Supplemental Digital Content is available in the text.
Keywords: acute lung injury, exhalation, pulmonary gas exchange, pulmonary ventilation, respiratory system, ventilators, mechanical
Objectives:
Lung-protective ventilation for acute respiratory distress syndrome aims for providing sufficient oxygenation and carbon dioxide clearance, while limiting the harmful effects of mechanical ventilation. “Flow-controlled ventilation”, providing a constant expiratory flow, has been suggested as a new lung-protective ventilation strategy. The aim of this study was to test whether flow-controlled ventilation attenuates lung injury in an animal model of acute respiratory distress syndrome.
Design:
Preclinical, randomized controlled animal study.
Setting:
Animal research facility.
Subjects:
Nineteen German landrace hybrid pigs.
Intervention:
Flow-controlled ventilation (intervention group) or volume-controlled ventilation (control group) with identical tidal volume (7 mL/kg) and positive end-expiratory pressure (9 cm H2O) after inducing acute respiratory distress syndrome with oleic acid.
Measurements and Main Results:
Pao2 and Paco2, minute volume, tracheal pressure, lung aeration measured via CT, alveolar wall thickness, cell infiltration, and surfactant protein A concentration in bronchoalveolar lavage fluid. Five pigs were excluded leaving n equals to 7 for each group. Compared with control, flow-controlled ventilation elevated Pao2 (154 ± 21 vs 105 ± 9 torr; 20.5 ± 2.8 vs 14.0 ± 1.2 kPa; p = 0.035) and achieved comparable Paco2 (57 ± 3 vs 54 ± 1 torr; 7.6 ± 0.4 vs 7.1 ± 0.1 kPa; p = 0.37) with a lower minute volume (6.4 ± 0.5 vs 8.7 ± 0.4 L/min; p < 0.001). Inspiratory plateau pressure was comparable in both groups (31 ± 2 vs 34 ± 2 cm H2O; p = 0.16). Flow-controlled ventilation increased normally aerated (24% ± 4% vs 10% ± 2%; p = 0.004) and decreased nonaerated lung volume (23% ± 6% vs 38% ± 5%; p = 0.033) in the dependent lung region. Alveolar walls were thinner (5.5 ± 0.1 vs 7.8 ± 0.2 µm; p < 0.0001), cell infiltration was lower (20 ± 2 vs 32 ± 2 n/field; p < 0.0001), and normalized surfactant protein A concentration was higher with flow-controlled ventilation (1.1 ± 0.04 vs 1.0 ± 0.03; p = 0.039).
Conclusions:
Flow-controlled ventilation enhances lung aeration in the dependent lung region and consequently improves gas exchange and attenuates lung injury. Control of the expiratory flow may provide a novel option for lung-protective ventilation.
Mechanical ventilation of patients suffering from acute respiratory distress syndrome (ARDS) may contribute to further lung injury (1). Modern ventilation management aims to avoid this by limiting tidal volume (VT) and end-inspiratory pressure (2–4)—where both are not independent, but rather conjoined via the respiratory system compliance. However, these lung-protective ventilation strategies are limited by the requirement to provide sufficient oxygenation and carbon dioxide clearance. Additionally, lung recruitment maneuvers and higher positive end-expiratory pressure (PEEP) support recruitment of lung tissue, but their role for lung-protective ventilation is currently under heavy debate (5–7).
Recently, we proposed ventilation with a constant expiratory flow as a new possibility to recruit lung tissue. In healthy pigs, we could demonstrate that a diminished expiratory derecruitment can be achieved with a constant expiratory flow and this was associated with an elevated Pao2 while compared with ventilation with conventional passive expiration with the same PEEP, peak inspiratory pressure, and VT (8). Furthermore, we could demonstrate an attenuation of lung injury by an almost constant expiratory flow in an animal model of ARDS. By controlling the expiratory flow, a significant reduction of PEEP and the end-inspiratory plateau pressure was possible while maintaining comparable Pao2 (9). However, whether the demonstrated attenuation of lung injury was caused by the reduced pressures or the constant expiratory flow remained unclear.
So far, only little attention has been paid to the expiratory flow and its potential role for lung-protective ventilation. One reason might be that standard ventilators do not control the expiratory flow. To our knowledge, there is only one new type of ventilator (Evone; Ventinova Medical B.V., Eindhoven, The Netherlands) that uses a constant inspiratory and expiratory flow. This ventilation mode was termed (bidirectional) “flow-controlled ventilation” (FCV) by the manufacturer. First clinical use for intraoperative ventilation was reported recently (10), but its use was not described in the setting of ARDS so far.
Taken together, our previous results suggest potential lung-protective properties of a constant expiratory flow beyond the limitation of VT and end-inspiratory pressure and led us to hypothesize that FCV attenuates lung injury during mechanical ventilation. In the work described below, we, therefore, compared FCV with volume-controlled ventilation (VCV) in terms of gas exchange, lung injury markers, lung aeration, and ventilation variables in a porcine model of oleic acid-induced ARDS.
METHODS
The study was approved by the appropriate governmental body (Regierungspräsidium Freiburg, file reference G-16/77) and conducted in accordance with the European law on the protection of animals used for scientific purposes (EU-Directive 2010/63).
A detailed description of the applied methods can be found in an online data supplement (Supplemental Digital Content 1, http://links.lww.com/CCM/F220). Briefly, 19 German landrace hybrid pigs (anesthetized, endotracheally intubated, ventilated, and fully instrumented, in the supine position) were subjected to oleic acid-induced lung injury (Fig. 1). All animals were initially ventilated with VCV using a standard ICU ventilator (Evita 4; Dräger medical, Lübeck, Germany) with Fio2 of 0.8, VT of 7 mL/kg body weight, PEEP of 9 cm H2O, and inspiratory to expiratory ratio of 1:1.2. The respiratory rate (RR) was adjusted to maintain the arterial blood pH above 7.2. The oleic acid was individually titrated until the Pao2-Fio2 ratio remained stable between 100 and 150 torr (13.3 and 20 kPa) for 20 minutes under constant ventilation.
Figure 1.
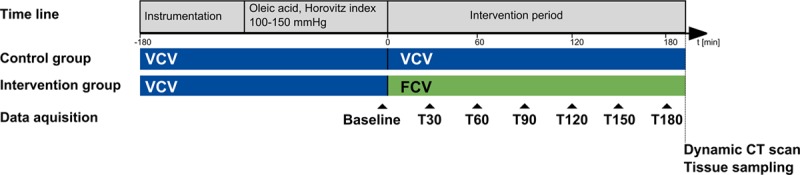
Timeline of experimental protocol. FCV = flow-controlled ventilation, VCV = volume-controlled ventilation.
The random allocation to either VCV (control) or FCV was disclosed, and the endotracheal tube was disconnected from the ventilator for 30 seconds and pulmonary edema fluid removed via active tracheal suction (all animals). The animals of the control group were reconnected to the standard ventilator and the animals of the FCV group were connected to a prototype ventilator capable of providing FCV (Evone; Ventinova Medical B.V., Eindhoven, The Netherlands). Ventilation was started with identical settings as mentioned above for both groups and maintained for 3 hours. A dynamic CT scan (60 axial images in 45 s on the same thoracic level) was then taken under the designated ventilation mode, and samples of lung tissue and bronchoalveolar lavage (BAL) fluid were collected.
The primary endpoint was the Pao2. Secondary endpoints included Paco2, tracheal pressure (Ptrach), VT, minute volume, RR, and dynamic compliance of the respiratory system. Recorded hemodynamic parameters were heart rate, mean arterial pressure, mean pulmonary arterial pressure, cardiac index, and the extravascular lung water index (ELWI). Each of these parameters was determined every 30 minutes. The CT scan data were analyzed separately for dependent and independent lung regions and divided into four compartments according to Gattinoni et al (11): 1) overinflated lung tissue; 2) normally aerated; 3) poorly aerated; and 4) nonaerated lung tissue. Lung tissue samples were hematoxylin and eosin stained for determination of alveolar wall thickness and cell infiltration. Concentrations of total protein (TPBAL) and surfactant protein A (SP-ABAL) were determined from the BAL fluid. Both protein measurements were normalized to the value of the control group.
An a priori sample size calculation based on the assumption of an effect size of 1.5 sds in the primary endpoint, an intended power of 80%, an α error of 5%, and an equal sample ratio resulted in n equals to 7 for each group. Data and statistical analyses were done offline with MATLAB (R2017b; MathWorks, Natick, MA). A linear mixed-effects model (12) was applied to the measurements, with the group allocation as a fixed effect and a random intercept by-subject. For repeated measurements, a random intercept by-time was added to the model. Statistical significance (p < 0.05) between the two groups was determined via a likelihood ratio test comparing the full model with a model reduced by the fixed effect. The goodness of fit of the applied linear mixed-effects model was assessed by visual inspection of the plotted residuals and by calculating adjusted R2 values (Supplemental Digital Content 2, http://links.lww.com/CCM/F220). Additionally, the se given for the estimate allows for assessment of the uncertainty of the modeled mean. For consistency and comparability, all data are reported as mean ± sem, if not declared otherwise.
RESULTS
In total, 19 animals were included in the study to obtain 14 evaluable datasets, seven in each group. Two animals were excluded before the randomized group allocation was disclosed due to insufficient oxygenation under baseline conditions (Pao2-Fio2 ratio, 85 torr [11.3 kPa] and 79 torr [10.5 kPa], respectively). In the FCV group, three animals were excluded due to software malfunction of the ventilator prototype (n = 2) and malignant arrhythmia (n = 1). Data for body weight, medication, and IV fluid substitution are provided as Supplemental Digital Content 3 (http://links.lww.com/CCM/F220). Data for the excluded animals can be found as Supplemental Digital Content 4 and 5 (http://links.lww.com/CCM/F220).
Ventilation and hemodynamic results are summarized for both groups in Figure 2 and Table 1. With FCV, Pao2 was higher (effect size, 49 torr [6.5 kPa]; 95% CI, 7–90 torr [0.9–12.0 kPa]) compared with the control group. Comparable Paco2 levels were achieved with a lower minute volume in the FCV group compared with the control (effect size, −2.4 L/min; 95% CI, −3.4 to −1.3 L/min), which was effected by a lower RR in the FCV group (effect size, −3/min; 95% CI, −5 to −1/min). The tracheal inspiratory plateau pressure (plateau Ptrach) and driving pressure (ΔPtrach) were comparable in both groups. Mean Ptrach (Ptrach mean) was higher in the FCV group (effect size, 1.4 cm H2O; 95% CI, 0.13–2.6 cm H2O). All hemodynamic variables were comparable in both groups.
Figure 2.
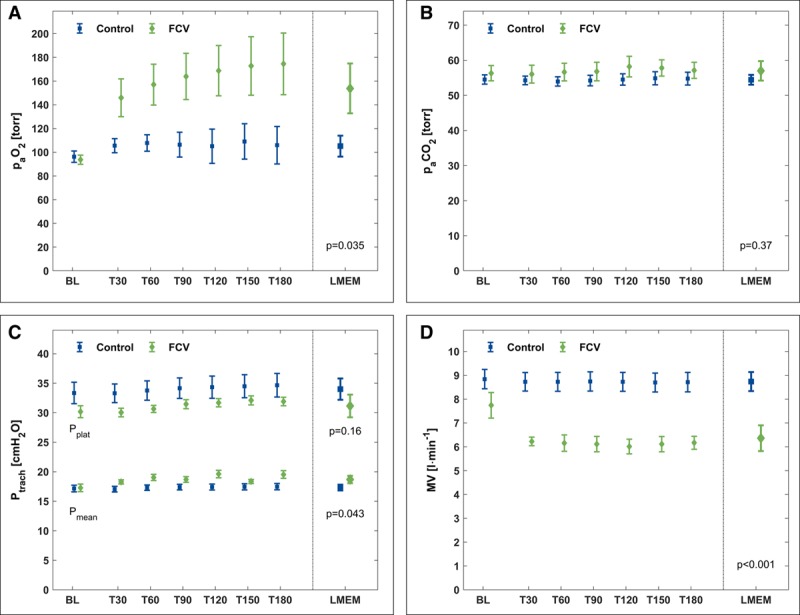
Measured (baseline [BL], T30–T180) and calculated (LMEM) variables for gas exchange and respiratory parameters. A, Pao2. B, Paco2. C, Tracheal plateau pressure (Pplat) and tracheal mean pressure (Pmean). D, Minute volume (MV). During BL, both groups were ventilated with a volume-controlled mode. FCV = flow-controlled ventilation, LMEM = calculated data from the linear mixed-effects model, Ptrach = tracheal pressure.
TABLE 1.
Summary of Respiratory, Hemodynamic, and Lung Injury Markers
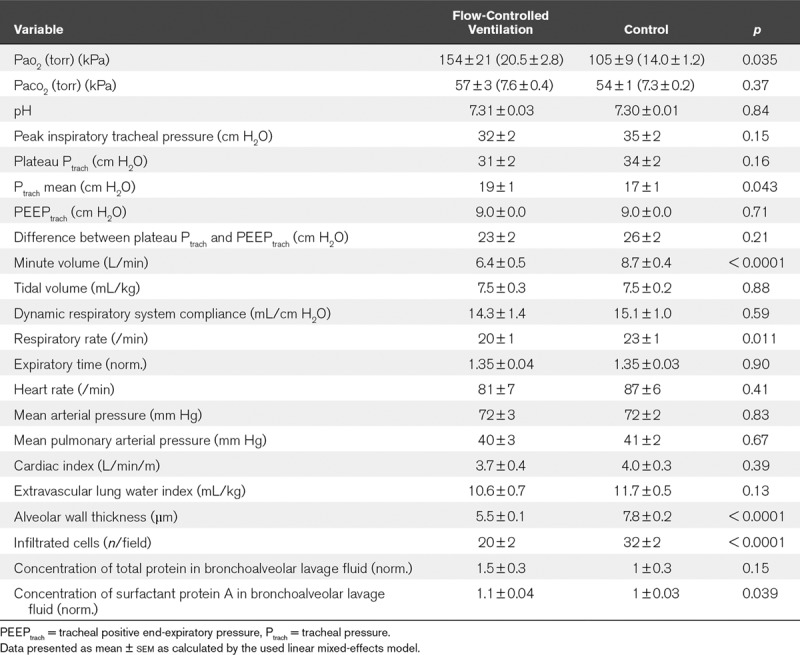
The lung tissue samples showed thinner alveolar walls (effect size, −2.3 µm; 95% CI, −2.7 to −1.8 µm) and a lower cell infiltration rate (effect size, −12 n/field; 95% CI, −16 to −7 n/field) after FCV compared with the control group (Table 1). Representative stained lung tissue samples can be found as Supplemental Digital Content 9 (http://links.lww.com/CCM/F224; legend, http://links.lww.com/CCM/F220). The concentration of TPBAL was comparable in both groups but was accompanied by a higher concentration of SP-ABAL after FCV (effect size, 0.10 [normalized]; 95% CI, 0.003–0.196).
TABLE 2.
Percentages of Four Lung Compartments of Independent and Dependent Lung Areas for Flow-Controlled Ventilation and Control Group
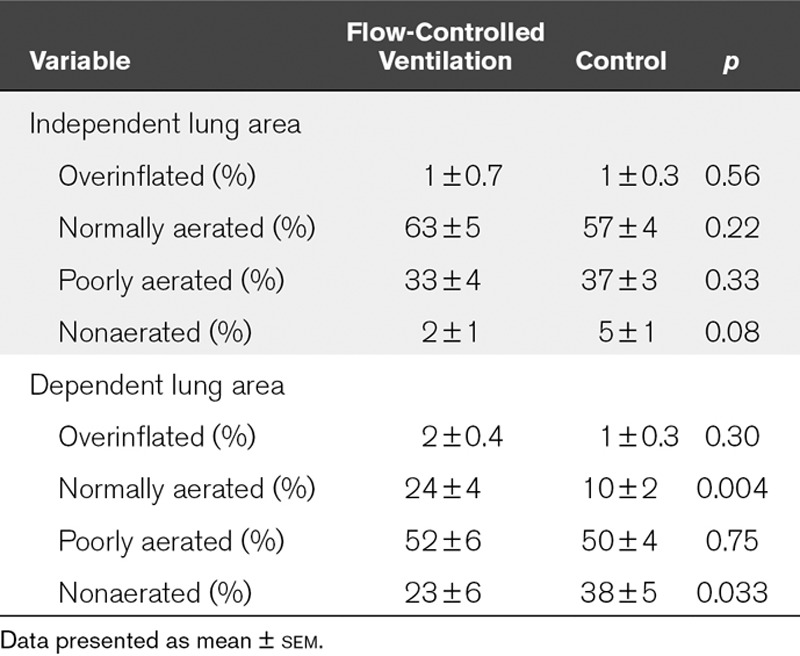
The dynamic CT scans revealed a left shifted histogram of CT density during FCV in the dependent lung areas (Fig. 3). Further analysis showed an increased percentage of normally aerated lung tissue (effect size, 13%; 95% CI, 5–21%) and a decreased percentage of nonaerated lung tissue (effect size, −15%; 95% CI, −28% to −1%) during FCV in the dependent lung areas compared with the control group (Table 2). Representative sequences of the dynamic CT scans are provided as additional movie files (Supplemental Digital Content 6, http://links.lww.com/CCM/F221; and Supplemental Digital Content 7, http://links.lww.com/CCM/F222—and legend, http://links.lww.com/CCM/F220).
Figure 3.
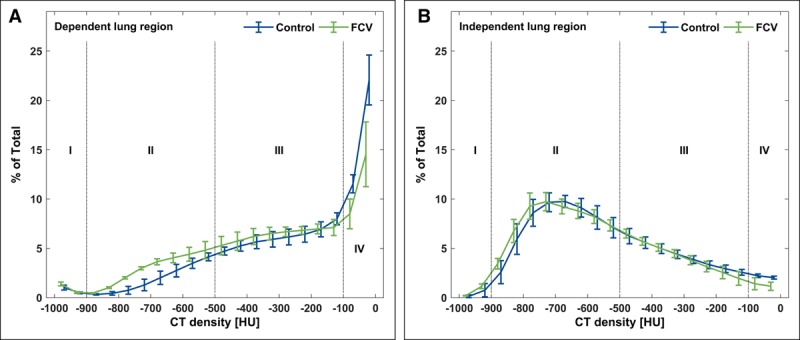
Histograms of CT densities for dependent (A) and independent (B) lung area in steps of 50 Hounsfield units (HU). Indicated are four distinctive lung compartments: 1) overinflated, 2) normally aerated, 3) poorly aerated, and 4) nonaerated lung tissue. FCV = flow-controlled ventilation.
DISCUSSION
The objective of this preclinical randomized study was to compare the effects of FCV with conventional mechanical ventilation in an ARDS model using pigs. The main findings are that, compared with VCV, with FCV, 1) the gas exchange was improved in terms of improved arterial oxygenation and lower minute volume required to achieve comparable Paco2, 2) the amount of aerated tissue in the dependent lung regions was higher, and 3) alveolar wall thickness and cell infiltration rate were lower, and the concentration of surfactant protein A in BAL fluid was higher.
Current lung-protective ventilation strategies for the treatment of ARDS focus mainly on limiting of VT and inspiratory pressure while still providing sufficient arterial oxygenation (13, 14). High VT and high inspiratory pressure support high Pao2 (15) but also increase the risk for ventilator-induced lung injury (16) and increase mortality (17). In this study, we found that by using FCV, we could improve gas exchange and attenuate lung injury compared with VCV—with comparable VT, PEEP, plateau pressure, and driving pressure. This suggests a potential therapeutic option for patients with ARDS which goes beyond VT and pressure limitation.
The main difference between the two ventilation modes we used is the expiratory flow profile with time (Supplemental Digital Content 8, http://links.lww.com/CCM/F223; and legend, http://links.lww.com/CCM/F220). The expiratory flow rate is constant and comparatively low during FCV, whereas during conventional, noncontrolled expiration, a high initial peak flow is followed by the well-known exponential decline. In accordance with a recent suggestion (18), we suspect this as the most influential mechanism on the obtained results.
Recruitment of Lung Tissue for Gas Exchange
The constant expiratory flow of FCV implies two possible mechanisms that lead to the observed improvements in gas exchange: First, mean Ptrach is elevated and consequently Pao2 rises (19). However, the time course of these two variables indicate an additional effect. Although mean Ptrach was higher at T30 and then drifted around the same level, oxygenation improved further. Hence, the second possible mechanism is the constant lung deflation, which has been shown to maintain oxygenation with significant lower levels of PEEP and to attenuate lung injury in a pig model with oleic acid-induced ARDS (9). In lung healthy pigs, FCV improved lung recruitment and oxygenation (8). The controlled deflation lengthens the time the alveoli are subjected to a pressure that exceeds closing pressure and the time until opening pressure is applied with the next inspiration is reduced. This recruiting effect can be seen in the dynamic CT scans in our study, which show a shift from nonaerated tissue to normally aerated tissue in the dependent lung regions.
One could argue that it may be possible to gain the desired effects by a simple increase of PEEP. However, increasing PEEP may increase overinflation in the independent lung regions (20, 21). This mechanism was suspected as a contributor to a higher mortality in ARDS patients treated with higher levels of PEEP in a recently published study (7). In our study, CT analyses did not indicate a relevant amount of overinflated lung tissue, in either ventilation mode. At this point it must be stressed, that the recruiting effect in this study was achieved without affecting PEEP and without recruitment maneuvers. Both were recently attributed to a higher mortality in ARDS patients in the aforementioned study (7).
The left shift in the histogram and the reduction of nonaerated lung tissue indicates a more homogenous ventilation in the dependent lung regions during FCV. This is supported by two investigations of ventilation with a controlled expiratory lung deflation which demonstrated a more homogeneous ventilation distribution using electrical impedance tomography in lung healthy patients (22) and in pigs with an oleic acid-induced ARDS (23).
We suspect that the steady deflation is a powerful mechanism that contributes substantially to the homogeneity of ventilation. Recently, Katira et al (24) showed that an abrupt deflation after sustained inflation causes lung injury. Of course, the setting of this study was different to ours and the comparison of results should be treated with caution. However, in presence of ARDS, compliance is substantially decreased and the lungs empty within a few hundred milliseconds. Consequently, abrupt deflation from end-inspiratory pressure to PEEP occurs in every conventional expiratory cycle. Additionally, the ARDS lung tissue inhomogeneity—with fast and slow emptying lung areas, which are characterized by different expiratory time constants—correlates with the severity of lung injury (25). Controlled expiration offers lung deflation independent of time constants and may contribute by this mechanism to an improved lung homogeneity. In consequence, improved lung homogeneity may attenuate lung injury as indicated in our study by alveolar wall thickness, cell infiltration rate, and SP-ABAL. The concentration of SP-A in BAL fluid from ARDS patients has been found to be lower than that in healthy volunteers (26) and correlates inversely to the severity of lung injury in polytraumatized patients (27). It was proposed as a key player for maintaining the integrity of the alveolar epithelium (28) by exerting antiapoptotic effects on pulmonary epithelial cells (29) and by modulating the response of inflammatory cells (30). Hence, the higher concentration of SP-A we found after FCV might have contributed to the thinner alveolar walls and the lower cell infiltration.
In summary, we propose that a constant lung deflation recruits lung tissue by improving lung tissue homogeneity without potential harmful side effects as higher minimal or maximal pressures of the respiratory system. The improved lung tissue homogeneity consequently attenuates lung injury.
Assessment of Lung Injury
We assessed the level of lung injury according to the recommendations of the American Thoracic Society (31), based on measurements in four different areas: 1) physiologic dysfunction, assessed by Pao2, Paco2, and corresponding minute volume, measured every 30 minutes; 2) histologic assessment of alveolar wall thickness; 3) evaluation of the integrity of the alveolar capillary barrier via concentration of total protein in BAL fluid as well as determination of ELWI; and 4) measurement of the inflammatory response via count of tissue infiltrated cells in stained lung tissue. Our results showed that lung injury was attenuated after FCV in three of these areas. Only the results for ELWI and TPBAL concentrations were inconclusive. However, a recent study showed a correlation between faster resolution of lung injury and higher TPBAL concentrations at an early stage of ARDS in ICU patients (32). In summary, we feel confident that our results provide evidence for an attenuated lung injury after FCV.
Limitations
The design and the experimental protocol for this study entail certain limitations. They were chosen to enable stable ventilation with fixed ventilator settings to examine the underlying mechanisms of the suspected lung-protective properties of a controlled expiration. However, we chose to adapt the RR continuously to the measured carbon dioxide partial pressure and the recruiting effects of FCV enabled ventilation with a lower minute volume by reducing the RR. However, ventilation of isolated rabbit lungs with a lower RR was attributed with an attenuation of lung injury (33) and hence, the reduced RR may have contributed to the observed results. However, the effect of the RR on outcome in ARDS patients was not studied yet. Although the conception of attenuation of lung injury due to a reduced cyclic opening and collapse of alveoli is widely accepted, an actual recommendation for ventilation of ARDS lungs merely state that a “balanced [RR] of 20–30/min” should be applied (34). However, adjusting the RR to the Paco2 and the pH, respectively, mirrors the actual recommendations of the ARDS Network. Additionally, the pH has a direct effect on the oxygen-binding affinity of hemoglobin (known as “Bohr effect”), hence a variation in Paco2 and consequently in the pH would have affected the primary endpoint. In summary, our experimental approach to adjust the RR to the pH reflected not only current clinical recommendations but also protected the primary endpoint from a potential influence in addition to the actual intervention.
FCV resulted in a higher mean Ptrach, which may have contributed to the higher Pao2, as noted above. Additionally, a previous study where a virtually constant expiratory flow was compared with a conventional expiration with comparable mean airway pressure (and a comparable RR) could demonstrate an attenuation of lung injury in a porcine model of ARDS (9). Regarding these previous results, it seems justified to propose the controlled expiration as the underlying cause for the demonstrated benefits. However, regarding exclusively the data of the presented study, we cannot exclude an influence of the lower RR and the higher mean Ptrach on the observed results.
We propose that the beneficial effects of a constant lung deflation are due to a recruiting effect which improves lung homogeneity. Obviously, the demonstrated effect size is depending on the amount of recruitable lung tissue and other settings (e.g., prone positioning) may result in different effect sizes.
Both groups were ventilated with comparable VT. However, it is worth noting that in our study the lungs received a volume load toward the upper end of the recommended range (4–8 mL/kg) (13). Having said that, we note that the mean VT in a recent large observational study in ARDS patients was 7.5 mL/kg predicted body weight (35). We, therefore, feel that our results mirror usual clinical practice and further suggest that FCV might achieve a comparable level of oxygenation with lower VT and/or lower inspiratory pressure.
The observational time of 3 hours was chosen to assess the short-term effects of two different ventilation modes. Although it was long enough to gain insights into the suspected underlying mechanisms, studies with longer observational periods are needed to evaluate long-term effects of FCV.
Our dynamic CT scans were recorded on one thoracic level and may not represent the whole lung. Our aim was to evaluate intratidal changes of the respective ventilation mode rather than to generate static images of end-inspiratory and end-expiratory intrathoracic gas volume. Additionally, the relevance of a dynamic CT scan was not addressed yet in a clinical setting. Regarding the considerable exposition to radiographs, a study in humans seems unfeasible in the near future.
We used two different ventilators, each with its own pressure and flow measuring system which poses a technical limitation of the study. Additionally, for the control group, Ptrach was continuously calculated from airway pressure and flow as described previously (36). However, there is no difference of airway and Ptrach for inspiratory plateau pressure and PEEP, as long as flow is absent. The difference of the calculated mean pressure is almost negligible for continuously recorded data, since airway pressure is higher during inspiration but is lower during expiration, compared with Ptrach. According to these considerations, we are confident that we report consistent data for Ptrach for both groups.
It is also worth considering the potential impact of FCV on the hemodynamics, reasoned by the elevated mean Ptrach which creates an elevated intrathoracic pressure and thus may impair venous return to the heart (37). However, we found a difference of only 2 cm H2O in mean Ptrach and no effects on hemodynamic variables; therefore, we estimate the effects on hemodynamics as marginal.
The translation of these results to the treatment of ARDS patients should be handled with care. FCV currently is depending on a Tritube (Ventinova Medical B.V., Eindhoven, The Netherlands), which does not allow for assisted spontaneous breathing and may affect patient-ventilator asynchrony. Additionally, if FCV still yields lung-protective properties with an individualized ventilator therapy (e.g., as proposed by the ARDS network) remains to be elucidated.
CONCLUSIONS
This study indicates lung-protective properties of a controlled expiration beyond VT and inspiratory pressure limitation in an animal model of ARDS. Further investigations of a controlled expiration and its therapeutic potential for the treatment of ARDS are necessary.
ACKNOWLEDGMENTS
We thank Dietmar Enk (University Hospital Münster), inventor of the “Flow-Controlled Ventilation” mode, for introducing us to the prototype used in this study. We would further like to gratefully acknowledge Tom Barnes (University of Greenwich) for his most helpful and critical comments on this work.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Presented as an abstract at Euroanaesthesia, Copenhagen, Denmark, June 3, 2018.
This work was carried out at the Department of Anesthesiology and Critical Care, Medical Center - University of Freiburg.
Drs. Schmidt’s, Wenzel’s, Spassov’s, Borgmann’s, Lin’s, Wirth’s, and Schumann’s institution received funding for the submitted work from the European Union’s Horizon 2020 research and innovation program, grant agreement No. 691519, in cooperation with Ventinova Medical B.V. (Eindhoven, The Netherlands), Unitron Group B.V. (IJzendijke, The Netherlands), and the Department of Anaesthesia, Rigshospitalet (Copenhagen, Denmark). Dr. Wollborn reports grants from Deutsche Forschungsgemeinschaft, outside the submitted work. Dr. Meckel reports personal fees from Acandis GmbH, Covidien, Medtronic, Microvention, and Stryker; grants from Bracco S.p.A.; and personal fees from Novartis, outside the submitted work. He received funding from Acandis, Medtronic, Stryker, Microvention, and Novartis Pharma. Dr. Schumann reports personal fees from Gründler medical and grants from Deutsche Forschungsgemeinschaft outside the submitted work. Dr. Schumann’s institution received grant funding from the Deutsche Forschungsgemeinschaft # SCHU 2499/4-1, # SCHU 2499/5-1, # SCHU 2499/7-1, and # SCHU 2499/8-1. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–2136 [DOI] [PubMed] [Google Scholar]
- 2.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–755 [DOI] [PubMed] [Google Scholar]
- 3.Hager DN. Recent advances in the management of the acute respiratory distress syndrome. Clin Chest Med 2015; 36:481–496 [DOI] [PubMed] [Google Scholar]
- 4.Rittayamai N, Brochard L. Recent advances in mechanical ventilation in patients with acute respiratory distress syndrome. Eur Respir Rev 2015; 24:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villar J, Suárez-Sipmann F, Kacmarek RM. Should the ART trial change our practice? J Thorac Dis 2017; 9:4871–4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahetya SK, Brower RG. Lung recruitment and titrated PEEP in moderate to severe ARDS: Is the door closing on the open lung? JAMA 2017; 318:1327–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of lung recruitment and titrated Positive End-Expiratory Pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: A Randomized Clinical Trial. JAMA 2017; 318:1335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt J, Wenzel C, Mahn M, et al. Improved lung recruitment and oxygenation during mandatory ventilation with a new expiratory ventilation assistance device: A controlled interventional trial in healthy pigs. Eur J Anaesthesiol 2018; 35:736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goebel U, Haberstroh J, Foerster K, et al. Flow-controlled expiration: a novel ventilation mode to attenuate experimental porcine lung injury. Br J Anaesth 2014; 113:474–483 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt J, Günther F, Weber J, et al. Flow-controlled ventilation during ear, nose and throat surgery: A prospective observational study. Eur J Anaesthesiol 2019; 36:327–334 [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L, Caironi P, Pelosi P, et al. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 2001; 164:1701–1711 [DOI] [PubMed] [Google Scholar]
- 12.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997; 16:2349–2380 [DOI] [PubMed] [Google Scholar]
- 13.Howell MD, Davis AM. Management of ARDS in adults. JAMA 2018; 319:711–712 [DOI] [PubMed] [Google Scholar]
- 14.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 15.Blanch L, Fernandez R, Vallés J, et al. Effect of two tidal volumes on oxygenation and respiratory system mechanics during the early stage of adult respiratory distress syndrome. J Crit Care 1994; 9:151–158 [DOI] [PubMed] [Google Scholar]
- 16.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med 2005; 31:922–926 [DOI] [PubMed] [Google Scholar]
- 17.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med 2000; 342:1301–8 [DOI] [PubMed] [Google Scholar]
- 18.Marini JJ, Gattinoni L. Protecting the ventilated lung: Vascular surge and deflation energetics. Am J Respir Crit Care Med 2018; 198:1112–1114 [DOI] [PubMed] [Google Scholar]
- 19.Marini JJ, Ravenscraft SA. Mean airway pressure: Physiologic determinants and clinical importance–part 1: Physiologic determinants and measurements. Crit Care Med 1992; 20:1461–1472 [PubMed] [Google Scholar]
- 20.Rouby JJ, Puybasset L, Nieszkowska A, et al. Acute respiratory distress syndrome: Lessons from computed tomography of the whole lung. Crit Care Med 2003; 31:S285–S295 [DOI] [PubMed] [Google Scholar]
- 21.Vieira SR, Puybasset L, Richecoeur J, et al. A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med 1998; 158:1571–1577 [DOI] [PubMed] [Google Scholar]
- 22.Wirth S, Springer S, Spaeth J, et al. Application of the novel ventilation mode FLow-Controlled EXpiration (FLEX): A Crossover Proof-of-Principle Study in Lung-Healthy Patients. Anesth Analg 2017; 125:1246–1252 [DOI] [PubMed] [Google Scholar]
- 23.Borgmann S, Schmidt J, Goebel U, et al. Dorsal recruitment with flow-controlled expiration (FLEX): An experimental study in mechanically ventilated lung-healthy and lung-injured pigs. Crit Care 2018; 22:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katira BH, Engelberts D, Otulakowski G, et al. Abrupt deflation after sustained inflation causes lung injury. Am J Respir Crit Care Med 2018; 198:1165–1176 [DOI] [PubMed] [Google Scholar]
- 25.Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2014; 189:149–158 [DOI] [PubMed] [Google Scholar]
- 26.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 1999; 160:1843–1850 [DOI] [PubMed] [Google Scholar]
- 27.Pison U, Obertacke U, Seeger W, et al. Surfactant protein A (SP-A) is decreased in acute parenchymal lung injury associated with polytrauma. Eur J Clin Invest 1992; 22:712–718 [DOI] [PubMed] [Google Scholar]
- 28.Goto H, Mitsuhashi A, Nishioka Y. Role of surfactant protein A in non-infectious lung diseases. J Med Invest 2014; 61:1–6 [DOI] [PubMed] [Google Scholar]
- 29.Goto H, Ledford JG, Mukherjee S, et al. The role of surfactant protein A in bleomycin-induced acute lung injury. Am J Respir Crit Care Med 2010; 181:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han S, Mallampalli RK. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc 2015; 12:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matute-Bello G, Downey G, Moore BB, et al. ; Acute Lung Injury in Animals Study Group: An official American thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011; 44:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrickson CM, Abbott J, Zhuo H, et al. ; NHLBI ARDS Network: Higher mini-BAL total protein concentration in early ARDS predicts faster resolution of lung injury measured by more ventilator-free days. Am J Physiol Lung Cell Mol Physiol 2017; 312:L579–L585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotchkiss JR, Jr, Blanch L, Murias G, et al. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med 2000; 161:463–468 [DOI] [PubMed] [Google Scholar]
- 34.Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: Ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med 2016; 42:699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 36.Guttmann J, Eberhard L, Fabry B, et al. Continuous calculation of intratracheal pressure in tracheally intubated patients. Anesthesiology 1993; 79:503–513 [DOI] [PubMed] [Google Scholar]
- 37.Hering R, Kreyer S, Putensen C. Effects of lung protective mechanical ventilation associated with permissive respiratory acidosis on regional extra-pulmonary blood flow in experimental ARDS. BMC Anesthesiol 2017; 17:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


