Abstract
Background
Sport-related concussions (SRCs) are known to have short-term effects on cognitive processes, which can result in diverse clinical presentations. The long-term effects of SRC and repeated exposure to head impacts that do not result in SRC on specific cognitive health outcomes remain unclear.
Objectives
To synthesize and appraise the evidence base regarding cognitive health in living retired athletes with a history of head-impact exposure or SRC.
Data Sources
A systematic search of the EMBASE, PsycINFO, MEDLINE/PubMed, CINAHL, Cochrane Central Register of Controlled Trials, and Web of Science databases was conducted from inception to April 2018 using common key words and medical subject headings related to 3 components: (1) the participant (eg, retired athlete), (2) the primary outcome measure (eg, cognitive test used), and (3) the secondary outcome measure (eg, history of sport concussion).
Study Selection
Cross-sectional studies of living retired male or female athletes in which at least 1 cognitive test was used as an outcome measure were included. Two reviewers independently screened studies.
Data Extraction
Data extraction was performed using Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Methodologic quality was assessed independently by 2 reviewers using the Downs and Black tool.
Data Synthesis
The search yielded 46 cross-sectional observational studies that were included in a qualitative synthesis. Most included studies (80%, n = 37) were published in the 5 years before our review. A large proportion of these studies (n = 20) included retired American National Football League players. The other research investigated professional, university, high school, and amateur retired athletes participating in sports such as American and Australian football, boxing, field and ice hockey, rugby, and soccer. The total sample consisted of 13 975 participants: 7387 collision-sport athletes, 662 contact-sport athletes, 3346 noncontact-sport athletes, and 2580 participants classified as controls. Compared with control participants or normative data, retired athletes displayed worse performance in 17 of 31 studies (55%) of memory, 6 of 11 studies (55%) of executive function, and 4 of 6 studies (67%) of psychomotor function and increased subjective concerns about cognitive function in 11 of 14 studies (79%). The authors of 13 of 46 investigations (28%) reported a frequency-response relationship, with poorer cognitive outcomes in athletes who had greater levels of exposure to head impacts or concussions. However, these results must be interpreted in light of the lack of methodologic rigor and moderate quality assessment of the included studies.
Conclusions
Evidence of poorer cognitive health among retired athletes with a history of concussion and head-impact exposure is evolving. Our results suggest that a history of SRC may more greatly affect the cognitive domains of memory, executive function, and psychomotor function. Retired athletes appeared to have increased self-reported cognitive difficulties, but the paucity of high-quality, prospective studies limited the conclusions that could be drawn regarding a cause-and-effect relationship between concussion and long-term health outcomes. Future researchers should consider a range of cognitive health outcomes, as well as premorbid ability, in diverse samples of athletes with or without a history of concussion or head-impact exposure to delineate the long-term effects of sport participation on cognitive functioning.
Keywords: mild traumatic brain injury, neurocognitive function, brain health
Key Points
The literature in this area has been largely dominated by investigations of retired American National Football League players, who are a unique cohort.
Evidence of decreased cognitive function in retired athletes was mixed. The greatest decreases were in memory, psychomotor function, executive function, and self-reported cognitive function.
Certain key cognitive domains, such as language, visuospatial processing, psychomotor function, perceptual abilities, and reaction time, have been underassessed.
A frequency-response relationship (ie, poorer cognitive outcomes in those with greater levels of exposure to head impacts or concussions) was demonstrated in only 13 of 46 studies.
The need to understand the relationship between a history of concussions or head-impact exposure and the development of later-life cognitive impairment is increasing. The current dialogue on this topic has largely stemmed from postmortem investigations of athletes, particularly retired American National Football League (NFL) players, with a history of substantial head-impact exposure throughout their careers. In the last decade, a dramatic shift in both public and scientific perceptions about the long-term consequences of concussion has been evident. An injury that was once viewed as a short-lived impairment of neurologic function and often trivialized is now implicated in a number of long-term neurologic sequelae.1–5 Research in non–sport-related traumatic brain injury (TBI) has supported a link between TBI and increased risks of cognitive impairment and dementias, such as Alzheimer disease in older adults,6 with data indicating that moderate and severe TBIs increased the risk of dementia between 2- and 4-fold.7 However, the long-term consequences of concussion, which is on the mild end of the TBI spectrum,8 remain poorly understood. Whereas a full recovery of cognitive functioning is generally the norm,9–11 the clinical pathway to recovery and return to sport is much more prolonged and less clear for a minority of athletes.8 Therefore, the purpose of our review was to investigate the literature on the long-term cognitive health status of retired athletes.
METHODS
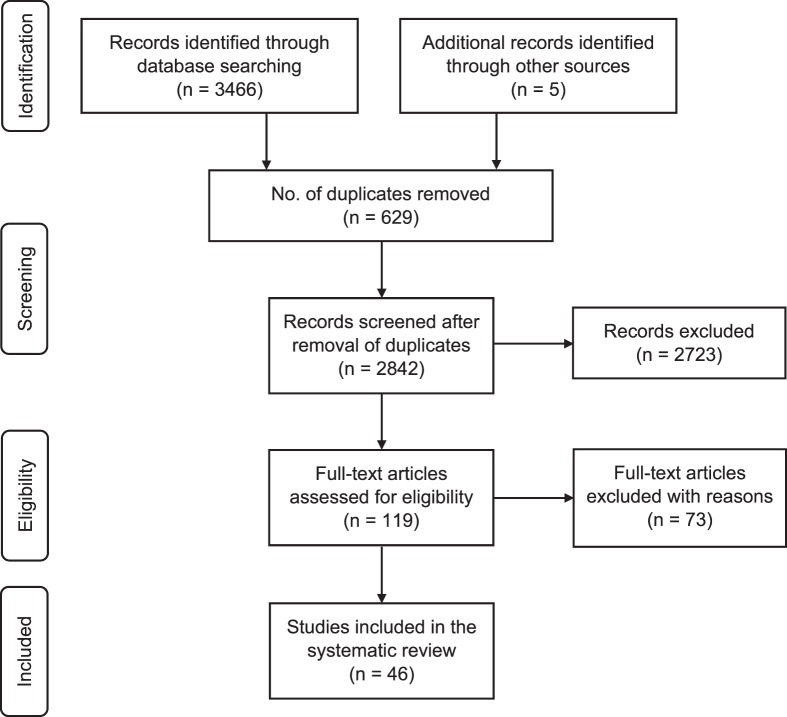
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA; www.prisma-statement.org) and was recorded in PROSPERO, a registry of systematic reviews. The flow diagram is presented in Figure 1. Registration is available at https://www.crd.york.ac.uk/prospero/ (registration number: CRD42016050750).
Figure 1.
Preferred reporting items for systematic reviews; flow diagram of study-selection process.
Eligibility Criteria
Studies were included if the authors evaluated retired male or female athletes who participated in organized sport at the amateur to professional level. At least 1 form of cognitive testing must have been used as an outcome measure. Studies were excluded if the investigators explored only athletes still actively involved in sport or did not report data on retired athletes as a subgroup or if they were case studies with 5 or fewer participants. Neurocognitive testing must have been conducted with and the results obtained from the participant (ie, rather than from friends or family). The primary outcomes of interest were a variety of cognitive domains: attention, memory, executive function, intelligence, processing speed, visuospatial abilities, and psychomotor speed. The secondary outcome variable of interest was a history of sport-related concussion (SRC).
Search Strategy
We searched the electronic databases EMBASE, PsycINFO, MEDLINE/PubMed, CINAHL, Cochrane Central Register of Controlled Trials, and Web of Science from their inception to April 2018 using the relevant database search engines. Common key words and medical subject headings were related to 3 components: (1) the participant (eg, retired athlete), (2) the primary outcome measure (eg, cognitive test used), and (3) the secondary outcome (eg, history of sport concussion). No search restrictions for date or language were imposed. The search strategy for each database and corresponding number of hits per database are presented in Appendix 1.
The electronic database searching was supplemented by searching the abstracts of the “International Conference on Concussion in Sports” consensus meetings (2001–2018) and conducting a gray-literature search and a hand search of the reference lists of the included studies. Two reviewers (J.C. and F.W.) independently screened the titles and abstracts to identify studies that potentially met the eligibility criteria. Full texts of these reports were retrieved and independently assessed for eligibility by the same 2 reviewers. Any disagreements on study inclusion were resolved through discussion and consultation with a third reviewer (S.P.B.) to reach a consensus. A total of 46 studies were included in this review.
Data Extraction and Analysis
A data-extraction template was used as a checklist of items that should be included in reports of cross-sectional studies based on the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.12 One reviewer (J.C.) recorded the study's aim, participant characteristics, details of concussion history, outcome measures used, and relevant outcome data (group means and standard deviations; Table 1).
Table 1.
Summary of Extracted Data of 46 Studies, Including Main Characteristics, Concussion Reports, Types of Cognitive Measures Used, and Primary Resultsa Continued on Next Page
| Study or Subgroup |
Objective(s) |
Participants |
No. of Concussions (Mean ± SDb) |
Cognitive Measures |
Primary Findings |
| Pearce et al13 (2018) | Investigate long-term neurophysiologic and cognitive effects of repeated concussion injuries in former professional rugby players | 25 former professional rugby athletes (mean age = 48.4 y [95% CI = 45.8, 51.0 y]) 25 controls (mean age = 48.8 y [95% CI = 45.9, 51.7 y]) | Rugby athletes: Mean (CI) = 8.5 (4.7, 11.3) Controls: NA | CANTAB–IED, CANTAB–PAL, CANTAB–RTI, CANTAB–SWM, CANTAB–VRT, O'Connor Finger Dexterity testc | Performance on cognitive testing showed that the performance across all tests was different between groups (CANTAB–IED: P < .01, d = 1.04; CANTAB–PAL: P < .01, d = 1.06; CANTAB–SWM: P = .02, d = 0.77). |
| Clark et al14 (2018) | Investigate the relationship between exposure to concussive/subconcussive head impacts, white-matter integrity, and functional task-related neural activity in former US football athletes | 61 players (age = 58.5 ± 3.66 y): 31 collegiate players, 30 professional players | Overall = 61 (3.87 ± 5.85) | MMSE, RBANS, WAIS | No group differences were observed across the concussion history–career duration stratifications for any neuropsychological test (all P > .05). |
| Lewis et al15 (2017) | Assess measures of corticomotor excitability and inhibition in retired rugby players | 23 elite rugby players (age = 43 ± 7 y) 28 community-level rugby (age = 45 ± 8 y) 22 retired controls (age = 44 ± 9 y) | Elite rugby players: 0 concussions (n = 0; 0%), 1–2 concussions (n = 3; 13%), ≥3 concussions (n = 20; 87%) Community-level rugby: 0 concussions (n = 1; 4%), 1–2 concussions (n = 3; 11%), ≥3 concussions (n = 23; 85%) Controls: 0 concussions (n = 16; 75%), 1–2 concussions (n = 5; 21%), ≥3 concussions (n = 1; 4%) | RPQ: predominantly early (RPQ–3) and late (RPQ–13) symptoms of brain injury | No between-groups differences in RPQ |
| Esopenko et al16 (2017) | Characterize retired professional ice hockey players' cognitive and psychosocial functioning in relation to concussion exposure and apolipoprotein E ε4 status | 33 retired professional ice hockey players (age = 54.3 ± 10.4 y) 18 controls (age = 53.5 ± 10.2 y) | Retired professional ice hockey players: 4.8 ± 2.7 Controls: 0.6 ± 0.8 | BVMT–R, Cambridge Brain Sciences, Cogstate,d CFQ, DQ, FAS, JLO, PASAT, RAVLT, ROCFT, SDMT, SOPT, TMT–A and –B, WASI, WCST | Reliable group differences in cognitive performance were observed on 1 test of executive (WCST) and 3 tests of intellectual function (WASI vocabulary, similarities, and matrix reasoning). |
| Gardner et al17 (2017) | Examine brain neurometabolite concentrations in retired rugby league players with a history of self-reported concussions | 16 retired rugby players (age = 38.3 ± 4.6 y) 16 controls (age = 37.9 ± 4.9 y) | Retired players Concussions: average = 33.44 (median = 20; IQR = 7–20; range = 3–100) Concussions with loss of consciousness sustained during career; average = 5.9 (median = 3.5; IQR = 3.5–6; range, 0–30) | ACS–TOPF, COWAT, GPT, RAVLT, RCFT, RPQ, TMT–A and –B, WAIS–IV | No between-groups differences for measures of depression, anxiety, or cognitive functioning |
| Deshpande et al18 (2017) | Estimate the association of playing high school football with cognitive impairment and depression at age 65 years | 3904 retired high school players (age = 64.4 ± 0.8 y) | Not specified | DWR, LF | Composite cognition scores did not differ between football players and all controls, including nonsport controls and non–collision-sport controls (−0.04; 97.5% CI = −0.14, 0.05; P = .37). |
| Kuhn et al19 (2017) | Investigate relationships among neuroimaging, neuropsychological testing, and symptoms in a cohort of retired NFL players | 45 NFL players (age = 46.7 ± 9.1 y) | Concussions = 6.9 ± 6.2 “Dings” = 13.0 ± 7.9 | BVMT–R, COWAT, CVLT-II, ImPACT, TMT–A and –B, TOMM, WAIS–III, WTAR | Correlations that were minimal and not different were present among the neuroimaging, neurocognitive, and symptom scores examined in this cohort of NFL retirees. |
| Montenigro et al20 (2017) | Develop a metric to quantify cumulative repeated head-impact exposure from football and to examine the association between repeated head-impact and long-term clinical outcomes | 93 former high school and collegiate football players (age = 47.3 ± 13.9 y) | Concussion history median ± IQR = 20 ± 3 | BRIEF–A, BTACT | Mean scores on the BTACT indicated that the entire sample was, on average, cognitively normal. |
| Strain et al21 (2017) | Assess the relationship of white-matter integrity and performance on the BNT in a group of retired professional football players and a control group | 25 retired NFL players (age = 59.4 ± 11.8 y) 22 controls (age = 61.1 ± 12.2 y) | Not specified | BNT | The mean BNT T score of the retired athletes was lower than that of the control group (P = .005). |
| McMillan et al22 (2017) | Investigate symptoms and a range of cognitive and health outcomes in retired rugby players with a history of repeated concussion | 52 retired international rugby players (age = 53.5 ± 13.0 y) 29 controls (age = 55.1 ± 9.0 y) | Retired international rugby players = 13.9 ± 18.9 Controls = 0.3 ± 0.5 | GPT, JLO, MOCA, RAVLT, RPQ, SDMT, SART, SF–36, TMT–B | Retired international rugby players performed poorer than controls on a test of verbal learning (P = .02). No differences on all other cognitive tests were found (P > .05). |
| Alosco et al23 (2017) | Examine olfactory function between former NFL players and controls Investigate the association between the B–SIT and behavioral/mood and neuropsychological tests in the former NFL players | 95/96 former NFL players (age = 55.29 ± 7.88 y; 1 player was excluded due to poor effort on testing) 28 controls (age = 57.14 ± 6.94 y) | Former NFL players Concussions: median = 50 Concussions resulting in loss of consciousness = 4.63 ± 16.45 | BRI, BRIEF–A, B–SIT, COWAT, D-KEFS, NAB–LL, ROCFT, TMT–A and –B, WAIS–R, WCST, Map Reading Test, Naming Test, Animal Fluency | In the former NFL players, lower olfactory test scores were correlated with worse neuropsychological and neuropsychiatric functioning. |
| Solomon et al24 (2016) | Investigate an association between years of exposure to pre–high school football (< 12 y) and neuroradiologic, neurologic, and neuropsychological outcomes in later life | 45 retired NFL players (age = 46.7 ± 9.1 y) | 6.9 ± 3.2 | BVMT–R, COWAT, CVLT–II, ImPACT, MMSE, TMT–A and –B, TOMM, WAIS–III, WTAR | Neurocognitive test scores did not demonstrate a relationship with years of exposure to pre–high school football. |
| Multani et al25 (2016) | Evaluate the effect of repetitive concussions in retired professional football players on white-matter tracts, self-reported symptoms, and neuropsychological assessment | 18 retired professional football players (age = 49.6 ± 12 y) 17 healthy male controls (age = 46.7 ± 10 y) | Retired professional male football players = 5.4 ± 4 Healthy male controls = 0 | Cognitive self-report questionnaire, RVDLT, WTAR | Retired players reported more neuropsychiatric and cognitive symptoms than healthy controls and worsening of these symptoms since their last concussion. |
| Koerte et al26 (2016) | Evaluate cortical thickness in former professional soccer players using high-resolution structural magnetic resonance imaging | 15 former soccer players (age = 49.3 ± 5.1 y) 15 controls (age = 49.6 ± 6.4 y) | Not specified | TMT–A and –B, ROCFT | All soccer players and controls tested within normal range for age for all cognitive tests. |
| Gardner et al27 (2016) | Characterize magnetic resonance imaging features of the septum pellucidum between retired NFL players with a history of repeated concussive/subconcussive head traumas and controls | 17 retired NFL players (age = 54.6 ± 15.8 y) 17 controls (age = 54.7 ± 15.8 y) | Not specified | MMSE | No difference in MMSE score (27.1 ± 1.7 versus 25.9 ± 3.3; P = .3) |
| Wright et al28 (2016) | Determine whether concussion history and cognitive reserve could be used to create an index to predict cognitive outcomes in retired American football players | 40 retired NFL players (age = 46.38 ± 10.75 y) | 3.93 ± 3.95 | AMNART, CVLT–II, FAS, ROCFT, SDMT, TMT–A and –B, | Retired NFL players displayed deficits in attention and processing speed, verbal memory, nonverbal memory, and executive ability. |
| Wilde et al29 (2016) | Evaluate the effects of boxing on brain structure and cognition | 10 boxers (8 retired, 2 active; age = 45.7 ± 9.71 y) 9 controls (age = 43.44 ± 9.11 y) | Not specified | SRTT, VSRT | Word-list recall was impaired in the boxers (P = .006), and implicit memory was preserved (P < .04). |
| Amen et al30 (2016) | Determine whether low perfusion in specific brain regions on neuroimaging can accurately separate professional football players from healthy controls | 161 retired NFL players (age = 52 ± 14.2 y) 124 for SPECT control group (age = 44 ± 16.6 y) | Not specified | CPT–II, MicroCog, or WebNeuro | Neuropsychological assessments showed 92% of players had decreased general cognitive proficiency, 86% had decreased information processing speed, 83% had memory loss, 83% had attentional deficits, and 85% had executive-function impairment. |
| Hume et al31 (2017) | Investigate cognitive function in former professional rugby players and assess the association between concussion history and cognitive function | 103 retired elite rugby players (age = 41.3 ± 7.5 y) 198 retired community rugby players (age = 44.9 ± 8.4 y) 65 retired noncontact sport players (age = 42.1 ± 7.7 y) | Elite rugby players = 3.5 ± 2.0 Community rugby players = 2.9 ± 2.2 Noncontact-sport players = 0.4 ± 0.8 | CNS Vital Signs | The elite rugby players performed worse than the noncontact-sport players on tests of complex attention, processing speed, executive functioning, and cognitive flexibility. |
| Vann Jones et al32 (2014) | Investigate the hypothesis that chronic low-level head trauma due to heading is associated with persistent cognitive decline | 92 former professional players (age = 67.45 ± 6.96 y) | Not specified | TYM | Compared with the only large United Kingdom-based MCI prevalence study of men, the authors demonstrated no difference between MCI among the sample of ex-professional footballers and a large sample of men in Wales. |
| Decq et al33 (2016) | Assess the prevalence of major depressive disorder, mild cognitive disorders, and headache in retired rugby players and explore the link between scores and the number of reported concussions | 239 retired rugby players (median age = 52 y; IQR = 49–55.75 y) 138 other retired sportsmen (median age = 52 y; IQR = 49–55 y) | Rugby players = 3.1 ± 5.01 Other retired sportsmen = 0.68 ± 1.83 | F–TICS–m | A higher rate of mild cognitive disorders was observed in retired rugby players (57%) than in other retired sportsmen (40%; P = .005). |
| Koerte et al34 (2016) | Characterize, neuroimaging features of cavum septi pellucidi between former NFL players who present with cognitive, mood, and behavioral symptoms and asymptomatic noncontact-sport athletes | 72 former NFL players (age = 54.53 ± 7.97 y) 14 former professional non-contact–sport athletes (age = 57.14 ± 7.35 y) | Not specified | BRI, BRIEF–A, NAB–LL, NAB–Map Reading, NAB–Naming, ROCFT, TMT–A and –B, WAIS–R, WCST, WRAT–4 | Former professional NFL players demonstrated lower outcome scores in most tests of cognitive functioning and higher scores in behavioral evaluations. |
| Meehan et al35 (2016) | Determine whether the exposure to the subconcussive blows that occur during National Collegiate Athletic Association Division III collision sports affect later-life neurobehavioral quality-of-life measures | 3656 men and women Age < 40 y = 538 (14.7%), 40–44 y = 452 (12.4%), 45–49 y = 571 (15.6%), 50–54 y = 744 (20.4%), 55–59 y = 551 (15.1%), 60–64 y = 382 (10.5%), 65–70 y = 367 (10.0%), >70 y = 47 (1.3%) | Diagnosed concussion in collision-sport athletes = 283 (7.6%), contact-sport athletes = 121 (3.3%), noncontact-sport athletes = 177 (4.8%), nonathletes = 255 (6.9%) Undiagnosed concussion in collision-sport athletes = 396 (10.7%), contact sport athletes = 134 (3.6%), noncontact sport athletes = 102 (2.8%), nonathletes = 113 (3.1%) | Neuro–QoL, PROMIS | Athletes with a history of concussion had lower scores, indicating worse self-reported health, on measures of general concerns regarding cognition, executive function, and positive affect. |
| Coughlin et al36 (2015) | Investigate cognitive, molecular, and structural markers in former NFL players | 9 retired NFL players (age = 64.81 y) 9 controls (age = 58.33 y) | Retired NFL players: n = 12.90 | CVLT–II | Former players had varied performance on a test of verbal learning and memory. |
| Stamm et al37 (2015) | Determine the relationship between exposure to repeated head impacts through tackle football before age 12 y and later-life executive function, memory, and estimated verbal intelligence quotient | 21 former NFL players AFE < 12 y (age = 51.95 ± 1.33 y) 21 former NFL players AFE ≥ 12 y (age = 52.33 ± 1.33 y) | AFE < 12 y = 392.00 ± 145.40 AFE ≥ 12 y = 370.30 ± 234.90 | NAB–LL, WCST, WRAT–4 | Former NFL players in the AFE <12 y group performed worse than the AFE ≥ 12 y group on all measures of the NAB–LL, WCST, and WRAT–4. |
| Koerte et al38 (2015) | Evaluate neurochemistry by using magnetic resonance spectroscopy and neurocognitive performance in former professional soccer players without a known history of concussion but with a history of extensive heading and former noncontact-sport athletes | 11 former soccer players (age = 52.0 ± 6.8 y) 14 former noncontact sport athletes (age = 46.9 ± 7.9 y) | Not specified | TMT–A and –B, ROCFT | All soccer players and controls tested within the normal range for age for TMT–A and –B and ROCFT. |
| Strain et al39 (2015) | Assess the relationship of memory performance with hippocampal volume and concussion history in retired NFL athletes with or without a diagnosis of MCI | 28 retired NFL athletes (MCI = 8; age = 58.1 ± 13 y) 27 controls (MCI = 6; age = 59 ± 12 y) | Retired NFL athletes MCI = 3.8 ± 3.5 Retired NFL athletes with MCI and concussion history = 4.6 ± 3.6 | BNT, CVLT–II, ROCFT, SORT | No differences were found on the ROCFT. The CVLT–II scores were worse in athletes than controls (P = .002), athletes with no MCI than athletes with MCI (P < .001), and athletes with MCI than controls (P < .001). |
| Terry et al40 (2015) | Examine neural activation, verbal memory, and behavioral scores on the fMRI paradigm between individuals who sustained at least 2 football-related concussions and controls | 25 football players (age = 52.0 ± 8.05 y) 16 controls (age = 49.1 ± 8.3 y) | 4.3 ± 3.7 | CVLT–II, WMS–IV, WTAR | Concussive history was not associated with worse memory functioning on neuropsychological tests or worse behavioral performance. |
| Casson et al41 (2014) | Perform clinical neurological, neuropsychological, and neuroradiologic examinations on a group of retired NFL players | 45 retired NFL players (age = 45.6 ± 8.9 y) | 6.9 ± 6.2 | MMSE, ImPACT | Most retired players had normal clinical mental status and CNS neurologic examinations. Neuropsychological testing revealed isolated impairments in 11 players (24%), but none had dementia. |
| Tremblay et al42 (2014) | Investigate white-matter integrity and cognitive and motor function in retired athletes with a history of concussions | 15 retired athletes (age = 60.87 ± 7.51 y) 15 controls (age = 58.13 ± 5.28 y) | Retired athletes = 2.08 ± 1.31 | EFT, MMSE, RAVLT, TMT–A and –B, SDMT, SRTT, TCFT | Most neuropsychological tests did not reveal clinically important differences between athletes and controls. Retired athletes showed reduced semantic verbal fluency (P = .04) and delayed recall (P = .03) and recognition conditions of the TCFT (P = .02). |
| Hart et al43 (2013) | Assess for the presence of cognitive impairment and depression in aging former NFL players and identify neuroimaging correlates of these dysfunctions | 34 retired NFL players (mean age = 61.8 y, range = 41–79 y) 26 controls (mean age = 60.1 y, range = 41–79 y) | 32/34 Retired NFL players ≥ 1 (average = 4.0) Mean per participant for Grade 1 = 2.0, Grade 2 = 0.5, Grade 3 = 1.6 | BNT, COWAT, CVLT–II, ROCFT, SORT, TMT–A and –B, WAIS–IV, WASI | Differences were found on BNT, CVLT–II, ROCFT, and SORT. No differences were found on the TMT, WAIS–IV, and COWAT. |
| Hampshire et al44 (2013) | Evaluate the performances and brain activation patterns of retired NFL players relative to controls using an fMRI-optimized neuropsychological test of executive function | 13 NFL alumni Controls were not specified | Not specified | CANTAB–TOTSPT | Behaviorally, the NFL alumni showed subclinical and modest performance deficits in executive function. |
| Pearce et al45 (2014) | Investigate corticomotor excitability and inhibition, cognitive functioning, and fine-motor dexterity in retired elite and amateur Australian football players with a history of concussions | 40 Australian football players: 20 elite players (age = 49.7 ± 5.7 y), 20 amateur players (age = 48.4 ± 6.9 y) 20 controls (age = 47.56 ± 6.85 y) | Elite players = 3.70 ± 2.89 Amateur players = 2.40 ± 1.56 | CANTAB–PAL, CANTAB–RTI, CANTAB–SWM | Reaction time showed differences between the healthy control group and the combined Australian football groups (P = .003), with no differences between the 2 Australian football groups (P = .06). No differences were observed when comparing the healthy control group and the combined football groups on the CANTABL–PAL and –SWM. However, within the Australian football players, the amateur player group performed worse than the elite group. |
| Seichepine et al46 (2013) | Examine executive function in current and retired collegiate and professional football players | 64 retired collegiate and professional football players (age = 47 ± 13.6 y, range = 25–81 y) | 56/64 Participants: 55 6/64 Participants: 100–140 1/64 Participants: approximately 350 1/64 Participants: approximately 20 000 | BRIEF–A | Group differences were observed between football players and normative data for healthy adults on the BRIEF–A in the following areas: Global Executive Composite (P < .05), Metacognition Index (P < .05), and Behavioral Regulation Index (P < .05). |
| Randolph et al47 (2013) | Explore the prevalence of cognitive impairment in a sample of retired NFL players | 41 retired NFL players selected from 513 players who completed surveys from previous study (age = 64.2 ± 5.5 y) 41 cognitively normal participants extracted from RBANS normative data (age = 64 ± 5.8 y) | Not specified | RBANS | RBANS total mean score was lower for the NFL players than the healthy control sample (P = .002). |
| Tremblay et al48 (2013) | Investigate the neuroimaging profile of former university athletes with concussions in relation to cognition | 30 former university athletes 15 experiment group (age = 60.87 ± 7.51 y), 15 controls (age = 58.13 ± 5.28 y) | Experimental group = 24.00 ± 4.55 Controls = no history of concussion | Color Trails test (forms A and B), MMSE, RAVLT, SDMT, TCFT | Relative to controls, former athletes showed reduced semantic verbal fluency (P = .040) and altered episodic memory on both delayed-recall (P = .026) and recognition (P = .015) conditions of the TCFT, whereas performance on the copy trial did not differ across groups (P > .15). |
| Ford et al49 (2013) | Examine long-term neural changes associated with multiple sport-related concussion using event-related fMRI | 27 retired NFL players: 15 with low-level concussion history (age = 64.1 ± 6.8 y), 12 with high-level concussion history (age = 62.6 ± 5.0 y) 14 controls (age = 62.2 ± 6.3 y) | Low-level concussion history (≤2 concussions) = 1.07 ± 0.96 High-level concussion history (≥3 concussions) = 6.5 ± 5.1 | BNT, COWAT, memory paradigm, MMSE, WAIS–III, WTAR, Telephone Interview for Cognitive Status, TMT–B | Both concussion groups demonstrated relational memory impairments relative to the age-matched group (P < .05). |
| Willeumier et al50 (2012) | Investigate the effects of body mass as measured by waist-to-height ratio on regional cerebral blood flow using SPECT | 76 retired NFL athletes: 38 healthy weight (age = 58 ± 9.6 y), 38 overweight (age = 58 ± 13.3 y) | Not specified | MicroCog | Between-groups differences were found in general cognitive proficiency and general cognitive function (P < .025). Overweight athletes had decreases in attention (P = .01326), general cognitive proficiency (P = .012), and memory (P = .005). |
| Amen et al51 (2011) | Determine whether, compared with a healthy control group, a retired NFL players group would exhibit decreased regional cerebral blood flow consistent with previous brain trauma and compromised neuropsychological functioning | 100 active and retired NFL players (age = 57.27 ± 12.37 y) 20 for SPECT control group (age = 50.0 ± 16.1 y) | Not specified Mean loss-of-consciousness episodes = 2.693 Loss-of-consciousness episodes: 0 episodes = 37, 1 episode = 15, 2 episodes = 15, 3–5 episodes = 18, >5 episodes = 14 | CPT–II, MCIS, MicroCog | Players scored in the bottom half of the percentile placements on all measures, including general cognitive functioning, general cognitive proficiency, processing speed, processing accuracy, attention, reasoning, and memory and excepting spatial processing and reaction time, which were both in the top half of the percentile placements. |
| Hinton et al52 (2011) | Determine the relative influence of current exercise and diet on the late-life cognitive health of former National Collegiate Athletic Association Division I collision-sport athletes (ie, football players) compared with non–collision-sport athletes and nonathletes | 214 former Division I collision-sport athletes (football players) 136 former Division I non–collision-sport athletes (other athletes) 50 former nonathletes (nonathletes) | Not specified | CDS | Former football players reported more cognitive difficulties and worse physical and mental health than controls. |
| De Beaumont et al53 (2009) | Investigate electrophysiological, motor, and cognitive measures in former athletes who sustained their last sport concussion > 30 y before the study | 19 former Canadian university-level athletes (age = 60.79 ± 5.16 y) 21 controls (age = 58.89 ± 9.07 y) | Former Canadian university-level athletes: range = 1–5 Controls: no history of concussion or neurologic insult | MMSE, ROCFT | Former athletes with concussion obtained an equivalent total score on the MMSE to that of former athletes with no history of concussion (P > .05). Every participant from both groups scored within the normal range on the MMSE. |
| Thornton et al54 (2008) | Examine the extent to which lifetime concussion exposure is associated with neurocognitive and symptomatic status in competitive versus recreational/retired rugby players and differential complications between groups | Male and female retired players 37 no heavy concussions (age = 32.14 ± 12.41 y), 29 1–2 heavy concussions (age = 28.72 ± 10.20 y), 35 ≥3 heavy concussions (age = 34.03 ± 12.06 y) | Divided participants into heavy concussion-exposure groups Sample size (female to male): grades 2 and 3: no heavy exposure to concussions = 37 (8:29), 1–2 heavy exposures = 39 (4:35), ≥3 heavy exposures = 35 (1:34) | CCFT, ETS Kit–V2 and –V3, PCSC, RAVLT, TMT–A and –B, WAIS–III, WCST, WMS–III | Participants with no heavy concussions reported fewer memory complaints (d = −0.68), less distress (d = −0.76), and fewer overall (total) postconcussion symptoms (d = −0.65) than those with ≥3 heavy concussions. |
| Guskiewicz et al55 (2005) | Investigate the association between recurrent concussion and late-life cognitive impairment in retired professional football players | 2552 retired professional footballers (age = 53.8 ± 13.4 y) | ≥ 1 = 1513 ≥3 = 597 | SF–36 (including MCS) | Retired players with ≥3 reported concussions had a 5-fold prevalence of MCI diagnosis and a 3-fold prevalence of reported substantial memory problems compared with retirees without a history of concussion. |
| Downs and Abwender56 (2002) | Investigate neuropsychological function between older and retired soccer players and swimmers | 32 male and female soccer players (15 men, 11 women; age = 19.81 ± 1.50 y) 6 older male soccer players (current/ retired professionals; age = 41.5 ± 9.77 y) Control groups: 22 male and female swimmers (7 men, 15 women; age = 19.50 ± 1.22 y), 7 adult male swimmers (age = 42.86 ± 15.09 y) | Not specified | CPT, PASAT, WCST | Soccer players performed worse than swimmers on tests of conceptual thinking. The older soccer group performed particularly poorly on measures of concentration, reaction time, and conceptual thinking. |
| Murelius and Haglund57 (1991) | Analyze possible chronic brain damage in 47 former amateur boxers who started their careers after the introduction of stricter Swedish amateur boxing rules | 25 high-match boxers (age = 30.5 ± 5.1 y) 25 low-match boxers (age = 32.3 ± 5.6 y) 25 soccer players (age = 33.0 ± 6.0 y) 25 track and field athletes (age = 33.4 ± 5.4 y) | Not specified | Synonyms test of verbal understanding, Block design test (Koh's cubes), TMT–A and –B, FTT, Claeson-Dahl Verbal Learning, Claeson-Dahl Verbal Retention, memory for designs, and motor functions of the hand | The groups differed on only 1 test. Boxers who had participated in a large number of bouts had slightly inferior finger-tapping performance. None of the boxers were considered to have definite signs of intellectual impairment. |
| Casson et al58 (1984) | Dispute the claims that chronic encephalopathy occurs in only an occasional poorly skilled fighter or those who are poorly educated or abusing drugs and alcohol | 18 Retired athletes: 13 former boxers, 2 active professional boxers, 3 active Golden Glove boxers (mean age = 36 y) | Not specified | BGT, DST, TMT, WMS | All former and active professional boxers had abnormal results on at least 2 of the 4 main test, and 8/17 had abnormal computed tomography scans; these had a higher mean impairment index on neuropsychological testing than those with normal computed tomography scans (P < .001). |
Abbreviations: AFE, age of first exposure; CI, confidence interval; fMRI, functional magnetic resonance imaging; IQR, interquartile range; MCI, mild cognitive impairment; NA, not applicable; NFL, National Football League; SPECT, single photon emission computed tomography.
Abbreviations of the cognitive measures are defined in Appendix 2.
Unless otherwise indicated.
Lafayette Instruments, Lafayette, IN.
Cogstate Ltd, New Haven, CT.
Methodologic Assessment
Two reviewers (J.C. and F.W.) independently evaluated the methodologic quality of the studies using an adapted Downs and Black checklist.59 Disagreements between the reviewers were resolved through discussion to achieve consensus. If agreement could not be reached, a third reviewer (S.P.B.) arbitrated. The checklist was modified to a maximum of 17 applicable questions that addressed the following methodologic components: reporting, external validity, internal validity (bias and confounding), and power. Seventeen items were rated on a 2-point scale (yes = 1 and no/unable to determine = 0), and 1 item was rated on a 3-point scale (yes = 2, partial = 1, and no = 0). The maximum achievable score was 18, with higher scores indicating better methodologic quality. Results were categorized according to the adapted Downs and Black checklist59 from Hartling et al60 and Hignett61 and were interpreted as follows: strong quality (≥14) represented the top 75%, moderate quality (scored 9–13) represented 50% to 74%, limited quality (5–8) represented 25% to 49%, and poor quality (<5) represented less than 25%.
RESULTS
Literature Search
After duplicates were removed, this review yielded a total of 2842 records. We screened the titles and abstracts of these records and identified 119 studies that potentially met the inclusion criteria and, hence, were subject to full review. Of these, 46 cross-sectional studies published between 1984 and 2018 met the criteria and were included in a quantitative synthesis. Given the heterogeneity of the study design and outcome measures, a meta-analysis could not be conducted for all studies. Where possible, we performed a meta-analysis depending on the homogeneity of the included studies.
Methodologic Assessment
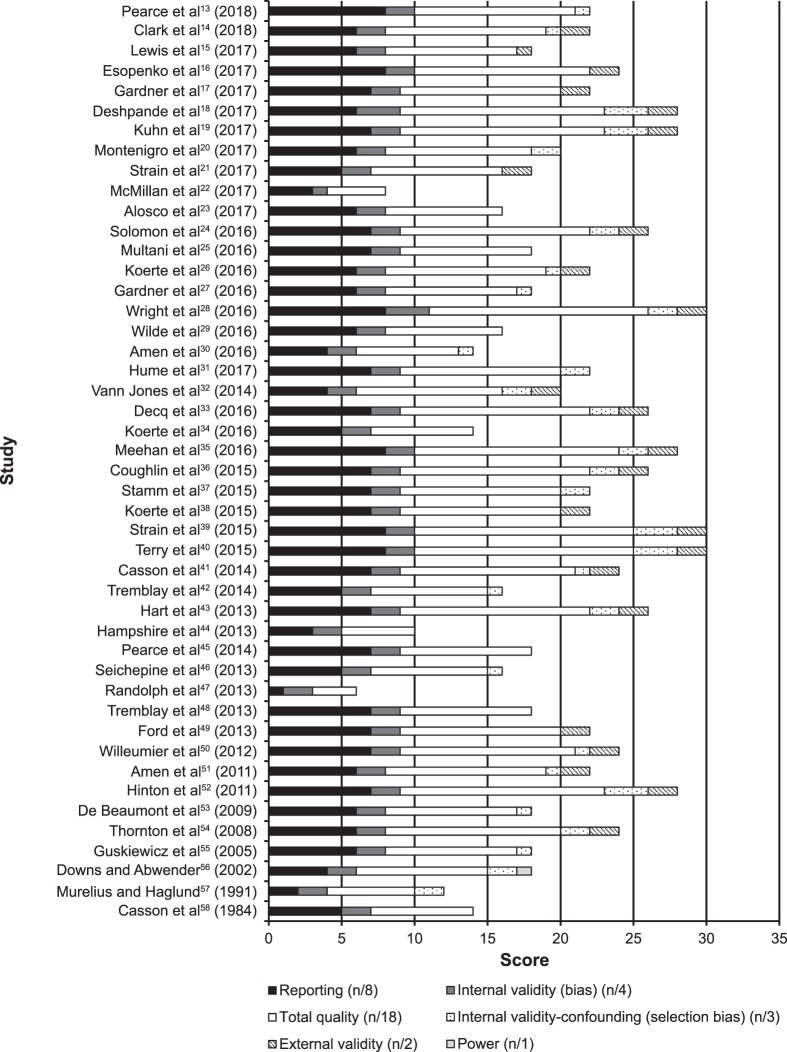
Detailed information on the quality appraisal of the 46 studies is presented in the Supplemental Table (273.9KB, pdf) (available online at http://dx.doi.org/10.4085/1062-6050-297-18.S1). A total of 11 (24%) studies had a methodologic quality score of poor or limited. The overall mean methodologic quality score was 10.3 ± 2.9 (out of a maximum of 18), which is considered moderate. A breakdown of the quality appraisal can be seen in Figure 2.
Figure 2.
Summary of quality appraisal of the included studies using the Downs and Black checklist.
Study Characteristics
The key study characteristics and findings of the 46 cross-sectional studies are summarized and presented in Table 1. The sample consisted of 13 975 participants: 7387 collision-sport athletes, 662 contact-sport athletes, 3346 noncontact-sport athletes, and 2580 participants classified as controls. Collision sports were boxing, football, ice hockey, and rugby. All contact-sport athletes were soccer players. Sports classified as noncontact were archery, athletics, badminton, ballroom dancing, canoeing, cricket, fencing, field hockey, gliding, golfing, horseback riding, paragliding, pelota, rock climbing, running, sailing, skiing, squash, swimming, table tennis, track and field, triathlon, and weightlifting. Only 3 (7%) of the 46 studies included female participants.35,54,56 Participants varied in age, medical history, socioeconomic background, concussion exposure, number of concussions reported, and types of sports played. A large proportion of these studies (n = 20) included retired NFL players.* In 7 studies, retired rugby players were investigated.13,15,17,22,31,33,54 Participants in the remaining studies included boxers (n = 3)29,57,58 and soccer athletes (n = 4),26,32,38,56 whereas other authors examined a combination of former professional and amateur athletes, including university and high school football and hockey athletes (n = 12).† Of the 46 studies, 33 included a control group,‡ and 13 did not.§
Types of Outcome Measures Used
Results from a wide variety of cognitive tests were reported. The tests and their corresponding cognitive domains are presented in Table 2. Objective neuropsychological tests alone were used in 31 studies‖ to assess different aspects of cognition. Six studies15,32,35,46,52,55 used subjective (self-reported) cognitive tests alone. The remaining 9 studies¶ used a combination of objective and subjective tests. Cognitive tests were categorized according to the predominant cognitive domain assessed. The 9 cognitive domains assessed were global cognitive ability, attention, memory, executive function, language, psychomotor function, intelligence, perception, and self-reported cognitive functioning (Figure 3). Memory was the most commonly studied cognitive health outcome (n = 31, 67%), followed by attention (n = 17, 37%) and global cognitive ability (n = 16, 35%). The studies by Kuhn et al19 and Tremblay et al42 were excluded from the analysis, as the cognitive results were duplicates of those reported by Solomon et al24 and Tremblay et al,48 respectively. The cognitive results are shown in Table 3.
Table 2.
Summary of Cognitive Tests Used Across Studies and Corresponding Cognitive Domains of Interesta
| Domain (No. of Studies) |
Domain Description |
Cognitive Testsb |
References |
| Global cognitive ability (16) | A broad array of cognitive domains | BTACT, Cambridge Brain Sciences, Cogstate,c CNS Vital Signs, F–TICS–m, ImPACT, MicroCog, MMSE, modified MMSE, MOCA, RBANS, WebNeuro | 14,16,20,22,24,27,30,31,33,41,47–51,53 |
| Attention (17) | Ability to concentrate and focus on specific stimuli; attention has multiple subprocesses specialized for different aspects of attentional processing and complex attention tasks, such as selective and divided attention | Color Trails Test (forms A and B), CPT–II, EFT, PASAT, SART, SDMT, SRTT, TMT, WAIS | 14,16,17,22–24,26,28,29,31,38,41,43,48, 49,54,57 |
| Memory (31) | Involves the registration, storage, recognition, and retrieval of information | Animal fluency, BVMT–R, CANTAB–PAL, CANTAB–SWM, CVLT–II, DWR, LF, NAB–LL, NAB–Map Reading test, RAVLT, ROCFT, RVDLT, SOPT, SORT, TCFT, TOMM, TYM, VSRT, WMS–IV | 13,16–18,22–26,28–32,34,36–41,43,45, 48–51,53,54,57,58 |
| Executive function (11) | Includes planning, decision making, working memory, responding to feedback, inhibition, and mental flexibility | CANTAB–IED, CANTAB–TOTSPT, D-KEFS, WCST | 13,16,23,28,31,34,37,41,44,54,56 |
| Language (8) | Includes object naming, word finding, fluency, grammar, syntax, and receptive language | BNT, COWAT, FAS, LF | 16,17,21,23,24,39,43,49 |
| Psychomotor function (6) | Relationship between cognitive functions and physical movements | CANTAB–VRT, CNS Vital Signs (motor speed domain), FTT, GPT, O'Connor Finger Dexterity testd | 13,17,22,31,45,56 |
| Intelligence (12) | Premorbid intelligence quotient refers to one's intellectual ability level before the onset of disorders, such as mild cognitive impairment and Alzheimer disease; estimating disease severity is important. | ACS–TOPF, CCFT, ETS Kit–V2 and –V3, WAIS–III and –IV, WASI, WRAT–4, WTAR | 14,16,17,24,25,34,37,40,41,43,49,54 |
| Perception (3) | Recognition and interpretation of sensory information from environment; includes response to this information for interacting with environment | B–SIT, JLO | 16,22,23 |
| Self-reported cognitive functioning (14) | The “self-experience” of cognition; a self-report is any method (eg, survey, questionnaire, or poll) that involves asking participant about feelings, attitudes, beliefs, etc | AD8, BRI, BRIEF–A, CDS, Cognitive Failures Questionnaire, cognitive self-report questionnaire, DQ, MCIS, Neuro–QoL, PCSC, PROMIS, RPQ, SF–36 (including MCS) | 15–17,20,22,23,25,34,35,46,47,52,54,55 |
The results of Kuhn et al19 and Tremblay et al42 were excluded, as the cognitive results were taken from Solomon et al24 and Tremblay et al,48 respectively.
Abbreviations are defined in Appendix 2.
Cogstate Ltd, New Haven, CT.
Lafayette Instruments, Lafayette, IN.
Figure 3.
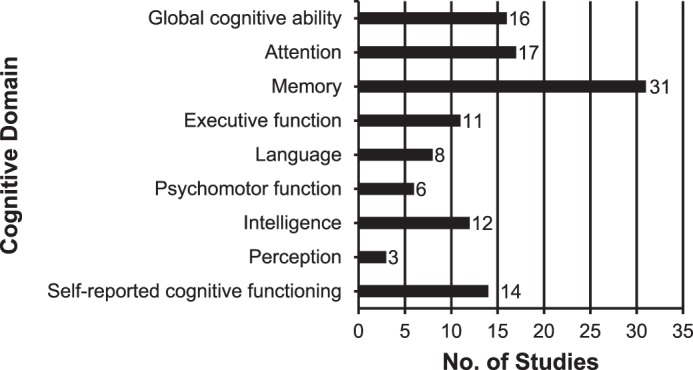
Summary of cognitive domains assessed in the included studies.
Table 3.
Evaluation of Cognitive Outcome Measures by Cognitive Domain Effect Size (Mean Difference Interval Variance, Random Effects Model [95% Confidence Interval])a Extended on Next Page
| Study or Subgroup |
No. of Participants (Retired Athletes/ Controls) |
Domain (Mean Difference [95% Confidence Interval]) |
||
| Global Cognitive Ability |
Attention |
Memory |
||
| Pearce et al13 (2018) | 25/25 | b | b | CANTAB–SWM: 2.20 (0.17, 4.23)c |
| Clark et al14 (2018) | 31/30 | RBANS: 0.70 (−6.21, 7.61)d | RBANS: 7.50 (−1.27, 16.27)d | b |
| Lewis et al15 (2017) | 51/22 | b | b | b |
| Esopenko et al16 (2017) | 33/18 | Data not providedd | Data not providedd | Data not providedd |
| Gardner et al17 (2017) | 16/16 | b | Composite cognition scored | Composite cognition scored |
| Deshpande et al18 (2017) | 3904/ND | b | b | No control groupd |
| Montenigro et al20 (2017) | 93/ND | No control groupd | b | b |
| Strain et al21 (2017) | 25/22 | b | b | b |
| McMillan et al22 (2017) | 52/29 | MOCA: −0.60 (−1.43, 0.23)d | TMT–B: 4.20 (−3.94, 12.34)d | RAVLT: −1.10 (−2.39, 0.19)c |
| Alosco et al23 (2017) | 95/28 | b | TMT–A: −5.17 (−9.68, −0.66)c | NAB–LL: −3.81 (−8.16, 0.54)d |
| Solomon et al24 (2016) | 45/ND | No control groupd | No control groupd | No control groupd |
| Multani et al25 (2016) | 18/17 | b | b | RVDLT: 0.35 (−1.47, 2.17)d |
| Koerte et al26 (2016) | 15/15 | b | TMT–B: −1.40 (−4.80, 2.00)d | ROCFT: −6.00 (−12.72, 0.72)c |
| Gardner et al27 (2016) | 17/17 | MMSE: 0.00 (−2.45, 2.45)d | b | b |
| Wright et al28 (2016) | 40/ND | b | No control groupc | No control groupc |
| Wilde et al29 (2016) | 10/9 | b | Data not providedd | Data not providedc |
| Amen et al30 (2016) | 161/ND | No control groupc | b | No control groupc |
| Hume et al31 (2017) | 301/65 | CNS Vital Signs: 0.00 (−5.24, 5.24)d | CNS Vital Signs: 1.00 (−2.91, 4.91)c | CNS Vital Signs: −1.00 (−5.59, 3.59)c |
| Vann Jones et al32 (2014) | 92/ND | b | b | No control groupd |
| Decq et al33 (2016) | 239/138 | F–TICS–m: −1.02 (−1.76, −0.28)c | b | b |
| Koerte et al34 (2016) | 72/14 | b | b | NAB–LL: −64.96 (−138.09, 8.17)c |
| Meehan et al35 (2016) | 1335/2321 | b | b | b |
| Coughlin et al36 (2015) | 9/9 | b | b | No control groupd |
| Stamm et al37 (2015) | 21/21 | b | b | NAB–LL: −2.48 (−4.44, −0.52)c |
| Koerte et al38 (2015) | 11/14 | b | TMT–B: 1.00 (−2.72, 4.72)d | ROCFT: −5.30 (−15.82, 5.22)d |
| Strain et al39 (2015) | 28/27 | b | b | CVLT–II: −7.74 (−11.71, −3.77)c |
| Terry et al40 (2015) | 25/16 | b | b | CVLT–II: −1.30 (−8.29, 5.69)d |
| Casson et al41 (2014) | 45/ND | No control groupd | No control groupd | No control groupd |
| Hart et al43 (2013) | 34/26 | b | TMT–B: −2.20 (−11.32, 6.92)d | ROCFT: −4.30 (−12.43, 3.83)c |
| Hampshire et al44 (2013) | 13/NS | b | b | b |
| Pearce et al45 (2014) | 20/20 | b | b | CANTAB–PAL: 1.07 (−1.27, 3.41)d |
| Seichepine et al46 (2013) | 64/ND | b | b | b |
| Randolph et al47 (2013) | 513/ND | No control groupc | b | b |
| Tremblay et al48 (2013) | 15/15 | MMSE: −0.20 (−0.91, 0.51)d | Color trails test (form B): 1.13 (−14.84, 17.10)d | RAVLT: −2.54 (−7.47, 2.39)c |
| Ford et al49 (2013) | 27/14 | MMSE: 0.50 (−0.89, 1.89)d | TMT–B: 13.00 (−7.90, 33.90)d | Memory paradigm: −0.07 (−0.10, −0.04)c |
| Willeumier et al50 (2012) | 38/38 | Data not providedc | b | Data not providedc |
| Amen et al51 (2011) | 100/ND | No control groupc | b | No control groupc |
| Hinton et al52 (2011) | 214/186 | b | b | b |
| De Beaumont et al53 (2009) | 19/21 | MMSE: 0.30 (−0.32, 0.92)d | b | ROCFT: −3.60 (−7.73, 0.53)c |
| Thornton et al54 (2008) | 74/37 | b | WAIS–III: 0.98 (−2.96, 4.92)d | RAVLT: −3.54 (−7.43, 0.35)d |
| Guskiewicz et al55 (2005) | 2552/ND | b | b | b |
| Downs and Abwender56 (2002) | 38/22 | b | b | b |
| Murelius and Haglund57 (1991) | 50/50 | b | Data not providedd | Data not providedd |
| Casson et al58 (1984) | 18/ND | b | b | No control groupc |
Table 3.
Extended From Previous Page
| Domain (Mean Difference [95% Confidence Interval]) | |||||
| Executive Function |
Language |
Psychomotor Function |
Intelligence |
Perception |
Self-Reported Cognitive Functioning |
| CANTAB–IED: 12.50 (4.64, 20.36)c | b | CANTAB–RTI: 15.46 (−22.39, 53.31)c | b | b | b |
| b | b | b | WAIS: −5.00 (−3.35, 13.35)d | b | b |
| b | b | b | b | b | RPQ: 3.50 (−2.51, 9.51)d |
| Data not providedc | Data not providedd | b | Data not providedc | Data not providedd | Data not providedc |
| b | Composite cognition scored | Composite cognition scorec | Composite cognition scored | b | Composite cognition scored |
| b | b | b | b | b | b |
| b | b | b | b | b | No control groupd |
| b | BNT: −9.40 (−15.58, −3.22)c | b | b | b | b |
| b | b | GPT: 6.20 (0.11,12.29)c | b | JLO: 0.10 (−0.88, 1.08)d | RPQ: 9.30 (4.14,14.46)c |
| D-KEFS: −1.40 (−2.55, −0.25)d | COWAT: −3.25 (−7.54, 1.04)d | b | b | Data not providedc | BRIEF-A: 1.32 (0.87, 1.78)c |
| b | No control groupd | b | No control groupd | b | b |
| b | b | b | WTAR: 1.93 (−3.02, 6.88)d | b | Data not providedc |
| b | b | b | b | b | b |
| b | b | b | b | b | b |
| No control groupc | b | b | b | b | b |
| b | b | b | b | b | b |
| b | b | b | b | b | b |
| CNS Vital Signs: −5.00 (−8.80, −1.20)c | b | CNS Vital Signs: −2.00 (−5.93, 1.93)d | b | b | b |
| b | b | b | b | b | b |
| b | b | b | b | b | b |
| WCST: −46.16 (−106.31, 13.99)d | b | b | WAIS–R: −51.71 (−107.44, 4.02)c | b | BRI: 162.76 (157.58, 167.94)c |
| b | b | b | b | b | Data not providedc |
| b | b | b | b | b | b |
| WCST: −7.52 (−8.94, −6.10)c | b | b | WRAT–4: −8.55 (−10.06, −7.04)c | b | b |
| b | b | b | b | b | b |
| b | b | b | b | b | b |
| b | b | b | WTAR: 2.30 (−5.33, 9.93)d | b | b |
| No control groupd | b | b | No control groupd | b | b |
| b | BNT: −4.80 (−12.37, 2.77)d | b | Data not providedd | b | b |
| Data not providedd | b | b | b | b | b |
| b | b | CANTAB–RTI: 41.44 (8.52, 74.36)c | b | b | b |
| b | b | b | b | b | No control groupc |
| b | b | b | b | b | No control groupc |
| b | b | b | b | b | b |
| b | BNT: 0.80 (−3.36, 4.96)d | b | WAIS–III: 1.60 (−11.22, 14.42)d | b | b |
| b | b | b | b | b | b |
| b | b | b | b | b | b |
| b | b | b | b | b | CDS: 0.19 (0.03, 0.35)c |
| b | b | b | b | b | b |
| WCST: −2.11 (−5.65, 1.43)d | b | b | WAIS–III: −0.17 (−2.01, 1.67)d | b | PCSC: 4.31 (1.18, 7.44)c |
| b | b | b | b | b | No control groupc |
| WCST: 27.50 (13.67, 41.33)c | b | PASAT: −10.60 (−31.10, 9.90)d | b | b | b |
| b | b | b | b | b | b |
| b | b | b | b | b | b |
Cognitive Findings
Global Cognitive Ability
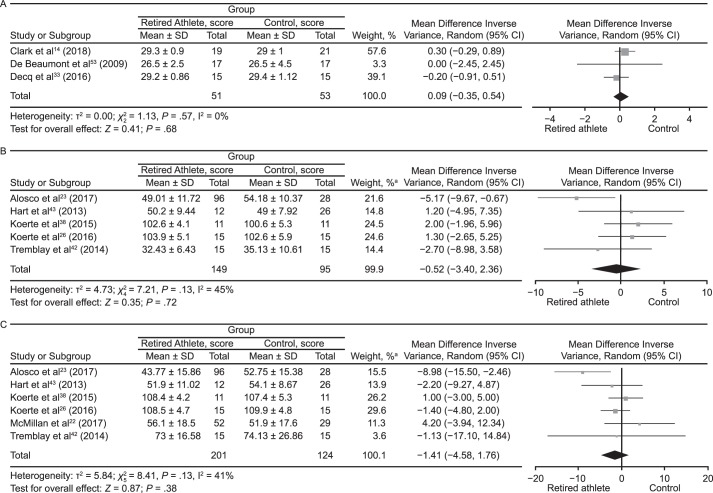
Global cognitive ability was assessed in 16 studies.# Individual cognitive tests are summarized in Table 2. Eleven of the 16 (69%) studies** showed no evidence for increased global cognitive difficulties in retired athletes compared with control participants or normative data, whereas the other 5 (31%) studies did.30,33,47,50,51 Decq et al33 found that retired rugby players had a higher rate of mild cognitive disorders on a modified version of the Telephone Interview for Cognitive Status than players from a variety of other sports. However, the cognitive results were not associated with reported concussions. Amen et al51 and Willeumier et al50 assessed the same cohort of retired NFL players and reported decreases from normal values in cognitive functioning and proficiency. Amen et al30 also studied a large number of the same participants and demonstrated relationships between position and body mass measured by waist-to-height ratio and cognitive function. Randolph et al47 observed that retired NFL players scored worse than the healthy control sample on the Repeatable Battery for the Assessment of Neuropsychological Status (P = .002). The meta-analysis of 3 studies measuring global cognition using the Mini-Mental State Examination showed no difference between groups for this outcome (mean difference [MD] = 0.09; 95% confidence interval [CI] = −0.35, 0.54; P = .68; Figure 4A). Overall, most studies did not support decreased global cognitive ability or proficiency in retired athletes.
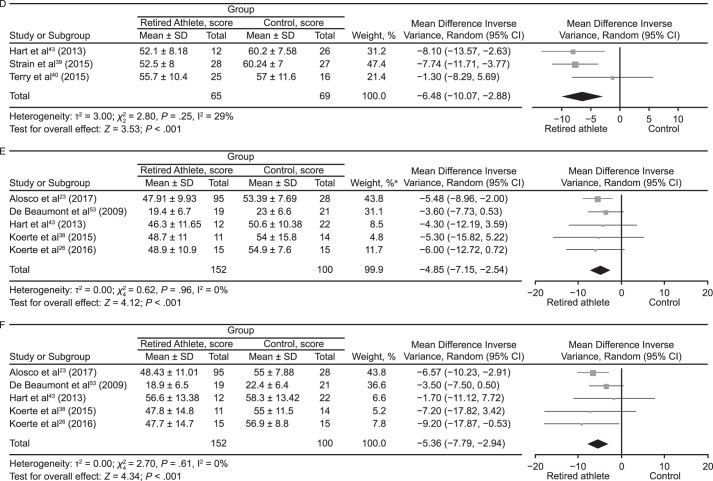
Figure 4.
Forest plots of between-groups comparisons for cognitive outcomes. Retired athletes compared with control participants using the following: A, Mini-Mental State Examination. B, Trail Making Test–A. C, Trail Making Test–B. a The total does not equal 100% because percentages were rounded. Continued on next page.
Figure 4.
Continued from previous page. D, California Verbal Learning Test. E, Rey-Osterreith Complex Figure Test Copy and Immediate Recall. F, Rey-Osterreith Complex Figure Test Delayed Recall. a The total does not equal 100% because percentages were rounded.
Attention
Tests of attention were administered in 17 (37%) of 46 investigations.†† In 3 studies,23,28,31 investigators found that retired athletes had decreased attention scores. Alosco et al23 reported that retired NFL players performed worse than controls on the Trail Making Test (TMT; P = .005). Wright et al28 determined that retired NFL players had attentional and processing-speed deficits on the TMT and the Symbol Digit Modalities Test compared with normative values. Hume et al31 noted that the elite rugby group performed worse on the Online Computerized Neurocognitive Assessment Software–Vital Signs test of complex attention than the noncontact-sport athletes (effect size = −0.67; 95% CI = −1.07, −0.26). Based on the variety of tests administered to investigate attention, the balance of the evidence did not support decreased functioning in retired athletes. The results from 6 studies that assessed attention using the TMT are illustrated in Figures 4B and 4C. Neither the TMT–A (MD = −0.52; 95% CI = −3.40, 2.36; P = .72) nor the TMT–B (MD = −1.41; 95% CI = −4.58, 1.76; P = .38) revealed between-groups differences when the findings were pooled.
Memory
Memory was assessed in 31 studies‡‡ using various tests (Table 2). The authors of 14 (45%) studies§§ reported normal functioning on memory tests. Seventeen (55%) studies‖‖ showed decreases in memory functioning among retired athletes compared with control participants or normative data. In 2 (6%) investigations,39,43 researchers determined that retired NFL players performed worse than control participants on the California Verbal Learning Test. McMillan et al22 identified worse performance on the Rey Auditory Verbal Learning Test by retired rugby players compared with the control group. Wilde et al29 found that word-list recall on the Verbal Selective Reminding Test was worse in boxers than in control participants, whereas Koerte et al34 observed that retired NFL players had decreased performance on a list-learning task. Tremblay et al48 described decreased scores by retired American football athletes on the Rey Auditory Verbal Learning Test and Taylor Complex Figure Test. Using the Spatial Working Memory and Paired Associates Learning subtests to assess memory and reported differences, Pearce et al13 noted that retired rugby players performed more poorly than the control group. Researchers in the remaining 4 studies28,36,37,58 reported deficits in memory tests; however, no control groups were included. The same group of retired NFL players studied by Amen et al51 and Willeumier et al50 had decreases in memory on the MicroCog memory subset. Similar results were shown by Amen et al,30 who studied a large proportion of the same players. In addressing relational memory impairments among retired athletes, Ford et al49 described the multiple-concussion group as worse at recognizing intact pairs as “old” (intact-pair hits) than the age-matched group (P < .05). Overall, the results were mixed. However, preliminary evidence of memory decline exists in retired athletes. Our meta-analysis of 3 studies using the California Verbal Learning Test outcome measure indicated that retired athletes performed worse than control participants (MD = −6.48; 95% CI = −10.07, −2.88; P < .001; Figure 4D). Our meta-analysis of 5 studies that used the Rey-Osterreith Complex Figure Test outcome measure also showed that the control group outperformed retired athletes (Rey-Osterreith Complex Figure Test Copy and Immediate Recall: MD = −4.85; 95% CI = −7.15, −2.54; P < .001; and Rey-Osterreith Complex Figure Test Delayed Recall: MD = −5.36; 95% CI = −7.79, −2.94; P < .001; Figures 4E and 4F, respectively).
Executive Function
Tests of executive function were used in 11 investigations.¶¶ The most commonly used instrument was the Wisconsin Card Sorting Test.16,34,37,54,56 Six (55%) studies13,16,28,31,37,56 demonstrated decreased executive function in retired athletes, whereas the remaining 5 (45%) studies did not.23,34,41,44,54 Three studies16,37,56 showed decreased performance on the Wisconsin Card Sorting Test in retired athletes compared with control participants. Wright et al28 found that retired NFL players displayed deficits in executive ability compared with normative data (Heaton system: 37.5%; Wechsler system: 20.0%). Pearce et al13 reported that retired rugby players performed worse than the control group on the Cambridge Neuropsychological Test Automated Battery–Intra-Extra Dimensional Set Shift subtest. Hume et al31 determined that the retired elite-rugby group performed worse on tests of executive function (effect size = −0.41; 95% CI = −0.80, −0.02) on the Online Computerized Neurocognitive Assessment Software–Vital Signs. Overall, the results were mixed. However, evidence of a decline in executive function in retired athletes exists.
Language
Eight groups16,17,21,23,24,39,43,49 reviewed language tests, including tests of naming, speech production, and verbal fluency. The Boston Naming Test was used in 4 (50%) studies.21,39,43,49 The mean Boston Naming Test T score of the retired athletes was lower than that of the control group in 2 of the 4 studies,21,43 and no differences were found in the other 2 studies.39,49 Esopenko et al16 used the Phonemic Word List Generation (verbal phonemic fluency test) and found no differences between retired contact-sport athletes and control participants. The Controlled Oral Word Association Test was used in 5 investigations,17,23,24,43,49 and no differences were identified between the retired athlete and control groups. Overall, our review showed mixed evidence of language deficits in retired athletes.
Psychomotor Function
Psychomotor function was assessed by 6 groups,13,17,22,31,45,56 with 4 sets of researchers13,17,22,45 reporting decreases in retired athletes compared with control participants. Pearce et al13 used the Cambridge Neuropsychological Test Automated Battery–Visuomotor Reaction Time subtest and found that retired rugby players reacted more slowly to the stimulus than the control group (P < .01). McMillan et al22 observed that retired rugby players had decreased fine motor coordination in the dominant hand on the Grooved Pegboard test, a measure of visual-motor coordination, whereas Gardner et al17 reported worse scores for the nondominant hand (P = .03). Pearce et al45 assessed fine motor control and learned that reaction time (both reaction to stimulus and movement time on the O'Connor Finger Dexterity Test) was better in the healthy control group than in the retired elite and amateur Australian football players (P = .003). The other 2 studies31,56 did not support decreases in psychomotor function in retired athletes compared with control individuals. Evidence of difficulties in psychomotor functioning exists in retired athletes.
Intelligence
Premorbid intelligence was assessed in 12 studies.## A total of 9 studies*** showed no difference in intellectual ability in retired athletes compared with control groups. In 2 investigations,16,34 retired athletes performed worse than control participants on intellectual function tests. Stamm et al37 demonstrated that former NFL players exposed to tackle football before age 12 years performed worse on the Wide Range Achievement Test 4 than former NFL players exposed at age 12 or later. Overall, we did not find evidence of decreased intellectual functioning in retired athletes.
Perception
Alosco et al23 used the Brief Smell Identification Test and reported scores that were lower among former NFL players than among noncontact control athletes. Visuospatial perception was assessed by 2 sets of researchers16,22 who used the Judgment of Line Orientation test. No group differences were detected between retired athletes and control individuals.
Self-Reported Cognitive Functioning
In 14 studies,††† a variety of subjective self-reported cognitive functioning tests were used to compare retired athletes and control groups or normative data (Table 2). Eleven (79%) studies‡‡‡ identified increased subjective reports of cognitive difficulties experienced by former athletes. Authors of the remaining 3 studies15,17,20 described no increase in self-reported concerns among retired athletes. We found evidence of increased sport-related cognitive concerns among retired athletes.
Rate of Mild Cognitive Impairment in Retired Athletes
Investigators in 3 studies32,47,55 implemented self-reports rather than formal diagnoses to examine mild cognitive impairment (MCI) in retired athletes. Guskiewicz et al55 determined that 35% of the retired NFL players self-reported cognitive difficulties, which were deemed consistent with MCI. Randolph et al47 noted indications of possible cognitive impairment in retired NFL players: a subsample of the players was compared with a clinical sample of patients with MCI, revealing similar profiles of impairments. Conversely, Vann Jones et al32 stated that the prevalence of possible MCI or dementia in former professional soccer players was not different from a large control sample. No association was demonstrated between low- and high-risk playing positions or length of playing career and a positive MCI screening result. Age was the only risk factor across both groups.
Pooled Summaries
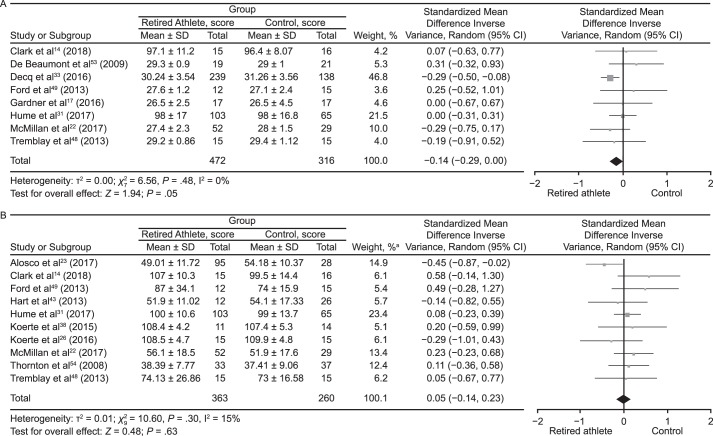
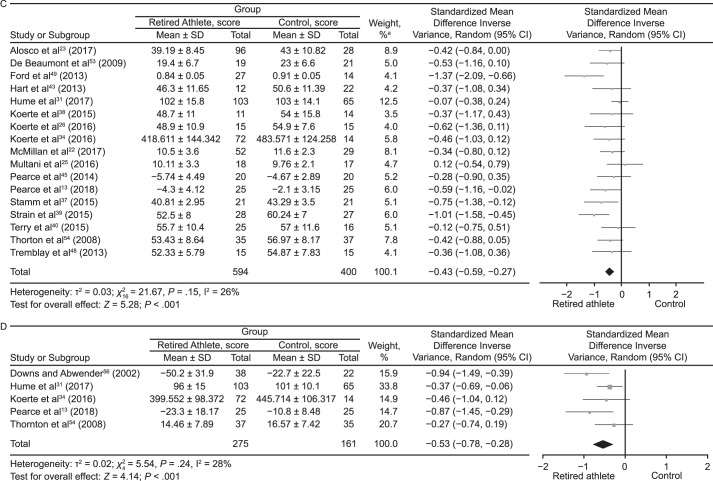
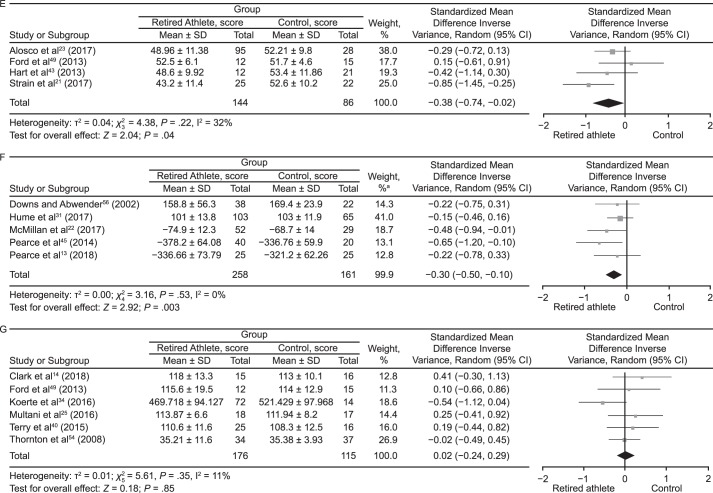
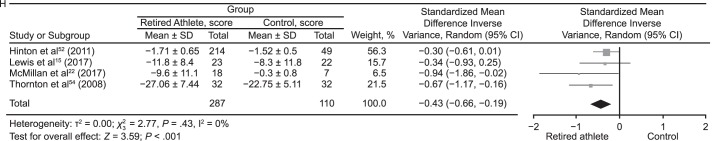
Meta-analyses were conducted where possible for outcome measures using different scales and tools to assess the cognitive domains (Figure 5). The data were pooled using the standardized MD (SMD) and a random-effects model according to the Cochrane guidelines.62 Global cognitive ability was almost different between groups (SMD = −0.14; 95% CI = −0.29, 0.00; P = .05; Figure 5A). The attention domain demonstrated no between-groups difference (SMD = 0.05; 95% CI = −0.14, 0.23; P = .63; Figure 5B). Retired players performed worse than control participants on tests of memory (SMD = −0.43; 95% CI = −0.59, −0.27; P < .001; Figure 5C). Assessment of executive function also revealed that control individuals outperformed retired athletes (SMD = −0.53; 95% CI = −0.78, −0.28; P < .001; Figure 5D). Tests of language (SMD = −0.38; 95% CI = −0.74, −0.02; P = .04; Figure 5E) and psychomotor function (SMD = −0.30; 95% CI = −0.50, −0.10; P = .003; Figure 5F) also favored control groups. The intelligence domain showed no between-groups difference (SMD = 0.01; 95% CI = −0.24, 0.26; P = .91; Figure 5G). Pooling of the self-report test results revealed that retired athletes reported more cognitive difficulties than control participants (SMD = −0.43; 95% CI = −0.66, −0.19; P < .001; Figure 5H).
Figure 5.
Forest plots of between-groups comparisons for cognitive domains. Retired athletes compared with control participants in the following areas: A, Global cognitive ability. B, Attention. a The total does not equal 100% because percentages were rounded. Continued on next page.
Figure 5.
Continued from previous page. C, Memory. D, Executive function. a The total does not equal 100% because percentages were rounded. Continued on next page.
Figure 5.
Continued from previous page. E, Language. F, Psychomotor function. G, Intelligence. a The total does not equal 100% because percentages were rounded. Continued on next page.
Figure 5.
Continued from previous page. H, Self-reported cognitive functioning.
Summary of Main Results
Our review focused on a qualitative synthesis of the included studies. The authors of 14 studies§§§ used a subjective, self-reported cognitive functioning test in mixed sporting populations, with 11 groups‖‖‖ reporting increased subjective cognitive difficulties experienced by former athletes compared with control individuals or normative data. A synthesis of studies demonstrated more evidence for cognitive deficits in the areas of memory, executive function, psychomotor function, and self-reported cognitive functioning.
The balance of evidence to date did not support an association between cognitive deficits in retirement and concussion history and exposure to head impacts. Most studies (n = 33) did not identify any association between concussion history or exposure to head impacts and cognitive deficits. However, investigators in 13 (28%) of 46 studies¶¶¶ described a frequency-response relationship, with greater cognitive impairments (subjective or objective) in athletes with greater levels of exposure to head impacts or concussions. The cross-sectional nature of the studies included in this review and an insufficient number of longitudinal studies limited our ability to make causal inferences about the relationship between concussion and long-term cognitive outcomes.
DISCUSSION
We appraised the literature regarding SRC and cognitive health outcomes in retired athletes. Our review was unique because we focused on clinical cognitive outcomes in living retired athletes. Findings suggested that certain areas of cognition may be affected by an SRC history. However, bias appeared to exist toward assessments of certain areas of cognition, such as global cognitive ability, attention, and memory, with neglect of other aspects of cognition, including language, psychomotor function, and perception. Furthermore, epidemiologic studies were confounded by individual differences in susceptibility to age-related neurocognitive decline or dementia-related pathologic conditions. Therefore, lifetime exposure to concussion is more than likely one of a myriad of environmental and predetermined risk factors for diminished cognitive reserve and early expression of neurocognitive decline. The researchers did not control for premorbid intellectual function and factors related to cognitive reserve. An important factor was the substantial overlap between a normal age-related neurocognitive downturn and the manifestation of SRC in later life.63
From this review, evidence of increased self-reported cognitive difficulties emerged. A substantial number of studies identified self-reported cognitive concerns among retired athletes (11 of 14 [79%] studies that tested for self-reported symptoms). However, conclusions drawn from self-reported data should be cautious, particularly in the absence of clear associations between self-reported symptoms and previous head-impact exposure or reported number of concussions and the potential recall bias that continues to be a limitation of all retrospective studies in this area. Furthermore, external factors, such as the media, may influence former players' reporting.64,65 Among the investigations of objective and subjective cognition in retired athletes,### a lack of clear agreement existed between the measures. Most authors (n = 6 studies) did not support16,17,20,22,25,54 the subjective reports with respect to neuropsychological test results. The clinical importance of the findings should be carefully considered in the overall context of the individual's performance on neuropsychological and cognitive testing and symptom self-report. The emerging disparity between subjective and objective tests may indicate that more sensitive cognitive test measures are required to recognize changes, which may then result in self-reported difficulties and translate to anomalies on cognitive tests.
Our meta-analysis of the cognitive domains of memory, executive function, language, psychomotor function, and self-reported cognitive functioning revealed that retired athletes performed worse than control participants. However, the magnitudes of the effect sizes were small; therefore, whether the effect sizes were clinically meaningful is unclear. An additional methodologic shortcoming of the reviewed studies was a paucity of studies that used a prospective, longitudinal design and biased recruitment. The study designs also raised important concerns along with retrospective recall bias and failure to control for confounding variables: namely, failure to control for a history of non–sport-related concussion. A further factor was the lack of suitable control groups. Important aspects associated with professional team sports, such as high levels of physical fitness, high income, and potential drug use (eg, opioid analgesics), were not considered. The late effect upper limb orthopaedic injuries may have had on psychomotor tests was also overlooked. A total of 13 studies**** did not include a control group, which greatly limited the conclusions that could be drawn because of a lack of context with respect to population normative data. Among the investigations that included a control group (n = 33), only a small number (n = 8) included an appropriate noncontact-athlete control cohort15,26,31,34,35,38,56,57; most researchers (n = 22 studies)†††† did not accurately match control individuals with retired noncontact athletes. In addition, a large proportion of investigators (n = 19) did not provide a definition of concussion,‡‡‡‡ which reduced the validity of player recall.66 Others67,68 noted both underreporting (eg, lack of understanding about concussion) and exaggeration of head impacts. The inability to accurately quantify participants' exposure to subconcussive blows was a further difficulty.
Just over 50% of the studies (n = 24)§§§§ controlled for a history of neurologic or psychiatric conditions. A history of head-impact exposure or concussion is one of myriad factors that may lead to neurocognitive decline in retired athletes; therefore, inclusion and exclusion criteria must be robust, and these factors must be appropriately controlled in research. Individuals with premorbid psychiatric or other health problems or life stressors are more likely to experience postconcussion syndrome.69,70 Similarly, only a minority of authors (n = 14 studies)‖‖‖‖ reported past or present alcohol or drug use. Downs and Abwender56 described screening for alcohol abuse but did not provide these findings in their “Results” section. Some investigators17,22,33,35 noted higher alcohol consumption among retired athletes than among control individuals, which could have negatively influenced cognitive performance on certain cognitive tests. Substance abuse has been associated with sustained deficits in executive functioning, especially inhibition.71 Long-term, high-dose anabolic steroid exposure may cause cognitive deficits, notably in visuospatial memory.72
Authors of epidemiologic and intervention studies73,74 have suggested that overall physical activity preserves or improves cognitive function during aging; therefore, the failure to control for current activity levels among retired players versus control groups in most research presented a potential bias. However, only a minority of studies26,35,38,42,48,52 controlled for the modifiable risk factors, such as diet and physical activity, that accounted for physical activity engagement and exercise frequency. Similarly, only a small proportion of investigators22,23,28,30,50–52 examined factors such as body mass index, weight-to-height ratio, or cardiovascular health. These factors may affect cognitive functioning: a meta-analysis75 indicated that being categorized in the overweight or obese range in midlife was a risk factor for dementia later in life. A large proportion of the studies (n = 20) included retired NFL players. Given the propensity toward being overweight in this population,76 the risk of cognitive impairment may be elevated.77 Hinton et al52 found that dietary fat intake was more associated with self-reported cognitive difficulties than was exposure to football alone among former collegiate football players. Wright et al28 reported that body mass index was associated with cognitive-reserve outcomes in retired NFL players.
Aside from the proposed negative relationship between concussion and cognitive function, the potential causes of cognitive concerns in retired athletes are diverse. Factors including genetics,78 diet and nutrition,79 exercise,80 obesity,81 chronic pain and life stress,82 childhood adversity,83 personality factors,84 family history of neurologic conditions,85 steroid use,86 drug and alcohol use,87 depression and anxiety,88 general medical history (eg, hypertension, diabetes, heart disease),89 and neurodegenerative diseases (eg, Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis)90–92 have been implicated in exacerbated cognitive decline with aging. Most authors did not control for these variables. Parental socioeconomic status, race, and medical history independently predicted baseline memory scores among collegiate athletes, whereas concussion history and years exposed to sport did not.93 None of the investigators accounted for socioeconomic status during childhood, which could affect cognitive reserve later in life and may account for differences among athlete groups with a history of concussion or head-impact exposure versus control participants or normative data. The importance of premorbid information; intellectual level; and learning disabilities, such as attention-deficit/hyperactivity disorder, which is known to exist at a high level among athletes, should also be considered. Athletes with a history of multiple concussions and a premorbid learning disability are vulnerable to neurocognitive impairment.94
Individual differences in baseline intelligence and education status have not been addressed in most studies. Stamm et al37 reported that 14% of the group younger than 12 years and 0% of the group older than 12 years had a learning disability and displayed differences in premorbid intellectual functioning (ie, Wechsler Test of Adult Reading scores), with the younger group representing those who were exposed to tackle football before age 12 and the older group representing those who were not exposed until a later age. This casts doubt on whether the group differences reflected premorbid impairment as opposed to the effects of concussion. Many authors included in control groups participants who were exposed to considerable concussion risk, albeit a lower risk of head impacts. The control groups of Alosco et al23 and Murelius and Haglund57 included participants with a history of playing soccer, and Alosco et al23 included 1 participant with a history of amateur wrestling; McMillan et al22 included a control group in which 34% of participants had a history of concussion and rugby participation. The inclusion of current and retired athletes in some studies, without distinguishing between them, may have skewed the results,46,54 as the results may have reflected the effects of recent concussions or current head-impact exposure.
The assessment of cognition should ideally capture all of its domains. The tests used varied greatly among studies, and only a small proportion of the authors used a comprehensive battery that explored all aspects of cognitive functioning. Most studies focused on specific domains, such as tests of attention, memory, and executive function. Furthermore, many of the assessments used were designed to detect gross cognitive impairments and may have failed to uncover subtle changes in cognitive function. Given the media interest in concussion and public perceptions, studies with negative findings are potentially less likely to be published. We did not assess publication bias in this review; it was not possible to perform a funnel plot due to the heterogeneity of the outcome measures used. Large-scale, prospective longitudinal studies with a high level of control of confounding factors are required to confirm the effects of aging with a history of concussion on cognitive functioning in retired athletes.
STUDY LIMITATIONS AND FUTURE WORK
The self-selected convenience samples limited the conclusions that could be drawn. This concern was exacerbated in some investigations because the inclusion criteria were limited to retired NFL players with a minimum 6-month history of self-reported complaints of cognitive, behavioral, and mood symptoms23,34,37 and players presenting to memory clinics with cognitive or behavioral symptoms.27 Self-selected participants may not have represented the retired athlete population. Given the retrospective nature of the studies, the possible long-term sequelae of concussion were influenced by methodologic biases, making it difficult to draw conclusions. A large proportion of the studies (n = 43) were based on retired male athletes; only 3 groups of authors35,54,56 recruited retired female athletes. Alumni of the NFL accounted for a large number of participants in many of the studies (n = 20). The career paths and levels of head-impact exposure make it impossible to infer results beyond this unique cohort. Aside from the level of head-impact exposure, a host of factors separate NFL players from the population at large, including income, education level, and various lifestyle aspects. Large gaps exist in our knowledge of the effect of concussion on female athletes (because of potential sex influences on concussion recovery95,96) and on people who participate at other sporting levels.
A further limitation was that concussions were often not well documented in the past. Therefore, all researchers relied on the athletes' self-reported history of concussion, making this information subject to retrospective recall bias. Only Wright et al28 corroborated patients' self-reported history with verifiable reports. Relying solely on players' self-reported history of concussion and retired athletes' responses to a survey-based questionnaire regarding subjective memory difficulties is potentially unreliable. This is compounded by the fact that SRCs that occurred in the past may have been overlooked by clinicians unless loss of consciousness occurred.97 A history of non–sport-related concussion, which accounts for most concussions,98,99 needs to be considered in future study designs.
CONCLUSIONS
A total of 46 studies evaluated 9 aspects of cognitive functioning. Relative to the control groups or normative data, 5 areas showed declines: memory, executive function, language, psychomotor function, and self-reported cognitive functioning. The 4 other areas—global cognitive ability, attention, intelligence, and perception—were, on balance, neutral. The preliminary evidence of a dose-response association between cognitive health outcomes and past concussion exposure warrants further examination to determine the complex interaction between previous head-impact exposure and factors that may influence cognitive health in the aging retired athlete. As detailed throughout this review, confounding variables, case ascertainment, recall bias on behalf of the participants, and publication bias in the SRC field at large may have inflated these findings.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Mockler (medical librarian), Trinity College Dublin.
SUPPLEMENTAL MATERIAL
Supplemental Table. Detailed quality appraisal for the 46 studies.
Found at DOI: http://dx.doi.org/10.4085/1062-6050-297-18.S1
Appendix 1.
Search Strategy Continued on Next Page
| Database |
Search Strategy |
Results |
| EMBASE | 1. “cognitive defect”/exp | 1792 |
| 2. “depression”/exp | ||
| 3. ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) NEAR/3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder*)):ti,ab | ||
| 4. (Neuropsychological NEAR/3 test*):ti,ab | ||
| 5. Depressi*:ti,ab | ||
| 6. 1 OR 2 OR 3 OR 4 OR 5 | ||
| 7. “athlete”/exp | ||
| 8. ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact-sport”) NEAR/5 (retire* OR former)):ti,ab | ||
| 9. 7 OR 8 | ||
| 10. 6 AND 9 | ||
| PsychINFO | 1. DE “Cognitive Impairment” OR DE “Depression (Emotion)” OR DE “Executive Function” | 783 |
| 2. TI ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) N3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder*)) OR AB ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) N3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder)) | ||
| 3. TI (Neuropsychological N3 test*) OR AB (Neuropsychological N3 test*) | ||
| 4. TI Depressi* OR AB Depressi* | ||
| 5. S1 OR S2 OR S3 OR S4 | ||
| 6. DE “Athletes” | ||
| 7. TI ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact- sport”) N5 (retire* OR former)) OR AB ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact-sport”) N5 (retire* OR former)) | ||
| 8. S6 OR S7 | ||
| 9. S5 AND S8 | ||
| MEDLINE/PubMed | 1. (MH “Cognition Disorders+”) OR (MH “Neurocognitive Disorders+”) OR (MH “Mild Cognitive Impairment”) OR (MH “Depression”) OR (MH “Depressive Disorder+”) | 459 |
| 2. TI ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) N3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder*)) OR AB ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) N3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder)) | ||
| 3. TI (Neuropsychological N3 test*) OR AB (Neuropsychological N3 test*) | ||
| 4. TI Depressi* OR AB Depressi* | ||
| 5. S1 OR S2 OR S3 OR S4 | ||
| 6. (MH “Athletes”) | ||
| 7. TI ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact-sport”) N5 (retire* OR former)) OR AB ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact-sport”) N5 (retire* OR former)) | ||
| 8. S6 OR S7 | ||
| 9. S5 AND S8 | ||
| CINAHL | 1. (MH “Cognition Disorders+”) OR (MH “Depression+”) | 239 |
| 2. TI ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) N3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder*)) OR AB ((Cogniti* OR neuropsychological OR neurocognitive OR executive OR brain) N3 (impairment OR defect* OR function* OR dysfunction* OR process* OR symptom* OR factor* OR Deficit* OR disorder)) | ||
| 3. TI (Neuropsychological N3 test*) OR AB (Neuropsychological N3 test*) | ||
| 4. TI Depressi* OR AB Depressi* | ||
| 5. S1 OR S2 OR S3 OR S4 | ||
| 6. (MH “Athletes”) | ||
| 7. TI ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact- sport”) N5 (retire* OR former)) OR AB ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact-sport”) N5 (retire* OR former)) | ||
| 8. S6 OR S7 | ||
| 9. S5 AND S8 | ||
| Cochrane Central Register of Controlled Trials | 1. [mh “Cognition Disorders”] OR [mh “Neurocognitive Disorders”] OR [mh “Mild Cognitive Impairment”] OR [mh “Depression”] OR [mh “Depressive Disorder”] | 14 |
| 2. ((Cogniti* or neuropsychological or neurocognitive or executive or brain) near/3 (impairment or defect* or function* or dysfunction* or process* or symptom* or factor* or Deficit* or disorder*)):ti,ab,kw | ||
| 3. (Neuropsychological NEAR/3 test*):ti,ab,kw | ||
| 4. Depressi*:ti,ab,kw | ||
| 5. {OR 1–4} | ||
| 6. [mh “Athletes”] | ||
| 7. ((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportmen OR rugby OR player* OR box* OR “contact- sport”) NEAR/5 (retire* OR former)):ti,ab,kw | ||
| 8. {OR 6, 7} | ||
| 9. {AND 5, 8} | ||
| Web of Science | 1. TS=(( Cogniti* or neuropsychological or neurocognitive or executive or brain) NEAR/3 (impairment or defect* or function* or dysfunction* or process* or symptom* or factor* or Deficit* or disorder*)) OR TS=((Neuropsychological NEAR/3 test*)) OR TS=( Depressi*) | 179 |
| 2. TS=((Athlete* OR “sports person” OR sportswomen OR sportswoman OR sportsman OR sportsmen OR rugby OR player* OR box* OR “contact-sport”) NEAR/5 (retire* OR former)) | ||
| 3. 2 AND 1 |
Appendix 2.
List of Abbreviations and Acronyms
| Abbreviation or Acronym |
Name |
| ACS–TOPF | Advanced Clinical Solutions Test of Premorbid Functioninga |
| AD8 | Eight-Item Informant Interview to Differentiate Aging and Dementiab |
| AMNART | American version of the National Adult Reading Test |
| BGT | Bender Visual-Motor Gestalt Testa |
| BNT | Boston Naming Testa |
| BRI | Behavioral Regulation Indexc |
| BRIEF–A | Behavior Rating Inventory of Executive Function–Adult Versionc |
| B–SIT | Brief Smell Identification Test |
| BTACT | Brief Test of Adult Cognition by Telephone |
| BVMT–R | Brief Visuospatial Memory Test–Revisedc |
| CANTAB–IED | Cambridge Neuropsychological Test Automated Battery–Intra-Extra Dimensional Set Shiftd |
| CANTAB–PAL | Cambridge Neuropsychological Test Automated Battery–Paired Associates Learningd |
| CANTAB–RTI | Cambridge Neuropsychological Test Automated Battery–Reaction Timed |
| CANTAB–SWM | Cambridge Neuropsychological Test Automated Battery–Spatial Working Memoryd |
| CANTAB–TOTSPT | Cambridge Neuropsychological Test Automated Battery–One Touch Spatial Planning Task |
| CANTAB–VRT | Cambridge Neuropsychological Test Automated Battery–Visuomotor Reaction Time |
| CCFT | Cattell Culture Fair Intelligence Test |
| CDS | Cognitive Difficulties Scale |
| CFQ | Cognitive Failures Questionnaire |
| CNS | Central nervous system |
| CNS Vital Signs | Online computerized neurocognitive assessment softwaree |
| COWAT | Controlled Oral Word Association Test |
| CPT | Conners Continuous Performance Testf |
| CPT–II | Conners Continuous Performance Test, second editionf |
| CVLT–II | California Verbal Learning Test, second editiona |
| D-KEFS | Delis-Kaplan Executive Function System |
| DQ | Dysexecutive Questionnaire |
| DST | Digit Symbol Test |
| DWR | Delayed Word Recall |
| EFT | Eriksen Flanker Task |
| ETS Kit–V2 and –V3 | Educational Testing Service Kit V2 and V3 vocabulary items |
| FAS | Verbal phonemic fluency test |
| F–TICS–m | French version of the modified Telephone Interview for Cognitive Status |
| FTT | Finger tapping test |
| GPT | Grooved Pegboard Testg |
| ImPACT | Immediate Post-Concussion Assessment and Cognitive Testingh |
| JLO | Judgment of Line Orientationc |
| LF | Letter Fluency task |
| MCIS | Mild Cognitive Impairment Screen |
| MCS | Mental Component Summary |
| MicroCog | MicroCog: Assessment of Cognitive Functioninga |
| MMSE | Mini-Mental State Examinationc |
| MOCA | Montreal Cognitive Assessmenti |
| NAB | Neuropsychological Assessment Batteryc |
| NAB–LL | Neuropsychological Assessment Battery List Learning test |
| Neuro–QoL | Quality of Life in Neurological Disorders |
| PASAT | Paced Auditory Serial Addition Test |
| PCSC | Postconcussion Syndrome Checklist |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| RAVLT | Rey Auditory Verbal Learning Test |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Statusa |
| RCFT | Rey Complex Figure Test |
| ROCFT | Rey-Osterreith Complex Figure Test |
| RPQ | Rivermead Post Concussion Symptoms Questionnaire |
| RVDLT | Rey Visual Design Learning Test |
| SART | Sustained Attention to Response Task |
| SDMT | Symbol Digit Modalities Testj |
| SF–36 (including MCS) | 36-Item Short Form Health Surveyk |
| SOPT | Self-Ordered Pointing Task |
| SORT | Semantic Object Retrieval Test |
| SRTT | Serial Reaction Time Test |
| TCFT | Taylor Complex Figure test |
| TMT | Trail Making Test |
| TMT–A | Trail Making Test part A |
| TMT–B | Trail Making Test part B |
| TOMM | Test of Memory Malingeringa |
| TYM | Test Your Memory |
| VRST | Verbal Selective Reminding Test |
| WAIS | Wechsler Adult Intelligence Scalea |
| WAIS–III | Wechsler Adult Intelligence Scale, third editiona |
| WAIS–IV | Wechsler Adult Intelligence Scale, fourth editiona |
| WAIS–R | Wechsler Adult Intelligence Scale, revised versiona |
| WASI | Wechsler Abbreviated Scale of Intelligencea |
| WCST | Wisconsin Card Sorting Testc |
| WMS | Wechsler Memory Scalea |
| WMS–III | Wechsler Memory Scale, third editiona |
| WMS–IV | Wechsler Memory Scale, fourth editiona |
| WRAT–4 | Wide Range Achievement Test, fourth editionj |
| WTAR | Wechsler Test of Adult Readinga |
Pearson Education, Inc, London, United Kingdom.
Washington University, St Louis, MO.
PAR, Inc, Lutz, FL.
Cambridge Cognition Ltd, Bottisham, Cambridge, United Kingdom.
CNS Vital Signs, Morrisville, NC.
MHS Inc, North Tonawanda, NY.
Lafayette Instrument, Lafayette, IN.
ImPACT Implications, Inc, Pittsburgh, PA.
Ziad Nasreddine, MoCA Test Inc, Greenfield Park, Québec, Canada.
WPS, Torrance, CA.
QualityMetric Incorporated, Lincoln, RI.
Footnotes
REFERENCES
- 1.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360–370. doi: 10.1001/jama.2017.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein TD, Alvarez VE, McKee AC. Concussion in chronic traumatic encephalopathy. Curr Pain Headache Rep. 2015;19(10):47. doi: 10.1007/s11916-015-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce N, Gallo V, McElvenny D. Head trauma in sport and neurodegenerative disease: an issue whose time has come? Neurobiol Aging. 2015;36(3):1383–1389. doi: 10.1016/j.neurobiolaging.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Manley G, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med. 2017;51(12):969–977. doi: 10.1136/bjsports-2017-097791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon G. Chronic traumatic encephalopathy in sports: a historical and narrative review. Dev Neuropsychol. 2018;43(4):279–311. doi: 10.1080/87565641.2018.1447575. [DOI] [PubMed] [Google Scholar]
- 6.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11(12):1103–1112. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- 7.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. 2012;69(10):1245–1251. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport: the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 9.Dougan BK, Horswill MS, Geffen GM. Athletes' age, sex, and years of education moderate the acute neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2014;20(1):64–80. doi: 10.1017/S1355617712001464. [DOI] [PubMed] [Google Scholar]
- 10.Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, Langhinrichsen-Rohling J. A meta-analysis of neuropsychological outcome after mild traumatic brain injury: re-analyses and reconsiderations of Binder et al (1997), Frencham et al (2005), and Pertab et al (2009) Clin Neuropsychol. 2011;25(4):608–623. doi: 10.1080/13854046.2011.565076. [DOI] [PubMed] [Google Scholar]
- 11.Broglio SP, Puetz TW. The effect of sport concussion on neurocognitive function, self-report symptoms and postural control: a meta-analysis. Sports Med. 2008;38(1):53–67. doi: 10.2165/00007256-200838010-00005. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce AJ, Rist B, Fraser CL, Cohen A, Maller JJ. Neurophysiological and cognitive impairment following repeated sports concussion injuries in retired professional rugby league players. Brain Inj. 2018;32(4):498–505. doi: 10.1080/02699052.2018.1430376. [DOI] [PubMed] [Google Scholar]
- 14.Clark MD, Varangis EML, Champagne AA, et al. Effects of career duration, concussion history, and playing position on white matter microstructure and functional neural recruitment in former college and professional football athletes. Radiology. 2018;286(3):967–977. doi: 10.1148/radiol.2017170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis GN, Hume PA, Stavric V, Brown SR, Taylor D. New Zealand rugby health study: motor cortex excitability in retired elite and community level rugby players. N Z Med J. 2017;130(1448):34–44. [PubMed] [Google Scholar]
- 16.Esopenko C, Chow TW, Tartaglia MC, et al. Cognitive and psychosocial function in retired professional hockey players. J Neurol Neurosurg Psychiatry. 2017;88(6):512–519. doi: 10.1136/jnnp-2016-315260. [DOI] [PubMed] [Google Scholar]
- 17.Gardner AJ, Iverson GL, Wojtowicz M, et al. MR spectroscopy findings in retired professional rugby league players. Int J Sports Med. 2017;38(3):241–252. doi: 10.1055/s-0042-120843. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande SK, Hasegawa RB, Rabinowitz AR, et al. Association of playing high school football with cognition and mental health later in life. JAMA Neurol. 2017;74(8):909–918. doi: 10.1001/jamaneurol.2017.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn AW, Zuckerman SL, Solomon GS, Casson IR, Viano DC. Interrelationships among neuroimaging biomarkers, neuropsychological test data, and symptom reporting in a cohort of retired National Football League players. Sports Health. 2017;9(1):30–40. doi: 10.1177/1941738116674006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montenigro PH, Alosco ML, Martin BM, et al. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma. 2017;34(2):328–340. doi: 10.1089/neu.2016.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strain JF, Didehbani N, Spence J, et al. White matter changes and confrontation naming in retired aging National Football League athletes. J Neurotrauma. 2017;34(2):372–379. doi: 10.1089/neu.2016.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88(6):505–511. doi: 10.1136/jnnp-2016-314279. [DOI] [PubMed] [Google Scholar]
- 23.Alosco ML, Jarnagin J, Tripodis Y, et al. Olfactory function and associated clinical correlates in former National Football League players. J Neurotrauma. 2017;34(4):772–780. doi: 10.1089/neu.2016.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon GS, Kuhn AW, Zuckerman SL, Casson IR, Viano DC, Lovell MR. Participation in pre-high school football and neurological, neuroradiological, and neuropsychological findings in later life: a study of 45 retired National Football League players. Am J Sports Med. 2016;44(5):1106–1115. doi: 10.1177/0363546515626164. [DOI] [PubMed] [Google Scholar]
- 25.Multani N, Goswami R, Khodadadi M, et al. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol. 2016;263(7):1332–1341. doi: 10.1007/s00415-016-8141-0. [DOI] [PubMed] [Google Scholar]
- 26.Koerte IK, Mayinger M, Muehlmann M, et al. Cortical thinning in former professional soccer players. Brain Imaging Behav. 2016;10(3):792–798. doi: 10.1007/s11682-015-9442-0. [DOI] [PubMed] [Google Scholar]
- 27.Gardner RC, Hess CP, Brus-Ramer M, et al. Cavum septum pellucidum in retired American pro-football players. J Neurotrauma. 2016;33(1):157–161. doi: 10.1089/neu.2014.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright MJ, Woo E, Birath JB, et al. An index predictive of cognitive outcome in retired professional American football players with a history of sports concussion. J Clin Exp Neuropsychol. 2016;38(5):561–571. doi: 10.1080/13803395.2016.1139057. [DOI] [PubMed] [Google Scholar]
- 29.Wilde EA, Hunter JV, Li X, et al. Chronic effects of boxing: diffusion tensor imaging and cognitive findings. J Neurotrauma. 2016;33(7):672–680. doi: 10.1089/neu.2015.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amen DG, Willeumier K, Omalu B, Newberg A, Raghavendra C, Raji CA. Perfusion neuroimaging abnormalities alone distinguish National Football League players from a healthy population. J Alzheimers Dis. 2016;53(1):237–241. doi: 10.3233/JAD-160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hume PA, Theadom A, Lewis GN, et al. A comparison of cognitive function in former rugby union players compared with former noncontact-sport players and the impact of concussion history. Sports Med. 2017;47(6):1209–1220. doi: 10.1007/s40279-016-0608-8. [DOI] [PubMed] [Google Scholar]
- 32.Vann Jones SA, Breakey RW, Evans PJ. Heading in football, long-term cognitive decline and dementia: evidence from screening retired professional footballers. Br J Sports Med. 2014;48(2):159–161. doi: 10.1136/bjsports-2013-092758. [DOI] [PubMed] [Google Scholar]
- 33.Decq P, Gault N, Blandeau M, et al. Long-term consequences of recurrent sports concussion. Acta Neurochir (Wien) 2016;158(2):289–300. doi: 10.1007/s00701-015-2681-4. [DOI] [PubMed] [Google Scholar]
- 34.Koerte IK, Hufschmidt J, Muehlmann M, et al. Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma. 2016;33(4):346–353. doi: 10.1089/neu.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meehan WP, III, Taylor AM, Berkner P, et al. Division III collision sports are not associated with neurobehavioral quality of life. J Neurotrauma. 2016;33(2):254–259. doi: 10.1089/neu.2015.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coughlin JM, Wang Y, Munro CA, et al. Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol Dis. 2015;74:58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamm JM, Bourlas AP, Baugh CM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015;84(11):1114–1120. doi: 10.1212/WNL.0000000000001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koerte IK, Lin AP, Muehlmann M, et al. Altered neurochemistry in former professional soccer players without a history of concussion. J Neurotrauma. 2015;32(17):1287–1293. doi: 10.1089/neu.2014.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strain JF, Womack KB, Didehbani N, et al. Imaging correlates of memory and concussion history in retired National Football League athletes. JAMA Neurol. 2015;72(7):773–780. doi: 10.1001/jamaneurol.2015.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terry DP, Adams TE, Ferrara MS, Miller LS. FMRI hypoactivation during verbal learning and memory in former high school football players with multiple concussions. Arch Clin Neuropsychol. 2015;30(4):341–355. doi: 10.1093/arclin/acv020. [DOI] [PubMed] [Google Scholar]
- 41.Casson IR, Viano DC, Haacke EM, Kou Z, LeStrange DG. Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology, and neurology examinations of 45 retired players. Sports Health. 2014;6(5):384–395. doi: 10.1177/1941738114540270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tremblay S, Henry LC, Bedetti C, et al. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137(pt 11):2997–3011. doi: 10.1093/brain/awu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart J, Jr, Kraut MA, Womack KB, et al. Neuroimaging of cognitive dysfunction and depression in aging retired NFL players: a cross-sectional study. JAMA Neurol. 2013;70(3):326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampshire A, MacDonald A, Owen AM. Hypoconnectivity and hyperfrontality in retired American football players. Sci Rep. 2013;3:2972. doi: 10.1038/srep02972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce AJ, Hoy K, Rogers MA, et al. The long-term effects of sports concussion on retired Australian football players: a study using transcranial magnetic stimulation. J Neurotrauma. 2014;31(13):1139–1145. doi: 10.1089/neu.2013.3219. [DOI] [PubMed] [Google Scholar]
- 46.Seichepine DR, Stamm JM, Daneshvar DH, et al. Profile of self-reported problems with executive functioning in college and professional football players. J Neurotrauma. 2013;30(14):1299–1304. doi: 10.1089/neu.2012.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randolph C, Karantzoulis S, Guskiewicz K. Prevalence and characterization of mild cognitive impairment in retired National Football League players. J Int Neuropsychol Soc. 2013;19(8):873–880. doi: 10.1017/S1355617713000805. [DOI] [PubMed] [Google Scholar]
- 48.Tremblay S, De Beaumont L, Henry LC, et al. Sports concussions and aging: a neuroimaging investigation. Cereb Cortex. 2013;23(5):1159–1166. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- 49.Ford JH, Giovanello KS, Guskiewicz KM. Episodic memory in former professional football players with a history of concussion: an event-related functional neuroimaging study. J Neurotrauma. 2013;30(20):1683–1701. doi: 10.1089/neu.2012.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willeumier K, Taylor DV, Amen DG. Elevated body mass in National Football League players linked to cognitive impairment and decreased prefrontal cortex and temporal pole activity. Transl Psychiatry. 2012;2:e68. doi: 10.1038/tp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amen DG, Newberg A, Thatcher R, et al. Impact of playing American professional football on long-term brain function. J Neuropsychiatry Clin Neurosci. 2011;23(1):98–106. doi: 10.1176/jnp.23.1.jnp98. [DOI] [PubMed] [Google Scholar]
- 52.Hinton PS, Johnstone B, Blaine E, Bodling A. Effects of current exercise and diet on late-life cognitive health of former college football players. Phys Sportsmed. 2011;39(3):11–22. doi: 10.3810/psm.2011.09.1916. [DOI] [PubMed] [Google Scholar]
- 53.De Beaumont L, Théoret H, Mongeon D, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132(pt 3):695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 54.Thornton AE, Cox DN, Whitfield K, Fouladi RT. Cumulative concussion exposure in rugby players: neurocognitive and symptomatic outcomes. J Clin Exp Neuropsychol. 2008;30(4):398–409. doi: 10.1080/13803390701443662. [DOI] [PubMed] [Google Scholar]
- 55.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 56.Downs DS, Abwender D. Neuropsychological impairment in soccer athletes. J Sports Med Phys Fitness. 2002;42(1):103–107. [PubMed] [Google Scholar]
- 57.Murelius O, Haglund Y. Does Swedish amateur boxing lead to chronic brain damage? 4. A retrospective neuropsychological study. Acta Neurol Scand. 1991;83(1):9–13. doi: 10.1111/j.1600-0404.1991.tb03952.x. [DOI] [PubMed] [Google Scholar]
- 58.Casson IR, Siegel O, Sham R, Campbell EA, Tarlau M, DiDomenico A. Brain damage in modern boxers. JAMA. 1984;251(20):2663–2667. [PubMed] [Google Scholar]
- 59.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartling L, Brison RJ, Crumley ET, Klassen TP, Pickett W. A systematic review of interventions to prevent childhood farm injuries. Pediatrics. 2004;114(4):e483–e496. doi: 10.1542/peds.2003-1038-L. [DOI] [PubMed] [Google Scholar]
- 61.Hignett S. Systematic review of patient handling activities starting in lying, sitting, and standing positions. J Adv Nurs. 2003;41(6):545–552. doi: 10.1046/j.1365-2648.2003.02566.x. [DOI] [PubMed] [Google Scholar]
- 62.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration Web site. eds. http://handbook-5-1.cochrane.org/ Updated March 2011. Accessed May 1, 2019.
- 63.Asken BM, Sullan MJ, Snyder AR, et al. Factors influencing clinical correlates of chronic traumatic encephalopathy (CTE): a review. Neuropsychol Rev. 2016;26(4):340–363. doi: 10.1007/s11065-016-9327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunstad J, Suhr JA. “Expectation as etiology” versus “the good old days”: postconcussion syndrome symptom reporting in athletes, headache sufferers, and depressed individuals. J Int Neuropsychol Soc. 2001;7(3):323–333. doi: 10.1017/s1355617701733061. [DOI] [PubMed] [Google Scholar]
- 65.Mittenberg W, DiGiulio DV, Perrin S, Bass AE. Symptoms following mild head injury: expectation as aetiology. J Neurol Neurosurg Psychiatry. 1992;55(3):200–204. doi: 10.1136/jnnp.55.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robbins CA, Daneshvar DH, Picano JD, et al. Self-reported concussion history: impact of providing a definition of concussion. Open Access J Sports Med. 2014;5:99–103. doi: 10.2147/OAJSM.S58005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Llewellyn T, Burdette GT, Joyner AB, Buckley TA. Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clin J Sport Med. 2014;24(1):76–79. doi: 10.1097/01.jsm.0000432853.77520.3d. [DOI] [PubMed] [Google Scholar]
- 68.Baugh CM, Kroshus E, Kiernan PT, Mendel D, Meehan WP., III Football players' perceptions of future risk of concussion and concussion-related health outcomes. J Neurotrauma. 2017;34(4):790–797. doi: 10.1089/neu.2016.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binder LM. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J Clin Exp Neuropsychol. 1986;8(4):323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]
- 70.Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A, Schonberger M. Predictors of postconcussive symptoms 3 months after mild traumatic brain injury. Neuropsychology. 2012;26(3):304–313. doi: 10.1037/a0027888. [DOI] [PubMed] [Google Scholar]
- 71.van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4(1):42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- 72.Kaufman MJ, Janes AC, Hudson JI, et al. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32(5):283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deslandes A, Moraes H, Ferreira C, et al. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59(4):191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- 75.Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tucker AM, Vogel RA, Lincoln AE, et al. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301(20):2111–2119. doi: 10.1001/jama.2009.716. [DOI] [PubMed] [Google Scholar]
- 77.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 78.Deary IJ, Wright AF, Harris SE, Whalley LJ, Starr JM. Searching for genetic influences on normal cognitive ageing. Trends Cogn Sci. 2004;8(4):178–184. doi: 10.1016/j.tics.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 79.Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci. 2014;17(6):241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hart RP, Wade JB, Martelli MF. Cognitive impairment in patients with chronic pain: the significance of stress. Curr Pain Headache Rep. 2003;7(2):116–126. doi: 10.1007/s11916-003-0021-5. [DOI] [PubMed] [Google Scholar]
- 83.Richards M, Wadsworth ME. Long-term effects of early adversity on cognitive function. Arch Dis Child. 2004;89(10):922–927. doi: 10.1136/adc.2003.032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luchetti M, Terracciano A, Stephan Y, Sutin AR. Personality and cognitive decline in older adults: data from a longitudinal sample and meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2016;71(4):591–601. doi: 10.1093/geronb/gbu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayden KM, Zandi PP, West NA, et al. Cache County Study Group. Effects of family history and apolipoprotein E epsilon4 status on cognitive decline in the absence of Alzheimer dementia: the Cache County Study. Arch Neurol. 2009;66(11):1378–1383. doi: 10.1001/archneurol.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanayama G, Hudson JI, Pope HG., Jr Illicit anabolic-androgenic steroid use. Horm Behav. 2010;58(1):111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5(2):4–14. [PMC free article] [PubMed] [Google Scholar]
- 88.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 89.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(4):323–334. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 90.Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13(4):217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- 92.Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurol Clin. 2007;25(3):577–609. doi: 10.1016/j.ncl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Houck Z, Asken B, Clugston J, Perlstein W, Bauer R. Socioeconomic status and race outperform concussion history and sport participation in predicting collegiate athlete baseline neurocognitive scores. J Int Neuropsychol Soc. 2018;24(1):1–10. doi: 10.1017/S1355617717000716. [DOI] [PubMed] [Google Scholar]
- 94.Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282(10):964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- 95.Baker JG, Leddy JJ, Darling SR, Shucard J, Makdissi M, Willer BS. Gender differences in recovery from sports-related concussion in adolescents. Clin Pediatr (Phila) 2015;55(8):771–775. doi: 10.1177/0009922815606417. [DOI] [PubMed] [Google Scholar]
- 96.Stone S, Lee B, Garrison JC, Blueitt D, Creed K. Sex differences in time to return-to-play progression after sport-related concussion. Sports Health. 2017;9(1):41–44. doi: 10.1177/1941738116672184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward AA., Jr The physiology of concussion. Clin Neurosurg. 1964;12:95–111. doi: 10.1093/neurosurgery/12.cn_suppl_1.95. [DOI] [PubMed] [Google Scholar]
- 98.Sojka P. “Sport” and “non-sport” concussions. CMAJ. 2011;183(8):887–888. doi: 10.1503/cmaj.110504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Browne GJ, Lam LT. Concussive head injury in children and adolescents related to sports and other leisure physical activities. Br J Sports Med. 2006;40(2):163–168. doi: 10.1136/bjsm.2005.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.