Abstract
Background
Overactive bladder syndrome is defined as urgency with or without urgency incontinence, usually with frequency and nocturia. Pharmacotherapy with anticholinergic drugs is often the first line medical therapy, either alone or as an adjunct to various non‐pharmacological therapies after conservative options such as reducing intake of caffeine drinks have been tried. Non‐pharmacologic therapies consist of bladder training, pelvic floor muscle training with or without biofeedback, behavioural modification, electrical stimulation and surgical interventions.
Objectives
To compare the effects of anticholinergic drugs with various non‐pharmacologic therapies for non‐neurogenic overactive bladder syndrome in adults.
Search methods
We searched the Cochrane Incontinence Group Specialised Register (searched 4 September 2012), which includes searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE, and the reference lists of relevant articles.
Selection criteria
All randomised or quasi‐randomised, controlled trials of treatment with anticholinergic drugs for overactive bladder syndrome or urgency urinary incontinence in adults in which at least one management arm involved a non‐drug therapy. Trials amongst patients with neurogenic bladder dysfunction were excluded.
Data collection and analysis
Two authors evaluated the trials for appropriateness for inclusion and risk of bias. Two authors were involved in the data extraction. Data extraction was based on predetermined criteria. Data analysis was based on standard statistical approaches used in Cochrane reviews.
Main results
Twenty three trials were included with a total of 3685 participants, one was a cross‐over trial and the other 22 were parallel group trials. The duration of follow up varied from two to 52 weeks. The trials were generally small and of poor methodological quality.
During treatment, symptomatic improvement was more common amongst those participants on anticholinergic drugs compared with bladder training in seven small trials (73/174, 42% versus 98/172, 57% not improved: risk ratio 0.74, 95% confidence interval 0.61 to 0.91). Augmentation of bladder training with anticholinergics was also associated with more improvements than bladder training alone in three small trials (23/85, 27% versus 37/79, 47% not improved: risk ratio 0.57, 95% confidence interval 0.38 to 0.88). However, it was less clear whether an anticholinergic combined with bladder training was better than the anticholinergic alone, in three trials (for example 74/296, 25% versus 95/306, 31% not improved: risk ratio 0.80, 95% confidence interval 0.62 to 1.04). The other information on whether combining behavioural modification strategies with an anticholinergic was better than the anticholinergic alone was scanty and inconclusive. Similarly, it was unclear whether these complex strategies alone were better than anticholinergics alone.
In this review, seven small trials comparing an anticholinergic to various types of electrical stimulation modalities such as Intravaginal Electrical Stimulation (IES), transcutaneous electrical nerve stimulation (TENS), the Stoller Afferent Nerve Stimulation System (SANS) neuromodulation and percutaneous posterior tibial nerve stimulation (PTNS) were identified. Subjective improvement rates tended to favour the electrical stimulation group in three small trials (54% not improved with the anticholinergic versus 28/86, 33% with electrical stimulation: risk ratio 0.64, 95% confidence interval 1.15 to 2.34). However, this was statistically significant only for one type of stimulation, percutaneous posterior tibial nerve stimulation (risk ratio 2.21, 95% confidence interval 1.13 to 4.33), and was not supported by significant differences in improvement, urinary frequency, urgency, nocturia, incontinence episodes or quality of life.
The most commonly reported adverse effect among anticholinergics was dry mouth, although this did not necessarily result in withdrawal from treatment. For all comparisons there were too few data to compare symptoms or side effects after treatment had ended. However, it is unlikely that the effects of anticholinergics persist after stopping treatment.
Authors' conclusions
The use of anticholinergic drugs in the management of overactive bladder syndrome is well established when compared to placebo treatment. During initial treatment of overactive bladder syndrome there was more symptomatic improvement when (a) anticholinergics were compared with bladder training alone, and (b) anticholinergics combined with bladder training were compared with bladder training alone. Limited evidence from small trials might suggest electrical stimulation is a better option in patients who are refractory to anticholinergic therapy, but more evidence comparing individual types of electrostimulation to the most effective types of anticholinergics is required to establish this. These results should be viewed with caution in view of the different classes and varying doses of individual anticholinergics used in this review. Anticholinergics had well recognised side effects, such as dry mouth.
Plain language summary
Anticholinergic drugs versus non‐drug active therapies for overactive bladder syndrome in adults without neurological problems
Overactive bladder syndrome occurs in adults who have urinary urgency with or without urgency urinary incontinence (leakage of urine). People usually empty their bladders frequently during the day and also at night (nocturia). It is a major problem affecting quality of life, in over 22 million people. It affects men and women, and is more common in women and in older people. It is also expensive for both patients and the health service. It is not clear exactly why it occurs, and there are many treatments including drugs and behavioural treatments such as bladder training and pelvic floor exercises. It is not clear which treatments work best, have the fewest side effects and which are most economical. Twenty three trials with 3685 participants were included in the review. Participants were more likely to improve if they were using an anticholinergic drug compared with bladder training alone, and also when using a combination of an anticholinergic drug plus bladder training. More people reported an improvement in their overactive bladder symptoms when using electrical stimulation than an anticholinergic drug, but this was only significant in one trial for one type of electrical stimulation, percutaneous posterior tibial nerve stimulation. These results have to be viewed with caution as different types and doses of the anticholinergic drugs were used in the trials. The main adverse effect reported was dry mouth, in about a third of the people taking an anticholinergic drug.
Background
Description of the condition
Overactive bladder syndrome is a symptom triad defined as "urgency, with or without urgency incontinence, usually with frequency and nocturia" (Abrams 2002). Alternative terminologies for overactive bladder syndrome are urge syndrome or urge‐frequency syndrome.
In a survey of 16,776 adults that was performed in Europe (France, Germany, Italy, Spain, Sweden, and the United Kingdom), 16.6% had overactive bladder syndrome. Urge incontinence was reported by 36% of those with overactive bladder symptoms (Milsom 2001). In a similar study in the United States, the National Overactive Bladder Evaluation (NOBLE) program estimated that 33 (16.5%) million people had overactive bladder syndrome. Of these, 12 million (37%) were incontinent (Stewart 2001). The estimated prevalence of an overactive bladder amongst people aged 40 years and above is 15.6% and 17.4% in men and women respectively, and the prevalence increases with age in both sexes. The symptoms of urgency or frequency, or both, are equally common in men and women but urgency incontinence is more prevalent in women (Milsom 2001). Patients with symptoms of overactive bladder syndrome who have demonstrated detrusor contractions during the filling phase of urodynamic assessment are termed as having detrusor overactivity. If detrusor overactivity is associated with a neurological condition it is termed as neurogenic detrusor overactivity. In the absence of a relevant neurological condition it is termed 'idiopathic detrusor overactivity'. In this review only idiopathic detrusor overactivity was considered.
Overactive bladder syndrome has economic and quality of life implications. It has been estimated that the economic cost of overactive bladder was USD 12.02 billion in 2000 in the United States (Hu 2003). It is also associated with poorer quality of life indices as shown by the Short Form (SF)‐36 questionnaire, King's Health Questionnaire, a higher depression score and a poorer quality of sleep (Stewart 2001).
The current European Association of Urology (EAU) guidelines suggest following a stepwise management pattern for the treatment of patients with overactive bladder and urgency urinary incontinence. The first step is conservative measures, which may include reducing the intake of caffeine drinks, optimising fluid intake, weight loss, usage of intravaginal oestrogens in postmenopausal women (Cody 2012), and short term usage of desmopressin in young adults with enuresis. The next option is bladder training (Wallace 2004), which can be combined with pelvic floor exercises in the presence of concomitant stress incontinence. The next step is the administration of anticholinergic medications. The options for patients refractory to conservative measures and anticholinergic treatment and with detrusor overactivity are intravesical botulinum toxin A injection (Duthie 2011), electrical stimulation, neuromodulation with implanted electrodes (Herbison 2009) and surgical interventions with high morbidity, such as clam ileocystoplasty and urinary diversion (Cody 2012a). In view of the complications associated with surgical intervention, it is commonly the last treatment option in patients with detrusor overactivity.
Description of the intervention
Pharmacotherapy is one of the main treatment options in the management of overactive bladder syndrome. Anticholinergics are classified based on pharmacokinetics (lipophilicity and molecular size) into tertiary and quaternary amines, or based on muscarinic receptor selectivity into non‐selective antagonists, M2‐M3 selective antagonists and M3 selective antagonists.
Tertiary amines are solifenacin, darifenacin, tolterodine, oxybutynin.
Qaternary amines are trospium and propantheline.
Non‐selective anticholinergics are tolterodine, trospium, oxybutynin, propiverine hydrochloride (HCl) and propantheline.
Solfenacin and darifenacin are M2‐M3 selective and M3 selective antagonists, respectively.
Quaternary amines do not cross the blood brain barrier and hence are thought to have fewer cognitive side effects in comparison with tertiary amines. In view of their receptor selectivity, M2‐M3 selective and M3 selective antagonists are thought to have fewer adverse effects. The number of anticholinergic drugs available on the market is increasing and their effectiveness has been assessed in both observational and randomised controlled trials (Madhuvrata 2012; Thuroff 1991; Van Kerrebroeck 1998). Anticholinergics are increasingly being used in primary and secondary care settings, particularly for the treatment of urgency urinary incontinence, and this has considerable resource implications (Kobelt 1997).
Comparators
Bladder training is often used as first line therapy in patients with overactive bladder syndrome whether or not they are incontinent. This modality involves encouraging patients to gradually increase the interval between voiding episodes. Employed in conjunction with a bladder chart, patients and clinicians can chart progress through both the volumes voided and the time interval between each void. It has been claimed that up to 50% of patients will achieve long term benefit with this approach (Holmes 1983).
Pelvic floor muscle training (PFMT) has been advocated as a means of improving pelvic floor control and reducing symptoms of overactive bladder and urgency urinary incontinence. PFMT is more commonly recommended for stress urinary incontinence than for symptoms of overactive bladder syndrome (Dumoulin 2010).
A combination of bladder training with biofeedback‐assisted PFMT, sometimes referred to as behavioural therapy, has been shown to improve bladder control by teaching patients new skills or habits and is a recognised form of non‐drug active therapy in the management of overactive bladder syndrome (Cardozo 1978).
Electrical stimulation involves the use of either implanted or external electrodes to stimulate reflex inhibition of pelvic efferents or activation of hypogastric efferents to down regulate detrusor muscle activity. This is mediated through stimulation of afferent input in the sacral root, S2 to S4 (Fall 1985; Shaker 1998).
External electrodes are broadly classified into endocavitary and percutaneous electrodes (Gameiro 2012). Endocavitary electrodes can be placed intravaginally or rectally. Percutanous approaches include transcutaneous electrical nerve stimulation (TENS) and posterior tibial nerve stimulation (PTNS).
When the electrodes are placed on the perineal skin surface it is termed TENS.
PTNS involves non‐invasive electrical stimulation of S2 to S4 sacral nerves via a 34‐G needle placed on the medial malleolus of the ankle. It was first described by Dr Marshall Stoller and the protocol was termed the Stoller Afferent Nerve Stimulation (SANS) protocol.
Sacral nerve stimulation is a technique which involves implanted electrodes. It is a two stage invasive procedure where an electrode is placed percutaneously alongside S3 in the sacral foramina (Herbison 2009).
Surgical management of overactive bladder and urgency urinary incontinence is uncommon and is generally reserved for the most severe cases that have not responded to conservative management (Cody 2012).
How the intervention might work
The pathophysiology of the overactive bladder remains to be fully elucidated. However, the involvement of the autonomic nervous system in bladder and detrusor function is recognised (de Groat 1997). The motor nerve supply to the bladder is via the parasympathetic nervous system (via sacral nerves S2, S3, S4) (Abrams 1988; Ouslander 1982; Ouslander 1986), which stimulates detrusor muscle contraction. This is mediated by acetylcholine acting on muscarinic (M) receptors at the level of the bladder. The bladder contains both M2 and M3 muscarinic receptor subtypes. Although the M2 subtype is more abundant, it is the M3 subtype which is mainly responsible for bladder contraction (Andersson 2002). The rationale for using anticholinergic drugs in the treatment of overactive bladder syndrome is to block the parasympathetic acetylcholine pathway and thus abolish or reduce the intensity of detrusor muscle contraction. For the purpose of this review, the term 'anticholinergic' refers to both anticholinergic and antimuscarinic drugs.
Why it is important to do this review
There are numerous drug and non‐drug treatments for overactive bladder. While anticholinergics are well established in practice, based on their efficacy versus no treatment or placebo (Nabi 2006), it is less certain how they compare to other established treatments such as bladder training and electrical stimulation.
This review compares anticholinergic drugs with other active (non‐drug) therapies which include bladder training (BT) and pelvic floor muscle training (PFMT). Anticholinergic drugs are also compared with various combinations of bladder training and PFMT with and without anticholinergics to assess if any particular combination shows superior efficacy. The review also compares anticholinergics with various forms of electrical stimulation including neuromodulation.
Other Cochrane reviews covering treatments for overactive bladder syndrome and urinary incontinence that might be of interest to the reader include:
anticholinergic drugs versus placebo (Nabi 2006);
which anticholinergic? (Madhuvrata 2012);
anticholinergic drugs versus other medications (Dublin 2004);
PFMT versus no treatment (Dumoulin 2010);
bladder training (Wallace 2004);
botulinum toxin injections (Duthie 2011); and
sacral neuromodulation with implanted devices (Herbison 2009).
Objectives
The following comparisons were made (for people with overactive bladder syndrome with or without urgency urinary incontinence).
1. Anticholinergic drugs versus bladder training (BT) alone.
2. Anticholinergic drugs versus pelvic floor muscle training (PFMT) alone.
3. Anticholinergic drugs versus external electrostimulation (endocavitary, percutaneous or sacral nerve modulation).
4. Anticholinergic drugs versus surgery.
5. Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone.
6. Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone.
7. Anticholinergic drugs versus combination non‐drug therapies.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials of anticholinergic drugs for the treatment of overactive bladder syndrome and urgency urinary incontinence.
Types of participants
All adult men and women with idiopathic overactive bladder syndrome or urgency urinary incontinence (symptomatic or urodynamic diagnosis), or both. Participants with likely neurogenic causes for their symptoms were excluded.
Types of interventions
At least one arm of the trial used an anticholinergic drug and at least one other arm used a non‐drug treatment (such as bladder training, PFMT or surgery).
Types of outcome measures
The primary measure of outcome was the number of participants whose symptoms were 'not cured' while on treatment. Data for the following outcome measures were sought.
A. Participants' observations
1. Number not cured during treatment (self‐reported, subjective)* 2. Number not cured after treatment (self‐reported, subjective)* 3. Number not improved (worse or unchanged) during treatment (self‐reported, subjective)* 4. Number not improved (worse or unchanged) after treatment (self‐reported, subjective)* 5. Number with nocturia during treatment 6. Number with nocturia after treatment
* Definitions based on criteria as reported by trialists for each trial
B. Quantification of symptoms
7. Number of pad changes over 24 hours (from self‐reported number of pads used) 8. Number of incontinent episodes over 24 hours (from self‐completed urinary diary) 9. Mean volume or weight of urine loss on pad test 10. Number of micturitions over 24 hours (from self‐completed urinary diary) 11. Frequency of sensation of urgency
C. Clinician's observations
12. Number not cured (worse, unchanged or improved) versus cured within first year (objective test) 13. Number not cured (worse, unchanged or improved) versus cured after first year (objective test) 14. Number not cured (worse, unchanged or improved) versus cured after 5 years (objective test) 15. Urodynamic‐diagnosed detrusor overactivity
D. Quality of life
16. General health status measures e.g. Short Form‐36 (Ware 1993) 17. Condition‐specific health measures (specific instruments designed to assess impact of urinary voiding problems) 18. Measures of psychological health
E. Socioeconomic measures
19. Health economic measures: costs of treatments; differential costs of treatment effect differences; formal cost effectiveness measures
F. Adverse events
20. Number experiencing adverse effects 21. Number withdrawing from treatment or trial arm 22. Number changing dose of treatment
G. Other outcomes
Non‐prespecified outcomes judged important when performing the review
Search methods for identification of studies
We did not impose any language or other limits on any of the searches.
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Group. Relevant trials were identified from the Incontinence Group Specialised Register, which is described under the Incontinence Group's module in The Cochrane Library. The register contains trials identified from MEDLINE, CINAHL, the Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings. The date of the most recent search of the register was 4 September 2012. The trials in the Incontinence Group Specialised Trials Register are also contained in CENTRAL.
The Incontinence Group Specialised Register was searched using the Group's own keyword system. The search terms used were:
{design.rct* or design.cct*} AND {TOPIC.URINE.INCON*} OR {TOPIC.URINE.overactivebladder*} AND {{INTVENT.CHEM.DRUG.ANTICHOLINERGIC} AND {INTVENT.*}} OR {relevant.review.anticholinergicVSnondrug} (All searches were of the keyword field of Reference Manager 12, Thomson Reuters).
Searching other resources
We checked all reference lists of identified trials and other relevant articles.
Data collection and analysis
Selection of studies
Reports of studies identified as possibly eligible for the review were evaluated for appropriateness for inclusion by the review authors working independently and without prior consideration of the results. Studies were excluded from the review if they were neither randomised nor quasi‐randomised trials.
Data extraction and management
Data extraction was undertaken independently by two review authors and cross‐checked. Where data may have been collected but were not reported, further clarification was sought from the researchers. Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Trial data were considered in relation to the seven main hypotheses. Any difference of opinion related to the data extracted was discussed and resolved with a third review author.
Assessment of risk of bias in included studies
At least two review authors assessed risk of bias using the Cochrane Collaboration assessment criteria:
quality of random allocation and concealment;
description of drop‐outs and withdrawals;
'blinding' at treatment and outcome assessment.
Any disagreements were resolved by discussion with a third review author.
Measures of treatment effect
For categorical outcomes we related the number of participants reporting an outcome to the numbers at risk in each group to derive a risk ratio (RR). For continuous variables we used means and standard deviations to derive a mean difference. For cross‐over trials and trials where continuous data were reported without measures of dispersion (for example, standard deviations) the data were entered into 'Other data' tables and comparisons made only on the direction of effect. Cross‐over trials were identified by the suffix '#'.
Assessment of heterogeneity
Differences between trials were further investigated when significant statistical heterogeneity was found at the 10% level, or from consideration of the I2 statistic (Higgins 2011) or appeared obvious from visual inspection of the results.
Data synthesis
When appropriate, meta‐analysis was undertaken. A fixed‐effect model was used for calculation of summary statistics (pooled estimates) and 95% confidence intervals (CIs).
Subgroup analysis and investigation of heterogeneity
Subcategories were identified according to the type(s) of drugs being compared or the different types of other interventions.
Results
Description of studies
Results of the search
Fifty studies were identified, of which 27 were excluded (see table 'Characteristics of excluded studies' for details). Of the 23 included trials, with 3685 participants, one was a cross‐over trial (Soomro 2001 #) and the other 22 were parallel group trials. The flow of literature through the assessment process is shown in Figure 1.
1.
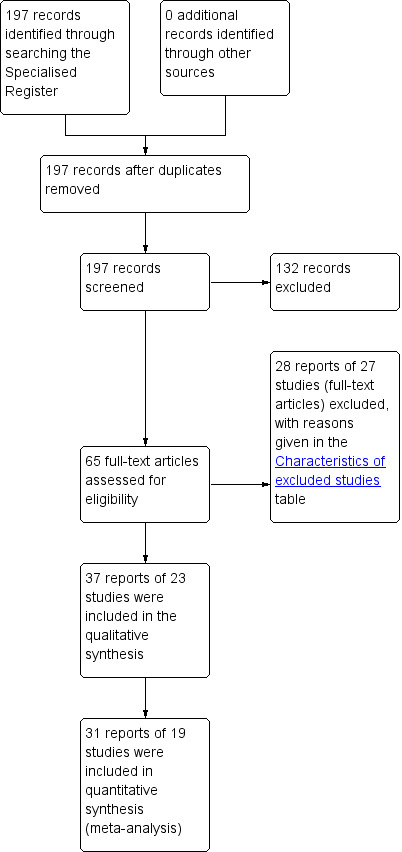
PRISMA study flow diagram.
Included studies
A detailed description of the 23 trials is given in the table 'Characteristics of included studies'. Four trials were in abstract form (Collas 1994; Milani 1987; Park 2002; Wise 1993), 18 were full publications (Burgio 1998; Burgio 2008; Burgio 2010; Burgio 2011; Chancellor 2008; Colombo 1995; Kaya 2011; Mattiasson 2003; Mattiasson 2009; Millard 2004; Ozdedeli 2010; Peters 2009; Smith 1996; Song 2006; Soomro 2001 #; Svihra 2002; Szonyi 1995; Wang 2006) and one was a doctor of medicine (MD) thesis (Macaulay 1988).
Comparisons
The trials made the following comparisons.
1. Seven trials assessed various anticholinergics versus bladder training (Collas 1994; Colombo 1995; Macaulay 1988; Milani 1987; Park 2002; Song 2006; Szonyi 1995).
2. Seven trials assessed various electrical stimulation modalities versus an anticholinergic drug:
two trials used probantheline (dose range 7.5 mg to 45 mg twice daily or three times daily) and one trial oxybutynin 5 mg bd daily (Wise 1993) versus intravaginal electrical stimulation (Smith 1996);
one trial used oxybutynin (dose range 2.5 mg twice daily to 5 mg three times daily) versus transcutaneous electrical nerve stimulation (TENS) (Soomro 2001 #), this was a cross‐over trial;
one trial used oxybutynin 5 mg three times daily versus Stoller Afferent Nerve Stimulation System neuromodulation (Svihra 2002);
one trial used trospium hydrochloride versus intravaginal electrical stimulation (Ozdedeli 2010);
one trial used oxybutynin 2 mg twice a day without dose titration versus intravaginal electrical stimulation (Wang 2006);
one trial used extended‐release tolterodine 4mg versus percutaneous tibial nerve stimulation (Peters 2009).
3. Two trials assessed a combination of an anticholinergic drug with bladder training versus bladder training alone (Park 2002; Szonyi 1995).
4. Four trials assessed a combination of an anticholinergic drug with bladder training versus the drug alone (Mattiasson 2003; Mattiasson 2009; Park 2002; Song 2006).
5. One trial assessed an anticholinergic drug (tolterodine) versus PFMT plus the anticholinergic drug (tolterodine) (Millard 2004).
6. One trial assessed an anticholinergic drug (oxybutynin) versus bladder training plus biofeedback‐assisted PFMT (Burgio 1998).
7. Four trials assessed anticholinergics versus anticholinergics and behavioural modification therapy (Burgio 2008; Burgio 2010; Burgio 2011; Chancellor 2008).
8. One trial compared anticholinergics versus physiotherapy (inferential current therapy plus pelvic floor exercises plus bladder training) versus combination therapy (Kaya 2011).
Participants
Six trials had both male and female participants (Chancellor 2008; Mattiasson 2003; Mattiasson 2009; Peters 2009; Soomro 2001 #; Szonyi 1995) and 15 had female participants only (Burgio 1998; Burgio 2010; Colombo 1995; Kaya 2011; Macaulay 1988; Milani 1987; Millard 2004; Ozdedeli 2010; Park 2002; Smith 1996; Song 2006; Svihra 2002; Wang 2006; Wise 1993), one trial included men only (Burgio 2011), while one did not specify the gender of the participants (Collas 1994). The trials all included participants with non‐neurogenic overactive bladder symptoms or did not specify the cause of the overactive bladder symptoms.
Follow up
The duration of follow up varied from two to 52 weeks.
Excluded studies
Details of the excluded studies are given in the table 'Characteristics of excluded studies'.
Risk of bias in included studies
The methodology and risk of bias of individual trials is summarised in the table 'Characteristics of included studies'.
The risk of bias for individual trials have also been summarised in Figure 2 and Figure 3.
2.
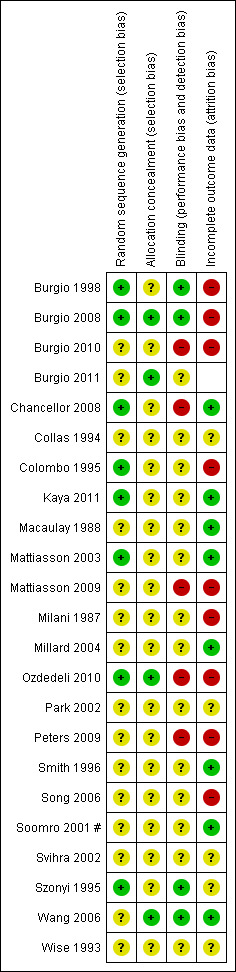
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
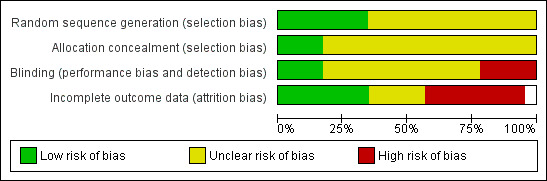
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
In one trial, outcome data were estimated manually from graphs (Macaulay 1988); in another, data were reported in a way that could not be interpreted for the purpose of the review (Wise 1993); and in two trials outcomes were reported as median percentage change and interquartile ranges, which could not be analysed in RevMan (Mattiasson 2003; Ozdedeli 2010). Standard deviations were not reported in two trials (Mattiasson 2009; Song 2006).
Allocation
In eight trials (Burgio 1998; Burgio 2008; Chancellor 2008; Colombo 1995; Kaya 2011; Mattiasson 2003; Ozdedeli 2010; Szonyi 1995) the random sequence generation was deemed at low risk of bias. In the remaining studies the authors were unable to judge the level of risk for selection bias as the reporting was inadequate. In three trials (Burgio 2008; Ozdedeli 2010; Wang 2006) the allocation concealment was deemed low risk. In the remaining trials the authors were unable to judge the level of risk for allocation concealment as the reporting was inadequate.
Blinding
In four trials (Burgio 1998; Burgio 2008; Szonyi 1995; Wang 2006) the blinding was deemed at low risk of bias. There was high risk of performance bias and detection bias in five trials (Burgio 2010; Chancellor 2008; Mattiasson 2009; Ozdedeli 2010; Peters 2009).
Incomplete outcome data
Drop‐outs and withdrawals were described in six trials (Chancellor 2008; Colombo 1995; Mattiasson 2009; Peters 2009; Song 2006; Wang 2006). In one trial (Svihra 2002) no actual numbers were provided for one group, and the trialists referred to "comparable therapeutic results" in their reporting. Similarly, no data were reported in another trial (Wise 1993).
Effects of interventions
Comparison 1: anticholinergic drugs versus bladder training (BT)
Seven trials addressed this comparison (Collas 1994; Colombo 1995; Macaulay 1988; Milani 1987; Park 2002; Song 2006; Szonyi 1995).
One trial compared tolterodine 4 mg daily versus bladder training (Park 2002).
Two trials compared oxybutynin 5 mg daily versus bladder training (Collas 1994; Szonyi 1995).
One trial compared oxybutynin 15 mg daily versus bladder training (Colombo 1995).
One trial compared oxybutynin 45 mg daily versus bladder training (Milani 1987).
One trial compared probantheline 45 mg daily versus bladder training (Macaulay 1988).
One trial compared tolterodine 2mg twice daily versus bladder training (Song 2006).
Participants' observations
Data describing cure rates during and after treatment were only available from two small trials (56 people). They tended to favour the anticholinergic groups but the differences were not statistically significant: not cured during treatment 7/28 (25%) versus 14/28 (50%) (RR 0.52, 95% CI 0.26 to 1.04; Analysis 1.1) (Colombo 1995; Macaulay 1988); not cured after treatment 9/28 (32%) versus 16 out of 28 (57%) (RR 0.58, 95% CI 0.16 to 2.21; Analysis 1.2) (Colombo 1995; Macaulay 1988). In the latter comparison there was statistical heterogeneity (I2 = 73%) as one trial suggested no difference whereas the other favoured the anticholinergic. However, in one trial oxybutynin 15 mg daily was used (Colombo 1995) and in the other probantheline 45 mg daily was used (Macaulay 1988). Variation in anticholinergic efficacy could have contributed to this finding.
1.1. Analysis.
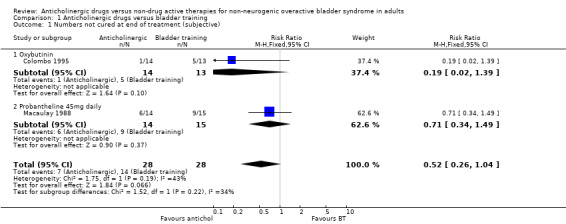
Comparison 1 Anticholinergic drugs versus bladder training, Outcome 1 Numbers not cured at end of treatment (subjective).
1.2. Analysis.
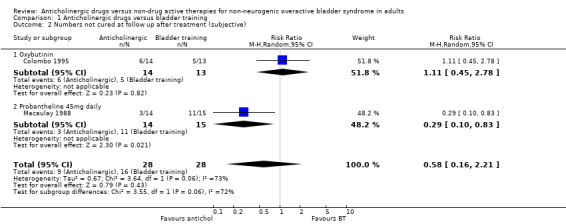
Comparison 1 Anticholinergic drugs versus bladder training, Outcome 2 Numbers not cured at follow up after treatment (subjective).
Data describing subjective improvement during treatment were available for all seven trials (346 participants), including three different anticholinergics. The trials favoured the anticholinergics in terms of number of participants not improved: 73/174 (42%) versus 98/172 (57%); this result was statistically significant (RR for number of people not improved 0.74, 95% CI 0.61 to 0.91; Analysis 1.3) (Collas 1994; Colombo 1995; Macaulay 1988; Milani 1987; Park 2002; Song 2006; Szonyi 1995).
1.3. Analysis.
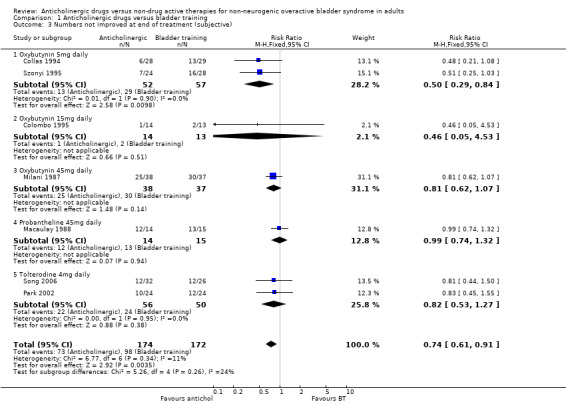
Comparison 1 Anticholinergic drugs versus bladder training, Outcome 3 Numbers not improved at end of treatment (subjective).
There were no useable data regarding whether this improvement continued after stopping treatment. Song et al (Song 2006) reported no change in nocturia between an anticholinergic and bladder training after treatment stopped.
Quantification of symptoms
Two trials (Macaulay 1988; Song 2006) reported frequency of micturition. There was no statistical difference between the two groups for number of micturitions per 24 hours (Analysis 1.10), but the trials were small.
1.10. Analysis.
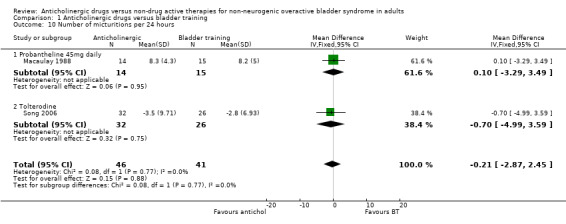
Comparison 1 Anticholinergic drugs versus bladder training, Outcome 10 Number of micturitions per 24 hours.
Quality of life and economic outcome
There were no data describing quality of life or economic outcomes.
Adverse events and withdrawals
Four trials (Colombo 1995; Milani 1987; Park 2002; Song 2006) reported this outcome and found more adverse events in the anticholinergic groups: overall 51/132 (39%) versus 2/126 (2%) (RR 14.50, 95% CI 5.02 to 41.87; Analysis 1.19). Data on withdrawals from treatment were available from two trials (Colombo 1995; Song 2006): 5/74 in the anticholinergic group compared with 2/65 in the bladder training group withdrew from treatment (RR 1.98, 95% CI 0.46 to 8.50. Analysis 1.20).
1.19. Analysis.
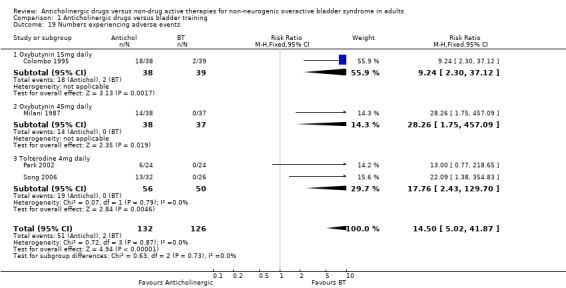
Comparison 1 Anticholinergic drugs versus bladder training, Outcome 19 Numbers experiencing adverse events.
1.20. Analysis.
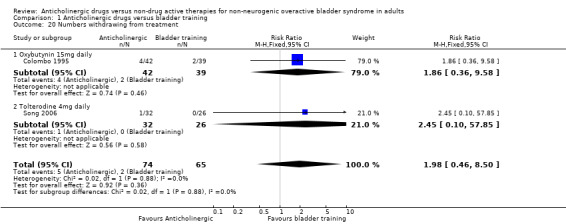
Comparison 1 Anticholinergic drugs versus bladder training, Outcome 20 Numbers withdrawing from treatment.
Long term outcome
No data were reported describing the long term outcome, although bladder training might be expected to continue to be effective after stopping instruction sessions.
Comparison 2: anticholinergic drugs versus pelvic floor muscle training (PFMT) alone
No eligible trials were identified.
Comparison 3: anticholinergic drugs versus external electrostimulation
Seven trials addressed this comparison (Ozdedeli 2010; Peters 2009; Smith 1996; Soomro 2001 #; Svihra 2002; Wang 2006; Wise 1993) but the Wise trial did not report usable data.
Four trials used different classes or doses of anticholinergics versus intravaginal electrical stimulation (Ozdedeli 2010; Smith 1996; Wang 2006; Wise 1993).
One trial used oxybutynin (dose range 2.5 mg twice daily to 5 mg three times a day) versus transcutaneous electrical nerve stimulation (TENS) (Soomro 2001 #). This was a cross‐over trial and the data could not be entered into the analysis.
One trial used oxybutynin 5 mg three times daily versus Stoller Afferent Nerve Stimulation System neuromodulation (Svihra 2002).
One trial used extended release tolterodine 4mg versus percutaneous tibial nerve stimulation (PTNS) (Peters 2009).
Participants' observations
Data from three trials compared the number of people subjectively not cured at the end of the treatment period (Peters 2009; Smith 1996; Wang 2006). There was no statistical difference between anticholinergic drugs and electrostimulation (RR 0.94, 95% CI 0.84 to 1.05; Analysis 3.1). One trial (Peters 2009) compared the number of people objectively not cured, showing no statistically significant difference between the two groups (RR 1.00, 95% CI 0.91 to 1.1; Analysis 3.2).
3.1. Analysis.
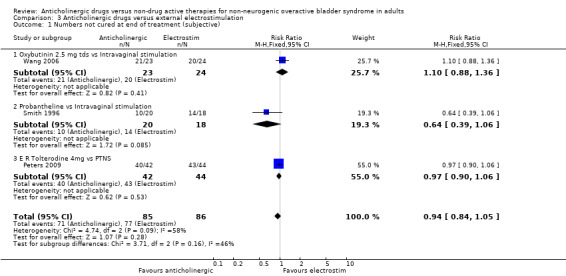
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 1 Numbers not cured at end of treatment (subjective).
3.2. Analysis.
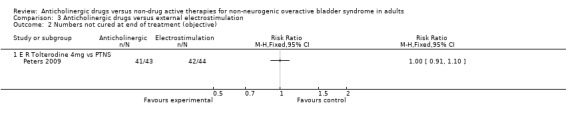
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 2 Numbers not cured at end of treatment (objective).
The combined data from three trials for number of people not improved at the end of the treatment period favoured the electrical stimulation group (RR 1.64, 95% CI 1.15 to 2.34; Analysis 3.3). The three trials compared different types of anticholinergic drugs (oxybutynin, probantheline and extended release tolterodine) with different types of electrostimulation: intravaginal (Smith 1996; Wang 2006) or PTNS (Peters 2009). This result is driven mainly by the result of the slightly larger Peters trial comparing extended release tolterodine against PTNS and demonstrating a statistically significant difference favouring PTNS.
3.3. Analysis.
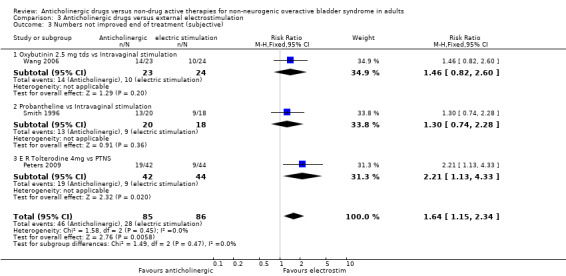
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 3 Numbers not improved end of treatment (subjective).
However, the Peters trial (Peters 2009) did not find a statistically significant difference between the groups for number of people objectively not improved (Analysis 3.4) or for for nocturia (Analysis 3.5).
3.4. Analysis.
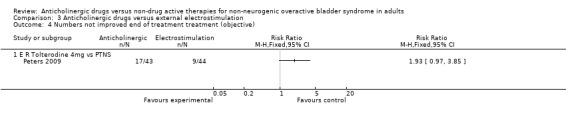
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 4 Numbers not improved end of treatment treatment (objective).
3.5. Analysis.
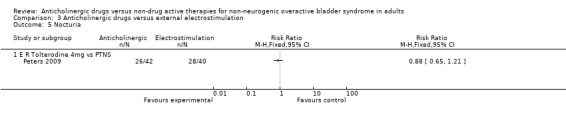
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 5 Nocturia.
Quantification of symptoms
The Peters trial (Peters 2009) found no statistically significant differences between extended release tolterodine and PTNS for number of micturitions per day (Analysis 3.6), sensation of urgency (Analysis 3.8) and the number of incontinence episodes per day (Analysis 3.9). One study reported there were no statistically significant differences between the groups in terms of number of pad changes per day comparing propantheline and intravaginal electrical stimulation ('Other data' Analysis 3.7) (Smith 1996).
3.6. Analysis.
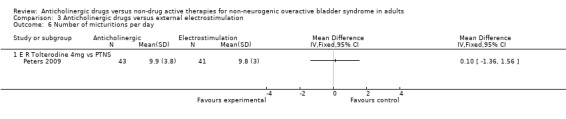
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 6 Number of micturitions per day.
3.8. Analysis.
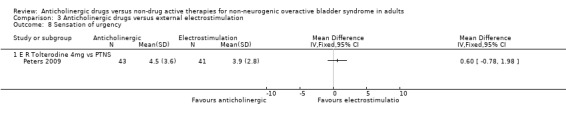
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 8 Sensation of urgency.
3.9. Analysis.
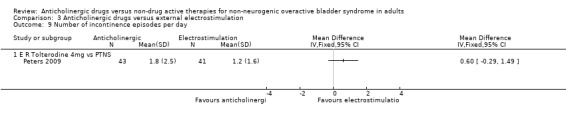
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 9 Number of incontinence episodes per day.
3.7. Analysis.
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 7 Number of pad changes per day‐IVS.
| Number of pad changes per day‐IVS | ||
|---|---|---|
| Study | Anticholinergic | Electric stimulation |
| Smith 1996 | 8.1, range 0‐16 | 6.5, range 0‐18 |
One small trial comparing trospium hydrochloride and intravaginal electrical stimulation reported that there were statistically significant differences from baseline to end of treatment for the number of micturitions per day, urgency and incontinence. The data were reported as medians (Analysis 3.10; Analysis 3.11; Analysis 3.12) and hence were not suitable for meta‐analysis to compare the groups at the end of treatment (Ozdedeli 2010).
3.10. Analysis.
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 10 Number of micturitions per day.
| Number of micturitions per day | ||
|---|---|---|
| Study | Trospium hydrochloride 45mg Median (Range) | Intravaginal Electrical Stimulation Median (Range) |
| Ozdedeli 2010 | 6 (3.3‐14.7) week 6 (17 participants) | 7 (0.6‐15) week 6 (18 participants) |
3.11. Analysis.
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 11 Sensation of urgency.
| Sensation of urgency | ||
|---|---|---|
| Study | Trospium hydrochloride 45mg Median (Range) | Intravaginal Electrical Stimulation Median (Range) |
| Ozdedeli 2010 | 2.7 (0‐8) week 6 (17 participants) | 1.7 (0‐13) week 6 (18 Participants) |
3.12. Analysis.
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 12 Number of incontinence episodes per day.
| Number of incontinence episodes per day | ||
|---|---|---|
| Study | Trospium hydrochloride 45mg Median (Range) | Intravaginal Electrical Stimulation Median (Range) |
| Ozdedeli 2010 | 1 (0‐5) week 6 (17 participants) | 1 (0‐5) 0.3 (0‐9) week 6 (18 participants) |
Quality of life assessment
The data from the Peters 2009 trial reported mean change from baseline for quality of life but there was no significant difference between the groups and the CI was wide. In the cross‐over trial of oxybutynin versus TENS, the trialists stated that there were no statistical differences in any of the parameters of the SF‐36 but they did not provide data (Soomro 2001 #). One trial (Ozdedeli 2010) reported no statistical difference between the two groups for the Beck Depression Inventory (BDI) and Incontinence Impact Questionnaire (IIQ‐7) scores. Health related quality of life scores were statistically similar for the two groups in the Peters 2009 trial (Peters 2009) (Analysis 3.15).
3.15. Analysis.
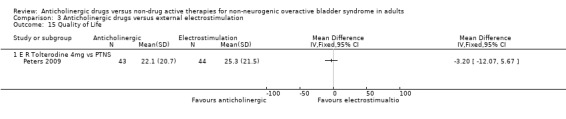
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 15 Quality of Life.
Adverse events and withdrawals
Two trials reported few adverse effects for the two groups (Ozdedeli 2010; Peters 2009) (Analysis 3.13). Two trials (Peters 2009; Wang 2006) reported on withdrawal rates and showed very few withdrawals from either group, and this was not statistically significant (Analysis 3.14).
3.13. Analysis.
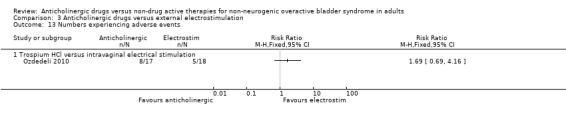
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 13 Numbers experiencing adverse events.
3.14. Analysis.
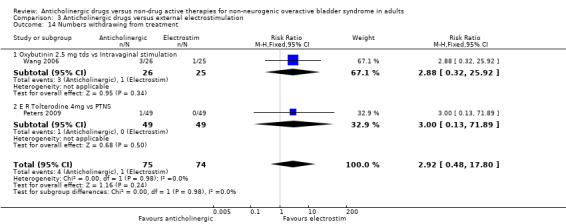
Comparison 3 Anticholinergic drugs versus external electrostimulation, Outcome 14 Numbers withdrawing from treatment.
Comparison 4: anticholinergic drugs versus surgery
No eligible trials were identified.
Comparison 5: anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone
Three trials addressed this comparison (Park 2002; Song 2006; Szonyi 1995).
Tolterodine plus bladder training versus bladder training alone (Park 2002; Song 2006).
Oxybutynin plus bladder training versus bladder training plus placebo (Szonyi 1995).
Participants' observations
In one small trial, the data were too few to assess differences in cure rates (Analysis 5.1) (Szonyi 1995). However, for subjective improvement at the end of treatment, the overall effect in the three small trials was in favour of a combination of an anticholinergic with bladder training compared with bladder training alone (RR 0.57, 95% CI 0.38 to 0.88; Analysis 5.2) (Park 2002; Song 2006; Szonyi 1995).
5.1. Analysis.
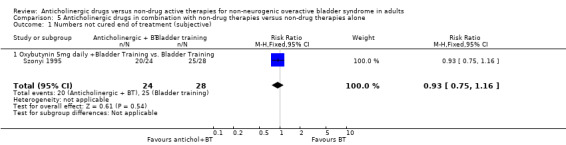
Comparison 5 Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone, Outcome 1 Numbers not cured end of treatment (subjective).
5.2. Analysis.
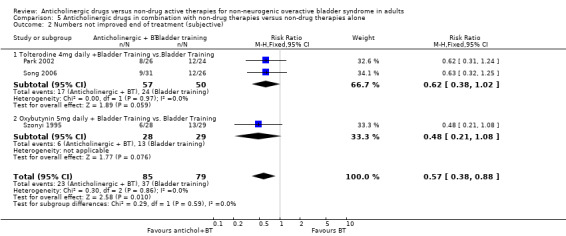
Comparison 5 Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone, Outcome 2 Numbers not improved end of treatment (subjective).
Quantification of symptoms
The percentage decrease from baseline in the number of voids per day for the anticholinergic plus bladder training versus bladder training alone arms were similar, at 30.3% and 32.6%, respectively, but statistical analysis was not possible ('Other data' Analysis 5.3.1) (Park 2002). Similarly, the percentage reduction in the sensation of urgency from baseline for the same groups was reported as 63.2% and 62.5%, respectively ('Other data' Analysis 5.4.1) (Park 2002).
5.3. Analysis.
Comparison 5 Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone, Outcome 3 Number of micturitions per day.
| Number of micturitions per day | |||
|---|---|---|---|
| Study | Antichol. + BT | BT ALONE | Average SD |
| percentage change from baseline | |||
| Park 2002 | 30.3% | 32.6% | 27.1% |
5.4. Analysis.
Comparison 5 Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone, Outcome 4 Sensation of urgency.
| Sensation of urgency | ||
|---|---|---|
| Study | Antichol + BT | Antichol. + BT |
| percentage change from baseline | ||
| Park 2002 | 63.2% | 48.4% |
Quality of life assessment
Quality of life and socioeconomic measures were not reported.
Adverse events and withdrawals
Two trials (Park 2002; Song 2006) reported that 19/57 (33%) participants had adverse events in the combination (anticholinergic plus bladder training) group compared to 0/50 with bladder training alone (Analysis 5.5) (Park 2002; Song 2006). Adverse events were mainly dry mouth, blurred vision, heartburn, constipation and dry skin.
5.5. Analysis.
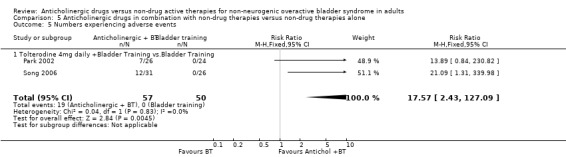
Comparison 5 Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone, Outcome 5 Numbers experiencing adverse events.
Comparison 6: anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone
Nine trials compared anticholinergic drugs plus a non‐drug treatment versus the anticholinergic on its own (Burgio 2008; Burgio 2010; Chancellor 2008; Kaya 2011; Mattiasson 2003; Mattiasson 2009; Millard 2004; Park 2002; Song 2006). However, the non‐drug treatments were considered too different to combine in meta‐analysis:
bladder training (Mattiasson 2003; Mattiasson 2009; Park 2002; Song 2006);
behavioural modification therapy (Burgio 2008; Burgio 2010; Chancellor 2008);
PFMT (Millard 2004);
interferential therapy plus PFMT plus bladder training (Kaya 2011).
Five of these trials included over 100 participants in each arm (Burgio 2008; Chancellor 2008; Mattiasson 2003; Mattiasson 2009; Millard 2004).
Anticholinergics in combination with bladder training (BT) versus anticholinergic alone
Three trials compared tolterodine 4mg daily in combination with bladder training with tolterodine alone (Mattiasson 2003; Park 2002; Song 2006). One of these trials compared flexible dosing of solifenacin 5 or 10mg with a combination of bladder training and flexible dosing of solifenacin 5 or 10mg (Mattiasson 2009).
Participants' observations
The three trials comparing the combination treatment of tolterodine and bladder training versus tolterodine alone all favoured the combination group, with higher improvement rates (RR 0.80, 95% CI 0.62 to 1.04; Analysis 6.2.1) (Mattiasson 2003; Park 2002; Song 2006) but the combined meta‐analysis was not statistically significant. The flexible dosing solifenacin trial (Mattiasson 2009) did not report any data from which overall cure or improvement rates could be calculated.
6.2. Analysis.
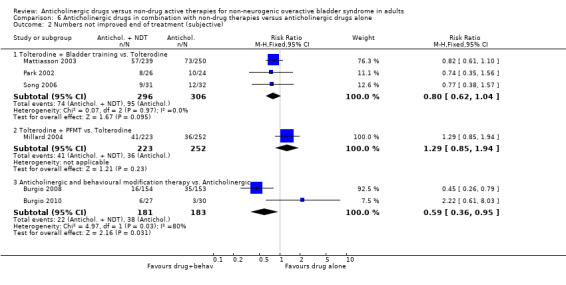
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 2 Numbers not improved end of treatment (subjective).
Quantification of symptoms
The flexible dosing solifenacin trial (Mattiasson 2009) showed a higher mean reduction in the frequency of micturition from baseline at eight and 16 weeks in the combination group, which was statistically significant at eight weeks favouring bladder training plus anticholinergic (MD ‐0.69, 95% CI ‐1.11 to ‐0.27; Analysis 6.4.1). However, there was no statistically significant difference in mean change in urgency (Analysis 6.5.1), number of pads used (Analysis 6.6.1) and number of incontinence episodes at the end of the treatment period (Analysis 6.7.1).
6.4. Analysis.
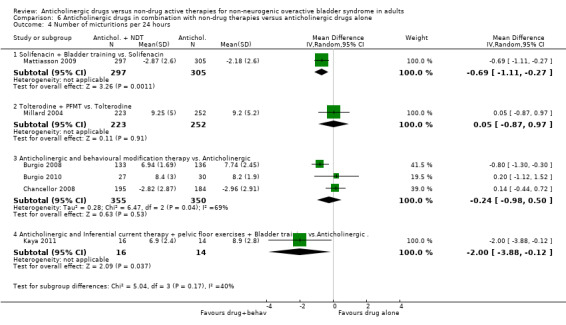
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 4 Number of micturitions per 24 hours.
6.5. Analysis.
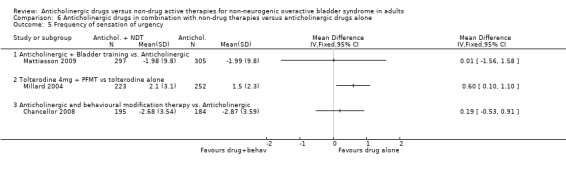
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 5 Frequency of sensation of urgency.
6.6. Analysis.
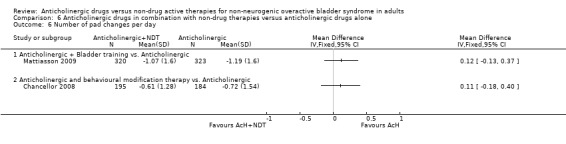
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 6 Number of pad changes per day.
6.7. Analysis.
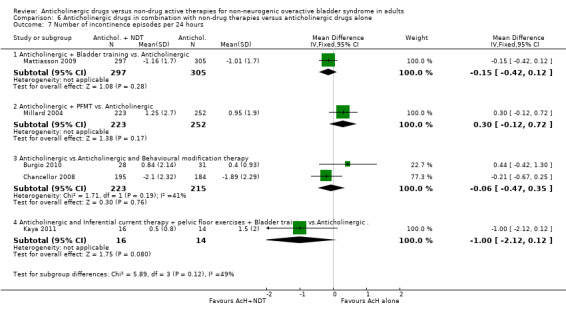
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 7 Number of incontinence episodes per 24 hours.
In one trial (Mattiasson 2003) the percentage change in incontinence episodes per day from baseline was reported as 87% for the combined anticholinergic plus bladder training arm and 81% for the anticholinergic arm ('Other data' Analysis 6.11.1).
6.11. Analysis.
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 11 Number of incontinence episodes per day.
| Number of incontinence episodes per day | ||
|---|---|---|
| Study | Anticholinergic + behavioural | Anticholinergic alone |
| Percentage change from baseline | ||
| Mattiasson 2003 | 87% | 81% |
Quality of life assessment
One large trial (Mattiasson 2009) did not find a statistically significant difference in incontinence quality of life (I‐QoL) scores between the two groups at eight and 16 weeks (MD ‐0.97, 95% CI ‐3.57 to 1.63; Analysis 6.12.1).
6.12. Analysis.

Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 12 Quality of Life.
Adverse events and withdrawals
The proportion of people experiencing adverse events was similar in the four trials (Mattiasson 2003; Mattiasson 2009; Park 2002; Song 2006) comparing bladder training and an anticholinergic versus anticholinergic alone (52.5% versus 54.4%; RR 0.97, 95% CI 0.88 to 1.07; Analysis 6.8.1). Two trials (Mattiasson 2009; Song 2006) compared the number of participants who withdrew from the study. There was no statistically significant difference between the two groups (4.8% versus 5.9%; RR 0.82, 95% CI 0.44 to 1.53; Analysis 6.9.1). One study (Mattiasson 2009) comparing the number of people changing doses between the two groups reported no statistical difference (Analysis 6.10.1).
6.8. Analysis.
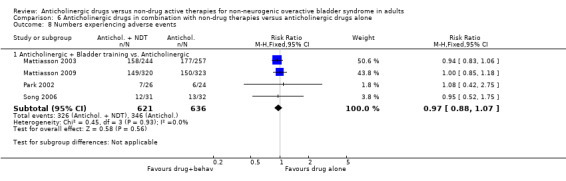
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 8 Numbers experiencing adverse events.
6.9. Analysis.
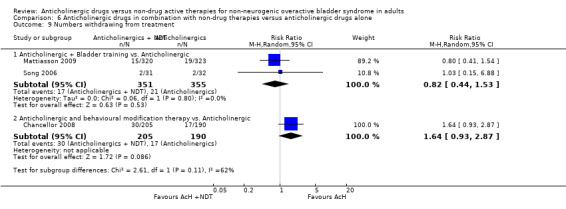
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 9 Numbers withdrawing from treatment.
6.10. Analysis.
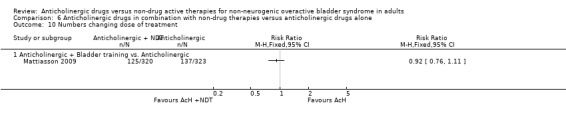
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 10 Numbers changing dose of treatment.
Anticholinergics in combination with pelvic floor muscle training (PFMT) versus anticholinergic alone
One relatively large trial, which had a total study population of 475 patients, addressed this comparison (Millard 2004), tolterodine 4 mg daily plus PFMT compared with tolterodine alone.
Participants' observations
There was no statistically significant difference between the groups in terms of subjective improvement when PFMT was added to anticholinergic treatment (Analysis 6.2.2). No data were provided for cure rates or nocturia.
Quantification of symptoms
There were no statistically significant differences in the number of micturitions per day (Analysis 6.4.2) or incontinence episodes per day (Analysis 6.7.2), but there were fewer reports of the sensation of urgency with tolterodine alone (MD 0.6, 95% CI 0.10 to 1.10; Analysis 6.5.2) (Millard 2004) compared with the drug supplemented with PFMT. There were no data on pad changes or pad tests, quality of life, socioeconomic outcome measures or adverse events and withdrawals.
Combination of anticholinergics and behavioural modification therapy
Three trials addressed this comparison (Burgio 2008; Burgio 2010; Chancellor 2008). Behavioural modification therapy included various combinations of PFMT, BT, urge suppression techniques, fluid management, timed or delayed voiding and lifestyle modification.
Participants' observation
Data from two trials (Burgio 2008; Burgio 2010) reported on subjective improvement: the result was statistically significant favouring the combined treatment group (RR 0.59, 95% CI 0.36 to 0.95; Analysis 6.2). However, there was statistical heterogeneity (P = 0.03) between the two studies with one trial favouring the combination group (Burgio 2008) and one trial showing no statistical difference between the two groups (Burgio 2010). When a random‐effects model was used the result was no longer statistically significant.
One trial (Chancellor 2008) reported on the frequency of nocturia for the two groups but found no statistical difference (Analysis 6.3.1).
6.3. Analysis.
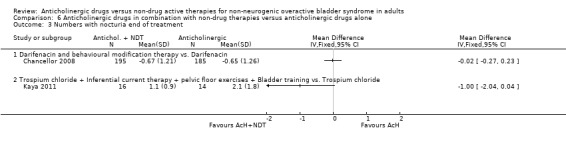
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 3 Numbers with nocturia end of treatment.
Quantification of symptoms
The meta‐analysis of the three trials (Burgio 2008; Burgio 2010; Chancellor 2008) reporting micturitions per day showed no statistical significant difference between the two groups (MD ‐0.24, 95% CI ‐0.98 to 0.50; Analysis 6.4.3). There were also no statistically significant differences in the one trial that reported urgency (Analysis 6.5.3) and number of pad changes per day (Analysis 6.6.2) (Chancellor 2008). Meta‐analysis of two trials (Burgio 2010; Chancellor 2008) showed no statistically significant difference between the two groups in terms of the number of incontinence episodes per day (MD ‐0.06, 95% CI ‐0.47 to 0.35; Analysis 6.7.3).
Adverse events and withdrawals
One trial (Chancellor 2008) stated that the adverse effects in the two groups were similar although exact figures were not reported. One trial (Chancellor 2008) compared the withdrawal rate for the two groups. This was higher in the combination group but the difference was not statistically significant (14.6% versus 8.9%; Analysis 6.9.2). This trial (Chancellor 2008) also reported improvement in health related quality of life in both groups, but there was no statistical difference between the groups.
Combination of anticholinergics plus electrical stimulation plus PFMT plus bladder training versus anticholinergic alone
One small trial compared trospium chloride and physiotherapy consisting of interferential current therapy, PFMT and bladder training versus trospium chloride alone (Kaya 2011).
Participants' observations
No data were available on cure or improvement. There was no statistically significant difference between the two groups for nocturia in this trial (Analysis 6.3.2).
Quantification of symptoms
This trial favoured the combination group in terms of two fewer micturitions per day (MD ‐2, 95% CI ‐3.88 to ‐0.12; Analysis 6.4.4); but not quite for incontinence episodes (Analysis 6.7.4).
Quality of life assessment
In this trial the combination group had statistically significantly better quality of life scores (Analysis 6.12.2).
Adverse events and withdrawals
In both arms, 7/31 (23%) of participants experienced adverse effects, mainly dry mouth. The trial did not give individual figures for each group. One patient in the anticholinergic group had blurred vision, however it was not clear if this was before or after administration of the anticholinergic.
Comparison 7: anticholinergic drugs versus combination non‐drug therapies
One small trial (Burgio 1998) compared oxybutynin 7.5 mg daily with behavioural treatment.
Another trial (Burgio 2011) compared oxybutynin (5 to 30 mg) with behavioural treatment for men who continued to have overactive bladder symptoms with alpha‐blocker therapy.
One small trial compared trospium chloride with a combination of inferential current therapy plus pelvic floor exercises plus bladder training (Kaya 2011).
Participants' observations
The Burgio trial (Burgio 1998), which compared anticholinergics with behavioural treatment, showed no statistically significant difference in subjective cure rates between the two groups (Analysis 7.1.1). However, for subjective improvement the result favoured the behavioural treatment therapy (RR 2.42, 95% CI 1.00 to 5.85; Analysis 7.2.1).
7.1. Analysis.
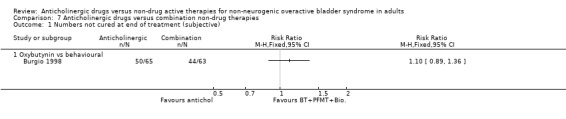
Comparison 7 Anticholinergic drugs versus combination non‐drug therapies, Outcome 1 Numbers not cured at end of treatment (subjective).
7.2. Analysis.
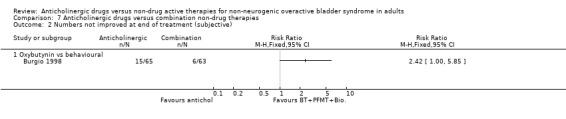
Comparison 7 Anticholinergic drugs versus combination non‐drug therapies, Outcome 2 Numbers not improved at end of treatment (subjective).
There were no data available for cure and improvement rates in the trial comparing an anticholinergic with the non‐drug combination of inferential current therapy plus pelvic floor exercises plus bladder training (Kaya 2011).
Quantification of symptoms
The Kaya trial (Kaya 2011) comparing an anticholinergic and a combination of inferential current therapy plus pelvic floor exercises plus bladder training showed no statistically significant difference between scores for nocturia (Analysis 7.3.1). For the number of micturitions per day there was no statistically significant difference between the two groups in either of the two trials (Analysis 7.4.1; Analysis 7.4.2). The combined result of three trials (Burgio 1998; Burgio 2011; Kaya 2011) for incontinence episodes per day showed fewer incontinence episodes in the combination therapy group compared with the anticholinergic drug alone (MD 0.41, 95% CI 0.11 to 0.70) (Analysis 7.5). However there was heterogeneity in the result, and when a random‐effects model was used the result was no longer statistically significant.
7.3. Analysis.

Comparison 7 Anticholinergic drugs versus combination non‐drug therapies, Outcome 3 Nocturia.
7.4. Analysis.
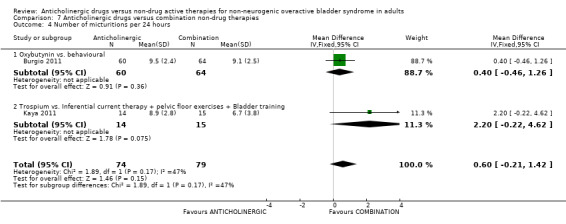
Comparison 7 Anticholinergic drugs versus combination non‐drug therapies, Outcome 4 Number of micturitions per 24 hours.
7.5. Analysis.
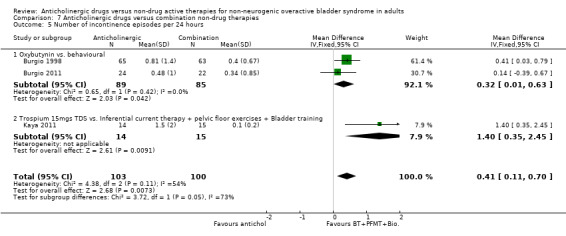
Comparison 7 Anticholinergic drugs versus combination non‐drug therapies, Outcome 5 Number of incontinence episodes per 24 hours.
Quality of life assessment
The quality of life score in the combination group was higher (better) in the non‐drug group in one small trial (13.70, 95% CI 0.94 to 26.46; Analysis 7.6.1).
7.6. Analysis.

Comparison 7 Anticholinergic drugs versus combination non‐drug therapies, Outcome 6 Quality of Life.
Discussion
Pharmacotherapy is one of the main treatment modalities in the management of overactive bladder syndrome, with or without the use of non‐drug active therapies such as bladder training (BT) or urge suppression techniques, pelvic floor muscle training (PFMT) (with or without biofeedback), lifestyle modification, fluid management and the various forms of electrical stimulation. All these recognised therapeutic modalities differ with respect to efficacy, tolerability and side effect profile.
Summary of main results
Bladder training (BT)
The current review suggests that patients are more likely to improve when treated with anticholinergics alone rather than bladder training alone. In the seven small trials comparing anticholinergics alone and bladder training alone, more people improved with drugs than with bladder training (RR for lack of improvement 0.74, 95% CI 0.61 to 0.91; Analysis 1.3). Interestingly, although the overall meta‐analysis favoured the anticholinergic group, with statistical significance, none of the individual trials reported a statistically significant difference in subjective improvement. The trials also used different anticholinergics, formulations and variations in dose. These results therefore have to be viewed with caution. The combination of an anticholinergic with bladder training versus bladder training alone favoured the combination group over bladder training alone, which was statistically significant (RR 0.57, 95% CI 0.38 to 0.88; Analysis 5.2). However, it was less clear whether an anticholinergic combined with bladder training was better than the anticholinergic alone, in three trials (for example RR for no improvement 0.80, 95% CI 0.62 to 1.04; Analysis 6.2.1).
External electrostimulation
In this review various types of electrical stimulation modalities such as Intravaginal Electrical Stimulation (IES), transcutaneous electrical nerve stimulation (TENS), the Stoller Afferent Nerve Stimulation System (SANS) neuromodulation and percutaneous posterior tibial nerve stimulation (PTNS) were identified. Cure rates between anticholinergic drug and electrical stimulation were similar for both therapies. Improvement rates tended to favour the electrostimulation group (RR for no improvement 0.64, 95% CI 1.15 to 2.34; Analysis 3.3). However, this was statistically significant only for one outcome in the PTNS trial (Peters 2009), not supported by the other outcomes, and needs to be confirmed in future research.
Pelvic floor muscle training (PFMT)
This review identified only one large study which compared anticholinergics individually against a combination of anticholinergic and PFMT (Millard 2004). Pelvic floor exercises alone did not appear to add any significant advantage to the overall improvement rates or individual symptoms of overactive bladder syndrome.
Behavioural modification therapy
The overall meta‐analysis of three trials in the review showed better subjective improvement rates with behaviour modification therapy, which could include behavioural strategies, urge suppression, bladder training and PFMT combined with anticholinergic therapy versus the anticholinergic therapy alone (Analysis 6.2.3). However, there was significant statistical heterogeneity between the three trials and the finding should therefore be interpreted with caution. The other information on whether combining these behavioural modification strategies with an anticholinergic was better than the anticholinergic alone was scanty and inconclusive. Similalry, it was unclear whether these complex strategies alone were better than anticholingerics alone.
Others
One small trial (Kaya 2011) compared three treatment protocols: anticholinergic alone, physiotherapy (Inferential current therapy plus pelvic floor exercises plus bladder training) and a combination of anticholinergic drug and physiotherapy. The physiotherapy protocol both individually and in combination with an anticholinergic reported significant reduction in the frequency of micturition per day and improvement in quality of life compared to the anticholinergic alone. However, this was a single small trial and definitive conclusion based on this are difficult to draw.
Adverse effects
Anticholinergics have well recognised adverse effects during treatment. Dry mouth was the most common followed by headache, constipation, dizziness, decreased visual acuity and tachycardia. There were too few data to assess withdrawal rates. The Cochrane review of anticholinergics (Nabi 2006) versus placebo found broadly similar rates of dry mouth but only a small difference in withdrawal rates, which was not statistically significant. As there were no data addressing long term performance, it is not possible to say if anticholinergics would continue to be effective even if the adverse effects were tolerable.
Overall completeness and applicability of evidence
The most common anticholinergic used in this review is oxybutynin followed by tolterodine at varying doses. A recent Cochrane review comparing various anticholinergics has shown oxybutynin and tolterodine to be similarly efficacious (Madhuvrata 2012). Newer generation long‐acting anticholinergics such as solifenacin and fesoterodine are becoming increasing popular in clinical practice and have been shown to have better efficacy than tolterodine (Madhuvrata 2012). This review identified only one study that used solifenacin and none using fesoterodine. It would be interesting to see how these compare to non‐drug therapies.
The length of follow up was also significantly variable between studies, ranging between two to 52 weeks. The authors would suggest viewing some of the evidence with caution due to potential variability between the different types of anticholinergics, formulation and dose differences between individual anticholinergics, and lack of evidence about long term use.
In contrast, it is possible that conservative physical therapies such as bladder training might continue to be effective even after the end of the period of intensive teaching. In the context of management of overactive bladder symptoms, it is appealing, to both the clinician and the patient, to avoid any form of pharmacotherapy, if possible, and to rely on alternatives such as bladder training, PFMT, biofeedback‐assisted therapies or electrostimulation. The latter are expensive in terms of resources needed to teach them and they may have limited availability in some healthcare settings. On the other hand, adverse effects resulting in poor patient compliance with drugs may compromise their effectiveness.
Quality of the evidence
The review was characterised by studies of varying size and having trials of moderate quality in each comparison. Some comparisons were only addressed by single trials. Different classes and types of anticholinergic drugs and varying doses of individual anticholinergics were used by the various trials making the meta‐analysis difficult and potentially causing clinical and statistical heterogeneity.
Some of the trials included patients with idiopathic overactive bladder syndrome, with clear exclusion of those with neurological diseases, but others did not clearly report such exclusions although we assumed that this was the case. This in itself could result in interpretation difficulties, given that it is known that people with neurogenic bladder problems respond differently to anticholinergic drugs. Therefore, a clearer identification of inclusion criteria to address selected subgroups could perhaps clarify this point.
Authors' conclusions
Implications for practice.
The use of anticholinergic drugs in the management of overactive bladder syndrome is well established. The evidence that is available suggests that anticholinergics are more effective than bladder training. However this should be viewed with caution as no individual study showed statistical significance although the overall meta‐analysis was statistically significant. Furthermore, the effect of bladder training might be expected to persist, which is not the case with anticholinergics, but this could not be assessed as there was little information about outcomes after active treatment stops. A combination of bladder training or behavioural modification therapy with anticholinergics may also be a useful strategy compared with such non‐drug treatments alone, or anticholinergic treatment alone, but more research is required to establish this as the evidence was limited.
The evidence suggesting that electrical stimulation may be better than anticholinergics was based on three small studies and needs to be confirmed. The limited evidence did suggest that non‐implantable electrical stimulation might be an option in patients who are refractory to anticholinergic therapy. There was, however, no evidence comparing sacral neuromodulation with anticholinergics.
The anticholinergics used in individual studies were of different types, formulations and doses, with varying lengths of follow up. It is currently unclear if there are certain anticholinergics that may more efficacious with a more acceptable side effect profile compared to bladder training. Newer generation drugs such as solifenacin and fesoterodine have been shown to be more efficacious than tolterodine in a recent Cochrane review (Madhuvrata 2012). In the current review only one study used solifenacin, and fesoterodine was not used at all. It is therefore possible that these anticholinergics will be more effective than bladder training, but there is currently no evidence to support this hypothesis.
There was not enough evidence to assess whether symptomatic improvement is sustained after stopping either treatment. This is important because the aim of bladder training is to achieve long‐term improvement. Anticholinergic treatment has well recognised side effects, such as dry mouth. These side effects are not uncommon and may lead to failure of treatment due to people stopping the use of the drugs.
Implications for research.
The most important clinical issue is whether to start treatment with anticholinergics, bladder training, electrostimulation or a combination of these treatments. There is a need for larger trials with longer study periods comparing these alternatives, particularly including follow up after treatment has ended. There is also a need for more trials comparing anticholinergics with various forms of implantable and non‐implantable electrostimulation modalities. The anticholinergic drugs tested should be those that are in current use and that have been shown to be the most effective with the least adverse effects.
The reporting of the constituent parts of non‐drug active therapies in trials need to be standardised to allow better interpretation of data and meta‐analyses. Assessment of cost effectiveness should be incorporated. Trials should use standardised terminology and outcome measures, and report outcomes in accordance with International Continence Society (ICS) standards and the CONSORT statement. In addition, more studies comparing anticholinergic drugs with various modalities of electrostimulation and botulinum toxin therapy are required to enable analysis and draw out meaningful conclusions.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2014 | Amended | Changed date under "Assessed as Up‐to‐date" to correspond with the "Date of Search" |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 13 November 2012 | New citation required and conclusions have changed | Nine new studies added |
| 13 November 2012 | New search has been performed | 9 new studies added: Burgio 2008; Burgio 2010; Chancellor 2008; Kaya 2011; Mattiasson 2009; Ozdedeli 2010; Peters 2009; Song 2006; Wang 2006 |
| 12 September 2008 | Amended | Converted to new review format. |
| 22 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Jonathan Cook for statistical advice.
Appendices
Appendix 1. Literature search by authors for the previous version of this review
The following searches were conducted by the review authors:
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (January 1966 to September 2004), PREMEDLINE, and Dissertation Abstracts were searched.
Data and analyses
Comparison 1. Anticholinergic drugs versus bladder training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers not cured at end of treatment (subjective) | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| 1.1 Oxybutinin | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.39] |
| 1.2 Probantheline 45mg daily | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.34, 1.49] |
| 2 Numbers not cured at follow up after treatment (subjective) | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.16, 2.21] |
| 2.1 Oxybutinin | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.45, 2.78] |
| 2.2 Probantheline 45mg daily | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.83] |
| 3 Numbers not improved at end of treatment (subjective) | 7 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.91] |
| 3.1 Oxybutynin 5mg daily | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.84] |
| 3.2 Oxybutynin 15mg daily | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.05, 4.53] |
| 3.3 Oxybutynin 45mg daily | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.62, 1.07] |
| 3.4 Probantheline 45mg daily | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.32] |
| 3.5 Tolterodine 4mg daily | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.27] |
| 4 Numbers not improved at follow up after treatment (subjective) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Numbers with nocturia at end of treatment | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Numbers with nocturia at follow up after treatment | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of pad changes per 24hrs | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number of incontinence episodes per 24 hours | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Mean weight of urine loss on pad test | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Number of micturitions per 24 hours | 2 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐2.87, 2.45] |
| 10.1 Probantheline 45mg daily | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐3.29, 3.49] |
| 10.2 Tolterodine | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐4.99, 3.59] |
| 11 Frequency of sensation of urgency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Numbers not cured within first year (objective) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Numbers not cured after first year (objective) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Number not cured after 5 years (objective) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Urodynamic diagnosed detrusor overactivity | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 General health status (SF‐36) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Condition‐specific health measures | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Socioeconomic measures | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Numbers experiencing adverse events | 4 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.50 [5.02, 41.87] |
| 19.1 Oxybutynin 15mg daily | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.24 [2.30, 37.12] |
| 19.2 Oxybutynin 45mg daily | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 28.26 [1.75, 457.09] |
| 19.3 Tolterodine 4mg daily | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.76 [2.43, 129.70] |
| 20 Numbers withdrawing from treatment | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.46, 8.50] |
| 20.1 Oxybutynin 15mg daily | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.36, 9.58] |
| 20.2 Tolterodine 4mg daily | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.10, 57.85] |
| 21 Numbers changing dose of treatment | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Other outcomes | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Anticholinergic drugs versus external electrostimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers not cured at end of treatment (subjective) | 3 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 1.1 Oxybutinin 2.5 mg tds vs Intravaginal stimulation | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.36] |
| 1.2 Probantheline vs Intravaginal stimulation | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.39, 1.06] |
| 1.3 E R Tolterodine 4mg vs PTNS | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.06] |
| 2 Numbers not cured at end of treatment (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 E R Tolterodine 4mg vs PTNS | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Numbers not improved end of treatment (subjective) | 3 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.15, 2.34] |
| 3.1 Oxybutinin 2.5 mg tds vs Intravaginal stimulation | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.82, 2.60] |
| 3.2 Probantheline vs Intravaginal stimulation | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.74, 2.28] |
| 3.3 E R Tolterodine 4mg vs PTNS | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.13, 4.33] |
| 4 Numbers not improved end of treatment treatment (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 E R Tolterodine 4mg vs PTNS | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Nocturia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 E R Tolterodine 4mg vs PTNS | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of micturitions per day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 E R Tolterodine 4mg vs PTNS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of pad changes per day‐IVS | Other data | No numeric data | ||
| 8 Sensation of urgency | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 E R Tolterodine 4mg vs PTNS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of incontinence episodes per day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 E R Tolterodine 4mg vs PTNS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of micturitions per day | Other data | No numeric data | ||
| 11 Sensation of urgency | Other data | No numeric data | ||
| 12 Number of incontinence episodes per day | Other data | No numeric data | ||
| 13 Numbers experiencing adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Trospium HCl versus intravaginal electrical stimulation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Numbers withdrawing from treatment | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.48, 17.80] |
| 14.1 Oxybutinin 2.5 mg tds vs Intravaginal stimulation | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.32, 25.92] |
| 14.2 E R Tolterodine 4mg vs PTNS | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.89] |
| 15 Quality of Life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 E R Tolterodine 4mg vs PTNS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Anticholinergic drugs in combination with non‐drug therapies versus non‐drug therapies alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers not cured end of treatment (subjective) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
| 1.1 Oxybutynin 5mg daily +Bladder Training vs. Bladder Training | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
| 2 Numbers not improved end of treatment (subjective) | 3 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.38, 0.88] |
| 2.1 Tolterodine 4mg daily +Bladder Training vs.Bladder Training | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 2.2 Oxybutynin 5mg daily + Bladder Training vs. Bladder Training | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.21, 1.08] |
| 3 Number of micturitions per day | Other data | No numeric data | ||
| 3.1 percentage change from baseline | Other data | No numeric data | ||
| 4 Sensation of urgency | Other data | No numeric data | ||
| 4.1 percentage change from baseline | Other data | No numeric data | ||
| 5 Numbers experiencing adverse events | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.57 [2.43, 127.09] |
| 5.1 Tolterodine 4mg daily +Bladder Training vs.Bladder Training | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.57 [2.43, 127.09] |
Comparison 6. Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers not cured at follow up after treatment (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Anticholinergic and behavioural modification therapy vs. Anticholinergic | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Numbers not improved end of treatment (subjective) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Tolterodine + Bladder training vs. Tolterodine | 3 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.62, 1.04] |
| 2.2 Tolterodine + PFMT vs. Tolterodine | 1 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.85, 1.94] |
| 2.3 Anticholinergic and behavioural modification therapy vs. Anticholinergic | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.95] |
| 3 Numbers with nocturia end of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Darifenacin and behavioural modification therapy vs. Darifenacin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Trospium chloride + Inferential current therapy + pelvic floor exercises + Bladder training vs. Trospium chloride | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of micturitions per 24 hours | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Solifenacin + Bladder training vs. Solifenacin | 1 | 602 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.11, ‐0.27] |
| 4.2 Tolterodine + PFMT vs. Tolterodine | 1 | 475 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.87, 0.97] |
| 4.3 Anticholinergic and behavioural modification therapy vs. Anticholinergic | 3 | 705 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.98, 0.50] |
| 4.4 Anticholinergic and Inferential current therapy + pelvic floor exercises + Bladder training vs.Anticholinergic . | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.88, ‐0.12] |
| 5 Frequency of sensation of urgency | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Anticholinergic + Bladder training vs. Anticholinergic | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Tolterodine 4mg + PFMT vs tolterodine alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Anticholinergic and behavioural modification therapy vs. Anticholinergic | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of pad changes per day | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Anticholinergic + Bladder training vs. Anticholinergic | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Anticholinergic and behavioural modification therapy vs. Anticholinergic | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of incontinence episodes per 24 hours | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Anticholinergic + Bladder training vs. Anticholinergic | 1 | 602 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 7.2 Anticholinergic + PFMT vs. Anticholinergic | 1 | 475 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.12, 0.72] |
| 7.3 Anticholinergic vs.Anticholinergic and Behavioural modification therapy | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.47, 0.35] |
| 7.4 Anticholinergic and Inferential current therapy + pelvic floor exercises + Bladder training vs.Anticholinergic . | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.12, 0.12] |
| 8 Numbers experiencing adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Anticholinergic + Bladder training vs. Anticholinergic | 4 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 9 Numbers withdrawing from treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Anticholinergic + Bladder training vs. Anticholinergic | 2 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 9.2 Anticholinergic and behavioural modification therapy vs. Anticholinergic | 1 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.93, 2.87] |
| 10 Numbers changing dose of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Anticholinergic + Bladder training vs. Anticholinergic | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Number of incontinence episodes per day | Other data | No numeric data | ||
| 11.1 Percentage change from baseline | Other data | No numeric data | ||
| 12 Quality of Life | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Anticholinergic + Bladder training vs. Anticholinergic | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Anticholinergic and Inferential current therapy + pelvic floor exercises + Bladder training vs.Anticholinergic . | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
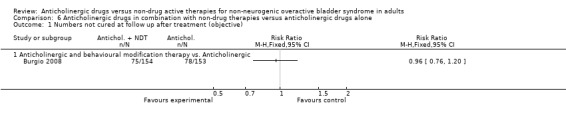
Comparison 6 Anticholinergic drugs in combination with non‐drug therapies versus anticholinergic drugs alone, Outcome 1 Numbers not cured at follow up after treatment (objective).
Comparison 7. Anticholinergic drugs versus combination non‐drug therapies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers not cured at end of treatment (subjective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Oxybutynin vs behavioural | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Numbers not improved at end of treatment (subjective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Oxybutynin vs behavioural | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Nocturia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Trospium vs. Inferential current therapy + pelvic floor exercises + Bladder training | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of micturitions per 24 hours | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.21, 1.42] |
| 4.1 Oxybutynin vs. behavioural | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.46, 1.26] |
| 4.2 Trospium vs. Inferential current therapy + pelvic floor exercises + Bladder training | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.22, 4.62] |
| 5 Number of incontinence episodes per 24 hours | 3 | 203 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.11, 0.70] |
| 5.1 Oxybutynin vs. behavioural | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.01, 0.63] |
| 5.2 Trospium 15mgs TDS vs. Inferential current therapy + pelvic floor exercises + Bladder training | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.35, 2.45] |
| 6 Quality of Life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Trospium vs. Inferential current therapy + pelvic floor exercises + Bladder training | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burgio 1998.
| Methods | RCT (double blinded between active drug and placebo) ITT | |
| Participants | n = 197 women Inclusion: 55yrs of age or older, ambulatory, urge incontinence occurring at least twice per week for at least 3 months Exclusion: continual leakage, postvoid residual urine volume more than 200mls, uterine prolapse past introitus, narrow‐angle glaucoma, unstable angina, decompensated congestive heart failure, history of malignant arrhythmias or impaired mental status mean age 67.7 (SD 7.5) | |
| Interventions | I ‐ (65) behavioural treatment: Visit 1 ‐ anorectal biofeedback used to help identify pelvic muscles and teach how to contract them while keeping abdominal muscles relaxed, Visit 2 ‐ teach how to respond adaptively to the sensation of urgency ("urge strategies"), participants were encouraged to pause, sit down if possible, relax the entire body and contract pelvic muscles repeatedly to diminish urgency and inhibit detrusor contraction and prevent urine loss, when urgency subsides they proceed to the toilet at normal pace, Visit 3 ‐ Pelvic muscle biofeedback was repeated for subjects who had not achieved at least 50% reduction in frequency of accidents as documented on bladder diaries. Combined bladder‐sphincter biofeedback was used to teach patients how to contract pelvic muscles against increasing volumes of fluid and during detrusor contraction, Visit 4 ‐ a review process "fine‐tune" home practice and encourage persistence: this include 45 pelvic muscle exercises every day (15 exercises 3 times per day). Patients were advised to practice in various positions, including lying, sitting and standing and also during activities. Finally patients were instructed to practice interrupting or slowing urinary stream during voiding once per day II ‐ (67) oxybutynin (2.5mg tds) III ‐ (65) placebo Duration: 8 weeks | |
| Outcomes | Numbers not cured (subjective): I: 44/63, II: 50/65
Numbers not improved (subjective): I: 6/63, II: 15/65
Incontinence episode/24hours ‐ mean (SD): I: 0.40 (0.67), n = 63, II: 0.81 (1.40), n = 65 Adverse outcomes: dry mouth I: 23/63 (34.9%), II: 65/65 (96.9%), III: 36/62 (54.8%), inability to void I: 4/63 (6.3%), II: 14/65 (21.5%), III: 2/62 (3.2%), constipation I: 14/63 (22.2%), II: 25/65 (38.5%), III: 23/62 (37.1%), blurred vision I: 6/63 (9.5%), II: 10/65 (15.4%), III: 6/62 (9.7%), confusion I: 4/63 (6.3%), II: 5/65 (7.7%), III: 7/62 (11.3%) |
|
| Notes | Ref ID: 5719 Combination (behavioural) treatment versus anticholinergic (oxybutynin) Full publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with computer‐generated random numbers using a block size of 6 |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the methodology |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assignment to drug treatment or the placebo control condition was double blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After 8 weeks 169 out of 197 participants completed the study |
Burgio 2008.
| Methods | Two stage multicentre randomised controlled trial 9 university based clinical centres in the US n = 307 |
|
| Participants | Community dwelling women with urge‐predominant incontinence. Most had symptoms of stress incontinence | |
| Interventions | Stage 1: drug therapy versus drug therapy + behavioural therapy for 10 weeks Stage 2: discontinuation of both drug and behavioral training sessions at end of stage 1. Instructed participants receiving combination therapy to continue their behavioural program after discontinuation of drug therapy. Participants were provided with a 2 month supply of the drug at no cost if they requested to resume drug therapy and did not have a urinary tract infection. Presented option of free drugs only after a patient requested to resume drug therapy. Follow‐up at 8 months Drug used ‐ extended release tolterodine tartrate at a dose of 4 mg per day. If not well tolerated, the dose could be decreased to 2 mg or another antimuscarinic medication could be substituted Behavioural therapy consisted of pelvic floor muscle training (using vaginal palpation); behavioural strategies to diminish urgency, suppress bladder contractions, and prevent both stress and urge incontinence; delayed voiding to increase voiding intervals for those who voided more than 8 times per day; and individualised fluid management for those with excessive urine output (>70oz/day) |
|
| Outcomes | Primary outcome at end of stage 2 (8 months) defined as not receiving drugs or any other therapy for urge incontinence and a 70% or greater reduction in frequency of incontinence episodes from baseline to end of stage 2 as recorded on the bladder diary Numbers not cured after stage 2 (at 8 months): 75 versus 78 Numbers not improved after stage 2 (at 8 months): 43 versus 85 Numbers not improved end of stage 1 (10 weeks treatment): 16 versus 35 Secondary outcomes (urgency and voids/day) were described in a second paper Improvement in score post‐treatment ‐ mean scores on the urgency severity scale decreased significantly from baseline to 10 weeks within both the drug alone and combined drug plus behavior groups, but the change between groups was not statistically significant, P = 0.30 Voids in 24 hours mean (SD) = 7.74 (2.45) n = 136 versus 6.94 (1.69) n = 133 |
|
| Notes | The study was supported by grants from National Institute of Diabetes and Kidney Diseases, whose program staff were involved in the design and conduct of the study, analysis and interpretation of data, and preparation and review of manuscript. Additional support, including provision of study drugs and funding, was contributed by Pfizer, whose staff reviewed and commented on the manuscript but were not involved in other aspects of the research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done at the coordinating centre through a web‐based automated randomisation system. A permuted block randomisation schedule, stratified by type of incontinence (urge only versus mixed), the number of incontinence episodes per week: 7 to 13 per week versus more than 14 per week |
| Allocation concealment (selection bias) | Low risk | Randomisation was done at the coordinating centre through a web‐based automated randomisation system. Clinical site ensured concealment of the allocation sequence* |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open label, study staff who performed evaluations, but not participants and interventionists, were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 220 out of 307 completed assessment at the end of 8 months |
Burgio 2010.
| Methods | RCT ITT |
|
| Participants | N = 64 Drug therapy = 32 Drug therapy plus behavioural therapy = 32 Community dwelling women with urge predominant incontinence |
|
| Interventions | Drug therapy versus drug therapy plus behavioural therapy (PFMT and urge suppression techniques) for 8 weeks (4 visits). Follow up at 6 and 12 months Drug used ‐ extended‐release oxybutynin (Individual dose titration between 5mg to 30mg) Behavioural therapy ‐ pelvic floor exercises and urge suppression techniques |
|
| Outcomes | 1) Not improved during treatment = 3/31 versus 6/28 2) Incontinence episode/24hours for 8 weeks ‐ mean (SD) ‐0.40(0.93), N = 31 (drug therapy) versus mean (SD) ‐0.84 (2.14), n = 28 (drug therapy + behavioural therapy) 3) Number of micturitions/24hrs mean (SD) = 8.2 (1.9) versus 8.4 (3.0) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified blocked randomisation, stratified by age, type of incontinence and severity of incontinence. Method of generation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | Questionnaires were completed by subjects before the visit and collected in a sealed envelope to ensure the nurse was blinded to these outcomes. Because participants were aware that behavioral treatment was provided in 1 study arm and they could potentially seek such treatment on their own, they were asked at each visit about any contact with other healthcare providers, new medications and hospitalisations open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31 out of 32 in drug group completed at 8 weeks compared with 27 out of 32 in the combined group 28 out of 32 in drug group completed at 6 months compared with 25 out of 32 in the combined group 28 out of 32 in drug group completed at 12 months compared with 22 out of 32 in the combined group |
Burgio 2011.
| Methods | Two site randomised controlled trial | |
| Participants | 143 community dwelling male veterans with over active bladder manifested by urgency and frequent urination, with or without urge incontinence | |
| Interventions | Nurse practitioners implemented behavioural and drug therapies over period of 8 weeks. Behavioural consisted of pelvic floor muscle training, delayed voiding, monitoring with bladder diaries and urge suppression techniques Drug group individually titrated extended release oxybutynin 5 to 30 mg/d |
|
| Outcomes | Assessed at baseline and 2 weeks after the fourth and final treatment visit (8 weeks) Primary outcome ‐ post‐treatment 24 hour voiding frequency measured according to 7 day bladder diary. Also measured nocturia, urgency, incontinence Secondary outcomes ‐ Global Perception of Improvement |
|
| Notes | Notes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified according to voiding frequency and presence or absence of incontinence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Research assistant blind to treatment group scored bladder diaries |
Chancellor 2008.
| Methods | Randomised, open label, parallel‐group, multicentre study ITT |
|
| Participants | n = 395 were randomised darifenacin alone = 190 darifenacin plus behavioural modification programme (BMP) = 205 |
|
| Interventions | Darifenacin treatment (with voluntary up‐titration from 7.5 mg once daily to 15 mg once daily at week 2) alone versus combination with a Behavioural Modification Programme (BMP) for men and women with dry or wet OAB BMP comprised a simple regimen for which training could be given in a primary physicians office, including timed voiding, dietary modifications and Kegel‐type exercises, and was meant to reflect real life clinical practice. Complex regimens that would require a trained physical therapist were avoided as would not be practical in primary care |
|
| Outcomes | Change in the number (per day) of micturitions
Urge urinary incontinence (UUI) episodes Urgency episodes Pads used Nocturnal voids Health related quality of life (HRQoL) Tolerability and safety assessments included adverse events and the number of discontinuations |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Validated, computer‐generated randomisation scheme used |
| Allocation concealment (selection bias) | Unclear risk | The randomisation scheme was reviewed by the sponsor's Quality Assurance group and locked after approval |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 173 out of 190 (91.1%) in drugs group completed compared with 175 out of 205 (85.4%). The proportion of patients discontinuing from the combination groups was higher (14.6% versus 8.9%) |
Collas 1994.
| Methods | RCT | |
| Participants | n = 57 mean age 82 (72‐98 years) | |
| Interventions | I (28) ‐ BT plus oxybutynin 2.5mg bd II (29) ‐ BT plus placebo Duration: 6 weeks | |
| Outcomes | Numbers NOT improved during treatment: I: 6/28, II: 13/29 | |
| Notes | Oxybutynin versus BT Abstract form, no details on methodology poor quality Ref ID: {7039} | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear from the methodology |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the methodology |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the publication |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention in the publication |
Colombo 1995.
| Methods | Randomised trial | |
| Participants | n = 81 women, severe (socially embarrassing urge urinary incontinence) Three patterns: detrusor instability, low compliance bladder and sensory bladder Exclusion: stable bladder, neurological disease, detrusor hyper‐reflexia, age > 65yrs, coexisting GSI, genital prolapse, post‐void residual > 50mls, previous gynae or urogynae surgery, previous drug therapy for urge urinary incontinence, urethral diverticulae, fistulae, urinary tract neoplasm, bacterial or interstitial cystitis, calculi or previous radiotherapy Mean age grp I 48yrs (31‐65), mean age grp II 49yrs (24‐65) | |
| Interventions | I ‐ (14) oxybutynin orally 5 mgs tds II ‐ (13) Bladder training: bladder training consisted of 6 weeks education, by simple but full explanation of the problem. At start of programme the maximum interval between two micturitions was identified and the patients were encouraged to initially hold their urine until this interval plus 30min had elapsed. Afterwards the women were asked to progressively increase the interval by 30min every 4 or 5 days. The goal was to achieve a delay between voids by up to 3 or 4 hours Duration: 6 weeks follow up at 6mths after end of study Cure was defined as reporting of total disappearance of urge incontinence and did not require protective pads or further therapies Clinical improvement was established when urge incontinence was subjectively less troublesome but the patient still wore protective pads and required further therapies All other cases were regarded as clinical failures | |
| Outcomes | Numbers not cured during treatment: I: 1/14, II: 2/13
Numbes not cured after treatment (at 6 months follow up): I: 5/14, II: 0/13
Numbers not improved during treatment: I: 14/14, II:10/13 Adverse events: dry mouth, constipation, nausea, dizziness, decrease in visual acuity and tachycardia. Numbers experiencing adverse events: I: 18/38, II: 2/39 Numbers withdrawn: I: 4/42, II: 2/39 (reasons for withdrawal: I: dry mouth x3, glaucoma x1, II: patient claimed treatment was time consuming) these cases we excluded from final analysis |
|
| Notes | Ref ID {6056} anticholinergic (oxybutynin) versus bladder training not clear in cure definition what authors meant by not needing further therapies (e.g. no need for second line treatment) Gave clinical results in subsets i.e. DI, low compliance and sensory urgency but not the same break down in cystometry results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the methodology |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in paper |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The treatment was discontinued in 6 cases (4 in the drug group because of side effects and 2 in bladder training because the patient claimed it was time consuming |
Kaya 2011.
| Methods | RCT | |
| Participants | N = 46 Women admitted to Dept of Obstetrics and Gynaecology with idiopathic detrusor overactivity |
|
| Interventions | A: Trospium chloride 15mg three times a day (N = 14) B: Physiotherapy (N = 15) C: Trospium chloride 15mg three times a day + physiotherapy (N = 16) Physiotherapy = bladder training + pelvic floor exercises + inferential therapy |
|
| Outcomes | Number of voids/day (mean (SD) = 8.9 (2.8) versus 6.7 (3.8) versus 6.9 (2.4) Nocturia before treatment (mean (SD) = 2.9 (1.8) versus 2.7 (2.7) versus 2.8 (1.5) Nocturia after treatment (mean (SD) = 2.1 (1.8) versus 0.4 (0.9) versus 1.1 (0.9) Incontinence per day (mean (SD) = 1.5 (2.0) versus 0.1 (0.20) versus 0.5 (0.8) QoL score (mean (SD) = 26.3 (19.5) versus 12.6 (15.1) versus 10.1 (12.2) Adverse effect: dry mouth was the most reported side effect (7/31, 22.58%) in the pharmacotherapy and combined therapy groups, 1 patient in the pharmacotherapy group withdrew from the study because of visual impairment |
|
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation list created by a statistician |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one patient in the pharmacotherapy group did not complete the study. All outcomes reported |
Macaulay 1988.
| Methods | Randomised trial | |
| Participants | n = 50 Inclusion: GSUI or DI at cystometry Exclusion: <20yrs, >65yrs, previous psychiatric illness, serious medical or renal illness, patients unable to attend regular follow up | |
| Interventions | I ‐ 19 psychotherapy II ‐ 16 bladder retraining BR. participants reviewed fortnightly for three months and again after one month, reaching a total of 7 sessions: participants were asked to keep a bladder diary and then learn to urinate at predetermined intervals. The time intervals were gradually increased throughout the study period. Participants were also taught pelvic floor muscle training to enable them to control as far as possible any symptoms of urgency III ‐ 15 probantheline 15 mgs tds, oral duration: 3 months | |
| Outcomes | Numbers not cured during treatment: I: 13/18, II: 9/15, III: 6/14 (data obtained manually from graph)
Numbers not cured after treatment (at follow up): I: 12/18, II: 11/15, III: 3/14 (data obtained manually from graph)
Numbers not improved during treatment: I: 13/18, II: 13/15, III: 12/14 (data obtained manually from graph)
Numbers not cured after treatment (at follow up ‐ period not less than 12 weeks and not over 20 weeks): I: 15/18, II: 12/15, III: 13/14 (data obtained manually from graph) Number of micturitions/24hrs‐ mean (SEM): I: 9.5 (7.5‐11.2), II: 6.8 (7.0‐10.2), III: 8.3 (8.0‐10.4). Bars represent SEM (data obtained manually from graph) Frequency of sensation of urgency ‐ mean (SEM): I: 1.0 (0.7‐1.4), II: 0.7 (0.2‐1.0), III: 0.3 (0.1‐0.6), P≤0.05. Bars represent SEM (data obtained manually from graph) |
|
| Notes | Ref ID: 16286 MD thesis Data estimated manually from graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient from each group withdrew and all the outcomes were reported |
Mattiasson 2003.
| Methods | Multicentre RCT ITT | |
| Participants | n = 501 Inclusion: women and men, > 18yrs age, urinary frequency > 8 x day and urgency (with or without urge urinary incontinence) Exclusion: any contraindication to antimuscarinics, electrotherapy or bladder training in last 3 months, catheterised patients or those using CISC, pregnancy or lactation, already on anticholinergic therapy or concomitant treatment for OAB (except oestrogen replacement included) | |
| Interventions | I (244) Tolterodine 2mg bd with bladder retraining. mean age grp I 62 yrs (range 19‐86) II ‐ (257) Tolterodine 2mg bd mean age group II 63 (range 22‐86) Duration: 24 weeks | |
| Outcomes | Numbers not improved: I: 57/239, II: 73/250 Number of Incontinence episodes/day ‐ median % change (Interqurtile range): I: ‐87 (‐100, ‐20), II: ‐81 (‐100, ‐41.8), P = 028 Number of micturitions/day ‐ median % change (interquartile range): I: ‐33 (‐42.3, 21.3), II: ‐25 (p38.8, ‐13.0), P<0.001 Adverse events: I: 158/244, II: 177/257 (dry mouth, headache and constipation) | |
| Notes | Ref ID {pending} Tolterodine versus tolterodine + bladder training Withdrawals not stated No usable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 391 patients (78%) completed 24 weeks of treatment, and completion rates were comparable for the two groups (tolterodine + BT, 77%; tolterodine alone, 79%). The main reasons for withdrawal were: adverse events (15%), lack of efficacy (3%), withdrawal of consent (2%), and protocol violations (1%). The two treatment groups were comparable in terms of the reasons for withdrawal, and 74% of patients in each treatment group were compliant in taking their medication throughout the study |
Mattiasson 2009.
| Methods | Multicentre, prospective, randomized, parallel‐group, open label study | |
| Participants | N = 643 Men or women over 18 with OAB |
|
| Interventions | Flexible‐dose solifenacin 5/10 mg (323 participants) versus solifenacin + simplified bladder training (320 participants) At week 8, patients in both groups could request a dose increase to solifenacin 10 mg od for the remaining 8 weeks of the study |
|
| Outcomes |
At 8 weeks using solifenacin 5 mg Mean number of micturitions/24h after 8 weeks = 9.32 versus 8.60 Change from baseline in micturitions/24h from baseline = ‐2.18 versus ‐2.87 Change from baseline in urgency from baseline = ‐1.99 versus ‐1.98 Change from baseline in urge incontinence episodes from baseline = ‐1.01 versus ‐1.16 Change in number of pads/24 from baseline = ‐1.19 versus ‐1.07 Change in patient perception of bladder condition from baseline = ‐1.24 versus ‐1.23 Change in treatment satisfaction VAS from baseline = 3.32 versus 3.50 Change in I‐QOL scores from baseline = 20.65 versus 19.68 At 16 weeks using solifenacin 5/10mg Mean number of micturitions/24h after 8 weeks = 9.32 versus 8.60 (SD not given) Change from baseline in micturitions/24h from baseline = ‐2.42 versus ‐3.11 Change from baseline in urgency from baseline = ‐2.20 versus ‐2.50 Change from baseline in urge incontinence episodes from baseline = ‐1.13 versus ‐1.38 Change in number of pads/24 from baseline = ‐1.29 versus ‐1.11 Change in patient perception of bladder condition from baseline = ‐1.58 versus ‐1.63 Change in treatment satisfaction VAS from baseline = 3.72 versus 4.18 Change in I‐QOL scores from baseline = 24.51 versus 25.34 Adverse effects solifenacin n = 323 versus solifenacin +simplified bladder training n = 320 Number experiencing adverse effects = 150 versus 149 Number withdrawing from treatment or trial arm = 19 versus 15 Number changing dose of treatment = 137 versus 125 |
|
| Notes | 81 centres in 16 countries in Europe and Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the paper |
| Allocation concealment (selection bias) | Unclear risk | No mention in the paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. Investigator or research nurse reviewed the bladder diaries with the patient to ensure accuracy of completion |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Full analysis set included all patients randomised at baseline who took one or more doses of study medication and who provided primary efficacy data at baseline and week 4 or 8". Safety population included all patients who took at least one dose of study medication |
Milani 1987.
| Methods | Randomised trial of idiopathic urge syndrome | |
| Participants | n = 81 women with idiopathic urge syndrome Exclusions; women >65, previous pelvic radiotherapy, pelvic masses or malignancy, urinary tract or renal pathology, central nervous system disease, 2nd or 3rd degree genital prolapse | |
| Interventions | I ‐ (37) bladder retraining BR II ‐ (38) oxybutynin 15mgs tds Duration: 4 weeks for oxybutynin. 12 weeks for bladder re‐training | |
| Outcomes | Numbers NOT cured during treatment: I: 10/37, II: 10/38 Numbers NOT improved during treatment: I: 30/37 II: 25/38 Numbers NOT cured after treatment: I: 13/37, II: 22/38 Adverse events: I: 0/37, II: 14/38 (dryness of mouth) | |
| Notes | Ref ID {9033} Oxybutynin versus bladder training Abstract form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 patients discontinued from the study (4 in the drug group because of side effects and 2 in bladder training because the patient claimed it was time consuming) |
Millard 2004.
| Methods | Multicentre RCT ITT | |
| Participants | n = 475 Females = 359 Males = 116 Age ‐ mean [SD] (range): 53.4 (17.4), (18‐90 years) Inclusion: males and females Urinary frequency > 8 times per day, urgency and urge urinary incontinence occurring at least once a day and all symptoms present for at least 6 months Exclusion: symptomatic GSI, significant residual volumes, neuropathy, glaucoma, UTI, positive urine culture | |
| Interventions | I (252): tolterodine, 2mg bd, orally II (223): tolterodine, 2mg bd, orally plus PFMT, 15 contractions in morning and afternoon and 20 contractions in evening Duration of study: 24 weeks PFMT programme consisted of a two‐page instructions which included: How to identify the correct muscle, the exercise programme, how to use pelvic floor muscle contraction to avoid leakage, and having realistic expectations. Patients were expected to contract their muscles for 10sec with rest of 10sec in between in the morning and afternoon, with 20 contractions in the evening, the number of contractions to increase up to 25 as muscle strength increased | |
| Outcomes | Numbers not improved during treatment: I: 36/252, II: 41/223 Number of incontinence episodes/day ‐ mean (SD): I: 0.95 (1.9), II: 1.25 (2.7) Number of micturitions/day ‐ mean (SD): 9.20 (5.2), II: 9.29 (5.0) Frequency of sensation of urgency/day ‐ mean (SD): I: 1.5 (2.3), II: 2.1 (3.1) Adverse events: 43/475 (headache, constipation, dizziness, dry mouth) | |
| Notes | Ref ID {pending} Tolterodine versus PFMT+tolterodine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the Group I ‐ 81.0 and 75.5% of subjects completed 12 and 24 weeks of treatment. The addition of pelvic floor exercises did not significantly impact continuation, with 79.7 and 71.4% completing 12 and 24 weeks (group II). Reasons for discontinuation were similar in both groups as follows; consent withdrawn and failure to take the medication 5.6%, protocol violation 5.2%, adverse event 9.4%. Only 1.9% discontinued due to lack of efficacy |
Ozdedeli 2010.
| Methods | RCT | |
| Participants | Females with OAB N = 35 |
|
| Interventions | Trospium hydrochloride 45mg versus intravaginal electrical stimulation | |
| Outcomes | Frequency of sensation of urgency/day at 6th week ‐ median (range) 2.7 (0‐8.0) versus 1.7 (0‐13.0) Number of incontinence episodes/day at 6th weeks ‐ 1 (0‐5) versus 0.3 (0‐9) Number of micturitions/day at 6th week ‐ 6 (3.3‐14.7) versus 7 (0.6 ‐15) IIQ‐7 at 18th week ‐ 51.2 (4.8‐68.1) versus 4.8 (0‐57.8) BDI at 18th week ‐ 10 (5‐40) versus 9.0 (6‐18) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The researcher randomised each patient into one of the two groups by opening sealed envelopes. The randomisation list was generated by a blinded researcher (the third author) using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | The randomisation results were kept in sealed envelopes, one for each patient |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no complete blindness in the study, since stimulation was applied by a separate researcher, while examination and data collection were carried out by a different researcher |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two patients in Group 1 (one patient discontinued the study when all her complaints were resolved and one patient developed haematuria) and 2 patients in Group 2 (due to menorrhagia in 1 patient and vaginal pain after vaginal probe placement in 1 patient) could not complete the study |
Park 2002.
| Methods | Randomised study | |
| Participants | n = 74 women with overactive bladder No exclusion criteria | |
| Interventions | I (24): bladder training
II (24): tolterodine 2mg bd, orally
III (26): tolterodine 2 mg bd, orally + bladder training
Duration of study: 12 weeks Bladder training consisted of explanation of pelvic anatomy and physiology, bladder inhibition by Kegal exercises whenever she feels urgency and bi‐weekly phone calls from an expert nurse to confirm that patient is doing it well |
|
| Outcomes | Numbers not improved during treatment: I: 12/24, II: 10/24, III: 8/26 Adverse events: I: 0/24, II: 6/24, III: 7/26 | |
| Notes | Ref ID [pending] tolterodine versus bladder training alone versus tolterodine + bladder training Abstract form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall discontinuation numbers not given |
Peters 2009.
| Methods | Multicentre RCT | |
| Participants | A total of 100 ambulatory adults with OAB symptoms,with or without a history of previous anticholinergic drug use, with at least 8 voids per 24 hours documented by history and physical and voiding diary were randomised to 1:1 to 12 weeks of treatment with weekly percutaneous tibial nerve stimulation or to 4 mg daily extended‐release tolterodine Exclusion criteria 1) OAB pharmacotherapy within the previous month 2) primary complaint of stress urinary incontinence 3) demonstrated sensitivity to tolterodine or its ingredients 4) pacemakers or implantable defibrillators 5) excessive bleeding, urinary or gastric retention, nerve damage or neuropathy 6) uncontrolled narrow angle glaucoma 7) positive urinalysis for infection 8) pregnancy, or current pregnancy or planning to become pregnant during the trial |
|
| Interventions | A (44): percutaneous tibial nerve stimulation (50) B (42): extended‐release tolterodine 4mg (40) for 12 weeks |
|
| Outcomes |
Participant observation percutaneous tibial nerve stimulation (44) versus extended‐release tolterodine 4mg (42) for 12 weeks Cured total (%) ‐ 1 (2.3) versus 2 (4.8) Improved total (%) ‐ 34 (77.3) versus 21 (50.0) No improvement/worsening total (%) ‐ 9 (20.5) versus 19 (45.2) Cured or improved total (%) 35 (79.5) versus 23 (54.8) Clinician observation percutaneous tibial nerve stimulation(44) versus extended‐release tolterodine 4mg (43) for 12 weeks Cured total (%) ‐ 2 (4.5) versus 2 (4.7) Improved total (%) ‐ 33 (75.0) versus 24 (55.8) No improvement/worsening total (%) ‐ 9 (20.5) versus 17 (39.5) Cured or improved total (%) ‐ 35 (79.5) versus 26 (60.5) Voids/day No./Total No. (%) = 30/41 (73.2) versus 32/43 (74.4) Nocturia No./Total No. (%) = 28/40 (70.0) versus 26/42 (61.0) Urge incontinence No./Total No. (%) = 24/30 (80.0) versus 27/37 (73.0) Moderate to severe urgency episodes/day No./Total No. (%) = 29/41 (70.7) versus 31/41 (75.6) Voided vol (cc) No./Total No. (%) = 31/41 (75.6) versus 28/43 (65.1) |
|
| Notes | Multicentre: 11 centres in US | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random block design stratified, stratified by investigational site, generation sequence not mentioned. Randomised 1:1 |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done at investigational site, however no mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blinded, bladder diary analysed by a biostatistician |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100 (50 in each group) adults were randomised. Of the patients who completed 12 weeks of therapy 41 receiving PTNS and 43 on tolterodine completed the voiding diary. Three patients in the drug group discontinued due to unsuccessful treatment |
Smith 1996.
| Methods | RCT ITT | |
| Participants | n = 38 females Age: 24‐82 years Inclusion: urinary incontinence Exclusion: Type 3 stress incontinence; pregnancy suspected; history of prolonged urinary retention; vaginal vault prolapse; diminished sensory perception; cardiac pacemaker; mixed incontinence with major and minor component indistinguishable | |
| Interventions | I (20): probantheline, 7.5 mg ‐ 45 mg bd ‐ tds, orally II (18): electrical stimulation, twice daily for 4 months, 12.5 and 5 MHz frequency: the intravaginal electric stimulation system was approved by FDA, electric pulse generator by a 9 volt alkaline battery with a 12.5 HZ and 50Hz frequency. Compliance monitored by a built‐in digital readout on the number of hours used (patient not aware of it) Duration of study: 4 months | |
| Outcomes | Numbers not cured during treatment: I: 10/20, II: 14/18 Numbers not improved during treatment: I: 10/20, II: 9/18 Numbers of pad changes/day ‐ average (range): I: 8.1 (0‐16), II: 6.5 (0‐18) Number of incontinence episodes/day ‐ average (range): I: 1.7 (0‐8), II: 1.4 (0‐8) | |
| Notes | Ref ID {2900} Anticholinergic (probantheline) versus electric stimulation (intravaginal) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one patient did not complete the study |
Song 2006.
| Methods | RCT | |
| Participants | n = 139 Women with symptoms of urgency and frequency, with or without urge incontinence |
|
| Interventions | Bladder training versus tolterodine 2 mg twice daily versus combination of bladder training and tolterodine 2mg twice daily treatment for 12 weeks | |
| Outcomes |
Bladder training (26) versus tolterodine 2 mg twice daily (32) versus combination (31) Number not improved = 12 versus 12 versus 9 Number of voids per day N, mean (SD): 8.1 versus 8.1 versus 7.9 Nocturia before treatment = 1.5 versus 1.7 versus 1.2 Nocturia after treatment = 0.6 versus 0.6 versus 0.6 Number withdrawing from tolterodine versus combination treatment = 2/32 versus 2/31 Adverse effects: tolterodine total number (%) versus combination total number (%) Dry mouth:7 (21.9%) versus 9 (28.9%) Hesitancy: 3 (9.4%) versus 2 (6.5%) Decreased appetite/constipation: 2 (6.3%) versus 2 (6.5%) Headache: 1 (3.1%) versus 0 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states 'randomly assigned' no mention of how this was done |
| Allocation concealment (selection bias) | Unclear risk | No mention in paper |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding of participants or care providers, no mention of blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Bladder training group 26 out of 46 (56%) completed. Tolterodine 32 out of 47 (68%) completed. Combination therapy 31 out of 46 (67%) completed. Out of 139 patients 89 completed the 12 week regimen |
Soomro 2001 #.
| Methods | RCT, ITT, cross‐over | |
| Participants | n = 43 Age‐ mean 50 ± 15 years Sex: F:M 30:13 Inclusion: patients with a history of frequency, urgency and urge incontinence who had not previously been treated at the department, including some who had previously received treatment from a general practitioner at least 6 months before Exclusion: not specified | |
| Interventions | I (43): oxybutynin, 2.5 mg BD, up to 5 mg TDS, orally
II (43): TENS, 20 HZ, 6 hours daily After 6 weeks of treatment in the first arm, patients were re‐assessed. Patients completed a post‐treatment side effects questionnaire. After a washout period of 2 weeks, patients were started on the second arm of treatment, and the same assessment was repeated after 6 weeks of treatment Total duration of study: 14 weeks |
|
| Outcomes | Numbers not improved during treatment: I: 20/40, II: 20/38 Number of voids per day N, mean (SD): I 43, 9 (SD 5), II 43, 9 (SD 4), P≤0.003 compared with baseline of 43, 11 (SD 5) Number of incontinence episodes: "no statistical difference between treatment arms" Number of micturitions per day: "statistically significant (p< 0.0001)" compared with baseline QoL: "No statistical difference found in any of the parameters of SF‐36" Adverse events: dry mouth, blurred vision, dry skin and skin irritation |
|
| Notes | Ref ID [ pending] Oxybutynin versus electric stimulation (TENS) Cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
Svihra 2002.
| Methods | Randomised trial ITT | |
| Participants | n = 28 females Age, mean (range): 54 years (45‐63) Inclusion: overactive bladder Exclusion: bladder neck obstruction | |
| Interventions | I ‐ (9) SANS, neuromodulation: modified non‐invasive SANS, using a surface electrode on the skin over the medial ankle and stimulated the tibial nerve with different values which cannot be set by the original SANS. Frequency of stimulation was 1Hz and duration of square impulse was 0.1ms. surface stimulation of 30min duration was repeated once a week for a period of 5 weeks II ‐ (10) oxybutynin orally 5mg tds III ‐ (9) control | |
| Outcomes | Numbers not cured during treatment: I: 2/9, II: "comparable therapeutic results" Numbers not improved during treatment: I: 7/9, II: "comparable therapeutic results" | |
| Notes | Ref ID: {15769} Results vague: actual data for the oxybutynin arm are not available,and only mention "comparable therapeutic results" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Szonyi 1995.
| Methods | Randomized double blind ITT | |
| Participants | n = 60 Mean age (SD) 82.19 (6.96), range 72‐98 years Inclusion: either sex, over 70, symptoms of urinary frequency, urgency and urge incontinence, mobile, able to attend opd, able to keep diary chart, willing to give consent Exclusion: UTI at time of recruitment, severe hepatic or renal disease, glaucoma, uncontrolled diabetes, concomitant anticholinergics (imipramine/propantheline) Patients in both groups well matched for age, sex (14:1 women:men), urodynamic characteristics | |
| Interventions | I (30) placebo + BR
II (30) oxybutynin 2.5mg bd + BR
Duration: 57 days Bladder training: patients were asked to delay micturition for as long as possible whenever they experience the need to pass urine and to try by this means to reduce their frequency |
|
| Outcomes | Numbers not cured during treatment: I: 25/28, II: 20/24 Numbers not improved during treatment: I: 16/28, II: 7/24 Adverse events: dry mouth, blurred vision, heartburn, constipation, dry skin. Poor compliance: I: 20%, II: 20% | |
| Notes | Ref ID {2906} anticholinergic (oxybutynin) + BR versus BR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was achieved by allocating tablets in random permutations of blocks of four patients at a time |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient in the placebo group and two randomised to active treatment failed to attend the first review and so no data were available for analysis. Not all patients kept the diary charts as requested, but diary data were available for analysis from 28 patients on placebo and 24 patients on active treatment. Five patients on placebo withdrew early , Eight patients on oxybutynin withdrew early |
Wang 2006.
| Methods | Placebo‐controlled RCT | |
| Participants | N = 68 Patients with OAB |
|
| Interventions | Intravaginal electrical stimulation (24 participants) versus oxybutynin 2.5mg without dose titration (23 participants) versus placebo (21 participants). 12 weeks duration | |
| Outcomes | Number cured after treatment ES versus oxybutynin = 4 versus 2 Number improved after treatment ES versus oxybutynin = 10 versus 7 Number cured after treatment ES versus oxybutynin =10 versus 14 Number of micturitions/24 hrs = 7.8 versus 7.4 Frequency of urgency episodes after treatment = 1 versus 6 Mean urine volume after treatment = 355 versus 336 Number withdrawing due to dry mouth drug group = 3 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The allocation of the three groups was undertaken by sequentially opening of sealed envelope, prepared by the Biostatistics Center for Chang Gung Medical College in blocks of six |
| Allocation concealment (selection bias) | Low risk | The allocation of the three groups was undertaken by sequentially opening of sealed envelope, prepared by the Biostatistics Center for Chang Gung Medical College in blocks of six |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Physiotherpists conducted treatments unaware of the progress and outcome of the interventions. Patients receiving pharmacotherapy and investigators were unaware of regimen from central pharmacy whether active drug or placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 patients dropout from the study, however all three groups had similar number of patients. All pre‐stated objectives reported |
Wise 1993.
| Methods | Randomised not placebo controlled | |
| Participants | n = 60 women with urodynamically proven detrusor instability | |
| Interventions | I ‐ (32) oxybutynin 5mg bd II ‐ (28) intravaginal maximal electrical stimulation 20HZ frequency with variable current of 0‐90mA | |
| Outcomes | Adverse events: I: 7/32, II: not specified No usable data for interpretation | |
| Notes | Ref ID {12049} Anticholinergic (oxybutynin) versus electric stimulation (intravaginal electric stimulation), no detailed methodology few actual figures /data listed no exclusion criteria Abstract form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention in the methods |
| Allocation concealment (selection bias) | Unclear risk | No mention in the methods |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention in the methods |
BD: Bidaily, BR: Bladder retraining, BT: Bladder training; CISC: Clean Intermittent Self Catheterisation; DI: Detrusor Instability, BT: Bladder Training, CISC: Clean Intermittent Self Catheterisation, ER: Extended Relase, GSUI: Genuine Stress Urinary Incontinence, ITT: Intention to treat, OPD: Out Patient Department, PFMT: Pelvic Floor Muscle Training, PV: Prompted Voiding, QoL: Quality of Life, RCT: Randomised Controlled Trial, SANS: Sacral Afferent Nerve Stimulation, TENS: Trancutaneous Electrical Nerve Stimulation, TDS: Tridaily, UI: Urinary Incontinence, UTI: Urinary Tract Infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Castleden 1986 # | This is a dose escalation study and comparison against placebo only and there is no non‐drug therapeutic arm |
| Enzelsberger 1991 | Emepronium bromide is a tricyclic antidepressant |
| Gelber 1997 | Study population: neuropathic bladder following stroke, with normal urodynamics |
| Goode 2011 | This was a part of BE‐DRI study and the objective was find factors that influence patient satisfaction |
| Henalla 1989 | Terodiline withdrawn from worldwide market because of serious cardiotoxicity |
| Henalla 1991 | Terodiline withdrawn from worldwide market because of serious cardiotoxicity |
| Jarvis 1981 | No good evidence of efficacy for flavoxate, and not recommended by WHO and is not in regular use anymore |
| Kelleher 1994 | Not electric stimulation |
| Kim 2008 | Objective of the study was to assess bladder training versus combination of propiverine + bladder training for urinary frequency not OAB |
| Klarskov 1981 | Terodiline withdrawn from worldwide market because of serious cardiotoxicity |
| Klarskov 1986 | Terodiline withdrawn from worldwide market because of serious cardiotoxicity |
| Koonings 1987 | Mixed urinary incontinence not relevant comparing surgery for SUI component versus surgery and anticholinergics |
| Koonings 1988 | Mixed urinary incontinence not relevant comparing surgery for sui component versus surgery and anticholinergics |
| Locher 2002 | Not a treatment trial testing anticholinergics but "to assess the effects of age and patients' attribution of incontinence to aging on health‐behaviours associated with incontinence" |
| MacDiarmid 2010a | Not compared with anticholinergics |
| Osman 2003 | Not OAB |
| Ouslander 1987 | Assessement of habit training in functionally impaired nursing home patients (dementia) |
| Ouslander 1988 | Assessement of habit training in functionally impaired nursing home patients (dementia) |
| Ouslander 1995 # | Prompted voiding is not bladder training and not part of our study protocol |
| Pennisi 1994 | Not RCT, only address urinary stress Incontinence |
| Peters 2010 | Not compared with anticholinergics |
| Visco 2011 | RCT comparing anticholinergics and Botilinum toxin. However study data is expected in mid‐2012 |
| Weil 1995 | No anticholinergic arm |
| Weil 2000 | No anticholinergic arm |
| Wise 1992 | Not confirmed to have been randomised |
| Wiseman 1990 | Terodiline withdrawn from worldwide market because of serious cardiotoxicity |
| Wiseman 1991 | Terodiline withdrawn from worldwide market because of serious cardiotoxicity |
OAB: Over Active Bladder
Contributions of authors
Bhavan Rai, June Cody and Ammar Alhasso conducted the review, including data abstraction, interpretation and writing the review. Ammar Alhasso and Laurence Stewart, the supervising consultant, reviewed and scrutinised the data collection, and were involved in the interpretation and the writing up of the original version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group
Declarations of interest
Mr L Stewart has been on consultation committees for tolterodine and oxybutynin. The remaining authors have no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Burgio 1998 {published data only}
- Burgio KL, Locher JL, Goode PS. Behavioural vs drug treatment for urge urinary incontinence in older women: A randomized controlled trial. JAMA 1998;280(23):1995‐2000. [DOI] [PubMed] [Google Scholar]
- Burgio KL, Locher JL, Goode PS. Combined behavioural and drug therapy for urge urinary incontinence in older women. Journal of the American Geriatrics Society 2000;48(4):370‐4. [DOI] [PubMed] [Google Scholar]
- Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Candib D. Behaviour vs drug therapy for urge incontinence in older women. Proceedings of the 15th Annual Scientific Meeting of the American Urogynecology Society. 1994:48.
- Burgio KL, Locher JL, Roth DL, Goode PS. Psychological improvements associated with behavioural and drug treatment of urge incontinence in older women. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2001;56B:46‐51. [DOI] [PubMed] [Google Scholar]
- Goode PS, Burgio KL, Locher JL, Umlauf MG, LLoyd KL, Roth DL. Urodynamic changes associated with behavioural and drug treatment of urge incontinence in older women. Journal of the American Geriatrics Society 2002;50:808‐16. [DOI] [PubMed] [Google Scholar]
Burgio 2008 {published data only}
- Burgio KL, Kraus SR, Borello‐France D, Chai TC, Kenton K, Goode PS, et al. The effects of drug and behavior therapy on urgency and voiding frequency. International Urogynecology Journal 2010;21(6):711‐9. [39603] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio KL, Kraus SR, Menefee S, Borello‐France D, Corton M, Johnson HW, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Annals of Internal Medicine 2008; Vol. 149, issue 3:161‐9. [27584] [DOI] [PMC free article] [PubMed]
Burgio 2010 {published data only}
- Burgio KL, Goode PS, Richter HE, Markland AD, Johnson TM, Redden DT. Combined behavioral and individualized drug therapy versus individualized drug therapy alone for urge urinary incontinence in women. Journal of Urology 2010;184(2):598‐603. [39902] [DOI] [PubMed] [Google Scholar]
Burgio 2011 {published data only}
- Burgio KL, Goode PS, Johnson II TM, Hammontree LN, Ouslander JG, Markland AD, et al. Behavioral vs. drug treatment for overactive bladder in men: The Motive Trial (Abstract number 1516). Journal of Urology 2010;183(4 Suppl 1):e585. [42519] [Google Scholar]
- Burgio KL, Goode PS, Johnson TM, Hammontree L, Ouslander JG, Markland AD, et al. Behavioral versus drug treatment for overactive bladder in men: the male overactive bladder treatment in veterans (MOTIVE) trial. Journal of the American Geriatrics Society 2011;59(12):2209‐16. [42742] [DOI] [PubMed] [Google Scholar]
Chancellor 2008 {published data only}
- Chancellor MB, Kianifard F, Beamer E, Mongay L, Ebinger U, Hicks G, et al. A comparison of the efficacy of darifenacin alone vs. darifenacin plus a Behavioural Modification Programme upon the symptoms of overactive bladder. International Journal of Clinical Practice 2008;62(4):606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collas 1994 {published data only}
- Collas DM, Szonyi G, Ding YY, Malone LJ. Oxybutynin with bladder retraining for detrusor instability in the elderly ‐ placebo controlled trial. Age and Ageing 1994;23S:9. [DOI] [PubMed] [Google Scholar]
Colombo 1995 {published data only}
- Colombo M, Zanetta G, Scalambrino S, Milani R. Oxybutynin and bladder training in the management of female urinary urge incontinence: A randomized study. International Urogynecology Journal and Pelvic Floor Dysfunction 1995;6:63‐7. [Google Scholar]
Kaya 2011 {published data only}
- Kaya S, Akbayrak T, Beksac S. Comparison of different treatment protocols in the treatment of idiopathic detrusor overactivity: a randomized controlled trial.. Clinical Rehabilitation 2011;25(4):327‐38. [DOI] [PubMed] [Google Scholar]
Macaulay 1988 {published data only}
- Macaulay AJ. Psychological factors in the aetiology and treatment of urinary symptoms in women. MD Thesis Charing Cross and Westminster Medical School 1988.
- Macaulay AJ, Holmes D, Stanton SL, Stern RS. A prospective randomised controlled trial of bladder retraining and brief psychotherapy for urinary frequency and urgency. Proceedings of the 15th Annual Meeting of the International Continence Society (ICS). 1985:184‐5.
- Macaulay AJ, Stern RS, Holmes DS, Stanton SL. Micturition and the mind: psychological factors in the aetiology and the treatment of urinary symptoms in women. BMJ (Clinical Research Edition) 1987;294(6571):540‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattiasson 2003 {published data only}
- Mattiasson A. Effect of simplified bladder training and tolterodine treatment in overactive bladder patients. Proceedings of 2nd International Consultation on Incontinence, 1‐3 July 2001 (Poster presentations). 2001:Abstract 59.
- Mattiasson A. Simplified bladder training augments tolterodine treatment in overactive bladder patients. Neurourology and Urodynamics 2001;20(4):403‐4. [Google Scholar]
- Mattiasson A, Blaakaer J, Hoye K, Wein AJ. Simplified bladder training augments the effectiveness of tolterodine in patients with an overactive bladder. BJU International 2003;91(1):54‐60. [DOI] [PubMed] [Google Scholar]
Mattiasson 2009 {published data only}
- Mattiasson A, Masala A, Morton R, Bolodeoku J. Efficacy of simplified bladder training in patients with overactive bladder receiving a solifenacin flexible‐dose regimen: results from a randomized study. BJU International 2009;105(8):1126‐35. [DOI] [PubMed] [Google Scholar]
Milani 1987 {published data only}
- Milani R, Scalambrino S, Carrera S Quadri G Riva D, Casolati E. A randomized trail of bladder retraining versus oxybutynin in the treatment of idiopathic urge syndrome, early results. Proceedings of 16th Annual Meeting of the International Continence Society (ICS). 1986:488‐90.
- Milani R, Scalambrino S, Quadri G, Carrera S, Riva D, Castolati E. Randomized drug therapy and bladder retraining in urge syndrome: late results. Proceedings of the 17th Annual Meeting of the International Continence Society (ICS) 1987;2:133‐4. [Google Scholar]
Millard 2004 {published data only}
- Millard RJ. Clinical efficacy of tolterodine with or without a simplified pelvic floor exercise regimen. Neurourology and Urodynamics 2004;23:48‐53. [DOI] [PubMed] [Google Scholar]
Ozdedeli 2010 {published data only}
- Ozdedeli S, Karapolat H, Akkoc Y. Comparison of intravaginal electrical stimulation and trospium hydrochloride in women with overactive bladder syndrome: a randomized controlled study. Clinical Rehabilitation 2010;24(4):342‐51. [DOI] [PubMed] [Google Scholar]
Park 2002 {published data only}
- Park TJ, Song C, Choo M. The effects of bladder retraining, tolterodine and bladder retraining and tolterodine in female patients with overactive bladder, a prospective randomised study. Neurourology and Urodynamics 2002;21(4):434‐5. [Google Scholar]
Peters 2009 {published data only}
- Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended‐release tolterodine: results from the overactive bladder innovative therapy trial. Journal of Urology 2009;182(3):1055‐61. [DOI] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Aaronson PS, Loehner D, Bingham W, Smith JJ. Intravaginal electrical stimulation in the treatment of genuine stress urinary incontinence and detrusor instability: a controlled study. Journal of Urology 1995;153(4 Suppl):491A. [Google Scholar]
- Smith JJ. Intravaginal stimulation randomized trial. Journal of Urology 1996;155(1):127‐30. [PubMed] [Google Scholar]
- Smith JJ, Loehner D, Bignham W. Intravaginal electrical stimulation in the treatment of GSUI and DI: a controlled study. Proceedings of the 15th Annual Scientific Meeting of the American Urogynecological Society. 1994:Abstract 25.
Song 2006 {published data only}
- Song C, Park JT, Heo KO, Lee KS, Choo MS. Effects of bladder training and/or tolterodine in female patients with overactive bladder syndrome: a prospective, randomized study. Journal of Korean Medical Science 2006;21(6):1060‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soomro 2001 # {published data only}
- Soomro NA, Khadra MH, Robson W, Neal DE. A crossover randomized crossover trial of transcutaneous electrical nerve stimulation and oxybutynin in patients with detrusor instability. Journal of Urology 2001;166(1):146‐9. [PubMed] [Google Scholar]
Svihra 2002 {published data only}
- Svihra J, Kurca E, Luptak J, Kliment J. Neuromodulative treatment of overactive bladder ‐ non‐invasive tibial nerve stimulation. Bratislavske Lekarske Listy 2002;103(12):480‐3. [PubMed] [Google Scholar]
Szonyi 1995 {published data only}
- Szonyi G, Collas DM, Ding YY, Malone‐Lee JG. Oxybutynin with bladder retraining for detrusor instability in elderly people: A randomized controlled trial. Age and Ageing 1995;24:287‐91. [DOI] [PubMed] [Google Scholar]
- Szonyi G, Ding YY, Malone‐Lee JG. Oxybutynin with bladder retraining for detrusor instability in elderly people. Proceedings of the 24th Annual Meeting of the International Continence Society (ICS). 1994:353‐4.
Wang 2006 {published data only}
- Wang AC, Chih SY, Chen MC. Comparison of electric stimulation and oxybutynin chloride in management of overactive bladder with special reference to urinary urgency: a randomized placebo‐controlled trial. Urology 2006;68(5):999‐1004. [DOI] [PubMed] [Google Scholar]
Wise 1993 {published data only}
- Wise BG, Cardozo LD, Plevnik S, Kelleher C, Abbott D. A comparative study of oxybutynin and maximal electrical stimulation in the treatment of detrusor instability. Proceedings of 23rd Meeting of ICS 1993;8:236. [Google Scholar]
References to studies excluded from this review
Castleden 1986 # {published data only}
- Castleden CM, Duffin HM, Millar AW. A controlled clinical pilot study of dicyclomine in detrusor instability. Proceedings of the 16th Annual Meeting of the International Continence Society (ICS). 1986:373‐5.
Enzelsberger 1991 {published data only}
- Enzelsberger H, Schatten C, Kurz C. Vergleich von Emeproniumbromid mit intravesikal appliziertem Lidocain‐Gel bei Frauen mit Urge‐Inkontinenz. Geburtsh u Frauenheilk 1991;51:54‐7. [DOI] [PubMed] [Google Scholar]
Gelber 1997 {published data only}
- Gelber DA, Swords L. Treatment of post‐stroke urinary incontinence. Journal of Neurologic Rehabilitation 1997;11(2):131. [Google Scholar]
Goode 2011 {published data only}
- Goode PS, Burgio KL, Kraus SR, Kenton K, Litman HJ, Richter HE, et al. Correlates and predictors of patient satisfaction with drug therapy and combined drug therapy and behavioral training for urgency urinary incontinence in women. International Urogynecology Journal 2011;22(3):327‐34. [SR‐INCONT41557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henalla 1989 {published data only}
- Henalla SM, Millar DR, Moon PV. The effects of terodiline and cystodistention combined with bladder drill for the treatment on the unstable bladder (preliminary results). Proceedings 19th Annual ICS meeting 1989;95:198‐9. [Google Scholar]
Henalla 1991 {published data only}
- Henalla SM, Millar DR, Moon PV. Medical or surgical augmentation of bladder drill for detrusor instability. Journal of Obstetrics and Gynaecology 1991;11(2):128‐30. [Google Scholar]
Jarvis 1981 {published data only}
- Jarvis GJ. A controlled trial of bladder drill and drug therapy in the management of detrusor instability. British Journal of Urology 1981;53:565‐6. [DOI] [PubMed] [Google Scholar]
- Jarvis GJ. The unstable bladder ‐ A psychosomatic disease?. Proceedings of 11th Annual Meeting of the International Continence Society (ICS). 1981:45‐6.
Kelleher 1994 {published data only}
- Kelleher CJ, Filschie J, Burton G, Khullar V, Cardozo LD. Acupuncture and the treatment of irritative bladder symptoms. Acupuncture in Medicine 1994;12:9‐12. [Google Scholar]
Kim 2008 {published data only}
- Kim SW, Song SH, Ku JH. Bladder training versus combination of propiverine with bladder training for female urinary frequency. A prospective, randomized, comparative study. Gynecologic and Obstetric Investigation 2008;65(2):123‐7. [DOI] [PubMed] [Google Scholar]
Klarskov 1981 {published data only}
- Klarskov P, Gerstenberg T, Hald T. Bladder retraining and terodiline on urge incontinence in females with stable detrusor function. Proceedings of the 14th Annual Meeting of the International Continence Society (ICS). 1984:404‐5.
Klarskov 1986 {published data only}
- Klarskov P, Gerstenberg TC, Hald T. Bladder training and terodiline in females with idiopathic urge incontinence and stable detrusor function. Scandinavian Journal of Urology and Nephrology 1986;20:41‐6. [DOI] [PubMed] [Google Scholar]
Koonings 1987 {published data only}
- Koonings PP, Bergman A. Urethral relaxation: Bladder instability and stress urinary incontinence. Where is the primary pathology?. Proceedings of the 8th Annual Meeting of the American Urogynecology Society. 1987.
Koonings 1988 {published data only}
- Koonings P, Bergman A, Ballard CA. Combined detrusor instability and stress urinary incontinence: Where is the primary pathology?. Gynecologic and Obstetric Investigation 1988;26:250‐6. [DOI] [PubMed] [Google Scholar]
Locher 2002 {published data only}
- Locher JL, Burgio KL, Goode PS, Roth DL, Rodriguez E. Effects of age and causal attribution to ageing on health related behaviours associated with urinary incontinence in older women. Gerentologist 2002;42(4):515‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDiarmid 2010a {published data only}
Osman 2003 {published data only}
- Osman T. Stress incontinence surgery for patients presenting with mixed incontinence and a normal cystogram. BJU International 2003;92(9):964‐8. [DOI] [PubMed] [Google Scholar]
Ouslander 1987 {published data only}
- Ouslander JG, Blaustein J, Connor A, Pitt A. Habit training and oxybutynin for incontinence in functionally disabled nursing home patients with detrusor hyperreflexia: A placebo controlled trial. Proceedings of the 17th Annual Meeting of the International Continence Society (ICS). 1987:153‐4.
Ouslander 1988 {published data only}
- OuslanderJG, Blaustein J, Connor A, Pitt A. Habit training and oxybutynin for incontinence in nursing home patients. Journal of the American Geriatrics Society 1988;36:40‐6. [DOI] [PubMed] [Google Scholar]
Ouslander 1995 # {published data only}
- Ouslander JG, Schnelle JF, Uman G, Fingold S, Glatler Nigam J, Thico E, et al. Does oxybutynin add to the effectiveness of prompted voiding for urinary incontinence among nursing home residents? A placebo controlled trial. Journal of the American Geriatrics Society 1995;43(6):610‐7. [DOI] [PubMed] [Google Scholar]
Pennisi 1994 {published data only}
- Pennisi M, Grasso‐Leanza Panella P, Pepe P. [Rehabilitation therapy in the treatment of female urinary incontinence. Our experience with 121 patients] [Italian] [La teapia riabilitativa nel trattamento dell'incontinenza urinaria femminile]. Minerav Urologica e Nefrologica 1994;46(4):245‐9. [PubMed] [Google Scholar]
Peters 2010 {published data only}
- Peters KM, Carrico DJ, Perez‐Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. Journal of Urology 20/02/2010;183(4):1438‐43. [DOI] [PubMed] [Google Scholar]
Visco 2011 {published data only}
- Visco A, Meikle S. Efficacy and impact of botulinum toxin A versus anticholinergic therapy for the treatment of bothersome urge urinary incontinence. www.ClinicalTrials.gov [accessed 23 June 2011] 2011. [SR‐INCONT41587]
Weil 1995 {published data only}
- Weil EHJ, Eerdmans PHA, Janknegt RA. A new randomized study on neuromodulation versus conventional treatment for incontinence or dysfunctional voiding patterns: a preliminary study. Proceedings of the 25th Annual Meeting of the International Continence Society (ICS). 1995:382‐3.
Weil 2000 {published data only}
- Weil EHJ, Ruiz‐Cerda JL, Eerdmans PHA, Janknegt RA, Bemelmans BLH, Kerrebroeck PhEV. Sacral root neuromodulation in the treatment of refractory urinary urge incontinence: A Prospective randomized clinical trial. European Urology 2000;37:161‐71. [DOI] [PubMed] [Google Scholar]
Wise 1992 {published data only}
- Wise BG, Cardozo LD, Cutner A, Kelleher C, Burton G. Maximal electrical stimulation: An acceptable alternative to anticholinergic therapy. Proceedings of the 13th Annual Meeting of the American Urogynecolgy Society. 1992:Abstract 32.
Wiseman 1990 {published data only}
- Wiseman P, Malone‐Lee JG, Rai G. A Study of terodiline with bladder retraining in the treatment of detrusor instability in the frail elderly. Neurourology and Urodynamics 1990;9(4):410‐1. [Google Scholar]
Wiseman 1991 {published data only}
- Wiseman PA, Malone‐Lee J, Rai GS. Terodoline with bladder retraining for treating detrusor instability in elderly people. BMJ 1991;302(6783):994‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abrams 1988
- Abrams PH, Blaivas JG, Stanton SL, Andersen JT. Standardisation of terminology of lower urinary tract function. Neurourology and Urodynamics 1988;7(5):403‐27. [Google Scholar]
Abrams 2002
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Subcommittee of the International Continence Society. Neurourology and Urodynamics 2002;21:167. [DOI] [PubMed] [Google Scholar]
Andersson 2002
- Waldeck K, Larsson B, Sandberg B, Andersson KE, Larsson B, Sandberg B, et al. Actions of the new antimuscarinic compound Lu 25‐109 on isolated human and pig detrusor. Neurourology and Urodynamics 2002;21(1):92‐8. [DOI] [PubMed] [Google Scholar]
Cardozo 1978
- Cardozo L, Stanton SL, Hafner J, Alan V. Biofeedback in the treatment of detrusor instability. British Journal of Urology 1978;50(4):250‐4. [DOI] [PubMed] [Google Scholar]
Cody 2012
- Cody JD, Richardson K, Moehrer B, Hextall A, Glazener CMA. Oestrogen therapy for urinary incontinence in post‐menopausal women.. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD001405.pub2] [DOI] [PubMed] [Google Scholar]
Cody 2012a
- Cody JD, Nabi G, Dublin N, McClinton S, Neal DE, Pickard R, Yong SM. Urinary diversion and bladder reconstruction/replacement using intestinal segments for intractable incontinence or following cystectomy. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003306.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Groat 1997
- Groat WC. A neurologic basis for the overactive bladder. Urology 1997;50(6A Suppl):36‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dublin 2004
- Dublin N, Alhasso AA, Stewart L. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003190.pub2] [DOI] [Google Scholar]
Dumoulin 2010
- Dumoulin C, Hay‐Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD005654.pub2] [DOI] [PubMed] [Google Scholar]
Duthie 2011
- Duthie James B, Vincent M, Herbison GP, Wilson DI, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD005493.pub3; CD005493] [DOI] [PubMed] [Google Scholar]
Fall 1985
- Fall M. Electrical pelvic floor stimulation for control of detrusor instability. Neurourology and Urodynamics 1984;4:329‐5. [Google Scholar]
Gameiro 2012
- Gameiro LFO, Dib RP, Gameiro MO, Amaro JL. Electrical stimulation with non‐implanted electrodes for overactive bladder in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010098] [DOI] [PubMed] [Google Scholar]
Herbison 2009
- Herbison GP, Arnold Edwin P. Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004202.pub2; CD004202] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons, Ltd.
Holmes 1983
- Holmes DM, Stone AR, Bary PR, et al. Bladder training ‐ 3 years on. BJU International 1983;55:640‐4. [DOI] [PubMed] [Google Scholar]
Hu 2003
- Hu Tw, Wagner TH, Bentkover JD, Leblanc K, Piancentini A, Stewart WF. Estimated economic costs of overactive bladder in the United States. Urology 2003;61:1123‐8. [DOI] [PubMed] [Google Scholar]
Kobelt 1997
- Kobelt G. Economic considerations and outcome measurement in urge incontinence. Urology 1997;50(6A Suppl):100‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Madhuvrata 2012
- Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay‐Smith EJC. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD005429.pub2] [DOI] [PubMed] [Google Scholar]
Milsom 2001
- Milson I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are symptoms of an overactive bladder and how are they managed? A population based prevalence study. BJU International 2001;87:760. [DOI] [PubMed] [Google Scholar]
Nabi 2006
- Nabi G, Cody J, Ellis G, Herbison P, Hay‐Smith J. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003781.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouslander 1982
- Ouslander JG, Kane RL, Abrass IB. Urinary incontinence in elderly nursing home patients. JAMA 1982;248(10):1194‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ouslander 1986
- Ouslander JG, Hepps K, Raz S, Su HL. Genitourinary dysfunction in a geriatric outpatient population. Journal of the American Geriatrics Society 1986;34(7):507‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaker 1998
- Shaker HS, Hassouna M. Sacral nerve root neuromodulation: an effective treatment of refractory urge incontinence. Journal of Urology 1998;159:1516‐9. [DOI] [PubMed] [Google Scholar]
Stewart 2001
- Stewart W, Herzog AR, Wein A, Abrams P, Payne C, Corey R, et al. Prevalence and impact of overactive bladder in US: results from NOBLE program. Neurourology and Urodynamics 2001;20:406. [Google Scholar]
Thuroff 1991
- Thuroff JW, Bunke B, Ebner A, Faber P, Geeter P, Hannappel J, et al. Randomized, double‐blind, multicenter trial on treatment of frequency, urgency and incontinence related to detrusor hyperactivity: oxybutynin versus propantheline versus placebo. Journal of Urology 1991;145(4):813‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Kerrebroeck 1998
- Kerrebroeck PE, Amarenco G, Thuroff JW, Madersbacher HG, Lock MT, Messelink EJ, et al. Dose‐ranging study of tolterodine in patients with detrusor hyperreflexia. Neurourology and Urodynamics 1998;17(5):499‐512. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wallace 2004
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001308.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


