Abstract
JD5037 is a novel peripherally restricted CB1 receptor (CB1R) inverse agonist being developed for the treatment of visceral obesity and its metabolic complications, including nonalcoholic fatty liver disease and dyslipidemia. JD5037 was administered by oral gavage at 10, 40, and 150 mg/kg/day dose levels for 34-days to Sprague Dawley rats, and at 5, 20, and 75 mg/kg/day dose levels for 28-days to Beagle dogs. In rats, higher incidences of stereotypic behaviors were observed in 10 mg/kg females and 40 mg/kg males, and slower responses for reflex and sensory tests were observed only in males at 10 and 40 mg/kg during neurobehavioral testing. Sporadic minimal incidences of decreased activity (males) and seizures (both sexes) were observed in rats during daily clinical observations, without any clear dose-relationship. Male dogs at 75 mg/kg during treatment period, but not recovery period, had an increased incidence of gut associated lymphoid tissue hyperplasia and inflammation in the intestine. In both species, highest dose resulted in lower AUCs indicative of non-linear kinetics. Free access to food increased the plasma AUG∞ by ~ 4.5-fold at 20 mg/kg in dogs, suggesting presence of food may help in systemic absorption of JD5037 in dogs. Based on the study results, 150 mg/kg/day in rats, and 20 and 75 mg/kg/day doses in male and female dogs, respectively, were determined to be the no-observed-adverse-effect-level.
Keywords: Peripheral cannabinoid receptor 1 (CB1R), inverse agonist, nonalcoholic steatohepatitis (NASH), JD5037, obesity, preclinical safety, toxicity
1.0. Introduction
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term for a variety of ailments associated with fat accumulation in the liver of people who drink little or no alcohol. NAFLD is one of the most common liver diseases occurring in people in their 40s-50s, mainly in the Western countries. About 20-30% of the adult population and 10% of children in Western countries are estimated to be suffering from NAFLD (Sanyal et al. 2015). Nonalcoholic steatohepatitis (NASH) is the most severe form of NAFLD, characterized by fat accumulation and inflammation in the liver which subsequently may result in liver cirrhosis. Currently there are no effective drugs available for treatment of NASH. Several drugs targeting various signaling pathways, including lipid metabolism, glucose metabolism, inflammation, fibrosis and apoptosis are under investigation for NASH therapy (Wattacheril et al. 2018). The endocannabinoid system plays a central role in regulation of food intake, lipid metabolism and energy expenditure in the body, mediated by G-protein coupled CB1 receptors (CB1R) (Kunz et al. 2008). CB1R are expressed in the brain as well as in the peripheral organs such as the liver, muscles, kidney, adipose tissues, gastrointestinal tract, thyroid, pancreas, and adrenals (Kirilly et al. 2012). Induction of CB1R has been associated with increased food consumption leading to obesity. Hence, research attention was focused on developing CB1R antagonists for the treatment of lipid disorders such as NASH, obesity, and metabolic syndrome.
Rimonabant, the first approved inverse agonist of CB1R showed reduction in food consumption, improved lipid profile, and reduced visceral and hepatic fat (Carai et al. 2006). However, due to its brain penetrant ability, neurologic and psychiatric effects like dizziness, seizures, depression, suicidal tendency, insomnia, and headache were observed (Sam et al. 2011). These observations resulted in withdrawal of rimonabant from the European and Brazilian markets in 2008. However, recent studies revealed that desired pharmacological effects on lipid control without having psychiatric and neurological side effects can be achieved by selectively blocking peripheral CB1R (Chen et al. 2017; Chorvat 2013; Hsiao et al. 2015; Shrestha et al. 2018; Tam et al. 2012). Hence, an inverse agonist of selective peripheral CB1R, JD5037, was developed jointly by United States National Institute of Health and Jenrin Discovery (Tam et al. 2012; Tam et al. 2017). JD5037 exhibited minimal to no (below level of detection) brain penetration in chow-fed mice (Chorvat et al. 2012; Tam et al. 2012). JD5037 showed zero percent occupancy at CB1R in mouse brain, while rimonabant (a brain penetrant CB1R inverse agonist) showed 87% occupancy at 30 mg/kg dose (Chorvat et al. 2012). JD5037 has been found effective in reducing appetite, body weight, hepatic steatosis and improving insulin resistance in diet induced obese mice (Chorvat et al. 2012; Cinar et al. 2014; Tam et al. 2012; Tam et al. 2017). JD5037 has been now permitted by the US-FDA for Phase-1 clinical trials for Nonalcoholic Steatohepatitis (NASH) indication in the United States, for which JD5037 has been licensed by Corbus Pharmaceuticals as CRB-4001. Here, we present the results of preclinical toxicology studies of JD5037 in Sprague Dawley rats and Beagle dogs that were conducted to support an Investigational New Drug (IND) application submission to US-FDA. The purpose of these studies was to evaluate toxicity resulting from repeat dose administration of JD5037 and possible reversal of toxicity findings after the recovery period. All the studies reported here were conducted in accordance with the principles of US-FDA Good Laboratory Practice guideline (21 CFR, Part 58).
2.0. Materials and methods
2.1. Experimental design
Sprague Dawley rats were obtained from Charles River Laboratories, Raleigh, NC (Charles River Nomenclature: CD® rats). The rats were approximately 8 to 9 weeks of age. The males weighed 286.1 to 344 g and the females weighed 192.6 to 236.5 g at the initiation of dose administration.
Beagle dogs were obtained from Covance, Cumberland, VA. The dogs were approximately 6 months of age at the initiation of dose administration. The males weighed 8.21 to 9.92 kg and the females weighed 6.57 to 9.86 kg at the initiation of dosing.
Both rats and dogs were randomized based on body weights per sex using a computer software program (Provantis, Instem, Stone, UK) and assigned to four dose groups with unique identification number. The study designs for rat and dog studies are presented in Tables 1 and 2.
Table 1.
Rat study design
| Group | Dose (mg/kg/day) | Formulation Concentration (mg/mL) | Number of Rats |
|||
|---|---|---|---|---|---|---|
| Corea/Recoveryb Cohort | TK Cohort | |||||
| M | F | M | F | |||
| 1- Control | 0 | 0 | 10 | 10 | 3 | 3 |
| 2- Low Dose | 10 | 1 | 10 | 10 | 9 | 9 |
| 3- Mid Dose | 40 | 4 | 10 | 10 | 9 | 9 |
| 4- High Dosec | 150 | 15 | 10 | 10 | 9 | 9 |
Core Groups (5 rats/sex/dose) Necropsy Day = Day 35
Dosing discontinued on Day 35. Recovery Groups (5 rats/sex/dose) Necropsy Day = Day 57
The 150 mg/kg/day dose was the maximum feasible dose based on the solubility of JD5037 in the vehicle.
Table 2.
Dog study design
| Group | Dose (mg/kg/day) | Formulation Concentration (mg/mL) | Number of Dogs |
|||
|---|---|---|---|---|---|---|
| Corea/Recoveryb Cohort | Fed-TK | |||||
| M | F | M | F | |||
| 1- Control | 0 | 0 | 5 | 5 | - | - |
| 2- Low Dose | 5 | 1 | 5 | 5 | - | - |
| 3- Mid Dose | 20 | 4 | 5 | 5 | - | - |
| 4- High Dose | 75c | 15 | 5 | 5 | - | - |
| 5- Mid Dose Fed TKd | 20 | 4 | - | - | 2 | 2 |
Core Groups (3 dogs/sex/group) Necropsy Day = Day 29
Dosing discontinued on Day 29. Recovery Groups (2 dogs/sex/group) Necropsy Day = Day 50
75 mg/kg/day dose was the maximum feasible dose based on the solubility of JD5037 in the formulation.
The dogs in the Fed-TK group were dosed only once on the last day of dose administration to evaluate the toxicokinetics of JD5037 under fed conditions. These dogs were fasted overnight prior to dose administration and on the day of dose administration these dogs had ad libitum access to food for 3 to 4 hours before dose administration and throughout the completion of TK blood collection
2.2. Housing and environmental conditions
The housing and animal care practices met current standards of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and current requirements stated in the Guide for the Care and Use of Laboratory Animals (Council 2011) and Animal Welfare Act regulations. The protocols were approved by Battelle’s Institutional Animal Care and Use Committee (IACUC). The dogs were acclimated to have access to their feed ration for a target of 2 to 5 hours for at least 3 days prior to Day 1 dose administration. Sham dosing of 40 mL of warm tap water (temperature of 35 to 40°C) was performed for at least 3 days prior to Day 1 dose administration to acclimate the dogs to the oral dosing procedure.
2.3. Food and water
Water from the Columbus municipal water supply was provided ad libitum. The city water conformed to the current United States Environmental Protection Agency (EPA) drinking water standards.
Rats
All rats had ad libitum access to certified feed (Purina Certified Rodent Diet, LabDiet 5002) except when fasted prior to scheduled necropsy and before clinical pathology blood collection. Due to gavage-related mortalities occurring in the first few days of the study, rats were fasted prior to dose administration for 2-3 hours from Days 4 and 5, and for approximately 5 hours from Day 7 onwards. Animals were sham dosed with saline on Day 5 (fasted) and Day 6 (non-fasted). Fasting in rats has been reported to help minimize risk of accidental gavage related injuries (Eichenbaum et al. 2011).
Dogs
The dogs were offered Purina Certified Canine Lab Diet 5007 once daily. Food enrichment (Hill’s prescription diet a/d canned food) was offered to some of the animals during the study period, due to low food consumption and thin appearance. Detailed feeding/fasting schedule is described under Section 2.8.
2.4. Formulation preparation and analysis
In both the rat and dog studies, formulation concentrations of 1, 4 and 15 mg/mL of JD5037 (Piramal Healthcare Ltd, Canada) were prepared in a vehicle constituted of 10.2% w/w Super Refined™ Polyethylene Glycol 600 (Croda Inc.), 33.2% w/w Labrasol® ALF (Gattefossé USA) and 56.0% w/w Gelucire® 44/14 (Gattefossé USA). The bulk JD5037 had a purity of 97.3%. The vehicle components were weighed into a container and stirred until a solution was formed. The prepared vehicle was maintained at approximately 70°C during formulation preparation. For preparation of test article formulations, an appropriate amount of the test article was weighed and transferred to the vehicle container. The formulation was stirred at approximately 70°C until incorporated and then homogenized for approximately 2 minutes. The formulation with the highest concentration, 15 mg/mL, was heated to approximately 90 to 97°C for 75 to 80 minutes, and 1 mg/mL and 4 mg/mL concentrations were heated to approximately 80°C and stirred until a visibly clear solution was achieved. The heating and stirring was essential for dissolution of the formulation components. The test article and control article formulations were then stored at room temperature until dose administration. Formulations were analyzed for concentration and homogeneity. The formulations were tested and found to be stable under the conditions of formulation preparation, storage and dose administration.
2.5. Dose administration
JD5037 and control article formulations were solidified when stored at room temperature. The formulations were heated up to 95 °C for maximum 30 minutes and then cooled to a target temperature of 37 to 40 °C. In order to keep the formulations in the liquid form, the formulations were continuously stirred on a hot plate to maintain 37 to 40 °C temperature during the period of dose administration. The syringes and gavage needles/catheter were also kept at the above mentioned temperature range when not in use. The formulations were viscous and hence the gavage needles were wiped out every time before dose administration.
Rat
Each rat was administered either the control article or formulated JD5037 (10, 40, and 150 mg/kg/day) once daily via oral gavage for up to 34 days (except Days 5 and 6) with dose volume of 10 mL/kg. Due to gavage related mortalities during initial days of the study, animals were not dosed with formulations on Days 5 and 6. On Days 5 and 6, animals were sham dosed with saline to keep animals acclimated with dosing procedure. Hence, dosing duration was extended from 28 days to 34 days to dose animals for 28 consecutive days. Due to difficulty with dose administration related to viscosity of the formulations and resistance of animals during dose administration, beginning on Day 7, all the animals were fasted for 2-6 hours prior to dose administration and dosed under light sedation with CO2/O2 inhalation. Fasting the rats helped keep the stomach empty during dose administration and light sedation avoided resistance of rats during dose administration. This approach reduced the gavage related mortalities later in the study.
Dog
During earlier pilot studies it was observed that the test formulation induced emesis in dogs immediately after dosing. Hence, to avoid accidental aspiration of feed content in the vomit, dogs were fasted prior to dose administration. The dogs (except Fed TK group dogs) were fasted for 7.5 to 18 hours. The feed was offered at 2 to 3 hours post-dose administration. The dogs from the Fed-TK group had full access to feed from 3 to 4 hours prior to dose administration until the 24-hour TK blood collection. Fed-TK arm was incorporated to study kinetics, particularly absorption, of JD5037 in the presence of food. Water was withheld for approximately 1 hour prior to dose administration to ensure that the stomach of the dogs was empty at the time of dosing. The dogs were allowed ad libitum access to water after dose administration.
All dogs were administered appropriate vehicle control or test article formulations at a dose volume of 5mL/kg by oral gavage (See Table 2). During dosing, 5 to 10 mL warm tap water was administered followed by dose formulation, a flush with warm control article (approximately 1 mL), and then warm tap water (approximately 10 mL) for all the dose group animals. This sequence of dose administration was followed to ensure that the formulation remains in the liquid form until it is completely administered into the stomach.
2.6. Clinical observations
For both rat and dog studies, cage-side clinical observations were performed on all study animals at least twice daily for the duration of treatment and once daily during the recovery phase.
2.7. Body weights
For both rats and dogs, body weights were recorded for group assignment, on Day 1 and weekly thereafter including the day prior to and the day of scheduled necropsy.
2.8. Food consumption
Rats
In the rat study, quantitative food consumption was recorded weekly.
Dogs
Beginning on Study Day 1, the dogs were fasted overnight (up to 18 hours) prior to each dose administration. On the days of dose administration, feed was offered at a target of 2 to 3 hours post-dose administration. Dogs had access to the feed for 2 to 5 hours. Due to low food consumption and clinical signs (e.g. emesis, decreased activity, and thin appearance), the dogs (except Fed-TK group) were fasted during Days 16 through 27 for a target of 7.5±1 hours prior to each dose administration. Feed was then offered at a target of 2 to 3 hours after dose administration. The dogs had overnight access to the food. Water was withheld for approximately one hour prior to dose administration. Supplemental food was offered to three 20 mg/kg/day animals and one 75 mg/kg/day animal due to low food consumption and decreased body weights. During recovery, animals had full access to their daily ration. The quantitative food consumption was measured daily throughout the study.
2.9. ECG examinations and blood pressure measurements in dogs
ECG tracings and blood pressure measurements were collected on all study dogs once during Week 4 before Day 28, at a target of 3 to 6 hours post-dose administration. Additionally, ECG and BP data were collected during recovery Week 7. The electrocardiogram (ECG) signals were collected using a Hewlett Packard PageWriter Xli Cardiography Model M1700A. The cardiograph was utilized to collect paper ECG waveform tracings of leads I, II, III, aVR, aVL, aVF, and V10 for each animal. The animals were placed into a restraint sling for ECG collection. Each animal’s ECG waveform tracing was recorded with 10 mm/mV (1mV) calibration pulse signals and collected at a paper speed of 10 mm/sec on leads I, aVF, V10 continuing with a paper speed of 25 mm/sec for leads I, aVF, V10, leads I, II, III and leads aVR, aVL, aVF. ECG intervals (RR, PR, QRS, and QT) were measured on the tracings. The QT interval was normalized for changes in heart rate (RR interval) by conversion to the corrected QT (QTc) interval using Fridericia’s formula: QTc = QT/(RR)1/3. Since baseline ECG were not collected, ECG recordings from treatment group animals were compared with concurrent control group animals.
Non-invasive blood pressure (BP) data were collected concurrently with the ECG collections utilizing a Cardell Max-12HD Vital Signs Monitor (Midmark, USA). An appropriate size SV4 small animal cuff was placed around the animal’s tail and three to four BP determinations were electronically captured for each animal.
2.10. Functional observational battery in rats
Functional observational battery (FOB) assessments were conducted in rats by an examiner blinded to group assignments on all animals in the Core and Recovery cohorts, during the final week of dose administration (4 to 6 hours post-dose administration) and during the final week of the recovery period. During prior dose-range finding studies, the maximum plasma concentration (Cmax) was observed at 4 to 6 hours post-dose (Tmax). Hence, FOB evaluation in this study was conducted at 4 to 6 hours post-dose.
2.11. Bioanalysis and toxicokinetics
Whole blood specimens were collected at pre-dose, and 0.5h, 1h, 2h, 4h, 8h, 12h and 24h post dose intervals in rats and dogs. The specimens were collected from retro-orbital venous plexus of rats after anesthetization with CO2/O2 and from cephalic or saphenous veins of dogs. The blood samples were collected into tubes containing K3EDTA as the anticoagulant and plasma specimens were collected. The extracts were analyzed by liquid chromatography with tandem mass spectrometry (LC-MS). Detailed bioanalytical method is presented in the Supplementary Method.
The concentration-time profiles were evaluated using the non-compartmental analysis (NCA) module in the Phoenix WinNonlin software program (Version 6.4, Certara L. P., Princeton, NJ). The mathematical calculations used to determine the toxicokinetic parameters were as follows:
• Cmax: Observed value
• Tmax: Observed value
• Elimination Rate Constant (λz): Estimated from the linear regression of time vs. log concentration associated with terminal portion of the curve
• Elimination half-life: ln(2)/ λz
• Clearance (Cl)= Dose/AUC∞
• Volume of Distribution (Vd)= Dose/(λz* AUC∞)
• AUClast is calculated using the linear trapezoidal rule
• AUC∞= AUClast + (Predicted Clast/ λz)
2.12. Clinical pathology
Clinical pathology analysis was performed on Days 15, 35 and 57 in rats, and at pretreatment and on Days 15, 29 and 50 in dogs. In rats, blood samples were collected from the retro-orbital venous plexus for hematology and clinical chemistry parameters, and via posterior vena cava for coagulation parameters. In dogs, blood samples were collected either from cephalic or saphenous veins. Animals were fasted overnight prior to sample collection. Various hematological (Advia 120, Siemens, Germany), clinical chemistry (Cobas c 501, Roche, Switzerland) and coagulation parameters (ACL 8000, Beckman Coulter, USA) were analyzed in both rats and dogs (see Supplementary Tables S1–S3 for list of parameters).
2.13. Necropsy
Rats
A complete necropsy was performed for all surviving animals in the Core and Recovery cohorts on Day 35 or 57, respectively. Rats were euthanized by CO2/O2 (70% / 30%) asphyxiation or exsanguination. A complete necropsy was also performed on moribund and found-dead animals.
Dogs
Dogs in the Core cohort (up to 3 per sex per group) were humanely euthanized by barbiturate overdose and necropsied on the day following their final dose administration (Day 29). The remaining dogs (2 per sex per group) were necropsied at the end of recovery period on Day 50. A complete necropsy was also performed on one moribund animal.
Tissues were placed in 10% neutral buffered formalin (NBF), with the exceptions of testes and epididymides, which were preserved in Modified Davidson’s fixative and subsequently transferred to 10% NBF, and the eyes with optic nerve which were fixed in Davidson’s fixative and subsequently transferred to 10% NBF. Complete necropsies were performed on all study animals that died or were terminated at an unscheduled interval.
2.14. Organ weights
Organs were collected and weighed at the time of scheduled sacrifice (see Supplementary Table S4 for complete list of organs).
2.15. Histopathology
In both rats and dogs, all the tissues from the Core cohort (Groups 1 through 4) and target tissues from the Recovery cohort were examined. Tissues were processed, stained with Hematoxylin and Eosin stains, and observed microscopically by a board-certified veterinary pathologist (see Supplementary Table S5 for complete list of organs).
2.16. Statistical analysis
All appropriate quantitative in-life data collected at Battelle was analyzed for test article effects by parametric or nonparametric analysis of variance (ANOVA) using the Provantis system. For parametric analysis, normality was determined by the Shapiro-Wilks test and homogeneity of variances was determined by Levene’s test. Both tests were conducted at the 0.05 level of significance. In some cases, data were log-transformed, where necessary, to meet parametric assumptions. For data determined to be normally distributed and homogenous among groups, an ANOVA F-test was used to determine whether there were differences among the group means. If the ANOVA F-test was significant at the 0.05 level, then tests for differences between the control and each of the comparison groups were conducted using Dunnett’s test, which adjusts for multiple comparisons. For data that were not normally-distributed and/or non-homogenous, analogous nonparametric methods were used. For the nonparametric analysis, a Kruskal-Wallis test was used to determine whether there were differences among the group means. If the Kruskal-Wallis test was significant at the 0.05 level, then tests for differences between the control and each of the comparison groups were conducted using Wilcoxon tests and the Bonferroni-Holm method to correct for multiple comparisons.
3.0. Results
3.1. Dose formulation analysis
All the formulations (1, 4, and 15 mg/mL concentrations) were homogeneous. The relative standard deviation (RSD), which indicates homogeneity of the formulations, was within 0.2 to 4.1% and 0.4 to 0.8% in the rat and dog study, respectively. The formulation concentrations were also within acceptable limit. Relative error for all formulations was within 0 to −5.9% (rat) and −1.8 to −6.2% (dog) of the target concentration (RE, relative error) at all the concentration levels. The formulations were stable at room temperature for up to 21 days.
3.2. Toxicokinetics of JD5037
Rats
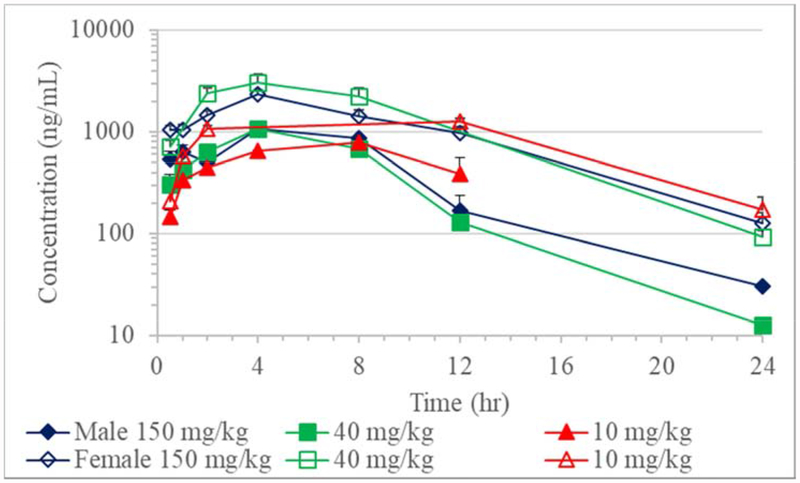
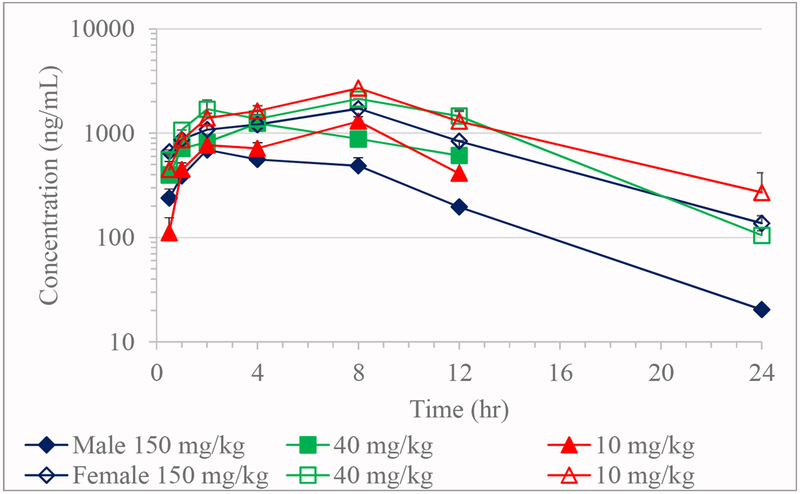
Absorption of JD5037 was slow with Tmax values on Day 1 ranging from 4 to 8 hours in males and 4 to 12 hours in females; while on Day 34, 2 to 8 hours in males and 8 hours in females (Table 8, Figures 3 and 4). Due to less than three time points with a measurable concentration after Tmax, the TK parameters such as elimination rate constant, elimination half-life, apparent clearance, and volume of distribution could not be calculated for most of the doses on Day 1 and Day 34.
Table 8.
Microscopic findings in dogs at the end of treatment period
| Tissue | Microscopic Observation | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 mg/kg N=3 | 5 mg/kg N=3 | 20 mg/kg N=3 | 75 mg/kg N=2 | 0 mg/kg N=3 | 5 mg/kg N=3 | 20 mg/kg N=3 | 75 mg/kg N=3 | ||
| AUClast (hr*ng/mL) on Day 28 | 0 | 2650 | 4250 | 3220 | 0 | 4940 | 3340 | 2680 | |
| Duodenum | Chronic-active inflammation | ||||||||
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Jejunum | Lymphoid tissue hyperplasia | ||||||||
| Minimal | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Chronic-active inflammation | |||||||||
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Ileum | Lymphoid tissue hyperplasia | ||||||||
| Minimal | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mild | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | |
| Chronic-active inflammation | |||||||||
| Mild | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Colon | Lymphoid tissue hyperplasia | ||||||||
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Pancreas | Single cell necrosis | ||||||||
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Thymus | Cortical Atrophy | ||||||||
| Minimal | 0 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | |
| Mild | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| Moderate | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Testes | Degeneration | NA | NA | NA | NA | ||||
| Minimal | 1 | 1 | 0 | 0 | |||||
| Mild | 0 | 0 | 1 | 1 | |||||
| Moderate | 0 | 0 | 2 | 0 | |||||
The numbers indicate number of animals affected in the group
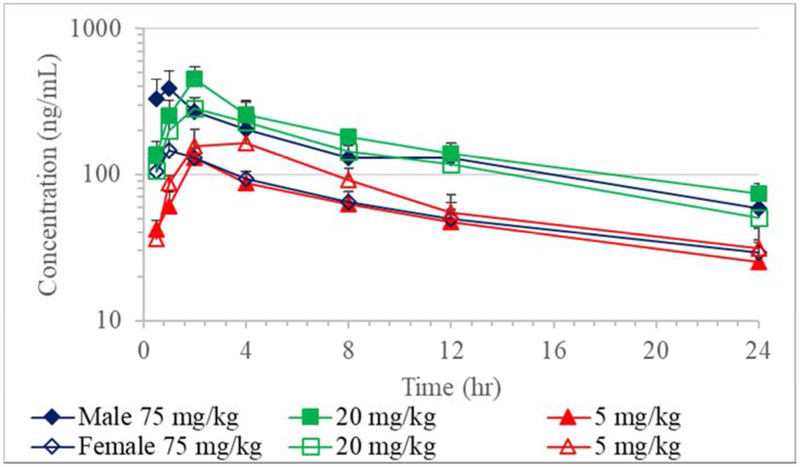
Figure 3.
Toxicokinetics of JD5037 in dogs on Day 1 (Mean + SE; n=5)
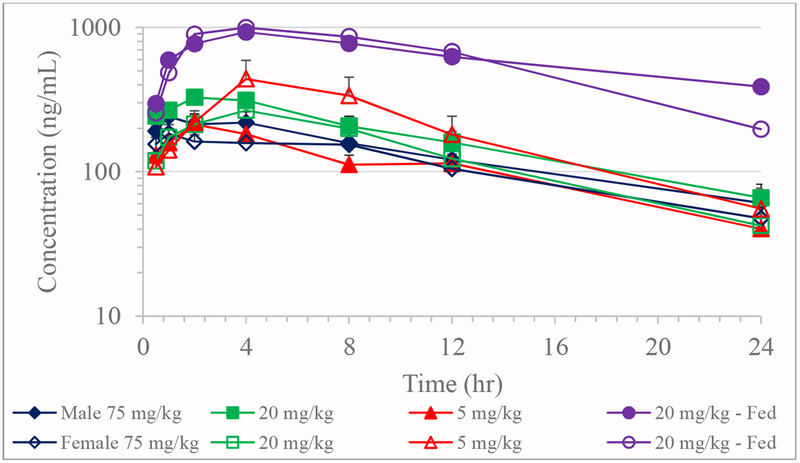
Figure 4.
Toxicokinetics of JD5037 in dogs on Day 28 (Mean + SE; n=5 except 75 mg/kg males where n=4)
Day 1:
The AUClast in males increased minimally with dose increment although the increase was not dose proportionate. Conversely, in females, there was a minimal decrease in AUClast at the 150 mg/kg dose compared to the 40 mg/kg dose. When normalized for dose, Cmax and AUClast both decreased as the dose increased on Day1 (Table 8). The lack of a discernable increase in systemic exposure with increasing dose levels suggested saturation of systemic exposure across the dose range of 10 to 150 mg/kg and, in particular, at the 40 and 150 mg/kg dose levels. When comparing males and females, females had consistently higher systemic exposure for a given dose. In females, Cmax values were 1.6- to 2.9-fold greater, and AUClast values were 2.6- to 4.4-fold higher than males on Day 1.
Day 34:
The AUClast in both the sexes at 150 mg/kg was lower than 10 and 40 mg/kg dose. Similar to Day 1, dose-normalized AUClast on Day 34 decreased exponentially with increasing dose. The Cmax values were 1.7- to 2.5-fold greater and AUClast values were 2.8- to 3.2-fold higher in females than males.
Compared to Day 1 the dose normalized Cmax on Day 34 was slightly decreased at 150 mg/kg in males, and 40 and 150 mg/kg/day dose in females, while increased at the 10 mg/kg dose. Dose normalized AUClast at 10 mg/kg dose was 1.4-fold greater in both the sexes on Day 34, while it was 1.4-fold and 1.2-fold lower at 150 mg/kg dose in males and females, respectively.
Dog
Absorption of JD5037 in dogs was varied with individual Tmax values ranging from 0.5 to 4 hours on Day 1 and 0.5 to 8 hours on Day 28 with no apparent dose or sex effects (Tables 4 and 5; Figures 3 and 4). The group mean elimination half-life for all groups ranged from 10.0 to 15.8 hours on Day 1 and 6.8 to 12.3 hours on Day 28. Compared to the fasted 20 mg/kg dose group on Day 28, fed group male dogs had a relatively longer elimination half-life of 16.5 hours than fasted males, while females had a comparable half-life of 7.7 hours. On both days, the apparent clearance and apparent volume of distribution were similar between males and females for a given dose. The apparent clearance and apparent volume of distribution values increased in both sexes as the dose increased from 5 to 75 mg/kg/day. However, the 20 mg/kg/day fed group dogs that were fed prior to dose administration, had a lower apparent clearance and apparent volume of distribution than the 20 mg/kg/day fasted group. On both days, dose normalized-Cmax and-AUC both decreased as the dose was increased from 5 to 75 mg/kg/day. The lack of a discernable increase in the systemic exposure as the dose was increased suggested potential saturation of absorption at 20 and 75 mg/kg/day dose levels. However, both male and female dogs that were fed prior to dose administration (20 mg/kg/day) had greater Cmax (2.4- to 6.2-fold) and AUC∞, (3.0- to 11.7-fold) than fasted dogs administered with 5, 20 or 75 mg/kg/day doses. The observed kinetics suggest the fed state exponentially increased the systemic exposure of JD5037 compared to the fasted state.
Table 4.
Toxicokinetics of JD5037 in dogs- Day 1
| Dose (mg/kg) | Sex | Cmax (ng/mL) | Cmax/Dose (ng/mL)/(mg/kg) | Tmax (hr) | Elimination Half-Life (hr) | Apparent Clearance (mL/hr/kg) | Apparent Volume of Distribution (mL/kg) | AUClast (hr*ng/mL) | AUC∞ (hr*ng/mL) | AUC∞/Dose (hr*ng/mL)/(mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg | Male | Mean | 135 | 26.9 | 2.20 | 13.6 | 3520 | 56800 | 1300 | 1890 | 378 |
| SE | 21.9 | 4.4 | 0.49 | 3.5 | 870 | 11200 | 300 | 530 | 105 | ||
| Female | Mean | 170 | 34.0 | 2.80 | 13.9 | 2800 | 48500 | 1800 | 2650 | 530 | |
| SE | 58.2 | 11.6 | 0.49 | 4.1 | 720 | 15000 | 500 | 890 | 179 | ||
| 20 mg/kg | Male | Mean | 387 | 19.3 | 1.80 | 15.8 | 3670 | 78900 | 3880 | 5770 | 288 |
| SE | 99.7 | 5.0 | 0.20 | 4.3 | 430 | 19100 | 530 | 690 | 34 | ||
| Female | Mean | 333 | 16.7 | 2.20 | 10.0 | 7600 | 112000 | 3120 | 3880 | 194 | |
| SE | 71.2 | 3.6 | 0.49 | 0.7 | 1960 | 35000 | 1070 | 1430 | 71 | ||
| 75 mg/kg | Male | Mean | 391 | 5.22 | 0.800 | 13.7 | 20200 | 406000 | 3350 | 4580 | 61.1 |
| SE | 126 | 1.67 | 0.122 | 1.8 | 4800 | 114000 | 710 | 940 | 12.6 | ||
| Female | Mean | 160 | 2.14 | 1.90 | 11.8 | 39100 | 649000 | 1470 | 2060 | 27.5 | |
| SE | 27.3 | 0.36 | 0.60 | 1.9 | 6400 | 121000 | 180 | 300 | 3.9 |
Table 5.
Toxicokinetics of JD5037 in dogs- Day 28
| Dose (mg/kg) | Sex | Cmax (ng/mL) | Cmax/Dose (ng/mL)/(mg/kg) | Tmax (hr) | Elimination Half-Life (hr) | Apparent Clearance (mL/hr/kg) | Apparent Volume of Distribution (mL/kg) | AUClast (hr*ng/mL) | AUC∞ (hr*ng/mL) | AUC∞/Dose (hr*ng/mL)/(mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg | Male | Mean | 221 | 44.2 | 2.40 | 11.4 | 1840 | 26300 | 2650 | 3460 | 691 |
| SE | 39 | 7.9 | 0.68 | 2.7 | 510 | 5500 | 540 | 700 | 140 | ||
| Female | Mean | 525 | 105 | 4.40 | 6.84 | 2010 | 18500 | 4940 | 4930 | 986 | |
| SE | 139 | 28 | 0.98 | 1.12 | 850 | 7700 | 1450 | 1980 | 396 | ||
| 20 mg/kg | Male | Mean | 373 | 18.6 | 3.20 | 9.85 | 4300 | 60700 | 4250 | 5250 | 262 |
| SE | 29 | 1.5 | 0.49 | 1.04 | 810 | 13800 | 580 | 830 | 42 | ||
| Female | Mean | 280 | 14.0 | 3.40 | 7.34 | 6120 | 60300 | 3340 | 3890 | 195 | |
| SE | 39 | 1.9 | 0.60 | 1.22 | 1180 | 10900 | 590 | 880 | 44 | ||
| 75 mg/kg | Male | Mean | 284 | 3.78 | 2.00 | 12.3 | 18900 | 320000 | 3220 | 4370 | 58.3 |
| SE | 46 | 0.61 | 0.71 | 2.4 | 3200 | 54000 | 480 | 800 | 10.6 | ||
| Female | Mean | 243 | 3.24 | 3.10 | 10.4 | 27400 | 392000 | 2680 | 2810 | 37.5 | |
| SE | 52 | 0.70 | 1.36 | 2.0 | 2500 | 46000 | 490 | 260 | 3.4 | ||
| 20 mg/kg (Fed) | Male | Mean | 927 | 46.4 | 4.00 | 16.5 | 837 | 20000 | 14900 | 24100 | 1200 |
| SE | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Female | Mean | 996 | 49.8 | 4.00 | 7.69 | 1200 | 13700 | 14900 | 17100 | 854 | |
| SE | NA | NA | NA | NA | NA | NA | NA | NA | NA |
3.3. Survival and clinical observations
Rats
There were 13 mortalities: four in the control females, one in the 10 mg/kg/day group females, three in the 40 mg/kg/day group females, and four and one in the 150 mg/kg/day group females and males, respectively. Clinical signs and/or necropsy examination indicated that these mortalities were related to gavage errors encountered probably as a result of the viscous nature of the formulation.
In male rats, seizures were observed once in one male animal in each treatment groups and not in the control group. Incidences of decreased activity were observed at 10 mg/kg/day (4 incidences in 3 animals), 40 mg/kg/day (1 incidence) and 150 mg/kg/day dose groups (2 incidences in 2 animals). However, the seizures and decreased activity were not considered treatment-related due to the low incidence and no dose correlation.
In female rats, seizures were observed once in 2, 1 and 3 animals from 0 mg//kg/day, 40 mg/kg/day and 150 mg/kg/day dose groups, respectively. They were not considered treatment-related as there was no dose-relationship and due to the sporadic nature of the occurrences.
Other clinical signs including excess salivation, soft feces, lethargy, red or clear nasal discharge were observed in control and all the treatment groups. Because of low incidence and/or absence of dose correlation these signs were considered unrelated to the test article administration.
At least one of the respiratory signs such as gasping/rasping/labored breathing/noisy breathing/sneezing were observed in all the groups including control. Respiratory abnormalities, in both sexes, were suspected to be due to aspiration of formulation contents during dose administration. Since these clinical signs were also observed in control animals or as isolated incidences, they were considered related to the viscosity of excipients in the formulations.
Dogs
All animals survived to scheduled termination with the exception of one male from the 75 mg/kg/day dose group that died on Day 16. A marked fibrinopurulent inflammation of lung pleura was thought to be the cause of moribundity of this animal, was considered unrelated to the systemic effect of JD5037 administration and likely consequence of inadvertent aspiration of viscous formulation.
In male dogs, there were no clinical signs attributed to the direct effect of the test article administration. In females, a decrease in activity was observed. The total incidences and number of females with decreased activity observed at 1 to 2 hour post dose administration were greater at 5 mg/kg/day (11 incidences in 5 out of 5 animals), 20 mg/kg/day (22 incidences in 4 out of 5 animals), and 75 mg/kg/day (11 incidences in 3 out of 5 animals) dose levels compared to the control group (a single incidence on Day 17). Clinical signs of decreased activity were not observed during the recovery phase. A change in fecal consistency (diarrhea, mucoid, soft) and nasal discharge were also observed in all the dose groups, including the controls in both sexes. Since gastrointestinal effects were also observed in control animals, this effect was not considered test-article related, and the vehicle excipients may have contributed to the effects like emesis and diarrhea. Emesis usually occurred within a few minutes of dosing. The low food consumption during initial days, and discomfort resulting from emesis and diarrhea may partly explain increased incidences of decreased activity observed in females.
3.4. Body weight
Rats
There were no test article-related changes in the group mean body weights or body weight gain for both sexes of rats during the treatment or during the recovery period.
Statistically significant reductions in body weight gains were apparent for male rats in all JD5037 treated groups compared to controls during the first week of treatment period only. As a result, group mean body weight in treatment groups remained significantly lower (up to 11% lower compared to concurrent control group) from Day 8 onwards except on Day 14 compared to control, while body weight gain in treatment groups during this duration was comparable to the control group. In females, there were no significant changes in group mean body weights except a 7.1% lower group mean body weight on Day 14 at 10 mg/kg/day compared to control. Similarly, there were no significant changes in body weight gain except a decrease at 40 mg/kg/day compared to the control group during the last week of treatment (0.742 g/day vs 1.424 g/day in control).
The decrease in body weight gain and/or group mean body weight in both sexes was neither dose nor plasma concentration-dependent and the observed reductions were also not consistent across study duration. Since body weight gain in males from Day 8 onwards was not changed, the lower group mean body weight in males from Day 8 onwards is likely a result of lower body weight gain during week 1. Hence, the changes in the body weights were unlikely related to the test article administration.
Dogs
There were no test article-related changes in the group mean body weights or body weight gain for both sexes of dogs during the treatment or recovery period.
3.5. Food consumption
Rats
In male rats, intermittent reductions (10-20%) in food consumption in the 10 and 40 mg/kg dose groups during the treatment period were statistically significant. Since the changes in food consumption were not dose or concentration dependent, and noted only during the first and third week of the dosing period, this effect was not considered test article related.
In female rats, there were no significant differences in the group mean food consumption during the study period.
Dogs
In male dogs, there were no statistically significant differences in mean daily food consumption in the JD5037-treated groups compared to concurrent control groups, except a decrease on Day 4, which was suspected to be spurious.
In female dogs, food consumption in the treatment group compared to control animals was reduced sporadically up to Day 16. However, food consumption in females remained comparable to the control group from Day 16 through the recovery period except on Days 21-22 at 75 mg/kg/day when access to food was increased to overnight. This suggests the dogs had increased food consumption possibly after they overcame a post-dose nauseating effect (indicated by salivation and emesis after dosing). Food consumption was increased when enrichment diet was offered and food access period was increased.
3.6. Neurobehavioral effects in rats
Neurobehavioral effects of JD5037 in rats were primarily noted at 10 and 40 mg/kg, with only a limited incidence observed at 150 mg/kg (Table 7). A higher incidence of stereotypic behaviors like repetitive grooming (including excessive / repetitive scratching or shaking of the head, and/or fast grooming) was seen in females at the 10 mg/kg and males at the 40 mg/kg. Additionally, slower reflex and sensory responses were observed in male rats at 10 and 40 mg/kg. Higher rectal temperatures were seen in female rats at all treatment levels compared to control.
Table 7.
Relevant FOB findings in rats during Week 5 (4-6 hours post-dose)
| Parameter | 0 mg/kg | 10 mg/kg | 40 mg/kg | 150 mg/kg | ||||
|---|---|---|---|---|---|---|---|---|
| M (N=10) |
F (N=7) |
M (N=10) |
F (N=10) |
M (N=10) |
F (N=8) |
M (N=9) |
F (N=6) |
|
| AUC (hr*ng/mL) on Day 34 | 0 | 0 | 9720 | 30700 | 10300 | 28600 | 6770 | 20600 |
| Mean plasma concentration (ng/mL) at 4 h post-dose on Day 34 | 0 | 0 | 716 | 1627 | 1237 | 1370 | 560 | 1207 |
| Repetitive groominga | 0 | 0 | 2 | 6 | 4 | 1 | 0 | 0 |
| Head shakingb | 0 | 0 | 0 | 6 | 1 | 1 | 0 | 1 |
| Scratching | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Slow or no response (approach, touch, tail pinch, or startle) | 0 | 0 | 4 | 1 | 5 | 0 | 1 | 1 |
| Retropulsion | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Reduced palpebral reflex | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Mean rectal temperature (°F) ± SD | 100.4±0.4 | 99.5±0.2 | 100.1±0.2 | 100.9*±0.3 | 99.6±0.5 | 100.6*±0.1 | 100.4±0.4 | 100.2*±0.2 |
includes excessive / repetitive scratching or shaking of the head, and/or fast grooming
With or without repetitive grooming.
p<0.05; M: Male; F: Female.
Numbers in the table, except rectal temperature, indicate ‘number of animals with the observation’.
During the recovery period, animals of both sexes at 40 mg/kg/day had statistically significant fewer rearing counts than the controls. There were no statistically significant differences between the controls and 10 and 150 mg/kg/day group rats during the recovery period.
3.7. Pathological evaluation
There were no test article-related findings noted in the coagulation, hematology or clinical chemistry analysis in both rats and dogs.
3.7.1. Organ weights
Rats
There were no statistically significant differences in organ weights for females at Core or Recovery necropsy. In males, there were some statistically significant changes in prostate weights at the end of treatment and pituitary weights at the end of recovery period. Group mean prostate weight (with seminal vesicles and coagulating gland) in 40 mg/kg/day (−20.4%) and 150 mg/kg/day (−16.3%) dose group males was statistically significantly decreased compared to the controls. However, prostate weights relative to terminal body weights in all the treatment groups were lower but not statistically significantly different. There was no clear plasma concentration-dependency or dose-dependency in prostate weight reduction. Additionally, there were no correlative histopathological findings in prostate. Hence, these minimal changes were not considered test article related. At the end of recovery period, there was a statistically significant decrease in male group mean pituitary weight (0.021g, 0.014g, 0.015g, and 0.013 g in control, 10 mg/kg, 40 mg/kg and 150 mg/kg, respectively) and pituitary:brain weight (0.96%, 0.67%, 0.75% and 0.61% in control, 10 mg/kg, 40 mg/kg and 150 mg/kg, respectively). There were no correlative microscopic alterations in the pituitary. Since hormonal analysis was not performed in this study, significance of reduced pituitary weights could not be determined with certainty. However, the differences were considered not likely to be treatment-related, given no clear dose-relationship, and absence of such a finding in males during the treatment period or in females during either the treatment or recovery period.
Dogs
Mean absolute thymus weight was statistically significantly decreased in males in 5 mg/kg/day (4.67±1.8 g) and 75 mg/kg/day (4.80±2.7%) dose group compared to control group (9.57±1.6 g). The reduction in thymus weights normalized to brain (thymus:brain) was also statistically significant at the 5 mg/kg/day (5.99±2.3%) and 75 mg/kg/day (6.05±3.2%) doses compared to control group (14.29±2.8%). The thymus weights normalized to body weights (thymus:terminal body weights) in males were not statistically significant. There was no clear correlation of reduction in thymus weight with plasma concentration of JD5037, hence, the changes were not considered test article related. There were no statistically significant changes in the organ weights of male dogs at the end of recovery period.
In female dogs, there were no statistically significant differences in mean absolute organ weight, mean organ-to-terminal body weight, or mean organ-to-brain weight in the JD5037 treated animals compared to the control group at the end of treatment or recovery period.
3.7.2. Gross pathology
There were no JD5037-related gross pathology findings in rats and dogs.
3.7.3. Histopathology
Rats
There were no noteworthy microscopic findings in the JD5037-treated rats.
Dogs
The tables 8 and 9 summarizes the microscopic findings in dogs at the end of treatment and recovery period, respectively. A male dog from the 75 mg/kg/day dose group which was euthanized on Day 16 due to moribundity showed marked fibrinopurulent inflammation of lung pleura which was considered the cause of death. Additionally, chronic-active inflammation in jejunum, minimal perivascular inflammation in aorta, minimal bone marrow hyperplasia, mild purulent inflammation in lungs, mild hemorrhages in the lung pleura, minimal prostate atrophy, mild testicular degeneration, mild thymic atrophy and minimal C-cell hyperplasia in thyroid glands were also observed. No other animals that survived for complete dosing period showed such lung lesions. Hence, this early mortality was considered unlikely related to the test article effect.
Table 9.
Microscopic findings in dogs at the end of recovery period.
| Tissue | Microscopic Observation | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 mg/kg N=2 | 5 mg/kg N=2 | 20 mg/kg N=2 | 75 mg/kg N=2 | 0 mg/kg N=2 | 5 mg/kg N=2 | 20 mg/kg N=2 | 75 mg/kg N=2 | ||
| AUClast (hr*ng/mL) on Day 28 | 0 | 2650 | 4250 | 3220 | 0 | 4940 | 3340 | 2680 | |
| Duodenum | Chronic-active inflammation | ||||||||
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Jejunum | Lymphoid tissue hyperplasia | ||||||||
| Minimal | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Chronic-active inflammation | |||||||||
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ileum | Lymphoid tissue hyperplasia | ||||||||
| Minimal | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Mild | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Chronic-active inflammation | |||||||||
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Colon | Lymphoid tissue hyperplasia | ||||||||
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pancreas | Single cell necrosis | ||||||||
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Thymus | Cortical Atrophy | ||||||||
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mild | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
The numbers indicate number of animals affected in the group
At terminal necropsy, one animal from 75 mg/kg/day dose had lesions of chronic-active inflammation in the duodenum, jejunum, and ileum, lymphoid hyperplasia of the Gut Associated Lymphoid Tissue (GALT) within the colon, ileum (2 animals) and jejunum, and single cell necrosis in pancreatic acinar cell. The inflammation in the intestinal tract and pancreatic necrosis were not seen in any of the males from 5 and 20 mg/kg/day dose groups, nor in any of the females. A mild lymphoid hyperplasia of GALT in the ileum was seen in a single male from 75 mg/kg/day dose group and a single female from 20 mg/kg/day dose group. A minimal hyperplasia was observed in lymphoid tissue of ileum, jejunum, mesenteric lymph nodes and bone marrow in a single male from the control group. At the end of recovery, the duodenum, colon, and pancreas appeared microscopically normal in all recovery animals from all the groups. Lymphoid hyperplasia was observed in the GALT of the ileum and/or jejunum of 1 out of 2 males from 75 mg/kg/day dose group, 2 out of 2 males from 20 mg/kg/day dose group, and 2 out of 2 males from control group. These findings are common and consistent with resolving intestinal inflammation; suspected to result in GALT reactivity (hyperplasia). There were no findings in the female animals. Given the increased incidence and/or severity of lesions in 75 mg/kg group males; intestinal inflammation, and lymphoid hyperplasia were interpreted as possible adverse effects related to JD5037 administration.
Thymus atrophy was observed at 5 mg/kg (2 out of 3 males; 3 out of 3 females), 20 mg/kg (2 out of 3 males; 3 out of 3 females), 75 mg/kg (1 out of 2 males; 1 out of 3 females) and in 1 out of 3 control females. Due to increased incidences in JD5307-treated animals, thymic atrophy was considered test article related. However, at the end of recovery period thymic atrophy was observed in only one male dog from 5 mg/kg dose group.
4.0. Discussion
The preclinical toxicology studies of JD5037 were conducted in rats and dogs. In dogs, the observed increase in the toxicokinetic parameters such as clearance and volume of distribution as the dose increased indicates non-linear kinetics of JD5037. The dose-normalized Cmax (Cmax/dose) and AUClast (AUClast/dose) values were decreased with dose increment in both sexes of both the species. The absolute Cmax or AUClast values did not increase dose proportionately. Additionally, the elimination half-life (t1/2) did not vary noticeably across the dose groups in dogs indicating the possibility of saturation of metabolizing enzymes was unlikely. Hence, the dose dependent increase in volume of distribution (Vd) and clearance, and dose-dependent decrease in dose-normalized AUC may be the result of either absorption saturation or sequestration of the drug in the circulation (e.g. plasma proteins) after absorption, or a combination of these factors. In vitro studies suggested that JD5037 has very high plasma protein binding in both rat (100%) and dog (99.97%) plasma (results not provided here). The AUC∞ in the fed dogs were exponentially higher (4.6-fold in males and 4.4-fold in females) compared to fasted dogs at 20 mg/kg dose. Similarly, dose-normalized AUC∞ in fed dogs was increased by 4.5-fold in males and 4.3-fold in females compared to fasted dogs. It is well documented that presence of food in the gastrointestinal tract, especially dietary lipids, increases absorption of lipophilic drugs (Charman et al. 1997; Koziolek et al. 2018). Canine food used in this study contained 7.21% total fatty acids which might have facilitated absorption of JD5037. Plasma albumin being the natural carrier of fatty acids has a high affinity for fatty acids (Spector 1975) and the presence of high post-prandial fatty acids in the circulation may displace the albumin-bound drug resulting in increased free drug concentration, which is partly indicated by 3 to 4 times lower Vd under fed conditions. A very high plasma protein binding of JD5037 may influence kinetics of other co-administered drugs, if any, during clinical trials. It is also important to note that the kinetics of JD5037 in dogs may also have been impacted by emesis (decreased internal dose) and diarrhea (increased intestinal motility and reduced time for drug absorption) observed within a few minutes of dose administration, which plausibly contributed to the wide range of individual Tmax values, and inter-animal variability in plasma concentrations and other TK parameters. Sex differences were observed in plasma exposure of JD5037 in rats. On both Days 1 and 34, JD5037 female rats had higher bioavailability (based on dose-normalized Cmax and AUClast) at all dose levels, which is reflected in Cmax and AUClast values. On both Days 1 and 34, Cmax and AUClast values in female rats were 1.6 to 4.4-fold greater than male rats in all treatment groups. There are multiple reasons for sex differences in kinetics of drugs such as differences in hormones, expression of CYP450 and drug transporters (Gochfeld 2017). However, the exact reasons for such sex differences in kinetics of JD5037 in rats are not clear. Such a trend was not evident in dogs indicating the sex differences in kinetics of JD5037 in rats may be a species-specific phenomenon.
The salivation during dose administration and emesis within few minutes of dose administration including vehicle in dogs may be due to stimulation of peripheral emetogenic signaling such as vagal afferents in gastric and duodenal mucosa (Elwood et al. 2010). Such peripheral emetogenic signals are generally transmitted to the nucleus of solitary tract, an ‘emesis center’ in the brain, resulting in emesis. The gastrointestinal related effects such as emesis, excess salivation, retching, and diarrhea might have caused discomfort contributing to the decreased activity of dogs which was evident in females only. The incidences of decreased activity were not correlated with dose- or plasma-concentration of JD5037.
During FOB evaluation in rats, the observed stereotypic behaviors including repetitive grooming (scratching, shaking of the head, and/or fast grooming) were considered related to effects of the test article. The FOB was performed at 4 to 6 hours post-dose administration which corresponds to the maximum plasma concentration (Cmax) observed between 2-8 hours post dose on Day 34. The incidence of stereotypic behavior was increased in the 10 mg/kg/day (females) and 40 mg/kg/day (males) dose groups which had the highest AUClast and plasma concentration at 4 hours post-dose and the least or no incidences of stereotypies in the 150 mg/kg/day dose (with the lowest AUClast and plasma concentration at 4 hours post dose) and control groups. All other types of stereotypic or unusual behavior observed were noted for individual animals only. Increase in stereotypic behaviors such as scratching in rats was reported after single dose administration of rimonabant, a CB1R antagonist (Kunz et al. 2008). It is not clear whether or how targeting peripheral CB1R by JD5037 would contribute to the stereotypic behaviors observed in this study.
A slight increase in body temperature (up to 1.38 °F) in female rats from treatment groups compared to control group had apparent correlation with Day 34 plasma exposure (AUClast) of JD5037. Considering the slight increase, the toxicological relevance of these changes is undetermined. Such elevated body temperature due to increased thermogenesis was reported in diet-induced obese mice after treatment with peripheral CB1R antagonist, BPR0912 (Hsiao et al. 2015) while no changes in temperature were reported after administration of another peripheral CB1R antagonist, TXX-522 in normal C57 mice (Chen et al. 2017).
The palpebral reflex (indicated by sluggish closing of eyelids) was slower in males, but not in females, at 40 mg/kg/day during Week 5. These observations correlated with AUClast and plasma concentration of JD5037 at 4 hours post-dose on Day 34, the time-point when FOB was performed. In all the cases, these effects were not observed during the recovery period. JD5037 has been shown not to penetrate the blood brain barrier and not target brain CB1R in mice (Chorvat et al. 2012; Tam et al. 2012). Hence, it is unclear whether an inverse agonism effect of JD5037 at peripheral CB1R would have contributed to the neurobehavioral effects such as the reduced response to tail-pinch, approach, touch or startle and reduced palpebral reflex. Such reduced responses were reported in global CB1R knockout mice (Zimmer et al. 1999). Central CB1R inverse agonists like rimonabant showed anxiogenic and cataleptic effects in mice (Tam et al. 2012). Such neurobehavioral effects were not clearly evident in this study after JD5037 administration.
There was increased incidence of chronic active inflammation and/or lymphoid tissue hyperplasia in the intestinal tract in male dogs at 75 mg/kg dose compared to control animals. Although these incidences were not correlated with systemic exposure of JD5037 (plasma exposure), it is possible that local irritation in the intestine may have resulted in the inflammatory lesions in the high dose which were recovered (either partially or completely) during the recovery period. Such local gastrointestinal irritation for long duration or at higher doses than used in this study may also affect food consumption and/or food absorption. The severity and incidences of thymic atrophy in dogs roughly correlate with JD5037 plasma concentrations in females, being lower at 75 mg/kg in which group the concentration was the lowest. There was no clear dose-response or plasma concentration-response in the reduction of thymus weights (maximum reductions were observed at 5 and 75 mg/kg/day doses). In clinical pathology analysis, there were no significant changes in either total leukocyte or absolute lymphocyte count in the peripheral blood of control and JD5037-treated animals. However, mature T-cell population was not analyzed in this study, hence functional impact of thymic atrophy on T-cell maturation is unknown. Thymic atrophy was noted in one control female dog, while no incidence was observed during the recovery period indicating the atrophy may have been resulted from stress associated with the treatment (Pearse 2006).
5.0. Conclusion
While gastrointestinal effects were observed in male dogs in this study, these appear to be either completely or partially reversible with discontinuation of treatment. Since these effects were not observed in female dogs and none of the rats, the possibility of translatability of this nonclinical finding to humans seems feeble. It will be worthwhile to consider non-linear kinetics of JD5037 observed in nonclinical studies during a Phase 1 clinical trial preparation. A special consideration should be given to the fed and fasting conditions of the subjects in the clinical trials since bioavailability of JD5037 can be many times greater under fed conditions. Based on the results obtained in these studies, 150 mg/kg/day was determined to be the no-observed-adverse-effect-level (NOAEL) in rats, while 20 mg/kg/day and 75 mg/kg/day in male and female dogs, respectively. Considering 150 mg/kg as the NOAEL in rat and 20 mg/kg in dog, the calculated human equivalent dose (HED) would be 24.2 mg/kg and 11 mg/kg for rat and dog NOAEL, respectively. Since the dog NOAEL resulted in the lowest HED, it will most likely be considered for calculating the first-in-human (FIH) dose. The average Cmax at 20 mg/kg (NOAEL) in dogs was 326.5 ng/mL which can be roughly converted as 570 nM. It thus appears that HED resulting from dog NOAEL dose will confer reasonable safety margin of 31.6, considering that in vitro IC50 (inhibitory concentration-50 for CB1R receptors) of JD5037 is much lower (IC50=18 nM; Chorvat et al. 2012), than the plasma concentration (570 nM) resulting from the NOAEL dose in dogs.
Supplementary Material
Figure 1.
Toxicokinetics of JD5037 in rats on Day 1 (Mean + SE; n≥2)
Figure 2.
Toxicokinetics of JD5037 in rats on Day 34 (Mean + SE; n≥2)
Table 3.
Cmax and AUClast values in rats on Day 1 and Day 34
| Sex | Dose (mg/kg) | Cmax (ng/mL) | Dose Normalized Cmax [(ng/mL)/(mg/kg)] | AUClast (hr*ng /mL) | Dose Normalized AUClast [(hr*ng/mL)/(mg/kg)] | Tmax (hr) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 34 | Day 1 | Day 34 | Day 1 | Day 34 | Day 1 | Day 34 | Day 1 | Day 34 | ||
| Male | 10 | 784 | 1300 | 78 | 130 | 6840 | 9720 | 684 | 972 | 8.0 | 8.0 |
| 40 | 1060 | 1240 | 27 | 31 | 8470 | 10300 | 212 | 258 | 4.0 | 4.0 | |
| 150 | 1060 | 691 | 7 | 5 | 9700 | 6770 | 65 | 45 | 4.0 | 2.0 | |
| Female | 10 | 1260 | 2700 | 126 | 270 | 21300 | 30700 | 2130 | 3070 | 12.0 | 8.0 |
| 40 | 3080 | 2130 | 77 | 53 | 37200 | 28600 | 930 | 715 | 4.0 | 8.0 | |
| 150 | 2350 | 1720 | 16 | 11 | 24800 | 20600 | 165 | 137 | 4.0 | 8.0 | |
Table 6.
Mortality incidences
| Dose (mg/kg) | Male | Female | Day(s) of Death |
|---|---|---|---|
| 0 | - | 4 | 2, 4 |
| 10 | - | 1 | 33 |
| 40 | - | 3 | 3, 7*, 9 |
| 150 | 1 | 4 | 2, 3, 4, 27 |
Note: All animals were found-dead, except a female from the 40 mg/kg dose group which was sacrificed due to moribundity on Day 7*.
Highlights:
Preclinical toxicology studies of JD5037, a peripheral CB1R inverse agonist, were conducted in rats and dogs.
JD5037 exhibited non-linear kinetics in both the species.
Fed state increased plasma exposure of JD5037 in dogs by 4.4 to 4.6-fold compared to fasting state.
NOAEL in rats was 150 mg/kg/day, while in male and female dogs it was 20 mg/kg/day and 75 mg/kg/day, respectively.
Acknowledgements
This project has been funded in whole or in part with Federal funds from the National Center for Advancing Translational Sciences, National Institutes of Health (Contract No. HHSN261200800001E) under BrIDGs/NCATS program. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: This abstract and poster of this research work have been presented at Society of Toxicology 2018 meeting.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES:
- Carai MA, Colombo G, Maccioni P, Gessa GL. 2006. Efficacy of rimonabant and other cannabinoid cb1 receptor antagonists in reducing food intake and body weight: Preclinical and clinical data. CNS Drug Rev. 12(2):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman WN, Porter CJH, Mithani S, Dressman JB. 1997. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and ph. J Pharm Sci-Us. 86(3):269–282. [DOI] [PubMed] [Google Scholar]
- Chen W, Shui F, Liu C, Zhou X, Li W, Zheng Z, Fu W, Wang L. 2017. Novel peripherally restricted cannabinoid 1 receptor selective antagonist txx-522 with prominent weight-loss efficacy in diet induced obese mice. Front Pharmacol. 8:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorvat RJ. 2013. Peripherally restricted cb1 receptor blockers. Bioorganic & medicinal chemistry letters. 23(17):4751–4760. [DOI] [PubMed] [Google Scholar]
- Chorvat RJ, Berbaum J, Seriacki K, McElroy JF. 2012. Jd-5006 and jd-5037: Peripherally restricted (pr) cannabinoid-1 receptor blockers related to slv-319 (ibipinabant) as metabolic disorder therapeutics devoid of cns liabilities. Bioorganic & medicinal chemistry letters. 22(19):6173–6180. [DOI] [PubMed] [Google Scholar]
- Cinar R, Godlewski G, Liu J, Tam J, Jourdan T, Mukhopadhyay B, Harvey-White J, Kunos G. 2014. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 59(1):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. 2011. Guide for the care and use of laboratory animals: Eighth edition Washington, DC: The National Academies Press. [Google Scholar]
- Eichenbaum G, Damsch S, Looszova A, Vandenberghe J, Van den Bulck K, Roels K, Megens A, Knight E, Hillsamer V, Feyen B et al. 2011. Impact of gavage dosing procedure and gastric content on adverse respiratory effects and mortality in rat toxicity studies. J Appl Toxicol. 31(4):342–354. [DOI] [PubMed] [Google Scholar]
- Elwood C, Devauchelle P, Elliott J, Freiche V, German AJ, Gualtieri M, Hall E, den Hertog E, Neiger R, Peeters D et al. 2010. Emesis in dogs: A review. J Small Anim Pract. 51 (1):4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M 2017. Sex differences in human and animal toxicology. Toxicol Pathol. 45(1):172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao WC, Shia KS, Wang YT, Yeh YN, Chang CP, Lin Y, Chen PH, Wu CH, Chao YS, Hung MS. 2015. A novel peripheral cannabinoid receptor 1 antagonist, bpr0912, reduces weight independently of food intake and modulates thermogenesis. Diabetes Obes Metab. 17(5):495–504. [DOI] [PubMed] [Google Scholar]
- Kirilly E, Gonda X, Bagdy G. 2012. Cb1 receptor antagonists: New discoveries leading to new perspectives. Acta Physiol (Oxf). 205(1):41–60. [DOI] [PubMed] [Google Scholar]
- Koziolek M, Carriere F, Porter CJH. 2018. Lipids in the stomach - implications for the evaluation of food effects on oral drug absorption. Pharm Res. 35(3):55. [DOI] [PubMed] [Google Scholar]
- Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W. 2008. Effects of rimonabant, acannabinoid cb1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond). 32(5):863–870. [DOI] [PubMed] [Google Scholar]
- Pearse G 2006. Histopathology of the thymus. Toxicol Pathol. 34(5): 515–547. [DOI] [PubMed] [Google Scholar]
- Sam AH, Salem V, Ghatei MA. 2011. Rimonabant: From rio to ban. Journal of obesity. 2011:432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L, American Association for the Study of Liver D, United States F, Drug A. 2015. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an american association for the study of liver diseases-u.S. Food and drug administration joint workshop. Hepatology. 61 (4): 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha N, Cuffe JSM, Hutchinson DS, Headrick JP, Perkins AV, McAinch AJ, Hryciw DH. 2018. Peripheral modulation of the endocannabinoid system in metabolic disease. Drug discovery today. 23(3):592–604. [DOI] [PubMed] [Google Scholar]
- Spector AA. 1975. Fatty acid binding to plasma albumin. Journal of lipid research. 16(3): 165–179. [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS et al. 2012. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell metabolism. 16(2):167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Szanda G, Drori A, Liu Z, Cinar R, Kashiwaya Y, Reitman ML, Kunos G. 2017. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Molecular metabolism. 6(10):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattacheril J, Issa D, Sanyal A. 2018. Nonalcoholic steatohepatitis (nash) and hepatic fibrosis: Emerging therapies. Annu Rev Pharmacol Toxicol. 58:649–662. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. 1999. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid cb1 receptor knockout mice. Proc Natl Acad Sci U S A. 96(10):5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.