Abstract
Purpose:
To identify the optimal dosing strategy for fluorescence guided surgery in patients with head and neck squamous cell carcinoma, we conducted a dose ranging study evaluating the anti-epidermal growth factor receptor (EGFR) therapeutic antibody, panitumumab, that was fluorescently labeled with the near-infrared dye IRDye800CW.
Procedures:
Patients (n=24) received either 0.5 or 1.0mg/kg panitumumab-IRDye800CW in the weight-based dosing group or 25 or 50 mg panitumumab-IRDye800CW in the fixed dosing group. Following surgery, whole primary specimens were imaged in a closed-field device and the mean fluorescence intensity (MFI) and tumor-to-background ratio (TBR) were assessed. Clinical variables, including dose, time of infusion-to-surgery, age, unlabeled dose, gender, primary tumor site and tumor size, were analyzed to evaluate the factors affecting the fluorescence intensity in order to identify the optimal dose for intraoperative fluorescence imaging.
Results.
A total of 24 primary tumor specimens were imaged and analyzed in this study. Although no correlations between TBR and dose of panitumumab-IRDye800CW were found, there were moderate-strong correlations between the primary tumor MFI and panitumumab-IRDye800CW dose for fixed dose (mg) (R2 = 0.42) and for dose/weight (mg/kg) (R2 = 0.54). Results indicated that the optimal MFI was at approximately 50mg for fixed dose and 0.75mg/kg for dose/weight. No significant differences were found for the primary tumor MFI and TBRs between the weight-based dosing and the fixed dosing groups. MFIs significantly increased when the infusion-to-surgery window was reduced to within 2 days (vs. 3 days or more, p < 0.05).
Conclusions:
Antibody-based imaging for surgical resection is under investigation in multiple clinical trials. Our data suggests that a fixed-dose of 50mg is an appropriate diagnostic dose for successful surgical fluorescence imaging.
Keywords: Fluorescence, Near-infrared imaging, Head and neck cancer, Optimal dosing strategy, Panitumumab, Image-guided surgery, EGFR, IRDye800CW
Introduction
Worldwide head and neck cancer is the sixth most common cancer by incidence, and causes almost 200,000 deaths each year [1]. Complete surgical resection of the primary tumor plays an integral role in head and neck cancer treatment, and it is well known that incomplete resections, including positive margins, are correlated with poor prognosis [2–4]. Fluorescence-guided surgery (FGS) has emerged as a promising technique to improve surgical precision during tumor resection. We and others have reported the feasibility and potential clinical benefits of FGS using antibody-based imaging for patients with solid tumors [5–17]. For example, the epidermal growth factor receptor (EGFR) is overexpressed in 90% of tumor tissue in patients with head and neck squamous cell carcinomas (HNSCC), making it an ideal candidate for targeting [18]. Using near-infrared fluorescence dye labeled anti-EGFR antibodies, we demonstrated the feasibility of both cetuximab and panitumumab-IRDye800CW for real-time, highly sensitive and specific imaging and complete resection of HNSCC [5–7].
Despite the number of reports on FGS and the successful application using antibodies for surgical imaging [8,10,17,19,20], the optimal dose, and optimal dosing approach has not been clearly defined. There is currently no consensus on the optimal dose, with reports anywhere between 5 mg and 100 mg of antibody for imaging purposes. Hence, determining the optimal dosing strategy for a particular patient remains challenging.
The optimal dose of panitumumab-IRDye800CW needs to balance a tolerable safety profile and provide adequate fluorescence intensity for detection. At the same time, also an appropriate tumor-to-background ratio (TBR) that discriminates the primary tumor from adjacent normal tissue should be achieved. Generally, the starting dose for a first-in-human trial is a microdose for safety purposes followed by dose escalation based on results from preclinical studies [21,22]. Although body size-dosing is appropriate for therapeutic agents, use for biologics and diagnostic agents remains controversial [23]. Fixed dosing has been recommended and adapted for simplicity of dose preparation, lower cost and reduction in dosing errors [24]. In our recent panitumumab-IRDye800CW diagnostic imaging trial, we initially decided to use weight-based dosing to ensure safety of our trial patients as the efficacy was unknown at higher doses in humans [5].
Because the field remains relatively young but is expanding rapidly, we propose some important parameters for identification of the appropriate dose. In the current study we reviewed our experience with dosing of panitumumab-IRDye800CW for FGS of HNSCC and present methodologies for identifying the optimal dose by comparing patient characteristics (e.g. dose) with tumor characteristics (e.g., mean fluorescence intensity (MFI), TBR).
Materials and Methods
Study Design
We performed a single center, non-randomized, prospective dose ranging study in patients with biopsy-proven HNSCC that were scheduled for surgical resection with curative intent. The study protocol was approved by the Stanford University Institutional Review Board and the FDA (). Informed consent was obtained from all individual participants included in the study. The study was performed in accordance with the Helsinki Declaration of 1975 and its amendments, FDA’s ICH-GCP guidelines, and the laws and regulations of the United States.
Between 09/2016–03/2018, 24 patients met eligibility criteria and were enrolled in the study. All 24 patients received an infusion with panitumumab-IRDye800CW 1–5 days prior to surgery. Four dose cohorts were evaluated: 0.5 and 1.0mg/kg in the weight-based dosing group and 25 and 50mg in the fixed dosing group. Patients in the 0.5 and 1.0 mg/kg cohort also received a 100mg loading dose of unlabeled panitumumab. On the day of surgery, fluorescence imaging of the primary tumor was performed, followed by resection in a manner similar to that previously described by Gao, et al [5].
Fluorescence imaging analysis of the primary tumor
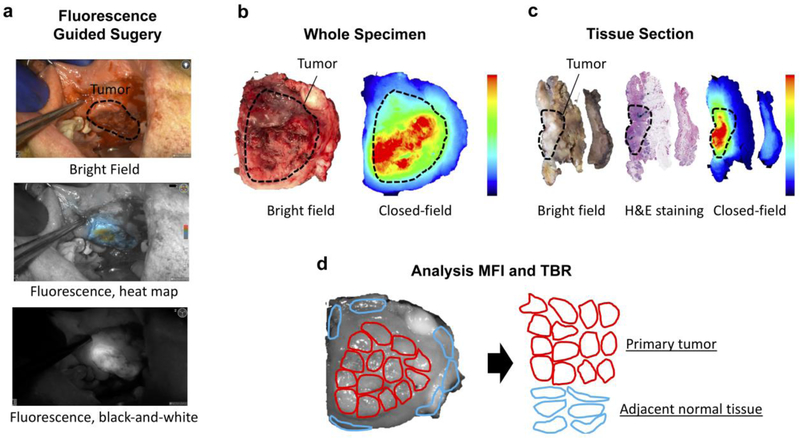
In the operation theatre, 24 primary surgical specimens were collected. Figure 1 provides an overview of the workflow followed for analysis of the fluorescence signal on the whole specimen. Upon gross inspection of the specimen by the surgeon, whereby the tumor area and the normal adjacent tissue were identified, the specimen was imaged in a closed-field fluorescence imaging device (Pearl Triology, LI-COR Biosciences Inc., Lincoln, NE, USA). Then the tissue was forwarded to pathology for formalin-fixation. Following formalin-fixation, the tumor specimen was processed by the pathologist for further histopathological evaluation. Briefly, the primary tumor was sliced into loafs at 5-mm intervals. Loafs, where needed, were subsequently cross-sectioned to fit into cassettes as per standard of care. The cassettes were then imaged with the same closed-field fluorescence imaging system in order to acquire representative images. Subsequently, cassettes were processed and tissue sections were paraffin embedded. A hematoxylin and eosin (H&E) slide was obtained from each tissue block for diagnostic evaluation. A board-certified pathologist delineated tumor and non-tumor areas on these H&E slides.
Figure 1:
a After panitumumab-IRDye800CW infusion 1–5 days prior to the day of surgery, on the day of surgery, intraoperative near-infrared fluorescence imaging is performed to visualize the tumor in situ using an open-field device. b Following primary tumor excision, the excised tissue specimen is imaged in a closed-field device. Hereafter the tissue is sent for pathology, formalin-fixed and processed per pathology standard of care whereby the primary tumor specimen is cut in loafs of approximately 5 mm thickness. Each loaf is re-imaged on the closed-field device, c after which the loafs are further cut to make them fit in cassettes as per standard of care and paraffin-embedded. Of each loaf, hematoxylin and eosin (H&E) stains are then obtained to allow for diagnosis.d To determine the mean fluorescence intensity (MFI) and tumor-to-background ratio (TBR) multiple regions of interest (ROIs) were drawn over the primary tumor and the adjacent normal tissue in the closed-field image. Subsequently, for all ROIs fluorescence intensities are determined as well as the size of the ROI. Primary tumor and adjacent normal tissue MFIs were then calculated by dividing the sum of the measured fluorescence intensities by the sum of the areas, for the primary tumor and for the adjacent normal tissue, respectively. The TBR were calculated by dividing the primary tumor MFI by the adjacent normal tissue MFI.
After matching the closed-field images with the H&E slides, regions of interest (ROIs) were drawn in the primary tumor and in the adjacent normal tissue on the fluorescence image obtained with the closed-field device using the system’s integrated software (ImageStudio, LICOR Biosciences Inc.). Multiple ROIs were drawn over the tumor and the adjacent peritumoral tissue, in order to account for the heterogeneous distribution of panitumumab-IRDye800CW in the tissue. After measuring the MFIs and area sizes for all ROIs, the primary tumor MFIs and adjacent normal tissue MFIs were defined by the following equation:
| (1) |
TBRs were subsequently calculated by dividing primary tumor MFI by the adjacent normal tissue MFI.
Data Analysis
The correlations between the primary tumor MFI/TBR and dose of panitumumab-IRDye800CW were analyzed, and a regression analysis was performed for total dose (mg) as well as dose/weight (mg/kg). The primary tumor MFIs and TBRs were compared among the patients between the weight-based dosing and fixed dosing groups. Clinical factors such as panitumumab-IRDye800CW dose (high dose: 1.0mg/kg and fixed 50mg, low dose: 0.5mg/kg and fixed 25mg), time of infusion-surgery (1–2 days,> 3 days), loading dose of unlabeled panitumumab, age (<= 60, > 60), gender, primary tumor site (tongue, other) and tumor size (<= 40mm, > 40mm) were assessed between two groups. Subsequently, within the weight-based dosing and fixed dosing group, MFIs and TBRs were compared in a similar way. A MannWhitney U test was used for comparison of the primary tumor MFIs and TBRs. A p-value of 0.05 or less was considered statistically significant.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. Patients received on average 47 mg of drug (range 25–95 mg) or a body weight adjusted average dose of 0.66 mg/kg (range 0.26–1.05 mg/kg). Of the total of 24 patients, five patients received 0.5 mg/kg, seven patients 1.0mg/kg, six patients a fixed 25mg dose and six patients a fixed 50 mg dose of panitumumab-IRDye800CW. The average dose patients received in 0.5 mg/kg cohort was 39 mg (range 28–47 mg), and in the 1.0 mg/kg cohort this was 69 mg (range 53–95 mg), as compared to the 25 and 50 mg in the fixed dosing cohort, respectively.
Table 1:
Patient Characteristics
| Weigh-based dosing group | Fixed dosing group | |||
|---|---|---|---|---|
| Characteristics | Low dose (0.5mg/kg) n=5 |
High dose (1.0mg/kg) n=7 |
Low dose (Fixed 25mg) n=6 |
High dose (Fixed 50mg) n=6 |
| Total Dose, average | 39mg | 69mg | 25mg | 50mg |
| Unlabeled dose | ||||
| 100mg | 5 | 7 | 0 | 0 |
| 0mg | 0 | 0 | 6 | 6 |
| Time of infusion-surgery | ||||
| <= 2 days | 4 | 3 | 4 | 6 |
| > 3 days | 1 | 4 | 2 | 0 |
| Age | ||||
| </= 60 years | 2 | 4 | 3 | 2 |
| > 60 years | 3 | 3 | 3 | 4 |
| Gender | ||||
| Male | 5 | 6 | 4 | 2 |
| Female | 0 | 1 | 2 | 4 |
| Primary site | ||||
| Tongue | 1 | 2 | 2 | 3 |
| Other | 4 | 5 | 4 | 3 |
| Tumor size | ||||
| </=40 mm | 4 | 4 | 4 | 4 |
| >40 mm | 1 | 3 | 2 | 2 |
The average body weight was 74kg (range 47–96), the average age at surgery was 62 years (range 32–85), and the majority of HNSCC patients presented with oral cavity SCC (88%). Tumor size ranged 6–55 mm (average 34mm) in maximum dimension. The average time of infusion to the start of surgery was 52 h (range 17–120). There were no infusion reactions to panitumumab or panitumumab-IRDye800CW.
Optimal Dosing Strategy of Panitumumab-IRDye800CW
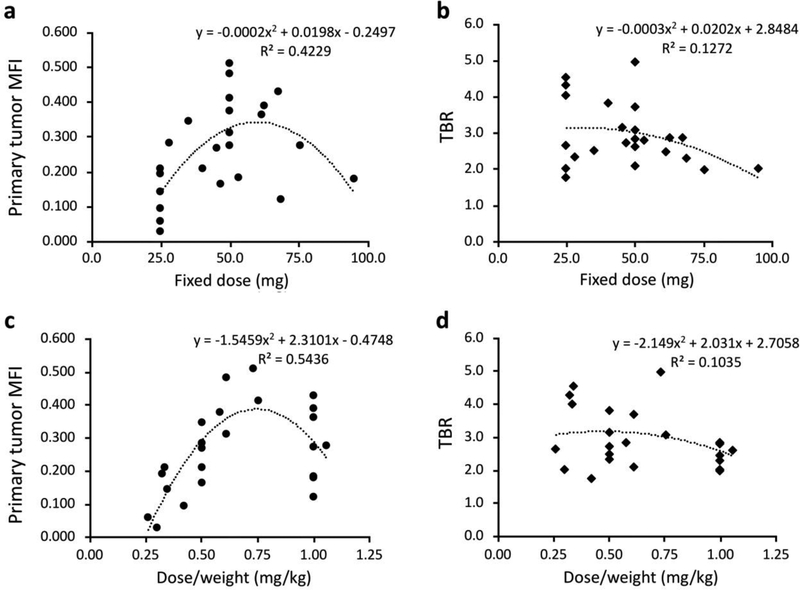
We evaluated the MFI measured on the primary tumor compared to that of histologically confirmed adjacent normal tissue and calculated the TBR. A total of 24 primary tumor specimens was evaluated. Primary tumors had a higher MFI than adjacent normal tissues (average 0.26 vs. 0.09, p < 0.01) and the average TBR was 3.0 (range 1.8–5.0). Quadratic polynomial regressions were performed between the primary tumor MFI/TBR and dose of panitumumab-IRDye800CW.The results of these regressions are shown in Fig. 2. There were moderate-strong correlations between the primary tumor MFI and dose of panitumumab-IRDye800CW for fixed dose (R2 = 0.42, p < 0.05) and for dose/weight (R2 = 0.54, p < 0.05). Results indicated that the optimal MFI was at approximately 50mg for fixed dose and 0.75 mg/kg for dose/weight. We qualified the optimal MFI as presence of a strong fluorescence signal allowing for fluorescence imaging and demarcation of the tumor during FGS. Considering the fact that 0.75 mg/kg for dose/weight resulted in 56 mg for fixed dose with the average body weight (74 kg), the fixed 50mg dose infusion seemed to lead to the appropriate dose for good primary tumor MFI. However, we failed to identify a correlation between TBR and dose of panitumumab-IRDye800CW for total dose (R2 = 0.12) and for dose/weight (R2 = 0.10). Between the weight-based dosing (n = 12) and fixed dosing groups (n = 12), no significant difference in primary tumor MFI was found. Similarly, no significant difference in TBR was found between two dosing groups.
Figure 2:
a Correlation between primary tumor mean fluorescence intensity (MFI) and the fixed dose panitumumab-IRDye800CW administered to the patient. b Correlation between the tumor-to-background ratio (TBR) and the fixed dose panitumumab-IRDye800CW administered to the patient. c Correlation between primary tumor MFI and the average dose panitumumab-IRDye800CW in mg/kg administered to the patient. d Correlation between the TBR and the average dose panitumumab-IRDye800CW in mg/kg administered to the patient.
Comparisons of Clinical Variables
Primary tumor MFIs significantly increased for the high dose groups compared to the low dose groups (0.33 vs. 0.18, p < 0.01), and when the infusion-to-surgery window was 1–2 days compared to >3 days (0.30 vs. 0.18, p < 0.05). Table 2 shows no significant differences between unlabeled dose, age, gender, tumor size, and tumor site for primary tumor MFIs or TBRs for the four cohorts evaluated (n = 24). TBRs were significantly higher in patients with primary tongue tumors compared to the other regions (3.6 vs. 2.6, p < 0.05). Also, no significant differences in TBRs were found when comparing dose, time of infusion-surgery, age, gender and tumor size.
Table 2:
Comparisons of clinical variables for primary tumor MFIs and TBRs
| Variable | MFI | TBR | |||
|---|---|---|---|---|---|
| Mean | p value | Mean | p value | ||
| Dose | High Low |
0.33 0.18 |
** | 2.8 3.1 |
N.S. |
| Time of infusion-surgery | <= 2 days > 3 days |
0.30 0.18 |
* | 3.1 2.5 |
N.S. |
| Unlabeled dose | 100 mg 0mg |
0.27 0.26 |
N.S. | 2.7 3.2 |
N.S. |
| Age | </= 60 years > 60 years |
0.25 0.27 |
N.S. | 3.0 2.9 |
N.S. |
| Gender | Male Female |
0.24 0.30 |
N.S. | 3.0 2.9 |
N.S. |
| Primary site | Tongue Other |
0.32 0.23 |
N.S. | 3.6 2.6 |
* |
| Tumor size | </= 40 mm >40 mm |
0.27 0.25 |
N.S. | 3.0 2.8 |
N.S. |
p<0.05
p<0.01
MFI=mean fluorescence intensity
TBR=tumor-to-background ratio
N.S.=not significant
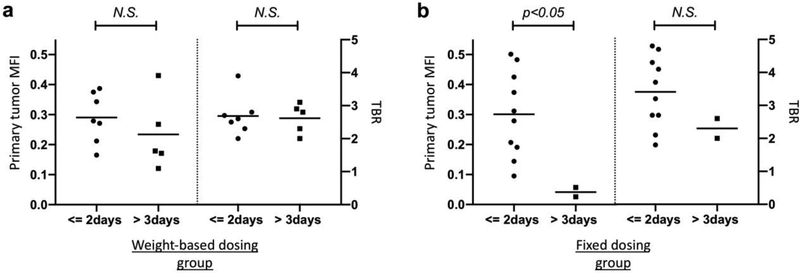
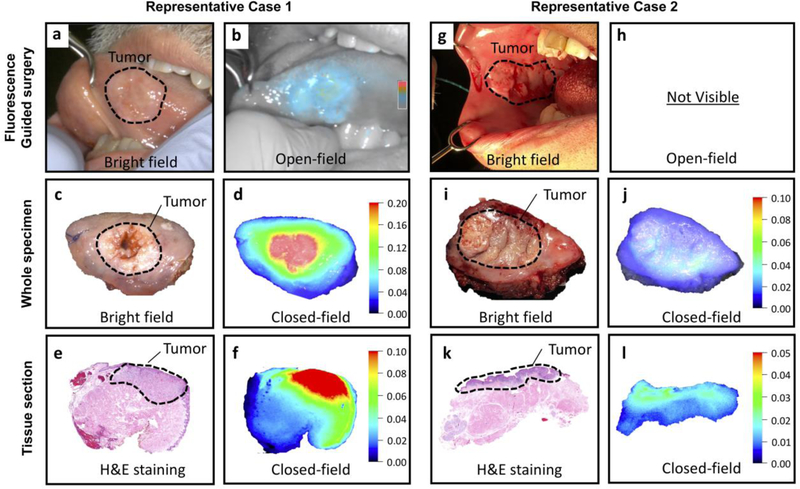
Within the weight-based dosing group, no significant differences of primary tumor MFIs and TBRs were identified when looking at variables such as time of infusion-surgery, age, primary tumor site and tumor size. In contrast to the weight-based dosing group, in the fixed dosing group significantly higher MFIs of the primary tumor were found when the infusion-to-surgery window was 1–2 days compared to >3 days in Fig. 3 (p<0.05). Figure 4 shows two representative cases in the fixed dosing group. Case 1 with SCC of the tongue received a fixed 25 mg dose of panitumumab-IRDye800CW with the time window of 1 day. At surgery, intraoperative open-field imaging indicated a good fluorescence signal for the primary tumor and could aid the decision of the margin assessment. For the tumor in which we found a good fluorescence signal, the primary tumor MFI was found 0.21 with a TBR of 4.1 (Fig 4a–f). Case 2 with buccal SCC received a fixed 25mg dose of panitumumab-IRDye800CW with the time window of 4 days. This in contrast to the other patient where the primary tumor MFI was found 0.03 with a TBR of 2.0. In this case, we could not get the good contrast of the primary tumor to the surrounding normal tissue (Fig. 4g–l).
Figure 3:
a Within the weight-based dosing group, no significant differences of primary tumor MFIs and TBRs were found when looking at time of infusion-surgery (1–2 days vs. >3 days). b In contrast to the weight-based dosing group, in the fixed dosing group significantly higher MFIs of the primary tumor were found when the infusion-to-surgery window was 1–2 days compared to>3 days (p<0.05)
Figure 4:
Left panel: a,b A patient with SCC of the tongue received 50 mg panitumumab-IRDye800CW 1 day before surgery. Intraoperative fluorescence imaging revealed clear tumor demarcation.Right panel: g,h A patient with buccal SCC was administered with 25 mg panitumumab-IRDye800CW 4 days before surgery. Via intraoperative fluorescence imaging it was hard to visualize the tumor due to the high background signal present. c-f, i-l Ex vivo closed-field imaging of the primary tumor specimen, and subsequent loafs, revealed that the fluorescence signal intensities of the primary tumor were up to several-folds lower in the patient that was administered with 25 mg panitumumab-IRDye800CW compared to the patient administered with 50 mg panitumumab-IRDye800CW.
Discussion
FGS is a new technique that has the potential to revolutionize oncology surgical precision. A clear understanding of the optimal dosing strategy is needed to implement FGS broadly. The current study reviewed the dosing strategy for FGS using panitumumab-IRDye800CW in 24 patients with HNSCC and showed that a fixed dose of 50 mg was the optimal dose. Higher doses did not significantly increase the primary tumor MFI, nor did it bring up the TBR. It remains unclear if the fluorescence intensity of the primary tumor scales with increasing dose of panitumumab-IRDye800CW.
The general lack of available clinical data makes it hard to determine whether a fixed dosing approach or body size-based dosing approach of a monoclonal antibody should be used [25]. However, a recent study suggested that, based on pharmacokinetic parameters of monoclonal antibodies, there is a rationale for fixed dosing of these drugs in oncology [23]. Specifically, for panitumumab, several authors evaluated the pharmacokinetics (PK) and pharmacodynamics (PD) and recommended the fixed dosing strategy for therapeutic purposes [23,24]. Results from our current study using panitumumab-IRDye800CW showed that there were no significant differences in primary tumor MFIs and TBRs between the weight-based dosing and the fixed dosing groups, even though the average given dose was almost twice as high in the weight-based dosing group. Moreover, the fixed dosing has several advantages; namely, it allows for reduction in patient wait times and improves the efficiency of drug compounding. Additional benefits can also be achieved, such as reduced potential for medication errors, reduced drug wastage, and prospective quality control of infusions [23,26]. Considering the PK/PD, safety, ease of preparation, and cost-effectiveness, in combination with our results, we propose that the dosing approach should be fixed and not weight dependent for our FGS studies. Our findings are in line with other studies that have shown that FGS trials that use small molecules and/or antibody fragments often take a body size-based dosing approach [27], whereas trials using antibody-dye complexes nowadays mainly focus on using fixed dosing [28]. For example, three clinical dose-finding studies on bevacizumab-IRDye800CW are underway, with fixed doses ranging from 4.5 to 50 mg in patients with familial adenomatous polyposis (), breast cancer () and pancreatic cancer () [17,29]. Similarly, fluorescent tracer SGM-101 targeting carcinoembryonic antigen (CEA) has been applied for detection of pancreatic cancer and colorectal cancer with fixed 5, 7.5 and 10 mg doses [16,20]. Although both groups have reported the safety and feasibility for imaging with fluorescent tracers, the optimal dosing strategy has yet to be established.
Although the TBRs in our current study were equivalent in fixed 25 mg and 50 mg cohorts, an increased antibody-dye dose was associated with and increased MFI and did not vary with patient characteristics. This finding is consistent with a previous report where panitumumab concentrations declined more rapidly when giving a low dose than after giving high doses in human studies [30]. Although the TBRs were equivalent, the low fluorescence signal (MFI) led to challenges in getting optimal signal with open-field devices intraoperatively at the 25mg fixed dose, which was remedied at the 50 mg fixed dose. Therefore, reporting should include both MFI and TBR to ensure that the signal will be appropriate for small amounts of disease. Ultimately, it appears that a fixed dose of 50mg panitumumab-IRDye800CW would be the preferred dose for successful intraoperative imaging of HNSCC within the ranges of doses we studied here. Yet, with the ongoing developments in the field, and the striving to using low doses eventually more sensitive cameras will be needed for the future as such to pick up weak fluorescence signals (low MFI).
Previously we had seen that infusion reactions frequently occurred toward cetuximab another anti-EGFR antibody [9]. Additionally in that trial, it was observed that the fluorescence signal in the tumor seemed enhanced upon the administration of a cetuximab loading dose prior to cetuximab-IRDye800CW [31]. Hence, when starting the phase I panitumumab-IRDye800 study, we included a loading dose of panitumumab as well. However, while progressing through the phase I panitumumab-IRDye800CW study, we did not see any infusion reactions, and therefor to study the effect of the loading dose on the MFI, we included additional no loading dose cohorts. Preloading of unlabeled antibody did not appear to add additional imaging value for FGS with panitumumab-IRDye800CW as we did not see any differences in primary tumor MFIs or TBRs, in contrast to earlier cetuximab-based studies [9]. In the fixed dosing group, however, we did find a significantly lower primary tumor MFI when surgery was performed >3 days post-infusion vs. 1–2 days post-infusion. This effect might be due to the lack of unlabeled dose as this effect was not noticeable in the group that received a loading dose of panitumumab. This suggests that preloading of unlabeled antibody may play an important role to maintain the prolonged imaging effect of the labeled antibodies, especially >3 days after infusion. Interestingly, for many other antibody-dye complexes that are currently undergoing clinical evaluation, including VEGF and SM101 targeted antibodies, the role of a ing dose has not been explored [13,14,16,20,28].
Conclusion
Antibody-based imaging for surgical resection is under investigation in multiple clinical trials. Our data suggests that a fixed dose of 50 mg of panitumumab-IRDye800CW is an appropriate diagnostic dose for successful surgical fluorescence imaging. Additional studies will be essential to delineate the clinical utility of this fluorescently tagged anti EGFR-antibody.
Acknowldegements
This work was supported in part by the Stanford Comprehensive Cancer Center, the Stanford University School of Medicine Medical Scholars Program, the Netherlands Organization for Scientific Research (Rubicon; 019.171LW.022), the National Institutes of Health and the National Cancer Institute (R01CA190306), and the Stanford Molecular Imaging Scholars (SMIS) program (NIT T32CA118681). Institutional equipment loans were received from Novadaq, SurgVision, and LI-COR Biosciences.
Footnotes
Conflicts of Interest
Eben Rosenthal is a consultant for LICOR Biosciences that manufactures IRDye800 and has equipment loans from this company.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Jemal A, Bray F, Center MM, et al. (2011) Global Cancer Statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- 2.Eldeeb H, Macmillan C, Elwell C, Hammod A (2012) The Effect of the Surgical Margins on the Outcome of Patients with Head and Neck Squamous Cell Carcinoma: Single Institution Experience. Cancer Biol Med 9:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettl T, El-Gindi A, Hautmann M, et al. (2016) Positive Frozen Section Margins Predict Local Recurrence in R0-Resected Squamous Cell Carcinoma of the Head and Neck. Oral Oncol 55:17–23 [DOI] [PubMed] [Google Scholar]
- 4.McMahon J, O’Brien C, Pathak I, et al. (2003) Influence of Condition of Surgical Margins on Local Recurrence and Disease-Specific Survival in Oral and Oropharyngeal Cancer. Br J Oral Maxillofac Surg 41:224–31 [DOI] [PubMed] [Google Scholar]
- 5.Gao RW, Teraphongphom NT, van den Berg NS, et al. (2018) Determination of Tumor Margins with Surgical Specimen Mapping Using Near-Infrared Fluorescence. Cancer Res 78:5144–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao RW, Teraphongphom N, de Boer E, et al. (2018) Safety of Panitumumab-IRDye800CW and Cetuximab-IRDye800CW for Fluorescence-Guided Surgical Navigation in Head and Neck Cancers. Theranostic 8:2488–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Keulen S, van den Berg NS, Nishio N, et al. (2019) Rapid, Non-Invasive Fluorescence Margin Assessment: Optical Specimen Mapping in Oral Squamous Cell Carcinoma. Oral Oncol 88:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore LS, Rosenthal EL, Chung TK, et al. (2017) Characterizing the Utility and Limitations of Repurposing an Open-Field Optical Imaging Device for Fluorescence-Guided Surgery in Head and Neck Cancer Patients. J Nucl Med 58:246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal EL, Warram JM, de Boer E, et al. (2015) Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res 21:3658–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tummers WS, Miller SE, Teraphongphom NT, et al. (2018) Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Ann Surg Oncol 25:1880–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller SE, Tummers WS, Teraphongphom N, et al. (2018) First-in-Human Intraoperative Near-Infrared Fluorescence Imaging of Glioblastoma Using Cetuximab-IRDye800. J Neurooncol 139:135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogstins CE, Tummers QR, Gaarenstroom KN, et al. (2016) A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin Cancer Res 22:2929–38 [DOI] [PubMed] [Google Scholar]
- 13.Lamberts LE, Koch M, de Jong JS, et al. (2017) Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin Cancer Res 23:2730–41 [DOI] [PubMed] [Google Scholar]
- 14.Nagengast WB, Hartmans E, Garcia-Allende PB, et al. (2017) Near-Infrared Fluorescence Molecular Endoscopy Detects Dysplastic Oesophageal Lesions Using Topical and Systemic Tracer of Vascular Endothelial Growth Factor A. Gut DOI: 10.1136/gutjnl-2017-314953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Oosten M, Crane LM, Bart J, van Leeuwen FW, van Dam GM (2011) Selecting Potential Targetable Biomarkers for Imaging Purposes in Colorectal Cancer Using TArget Selection Criteria (TASC): A Novel Target Identification Tool. Transl Oncol 4:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogstins CES, Boogerd LSF, Sibinga Mulder BG, et al. (2018) Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann Surg Oncol 25:3350–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller M, Qiu SQ, Linssen MD, et al. (2018) Implementation and Benchmarking of a Novel Analytical Framework to Clinically Evaluate Tumor-Specific Fluorescent Tracers. Nat Commun 9:3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandis JR, Tweardy DJ (1993) Elevated Levels of Transforming Growth Factor a and Epidermal Growth Factor Receptor Messenger RNA Are Early Markers of Carcinogenesis in Head and Neck Cancer. Cancer Res 53:3579–84 [PubMed] [Google Scholar]
- 19.Hekman MC, Boerman OC, de Weijert M, et al. (2016) Targeted Dual-Modality Imaging in Renal Cell Carcinoma: An Ex Vivo Kidney Perfusion Study. Clin Cancer Res 22:4634–42 [DOI] [PubMed] [Google Scholar]
- 20.Boogerd LSF, Hoogstins CES, Schaap DP, et al. (2018) Safety and Effectiveness of SGM-101, a Fluorescent Antibody Targeting Carcinoembryonic Antigen, for Intraoperative Detection of Colorectal Cancer: a Dose-Escalation Pilot Study. Lancet Gastroenterol Hepatol 3:181–91 [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal EL, Warram JM, de Boer E, et al. (2016) Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J Nucl Med 57:144–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tummers WS, Warram JM, Tipirneni KE, et al. (2017) Regulatory Aspects of Optical Methods and Exogenous Targets for Cancer Detection. Cancer Res 77:2197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrikx JJMA,Haanen JBAG, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH (2017) Fixed dosing of Monoclonal Antibodies in Oncology. Oncologist 22:1212–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DD, Zhang S, Zhao H, Men AY, Parivar K (2009) Fixed dosing Versus Body Size-Based Dosing of Monoclonal Antibodies in Adult Clinical Trials. J Clin Pharmacol 49:1012–24 [DOI] [PubMed] [Google Scholar]
- 25.Mathijssen RH, de Jong FA, Loos WJ, van der Bol JM, Verweij J, Sparreboom A (2007) Flat-Fixed dosing Versus Body Surface Area Based Dosing of Anticancer Drugs in Adults: Does It Make a Difference? Oncologist 12:913–23 [DOI] [PubMed] [Google Scholar]
- 26.Pouliquen AL, Escalup L, Jourdan N, Cottu P, Faure P, Madelaine-Chambrin I (2011) Dose Standardisation of Anticancer Drugs. Int J Clin Pharm 33:221–8 [DOI] [PubMed] [Google Scholar]
- 27.Boogerd LSF, Hoogstins CES, Gaarenstroom KN, et al. (2017) Folate Receptor-α Targeted Near-Infrared Fluorescence Imaging in High-Risk Endometrial Cancer Patients: a Tissue Microarray and Clinical Feasibility Study. Oncotarget 9:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlaar NJ, Koller M, de Jongh SJ, et al. (2016) Molecular Fluorescence-Guided Surgery of Peritoneal Carcinomatosis of Colorectal Origin: A Single-Centre Feasibility Study. Lancet Gastroenterol Hepatol 1:283–90 [DOI] [PubMed] [Google Scholar]
- 29.Hartmans E, Tjalma JJJ, Linssen MD, et al. (2018) Potential Red-Flag Identification of Colorectal Adenomas with Wide-Field Fluorescence Molecular Endoscopy. Theranostics 8: 1458–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang BB, Lum P, Chen A, et al. (2010) Pharmacokinetic and Pharmacodynamic Perspectives on the Clinical Drug Development of Panitumumab: Clin Pharmacokinet 49:729–40 [DOI] [PubMed] [Google Scholar]
- 31.Moore LS, Rosenthal EL, de Boer E, et al. (2017) Effects of an Unlabeled Loading Dose on Tumor-Specific Uptake of a Fluorescently Labeled Antibody for Optical Surgical Navigation. Mol Imaging Biol 19:610–6 [DOI] [PMC free article] [PubMed] [Google Scholar]