enhanced oxidative metabolism (167,168,25,163). Since the mitochondrial architecture was markedly altered to an enlarged hyperfused mitochondrial phenotype as a consequence of a muscle-selective ERα deletion (81), the Hevener laboratory has engaged in interrogating how ERα regulates these mitochondrial remodeling processes. Studies in vitro show that treatment of murine myotubes with ERα agonists promotes mitochondrial fission achieved by rings of high order dynamin-related protein (Drp) 1 oligomers. Interestingly, ERα activation drives mitochondrial fission via coordinated activation of the fission controlling enzymes Drp1 and calcineurin, as well as direct repression of the calcineurin inhibitor Rcan1 (Figs. 4 and 5) (81). Because mitochondria from both female and male MERKO mouse muscle were enlarged, elongated, and hyperfused it was hypothesized that a reduction in fission–fusion dynamics was a primary consequence of muscle ERα deletion (81).
Internally consistent with the morphological data obtained by transmission electron microscopy, analysis of mitochondrial dynamics signaling showed reduced fission signaling by Drp1 (including increased phosphorylation at the inhibitory Ser637 site and reduced total Drp1 protein on the outer mitochondrial membrane) as well as increased abundance of the inner and outer mitochondrial membrane fusion proteins OPA1 and Mfn2, respectively, in MERKO muscle (81) (Fig. 4). Ribas et al. observed a marked increase in expression of the mitochondrial fission inhibitor Rcan1 in Esr1-KD myotubes, female MERKO muscle, and muscle from women displaying clinical features of the metabolic syndrome. Lentiviral overexpression of Rcan1 in myotubes to levels seen in MERKO mouse muscle impaired insulin action (81). Moreover, Ribas et al. confirmed that impairment of muscle mitochondrial fission led to dysfunction in mitochondrial respiration and insulin resistance in primary mytobues from female mice with Dnm1L deletion, and in C2C12 myotubes with lentiviral-mediated Dnm1L knockdown (81). Therefore it is hypothesized that a reduction in the direct effects of ERα on muscle insulin signaling as well as indirect effects of ERα on muscle insulin action mediated by mitochondrial dysfunction contribute to the development of global disturbances in insulin sensitivity and metabolic health (Fig. 4).
In light of the observation that Rcan1 was induced in ERα-deficient muscle from female mice only (not in males), despite a similar impairment in fission signaling in both sexes of MERKO mice, additional studies to flesh out the sex-specific mechanisms that underlie the impairment in mitochondrial dynamics and function in the context of ERα insufficiency are required. These studies will be viewed of translational importance since it is well known that sex is an important biological variable contributing to differences in disease incidence and pathobiology. It will also be important to discern whether the impairment in mitochondrial quality control and turnover seen in MERKO muscle is a consequence or causal of the stall in mtDNA replication and contributory or resultant of insulin resistance. The use of broad transcriptomic, proteomic, and metabolomic approaches in rodents harboring conditional ERα deletion alleles coupled with more targeted chromatin immunoprecipitation analyses in ERα ligand-treated animals will allow for the identification of novel ERα target genes and reveal new signaling nodes controlling metabolic function and insulin action specifically in muscle.
Conclusions and Perspectives
In recent years novel molecular targets have emerged offering the prospect of pharmacological intervention to restore metabolic homeostasis and insulin action, as well as ameliorate complications associated with type 2 diabetes and obesity. The inherent beauty of targeting ERα therapeutically is underscored by decades of research and in-depth knowledge related to biological/clinical efficacy and toxicity profiles obtained for estradiol replacement/selective estrogen receptor modulators during preclinical and clinical studies in animal models and human subjects. Estrogens are shown to promote energy homeostasis, improve body fat distribution, and diminish insulin resistance, β-cell dysfunction, and inflammation. The challenge with estrogens, however, is their relatively narrow therapeutic index when used chronically. Thus, the translation of the basic advances in diabetes and obesity treatment described in this review, although successful in rodents, is problematic when extending to clinical practice. Therefore, it is imperative that we determine how to modulate specific ER-controlled pathways involved in energy balance and glucose homeostasis, and develop estradiol mimetics that initiate specific cellular events promoting metabolic benefit without unwanted side effects.
With regard to whole body metabolism, obesity, and insulin sensitivity, future studies should focus on identifying the critical nodes of ERα-mediated metabolic crosstalk between all glucoregulatory tissues and determine the overlap of ERα-regulated networks, especially mitochondrial targets, as these studies may reveal new pharmacological targets for further therapeutic exploitation. Defining and then selectively targeting the ERα–mitochondrial axis may provide the required therapeutic selectively to achieve the desired therapeutic effectiveness. Now that novel technologies allow us to study this complex organelle in a more precise and comprehensive way, a new era of mitochondrial biology has emerged. A major area of focus for diabetes researchers is to understand the genes that regulate key aspects of mitochondrial function and determine how this organelle controls other pathways including insulin action, substrate metabolism, inflammation, and tissue mass.
Acknowledgments
We would like to acknowledge all of the terrific research performed by many of our esteemed colleagues in the fields of nuclear receptor biology and integrative metabolism; however, due to page limits, we were unable to cite a large number of outstanding studies. We would like to take this opportunity to thank Dr. Kenneth Korach for his generous support of our research and for providing us with the ERα floxed mouse as well as a variety of powerful molecular tools. We would also like to thank Drs. Ronald Evans, Christopher Glass, and Jerrold Olefsky for helpful discussions and intellectual contributions to our research.
Financial Support: This work was supported by grants from the National Institutes of Health (DK89109 and DK063491), the NIH Nuclear Receptor Signaling Atlas (NURSA NDSP, parent award U24DK097748), UCLA Department of Medicine, Iris Cantor-UCLA Women’s Health Research Foundation, and the UCLA Jonsson Comprehensive Cancer Center.
Glossary
Abbreviations
- AMP 5′
adenosine monophosphate
- AMPK
5′ adenosine monophosphate-activated protein kinase
- AF1
activation function 1
- AF2
activation function 2
- AP1
activation protein 1
- COX1
mitochondrial cytochrome C oxidase 1
- Cyp19
aromatase cytochrome p450
- DBD
DNA binding domain
- Drp1
dynamin related protein 1
- E2
estradiol
- ER
estrogen receptor
- ERE
estrogen response element
- ERK
extracellular signal regulated kinase
- Esr1
mouse gene encoding the estrogen receptor alpha
- FABP
fatty acid binding protein
- FAT/CD36
plasma membrane inducible long chain fatty acid translocase shown to transport fatty acids to the mitochondria
- Foxo
subgroup of the forkhead family of transcription factors with a conserved forkhead box, DNA-binding domain
- GPCR
G-protein coupled receptors
- GPER
G protein-coupled estrogen receptor
- HRT
hormone replacement therapy
- KD
knockdown
- KO
knockout
- LBD
ligand binding domain
- mt
mitochondrial
- MAPK
mitogen-activated protein kinase
- MEF2
myocyte enhancer factor 2
- MERKO
muscle specific ERα knockout
- MetSyn
Metabolic Syndrome
- Mfn
Mitochrondrial mitofusin
- MNAR
modulator of nongenomic estrogen receptors
- mt
mitochondrial
- OPA1
optic atrophy 1
- OVX
ovariectomized
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PPAR
peroxisome proliferator activated receptor
- PolG
heterodimeric enzyme polymerase gamma that controls mtDNA replication
- Polg1
the gene that encodes the catalytic subunit of the mtDNA polymerase PolG
- Rcan1
regulator of calcineurin 1, a phosphatase that (among many functions) controls Drp1 action and mitochondrial fission
- ROS
reactive oxygen species
- RR
relative risk
- SF1
steroidogenic factor 1
- T2D
type 2 diabetes mellitus
- TFAM
mitochondrial transcription factor A
- UCP
uncoupling protein
Additional Information
Disclosure Statement: The authors have nothing to disclose.
References
- 1. Kenneth D. Kochanek M.A., Sherry L. Murphy B.S., Jiaquan Xu M.D., and Elizabeth Arias Ph.D., Division of Vital Statistics National Vital Statistics Reports, Vol. 68, No. 9, June 24, 2019 [PubMed] [Google Scholar]
- 2. Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia. 2006;49(3):459–468. [DOI] [PubMed] [Google Scholar]
- 3. Kanaya AM, Herrington D, Vittinghoff E, et al. ; Heart and Estrogen/progestin Replacement Study Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. [DOI] [PubMed] [Google Scholar]
- 4. Margolis KL, Bonds DE, Rodabough RJ, et al. ; Women’s Health Initiative Investigators Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. [DOI] [PubMed] [Google Scholar]
- 5. Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5(10):553–558. [DOI] [PubMed] [Google Scholar]
- 6. Pentti K, Tuppurainen MT, Honkanen R, et al. Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. Eur J Endocrinol. 2009;160(6):979–983. [DOI] [PubMed] [Google Scholar]
- 7. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. [DOI] [PubMed] [Google Scholar]
- 9. Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98(10):5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Malley BW. Mechanisms of action of steroid hormones. N Engl J Med. 1971;284:370–377. [DOI] [PubMed] [Google Scholar]
- 11. Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. [DOI] [PubMed] [Google Scholar]
- 12. Safe S, Kim K, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41(5):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol. 2010;24(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. [DOI] [PubMed] [Google Scholar]
- 15. Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. [DOI] [PubMed] [Google Scholar]
- 16. Levin ER. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med. 2015;66:271–280. [DOI] [PubMed] [Google Scholar]
- 17. Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–51. [DOI] [PubMed] [Google Scholar]
- 18. Ronda AC, Boland RL. Intracellular Distribution and Involvement of GPR30 in the Actions of E2 on C2C12 Cells. J Cell Biochem. 2016;117(3):793–805. [DOI] [PubMed] [Google Scholar]
- 19. Liu S, Mauvais-Jarvis F. Minireview: estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151(3):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pedram A, Razandi M, Blumberg B, Levin ER. Membrane and nuclear estrogen receptor α collaborate to suppress adipogenesis but not triglyceride content. Faseb J. 2016;30(1):230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20(8):1707–1714. [DOI] [PubMed] [Google Scholar]
- 22. Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. [DOI] [PubMed] [Google Scholar]
- 23. Lin CY, Vega VB, Thomsen JS, et al. Whole-genome cartography of estrogen receptor alpha binding sites. Plos Genet. 2007;3(6):e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seiler-Tuyns A, Walker P, Martinez E, Mérillat AM, Givel F, Wahli W. Identification of estrogen-responsive DNA sequences by transient expression experiments in a human breast cancer cell line. Nucleic Acids Res. 1986;14(22):8755–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hewitt SC, Korach KS. Estrogen receptors: new directions in the new millennium. Endocr Rev. 2018;39(5):664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bourdeau V, Deschênes J, Métivier R, et al. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18(6):1411–1427. [DOI] [PubMed] [Google Scholar]
- 27. Fullwood MJ, Liu MH, Pan YF, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462(7269):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carleton JB, Berrett KC, Gertz J. Multiplex enhancer interference reveals collaborative control of gene regulation by estrogen receptor alpha-bound enhancers. Cell Syst. 2017;5:333–344 e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao H, Fält S, Sandelin A, Gustafsson JA, Dahlman-Wright K. Genome-wide identification of estrogen receptor alpha-binding sites in mouse liver. Mol Endocrinol. 2008;22(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Droog M, Mensink M, Zwart W. The estrogen receptor α-Cistrome beyond breast cancer. Mol Endocrinol. 2016;30(10):1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welboren WJ, Sweep FC, Span PN, Stunnenberg HG. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer. 2009;16(4):1073–1089. [DOI] [PubMed] [Google Scholar]
- 32. Welboren WJ, van Driel MA, Janssen-Megens EM, et al. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. Embo J. 2009;28(10):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andersson R, Gebhard C, Miguel-Escalada I, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507(7493):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Z, Merkurjev D, Yang F, et al. Enhancer activation requires trans-recruitment of a mega transcription factor complex. Cell. 2014;159(2):358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. [DOI] [PubMed] [Google Scholar]
- 36. Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27(9):1020–1027. [DOI] [PubMed] [Google Scholar]
- 37. Nilsson M, Dahlman I, Rydén M, et al. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond). 2007;31(6):900–907. [DOI] [PubMed] [Google Scholar]
- 38. Deng HW, Li J, Li JL, et al. Association of estrogen receptor-alpha genotypes with body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab. 2000;85(8):2748–2751. [DOI] [PubMed] [Google Scholar]
- 39. Casazza K, Page GP, Fernandez JR. The association between the rs2234693 and rs9340799 estrogen receptor alpha gene polymorphisms and risk factors for cardiovascular disease: a review. Biol Res Nurs. 2010;12(1):84–97. [DOI] [PubMed] [Google Scholar]
- 40. Okura T, Koda M, Ando F, Niino N, Shimokata H. Relationships of resting energy expenditure with body fat distribution and abdominal fatness in Japanese population. J Physiol Anthropol Appl Human Sci. 2003;22(1):47–52. [DOI] [PubMed] [Google Scholar]
- 41. Okura T, Koda M, Ando F, Niino N, Tanaka M, Shimokata H. Association of the mitochondrial DNA 15497G/A polymorphism with obesity in a middle-aged and elderly Japanese population. Hum Genet. 2003;113(5):432–436. [DOI] [PubMed] [Google Scholar]
- 42. Yamada Y, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density of the femoral neck in elderly Japanese women. J Mol Med (Berl). 2002;80(7):452–460. [DOI] [PubMed] [Google Scholar]
- 43. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298(2):E304–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–597. [DOI] [PubMed] [Google Scholar]
- 46. Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001;178(1-2):147–154. [DOI] [PubMed] [Google Scholar]
- 47. Villablanca A, Lubahn D, Shelby L, Lloyd K, Barthold S. Susceptibility to early atherosclerosis in male mice is mediated by estrogen receptor alpha. Arterioscler Thromb Vasc Biol. 2004;24(6):1055–1061. [DOI] [PubMed] [Google Scholar]
- 48. Couse JF, Curtis SW, Washburn TF, Eddy EM, Schomberg DW, Korach KS. Disruption of the mouse oestrogen receptor gene: resulting phenotypes and experimental findings. Biochem Soc Trans. 1995;23(4):929–935. [DOI] [PubMed] [Google Scholar]
- 49. DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15(3):318–368. [DOI] [PubMed] [Google Scholar]
- 50. Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149(1):33–45. [DOI] [PubMed] [Google Scholar]
- 51. Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 53. Yki-Järvinen H. Sex and insulin sensitivity. Metabolism. 1984;33(11):1011–1015. [DOI] [PubMed] [Google Scholar]
- 54. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117(6):1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choi CS, Fillmore JJ, Kim JK, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117(7):1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51(6):1907–1912. [DOI] [PubMed] [Google Scholar]
- 58. Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50(6):1344–1350. [DOI] [PubMed] [Google Scholar]
- 59. Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282(5):E1139–E1146. [DOI] [PubMed] [Google Scholar]
- 60. Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51(7):861–870. [DOI] [PubMed] [Google Scholar]
- 61. Hamilton DJ, Minze LJ, Kumar T, et al. Estrogen receptor alpha activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Physiol Rep. 2016;4:e12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor alpha increases insulin-stimulated skeletal muscle glucose uptake. J Physiol. 2011;589(8):2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382(4):646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285(2):E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alonso A, González-Pardo H, Garrido P, et al. Acute effects of 17 β-estradiol and genistein on insulin sensitivity and spatial memory in aged ovariectomized female rats. Age (Dordr). 2010;32(4):421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Salehzadeh F, Rune A, Osler M, Al-Khalili L. Testosterone or 17{beta}-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J Endocrinol. 2011;210(2):219–229. [DOI] [PubMed] [Google Scholar]
- 67. Park YM, Pereira RI, Erickson CB, Swibas TA, Kang C, Van Pelt RE. Time since menopause and skeletal muscle estrogen receptors, PGC-1α, and AMPK. Menopause. 2017;24(7):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabet Med. 2007;24(12):1491–1495. [DOI] [PubMed] [Google Scholar]
- 69. Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97(23):12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guercio G, Di Palma MI, Pepe C, et al. Metformin, estrogen replacement therapy and gonadotropin inhibition fail to improve insulin sensitivity in a girl with aromatase deficiency. Horm Res. 2009;72(6):370–376. [DOI] [PubMed] [Google Scholar]
- 71. Takeda K, Toda K, Saibara T, et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol. 2003;176(2):237–246. [DOI] [PubMed] [Google Scholar]
- 72. Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61–70. [DOI] [PubMed] [Google Scholar]
- 73. Maffei L, Rochira V, Zirilli L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf). 2007;67(2):218–224. [DOI] [PubMed] [Google Scholar]
- 74. Jones ME, McInnes KJ, Boon WC, Simpson ER. Estrogen and adiposity–utilizing models of aromatase deficiency to explore the relationship. J Steroid Biochem Mol Biol. 2007;106(1-5):3–7. [DOI] [PubMed] [Google Scholar]
- 75. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. [DOI] [PubMed] [Google Scholar]
- 76. Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1-2):63–68. [DOI] [PubMed] [Google Scholar]
- 77. Barros RP, Morani A, Moriscot A, Machado UF. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol Cell Endocrinol. 2008;295(1-2):24–31. [DOI] [PubMed] [Google Scholar]
- 78. Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076–2084. [DOI] [PubMed] [Google Scholar]
- 79. Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 81. Ribas V, Drew BG, Zhou Z, et al. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. 2016;8:334ra354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wiik A, Gustafsson T, Esbjörnsson M, et al. Expression of oestrogen receptor alpha and beta is higher in skeletal muscle of highly endurance-trained than of moderately active men. Acta Physiol Scand. 2005;184(2):105–112. [DOI] [PubMed] [Google Scholar]
- 83. Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. Plos One. 2010;5(4):e10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640–645. [DOI] [PubMed] [Google Scholar]
- 85. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. [DOI] [PubMed] [Google Scholar]
- 86. Ribas V, Drew BG, Soleymani T, Daraei P, Hevener A. Skeletal muscle specific ER alpha deletion is causal for the metabolic syndrome. Endocr Rev. 2010;31:S5. [Google Scholar]
- 87. Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006;103(5):1605–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17beta-estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. J Mol Endocrinol. 2003;31(1):37–45. [DOI] [PubMed] [Google Scholar]
- 89. Alonso A, Ordóñez P, Fernández R, et al. 17beta-estradiol treatment is unable to reproduce p85 alpha redistribution associated with gestational insulin resistance in rats. J Steroid Biochem Mol Biol. 2009;116(3–5):160–170. [DOI] [PubMed] [Google Scholar]
- 90. Hansen PA, McCarthy TJ, Pasia EN, Spina RJ, Gulve EA. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J Appl Physiol (1985). 1996;80(5):1605–1611. [DOI] [PubMed] [Google Scholar]
- 91. Barros RP, Gustafsson JÅ. Estrogen receptors and the metabolic network. Cell Metab. 2011;14(3):289–299. [DOI] [PubMed] [Google Scholar]
- 92. Murgia M, Jensen TE, Cusinato M, Garcia M, Richter EA, Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J Physiol. 2009;587(Pt 17):4319–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zorzano A, Palacín M, Gumà A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. 2005;183(1):43–58. [DOI] [PubMed] [Google Scholar]
- 94. Fu MH, Maher AC, Hamadeh MJ, Ye C, Tarnopolsky MA. Exercise, sex, menstrual cycle phase, and 17beta-estradiol influence metabolism-related genes in human skeletal muscle. Physiol Genomics. 2009;40(1):34–47. [DOI] [PubMed] [Google Scholar]
- 95. Høeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol (1985). 2009;107(3):824–831. [DOI] [PubMed] [Google Scholar]
- 96. Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101(11):2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992;41(4):465–475. [DOI] [PubMed] [Google Scholar]
- 98. Banks EA, Brozinick JT Jr, Yaspelkis BB 3rd, Kang HY, Ivy JL. Muscle glucose transport, GLUT-4 content, and degree of exercise training in obese Zucker rats. Am J Physiol. 1992;263(5 Pt 1):E1010–E1015. [DOI] [PubMed] [Google Scholar]
- 99. Brozinick JT Jr, Etgen GJ Jr, Yaspelkis BB 3rd, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT-4 protein content and translocation in obese Zucker rats. Am J Physiol. 1993;265(3):E419–427. [DOI] [PubMed] [Google Scholar]
- 100. Brozinick JT Jr, Etgen GJ Jr, Yaspelkis BB 3rd, Ivy JL. Glucose uptake and GLUT-4 protein distribution in skeletal muscle of the obese Zucker rat. Am J Physiol. 1994;267(1 Pt 2):R236–R243. [DOI] [PubMed] [Google Scholar]
- 101. Hevener AL, Reichart D, Olefsky J. Exercise and thiazolidinedione therapy normalize insulin action in the obese Zucker fatty rat. Diabetes. 2000;49(12):2154–2159. [DOI] [PubMed] [Google Scholar]
- 102. Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43(7):862–865. [DOI] [PubMed] [Google Scholar]
- 103. Rodnick KJ, Holloszy JO, Mondon CE, James DE. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes. 1990;39(11):1425–1429. [DOI] [PubMed] [Google Scholar]
- 104. Lemoine S, Granier P, Tiffoche C, Berthon PM, Thieulant ML, Carré F, Delamarche P. Effect of endurance training on oestrogen receptor alpha expression in different rat skeletal muscle type. Acta Physiol Scand. 2002;175(3):211–217. [DOI] [PubMed] [Google Scholar]
- 105. Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor alpha transcripts in rat skeletal muscle. Acta Physiol Scand. 2002;174(3):283–289. [DOI] [PubMed] [Google Scholar]
- 106. Mora S, Pessin JE. The MEF2A isoform is required for striated muscle-specific expression of the insulin-responsive GLUT4 glucose transporter. J Biol Chem. 2000;275(21):16323–16328. [DOI] [PubMed] [Google Scholar]
- 107. van Rooij E, Fielitz J, Sutherland LB, et al. Myocyte enhancer factor 2 and class II histone deacetylases control a gender-specific pathway of cardioprotection mediated by the estrogen receptor. Circ Res. 2010;106(1):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Moreno H, Serrano AL, Santalucía T, et al. Differential regulation of the muscle-specific GLUT4 enhancer in regenerating and adult skeletal muscle. J Biol Chem. 2003;278(42):40557–40564. [DOI] [PubMed] [Google Scholar]
- 109. Gan Z, Burkart-Hartman EM, Han DH et al. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011;25(24):2619–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Oshel KM, Knight JB, Cao KT, Thai MV, Olson AL. Identification of a 30-base pair regulatory element and novel DNA binding protein that regulates the human GLUT4 promoter in transgenic mice. J Biol Chem. 2000;275(31):23666–23673. [DOI] [PubMed] [Google Scholar]
- 111. Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab. 2008;295(3):E698–E704. [DOI] [PubMed] [Google Scholar]
- 112. Gong H, Xie J, Zhang N, Yao L, Zhang Y. MEF2A binding to the Glut4 promoter occurs via an AMPKα2-dependent mechanism. Med Sci Sports Exerc. 2011;43(8):1441–1450. [DOI] [PubMed] [Google Scholar]
- 113. Collins BC, Mader TL, Cabelka CA, Inigo MR, Spangenburg EE, Lowe DA. Deletion of estrogen receptor alpha in skeletal muscle results in impaired contractility in female mice. J Appl Physiol (1985). 2018;124:980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A. 2003;100(4):1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ordóñez P, Moreno M, Alonso A, Llaneza P, Díaz F, González C. 17beta-Estradiol and/or progesterone protect from insulin resistance in STZ-induced diabetic rats. J Steroid Biochem Mol Biol. 2008;111(3-5):287–294. [DOI] [PubMed] [Google Scholar]
- 116. Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17beta-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-alpha-mediated signals. Am J Physiol Cell Physiol. 2009;297(5):C1249–C1262. [DOI] [PubMed] [Google Scholar]
- 117. Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403. [DOI] [PubMed] [Google Scholar]
- 118. Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf). 2008;194(1):81–93. [DOI] [PubMed] [Google Scholar]
- 119. Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol (1985). 2008;104(2):347–353. [DOI] [PubMed] [Google Scholar]
- 120. Thomas A, Bunyan K, Tiidus PM. Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf). 2010;198(1):81–89. [DOI] [PubMed] [Google Scholar]
- 121. Kamanga-Sollo E, White ME, Hathaway MR, Weber WJ, Dayton WR. Effect of Estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol. 2010;39(1):54–62. [DOI] [PubMed] [Google Scholar]
- 122. Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Up-regulation of PI3K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha, but not estrogen receptor-beta, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun. 2005;336(4):1221–1226. [DOI] [PubMed] [Google Scholar]
- 123. Noh EM, Lee YR, Chay KO, et al. Estrogen receptor α induces down-regulation of PTEN through PI3-kinase activation in breast cancer cells. Mol Med Rep. 2011;4(2):215–219. [DOI] [PubMed] [Google Scholar]
- 124. Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Simoncini T, Fornari L, Mannella P, Varone G, Caruso A, Liao JK, Genazzani AR. Novel non-transcriptional mechanisms for estrogen receptor signaling in the cardiovascular system. Interaction of estrogen receptor alpha with phosphatidylinositol 3-OH kinase. Steroids. 2002;67(12):935–939. [DOI] [PubMed] [Google Scholar]
- 126. Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase ½ in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26(37):9439–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ronda AC, Vasconsuelo A, Boland R. Extracellular-regulated kinase and p38 mitogen-activated protein kinases are involved in the antiapoptotic action of 17beta-estradiol in skeletal muscle cells. J Endocrinol. 2010;206(2):235–246. [DOI] [PubMed] [Google Scholar]
- 128. Ronda AC, Buitrago C, Boland R. Role of estrogen receptors, PKC and Src in ERK2 and p38 MAPK signaling triggered by 17β-estradiol in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;122(4):287–294. [DOI] [PubMed] [Google Scholar]
- 129. Niu W, Huang C, Nawaz Z, et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem. 2003;278(20):17953–17962. [DOI] [PubMed] [Google Scholar]
- 130. Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80(5):569–578. [DOI] [PubMed] [Google Scholar]
- 131. Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274(15):10071–10078. [DOI] [PubMed] [Google Scholar]
- 132. Sørensen MB, Rosenfalck AM, Højgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9(10):622–626. [DOI] [PubMed] [Google Scholar]
- 133. Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163–175B. [DOI] [PubMed] [Google Scholar]
- 134. Tiidus PM. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J Appl Physiol. 2000;25(4):274–287. [DOI] [PubMed] [Google Scholar]
- 135. Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. 2011;68(4):331–336. [DOI] [PubMed] [Google Scholar]
- 136. Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition – a substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):651–656. [DOI] [PubMed] [Google Scholar]
- 137. Sipilä S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond). 2001;101(2):147–157. [PubMed] [Google Scholar]
- 138. Teixeira PJ, Going SB, Houtkooper LB, et al. Resistance training in postmenopausal women with and without hormone therapy. Med Sci Sports Exerc. 2003;35(4):555–562. [DOI] [PubMed] [Google Scholar]
- 139. Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol (1985). 2009;107(3):853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sotiriadou S, Kyparos A, Albani M, et al. Soleus muscle force following downhill running in ovariectomized rats treated with estrogen. Appl Physiol Nutr Metab. 2006;31(4):449–459. [DOI] [PubMed] [Google Scholar]
- 141. Boland R, Vasconsuelo A, Milanesi L, Ronda AC, de Boland AR. 17beta-estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids. 2008;73(9-10):859–863. [DOI] [PubMed] [Google Scholar]
- 142. Vasconsuelo A, Milanesi L, Boland R. 17Beta-estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2008;196(2):385–397. [DOI] [PubMed] [Google Scholar]
- 143. Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55(5):1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am J Physiol Cell Physiol. 2009;297(3):C548–C555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Høeg LD, Sjøberg KA, Jeppesen J, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cortright RN, Koves TR. Sex differences in substrate metabolism and energy homeostasis. Can J Appl Physiol. 2000;25(4):288–311. [DOI] [PubMed] [Google Scholar]
- 147. Amati F, Dubé JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60(10):2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maher AC, Akhtar M, Vockley J, Tarnopolsky MA. Women have higher protein content of beta-oxidation enzymes in skeletal muscle than men. Plos One. 2010;5(8):e12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262(6 Pt 1):E791–E799. [DOI] [PubMed] [Google Scholar]
- 150. Hamadeh MJ, Devries MC, Tarnopolsky MA. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J Clin Endocrinol Metab. 2005;90(6):3592–3599. [DOI] [PubMed] [Google Scholar]
- 151. Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics. 2010;42(3):342–347. [DOI] [PubMed] [Google Scholar]
- 152. D’Eon TM, Rogers NH, Stancheva ZS, Greenberg AS. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring). 2008;16(6):1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kim JY, Jo KJ, Kim OS, et al. Parenteral 17beta-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci. 2010;87(11-12):358–366. [DOI] [PubMed] [Google Scholar]
- 156. Lipovka Y, Chen H, Vagner J, Price TJ, Tsao TS, Konhilas JP. Oestrogen receptors interact with the alpha-catalytic subunit of AMP-activated protein kinase. Biosci Rep. 2015;35:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Torres MJ, Ryan TE, Lin CT, Zeczycki TN, Neufer PD. Impact of 17β-estradiol on complex I kinetics and H2O2 production in liver and skeletal muscle mitochondria. J Biol Chem. 2018;293(43):16889–16898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Torres MJ, Kew KA, Ryan TE, et al. 17beta-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 2018;27:167–179 e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chandel NS. Mitochondria: back to the future. Nat Rev Mol Cell Biol. 2018;19(2):76. [DOI] [PubMed] [Google Scholar]
- 160. Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74(3):456–465. [DOI] [PubMed] [Google Scholar]
- 161. Català-Niell A, Estrany ME, Proenza AM, Gianotti M, Lladó I. Skeletal muscle and liver oxidative metabolism in response to a voluntary isocaloric intake of a high fat diet in male and female rats. Cell Physiol Biochem. 2008;22(1-4):327–336. [DOI] [PubMed] [Google Scholar]
- 162. Gómez-Pérez Y, Amengual-Cladera E, Català-Niell A, et al. Gender dimorphism in high-fat-diet-induced insulin resistance in skeletal muscle of aged rats. Cell Physiol Biochem. 2008;22(5-6):539–548. [DOI] [PubMed] [Google Scholar]
- 163. Capllonch-Amer G, Lladó I, Proenza AM, García-Palmer FJ, Gianotti M. Opposite effects of 17-β estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes. J Mol Endocrinol. 2014;52(2):203–214. [DOI] [PubMed] [Google Scholar]
- 164. Capllonch-Amer G, Sbert-Roig M, Galmés-Pascual BM, Proenza AM, Lladó I, Gianotti M, García-Palmer FJ. Estradiol stimulates mitochondrial biogenesis and adiponectin expression in skeletal muscle. J Endocrinol. 2014;221(3):391–403. [DOI] [PubMed] [Google Scholar]
- 165. Moreno AJ, Moreira PI, Custódio JB, Santos MS. Mechanism of inhibition of mitochondrial ATP synthase by 17β-estradiol. J Bioenerg Biomembr. 2013;45(3):261–270. [DOI] [PubMed] [Google Scholar]
- 166. Wang J, Green PS, Simpkins JW. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem. 2001;77(3):804–811. [DOI] [PubMed] [Google Scholar]
- 167. Lewis SC, Uchiyama LF, Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 2016;353:aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mahdaviani K, Benador IY, Su S, et al. Mfn2 deletion in brown adipose tissue protects from insulin resistance and impairs thermogenesis. EMBO Rep. 2017;18(7):1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]