Supplemental Digital Content is available in the text.
Keywords: body mass index, cardiomyopathy, obesity, overweight, population, women
Background:
Incidence rates of cardiomyopathies, which are a common cause of heart failure in young people, have increased during the last decades. An association between body weight in adolescence and future cardiomyopathy among men was recently identified. Whether or not this holds true also for women is unknown. The aim was therefore to determine whether for young women being overweight or obese is associated with a higher risk of developing cardiomyopathy.
Methods:
This was a registry-based national prospective cohort study with data collected from the Swedish Medical Birth Register, 1982 to 2014, with up to 33 years of follow-up. Included women were of childbearing age (18–45 years) during the initial antenatal visit in their first or second pregnancy (n=1 393 346). We obtained baseline data on body mass index (BMI), smoking, education, and previous disorders. After exclusions, mainly because of previous disorders, the final sample was composed of 1 388 571 women. Cardiomyopathy cases were identified by linking the Medical Birth Register to the National Patient and Cause of Death registers.
Results:
In total, we identified 1699 cases of cardiomyopathy (mean age at diagnosis, 46.2 [SD 9.1] years) during the follow-up with an incidence rate of 5.9 per 100 000 observation years. Of these, 481 were diagnosed with dilated cardiomyopathy, 246 had hypertrophic cardiomyopathy, 61 had alcohol/drug-induced cardiomyopathy, and 509 had other forms. The lowest risk for being diagnosed with a cardiomyopathy was detected at a BMI of 21 kg/m2, with a gradual increase in risk with higher BMI, particularly for dilated cardiomyopathy, where a hazard ratio of 4.71 (95% CI, 2.81–7.89) was found for severely obese subjects (BMI ≥35 kg/m2), as compared with BMI 20 to <22.5.
Conclusions:
Elevated BMI among young women was associated with an increased risk of being diagnosed with a subsequent cardiomyopathy, especially dilated cardiomyopathy, starting already at mildly elevated body weight, whereas severe obesity entailed an almost 5-fold increase in risk. With the increasing numbers of persons who are overweight or obese, higher rates of cardiomyopathy can be expected in the future, along with an altered disease burden related to adiposity.
Clinical Perspective.
What Is New?
Cardiomyopathies are increasingly common as underlying cause of heart failure in the young, and risk factors for developing these disorders are insufficiently studied, especially among women.
The present study demonstrates that elevated body mass index in young women is strongly associated with an increased risk of subsequent cardiomyopathy, starting already at mildly elevated body weight.
What Are the Clinical Implications?
Our results emphasize the importance of preventing overweight and obesity in young people to protect from future cardiac remodeling, independently of ischemic heart disease.
Along with the rising rates of overweight and obesity in both women and men, a potential increase in cardiomyopathy cases is to be expected.
In contrast to declining incidence rates of heart failure observed for elderly persons, hospitalizations for heart failure in young people are on the rise in Sweden and Denmark.1–3 Although cardiomyopathy is increasingly common as the underlying condition for heart failure among young persons, the most frequent causes in older people are coronary heart disease, hypertension, and diabetes mellitus.1,3,4 Cardiomyopathies are a diverse group of disorders, with varying classifications proposed during the last decades. However, a position statement issued by the Working Group on Pericardial and Myocardial Diseases of the European Society of Cardiology categorized cardiomyopathies into 5 main groups: dilated, hypertrophic, restrictive, arrhythmogenic right ventricular, and unclassified.5 Discharge diagnoses of different types of cardiomyopathies in the Swedish National Hospital Register have been shown to be accurate in at least 85% of the cases.6
Compared with other causes of heart failure, cardiomyopathies are rare, and risk factors for these conditions are as yet scarcely explored. However, we recently found that higher body mass index (BMI) in adolescent men was associated with an increased risk for cardiomyopathy in midlife,7 especially for dilated cardiomyopathy. This heightened risk appeared at levels that are considered high-normal (BMI 22.5 to <25 kg/m2), and the risk was increased more than 9-fold for the severely obese (BMI ≥35 kg/m2), as compared with BMI 18.5 to <20 kg/m2. The prevalence of overweight and obesity among young Swedish men and women has increased significantly during the last decades,8,9 which coincides with a rise in the number of cardiomyopathy cases in the Swedish population.1 In light of the association that we discovered recently between BMI and cardiomyopathy in young men,7 we wanted to investigate whether this also applied to young women, in whom rates of cardiomyopathy have been shown to be lower than in men.10 Compared with the causes in men, stress-induced cardiomyopathy and cardiotoxic drug exposure, for instance chemotherapy for breast cancer, are more prevalent in women, and peripartum cardiomyopathy is exclusive to women only.11,12 To study the long-term risks of developing specific types of cardiomyopathies (excluding cases considered to be pregnancy-related cardiomyopathies) across categories of BMI in young women, we used the Medical Birth Register, which contains maternal information from 99% of the deliveries performed in Sweden,13 including more than 85% of all women in the Swedish population.14
Methods
The raw data for our analyses are potentially identifiable. Access to individual-level data is possible but requires permission through the Swedish Ethical Review Authority and from the primary data providers: the Swedish National Board of Health and Welfare, and Statistics Sweden.
Study Population
The study comprised a cohort of women of childbearing age (18–45 years) in the Swedish Medical Birth Register who gave birth between January 1, 1982, and December 31, 2014, and who had BMI registered at the initial antenatal visit (generally early in the first trimester before any appreciable pregnancy-related weight gain) during their first or second pregnancy (n=1 393 346). The Medical Birth Register includes data on deliveries in Sweden since 1973, with an estimated coverage of 99%. All healthcare providers submit information from the medical records regarding prenatal, delivery, and neonatal care to the register.13 Data on maternal height and weight have been collected for all deliveries since 1983, excluding 1990 and 1991 (no data are available). Until 1990, early pregnancy weight was estimated by subtracting gestational weight gain from delivery weight. This was recorded with 2 digits, which means that all women who weighed 100 kg or more were registered as weighing 99 kg during these years. From 1992 onwards, height was self-reported and weight was measured during the first antenatal visit (within 12 weeks of gestation in 90% of cases). For all the years, valid information on height is available for 80%, and weight for 70%.13 Visual inspection of annual body weight deciles revealed a larger than expected increase in body weight between 1989 and 1992. Therefore, the weight variable for 1982 to 1989 was adjusted by estimating annual weight gain within deciles from 1992 to 2003, creating an almost linear result, as previously described.15 In the present study, overweight was defined as BMI ≥25 kg/m2, and obesity as BMI ≥30 kg/m2. We also obtained data on self-reported smoking, hypertension, and diabetes mellitus from the register. Information regarding highest maternal education level was acquired from the Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA), which includes information on all residents of Sweden from the age of 16 years (80% coverage); this was made possible by linking the registers using the Swedish personal identification numbers.
In the final study sample, we excluded participants with BMI <15 or >60 (n=1077); height <140 cm or >200 cm (n=370); previous pregnancy-related cardiomyopathy (n=35); other previous cardiomyopathy (n=172); congenital heart disease (n=3084); and previous ischemic heart disease (n=37; Figure I in the online-only Data Supplement).
Outcomes
The national health system in Sweden is publicly funded and provides specialist hospital inpatient and outpatient care at low cost to all residents. Diagnostic codes from visits or discharges are recorded in the National Patient Register, and information on cause-specific deaths is reported to the Cause of Death Register. Inpatient discharge data coverage increased gradually from 1970 to 1986, and is complete from 1987, with outpatient visits registered from 2001. Primary care data are not included. Cardiomyopathy cases, defined according to the International Classification of Diseases, 8th, 9th, and 10th Revisions, were identified through linkage to the National Patient and Cause of Death registers (Methods and Table I in the online-only Data Supplement), and categorized as (1) dilated cardiomyopathy; (2) hypertrophic cardiomyopathy; (3) alcohol/drug-induced cardiomyopathy; (4) pregnancy-related cardiomyopathy; and (5) other cardiomyopathies (a composite of specified less-common and unspecified forms). Pregnancy-related cardiomyopathy was defined as being diagnosed with either new-onset heart failure or cardiomyopathy in the period covering 90 days before and 180 days after delivery, or as having a peripartum cardiomyopathy diagnostic code (O90.3, International Classification of Diseases, Tenth Revision; Table I in the online-only Data Supplement); these were not considered as outcomes in the present study. Information about disorders present at baseline included the following diagnoses: diabetes mellitus, hypertension, valvular disease, atrial fibrillation, ischemic stroke, hemorrhagic stroke, cancer, preeclampsia, gestational hypertension, and gestational diabetes mellitus (Methods in the online-only Data Supplement). Cases of diabetes mellitus and hypertension were additionally identified by self-reported data from the Medical Birth Register.
Statistical Analysis
Statistical calculations were performed with R ver. 3.6.0 software (Planting of a Tree; http://www.R-project.org). The follow-up period started on the date of the first antenatal visit (baseline), and subjects were followed until a first cardiomyopathy diagnosis, including pregnancy-related cardiomyopathy; death; a myocardial infarction; or the end of follow-up (December 31, 2014), whichever occurred first. Women with a pregnancy-related cardiomyopathy were censored at the date of diagnosis and were not included as events, because the risk for this condition is present exclusively around pregnancy, which constitutes a small fraction of the follow-up period. Women with a myocardial infarction before a cardiomyopathy diagnosis were considered as having heart failure of ischemic origin and were therefore censored at the time of the infarction, and excluded from all analyses. A sensitivity analysis was performed with the Fine-Gray method to study competing risk, in which death was treated as a competing event and fatal cardiomyopathies were coded as cardiomyopathy events rather than deaths. Incidence rates and corresponding 95% CIs were calculated using Poisson regression. Cox proportional hazards models were used to estimate cause-specific hazard ratios (HR) with 95% CI, to assess the influence of BMI on cardiomyopathy. BMI (kg/m2) was categorized into 8 groups (15 to <18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25 to <27.5, 27.5 to <30, 30 to <35, 35 to <60), of which BMI 20 to <22.5 was set as the reference. Education was categorized into 3 groups (<9 years, 9–12 years, >12 years of studies). The assumptions of proportional hazards for the regression models were examined using tests based on weighted residuals.16 Smoking and education did not fulfill the assumptions; hence, we stratified the model for these variables. Model 1 was adjusted for age, year of pregnancy, and parity. Model 2 was additionally adjusted for baseline disorders (diabetes mellitus, hypertension, valvular disease, atrial fibrillation, ischemic stroke, hemorrhagic stroke, and cancer). Model 3 was further adjusted for smoking and education. Spline plots were generated based on Model 3, with BMI as a restricted cubic spline with degree 3 and 4 knots placed at 5% (18.9), 35% (21.7), 65% (24.2), and 95% (31.6). BMI values between 15 and 40 were plotted against the reference (BMI 21).
The study was approved by the Regional Ethical Review Board in Gothenburg (Dnr. 103–15).
Results
Study Population and Follow-Up
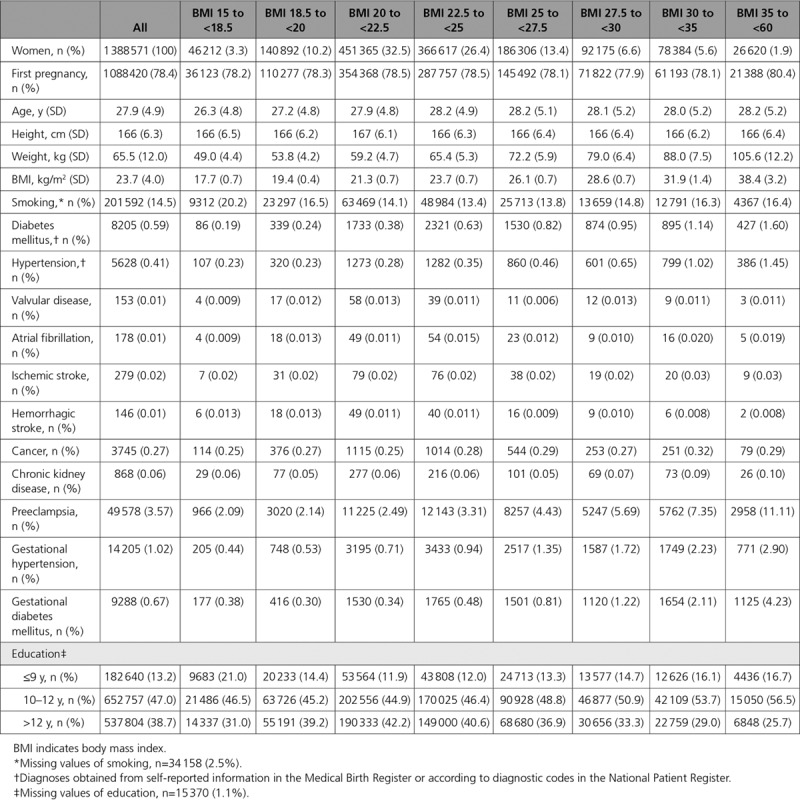
The final study sample after exclusions contained 1 388 571 women (mean age, 27.9, SD 4.9 years) with a mean BMI of 23.7 (SD 4.0) kg/m2. Of these, 69.1% were of normal weight (BMI 18.5 to <25 kg/m2), 20.1% were overweight (BMI 25 to <30 kg/m2), 5.6% were obese (BMI 30 to <35 kg/m2), and 1.9% were severely obese (BMI ≥35 kg/m2). Women with a first-time pregnancy represented 78.4% (n=1 088 420) of the study population. Approximately 15% were smokers at the initial antenatal visit, with higher percentages of smokers among the leanest and the obese subjects. Concomitant or preexisting disorders were, overall, very few in this young population, with diabetes mellitus (0.59%, n= 8205) and hypertension (0.41%, n= 5628) the most frequent, and rising with increasing BMI. Preeclampsia, gestational hypertension, and gestational diabetes mellitus at the index pregnancy were also more common with elevated BMI. The highest proportion of women with more than 12 years of studies was seen within the midrange BMI categories (Table 1).
Table 1.
Baseline Characteristics of the Study Population by BMI Category
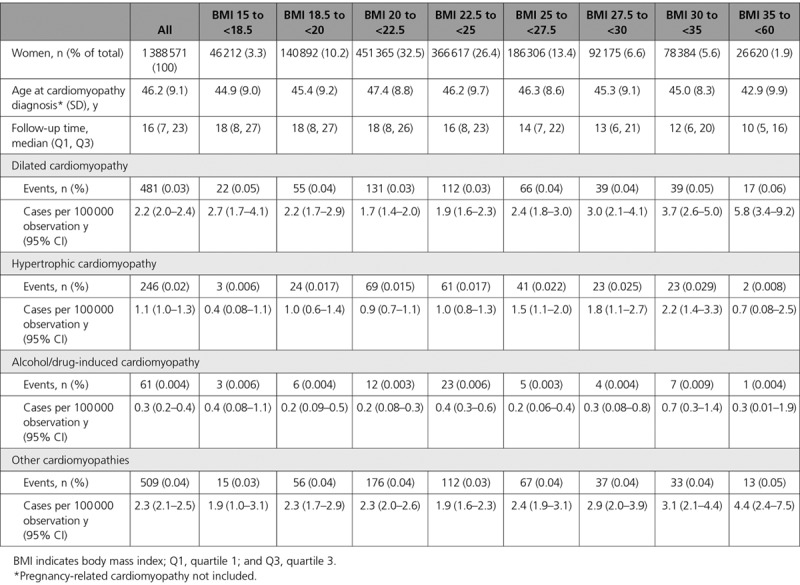
During the up to 33 years of follow-up (median, 16 years; interquartile interval 7–23 years), 1699 women (0.1% of the study population) were diagnosed with a cardiomyopathy at a mean age of 46.2 (SD 9.1) years (5.9 per 100 000 observation years; Table 2). Of these, 481 (28.3%) were diagnosed with a dilated cardiomyopathy (2.2 per 100,000 observation years), 246 (14.5%) with a hypertrophic cardiomyopathy (1.1 per 100,000 observation years), 61 (3.6%) with an alcohol/drug-induced cardiomyopathy (0.3 per 100,000 observation years), and 509 (30.0%) with other cardiomyopathies (2.3 per 100,000 observation years; Table 2). This last group consisted of 438 unspecified cardiomyopathies and 71 specified, less common forms (Table I in the online-only Data Supplement). Of the 1699 cardiomyopathy cases, 374 (22.0%) were pregnancy-related cardiomyopathies that occurred at the index or any future pregnancy (14.6 and 14.1 per 100 000 pregnancies, respectively), and these were censored at the date of diagnosis.
Table 2.
Event Rates per 100 000 Observation Years for Dilated Cardiomyopathy, Hypertrophic Cardiomyopathy, Alcohol/Drug-Induced Cardiomyopathy, and Other Cardiomyopathies by BMI Category
Risk for Cardiomyopathy
The lowest risk for developing a cardiomyopathy diagnosis was found at a baseline BMI of 21 kg/m2 (Figure). At higher BMI values, the risk of cardiomyopathy rose incrementally, particularly in the case of dilated cardiomyopathy. The highest incidence rate for dilated cardiomyopathy was seen among the severely obese (5.8 per 100 000 observation years), at a mean age at diagnosis of 42.8 years (Table 2). At BMI 25 to <27.5 kg/m2, the HR for dilated cardiomyopathy was 1.55 (95% CI, 1.14–2.11), as compared with BMI 20 to <22.5, when adjusted for age, year, parity, baseline disorders, smoking, and education. The adjusted HR at BMI ≥35 was 4.71 (95% CI, 2.81–7.89; Table 3). Corresponding HRs for hypertrophic cardiomyopathy followed a similar pattern, except in the highest BMI category, where there were only two cases. Alcohol/drug-induced cardiomyopathies were rare, with resulting wide CIs (Table 3). For each unit of increment in BMI, the HR for dilated cardiomyopathy, in the fully adjusted model, was 1.09 (95% CI, 1.06–1.12), for hypertrophic cardiomyopathy, 1.06 (95% CI, 1.01–1.10), for alcohol/drug-induced cardiomyopathy, 1.03 (95% CI, 0.95–1.13), and 1.06 (95% CI, 1.03–1.10) for other cardiomyopathies (Table 3). The competing risk analysis, in which death was treated as a competing event, only changed the results marginally (Table II in the online-only Data Supplement).
Table 3.
Hazard Ratios (95% CIs) for Cases With Dilated Cardiomyopathy, Hypertrophic Cardiomyopathy, Alcohol/Drug-Induced Cardiomyopathy, and Other Cardiomyopathies by BMI Category
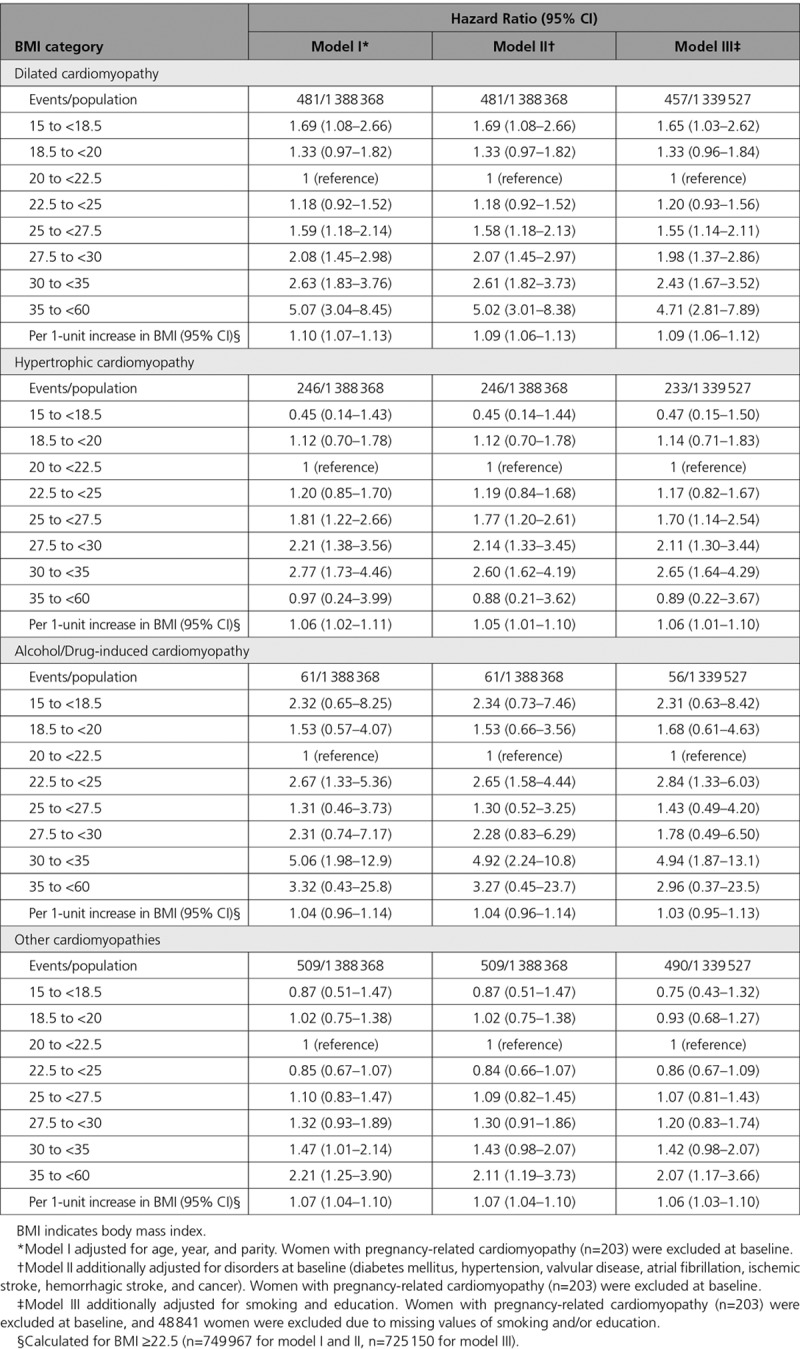
Figure.
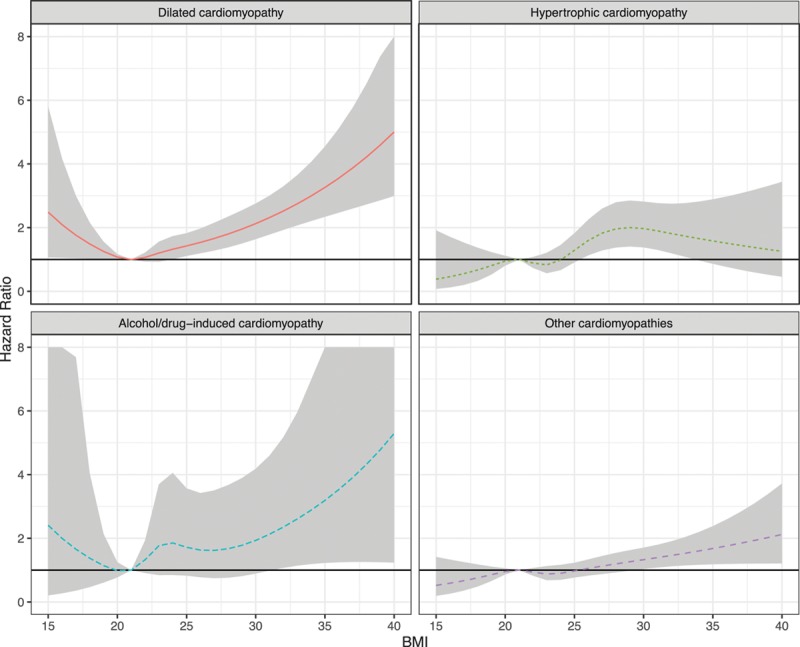
Association between body mass index (BMI) in young women and risk for subsequent cardiomyopathy. The model is adjusted for age, year, parity, comorbidities at baseline, smoking, and level of education (n=1 339 527). BMI is restricted to the range of 15 to 40, and modeled as a restricted cubic spline with knots at 5%, 35%, 65%, and 95% (i.e., 18.9, 21.7, 24.2, and 31.6, respectively), with a BMI of 21 as reference.
Discussion
In this nationwide cohort study of almost 1.4 million women of childbearing age, we found that high BMI was associated with an increased risk of being diagnosed subsequently with cardiomyopathy, particularly the dilated form, independently of preexisting disorders, smoking, and education. The risk increased gradually as BMI increased across and beyond the overweight and obese ranges, with the highest risk seen for severely obese women.
Recently, we reported on a similar association between high BMI and subsequent cardiomyopathy in men,7 with BMI being measured in late adolescence at the mandatory assessment for military service that was undertaken by all 18-y-old males in Sweden. An equivalent mandatory assessment of body weight for young Swedish women is not in place. However, the vast majority of women in Sweden (>85%) give birth at some point in life,14 and anthropometric variables are then registered in the Medical Birth Register.13 In this register, rising rates of obesity have been documented,9 as is also the case in other national8 and global17 studies. In the current study, we found that 7.5% of the young women in the study population were obese, which is somewhat lower than in other Swedish reports that included older age categories, and with data from the later time period of the present study.18,19 However, according to a recent publication from the National Board of Health and Welfare in Sweden, the prevalence of maternal obesity continues to increase and was as high as 15.1% in 2017.20
An association between obesity in middle-aged women and heart failure has previously been shown in a report based on the Prospective Population Study of Women and H70 Studies in Sweden.21 In the current study, we investigated a more specific epidemiological association, considering the different forms of cardiomyopathy. Although it is true that heart failure and cardiomyopathy overlap, they are not interchangeable, which becomes even more clear when the term “cardiomyopathy” is used correctly.5 The lack of previous knowledge about the relationship between adiposity in young women and different types of cardiomyopathy highlights the novelty of these findings. Apart from our study of men,7 we have found only 1 previous study relevant to this topic, based on a cohort of 2.3 million Israeli adolescents,22 demonstrating a gradual increase in risk of cardiomyopathy-related death with increasing BMI. That study, however, identified only 121 cardiomyopathy deaths and was not sufficiently powered to investigate specific forms of cardiomyopathy or the association with severe obesity, which constitutes an increasing proportion of overweight persons.8
Compared with our results for adolescent men,7 the present incidence rates of dilated and hypertrophic cardiomyopathies for females were lower, in accordance with previous findings,23–25 showing a prevalence of idiopathic dilated cardiomyopathy between 1:1.9 and 1:4.3 for the female-to-male ratios, whereas hypertrophic cardiomyopathy has been somewhat more equally distributed between the sexes.23,24 Reports on alcoholic cardiomyopathy have been inconclusive.26,27 However, we noted a lower incidence of alcohol/drug-induced cardiomyopathy among women than in our study of men.7 Further, we found a slightly higher BMI threshold for women, who were on average a decade older than the men. Notably, men are known to have higher risks for diabetes mellitus,28 and mortality29 for a given BMI than women, so our results appear to fit with the slight sex differences observed for the association between adiposity and adverse health outcomes. In the current study, the risk for dilated cardiomyopathy, in particular, was associated with higher BMI, a pattern similar to that of men.7 Our finding of an increased risk for dilated cardiomyopathy at the lower end of the BMI spectrum must be interpreted with caution, because this group only comprised a small number of events. Speculatively, higher rates of smoking, poor nutrition status, as well as alcohol or substance abuse may have predisposed underweight women to future cardiac disease, but in the absence of data, this cannot be confirmed. Although there were too few cases to allow solid conclusions, the association between BMI and hypertrophic cardiomyopathy followed the same trend as for dilated cardiomyopathy, even though most cases are thought to be hereditary.30 This suggests that high BMI exacerbates or synergizes with genetic defects in cardiac function, and thus, probably results in earlier expression of symptoms. However, as genetic testing has not been routinely available on a national basis until recently, this hypothesis could not be investigated in the present study.
The association that we found between BMI and cardiomyopathy may be derived from myocardial remodeling in obese subjects, as shown in a report on young otherwise healthy women.31 This indicates an early onset of potentially adverse alterations in the myocardium that is related to obesity at a young age. Elevated body weight entails an increased metabolic demand that requires hemodynamic changes including increased blood volume and cardiac output. Over time, this results in ventricular dilation and hypertrophy along with impaired cardiac function.32 Another contributing mechanism is the increased secretion of adipocyte-derived molecules that comes with excess adipose tissue, for instance, leptin, neprilysin, and aldosterone.33 This has been proposed to promote a proinflammatory state, which may lead to cardiac fibrosis and microvascular abnormalities. Accordingly, there is a growing body of evidence suggesting that systemic inflammation may play a major role in the pathogenesis of heart failure.34 Other postulated mechanisms that link adiposity to cardiac remodeling and dysfunction include high levels of oxidative stress, as well as myocardial lipotoxicity, in which intracellular accumulation of excess fatty acid and triglycerides leads to apoptosis of the cardiac myocytes.35 Taken together, the pathophysiology of obesity-related cardiomyopathy is not fully understood, but appears to involve a multifaceted interplay between increased hemodynamic load, neurohormonal dysregulation, inflammation, and lipotoxicity among others.
Strengths and Limitations
The present nationwide cohort study included almost 1.4 million women for up to 33 years of follow-up. This enabled an analysis with adequate statistical precision, despite a relatively low rate of female cardiomyopathy events in midlife. Almost all (99%) of the women who gave birth in Sweden during the study period, representing a large majority of Swedish women,14 were included. This indicates that the study population was adequately representative of women in Sweden. However, involuntary childlessness is known to be more common among obese women,36 so it is possible that the incidences of cardiomyopathies may have been underestimated.
Along with our previous results for men,7 the present study showed that being overweight or obese is associated with cardiomyopathy regardless of sex. It is likely that our findings can be generalized to other ethnicities, although this needs to be confirmed in future studies. Furthermore, a validation study of cardiomyopathy diagnoses in the National Hospital Register during an overlapping period with this report showed a high diagnostic accuracy (>85%), which did not change during the study period, with almost uniform use of echocardiography in investigations of suspected cardiomyopathy throughout those years.6 Thus, we believe that most of the cardiomyopathy cases in the current study were accurately diagnosed.
Moreover, in the Medical Birth Register, height was occasionally self-reported, and as women tend to overestimate this measurement,37 BMI values may have been underestimated in some cases. We considered measured weight at the first antenatal visit, most of which occurred within 12 weeks of gestation,13 to be a satisfactory estimate of prepregnancy weight, as weight gain during the first trimester has been reported to be negligible.38–40 Anthropometric variables other than BMI, such as waist circumference and waist-to-hip ratio, were not available in the dataset, although we recognize central fat accumulation appears more strongly linked to adverse metabolic outcomes in women than in men.41 Further, diabetes mellitus and hypertension were probably underestimated in this study population because of lack of primary care data. Another limitation was the inaccessibility of information on body weight and other potential risk factors during the 33-y follow-up. Although repeated individual measurements of BMI could be obtained from women with subsequent pregnancies, these would mostly be confined to a decade, which only represents the first years of the follow-up period. Data on BMI from the later part of the follow-up (closer to the cardiomyopathy event) would still be missing. Thus, we believe that including repeated measurements of BMI in pregnant women would not meaningfully enhance our analyses as only minimal additional information on the longitudinal relationship between BMI and cardiomyopathy risk would be provided. Even so, BMI has been demonstrated to be highly consistent in terms of tracking during life,42 and accordingly any cardiac abnormalities may well result from a continuing effect of elevated body weight. It seems unlikely that repeated measurements would have changed our conclusions.
Conclusions
Higher BMI among young women was associated with an increased risk of being diagnosed with a subsequent cardiomyopathy, especially dilated cardiomyopathy, starting already at mildly elevated body weight, whereas severe obesity entailed an almost 5-fold increase in risk. This is in line with previous findings for young men. If these associations are at least partially causal, with increasing numbers of people who are overweight or obese, higher rates of cardiomyopathies, along with an altered disease burden related to adiposity, can be expected in the future.
Sources of Funding
This study was supported by grants from the Swedish Government under an agreement concerning economic support for research and education of doctors (ALFGBG-717211, 813511), the Swedish Research Council 2013-5187 (Swedish Initiative for Microdata Research in the Social and Medical Sciences), and 2018-02527, the Swedish Heart and Lung Foundation (2017-0244, 2018-0366, 2018-0589), and the Swedish Council for Health, Working Life and Welfare (FORTE; 2013-0325). Dr Sattar’s work is supported by a British Heart Foundation Research Excellence Award – RE/18/6/34217.
Disclosures
None.
Supplementary Material
Footnotes
This article is part of the Science Goes Red™ collection. Science Goes Red™ is an initiative of Go Red for Women®, the American Heart Association’s global movement to end heart disease and stroke in women.
Sources of Funding, see page 528
Guest Editor for this article was Eileen Hsich, MD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.119.044056.
References
- 1.Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2014;35:25–32. doi: 10.1093/eurheartj/eht278. doi: 10.1093/eurheartj/eht278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen MN, Kober L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp-Pedersen C, Andersson C. Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223. doi: 10.1161/CIRCULATIONAHA.116.025941. doi: 10.1161/CIRCULATIONAHA.116.025941. [DOI] [PubMed] [Google Scholar]
- 3.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/nrcardio.2017.154. doi: 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 4.Wong CM, Hawkins NM, Petrie MC, Jhund PS, Gardner RS, Ariti CA, Poppe KK, Earle N, Whalley GA, Squire IB, et al. MAGGIC Investigators. Heart failure in younger patients: the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). Eur Heart J. 2014;35:2714–2721. doi: 10.1093/eurheartj/ehu216. doi: 10.1093/eurheartj/ehu216. [DOI] [PubMed] [Google Scholar]
- 5.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 6.Basic C, Rosengren A, Lindstrom S, Schaufelberger M. High validity of cardiomyopathy diagnoses in western Sweden (1989-2009). ESC Heart Fail. 2018;5:233–240. doi: 10.1002/ehf2.12224. doi: 10.1002/ehf2.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson J, Schaufelberger M, Lindgren M, Adiels M, Schiöler L, Torén K, McMurray J, Sattar N, Åberg M, Rosengren A. Higher body mass index in adolescence predicts cardiomyopathy risk in midlife. Circulation. 2019;140:117–125. doi: 10.1161/CIRCULATIONAHA.118.039132. doi: 10.1161/CIRCULATIONAHA.118.039132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neovius M, Teixeira-Pinto A, Rasmussen F. Shift in the composition of obesity in young adult men in Sweden over a third of a century. Int J Obes (Lond) 2008;32:832–836. doi: 10.1038/sj.ijo.0803784. doi: 10.1038/sj.ijo.0803784. [DOI] [PubMed] [Google Scholar]
- 9.Chaparro MP, Ivarsson A, Koupil I, Nilsson K, Haggstrom J, de Luna X, Lindgren U. Regional inequalities in pre-pregnancy overweight and obesity in Sweden, 1992, 2000, and 2010. Scand J Public Health. 2015;43:534–539. doi: 10.1177/1403494815579478. doi: 10.1177/1403494815579478. [DOI] [PubMed] [Google Scholar]
- 10.Charron P, Elliott PM, Gimeno JR, Caforio ALP, Kaski JP, Tavazzi L, Tendera M, Maupain C, Laroche C, Rubis P, et al. EORP Cardiomyopathy Registry Investigators. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J. 2018;39:1784–1793. doi: 10.1093/eurheartj/ehx819. doi: 10.1093/eurheartj/ehx819. [DOI] [PubMed] [Google Scholar]
- 11.Meyer S, van der Meer P, van Tintelen JP, van den Berg MP. Sex differences in cardiomyopathies. Eur J Heart Fail. 2014;16:238–247. doi: 10.1002/ejhf.15. doi: 10.1002/ejhf.15. [DOI] [PubMed] [Google Scholar]
- 12.De Bellis A, De Angelis G, Fabris E, Cannata A, Merlo M, Sinagra G. Gender-related differences in heart failure: beyond the “one-size-fits-all” paradigm. Heart Fail Rev. 2019 doi: 10.1007/s10741-019-09824-y. doi: 10.1007/s10741-019-09824-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.The Centre for Epidemiology (EpC) at the National Board of Health and Welfare. The Swedish Medical Birth Register: A summary of content and quality. 2003. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2003-112-3_20031123.pdf Accessed May 4, 2018.
- 14.Statistics Sweden. Olika generationers barnafödande. Demografiska rapporter. 2011;3 http://share.scb.se/ov9993/data/publikationer/statistik/_publikationer/be0701_2011a01_br_be51br1103.pdf Accessed June 27, 2019. [Google Scholar]
- 15.Persson CE, Adiels M, Björck L, Rosengren A. Young women, body size and risk of atrial fibrillation. Eur J Prev Cardiol. 2018;25:173–180. doi: 10.1177/2047487317740644. doi: 10.1177/2047487317740644. [DOI] [PubMed] [Google Scholar]
- 16.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515. [Google Scholar]
- 17.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. doi: 10.1016/s0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neovius K, Johansson K, Kark M, Tynelius P, Rasmussen F. Trends in self-reported BMI and prevalence of obesity 2002-10 in Stockholm County, Sweden. Eur J Public Health. 2013;23:312–315. doi: 10.1093/eurpub/cks128. doi: 10.1093/eurpub/cks128. [DOI] [PubMed] [Google Scholar]
- 19.Sundquist J, Johansson SE, Sundquist K. Levelling off of prevalence of obesity in the adult population of Sweden between 2000/01 and 2004/05. BMC Public Health. 2010;10:119. doi: 10.1186/1471-2458-10-119. doi: 10.1186/1471-2458-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics Sweden. Statistik om graviditeter, förlossningar och nyfödda barn 2017. Sveriges officiella statistik. 2019 https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2019-5-2.pdfAccessed May 8, 2019. [Google Scholar]
- 21.Halldin AK, Schaufelberger M, Lernfelt B, Björck L, Rosengren A, Lissner L, Björkelund C. Obesity in middle age increases risk of later heart failure in women-results from the Prospective Population Study of Women and H70 Studies in Gothenburg, Sweden. J Card Fail. 2017;23:363–369. doi: 10.1016/j.cardfail.2016.12.003. doi: 10.1016/j.cardfail.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Twig G, Ben-Ami Shor D, Furer A, Levine H, Derazne E, Goldberger N, Haklai Z, Levy M, Afek A, Leiba A, et al. Adolescent body mass index and cardiovascular disease-specific mortality by midlife. J Clin Endocrinol Metab. 2017;102:3011–3020. doi: 10.1210/jc.2017-00329. doi: 10.1210/jc.2017-00329. [DOI] [PubMed] [Google Scholar]
- 23.Bagger JP, Baandrup U, Rasmussen K, Møller M, Vesterlund T. Cardiomyopathy in western Denmark. Br Heart J. 1984;52:327–331. doi: 10.1136/hrt.52.3.327. doi: 10.1136/hrt.52.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Codd MB, Sugrue DD, Gersh BJ, Melton LJ., 3rd. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation. 1989;80:564–572. doi: 10.1161/01.cir.80.3.564. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- 25.Torp A. Incidence of congestive cardiomyopathy. Postgrad Med J. 1978;54:435–439. doi: 10.1136/pgmj.54.633.435. doi: 10.1136/pgmj.54.633.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbano-Márquez A, Estruch R, Fernández-Solá J, Nicolás JM, Paré JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA. 1995;274:149–154. doi: 10.1001/jama.1995.03530020067034. doi: 10.1001/jama.1995.03530020067034. [DOI] [PubMed] [Google Scholar]
- 27.Andersson B, Waagstein F. Spectrum and outcome of congestive heart failure in a hospitalized population. Am Heart J. 1993;126(3 p)(t 1):632–640. doi: 10.1016/0002-8703(93)90414-5. doi: 10.1016/0002-8703(93)90414-5. [DOI] [PubMed] [Google Scholar]
- 28.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, et al. Scottish Diabetes Research Network Epidemiology Group. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54:3003–3006. doi: 10.1007/s00125-011-2313-3. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson Ch L, et al. Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. doi: 10.1016/s0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 31.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Dávila-Román VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 32.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Packer M. Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur J Heart Fail. 2018;20:873–878. doi: 10.1002/ejhf.1167. doi: 10.1002/ejhf.1167. [DOI] [PubMed] [Google Scholar]
- 34.Rajendiran KS, Ananthanarayanan RH, Satheesh S, Rajappa M. Elevated levels of serum sialic acid and high-sensitivity C-reactive protein: markers of systemic inflammation in patients with chronic heart failure. Br J Biomed Sci. 2014;71:29–32. doi: 10.1080/09674845.2014.11669959. doi: 10.1080/09674845.2014.11669959. [DOI] [PubMed] [Google Scholar]
- 35.Sletten AC, Peterson LR, Schaffer JE. Manifestations and mechanisms of myocardial lipotoxicity in obesity. J Intern Med. 2018;284:478–491. doi: 10.1111/joim.12728. doi: 10.1111/joim.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 37.Nyholm M, Gullberg B, Merlo J, Lundqvist-Persson C, Råstam L, Lindblad U. The validity of obesity based on self-reported weight and height: implications for population studies. Obesity (Silver Spring) 2007;15:197–208. doi: 10.1038/oby.2007.536. doi: 10.1038/oby.2007.536. [DOI] [PubMed] [Google Scholar]
- 38.Carmichael S, Abrams B, Selvin S. The pattern of maternal weight gain in women with good pregnancy outcomes. Am J Public Health. 1997;87:1984–1988. doi: 10.2105/ajph.87.12.1984. doi: 10.2105/ajph.87.12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siega-Riz AM, Adair LS, Hobel CJ. Institute of Medicine maternal weight gain recommendations and pregnancy outcome in a predominantly Hispanic population. Obstet Gynecol. 1994;84:565–573. [PubMed] [Google Scholar]
- 40.Abrams B, Carmichael S, Selvin S. Factors associated with the pattern of maternal weight gain during pregnancy. Obstet Gynecol. 1995;86:170–176. doi: 10.1016/0029-7844(95)00119-c. doi: 10.1016/0029-7844(95)00119-c. [DOI] [PubMed] [Google Scholar]
- 41.Wannamethee SG, Papacosta O, Whincup PH, Carson C, Thomas MC, Lawlor DA, Ebrahim S, Sattar N. Assessing prediction of diabetes in older adults using different adiposity measures: a 7 year prospective study in 6,923 older men and women. Diabetologia. 2010;53:890–898. doi: 10.1007/s00125-010-1670-7. doi: 10.1007/s00125-010-1670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer O, Krüger H, von Kries R, Toschke AM. Factors associated with tracking of BMI: a meta-regression analysis on BMI tracking. Obesity (Silver Spring) 2011;19:1069–1076. doi: 10.1038/oby.2010.250. doi: 10.1038/oby.2010.250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


