Supplemental Digital Content is Available in the Text.
Diabetic polyneuropathy and painful diabetic polyneuropathy are frequent in early type 2 diabetes, associated with modifiable risk factors, and have major impact on mental health.
Keywords: Painful diabetic polyneuropathy, Polyneuropathy, Neuropathic pain, Prevalence, Patient characteristics, Quality of life, Mental health
Abstract
Most studies of diabetic polyneuropathy (DPN) and painful DPN are conducted in persons with longstanding diabetes. This cross-sectional study aimed to estimate the prevalence of DPN and painful DPN, important risk factors, and the association with mental health in recently diagnosed type 2 diabetes. A total of 5514 (82%) patients (median diabetes duration 4.6 years) enrolled in the Danish Centre for Strategic Research in Type 2 Diabetes cohort responded to a detailed questionnaire on neuropathy and pain. A score ≥4 on the MNSI questionnaire determined possible DPN, whereas pain presence in both feet together with a score ≥3 on the DN4 questionnaire determined possible painful DPN. The prevalence of possible DPN and possible painful DPN was 18% and 10%, respectively. Female sex, age, diabetes duration, body mass index, and smoking were associated with possible DPN, whereas only smoking showed a clear association with possible painful DPN (odds ratio 1.52 [95% confidence interval: 1.20-1.93]). Possible DPN and painful DPN were independently and additively associated with lower quality of life, poorer sleep, and symptoms of depression and anxiety. Possible DPN itself had greater impact on mental health than neuropathic pain. This large study emphasizes the importance of careful screening for DPN and pain early in the course of type 2 diabetes.
1. Introduction
Diabetic polyneuropathy (DPN) is a serious diabetes complication. Previous studies have reported a wide range of prevalence from 26% to 50% for DPN,1,25,37,38,44 and between 8% and 30% for painful DPN.1,3,7,12,13,37,43 This variation may be explained by different assessment methods and definitions of DPN, and differentially selected study populations.28,31,34,40,42 Most studies have examined patients with long duration of diabetes, ie, 8 to 17 years,3,7,13,30,31,35,37,38,44 whereas little is known about the prevalence of DPN and painful DPN in recently diagnosed diabetes.
Accumulating evidence suggest that not only hyperglycemia, but also factors, such as increasing diabetes duration, type 2 vs type 1 diabetes, obesity, smoking, and female sex,2,3,7,11,13,23,30,31,35,37,38,44 may be linked to DPN and painful DPN, which particularly may be true in type 2 diabetes. However, existing studies are old,44 based on mixed population (eg, nondiabetes, type 1 diabetes, and type 2 diabetes3,7,12,13,38,44) include patients with longstanding diabetes,3,7,13,30,35,37,38,44 are of smaller size,3,7,12,30,35 or only investigate painful DPN.1,3,12 Less evidence on factors associated with DPN and painful DPN in recently diagnosed type 2 diabetes patients is available from large-scale studies.
In patients with diabetes, chronic neuropathic pain has been related to decreased quality of life (QoL), poor sleep, and symptoms of anxiety and depression.4,7,11,13,18,19,21,35,38,41 By contrast, the impact of DPN itself—regardless of pain—on QoL and mental health comorbidities is uncertain in type 2 diabetes. A study suggested that having DPN without painful symptoms had no effect on mental health-related measures,38 whereas other studies found depression to be more common both among patients with diabetes with painless and painful DPN.4,11
To fill these knowledge gaps, we conducted a questionnaire survey on neuropathy and pain in the large Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort that enrolls patients with recently diagnosed type 2 diabetes throughout Denmark. The aims of this article are (1) to explore the prevalence of possible DPN and painful DPN in recently diagnosed type 2 diabetes patients, (2) to investigate patient characteristics and lifestyle factors associated with possible DPN and painful DPN, and (3) to examine the impact of possible DPN and painful DPN on mental health in recently diagnosed type 2 diabetes.
2. Methods
2.1. Setting and patients
This study is based on the 7011 patients with type 2 diabetes consecutively enrolled in the DD2 cohort by February 2016. Detailed information on the logistics and characteristics of this cohort has previously been reported.10,26 In brief, the DD2 cohort began enrolment in November 2010, and the project is ongoing. Enrolment of newly or recently diagnosed (median diabetes duration at time of enrolment 1.3 year, interquartile range [IQR] 0.3-2.9 years) type 2 diabetes patients takes place at the general practitioner's office and outpatient hospital clinics (Departments of Endocrinology) in Denmark. During the DD2 enrolment period, all patients have been diagnosed with diabetes according to the WHO criteria.10
2.2. Questionnaire
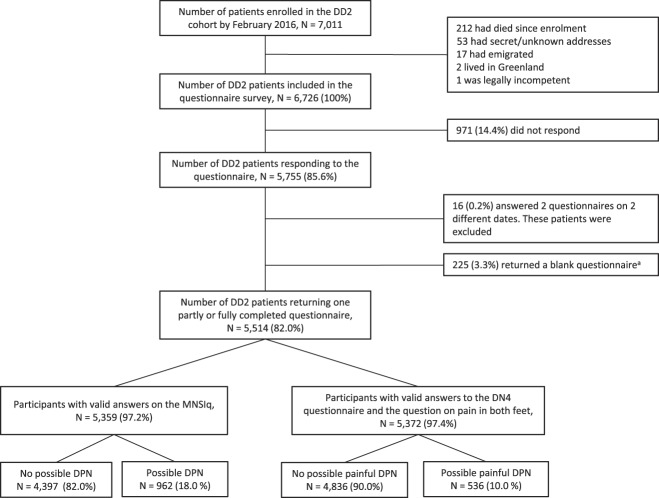
By June 7, 2016, a detailed questionnaire consisting of 41 questions was sent out to all patients alive and living in Denmark with a known address enrolled into DD2 (N = 6726) (Fig. 1). A complete version of the questionnaire is available in the supplementary digital content (supplementary Table 1, available as supplemental digital content at http://links.lww.com/PAIN/A903). In September 2016 and again in October 2016, a reminder was sent to those who had not provided a response. All patients were sent a paper version and a link to an electronic version allowing them to answer in their preferred way. All patients were asked to return a blank questionnaire including a note of the reason if they did not want to participate in the questionnaire survey. A subsample of the cohort was invited for a detailed clinical examination, and these results will be presented in a separate publication.
Figure 1.
Flowchart of study population. DD2, Danish Centre for strategic research in type 2 diabetes; MNSIq, Michigan Neuropathy Screening Instrument questionnaire, DN4, Douleur Neuropathique en 4 questions. aReason for nonparticipation: No reason provided: 89 (39.6%), no surplus energy because of other comorbidity: 18 (8.0%), no surplus energy because of death/illness among near relative: 3 (1.3%), dementia and other conditions hindering adequate answers to the questionnaire: 21 (9.3%), too busy/no free time: 4 (1.8%), well-regulated/solely diet-treated thus feeling the questionnaire is not relevant: 25 (11.1%), mail delivery not possible (invalid address, full or locked mailbox): 31 (13.8%), died in the period February to end of questionnaire survey: 9 (4.0%), and other single reasons: 25 (11.1%).
2.3. Patient characteristics
Patient demographics included in the questionnaire were age, sex, height, and weight. Lifestyle factors included smoking habits, alcohol consumption (>7/14 units of alcohol [women/men], which is the maximum safe amount recommended by the Danish Health Authority), and questions on physical activity level.
2.4. Diabetic polyneuropathy
There is no gold standard for identifying polyneuropathy for epidemiological research purposes, but the Michigan Neuropathy Screening Instrument questionnaire part (MNSIq)15 is a commonly used symptom-based screening tool for identifying DPN.2,8,43 We used the MNSIq and the validated cutoff of ≥4/13 abnormal responses to define possible DPN.15,33 This cutoff had a sensitivity of 40% and a specificity of 92% for detecting confirmed clinical neuropathy in a selected group of patients with longstanding type 1 diabetes.24
Questions on gait instability and falls, as well as frequency and severity of falls, were also included in the questionnaire.
2.5. Painful diabetic polyneuropathy and other pain
The questionnaire contained questions on general pain (any constant or recurrent pain and location of pain) and pain in both feet. Patients reporting pain in both feet were given more detailed questions about the pain. They filled out the 7-item Douleur Neuropathique en 4 Questions (DN4) that is a screening tool for neuropathic pain and with a high performance in DPN.32,35 The DN4 questionnaire comprises 7 “yes” or “no” items related to pain quality; 4 sensory descriptors (tingling, pins and needles, numbness, and itching) and 3 pain descriptors (burning, painful cold, and electric shock sensation). Only patients with pain in the feet completed the DN4, and it was specified that it concerned characteristics of the pain in their feet (Supplementary table 1, available as supplemental digital content at http://links.lww.com/PAIN/A903). A DN4 score of ≥3/7 has a sensitivity and specificity of 84% for identifying clinically confirmed painful DPN.32 Patients with pain in both feet and a DN4 score ≥3 were considered to have possible painful DPN6,17,32,35 regardless of MNSIq score (Fig. 2). Our neuropathic pain definition was in accordance with the consensus statement (NeuroPPIC) from the Neuropathic Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP) for the basic “entry level” to identify possible neuropathic pain in questionnaire studies.39 We included additional questions on pain quality, use of pain medications, and pain duration and pain intensity within the previous 24 hours and 7 days at the time of evaluation were recorded. For the latter, we used a numeric rating scale (NRS) ranging from 0 to 10, with 0 denoting no pain and 10 the worst possible pain. We used the Patient-Reported Outcome Measurement Information System (PROMIS) short form v1.0—Pain Interference 4a to assess pain interference with daily activities, household, and social activities within the previous 7 days.22
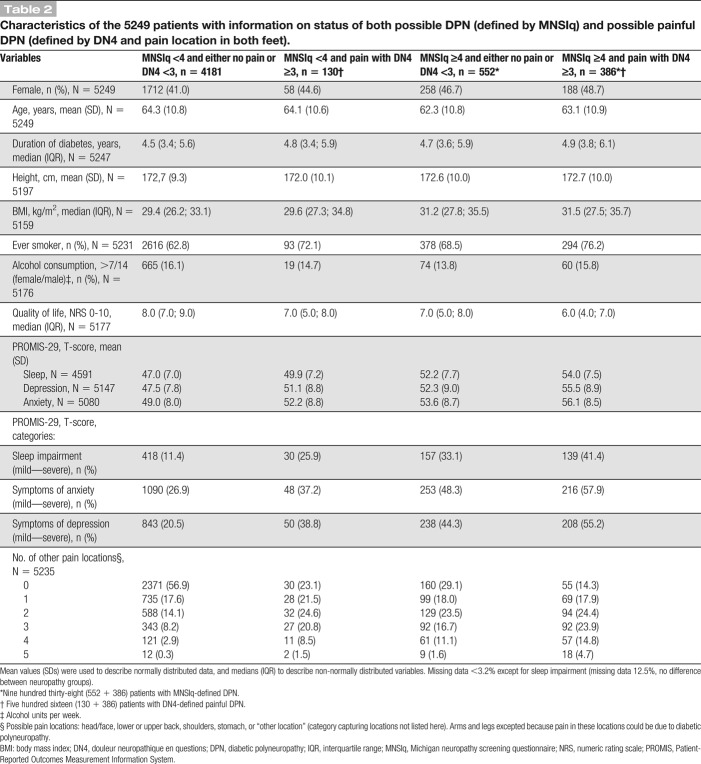
Figure 2.
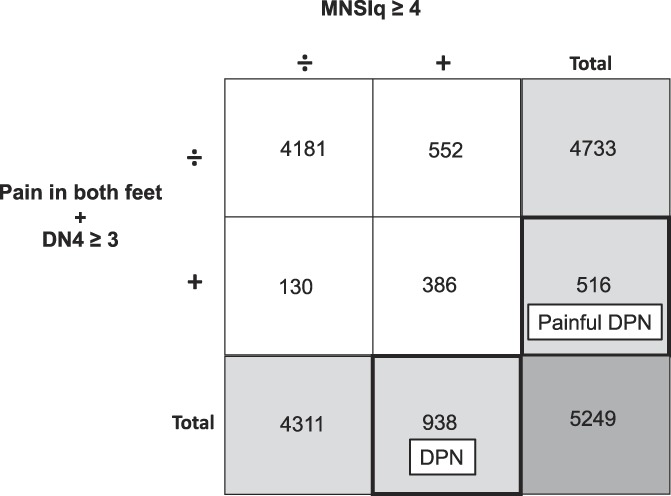
Possible DPN and possible painful DPN definitions. MNSIq, Michigan Neuropathy Screening Instrument questionnaire, DN4, Douleur Neuropathique en 4 questions. The numbers in the figure corresponds to the distribution of patients in the cohort of patients with available data on the criteria for both possible DPN and painful DPN (N = 5249). The numbers are evident from Table 2. DPN, diabetic polyneuropathy.
2.6. Mental health
The patients rated their QoL in the previous 7 days using an NRS ranging from 0 to 10, with 10 being the best QoL possible and 0 the worst.20 Sleep disturbance and symptoms of depression and anxiety were assessed using the PROMIS Short Forms 4a. The instruments grade symptoms experienced during the previous 7 days with a frequency or severity grading of symptoms from “never” to “always” or from “bad” to “very good” with 5 options. The scores are converted into PROMIS T-scores, which are standardized relative to an American/US reference population and are used to categorize the level of impairment/symptoms (normal, mild, moderate, and severe).22,29
2.7. Ethical considerations
All DD2 patients volunteered to participate in the DD2 study and gave written informed consent. The Danish National Committee on Health Research Ethics (record number S-20100082) has approved the DD2 project. The Danish Data Protection Agency (record number 2008‐58‐0035) has approved the DD2 project, and the study is registered at Aarhus University internal notification no. 62908-250.
2.8. Statistical analyses
Mean values (±SD) were used to describe normally distributed data, and medians (IQR) to describe non-normally distributed variables. Information on both DPN (defined based on MNSIq) and painful DPN (defined based on DN4 and pain location in both feet) status was available for a subpopulation of 5249 patients. Combination of MNSIq-defined possible DPN and DN4-defined possible painful DPN status yielded 4 distinct groups (Fig. 2) for which descriptive data were provided. Finally, descriptive data on age, sex, and diabetes duration were provided for responders and nonresponders.
We calculated the prevalence of DPN and painful DPN with 95% confidence intervals (CIs) using the exact method for binomial distributions.
We used multivariable linear (age, body mass index [BMI], diabetes duration, and height) and logistic (sex, smoking status [ever (former + current) vs never], and alcohol consumption) regressions and modeled each patient characteristic as a function of possible DPN and possible painful DPN and an interaction term of DPN and painful DPN, while controlling for age, sex, and diabetes duration. If no significant interaction was observed between DPN and painful DPN, each regression was rerun without the interaction term. The significance level was chosen at <0.05. The cross-sectional study design facilitates an investigation of associations, not of temporal relationships. Therefore, possible DPN and possible painful DPN could be included as the independent variables enabling us to include both DPN and painful DPN simultaneously in the multivariable regression models used to examine associations with patient characteristics. This approach allowed us (1) to investigate the association of the evaluated patient characteristics and possible DPN defined by DN4 and pain in both feet separately from the association with possible DPN defined by MNSIq, and (2) to handle the fact that some patients had possible painful DPN but had an MNSIq score <4 (Fig. 2), including the evaluation of possible interaction between possible DPN defined by MNSIq and possible painful DPN defined by DN4.
To evaluate the impact of DPN and painful DPN on mental health, we used the same approach as described above, a multivariable linear regression to model QoL and T-scores for sleep, depression, and anxiety as functions of DPN and painful DPN and adjusted for age, sex, diabetes duration, and BMI (model 1). To control for possible confounding by pain other than neuropathic pain in the feet, the regressions were rerun including a variable of the number of pain locations other than extremities (model 2). Because obesity is strongly associated with mental health outcomes, we adjusted for BMI. However, the direction of the association of BMI-mental health could be bidirectional, and thus, we performed a sensitivity analysis in which we left out BMI in the regressions. All regressions were first run including an interaction term between DPN and painful DPN, and if no interaction was observed, the regressions were rerun without the interaction term. We used a Wald test to compare the sizes of the associations of DPN and painful DPN with mental health outcomes in the regression models without interaction term.
Finally, the correlation between mental health and pain intensity in the feet was estimated using Spearman's rho.
There were few missing data, and all analyses were performed as complete case analyses.
Data were analyzed using STATA version 14.
3. Results
3.1. Patient population
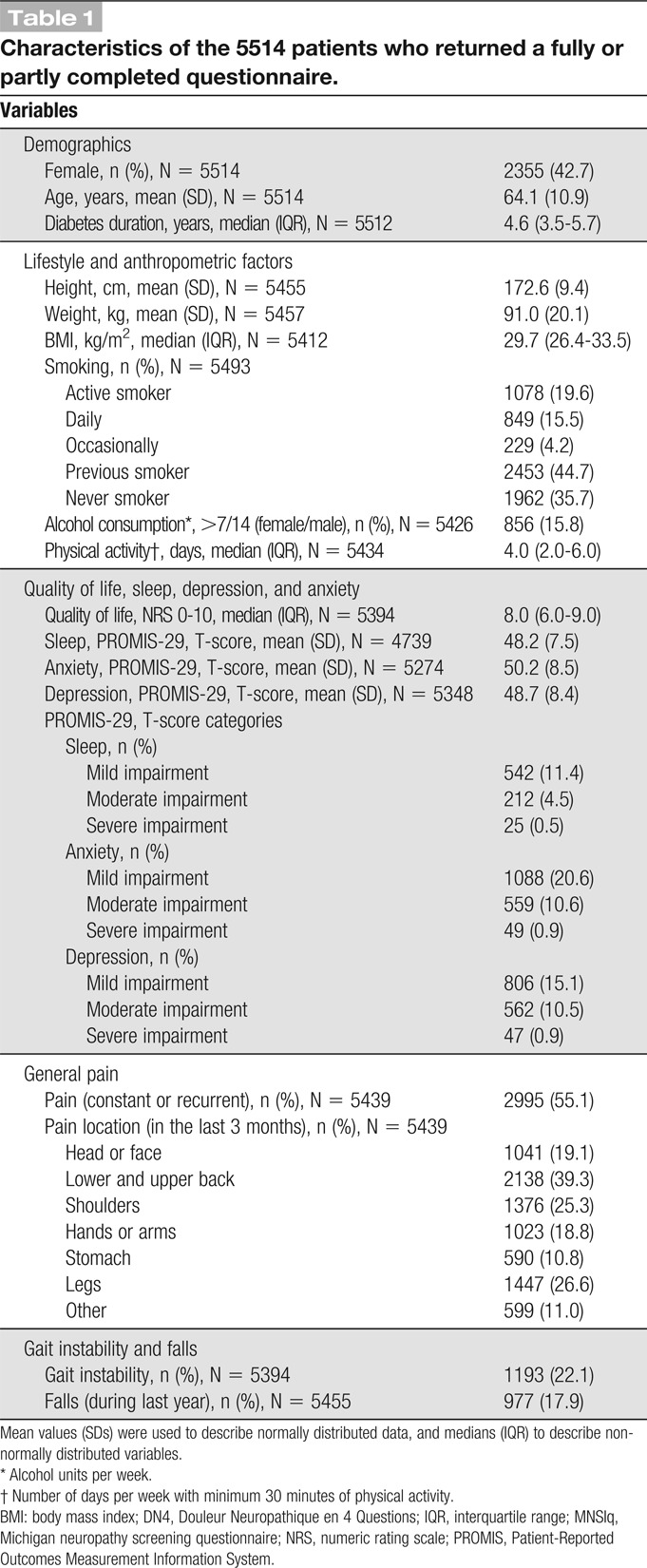
As seen in Figure 1, the number of patients responding to the questionnaire was 5755 (85.6%). Of these, 225 (3.3%) returned a blank questionnaire (136 [60.4%] patients provided a reason for nonparticipating), and 16 (0.2%) patients were excluded because they answered the questionnaire multiple times. Of the remaining 5514 patients (82% of those who initially received a questionnaire), 42.7% were women, mean (±SD) age was 64.1 (10.9) years, and median duration of diabetes (IQR) was 4.6 (3.5-5.7) years. Further patient characteristics are provided in Table 1. Diabetes duration and sex distribution were similar among responders and nonresponders (supplementary Table 2, available as supplemental digital content at http://links.lww.com/PAIN/A903), but nonresponders were slightly younger than responders (mean age [±SD] 59.6 [12.8] vs 64.1 [10.9]).
Table 1.
Characteristics of the 5514 patients who returned a fully or partly completed questionnaire.
3.2. Prevalence
Of the 5359 patients with valid answers on the MNSIq, 962 had a score ≥4, suggesting a prevalence of possible DPN of 18.0% (95% CI: 16.9%-19.0%) (Fig. 1; supplementary Table 3, available as supplemental digital content at http://links.lww.com/PAIN/A903).
Of the 5372 patients with valid data to assess painful DPN, 536 reported pain in both feet and had a DN4 score ≥3, corresponding to a prevalence of possible painful DPN of 10.0% (95% CI 9.2%-10.8%) (Fig. 1; supplementary Table 3, available as supplemental digital content at http://links.lww.com/PAIN/A903). Of those with painful DPN, 130 (28.0%) did not fulfill the MNSIq criteria for DPN (Table 2).
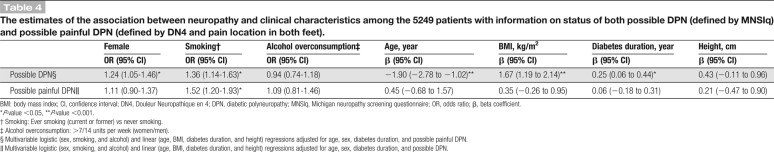
Table 2.
Characteristics of the 5249 patients with information on status of both possible DPN (defined by MNSIq) and possible painful DPN (defined by DN4 and pain location in both feet).
Prevalence was stable across questionnaire intervals (supplementary Table 4, available as supplemental digital content at http://links.lww.com/PAIN/A903).
3.3. Pain: painful diabetic polyneuropathy
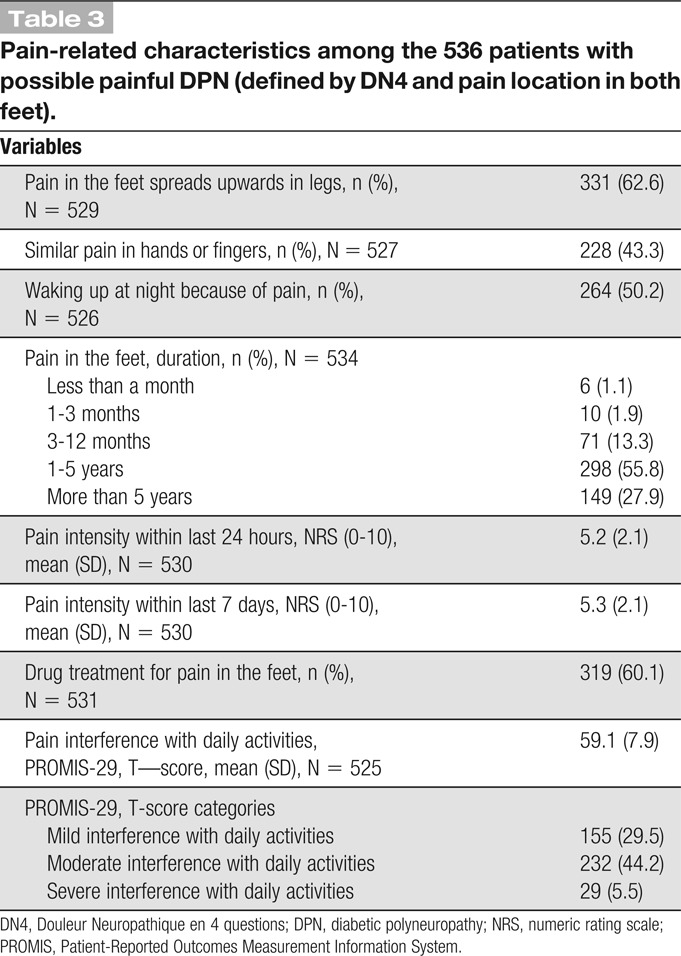
As shown in Table 3, more than 80% of the patients with painful DPN had pain in the feet for more than 1 year. Pain often interfered with daily activities, including household chores and social activities (79.2%), and 60.1% reported concomitant drug treatment for their pain. The average (±SD) pain intensity in the feet was 5.3 (2.1) the last 7 days on an NRS (0-10), and 76.2% had moderate to severe pain intensity (NRS ≥4). The most common pain description from the DN4 was burning pain (71.8%), 36.4% reported cold pain, and 38.2% had electric shock like pain (data not shown). There was a negative correlation between reported QoL and the intensity of pain (Spearman's rho −0.24, P < 001) and a positive but weak correlation between reported symptoms of anxiety, depression, and poor sleep and pain in the feet within the last 7 days (Spearman's rho 0.25, 0.23, and 0.26, P < 0.001) (data not shown).
Table 3.
Pain-related characteristics among the 536 patients with possible painful DPN (defined by DN4 and pain location in both feet).
The small group of patients with painful DPN that did not fulfill the MNSIq criteria for DPN (N = 130) did not differ from those with painful DPN fulfilling the MNSIq criteria (N = 386) regarding age, sex, duration of diabetes, and use of pain medications (Table 2). However, they reported lower mean (±SD) pain intensity (average 7 days: 4.3 [2.1] vs 5.6 [2.1] [data not shown]). The most common pain descriptors on the MNSIq were prickling feeling, burning pain, and leg pain in both groups (supplementary Fig. 1A, available as supplemental digital content at http://links.lww.com/PAIN/A903).
3.4. Pain: pain other than painful diabetic polyneuropathy
A higher proportion of patients with possible DPN and possible painful DPN had complaints of pain in various body sites compared to those with no DPN (Table 2). The proportion of patients reporting pain at 2 or more locations other than the extremities was 24.5% in those without DPN, 55.4% in those with painful DPN not fulfilling the MNSIq criteria for DPN, 52.7% in those with DPN not fulfilling the criteria for painful DPN, and 67.6% in those with painful DPN fulfilling the MNSIq criteria for DPN (Table 2).
3.5. Association between diabetic polyneuropathy and painful diabetic polyneuropathy and patient characteristics
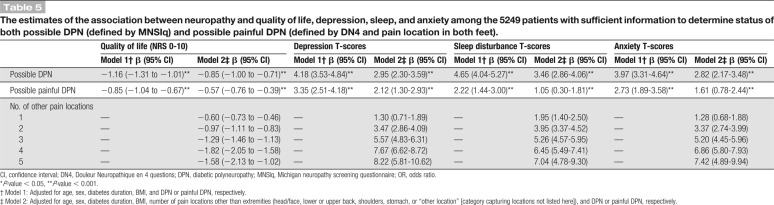
We found no statistically significant interaction between possible DPN defined by MNSIq and possible painful DPN defined by DN4 and pain in both feet, suggesting that the estimates of association between possible DPN and patient characteristics were independent of the presence of possible painful DPN, and vice versa.
After correction for age, sex, diabetes duration, and painful DPN, DPN was statistically significantly associated with younger age, longer duration of diabetes, higher BMI, female sex, and presence of ever tobacco smoking (Table 4). Associations were generally weaker for painful DPN except for ever tobacco smoking that was statistically significant associated with painful DPN (odds ratio: 1.52 [1.20-1.93]) (Table 4).
Table 4.
The estimates of the association between neuropathy and clinical characteristics among the 5249 patients with information on status of both possible DPN (defined by MNSIq) and possible painful DPN (defined by DN4 and pain location in both feet).
3.6. Association between diabetic polyneuropathy, painful diabetic polyneuropathy, and mental health
Again, we found no statistically significant interaction between possible DPN defined by MNSIq and possible painful DPN defined by DN4 and pain in both feet, suggesting that the estimates of association between possible DPN and mental health outcomes were independent of the estimates of association of possible painful DPN, and vice versa.
Both DPN and painful DPN were independently and additively associated with lower QoL (DPN: −1.16 [−1.31 to −1.01], painful DPN: −0.85 [−1.04 to −0.67]), and higher T-scores of depression (DPN: 4.18 [3.53-4.84], painful DPN: 3.35 [2.51-4.18]), poor sleep (DPN: 4.65 [4.04-5.27], painful DPN: 2.22 [1.44-3.00]), and anxiety (DPN: 3.97 [3.31-4.64], painful DPN: 2.73 [1.89-3.58]) after controlling for age, sex, diabetes duration, BMI, and DPN or painful DPN status (Table 5). The size of the effect of DPN on mental health outcomes was in general higher than that of painful DPN (Supplementary Table 5, supplementary Fig. 2, available as supplemental digital content at http://links.lww.com/PAIN/A903).
Table 5.
The estimates of the association between neuropathy and quality of life, depression, sleep, and anxiety among the 5249 patients with sufficient information to determine status of both possible DPN (defined by MNSIq) and possible painful DPN (defined by DN4 and pain location in both feet).
Further controlling for pain in other bodily localizations reduced the effect size of the associations, eg, for depression (DPN: 2.95 [2.30-3.59], painful DPN: 2.12 [1.30-2.93]). The total effect of fulfilling both the criteria for DPN and painful DPN on eg, QoL score (−0.85 + −0.57 = −1.42) was of the same order of magnitude as having pain in 3 other areas/locations (−1.29), eg, headache, back pain, and stomach pain (Table 5).
Leaving BMI out of the models resulted in slightly higher DPN and painful DPN estimates for all mental health outcomes, thus not changing any conclusions (supplementary Table 6, available as supplemental digital content at http://links.lww.com/PAIN/A903).
4. Discussion
In this large study of a nationwide cohort with recently diagnosed type 2 diabetes patients, the prevalence of possible DPN was 18% and the prevalence of possible painful DPN was 10%. We found an association between possible DPN and female sex, smoking, longer diabetes duration, lower age, and higher BMI, whereas most relations were weaker for possible painful DPN that was only statistically significant associated with smoking. By contrast, both possible DPN and painful DPN were independently and additively associated with decreased QoL and increased symptoms of depression, anxiety, and poor sleep. Moreover, possible DPN had greater impact on mental health than possible neuropathic pain.
This is the largest questionnaire study to date that examines the prevalence and clinical characteristics of possible DPN and painful DPN in a cohort of recently diagnosed type 2 diabetes patients using validated screening tools. The prevalence of DPN (18%) and painful DPN (10%) found in this study are similar to the prevalence reported in 2 survey studies using the MNSIq for the diagnosis of DPN and the MNSIq in combination with brief pain inventory (BPI) to diagnose painful DPN.2,43 In the ADDITION Denmark cohort study consisting of 1445 screenings-detected type 2 diabetes patients, the prevalence of DPN at time of diabetes diagnosis was of 13.1%,2 whereas in a French nationwide cohort study consisting of 1023 patients with type 1 and 2 diabetes with a mean duration of diabetes of 15 years, the prevalence of painful DPN was 8% using a MNSIq cutoff of 7 as compared to 4 in our study.43 In a large UK study of patients with diabetes in a community health care setting, the duration of diabetes was similar to our study (median 5 years), but the prevalence estimate of painful DPN was twice as high or 21%.1 This difference may be related to painful DPN being defined based on a clinical evaluation in the UK study. Other studies of more longstanding diabetes have likewise reported higher prevalence of both DPN and painful DPN than our study.1,3,25,31,37,38 These differences may be partly explained by the longer diabetes duration, but also by the different diagnostic criteria used for DPN and painful DPN. Thus, our use of questionnaire-based tools to determine DPN and painful DPN in the absence of clinical examination and confirmatory tests reduces the level of certainty of the DPN diagnoses.14,17,33 Moreover, the sensitivity of an MNSIq score ≥4 was 40% compared with clinically defined DPN in a study of younger patients with longstanding type 1 diabetes, and thus, we likely also underestimate DPN prevalence in our cohort.
Our associations of female sex, smoking, higher BMI, and longer duration of diabetes with DPN in recently diagnosed type 2 diabetes corroborate previous studies of patients with longstanding diabetes.23,27,44 However, in contrast to some previous studies, we only observed an association of painful DPN with smoking status and not with, eg, sex, age, and BMI.1,23,31,38 An explanation may be our analytical approach which—in comparison with most previous studies—allowed us to disentangle the effect of the risk factor on pain occurrence in DPN independent from that on DPN risk itself.23,27,44 Moreover, power was reduced for painful DPN due to the lower prevalence; however, the estimates were smaller for painful DPN than DPN. We did not observe an association between body height and DPN, although it has been proposed that tall stature is a risk factor for peripheral neuropathy due to increased nerve length and nerve surface area.9 Surprisingly, we observed that DPN was negatively associated with age. Increasing age is generally a marker of longer diabetes duration; however, the DD2 enrolls patients with type 2 diabetes around time of diabetes diagnosis. A younger age at time of diagnosis is a marker of a worse phenotype,5 which may explain our observation of a negative association of age and DPN. Moreover, nonresponders were in general younger, and we cannot exclude that part of the age association may be explained by a responder bias if nonresponders have DPN to a lesser extent than responders.
Our observation that painful DPN was associated with lower QoL and symptoms of depression, anxiety, and poor sleep is consistent with previous studies of diabetes.7,35,38 However, we also observed a tendency towards that DPN itself was associated with worse mental health independent of neuropathic pain, which has been observed in some4,11 but not all studies,38 and we even observed that DPN itself (MNSIq-defined) had a stronger association with worse mental health outcomes than neuropathic pain. In accordance, the correlation between pain intensity and mental health outcomes was weak. The effect of DPN and painful DPN on mental health measures was additive, and thus, those fulfilling both the DPN and painful DPN criteria had the most severe symptoms, which is in accordance with an Italian study showing more severe depressive symptoms among those with painful DPN as compared to those with nonpainful using Beck depression inventory II.11 In concert with other studies, many of the patients in all 3 neuropathy groups had complaints of general pain (eg, back and neck pain, headache, and stomachache).21,35 The effect size of general pain in 2 bodily localizations on QoL, depression, anxiety, and sleep scores was of a similar order of magnitude as that of DPN and painful DNP. Patients with possible DPN and painful DPN more often had pain at other locations than the group without any DPN, also suggesting that positive answers to the MNSIq and pain in the feet could be due to other causes than DPN.
A large proportion (3/4) of the patients with painful DPN reported neuropathic pain of moderate to severe intensity (NRS ≥4) and 60.1% reported use of pain medication. This is similar to results published before.7,21 Pain intensity was positively correlated with symptoms of anxiety, depression, and sleep disturbance. The fact that many of the patients had moderate to severe pain intensity despite taking drugs for their pain may indicate either inappropriate treatment or a lack of effective neuropathic drug treatments.16
The main strength of this questionnaire study is the large sample size, the high response rate (85.6%), and the low level of missing data. Reassuringly, similar estimates of the prevalence were observed across questionnaire intervals.
The DD2 cohort enrolls patients from primary care and hospital outpatient clinics. Since around half of the patients have been enrolled from hospital outpatient clinics, the DD2 cohort may hold patients with more severe diabetes than the average type 2 diabetes population in Denmark. However, baseline data from the DD2 cohort are similar to data from a cohort of type 2 patients receiving their first glucose-lowering drug indicating that the DD2 cohort is representative of recently diagnosed type 2 diabetes patients in Denmark.10,36 The cross-sectional design of this study has some innate limitations including the inability to determine temporal relationships. Finally, we lack information on other diabetes complications and comorbidity, which can affect QoL-related outcome measures.
In conclusion, in this largest questionnaire study of possible DPN in recently diagnosed type 2 diabetes patients, a significant proportion of patients had possible DPN and possible painful DPN. The presence of possible DPN was associated with female sex, longer diabetes duration, higher BMI, and smoking, whereas smoking was the only factor clearly associated with painful DPN. Patients with possible DPN and painful DPN reported lower QoL and more symptoms of anxiety, depression, and poor sleep. Since DPN in recently diagnosed diabetes patients is associated with modifiable risk factors and has major impact on QoL, it is important to carefully screen for this early complication in type 2 diabetes.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A903.
Supplementary Material
Acknowledgments
The authors sincerely thank all DD2 study participants including the patients and the staff in private practices and outpatient hospital clinics. The authors are very grateful to Professor E. Feldman, University of Michigan, for valuable comments on the manuscript.
The Danish Centre for Strategic Research in Type 2 Diabetes Project (DD2) is supported by the Danish Agency for Science [grant number 09-067009, 09-075724], the Danish Health and Medicines Authority, the Danish Diabetes Association, and an unrestricted donation from Novo Nordisk A/S. Project partners are listed on the website (www.dd2.nu). The International Diabetic Neuropathy Consortium (IDNC) research programme is supported by a Novo Nordisk Foundation Challenge Programme grant (Grant number NNF14OC0011633). None of the study funders were involved in the design of the study; the collection, analysis, and interpretation of data; writing the manuscript; or the decision to submit the manuscript for publication. T.S. Jensen and N.B. Finnerup are members of the DOLORisk consortium funded by the European Commission Horizon 2020 (ID633491).
Data availability: More information about the DD2 cohort can be found at the Danish DD2 website www.dd2.nu. The DD2 national advisory forum strongly encourages national and international collaboration and application form can be found through this link https://dd2.nu/media/1264/standard-dd2-protocol_final.doc. Interested researchers can contact DD2 through Assistant Professor Jens Steen Nielsen at: jsn@rsyd.dk.
Preliminary results were presented at the annual SASP (the Scandinavian Association for the Study of Pain) April 27-28, 2017, Aalborg, Denmark, and the 6th International Congress on Neuropathic Pain (NeuPSIG 2017), June 15-18, Gothenburg, Sweden.
Author contributions: S.S. Gylfadottir designed the study, performed the statistical analyses, drafted the manuscript, contributed to the discussion, and approved the final manuscript. D.H. Christensen collected the data, designed the study, performed the statistical analyses, drafted the manuscript, contributed to the discussion, and approved the final manuscript. S.K. Nicolaisen designed the study, researched the data, revised the manuscript critically, contributed to the discussion, and approved the final manuscript. N.T. Andersen, R.W. Thomsen, N.B. Finnerup, B.C. Callaghan, J.S. Nielsen, S.H. Sindrup, H. Andersen, M. Itani, K.S. Khan, A.G. Kristensen, and T.S. Jensen designed the study, revised the manuscript critically, contributed to the discussion, and approved the final manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
S.S. Gylfadottir and D.H. Christensen are shared first authors. R.W. Thomsen and N.B. Finnerup are shared last authors.
References
- [1].Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes care 2011;34:2220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, Jensen TM, Finnerup NB, Jensen TS, Lauritzen T, Feldman EL, Callaghan BC, Charles M. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes care 2018;41:1068–75. [DOI] [PubMed] [Google Scholar]
- [3].Aslam A, Singh J, Rajbhandari S. Prevalence of painful diabetic neuropathy using the self-completed Leeds assessment of neuropathic symptoms and signs questionnaire in a population with diabetes. Can J Diabetes 2015;39:285–95. [DOI] [PubMed] [Google Scholar]
- [4].Bai JW, Lovblom LE, Cardinez M, Weisman A, Farooqi MA, Halpern EM, Boulet G, Eldelekli D, Lovshin JA, Lytvyn Y, Keenan HA, Brent MH, Paul N, Bril V, Cherney DZI, Perkins BA. Neuropathy and presence of emotional distress and depression in longstanding diabetes: results from the Canadian study of longevity in type 1 diabetes. J Diabetes Complications 2017;31:1318–24. [DOI] [PubMed] [Google Scholar]
- [5].Bo A, Thomsen RW, Nielsen JS, Nicolaisen SK, Beck-Nielsen H, Rungby J, Sorensen HT, Hansen TK, Sondergaard J, Friborg S, Lauritzen T, Maindal HT. Early-onset type 2 diabetes: age gradient in clinical and behavioural risk factors in 5115 persons with newly diagnosed type 2 diabetes-results from the DD2 study. Diabetes Metab Res Rev 2018;34:e2968. [DOI] [PubMed] [Google Scholar]
- [6].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [7].Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One 2013;8:e74195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cardinez N, Lovblom LE, Bai JW, Lewis E, Abraham A, Scarr D, Lovshin JA, Lytvyn Y, Boulet G, Farooqi MA, Orszag A, Weisman A, Keenan HA, Brent MH, Paul N, Bril V, Cherney DZ, Perkins BA. Sex differences in neuropathic pain in longstanding diabetes: results from the Canadian Study of Longevity in type 1 diabetes. J Diabetes Complications 2018;32:660–4. [DOI] [PubMed] [Google Scholar]
- [9].Cheng YJ, Gregg EW, Kahn HS, Williams DE, De Rekeneire N, Narayan KM. Peripheral insensate neuropathy—a tall problem for US adults? Am J Epidemiol 2006;164:873–80. [DOI] [PubMed] [Google Scholar]
- [10].Christensen DH, Nicolaisen SK, Berencsi K, Beck-Nielsen H, Rungby J, Friborg S, Brandslund I, Christiansen JS, Vaag A, Sorensen HT, Nielsen JS, Thomsen RW. Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open 2018;8:e017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].D'Amato C, Morganti R, Greco C, Di Gennaro F, Cacciotti L, Longo S, Mataluni G, Lauro D, Marfia GA, Spallone V. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diabetes Vasc Dis Res 2016;13:418–28. [DOI] [PubMed] [Google Scholar]
- [12].Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabetic Med 2004;21:976–82. [DOI] [PubMed] [Google Scholar]
- [13].Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes care 2006;29:1518–22. [DOI] [PubMed] [Google Scholar]
- [14].England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetrical polyneuropathy: a definition for clinical research. A report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Arch Phys Med Rehabil 2005;86:167–74. [DOI] [PubMed] [Google Scholar]
- [15].Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes care 1994;17:1281–9. [DOI] [PubMed] [Google Scholar]
- [16].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 2000;47:123–8. [DOI] [PubMed] [Google Scholar]
- [19].Geelen CC, Smeets R, Schmitz S, van den Bergh JP, Goossens M, Verbunt JA. Anxiety affects disability and quality of life in patients with painful diabetic neuropathy. Eur J Pain 2017;21:1632–41. [DOI] [PubMed] [Google Scholar]
- [20].Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA 1994;272:619–26. [PubMed] [Google Scholar]
- [21].Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain 2006;7:892–900. [DOI] [PubMed] [Google Scholar]
- [22].HealthMeasures. PROMIS health measures. Availale at: http://www.healthmeasures.net/explore-measurement-systems/promis. Accessed May 1, 2019.
- [23].Hebert HL, Veluchamy A, Torrance N, Smith BH. Risk factors for neuropathic pain in diabetes mellitus. PAIN 2017;158:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, Feldman EL. Use of the Michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabetic Med 2012;29:937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, Lim J, Malik RA, Alam U. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther 2018;40:828–49. [DOI] [PubMed] [Google Scholar]
- [26].Nielsen JS, Thomsen RW, Steffensen C, Christiansen JS. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) study: implementation of a nationwide patient enrollment system. Clin Epidemiol 2012;4(suppl 1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabet Stud 2015;12:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ 2014;348:g1799. [DOI] [PubMed] [Google Scholar]
- [29].Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 2011;18:263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Raputova J, Srotova I, Vlckova E, Sommer C, Uceyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, Forer L, Belobradkova J, Olsovsky J, Weber P, Dusek L, Jarkovsky J, Bednarik J. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. PAIN 2017;158:2340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Curr Diabetes Rep 2013;13:533–49. [DOI] [PubMed] [Google Scholar]
- [32].Spallone V, Morganti R, D'Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabetic Med 2012;29:578–85. [DOI] [PubMed] [Google Scholar]
- [33].Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012;28(suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- [35].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice AS, Bennett DL. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. PAIN 2016;157:1132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thomsen RW, Baggesen LM, Svensson E, Pedersen L, Norrelund H, Buhl ES, Haase CL, Johnsen SP. Early glycaemic control among patients with type 2 diabetes and initial glucose-lowering treatment: a 13-year population-based cohort study. Diabetes Obes Metab 2015;17:771–80. [DOI] [PubMed] [Google Scholar]
- [37].Truini A, Spallone V, Morganti R, Tamburin S, Zanette G, Schenone A, De Michelis C, Tugnoli V, Simioni V, Manganelli F, Dubbioso R, Lauria G, Lombardi R, Jann S, De Toni Franceschini L, Tesfaye S, Fiorelli M, Spagnoli A, Cruccu G. A cross sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. PAIN 2018;159:2658–66. [DOI] [PubMed] [Google Scholar]
- [38].Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, Mathieu C, Colin IM. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–13. [DOI] [PubMed] [Google Scholar]
- [39].van Hecke O, Kamerman PR, Attal N, Baron R, Bjornsdottir G, Bennett DL, Bennett MI, Bouhassira D, Diatchenko L, Freeman R, Freynhagen R, Haanpaa M, Jensen TS, Raja SN, Rice AS, Seltzer Z, Thorgeirsson TE, Yarnitsky D, Smith BH. Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies: a NeuPSIG systematic review, Delphi survey, and expert panel recommendations. PAIN 2015;156:2337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med 2008;9:660–74. [DOI] [PubMed] [Google Scholar]
- [41].Vileikyte L, Peyrot M, Gonzalez JS, Rubin RR, Garrow AP, Stickings D, Waterman C, Ulbrecht JS, Cavanagh PR, Boulton AJ. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia 2009;52:1265–73. [DOI] [PubMed] [Google Scholar]
- [42].Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am 2013;42:747–87. [DOI] [PubMed] [Google Scholar]
- [43].Wu EQ, Borton J, Said G, Le TK, Monz B, Rosilio M, Avoinet S. Estimated prevalence of peripheral neuropathy and associated pain in adults with diabetes in France. Curr Med Res Opin 2007;23:2035–42. [DOI] [PubMed] [Google Scholar]
- [44].Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A903.