Abstract
Background
Atrial fibrillation (AF) is initiated through arrhythmic atrial excitation from outside the sinus node or remodeling of atrial tissue that allows reentry of excitation. Angiotensin II (AngII) has been implicated in initiation and maintenance of AF through changes in Ca2+ handling and production of reactive oxygen species (ROS).
Objective
We aimed to determine the role of Pak1, a downstream target in the AngII signaling cascade, in atrial electrophysiology and arrhythmia.
Method
WT and Pak1−/− mice were used to determine atrial function in vivo, on the organ and cellular level by quantification of electrophysiological and Ca2+-handling properties.
Results
We demonstrate that reduced Pak1 activity increases the inducibility of atrial arrhythmia in vivo and in vitro. On the cellular level, Pak1−/− atrial myocytes (AMs) exhibit increased basal and AngII (1 µM)-induced ROS production, sensitivity to the NOX2 inhibitors gp91ds-tat and apocynin (1 µM), and enhanced membrane translocation of Rac1 that is part of the multi-molecular NOX2 complex. Upon stimulation with AngII, Pak1−/− AMs exhibit an exaggerated increase in [Ca2+]i, and arrhythmic events that were sensitive to NCX inhibitors (KB-R7943, SEA0400; 1 µM) and suppressed in AMs from NOX2 deficient (gp91phox−/−) mice. Pak1 stimulation (FTY720: 200 nM) in WT AMs and AMs from a canine model of ventricular tachypacing-induced AF, prevented AngII-induced arrhythmic Ca2+ overload, by attenuating NCX activity in a NOX2 dependent manner.
Conclusion
The experiments support that Pak1 stimulation can attenuate NCX-dependent Ca2+ overload and prevent triggered arrhythmic activity by suppressing NOX2-dependent ROS production.
Keywords: p21-activated kinase, atrial fibrillation, NADPH-oxidase 2, excitation- (ECC) coupling, reactive oxygen species
Introduction
The pre-disposition for atrial fibrillation (AF) increases with age and in the presence of cardiovascular morbidities such as hypertension and hypertrophy. This makes AF the most common sustained arrhythmia with high morbidity and mortality based on thromboembolic events and stroke1. AF occurs when non-pacemaker cells focally induce rapid, triggered, propagating electrical impulses, or when ectopic beats trigger re-entry2. Repeated AF episodes promote electrical and structural remodeling of the atrial tissue creating a ‘substrate’ with increased propensity for recurrence and prolonged AF episodes1.
Angiotensin II (AngII) has been identified as one prominent signaling component in the induction of AF and tissue remodeling. Increased plasma concentrations and expression levels of AngII in atrial tissue were detected in animal models with increased propensity for AF and patients with persistent AF3,4. An increase of AngII concentrations through cardiac specific overexpression of angiotensin converting enzyme5 or increased activation of the AngII signal transduction cascade e.g. by Rac1 overexpression, lead to enlarged atria with increased propensity for AF2,6.
ROS production is a prominent effector of the AngII-induced signaling cascade. Models of AngII and pacing-induced heart failure depend on AngII receptor type 1 (AT1R) mediated ROS production7 and in models of AF, ROS scavengers reduce arrhythmic trigger formation8. In the heart NOX2 is implicated in AngII-induced apoptosis and hypertrophy9. In atrial tissue a connection between AngII and NOX2 dependent ROS production has been proposed10 and NOX2 expression was shown to be increased in humans with persistent and post-operative AF11.
Downstream target in the AngII signaling cascade is p21-activated kinase (Pak1). Pak1 is a serine/threonine kinase that is expressed in the cardiac muscle where its deficiency exaggerates the cardiac hypertrophic response and tissue damage after ischemia-reperfusion injury12,13. On the other hand Pak1 stimulation was shown to antagonize AngII-induced hypertrophic remodeling14 and in ventricular myocytes (VMs) we demonstrated that Pak1 is critical for maintenance of the T-tubular structure and the integrity of the Ca2+ release unit of L-type Ca2+ channel (LTCC) and ryanodine receptor (RYR)15. Under basal conditions no increase in arrhythmic activity was identified in Pak1−/− VMs, however, the cells were highly sensitive to pacing, ischemia-reperfusion, and glycoside- induced Ca2+ overload15,16. In atrial tissue the role of Pak1 has not yet been examined but given its pivotal position in the AngII/Rac1 signaling cascade, we aimed to test the hypothesis that modulation of Pak1 activity could critically influence the occurrence of arrhythmic events in atrial tissue.
Method
Experimental details on animal models used, cell isolation, cell fractionation, and measurements of [Ca2+]i and ROS are presented in the Supplements. Animal procedures were performed with the approval of the IACUC of Rush University and Northwestern University and in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals.
Results
A Role for Pak1 Regulation in Atrial Excitability
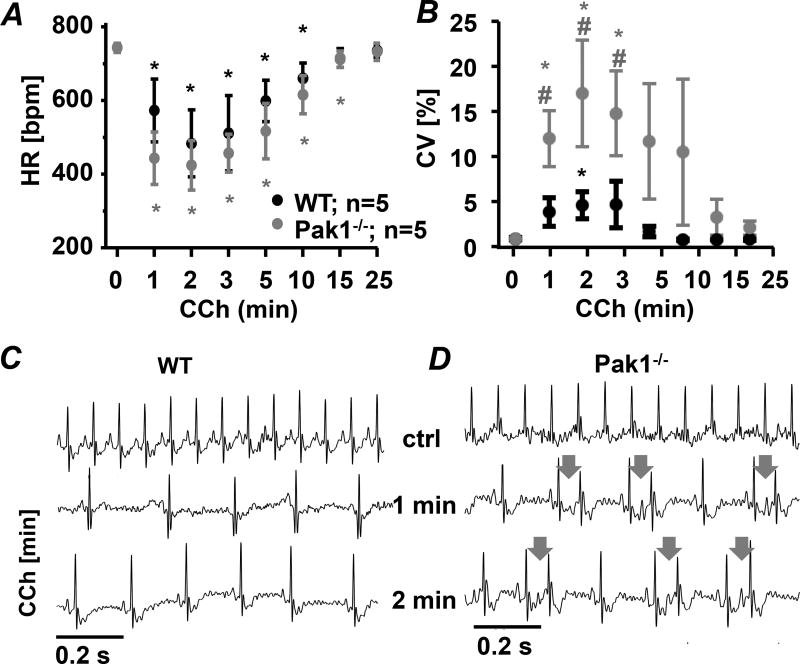
In VMs we demonstrated that decreased Pak1 expression increases the propensity for arrhythmic events16. To determine the consequences of reduced Pak1 activity on atrial electrophysiology we obtained ECG recordings from conscious WT and Pak1−/− mice. Under control conditions ECGs did not reveal a difference in heart rate, atrial and ventricular excitation or repolarization. Intraperitoneal injection of carbachol (CCh 150 ng/g) which facilitates the inducibility of atrial arrhythmia17, resulted in a comparable, transient decrease of heart rate (HR) in WT and Pak1−/− mice (Fig. 1A). After CCh injection however, the coefficient of variation of the R-R interval (CV: Fig. 1B)18 increased significantly in Pak1−/− mice compared to baseline, to WT-Ctrl, and to WT-CCh treated mice (Fig. 1B). Analysis of ECG traces showed the occurrence of frequent, spontaneous premature atrial contractions (PACs) in Pak1−/− mice (Fig. 1CD). Such frequent PACs were not observed in WT mice.
Figure 1. Reduced Pak1 activity enhances the propensity for atrial arrhythmic events.
(A) Heart rate (HR, beats per minute: bpm) and (B) Coefficient of variation (CV) recorded in conscious WT and Pak1−/− mice after intraperitoneal injection of carbachol (CCh: 150 ng/g). Representative ECG recording in WT (C) and Pak1−/− (D) mice show PACs in Pak1−/− hearts (arrows indicate P-wave). (*p < 0.05 compared to t = 0 min; #p < 0.05 compared to WT)
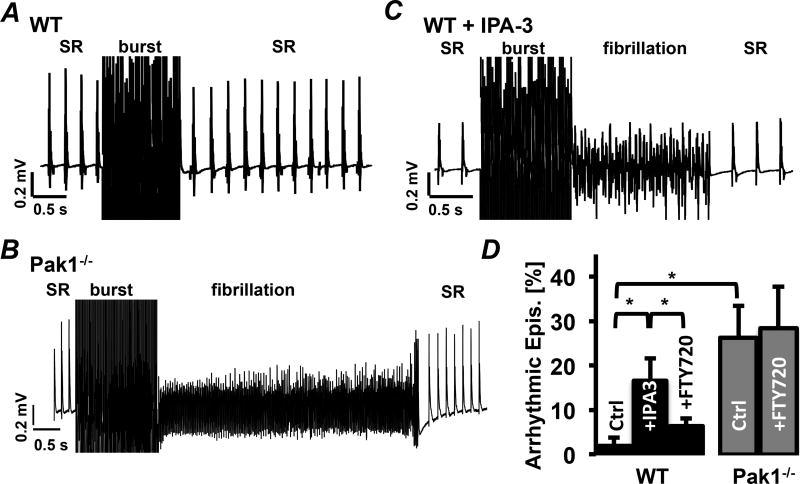
Increased arrhythmicity was also observed in isolated Langendorff perfused Pak1−/− hearts during recordings of left-atrial electrograms (Fig. 2AB). Atrial burst pacing during sinus rhythm (SR) induced arrhythmic activity in 6 out of 6 Pak1−/− hearts, compared to 1 out of 5 WT hearts (χ2 = 5.76; p = 0.01). Arrhythmic episodes in Pak1−/− occurred after 26.7 ± 7.2 % of the burst pacing episodes compared to only 1.9 ± 1.9 % in WT mice (Fig. 2D). In all other cases WT and Pak1−/− hearts went back to SR after burst pacing.
Figure 2. Acute Pak1 inhibition increases atrial arrhythmic events.
Examples of left atrial electrograms showing spontaneous activity (SR: sinus rhythm) and burst pacing (burst) induced arrhythmia in WT (A), Pak1−/− (B), and WT hearts perfused with IPA-3 (10 µmol/L) (C). (D) Percent of burst pacing episodes that induced arrhythmic events in Pak1−/− and WT hearts during Ctrl conditions, treatment with IPA-3, or FTY720 (1 µmol/L). (*p < 0.05).
To determine if arrhythmicity of Pak1−/− hearts can be mimicked by acute Pak1 inhibition, WT hearts were perfused with allosteric Pak1 inhibitor IPA-3 (10 µmol/L)19. After IPA-3 arrhythmia were determined in 69 % of WT+IPA-3 treated hearts (χ2 = 6.24; p = 0.01; Fig. 2CD). This increase could be attenuated with FTY720 (1 µmol/L) a sphingosine-1 phosphate receptor (S1PR) agonist known to stimulate Pak120. In the presence of IPA-3+FTY720 WT atria showed arrhythmia after only 6.36 ± 3.4 % of the burst pacing events (n = 3). In Pak1−/− hearts FTY720 failed to attenuate arrhythmic activity (Pak1−/−FTY: 28.2 ± 14 %; n = 3; Fig. 2D) supporting that Pak1 expression is required for its anti-arrhythmic benefit.
Arrhythmic episodes in Pak1−/− atria were short in duration, with 80 % shorter than 1 s. The experimental results support that in vivo decreased Pak1 activity increases the propensity for arrhythmic atrial trigger events.
Contribution of ROS to Arrhythmic Activity
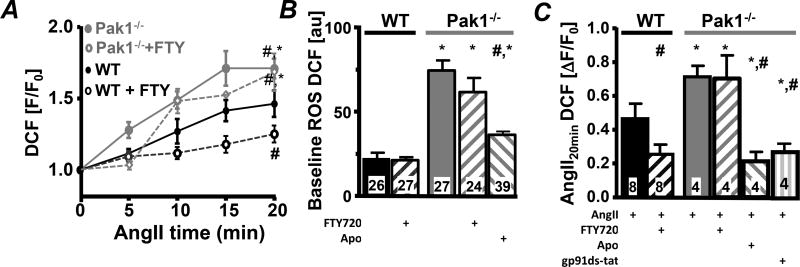
We have previously shown in VMs that decreased Pak1 activity enhances ROS production16. To determine the impact of attenuated Pak1 activity on atrial ROS production, DCF fluorescence was monitored in WT and Pak1−/− AMs at basal conditions and during AngII superfusion (1 µmol/L)16. Under basal conditions DCF fluorescence was elevated in Pak1−/− compared to WT AMs (Fig. 3B) and exaggerated ROS production was determined during AngII superfusion (Fig. 3AC). Basal ROS levels in Pak1−/− AM and AngII-induced ROS production were attenuated by the unspecific NADPH oxidase blocker apocynin (Fig. 3BC)16 and the NOX2 specific inhibitor gp91ds-tat in Pak1−/− AMs (Fig. 3C; Supplemental Fig. 1AB (SFig. 1AB)) as previously shown in WT myocytes16. Pre-treatment of WT and Pak1−/− AMs with Pak1 stimulator FTY720 (200 nmol/L) did not affect basal ROS levels in WT AMs but suppressed the rate of AngII-induced ROS production (Fig. 3ABC). In Pak1−/− AMs FTY720 remained without effect indicating the necessity of Pak1 protein for attenuation of ROS production.
Fig. 3. Attenuated Pak1 activity increases ROS.
(A) Change in fluorescence recorded in WT (black) and Pak1−/− (grey) AMs loaded with DCF during stimulation with AngII in the absence or presence of FTY720 (open symbols). Bar graphs show DCF fluorescence at baseline (B) and (C) 20 min after AngII, AngII+FTY720, AngII+Apo (Apo: 1 µmol/L) or AngII+gp91ds-tat (1 µmol/L) superfusion in WT and Pak1−/− cells. (*: p < 0.05 compared to WT; #:p < 0.05 compared to Ctrl).
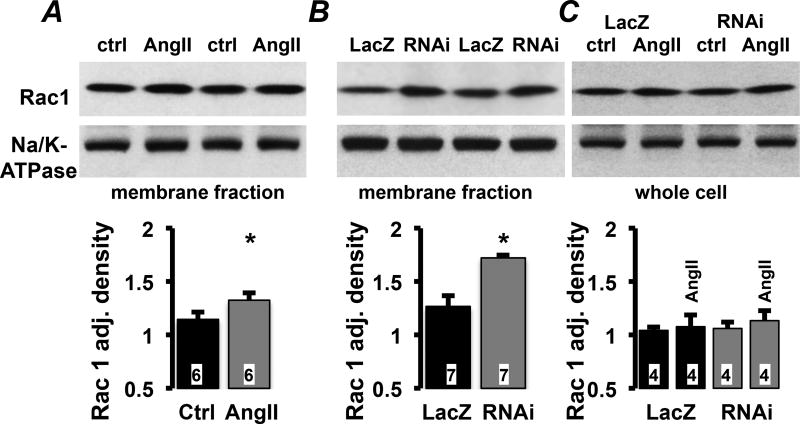
A prerequisite for NOX2 activation is the association of Rac1 with p67phox at the plasma membrane30. AngII stimulation (30 min) or Pak1-RNAi treatment (24 h) of HL-1 cells, resulted in significantly increased levels of Rac1 in the plasma membrane fraction compared to control or LacZ treated HL-1 cells (Fig. 4AB). The total amount of Rac1 protein in whole cell lysate of HL-1 cells treated with LacZ or Pak1-RNAi in the presence and absence of AngII (Fig. 4C), remained constant. The results support that Pak1 is a negative regulator of Rac1 membrane translocation and ROS production through NOX2.
Fig. 4. Attenuated Pak1 promotes Rac1 plasma membrane translocation.
Western blotting results from membrane fractions or whole cell lysate of HL-1 treated with AngII (A), Pak1-RNAi (B), and (C) LacZ or Pak1-RNAi after stimulation with AngII. Densitometric quantification (bottom) presented as relative density adjusted to Na+/K+-ATPase (NKA) loading control. (*: p < 0.05 compared to Ctrl or LacZ, respectively).
Basal Ca2+ Handling Properties of Pak1−/− AMs
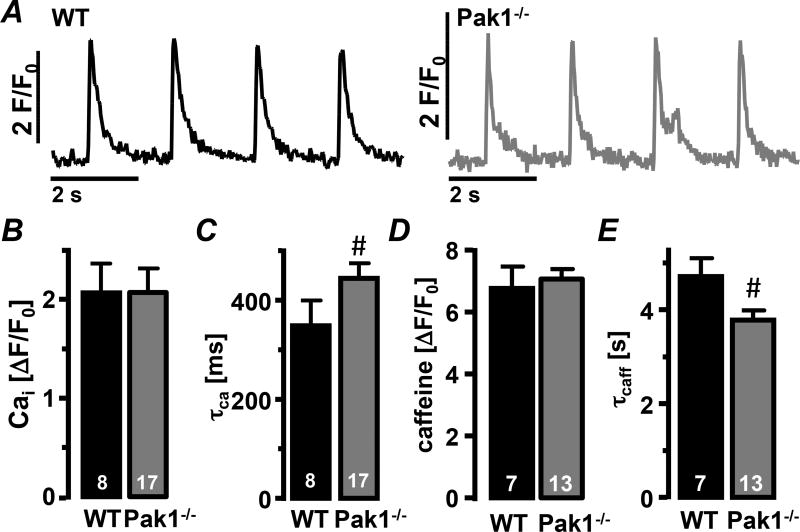
In myocytes enhanced ROS production can lead to the occurrence of arrhythmic events due to the deregulation of Ca2+ handling properties10. Despite higher ROS levels in Pak1−/− AMs, Ca2+ transient amplitudes were not different from WT AMs (Fig. 5AB). However, the Ca2+ transient decay constant (τCa) was prolonged in Pak1−/− AMs (Fig. 5C). Caffeine (10 mmol/L) induced Ca2+ release from the intracellular stores revealed no difference in Ca2+ load of the sarcoplasmic reticulum (SR) between WT and Pak1−/− AMs. But Ca2+ removal in the presence of caffeine (τcaff), which reflects the activity of NCX, was accelerated in Pak1−/− AMs (Fig. 5DE). The data indicate an increased contribution of NCX to Ca2+ extrusion in Pak1−/− AMs, consistent with the increased number of spontaneous Ca2+ transients observed (Fig. 6H). No differences were determined in the Ca2+ wave propagation velocity (SFig. 2A), or the mRNA levels for the Ca2+ handling proteins SERCA, NCX, RYR2, and phospholamban (PLN; SFig. 2B).
Figure 5. Ca2+ removal shifts from SERCA to NCX in Pak1−/− myocytes.
(A) Field stimulation-induced Ca2+ transients recorded in WT (black) and Pak1−/− (grey) AMs under control conditions. Bar graphs show (B) the Ca2+ transient amplitude and (C) decay constant (τCa), the caffeine transient amplitude (D) and (E) decay constant (τcaff). (#: p < 0.05 compared to WT).
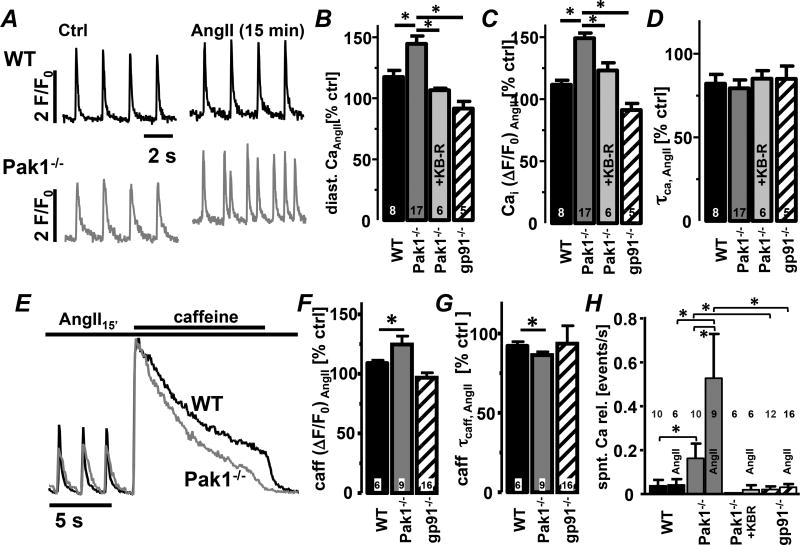
Fig. 6. Pak1−/− AMs exhibit exaggerated AngII-induced spontaneous Ca2+ transients.
(A) Field stimulation-induced Ca2+ transients from WT and Pak1−/− (grey) AMs. Bar graphs represent percent change after 15 min of AngII superfusion of (B) diastolic Ca2+, (C) Ca2+ transient amplitude and (D) decay constant (τcaff) in AMs from WT, Pak1−/−, gp91phox−/−, and Pak1−/− AMs treated with KB-R7943 (KBR: 1 µmol/L). (E) Normalized caffeine transients (10 mmol/L) after 15 min of AngII superfusion. Bar graphs show (F) caffeine transient amplitude and (G) decay constant. (H) Mean number of spontaneous Ca2+ transients (events/s) in AMs before and after AngII superfusion for treatment conditions described in A–D. (*: p < 0.05)
Superfusion of WT and Pak1−/− AMs with AngII (1 µmol/L)-induced an increase in diastolic [Ca2+]i and Ca2+ transient amplitude that was significantly more pronounced in Pak1−/− AMs (Fig. 6A–C). Also the load of the SR was increased and τcaff was accelerated in Pak1−/− AMs with spontaneous Ca2+ transients remaining more numerous in Pak1−/− AMs (Fig. 6E–G). In mice deficient for NOX2 expression (gp91phox−/−: Fig. 6E–H) or WT AMs treated with apocynin (SFig. 3) AngII failed to increase [Ca2+]i and the number of spontaneous Ca2+ transients. The data support the hypothesis that attenuation of Pak1 signaling enhances AngII-induced Ca2+ deregulation by increasing NOX2/ROS production.
In the presence of the reverse mode NCX blocker KB-R7943 (1 µmol/L) the AngII dependent increase in diastolic [Ca2+]i and Ca2+ transient amplitude in Pak1−/− cells was attenuated (Fig. 6B–D). Also the occurrence of spontaneous Ca2+ transients was almost eliminated (Fig. 6H). Due to off-target effects of KB-R7943, experiments were repeated with the NCX specific blocker SEA0400 (SFig.4). The results with KB-R7943 and SEA0400 were comparable. The data support that loss of Pak1 increases NOX2/ROS production leading to increased NCX activity in combination with more frequent spontaneous Ca2+ transients.
Pak1 Attenuates AngII/ROS Production and Spontaneous Ca2+ transients
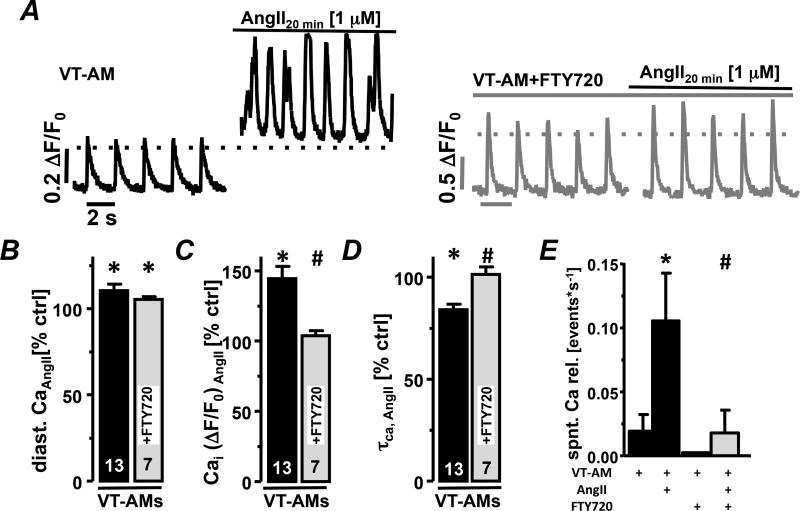
Atrial arrhythmias are often linked to enhanced ROS production and increased NOX2 derived ROS was described in animal models of rapid atrial pacing-induced AF and tissue from AF patients10. Our experiments identify Pak1 as a negative regulator of NOX2/ROS. We therefore tested the hypothesis that Pak1 stimulation can attenuate ROS-induced Ca2+ deregulation in AMs from a canine model of ventricular tachypacing-induced AF (VT-AMs)21. Diastolic [Ca2+]i and Ca2+ transient amplitude were increased in isolated canine AMs during AngII superfusion (Fig. 7A–C), accompanied by an increase in spontaneous Ca2+ transients (Fig. 7E). Pak1 stimulation (FTY720: 200 nM) in VT-AMs, attenuated AngII-induced [Ca2+]i increase as well as spontaneous events (Fig. 7A–E).
Fig. 7. Pak1 stimulation prevents arrhythmic events in AMs from a canine AF model.
(A) Field stimulation-induced Ca2+ transients in VT-AMs before and 20 min after AngII stimulation under Ctrl conditions (black) and in presence of FTY720 (200 nmol/L; grey). Bar graphs show percent change in (B) diastolic [Ca2+]i, Ca2+ transient amplitude (C) and decay constant (τCa,(D)) after AngII stimulation under Ctrl conditions and in presence of FTY720. (E) Mean number of spontaneous Ca2+ transients (events/s) in VT-AMs under indicated treatment conditions (*: p < 0.05 compared to Ctrl, #: p< 0.05 compared to VT-AMs+AngII).
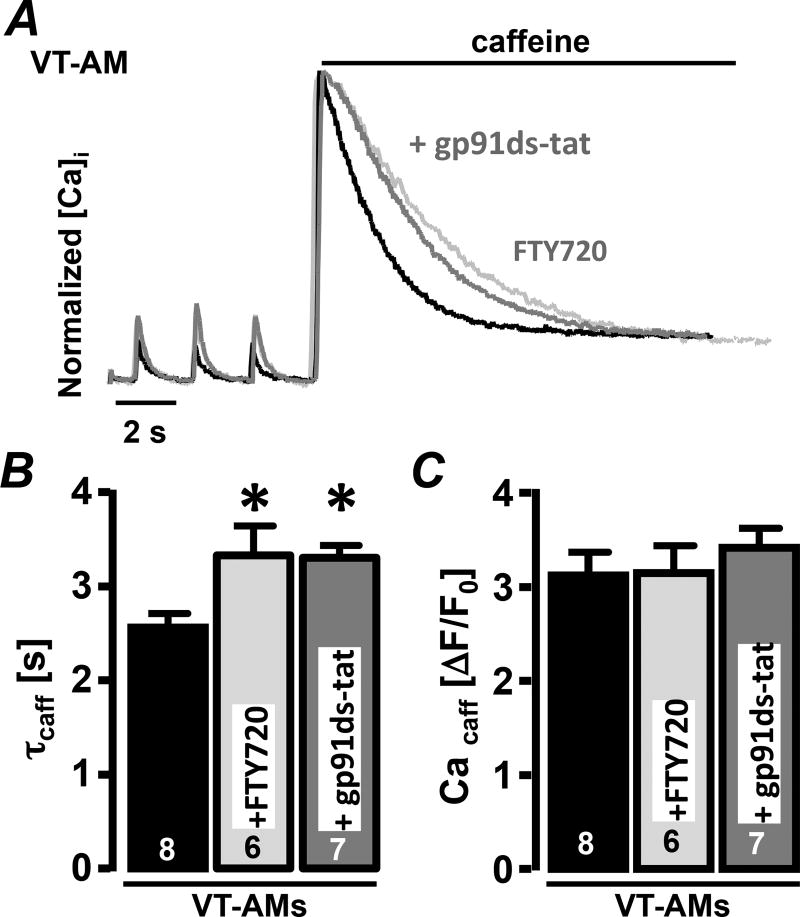
Increased NCX activity was reported in these canine models of ventricular tachypacing-induced AF22. To determine the impact of Pak1 stimulation on NCX activity, VT-AMs were treated with FTY720. Pak1 stimulation did not impact the load of the SR but significantly prolonged τcaff supporting a Pak1 mediated decrease in NCX activity (Fig. 8A–C). Our prior experiments showed that FTY720 also attenuates NOX2/ROS production. To determine the role of ROS in FTY720 signaling, VT-AMs were treated with the NOX2 inhibitor gp91ds-tat (1 µM). gp91ds-tat attenuated NCX activity comparable to FTY720 (Fig. 8A–C).
Fig. 8. Pak1 stimulation and NOX2 inhibition attenuate NCX activity in VT-AMs.
(A) Normalized caffeine-induced Ca2+ transients before and after AngII stimulation (20 min) in Ctrl (black), in presence of gp91ds-tat (1 µmol/L, grey) or FTY720 (200 nmol/L, light-grey). Bar graphs show (B) caffeine transient decay constant (τcaff) and (C) amplitude under Ctrl conditions and in presence of gp91ds-tat or FTY720. (*: p < 0.05 compared to CtrI).
Overall our data support that Pak1 stimulation in atrial tissue could be a pharmacological tool to attenuate NOX2 dependent ROS production and prevent Ca2+ overload through attenuation of NCX activity.
Discussion
Our data demonstrate for the first time that regulation of Pak1 activity significantly influences the propensity for atrial arrhythmic trigger events and that stimulation of Pak1 activity can prevent cellular Ca2+ overload by suppressing the NOX2/ROS dependent stimulation of NCX activity.
Physiological Consequences of Changed Pak1 Expression
Previous experiments in Pak1−/− mice have shown that cardiac contractile function is maintained13 but that hearts exhibit an increased sensitivity for hypertrophic remodeling when challenged by banding, β-adrenergic, or AngII stimulation13,14. Under control conditions the atria of Pak1−/− mice do not exhibit an AF phenotype given their ECGs don’t exhibit prolongations of P-wave, PQ- and QRS durations as described in other mouse models of AF23. Pak1−/− atria also did not exhibit tissue fibrosis that would support the formation of an arrhythmic substrate14. However, Pak1−/− mice demonstrate frequent spontaneous PACs, consistent with the increased incidence of spontaneous Ca2+ release events in isolated AMs from Pak1−/− mice. In light of clinical studies that show a correlation between PAC frequency and risk of atrial fibrillation24 it is not surprising that Pak1−/− mice had increased AF inducibility. In the absence of fibrosis, the arrhythmia noted in Pak1−/− are likely because of electrical changes, not unlike what is noted in patients with lone AF25. A complete characterization of the electrophysiologial phenotype in the intact atrium is beyond the scope of the current study, but will be examined in the future2.
Comparable to VMs, AMs of Pak1−/− mice exhibit increased basal ROS production that is sensitive to NOX2 inhibitors16. Increased ROS production upon Pak1 inhibition has now been demonstrated in AMs and VMs, HL-1 cells16 and microglia26. Nox2 activation requires the assembly of cytosolic (Rac1, p47phox, p67phox) and membrane bound (p22phox, gp91phox) proteins into a multi-cellular complex27. A critical step in this is the translocation of Rac1 to the plasma membrane27. Increased Rac1 levels at the membrane upon AngII stimulation or loss of Pak1 support that Pak1 antagonizes Rac1 activation. The mechanism by which Pak1 negatively regulates Rac1 and NOX2 activation remains to be determined27. Since Pak1 stimulation can attenuate ROS production, the regulation of NOX2 seems in part mediated by Pak1 kinase activity. Pak1 dependent PP2A activation could also enhance p47phox de-phosphorylation and NOX2 inactivation26. However, in WT-AMs inhibition of PP2A by okadaic acid was insufficient to increase p47phox phosphorylation (SFig. 5G).
Mechanism of Arrhythmicity
Arrhythmic activity in the atria is often related to deregulation of the Ca2+ handling properties28 and coincides with increased NCX activity. NCX, whose activity depends on the membrane potential and the ion gradient for Na+ and Ca2+, can promote the occurrence of spontaneous after-depolarization. During diastole NCX removes Ca2+ from the cytoplasm, which conjoins with a depolarization of the membrane potential that can trigger action potentials. However, during increased [Na+]i NCX can operate in reverse where, during the upstroke and plateau of the action potential, it can contribute to Ca2+ influx and Ca2+ over-load29.
In Pak1−/− myocytes, Ca2+ removal is shifted from reuptake, to extrusion via NCX16. The Ca2+ load of the SR did not change despite increased NCX activity consistent with reports from mouse myocytes overexpressing NCX30. Increased NCX activity here coincided with maintained and even increased load of the SR where increased Ca2+ extrusion was counter-balanced by Ca2+ entry through NCX reverse-mode30. In Pak1−/− AMs NCX-dependent Ca2+ entry is reflected in the sensitivity of the AngII-induced increase in diastolic and systolic [Ca2+]i to two different NCX inhibitors.
Increased NCX activity, as in Pak1−/− myocytes, is not always linked to increased protein expression. Increased [Na+]i would facilitate Ca2+ entry through reverse mode activity29 but in contrast to our results in Pak1−/− myocytes, also attenuate Ca2+ extrusion. A stimulation of extrusion by increased [Ca2+]i is not supported by our data. While no direct phosphorylation of NCX through Pak1 was described, NCX activity could increase in Pak1−/− due to enhanced PKC phosphorylation based on attenuated PP2A activity. PP2A inhibition in WT AMs however, was insufficient to increase NCX activity (SFig.5).
An alternative explanation for enhanced NCX activity is its stimulation through ROS that has been previously reported31,32. In Pak1−/− VMs we demonstrated increased NCX activity that was reversible with ROS scavengers and NOX2 inhibitors16. Here we further demonstrate that increased NCX activity in Pak1−/− AMs correlates with increased basal and AngII-induced ROS production and present evidence that in WT and VT-AMs NOX2 inhibition or stimulation of Pak1 attenuated basal and AngII induced increase in [Ca2+]i, NCX-dependent Ca2+ extrusion and spontaneous Ca2+ transients. Overall we propose that in AMs increased levels of ROS contribute to enhanced NCX activity. ROS could directly effect NCX or the response is mediated through post-translational protein modifications.
Pak1 Stimulation Attenuates Arrhythmic Activity in AF
Modulation of the AngII signaling pathway was shown to impact arrhythmic activity in AF animal models. AngII receptor blocker, enhanced AngII degradation, or depressed AngII synthesis all attenuated tissue remodeling and arrhythmic activity6. Overexpression of Rac1 itself creates an AF phenotype, while Rac1 inhibitors can suppress it6. NOX2 is the downstream target of the AngII/Rac1 signaling cascade. Its activity is increased in patients with AF and suppression of NOX2 in AF patient’s decreased arrhythmic activity10. To stimulate Pak1 activity we used FTY720. FTY720 activates Pak1 and promotes cardio-protective signaling20. We demonstrate on a cellular level that FTY720 prevents and reverses AngII-induced ROS production in AMs. This is in contrast to prior reports showing FTY720-induced ROS production33. However, higher concentrations and longer treatment times used could explain the discrepancy. FTY720 activates a variety of signaling pathways20 but no changes in atrial electrophysiology, or changes in ECC of Pak1−/− myocytes was observed. We therefore propose that its primary mechanism of action is through the Pak1-dependent suppression of NOX2/ROS production.
We used isolated myocytes from a canine model of ventricular tachypacing-induced AF. The arrhythmic activity in this model is based on the remodeling of the atrial substrate and reentry21. The enhanced NCX activity and ROS production described in this model made it a good target to identify the benefit of Pak1 stimulation. In vivo, Pak1 dependent attenuation of NOX2 and NCX activity might be insufficient to reverse the arrhythmia during persistent AF. But under conditions of paroxysmal trigger-induced AF, Pak1 stimulation could reduce arrhythmic events and prevent further atrial remodeling.
Limitation
The NCX reverse mode blocker KB-R7943 was reported to have unspecific effects, e.g. block of RYRs, Ca2+-activated potassium channels, and mitochondrial uniporter. However, experimental results were comparable to those obtained with the more specific NCX blocker SEA0400 supporting the validity of the results.
Conclusion
We demonstrated for the first time that a negative regulation of Pak1 results in an increased chance for atrial arrhythmic trigger events. Pak1 stimulation on the other hand attenuates Rac1 mediated NOX2/ROS production and thereby NCX dependent Ca2+ overload. The anti-arrhythmic effect of Pak1 makes it an interesting pharmacological target to attenuate baseline and AngII-induced ROS production and arrhythmic activity. Whether Pak1 signaling is able to attenuate AngII-induced fibrotic remodeling and AF substrate formation remains to be determined.
Supplementary Material
Acknowledgments
The work was supported by grants from the American Heart Association GRNT25700257, the National Institutes of Health HL128330, and HL132871 to KB, HL093490 to RA, and PO1 HL 62426 and to RJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest has to be disclosed.
Disclosures
None
Contributor Information
Jaime DeSantiago, Email: Jaime_DeSantiago@rush.edu.
Dan J. Bare, Email: Dan_Bare@rush.edu.
Disha Varma, Email: Disha_Varma@rush.edu.
R. John Solaro, Email: SolaroRJ@uic.edu.
Rishi Arora, Email: Rishi.Arora@nm.org.
Kathrin Banach, Email: Kathrin_Banach@rush.edu.
References
- 1.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 3.Cardin S, Li D, Thorin-Trescases N, Leung T-K, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 5.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adam O, Frost G, Custodis F, Sussman MA, Schäfers H-J, Böhm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 7.Kakishita M, Nakamura K, Asanuma M, Morita H, Saito H, Kusano K, Nakamura Y, Emori T, Matsubara H, Sugaya T, Ogawa N, Ohe T. Direct evidence for increased hydroxyl radicals in angiotensin II-induced cardiac hypertrophy through angiotensin II type 1a receptor. J Cardiovasc Pharmacol. 2003;42:S67–S70. doi: 10.1097/00005344-200312001-00015. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie L-H. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol. 2011;50:128–136. doi: 10.1016/j.yjmcc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 10.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 11.Chang J-P, Chen M-C, Liu W-H, Yang C-H, Chen C-J, Chen Y-L, Pan K-L, Tsai T-H, Chang H-W. Atrial myocardial nox2 containing NADPH oxidase activity contribution to oxidative stress in mitral regurgitation: potential mechanism for atrial remodeling. Cardiovasc Pathol. 2011;20:99–106. doi: 10.1016/j.carpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Monasky MM, Taglieri DM, Patel BG, Chernoff J, Wolska BM, Ke Y, Solaro RJ. P21-Activated Kinase Improves Cardiac Contractility during Ischemia-Reperfusion concomitant with changes in Troponin-T and Myosin Light Chain 2 Phosphorylation. Am J Physiol Heart Circ Physiol. 2011;302:H224–H230. doi: 10.1152/ajpheart.00612.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taglieri DM, Monasky MM, Knezevic I, Sheehan KA, Lei M, Wang X, Chernoff J, Wolska BM, Ke Y, Solaro RJ. Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J Mol Cell Cardiol. 2011;51:988–996. doi: 10.1016/j.yjmcc.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Zi M, Naumann R, et al. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation American Heart Association, Inc. 2011;124:2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desantiago J, Bare DJ, Ke Y, Sheehan KA, Solaro RJ, Banach K. Functional integrity of the T-tubular system in cardiomyocytes depends on p21-activated kinase 1. J Mol Cell Cardiol. 2013;60:121–128. doi: 10.1016/j.yjmcc.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desantiago J, Bare DJ, Xiao L, Ke Y, Solaro RJ, Banach K. p21-Activated kinase1 (Pak1) is a negative regulator of NADPH-oxidase 2 in ventricular myocytes. J Mol Cell Cardiol. 2014;67:77–85. doi: 10.1016/j.yjmcc.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI, Clapham DE. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol. 2001;37:2136–2143. doi: 10.1016/s0735-1097(01)01304-3. [DOI] [PubMed] [Google Scholar]
- 18.Tateno K, Glass L. Automatic detection of atrial fibrillation using the coefficient of variation and density histograms of RR and deltaRR intervals. Med Biol Eng Comput. 2001;39:664–671. doi: 10.1007/BF02345439. [DOI] [PubMed] [Google Scholar]
- 19.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egom EE, Mohamed TM, Mamas MA, et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301:H1487–H1495. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng J, Villuendas R, Cokic I, et al. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–396. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 23.Reil JC, Hohl M, Oberhofer M, et al. Cardiac Rac1 overexpression in mice creates a substrate for atrial arrhythmias characterized by structural remodeling. Cardiovasc Res. 2010;87:485–493. doi: 10.1093/cvr/cvq079. [DOI] [PubMed] [Google Scholar]
- 24.Chong B-H, Pong V, Lam K-F, Liu S, Zuo M-L, Lau Y-F, Lau C-P, Tse H-F, Siu C-W. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 25.Wyse DG, Van Gelder IC, Ellinor PT, Go AS, Kalman JM, Narayan SM, Nattel S, Schotten U, Rienstra M. Lone atrial fibrillation: does it exist? J Am Coll Cardiol. 2014;63:1715–1723. doi: 10.1016/j.jacc.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roepstorff K, Rasmussen I, Sawada M, Cudre-Maroux C, Salmon P, Bokoch G, van Deurs B, Vilhardt F. Stimulus-dependent regulation of the phagocyte NADPH oxidase by a VAV1, Rac1, and PAK1 signaling axis. J Biol Chem. 2008;283:7983–7993. doi: 10.1074/jbc.M708281200. [DOI] [PubMed] [Google Scholar]
- 27.Sigal N, Gorzalczany Y, Pick E. Two pathways of activation of the superoxide-generating NADPH oxidase of phagocytes in vitro-distinctive effects of inhibitors. Inflammation. 2003;27:147–159. doi: 10.1023/a:1023869828688. [DOI] [PubMed] [Google Scholar]
- 28.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na(+) concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 30.Terracciano CM, Souza AI, Philipson KD, MacLeod KT. Na+-Ca2+ exchange and sarcoplasmic reticular Ca2+ regulation in ventricular myocytes from transgenic mice overexpressing the Na+-Ca2+ exchanger. J Physiol. 1998;512:651–667. doi: 10.1111/j.1469-7793.1998.651bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves JP, Bailey CA, Hale CC. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986;261:4948–4955. [PubMed] [Google Scholar]
- 32.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–H833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 33.Omar HA, Chou C-C, Berman-Booty LD, et al. Antitumor effects of OSU-2S, a nonimmunosuppressive analogue of FTY720, in hepatocellular carcinoma. Hepatology. 2011;53:1943–1958. doi: 10.1002/hep.24293. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.