Abstract
Background
About 350 million people are chronically infected carriers of hepatitis B virus and are at a higher risk of serious illness and death from cirrhosis of the liver and liver cancer. Chinese medicinal herbs have been used widely for more than 2000 years to treat chronic liver disease.
Objectives
To assess whether Chinese medicinal herbs are effective and safe for treating asymptomatic carriers of hepatitis B virus.
Search methods
The trials registers of The Cochrane Hepato‐Biliary Group, The Cochrane Library, and The Cochrane Complementary Medicine Field were searched in combination with MEDLINE, EMBASE, and handsearches of Chinese journals and conference proceedings (October 2000).
Selection criteria
Randomised or quasi‐randomised trials (minimum follow‐up three months) in asymptomatic carriers of hepatitis B virus. Chinese medicinal herbs (single herb or compound of herbs) compared with placebo, no intervention, general non‐specific treatment, or interferon treatment. Trials of Chinese medicinal herbs plus interferon versus interferon alone were also included.
Data collection and analysis
Data were extracted independently by two authors. Analysis was performed by intention‐to‐treat where possible. Pre‐specified subgroup analyses were: ethnic origin, age at time of infection, and single herb or compound of herbs.
Main results
Three randomised clinical trials (307 patients) that followed patients for three months or more after the end of treatment were included. The methodological quality was poor. The herbal compound 'Jianpi Wenshen recipe' had significant effects on viral markers compared to interferon: relative risk 2.40 (95% CI 1.01 to 5.72) for clearance of serum HBsAg, 2.03 (95% CI 0.98 to 4.20) for clearance of HBeAg, and 2.54 (95% CI 1.13 to 5.70) for seroconversion of HBeAg to anti‐HBe. Phyllanthus amarus and Astragalus membranaceus showed no significant antiviral effect compared with placebo. Analysis of pooling eight randomised clinical trials with less than three months follow‐up did not show a significant benefit of Chinese medicinal herbs on viral markers. Data on long‐term clinical outcomes and quality of life were lacking.
Authors' conclusions
Based on one low quality trial, the medicinal herb 'Jianpi Wenshen recipe' may have an antiviral activity in asymptomatic carriers of hepatitis B virus. However, rigorous randomised, double‐blind, placebo‐controlled trials are needed before herbs should be used for this condition.
Plain language summary
Firm evidence for effect of Chinese medicinal herbs for asymptomatic hepatitis B virus carriers is still awaited
Chinese medicinal herbs have a long history in the treatment of liver diseases. Three randomised clinical trials were included. Due to their poor methodologic quality and the existing small number of trials, there is currently insufficient evidence for treating asymptomatic hepatitis B virus carriers with Chinese medicinal herbs like the herbal compound 'Jianpi Wenshen recipe', Phyllanthus amarus and Astragalus membranaceus. Methodologically better and larger randomised trials are needed comparing medicinal herbs versus placebo.
Background
Hepatitis B is a liver disease caused by hepatitis B virus (HBV) infection. Worldwide more than two billion people alive today have been infected (WHO 2000). About 350 million people are chronically infected carriers of the virus (WHO 2000) and are at a higher risk of development of serious sequelae. More than one million carriers worldwide die from cirrhosis of the liver and liver cancer each year (WHO 2000). China alone bears a heavy burden of hepatitis B with an estimated 120 million infected carriers, and 300,000 people die each year (CMH 1999). HBV infection is, therefore, an important public health problem in China. Alone in this country treatment costs amount from 30 to 50 billion RMB Yuan (3.57˜5.95 billion US $) every year (CMH 1999).
Most chronic HBV carriers are asymptomatic and constitute the reservoir of HBV infection to the non‐immune population. Progressive disease is associated with persistence of viral replication whereas remission is associated with loss of active viral replication (Dusheiko 1999). Eradication of the carrier state is an important step in the control of HBV infection.
HBV is transmitted by body fluids, such as blood and serum, and can be passed from mother to child. The virus belongs to the family hepadnaviridae (Dusheiko 1999). The virus has a round structure (42 nm diameter) called the Dane particle and has an outer coat and an inner core. The protein on the surface, called hepatitis B surface antigen (HBsAg), is a standard marker of infection when found in the serum. The inner core is made of nucleocapsid protein (HBcAg) which encloses the virus DNA (HBV DNA). Another core protein, called hepatitis B 'e' antigen (HBeAg), is used in the diagnosis as a marker of infection and virus replication.
With the availability of a safe and effective vaccine, the incidence of new HBV carriers in children has decreased in high endemic areas. However, in areas with low endemicity a 'high risk group' immunization strategy will not lead to a significant reduction of HBV infection on a national or international scale (WHO 2000). Most adult patients with chronic HBV infection were born before vaccination was available and hence millions of patients are awaiting improvement in the treatment of this disease. Currently, interferon (IFN) and lamivudine are the only two drugs licensed worldwide for treatment of chronic HBV infection. Treating adults who have both raised alanine aminotransferase (ALT) levels and are HBeAg and/or HBV DNA positive with alpha IFN results in viral, biochemical, and histological remission in about 30˜40 per cent of cases (Sherlock 1997). However, available information suggests that patients with normal ALT respond poorly to IFN and lamivudine (Lin 1999). Consequently, no drug treatment is currently recommended for asymptomatic HBV carriers.
Chinese herbal medicine is part of Traditional Chinese Medicine (TCM), which is a 3000‐year‐old holistic system of medicine combining the use of medicinal herbs, acupuncture, food therapy, massage, and therapeutic exercise for both treatment and prevention of disease (Fulder 1996). TCM has its unique theories for concepts of etiology, systems of diagnosis and treatment which are vital to its practice. TCM drug treatment consists typically of complex prescriptions of a combination of several components. The combination based on the Chinese diagnostic patterns (i.e., inspection, listening, smelling, inquiry, and palpation) follows a completely different rationale than many western drug treatments. In this review Chinese medicinal herbs are considered collectively as a special experimental treatment for HBV infection.
Chinese medicinal herbs have been used widely for more than 2000 years to treat chronic liver diseases, including chronic HBV infection (Wang 2000). For hepatitis B, the TCM diagnostic process includes laboratory measures of liver function indices and viral markers. Many controlled trials have been done on treatment with the medicinal herbs, but efficacy has not been assessed systematically. The spontaneous evolution of chronic hepatitis B disease is difficult to predict and a definitive assessment of the effects of treatment requires long‐term follow‐up (Liaw 1997).
Objectives
The primary objective was to assess the antiviral efficacy of Chinese medicinal herbs as a special experimental treatment for asymptomatic carriers of HBV infection compared with placebo, no intervention, non‐specific treatment, or IFN treatment. Where possible, meta‐analysis on subcategories of herbal medicines according to the organisation of the materia medica was performed.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCTs) or quasi‐randomised clinical trials were included irrespective of blinding, publication status and language. Only trials with a minimum follow‐up of three months were included.
Types of participants
Male or female patients, of any age or ethnic origin, who were asymptomatic carriers of HBV (serum HBsAg positive and/or HBeAg positive for more than six months, no symptoms and signs of hepatitis, with serum levels of ALT or aspartate aminotransferase (AST) within the normal range). Patients with superinfection or coinfection of HBV with another hepatitis virus were excluded. Clinical diagnosis included disease history, symptoms and signs, viral replication markers (HBsAg, HBeAg, HBV DNA), and liver biochemistry.
Types of interventions
Chinese medicinal herbs (single herb or compound) compared with placebo, no intervention, general non‐specific treatments such as vitamins, or IFN treatment (no limitation regarding IFN type or IFN treatment regimen). Trials of Chinese medicinal herbs plus IFN versus IFN alone were also included.
Types of outcome measures
The following outcome measures were sought at three, six and 12 months after completion of the treatment and at maximal follow‐up:
Antiviral response:
Loss of serum HBsAg
Loss of serum HBeAg
Loss of serum HBV DNA
Seroconversion from HBeAg to anti‐HBe (antibody against HBeAg).
Viral response should be measured by validated methods as follows: HBsAg, anti‐HBs, anti‐HBe, and HBeAg were measured by either enzyme immunoassay or radial immunoassay. HBV DNA was measured by direct molecular hybridisation or polymerase chain reaction assay.
Biochemical response: Normal level or no changes of ALT and AST levels (less than 40 IU/L).
Other outcome measures: Important outcomes such as incidence of liver cirrhosis, hepatocellular carcinoma (HCC), death, and quality of life were also assessed.
Adverse events: Serious adverse events (death, hospitalisation or prolongation of hospitalisation, persistent or significant disability or events that may jeopardise the patient or may require intervention to prevent one of the former serious adverse events) (ICH 1997). Less serious adverse events (fever, headache, allergy, gastrointestinal reaction, decreased white blood cell count).
Search methods for identification of studies
Electronic searches The trials registers of The Cochrane Hepato‐Biliary Group, The Cochrane Library, and of The Cochrane Complementary Medicine Field were searched in combination with MEDLINE (March 1966 to Obtober 2000) and EMBASE (January 1980 to October 2000). A search strategy for the Cochrane Library was developed by the Cochrane Hepato‐Biliary Group. MEDLINE and EMBASE were searched using the MeSH terms: hepatitis‐B, carrier‐state, medicine‐Chinese‐traditional, plants‐medicinal, drugs‐Chinese‐herbal, plants extracts, and herbs. No language restrictions were applied.
Handsearches Chinese Journal of Infectious Diseases, Chinese Journal of Hepatology, Chinese Journal of Clinical Hepatology, Chinese Journal of Integrated Traditional and Western Medicine, and Journal of Integrated Traditional and Western Medicine on Liver Diseases were handsearched from the first publication date onwards (October 2000). Conference proceedings in Chinese were also handsearched.
Data collection and analysis
Selection of trials for inclusion Two authors independently selected the trials according to the pre‐specified selection criteria. Disagreement was resolved by discussion.
Assessment of methodological quality The quality of included trials was assessed using the instrument developed by Jadad et al. (Jadad 1996). In addition, concealment of the allocation sequence was scored as A (adequate), B (unclear) or C (inadequate), following the criteria adopted from The Cochrane Handbook and Schulz et al. (Schulz 1995) as follows:
A ‐ Adequate measures to conceal allocations such as central randomisation; serially numbered, opaque, sealed envelopes; or other description that contained convincing elements of concealment. B ‐ Unclearly concealed trials, in which the authors either did not report an allocation concealment approach at all, or reported an approach that did not fall into one of the categories in (A). C ‐ Inadequately concealed trials, in which method of allocation was not concealed, such as alternation methods or use of case record numbers.
Data extraction Data were extracted independently by two authors using a self‐developed data extraction form. Disagreement was resolved by discussion. Papers not in English or Chinese were translated with the help of the Cochrane Hepato‐Biliary Group.
Data on the number of allocated patients, irrespective of compliance or follow‐up, were sought to allow an intention‐to‐treat analysis. If the above data were not available in the trial reports further information was sought by correspondence with the principal investigator.
Data synthesis Dichotomous data were presented as relative risk (RR) and continuous outcomes as weighted mean difference (WMD), both with 95% confidence intervals (CI). Intention‐to‐treat analysis was performed if possible. For dichotomous outcomes, patients with incomplete or missing data were included in sensitivity analyses by counting them as treatment failures ('worst‐case' scenario analysis).
We intended to display studies as comparisons of traditional Chinese medicinal herbs versus: ‐ placebo ‐ no treatment ‐ general non‐specific treatment (such as vitamins) ‐ IFN treatment.
Trials of Chinese medicinal herbs plus IFN treatment versus IFN treatment alone were presented as a separate comparison.
Trials within these comparisons were presented as pre‐specified sub‐categories according to whether the herbal therapy intervention was: ‐ single herb (or component from single herb), or ‐ compound of herbs.
Meta‐analysis was only performed within comparisons where individual trials compared similar treatment and control interventions.
We pre‐specified the following subgroup analyses: ‐ ethnic origin ‐ age at time of infection. Age is a major factor in determining the outcome of HBV infection. Children and adults exhibit different patterns of disease outcome. Young children rarely develop symptomatic HBV infection, but about 25 per cent infected under the age of seven will become carriers. After the age of seven, children exhibit an adult pattern of disease outcome with about 5 to 10 per cent becoming carriers (WHO 1999). ‐ single herb or compound of herbs.
Sensitivity analysis If a sufficient number of trials was found, sensitivity analyses were pre‐specified as follows: ‐ excluding quasi‐randomised trials (such as alternate allocation or systematic allocation) ‐ excluding studies with inadequate concealment of allocation (Schulz 1995) ‐ excluding unblinded studies.
Potential biases (Vickers 1998) were investigated according to Egger et al. (Egger 1997).
Results
Description of studies
Our initial searches (October 2000) identified 95 articles, 56 from the electronic searches and 39 from handsearching. After reading titles and abstracts, 53 of these articles were excluded because they were duplicates, non‐clinical studies or had study objectives different from this review. A total of 42 articles published in Chinese and English were retrieved for further assessment. Of these, 31 articles were excluded because they did not meet our inclusion criteria. The reasons for exclusion are listed under 'Characteristics of excluded studies'. None of the studies were excluded due to superinfection or coinfection with other hepatitis virus.
In total, 11 articles, each describing a RCT, were identified. They reported random allocation of asymptomatic HBV carriers (n = 932) to Chinese medicinal herb versus placebo, no treatment, or IFN.
These trials tested eight different herbal medicines. Two single herbs versus placebo: Phyllanthus amarus (Leelarasamee 1990; Milne 1994; Peng 1993; Thamlikitkul 1991) and Astragalus membranaceus (Peng 1997). One single herb (Geshanxiao) versus no intervention (Yang 1995). One compound of herbs (Ganling Wan) versus placebo (Qiu 1993). And four compounds of herbs versus IFN: Toad‐ant capsule (Dang 1997), Jianpi Wenshen recipe (Song 1994), Ganbifu (Xu 1999), and Yiganling (Yang 1996a).
Of the 11 trials, eight had a follow‐up duration of less than three months after the end of treatment. According to our inclusion criteria, trials had to report a minimum of three months follow‐up. However, in order to investigate immediate therapeutic effects, we decided to present the eight trials with shorter follow‐up in a separate analysis. These eight trials are described in 'Additional table: Trials with less than three months follow‐up' (Table 3).
1. Trials with less than three months follow‐up.
| Study ID | Methods | Participants | Interventions | Outcomes | Methodology |
| Dang 1997 | Randomised clinical trial. Generation of allocation sequence: using random rank table. Allocation concealment: no information. Blinding: no blinding. Estimation of sample size: yes, by formula. Withdrawal/ drop‐out: unstated. Jadad score=2. | Ethnic: Chinese; 72 patients (36 in the treatment group, 24 males and 12 females, nine with age less than or equal to 18 yrs, 27 with lrager than 18 yrs; 36 in control group, 23 males and 13 females, eight with age less than or equal to 18 yrs, 28 with age larger than 18 yrs). Setting: unstated. Inclusion criteria: carriers being HBsAg positive for more than six months, with normal liver function and no symptoms. Exclusion criteria: age less than 12 yrs or larger than 65 yrs; pregnant or breast feeding; allergic to test drugs. | Experimental: Toad‐ant capsule (a self‐prescribed compound) orally, 10˜12 capsules (3˜3.6 g) for adults, 0.9˜1.5 g for children, three times daily (t.i.d), for six to nine months. Control: alfa‐interferon tablet, one tablet (no data on unit) orally, daily, for six to nine months. | HBsAg, HBeAg, and adverse effects. The outcomes were assessed at the end of treatment. | Low |
| Milne 1994 | Randomised placebo‐ controlled trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: not stated. Estimation of sample size: no information. Withdrawal/ drop‐out: yes, two in placebo group. Jadad‐score=2. | Ethnic: the treatment group included 22 Maoris, 21 Europeans, five Pacific Islanders and four Asians; the placebo group included 22 Maoris, 22 Europeans, five Pacific Islanders and four Asians. 105 patients (52 in the treatment group, all males, average age 31 yrs, ranging 18‐66 yrs ; 53 in placebo group, all males, average age 31 yrs, ranging 18‐68 yrs). Setting: unstated. Inclusion criteria: blood donors being HBsAg positive for more than six months and with normal ALT level. Exclusion criteria: unstated. | Experimental: Phyllanthus amarus, one capsule (290 mg) orally, t.i.d, for eight weeks. Control: Placebo (glucose), one capsule (300 mg) orally, t.i.d, for 30 days. | HBsAg, HBeAg; ALT, count of blood cells, serum urea and serum creatinine. Adverse effects. Follow‐up: till the end of treatment. The outcomes were assessed at the end of two months treatment. | Low |
| Peng 1993 | Randomised, double blind, placebo‐controlled trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: double blind; the placebo was glucose made as the same weight and appearance as the tested drug. Estimation of sample size: no information. Withdrawal/ drop‐out: yes, one in both group respectively. Jadad‐score=3. | Ethnic: Chinese; 30 patients (23 males and seven females, age from 21˜58 yrs. 16 in treatment group, 14 in control group). Setting: unstated. Inclusion criteria: carriers being HBsAg positive for more than six months and with normal liver function. Exclusion criteria: unstated. | Experimental: Phyllanthus urinaria (a herb), two capsule (400 mg) orally, t.i.d, for 60 days. Control: Placebo (glucose powder), two capsules orally, t.i.d, for 60 days. | HBsAg, HBeAg. Follow‐up: one month after the end of treatment. The outcomes were assessed at the end of 60 days of treatment and one month after the treatment. | High |
| Qiu 1993 | Randomised, double blind, placebo‐controlled trial. Generation of allocation sequence: random number table. Allocation concealment: sealed, labelled drugs. Blinding: double blind; the placebo was composed of 1/10 of the tested drug with same appearance. Estimation of sample size: no information. Withdrawal/ drop‐out: unstated. Jadad‐score=3. | Ethnic: Chinese; 169 patients (including 93 acute and chronic active hepatitis, and 76 asymptomatic HBV carriers. No details on age and gender. Only data of 76 carriers were used in this review). Setting: inpatients and outpatients. Inclusion criteria: asymptomatic HBV carriers being HBsAg and HBeAg both positive for more than six months, with normal liver function. Exclusion criteria: unstated. | Experimental: Ganling Wan (a herbal compound), two pills orally, t.i.d, for three months. Control: Placebo (composed of 1/10 amount of Ganling Wan), two pills orally, t.i.d, for three months. | HBsAg, HBeAg, and adverse events. The outcomes were assessed at the end of three months treatment. | High |
| Thamlikitkul 1991 | Randomised placebo‐ controlled trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: how the placebo applied was not stated. Estimation of sample size: yes. Withdrawal/ drop‐out: none. Jadad‐score=2. | Ethnic: Thailanders; 65 patients (34 in the treatment group, 19 males and 15 females, average age 25 yrs, ranging 16‐36 yrs ; 31 in placebo group, 16 males and 15 females, average age 26 yrs, ranging 19‐35 yrs). Setting: outpatients. Inclusion criteria: blood donors and health care personnel being HBsAg positive for more than six months and with normal ALT level. Exclusion criteria: subjects with known hematologic, liver, lung and renal diseases as well as pregnant women were excluded. | Experimental: Phyllanthus amarus, one capsule (200 mg) orally, t.i.d, for 30 days. Subjects who fully complied with the drug were given Phyllanthus amarus 200 mg orally t.i.d for another 30 days. Control: Placebo (starch powder), one capsule orally, t.i.d, for 30 days. Subjects who fully complied with placebo were given two capsules (400 mg) of Phyllanthus amarus orally t.i.d, for another 30 days. | HBsAg, HBeAg; liver function test and renal function test; symptoms and signs. Adverse effects. Follow‐up: till the end of treatment. The outcomes were assessed at the end of 30 and 60 days of treatment. Only data from the first study period of 30 days were extracted. | Low |
| Xu 1999 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Estimation of sample size: no information. Withdrawal/ drop‐out: unstated. Jadad score=1. | Ethnic: Chinese; 44 patients (23 in the treatment group, 19 males and four females, age from 18‐44 yrs; 21 in control group, 18 males and three females, age from 17‐48 yrs). Setting: unstated. Inclusion criteria: carriers being HBsAg positive for more than six months and with normal liver function. Exclusion criteria: unstated. | Experimental: Ganbifu capsule (mixture of herbs), two capsules (500 mg) orally, t.i.d, for six months. Control: Interferon, 20,000˜50,000 units, acupoint injection, every other day, for 20 days, then stop for one week. This treatment schedule was repeated 6.6 times during six months. | HBsAg, HBeAg. The outcomes were assessed at the end of six months treatment. | Low |
| Yang 1995 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Estimation of sample size: no information. Withdrawal/ drop‐out: unstated. Jadad score=1. | Ethnic: Chinese; 60 patients (30 in the treatment group, 19 males and 11 females, average age 36 yrs, from 12‐51 yrs; 30 in control group, 17 males and 13 females, average age 39 yrs, from 16‐47 yrs). Setting: unstated. Inclusion criteria: asymptomatic carriers being HBsAg, HBeAg and anti‐HBc positive for more than six months, with normal liver function and no clinical symptoms and signs of liver disease. Exclusion criteria: unstated. | Experimental: 'Geshanxiao' (Chinese herbal medicine) 16 g decoction divided into three to five times orally, for six months. Control: no treatment. | HBsAg, anti‐HBs, HBeAg, anti‐HBe, anti‐HBc, and adverse events. The outcomes were assessed at the end of three months treatment. | Low |
| Yang 1996 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Estimation of sample size: no information. Withdrawal/ drop‐out: unstated. Jadad score=1. | Ethnic: Chinese; 80 patients (40 in the treatment group, 31 males and nine females, average age 25 yrs, from 11‐47 yrs; 40 in control group, 28 males and 12 females, average age 24 yrs, from 13‐44 yrs). Setting: unstated. Inclusion criteria: carriers being HBsAg, HBeAg and/or anti‐HBc positive for more than six months, with normal liver function and no clinical symptoms and signs of liver disease. Exclusion criteria: unstated. | Experimental: Yiganling decoction (mixture of 15 herbs) one dose orally, daily, for two to three months. Co‐interventions included vitamin B co (two tablets) plus vitamin C (0.2 g) plus hypoxanthosine (0.2 g) orally, t.i.d, for three months. Control: Interferon, one MU, i.m., every other day, plus the same co‐interventions as treatment group; all for three months. | HBsAg, HBeAg. The outcomes were assessed at the end of three months treatment. | Low |
The remaining three trials involving 307 patients met all the inclusion criteria (Leelarasamee 1990; Peng 1997; Song 1994). They followed patients for three to 12 months (median six months) after the end of treatment. One trial included Thailanders (n = 116), but did not describe their sex or age (Leelarasamee 1990). The other two trials included Chinese children and adults, with a male to female ratio of 2:1 (130:61) (Peng 1997; Song 1994). The average sample size of the trials was 102 patients, ranging from 80 to 116. The median duration of treatment was three months (range one to three months). The primary outcomes reported were viral responses (including serum HBsAg, HBeAg, anti‐HBe). Only one trial described adverse events (Leelarasamee 1990).
Risk of bias in included studies
The three included trials had low methodological quality (Jadad‐score two for Leelarasamee 1990 and Peng 1997, and one for Song 1994). Allocation concealment and generation of the allocation sequence were not described in these trials. Only one trial was described as blinded, but no details on the degree of blindness were given (Leelarasamee 1990).
The eight trials with follow‐up of less than three months also had general low methodological quality, but two trials had high methodological quality (Jadad‐score three; Peng 1993; Qiu 1993). The Jadad‐scores were two for Dang 1997, Milne 1994, and Thamlikitkul 1991; one for Xu 1999, Yang 1995, and Yang 1996a. Adequate allocation concealment was described in one trial (Qiu 1993) and was not stated in the other trials. Four trials were placebo‐controlled (Milne 1994; Peng 1993; Qiu 1993; Thamlikitkul 1991), but two gave no information on how the placebo was applied (Milne 1994; Thamlikitkul 1991).
Three trials reported sample size calculation (Dang 1997; Leelarasamee 1990; Milne 1994). Two trials reported numbers lost to follow‐up (Leelarasamee 1990; Peng 1993), and another trial described the withdrawal of patients (Milne 1994). No trial reported using intention‐to‐treat analysis.
An insufficient number of trials was found to perform meaningful sensitivity analysis excluding studies with inadequate methodological quality.
Effects of interventions
Antiviral response One trial (Song 1994) involving 80 patients showed a significantly better effect of a herbal compound 'Jianpi Wenshen recipe' on clearance of serum HBsAg compared with IFN (RR 2.40, 95% CI 1.01 to 5.72, P = 0.05). RRs were 2.54 (95% CI 1.13 to 5.70, P = 0.02) for seroconversion of HBeAg to anti‐HBe and 2.03 (95% CI 0.98 to 4.20, P = 0.06) for clearance of serum HBeAg.
Another trial (n = 101) (Peng 1997) showed no antiviral effect of a herbal extract of Astragalus membranaceus on clearance of serum HBsAg (RR 7.84, 95% CI 0.49 to 124.12, P = 0.14), on clearance of serum HBeAg (RR 2.77, 95% CI 0.97 to 7.90) and on seroconversion of HBeAg to antiHBe (RR 1.55, 95% CI 0.52 to 4.59, P = 0.4) when comparing with placebo. One trial (Leelarasamee 1990) reported outcome of serum HBeAg level in patients treated with herbal medicine Phyllanthus amarus versus placebo, but the difference was not significant (WMD ‐0.01, 95% CI ‐0.24 to 0.22). Phyllanthus amarus showed no effect on clearance of serum HBsAg in this trial.
None of the included trials reported serum HBV DNA levels.
Eight trials with less than three months follow‐up were examined to see how their findings were compared with three trials with a minimum of three months follow‐up after the end of treatment. Data were available for clearance of serum HBsAg (n = 532), HBeAg (n = 380) and seroconversion of HBeAg to anti‐HBe (n = 104). The RR for serum HBsAg clearance was 1.22 (95% CI 0.57 to 2.60) using random effects model because of significant heterogeneity (Chi‐square = 11.1, df = 5, P = 0.05). The RR for serum HBeAg clearance was 1.06 (95% CI 0.70 to 1.62) using random effects model (test for heterogeneity Chi‐square = 13.1, df = 6, P = 0.04). Seroconversion from HBeAg to anti‐HBe was reported in two trials and the RR was 2.47 (95% CI 0.61 to 10.04).
No trials were found examining medicinal herbs plus IFN versus IFN alone in asymptomatic carriers of HBV.
Biochemical response One trial reported assessment of liver biochemistry after the end of therapy and showed no abnormal values (Leelarasamee 1990).
Other outcomes None of the included trials reported on death, liver cirrhosis, HCC, or quality of life.
Adverse events Only one out of three included trials reported monitoring adverse events through biochemical and haematological analyses and showed no significant abnormal values during one month treatment with Phyllanthus amarus (Leelarasamee 1990). Five out of eight trials with follow‐up less than three months reported adverse events (Dang 1997; Milne 1994; Qiu 1993; Thamlikitkul 1991; Yang 1995). One of them reported appearance of the symptoms including nausea, gastric discomfort, sore mouth, tiredness, flu‐like symptoms, dizziness and headache in 18/52 Phyllanthus amarus recipients and in a similar proportion of 53 placebo treated patients (Milne 1994). This trial also reported an erythematous truncal rash in one Phyllanthus amarus recipient. Four patients in the Phyllanthus amarus group complained of plant‐smell after belching and at urination in another trial (Thamlikitkul 1991).
No serious adverse events were reported.
Publication bias There was an insufficient number of trials for us to investigate publication bias.
Discussion
Based on three RCTs, it appears that herbal compound 'Jianpi Wenshen recipe' may have positive effects on the clearance of serum HBsAg and on the seroconversion of HBeAg to anti‐HBe in asymptomatic HBV carriers.
Strength of the evidence The three trials included in this review had poor methodological quality and provided only limited descriptions of how the allocation sequence was generated or how allocation was concealed. One placebo‐controlled trial was described as blinded but no details were given about the appearance, taste and smell of the placebo or how blinding was applied (Leelarasamee 1990). The other two trials were not blinded, and they reported extraordinary large difference in the distribution of patients between the arms in the trials, but gave no explanation on how this happened (Peng 1997; Song 1994). Methodologically less rigorous trials show larger differences between experimental and control groups and larger treatment effects than trials conducted with more rigor (Schulz 1995; Moher 1998; Kjaergard 1999). The small number of trials identified and their general low methodological quality prohibited meaningful sensitivity analysis to explore how robust the results of this review are to exclusion of the trials with inadequate methodology. We cannot, therefore, exclude potential bias in selection of patients, their subsequent treatment, and judgement of outcomes in the included trials. No multi centre, large scale RCTs with long‐term follow‐up were identified.
Population The small number of trials made it impossible to conduct subgroup analysis for ethnic origin or age at infection. One included trial was in Thai patients and the other two in Chinese patients.
Category of Chinese medicinal herbs The included trials tested different herbs against different control interventions. Two single herbs, Phyllanthus amarus and Astragalus membranaceus were compared with placebo, and one compound of herbs ('Jianpi Wenshen recipe') was compared with IFN in the included trials. The herbs were given in different formulations (capsule, injection, and decoction) and in different ways (orally, acupoint injection, and intramuscular injection). Ideally, we should consider differences in herbal constituents, dosage, duration, and control intervention in each trial when evaluating the efficacy of Chinese medicinal herbs. We were limited in this respect by the small number of trials.
Efficacy Based on three trials with adequate follow‐up, only one herb compound 'Jianpi Wenshen recipe' may have a positive effect on clearance of HBV compared to IFN in asymptomatic HBV carriers. Eight trials with less than three months follow‐up, however, did not show a beneficial viral response to Chinese medicinal herbs. Data from RCTs on clinical relevant outcomes from long‐term follow‐up are lacking.
Among the herbs tested in this review, including the trials with less than three months follow‐up, the single herb Phyllanthus amarus tested in four trials showed no significant antiviral effect compared with placebo. In our recent review of chronic hepatitis B infection Phyllanthus amarus demonstrated significant antiviral activity (clearance of HBeAg and seroconversion of HBeAg to anti‐HBe) compared with non‐specific treatment or no intervention (Liu 2001). One explanation for the difference could be that chronic hepatitis B and asymptomatic HBV carriers differ in their response to Phyllanthus amarus. Other possible explanations include the difference of ethnic origin of the participants, the source of the herb, the processing and preparation of the herb, and the duration of treatment. One of the trials in this review of asymptomatic carriers compared the herbal compound 'Jianpi Wenshen recipe' with IFN and showed a tendency towards a better effect with the herbs than of IFN among the participants, who were mainly children (Song 1994).
The goal of treatment of asymptomatic HBV carriers is to prevent progression to hepatic fibrosis, liver cirrhosis and HCC. Long‐term follow‐up studies suggest that HBeAg seroconversion, whether induced by IFN therapy or spontaneously, is beneficial in terms of survival, liver failure and HCC (Niederau 1996; Lin 1999). However, asymptomatic HBV carriers are not suitable candidates for IFN or lamivudine treatment because of poor response rates. There are two possible reasons for the poor response: the immune tolerance to HBV after infection at birth or during early childhood (Lai 1998) and persistence of the viral DNA in the infected person's liver (Lin 1998). Searches on The Cochrane Library and MEDLINE did not find evidence supporting the use of IFN treatment in asymptomatic HBV carriers. Three RCTs (Lai 1987; Lai 1991; Rodriguez 1997) have compared alpha‐IFN versus placebo involving 143 asymptomatic HBV carriers, but no significant effects were found. The combined odds ratios were 4.20 (95% CI 0.48 to 95.14, P = 0.15) for loss of serum HBeAg, 2.82 (95% CI 0.52 to 20.05, P = 0.16) for loss of HBV DNA, and no patients lost their serum HBsAg by IFN treatment (Liu 2000).
Adverse events A conclusion on adverse events associated with Chinese medicinal herbs cannot be drawn from this review due to the limited number of trials identified, the duration of treatment and follow‐up, and inadequate recording and reporting of adverse outcomes. Only one included trial reported adverse events (Leelarasamee 1990). In China, clinical trials of medicinal herbs seldom report adverse events, and the reason for this is possibly that Chinese practitioners perceive medicinal herbs as free of side effects (Liu 1999b). However, there are reports of liver toxicity and even cancer associated with using Chinese herbal medicines (Melchart 1999; Nortier 2000; Tomlinson 2000). Safety of complementary medicines needs to be monitored (Marrone 1999). In clinical trials efficacy and safety should receive equal attention and occasional and severe adverse events need to be investigated in large scale epidemiological studies.
Authors' conclusions
Implications for practice.
Based on this systematic review, herbal compound 'Jianpi Wenshen recipe' may have antiviral activity in asymptomatic HBV carriers. However, due to the poor methodological quality of the trials and the small number of trials conducted, there is currently insufficient evidence for treating asymptomatic HBV carriers with any of these Chinese medicinal herbs.
Implications for research.
The methodological quality of clinical trials of Chinese medicinal herbs needs to be improved (Tang 1999). The following is necessary: (i) detailed reporting of the generation of the allocation sequence and of allocation concealment, (ii) application of blinding and placebo control, (iii) clear description of withdrawals/dropouts during the trial, (iv) reporting of clinically important outcome measures from long‐term follow‐up.
Rigorously designed, multicentre, randomised, double‐blind, placebo‐controlled trials are required to evaluate Chinese medicinal herbs for asymptomatic HBV carriers. Outcome measures should include liver histopathology (such as hepatic fibrosis, cirrhosis and liver cancer), quality of life and mortality as well as virological and biochemical outcomes. Adverse events should be critically assessed by standardised monitoring or an effective self‐report system, and attention should be paid to long‐term adverse effects of Chinese medicinal herbs.
What's new
| Date | Event | Description |
|---|---|---|
| 9 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Klaus Linde for his advice and constructive comments on our protocol. We are grateful to Christian Gluud for expert suggestions and corrections.
Data and analyses
Comparison 1. Chinese medicinal herbs versus placebo or IFN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Phyllanthus amarus versus placebo | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Astragalus membranaceus versus saline | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.84 [0.49, 124.12] |
| 1.3 Jianpi Wenshen compound versus IFN | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.01, 5.72] |
| 2 Loss of HBeAg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Astragalus membranaceus versus saline | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.97, 7.90] |
| 2.2 Jianpi Wenshen compound versus IFN | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.98, 4.20] |
| 3 Decrease of serum HBeAg level | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Phyllanthus amarus versus placebo | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.24, 0.22] |
| 4 Seroconversion of HBeAg to anti‐HBe | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Astragalus membranaceus versus saline | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.52, 4.59] |
| 4.2 Jianpi Wenshen compound versus IFN | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.13, 5.70] |
1.1. Analysis.
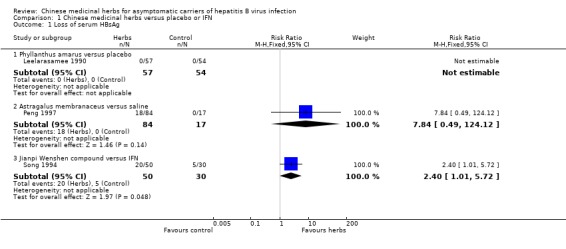
Comparison 1 Chinese medicinal herbs versus placebo or IFN, Outcome 1 Loss of serum HBsAg.
1.2. Analysis.
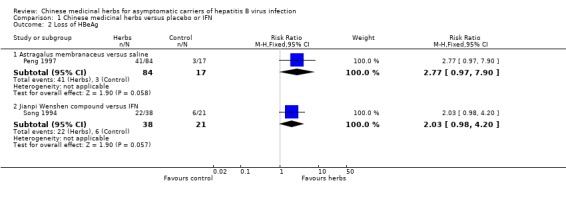
Comparison 1 Chinese medicinal herbs versus placebo or IFN, Outcome 2 Loss of HBeAg.
1.3. Analysis.
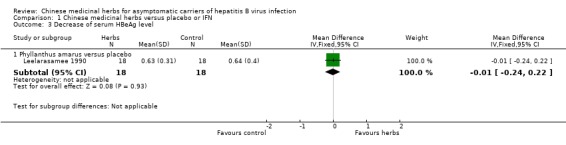
Comparison 1 Chinese medicinal herbs versus placebo or IFN, Outcome 3 Decrease of serum HBeAg level.
1.4. Analysis.
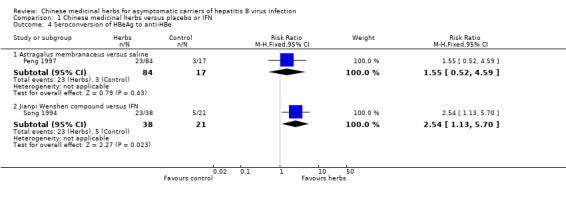
Comparison 1 Chinese medicinal herbs versus placebo or IFN, Outcome 4 Seroconversion of HBeAg to anti‐HBe.
Comparison 2. Duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow‐up < three months | 8 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.92, 1.98] |
| 1.2 Follow‐up = or > three months | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [1.29, 7.15] |
| 2 Loss of serum HBeAg | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow‐up < three months | 7 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.90, 1.42] |
| 2.2 Follow‐up = or > three months | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.26, 4.27] |
| 3 Seroconversion of HBeAg to anti‐HBe | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Follow‐up < three months | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.61, 10.04] |
| 3.2 Follow‐up = or > three months | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.11, 4.01] |
2.1. Analysis.
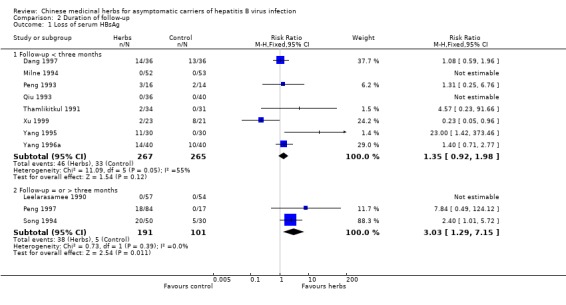
Comparison 2 Duration of follow‐up, Outcome 1 Loss of serum HBsAg.
2.2. Analysis.
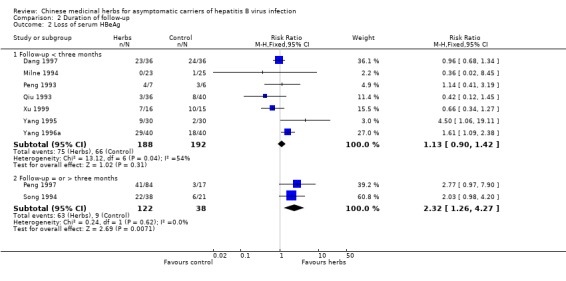
Comparison 2 Duration of follow‐up, Outcome 2 Loss of serum HBeAg.
2.3. Analysis.
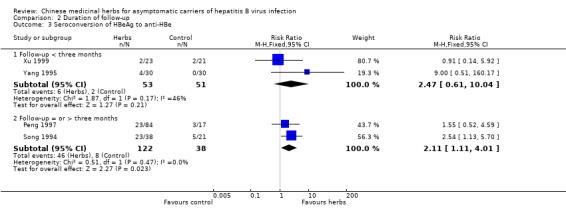
Comparison 2 Duration of follow‐up, Outcome 3 Seroconversion of HBeAg to anti‐HBe.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dang 1997.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Leelarasamee 1990.
| Methods | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: the trial was described as blinded, but no detail was given on appearance, taste and smell of the placebo. Estimation of sample size: yes. Loss during follow‐up: Ten patients in treatment group and 15 in placebo group were lost at six months follow‐up. Jadad‐score = 2. | |
| Participants | Ethnic: Thailanders; 116 patients (59 in the treatment group; 57 in placebo group), no data on gender and age. Setting: outpatients. Inclusion criteria: blood donors being HBsAg positive for six to 12 months and with normal biochemical and haematological tests, and with informed consent. Exclusion criteria: unstated. | |
| Interventions | Experimental: Phyllanthus amarus, two capsules (400mg), orally, three times daily, for 30 days. Control: Placebo (glucose), orally, three times daily, for 30 days. | |
| Outcomes | HBsAg, HBeAg; Symptoms; Biochemical and haematological tests. Adverse effects. Maximum follow‐up: six months. The outcomes were assessed at one, two, and six months after the end of treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Milne 1994.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peng 1993.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peng 1997.
| Methods | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: not blinded. Estimation of sample size: no information. Loss during follow‐up: four patients in treatment group and two in control group were lost. Jadad score = 2. | |
| Participants | Ethnic: Chinese; 111 patients (72 in the experimental group I, 59 males and 13 females, average age 34 yrs, from 8‐54 yrs; 20 in experimental group II, 15 males and five females, average age 31 yrs, from 17‐48 yrs; 19 in control group, 13 males and six females, average age 35 yrs, from 13‐61 yrs). Setting: outpatients. Inclusion criteria: carriers of HBsAg for more than six months, with normal liver function, and no clinical symptoms and signs of liver disease, confirmation made after six months' observation. Exclusion criteria: unstated. | |
| Interventions | Experimental: Group I: Astragalus membranaceus, one ml, acupoint injection, thrice weekly, for three months. Group II: Astragalus membranaceus, four ml, i.m., thrice weekly, for three months. Control: Saline, one ml, acupoint injection, thrice weekly, for three months. Data of group I and II were combined and compared with the control group. | |
| Outcomes | HBsAg, anti‐HBs, HBeAg, anti‐HBe and anti‐HBc. Maximum follow‐up: six months. The outcomes were assessed at the end of three months treatment and at six months of follow‐up. | |
| Notes | The distribution of patients in the three arms of the trial showed a ratio of 3.8:1.1:1.0, and the trial report did not explain how this happened. We have sent a letter, requesting an explanation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Qiu 1993.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Song 1994.
| Methods | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: not blinded. Estimation of sample size: no information. Loss during follow‐up: unstated. Jadad score=1. | |
| Participants | Ethnic: Chinese; 80 patients (50 in the treatment group, 27 males and 23 females, average age 15 yrs, from 7‐32 yrs; 30 in control group, 16 males and 14 females, average age 15 yrs, from 8‐31 yrs). Setting: outpatients and inpatients. Inclusion criteria: carriers of HBsAg for more than six months, with normal liver function, and no clinical symptoms and signs of liver disease. Exclusion criteria: unstated. | |
| Interventions | Experimental: Jianpi Wenshen recipe (self‐prescription of 11 herbs), one dose decoction orally, two times daily, for three months. Control: interferon‐alpha, one mega unit (MU) i.m., two times daily (for children, one time daily), for three months. | |
| Outcomes | HBsAg, HBeAg, anti‐HBe and anti‐HBc IgM. Follow‐up: at three, six and 12 months after the end of treatment. The outcomes were assessed at three, six and 12 months of follow‐up. | |
| Notes | The distribution of patients in the two arms of the trial showed a ratio of 1.7:1 and the trial report did not explain how this happened. We have sent a letter, requesting an explanation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Thamlikitkul 1991.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Xu 1999.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yang 1995.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yang 1996a.
| Methods | See Additional table: Trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bodenheimer 1983 | Randomised clinical trial comparing quinacrine versus placebo in patients with chronic hepatitis B. |
| Cao 1996 | Comparison between two case series of 143 patients treated with either Phyllanthus urinaria (a herb) or a compound of three herbs (Rhizoma Polygoni cuspidati, Chinese Taxillus twig, Astragalus membranaceus). The patients were 102 asymptomatic HBV carriers, 39 chronic hepatitis B, and two HBV‐induced liver cirrhosis. The outcomes were not reported separately. |
| Chen 1992 | Comparison between two case series of 89 asymptomatic HBV carriers treated with either Jian Qi Ling (compound of herbs) or Ganbifu (compound of herbs) plus Mieaoling (compound of herbs). |
| Chen 2000 | Randomised clinical trial comparing a compound of herbs (Chaoyang Wan) versus cinobufacin (extract of herb) versus non‐specific treatment in three groups. The participants were a mixture of chronic hepatitis B (n=160) and asymptomatic HBV carriers (n=20). The results were not reported separately. |
| Cong 1999 | Randomised clinical trial comparing two herbal compounds (Yigan Qingjie recipe plus Yigan Fubu recipe) versus Weilining (commercial Chinese spelling name of a western medicine, no detail on its pharmaceutical name) plus anti‐hepatitis B immune ribonucleic acid (iRNA, an immunoregulatory agent) in 43 cases of HBV carriers. |
| Doshi 1994 | Observational study on Phyllanthus amarus without control group in 30 asymptomatic HBV carriers. No patients lost their HBsAg during four to eight weeks treatment. |
| Guo 1998 | Comparison between two case series of 25 asymptomatic HBV carriers treated with either Huangqi Jianzhong Tang (compound of herbs) plus Qixie granule (compound of herbs) or Huangqi Jianzhong Tang alone. |
| Lei 1999 | Comparison between two case series of asymptomatic HBV carriers treated with either Shennongsu Ganbao (compound of herbs) or glycyrrhizin (a herbal ingredient from liquorice root). |
| Li 1997 | Randomised clinical trial comparing Yigan Wan (compound of 28 herbs) versus polyinosinic and cytidylic acid (Poly I:C) in 60 asymptomatic HBV carriers. Both groups received the same co‐intervention (multivitamins). The trial showed better effects of the herbal compound on loss of HBsAg (16/30), HBeAg (23/30), and conversion of HBeAg to anti‐HBe (22/30) than of Poly I:C alone (7/30, 14/30, and 14/30, respectively) for six months treatment. The control intervention did not fulfil our inclusion criteria. |
| Li 1999 | Comparison between two case series of 59 patients with chronic hepatitis B and 11 asymptomatic HBV carriers treated with either composita genus Phyllanthus (a compound consisted mainly of Phyllanthus) or IFN. |
| Li 1999b | Randomised clinical trial comparing Yigan Kangte capsule (herbal compound) plus thymosin versus Yigan Kangte capsule alone and versus thymosin alone in 90 cases of asymptomatic HBV carriers. The intervention with thymosin led to the exclusion. The comparison of Yigan Kangte plus thymosin versus thymosin alone showed no significant effects of the combination regarding loss of HBsAg (5/30), HBeAg (13/30) and HBV DNA (9/25) than thymosin alone (3/30, 7/30, 4/20, respectively). |
| Ma 1993 | Randomised clinical trial comparing two different herbal compounds (composita Phyllanthus amarus tablet versus Mieaoling tablet) in 60 asymptomatic HBV carriers. |
| Mi 1999 | Randomised clinical trial comparing Fuzheng Jiedu Tang (decoction of herbal compound) plus Poly I:C versus Poly I:C in 128 cases of asymptomatic HBV carriers. The control intervention did not fulfil our inclusion criteria. The trial showed better effects of the combination than of Poly I:C alone on loss of HBsAg (18/66 versus 2/62, P=0.003), HBeAg (16/34 versus 8/34, P=0.05) and seroconversion of HBeAg to anti‐HBe (8/34 versus 0/34, P=0.05). |
| Shang 1996 | Randomised clinical trial comparing anti‐hepatitis B iRNA (an immunoregulatory agent) plus Qiang Lichun (herbal preparation) versus anti‐hepatitis B iRNA alone. The patients were 10 asymptomatic HBV carriers, 15 chronic active hepatitis B and 30 chronic persistent hepatitis B, but the outcomes were not reported separately in the two groups of patients. The trial showed significantly better effects of the combination than of anti‐hepatitis B iRNA alone on loss of HBsAg (14/26 versus 6/24, P=0.05), HBeAg (12/24 versus 3/20, P=0.03), and HBV DNA (12/28 versus 0/27, P=0.02). |
| Shen 1996 | Randomised clinical trial comparing herbal compound (Hepatitis B II) versus other herbal interventions including Yunzhi Gantai, plus Aotaile or Yiganling (silymarin preparation) in 328 cases of asymptomatic HBV carriers and chronic hepatitis B. The trial showed significantly better effects of the herbal compound than other three herbs on loss of HBsAg (160/218 versus 28/100, P<0.00001), but not on HBeAg (120/184 versus 38/74, P=0.06). |
| Tajiri 1991 | Observational study on Sho‐Saiko‐to (compound of seven herbs) without control group in children with chronic hepatitis B infection with elevated serum alanine aminotransferase. |
| Thyagarajan 1988 | Randomised, double‐blind, placebo‐controlled trial comparing Phyllanthus amarus versus placebo. The participants were 28 asymptomatic HBV carriers, 14 HBV carriers with symptoms and 18 HBV carriers with glomerular nephritis, but the outcomes were not reported separately. A letter has been sent to the authors and if we get useful information, it will be included in this review. |
| Wang 1999 | Randomised clinical trial comparing cinobufacin (herbal extract) plus co‐interventions (compound Yiganling, Gantaile, vitamin B compound and C) versus the same co‐interventions. The participants were asymptomatic HBV carriers and patients with chronic hepatitis B, but the results were not reported separately. |
| Wang 1999b | Comparison between two case series of 72 asymptomatic HBV carriers treated with either a herb extract (Astragalus membranaceus) or saline. |
| Xu 1996 | Comparison between two case series of 130 asymptomatic HBV carriers treated with integrated traditional Chinese medicine and western medicine. |
| Xue 1991 | Comparison between two case series of 50 asymptomatic HBV carriers treated with either Chinese herbal medicine or IFN plus transfer factor (an immunoregulatory agent). |
| Yang 1992 | Comparison between two case series of 130 asymptomatic HBV carriers treated with either Caiguang decoction (compound of herbs) or Shushe tablet (compound of herbs). |
| Yang 1996b | Non‐randomised study comparing a compound of 12 herbs versus another herbal compound (Fufang Shushe tablets) plus non‐specific drugs in 162 HBV carriers. |
| Zhang 1992 | Randomised clinical trial comparing Phyllanthus amarus plus basic treatment versus Indigowood root plus basic treatment in chronic HBV infection. |
| Zhang 1994a | Randomised clinical trial comparing three different herbal medicines: Aotaile (herbal compound) granule versus Fugankang granule versus glycyrrhizin tablet (a herbal extract) in 90 cases of asymptomatic HBV carriers. |
| Zhang 1994b | Randomised clinical trial comparing a herbal compound (Kang Ao Yigan granule) versus Yunzhi Gantai (a herbal extract) in 334 asymptomatic HBV carriers. |
| Zhang 1995 | Randomised clinical trial comparing a herbal compound versus Polyporus umbellatus polysaccharide (a herbal extract) in 60 cases of asymptomatic HBV carriers. |
| Zhang 1996 | Randomised clinical trial comparing a herbal compound (Yigan tablet No 1) versus anti‐hepatitis B immune ribonucleic acid (an immuno moderator) in 150 asymptomatic HBV carriers. |
| Zhang 1999 | Randomised clinical trials comparing ultra‐red irradiation therapy plus levamisole smear versus ultra‐red irradiation therapy alone in 75 asymptomatic HBV carriers. |
| Zhao 1991 | Randomised clinical trial comparing different medicinal herbs and western medicines: agaric (a herb) plus agacore (another herb) plus hepatitis B vaccine versus agaric plus agacore plus BCG (Bacille Calmette‐Guerin) vaccine in asymptomatic HBV carriers. |
| Zhuo 1985 | Non‐randomised trial comparing herbal compound 848 pill versus no treatment in two case series of 39 asymptomatic HBV carriers. |
Contributions of authors
Jianping Liu designed, drafted and revised the protocol, performed searches, selected trials, extracted data, wrote and revised the review. Heather McIntosh developed the search strategy, provided methodological perspective, and revised the protocol and the review. Hui Lin handsearched journals, retrieved papers, and extracted data.
Sources of support
Internal sources
The Copenhagen Trial Unit, Denmark.
External sources
The 1991 Pharmacy Foundation, Denmark.
The Danish Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
The Copenhagen Hospital Coporation's Research Council Grant on Getting Research into Practice (GRIP), Denmark.
The Danish Council for Development Research, Denmark.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g., employment, consultancy, stock ownership, honoraria, or expert testimony).
Edited (no change to conclusions)
References
References to studies included in this review
Dang 1997 {published data only}
- Dang ZF. Clinical research of 'toad‐ant capsule' in the treatment of asymptomatic HBV carriers. Henan Journal of Traditional Chinese Medicine 1997;17(3):164‐165. [Google Scholar]
Leelarasamee 1990 {published data only}
- Leelarasamee A, Trakulsomboon S, Maunwongyathi P, Somanabandhu A, Pidetcha P, Matrakool B, et al. Failure of Phyllanthus amarus to eradicate hepatitis B surface antigen from symptomless carriers. Lancet 1990;335(8705):1600‐1. [DOI] [PubMed] [Google Scholar]
Milne 1994 {published data only}
- Milne A, Hopkirk N, Lucas CR, Waldon J, Foo Y. Failure of New Zealand hepatitis B carriers to respond to Phyllanthus amarus. New Zealand Medical Journal 1994;107(980):243. [PubMed] [Google Scholar]
Peng 1993 {published data only}
- Peng XC, Liu XL, Liu SC, Yang DZ, Wang WJ, Zhang HJ. Preliminary observation on Phyllanthus urinaria for treatment of HBsAg carriers. Chinese Journal of Experimental and Clinical Virology 1993;7(3):306‐307. [Google Scholar]
Peng 1997 {published data only}
- Peng MH, Wang XY, Sun FY, Du XZ, Xiang DP, Wang WS. [Observation of therapeutic effect of Astragalus membranaceus by acupoint injection for treatment of hepatitis B virus carriers]. Chinese Journal of Chinese Medicine and Pharmacology Information 1997;4(4):22‐3. [Google Scholar]
Qiu 1993 {published data only}
- Qiu YQ, Liu HR, Lei BJ, Yi SF, Xi YM. Ganling Wan for viral hepatitis and chronic HBV carriers: a randomised double blind controlled trial. West China Medical Journal 1993;8(4):380‐381. [Google Scholar]
Song 1994 {published data only}
- Song YW, Song YZ. [Observation of Chinese herbal treatment based on 'spleen' and 'kidney' on asymptomatic carriers of hepatitis B virus]. Zhejiang Journal of Traditional Chinese Medicine 1994;12(5):487‐8. [Google Scholar]
Thamlikitkul 1991 {published data only}
- Thamlikitkul V, Wasuwat S, Kanchanapee P. Efficacy of Phyllanthus amarus for eradication of hepatitis B virus in chronic carriers. Journal of the Medical Association of Thailand 1991;74(9):381‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Xu 1999 {published data only}
- Xu JC. [Observation on therapeutic effect of acupoint injection in hepatitis B virus carriers]. Journal of Modern Integrated Traditional and Western Medicine 1999;8(8):1340‐1. [Google Scholar]
Yang 1995 {published data only}
- Yang SP, Ren JJ, Wang DZ, Li QY, Zhang YP, Liu YM. The effect of Tu Jia Zu herbs 'Ge Shan Xiao' to treat HBV carriers. Chinese Journal of Ethnomedicine and Ethnopharmacy 1995;1(2):33‐34. [Google Scholar]
Yang 1996a {published data only}
- Yang M, Dong GN, Liang HQ. [Clinical observation on 40 cases of hepatitis B virus carriers by using integrated traditional and western medicines]. Anhui Clinical Journal of Traditional Chinese Medicine 1996;8(4):152. [Google Scholar]
References to studies excluded from this review
Bodenheimer 1983 {published data only}
- Bodenheimer HC, Schaffner F, Vernace S, Hirschman SZ, Goldberg JD, Chalmers T. Randomized controlled trial of quinacrine for the treatment of HBsAg‐positive chronic hepatitis. Hepatology 1983;3(6):936‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cao 1996 {published data only}
- Cao WZ, Liu JQ, Cao DY, Su F, Liu HM, Wu RZ, et al. Clinical study on genus Phyllanthus for treatment of chronic hepatitis B virus carriers. Shanghai Journal of Chinese Medicine and Pharmacology 1996;30(5):8‐9. [Google Scholar]
Chen 1992 {published data only}
- Chen DY, Chen HZ, Li ZX, Liang YC, Sheng SY, He SY, et al. Jian Qi Ling for treating 52 cases of HBsAg carriers. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1992;2(1):36‐7. [Google Scholar]
Chen 2000 {published data only}
- Chen XM. [Clinical efficacy of Chaoyang pill and Cinobufacin for treatment of hepatitis B]. Hubei Journal of Traditional Chinese Medicine 2000;22(6):28. [Google Scholar]
Cong 1999 {published data only}
- Cong C. [Yigan Qingjie recipe plus Yigan Fubu recipe for treating 33 cases of hepatitis B virus infection]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1999;9(1):33‐4. [Google Scholar]
Doshi 1994 {published data only}
- Doshi JC, Vaidya AB, Antarkar DS, Deolalikar R, Antani DH. A two‐stage clinical trial of Phyllanthus amarus in hepatitis B carriers: failure to eradicate the surface antigen. Indian Journal of Gastroenterology 1994;13(1):7‐8. [PubMed] [Google Scholar]
Guo 1998 {published data only}
- Guo XX. [Jianpi Wenyang recipe for treatment of 25 cases of asymptomatic hepatitis B virus infection]. Traditional Chinese Medicine Research 1998;11(6):40‐1. [Google Scholar]
Lei 1999 {published data only}
- Lei L, Yang ZH, Yang DW, Hu WZ. [Shennongsu Ganbao for treating chronic asymptomatic carriers of hepatitis B virus]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1999;9(3):33. [Google Scholar]
Li 1997 {published data only}
- Li PY, Li SY. [Compound Yigan Wan for treatment of 30 cases of asymptomatic carriers of hepatitis B virus]. Clinical Focus 1997;13(6):271‐2. [Google Scholar]
Li 1999 {published data only}
- Li GY, Zhang WX, Mi ZB. Compound genus Phyllanthus for treating 38 cases of hepatitis B. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1999;9(1):32. [Google Scholar]
Li 1999b {published data only}
- Li P, Huang JL, Xu Y, Liang Y. [Yigan Kangte capsule plus thymosin for treatment of 30 cases of chronic hepatitis B virus carriers]. Chinese Journal of Integrated Traditional and Western Medicine in Spleen and Stomach 1999;7(1):42. [Google Scholar]
Ma 1993 {published data only}
- Ma FX, Zhang Y. Clinical observation on compound Phyllanthus amarus tablet for treatment of asymptomatic hepatitis B virus carriers. Shanghai Journal of Chinese Medicine and Pharmacology 1993;27(5):8‐9. [Google Scholar]
Mi 1999 {published data only}
- Mi ZX. [Integration of traditional Chinese medicine and western medicine for treatment of hepatitis B surface antigen carriers]. Research of Traditional Chinese Medicine 1999;15(1):45‐6. [Google Scholar]
Shang 1996 {published data only}
- Shang YZ, Feng YX, Chen QY. [Effects of anti‐hepatitis B viral immune ribonucleic acid combined Qiang Lichun oral juice on hepatitis B viral replicative marker]. Acta Academiae Medicinae Hubei 1996;17(1):81‐2. [MEDLINE: 96122068] [Google Scholar]
Shen 1996 {published data only}
- Shen G. Clinical observation on 228 cases of hepatitis B and its virus carriers treated by drug 'Hepatitis II'. Hunan Journal of Traditional Chinese Medicine 1996;12(1):4‐5. [Google Scholar]
Tajiri 1991 {published data only}
- Tajiri H, Kozaiwa K, Ozaki Y, Miki K, Shimuzu K, Okada S. Effect of Sho‐Saiko‐to Xiao‐Chai‐Hu‐Tang on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. American Journal of Chinese Medicine 1991;19(2):121‐9. [DOI] [PubMed] [Google Scholar]
Thyagarajan 1988 {published data only}
- Thyagarajan SP, Subramanian S, Thirunala‐Sundari T, Venkateswaran PS, Blumberg BS. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet 1988;2(8614):764‐6. [DOI] [PubMed] [Google Scholar]
Wang 1999 {published data only}
- Wang SY, Zhu XY, Xing BC. The effect of Cinobufacin on chronic hepatitis B virus carriers and chronic hepatitis B. Chinese Journal of Clinical Hepatology 1999;15(2):111‐2. [Google Scholar]
Wang 1999b {published data only}
- Wang XY, Peng MH, Sun FY, Du XY, Xiang PD, Wang WS. Clinical study on Astragalus membranaceus by acupoint injection in treatment of chronic HBsAg carriers. Chinese Accupuncture and Moxibustion 1999;19(4):223‐5. [Google Scholar]
Xu 1996 {published data only}
- Xu AB. Analysis of therapeutic effect on 130 cases of asymptomatic carriers of hepatitis B virus by integrated traditional Chinese and western medicine. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1996;6(4):36. [Google Scholar]
Xue 1991 {published data only}
- Xue Q, Wang SC, Ren ZS. Observation of therapeutic effect on Chinese medicine and medicinal herbs for asymptomatic HBsAg carriers. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1991;1(2):43‐4. [Google Scholar]
Yang 1992 {published data only}
- Yang YF. Compound Caiguang preparation for treating 65 cases of asymptomatic HBsAg carriers. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1992;2(4):33, 46. [Google Scholar]
Yang 1996b {published data only}
- Yang H. Treatment of HBV carriers by 'ErXian translating‐negative': a report of 83 cases. New Journal of Traditional Chinese Medicine 1996;28(2):51. [Google Scholar]
Zhang 1992 {published data only}
- Zhang JL, He WN, Ye P. Clinical observation on Phyllanthus amarus for treating chronic hepatitis B virus infection. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1992;2(1):8‐10. [Google Scholar]
Zhang 1994a {published data only}
- Zhang DM. [Observation of therapeutic effects of Aotaile, Fugankang and glycyrrhizin tablet on 90 cases of hepatitis B virus carriers]. Fujian Journal of Traditional Chinese Medicine 1994;25(4):12‐3. [Google Scholar]
Zhang 1994b {published data only}
- Zhang YL, Shi ZC, Ji YQ, Shi PC. The effect of 'anti‐HBs benefiting liver granule' to treat 240 cases with asymptomatic HBV carriers. Hebei Journal of Traditional Chinese Medicine 1994;16(3):12‐13. [Google Scholar]
Zhang 1995 {published data only}
- Zhang J. [Application of Qingre Jiedu recipe to hepatitis B virus carriers]. Anhui Clinical Journal of Traditional Chinese Medicine 1995;7(4):26‐7. [Google Scholar]
Zhang 1996 {published data only}
- Zhang CL, Xu WJ, Wang RF, Chen SZ. [Clinical observation of Yigan tablet No 1 for treatment of 86 cases of chronic hepatitis B virus carriers]. Practical Journal of Integrated Traditional Chinese and Western Medicine 1996;9(7):427. [Google Scholar]
Zhang 1999 {published data only}
- Zhang F, Liu JH, Xin Y. [HD‐91‐II type instrument for therapy of liver diseases combined with levamisole smear for treatment of 48 hepatitis B virus carriers]. Chinese Journal of Integrated Traditional Chinese and Western Medicine on Liver Diseases 1999;9(3):32. [Google Scholar]
Zhao 1991 {published data only}
- Zhao HT, Lu KF, Wang WB. [A report on therapeutic effect using integrated traditional Chinese medicine and western medicine in treating asymptomatic carriers of HBV]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1991;1(2):37‐8. [Google Scholar]
Zhuo 1985 {published data only}
- Zhuo HC, Wang BE, Yin WY, Wang HZ, Hu ZH, Song ZZ, et al. [A preliminary clinical study of the effect of complex TCM pills (848) on chronic HBV carriers]. Chinese Journal of Integrated Traditional and Western Medicine 1985;5(10):606‐8. [PubMed] [Google Scholar]
Additional references
CMH 1999
- Chinese Ministry of Health. Health News. Newspaper of Health News 22/01/1999:4th Edition.
Dusheiko 1999
- Dusheiko G. Hepatitis B. In: Bircher J, Benhamon JP, McIntyre N, Rizzetto M, Rodes J editor(s). Oxford Textbook of Clinical Hepatology. Second Edition. Vol. 1, Oxford: Oxford University Press, 1999:891. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fulder 1996
- Fulder S. The Handbook of Alternative and Complementary Medicine. Oxford: Oxford University Press, 1996. [Google Scholar]
ICH 1997
- International conference on harmonisation expert working group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. 1. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kjaergard 1999
- Kjaergard LL, Villumsen J, Gluud C. Quality of randomised clinical trials affects estimates of intervention efficacy (abstract). VII Cochrane Colloquium. Rome, 1999:57.
Lai 1987
- Lai CL, Lok AS, Lin HJ, Wu PC, Yeoh EK, Yeung CY. Placebo‐controlled trial of recombinant alpha 2‐interferon in Chinese HBsAg‐carrier children. Lancet 1987;2(8564):877‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lai 1991
- Lai CL, Lin HJ, Lau JN, Flok AS, Wu PC, Chung HT, et al. Effect of recombinant alpha 2 interferon with or without prednisone in Chinese HBsAg carrier children. Q J Med 1991;78(286):155‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Lai 1998
- Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai RI, et al. A 1‐year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998;339(2):61‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liaw 1997
- Liaw YF, Tsai SL. Pathogenesis and clinical significance of acute exacerbation and remissions in patients with chronic hepatitis B virus infection. Viral Hep Rev 1997;3:143‐54. [Google Scholar]
Lin 1998
- Lin E, Luscombe C, Colledge D, Wang YY, Locarnini S. Long‐term therapy with the quanine nucleoside analog penciclovir controls chronic duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother 1998;42:2132‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 1999
- Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long term beneficial effects of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology 1999;29(3):971‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 1999b
- Liu JP, Liu C, Leng TJ. Evaluation of clinical trials for viral hepatitis in the past nine years in Chinese medical literatures. West China Medical Journal 1999;14(3):264‐5. [Google Scholar]
Liu 2000
- Liu JP, Gluud C. Interferon for asymptomatic hepatitis B virus carriers. Unpublished data 2000.
Liu 2001
- Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marrone 1999
- Marrone CM. Safety issues with herbal products. Ann Pharmacother 1999;33(12):1359‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Melchart 1999
- Melchart D, Linde K, Weidenhammer W, Hager S, Shaw D, Bayer R. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA 1999;282(1):28‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Niederau 1996
- Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, et al. Long term follow‐up of HBeAg positive patients treated with interferon alpha for chronic hepatitis B. N Engl J Med 1996;334(22):1422‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nortier 2000
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 2000;342(23):1686‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodriguez 1997
- Rodriguez‐Inigo E, Bartolome J, Lopez‐Alcorocho JM, Contonat T, Oliva H, Carreno V. Activation of liver disease in healthy hepatitis B surface antigen carriers during interferon‐alpha treatment. J Med Virol 1997;53(1):76‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherlock 1997
- Sherlock S, Dooley J. Treatment of chronic hepatitis B. Diseases of the liver and biliary system. Tenth. Oxford: Blackwell Science, 1997:371. [Google Scholar]
Tang 1999
- Tang JL, Zhan SY, Ernst E. Review of randomised controlled trials of traditional Chinese medicine. BMJ 1999;319:160‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomlinson 2000
- Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. J Clin Pharmacol 2000;40(5):451‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19:159‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2000
- Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol 2000;15(Suppl.):E67‐E70. [DOI] [PubMed] [Google Scholar]
WHO 1999
- WHO 1999. Diseases and Vaccines. Hepatitis B. www.who.int/gpv‐dvacc/diseases/hepatitis_b.htm 1999, issue March.
WHO 2000
- WHO 2000. Hepatitis B. Fact Sheet WHO/204 2000.


