Abstract
Background
Minocycline is an oral antibiotic used for acne vulgaris. Its use has lessened due to safety concerns (including potentially irreversible pigmentation), a relatively high cost, and no evidence of any greater benefit than other acne treatments. A modified‐release version of minocycline is being promoted as having fewer side‐effects.
Objectives
To assess new evidence on the effects of minocycline for acne vulgaris.
Search methods
Searches were updated in the following databases to November 2011: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library, MEDLINE (from 1946), EMBASE (from 1974), and LILACS (from 1982). We also searched trials registers and checked reference lists for further references to relevant randomised controlled trials (RCTs).
The Cochrane Skin Group's Trials Search Co‐ordinator undertook searches exploring minocycline's adverse effects in EMBASE and MEDLINE in February 2012.
Selection criteria
We selected randomised controlled trials (RCTs) comparing minocycline, at any dose, to an active or a placebo control, in participants with inflammatory acne vulgaris. For adverse effects, we selected additional studies that reported the number of adverse effects and the number of participants treated.
Data collection and analysis
Outcome measures used in the trials included lesion counts, acne grades/severity scores, doctors' and participants' global assessments, adverse effects, and dropout rates. Two authors independently assessed the quality of each study. Effect sizes were calculated, and meta‐analyses were undertaken where possible.
Sixteen studies met the inclusion criteria for the review of adverse effects.
Main results
We included 12 new RCTs for this update, giving a total of 39 RCTs (6013 participants). These additional 12 RCTs have not changed the original conclusions about the clinical efficacy of minocycline.
The identified RCTs were generally small and poor quality. Meta‐analysis was rarely possible because of the lack of data and different outcome measures and trial durations. Although minocycline was shown to be an effective treatment for moderate to moderately‐severe acne vulgaris, there was no evidence that it is better than any of the other commonly‐used acne treatments. One company‐sponsored RCT found minocycline to be less effective than combination treatment with topical erythromycin and zinc. No trials have been conducted using minocycline in those participants whose acne is resistant to other therapies. Also, there is no evidence to guide what dose should be used.
The adverse effects studies must be interpreted with caution. The evidence suggests that minocycline is associated with more severe adverse effects than doxycycline. Minocycline, but not other tetracyclines, is associated with lupus erythematosus, but the risk is small: 8.8 cases per 100,000 person‐years. The risk of autoimmune reactions increases with duration of use. The evidence does not support the conclusion that the more expensive extended‐release preparation is safer than standard minocycline preparations.
Authors' conclusions
Minocycline is an effective treatment for moderate to moderately‐severe inflammatory acne vulgaris, but there is still no evidence that it is superior to other commonly‐used therapies. This review found no reliable evidence to justify the reinstatement of its first‐line use, even though the price‐differential is less than it was 10 years ago. Concerns remain about its safety compared to other tetracyclines.
Plain language summary
Minocycline for acne vulgaris: efficacy and safety
Acne is the most common skin disease of adolescence, and in most cases it clears spontaneously. However, in some people it persists in to adulthood. There are many different treatment options, but there is little good evidence to inform doctors and individuals about which to choose.
Minocycline was the most prescribed antibiotic used to treat acne because it was thought to be better than other options, despite the original version of this review finding no reliable evidence that it was any better than other treatments. Over recent years it has been used less, which was due to serious concerns about its safety, including skin pigmentation, which in some cases is irreversible. It was also more expensive than other treatments.
Since the first version of this review, minocycline's cost has fallen. In the UK, the daily cost of generic minocycline is now one third the cost of tetracycline. This update was undertaken to identify whether there was any new evidence that might change the conclusions of the original review or provide information on risks associated with minocycline therapy. Twelve new RCTs were identified, making a total of 39 RCTs (6013 participants).
In summary, there is no evidence to support the first‐line use of minocycline in the treatment of acne. All of the trials showed that, on average, people treated with minocycline experienced an improvement in their acne. However, no study conclusively showed any important clinical difference between minocycline or other commonly‐used therapies. The analysis found that minocycline may act more quickly than oxytetracycline or tetracycline, but there is no overall difference in the end. There is no evidence that it is more effective in acne that is resistant to other therapies, or that the effects last longer. Although it is often claimed that the more expensive once‐daily slow‐release preparation is a more attractive option to teenagers with acne, the evidence in this review does not show it to be any better or safer compared to other oral antibiotics that have to be taken more frequently.
Despite a thorough search for evidence, it is still not known which of the tetracyclines are the safest to take overall as they are all associated with side‐effects. The only conclusion that we could make was that people treated with minocycline for acne are at a significantly greater risk of developing an autoimmune (lupus‐like) syndrome than those given tetracycline or no treatment.
Background
Please see the Glossary in Table 23 for an explanation of terms used in this review.
1. Glossary of terms.
| Medical term | Explanation/description |
| Antineutrophil antibody (ANA) positivity | Antineutrophil antibodies are a group of autoantibodies. They are detected in a blood test in a number of autoimmune disorders |
| Autoimmune hepatitis | A disease of the liver that occurs when the body's immune system attacks cells of the liver |
| Benign intracranial hypertension | Also known as 'pseudotumour cerebri', this is a syndrome that shows increased pressure in the brain that is not caused by tumours. Symptoms are the same as those that result from brain tumours and other types of intracranial hypertension. They include headaches, nausea, double vision, and loss of vision. There is some controversy between different groups about the causes, but there are some known causes, including several prescription medications |
| Dual‐flow cytometry analysis | An analytical method that is laser‐based and used to count cells and detect biomarkers |
| Eosinophilia | An increase in the number of a type of white blood cells known as eosinophils |
| Matrix metalloproteinase inhibitors | A drug that stops the action of zinc‐dependent proteases (enzymes that break down proteins) |
| Nephritis | Nephritis is inflammation of the nephrons in the kidneys |
| Pneumonitis | Inflammation of lung tissue |
| Polyarteritis nodosa | A disease of unknown cause that affects arteries |
| Proteolytic tissue damage | Tissue damage caused by proteolysis (the breakdown of proteins into smaller polypeptides or amino acids) |
| Serological marker | Serology is the science that deals with the characterisation of serum, the non‐cellular component of blood. Serological markers are used to distinguish specific diseases in individuals. These markers are invaluable in the detection of some cancers, especially due to their potential in identifying the early stages of the disease, prior to the onset of symptoms |
| Serum‐sickness‐like syndrome | Serum‐sickness‐like reactions are specific drug reactions that cause a range of symptoms, including fever, skin rash, swelling of the mouth and lymph nodes, joint and muscle pain and protein in the urine |
| Systemic lupus erythematosus‐like syndrome | Systemic lupus erythematosus often abbreviated to 'SLE' or 'lupus', is a systemic autoimmune disease (or autoimmune connective tissue disease) that can affect any part of the body. As occurs in other autoimmune diseases, the immune system attacks the body's cells and tissue, resulting in inflammation and tissue damage |
Description of the condition
Acne is the most common skin disease of adolescence, and few teenagers escape the experience (Williams 2012). The severity of acne varies considerably, and in some individuals, acne persists well beyond the teens for reasons that are not yet clear (Goulden 1997). Acne usually begins before or during puberty when the output of sebum (grease) by tiny hair follicles on the face and upper trunk increases substantially (Rothman 1993). The production of sebum is controlled by male hormones (androgens) in both sexes. Spots form in follicles that respond abnormally to the hormones producing sebum. At the same time that sebum production increases, some of the openings through which the sebum flows (pores) become blocked by horny impactions made up of dead skin cells. The sebum acts as a nutrient for a resident skin bacterium called Propionibacterium acnes (P. acnes), which colonises both healthy and diseased follicles. The role of P. acnes in the pathogenesis of acne has never been formally proven, and there is some doubt that it has any role to play (Shaheen 2011).
In the absence of effective treatment, acne persists for an average of 8 to 12 years in most sufferers (Cunliffe 1989; Cunliffe 1996), before it resolves spontaneously, usually but not always, by the early 20s.
Description of the intervention
Conventional treatments act by interfering with one or more of the factors (described above) that cause spots to form. Thus, drug treatments that reduce sebum production or the blockage of the pores, inhibit the growth of the acne bacillus, or both, are commonly used (Leyden 1997). Alternative approaches, such as dietary manipulation, relaxation therapy, homeopathy, Chinese herbs, and counselling, have been tried in acne management, especially in those people who do not want to use conventional methods for extended periods. Most available treatments, such as antibiotics, antiandrogens (including the combined oral contraceptive), and agents that unblock pores, only stop spots forming whilst the drug is being used, and therefore must be used extensively and continuously. The only potential cure for acne is oral isotretinoin (Roaccutane™), which reduces sebum production permanently (Saurat 1997). However, oral isotretinoin is a teratogen (a drug that, like thalidomide, may cause abnormalities in unborn babies) and causes significant side‐effects; therefore, prescribing is limited to individuals whose acne is severe, persistent, or unresponsive to alternative medications. It is also recommended in people who scar easily as well as those who are emotionally distressed (Ortonne 1997). Thus, oral and topical antibiotics continue to be widely prescribed.
Minocycline is an orally‐taken antibiotic that belongs to a class of drugs known as the tetracyclines. These can be subdivided into two classes: the original or 'first‐generation' tetracyclines (oxytetracycline and tetracycline) and the 'second‐generation' tetracyclines (such as minocycline, doxycycline, and lymecycline), which were chemically adapted to provide additional benefits. Historically, the preferential use of minocycline in the treatment of acne arose because of several perceived advantages over the other tetracyclines (that were fostered by a very successful marketing strategy).
One of the well‐publicised benefits of minocycline was its convenience ‐ because of its extended half‐life, it only needs to be taken orally once‐daily, and absorption is not affected by food. This is in contrast to tetracycline and oxytetracycline, which need to be taken on an empty stomach up to four times a day. It is also widely perceived by clinicians to have a faster onset of action than tetracycline or oxytetracycline and to be beneficial in acne that does not respond to other therapy (Knaggs 1993). In addition, although the exact relationship between bacterial levels and acne severity has not been clearly defined, in vivo studies have shown that minocycline produces a greater reduction of skin P. acnes levels compared to tetracycline (Eady 1990a), and there is a lower level of bacterial resistance to it (Eady 1993). The effects of minocycline are also commonly believed to persist post‐treatment because of its high lipophilicity (fat solubility) and resultant distribution within the body (Chopra 1992; Leyden 1982).
Many of the pharmacological advantages of minocycline over the first‐generation tetracyclines (oxytetracycline and tetracycline) have been ascribed to its increased lipid solubility (Colaizzi 1969). A greater per cent of the drug is absorbed from the intestinal tract, and the serum half‐life is extended by several hours (Agruh 2006). The sustained blood levels are thought to translate biologically into higher skin concentrations and enhanced sebum penetration (Luderschmidt 1985; Macdonald 1973), although this view has been challenged (Aubin 1989). The absorption profile and steady state concentration also varies significantly between individuals, which cannot be explained by participant size and weight (Leyden 1985). There is considerable variation and overlap in serum concentrations that are achieved following doses of 100 mg or 200 mg per day (Eady 1993; Gardner 1997). The observation that the absorption profile of minocycline is minimally affected by the stomach contents (Leyden 1985) has been disputed by a later study, which showed that the presence of food in the stomach reduced minocycline absorption between 2% and 51% (Meyer 1996).
As a result of the enhanced absorption of minocycline, lower doses are required and less of the active drug remains in the gastro‐intestinal tract, minimising the disturbances to the resident microflora that often result in gastro‐intestinal upset (Fanning 1977). However, minocycline and other second‐generation tetracyclines exhibit an increased spectrum and incidence of adverse effects, which has been linked to their accumulation in fatty tissue and to their longer half‐life (Ruef 1996).
The once‐daily dosage advantage of minocycline is not unique; lymecycline and doxycycline are typically prescribed as a single‐daily dose. In recent years, lymecycline has gained in popularity following the publication of a series of manufacturer‐sponsored trials attesting to its efficacy in acne.
Initially, attention focused on the vestibular side‐effects of minocycline (Gump 1977; Williams 1974). The vestibular system is in the inner ear and contributes to balance and the sense of spatial orientation. Diseases of the vestibular system usually induce vertigo and instability, and they are often accompanied by nausea. Prior to 1974, reports of these side‐effects were rare, but that year saw a marked increase from less than 10% to over 70% of individuals in the U.S. who were treated. A similar increase was not evident in other countries (Allen 1976). With widespread and continued use, other side‐effects have become apparent, leading to periodic debate over the safety of minocycline use for acne (Basler 1979; Davies 1989; Wright 1988). All tetracyclines bind to calcified tissues and are deposited and persist where normal bone forms. Minocycline causes pigmentation in a variety of tissues including skin, thyroid, nails, sclera, teeth, conjunctiva, tongue, and bone. The pigmentation can be irreversible.
Three patterns of serious reactions to minocycline have been described:
early onset dose‐related toxicity reactions resulting in single organ dysfunction;
hypersensitivity reactions (presenting as pneumonitis, eosinophilia, nephritis, and serum‐sickness‐like syndrome); and
autoimmune disorders (systemic lupus erythematosus‐like syndrome, autoimmune hepatitis, and polyarteritis nodosa).
Safety concerns increased markedly following the publication of an article in the BMJ that highlighted the risk of potentially fatal liver failure with two documented deaths (Gough 1996). Further reports followed (Beneton 1997; Crosson 1997; Knowles 1996; MacNeil 1997; Shapiro 1997), and consequently, the level of minocycline prescribing fell by 38% in the UK (Ferguson 1998; Walsh 2012). The ensuing controversy over the safety of minocycline provoked several articles, with differing opinions amongst dermatologists as to the relative risks and benefits of minocycline, tetracycline or oxytetracycline for the treatment of acne (Beneton 1997; Cunliffe 1996; Ferner 1996; Fessler 1996; Seukeran 1997).
In 2010 the U.S. Food and Drug Administration (FDA) approved an extended‐release (ER) formulation of minocycline (Solodyn® by Medicis pharmaceutical Corporation) for once‐daily treatment of non‐nodular moderate to severe acne at a dose of 1 mg/kg. The packaging insert states that a 135 mg dose has a longer T max (time to maximum dose in the blood) (3.5 to 4 hours versus (vs) 2.25 to 3 hours), a lower C max (maximum blood concentration) (2.63 mcg/ml vs 2.92 mcg/ml), and a smaller AUC (area under the curve, which represents how much of the drug is in the body over time) (33.32 vs 46.35 mcg/hr/ml). It is claimed that the extended‐release formulation has reduced side‐effects, particularly vertigo. Three included studies tested this formulation (Fleisch 2006a (MP010404); Fleisch 2006b (MP010405); Stewart 2006 (MP010401)).
As well as considering the relative risks and benefits of interventions, unfortunately, in today's climate of rising healthcare expenditure, the comparative cost‐benefit must be considered. Since the original publication of the review, the relative costs of antibiotics have changed dramatically. Minocycline is no longer the most expensive (Table 24).
2. Relative costs of oral antibiotics for acne (BNF April 2012).
| Antibiotic | Dose unit | Number of capsules/tablets | Cost per pack (£) |
7‐day cost (1 g or 100 mg) |
| Tetracycline | 250 mg | 28 | 13.35 | 13.35 |
| Oxytetracycline generic | 250 mg | 28 | 1.19 | 1.19 |
| Lymecycline (Tetralysal) | 300 mg | 28 | 7.77 | 7.77 |
| ‐ | 300 mg | 56 | 14.97 | 7.49 |
| Doxycycline generic | 50 mg | 28 | 1.70 | 1.70 |
| ‐ | 100 mg | 8 | 1.03 | 0.90 |
| Doxycycline (Vibramycin) | 100 mg | 8 | 4.91 | 4.30 |
| Doxycycline ER (Efracea) | 40 mg | 56 | 29.78 | Not acne |
| Minocycline generic | 50 mg | 56 capsules | 15.27 | 3.82 |
| ‐ | 100 mg | 28 capsules | 13.09 | 3.27 |
| ‐ | 50 mg | 28 tablets | 4.76 | 2.38 |
| ‐ | 100 mg | 28 tablets | 10.97 | 2.74 |
| Minocycline ER generic | 100 mg | 56 capsules | 20.08 | 2.51 |
| Erythromycin generic | ‐ | ‐ | ‐ | ‐ |
| Erythrocin | 250 mg | 100 tablets | 18.20 | 5.10 |
| ‐ | 500 mg | 100 tablets | 36.40 | 5.10 |
| Erymax | 250 mg | 28 capsules | 5.61 | 5.61 |
| ‐ | ‐ | 112 capsules | 22.44 | 5.61 |
| Erythroped A | 500 mg | 28 | 10.78 | 5.39 |
| Trimethoprim generic | 100 mg | 28 | 0.88 | 0.44 |
| ‐ | 200 mg | 14 | 0.82 | n/a |
How the intervention might work
The fact that acne responds to antibiotics is one of the strongest pieces of evidence that acne is a bacterial disease caused by the bacterium P.acnes. However, all of the antibiotics used to treat acne, including minocycline, also exhibit multiple anti‐inflammatory effects. For example, the tetracyclines as a group are matrix metalloproteinase inhibitors, and this action may contribute to therapeutic efficacy in acne by limiting proteolytic tissue damage (Soory 2008). Hence, the relative contributions of antibacterial activity and anti‐inflammatory activity to clinical efficacy of the tetracyclines, including minocycline, is not known.
Why it is important to do this review
Prescribing of minocycline for acne has fallen dramatically in the last decade since the publication of the original review (Walsh 2012). However, its use is potentially increasing again due to the recent licensing in the United States of a extended‐release version (Solodyn®). There appears to have been a non‐evidence based switch back to first‐generation tetracyclines (such as oxytetracycline or tetracycline) or to the second‐generation tetracycline, lymecycline. The main reason for updating this review was to examine new data on the relative efficacy of the tetracyclines in acne and, especially, any head‐to‐head comparisons of minocycline with lymecycline. Whilst the emphasis was on clinical efficacy, we also sought to examine new safety data, especially any that shed light on the relative risks of the tetracyclines when used chronically, as in acne management.
Objectives
The primary aim of updating this review was to determine whether evidence from newer studies was persuasive enough to justify amending our original conclusions about the efficacy or safety of minocycline for acne, or both. Specifically the objectives were as follows: 1. To identify any new RCTs comparing the efficacy of minocycline against placebo and other drug treatments for acne (both oral and topical) with the aim of undertaking meta‐analysis. 2. To examine any new safety data on the incidence of adverse effects associated with minocycline therapy, and to determine whether the risk increases with dose or duration of therapy.
Methods
Criteria for considering studies for this review
Types of studies
All prospective randomised controlled trials (RCTs) in which minocycline was compared either to placebo or to another active therapy in participants with acne vulgaris were eligible for inclusion, if at least one generally‐accepted outcome measure was used. We did not exclude trials on the basis of language, and we included open trials.
It is recognised that rare adverse drug reactions (ADRs) are unlikely to occur in clinical trials involving relatively‐small numbers of participants with short follow‐up periods; therefore, estimates of the frequency of such events cannot be obtained by pooling data from several small trials. In addition, spontaneous report systems and case reports are not reliable sources of evidence, as the actual number of events that individuals experience is uncertain because of selective reporting and the fact that the number of participants who received the therapy overall is not known. Therefore, information on the incidence of the less common and more severe ADRs associated with minocycline was sought from systematic reviews, cohort studies, or case‐control studies that provided a clear indication on the numerator (i.e. the number of adverse effects) and the denominator (the number of participants treated).
Types of participants
Participants with a diagnosis of acne vulgaris on the face, upper trunk, or both. We accepted studies that used the diagnosis of papulopustular, inflammatory, juvenile, or polymorphic acne. Restrictions were not made on age, gender, or acne severity. We included trials that recruited only participants with nodular acne, but they were considered separately.
Types of interventions
Studies that examined minocycline at any dose, compared either to placebo or another active therapy (topical or oral). We included studies that permitted the use of concomitant topical or oral antiacne medications if both treatment groups were treated equivalently, and the results of the study were interpreted accordingly.
Types of outcome measures
In accordance with the methods used in the original version of the review, this update did not select primary and secondary outcome measures. This is because there is no evidence on which to differentiate the reliability and validity of measures. The outcome measures of interest were those that estimated clinical efficacy, participant acceptability, or both, in a defined way. There are many different methods used to assess clinical efficacy, and there is no evidence at present on their relative validity, reliability, or responsiveness. Therefore, we included lesion counts (total, inflamed, and non‐inflamed, separately), acne severity scores, physicians' global evaluation, and participants' self‐assessment. The abbreviations used throughout this review are non‐inflamed lesion (NIL), inflamed lesion (IL), and total lesion (TL).
Data on the overall incidence of ADRs, the incidence of gastro‐intestinal (GI) disturbances, and the incidence of ADRs necessitating withdrawal of therapy were analysed for each study to assess the relative safety of each intervention. We judged the acceptability of each therapy either directly or through evidence of compliance and overall dropout rates. We excluded studies that used only surrogate markers of efficacy (such as numbers of cutaneous propionibacteria).
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised the search strategies for the databases below and searched up to 8th November 2011:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library using the strategy in Appendix 2;
MEDLINE (from 1946) using the strategy in Appendix 3;
EMBASE (from 1974) using the search strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information Database, from 1982) using the search strategy in Appendix 5.
Trials registers
We searched the following trials registers, using the terms 'minocycline' and 'acne', on 16th April 2012.
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The Ongoing Skin Trials Register on (www.nottingham.ac.uk/ongoingskintrials).
Searching other resources
Adverse effects
For this update, we revised the adverse effects search strategy for MEDLINE and developed a new strategy for EMBASE, in order to make our search more comprehensive. We ran our searches up to 9th February 2012.
EMBASE (from 1974) using the strategy in Appendix 6.
MEDLINE (from 1946) using the strategy in Appendix 7.
Reference lists
We checked the bibliographies of included studies and review articles for further references to relevant trials.
Correspondence
For the original review, a list of the identified RCTs was sent to the first author of each study and 42 acne experts to enquire about their knowledge of any further published or unpublished trials. This list was also sent to Wyeth‐Lederle, the original developers of minocycline; and Medicis, who market minocycline in the United States. We also contacted the drug information departments of 16 pharmaceutical companies who manufactured other acne therapies.
For the 2012 update, we contacted 15 companies and authors for data that had not been included in the published article.
Data collection and analysis
Selection of studies
Once a study had been identified, we recorded the citation and located a copy of the report. We translated studies written in languages other than English if it could not be ascertained that it was randomised or controlled from the initial inspection. Two reviewers (SEG and EAE for the original; SEG and CB for the update) then independently assessed each study to see whether it met the inclusion criteria for the review. We resolved any discrepancies, other than those of simple error, by discussion. Where resolving disagreement by discussion was not possible, we added the article to those awaiting assessment and contacted the authors of the study for clarification. We considered duplicate publications in tandem.
Data extraction and management
We designed and piloted data extraction forms on a sample of five trials to detect any confusing or incomplete coding instructions. After we made revisions, we re‐piloted the resulting forms. The team performed a double abstraction process, with EAE and SEG (SEG and CB for the update) independently extracting data. We resolved disagreements other than those of simple error by discussion or contacted the authors of the studies.
We extracted from each study the following information on key variables characterising participants, interventions, and outcomes, entering it into the tables of included studies:
study information ‐ author, publication status (full report/abstract/unpublished data, publication date, sponsorship, setting, dual publication);
key variables characterising the study ‐ overall design, trial type (parallel or cross‐over), number of participants allocated to each treatment group, study duration, participant/provider and outcome assessor blinding, number and reason for dropouts, method of analysis;
key variables characterising the intervention ‐ dose, duration, use of concomitant therapy, skin hygiene, previous treatment withdrawal (oral and topical), control for ultraviolet light exposure, instructions to the participant, whether the dose was taken on an empty stomach, evidence of compliance monitoring; and
key variables characterising the participants ‐ number of participants enrolled, recruitment method, overall mean age, initial acne severity, comparability of study groups at entry, number at final assessment, percentage of participants not accounted for, and inclusion and exclusion criteria.
outcome data ‐ For continuous outcome measures, such as lesion count or grade, we extracted raw data on means and dispersion. Where categorical outcomes had been used, we recorded the number of participants in each group and the denominator.
adverse effects ‐ We sought data on the number and type and how the information was obtained. In some cases, information was only available on the number of events, so no denominator could be determined; therefore, we considered this information separately.
other information ‐ We examined publication bias by comparing the outcomes of published and unpublished trials. We assumed expectation bias in all open trials and in those in which the principal investigators were responsible for the collection of subjective data.
We collected 'Risk of bias' information for all studies for the 2012 update.
Assessment of risk of bias in included studies
We assessed each study for specific methodological and substantive components that may have influenced the validity of the results. Methodological components relate to the overall trial design and execution, whilst substantive components are specific to the topic under consideration (Glass 1981). We used the following criteria:
(a) methodological components (The Standards of Reporting Trials Group 1994), including whether an adequate sample size enrolled, whether the correct randomisation protocol followed, whether allocation was concealed, whether there was baseline comparability of the groups, whether the reliability and validity of outcome measures were examined, whether the withdrawals (number and reason) were clearly stated, whether all participants were accounted for, and whether the appropriate method of analysis was used; and
(b) substantive components (Eady 1990b), including whether there was adequate study duration, whether the acne severity at inclusion was clearly stated, whether there were explicit and appropriate inclusion/exclusion criteria, whether there was adequate withdrawal of the previous therapy (four weeks oral and two weeks topical), whether the use of concomitant medication was prohibited, whether there was monitoring of participant compliance, whether the tetracycline was taken on an empty stomach, whether clear instructions were given to participants, whether there was standardisation of the skin hygiene routine, whether there was control of exposure to UV light, whether there was a uniform site of evaluation, whether the number and timing of assessments were standardised, and whether there was evaluation of inter‐assessor variability.
Two reviewers (EAE and SEG for the original; SEG and CB for the update) independently assessed the trials, resolving disagreements by discussion. We did not exclude open and single‐blind studies from the review, but we took the degree of blinding and the resultant potential for bias into consideration in the interpretation of the results.
Measures of treatment effect
In this review, our analyses attempted to include all people who had been randomised to minocycline or control treatments (an intention‐to‐treat analysis).
Where continuous data, such as lesion counts or grades from baseline, were used, we extracted the mean and standard error/deviation of the change from baseline to each assessment and calculated the mean difference. In most cases, it was not possible to extract these data directly from the trial report or obtain them from the authors/trial sponsors. If the calculated P value or T statistic was given, we used it to estimate the effect size; otherwise, we used the authors' report of significance.
If the authors of the study had designated a cut‐off point for determination of clinical effectiveness (e.g. a 40% reduction in inflamed lesion counts, or attainment of grade 2 on a grading scale), we used this to calculate the risk ratio (RR). Similarly, we dichotomised the results of the participant and doctor evaluations where necessary (e.g. into either 'improved' or 'not improved') and calculated RRs. The RR compares the risk of the event in people receiving minocycline versus people who are receiving an alternative treatment.
A RR of 1 means there is no difference in risk between the 2 groups.
A RR of < 1 means the event is less likely to occur in the experimental group than in the control group.
A RR of > 1 means the event is more likely to occur in the experimental group than in the control group.
We summarised data on the more common adverse effects as number of participants experiencing an event compared to the number of participants treated with the drug. We calculated RRs for the overall incidence of any adverse effect, for adverse effects necessitating withdrawal, and for the incidence of gastro‐intestinal complaints.
Unit of analysis issues
We collected data from only the first stage of cross‐over trials to exclude potential additive effects in the second phase.
Dealing with missing data
Where possible, we used results from intention‐to‐treat analyses, rather than those from the per‐protocol/efficacy analyses. If the data were not clear or not included in the trial report, we contacted the primary author of the paper for assistance and clarification. If the data could not be attained by any method and only the partial data from the report were available, we calculated values where possible, and if not, we reported the authors' report of significance in tabular form.
Data synthesis
We reported fixed‐effect meta‐analyses as the default. Where significant heterogeneity was detected, we also undertook random‐effects analyses.
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was possible because of the lack of data. We quantified the levels of heterogeneity using I² statistic.
Sensitivity analysis
We undertook sensitivity analyses where possible by comparing the per‐protocol analyses with the intention‐to‐treat. Where data were not available for dichotomous outcomes, we made a 'worse case' assumption, i.e. we assumed that the people with missing data had poor outcomes.
For the analysis of adverse effects in the trials, we re‐ran the meta‐analysis excluding open (non‐blinded) studies where the participant and investigator knew their treatment allocation.
Results
Description of studies
Results of the search
The original review contained 27 RCTs. The searches for the update identified a further 12 RCTs. Thirty‐nine RCTs in total were identified comparing minocycline to another comparator, with a total of 6013 participants. All but seven were published in English: Two studies were published in German (Blecschmidt 1987; Laux 1989), three studies were published in French (Dreno 1998 [pers comm]; Lorette 1994; Waskiewicz 1992), and one study each were published in Italian (Fallica 1985) and Spanish (Campo 2003).
Included studies
Design
The duration of the trials ranged from 4 to 24 weeks, with a median of 12 weeks. There is no agreed minimum duration of acne trials, but 12 weeks is commonly used. Nineteen of the trials were conducted under double‐blind conditions where neither the assessor nor the participant knew the treatment allocation. In another six, the investigator was unaware of the treatment allocation, but the participant was. Both the participant and the investigator knew the treatment allocation in 14 RCTs. One cross‐over trial was identified (Hersle 1976).
Sample sizes
The numbers of participants included in the individual RCTs ranged from 18 (Smit 1978) to 649 (Ozolins 2005). The median number of participants was 100. Twenty‐four of the studies included 109 individuals or fewer.
Only four RCTs ‐ all conducted in the UK ‐ stated that a power calculation had been undertaken to ensure sufficient numbers of participants had been included to exclude the effects of chance (Bossuyt 2003 (TETRABUK); Cunliffe 1998; Darrah 1996; Ozolins 2005).
Bossuyt 2003 (TETRABUK) used a non‐inferiority design (80% probability, using a one‐sided test performed at the 0.025 level of significance and a maximum difference of 15% in inflamed lesions). Non‐inferiority trials are designed to demonstrate that the efficacy of a new treatment is not worse than the chosen control by more than a specified margin (in this case a 15% difference in inflamed lesion count). There are a number of inherent weaknesses in this type of design.
Cunliffe 1998 based calculations on the ability to detect a 15% difference in the per cent reduction in inflammatory lesion count with 80% probability, using a two‐sided test performed at the 0.05 significance level. They estimated that 67 evaluable participants per treatment group were required.
Darrah 1996 estimated that 150 evaluable participants were required to demonstrate with 80% probability that the 95% confidence interval of the true difference in response rates was within ± 15% in respect of the percentage of participants who achieved at least a 40% reduction from baseline to the end of treatment in the number of acne lesions.
Ozolins 2005 estimated that based on participants' evaluation of overall improvement, 132 would be needed for a 20% relative treatment effect between the test regimen and 5% benzoyl peroxide to be detected with a 75% response rate (80% probability, using a two‐sided test at the 0.05 level of significance) on the assumption of a 23% dropout rate.
Setting
In total, 28 studies were conducted in more than 1 centre. Of the 39 RCTs, 36 were conducted in dermatology clinics and only 3 in general practice, which were all in the UK (Darrah 1996; Ozolins 2005; Peacock 1990).
The RCTs were conducted in different countries: U.S. (nine), UK (seven), France (six), Germany (three), Italy (three), Chile (one), Columbia (one), Japan (one), Sweden (one), India (one), Iceland (one), Netherlands (one), and Belgium (one). Three further RCTs were conducted over three sites in different EU countries.
Participants
The 39 RCTs enrolled a total of 6013 participants. The ages ranged from 9 to 47, although most trials (29) insisted on a minimum age of between 12 to 17 years. Where it was stated in 29 of the RCTs, the maximum age was above 24 and generally over 30. Post‐adolescent acne is generally regarded as harder to treat, so the degree to which the results can be generalised to adolescents is questionable. We didn't identify any subgroup analyses by age.
Two of the RCTs enrolled only men (Gollnick 1997; Pigatto 1986) because they were comparing minocycline with oral isotretinoin, which is known to cause foetal abnormalities. One further RCT of an oral contraceptive inevitably only included women (Monk 1987). Otherwise, there was a fairly even distribution of men and women or boys and girls across the studies, with a few notable exceptions (Blecschmidt 1987; Cullen 1976; Darrah 1996; Dreno 1998 [pers comm]; Dreno 2001; Fallica 1985; Hersle 1976; Fleisch 2006a (MP010404); Fleisch 2006b (MP010405); Ozolins 2005; Peacock 1990; Pelfini 1989).
All trials reported the entry and exclusion criteria, but there was a lot of variation. The severity of acne varied from mild to severe, but most RCTs included mainly participants with 'moderate to moderately severe inflammatory disease'. Only four trials included participants with mild as well as moderate acne (Darrah 1996; Hersle 1976; Ozolins 2005; Revuz 1985), and none only included non‐inflammatory acne. Almost all included moderate acne (33), and 24 included severe acne. Three trials only included severe acne, two compared minocycline to oral isotretinoin (Gollnick 1997; Pigatto 1986), and one assessed combination treatment with adapalene (Smit 1978).
Exclusion criteria almost always included hypersensitivity to tetracyclines or the comparator as well as pregnancy and lactation. In addition, some authors specifically excluded participants with renal or hepatic dysfunction, vertigo (a common side‐effect of minocycline therapy), or any intercurrent illness. Participants with secondary acne and acne conglobata or fulminans were specifically mentioned as excluded by a minority of authors. Such participants should not have been included in any of the trials.
Interventions
Among the trials identified, there was considerable variation in the choice of comparator and in the dose of minocycline. Treatment regimens varied from 100 mg per day in 1 or 2 divided doses, to 100 mg or 200 mg initially followed by 50 mg or 100 mg after the first 4 weeks. In 1 instance, 100 mg was given on alternate days after the first 2 weeks, as is recommended in France by the manufacturer (Cunliffe 1998).
Six RCTs included a placebo comparison, and three RCTs examined different doses of minocycline. The following oral antibiotics were compared with minocycline: oxytetracycline (2 RCTs), tetracycline (7), doxycycline (5), lymecycline (4), roxithromycin (1), faropenem (1), and josamycin (1). Minocycline was compared against zinc gluconate in one RCT and two different hormonal treatments; cyproterone acetate/ethinyloestradiol (1 RCT) and a type 1 5‐alpha reductase inhibitor (1 RCT). The comparisons against topical treatments were topical clindamycin (3 RCTs), topical erythromycin/zinc(1), topical fusidic acid (1) and benzoyl peroxide and benzoyl peroxide/erythromycin (1). Combination treatments were evaluated in four RCTs, and two RCTs compared minocycline with isotretinoin. Finally, one RCT evaluated minocycline as a maintenance therapy.
Minocycline was compared with the following: zinc gluconate in one RCT, two different hormonal treatments (cyproterone acetate/ethinyloestradiol) in one RCT, a type 1 5‐alpha reductase inhibitor in one RCT. The comparisons against topical treatments were as follows: topical clindamycin in three RCTs, topical erythromycin/zinc in one RCT, topical fusidic acid in one RCT, and benzoyl peroxide and benzoyl peroxide/erythromycin in one RCT. Combination treatments were evaluated in four RCTs, and two RCTs compared minocycline with isotretinoin. Finally, one RCT evaluated minocycline as a maintenance therapy.
Outcomes
Almost all the trials used more than one outcome measure, and over 50 different methods were used. Thirty‐five RCTs counted various combinations of lesions, but there was once again considerable variation in how this was carried out, what was included, and how it was reported (e.g. separate and total lesion counts, absolute changes from baseline, and percentage lesion counts). Although most authors reported mean reductions in grade or lesion count, a minority reported medians instead or in addition (Gollnick 1997; Monk 1987). Four used grades only (Blecschmidt 1987; Fallica 1985; Hubbell 1982; Samuelson 1985), and only 17 included any assessment made by the participants.
A variety of significance tests were used with both acne grades and lesion counts: the commonest being the student's t‐test, the Mann‐Whitney U test, and analysis of covariance. In two instances, non‐parametric tests, such as Mann‐Whitney or Wilcoxon, had been carried out on means instead of medians (Smit 1978;Stainforth 1993). As far as it could be ascertained, most studies were analysed on a per‐protocol basis. A minority of trials used intention‐to‐treat analysis. It was not always clear how withdrawals and participants who failed to attend at one or more visits had been dealt with, and few studies specified how many of the participants enrolled had been included in the final analysis.
Two studies used a simple quality of life (QOL) questionnaire (Dreno 1998 [pers comm]; Peacock 1990), and one included recognised QOL instruments suitable for cost‐utility analysis (Ozolins 2005).
Adverse effects
All evaluable trials collected data on unwanted effects, and all but one reported these data in some form. However, some collected data on adverse events, some on side‐effects, and some on tolerance. Details of how unwanted effects were identified were given only occasionally and were rarely adequate. Twelve studies gave no detail whatsoever, and five merely stated that they asked the participant. Revuz 1985 recorded those adverse effects that were spontaneously reported by participants or observed by the doctor. Khanna 1993 questioned participants about four specific categories of side‐effect (photosensitivity, signs of benign intracranial hypertension, hyperpigmentation, and vaginal candidiasis). Ruping 1985 reported tolerance on a five‐point scale as separately assessed by participants and physicians. Harrison 1988 asked the specific question, 'Has the treatment upset you in any way?'. Waskiewicz 1992 and Lorette 1994 assessed tolerance on the basis of subjective criteria (e.g. dizziness) and objective signs (e.g. urticaria). Darrah 1996 recorded adverse effects as observed by the physician and as reported spontaneously by the participant in response to a non‐leading question. There was clearly confusion about definitions, and some authors had apparently made quite arbitrary decisions about which adverse effects were possibly drug‐related. For example, Cullen 1976 ruled that joint pain and swelling of the fingers in a minocycline‐treated participant was unlikely to be drug‐related. In the vast majority of studies, it was impossible to ascertain what proportion of participants had been included in the safety analysis. In 6/26 studies, side‐effects were only reported if they led to withdrawal of the participant. Only one of these six studies actually made it clear that participants who hadn't been withdrawn did not report any side‐effects.
For the additional review of adverse effects, we identified 16 studies (please see Table 25), of which 3 used a number of different designs simultaneously. Three studies used nationally‐reported pharmacovigilence data, and four examined cohorts of consecutive participants attending clinics. Large prescribing databases were the subject of two cohort studies and three case‐control studies. Finally, we identified seven systematic reviews.
3. Minocyline adverse events.
| Author | Adverse event | Methods | Population | Case Definition | Number of cases | Interventions | Outcomes | Results | Authors' conclusions | Comment |
| Angulo 1998 | Coexisting systemic lupus erythematosus (SLE) and autoimmune hepatitis | A systematic review of MEDLINE ‐ 1966 to April 1998. Not all search terms were stated. Bibliographies were searched |
Any patients treated with tetracycline | SLE, Autoimmune hepatitis, arthritis, lupus, chronic hepatitis, antimyeloperoxidase, vasculitis, and toxicity | There were 60 cases of systematic lupus erythematosus, and 24 cases of minocycline‐induced autoimmune hepatitis. 13/84 had both conditions |
Minocycline 'long‐term' therapy |
Clincial symptoms, laboratory tests for liver involvement and autoantibodies | The 13 patients were characterised by symmetrical polyarthralgias/polyarthritis, elevated liver enzymes, and positive antinuclear antibodies. Minocycline withdrawal resulted in symptom resolution and improvement of laboratory results | The association of drug‐induced lupus and autoimmune hepatitis likely represented only 1 component of a broad clinical spectrum of minocycline‐induced autoimmune disorders. The actual incidence of that association was probably underestimated. Baseline and periodic liver function and ANA tests should be preformed on those receiving long‐term minocycline therapy | These are only the cases published in the literature; therefore, we will be under‐reporting the true incidence |
| Fay 2008 | Hyperpigmentation | Retrospective medical record review of patients with rheumatoid arthritis (RA) visiting 2 centres (1992 to 2005) | Rheumatoid arthritis | 4/7 had American College of Rheumatologists criteria for RA or diagnosis from a board‐certified rheumatologist. Hyperpigmentiation when the rheumatologist, physical exam, or both consistent with this finding | 44/121 (36%) participants receiving at least 1 course of minocycline of 30 days or more | Minocycline | Bluish‐grey or muddy‐brown discolouration, non‐blanching, non‐transient | 79 (65%) were women. The distribution was greatest in the lower and upper extremities, with head and neck commonly seen. In multivariable regression, age was found to be the only independent determinant | Although common, it infrequently led to dose reduction or discontinuation. It seems to increase with age | The results reflect the demographics of RA population and are possibly not able to be generalised to a younger population without comorbid conditions. The population was not randomly drawn |
| Goulden 1996 | All adverse events | Cohort study to estimate the absolute incidence of common ADRs | Acne | ‐ | 95/700 patients (13.6%) experienced a side‐effect attributable to minocycline.The mean duration of treatment was 10.5 months (range 2 weeks to 4 years) | Minocycline | ‐ | No cases of autoimmune disorders were reported, although there were 22 cases of gastro‐intestinal disturbance, 24 cases of vestibular disturbance, 14 cases of headache and visual disturbances, 7 cases of cutaneous reactions, and 17 cases of abnormal pigmentation | The incidence was found to be greater in women than men (13.5% compared to 7.5%) and in those over the age of 35 (27% compared to 11.8%). The incidence did not significantly rise with increased dose, except in the case of pigmentation, which occurred after a minimum period of 8 months and a total cumulative dose of 70 g | These results must be interpreted with caution as the study was not large enough to detect rare ADRs and was not controlled |
| Grasset 2003 | All ‐ systemic disease, drug‐induced lupus, hepatitis, autoimmune vasculitis, hypersensitivity reactions (DRESS: drug reaction with eosinophilia and systemic symptoms), pseudodisease serum, intracranial hypertension, abnormal pigmentation, and various other side‐effects | Comprehensive systematic literature review (search methods reported): MEDLINE, IPA (International Pharmaceutical Abstracts), EMBASE (current contents, 1997‐ 2001). The search resulted in 96 papers, of which 70 were eligible for review |
Acne | ‐ | ‐ | All tetracyclines | Clinical symptoms and laboratory tests for autoimmune disease markers | 72 cases of autoimmune disorder, 5 cases of vasculitis, 15 cases of hypersensitivity syndrome, 3 cases of serum sickness, 24 cases of pseudomotor cerebri, and 123 cases of abnormal pigmentation |
Adverse effects of tetracyclines might be serious and sometimes unknown. Long‐term treatment by tetracyclines must be researched in people presenting such symptoms. Moreover, several adverse drug reactions might be avoided by an optimal use of the drug (oesophageal ulcerations, photosensitivity) or by shorter periods of treatment (autoimmune disorders, pigmentations); only drug reaction with eosinophilia and systemic symptoms are drug adverse reactions unpredictable and sometimes severe | Review of the literature |
| Ten Holder 2002 | Cutaneous and systemic manifestations of drug‐induced vasculitis | MEDLINE 1965 to December 1999. Not all search terms were stated (English‐language only). The results were not systematically stated. Bibliography review |
All | Included drug‐induced vasculitis, Churg‐Strauss syndrome, Good pastures syndrome, Henoch‐Schonlein purpura, polyarteritis nodosa, Wegeners granulomatosus, hypersensitivity vasculitis, microscopic polyangiitis, serum‐sickness, and cryoglobulinaemia | 13 cases of vasculitis in a second course of therapy following an uneventful first course | All drugs | Death, laboratory measures of autoimmune disease markers | Cutaneous and systemic manifestations of drug‐induced vasculitis | ‐ | ‐ |
| Lawrenson 2000 | Liver damage | Systematic review. MEDLINE CINAHL Cochrane EMBASE Current Contents TOXLINE (earliest available to December 1998). The search terms were stated. English, French, German, Swedish and Spanish Bibliographic databases Grey literature A Citation search was undertaken using the BIDS database. Data on the number of adverse events reported was taken from the Uppssala Monitoring Center for the time period 1968 ‐ October 1998. Sales data were obtained from the company 'Intercontinental Medical Statistics'. All references of the retrieved articles were searched for further relevant publications. |
Literature review: acne WHO: all reactions |
Liver damage: 1) liver disease (fatty liver, liver failure, liver function tests, liver transplantation, hepatic dysfunction); 2) Hepatitis (hepatitis, autoimmune hepatitis, chronic hepatitis, chronic drug‐induced hepatitis, toxic hepatitis); and 3) jaundice |
65 case reports in the literature review WHO: 493/8025 (6%) reactions recorded as involving the liver |
Minocycline | Altered liver enzyme levels, positive ANA, histological evidence of chronic active hepatitis. Autoimmune hepatitis, mortality due to minocycline‐related hepatotoxicity |
Literature review: Cases of autoimmune were generally associated with prolonged minocycline courses, the presence of autoantibodies and symptoms of arthritis, arthralgia, or both. Recovery on cessation of the drug. 16 cases of hepatic damage attributable to hypersensitivity with 3 deaths. Unspecified arthritis in remaining. 1 further death. WHO: 22 different types of hepatic reactions. Even gender distribution. Mean age 30 for women and 30 for men |
2 different types: 1) hypersensitivity with rapid onset usually within 1 month; and 2) autoimmune hepatitis generally after a year or more of therapy ‐ it is more common in women. Further that it is inappropriate to make any comment with regard to monitoring |
All cases entered into a database and independently reviewed. The authors also report an un‐validated exploration of the GPRD, which suggests people with acne may be more prone to hepatic illness. This type of review cannot quantify the absolute or attributable risk of liver dysfunction |
| Lebrun‐Vignes 2012 (AFSSAPS 2009) | All | Review of spontaneous reports to the French National Pharmacovigilence Committee. Data from Marketing Authorisation Holders. Sales figures taken into account |
All patients receiving tetracycline therapy | Any adverse event reported | 924 | Minocycline, doxycycline, metacycline and lymecycline | Adverse events Serious adverse events |
Data from 1985 to 2008 identified 2099 events; 1083(51%) with doxycycline and 921 (44%) with minocycline. The proportion of severe events was higher than with minocycline than doxycycline: 268 (29.5%) versus 211 (19.5%) (P < 0.01, Chi² test). Compared to doxycycline, the minocycline group were noted as experiencing 'significantly more frequent', cutaneous reactions including pigmentation, neurological disorders relating intracranial hypertension, respiratory disorders, hypersensitivity reactions; eosinophilia, autoimmune disorders, and DRESS. In the minocycline group, the most common were cutaneous disorders (including pigmentation) (42%), and neurological (12.5%), including intracranial hypertension in one third of cases. There were 41 cases of hypersensitivity DRESS reactions(compared to 5 with doxycycline), and 95% were serious (5% fatal). Other hypersensitivity reactions were also more common in minocycline (4% vs 1.6%), including lupus. Data reported to marketing authorisation holders suggest that serious adverse drug reactions were 4 times more frequent for minocycline than doxycycline (141 vs 33). | In practice, minocycline has a less favourable risk‐benefit balance than doxycycline, particularly in the treatment of acne | These data arise from spontaneous reports to the safety monitoring system in France and to the manufacturers. They are therefore likely to be under‐estimates. The increased frequency of minocycline reports may be influenced by the publicity around the risks of minocycline. Sales data used as a proxy denominator indicated that minocycline was used 1.5 times less than doxycycline [AFSSAPS report not accessed] |
| Margolis 2007 | Lupus erythematosus | Retrospective cohort study. United Kingdom Health Improvement Network database |
Acne | History of acne; at least one year of follow‐up; between age of 15 to 35. Diagnosis of LE; systemic or cutaneous as determined by the GP. | 97 694 individuals with acne. 24 282 exposed to minocycline 5% random sample of age‐matched individuals from entire database used to provide estimate of incidence in general population. |
Tetracyclines | Diagnosis of LE; systemic or cutaneous as determined by the GP. | 51 cases if LE (0.05% of acne cohort); of these 24 had been exposed to minocycline. HR for association of minocycline to LE was 2.64 (95% CI: 1.51 to 4.66) and when adjusted for age and gender 3.11 (95% CI: 1.77, 5.48). A strong relationship between the duration of exposure and LE was noted. But it still occurs with exposures of less than 6 months. Frequency estimated at 8.8 cases per 100,000 person‐years. | The use of minocycline and not the other tetracyclines is associated with LE. The event is uncommon but the risk and benefit of minocycline must be carefully considered. | The overall frequency was noted as 'rare'; but matched controls were not used. Reliance on accuracy of GP recording which was not verified by researchers but they did 3 sensitivity analyses. Potential for confounding by indication noted. |
| Margolis 2010 | Inflammatory bowel disease (IBD) | Retrospective cohort study United Kingdom Health Improvement Nework database |
Acne | History of acne; at least one year of follow‐up; between age of 15 to 35. Diagnosis of LE; systemic or cutaneous as determined by the GP. | 94,487 individuals with acne. 24,085 individuals with a minocycline prescription, 41 of whom developed IBD. | Tetracyclines | Diagnosis of IBD as determined by GP. | IBD noted in 41/24,085 individuals exposed to minocycline. HR for developing IBD following exposure to minocycline 1.19 (95% CI: 0.79, 1.79). For ulcerative colitis the associations were 1.10 (0.76, 1.82) and Chrons 1.28 (0.72, 2.30). | Tetracycline antibiotics and in particular doxycycline may be associated with the development of IBD particularly CD. | ‐ |
| Marzo‐Ortega 2007 | Antineutorophil antibody (ANA) and Antineutrophil cytoplasmic antibody (ANCA) positivity. | Cross sectional study of consecutive patients attending United Kingdom acne clinic June 1998 and Oct 1999. | Acne | Acne patients who agreed to participate in study. Retrospective review of experience | 252 consecutive patients with acne who agreed to participate. 174 (69%) exposed to minocycline | Mnocycline | Blood test for ANA, ANCA, liver function tests and HLA analysis. | No statistically significant difference in the prevalence of ANA positivity between minocycline exposed and unexposed groups. Higher titres in the minocycline exposed group. ANCA positivity in 7 % of exposed group but not in the unexposed cohort. Positive ANA occurs in around 10% of patients with acne regardless as to MN exposure. |
ANA positivity is seen in patients with acne irrespective of exposure to MN. However p‐ANCA appear to be a serological marker for developing autoimmune disease in patients receiving MN. | ‐ |
| Schlienger 2000 | Lupus | Systematic review. MEDLINE 1966 to October 1999 EMBASE Search terms stated: minocycline, arthritis, arthralgia, lupus, SLE. English and non‐English Bibliographies searched Cases screened, |
All | 1) No history of SLE before minocycline started. 2) positive ANA along with at least one clinical feature of SLE 3) recovery after minocycline withdrawal. |
57 cases (27 publications) | Minocycline | Clinical manifestations, time to exposure, laboratory manifestations | 47 women and 8 men (1 data not available). The median time of exposure was 19 months (range 3 days to 6 years). All patients had polyarthralgia/poly arthritis often associated with myalgia. Musculo skeletal symptoms frequently accompanied by constitutional symptoms such as fever, malaise, fatigue, anorexia or weight loss. Liver involvement in 31 patients. 12 patients with dermatological manifestations. Therapy stopped in all cases which resulted in improvement. | Long‐term exposure to minocycline may be associated with drug‐induced lupus. Baseline and periodic liver function and ANA tests accompanied by appropriate clinical monitoring are suggested for patients receiving long‐term minocycline therapy. | ‐ |
| Schoonen 2010 | Lupus | Matched case‐control study using the general practice research database (GPRD) between 1987 and 2001. | All | SLE or drug‐induced lupus. | 875 cases with no comorbid autoimmune condition and 3632 controls matched for age, gender and the medical practice attended. | A number of drugs, including minocycline | Diagnosis of lupus. | Of the 875 incident cases (of which 83 % were women), 12% (n = 107) had evidence of a prescription for one or more of the drugs investigated. Fifty of the lupus cases had one or more minocycline prescriptions giving an OR of 4.23 (95% CI: 1.03, 42.74) compared to controls. | The authors reported a clear trend of increasing risk with increasing numbers of prescriptions. The findings support a causal relationship. | ‐ |
| Seaman 2001 | Liver damage | Cohort analysis and case‐control study using the United Kingdom General Practice Research database which contains the anonymous records of approximately 8 million people. | All | Cohort: new users of minocycline compared with new users of oxytetracycline, tetracycline Case‐control study assessing antibiotic exposure in new cases of liver dysfunction in those exposed to minocycline |
Cohort: 153,530 with a diagnosis of acne. 29,332 (19.1%) exposed to minocycline ‐ who had not previously been exposed. 13 new users with liver‐dysfunction. Case‐control: 250 cases of liver dysfunction. |
Minocycline oxytetracycline tetracycline |
Liver dysfunction: Raised liver enzymes, jaundice, liver dysfunction, hepatitis, liver failure. | Cohort: The incidence of liver dysfunction was rare:1.04 cases/10,000 exposed person months (EPM) for minocycline and 0.69 cases/10,000 EPM in those exposed to oxytetracycline/tetracycline (relative risk 1.51 (CI95: 0.63, 3.65). The risk in both groups was greatest in the first month of use. Case‐control: The adjusted odds ratio (ORadj) of liver dysfunction associated with exposure to minocycline compared with non use was 2.10 (CI95: 1.30, 3.40); for oxytetracycline/tetracycline, the ORadj was 1.46 (CI95: 0.81, 2.64); and for exposure to erythromycin, the ORadj was 1.64 (CI95: 0.71, 3.80). | The authors concluded that individuals with newly prescribed minocycline the incidence of liver dysfunction was rare. The authors thus support a weak association between the use of oral antibiotics and liver dysfunction in patients with acne. The risk associated with exposure to minocycline appears to be very small. The cohort analysis demonstrated that any risk associated with minocycline was not significantly greater than that associated with oxytetracycline/tetracycline exposure. | ‐ |
| Shapiro 1997 | Hypersensitivity syndrome reaction (HSR), serum‐sickness like reaction (SSLR), single organ dysfunction (SOD) and drug induced lupus (DIL) | Case series. MEDLINE (1966 to October 1996): search terms not all stated. Ontario drug safety clinic database Adverse Drug Reaction Monitoring Division of the Canadian Heath Protection Branch. Utilisation data from IMS to identify prescribing patterns. |
All | Separate definitions provided for each condition. | 19 reports of HSR, 11 reports of SSLR, 40 reports of SOD, 33 reports of DIL attributable to minocycline. | Tetracycline Minocycline Doxycycline |
Autoimmune drug‐induced reactions, hypersensitivity. | No difference in the average daily doses of minocycline causing the reactions. | Early serious events occurring during the course of tetracycline antibiotic treatment include HSR, SSLR and SOD. Drug‐induced lupus, which occurs late ni the course of therapy, is reported only with minocycline. We theorize that minocycline metabolism may account for the increased frequency of serious adverse events with this drug. | ‐ |
| Smith 2005 | All | Systematic review, of safety of doxycycline and minocycline. MEDLINE Embase Biosis 1966 and August 2003 Search terms stated FDA MedWatch data. English language only number of new prescriptions Jan 1998 to Aug 2003. |
All | Adverse event, adverse reaction, side‐effect. | 333 AEs with minocycline and 130 with doxycycline MEDWATCH: 628 with doxycyline and 1099 minocycline Approx 47.63 million doxycycline prescriptions and 15.235 million minocycline prescriptions |
Doxycycline Minocycline | n/a | Whole body, digestive system (oesophageal erosion), skin (photosensitivity, photo‐onycholysis, rash), CNS (intracranial hypertension)and other reactions (hypoglycaemia, anosmia) were reported. | Event rates estimated to be 13 per million with doxycycline and 72 per million with minocycline based on FDA data. The incidence of AE's with either drug is very low, but doxycyline has fewer. Gastro‐intestinal reactions were the most common with doxycycline and CNS and gastro‐intestinal with minocycline. |
The authors also note the discrepancy between the types of minocycline adverse events in the trails and case reports. With immunologic events being reported many years after clinical trials were completed. |
| Sturkenboom 1999 | Drug‐induced SLE | Case‐control study. Cohort of participants identified by the General Practice Research Database | Acne | Cases were included if they had negative findings in a rheumatoid arthritis test or latex agglutination test, positive or unmeasured anti‐nuclear antibodies, elevated or unmeasured ESR or absent or unmeasured anti‐DNA antibodies | 27688 acne patients aged 15 to 29. Each case identified matched with 8 controls. 29 participants with lupus like syndrome matched with 152 controls. 27 of whom were women |
Minocycline or other tetracyclines. | ‐ | From matched controls selected from the same cohort it was estimated that the risk of developing SLE‐like syndrome following tetracycline exposure is increased 3.5 times (95% CI 1.3 to 7.0). The same study also demonstrated that 85 % of the cases were women and they have a 14 fold relative risk (95% CI: 1.8 to 111) of developing the disorder in comparison to men. The absolute risk is 52.8 cases per 100 000 prescriptions and minocycline increases the risk 8.5 times (95% CI: 2.1 to 35) compared to other tetracyclines which carry a risk of 1.7 (95% CI: 0.4 to 8.1) |
The authors conclude a 8.5 fold greater risk of lupus‐like syndrome in young women currently using minocycline for acne compared with non‐users or past users and that this effect is strongest for longer‐term use. Since lupus‐like syndrome is uncommon and reversible after stopping minocycline treatment, the increased risk associated with minocycline use only moderately affects the risk/benefit balance |
There also appears to be a strong effect of cumulative minocycline dose and prolonged (> 100 days) exposure |
Excluded studies
We excluded 64 studies that are commonly cited as evidence of the effectiveness of minocycline: Most were uncontrolled cohort studies. However, six were actually RCTs, but they did not meet the inclusion criteria for this review, primarily due to the non‐clinical outcomes used in the studies (Bodokh 1997; Goulden 1996; Kligman 1998; Leyden 1997a; Nishijima 1996; Pablo 1975). In one trial, all participants were given minocycline and then randomised to receive either streptokinase (Varidase) or placebo (Randazzo 1981); therefore, this trial was excluded. Please see the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
It is clear from the above description of the included trials that there was considerable variation between them with respect to numerous factors, which might affect study quality or introduce bias, or both. We sought further information from trial investigators when there was insufficient information in the trial report to make a judgement.
Allocation
Only six of the RCT reports (published and unpublished) mentioned any specifics about how the randomisation was carried out and were rated as low risk of bias (Blecschmidt 1987; Cunliffe 1998; Darrah 1996; Leyden 2006 (Part 2); Ozolins 2005; Peacock 1990). The remainder of the included studies did not provide additional information beyond the fact that the study was randomised. One study matched pairs of participants in the treatment groups prior to randomisation on the basis of age, sex, and baseline acne severity (Sheehan‐Dare 1989). Pelfini 1989 and Waskiewicz 1992 were rated as high risk of bias. In Pelfini 1989, the trial design was compromised by a number of participants who were also given 5% benzoyl peroxide, which is very active. Also, two different treatments schedules and the method of randomisation was unclear and possibly based on severity. In Waskiewicz 1992, the investigators stated that three participants dropped out and were re‐included in the trial three to six months after their dropout. In the meantime, their acne did not improve spontaneously or with other treatments. The re‐inclusion of dropouts was judged to have compromised the randomisation.
Four RCTs provided adequate descriptions of allocation concealment (Cunliffe 1998; Hayashi 2011; Leyden 2006 (Part 2); Ozolins 2005). We rated 10 studies as high risk (Blecschmidt 1987; Cabezas 1993; Fallica 1985; Gollnick 1997; Laux 1989; Monk 1987; Pigatto 1986; Ruping 1985; Schollhammer 1994; Waskiewicz 1992) as no allocation was attempted or it was judged to be inadequate. We rated the remainder of the studies (25) as unclear.
Blinding
We included 13 open trials (Blecschmidt 1987; Darrah 1996; Fallica 1985;Gollnick 1997; Hayashi 2011; Khanna 1993; Laux 1989; Monk 1987; Pelfini 1989, Pigatto 1986; Ruping 1985; Schollhammer 1994; Waskiewicz 1992) in this review and rated them as high risk of bias. We decided not to exclude these studies but to interpret the results in consideration of the bias that is often associated with open trials.
In total, we described 20 trials as 'double‐blind', which were therefore classified as at low risk of bias: Cabezas 1993;Cullen 1976;Cunliffe 1998;Drake 1990;Dreno 1998 [pers comm];Dreno 2001; Hersle 1976;Hubbell 1982;Leyden 2004;Leyden 2006 (Part 2);Lorette 1994; Stewart 2006 (MP010401);Fleisch 2006a (MP010404); Fleisch 2006b (MP010405);Olafsson 1989;Pierard 2002; Revuz 1985; Samuelson 1985; Sheehan‐Dare 1989; Smit 1978. In a further six RCTs, the participant knew what treatment they were allocated to, but the assessor did not. Five of these (Bossuyt 2003 (TETRABUK); Campo 2003; Harrison 1988; Peacock 1990; Stainforth 1993) were rated as unclear as they are more open to biases. The sixth (Ozolins 2005) was classed as low risk of bias because it stated, "Participants were given specific written and spoken instructions not to discuss the nature of their medication with assessors. Instances of treatment unmasking to assessors during the study were recorded."
Incomplete outcome data
Only 19 of the 39 included studies provided sufficient information to assess them as being at low risk of attrition bias, with reasons for dropouts fully accounted for and dropouts balanced between the groups (Cullen 1976; Cunliffe 1998; Darrah 1996; Dreno 2001; Fallica 1985; Gollnick 1997; Harrison 1988; Hayashi 2011; Khanna 1993; Leyden 2006 (Part 2); Stewart 2006 (MP010401); Olafsson 1989; Ozolins 2005; Peacock 1990; Pelfini 1989; Pierard 2002; Pigatto 1986; Smit 1978; Stainforth 1993). We judged 14 studies to be at high risk of bias due to the proportion of dropouts, incomplete reporting of dropouts, or imbalanced rates of dropout between the groups, with the remaining 5 studies being judged as unclear due to the lack of information.
Selective reporting
We only judged three studies (Leyden 2004; Lorette 1994; Waskiewicz 1992) to be at high risk of bias. Leyden 2004 only reported inflammatory lesions from prespecified outcomes that included lesion counts, investigator and participant assessments, and photographs. It may be that these data were collected but not included in the published report of the study. Lorette 1994 did not provide the number of participants in each group who experienced adverse effects. Although the types of adverse effects were listed, clinical tolerability is described only as 'satisfactory'. Waskiewicz 1992 provided only percentage improvements and acne count.
We rated nine studies as unclear risk of bias as we were unable to assess, from the report of the study, whether all outcomes had been fully reported or where further information was not available from the trial investigator (Campo 2003; Dreno 1998 [pers comm]; Gollnick 1997; Hubbell 1982; Monk 1987; Ozolins 2005; Ruping 1985; Samuelson 1985; Schollhammer 1994).
The remaining 27 studies reported all prespecified outcomes and therefore were rated as low risk of bias.
Effects of interventions
The outcome measures of interest were those that estimated clinical efficacy, participant acceptability, or both, in a defined way. There are many different methods used to assess clinical efficacy, and there is no evidence at present on their relative validity, reliability, or responsiveness. Therefore, lesion counts (total, inflamed, and non‐inflamed, separately), acne severity scores, physicians' global evaluation, and participants' self assessment have all been included in the description of the effects of interventions.
1. Minocycline versus placebo
Six RCTs included a placebo comparison (Cabezas 1993; Hersle 1976; Leyden 2004; Stewart 2006 (MP010401); Fleisch 2006a (MP010404); Fleisch 2006b (MP010405)).
We identified one trial that used a cross‐over design, comprising two five‐week treatment phases (Hersle 1976). It is not clear whether the 43 participants completing the study were aware of the cross‐over, as the tablets in each phase were identical. There was no wash‐out period between the two phases, which meant that the results for placebo in the second phase could not be considered reliable. A summed weighted acne lesion score was used as the sole outcome measure, and during the first phase, the minocycline group demonstrated a significant reduction (P < 0.05, paired student's t‐test), whilst the placebo‐treated group did not. No measures of dispersion were presented, and no statistical comparison was performed between the groups.
A placebo arm was included in a trial that compared minocycline to a drug in the experimental stages (see comparison 4b). The 34 participants treated with minocycline were reported to have a 49.2% reduction in inflamed lesion counts compared to 26.8% in the 37 participants treated with placebo (Analysis 1.1) (means difference (MD) 22.40, 95% CI 4.34 to 40.46) (Leyden 2004). Non‐inflamed lesion counts did not appear to be undertaken, and no usable data on adverse effects were reported.
1.1. Analysis.
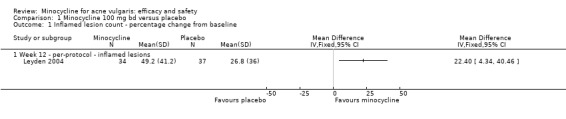
Comparison 1 Minocycline 100 mg bd versus placebo, Outcome 1 Inflamed lesion count ‐ percentage change from baseline.
Three RCTs evaluated a extended‐release formulation of minocycline and were all sponsored by Medicis Pharmaceutical Corporation. The two 3‐month phase 3 trials (Fleisch 2006a (MP010404); Fleisch 2006b (MP010405)) included a total of 615 participants receiving minocycline 1 mg/kg and 309 participants receiving placebo. The results of the phase 2 dose‐finding trial are further discussed under '2. Dose response' (Stewart 2006 (MP010401)). The similarities between the trial designs in the three RCTs meant that meta‐analysis could be undertaken. Minocycline ER resulted in a statistically significant greater percentage reduction in inflamed lesion counts (45.5% reduction versus 32.4%) in Analysis 2.1 (MD 13.43, 95% CI 7.10 to 19.76) and total lesion counts in Analysis 2.2 (MD 9.84, 95% CI 4.84 to 14.84), but not non‐inflamed lesion counts (14.9 vs 6.3 mean per cent reduction from baseline ‐ data not presented in poolable format); the authors stated it as being 'not inferior' and not causing an exacerbation. Pooled treatment success as evaluated by the investigator was Analysis 2.3 (RR 1.89, 95% CI 1.26 to 2.82). For all three analyses I² statistic = 0%. There was no statistically significant difference between the numbers of participants at different doses of minocycline and the placebo group whose skin was clear or almost clear after 12 weeks in Analysis 2.4 (RR 1.62, 95% CI 0.81 to 3.24). Adverse effect data were not presented for the individual trials.
2.1. Analysis.
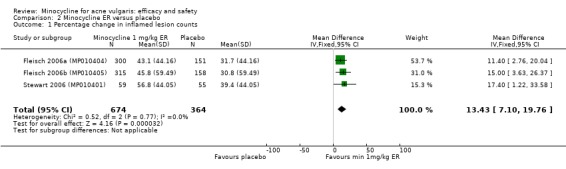
Comparison 2 Minocycline ER versus placebo, Outcome 1 Percentage change in inflamed lesion counts.
2.2. Analysis.
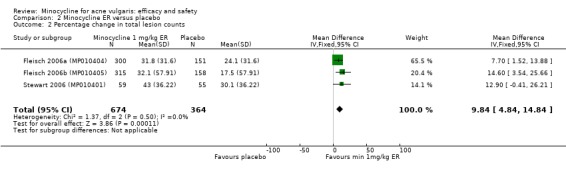
Comparison 2 Minocycline ER versus placebo, Outcome 2 Percentage change in total lesion counts.
2.3. Analysis.
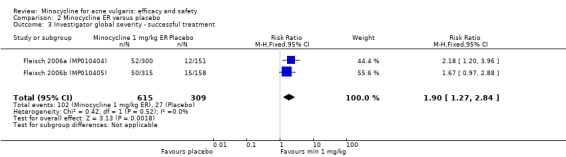
Comparison 2 Minocycline ER versus placebo, Outcome 3 Investigator global severity ‐ successful treatment.
2.4. Analysis.
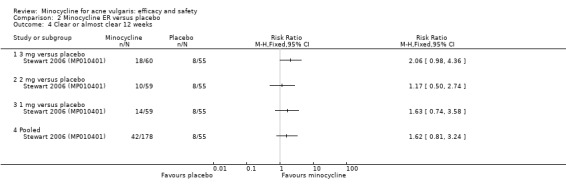
Comparison 2 Minocycline ER versus placebo, Outcome 4 Clear or almost clear 12 weeks.
One small Spanish‐language study conducted in Chile (80 participants) compared minocycline to tetracycline and placebo (Cabezas 1993). Very sparse data on the trial methodology and results were available in this publication. The authors did not report any statistical comparison between the minocycline and placebo group. No further data could be obtained from either the trial authors or sponsors.
2. Dose response
In most countries, the manufacturer's recommended dose of minocycline for acne is 100 mg per day, which is half the normal therapeutic dose used for other indications. Therefore, it is surprising that no study was located that compared 100 mg to 200 mg per day in the treatment of acne.
Pierard 2002 compared 50 mg a day for 12 weeks with 50 mg twice‐daily for 4 weeks, followed by 50 mg a day for 8 weeks. There were only 28 and 31 participants randomised to each group, respectively; very few methodological details were provided; and there were no baseline assessments of equivalence. The authors concluded that the inflammatory papule counts in the 100/50 mg group showed a statistically significant greater reduction compared to the 50 mg daily group (P < 0.05, non‐parametric pairwise Wilcoxon rank sum test). No details were provided on open comedones, closed comedones, and pustules. There was also a significant difference in the investigator and participant global assessments (P < 0.05, Chi² test). The trial was primarily an evaluation of in vivo antibacterial efficacy, and therefore few protocol details were presented and few clinical data were available from the publication. The authors did not respond to requests for additional information. The authors concluded that the "microbial response to minocycline 100/50 mg was also superior", although no data were provided for further assessment, only a graph. There was insufficient information mentioned in the trial report to allow for proper assessment.
A publication of a double‐blind, phase two, dose‐ranging trial that explored the appropriate dose of a extended‐release form of minocycline was identified (Stewart 2006 (MP010401)). The randomisation was stratified by body weight with 233 participants with moderate to severe acne given either placebo or 1 mg/kg, 2 mg/kg, or 3 mg/kg minocycline ER for 12 weeks. There was a high dropout of 57 participants (24%). By day 84, the number of inflammatory lesions had decreased by 39% in the placebo group and 57%, 49%, and 47%, respectively, in the minocycline groups. The authors concluded that no dose‐dependent effect was observed in either global assessment scores, inflamed lesion counts, non‐inflamed lesions, or total lesion counts. Although it is probable that insufficient numbers of participants were recruited to ensure the trial was adequately powered, no power calculation was included in the publication. Compared to placebo, the only difference in per cent change from baseline in inflammatory lesions that reached statistical significance was the 1 mg/kg group at 84 days. There were no statistically significant differences between any of the groups for the changes in the number of non‐inflamed lesions, total lesion counts, or number of participants who were clear/almost clear as rated by the assessor (Analysis 3.1). Subgroup analyses were conducted (it was not stated whether they were planned or post hoc). The analyses indicated that minocycline ER "seemed to be somewhat less effective in the heaviest subjects".
3.1. Analysis.
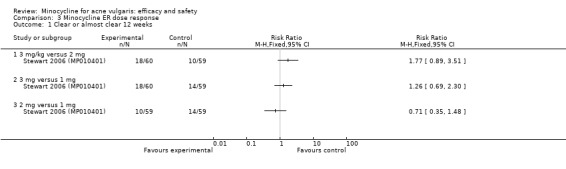
Comparison 3 Minocycline ER dose response, Outcome 1 Clear or almost clear 12 weeks.
One RCT compared minocycline at a continuous dose of 100 mg per day for 8 weeks with the same initial regimen, but at a reduced dose of only 50 mg per day after 2 weeks. The lead investigator supplied the full report of outcomes from the trial report, but it is as yet unpublished (Dreno 1998 [pers comm]). A total of 325 participants were included in the tolerance analysis: 307 in the intention‐to‐treat and 214 in the per‐protocol. After eight weeks, no significant differences between the dosage regimens were noted in any of the outcome measures using either per‐protocol or intention‐to‐treat analysis (Analysis 4.1; Analysis 4.2; Analysis 4.3). However, because of the short duration of the study, inferences cannot be made concerning their relative efficacies in long‐term treatment.
4.1. Analysis.
Comparison 4 Minocycline 100 mg od versus 100 mg/50 mg od, Outcome 1 Lesion counts ‐ reduction from baseline after 60 days therapy.
4.2. Analysis.
Comparison 4 Minocycline 100 mg od versus 100 mg/50 mg od, Outcome 2 Overall clinical improvement ‐ dr‐assessed.
4.3. Analysis.
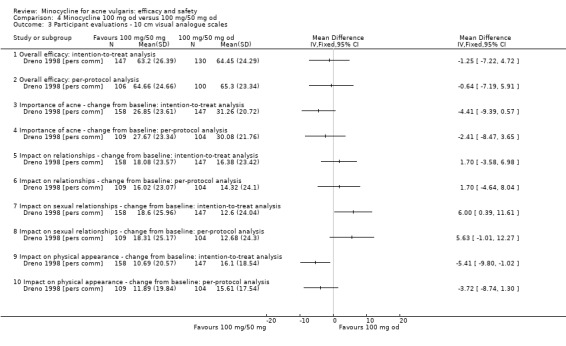
Comparison 4 Minocycline 100 mg od versus 100 mg/50 mg od, Outcome 3 Participant evaluations ‐ 10 cm visual analogue scales.
3 Minocycline versus other oral antibiotics
a. Minocycline versus tetracycline or oxytetracycline (abbreviated as (oxy)tetracycline)
Tetracycline and oxytetracycline are in the first‐generation class of tetracyclines.
The original version of the review included 7 RCTs, and 2 further RCTs were identified in the 2012 update. Of these nine studies, the authors of five reported no statistically significant differences between minocycline and (oxy)tetracycline: The methodology of these five studies reflected standards at the time, but by today's standards, it would not be considered robust. They were also likely to be underpowered due to the small total numbers of participants included: Cullen 1976 included 100 participants (Analysis 5.8), Fallica 1985 included 100 participants, Hubbell 1982 included 104 participants (Analysis 5.7), Samuelson 1985 included 62 participants (Analysis 5.4), and Khanna 1993 included 44 participants (Analysis 5.9).
5.8. Analysis.
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 8 Overall improvement.
5.7. Analysis.
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 7 Number of participants converting to Pillsbury grade I.
5.4. Analysis.
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 4 At least moderate improvement according to assessor.
5.9. Analysis.
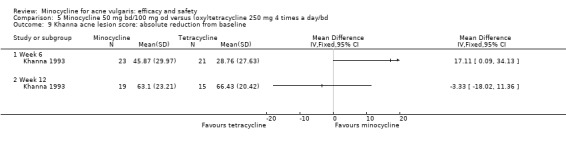
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 9 Khanna acne lesion score: absolute reduction from baseline.
The sixth study was more recent, well‐designed, and a larger observer‐blind study conducted in those with mild to moderate acne in primary care in the UK (Ozolins 2005). The trial was funded independently. The initial plan was to compare 11 treatments, but slow‐recruitment meant that 6 arms were discontinued. Power calculations were undertaken at the start of the study, and approximately 130 participants were randomised to each arm (649 participants in total). Minocycline and oxytetracycline obtained comparable results at 18 weeks with a decrease in inflamed lesion count from baseline (MD 3.60, 95% CI ‐1.56 to 8.76) (Analysis 5.2) and in those participants with at least moderate improvement, as assessed by the participants themselves (Analysis 5.3) and by the assessors (Analysis 5.4). However, at 12 weeks the minocycline‐treated group had a statistically significantly greater reduction in the mean number of inflamed lesions (RR 7.30, 95% CI 1.12 to 13.48) (Analysis 5.2).
5.2. Analysis.
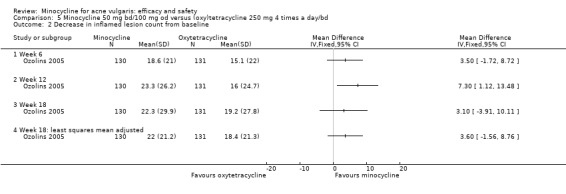
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 2 Decrease in inflamed lesion count from baseline.
5.3. Analysis.
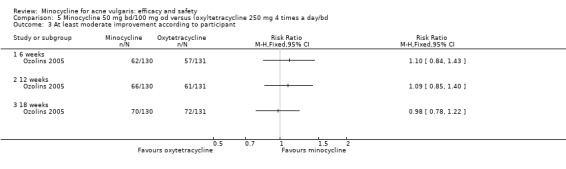
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 3 At least moderate improvement according to participant.
The overall numbers of participants experiencing adverse effects were not reported, and there were no differences in the numbers of participants withdrawing because of adverse effects. The oral antibiotic‐treated groups experienced more gastro‐intestinal and musculoskeletal events at week six. Using 2005 acquisition costs, oxytetracycline was reported as being more cost‐effective and of similar effectiveness at approximately 1/7th of the cost at that time. The acquisition costs have now changed as generic minocycline has become available. The clinical efficacy of both tetracyclines was reported as being compromised in individuals who were colonised by tetracycline‐resistant propionibacteria. Most of the treatment effect was seen after the first six weeks of treatment.
Three studies found minocycline to be superior to (oxy)tetracycline (Blecschmidt 1987 (Analysis 5.1); Cabezas 1993; Ruping 1985). The first two RCTs were however conducted under open conditions and had a number of serious methodological flaws. Therefore, the results must be interpreted with extreme caution. Ruping 1985 reported that minocycline was significantly superior to tetracycline in terms of the reduction in inflamed lesion count at week 12, but presented the results only in graphical form and did not include any measures of dispersion or state the method used to calculate its P values. Visual inspection of the graphical data does not support the conclusion that minocycline is superior. The study included participants with acne conglobata, and assessments were performed only on one side of the face, back, or chest, with the assessment intervals grouped into 14‐day periods without standardisation. The trial also reported that 36 participants could not be evaluated because of faulty or inadequate statements, with no details as to which treatment they were randomised to, and no information was provided on dropouts or the number of participants included in the analysis. The total number of adverse effects in each group was not reported, and the text is contradictory about the numbers withdrawing due to adverse effects. The second study (Blecschmidt 1987) enrolled 237 participants and had similar design faults, with 43 participants being excluded from the analysis and duration of treatment varying between 12 and 20 weeks. The outcome measure used was the number of participants improving by at least two grades at each assessment point, with no indication of what grade they improved from or to.
5.1. Analysis.
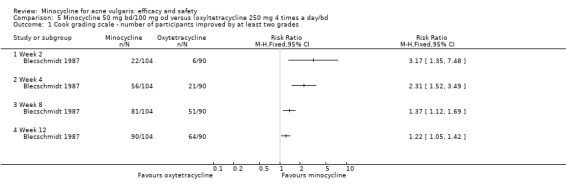
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 1 Cook grading scale ‐ number of participants improved by at least two grades.
Cabezas 1993 was a small (80 participants) placebo‐controlled, double‐blind study. It was conducted in Chile, and a very brief report was published in Spanish with few methodological details. Neither the company nor the authors responded to requests for more information. There were no statistical comparisons with the placebo group, but the authors reported that there was a statistically significant difference in favour of minocycline in the average numbers of 'lesions', papules, and pustules after 45 days of treatment.
It has been suggested that minocycline has a faster onset of action than first‐generation tetracyclines because it is more fat‐soluble, and therefore higher levels are obtained in sebum. Khanna 1993 recorded a statistically significant difference in favour of minocycline compared to tetracycline in the 'acne lesion score' at six weeks but not 12 weeks (Analysis 5.9). This effect was also reported by Hubbell 1982 in the number of participants converting to grade 1 acne (indicating mild acne), although data for only 55 of 104 enrolled participants were included in the data analysis (Analysis 5.7). Compared to tetracycline Samuelson 1985 reported statistically significant differences in the change in acne grade from baseline in favour of minocycline at weeks two and eight, but only as assessed by the investigator and not when self‐assessed by participants. However, the only data provided were the mean grade at each time point, which showed no difference between the groups (Analysis 5.5; Analysis 5.6).
5.5. Analysis.
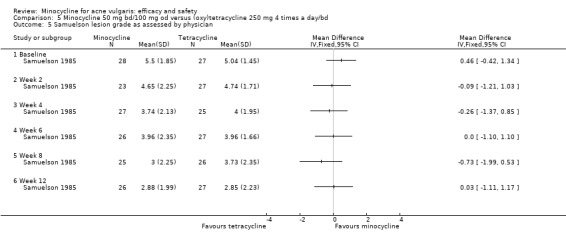
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 5 Samuelson lesion grade as assessed by physician.
5.6. Analysis.
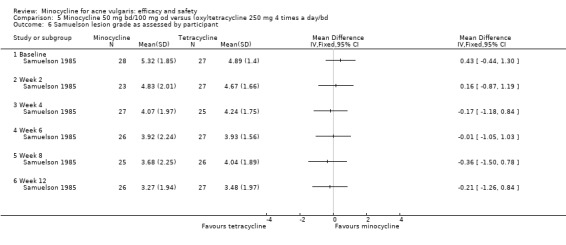
Comparison 5 Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd, Outcome 6 Samuelson lesion grade as assessed by participant.
Blecschmidt 1987 found that, statistically significantly, more participants treated with minocycline had improved by 2 or more grades by the end of weeks 2, 4, 8, and 12 than those treated with oxytetracycline (Analysis 5.1). Visual inspection of the survival curves presented in Ruping 1985 also suggested minocycline had a faster onset of action against papules and pustules (with the caveat about the methodology as described in the paragraph). In all cases where the initial response to minocycline was faster, the magnitude of the reduction in acne severity produced by both drugs at the end of the treatment period was similar (Analysis 5.3; Analysis 5.4).
The concerns about the robustness of the RCTs not withstanding, some limited meta‐analysis of the 'overall improvement' results could be undertaken (Analysis 5.8). This indicated that, as assessed by the investigator, more participants receiving minocycline had improved compared to those receiving tetracycline at week 6 (RR 1.43, 95% CI 1.04 to 1.96, I² statistic = 72%), but not week 12 (RR 0.97, 95% CI 0.72 to 1.31, I² statistic = 0%). However, this difference was not evident when a random‐effects meta‐analysis was undertaken, reflecting the heterogeneity identified.
b. Minocycline versus lymecycline (Tetralysal)
Lymecycline is a second‐generation tetracycline, which, like minocycline, is typically given as a single‐daily dose. It has become the most commonly prescribed first‐line antibiotic for acne in Europe.
The original version of the review included two RCTs that compared lymecycline with minocycline, both of which used reducing doses of each drug (as is common practice in France). The first was a 144‐participant double‐blind, double‐dummy trial in 5 centres (Cunliffe 1998), which was sponsored by Galderma, who manufacture lymecycline. A variety of outcome measures were used, but the primary end point was declared in advance as the per cent reduction in inflamed lesion count at week 12. Using both intention‐to‐treat (ITT) and per‐protocol analyses, no significant difference was found between minocycline and lymecycline for this or any other outcome measure (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5). The sample size had been calculated to enable the study to detect a 15% difference in the percentage reduction in the inflamed lesion count with an 80% probability using a two‐sided test performed at the 0.05 significance level. The second study was a small (71 participants) 12‐week open study and additionally included participants treated with doxycycline (Schollhammer 1994). The authors reported a 68.4%, 72.7%, and 62.4% reduction in inflamed lesion counts in the minocycline, lymecycline, and doxycycline groups, respectively. These differences were not found to be statistically significant (P > 0.05, test not reported); however, the trial was likely to be very underpowered.
6.1. Analysis.
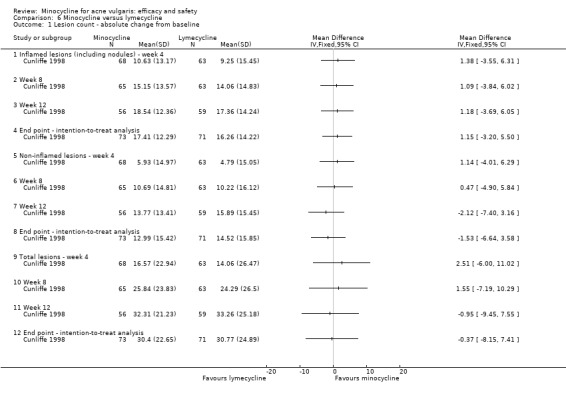
Comparison 6 Minocycline versus lymecycline, Outcome 1 Lesion count ‐ absolute change from baseline.
6.2. Analysis.
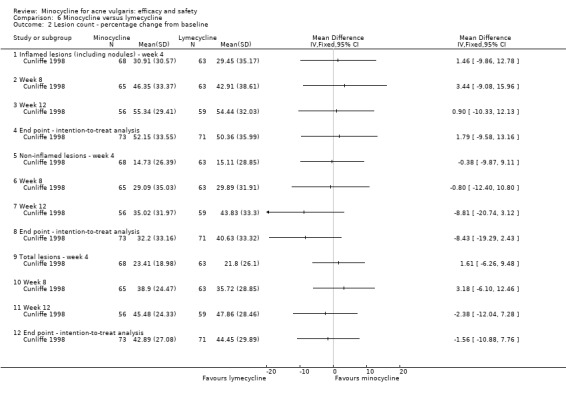
Comparison 6 Minocycline versus lymecycline, Outcome 2 Lesion count ‐ percentage change from baseline.
6.3. Analysis.
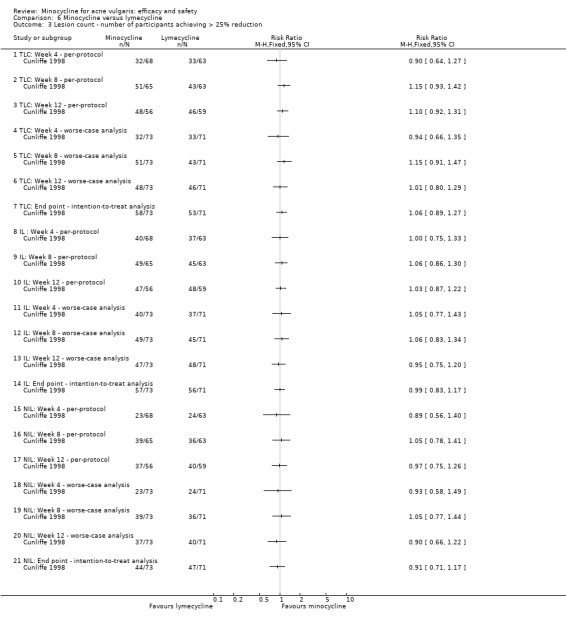
Comparison 6 Minocycline versus lymecycline, Outcome 3 Lesion count ‐ number of participants achieving > 25% reduction.
6.4. Analysis.
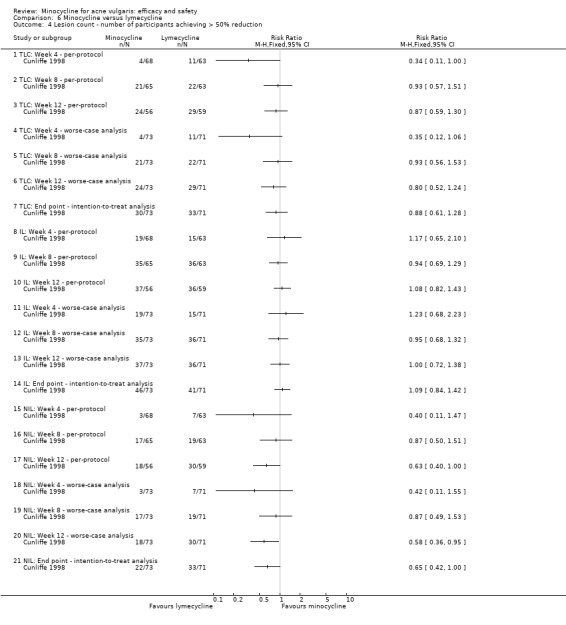
Comparison 6 Minocycline versus lymecycline, Outcome 4 Lesion count ‐ number of participants achieving > 50% reduction.
6.5. Analysis.
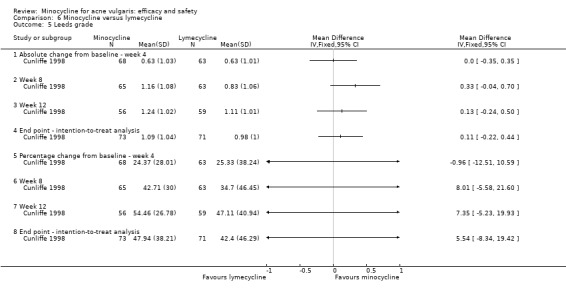
Comparison 6 Minocycline versus lymecycline, Outcome 5 Leeds grade.
The 2012 update identified a further two 12‐week RCTs trials that both compared 300 mg lymecycline daily with 2 different minocycline regimens.
Pierard 2002 compared 300 mg lymecycline per day for 12 weeks with 2 different minocycline regimens (50 mg a day for 12 weeks or, alternatively, 50 mg twice‐daily for 4 weeks, followed by 50 mg a day for 8 weeks). Double‐dummy treatments were used. One of the authors was affiliated with Wyeth‐Lederle. There were only 28 and 31 participants randomised to each group, respectively; very few methodological details were provided; and there were no baseline assessments of equivalence. The trial was primarily an evaluation of in vivo antibacterial efficacy; therefore, few protocol details were presented, and few clinical data were available from the publication. The authors concluded that the 100/50 mg group compared to the lymecycline group showed statistically significant (P < 0.05, non‐parametric pair‐wise Wilcoxon rank sum test) "less severe" acne lesions, a greater reduction in inflammatory papule counts, and "significantly less lesions" at week 12. "Approximately one third" of participants in the lymecycline and minocycline 50 mg groups reported "the clearance of acne lesions" compared to "over half" in the minocycline 100 mg/50 mg group. Total lesions counts are not a good measure of outcome because inflamed and non‐inflamed lesions respond differently to treatments. There was also a significant difference in the investigator and participant global assessments (P < 0.05, Chi² test). The authors concluded that the "microbial response to minocycline 100/50 mg was also superior" as assessed by percentage of live bacteria and percentage of dead bacteria and debris, which were assessed by dual‐flow cytometry analysis. The authors did not respond to requests for additional information.
Bossuyt 2003 (TETRABUK) was sponsored and organised by Galderma, who manufacture lymecycline. The study compared extended‐release minocycline 100 mg with 300 mg lymecycline daily for 12 weeks in 68 and 66 participants, respectively. At the end of 12 weeks, there was no significant difference between the 2 groups in the lesion count data (IL, NIL, TLC). There were similarly no difference in global severity grade or participant or physician global assessment of improvement. There were some discrepancies in the publication with regard to the numbers of participants included in the per‐protocol analysis, which potentially indicated that approximately one third of participants randomised to each group did not complete the study. The "economic evaluation" included in the paper was a cost‐minimisation analysis that compared the most expensive minocycline preparation (extended‐release) with lymecycline. The prices of both minocycline and tetracycline have changed since 2003.
Limited pooling of data could be undertaken from Cunliffe 1998 and Bossuyt 2003 (TETRABUK); lymecycline appeared to have a greater effect on both the participant and doctor global assessments of overall improvement using intention‐to‐treat analysis, but the result was not significant (Analysis 6.6). Further data would increase the statistical power of this analysis.
6.6. Analysis.
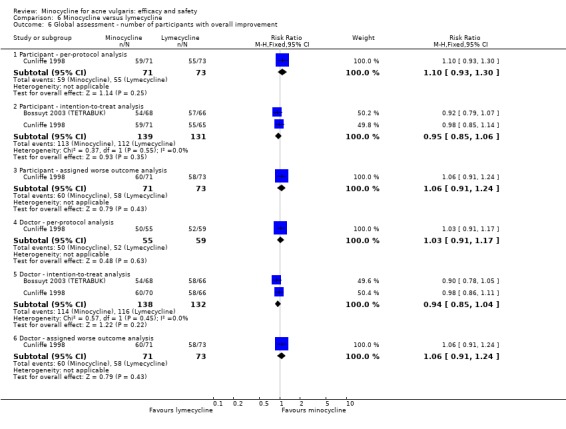
Comparison 6 Minocycline versus lymecycline, Outcome 6 Global assessment ‐ number of participants with overall improvement.
c. Minocycline versus doxycycline
Doxycycline is a second‐generation tetracycline.
All 5 trials of minocycline versus doxycycline contained less than 80 participants. Therefore, not surprisingly, because of inadequate statistical power, they did not detect any overall difference between the drugs. Four were 12 weeks, with Lorette 1994 being 17 weeks. All three open studies had deficiencies in either the outcome measures used, the trial design, or both, (Laux 1989; Schollhammer 1994; Waskiewicz 1992) and included very small numbers of participants: 74, 50, and 77, respectively. Neither of the two double‐blind studies contained sufficient information to allow effect sizes to be calculated directly (Lorette 1994; Olafsson 1989). The included participant numbers were 79 and 71, respectively.
The physician‐assessed improvement could be pooled in three trials (RR 0.99, 95% CI 0.88 to 1.12, I² statistic = 0%) (Analysis 7.2).
7.2. Analysis.
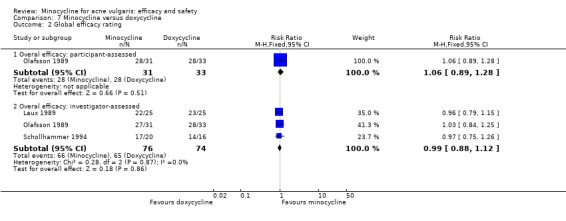
Comparison 7 Minocycline versus doxycycline, Outcome 2 Global efficacy rating.
d. Minocycline versus roxithromycin
Roxithromycin is a semi‐synthetic macrolide antibiotic derived from erythromycin and with a similar mode of action. Roxithromycin is licensed for acne in Japan, but it is not licensed in the United States or the United Kingdom.
A Japanese RCT compared 4‐week treatment with 100 mg daily minocycline (given as single dose once a day or 50 mg twice a day) with 150 mg roxithromycin daily (Hayashi 2011) in 49 and 50 participants with moderate acne. A third trial group of 51 participants received faropenem 200 mg three times a day. A second four‐week follow‐up period occurred in which no treatment was given. The only acne‐treatment‐related exclusion was if participants had received oral antibiotics within the past month. Concomitant use of acne treatments was prohibited in the treatment period, but permitted during the observational follow‐up period. Unsurprisingly, given the small numbers of participants treated and the short (four‐week) treatment duration, the authors reported no significant differences between the reductions in inflammatory and non‐inflammatory lesion counts from baseline, or quality of life. A significant reduction in all groups was seen for all outcomes from baseline at all time points. Data were reported in graphical format only. The microbiological outcomes suggested resistance had developed to roxithromycin in some participants. Dizziness and nausea were reported in two minocycline‐treated participants.
e. Minocycline versus faropenem
Faropenem is a member of the class of antibiotics known as penems. These antibiotics are broad spectrum and related to penicillins and cephalosporins. Faropenem is licensed for acne in Japan, but to date, the FDA have not approved it for any indication because of a lack of efficacy data. It is not licensed in the UK. One small trial compared minocycline versus faropenem (Hayashi 2011). The results are described in the section on minocycline versus roxithromycin. Three participants in the faropenem group had diarrhoea.
f. Minocycline versus josamycin
Josamycin is a macrolide antibiotic related to erythromycin and with a similar mode of action. It is not approved in the UK or US.
122 participants with severe or refractory acne were allocated to 2 different treatment schedules depending on severity (Pelfini 1989). The first group received 500 mg josamycin or 100 mg minocycline once daily for 8 weeks. The second received 1000 mg josamycin and 200 mg minocycline. Some participants also received topical 5% benzoyl peroxide. It is unclear how the groups were allocated. The authors reported a statistically significant reduction in the number of papulopustules, comedones, and nodulo‐cysts, and the intensity of seborrhoea and erythema. They reported that josamycin was more effective than minocycline (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5). The standard of the publication was however very poor, and the results cannot therefore be substantiated given the potential biases introduced by the likelihood of the trial being open, the dosing schedule, and use of benzoyl peroxide. One participant receiving josamycin had mild gastric discomfort, one minocycline‐treated participant had a rash, and a third had to discontinue minocycline due to severe gastric intolerance.
8.1. Analysis.
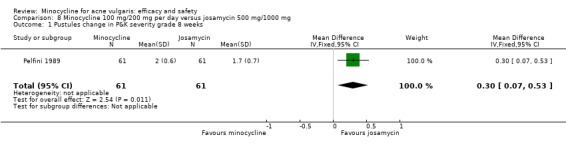
Comparison 8 Minocycline 100 mg/200 mg per day versus josamycin 500 mg/1000 mg, Outcome 1 Pustules change in P&K severity grade 8 weeks.
8.2. Analysis.
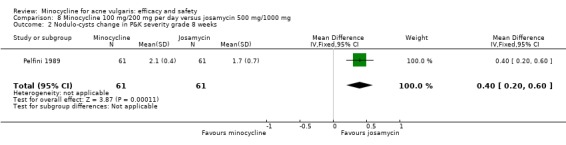
Comparison 8 Minocycline 100 mg/200 mg per day versus josamycin 500 mg/1000 mg, Outcome 2 Nodulo‐cysts change in P&K severity grade 8 weeks.
8.3. Analysis.
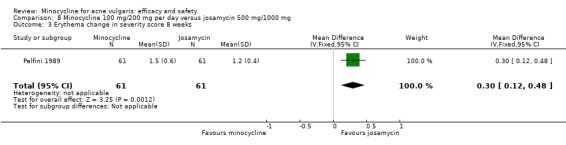
Comparison 8 Minocycline 100 mg/200 mg per day versus josamycin 500 mg/1000 mg, Outcome 3 Erythema change in severity score 8 weeks.
8.4. Analysis.
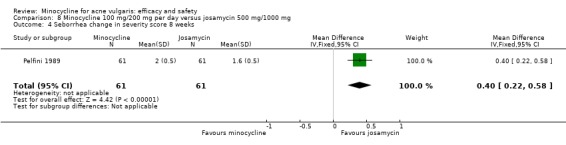
Comparison 8 Minocycline 100 mg/200 mg per day versus josamycin 500 mg/1000 mg, Outcome 4 Seborrhea change in severity score 8 weeks.
8.5. Analysis.

Comparison 8 Minocycline 100 mg/200 mg per day versus josamycin 500 mg/1000 mg, Outcome 5 Evaluation of clinical efficacy 8 weeks.
4. Minocycline versus hormonal treatments
Excessive oil production in the skin contributes to acne. The oil‐producing glands in the skin are controlled by hormones called androgens (for example, testosterone). One potential mechanism of treating acne is to manipulate the hormones.
a. Minocycline versus cyproterone acetate/ethinyloestradiol (Diane™)
In the UK, the oral contraceptive Dianette™ containing 2 mg of cyproterone acetate (an antiandrogenic progestogen that competes with dihydrotestosterone) and 0.035 mg ethinyloestradiol offers an alternative to antibiotics in women with moderately‐severe inflammatory acne. Diane™, the product compared with minocycline in the open trial of Monk 1987, contained a higher concentration of ethinyloestradiol (0.05 mg) than in the currently available product known as Dianette™. The authors found no overall difference between the treatments after 24 weeks, but as the trial only included 98 participants, it was likely to be inadequately statistically powered to conclude that the treatments are equivalent. 17% (6 out of 36 participants) of the Diane™‐treated group and 20% (7 out of 35 participants) of the minocycline group thought their acne had completely cleared (RR 1.20, 95% CI 0.45 to 3.22) (Analysis 9.1). The methodology used reflects the standards in 1987 when the trial was conducted; there were serious flaws by today's standards. There is a Cochrane review of the use of hormonal treatments to treat acne (Arowojolu 2009).
9.1. Analysis.
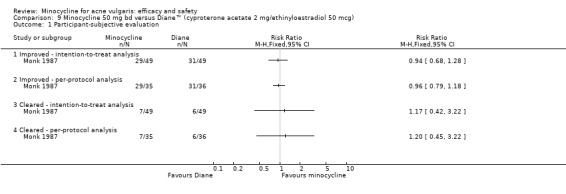
Comparison 9 Minocycline 50 mg bd versus Diane™ (cyproterone acetate 2 mg/ethinyloestradiol 50 mcg), Outcome 1 Participant‐subjective evaluation.
b. Minocycline versus compound A: inhibitor of type I 5‐alpha reductase
It is unusual for companies and journals to publish trials with negative findings as in the case of an experimental drug code‐named Compound A, which was compared to minocycline. Compound A inhibits the enzyme (called 5‐alpha reductase type 1) in the hair follicles that converts testosterone into the active hormone, dihydrotestosterone (DHT). This is one of the chemicals that causes sebum to be produced by the hair follicles. The experimental drug reduces the amount of DHT in the blood and sebum, but was shown to be ineffective in treating acne; therefore, the authors concluded that more research is required into sebaceous gland control at the cellular level (Leyden 2004). The trial was intended to last six months, but only three‐month data were reported. It is probable that the trial was stopped early due to lack of efficacy in the group receiving compound A.
After 3 months, the 34 men treated with 100 mg minocycline twice a day were reported to have a 49.2% reduction in inflamed lesion counts compared to 25.7% in the 37 men treated with 25 mg of compound A daily (MD 23.50, 95% CI 3.8 to 43.2) (Analysis 10.1). Two other arms in the study included a total 74 participants who received a combination of Compound A and minocycline, and they achieved similar results to those treated with minocycline alone. No usable data on overall adverse effects were reported, but one man receiving minocycline was reported as having a transient elevation in liver enzymes.
10.1. Analysis.
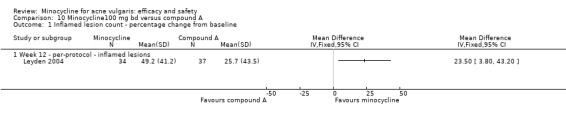
Comparison 10 Minocycline100 mg bd versus compound A, Outcome 1 Inflamed lesion count ‐ percentage change from baseline.
5. Minocycline versus zinc gluconate
Oral zinc salts (sulphate, citrate, and gluconate) have been used to treat acne since 1970. Their mechanism of action is poorly understood.
The 2012 update identified 1 double‐blind 'equivalence' RCT that compared 100 mg minocycline a day with 30 mg of zinc gluconate in 332 participants (Dreno 2001). The sponsoring company (Labcatal Pharmaceuticals) also kindly supplied additional information. Minocycline produced a greater reduction in both inflamed and non‐inflamed lesion counts, at all time points, and was evaluated as more effective by both clinicians and participants. Interestingly, for both treatment groups, significantly more women were reported as responding. After 90 days of treatment, the mean difference between the percentage change in inflamed lesions from baseline was as follows: MD ‐16.42, 95% CI ‐25.10 to ‐7.74 (Analysis 11.1). The clinician assessed 102/161 minocycline‐treated participants as having a successful treatment (defined as a two thirds reduction in IL) compared to 49/157 of those treated with zinc (RR 2.03, 95% CI 1.56 to 2.63) (Analysis 11.2). The results for the doctor and participant overall assessment of effectiveness were similar (Analysis 11.3).
11.1. Analysis.
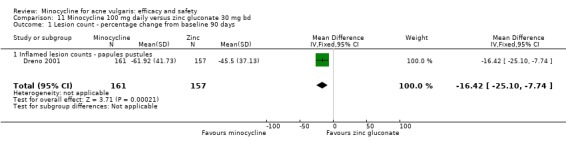
Comparison 11 Minocycline 100 mg daily versus zinc gluconate 30 mg bd, Outcome 1 Lesion count ‐ percentage change from baseline 90 days.
11.2. Analysis.
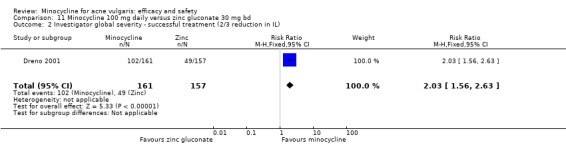
Comparison 11 Minocycline 100 mg daily versus zinc gluconate 30 mg bd, Outcome 2 Investigator global severity ‐ successful treatment (2/3 reduction in IL).
11.3. Analysis.
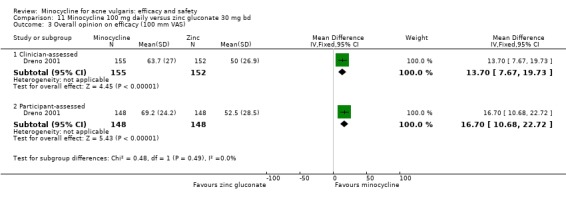
Comparison 11 Minocycline 100 mg daily versus zinc gluconate 30 mg bd, Outcome 3 Overall opinion on efficacy (100 mm VAS).
Two minocycline‐treated participants had to withdraw for GI disturbances compared to 4 zinc‐treated participants (RR 0.04, 95% CI ‐0.01 to 0.09). 36/169 participants treated with minocycline experienced an adverse effect compared to 55/163 treated with zinc (RR ‐0.12, 95% CI ‐0.22 to ‐0.03). These led to the withdrawal of 4 participants in the minocycline group and 5 in the zinc group (RR ‐0.01, 95% CI ‐0.04 to 0.03).
6. Minocycline versus topical acne treatments
a. Clindamycin
Three trials compared minocycline 50 mg twice‐daily with 1% clindamycin applied topically twice‐daily (Drake 1990;Peacock 1990; Sheehan‐Dare 1989). The trials were of similar design and duration, and all included lesion counts. However, data could not be pooled as the results were presented graphically, and no further information could be obtained from the authors or sponsors.
In all three trials, there was a statistically significant decrease in lesion counts in all groups. Although there was conflicting data in the two Sheehan reports with one saying there was no statistically significant reduction in non‐inflamed lesions, there was a definite trend in Sheehan‐Dare 1989 for superiority of topical clindamycin. However, this did not reach statistical significance, probably due to the large range of lesion counts included, and the small number of participants (66). The other two trials obtained virtually identical results for both minocycline and clindamycin. This is to be expected given both trials were very small (52 participants in Drake 1990 and 80 in the Peacock 1990 trial completed the trial). Peacock 1990 also reported on aspects of quality of life obtained via participant questionnaires, with no significant difference between the groups (Analysis 12.1).
12.1. Analysis.
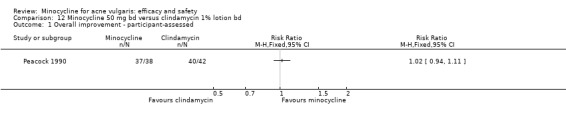
Comparison 12 Minocycline 50 mg bd versus clindamycin 1% lotion bd, Outcome 1 Overall improvement ‐ participant‐assessed.
Both treatments were reported as well‐tolerated.
b. Fusidic acid
Fusidic acid is an antibiotic that is especially useful in the treatment of staphylococcal infections. Fusidic acid is widely‐available internationally, but not in the United States. Topical use for a chronic condition like acne is contra‐indicated because fusidic acid promotes resistance when used alone. Awareness of this issue was low when the trial was conducted.
Twice‐daily 2% fusidic acid (Darrah 1996) was compared with 50 mg oral minocycline twice a day in participants with mild to moderate facial acne in UK general practice. Although spots were always counted on the right side of the face, participants were instructed to apply the topical medication to the affected area only. This trial included both a power calculation and declared the primary end point in advance. However, the end point defined was rather loose, namely a 40% reduction in either total lesion count or total non‐inflamed lesion count or total inflamed lesion count by the end of the treatment period. Using this criterion, no significant difference was found between the treatments; 188 participants were included in the non‐inferiority design (Analysis 13.1). At the end of treatment, the overall reduction in the inflamed lesion count due to minocycline was significantly superior (P = 0.04, Chi² test with Yate's continuity correction) than that due to fusidic acid (MD 3.00, 95% CI 0.06 to 5.94) (Analysis 13.2). The authors' concluded this was unlikely to be of clinical significance. There was a trend for fusidic acid to be superior against non‐inflamed lesions. The fusidic acid‐treated participants may have done better by applying treatment to the whole face to prevent new lesions forming. As to be expected, topical fusidic acid was associated with more skin adverse effects: 13/90 compared to 3/84. Minocycline was associated with more gastro‐intestinal disturbances: 4/84 compared to 1/90.
13.1. Analysis.
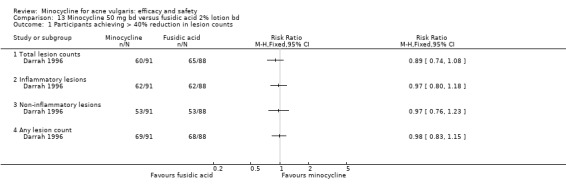
Comparison 13 Minocycline 50 mg bd versus fusidic acid 2% lotion bd, Outcome 1 Participants achieving > 40% reduction in lesion counts.
13.2. Analysis.
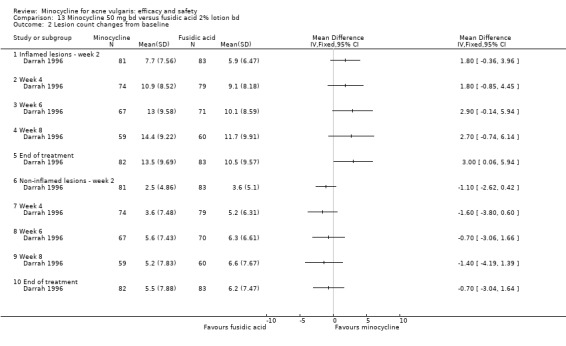
Comparison 13 Minocycline 50 mg bd versus fusidic acid 2% lotion bd, Outcome 2 Lesion count changes from baseline.
c. Erythromycin/zinc
Stainforth 1993 evaluated a twice‐daily topical application of 4% erythromycin and 1.2% zinc acetate against minocycline 50 mg twice a day in 109 participants. There was a significant reduction in lesion counts in both groups at 12 weeks compared to baseline, with good results seen after 2 weeks. The combination topical was significantly better than minocycline against both inflamed and non‐inflamed lesions at the end of the 12‐week treatment period (Analysis 14.1; Analysis 14.3; Analysis 14.2). However, there was no significant difference between the groups in terms of acne grade at any time point nor in the participants' self‐assessment using a visual analogue scale (Analysis 14.4). The minocycline‐treated participants recorded the lowest percentage reductions in inflamed and non‐inflamed lesions of all the trials that presented data in this form. After 12 weeks, there was only a 23% reduction in non‐inflamed lesions and 36% reduction in inflamed lesions. It is possible that the results may be biased as the authors admit that, in at least seven cases, therapy became known to the assessor during the trial. The topical treatment was associated with dryness and irritation in five participants. Four participants receiving minocycline reported transient headache, with one having symptoms suggestive of benign intracranial hypertension.
14.1. Analysis.
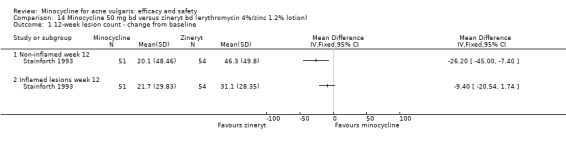
Comparison 14 Minocycline 50 mg bd versus zineryt bd (erythromycin 4%/zinc 1.2% lotion), Outcome 1 12‐week lesion count ‐ change from baseline.
14.3. Analysis.
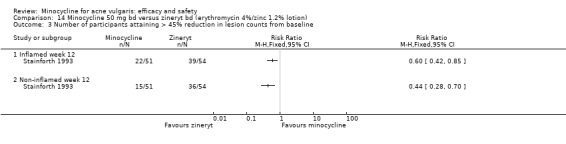
Comparison 14 Minocycline 50 mg bd versus zineryt bd (erythromycin 4%/zinc 1.2% lotion), Outcome 3 Number of participants attaining > 45% reduction in lesion counts from baseline.
14.2. Analysis.
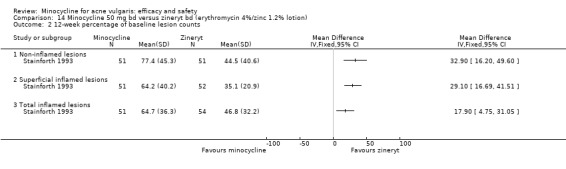
Comparison 14 Minocycline 50 mg bd versus zineryt bd (erythromycin 4%/zinc 1.2% lotion), Outcome 2 12‐week percentage of baseline lesion counts.
14.4. Analysis.
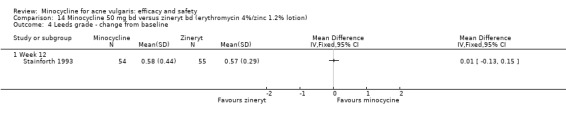
Comparison 14 Minocycline 50 mg bd versus zineryt bd (erythromycin 4%/zinc 1.2% lotion), Outcome 4 Leeds grade ‐ change from baseline.
d. Benzoyl peroxide or benzoyl peroxide/erythromycin
Extended‐release minocycline was compared to 5% benzoyl peroxide and 2 different combination regimens of benzoyl peroxide and erythromycin in a large, 18‐week, community‐based, independently‐funded study in the UK (see the oxytetracycline comparison for more details). The combination treatments were (a) separate formulations of 2% erythromycin in the morning, 5% benzoyl peroxide at night; and (b) a single formulation containing 3% erythromycin and 5% benzoyl peroxide to be applied morning and night. The participants had mild to moderate acne (Ozolins 2005). The outcomes were change in inflammatory lesion count from baseline and the number of participants assessed by participants having at least 'moderate improvement' as determined by investigators and the participants themselves.
Minocycline and 5% benzoyl peroxide produced similar results, with an average of 22.3 less inflamed lesions (Analysis 15.3) and similar improvements (Analysis 15.1; Analysis 15.2). The results for the two different combination regimens were similar: a trend towards the combination treatments being more effective, which was not statistically significant (Analysis 16.1; Analysis 16.2; Analysis 16.3; Analysis 17.1; Analysis 17.2; Analysis 17.3). The numbers of adverse effects at each time point were reported. As to be expected, there were more systemic adverse effects in the minocycline group and more local irritation in the topical groups. The two erythromycin‐containing regimens produced the largest reductions in the frequency and population density of viable organisms. The three topical regimens significantly lowered the frequency and population density of erythromycin‐resistant propionibacteria.
15.3. Analysis.
Comparison 15 Minocycline 100 mg ER od versus benzoyl peroxide bd, Outcome 3 Lesion count ‐ change from baseline.
15.1. Analysis.
Comparison 15 Minocycline 100 mg ER od versus benzoyl peroxide bd, Outcome 1 Overall improvement ‐ participant assessed at least moderate improvement at 18 weeks.
15.2. Analysis.
Comparison 15 Minocycline 100 mg ER od versus benzoyl peroxide bd, Outcome 2 Overall improvement ‐ assessor at least moderate improvement at 18 weeks.
16.1. Analysis.
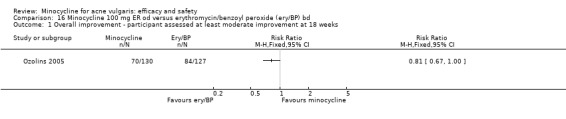
Comparison 16 Minocycline 100 mg ER od versus erythromycin/benzoyl peroxide (ery/BP) bd, Outcome 1 Overall improvement ‐ participant assessed at least moderate improvement at 18 weeks.
16.2. Analysis.
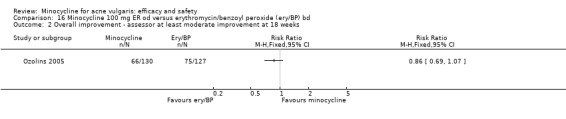
Comparison 16 Minocycline 100 mg ER od versus erythromycin/benzoyl peroxide (ery/BP) bd, Outcome 2 Overall improvement ‐ assessor at least moderate improvement at 18 weeks.
16.3. Analysis.
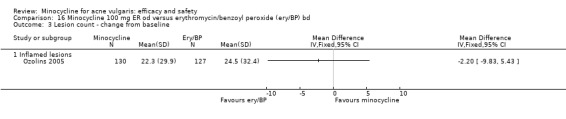
Comparison 16 Minocycline 100 mg ER od versus erythromycin/benzoyl peroxide (ery/BP) bd, Outcome 3 Lesion count ‐ change from baseline.
17.1. Analysis.
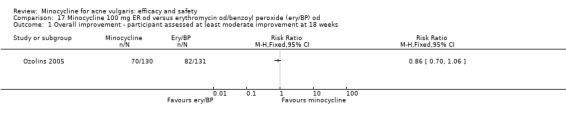
Comparison 17 Minocycline 100 mg ER od versus erythromycin od/benzoyl peroxide (ery/BP) od, Outcome 1 Overall improvement ‐ participant assessed at least moderate improvement at 18 weeks.
17.2. Analysis.

Comparison 17 Minocycline 100 mg ER od versus erythromycin od/benzoyl peroxide (ery/BP) od, Outcome 2 Overall improvement ‐ assessor at least moderate improvement at 18 weeks.
17.3. Analysis.
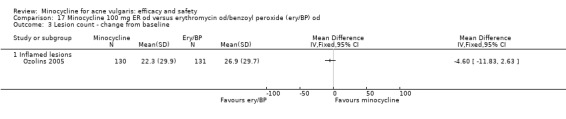
Comparison 17 Minocycline 100 mg ER od versus erythromycin od/benzoyl peroxide (ery/BP) od, Outcome 3 Lesion count ‐ change from baseline.
7. Combination therapy
Harrison 1988 compared 50 mg minocycline twice‐daily versus 50 mg doxycycline once‐daily in a group of 43 participants with acne of unspecified severity, who also received separate formulations of 4% chlorhexidine and 5% benzoyl peroxide. After 12 weeks, only 34 participants remained. Unsurprisingly, because of the very small number of participants enrolled and the additional active treatments used in both groups, no significant differences were detected in any of the reductions in lesion counts: A 59% reduction in total lesion counts was seen for each group, with a 67% to 84% decrease in the number of papules and pustules (Analysis 18.1). There was some apparent discrepancy between the adverse effect data, with tolerance being reported as less than good or excellent in 7% of participants receiving doxycycline and 21% receiving minocycline. The study authors reported that four participants in the doxycycline group and three in the minocycline group experienced side‐effects, but it was unclear what they were as only those designated as treatment‐related were reported.
18.1. Analysis.
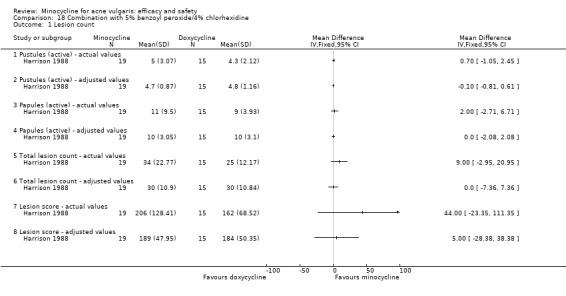
Comparison 18 Combination with 5% benzoyl peroxide/4% chlorhexidine, Outcome 1 Lesion count.
Revuz 1985 compared minocycline against placebo over 60 days in 90 participants who were also applying topical erythromycin/tretinoin gel (of unknown strength). Reductions in the number of lesions in both groups were reported, with minocycline reported as having a statistically significant greater effect against the total number of 'retentional' lesions (thought to be cysts, plus inflamed lesions), but not non‐inflamed lesions. Significant reductions in the number of papules and pustules were noted with no significant differences between the two groups. 69% of participants reported good or very good response in the minocycline group compared to 57% in the placebo group (RR 1.20, 95% CI 0.84 to 1.72) (Analysis 19.1). The data for the physician‐reported good/very good response was 77% versus 54% (RR 1.41, 95% CI 0.99 to 2.01) (Analysis 19.1). The latter was reported as statistically significant. Unsurprisingly, the rate of skin adverse effects was similar, although more burning was reported in the placebo group (26/34 compared to 21/39).
19.1. Analysis.
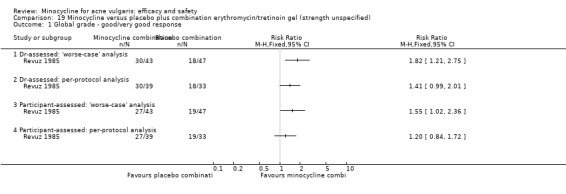
Comparison 19 Minocycline versus placebo plus combination erythromycin/tretinoin gel (strength unspecified), Outcome 1 Global grade ‐ good/very good response.
Smit 1978 randomised 18 participants with severe acne to either 100 mg minocycline or 100 mg doxycycline. Both groups received a topical 5% salicylic acid and 5% resorcinol preparation to be applied twice daily for the 12‐week duration of the RCT. The trial did not find any difference between the two groups in the change in overall score from baseline. However, too few participants were included to infer anything about the relative effectiveness.
The 2012 update identified the abstract of a 24‐week investigator‐blinded RCT that compared 100 mg minocycline daily to 300 mg lymecycline for 2 weeks, followed by 150 mg for another 8 weeks in 152 participants who were also being treated with 0.1% adapalene gel once daily (Campo 2003). Most, but not all, of the RCTs included in this review found a minimal effect of minocycline (and other antibiotics) on non‐inflamed lesions; however, this study used adapalene in both groups, which is active, against non‐inflamed lesions. Lymecycline was reported as having a statistically significant greater reduction than minocycline in total lesion counts (67% and 77%, respectively) and non‐inflamed lesions (64% versus 77%), but there was no difference in inflamed lesions or global assessments. There were however some contradictions in the abstract, so clarification is required. Data appeared to only be reported from the 122/152 evaluable participants; therefore, the study was likely not to have included sufficient numbers of participants to be able to conclude there was no difference between the groups. Further information was requested from the company, but no response was received. The treatments were reported as 'well tolerated'.
8. Minocycline versus oral isotretinoin in nodular acne
Isotretinoin is the only treatment that often cures acne after a single course. It works primarily by stopping the production of sebum. However, it is associated with severe side‐effects; therefore, it is reserved for severe or refractory acne. It can also harm the foetus, so it is not used in women of childbearing age without adequate contraception.
Two open trials compared oral isotretinoin with minocycline in nodular acne; participants and investigators will always be able to tell who receives isotretinoin due to its distinctive side‐effect profile.
In one study, the minocycline‐treated group of 50 men also received topical azelaic acid (Gollnick 1997); the second group of 35 men were treated with isotretinoin dosed according to their weight. The authors stated that the study was randomised, but there were unequal numbers of participants in each group. Furthermore, whilst at the start of the trial the two groups were demographically comparable for age, there appeared to be other differences. Only the abstract was available for the original review, but the trial had been published in full by the update, and there were some minor differences in the reported results. The study had two phases; an initial six‐month study phase followed by a second three‐month maintenance phase (topical azelaic acid only in the minocycline group). The full publication notes that, "Participants in whom a very good clinical improvement was achieved prematurely, i.e. before completing the 6 months of study phase 1, were transferred early to study phase 2". This makes the findings difficult to interpret.
Very high percentage reductions in all types of lesion count were reported for both isotretinoin and the minocycline/azelaic acid combination. The authors reported a statistically significant difference in favour of oral isotretinoin against non‐inflamed lesions (66% reduction from baseline vs 80%) (Analysis 20.2) and papules and pustules (88% vs 97%) after 24 weeks of treatment (Analysis 20.3). The clinical significance of these differences is unclear. Changes in the nodule count were 100% in both groups. The investigator reported that 100% of participants in both groups improved. The speed of onset of improvement in this study with the minocycline/azelaic acid combination was rapid and equivalent to oral isotretinoin after just one month of treatment. The adverse effect rates reflected the better tolerance of minocycline/azelaic acid, which is to be expected. In the maintenance phase, 37% of participants who continued on azelaic acid (AA) but had their minocycline stopped showed marked deterioration compared to 4% in the isotretinoin group who stopped treatment. These findings warrant further investigation and a robust trial being undertaken. Because of the side‐effects associated with isotretinoin, the combination would be a good option particularly in female participants with nodular acne.
20.2. Analysis.
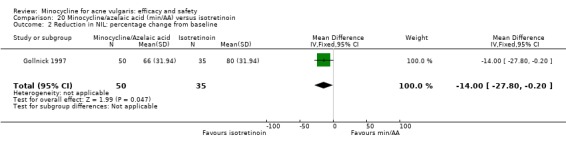
Comparison 20 Minocycline/azelaic acid (min/AA) versus isotretinoin, Outcome 2 Reduction in NIL: percentage change from baseline.
20.3. Analysis.
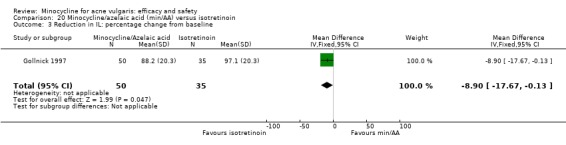
Comparison 20 Minocycline/azelaic acid (min/AA) versus isotretinoin, Outcome 3 Reduction in IL: percentage change from baseline.
The second study focused on the biochemical impact of isotretinoin, included only 24 men (Pigatto 1986), and reported few clinical outcomes. The results showed that oral isotretinoin was significantly superior to minocycline with respect to reductions in the number and diameter of nodular lesions. This study is of note because it recorded a total of 16 adverse effects in 7 of 12 minocycline‐treated participants, which is a much higher rate than in any other trial. Laboratory testing was undertaken as part of the study‐examined changes in the metabolism of lipids: Three participants treated with minocycline had slight but persistent abnormal elevations of alkaline phosphatase during therapy, and five had initial transient minor abnormal elevations of the liver enzymes aspartate aminotransferase (AST or SGOT) and alanine aminotransferase (ALT or SGPT).
9. Minocycline as a maintenance therapy
The 2012 update found 1 randomised double‐blind 12‐week trial that evaluated minocycline as a maintenance therapy after participants with moderately‐severe acne had shown 75% or greater global improvement after 12 weeks of treatment with 0.1% tazarotene gel each evening, plus 100 mg minocycline twice daily (Leyden 2006 (Part 2)). Tazarotene gel is not available in the UK. The three regimens that were compared (minocycline 100 mg twice daily, 0.1% tazarotene each evening, or a combination of the 2 regimens) were all effective at maintaining improvement over a 12‐week period, and there were no statistically significant differences between the groups for the following outcomes: severity score, maintenance of a 50% or greater global improvement, maintenance of a 75% or greater global improvement, mean percentage change in inflamed lesion count or non‐inflamed lesion count, or percentage of participants showing good or excellent maintenance. There was however a gradual increase in the number of inflamed lesions whilst the non‐inflamed lesions continued to reduce in number. However, the trial was not powered to detect differences, and no power calculation was reported. The data suggest that the minocycline‐containing regimens may be more effective at maintaining a reduction in inflamed lesions compared to tazarotene alone, and that tazarotene‐containing regimens had more impact on non‐inflamed lesions. Both findings are consistent with their mechanisms of action. However, further evaluation is required in a larger sample of participants before any conclusions can be drawn. All regimens were reported as being well‐tolerated (Analysis 21.1; Analysis 21.2; Analysis 21.3; Analysis 21.4; Analysis 21.5; Analysis 21.6).
21.1. Analysis.
Comparison 21 Minocycline 100 mg od maintenance, Outcome 1 Lesion count ‐ percentage change from baseline versus tazarotene.
21.2. Analysis.
Comparison 21 Minocycline 100 mg od maintenance, Outcome 2 Overall disease severity score versus tazarotene.
21.3. Analysis.
Comparison 21 Minocycline 100 mg od maintenance, Outcome 3 Overall clinical improvement ‐ Dr‐assessed versus tazarotene.
21.4. Analysis.
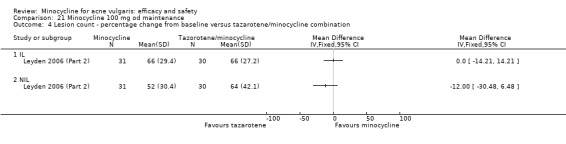
Comparison 21 Minocycline 100 mg od maintenance, Outcome 4 Lesion count ‐ percentage change from baseline versus tazarotene/minocycline combination.
21.5. Analysis.
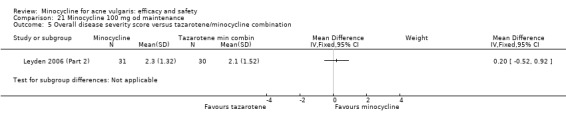
Comparison 21 Minocycline 100 mg od maintenance, Outcome 5 Overall disease severity score versus tazarotene/minocycline combination.
21.6. Analysis.
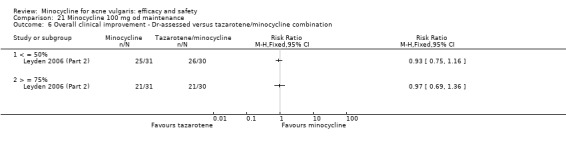
Comparison 21 Minocycline 100 mg od maintenance, Outcome 6 Overall clinical improvement ‐ Dr‐assessed versus tazarotene/minocycline combination.
10. Minocycline in tetracycline recalcitrant acne
No RCTs were located that evaluated minocycline in therapy‐resistant acne. The only evidence available was from five open, uncontrolled studies, which did not meet the inclusion criteria for this review because the results were considered unreliable due to the inadequacy of the study design (Becker 1974; Cullen 1978; Degreef 1983; Knaggs 1993; Rossman 1981).
Adverse effects
a) Results from the included RCTs
There were numerous differences between the RCTs in the way adverse effect data were collected, interpreted, reported, and analysed, which means that pooled estimates must be interpreted with caution. Accurate denominators could not be ascertained for many of the studies. Most trials only reported the most significant adverse effects; others did not report numbers or percentages of adverse effects. Furthermore, many trials were conducted under 'open' conditions.
Twenty‐nine of the RCTs reported adverse events that they attributed to minocycline therapy; of the 1906 participants treated, 332 (17.4%) experienced 1 or more events.
Thirty‐four RCTs reported the number of participants who withdrew due to adverse events; this was, in total, 79 (3.6%) out of 2143 treated.
There was a trend for minocycline to be associated with more adverse effects than placebo (RR 1.25, 95% CI 0.95 to 1.65, I² statistic = 24%) (Analysis 22.1.1) and higher‐dose minocycline more than lower‐dose (Analysis 22.1). Meta‐analysis indicated that the rates of adverse effects in minocycline‐treated participants were less than in those who were treated with (oxy)tetracycline (RR 0.73, 95% CI 0.53 to 1.01, I² statistic = 28%) (Analysis 22.1.5). However, a sensitivity analysis that removed the 4 'open'‐label studies (in which the reporting could have been influenced by the participants' and investigators' knowledge of treatment assignment) removed this difference (RR 1.07, 95% CI 0.59 to 1.95, I² statistic = 34%) (Analysis 22.1.6). The largest study (Ozolins 2005) did not report data on overall adverse effects in a comparable format. The only other statistically significant differences identified were that minocycline‐treated participants experienced fewer adverse effects than those receiving zinc, as shown in Dreno 2001 (RR 0.63, 95% CI 0.44 to 0.91) (Analysis 22.1.15). Minocycline alone or in combination with azelaic acid also produced fewer side‐effects than isotretinoin, as shown in Pigatto 1986 (RR 0.60, 95% CI 0.37 to 0.97) (Analysis 22.1.21) and Gollnick 1997 (RR 0.55, 95% CI 0.35 to 0.85) (Analysis 22.1.20). The results for those adverse effects necessitating treatment withdrawal when being treated with minocycline were similar to those for all adverse effects. Minocycline was associated with more withdrawals, due to adverse effects, than placebo (risk difference (RD) 0.08, 95% CI 0.03 to 0.13, I² statistic = 0%) (Analysis 22.2.1) and less than (oxy)tetracycline (RD ‐0.03, 95% CI ‐0.06 to ‐0.00, I² statistic = 44% ) (Analysis 22.2.8). There were no other notable differences (Analysis 22.2).
22.1. Analysis.
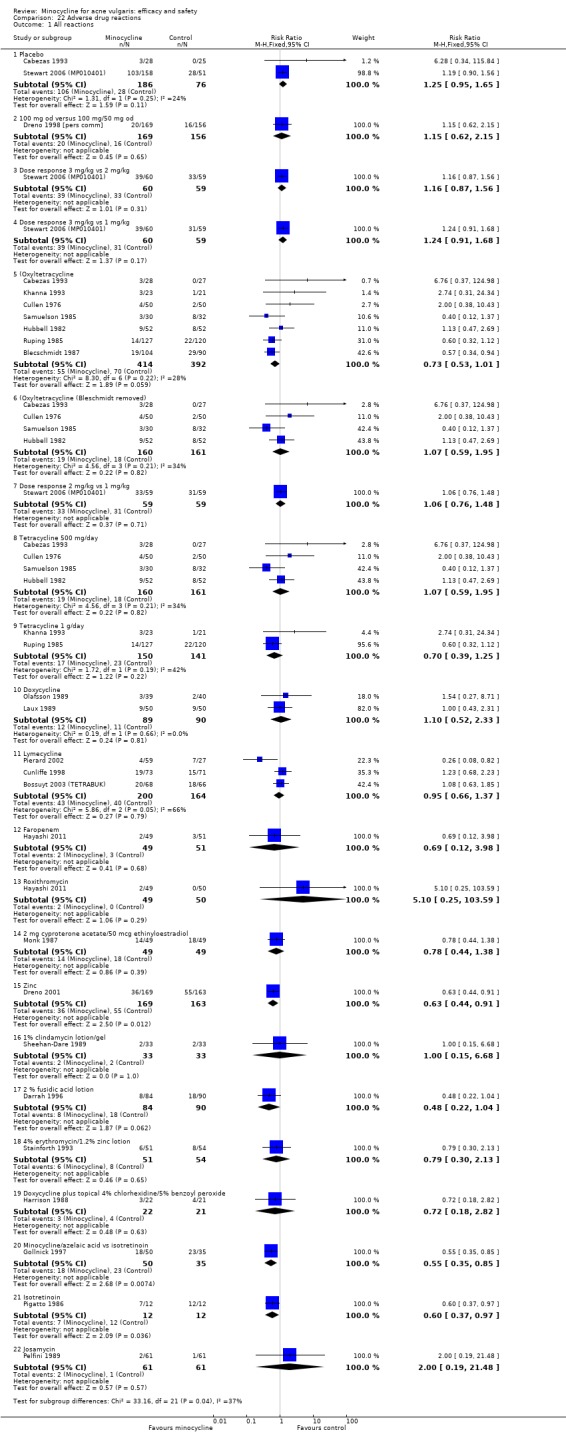
Comparison 22 Adverse drug reactions, Outcome 1 All reactions.
22.2. Analysis.
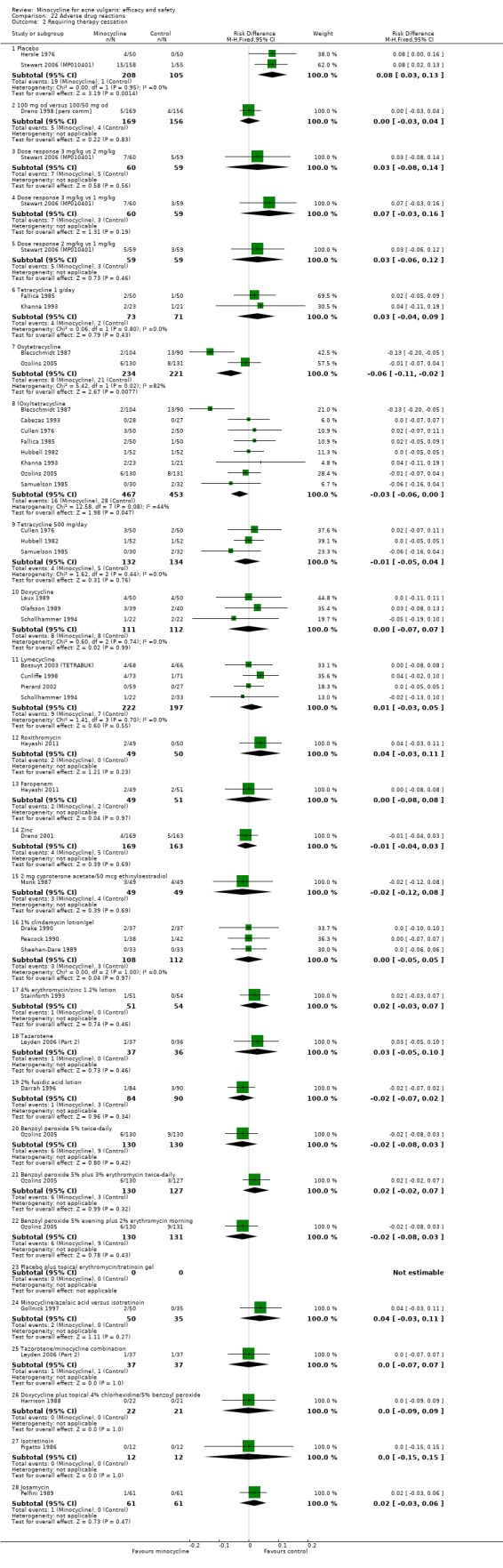
Comparison 22 Adverse drug reactions, Outcome 2 Requiring therapy cessation.
The focus of the 233‐participant phase 2 study, Stewart 2006 (MP010401), was on acute vestibular adverse effects, which are hypothesised as being dose‐related. The researchers defined vestibular events as one or more of the following symptoms: nausea, vomiting, dizziness, vertigo, and ringing in the ears; the symptoms being attributed to the rapid rise in serum minocycline levels with standard minocycline. The authors reported that the results of the trial indicated this was dose‐dependent (10%, 24%, and 28% in the 1, 2, and 3 mg/kg minocycline groups, respectively, compared to 16% in the placebo group). These differences were non‐significant statistically, although the study may not have been powered adequately. However, it is not clear whether the symptoms included in the composite measure were actually due to vestibular effects; nausea and vomiting were common symptoms. These adverse effects were more common in the first five days of treatment and in the heaviest participants. In summary, the data reported do not support the study authors conclusions that "the key benefit of this new minocycline preparation is safety", because no standard‐release formulation was included as a comparison.
b) Results from other studies of adverse events
It is recognised that rare adverse effects are unlikely to occur in clinical trials involving relatively small numbers of participants with short follow‐up periods. Estimates of the frequency of such events cannot be obtained by pooling data from several small trials. In addition, spontaneous report systems and case reports are not reliable sources; the number of adverse effects is uncertain because of selective reporting, and the number of participants who received the therapy overall is not known. Therefore, information on the incidence of the less common and more severe adverse effects associated with minocycline in any condition was sought from controlled studies that provided a clear indication on the numerator (i.e. the number of adverse effects) and the denominator (the number of participants treated). These studies are subject to different biases than RCTs.
The results of the 16 studies that met the inclusion criteria for the review of adverse effects are summarised in Table 25.
The notable findings of the studies were as follows.
FDA data on prescription rates and spontaneous reports of adverse effects estimated that rates of any adverse effect is 13 per million with doxycycline and 72 per million with minocycline. Gastro‐intestinal reactions were the most common with doxycycline, whereas changes affecting the central nervous system and gastro‐intestinal disturbances were more common with minocycline (Smith 2005).
The proportion of severe adverse effects were higher with minocycline than doxycycline (Lebrun‐Vignes 2012) (29.5% of events versus 19.5%).
The most common adverse effects of minocycline were cutaneous disorders (42%) and neurological disorders (12.5%) (Lebrun‐Vignes 2012).
Hypersensitivity reactions were more common with minocycline compared to doxycyline (4% versus 1.6%) (Lebrun‐Vignes 2012).
The minocycline‐associated pigmentation in rheumatoid arthritis participants seemed to increase with age (Fay 2008).
The incidence of adverse effects was greater in women compared to men (13.5% compared to 7.5%) (Goulden 1997).
The incidence of adverse effects was greater in those over the age of 35 (27% compared to 11.8%) (Goulden 1997).
The incidence of adverse effects did not seem to rise significantly with dose, with the exception of pigmentation (Goulden 1997).
There were two different types of liver damage associated with minocycline: hypersensitivity with rapid onset (usually within one month) and autoimmune hepatitis generally after a year or more of therapy (more common in women). The authors noted that they could not make any recommendations about whether or not participants should have routine liver function monitoring (Lawrenson 2000).
There were 51 cases of lupus (0.05% of acne cohort of 97,694 participants); of these, 24 had been exposed to minocycline (Margolis 2007). The hazard ratio (HR) for association of minocycline and lupus erythematosus (LE) was 2.64 (95% CI 1.51 to 4.66) and when adjusted for age and gender, the hazard ratio was 3.11 (95% CI 1.77 to 5.48). A strong relationship between the duration of exposure and LE was noted, but cases have still occurred with exposures of less than six months. The frequency of LE in people treated with minocycline was estimated at 8.8 cases per 100,000 person‐years (Margolis 2007).
Antineutrophil antibody (ANA) positivity was seen in participants with acne, irrespective of exposure to minocycline. However, antineutrophil cytoplasmic antibody positivity appeared to be a serological marker for developing autoimmune disease in participants receiving minocycline (Marzo‐Ortega 2007).
Minocycline was associated with a greater risk of lupus than controls with an OR of 4.23 (95% CI 1.03 to 42.74) (Schoonen 2010).
There was an 8.5 fold greater risk of lupus‐like syndrome in young women currently using minocycline for acne compared with non‐users or past users, and this effect is strongest for longer‐term use. The absolute risk of lupus‐like syndrome is 52.8 cases per 100,000 prescriptions, and minocycline increases the risk 8.5 times (95% CI 2.1 to 35) compared to other tetracyclines, which carry a risk of 1.7 (95% CI 0.4 to 8.1) (Sturkenboom 1999).
The adjusted odds ratio of liver dysfunction associated with exposure to minocycline compared with non‐use was 2.10 (95% CI 1.30 to 3.40), and for oxytetracycline/tetracycline it was 1.46 (95% CI 0.81 to 2.64). Overall, the incidence of liver dysfunction was rare: 1.04 cases/10,000 exposed person months (EPM) for minocycline and 0.69 cases/10,000 EPM in those exposed to oxytetracycline/tetracycline (RR 1.51, 95% CI 0.63 to 3.65) (Seaman 2001).
Recommendations were as follows:
periodic liver function tests and ANA tests should be performed on those receiving long‐term minocycline therapy (Angulo 1998; Schlienger 2000); and
since lupus‐like syndrome is uncommon and reversible after stopping minocycline treatment, the increased risk associated with minocycline use only moderately affects the risk/benefit balance (Sturkenboom 1999).
Discussion
Summary of main results
Clinical efficacy
The 39 RCTs that were included in this updated systematic review demonstrated that minocycline is active against both inflamed and non‐inflamed lesions, although there were large variations between trials in both the absolute and percentage decreases attained. Per cent reductions in lesion counts were the most frequently‐used outcome measure.
The most robust data from the 6 placebo‐controlled trials indicated that minocycline is more active than placebo against inflamed lesions, producing a 45.5% reduction compared to 32% after 12 weeks but with large in‐group variations in response (Analysis 2.1). There is no evidence to suggest this is a clinically meaningful difference or whether participants were more satisfied with their treatment. The effect against non‐inflamed lesions was smaller (14.9% versus 6.3%; no standard deviations were reported). There was no difference in the number of participants ascertained to be 'clear' or 'almost clear' at the end of 12 weeks of treatment (Analysis 2.4).
There is no robust evidence to indicate whether or not the effects of minocycline are dose‐dependent; it is likely that all three of the RCTs that looked at this issue contained insufficient numbers of participants. However, one indicated that after 8 weeks of therapy, there was no difference between a 100 mg a day dose compared to 100 mg a day for 2 weeks, followed by 50 mg a day.
The most robust data from an independently‐funded RCT in UK general practice (Ozolins 2005) suggested that, when used to treat facial acne, minocycline produces similar results to oxytetracycline, benzoyl peroxide, and combination treatment with erythromycin/benzoyl peroxide. Out of the nine RCTs that compared minocycline to a first‐generation tetracycline, five did not find any difference in efficacy, but did not contain sufficient numbers of participants to conclude that there is no difference. The most robust study (Ozolins 2005) found minocycline had a greater effect than oxytetracycline against inflamed lesions at 12 weeks, but not 18 weeks (Analysis 5.2). Three other studies found minocycline to be superior, but all had serious flaws (Blecschmidt 1987; Cabezas 1993; Ruping 1985). Seven of the nine RCTs that compared minocycline to other second‐generation tetracyclines had serious methodological flaws. None of these trials found any difference between the treatments, but all but two (Bossuyt 2003 (TETRABUK); Cunliffe 1998) were unlikely to contain sufficient numbers of participants. The former was a non‐inferiority design. Minocycline was compared to three other non‐tetracycline antibiotics (roxithromycin, faropenem, and josamycin) that are not licensed in the UK or U.S. As with the tetracycline RCTS, all had methodological flaws. There is some evidence to suggest that minocycline may have a more rapid onset of action than low‐dose (500 mg/day) tetracycline; further evaluation is required, including the clinical significance of this.
Only three other studies were able to detect any difference between treatments; minocycline was found to have greater activity against inflammatory lesions than 2% topical fusidic acid (Darrah 1996) and oral zinc gluconate (Dreno 2001). It was also superior to zinc against non‐inflamed lesions (Dreno 2001). Minocycline was found to be inferior to a topically applied combination of 4% erythromycin and 1.2% zinc against both inflamed and non‐inflamed lesions (Stainforth 1993), although the mean lesion counts did not change in the minocycline group after the second week. Another finding of note that warrants further evaluation is that a minocycline/azelaic acid combination may produce good results in nodular acne.
It is now widely‐accepted that tetracycline and erythromycin should be given for acne in full therapeutic doses, and yet minocycline and doxycycline are still given at lower doses (usually one half the full therapeutic dose). It is surprising that no adequate dose‐response study has been done to confirm that doses of 200 mg and 100 mg are equivalent in terms of clinical efficacy. Therefore, no recommendations can be made concerning the appropriate dose of minocycline that should be used.
The efficacy of minocycline in tetracycline‐recalcitrant acne cannot be confirmed or refuted as no RCT was located, and similarly, no evaluation of the rate of relapse of participants treated with minocycline was retrieved. Only three studies attempted any measure of the impact of treatment of participant quality of life (Dreno 1998 [pers comm]; Ozolins 2005; Peacock 1990). There was no indication from the trials as to whether minocycline was more acceptable to participants than other forms of acne therapy, and the five trials that monitored compliance did not report the results. It is important that some measure of compliance is included in clinical trials as differences between treatment and control groups can seriously distort the outcome, and where compliance is poor, the sample size will have to be increased to detect the true treatment effect. In the case of acne therapy, compliance and acceptability are important issues and will impact on the clinical results seen.
There is one very important issue that this review has not addressed and that is the emerging problem of antibiotic resistance inP. acnes. Available data suggests that up to one in four antibiotic‐treated acne participants are colonised by tetracycline‐resistant strains of propionibacteria (Coates 1999). The resistant strains may or may not show decreased sensitivity to minocycline (Eady 1993). However, the minimum inhibitory concentration of minocycline for all of them is within the National Committee for Clinical Laboratory Standards guidelines for sensitive strains (less than or equal to 4 mg/ml), and all could potentially be inhibited by serum levels of the drug achieved on a higher dose of 200 mg. The question therefore arises as to whether minocycline is the drug of choice when tetracycline‐resistant strains are present on the skin.
Adverse effects
Prescribers should inform people that there are extremely rare cases of hypersensitivity to minocycline and tell them what signs and symptoms to look out for. If hypersensitivity occurs, it may be fatal, so medical help should be obtained immediately.
Despite the large volume of data collected, it is still not possible to produce a reliable estimate of the likelihood of experiencing an adverse effect during a course of minocycline for acne. Moreover, it is not possible to predict who might be at increased risk of a serious adverse event on the basis of age, gender, pre‐existing health conditions, or dose or duration of minocycline therapy (except in the case of lupus and skin pigmentation ‐ see below).
The most robust study that used spontaneous adverse effect reports coupled with sales data suggested that the overall rate of adverse effects is extremely low at 72 per million people treated with minocycline (0.0072%), but is higher than for doxycycline (Smith 2005). This is likely to be a gross underestimate as there is no mandate for clinicians, pharmacists, or people to report adverse effects when receiving therapy; reporting is done on a voluntary basis. The inadequacy of this system is highlighted by the fact that 332 of the 1906 (17.4%) participants receiving minocycline in the 39 included RCTs reported an adverse effect, a rate that is over 2400 times higher.
The studies do support a conclusion of an increased risk of lupus associated with minocycline that is not seen with other tetracyclines, and which increases with duration of treatment (Margolis 2007; Marzo‐Ortega 2007; Schoonen 2010; Sturkenboom 1999). It should however be noted that the absolute risk is small: One study estimated it to be 52.8 cases per 100,000 prescriptions (Sturkenboom 1999). Similarly, there is an increase in the risk of liver dysfunction associated with minocycline use, but the incidence is rare: 1.04 cases/10,000 exposed person months (Seaman 2001). This would support monitoring and periodic liver function tests and ANA tests in those receiving long‐term treatment.
Relating to the extended‐release version of minocycline, the RCT data reported do not support the conclusions that "the key benefit of this new minocycline preparation is safety", because no standard‐release formulation was included as a comparison, and the placebo‐controlled trial was likely to be underpowered (Fleisch 2006a (MP010404); Fleisch 2006b (MP010405); Stewart 2006 (MP010401)).
Overall completeness and applicability of evidence
Systematic reviewing is a retrospective study, and the conclusions are therefore dependent on the primary studies that have actually been conducted, are successfully identified, and then included. In order to prevent any bias arising from the inherent observational nature of the review, a strict systematic review protocol was developed prior to the onset of the review. This was not published as a Cochrane Protocol as this was not a requirement when the review was initially undertaken.
Two authors independently assessed each study. An exhaustive search was conducted and successfully located three unpublished RCTs, Cunliffe 1998 (which has since been published) and Drake 1990 and Dreno 1998 [pers comm], all of which met the inclusion criteria for the review. It is unlikely that any publication bias existed, as the majority of the studies failed to find any differences between the comparators. And any positive study, in either direction, would probably have been widely‐publicised and cited in the retrieved studies. Language bias was avoided by inclusion of any RCT regardless of language of publication.
In many cases, the individual study results were not analysed by intention‐to‐treat, and therefore sensitivity analysis was used to compare the results obtained from efficacy analysis, intention‐to‐treat, or when dropouts were treated as non‐responders. In some cases where categorical outcomes were used, the results had to be dichotomised; therefore, sensitivity analyses were used to validate these assumptions by examining the effect that different cut‐off points had on the overall results. In no case were these different analyses found to affect the outcome of any study. Similarly, no study that used both intention‐to‐treat analysis and per‐protocol/efficacy analysis found any difference in outcome.
Quality of the evidence
Please see Figure 1.
1.
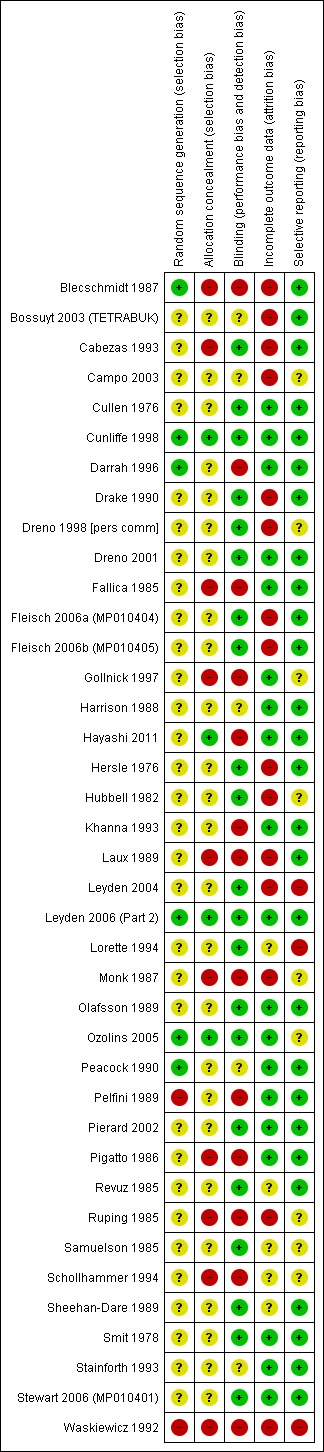
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The objective of this review was to evaluate the efficacy and safety of minocycline in acne vulgaris by systematically reviewing the evidence from randomised controlled trials (RCTs). The intention was to pool the results of individual trials to produce overall summary measures of effect. In practice, this was hard to do because the internal validity of most of the studies was severely compromised on account of inadequate design. As well as the use of many disparate outcome measures, there were numerous methodological differences between the retrieved studies that could not be reconciled. This meant that pooling was not appropriate. In addition, the methodological reporting in many of the trial reports was poor, and as a result, the legitimacy of their conclusions could not be properly evaluated.
Only 1 trial conclusively stated funding sources independent from any sponsor (Ozolins 2005): 28 cited industry‐sponsorship, and 10 made no declaration.
Most of the trialists made some attempt to show that the different treatment groups were comparable at baseline. However, a variety of criteria were used, and some of them were of questionable validity in this context (e.g. height, age of onset of acne). The following criteria were used by a majority of trialists: age, gender, lesion count, or lesion score. In addition, a minority of trialists used weight, duration of acne, and acne grade/severity.
Most trialists specified how long previous acne treatments or other therapies that might have affected acne severity, should have been stopped prior to entry in the trial. However, there was absolutely no consensus on how long this should be. The specified wash‐out period for antibiotics was as short as 48 hours (Revuz 1985) and up to 4 months (Hubbell 1982). For retinoids and hormonal therapy, wash‐out periods varied from 14 days (Peacock 1990) to 1 year (Darrah 1996). Some studies used similar wash‐out periods for oral and topical acne therapy (Samuelson 1985; Sheehan‐Dare 1989); others used shorter periods for topical therapy (Drake 1990; Lorette 1994). Approximately a third of reports mentioned that the trial was not conducted during the summer to avoid the beneficial/camouflaging effects of ultraviolet light.
Concomitant therapy that might affect acne severity was specifically mentioned as being disallowed in nine trials; three others mentioned that no prescribed topical therapy was permitted. It was unclear in the majority of cases whether participants taking potentially interfering medications were excluded from the per‐protocol analysis or continued to be included in the study. It was not usually possible to tell whether concomitant medications had been recorded. Skin hygiene regimens were standardised, usually by the provision of a simple non‐mediated soap, in seven trials. Two trials (Dreno 1998 [pers comm]; Ozolins 2005) additionally provided a moisturiser. Compliance is a significant problem in acne, and only a minority of the authors indicated what instructions were given to participants. Only five trials apparently included some form of compliance monitoring. Of the studies for which details were available, in one instance, medication was taken under supervision (Samuelson 1985); two studies counted unused medication (Cunliffe 1998; Peacock 1990); and two studies used a participant‐completed diary card (Ozolins 2005; Stainforth 1993).
In some cases, minocycline was taken on an empty stomach, in others before, with, or after a meal. Six reports mentioned that antacids were among the non‐permitted medications, and only four specified exclusion of participants taking divalent metal ions (three iron and one zinc).
Potential biases in the review process
The limitations of this review stemmed from the methodological insufficiencies and the subsequent heterogeneity in the primary studies, and the inadequacies of the reported data. The studies generally included insufficient numbers of participants, and the majority were only of 12 weeks duration, so assumptions could not be made about the impact of longer‐term therapy. Although additional data were obtained from several authors, the manufacturers of minocycline, who sponsored many of the studies, failed to provide any of the requested information, despite an initial agreement (Brock 1998 [pers comm]). Subgroup analysis was also impossible due to the poor characterisation of participant groups and lack of adequate outcome data. It was also not possible to examine the impact of study design on the results, particularly with reference to the degree of blinding, as many studies were inconclusive.
Authors' conclusions
Implications for practice.
The additional 12 RCTs that were located for the update have not changed the original conclusions about the clinical efficacy of minocycline. The 39 RCTs now included in this review do not provide any evidence to support the first‐line use of minocycline in the treatment of acne. Although it has been shown to be an effective treatment for moderate to moderately‐severe acne vulgaris at a dose of 100 mg per day, no study has conclusively shown any important clinical difference between minocycline and other commonly‐used therapies. Meta‐analysis indicated that minocycline may have a more rapid onset of action than tetracycline or oxytetracycline, but overall efficacy in the longer‐term is similar. There is no evidence that minocycline is more effective in acne resistant to other therapies, or that it has a more prolonged effect. Insufficient information was found to make any recommendations concerning the appropriate dose that should be used.
The relative safeties of the tetracyclines have still not been adequately determined, and little further information could be derived from the included RCTs because of their inherent inability to detect rare events. Recent reviews of case reports and case series (Gough 1996; Shapiro 1997) suggest that minocycline therapy for acne may be associated with a broader spectrum and a higher incidence of severe adverse effects than other tetracyclines. The lack of a denominator in nearly all of the studies means that the risks for minocycline compared to other tetracyclines cannot be compared. Only in the case of lupus‐like syndrome has it been conclusively shown that acne participants treated with minocycline are at a significantly greater risk than those given tetracycline or no treatment (Sturkenboom 1999). The risk of developing pigmentation (which can be irreversible) and lupus‐like syndrome increases with cumulative dose.
Implications for research.
The poor methodological quality of the acne trials was highlighted in the original version of this review. This was also the case for many of the trials identified in this update, with a few notable exceptions. In order to enable comparison of acne treatment, either directly or indirectly through modelling, an agreed set of core outcome measures should be developed. Until this has been done, trialists are encouraged to include lesion counts and quality of life as outcome measures.
It is surprising that very basic information about acne therapy with the tetracycline group of antibiotics is still unavailable.
What's new
| Date | Event | Description |
|---|---|---|
| 19 July 2013 | Amended | The abbreviation "qd" was replaced with the proper term 'four times a day', as requested by the Cochrane Editorial Unit. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 2 August 2012 | Amended | Published note added |
| 6 July 2012 | New search has been performed | New search for studies |
| 6 July 2012 | New citation required but conclusions have not changed | A substantial amount of new information has been added in the form of 12 newly‐included studies. |
| 30 September 2009 | Amended | Converted to new review format. |
| 1 November 2006 | New citation required and conclusions have changed | Substantive amendment |
| 18 November 2002 | Amended | New studies found and included or excluded. |
| 1 June 2000 | Amended | Minor update. |
Notes
The views expressed here are those of the authors and do not necessarily represent the views or official positions of the institutions they are employed at.
Acknowledgements
We would like to thank the following people and companies for providing unpublished or additional information: Professor WJ Cunliffe (Cunliffe 1998); Professor B Dréno (Dreno 1998 [pers comm]); Dr N Khanna (Khanna 1993); Laboratoires Galderma (Cunliffe 1998); Leo Laboratories Ltd (Darrah 1996); Pharmacia & Upjohn Ltd (Drake 1990); Pfizer (Harrison 1988); and Yamanouchi (Stainforth 1993).
The authors are grateful to the UK's National Institute for Health Research for providing a grant under the 2012 Cochrane Review Incentive Scheme to facilitate the update of this review.
The Cochrane Skin Group editorial base wishes to thank Hywel Williams, who was the Key Editor for the 2012 update of this review; Jo Leonardi‐Bee, who was the Statistical Editor; and Olivier Chosidow, who was the clinical referee.
Appendices
Appendix 1. Skin Group Specialised Register search strategy
(Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin or “Mino‐Tabs” or “aknin‐mino” or “akne‐puren” or “mino wolff” or “mino‐wolff” or “icht oral” or “akne puren” or “icht‐oral” or “aknin mino” or “7 dimethylamino 6 demethyl 6 deoxytetracycline” or “mino‐50”) AND acne
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor Acne Vulgaris explode all trees #2 (acne):ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Minocycline explode all trees #5 (Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin or "Mino‐Tabs" or "aknin‐mino" or "akne‐puren" or "mino wolff" or "mino‐wolff" or "icht oral" or "akne puren" or "icht‐oral" or "aknin mino" or "7 dimethylamino 6 demethyl 6 deoxytetracycline" or "mino‐50") #6 (#4 OR #5) #7 (#3 AND #6)
Appendix 3. MEDLINE (OVID) search strategy
1. exp Minocycline/ 2. (Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin).mp. 3. (Mino‐Tabs or aknin‐mino or akne‐puren or mino wolff or mino‐wolff or icht oral or akne puren or icht‐oral or aknin mino or mino‐50).mp. 4. "7 dimethylamino 6 demethyl 6 deoxytetracycline".mp. 5. 10118‐90‐8.rn. 6. 1 or 2 or 3 or 4 or 5 7. acne.mp. or exp Acne Vulgaris/ 8. randomized controlled trial.pt. 9. controlled clinical trial.pt. 10. randomized.ab. 11. placebo.ab. 12. clinical trials as topic.sh. 13. randomly.ab. 14. trial.ti. 15. 8 or 9 or 10 or 11 or 12 or 13 or 14 16. (animals not (human and animals)).sh. 17. 15 not 16 18. 6 and 7 and 17
Appendix 4. EMBASE (OVID) search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp acne vulgaris/ 15. acne.ti,ab. 16. 14 or 15 17. exp minocycline/ 18. 10118‐90‐8.rn. 19. "7 dimethylamino 6 demethyl 6 deoxytetracycline".mp. 20. (Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin).mp. 21. (Mino‐Tabs or aknin‐mino or akne‐puren or mino wolff or mino‐wolff or icht oral or akne puren or icht‐oral or aknin mino or mino‐50).mp. 22. 17 or 18 or 19 or 20 or 21 23. 13 and 16 and 22
Appendix 5. LILACS search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Words] and acne [Words] and “Mino‐Tabs” or “aknin‐mino” or “akne‐puren” or “mino wolff” or “mino‐wolff” or “icht oral” or “akne puren” or “icht‐oral” or “aknin mino” or “7 dimethylamino 6 demethyl 6 deoxytetracycline” or “mino‐50” or Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin [Words]
Appendix 6. Adverse effects search strategy EMBASE (OVID)
1. side effect$.ti,ab. 2. metabolite$.ti,ab. 3. photoallergic reaction$.ti,ab. 4. phototoxicit$.ti,ab. 5. (sensitization or sensitisation).ti,ab. 6. stinging.ti,ab. 7. burning.ti,ab. 8. fetal abnormalit$.ti,ab. 9. (toxic effect$ or drug effect$).ti,ab. 10. (safe or safety).ti,ab. 11. toxicity.ti,ab. 12. noxious.ti,ab. 13. complication$.ti,ab. 14. tolerability.ti,ab. 15. treatment emergent.ti,ab. 16. tolerability.ti,ab. 17. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab. 18. rebound.ti,ab. 19. skin thinning.ti,ab. 20. lupus induced hepatitis.ti,ab. 21. exp postmarketing surveillance/ 22. exp drug surveillance program/ 23. exp drug hypersensitivity/ or exp hypersensitivity reaction/ or exp delayed hypersensitivity/ or exp hypersensitivity/ or exp immediate type hypersensitivity/ 24. exp drug eruption/ 25. exp anaphylaxis/ 26. exp allergic conjunctivitis/ 27. exp atopic dermatitis/ 28. exp food allergy/ 29. exp respiratory tract allergy/ 30. exp urticaria/ 31. exp intoxication/ 32. exp toxic hepatitis/ 33. exp addiction/ 34. exp drug toxicity/ 35. exp teratogenic agent/ 36. exp mutagenic agent/ 37. exp carcinogen/ 38. exp contact dermatitis/ 39. exp skin allergy/ 40. exp irritant dermatitis/ 41. exp phototoxicity/ 42. exp photodermatosis/ or exp photoallergy/ 43. exp burning mouth syndrome/ 44. exp drug monitoring/ 45. exp sleep apnea syndrome/ 46. exp heart arrhythmia/ 47. hypercalcemia/ 48. urolithiasis/ 49. tachyphylaxis/ 50. withdrawal syndrome/ 51. atrophy/ 52. telangiectasia/ 53. liver disease/ 54. kidney disease/ 55. disseminated intravascular clotting/ 56. multiple organ failure/ 57. Stevens Johnson syndrome/ 58. toxic epidermal necrolysis/ 59. heart block/ 60. coma/ 61. paralysis/ 62. nausea/ 63. vomiting/ 64. benign intracranial hypertension.ti,ab. or exp brain pseudotumor/ 65. exp pigment disorder/ 66. exp pigmentation/ 67. pigmentation.ti,ab. 68. exp adverse drug reaction/ 69. exp drug safety/ 70. exp phase 4 clinical trial/ 71. (ae or to).fs. 72. exp minocycline/ 73. 10118‐90‐8.ti,ab. 74. "7 dimethylamino 6 demethyl 6 deoxytetracycline".ti,ab. 75. (Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin).ti,ab. 76. (Mino‐Tabs or aknin‐mino or akne‐puren or mino wolff or mino‐wolff or icht oral or akne puren or icht‐oral or aknin mino or mino‐50).ti,ab. 77. or/72‐76 78. 71 and 77 79. or/1‐70 80. 77 and 79 81. 78 or 80 82. limit 81 to human
Appendix 7. Adverse effects search strategy MEDLINE (OVID)
1. exp product surveillance, postmarketing/ or exp adverse drug reaction reporting systems/ or exp clinical trials, phase iv/ 2. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab. 3. exp hypersensitivity/ or exp drug hypersensitivity/ or exp drug eruptions/ or exp hypersensitivity, delayed/ or exp hypersensitivity, immediate/ 4. exp anaphylaxis/ or exp conjunctivitis, allergic/ or exp dermatitis, atopic/ or exp food hypersensitivity/ or exp respiratory hypersensitivity/ or exp urticaria/ 5. side effect$.ti,ab. 6. exp Poisoning/ 7. exp hepatitis, toxic/ or exp hepatitis, chronic, drug‐induced/ 8. exp Substance‐Related Disorders/ 9. exp Drug Toxicity/ 10. exp Abnormalities, Drug‐Induced/ 11. exp Teratogens/ 12. exp Mutagens/ 13. exp Carcinogens/ 14. metabolite$.ti,ab. 15. exp dermatitis, contact/ or exp dermatitis, allergic contact/ or exp dermatitis, irritant/ or exp dermatitis, phototoxic/ 16. photoallergic reaction$.ti,ab. 17. exp dermatitis, allergic contact/ or exp dermatitis, photoallergic/ 18. phototoxicit$.ti,ab. 19. (sensitization or sensitisation).ti,ab. 20. exp Burning Mouth Syndrome/ 21. stinging.ti,ab. 22. burning.ti,ab. 23. fetal abnormalit$.ti,ab. 24. exp Drug Monitoring/ 25. drug effect$.ti,ab. 26. Sleep Apnea, Obstructive/ 27. ARRHYTHMIA/ 28. (safe or safety).ti,ab. 29. toxicity.ti,ab. 30. noxious.ti,ab. 31. complication$.ti,ab. 32. treatment emergent.ti,ab. 33. tolerability.ti,ab. 34. rebound.ti,ab. 35. Hypercalcemia/ci [Chemically Induced] 36. Urinary Calculi/ci [Chemically Induced] 37. Tachyphylaxis/ci, de [Chemically Induced, Drug Effects] 38. Substance Withdrawal Syndrome/ci, de [Chemically Induced, Drug Effects] 39. ATROPHY/ci [Chemically Induced] 40. TELANGIECTASIS/ci [Chemically Induced] 41. skin thinning.ti,ab. 42. Liver Diseases/ci [Chemically Induced] 43. Kidney Diseases/ci [Chemically Induced] 44. Disseminated Intravascular Coagulation/ci [Chemically Induced] 45. Multiple Organ Failure/ci [Chemically Induced] 46. Stevens‐Johnson Syndrome/ci [Chemically Induced] 47. Epidermal Necrolysis, Toxic/ci [Chemically Induced] 48. Heart Block/ci [Chemically Induced] 49. COMA/ci [Chemically Induced] 50. PARALYSIS/ci [Chemically Induced] 51. exp Nausea/ 52. exp Vomiting/ 53. benign intracranial hypertension.ti,ab. or exp Pseudotumor Cerebri/ 54. exp Pigmentation Disorders/ or pigmentation.ti,ab. or exp Pigmentation/ 55. lupus induced hepatitis.ti,ab. 56. or/1‐55 57. (Minomycin or Akamin or Minocin or Minoderm or Cyclimycin or Aknemin or Solodyn or Dynacin or Sebomin or Acnamino or Minopen or Maracyn or minocycline or cyclomin or mynocine or mestacine or arestin or minakne or minox or lederderm or minoplus or blemix or dentomycin or minotab or minolis or Cyclops or aknosan or minoclir or klinomycin or Minocyclin or Minocyclinum or Minocyklin or Minocyklina or Minosiklin or Minosykliini or Minociclina or borymycin or cynomycin or lederderm or logryx or menocycline or mestacine or micromycin or minaxen or Minoclin or minoclir or minocyn or minogalen or minoline or minomax or mirosin or mynocine or romin or skinocyclin or spicline or vectran or vectrin).ti,ab. 58. exp Minocycline/ 59. (Mino‐Tabs or aknin‐mino or akne‐puren or mino wolff or mino‐wolff or icht oral or akne puren or icht‐oral or aknin mino or mino‐50).ti,ab. 60. "7 dimethylamino 6 demethyl 6 deoxytetracycline".ti,ab. 61. 10118‐90‐8.rn. 62. or/57‐61 63. 56 and 62 64. ae.fs. 65. to.fs. 66. co.fs. 67. po.fs. 68. or/64‐67 69. 62 and 68 70. 63 or 69 71. limit 70 to humans
Data and analyses
Comparison 1. Minocycline 100 mg bd versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inflamed lesion count ‐ percentage change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Week 12 ‐ per‐protocol ‐ inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Minocycline ER versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage change in inflamed lesion counts | 3 | 1038 | Mean Difference (IV, Fixed, 95% CI) | 13.43 [7.10, 19.76] |
| 2 Percentage change in total lesion counts | 3 | 1038 | Mean Difference (IV, Fixed, 95% CI) | 9.84 [4.84, 14.84] |
| 3 Investigator global severity ‐ successful treatment | 2 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.27, 2.84] |
| 4 Clear or almost clear 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 3 mg versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 2 mg versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 1 mg versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pooled | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Minocycline ER dose response.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clear or almost clear 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 3 mg/kg versus 2 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3 mg versus 1 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 mg versus 1 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Minocycline 100 mg od versus 100 mg/50 mg od.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lesion counts ‐ reduction from baseline after 60 days therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Non‐inflamed: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐inflamed lesions: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Inflamed lesions: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Inflamed lesions: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Total lesions: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Total lesions: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall clinical improvement ‐ dr‐assessed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Any improvement: intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Any improvement: per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Any improvement: worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Moderate or excellent improvement: intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Moderate or excellent improvement: per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Moderate or excellent improvement: worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participant evaluations ‐ 10 cm visual analogue scales | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Overall efficacy: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Overall efficacy: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Importance of acne ‐ change from baseline: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Importance of acne ‐ change from baseline: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Impact on relationships ‐ change from baseline: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Impact on relationships ‐ change from baseline: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Impact on sexual relationships ‐ change from baseline: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Impact on sexual relationships ‐ change from baseline: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Impact on physical appearance ‐ change from baseline: intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Impact on physical appearance ‐ change from baseline: per‐protocol analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Minocycline 50 mg bd/100 mg od versus (oxy)tetracycline 250 mg 4 times a day/bd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cook grading scale ‐ number of participants improved by at least two grades | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Week 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Week 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Week 12 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Decrease in inflamed lesion count from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Week 18 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Week 18: least squares mean adjusted | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 At least moderate improvement according to participant | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 At least moderate improvement according to assessor | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Samuelson lesion grade as assessed by physician | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Samuelson lesion grade as assessed by participant | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of participants converting to Pillsbury grade I | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Week 6: intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Week 6: per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Week 24: intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Week 24: per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Overall improvement | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Week 6: investigator‐assessed | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.04, 1.96] |
| 8.2 Week 12: investigator‐assessed | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 8.3 Week 24: investigator‐assessed | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.79, 2.07] |
| 8.4 Week 12: participant‐assessed | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 8.5 Week 18: satisfactory overall clinical response: participant‐assessed | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 8.6 Week 18: participant‐assessed | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 8.7 Week 18: investigator‐assessed | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 9 Khanna acne lesion score: absolute reduction from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Minocycline versus lymecycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lesion count ‐ absolute change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Inflamed lesions (including nodules) ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Non‐inflamed lesions ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Total lesions ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Lesion count ‐ percentage change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Inflamed lesions (including nodules) ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Non‐inflamed lesions ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Total lesions ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Lesion count ‐ number of participants achieving > 25% reduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 TLC: Week 4 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 TLC: Week 8 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 TLC: Week 12 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 TLC: Week 4 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 TLC: Week 8 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 TLC: Week 12 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 TLC: End point ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 IL : Week 4 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 IL: Week 8 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 IL: Week 12 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 IL: Week 4 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 IL: Week 8 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 IL: Week 12 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 IL: End point ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 NIL: Week 4 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 NIL: Week 8 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 NIL: Week 12 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 NIL: Week 4 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 NIL: Week 8 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 NIL: Week 12 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 NIL: End point ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Lesion count ‐ number of participants achieving > 50% reduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 TLC: Week 4 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 TLC: Week 8 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 TLC: Week 12 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 TLC: Week 4 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 TLC: Week 8 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 TLC: Week 12 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 TLC: End point ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 IL: Week 4 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 IL: Week 8 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 IL: Week 12 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 IL: Week 4 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 IL: Week 8 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 IL: Week 12 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 IL: End point ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 NIL: Week 4 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 NIL: Week 8 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 NIL: Week 12 ‐ per‐protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 NIL: Week 4 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 NIL: Week 8 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 NIL: Week 12 ‐ worse‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 NIL: End point ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Leeds grade | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Absolute change from baseline ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Percentage change from baseline ‐ week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 End point ‐ intention‐to‐treat analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Global assessment ‐ number of participants with overall improvement | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Participant ‐ per‐protocol analysis | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.93, 1.30] |
| 6.2 Participant ‐ intention‐to‐treat analysis | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| 6.3 Participant ‐ assigned worse outcome analysis | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.24] |
| 6.4 Doctor ‐ per‐protocol analysis | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 6.5 Doctor ‐ intention‐to‐treat analysis | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 6.6 Doctor ‐ assigned worse outcome analysis | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.24] |
Comparison 7. Minocycline versus doxycycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with > 50% reduction in IL count | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Global efficacy rating | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Overal efficacy: participant‐assessed | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.28] |
| 2.2 Overal efficacy: investigator‐assessed | 3 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3 Cure | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.49] |
| 4 Other outcome | Other data | No numeric data |
7.1. Analysis.
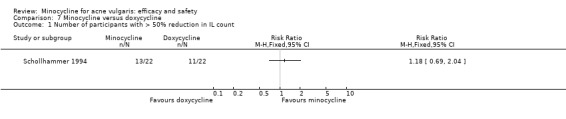
Comparison 7 Minocycline versus doxycycline, Outcome 1 Number of participants with > 50% reduction in IL count.
7.3. Analysis.
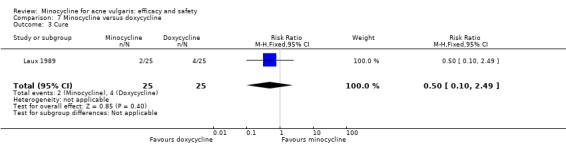
Comparison 7 Minocycline versus doxycycline, Outcome 3 Cure.
7.4. Analysis.
Comparison 7 Minocycline versus doxycycline, Outcome 4 Other outcome.
| Other outcome | ||||
|---|---|---|---|---|
| Study | Outcome | Minocycline | Doxycycline | Inter‐group analysis |
| Lorette 1994 | Per cent reduction in lesion counts from baseline ‐ day 120 | N = 23 Comedones: 77.4% Papules: 69.6% Pustules: 65.5% Score: 68.4% | N = 31 Comedones: 69.3% Papules: 84.7% Pustules: 86.9% Score: 79.4% | Comedones: P = 0.516 (Chi² test); Effect Size = 0.156 Pustules: P = 0.187 (Chi² test); Effect Size = 0.313 Papules: P = 0.064 (Chi² test); Effect Size = 0.44 Score: P = 0.36 (Chi² test); Effect Size = 0.253 |
| Schollhammer 1994 | Week 12 per cent reduction in inflamed lesion count | N = 13 68.4% | N = 11 62.4% | P > 0.05 (test unknown) |
| Waskiewicz 1992 | Per cent reduction in lesion counts from baseline | N = 30 Closed comedones: 67.7% Open comedones: 59.2% Papules: 64.2% Pustules: 76.1% Total lesion count: 66.3% SCORE: 67.1% | N = 30 Closed comedones: 66.7% Open comedones: 67.0% Papules: 65.4% Pustules: 76.8% Total lesion count: 67.7% SCORE: 68.8% | No statistical analysis performed |
Comparison 8. Minocycline 100 mg/200 mg per day versus josamycin 500 mg/1000 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pustules change in P&K severity grade 8 weeks | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.07, 0.53] |
| 2 Nodulo‐cysts change in P&K severity grade 8 weeks | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.20, 0.60] |
| 3 Erythema change in severity score 8 weeks | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.12, 0.48] |
| 4 Seborrhea change in severity score 8 weeks | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.22, 0.58] |
| 5 Evaluation of clinical efficacy 8 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Markedly effective | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Effective | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Moderately effective | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Combined effective or markedly effective | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Ineffective | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Minocycline 50 mg bd versus Diane™ (cyproterone acetate 2 mg/ethinyloestradiol 50 mcg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐subjective evaluation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Improved ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Improved ‐ per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Cleared ‐ intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Cleared ‐ per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Minocycline100 mg bd versus compound A.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inflamed lesion count ‐ percentage change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Week 12 ‐ per‐protocol ‐ inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Minocycline 100 mg daily versus zinc gluconate 30 mg bd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lesion count ‐ percentage change from baseline 90 days | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐16.42 [‐25.10, ‐7.74] |
| 1.1 Inflamed lesion counts ‐ papules pustules | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐16.42 [‐25.10, ‐7.74] |
| 2 Investigator global severity ‐ successful treatment (2/3 reduction in IL) | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.56, 2.63] |
| 3 Overall opinion on efficacy (100 mm VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Clinician‐assessed | 1 | 307 | Mean Difference (IV, Fixed, 95% CI) | 13.70 [7.67, 19.73] |
| 3.2 Participant‐assessed | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | 16.70 [10.68, 22.72] |
Comparison 12. Minocycline 50 mg bd versus clindamycin 1% lotion bd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement ‐ participant‐assessed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 13. Minocycline 50 mg bd versus fusidic acid 2% lotion bd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants achieving > 40% reduction in lesion counts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Total lesion counts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Inflammatory lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Non‐inflammatory lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Any lesion count | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Lesion count changes from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Inflamed lesions ‐ week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 End of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Non‐inflamed lesions ‐ week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 End of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Overall clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Participants with average or greater response ‐ week 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Week 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Week 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 End of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Participants with good or very good response ‐ week 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Week 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Week 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 End of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.3. Analysis.
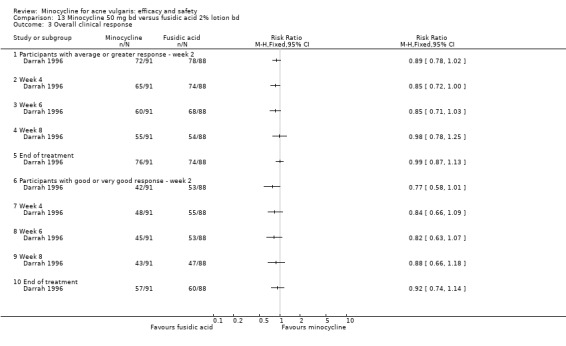
Comparison 13 Minocycline 50 mg bd versus fusidic acid 2% lotion bd, Outcome 3 Overall clinical response.
Comparison 14. Minocycline 50 mg bd versus zineryt bd (erythromycin 4%/zinc 1.2% lotion).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 12‐week lesion count ‐ change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Non‐inflamed week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Inflamed lesions week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 12‐week percentage of baseline lesion counts | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Non‐inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Superficial inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Total inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants attaining > 45% reduction in lesion counts from baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Inflamed week 12 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Non‐inflamed week 12 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Leeds grade ‐ change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Minocycline 100 mg ER od versus benzoyl peroxide bd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement ‐ participant assessed at least moderate improvement at 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Overall improvement ‐ assessor at least moderate improvement at 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lesion count ‐ change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Minocycline 100 mg ER od versus erythromycin/benzoyl peroxide (ery/BP) bd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement ‐ participant assessed at least moderate improvement at 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Overall improvement ‐ assessor at least moderate improvement at 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lesion count ‐ change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Minocycline 100 mg ER od versus erythromycin od/benzoyl peroxide (ery/BP) od.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement ‐ participant assessed at least moderate improvement at 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Overall improvement ‐ assessor at least moderate improvement at 18 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lesion count ‐ change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Inflamed lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Combination with 5% benzoyl peroxide/4% chlorhexidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lesion count | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pustules (active) ‐ actual values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pustules (active) ‐ adjusted values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Papules (active) ‐ actual values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Papules (active) ‐ adjusted values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Total lesion count ‐ actual values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Total lesion count ‐ adjusted values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Lesion score ‐ actual values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Lesion score ‐ adjusted values | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. Minocycline versus placebo plus combination erythromycin/tretinoin gel (strength unspecified).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global grade ‐ good/very good response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Dr‐assessed: 'worse‐case' analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Dr‐assessed: per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Participant‐assessed: 'worse‐case' analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Participant‐assessed: per‐protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 20. Minocycline/azelaic acid (min/AA) versus isotretinoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with good or very good response after 6 months | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.03] |
| 2 Reduction in NIL: percentage change from baseline | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐27.80, ‐0.20] |
| 3 Reduction in IL: percentage change from baseline | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐8.90 [‐17.67, ‐0.13] |
20.1. Analysis.
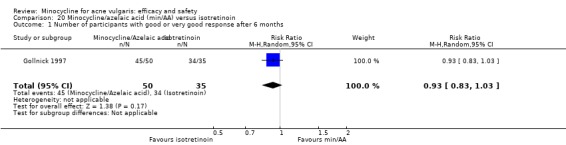
Comparison 20 Minocycline/azelaic acid (min/AA) versus isotretinoin, Outcome 1 Number of participants with good or very good response after 6 months.
Comparison 21. Minocycline 100 mg od maintenance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lesion count ‐ percentage change from baseline versus tazarotene | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 NIL | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 IL | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall disease severity score versus tazarotene | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Overall clinical improvement ‐ Dr‐assessed versus tazarotene | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 < = 50% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 > = 75% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Lesion count ‐ percentage change from baseline versus tazarotene/minocycline combination | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 IL | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 NIL | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Overall disease severity score versus tazarotene/minocycline combination | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Overall clinical improvement ‐ Dr‐assessed versus tazarotene/minocycline combination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 < = 50% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 > = 75% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 22. Adverse drug reactions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All reactions | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Placebo | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.95, 1.65] |
| 1.2 100 mg od versus 100 mg/50 mg od | 1 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.62, 2.15] |
| 1.3 Dose response 3 mg/kg vs 2 mg/kg | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.56] |
| 1.4 Dose response 3 mg/kg vs 1 mg/kg | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.91, 1.68] |
| 1.5 (Oxy)tetracycline | 7 | 806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.01] |
| 1.6 (Oxy)tetracycline (Bleschmidt removed) | 4 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.59, 1.95] |
| 1.7 Dose response 2 mg/kg vs 1 mg/kg | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.48] |
| 1.8 Tetracycline 500 mg/day | 4 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.59, 1.95] |
| 1.9 Tetracycline 1 g/day | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.39, 1.25] |
| 1.10 Doxycycline | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.52, 2.33] |
| 1.11 Lymecycline | 3 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 1.12 Faropenem | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 1.13 Roxithromycin | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.10 [0.25, 103.59] |
| 1.14 2 mg cyproterone acetate/50 mcg ethinyloestradiol | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.44, 1.38] |
| 1.15 Zinc | 1 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.91] |
| 1.16 1% clindamycin lotion/gel | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.68] |
| 1.17 2 % fusidic acid lotion | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.22, 1.04] |
| 1.18 4% erythromycin/1.2% zinc lotion | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.13] |
| 1.19 Doxycycline plus topical 4% chlorhexidine/5% benzoyl peroxide | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.18, 2.82] |
| 1.20 Minocycline/azelaic acid vs isotretinoin | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.85] |
| 1.21 Isotretinoin | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.37, 0.97] |
| 1.22 Josamycin | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.48] |
| 2 Requiring therapy cessation | 30 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Placebo | 2 | 313 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.13] |
| 2.2 100 mg od versus 100/50 mg od | 1 | 325 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.04] |
| 2.3 Dose response 3 mg/kg vs 2 mg/kg | 1 | 119 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.08, 0.14] |
| 2.4 Dose response 3 mg/kg vs 1 mg/kg | 1 | 119 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.03, 0.16] |
| 2.5 Dose response 2 mg/kg vs 1 mg/kg | 1 | 118 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.06, 0.12] |
| 2.6 Tetracycline 1 g/day | 2 | 144 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
| 2.7 Oxytetracycline | 2 | 455 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.06 [‐0.11, ‐0.02] |
| 2.8 (Oxy)tetracycline | 8 | 920 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 2.9 Tetracycline 500 mg/day | 3 | 266 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 2.10 Doxycycline | 3 | 223 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.07, 0.07] |
| 2.11 Lymecycline | 4 | 419 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.12 Roxithromycin | 1 | 99 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.03, 0.11] |
| 2.13 Faropenem | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.08, 0.08] |
| 2.14 Zinc | 1 | 332 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.03] |
| 2.15 2 mg cyproterone acetate/50 mcg ethinyloestradiol | 1 | 98 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 2.16 1% clindamycin lotion/gel | 3 | 220 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 2.17 4% erythromycin/zinc 1.2% lotion | 1 | 105 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 2.18 Tazarotene | 1 | 73 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.05, 0.10] |
| 2.19 2% fusidic acid lotion | 1 | 174 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.02] |
| 2.20 Benzoyl peroxide 5% twice‐daily | 1 | 260 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 2.21 Benzoyl peroxide 5% plus 3% erythromycin twice‐daily | 1 | 257 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.02, 0.07] |
| 2.22 Benzoyl peroxide 5% evening plus 2% erythromycin morning | 1 | 261 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 2.23 Placebo plus topical erythromycin/tretinoin gel | 0 | 0 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.24 Minocycline/azelaic acid versus isotretinoin | 1 | 85 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.03, 0.11] |
| 2.25 Tazorotene/minocycline combination | 1 | 74 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 2.26 Doxycycline plus topical 4% chlorhexidine/5% benzoyl peroxide | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 2.27 Isotretinoin | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.15, 0.15] |
| 2.28 Josamycin | 1 | 122 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.03, 0.06] |
| 3 Gastro‐intestinal disturbances | 19 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Placebo | 2 | 262 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.06] |
| 3.2 100 mg od versus 100 mg/50 mg od | 1 | 325 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.04, 0.05] |
| 3.3 50 mg bd for 4 weeks then 50 mg od for 8 weeks versus 50 mg od for 8 weeks | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.13, 0.06] |
| 3.4 Dose response 3 mg/kg vs 2 mg/kg | 1 | 119 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [‐0.07, 0.26] |
| 3.5 Dose response 3 mg/kg vs 1 mg/kg | 1 | 119 | Risk Difference (M‐H, Fixed, 95% CI) | 0.18 [0.03, 0.33] |
| 3.6 Dose response 2 mg/kg vs 1 mg/kg | 1 | 118 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.06, 0.23] |
| 3.7 Tetracycline 500 mg/day | 4 | 319 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 3.8 Tetracycline 1 g/day | 2 | 144 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 3.9 (Oxy)tetracycline | 5 | 365 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 3.10 Doxycycline | 2 | 179 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 3.11 Lymecycline | 2 | 230 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 3.12 Faropenem | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.06 [‐0.13, 0.01] |
| 3.13 Roxithromycin | 1 | 99 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 3.14 Josamycin | 1 | 122 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 3.15 Zinc | 0 | 0 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.16 2 mg cyproterone acetate/50 mcg ethinyloestradiol | 1 | 98 | Risk Difference (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.31] |
| 3.17 1% clindamycin lotion/gel | 1 | 66 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.05, 0.11] |
| 3.18 4% erythromycin/1.2% zinc lotion | 1 | 105 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 3.19 2% fusidic acid lotion | 1 | 174 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.01, 0.09] |
| 3.20 Doxycycline plus topical 4% chlorhexidine/ 5% benzoyl peroxide | 1 | 43 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 4 Acute vestibular disturbances | 1 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.09, 1.72] |
| 4.1 Dose response 3 mg/kg vs placebo | 1 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.95, 2.82] |
| 4.2 Dose response 2 mg/kg vs placebo | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.71, 2.27] |
| 4.3 Dose response 1 mg/kg vs placebo | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.49, 1.77] |
| 4.4 Dose response 3 mg/kg vs 2 mg/kg | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.80, 2.08] |
| 4.5 Dose response 3 mg/kg vs 1 mg/kg | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.02, 3.03] |
| 4.6 Dose response 2 mg/kg vs 1 mg/kg | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.75, 2.44] |
22.3. Analysis.
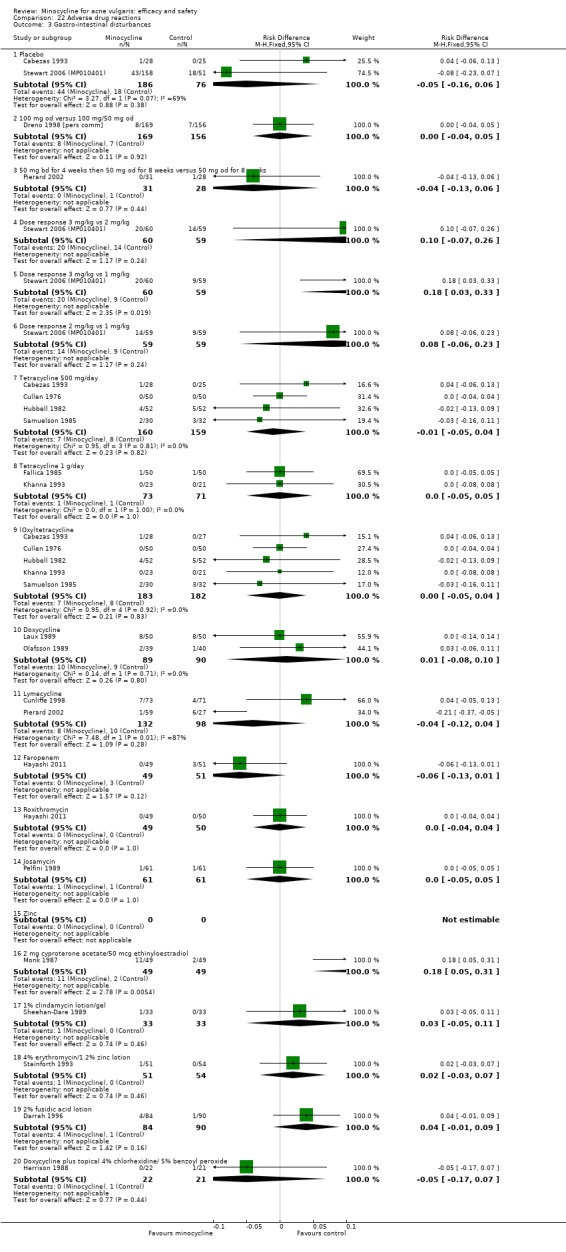
Comparison 22 Adverse drug reactions, Outcome 3 Gastro‐intestinal disturbances.
22.4. Analysis.
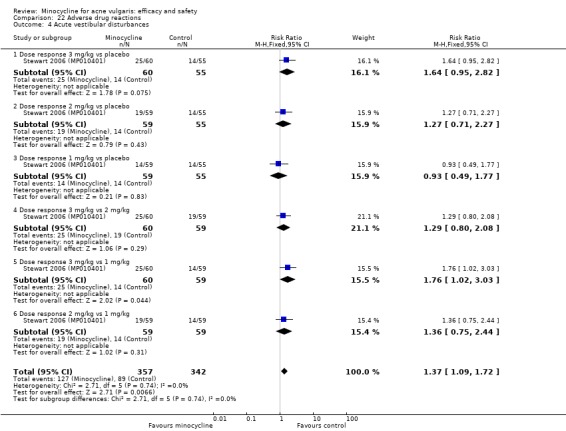
Comparison 22 Adverse drug reactions, Outcome 4 Acute vestibular disturbances.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blecschmidt 1987.
| Methods |
|
|
| Participants | 194 participants were enrolled.
104 participants were randomised in the minocycline group, and 90 participants were randomised in the oxytetracycline group. (For 43 there were no data.)
There were at least 4 dropouts.
The mean age was 20.9 +/‐ 5 (range = 13 to 45).
Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Germany
Language: German Review version: 2002 The trial report was inadequate for 'Risk of bias' assessment. The outcome measures were inappropriate. The dose reduction was not adhered to in 46 participants. There was variable duration of treatment. There was unclear dropout reporting. No further information was obtainable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred according to a prepared randomisation schedule. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of dropouts in each group was unclear; 43 participants were not included in the efficacy analysis. There were 18 dropouts in the minocycline group, and 23 in the oxytetracycline group were treated for only 12 weeks because of the side‐effects and low compliance. 6 participants were lost to follow up: 6 dropped out due to unspecified treatment, and 31 dropped out due to the use of topical treatment. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Bossuyt 2003 (TETRABUK).
| Methods |
|
|
| Participants | 136 participants were enrolled.
68 participants were randomised in the minocycline group, and 66 participants were randomised in the lymecycline group.
There were 30 dropouts, plus 2 were screened but not treated.
The mean age was 18.6 (range = 12 to 29).
Recruitment was fulfilled by hospital out‐patients. Power calculation: To demonstrate the non‐inferiority of lymecycline compared to minocycline ‐ 80% power, based on a 1‐tailed alpha risk of 0.025, the difference of at most 15% in the reduction in ILC ‐ 64 participants per group would be required. There was baseline comparability for demographic data and baseline severity. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
No other antiacne/anti‐inflammatory, topical, oral, or systemic antibiotics, with the exception of short penicillin courses; corticosteroids; or any other treatment likely to interfere with tetracyclines were allowed. Appearance: standard Instructions: taken before, during, or after meals at the same time of day Skin hygiene: not specified ‐ cosmetics were allowed but listed as concomitant Empty stomach: no Compliance: not reported |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: UK and Belgium
Language: English Review version: 2012 There were discrepancies in the reporting of the participant numbers: 136 enrolled, 2 were not treated, 66 were assigned to lymecycline, and 68 were assigned to minocycline, so the total number was 134. Per‐protocol figures stated in the tables (42 minocycline and 44 lymecycline) did not match the numbers of withdrawals (16 and 14, respectively). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 131): "Randomization was balanced by center and by block." Comment: This was unclear; it was stated to be randomised, but no details were given. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was probably done as the study is described as "investigator masked", but no details were given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 136 participants enrolled: 2 were not treated, 66 were assigned to lymecycline, 68 were assigned to minocycline, so the total number was 134 (Table 25 page 132). Per‐protocol figures stated in the tables (42 minocycline and 44 lymecycline) did not match the number of withdrawals (16 and 14, respectively, Table II). The text stated that 56 lymecycline and 53 minocycline participants were evaluable for the per‐protocol efficacy analysis (total n = 109) (number of inflammatory lesions, see statistical methods, page 131). This does not match the number of dropouts in table II, which was 14 lymecycline participants and 16 minocycline participants, so the total number of participants left was 130, or the total number of participants in table 3 was 86 (44 lymecycline participants and 42 lymecycline participants). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported on: inflammatory lesions count, assessor‐rated overall improvement, participant‐rated overall improvement, adverse drug reactions, tolerance, and economics at 12 weeks. |
Cabezas 1993.
| Methods |
|
|
| Participants | 80 participants were enrolled.
28 participants were randomised in the minocycline group, 27 participants were randomised in the tetracycline group, and 25 participants were randomised in the placebo group.
No dropouts were stated.
Recruitment was fulfilled by students using the medical service at the University of Chile. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
All were identical capsules. Concomitant therapy: none Appearance: identical Instructions: none Skin hygiene: no details Empty stomach: no details Compliance: no details |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Chile, participants of the student medical service of the University of Chile
Language: Spanish Review version: 2012 Additional information was sought from the authors, but it was not obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There were no details; it was only stated to be 'double blind'. |
| Allocation concealment (selection bias) | High risk | No allocation concealment was employed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Administation of the medicines was carried out under the following conditions: Packets of medicine were coded using a key. The dermatologist, who did not know the contents of the packets or the coding key, gave the participant 2 packets of pills in the first consultation; the order of delivery was determined by the numerical key on the packaging. It was unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details were given about the dropouts. There was no apparent dropout of any participants. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Campo 2003.
| Methods |
|
|
| Participants | 152 participants were enrolled.
58 participants were randomised in the minocycline group, and 64 participants were randomised in the lymecycline group.
There were 30 dropouts.
The ages of the participants were not specified.
It was not stated where participants were recruited from. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: adapalene gel 0.1% once daily Appearance: not specified Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Colombia
Language: English Review version: 2012 Only the abstract was available. Galderma Columbia were contacted for more information, but there was no response. The time points for the assessment of outcomes were unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given; it was stated to be randomised. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details were given; it was stated to be investigator‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 152 participants were recruited: 122 were evaluable. No further details were given. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were the mean number of total inflammatory lesions and mean number of lesions at each time point. Both were reported. |
Cullen 1976.
| Methods |
|
|
| Participants | 100 participants were enrolled.
50 participants were randomised in the minocycline group, and 50 participants were randomised in the tetracycline group.
There were 8 (16%) dropouts in the minocycline group, and 10 (20%) in the tetracycline group.
The mean age was 20 (range = 14 to 31).
Recruitment was fulfilled by students at a private dermatology clinic. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: identical Instructions: empty stomach Skin hygiene: non‐medicated soap Empty stomach: yes Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2002 The assessment intervals were variable. There was a contradiction in terms of the numbers of participants completing the trial. 2 participants with acne conglobata were included. There was no statistical analysis of the results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated as 'random distribution' and 'random assignment'. There were no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote (page 1209): "Neither participant nor physician knew the identity of the medication dispensed." Comment: It was stated as double‐blind. There were identical medications, which were coded, and the identity of the medication was only revealed at the end of the study. (All lesion counts and evaluations were made only by a senior trialist.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/100 participants dropped out; reasons were given. This was unlikely to introduce bias. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Cunliffe 1998.
| Methods |
|
|
| Participants | 144 participants were enrolled.
73 participants were randomised in the minocycline group, and 71 participants were randomised in the lymecycline group.
There were 14 (20%) dropouts in the minocycline group, and 15 (21%) in the lymecycline group.
The mean age was 19.0 (range = 12 to 32).
Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: double‐dummy Instructions: avoid extensive exposure to sun/UV; ingest with sufficient liquid; avoid concomitant dairy products, iron, and calcium; antacids within 2 hours prior‐ or post‐caps Skin hygiene: Cetaphil cleansing lotion Empty stomach: no Compliance: capsule count at each visit |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: UK and France
Language: English Review version: 2002 Power calculation: In order to demonstrate with 80% probability using a 2‐sided test performed at the 0.05 significance level that the true response was within +/‐ 15% in respect of the percentage reduction in the ILC from baseline to the end of treatment, 144 participants would be needed (67 evaluable per group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The following was stated: "Within each center participants were assigned to one of the 2 treatment groups using a randomisation procedure by blocks of 4 assuring therefore that treatments are balanced every 4 consecutive participants." |
| Allocation concealment (selection bias) | Low risk | The identification of each participant's treatment was inserted in a sealed envelope provided by the sponsor and retained by the investigator. In an emergency, the investigator had access to the code of the concerned participant. In practice, no such event occurred. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote (page 2): "A double‐dummy technique involving administration of placebo minocycline capsules with lymecycline and placebo lymecycline capsules with minocycline was employed to ensure the blinding of the study." Comment: It was stated as double‐blind. Participants were blinded to treatment type by use of a placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for dropouts were reported, and results were reported as ITT and per‐protocol. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point as planned. There was access to the trial report. |
Darrah 1996.
| Methods |
|
|
| Participants | 188 participants were enrolled.
93 participants were randomised in the minocycline group, and 95 participants were randomised in the fusidic acid group.
There were 19 (20%) dropouts in the minocycline group, and 18 (19%) dropouts in the fusidic acid group.
The mean age was 17.8 (range = 11 to 29).
Recruitment was fulfilled through general practice. Groups were matched for age, gender, duration of acne, and previous treatment. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Instructions: apply to acne‐affected area after washing with provided soap Skin hygiene: non‐medicated soap Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: UK
Language: English Review version: 2002 Power calculation: In order to demonstrate with 80% probability that the 95% confidence interval (CI) of the true response was within +/‐ 15% in respect of the percentage of participants achieving at least a 40% reduction in the number of acne lesions from baseline to the end of treatment, 150 participants would be needed (75 per group). The primary outcome measure was inappropriate. Lotion was applied to the acne‐affected area only. There were 38 centres, but no reporting of inter‐assessor variability. The full trial report was made available by the manufacturer. Adverse effects were reported in 84 minocycline participants and 90 fusidic acid participants. This was a well‐conducted study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred according to a computer‐generated, random numbers table in balanced blocks of 4. Each block of 4 treatments contained 2 treatments with fusidic acid and 2 with minocycline. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was not used ‐ open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for the dropouts were described in full (in the results on page 101). |
| Selective reporting (reporting bias) | Low risk | The outcomes described were as follows:
|
Drake 1990.
| Methods |
|
|
| Participants | 74 participants were enrolled.
The number of randomised participants was not specified.
There were 22 (30%) dropouts (allocation unknown). Baseline comparability of groups: demographics and baseline variables. The age range was 14 to 35. Recruitment was fulfilled through a university clinic. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: double‐dummy Instructions: wash face apply gel morning/evening Skin hygiene: non‐medicated soap wash face morning and evening Empty stomach: not specified Compliance: not specified Duration of therapy: unclear ‐ at least 6 weeks to be included in the analyses |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2002 Unpublished data were only available in the form of a technical report. There were no details of the number of participants randomised to each group, or dropouts. The results were only presented graphically with no measures of dispersion. The manufacturers were unable to supply further details about the unpublished data. The duration of therapy was unclear; each participant needed at least 6 weeks of therapy to be included in the analysis. The numbers of participants allocated to each group or dropouts in each group, were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was described as randomised. No detail was given about the generation of the sequence other than that "separate randomisation lists for males and females were used to assure that there were equal numbers of participants of each sex." (Page 3) |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind (abstract), but no details were given. A double‐dummy was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (page 4): "Of the 74 participants that enrolled into this study, 52 were deemed evaluable for efficacy analysis; the other 22 participants did not complete at least 6 weeks of treatment." Comment: No reasons were given for the discontinuation other than that 4 participants withdrew due to adverse effects (reasons given: vaginitis in 2 receiving clindamycin, gastro‐intestinal distress in 1 receiving minocycline, and another minocycline participant developed a rash and abdominal distress). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point, but most outcomes were reported graphically. |
Dreno 1998 [pers comm].
| Methods |
|
|
| Participants | 325 participants were enrolled. 169 participants were randomised in the constant‐dose group, and 156 participants were randomised in the reducing‐dose group. ITT: 160/147 There were 59 (35%) dropouts in the constant dose group, and 52 (33%) in the reducing dose group. The mean age was 19.54 +/‐ 4.58 (range = 12 to 41). Recruitment was fulfilled through hospitals and clinics. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: identical Instructions: with breakfast but not with milk Skin hygiene: non‐medicated soap and moisturiser Empty stomach: no (during breakfast but not with milk) Compliance: monitored |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: France
Language: not published Review version: 2002 The full trial report of the results section was provided by the lead investigator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated to be randomised, but the details supplied by the investigator did not clarify the method used. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind; it was unclear how this was achieved. Medications were identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropouts were as follows: 59/169 participants on a constant dose and 52/156 on a reducing dose. The reasons for dropout were unclear. Although dropouts were evenly distributed between the groups, the dropout rate was high. |
| Selective reporting (reporting bias) | Unclear risk | This was unclear; insufficient data were available. There was potential for large inter‐assessor variability. |
Dreno 2001.
| Methods |
|
|
| Participants | 332 participants were enrolled.
169 participants were randomised in the minocycline group, and 163 participants were randomised in the zinc group.
There were 44 (13%) dropouts (24 in the minocycline group and 20 in the zinc group).
Age: 19.2 mean range
Recruitment was fulfilled through private dermatologists, care units, and hospitals. Baseline comparability of groups tested for age, gender, family history of acne, age at onset, use of concomitant treatment, Oc, Cc, PA, PU, previous topical or systemic use. Minocycline was previously prescribed for 30% of participants in the minocycline group and 19% in the zinc group. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not reported Appearance: identical and placebo used for second dose of minocycline Instructions: not reported Skin hygiene: only those provided in trial permitted and no facial cosmetics with antiseptic properties; high‐fat soap and moisturiser Empty stomach: yes, unless GI disturbance then could be taken at night Compliance: very good and comparable: 95.1% +/‐ 6.2% zinc versus 95.9% +/‐ 6.5% ‐ no details of how evaluated |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: France Language: English Review version: 2012 Additional data were provided by Labcatal. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated to be multicentre randomisation, but no details were given. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear; no details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind, but there were no further details other than the following quote on page 136: "Both groups received look‐alike capsules." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for each time point (please see Figure 1, page 137). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point. |
Fallica 1985.
| Methods |
|
|
| Participants | 100 participants were enrolled. 50 participants were randomised in the minocycline group, and 50 participants were randomised in the tetracycline group. There were 4 (8%) dropouts in the minocycline group and 4 (8%) in the tetracycline group. The mean age was 19.22 (range = 12 to 36). Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Instructions: minocycline to be taken 1 hour before main meal Skin hygiene: not specified Empty stomach: yes Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Italy
Language: Italian and English Review version: 2002 The results were presented graphically and as statistical analysis values. The report was inadequate for 'Risk of bias' assessment. The age and sex distribution with the 2 groups after randomisation was homogeneous. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was unclear; it was described as controlled randomised. There were no further details in the published report. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the minocycline group, 3 participants did not present at the 7th control due to good improvement, while 1 was suspended for nausea; 4 were not present at the 6th week, due to optimal improvement; and 2 were suspended after 4 weeks (of these, 1 was for complete resolution of acne and 1 was for refusal to continue). In the tetracycline group, 4 were absent at the 7th control for good or modest improvement, and 6 did not attend for the 6th visit (of these, 1 was for optimal improvement, 1 was for gastralgia, and 4 were for refusal to continue). |
| Selective reporting (reporting bias) | Low risk | The outcomes were reported at each time point for each group. |
Fleisch 2006a (MP010404).
| Methods |
|
|
| Participants | 451 participants were enrolled (n = 300 minocycline, n = 151 placebo).
After screening and baseline evaluations in the phase 3 studies, participants were randomised in a 2:1 ratio to 2 treatment groups (ER‐minocycline 1 mg/kg [n = 615] or placebo [n = 309]). Each participant's study drug supply was determined by body weight and available tablet strength (Table 2). Assignment to treatment groups was stratified by the severity of acne (moderate or severe). The number of dropouts was not given by group (21% in both phase RCTs). The mean age was 19.2 years in the minocycline group and 21.3 years in the placebo group. It was not stated where participants were recruited from. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
"Each subject's study drug supply was determined by body weight and available tablet strength." Concomitant therapy: none Appearance: not specified Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2012 The blinding was potentially negated: "Each subject's study drug supply was determined by body weight and available tablet strength." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | For the phase 3 study, participants were randomised in a 2:1 ratio to 2 treatment groups stratified by severity of acne (moderate or severe) (page 22). Comment: This was probably done. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. "Each subject's study drug supply was determined by body weight and available tablet strength." This suggests that the blinding may have been potentially negated; it was not stated how this was dealt with. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind. "Each subject's study drug supply was determined by body weight and available tablet strength." This suggests that the blinding may have been potentially negated; it was not stated how this was dealt with. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 451 participants were recruited (Table 3, page 25). The table on page 26 stated that the number for a pooled analysis was 674 for the treatment group and 364 for the placebo group at all time points, suggesting that there were no dropouts. Quote (page 24): "89% of subjects completed the 84‐day treatment phase. The most frequent reasons for premature withdrawal in the extended‐release minocycline group were loss to follow up (3.3%) and adverse experiences (3.0%); the most frequent reasons for premature withdrawal in the placebo group were loss to follow‐up (4.1%) and withdrawal of consent (4.9%)." Comment: 89% completed treatment, but dropouts and withdrawals totaled 15.3%. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point. |
Fleisch 2006b (MP010405).
| Methods |
|
|
| Participants | The mean age was 20.0 years in the minocycline group and 19.6 years in the placebo group. After screening and baseline evaluations in the phase 3 studies, participants were randomised in a 2:1 ratio to 2 treatment groups (ER‐minocycline 1 mg/kg [n = 615] or placebo [n = 309). Each participant's study drug supply was determined by body weight and available tablet strength (Table 2). Assignment to treatment groups was stratified by the severity of acne (moderate or severe). The number of dropouts was not given by group (21% in both phase RCTs). The mean age was 19.2 years in the minocycline group and 21.3 years in the placebo group. It was not stated where participants were recruited from. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
"Each subject's study drug supply was determined by body weight and available tablet strength." Concomitant therapy: none Appearance: not specified Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Review version: 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | For the phase 3 study, participants were randomised in a 2:1 ratio to 2 treatment groups stratified by severity of acne (moderate or severe) (page 22). |
| Allocation concealment (selection bias) | Unclear risk | No details were given. "Each subject's study drug supply was determined by body weight and available tablet strength." This suggests that the blinding may have been potentially negated; it was not stated how this was dealt with. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind. "Each subject's study drug supply was determined by body weight and available tablet strength." This suggests that the blinding may have been potentially negated; it was not stated how this was dealt with. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 451 participants were recruited (Table 3, page 25). The table on page 26 states that the number for a pooled analysis was 674 for the treatment group and 364 for the placebo group at all time points, suggesting that there were no dropouts. Quote (page 24): "89% of subjects completed the 84‐day treatment phase. The most frequent reasons for premature withdrawal in the extended‐release minocycline group were loss to follow up (3.3%) and adverse experiences (3.0%); the most frequent reasons for premature withdrawal in the placebo group were loss to follow‐up (4.1%) and withdrawal of consent (4.9%)." Comment: 89% completed treatment, but dropouts and withdrawals totaled 15.3%. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point. |
Gollnick 1997.
| Methods |
|
|
| Participants | 85 men were enrolled.
The number of randomised participants was not specified, but it was unequal as 50 were assigned to minocycline/azelaic acid and 35 were assigned to isotretinoin. 50 participants were randomised to the minocycline/azelaic acid group and 35 to the isotretinoin group. There were 8 (9%) dropouts. The mean age was 19 (range = 15 to 31). Recruitment was fulfilled by hospital out‐patients. At baseline the 2 groups were comparable for age. There were however differences between the groups in the duration of acne, the numbers of individuals who had received pre‐treatment, those who had acne on their face and trunk, and those who had papulopustular acne. It was not reported whether these differences had been subjected to statistical analyses. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Instructions: use 1 inch of cream If there was pronounced local irritation, the frequency of applications reduced temporarily to once‐daily. It was stopped where necessary until symptoms had disappeared. The isotretinoin group had regular liver function test monitoring. Concomitant therapy: not specified Appearance: standard‐open trial Instructions: not specified Skin hygiene: vehicle of AA cream for individuals in isotretinoin group as a moisturiser. Otherwise, not stated Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Germany, Austria, and Switzerland
Lanugage: English Review version: 2002 abstract, 2012 full This was open necessarily due to the adverse effect profile of isotretinoin. In the initial version of this review, the only information that could be obtained was a brief summary of the study that was published in a review article. The 2006 update included the information included in the full publication published in 2001. Primary outcomes were presented graphically and a median was given. "Participants who during study phase 1 had achieved a very good therapeutic improvement were eligible for admission to the second, 3 month study phase (maintenance treatment). Participants in whom a very good clinical improvement was achieved prematurely, i.e. before completing the 6 months of study phase 1, were transferred early to phase 2. The participants of the initial AAMino group admitted to the second study phase used AA cream twice daily as maintenance therapy over a period of 3 months. Participants in the Iso group did not receive any further maintenance therapy." This trial was a 2‐phase trial: 6 months of treatment and then 3 months of maintenance. Participants in whom a very good clinical improvement was achieved prematurely, i.e. before completing the 6 months of study phase 1, were transferred early to study phase 2. The paper stated that all 85 participants were included in the analysis of efficacy. The therapy was regarded as completed before the end of the 6 months in 10% of the participants in the minocycline/azelaic group, while 78% of the participants of this group finished the first study phase as per the schedule after 6 months. The corresponding figures in the isotretinoin group were 14.3% and 77.1%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was randomised and open‐label. No details were given. There were unequal numbers of participants, which suggested a problem with the randomisation as did the differences reported at baseline. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was open‐label. It was not possible to blind due to the side‐effect profile of isotretinoin. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 541): "All 85 participants were included in the analysis of efficacy." Comment: All participants were accounted for: 85 were recruited and 77 completed. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were reported at each time point. Secondary outcomes were investigator and participant global assessments of the therapeutic result. It was unclear if participant‐rated global outcomes were reported; Figure 3 gave global outcomes, but it was unclear if this included participant‐reported outcomes. |
Harrison 1988.
| Methods |
|
|
| Participants | 43 participants were enrolled.
22 participants were randomised in the minocycline group, and 21 participants were randomised in the doxycycline group.
There were 3 (14%) dropouts in the minocycline group, and 6 (29%) in the doxycycline group.
The mean age was 20 (range = 16 to 35).
Recruitment was fulfilled by hospital out‐patients. The groups were equivalent at baseline for gender and duration of acne. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: recorded, but no details were given Appearance: standard Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: UK
Language: English Review version: 2002 Additional data were provided by Pfizer. We cannot attribute any changes/alterations to oral therapy as benzoyl peroxide is highly active, particularly against non‐inflamed lesions. Division of lesions into active and less active was likely to be highly subjective. Lesion counts and scores were adjusted to account for different baseline values; no further details were given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 242): "The participants were randomised." Comment: No details were given. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was observer‐blinded; there were no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 participants entered the study: 21 received doxycycline and 22 received minocycline. 15 in the doxycycline group completed and were analysed; 19 in the minocycline group completed and were analysed. Analysis was per‐protocol. |
| Selective reporting (reporting bias) | Low risk | All the lesion count data were presented. The participants' assessment of severity on the visual analogue scale and score 0 to 100 was found to be inconsistent, and it was felt that these types of data were insufficiently accurate to compare the effects of the 2 drugs. |
Hayashi 2011.
| Methods |
|
|
| Participants | 50 participants were enrolled.
49 participants were randomised in the minocycline group, 50 participants were randomised in the roxithromycin group, and 51 participants were randomised in the faropenem group.
There were 9, 7, and 8 dropouts, respectively. All were accounted for.
The mean age was 26.5 (26.1, 26.2, 27,1).
Recruitment was not specified. There were no baseline differences in age, gender, duration of disease or severity. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: those with indication for acne or influence on acne prohibited. Permitted: treatment for complications that did not affect acne temporary use of antibiotics to treat incidental infections except azithromycin, external non‐comedogenic moisturisers, and vitamin B2, B6, C and E preparations permitted. Hormone therapy and physical treatments prohibited. Topical medication permitted during the second 4‐week 'observation' period: 34 participants used nadifloxacin or clindamycin "when participants were treated with any concomitant drugs or therapies, the name of the drug or therapy, route of administration, daily dose, treatment duration and reason for concomitant use were recorded in the case report form." Appearance: standard‐open trial Instructions: not specified Skin hygiene: external non‐comedogenic moisturisers permitted Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Japan Language: English Review version: 2012 The first 4 weeks only were eligible for inclusion because oral therapy stopped and topical clindamycin or nadifloxacin was given. Many variables affecting acne treatment were not controlled. Physicians were permitted to exclude patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | The envelope method was stated, but no details were provided. Comment: This was probably done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was an open‐label study; there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for, even with regard to distribution of dropouts between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes were included, but no data were provided. The results were presented in graphical form only. |
Hersle 1976.
| Methods |
|
|
| Participants | 50 participants were enrolled.
25 participants were randomised in the minocycline group, and 25 participants were randomised in the placebo group.
There were 9 (24%) dropouts (all in the minocycline group). The age range was 14 to 34. Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: identical Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Sweden
Language: English Review version: 2002 The report was too inadequate to permit accurate validity assessment. There was no wash‐out period between the study arms. The outcome measures were inappropriate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given; it was stated to be randomised. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind. The tablets were identical and filled in coded bottles. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All dropouts were accounted for, but all were in the minocycline group (7/50). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported, but there was insufficient data to calculate the effect size. |
Hubbell 1982.
| Methods |
|
|
| Participants | 104 participants were enrolled.
52 participants were randomised in the minocycline group, and 52 participants were randomised in the tetracycline group.
There were 27 (52%) dropouts in the minocycline group and 28 (54)* in the tetracycline group.
The mean age was 17.4 (range = 14 to 35).
Recruitment was fulfilled through the Airforce. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: identical Instructions: take on empty stomach Skin hygiene: wash bd with common soap Empty stomach: yes Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2002 *25 minocycline participants and 26 tetracycline participants did not attend a minimum of 6 visits, although no reasons were given for this. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was described as randomised, but no details were given about generation of the sequence. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear if the allocation was concealed. It stated that each participant "was randomly assigned a numbered medication." (page 989) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind, but no details were given. Appearance of the capsules was identical. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Yes ‐ all participants were accounted for, although no reasons were given for the 55 who dropped out. There were contradictory report numbers regarding dropouts and side‐effects. There was a very high dropout rate: 104 commenced, 55 dropped out, and 51 did not fulfil the visit requirements. 4 dropped out as follows: In the tetracycline group, 1 dropped out due to side‐effects, and 1 due to worsening of acne. In the minocycline group, 1 participant dropped out because of unsatisfactory results, and 1 because of a severe flare of acne. |
| Selective reporting (reporting bias) | Unclear risk | Yes ‐ all outcomes were reported at each time point. |
Khanna 1993.
| Methods |
|
|
| Participants | 44 participants were enrolled. 23 participants were randomised in the minocycline group, and 21 participants were randomised in the tetracycline group. There were 4 (17%) dropouts in the minocycline group and 6 (29%) in the tetracycline group. The mean age was 20.7 (range = 14 to 24). Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Instructions: empty stomach for tetracycline Skin hygiene: normal soap and water Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: India
Language: English Review version: 2002 It was not clear whether large IL referred to nodules. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated "participants were randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was an open trial (confirmed by the author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts were accounted for, but the analysis was per‐protocol. 19/23 participants completed in the minocycline group; 15/21 participants completed in the tetracycline group (completed at 12 weeks). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Laux 1989.
| Methods |
|
|
| Participants | 100 participants were enrolled.
50 participants were randomised in the minocycline group, and 50 participants were randomised in the doxycyline group.
The number of dropouts was not specified.
The mean age was 21 (range = 15 to 36).
Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: standard Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Germany
Language: German Review version: 2002 Translation was only available for interim analysis. No denominators were given for the results. There was no information on dropouts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not specified. It was described as a "randomised comparative clinical study". |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was an open trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was no information on dropouts. No denominators were given for the results, and it was unclear how many participants were in each group at each time point. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
Leyden 2004.
| Methods |
|
|
| Participants | Only men were enrolled. The number of participants enrolled was not specified: "182 evaluable", but 269 had safety data suggesting the number randomised was > 182. The randomisation of participants was not specified. The number of dropouts was not specified. Recruitment was fulfilled through hospitals and medical centres. There was no difference between the groups in lesion counts at baseline. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: double‐dummy Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2012 The publication provided a brief summary of the trial and insufficient information for proper analysis. There were inconsistencies in the trial report about duration and the numbers of participants included. We suspect that the trial was stopped early due to lack of efficacy. No demographic data were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote (page 444): "Treatment groups were blinded by adding placebo tablets in a double‐dummy design." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants assigned to each group was not reported. The number of dropouts or participants lost to follow up were not reported. Data reported on 182 "evaluable " participants, yet 269 were available for safety data. |
| Selective reporting (reporting bias) | High risk | The specified outcomes were ILC, TLC, investigator and participant assessment, and photographs. Only inflammatory lesions were reported. Adverse drug reactions were briefly summarised: Only serious adverse drug reactions were reported. |
Leyden 2006 (Part 2).
| Methods |
|
|
| Participants |
The following are taken from page 606 of the trial. "189 participants enrolled, 137 completed open label phase, 110 [were] randomised to [the] maintenance phase." Randomised: 0.1% tazarotene gel each evening plus a placebo capsule twice‐daily, vehicle gel each evening plus minocycline capsule twice‐daily, or 0.1% tazarotene gel each evening plus a minocycline capsule twice‐daily. 20 participants did not complete the randomised phase of the study. The mean age was 22 years. Participants "enrolled from 5 investigational sites in the United States. The sites were referral or research centres, and enrolment was generally performed by investigators who recruited their existing participants or participants who responded to an advertisement." There were comparable demographics at baseline. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: none permitted Appearance: standard (open‐label) Instructions: pea‐sized amount to the face in a thin film 15 to 20 minutes after washing with a mild, non‐medicated cleanser and drying with a soft towel Skin hygiene: Quote (page 606): "Washing with a mild non‐medicated cleanser and drying with a soft towel. Participants were supplied with a noncomedogenic moisturiser to use if facial dryness developed. No other lotions, creams, medicated powders, or solutions were allowed on the treatment area." Empty stomach: no Compliance: measured as reported in Figure 1, page 607, but method of assessment was not specified Wash‐out periods: 14 days topical acne medications, 30 days oral antibiotics and investigational drugs, 12 weeks oestrogens or birth control pills if used for less than 12 weeks, 2 years for oral retinoids |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2012 The trial was financially sponsored, but disclosure was made. Quote (page 612): "The initial draft of the manuscript was reviewed by Allergan Inc, but the company did not prepare the manuscript or have the opportunity to approve the final version." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 606): "Participants who achieved at least 75% global improvement at week 12 were assigned a unique participant number obtained from a computer‐generated randomisation schedule (using a block size of 6) provided by the sponsor. The assignment of numbers was not necessarily continuous (because 1 investigator may have received noncontiguous blocks of numbers) but was always in blocks of 6." Comment: Yes, this was randomised. |
| Allocation concealment (selection bias) | Low risk | Small block randomisation was employed. Comment: It was possible to predict sequence in small block randomisation, but this was judged as probably low risk. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote (page 606): "The labels on the medication containers were concealed." It was stated as double‐blind, but it was unclear if the appearance of products was identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for at each time point (please see Figure 1, page 607). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
Lorette 1994.
| Methods |
|
|
| Participants | 71 participants were enrolled.
35 participants were randomised in the minocycline group, and 36 participants were randomised in the doxycycline group.
There were 12 dropouts (34%) in the minocycline group and 5 dropouts (14%) in the doxycycline group. Plus, 1 was unspecified.
The mean age was 18.6.
Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: not specified Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: France
Language: French Review version: 2002 The baseline mean Cc count was significantly different (at 5%): doxycycline 8.5, minocycline 4.1. Side‐effects were not documented. Results were expressed as percentages only with no dispersion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not described in detail; it was only stated that it was a multicentre (4 centres), phase IV, double‐blind, 2 parallel‐group study |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This was described as double‐blind, but no details were given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | From the 71 participants recruited, 1 was excluded due to taking a vitamin A therapy concurrently, 7 were lost to follow up (2 in the doxycyline group and 5 in the minocycline group), and 10 dropped out (3 in the doxycycline group and 7 in the minocycline group). Howver, results were expressed as percentages only with no participant numbers; therefore, this was unclear. |
| Selective reporting (reporting bias) | High risk | Adverse effects were reported in that clinical tolerability was described as "satisfactory". Adverse effects included gastro‐intestinal disturbances, headaches, and dizziness, but the numbers of each participants reporting adverse events were not given for each time point or for each group (minocycline or doxycycline). |
Monk 1987.
| Methods |
|
|
| Participants | 98 women were enrolled.
49 participants were randomised in the minocycline group, and 49 participants were randomised in the cyproterone group.
There were 10 (20%) dropouts in the minocycline group and 10 (20%) in the cyproterone group.
The mean age was 23.5.
Recruitment was fulfilled through hospitals. With regard to baseline characteristics, there was no difference between groups in terms of age, weight, blood pressure, cycle length, or face lesions, but the minocycline group at baseline had a larger number of comedones. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified but no other oral contraceptive or steroid during study period Appearance: standard Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United Kingdom
Language: English Review version: 2002 The study results were quoted as median and range. No further details were available from the manufacturers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was unclear; no details were given. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was intentionally not blinded due to a difference in the dose regime and contraceptive advice. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The reasons for dropout were recorded (Table 25, page 320). 78 completed 24 weeks of treatment: 39 from each group. 10 from each group failed to complete the study. However, most data were presented graphically or as percentages of participants. There was tabulated total lesion count data for 36 and 35 participants in the Diane™ and minocycline groups, respectively. |
| Selective reporting (reporting bias) | Unclear risk | All acne outcomes (lesion counts and subjective assessments, side‐effects) were reported at each time point. Weight and blood pressure were recorded at each visit, but these measurements did not appear in the published report of the trial. |
Olafsson 1989.
| Methods |
|
|
| Participants | 79 participants were enrolled.
39 participants were randomised in the minocycline group, and 40 participants were randomised in the doxycycline group.
There were 8 (21%) dropouts in the minocycline group and 7 (18%) in the doxycycline group.
The mean age was 20.5 (range = 14 to 37).
Recruitment was fulfilled by hospital out‐patients. Compliance was not specified, but publication notes that 1 dropped out due to poor compliance (doxycycline). Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: double‐dummy Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Iceland
Language: English Review version: 2002 The results were given in graphical form ‐ there were no data. Overall effectiveness: number of participants given as per cent with no indication of denominator (i.e. the total numbers of participants). There was no indication of participant numbers overall. The minocycline group had more lesions at baseline, but this was not significant. The manufacturers were unable to supply further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 15): "The participants were randomly allocated to two groups." No further details about the method of randomisation were given. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. No details were given in the published report. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This was described as double‐blind and double‐dummy. Matching placebos were supplied for both the doxycycline and the minocycline tablets (see page 17 "Acknowledgements"). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participants dropped out, with reasons given in the published report. 8 did not complete the study from the minocycline group, 7 did not complete the study from the doxycycline group, but the number of participants was given as per cent with no indication of denominator, and there is no indication of participant numbers for the overall dropout. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point. |
Ozolins 2005.
| Methods |
|
|
| Participants | 649 participants were enrolled.
The mean age was 19.7 (SD 6.07) (range = 11 to 42). Recruitment was fulfilled from GP surgeries in Leeds and Nottingham, United Kingdom, and colleges in the United Kingdom. A letter was sent from GPs to patients requesting participation. There was baseline equivalence between the groups, except that participants in the erythromycin + BP group had more tetracycline‐resistant propionibacteria. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
7 treatment comparisons were made. Concomitant therapy: not permitted Appearance: vehicle control plus low‐dose vitamin C, not matching Instructions: thorough and adequate Skin hygiene: controlled‐unperfumed soap and E45 cream or own unmedicated products Empty stomach: clear instructions for proper use (and storage) of medication given Compliance: return of unused mediation and diary cards |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United Kingdom
Language: English Review version: 2012 The HTA report was available. Due to poor recruitment, 6 treatment groups were discontinued (112 randomised):
The trial was independent of industry sponsorship. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2189) (Lancet 2004;364:2188‐95): "Participants were randomly allocated to one of five antimicrobial treatment groups by use of a computer‐generated randomisation code, generated using SAS PROC PLAN (HTA report)." |
| Allocation concealment (selection bias) | Low risk | Quote (page 2189) (Lancet 2004;364:2188‐95): "...randomly allocated to one of five antimicrobial treatment groups by use of a computer‐generated randomisation code known only to the trial coordinator and pharmacy staff at Queen's Medical Centre, Nottingham, United Kingdom. The randomisation was in blocks of 11, without stratification." "Each participant received from the assessor a sealed opaque box labelled with his or her unique identification number. Each box contained both oral and topical formulations with detailed instructions for their proper use and storage." Comment: Particpants were enrolled and allocated treatment numbers by the clinical assessors, who had no knowledge of which treatment they were allocating to the participant (HTA report). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Observer‐masked participants received medications in their original packaging, so participants could have been aware of their treatment assignment. Quote (page 2189) (Lancet 2004;364:2188‐95): "Participants were given specific written and spoken instructions not to discuss the nature of their medication with assessors. Instances of treatment unmasking to assessors during the study were recorded." GPs of participants were not involved in the assessment of the trial, but they were still kept blind to the treatment as they could withdraw participants from the trial (HTA report). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for at each time point with reasons (please see Figure, page 2190) (Lancet 2004;364:2188‐95). In the oxytetracycline 500 mg bd group, 37/131 dropped out. In the minocycline 100 mg od group, 40/130 dropped out. In the erythromycin 3% and BP 5% bd group, 38/130 dropped out. In the erythromycin 2% od and BP 5% od group, 25/127 dropped out. In the BP 5% od group, 38/131 dropped out. |
| Selective reporting (reporting bias) | Unclear risk | The 2 primary outcomes for efficacy were both reported at the 18‐week end point only. The 2 grading methods reported as secondary outcomes were reported at 18 weeks (internet version only: Lancet 2004;364:2188‐95). Quality of life estimates were reported elsewhere (HTA report), using the Dermatology Life quality Index (DLQI) or the children's version (CDQLI). All participants and dropouts were accounted for. Reductions in bacterial growth score and proportion of participants with viable Proprionibacteria were reported at weeks 6, 12, and 18 for all treatment arms. Skin colonisation by antibiotic‐resistant propionibacteria was monitored at weeks 6, 12, and 18. Features of local irritate and adverse events were recorded at weeks 6, 12, and 18. Cost effectiveness and 'willingness to pay' measures were reported at the 18‐week end point. Numbers were analysed as intention‐to‐treat (HTA report). |
Peacock 1990.
| Methods |
|
|
| Participants | 80 participants were enrolled.
38 participants were randomised in the minocycline group, and 42 participants were randomised in the clindamycin group.
There were 9 (24%) dropouts in the minocycline group and 8 (14%) in the clindamycin group.
The mean age was 21 (range = 18 to 34).
Recruitment was fulfilled though students at 3 university health centres. There was baseline comparability between the groups in terms of demographics, severity, duration, and previous medication. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Instructions: women were not to use new cosmetics Skin hygiene: wash bd with non‐medicated soap Empty stomach: not specified Compliance: count/measure of unused medication |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United Kingdom
Language: English Review version: 2002 All participants were included in the analysis if they received a minimum of 4 weeks treatment and attended week 4 and final assessments. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used. No details were given about the randomisation method. Comment: This was probably done. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear; no details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Observer nurses were blind to allocation; dispenser nurses were aware of allocation. It was unclear if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/42 clindamycin participants did not complete, and 9/38 minocycline participants did not complete (reasons were given). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Pelfini 1989.
| Methods |
|
|
| Participants | 122 participants were enrolled.
61 participants were randomised in the minocycline group, and 61 participants were randomised in the josamycin group.
There was 1 dropout (minocycline participant).
The mean age was 21.3 in the minocycline group (range = 14 to 34) and 20.3 in the josamycin group (range = 14 to 33).
Participants were recruited from the university dermatology departments. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: "In 69 patients, 35 in the josamycin group, 34 in the minocycline group, the clinical presentation suggested the association of a topical medication 5% benzoyl peroxide ointment to the oral treatment." Appearance: standard Instructions: orally once‐daily for 2 months Skin hygiene: no details Empty stomach: no details Compliance: no details |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Italy Language: English Review version: 2012 35/61 josamycin participants and 34/61 minocycline participants suggested concomitant use of 5% benzoyl peroxide treatment at presentation. There was concomitant use of benzoyl peroxide in some participants. There were 2 different regimens, and it was unclear how participants were allocated to each. This was a very poor trial write‐up due to the English not being the first language of the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...randomly allocated." Comment: The report suggests that a number of participants were also given 5% benzoyl peroxide, which is very active. These were not randomised. Also, there were 2 different treatments schedules, and it was unclear how they were randomised (possibly based on severity). |
| Allocation concealment (selection bias) | Unclear risk | This was unclear; no details were given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'Previous open studies...' was stated, implying that this was also open. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/61 participants discontinued in the minocycline group due to severe gastric intolerance. It appears all other participants provided data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. The results were presented graphically only. |
Pierard 2002.
| Methods |
|
|
| Participants | 86 participants were enrolled.
The following were randomised: 31 minocycline 100 mg then 50 mg/28 minocycline 50 mg/27 lymecycline 300 mg.
There were 7, 4, and 7 dropouts, respectively (18 in total).
Age was 24 +/‐ 3 years; the mean was not stated, but the range was 17 to 35.
Recruitment was fulfilled by hospital out‐patients. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not stated Appearance: identical, double‐dummy Instructions: to be taken with meals and water in the morning and evening Skin hygiene: not stated Empty stomach: no Compliance: not stated |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Sponsorship: Wyeth Lederle
Country: Belgium Review version: 2012 Bacterial viability assessments were undertaken using cyanoacrylate skin surface stripping. Dual flow cytometry was used to obtain fluorescence data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated to be randomised, but no details were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind and double‐dummy. No details were given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants in each treatment group was reported at the start and the end (after 12 weeks) of the study, but the reasons for dropouts were not given. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
Pigatto 1986.
| Methods |
|
|
| Participants | 24 men were enrolled.
12 participants were randomised in the minocycline group, and 12 participants were randomised in the isotretinoin group.
There were no dropouts.
The mean age was 23 +/‐ 3 (range = 20 to 29).
Recruitment was fulfilled though a university hospital. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: standard Instructions: not specified Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Italy
Language: English Review version: 2002 There was insufficient information in report to permit adequate validity assessment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly divided' was stated. No details were given. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was not possible due to the side‐effect profile of isotretinoin. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 24 participants completed the study. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. Data were presented in graphical form only. |
Revuz 1985.
| Methods |
|
|
| Participants | 91 participants were enrolled.
43 participants were randomised in the minocycline group, and 47 participants were randomised in the placebo group.
There were 4 (9%) dropouts in the minocycline group and 13 (28%) in the placebo group.
The mean age was 22.4 +/‐ 4.7 (range = 14 to 37).
Recruitment was fulfilled by hospital out‐patients. There was baseline comparability in terms of type of acne, number of features, severity, assessment site, gender, and age of the participants. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: identical Instructions: take in the evening Skin hygiene: "washing and drying" Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: France
Language: English Review version: 2002 The concomitant therapy was very active; no strength was given. There was confusion in terms of the participant numbers. No further information was obtained after requests to the author/manufacturer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was unclear; it was stated to be randomised, but no details were given. It was noted that a "code" was used. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear; no details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This was unclear; it was stated to be double‐blind, but no details were given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 91 participants were included in the trial, but 1 was excluded from the analysis as the acne was located solely on the back. Demographic details were given for 90 participants in table 1, page 105 of the published report; however, on page 103, it was unclear whether 89 or 90 participants started the trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported at each time point. |
Ruping 1985.
| Methods |
|
|
| Participants | The number of enrolled participants was unclear: 283 participants were available for evaluation at the end of the study (15 therapeutic centres). 127 participants were randomised in the minocycline group, and 120 participants were randomised in the tetracycline group. For 36 participants, randomisation was unspecified. The number of dropouts was not specified. The age of the participants was not specified. It was not stated where participants were recruited from. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: standard Instructions: minocycline to be taken 30 minutes before meals Skin hygiene: not specified Empty stomach: minocycline = yes, tetracycline = no Compliance: 'largely satisfactory' |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: Germany
Language: English Review version: 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated to be 'randomised', and participants were divided in to 2 groups. No details were given. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was an open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It reported only the participants available at the end of the study; the number randomised was not given. There was no information on 36 participants whose 'information was inadequate or faulty'. There were no details on dropouts or indication of denominator in outcome measures. |
| Selective reporting (reporting bias) | Unclear risk | The results were presented graphically; all outcomes were reported. The adverse event data were very sparse. There were non‐standardised follow‐up periods. |
Samuelson 1985.
| Methods |
|
|
| Participants | 62 participants were enrolled.
30 participants were randomised in the minocycline group, and 32 participants were randomised in the tetracycline group.
The number of dropouts was 4 (14%) in the minocycline group and 6 (19%) in the tetracycline group.
The mean age was 19 (range = 17 to 30).
Recruitment was fulfilled through students attending a private clinic. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: identical Skin hygiene: not specified Empty stomach: not specified Compliance: taken under nurse supervision |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States
Language: English Review version: 2002 62 participants were randomised, but only 55 were included in the efficacy analysis as the others had grade 3 acne. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 463): "...fully randomised double blind format." |
| Allocation concealment (selection bias) | Unclear risk | This was not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This was described as double‐blind; it was unclear who was blinded ‐ whether it was the investigator, participants, or an independent dermatologist. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were accounted for at each time point. 2/30 minocycline participants dropped out due to attendance or psychiatric problems; 2/32 tetracycline participants dropped out due to adverse events. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported at the specified time points. |
Schollhammer 1994.
| Methods |
|
|
| Participants | 77 participants were enrolled.
22 participants were randomised in the minocycline group, 33 participants were randomised in the lymecycline group, and 22 participants were randomised in the doxycycline group.
There were 2 (9%) dropouts in the minocycline group, 4 (12%) dropouts in the lymecycline group, and 6 (27%) in the doxycycline group.
The age range was 9 to 30.
Recruitment was fulfilled by hospital out‐patients. Baseline severity was equivalent between the groups. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: France
Language: French Review version: 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was described as randomised (3‐arm). There were no details of the randomisation method. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was not specified (not apparently blinded). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This was unclear. The dropouts and reasons for dropout in each group were not given. And for the analyses in this review, the number of participants at each time point were estimated by calculating from the percentages of the numbers in each group (Table 25, page 25). |
| Selective reporting (reporting bias) | Unclear risk | This was unclear. The outcomes were reported at time point 0 and at 12 weeks for the average number of lesions (week 0), % reduction of lesions by week 12, and percentage of participants with more than 50% reduction in lesions at week 12. No denominators were given for the number of participants with 50% lesion reduction. |
Sheehan‐Dare 1989.
| Methods |
|
|
| Participants | 66 participants were enrolled.
33 participants were randomised to the minocycline group; 33 participants were randomised to the clindamycin group.
There was 1 (6%) dropout in the minocycline group and 6 (18%) in the clindamycin group.
The age range was 14 to 35.
Recruitment was fulfilled by hospital out‐patients. There was baseline comparability (age, sex, grade, and count). Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Appearance: standard but double‐dummy used Concomitant therapy: not specified Instructions: capsules to be taken before meals, apply lotion to whole face Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United Kingdom
Language: English Review version: 2002 Data were presented in graphical form only. Macules were included in the ILC. Grades and counts were log transformed prior to analysis. The manufacturers or authors couldn't supply further information. The numbers of participants in each group were not reported. There were differences between the 2 reports of the same trial, including whether or not there was a statistically significant reduction in non‐inflamed lesion counts, and in the type of statistical tests used. There were differences between 2 publications of this trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stated to be by matched pairs on the basis of age, sex, acne grade, and numbers of both inflamed and non‐inflamed lesions, but no details were given about the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear; no details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be "double blind, double dummy" (page 25 of the published report). No further details were given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 66 participants were enrolled, but there were no details on how many participants were randomised to each group: It was assumed to be 33 due to matched pairs. 6 dropped out from the clindamycin arm and 1 from the minocycline arm, but it was unclear at which time point these dropouts occurred. |
| Selective reporting (reporting bias) | Low risk | All outcomes, including adverse events, were reported at each time point. No data were reported (graphical only). |
Smit 1978.
| Methods |
|
|
| Participants | 18 participants were enrolled.
9 participants were randomised in the minocycline group, and 9 participants were randomised in the doxycycline group.
There was 1 (11%) dropout in the minocycline group and 1 (11%) in the doxycycline group.
The age of the participants was not specified.
Recruitment was fulfilled through a hospital. Baseline comparability was not specified. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: not specified Instructions: capsules to be taken after dinner Skin hygiene: not specified Empty stomach: no Compliance: not specified |
|
| Outcomes |
Outcomes of the trial 1. Score = E x (S + C + P + I + A) E = Extent of symptoms (1 to 5) S = Seborrohea (0 to 4) C = Comedones (0 to 4) P = Papules/pustules (0 to 4) I = Infiltration (0 to 4) A = Abscess (0 to 4) 2. Change in score from baseline (primary outcome) 3. Laboratory tests 4. Adverse drug reactions as reported by the participants |
|
| Notes | Country: Netherlands
Language: English Review version: 2002 There were very small numbers of participants. Concommitant therapy was likely to mask treatment effect. The trial report was very brief; it was inadequate for validity assessment. Individual participant results were given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 187): "The participant was given either doxycycline or minocycline according to a prearranged system of allocation." |
| Allocation concealment (selection bias) | Unclear risk | This was unclear. No details were given in the published report. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This was described as "double blind", but no details were given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 18 participants who entered the trial, "2 were lost to follow up due to non‐medical reasons." (page 187) |
| Selective reporting (reporting bias) | Low risk | All outcomes were recorded at each time point, only the before and after (at 3 months) scores were reported in Table 25 of the published report. |
Stainforth 1993.
| Methods |
|
|
| Participants | 109 participants were enrolled.
54 participants were randomised in the minocycline group, and 55 participants were randomised in the erythromycin group.
There were 9 (17%) dropouts in the minocycline group and 7 (13%) in the erythromycin group.
The mean age was 20.2 (range = 14 to 47).
Recruitment was fulfilled through a hospital. There was baseline comparability for age, sex, extent of disease, NILC, superficial ILC, and ILC. The mean acne grade was slightly greater in minocycline participants: 1.18 (range 0.5 to 2.5) versus 0.89 (0.5 to 2.0). Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not specified Appearance: standard Instructions: lotion to be applied after washing morning and evening, 2 tablets to be taken 12 hours apart Skin hygiene: not specified Empty stomach: not specified Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United Kingdom
Language: English Review version: 2002 The minocycline counts were static after 2 weeks of therapy. The total inflamed lesion count were not valid as it included macules. The assessor guessed the therapy allocation in 7 cases. The results were presented in graphical form only, but additional information was supplied by the manufacturer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 119): "Patients were allocated randomly, without stratification." No further details were given. |
| Allocation concealment (selection bias) | Unclear risk | This was unclear; no further details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was single‐blind. Quote: "The investigator clinically assessing a patent was not informed of which treatment that participant was on." But it was observed that the participant notes indicated that in 7 cases the assignment was guessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes, all participants, dropouts, and losses to follow up were accounted for. |
| Selective reporting (reporting bias) | Low risk | Yes, all outcomes were reported at each time point (assessments not planned at week 8 of study). |
Stewart 2006 (MP010401).
| Methods |
|
|
| Participants | 241 participants were enrolled, and 233 received study medication.
Randomised was as follows: Minocycline 1 mg/kg n = 59, 2 mg/kg n = 59, 3 mg/kg n = 60; placebo n = 55. It was stratified based on participant weight. There were 49 dropouts: 8 participants were randomised but not given medication, 16 discontinued due to adverse events, 9 withdrew consent, 8 were lost to follow up, and for 16 no reasons were given (described as "other" on page 13). The dropouts were not stated by group. A total of 57 failed to complete trials to follow up, leaving 184 evaluable out of 241 randomised, or n = 231 who received medication. The mean age was 17.7 years (range = 17 to 19 year). It was not stated where participants were recruited from. The groups were comparable at baseline in terms of demographics and baseline lesion counts. Participants had moderate to severe acne. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not stated Appearance: not stated Instructions: to be taken in the morning Skin hygiene: not stated Empty stomach: not stated Compliance: not stated |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: United States multicentre Language: English Review version: 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was described as in the following quote (page 12): "Subjects randomly were assigned to 1 of 3 active treatments (1, 2 or 3 mg/kg daily) or placebo). No further details given about method of randomisation. Randomisation was stratified by participant's weight." Comment: This was probably done. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. Comment: This was probably done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated to be double‐blind. Comment: This was probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 241 participants were randomised; 233 received treatment. 49 dropped out or were lost to follow up with reasons given in the Results (page 13). Analsyses were performed as ITT (on participants who received the study drug). "Last observation carried forward" was used to impute missing data. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes: There was a reduction in the number of inflammatory lesions from day 1 to day 84. Secondary outcomes: There was a reduction in the number of inflammatory lesions at interim visits, changes in non‐inflammatory (open and closed comedones) and total inflammatory and non‐inflammatory lesion counts and changes in the investigator's static global evaluation of acne severity. Safety assessments. All outcomes were reported at given time points. |
Waskiewicz 1992.
| Methods |
|
|
| Participants | 74 participants were enrolled.
38 participants were randomised in the minocycline group, and 36 participants were randomised in the doxycycline group.
There were 8 (21%) dropouts in the minocycline group and 6 (17%) in the doxycycline group.
The age of the participants was over 15 years.
Recruitment was fulfilled by hospital out‐patients. At baseline, the groups were compared to find differences between their TLC and acne score. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
Concomitant therapy: not permitted Appearance: standard Instructions: with main meal Skin hygiene: not specified Empty stomach: no Compliance: not specified |
|
| Outcomes |
Outcomes of the trial
|
|
| Notes | Country: France
Language: French Review version: 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The investigators stated that the trial "was performed on 74 participants, randomly divided into 2 groups. In the course of the study 14 participants gave up and each of them was replaced by a new participant to maintain finally the number of 30 in each group. 3 participants dropped out and were re‐included in the trial, 3 to 6 months after their dropout. In the meantime their acne did not improve spontaneously or with other treatments." Comment: It was unclear if the randomisation was adequate, but it was probably high risk of bias due to unusual randomisation method. |
| Allocation concealment (selection bias) | High risk | This was not used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This was an open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This was deemed high risk due to the re‐inclusion of dropouts. 60/74 competed, three who withdrew were re‐included after 3 to 6 months. |
| Selective reporting (reporting bias) | High risk | Results were given as percentage improvement and acne counts. No standard deviations were given. |
Abbreviations: AA = azelaic acid; ACID = concomitant antacids; ADR = adverse drug reactions; BF = lactating/breast feeding; Cc = closed comedone; Co = open comedone; Dr‐assessed = doctor (physician)‐assessed; ECLA = Echelle de Cotation des Lésions d'Acné or Acne Lesion Score Scale; ER= extended‐release; IL = inflammatory lesion; ILC = inflammatory lesion count; ILL = significant systemic illness; IRON = concomitant iron supplements; ITT = intention‐to‐treat analysis; LOCF = last observation carried forward; MAC = macule; MDR = history of multiple drug reactions; n = number; NA = not applicable; NIL = non‐inflammatory lesion; NILC = non‐inflammatory lesion count; NOD = nodule; OC = taking oral contraceptives; PA = papule; PREG = pregnancy; PU = pustule; RCT = Randomised controlled trial; SENS = history of sensitivity to tetracyclines; TLC = total lesion count; VAS = visual analogue scale; VERT = vertigo
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alberto 1990 | This was an uncontrolled clinical study. |
| Altieri 1989 | This was an uncontrolled clinical study. |
| Anonymous 2006 | This was a summary paper. |
| Arata 1969 | This undertook bacteriological evaluation, and there was no clinical data. |
| Arrese 1998 | This undertook bacterial viability evaluation, comparing minocycline and lymecycline. There was no clinical data. |
| Barba 1989 | This was an uncontrolled clinical study. |
| Barba Gomez 1990 | This was an uncontrolled clinical study. |
| Becker 1974 | This was an uncontrolled clinical study in participants with tetracycline‐recalcitrant acne. |
| Bodokh 1997 | This was an RCT on the evaluation of impact of minocycline on pilosebaceous follicles. |
| Bok 1985 | This was an uncontrolled, retrospective clinical study. |
| Clerico 1984 | This was an uncontrolled clinical study. |
| Cohen1985 | This was an uncontrolled clinical study in participants with antibiotic‐recalcitrant acne. |
| Coskey 1976 | This was an uncontrolled clinical study in participants with antibiotic‐recalcitrant acne. |
| Cullen 1978 | This was an uncontrolled clinical study in participants with tetracycline‐recalcitrant acne. |
| Degitz 2008 | This was a review. |
| Degreef 1983 | This was an uncontrolled clinical study in participants with antibiotic‐recalcitrant acne. |
| Del Rosso 2004 | This was a review. |
| Donadini 1989 | All participants were given minocycline then they were randomised to topical treatment with either meclocycline or placebo. |
| Eady 1990 | This was a bacteriological investigation with additional clinical data. |
| Eady 1993 | This was a bacteriological investigation with additional clinical data. |
| Fernandez‐Obregon 2000 | This was a retrospective study. |
| Funt 1985 | This was an uncontrolled clinical investigation of combination therapy with minocycline and ibuprofen. |
| Goto 1969 | This was a bacteriological investigation. |
| Goulden 1996 | This was a non‐randomised uncontrolled study. |
| Gruber 1998 | This was a non‐randomised controlled clinical trial. |
| Hughes 1989 | This was a non‐randomised study in participants with acne recalcitrant to erythromycin/benzoyl peroxide combination therapy. |
| Jeanmougin 1987 | This was an uncontrolled clinical evaluation of combination therapy with minocycline and benzoyl peroxide. |
| Ketelbey 1988 | This was an uncontrolled clinical study. |
| Kircik 2010 | This was a review. |
| Kircik 2011 | This was a commentary of minocycline therapy for acne. |
| Kligman 1998 | This was a RCT of microbiological evaluation. |
| Knaggs 1993 | This was a retrospective study in participants with tetracycline‐recalcitrant acne. |
| Kurka 1976 | This was an uncontrolled clinical study. |
| Laux 1987 | This was an interim analysis of Laux 1989. |
| Layton 1992 | This was an abstract of Knaggs 1993. |
| Leyden 1982 | This was a cross‐over study with all participants receiving tetracycline 500 mg bd followed by minocycline 100 mg bd. This was a bacteriological investigation with additional clinical data. |
| Leyden 1996 | There were no clinical outcomes. |
| Leyden 1997a | There were microbial outcomes only. |
| Leyden 2006(Part 1) | The randomisation was broken. |
| Lowy 1982 | This was an uncontrolled clinical study. |
| Luderschimidt 1985 | There were no clinical outcomes; they were microbiological only. |
| Millar 1987 | This was an uncontrolled clinical study. |
| Minami 1969 | This was a bacteriological investigation. |
| Miura 1969 | This was a bacteriological investigation. |
| Mizuno 1980 | This was an uncontrolled clinical study. |
| Mobacken 1993 | This assessed lymecycline, not minocycline. |
| Monk 2011 | This was a review. |
| Montero 1972 | This was an uncontrolled clinical study. |
| Ng 2002 | This was a non‐randomised prospective cohort looking at depressive symptoms in people treated with isotretinoin compared to antibiotics and topical. |
| Nishijima 1996 | This was a controlled clinical study with microbiological outcomes. |
| Ochsendorf 2010a | This was a review. |
| Pablo 1975 | This was a RCT, but it analysed sebum by spectroscopy. |
| Pavone 1994 | This was a non‐randomised, controlled, open‐label study. |
| Randazzo 1981 | There was no control group for minocycline as all participants were allocated to minocycline then randomly assigned Varidase or placebo. |
| Reisner 1983 | This was a review. |
| Rocco 1998 | This trial entailed non‐randomised microbiological evaluation with additional clinical data. |
| Rossman 1981 | This was an open‐label, controlled, cross‐over trial with no wash‐out period. Participants were assigned to tetracycline 250 mg 4 times a day for 6 weeks followed by minocycline 50 mg tds for 6 weeks. |
| Sanchez 2006 | This was an uncontrolled trial. |
| Savage 2010 | This was a review. |
| Schulz 1984 | This was an uncontrolled clinical study. |
| Shalita 2011 | This was a review. |
| Sloan 2008 | This was a review of safety. |
| Takeuchi 1980 | This was an uncontrolled clinical study. |
| Thiboutot 2011 | This was a review. |
| Thielitz 2009 | This was a review. |
| Unna 1989 | This was an uncontrolled clinical study. |
| Villano 1984 | This was an uncontrolled clinical study. |
| Zaenglein 2006 | This was a review. |
Characteristics of studies awaiting assessment [ordered by study ID]
Kawana 2007.
| Methods | We are not able to complete this cell; please see the 'Notes' cell. |
| Participants | Acne |
| Interventions |
|
| Outcomes |
|
| Notes | This paper could not be supplied by the British Library. |
Revuz 1990.
| Methods | We are not able to complete this cell; please see the 'Notes' cell. |
| Participants | Acne |
| Interventions |
|
| Outcomes |
|
| Notes | This paper could not be ordered. The authors were contacted, but they could not supply data. |
Revuz 1993 [pers comm].
| Methods | This was an double‐blind RCT in a hospital setting (17 centres). The duration of the trial was 28 weeks. Industrial support came from Lederle. |
| Participants | 300 participants were enrolled. 98, 96, and 100 participants were randomised, respectively. Recruitment was fulfilled by hospital out‐patients. |
| Interventions |
The appearance of the capsules were identical. |
| Outcomes | These were not known. |
| Notes | Country: France Language: English Review version: 2002 The strength of the benzoyl peroxide was not known. The authors and sponsors were contacted for additional information. The author could not supply additional data. |
Yoon 2005.
| Methods | We are not able to complete this cell; please see the 'Notes' cell. |
| Participants | Acne |
| Interventions |
|
| Outcomes |
|
| Notes | This trial was written in Japanese, and it is awaiting translation. It was not clear if it was randomised. |
Characteristics of ongoing studies [ordered by study ID]
EUCTR2008‐002642‐32‐GB.
| Trial name or title | A Placebo Controlled, Single‐Blind, Pilot Clinical Evaluation of the Effect of a Novel Antibiotic Preparation on the Cutaneous Microflora and Clinical Signs in Acne Patients |
| Methods | This is a randomised, single‐blind trial. |
| Participants | Mild to moderate facial acne vulgaris: grade = 4 |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2009 |
| Contact information | Warner Chilcott United Kingdom |
| Notes | It is not clear whether this trial has completed. |
NCT00240513.
| Trial name or title | A Randomized Study to Compare the Acne Relapse Rate After a 3‐mo Course of Oral Minocycline, to a 3‐mo Course of Oral Minocycline in Combination With a Daily Dose of Topical Tretinoin 0.01% Followed by 3 mo of Topical Tretinoin Alone [sic] |
| Methods | This is a RCT. |
| Participants | Diagnosis of acne vulgaris with a minimum of 20 IL on the face |
| Interventions |
|
| Outcomes |
|
| Starting date | 2004 |
| Contact information | Richard Thomas (Principal Investigator) DermResearch @888 Inc Canada |
| Notes | The Clinical trials register states that this has been terminated. |
NCT00392223.
| Trial name or title | A Phase III, Multicenter, Randomized, Double Blind‐Double Dummy Study, To Evaluate Efficacy And Safety Of Treatment With Azithromycin, Microspheres, Oral Powder For Suspension, 2 G, In One Administration A Week, For 8 Weeks, Compared With Treatment With Minocycline Capsules, 100 Mg Die For 8 Weeks, In Outpatients With Moderate To Severe Inflammatory Acne |
| Methods | This is a randomised, double‐blind, phase III trial in a multicentre setting (8 weeks). |
| Participants | Moderate to severe acne vulgaris |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2007 |
| Contact information | Pfizer Italy |
| Notes | This was terminated. |
NCT00988026.
| Trial name or title | Safety and Efficacy Comparison of Minocycline Microgranules vs Lymecycline in the Treatment of Mild to Moderate Acne. Randomized, Double Blind, Parallel and Prospective Clinical Trial for 8 Weeks |
| Methods | This is a randomised, parallel‐assignment, double‐blind, phase IV trial (8 weeks). |
| Participants | Mild to moderate acne: > 20 NIL and > 15 IL |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2009 |
| Contact information | Luis Leobardo Velazequez‐Arenas leovel2002@yahoo.com.mx |
| Notes | ‐ |
NCT01206348.
| Trial name or title | A Phase IV, Open‐Label Study Evaluating the Use of Solodyn® (Minocycline HCL Extended‐Release Tablets), Ziana, and Triaz Foaming Cloths as Combination Acne Therapy Prior to Treatment With Isotretinoin |
| Methods | This is a phase IV, open‐label RCT (12 weeks). |
| Participants | Moderate to severe acne |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2010 |
| Contact information | Medicis Global Service Corporation |
| Notes | This has been completed. |
NCT01362010.
| Trial name or title | Pilot, Multicenter, Randomized, Double Blind, Placebo Controlled, Parallel Group, Dose Range Finding Study, to Evaluate the Tolerability and Safety of FXFM244 Antibiotic Foam and to Monitor Its Clinical Effect in Acne Vulgaris Patients |
| Methods | This is a pilot, randomised, double‐blind, placebo‐controlled, parallel‐group, dose‐range‐finding trial in a multicentre setting. |
| Participants | Acne vulgaris: minimum of 20, but not more than 50, inflammatory lesions, and 20 to 100 non‐inflammatory lesions |
| Interventions |
|
| Outcomes |
|
| Starting date | July 2011 (but stated as not yet recruiting) |
| Contact information | Avner Shemer Tel‐Nordau Clalit health services |
| Notes | ‐ |
NA = not applicable
Differences between protocol and review
This review was first published in 2000 when there was no requirement to publish a Cochrane Protocol. It was however conducted according to a prespecified systematic review protocol according to best methodological practice. There have been no subsequent amendments to this protocol.
For the 2012 update, an additional 12 RCTs were included, and 16 studies were reviewed to evaluate adverse effects. Changes were made to most sections of the review to reflect the impact of the included studies. Two additional tables were added: Table 24 documented the relative costs of oral antibiotics for acne (BNF April 2012), and Table 25 summarised the included adverse effect studies.
Contributions of authors
SEG was the contact person with the editorial base; SEG coordinated contributions from the co‐authors and wrote the final draft of the review. SEG and CB screened papers against eligibility criteria. SEG obtained data on ongoing and unpublished studies. SEG and CB appraised the quality of papers. SEG and CB extracted data for the review and sought additional information about papers. SEG and CB entered data into RevMan. SEG analysed and interpreted data. SEG and EAE worked on the methods sections. SEG and EAE checked the manuscript for inconsistencies. SEG, CP, and JN drafted the clinical sections of the background and responded to the clinical comments of the referees. SEG responded to the methodology and statistics comments of the referees. KT was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers. SEG is the guarantor of the update.
Sources of support
Internal sources
Leeds Foundation for Dermatological Research, UK.
National Institute for Health and Clinical Excellence, UK.
External sources
-
National Institute for Health Research Cochrane Review Incentive Scheme 2011, UK.
Grant to enable the update to be undertaken
Declarations of interest
One of the reviewers (EAE) has previously received research funds from one of the manufacturers of minocycline, and she was the co‐author of published studies on minocycline, including the following included study: Ozolins M, Eady EA, Avery AJ, Cunliffe PWJ, Wan Po PAL, O'Neill PC, et al. Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet 2004;364(9452):2188‐95.
Associate Professor Catalin Popescu has received honoraria for speaking at Astellas Pharma‐sponsered meetings and symposia. Astellas Pharma is the producer of Unidox (doxycycline) tablets, but none of Associate Professor Popescu's talks and none of these meetings were about Unidox. Unidox is not actively promoted by Astellas, as there are lots of generic doxycycline products. Moreover, minocycline has never been available in Romania, which is the country in which Associate Professor Popescu works.
Edited (no change to conclusions)
References
References to studies included in this review
Blecschmidt 1987 {published data only}
- Blechschmidt J, Engst R, Hoting E, Klovekorn W, Maas B, Meinhof W, et al. Treatment of papulo‐pustular acne‐ comparison of the efficacy and tolerance of minocycline and oxytetracycline [Behandlung der acne papulopustulosa ‐vergleich der wirksamkeit und verträglichkeit von minocyclin und oxytetracyclin]. Munchener Medizinische Wochenschrift 1987;129(29/30):562‐4. [Google Scholar]
Bossuyt 2003 (TETRABUK) {published data only (unpublished sought but not used)}
- Bossuyt L, Bosschaert J, Richert B, Cromphaut P, Mitchell, T, Al Abadie M, et al. Lymecycline in the treatment of acne: an efficacious, safe and cost‐effective alternative to minocycline. European Journal of Dermatology 2003;13(2):130‐5. [PubMed] [Google Scholar]
- Bossuyt L, Richert B, Al Abadie M, Henry I, Bewley AP, Czernielewski J. Safety and efficacy comparison of lymecycline versus minocycline in the treatment of acne vulgaris [Abstract]. 11th Congress of the European Academy of Dermatology & Venereology; 2‐6 October 2002, Prague. 2002:1‐41.
- Czernielewski J, Bossuyt L, Richert B, Al Abadie M, Henry I, Bewley AP. Safety and efficacy comparison of lymecycline versus minocycline in the treatment of acne vulgaris [Poster P0010]. Proceedings of 20th World Congress of Dermatology; 1‐5 July 2002, Paris. 2002:1S372.
Cabezas 1993 {published data only}
- Cabezas AM, Jacial A, Rojas H. A double blind study of the effectiveness of minocycline compared with tetracycline or placebo in the treatment of inflammatory acne [Ensayo terapeutico con minocin]. Revista Chilena De Dermatologia 1993;9(1):6‐11. [Google Scholar]
Campo 2003 {published data only}
- Campo M, Zuluaga A, Escobar P, Motta A, Argote A, Jaramillo C, et al. A comparative study on the effectiveness of lymecycline and minocycline and adapalene in the treatment of acne vulgaris [Poster P0005]. Proceedings of 20th World Congress of Dermatology; 1‐5 July 2002, Paris. 2002:1S371.
- Campo MH, Zuluaga A, Escobar P, Motta A, Argote A, Jaramillot C, et al. Efficacy and safety of lymecycline combined with adapalene gel and minocycline combined with adapalene in the treatment of acne vulgaris. [Abstract P1‐15]. 12th Congress of the European Academy of Dermatology and Venereology. Barcelona, Spain 15‐18th October 2003. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):168. [Google Scholar]
Cullen 1976 {published data only}
- Cullen SI, Cohan RH. Minocycline therapy in acne vulgaris. Cutis 1976;17(6):1208‐10, 1214. [PubMed] [Google Scholar]
Cunliffe 1998 {published and unpublished data}
- Cunliffe WJ, Grosshans E, Belaich S, Meynadier J, Alirezai M, Thomas L. A comparison of the efficacy and safety of lymecycline and minocycline in patients with moderately severe acne vulgaris. European Journal of Dermatology 1998;8(3):161‐6. [PubMed] [Google Scholar]
- Cunliffe WJ, et al. A comparison of the efficacy and safety of lymecycline and minocycline in acne [Abstract 5104]. The 19th World Congress of Dermatology 15‐20 June, Sydney,1997. Australasian Journal of Dermatology 1997;38(Suppl 2):261. [Google Scholar]
Darrah 1996 {published and unpublished data}
- Darrah AJ, Gray PL. An open multicentre study to compare fusidic acid lotion and oral minocycline in the treatment of mild to moderate acne vulgaris of the face. European Journal of Clinical Research 1996;8:97‐107. [Google Scholar]
Drake 1990 {unpublished data only}
- Drake L. Comparative efficacy and tolerance of Cleocin T topical gel (clindamycin phosphate topical gel) versus oral minocycline in the treatment of acne vulgaris. Data on file (Technical Report from Pharmacia and Upjohn Ltd) 1990.
Dreno 1998 [pers comm] {unpublished data only}
- Dreno B ( Department of Cancero‐Dermatology, Hôtel Dieu, Nantes Cedex, France). Trial report [personal communication]. Letter to S Garner (NICE, London, UK) 22 October 1998.
Dreno 2001 {published data only}
- Dreno B, Moyse D, Alirezai M, Amblard P, Auffret N, Beylot C, et al. Multicenter randomized comparative double‐blind controlled clinical trial of the safety and efficacy of zinc gluconate versus minocycline hydrochloride in the treatment of inflammatory acne vulgaris. Dermatology 2001;203(2):135‐40. [DOI] [PubMed] [Google Scholar]
Fallica 1985 {published data only}
- Fallica L, Vignini M, Rodeghiero R. Minocycline and tetracycline hydrochloride in the treatment of acne vulagris: comparative data [La minociclina e la tetraciclina cloridrato nel trattamento dell'acne volgare:dati comparativi]. Dermatologica Clinicia 1985;5(2):145‐50. [Google Scholar]
- Rabbiosi G, Marson G, Sapuppo A. Acne: minocycline and tetracycline treatment. Royal Society of Medicine Services International Congress & Symposium Series 1985;95:121‐5. [Google Scholar]
Fleisch 2006a (MP010404) {published data only}
- Fleischer AB, Dinehart S, Stough D, Plott RT. Safety and efficacy of a new extended‐release formulation of minocycline. Solodyn Phase, 2. Study Group, and Solodyn Phase, 3. Study Group. Cutis 2006;78(4 Suppl):21‐31. [PubMed] [Google Scholar]
Fleisch 2006b (MP010405) {published data only}
- Fleischer AB, Dinehart S, Stough D, Plott RT. Safety and efficacy of a new extended‐release formulation of minocycline. Solodyn Phase, 2. Study Group, and Solodyn Phase, 3. Study Group. Cutis 2006;78(4 Suppl):21‐31. [PubMed] [Google Scholar]
Gollnick 1997 {published and unpublished data}
- Gollnick HPM, Graup K, Zaumseil RP. Comparison of combined azelaic acid cream plus oral minocycline with oral isotretinoin in severe acne. European Journal of Dermatology 2001;11(6):538‐44. [PubMed] [Google Scholar]
- Graupe K, Cunliffe WJ, Gollnick HP, Zaumseil, RP. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis 1996;57(1 Suppl):20‐35. [PubMed] [Google Scholar]
Harrison 1988 {published and unpublished data}
- Harrison PV. A comparison of doxycycline and minocycline in the treatment of acne vulgaris. Clinical & Experimental Dermatology 1988;13(4):242‐4. [DOI] [PubMed] [Google Scholar]
Hayashi 2011 {published data only}
- Hayashi N, Kawashima M. Efficacy of oral antibiotics on acne vulgaris and their effects on quality of life: a multicenter randomized controlled trial using minocycline, roxithromycin and faropenem. Journal of Dermatology 2011;38(2):111‐9. [DOI] [PubMed] [Google Scholar]
Hersle 1976 {published data only}
- Hersle K, Gisslen H. Minocycline in acne vulgaris: a double‐blind study. Current Therapeutic Research 1976;19(3):339‐42. [PubMed] [Google Scholar]
Hubbell 1982 {published data only}
- Hubbell CG, Hobbs ER, Rist T, White JW. Efficacy of minocycline compared with tetracycline in treatment of acne vulgaris. Archives of Dermatology 1982;118(12):989‐92. [PubMed] [Google Scholar]
Khanna 1993 {published and unpublished data}
- Khanna N. Treatment of acne vulgaris with oral tetracyclines. Indian Journal of Dermatology, Venereology & Leprology 1993;59(2):74‐6. [PubMed] [Google Scholar]
Laux 1989 {published data only}
- Laux B. Treatment of acne vulgaris. A comparison of doxycycline versus minocycline [Behandlung der acne vulgaris‐ein vergleich von doxycyclin versus minocyclin]. Hautarzt 1989;40(9):577‐81. [PubMed] [Google Scholar]
Leyden 2004 {published data only}
- Leyden J, Bergfeld W, Drake L, Dunlap F, Goldman MP, Gottlieb AB, et al. A systemic type I 5 alpha‐reductase inhibitor is ineffective in the treatment of acne vulgaris. Journal of the American Academy of Dermatology 2004;50(3):443‐7. [DOI] [PubMed] [Google Scholar]
Leyden 2006 (Part 2) {published data only}
- Leyden J, Thiboutot DM, Shalita A, Webster G, Washenik K, Strober BE, et al. Comparison of tazarotene and minocycline maintenance therapies in acne vulgaris: a multicentre, double‐blind, randomised parallel‐group study. Archives of Dermatology 2006;142(5):605‐12. [DOI] [PubMed] [Google Scholar]
Lorette 1994 {published data only}
- Lorette G, Belaich S, Beylot MC, Ortonne JP. Doxycycline (Tolexine) 50 mg/day versus minocycline 100 mg/day in the treatment of acne vulgaris. Evaluation on four months of treatment [Doxycycline (Tolexine) 50mg/jour versus minocycline 100mg/jour dans le traitement de l'acné juvénile. Evaluation sur quatr mois de traitement]. Nouvelles Dermatologiques 1994;13(9):662‐5. [Google Scholar]
Monk 1987 {published data only}
- Monk BE, Almeyda JA, Caldwell IW. Efficacy of low‐dose cyproterone acetate compared with minocycline in the treatment of acne vulgaris. Clinical & Experimental Dermatology 1987;12(5):319‐22. [DOI] [PubMed] [Google Scholar]
Olafsson 1989 {published data only}
- Ólafsson JH, Gudgeirsson J, Eggertsdóttir GE, Kristjánsson F. Doxycycline versus minocycline in the treatment of acne vulgaris: a double‐blind study. Journal of Dermatological Treatment 1989;1(1):15‐7. [Google Scholar]
Ozolins 2005 {published and unpublished data}
- Kerst AJF. Acne vulgaris: comparison of five antimicrobial regimens [Acne vulgaris: vergelijking van vijf antimicrobiele regimens]. Geneesmiddelenbulletin 2005;39(6):68‐9. [Google Scholar]
- Ozolins M, Eady EA, Avery A, Cunliffe WJ, Li Wan Po A, O'Neill C, et al. A cost‐effective rationale for the selection of antimicrobial therapy in acne: a randomized controlled trial [Abstract]. 11th Congress of the European Academy of Dermatology and Venereology; 2‐6 October, Prague. 2002:P1‐1.
- Ozolins M, Eady EA, Avery A, Cunliffe WJ, Li Wan Po A, O'Neill C, et al. A cost‐effectiveness rationale for the selection of antimicrobial therapy in acne: a randomized controlled trial [Abstract RF‐2]. British Journal of Dermatology 2002;147(Suppl 62):13. [Google Scholar]
- Ozolins M, Eady EA, Avery A, Cunliffe WJ, O'Neill C, Simpson NB, et al. Randomised controlled multiple treatment comparison to provide a cost‐effectiveness rationale for the selection of antimicrobial therapy in acne. Health Technology Assessment (Final HTA acne study report MO 24/03/04) 2005; Vol. 9, issue 1:iii‐100. [DOI] [PubMed]
- Ozolins M, Eady EA, Avery AJ, Cunliffe PWJ, Wan Po PAL, O'Neill PC, et al. Comparison of five antmicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet 2004;364(9452):2188‐95. [DOI] [PubMed] [Google Scholar]
Peacock 1990 {published data only}
- Peacock CE, Price C, Ryan BE, Mitchell AD. Topical clindamycin (Dalacin T) compared to oral minocycline (Minocin 50) in treatment of acne vulgaris. A randomized observer‐blind controlled trial in three university student health centres. Clinical Trials Journal 1990;27(3):219‐28. [Google Scholar]
Pelfini 1989 {published data only}
- Pelfini C, Bonino S, Privitera G, Cerimele D, Venier A, Serri F. Josamycin versus minocycline in the treatment of papulopustular acne. Journal of Chemotherapy 1989;1(4 Suppl):921‐3. [PubMed] [Google Scholar]
Pierard 2002 {published data only}
- Pierard‐Franchimont C, Goffin V, Arrese JE, Martalo O, Braham C, Slachmuylders P, et al. Lymecycline and minocycline in inflammatory acne: a randomized, double‐blind intent‐to‐treat study on clinical and in vivo antibacterial efficacy. Skin Pharmacology & Applied Skin Physiology 2002;15(2):112‐9. [DOI] [PubMed] [Google Scholar]
Pigatto 1986 {published data only}
- Pigatto PD, Finzi AF, Altomare GF. Isotretinoin versus minocycline in cystic acne: a study of lipid metabolism. Dermatologica 1986;172(3):154‐9. [DOI] [PubMed] [Google Scholar]
Revuz 1985 {published data only}
- Revuz JE, Guillaume JC, Poli F, Pouget F, Zeller J, Boitier F. Controlled trial of minocycline in acne patients. Royal Society of Medicine Services International Congress & Symposium Series 1985;95:103‐8. [Google Scholar]
Ruping 1985 {published data only}
- Ruping KW, Tronnier H. Acne therapy: results of a clinical multi‐centre study with minocycline. Royal Society of Medicine Services International Congress & Symposium Series 1985;95:109‐19. [Google Scholar]
Samuelson 1985 {published data only}
- Samuelson JS. An accurate photographic method for grading acne: initial use in a double‐blind clinical comparison of minocycline and tetracycline. Journal of the American Academy of Dermatology 1985;12(3):461‐7. [DOI] [PubMed] [Google Scholar]
Schollhammer 1994 {published data only}
- Schollhammer M, Alirezai M. Comparative study of lymecycline, minocycline and doxycycline in the treatment of acne vulgaris [Etude comparative de la lymécycline, de la minocycline et de la doxycycline dans le traitement de l'acné vulgaire]. Réalités Thérapeutiques en Dermato‐Vénérologie 1994;42:24‐6. [Google Scholar]
Sheehan‐Dare 1989 {published data only}
- Sheehan‐Dare RA, Papworth‐Smith J, Cunliffe WJ. A comparative study between topical clindamycin and oral minocycline in the treatment of acne vulgaris. Round Table Series ‐ Royal Society of Medicine 1989;19:24‐8. [Google Scholar]
- Sheehan‐Dare RA, Papworth‐Smith J, Cunliffe WJ. A double‐blind comparison of topical clindamycin and oral minocycline in the treatment of acne vulgaris. Acta Dermato‐Venereologica 1990;70(6):534‐7. [PubMed] [Google Scholar]
Smit 1978 {published data only}
- Smit F. Minocycline versus doxycycline in the treatment of acne vulgaris. Dermatologica 1978;157(3):186‐90. [DOI] [PubMed] [Google Scholar]
Stainforth 1993 {published and unpublished data}
- Stainforth J, MacDonald‐Hull S, Papworth‐Smith JW, Eady EA, Cunliffe WJ, Norris JFB, et al. A single‐blind comparison of topical erythromycin/zinc lotion and oral minocycline in the treatment of acne vulgaris. Journal of Dermatological Treatment 1993;4(3):119‐22. [Google Scholar]
Stewart 2006 (MP010401) {published data only}
- Stewart DM, Torok HM, Weiss JS, Plott RT. Dose‐ranging efficacy of new once‐daily extended‐release minocycline for acne vulgaris. Cutis 2006;78(4 Suppl):11‐20. [PubMed] [Google Scholar]
Waskiewicz 1992 {published data only}
- Waskiewicz W, Grosshans E. Treatment of acne vulgaris with cyclines of second generation: a comparison of doxycyline 50mg daily versus minocycline 100mg daily [Traitement de l'acné juvénile par les cyclines de deuxième génération: étude comparative randomisée doxycycline 50mg/jour versus minocycline 100mg/ jour]. Nouvelles Dermatologiques 1992;11:8‐11. [Google Scholar]
References to studies excluded from this review
Alberto 1990 {published data only}
- Alberto N, Abubakar AA. Clinical trial of minocycline in the treatment of Filipino patients with acne vulgaris. Philippine Journal of Internal Medicine 1990;28(6):457‐61. [Google Scholar]
Altieri 1989 {published data only}
- Altieri E, Venturini D. Clinical findings on the therapeutic activity of minocycline in acne vulgaris [Rilievi clinici sull'attività terapeutica della minociclina nell'acne giovanile]. Dermatologia Clinica 1989;9(2):107‐15. [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. Extended‐release minocycline (Solodyn) for acne. Medical Letter on Drugs & Therapeutics 2006;48(1248):95‐6. [PubMed] [Google Scholar]
Arata 1969 {published data only}
- Arata J, Fujita S, Tokumaru S, Kodama H. Clinical experience with minocycline in the dermatological field (Japanese). Japanese Journal of Antibiotics 1969;22(6):480‐2. [PubMed] [Google Scholar]
Arrese 1998 {published data only}
- Arrese JE, Goffin V, Avila‐Camacho M, Greimers R, Pierard GE. A pilot study on bacterial variability in acne. Assessment using dual flow cytometry on microbials present in follicular casts and comedones. International Journal of Dermatology 1998;37(6):461‐464. [DOI] [PubMed] [Google Scholar]
Barba 1989 {published data only}
- Barba JF, Jimenez Brito G, Hermoso I, Morales Ortiz R, Altamirano L. Effectiveness and safety of oral minocycline in treating acne vulgaris [Evaluación de la minociclina (minocin) en acné vulgaris inflamatorio]. Compendium de Investigaciones Clinicas Latinoamericanas 1989;9(3):99‐107. [Google Scholar]
Barba Gomez 1990 {published data only}
- Barba Gómez JF, Morales Ortiz R. An evaluation of minocycline (minocin) in inflammatory acne vulgaris [Evaluación de la minociclina (minocin) en acné vulagris inflamatorio]. Investigación Médica Internacional 1990;16(4):240‐4. [Google Scholar]
Becker 1974 {published data only}
- Becker FT. Treatment of tetracycline‐resistant acne vulgaris. Cutis 1974;14(4):610‐13. [Google Scholar]
Bodokh 1997 {published data only}
- Bodokh I, Jacomet Y, Lacour JP, Ortonne JP. Minocycline induces an increase in the number of excreting pilosebaceous follicles in acne vulgaris. A randomised study. Acta Dermato‐Venereologica 1997;77(4):255‐259. [DOI] [PubMed] [Google Scholar]
Bok 1985 {published data only}
- Bok LB. Practical experience with minocycline. Royal Society of Medicine Services International Congress & Symposium Series 1985;95:83‐6. [Google Scholar]
Clerico 1984 {published data only}
- Clerico R, Cantoresi F, Bianchini D. Therapeutic activity of minocycline in patients with acne vulgaris [Attività terapeutica della minociclina in pazienti affetti da acne volgare]. Dermatologia Clinica 1984;4(4):341‐9. [Google Scholar]
Cohen1985 {published data only}
- Cohen PM. A general practice study investigating the effect of Minocin 50 mg b.d. for 12 weeks in the treatment of acne vulgaris. Journal of International Medical Research 1985;13(4):214‐21. [DOI] [PubMed] [Google Scholar]
Coskey 1976 {published data only}
- Coskey RJ. Acne: treatment with minocycline. Cutis 1976;17(4):799‐801. [PubMed] [Google Scholar]
Cullen 1978 {published data only}
- Cullen SI. Low‐dose minocycline therapy in tetracycline‐recalcitrant acne vulgaris. Cutis 1978;21(1):101‐4. [PubMed] [Google Scholar]
- Cullen SI. Treatment of tetracycline resistant acne vulgaris with minocycline in a low dosage. Therapiewoche 1982;32(44):5347‐51. [Google Scholar]
Degitz 2008 {published data only}
- Degitz K, Ochsendorf F. Pharmacotherapy of acne. Expert Opinion on Pharmacotherapy 2008;9(6):955‐71. [DOI] [PubMed] [Google Scholar]
Degreef 1983 {published data only}
- Degreef H. Minocycline in the treatment of acne vulagris. Current Therapeutic Research 1983;33(1):8‐13. [Google Scholar]
Del Rosso 2004 {published data only}
- Rosso JQ. A status report on the use of subantimicrobial‐dose doxycycline: a review of the biologic and antimicrobial effects of the tetracyclines. Cutis 2004;74(2):118‐22. [PubMed] [Google Scholar]
Donadini 1989 {published data only}
- Donadini A. Is topical antibiotic therapy associated with the same oral treatment useful in patients with acne? [Ha significato nell'acne l'impiego di un antibiotico topico nel corso di analoga terapia sistemica?]. Annali Italiani di Dermatologia Clinica e Sperimentale 1989;43(2):153‐69. [Google Scholar]
Eady 1990 {published data only}
- Eady EA, Cove JH, Holland KT, Cunliffe WJ. Superior antibacterial action and reduced incidence of bacterial resistance in minocycline compared to tetracycline‐treated acne patients. British Journal of Dermatology 1990;122(2):233‐44. [DOI] [PubMed] [Google Scholar]
Eady 1993 {published data only}
- Eady EA, Jones CE, Gardner KJ, Taylor JP, Cove JH, Cunliffe WJ. Tetracycline‐resistant propionibacteria from acne patients are cross resistant to doxycycline but sensitive to minocycline. British Journal of Dermatology 1993;128(5):556‐60. [DOI] [PubMed] [Google Scholar]
Fernandez‐Obregon 2000 {published data only}
- Fernandez‐Obregon AC. Azithromycin for the treatment of acne. International Journal of Dermatology 2000;39(1):45‐50. [DOI] [PubMed] [Google Scholar]
Funt 1985 {published data only}
- Funt LS. Oral ibuprofen and minocycline for the treatment of resistant acne vulgaris. Journal of the American Academy of Dermatology 1985;13(3):524‐5. [DOI] [PubMed] [Google Scholar]
Goto 1969 {published data only}
- Goto M. Evaluation of minocycline (7‐dimethylamino‐6‐demethyl‐6‐deoxytetracycline) in the dermatological field (Japanese). Japanese Journal of Antibiotics 1969;22(6):488‐92. [PubMed] [Google Scholar]
Goulden 1996 {published data only}
- Goulden V, Glass D, Cunliffe WJ. Safety of long‐term high‐dose minocycline in the treatment of acne. British Journal of Dermatology 1996;134(4):693‐695. [DOI] [PubMed] [Google Scholar]
Gruber 1998 {published data only}
- Gruber F, Grubisic‐Greblo H, Kastelan M, Brajac I, Lenkovic M, Zamolo G. Azithromycin compared with minocycline in the treatment of acne comedonica and papulo‐pustulosa. Journal of Chemotherapy 1998;10(6):469‐73. [DOI] [PubMed] [Google Scholar]
Hughes 1989 {published data only}
- Hughes BR, Murphy CE, Barnett J, Cunliffe WJ. Strategy of acne therapy with long‐term antibiotics. British Journal of Dermatology 1989;121(5):623‐8. [DOI] [PubMed] [Google Scholar]
Jeanmougin 1987 {published data only}
- Jeanmougin M. Minocycline and benzoyl peroxide in the treatment of acne vulgaris: Results of a multicentre trial with 256 patients [Minocycline et peroxyde de benzoyle dans le traitement de l'acné: résultats d'une étude multicentrique sur 256 patients]. Comptes Rendus de Thérapeutique et de Pharmacologie Clinique 1987;5(50):3,5,7‐9,11. [Google Scholar]
Ketelbey 1988 {unpublished data only}
- Ketelbey JW. Acne and minocycline. New Zealand Medical Journal 1988;101(851):520. [PubMed] [Google Scholar]
Kircik 2010 {published data only}
- Kircik LH. Doxycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. Journal of Drugs in Dermatology 2010;9(11):1407‐1411. [PubMed] [Google Scholar]
Kircik 2011 {published data only}
- Kircik LH. Commentary: Doxycycline vs. minocycline for the management of acne. Journal of Drugs in Dermatology 2011;10(9):966. [PubMed] [Google Scholar]
Kligman 1998 {published data only}
- Kligman AM. Comparison of a topical benzoyl peroxide gel, oral minocycline, oral doxycycline and a combination for suppression of P. acnes in acne patients. Journal of Dermatological Treatment 1998;9(3):187‐191. [Google Scholar]
Knaggs 1993 {published data only}
- Knaggs HE, Layton AM, Cunliffe WJ. The role of oral minocycline and erythromycin in tetracycline therapy‐resistant acne ‐ a retrospective study and a review. Journal of Dermatological Treatment 1993;4(2):53‐6. [Google Scholar]
Kurka 1976 {published data only}
- Kurka M, Orfanos CE. Which antibiotics are helpful in acne? A review of the literature and personal test results with minocyclin (klinomycin) [Welche antibiotika helfen bei akne?]. Zeitschrift fur Hautkrankheiten 1976;51(2):45‐54. [PubMed] [Google Scholar]
Laux 1987 {published data only}
- Laux B. Treatment of acne vulgaris [Behandlung der acne vulgaris: vergleichende klinische studie mit doxycyclin 50mg/d versus minocyclin 100mg/d]. Ärztliche Kosmetologie 1987;17(5):366‐72. [Google Scholar]
Layton 1992 {published data only}
- Layton AM, Cunliffe WJ, Knaggs HE, MacDonald‐Hull SP. The role of minocycline in therapy resistant acne (Abstract). British Journal of Dermatology 1992;127(suppl. 40):31. [Google Scholar]
Leyden 1982 {published data only}
- Leyden JJ, McGinley KJ, Kligman AM. Tetracycline and minocycline treatment. Effects on skin‐surface lipid levels and Propionibacterium acnes. Archives of Dermatology 1982;118(1):19‐22. [PubMed] [Google Scholar]
Leyden 1996 {published data only}
- Leyden JJ, Kaidbey K, Gans EH. The antimicrobial effects in vivo of minocycline, doxycycline and tetracycline in humans. Journal of Dermatological Treatment 1996;7(4):223‐225. [Google Scholar]
Leyden 1997a {published data only}
- Leyden JJ, Gans EH. The human in vivo antimicrobial effects of dual acne therapy: Oral Dynacin (minocycline HCl) plus topical Triaz (benzoyl peroxide special gel). Journal of Dermatological Treatment 1997;8(Suppl 2):S3‐6. [Google Scholar]
Leyden 2006(Part 1) {published data only}
- Leyden J, Thiboutot DM, Shalita A, Webster G, Washenik K, Strober BE, et al. Comparison of tazarotene and minocycline maintenance therapies in acne vulgaris: a multicentre, double‐blind, randomised parallel‐group study. Archives of Dermatology 2006;142(5):605‐612. [DOI] [PubMed] [Google Scholar]
Lowy 1982 {published data only}
- Lowy G. Minocycline and acne [Minociclina e acne]. Folha Medica 1982;85(1):539‐40. [Google Scholar]
Luderschimidt 1985 {published data only}
- Luderschmidt C, Nissen H, Neubert U, Knuechel M. Newer methods for measuring therapeutic response in acne. Royal Society of Medicine Services International Congress & Symposium Series 1985;95:131‐40. [Google Scholar]
Millar 1987 {published data only}
- Millar ED, Jolliffe DS, Leigh AP. A general practice study investigating the effect of minocycline (Minocin) 50 mg bd for 12 weeks in the treatment of acne vulgaris. British Journal of Clinical Practice 1987;41(8):882‐6. [PubMed] [Google Scholar]
Minami 1969 {published data only}
- Minami K, Tashiro M. Comparison of clinical effects of minocycline and demethylchlortetracycline in the dermatological field (Japanese). Japanese Journal of Antibiotics 1969;22(6):493‐500. [PubMed] [Google Scholar]
Miura 1969 {published data only}
- Miura Y, Mizumoto T, Shibaki H. Minocycline (Japanese). Japanese Journal of Antibiotics 1969;22(6):483‐7. [PubMed] [Google Scholar]
Mizuno 1980 {published data only}
- Mizuno K. Clinical experiences of minocycline on acne (Japanese). Nishinihon Journal of Dermatology 1980;42(5):874‐7. [Google Scholar]
Mobacken 1993 {published data only}
- Mobacken H. Oral tetracycline‐treatment of acne. Rapid facial improvement, but back lesions are more difficult to treat (Swedish). Lakartidningen 1993;90(34):2755‐7. [PubMed] [Google Scholar]
Monk 2011 {published data only}
- Monk E, Shalita A, Siegel DM. Clinical applications of non‐antimicrobial tetracyclines in dermatology. Pharmacological Research 2011;63(2):130‐45. [DOI] [PubMed] [Google Scholar]
Montero 1972 {published data only}
- Montero ED. Minocycline (therapeutic evaluation in various skin conditions) [Minociclina (ensayo therapeutico en diversas dermatosis)]. Medicina Cutanea 1972;6:105. [Google Scholar]
Ng 2002 {published data only}
- Ng CH, Tam MM, Celi E, Tate B, Schweitzer I. Prospective study of depressive symptoms and quality of life in acne vulgaris patients treated with isotretinoin compared to antibiotic and topical therapy. Australasian Journal of Dermatology 2002;43(4):262‐8. [DOI] [PubMed] [Google Scholar]
Nishijima 1996 {published data only}
- Nishijima S, Kurokawa I, Kawabata S. Sensitivity of Propionibacterium acnes isolated from acne patients: comparative study of antimicrobial agents. Journal of International Medical Research 1996;24(6):473‐7. [DOI] [PubMed] [Google Scholar]
Ochsendorf 2010a {published data only}
- Ochsendorf F. Minocycline in acne vulgaris: benefits and risks. American Journal of Clinical Dermatology 2010;11(5):327‐41. [DOI] [PubMed] [Google Scholar]
Pablo 1975 {published data only}
- Pablo GM, Fulton JE. Sebum: analysis by infrared spectroscopy. II. The suppression of fatty acids by systemically administered antibiotics. Archives of Dermatology 1975;111(6):734‐5. [DOI] [PubMed] [Google Scholar]
Pavone 1994 {published data only}
- Pavone P. Azithromycin versus minocycline in the treatment of acne vulgaris [Azitromicina versus minociclina nel trattamento dell' acne volgare]. Chronica Dermatologica 1994;4(5):867‐72. [Google Scholar]
Randazzo 1981 {published data only}
- Randazzo SD, Lo Presti V, Caruso A. Clinical evaluation of combined minocycline streptokinase‐streptodornase treatment in acne [Valutazione clinica del trattamento con minociclina piu streptochinasi‐streptodornasi nell'acne volgare]. Annali Italiani di Dermatologia Clinica e Sperimentale 1981;35(1):67‐77. [Google Scholar]
Reisner 1983 {published data only}
- Reisner RM. Antibiotic and anti‐inflammatory therapy of acne. Dermatologic Clinics 1983;1(3):385‐97. [Google Scholar]
Rocco 1998 {published data only}
- Rocco D, Pagnini U, Rocco A. Effect of cefuroxime axetil in the treatment of papulo‐pustular acne [L'acetossietilcefuroxime nel trattamento dell'acne papulo‐pustulosa]. Giornale Italiano di Dermatologia e Venereologia 1998;133(6):465‐8. [Google Scholar]
Rossman 1981 {published data only}
- Rossman RE. Minocycline treatment of tetracycline‐resistant and tetracycline‐responsive acne vulgaris. Cutis 1981;27(2):196‐7, 201, 207. [PubMed] [Google Scholar]
Sanchez 2006 {published data only}
- Sanchez AT, Olivera RMP, Sanchez GMDO, Dorantes GL. Quality of life and psychological symptoms in patients with severe acne in treatment with isotretinoin [Calidad de vida y sintomas psicologicos en pacientes con acne severo tratados con isotretinoina]. Dermatologia Revista Mexicana 2006;50(4):121‐6. [Google Scholar]
Savage 2010 {published data only}
- Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Review of Clinical Pharmacology 2010;3(4):563‐80. [DOI] [PubMed] [Google Scholar]
Schulz 1984 {published data only}
- Schulz H, Stauder G. Oral minocycline treatment for acne vulagris and for rosacea [Orale minocyclintherapie der akne vulgaris und der rosacea]. Zeitschrift für Allgemeinmedizin 1984;60(24):1033‐7. [Google Scholar]
Shalita 2011 {published data only}
- Shalita AR, Webster GF, Wortzman MS, Nelson DB. Doxycycline vs. minocycline for the management of acne. Journal of Drugs in Dermatology 2011;10(9):965‐6. [PubMed] [Google Scholar]
Sloan 2008 {published data only}
- Sloan B, Scheinfeld N. The use and safety of doxycycline hyclate and other second‐generation tetracyclines. Expert Opinion on Drug Safety 2008;7(5):571‐7. [DOI] [PubMed] [Google Scholar]
Takeuchi 1980 {published data only}
- Takeuchi S, Morishita M. Treatment of acne vulgaris with minomycin capsules (50mg) (Japanese). Nishinihon Journal of Dermatology 1980;42(5):870‐3. [Google Scholar]
Thiboutot 2011 {published data only}
- Thiboutot D. Rethinking treatment of acne in the severe patient. Journal of Drugs in Dermatology 2011;10(6):s8‐s12. [PubMed] [Google Scholar]
Thielitz 2009 {published data only}
- Thielitz A, Gollnick H. Overview of new therapeutic developments for acne. Expert Review of Dermatology 2009;4(1):55‐65. [Google Scholar]
Unna 1989 {published data only}
- Unna PJ, Ischler T. Pragmatic treatment of juvenile acne [Pragmatische therapie der acne juvenilis]. Zeitschrift für Allgemeinmedizin 1989;65(34):860‐4. [Google Scholar]
Villano 1984 {published data only}
- Villano PA. Minocycline in the treatment of acne vulgaris [La minociclina nel trattamento dell'acne volgare]. Dermatologia Clinica 1984;4(1):71‐4. [Google Scholar]
Zaenglein 2006 {published data only}
- Zaenglein AL, Thiboutot DM. Expert committee recommendations for acne management. Pediatrics 2006;118(3):1188‐99. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kawana 2007 {published data only}
- Kawana S, Ueno T, Shimoda T. Efficacy of long‐term administration of roxithromycin (Rulid) in treatment of acne: comparison with minocycline hydrochloride. Nishinihon Journal of Dermatology 2007;69(4):424‐7. [Google Scholar]
Revuz 1990 {published data only}
- Revuz J, Amblard P, Dreno B, Lorette G, Ortonne JP, Puissant A. Efficacy of zinc gluconate in the treatment of inflammatory acne [Efficacite du gluconate de zinc dans le traitement de l'acne inflammatoire]. Abstr Dermatol 1990;77:2‐4. [Google Scholar]
Revuz 1993 [pers comm] {unpublished data only}
- Revuz J ( 11, Chaussée de la Muette, Paris, France). [personal communication] [Evaluation comparative en double aveugle de trois strategies de traitement de l'acne avec minocycline microgranules 100mg/jour associee au peroxyde de benzoyle (unpublished)]. Letter to S Garner (NICE, London, UK) 11 October 1993.
Yoon 2005 {published data only}
- Yoon YH, Ro BI, Seo SJ, Kim MN, Hong CK. Comparison of cost and effectiveness between isotretinoin versus minocycline in the treatment of patients with acne. Korean Journal of Dermatology 2005;43(9):1200‐6. [Google Scholar]
References to ongoing studies
EUCTR2008‐002642‐32‐GB {published data only}
- EUCTR2008‐002642‐32‐GB. A Placebo Controlled, Single‐Blind, Pilot Clinical Evaluation of the Effect of a Novel Antibiotic Preparation on the Cutaneous Microflora and Clinical Signs in Acne Patients. //apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2008‐002642‐32‐GB Accessed 16 April 2012.
NCT00240513 {published data only}
- NCT00240513. Study Comparing Acne in Patients Taking Oral Minocycline to Patients Taking Minocycline Plus Topical Tretinoin. clinicaltrials.gov/ct2/show/NCT00240513?term=NCT00240513&rank=1 Accessed 16 April 2012.
NCT00392223 {published data only}
- NCT00392223. Efficacy And Safety Of Azithromycin SR Compared With Minocycline In Acne. clinicaltrials.gov/ct2/show/NCT00392223?term=NCT00392223&rank=1 Accessed 16 April 2012.
NCT00988026 {published data only}
- NCT00988026. Safety and Efficacy Comparison of Minocycline Microgranules Versus Lymecycline in the Treatment of Mild to Moderate Acne (MXMIN‐001). clinicaltrials.gov/ct2/show/NCT00988026 Accessed 16 April 2012.
NCT01206348 {published data only}
- NCT01206348. Combination Treatment for Moderate to Severe Acne. clinicaltrials.gov/ct2/show/NCT01206348?term=NCT01206348&rank=1 Accessed 16 April 2012.
NCT01362010 {published data only}
- NCT01362010. Clinical Study to Evaluate Tolerability and Safety of FXFM244 and to Monitor Clinical Effect in Acne Vulgaris Patients. clinicaltrials.gov/ct2/show/NCT01362010?term=NCT01362010&rank=1 Accessed 16 April 2012.
Additional references
AFSSAPS 2009
- Editorial. Fewer adverse effects with doxycycline than with minocycline. Prescrire International 2009;18(103):213. [PubMed] [Google Scholar]
Agruh 2006
- Agruh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycyclines. Journal of Antimicrobial Chemotherapy 2006;58(2):256‐265. [DOI] [PubMed] [Google Scholar]
Allen 1976
- Allen JC. Minocycline. Annals of Internal Medicine 1976;85(4):482‐7. [DOI] [PubMed] [Google Scholar]
Angulo 1998
- Angulo JM, Sigal LH, Espinoza LR. Coexistent minocycline‐induced systematic lupus erythematosus and autoimmune hepatitis. Seminars in Arthritis & Rheumatism 1998;28(3):187‐92. [DOI] [PubMed] [Google Scholar]
Arowojolu 2009
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA, Garner SE. Combined oral contraceptive pills for treatment of acne. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004425.pub4] [DOI] [PubMed] [Google Scholar]
Aubin 1989
- Aubin F, Blanc D, Guinchard C, Agache P. Absence of minocycline in sebum?. Journal of Dermatology 1989;16(5):369‐73. [DOI] [PubMed] [Google Scholar]
Basler 1979
- Basler RSW. Minocycline therapy for acne. Archives of Dermatology 1979;115(12):1391. [PubMed] [Google Scholar]
Beneton 1997
- Beneton N, Bocquet H, Cosnes A, Revuz J, Roujeau JC. Benefit‐risk assessment of acne therapies. Lancet 1997;349(9060):1252. [DOI] [PubMed] [Google Scholar]
Brock 1998 [pers comm]
- Brock P (Wyeth Laboratories, Havant, UK). [[personal communication]]. Letter to S Garner (NICE, London UK) 21 October 1998.
Burke 1984
- Burke BM, Cunliffe WJ. The assessment of acne vulgaris‐ the Leeds technique. British Journal of Dermatology 1984;111(1):83‐92. [DOI] [PubMed] [Google Scholar]
Chopra 1992
- Chopra I, Hawkey PM, Hinton M. Tetracyclines, molecular and clinical aspects. Journal of Antimicrobial Chemotherapy 1992;29(3):245‐77. [DOI] [PubMed] [Google Scholar]
Coates 1999
- Coates P, Eady EA, Cove JH. Antibiotic‐resistant acne. Current Practice of Medicine 1999;2(6):121‐3. [Google Scholar]
Colaizzi 1969
- Colaizzi JL, Klink PR. pH‐partition behaviour of tetracyclines. Journal of Pharmaceutical Sciences 1969;58(10):1184‐9. [DOI] [PubMed] [Google Scholar]
Cook 1979
- Cook CH, Centner RL, Michaels SE. An acne grading method using photographic standards. Archives of Dermatology 1979;115(5):571‐5. [PubMed] [Google Scholar]
Crosson 1997
- Crosson J, Stillman MT. Minocycline‐related lupus erythematosus with associated liver disease. Journal of the American Academy of Dermatology 1997;36(5 Suppl 2):867‐8. [DOI] [PubMed] [Google Scholar]
Cunliffe 1989
- Cunliffe WJ. Acne (Focal Points in Dermatology). London: Martin Dunitz, 1989. [Google Scholar]
Cunliffe 1996
- Cunliffe WJ. Minocycline for acne. Doctors should not change the way they prescribe for acne. BMJ 1996;312(7038):1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 1989
- Davies MG, Kersey PJW. Acute hepatitis and exfoliative dermatitis associated with minocycline. BMJ 1989;298(6686):1523‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eady 1990a
- Eady EA, Cove JH, Holland KT, Cunliffe WJ. Superior antibacterial action and reduced incidence of bacterial resistance in minocycline compared to tetracycline‐treated acne patients. British Journal of Dermatology 1990;122(2):233‐44. [DOI] [PubMed] [Google Scholar]
Eady 1990b
- Eady EA, Cove JH, Joanes DN, Cunliffe WJ. Topical antibiotics for the treatment of acne vulgaris: a critical evaluation of the literature on their clinical benefit and comparative efficacy. Journal of Dermatological Treatment 1990;1(4):215‐26. [Google Scholar]
Fanning 1977
- Fanning WL, Gump DW, Sofferman RA. Side effects of minocycline: a double blind study. Antimicrobial Agents and Chemotherapy 1977;11(4):712‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fay 2008
- Fay BT, Whiddon AP, Puumala S, Black N, O'Dell JR, Mikuls TR. Minocycline‐induced hyperpigmentation in rheumatoid arthritis. Journal of Clinical Rheumatology 2008;14(1):17‐20. [DOI] [PubMed] [Google Scholar]
Ferguson 1998
- Ferguson JJ, Jenkins MGV, Field J. Paper in BMJ influenced prescribing of minocycline. BMJ 1998;316(7124):72‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferner 1996
- Ferner RE, Moss C. Minocycline for acne. BMJ 1996;312(7024):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fessler 1996
- Fessler B. Tetracycline instead of minocycline in antibiotic therapy of acne? [Antibiotische Aknetherapie. Tetracyclin statt Minocyclin?]. Deutsche Apotheker Zeitung 1996;136(31):35‐6. [Google Scholar]
Gardner 1997
- Gardner KJ, Eady EA, Cove JH, Taylor JP, Cunliffe WJ. Comparison of serum antibiotic levels in acne patients receiving the standard or a modified release formulation of minocycline hydrochloride. Clinical & Experimental Dermatology 1997;22(2):72‐6. [PubMed] [Google Scholar]
Glass 1981
- Glass GV, McGaw B, Smith, ML. Meta‐analysis in social research: individual and neighbourhood reactions. Beverly Hills, California: Sage Publications, Inc, 1981:77‐91. [Google Scholar]
Gough 1996
- Gough A, Chapman S, Wagstaff K, Emery P, Elias E. Minocycline‐induced autoimmune hepatitis and systemic lupus erythematosus‐like syndrome. BMJ 1996;312(7024):169‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goulden 1997
- Goulden V, Clarke SM, Cunliffe WJ. Post‐adolescent acne: a review of clinical features. British Journal of Dermatology 1997;136(1):66‐70. [PubMed] [Google Scholar]
Grasset 2003
Gump 1977
- Gump DW, Ashikaga T, Fink TJ, Radin AM. Side effects of minocycine: different dosage regimens. Antimicrobial Agents & Chemotherapy 1977;12(5):642‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knowles 1996
- Knowles SR, Shapiro L, Shear NH. Serious adverse reactions induced by minocycline. Report of 13 patients and review of the literature. Archives of Dermatology 1996;132(8):934‐9. [PubMed] [Google Scholar]
Lawrenson 2000
- Lawrenson RA, Seaman HE, Sundstrom A, Williams TJ, Farmer RDT. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilence data. Drug Safety 2000;23(4):333‐49. [DOI] [PubMed] [Google Scholar]
Lebrun‐Vignes 2012
- Lebrun‐Vignes B, Kreft‐Jais C, Castot A, Chosidow O. Comparative analysis of adverse drug reactions to tetracyclines: results of a French national survey and review of the literature. British Journal of Dermatology 2012;166:1333‐41. [DOI] [PubMed] [Google Scholar]
Leyden 1985
- Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk and iron. Journal of the American Academy of Dermatology 1985;12(2 1):308‐12. [DOI] [PubMed] [Google Scholar]
Leyden 1997
- Leyden JJ. Therapy for acne vulgaris. New England Journal of Medicine 1997;336(16):1156‐62. [DOI] [PubMed] [Google Scholar]
Luderschmidt 1985
- Luderschmidt C, Nissen H, Neubert U, Knuechel M. Newer methods for measuring therapeutic response in acne. Royal Society of Medicine Services International Congress & Symposium Series 1985;95:131‐40. [Google Scholar]
Macdonald 1973
- Macdonald H, Kelly RG, Allen ES. Pharmacokinetic studies on minocycline in man. Clinical Pharmacology & Therapeutics 1973;14(5):852‐61. [DOI] [PubMed] [Google Scholar]
MacNeil 1997
- MacNeil M, Haase DA, Tremaine R, Marrie TJ. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. Journal of the American Academy of Dermatology 1997;36(2 Suppl):347‐50. [DOI] [PubMed] [Google Scholar]
Margolis 2007
- Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. British Journal of Dermatology 2007;157(3):540‐6. [DOI] [PubMed] [Google Scholar]
Margolis 2010
- Margolis DJ, Fanelli M, Hoffstad O, Lewis JD. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. American Journal of Gastroenterology 2010;105(12):2610‐6. [DOI] [PubMed] [Google Scholar]
Marzo‐Ortega 2007
- Marzo‐Ortega H, Baxter K, Strauss RM, Drysdale S, Griffiths B, Misbah SA, et al. Is minocycline therapy in acne associated with antineutrophil cytoplasmic antibody positivity? A cross‐sectional study. British Journal of Dermatology 2007;156(5):1005‐9. [DOI] [PubMed] [Google Scholar]
Meyer 1996
- Meyer FP. Minocycline for acne. Food reduces minocycline's bioavailability. BMJ 1996;312(7038):1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortonne 1997
- Ortonne JP. Oral isotretinoin treatment policy. Do we all agree?. Dermatology 1997;195(Suppl 1):34‐7. [DOI] [PubMed] [Google Scholar]
Rothman 1993
- Rothman KF, Lucky AW. Acne vulgaris. Advances in Dermatology 1993;8:347‐74, 75. [PubMed] [Google Scholar]
Ruef 1996
- Ruef C, Blaser J, Maurer P, Keller H, Follath F. Miscellaneous antibiotics. In: Dukes MNG editor(s). Meyler's Side Effects of Drugs. 13. B.V: Elsevier Science, 1996:725‐63. [Google Scholar]
Saurat 1997
- Saurat JH. Oral isotretinoin. Where now, where next!. Dermatology 1997;195(Suppl 1):1‐3. [DOI] [PubMed] [Google Scholar]
Schlienger 2000
- Schlienger RG, Bircher AJ, Meier CR. Minocycline‐induced lupus: a systematic review. Dermatology 2000;200(3):223‐31. [DOI] [PubMed] [Google Scholar]
Schoonen 2010
- Schoonen WM, Thomas SL, Somers EC, Smeeth L, Kim J, Evans S, et al. Do selected drugs increase the risk of lupus? A matched case‐control study. British Journal of Clinical Pharmacology 2010;70(4):588‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seaman 2001
- Seaman HE, Lawrenson RA, Williams TJ, MacRae KD, Farmer RDT. The risk of liver damage associated with minocycline: a comparative study. Journal of Clinical Pharmacology 2001;41(8):852‐60. [DOI] [PubMed] [Google Scholar]
Seukeran 1997
- Seukeran DC, Eady EA, Cunliffe WJ, Beneton N, Bocquet H, Cosnes A, et al. Benefit‐risk assessment of acne therapies. Lancet 1997;349(9060):1251‐2. [DOI] [PubMed] [Google Scholar]
Shaheen 2011
- Shaheen B, Gonzalez M. A microbial aetiology of acne: what is the evidence?. British Journal of Dermatology 2011;165(3):474‐85. [DOI] [PubMed] [Google Scholar]
Shapiro 1997
- Shapiro LE, Knowles SR, Shear NH. Comparative safety of tetracycline, minocycline, and doxycycline. Archives of Dermatology 1997; Vol. 133, issue 10:1224‐30. [PubMed]
Smith 2005
Soory 2008
- Soory M. A role for non‐antimicrobial actions of tetracyclines in combating oxidative stress in periodontal and metabolic diseases: a literature review. Open Dent J 2008;2:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturkenboom 1999
- Sturkenboom MCJM, Meier CR, Jick H, Stricker BHC. Minocycline and lupuslike syndrome in acne patients. Archives of Internal Medicine 1999;159(5):493‐7. [DOI] [PubMed] [Google Scholar]
Ten Holder 2002
- Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug‐induced vasculitis. Annals of Pharmacotherapy 2002;36(1):130‐47. [PUBMED: 11816242] [DOI] [PubMed] [Google Scholar]
The Standards of Reporting Trials Group 1994
- Anonymous. A proposal for the structured reporting of randomised controlled trials. The Standards of Reporting Trials Group. JAMA 1994;272(24):1926‐31. [PubMed] [Google Scholar]
Walsh 2012
- Walsh S, Creamer D. Minocycline in the management of acne vulgaris: the challenge of conveying pharmacovigilence data to primary care. British Journal of Dermatology 2012;166:1155‐1159. [DOI] [PubMed] [Google Scholar]
Williams 1974
- Williams DN, Laughlin LW, Lee YH. Minocycline: possible vestibular side‐effects. Lancet 1974;2(7883):744‐6. [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;379(9813):361‐72. [DOI] [PubMed] [Google Scholar]
Wright 1988
- Wright AL, Colver GB. Tetracyclines ‐ How safe are they?. Clinical & Experimental Dermatology 1988;13(2):57‐61. [DOI] [PubMed] [Google Scholar]


