ABSTRACT
Background:
Resuscitation from hemorrhagic shock (HS) by blood transfusion restores oxygen (O2) delivery and provides hemodynamic stability. Current regulations allow red blood cells (RBCs) to be stored and used for up to 42 days. During storage, RBCs undergo many structural and functional changes. These storage lesions have been associated with adverse events and increased mortality after transfusion, increasing the need for improved RBC storage protocols. This study evaluates the efficacy of anaerobically stored RBCs to resuscitate rats from severe HS compared with conventionally stored RBCs.
Methods and results:
Rat RBCs were stored under anaerobic, anaerobic/hypercapnic, or conventional conditions for a period of 3 weeks. Hemorrhage was induced by controlled bleeding, shock was maintained for 30 min, and RBCs were transfused to restore and maintain blood pressure near the prhemorrhage level. All storage conditions met current regulatory 24-h posttransfusion recovery requirements. Transfusion of anaerobically stored RBCs required significantly less RBC volume to restore and maintain hemodynamics. Anaerobic or anaerobic/hypercapnic RBCs restored hemodynamics better than conventionally stored RBCs. Resuscitation with conventionally stored RBCs impaired indices of left ventricular cardiac function, increased hypoxic tissue staining and inflammatory markers, and affected organ function compared with anaerobically stored RBCs.
Conclusions:
Resuscitation from HS via transfusion of anaerobically stored RBCs recovered cardiac function, restored hemodynamic stability, and improved outcomes.
Keywords: Trauma, transfusion, shock, blood storage, storage lesion
INTRODUCTION
Trauma accounts for 10% of deaths worldwide, and represents the third most common cause of death in the United States (1, 2). Excluding other causes of trauma, like traumatic brain injury, sepsis, and multiple organ failure, hemorrhagic shock (HS) from exsanguination accounts for roughly half of trauma deaths (3). HS decreases oxygen (O2)-carrying capacity and induces cardiovascular collapse, limiting O2 delivery to tissues and the washout of metabolic waste, and can result in multiorgan failure if not corrected in time (4–6). In addition to stopping the bleeding, severe HS is frequently corrected by infusing large volumes of red blood cells (RBCs) to restore volume, O2-carrying capacity, and to recover blood flow and hemodynamic stability (7).
Transfusion-associated infections, transfusion-related acute lung injury (TRALI), and acellular hemoglobin (Hb) toxicity have been partly responsible for increasingly restrictive transfusion approaches (8–10). Although there is no debate about the need for blood transfusions, as blood products remain a vital resource for clinical care, transfusion of stored RBCs is associated with adverse events and increased mortality (11). During storage, RBCs undergo many physical, functional, and morphological changes, known as “storage lesions” (12–14). The Food and Drug Administration (FDA) limits the RBC storage to 42 days in approved additive solutions, based on 24-h posttransfusion recovery of RBCs and hemolysis measurements (15). The 24-h posttransfusion recovery is a limited metric of functionality of transfused RBCs, as it only considers the number of surviving RBCs after 24 h in healthy volunteers, and it does not consider the hemodynamic stability, O2 delivery, or any other response in the transfusion recipient. Transfusion-related adverse short- and long-term events, including infection, stroke, and multiorgan failure, among others, are among the costliest contributors to healthcare expenditures, accounting for up to $64 billion per year in the United States alone (16, 17).
New additive solutions have been shown to improve in vitro quality of stored RBCs, and new methods are being explored to decrease storage lesions (18–20). Recent studies show that the quality of RBCs degrades at varying rates (21, 22), possibly due to nonuniform Hb O2 saturation during storage (23). Anaerobic storage of blood has been proposed to improve the quality and uniformity of RBC units by decreasing oxidative changes occurring during storage (RBC deformability, metHb formation, and metabolic changes, such as pentose phosphate pathway upregulation) (24, 25). In addition, anaerobic storage with a small amount of CO2 has also been explored as a method of preserving ATP balance in stored RBCs (26).
No in vivo study has been performed before to assess the functionality of anaerobically stored RBCs. This study aims to evaluate the implications of goal-directed resuscitation from severe HS with anaerobically stored RBCs. In efforts to translate these results from rats to humans, we chose to use the 24-h posttransfusion recovery of stored RBCs as the parameter to determine the age of the rat RBCs transfused. A goal-directed resuscitation with aggressive administration of RBCs is a more clinically relevant model, as animals are transfused until a set central hemodynamic goal is met based on mean arterial pressure (MAP) rather than transfusing an arbitrary infusion volume, according to the Advanced Trauma Life Support guidelines based on the recommendations of the American College of Surgeons (27).
MATERIALS AND METHODS
Animal preparation
Studies were performed in male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, Ind) weighing 200 to 250 g. Animal handling and care followed the NIH Guide for Care and Use of Laboratory Animals. The experimental protocol was approved by the UCSD Institutional Animal Care and Use Committee. Animals were anesthetized with isoflurane in compressed room air (Drägerwerk AG, Lübeck, Germany) and placed on a heating pad to maintain core body temperature at 37°C for the duration of the experiment (for more details, see supplemental methods, Supplemental Digital Content 1).
Cardiac function
A 2F pressure–volume (PV) conductance catheter (SPR-858; Millar Instruments, Houston, Tex) was inserted into the left ventricle (LV) using the closed chested method and the signal acquired continuously (MPVS300; Millar Instruments, and PowerLab 8/30, ADInstruments, Colorado Springs, Colo) (28). For the closed chest method, the right carotid artery is isolated with blunt dissection, and the PV catheter is advanced through the aorta until a PV loop is acquired.
Cardiac pressure–volume indices
Cardiac function parameters were calculated from 15 to 20 cardiac cycles at each time point. Stroke volume (SV), stroke work (SW), cardiac output (CO), ejection fraction (EF), cardiac contractility (dP/dt/Ved), and arterial elastance (Ea) were directly calculated in the PowerLab software. Systemic vascular resistance (SVR) was calculated as: 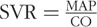 . The internal energy utilization (IEU) was used as a measure of internal metabolism of the LV (29) and was calculated as
. The internal energy utilization (IEU) was used as a measure of internal metabolism of the LV (29) and was calculated as 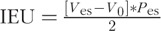 .
.
Hemorrhagic shock resuscitation protocol
Anesthetized rats were hemorrhaged by withdrawing 50% of the animal's blood volume (BV; estimated as 7% body weight) via the femoral artery catheter over 30 min. Hypovolemia was maintained for 30 min before resuscitation. Resuscitation was implemented by infusing the 3-week-old stored blood through the jugular vein catheter at a rate of 300 μL/min, until the animal reached 90% baseline MAP. If the animal's MAP fell below 80% baseline MAP, additional blood was infused (Fig. 1B). The total resuscitation time did not exceed 1 h.
Fig. 1.
Experimental setup.
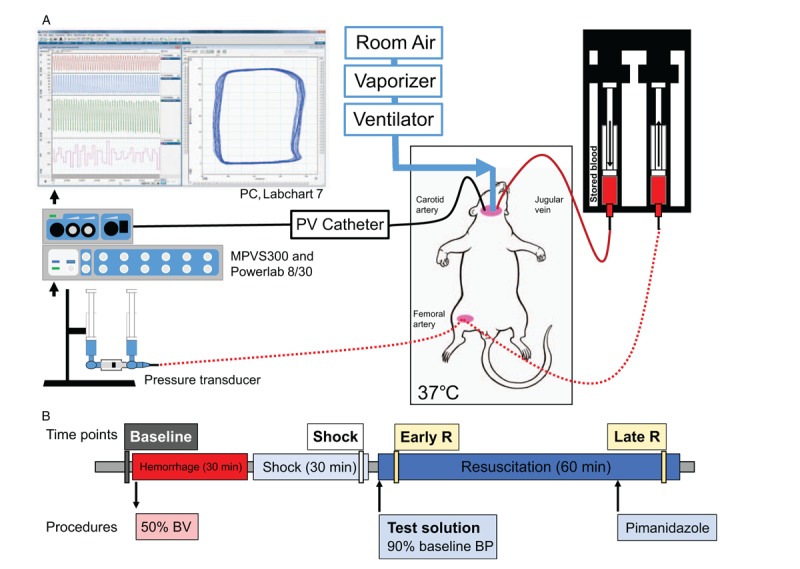
(A) Rats were instrumented with catheters in the left femoral artery and left jugular vein, a tracheal cannula, and a miniaturized pressure–volume catheter introduced through the right carotid artery. (B) Animals were hemorrhaged for half their blood volume, held in shock for 30 min, and then transfused to a goal blood pressure. Resuscitation phase was 1 h long, and pimonidazo le (stain for hypoxic tissues) was injected 15 min before the end of the resuscitation period.
Blood collection and preparation
Briefly, male Sprague-Dawley rats (Harlan Laboratories) weighing 300 to 450 g were anesthetized with a ketamine/xylazine cocktail, and a femoral artery catheter was implanted. Animals bled freely into CP2D (taken from AS-3 blood preparation kit; Haemonetics Corporation, Braintree, Mass), and final CP2D concentration was adjusted to the manufacturer's recommended concentration, and was pooled. Pooled blood was then centrifuged and the supernatant was removed. Finally, AS-3 was added to the manufacturer's recommended concentration, and units were passed through a neonatal leukocyte reduction filter (Haemonetics Corporation). RBC units intended for conventional storage (conventional) were stored at 4°C. Units for anaerobic storage (anaerobic) and anaerobic + CO2 storage (AN+CO2) were deoxygenated by filling the storage bag with nitrogen (or 5% CO2, balance nitrogen), and mixing by rotation at approximately 30 RPM and then stored at 4°C. All blood units were gently mixed and brought to room temperature before infusion.
24-h recovery
Donor rat blood from each group was radiolabeled with Technetium-99 (Tc99) after 3 and 4 weeks of storage, as described by Zink et al. (30). 200 μL of Tc99 radiolabeled blood (approximately 2% of BV) was delivered i.v. to male Sprague-Dawley rats (n = 2 per pool, per storage condition) and samples were drawn at 5 min and 24-h postinjection via tail clip. Samples were all run for radioactivity on a Cobra II gamma counter (Packard Instrument Co., Meriden, Conn) at the same time, so counts reported are independent of sample time.
UHPLC-MS metabolomics
Metabolomics analyses were performed on rat plasma at baseline and after transfusion of 3-week old conventionally or anaerobically stored blood. Detailed descriptions of the metabolomics extraction protocols and analyses have been recently published (31, 32).
Statistical analysis
Results are presented as median with 95% confidence interval of the median. The Grubbs’ method was used to assess closeness for all measured parameters at baseline and shock. Sample size was calculated using an α of 0.05 and a 1−β of 0.9, resulting in a minimum acceptable sample size of 5 per group. Additional animals were included due to the complexity of the experimental setup. Statistically significant changes between solutions and time points were analyzed using two-way analysis of variance (ANOVA), followed by post hoc analyses using Tukey's multiple comparisons test when appropriate. All statistics were calculated using GraphPad Prism 6 (GraphPad, San Diego, Calif). Results were considered statistically significant if P < 0.05.
RESULTS
Stored RBC characterization
In vitro analysis of stored RBCs was performed in two pools of blood, split three ways each. Hemolysis during storage occurred at similar rates. After the 4th week of storage the anaerobic RBCs (anaerobic, AN+CO2) had significantly higher hemolysis compared with conventional storage (Fig. 2A). ATP decreased for all methods, but after the 4th week of storage AN+CO2 and anaerobic RBCs had 51% and 34% higher ATP than conventionally stored RBCs, respectively (Fig. S1B, Supplemental Digital Content 2). After 1 week of storage, anaerobic RBCs had 81% higher 2,3-DPG (P<0.05) than conventional or AN+CO2, but 2,3-DPG was depleted by week 2 of storage (Fig. S1C, Supplemental Digital Content 2). The viscosity of all units increased during storage, and after 4 weeks storage, conventional RBCs had significantly higher viscosity than anaerobically stored RBCs (Fig. 2B). At 3 weeks of storage, anaerobic RBC units had significantly higher lactate (31.0 [95% CI, 26.2–35.8 mmol/L]) compared with conventional (22.0 mmol/L [95% CI, 20.5–23.4 mmol/L]) or AN+CO2 units (24.0 mmol/L [95% CI, 22.6–25.4 mmol/L], respectively).
Fig. 2.
Properties of stored rat RBCs change during storage (two pools of blood, split three ways each).
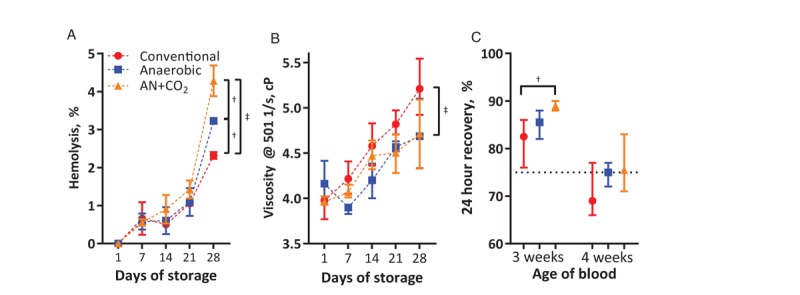
(A) Hemolysis relative to day 1—hemolysis increased during storage (n = 2 per group). (B) Viscosity (measured at 500/s) increased for all units during storage (n = 4 per group). (C) At 3 weeks of storage, all units showed >75% 24-hour recovery. At 4 weeks of storage, 24-h recovery decreased from 3 weeks of storage. Data are presented as median ±95% confidence interval. †P<0.05; ‡P<0.01.
After 3 weeks of storage, 24-h posttransfusion recovery was more than 75% for all RBCs (n = 4 per storage condition). AN+CO2 RBCs showed statistically significantly higher 24-h posttransfusion recovery than conventional RBCs. After 4 weeks of storage, 24-h posttransfusion recovery was below 75% for conventionally stored RBCs, whereas recovery of anaerobically stored RBCs was above 75% (Fig. 2C). No significant correlation was observed between the 24-h posttransfusion recovery and the ATP, 2,3-DPG, hemolysis, or viscosity. HS and resuscitation studies were completed with RBCs stored for 3 weeks, as the 4 weeks 24-h posttransfusion recovery was below FDA requirements (75% recovery).
In vivo studies
A total of 19 animals were entered into the HS/resuscitation study. The animals were randomly assigned to the following groups: conventional (n = 6), anaerobic (n = 7), and AN+CO2 (n = 6). All animals survived the protocol. All groups were similar at baseline determined by Grubb's method.
Systemic hemodynamics
All animals had similar MAP at baseline and at the end of the shock period (Fig. 3A). Early in resuscitation, when similar volumes of stored blood had been given to all animals, MAP was significantly higher for animals that received anaerobically stored RBCs than conventionally stored RBCs. Despite continued infusion, infusion of conventionally stored RBCs did not restore MAP, whereas anaerobically stored RBCs restored MAP to near baseline. Hemorrhage induced bradycardia in all animals (Fig. 3B) and transfusion did not revert heart rate changes in any of the groups. However, conventionally stored RBCs resulted in more severe bradycardia. SVR decreased during shock (Fig. 3C). Conventionally stored RBCs were not able to restore SVR to baseline levels, whereas anaerobically stored RBCs resulted in increased SVR compared with conventionally stored RBCs.
Fig. 3.
Hemodynamics and cardiac function during the hemorrhagic shock/resuscitation protocol.
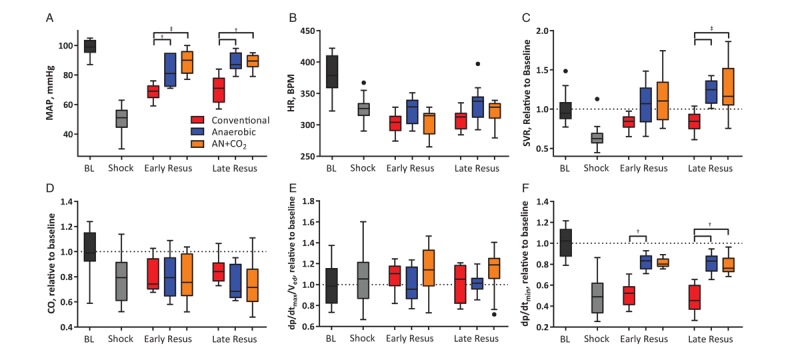
(A) Mean arterial pressure was not restored after transfusion of conventionally stored cells. (B) Heart rate decreased during shock and stayed depressed through the remainder of the protocol. (C) Systemic vascular resistance increased after transfusion of anaerobic and AN+CO2 stored cells. (D) Cardiac output did not increase after resuscitation. (E) Isovolemic contraction normalized to end diastolic volume shows that preload independent contraction increases after hemorrhagic shock. (F) Isovolemic relaxation was slowed during hemorrhagic shock and transfusion of anaerobically stored blood restored isovolemic relaxation rate better than conventionally stored blood. n = 6 for conventional, n = 7 for anaerobic, and n = 6 for AN+CO2 for all in vivo studies. †P<0.05; ‡P<0.01.
Cardiac function
SV decreased after the hemorrhage and recovered with transfusion. As a result of the goal-based resuscitation, cardiac function during resuscitation was similar between groups (Fig. S2, Supplemental Digital Content 3). Conventionally stored RBCs maintained SV after resuscitation compared with baseline, whereas anaerobically stored RBCs slightly decreased SV compared with conventional storage (Fig. S2A, Supplemental Digital Content 3). CO decreased during shock, and increased after transfusion, although baseline CO was not achieved during resuscitation (Fig. 3D). Preload-independent cardiac contractility (dp/dtmax normalized to end diastolic volume, i.e., dp/dtmax/Ved) increased during shock and remained elevated for the duration of the protocol (Fig. 3E). Isovolemic relaxation rate (dp/dtmin) decreased with shock and did not recover with conventionally stored RBCs, whereas it recovered with anaerobically stored RBCs (Fig. 3F).
Hematology
Hemorrhage reduced hematocrit to a similar level in all groups (Table 1). Following the goal-based protocol, animals were transfused until MAP reached 90% of the baseline MAP. A significantly higher volume of conventionally stored RBCs was transfused in attempts to reach this blood pressure goal (Fig. 4). At the end of the observation period, animals transfused with anaerobically stored RBCs required half the volume of those transfused with conventionally stored RBCs. This corresponded to an infusion of 58% [95% CI, 46%–70%], 29% [95% CI, 17%–42%], and 27% [95% CI, 17%–38%] of the baseline BV for conventional, anaerobic, and AN+CO2, respectively. Therefore, animals receiving conventionally stored RBCs had significantly higher hematocrit and plasma Hb concentration at the end of resuscitation (Table 1).
Table 1.
Hematology of rats during the hemorrhagic shock/resuscitation protocol
| Time point | Group | Hct, % | Hb, g/dL | pHb, g/dL |
| BL | 43 [42–43] | 13.8 [13.4–14.0] | ||
| Shock | 28 [27–29] | 8.9 [8.5–9.4] | ||
| Early resuscitation | Conventional | 40 [36–42] | 13.1 [11.8–14] | 0.1 [0.0–0.1] |
| Anaerobic | *35 [33–37] | †11.9 [10.6–11.9] | 0.1 [0.0–0.2] | |
| AN+CO2 | 37 [35–39] | 12.6 [12.1–13.3] | 0.1 [0.1–0.2] | |
| Late resuscitation | Conventional | 54 [42–57] | 18.4 [15.1–18.5] | 0.4 [0.2–0.4] |
| Anaerobic | †39 [35–46] | †13.5 [11.1–16.4] | †0.1 [0.0–0.1] | |
| AN+CO2 | †40 [35–42] | †13.7 [11.6–15.0] | †0.1 [0.0–0.1] |
*P<0.05 vs. conventional.
†P<0.01 vs. conventional.
Data are presented as median with 95% confidence interval.
Fig. 4.
Volume of blood (in relative number of blood units) delivered to the animals throughout the protocol.
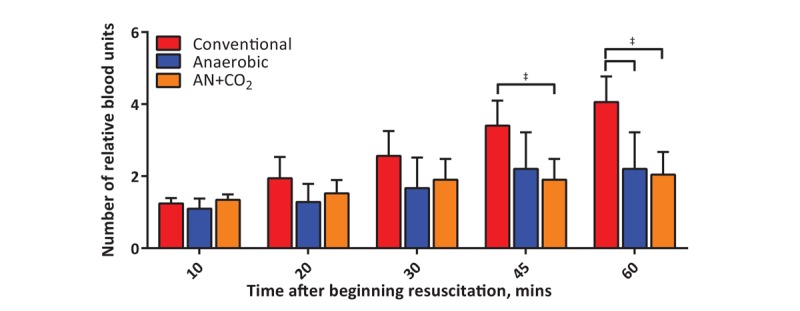
Significantly more conventionally stored blood than anaerobically stored blood was transfused while attempting to reach the hemodynamic goal. †P<0.05; ‡P<0.01.
Blood chemistry
Shock induced systemic acidosis for all groups, and transfusion of conventionally stored RBCs worsened the acidosis (Table 2). Anaerobic and AN+CO2 RBCs restored blood pH by the end of the experimental protocol. Potassium was significantly lower after transfusion of anaerobic and AN+CO2 stored RBCs compared with conventionally stored RBCs. Calcium was significantly higher after transfusion of anaerobically stored RBCs than conventionally stored RBCs. Shock increased glucose levels, which remained elevated during resuscitation. Lactate also increased during shock. Resuscitation with conventionally stored RBCs initially increased lactate levels, whereas anaerobically stored RBCs slightly decreased lactate levels (Fig. 5A). Lactate levels were elevated at the end of resuscitation. Animals transfused with conventionally stored RBCs had higher lactate compared with animals transfused with anaerobically stored RBCs. Metabolic reprogramming secondary to HS has been extensively described in humans and animal models (31, 33). Shock promoted significant metabolic changes (extensively reported in Supplemental Digital Content 4). Anaerobically stored RBCs were able to correct the metabolic derangements observed during shock compared with conventionally stored RBCs (Fig. 5).
Table 2.
Blood gasses and chemistry of rats during hemorrhagic shock/resuscitation protocol
| Conventional | Anaerobic | AN+CO2 | ||
| pH | BL | 7.43 [7.39–7.47] | ||
| Shock | 7.25 [7.15–7.28] | |||
| Early resuscitation | 7.05 [6.96–7.18] | †7.23 [7.09–7.36] | †7.25 [7.13–7.31] | |
| Late resuscitation | 7.13 [6.97–7.32] | †7.36 [7.27–7.41] | †7.37 [7.33–7.42] | |
| PCO2, mmHg | BL | 38.7 [35.7–44.0] | ||
| Shock | 30.2 [27.8–33.1] | |||
| Early resuscitation | 36.8 [32.8–43.7] | 36.9 [34.1–64.8] | 33.7 [31.4–39.7] | |
| Late resuscitation | 40.4 [37.0–48.8] | 41.2 [34.3–53.5] | 37.9 [31.2–40.4] | |
| PO2, mmHg | BL | 93.8 [87.0–101.0] | ||
| Shock | 118.0 [106.0–128.0] | |||
| Early resuscitation | 91.9 [87.5–96.3] | 90.2 [69.0–108.0] | *,‡109.0 [102.0–125.0] | |
| Late resuscitation | 56.6 [44.0–68.1] | †82.4 [65.9–89.7] | †89.7 [78.9–114.0] | |
| Glucose, mg/dL | BL | 199 [176–230] | ||
| Shock | 450 [328–556] | |||
| Early resuscitation | 415 [267–450] | 392 [344–577] | 423 [170–529] | |
| Late resuscitation | 315 [186–330] | 294 [221–459] | 350 [183–459] | |
| K+, mEq/L | BL | 4.3 [4.1–4.7] | ||
| Shock | 5.1 [4.6–5.7] | |||
| Early resuscitation | 6.0 [5.5–6.3] | †5.2 [4.8–5.5] | †4.5 [4.3–5.2] | |
| Late resuscitation | 6.4 [5.4–7.1] | †4.8 [4.6–5.3] | †4.7 [3.9–5.1] | |
| Na+, mEq/L | BL | 138 [136–139] | ||
| Shock | 134 [133–136] | |||
| Early resuscitation | 136 [135–140] | 134 [130–136] | 136 [133–140] | |
| Late resuscitation | 137 [136–141] | 137 [134–139] | 137 [134–138] | |
| Ca2+, mEq/L | BL | 2.03 [1.80–2.19] | ||
| Shock | 2.36 [2.11–2.48] | |||
| Early resuscitation | 1.94 [1.83–1.97] | 1.95 [1.68–2.14] | 2.12 [1.8–2.23] | |
| Late resuscitation | 1.64 [1.57–2.00] | *2.01 [1.87–2.43] | *1.89 [1.80–2.35] | |
| Cl−, mEq/L | BL | 102 [101–103] | ||
| Shock | 100 [99–104] | |||
| Early resuscitation | 101 [99–103] | 100 [95–103] | 100 [98–103] | |
| Late resuscitation | 103 [99–107] | 103 [99–107] | 102 [100–105] |
*P<0.05 vs. conventional.
†P<0.01 vs. conventional.
‡Is used to denote the significant difference (P<0.01) in PO2 between AN+CO2 and AN at the Early resuscitation timepoint.
Data are presented as median with 95% confidence interval.
Fig. 5.
Blood chemistry and metabolomics during the hemorrhagic shock/resuscitation protocol.
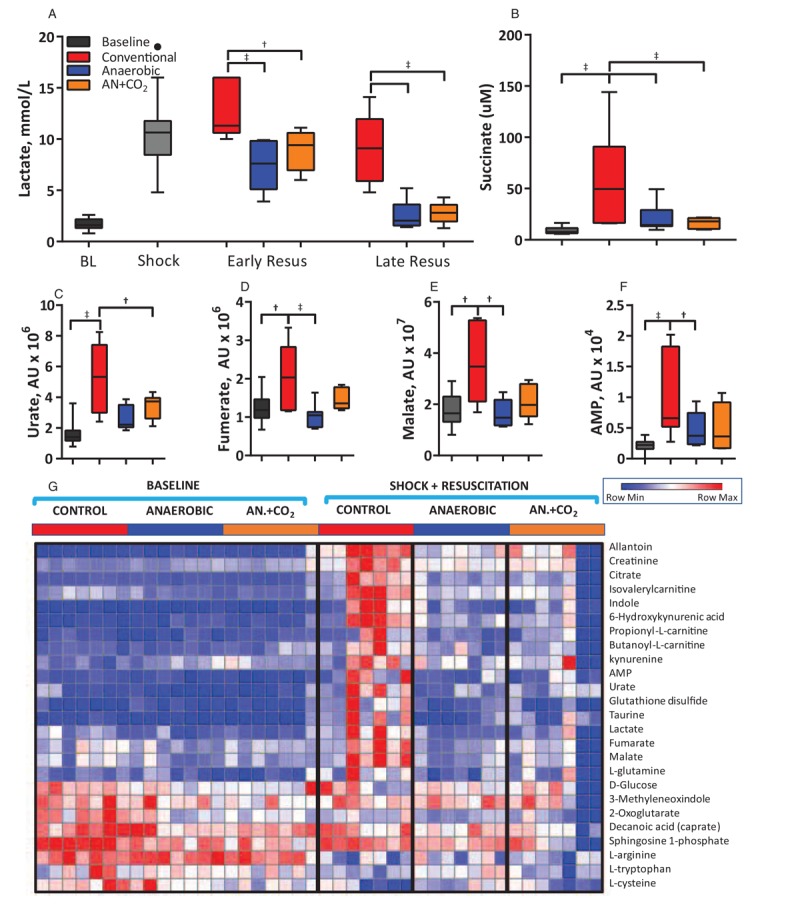
(A) Lactate increased during shock and increased further upon transfusion of conventionally stored cells. (B) Succinate was significantly higher for animal transfused with conventionally stored cells than anaerobically stored cells. (C–F) Concentration of urate, fumarate, malate, and AMP in the plasma at baseline and the end of resuscitation for each group. (G) Heatmap of metabolites. †P<0.05; ‡P<0.01.
Oxygen delivery
Shock increased arterial Hb O2 saturation. Resuscitation resulted in a decrease in arterial O2 saturation. Conventionally stored RBC transfusion resulted in a dose-dependent decrease in arterial O2 saturation (Fig. 6A). Oxygen delivery (DO2) decreased during shock and was partially restored upon transfusion in all experimental groups (Fig. 6B). Despite a decreased arterial O2 saturation, conventionally stored RBCs restored DO2 due to an increase in O2-carrying capacity (hematocrit and Hb). No difference in DO2 normalized by Hb was observed among resuscitation groups (Fig. 6C). The ratio of DO2 to oxygen consumption (DO2/VO2) increased after transfusion and remained increased by the end of protocol (Fig. 6D).
Fig. 6.
Oxygen delivery during the hemorrhagic shock/resuscitation.
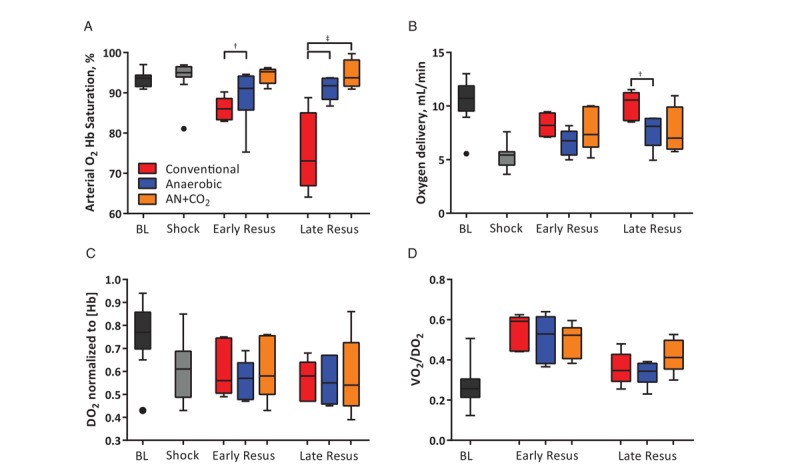
(A) Arterial Hb O2 saturation decreased upon administration of conventionally stored cells, but not anaerobically stored cells. (B) Oxygen delivery increased after transfusion of stored RBCs. (C) When normalized to Hb content, there are no differences in oxygen delivery among groups. (D) A higher ratio of oxygen was extracted after resuscitation. †P<0.05; ‡P<0.01.
Organ damage, function, and hypoxia
Markers of organ injury, function, and inflammation are displayed in Figure 7, A–J. Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT), markers of liver damage, were elevated in animals transfused with conventionally stored RBCs compared with anaerobically stored RBCs. Serum creatinine and blood urea nitrogen (BUN), markers of kidney function, were slightly higher in the animals transfused with conventionally stored RBCs compared with anaerobically stored RBCs, but there were no significant differences in serum creatinine. Urinary neutrophil gelatinase-associated lipocalin (U-NGAL), a marker of acute kidney damage, was also elevated in animals given conventionally stored RBCs compared with anaerobically stored RBCs. CXCL1, a neutrophil activating protein, was elevated in the liver, spleen, and lung for animals receiving conventionally stored RBCs compared with anaerobically stored RBCs. Other markers of inflammation, such as % of CD45+ neutrophils and interleukin-6 concentration, were also elevated in response to transfusion of conventionally stored RBCs compared with anaerobically stored RBCs. Organ hypoxia, as measured by pimonidazole staining, is displayed in Figure 7K. All organs other than the kidney showed higher levels of hypoxia in animals given conventionally stored RBCs compared with anaerobic stored RBCs.
Fig. 7.
Markers of organ damage, function, inflammation, and hypoxia postmortem.
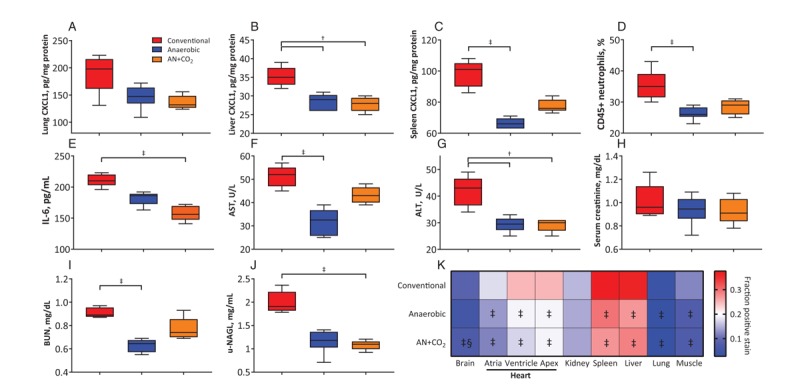
All hemorrhaged animals showed significant organ damage, function, and inflammation relative to sham controls (not shown). (A–C) Markers of neutrophil activation in individual organs. (D) Percentage of CD45+ neutrophils. (E) Interleukin-6 levels. (F) Aspartate aminotransferase (AST) and (G) Alanine aminotransferase (ALT) are markers of liver damage. (H, I) Markers of kidney function. (J) Urinary neutrophil gelatinase-associated lipocalin (u-NGAL) is a marker of acute kidney damage. (K) Organ hypoxia was quantified via pimonidazole staining 15 min before sacrifice and are expressed as a fraction of positive staining. †P<0.05 vs. conventional; ‡P<0.01 vs. conventional, §P<0.05 vs. anaerobic.
DISCUSSION
The principal finding of this study is that transfusion of stored RBCs under anaerobic and anaerobic/hypercapnic conditions after severe hemorrhage required lower transfusion volumes compared with conventionally stored RBCs. Anaerobically stored RBCs restored O2 delivery and cardiac function while improving systemic hemodynamics, and metabolism compared with transfusion of conventionally stored RBCs. Furthermore, transfusion of RBCs stored in anaerobic conditions produced a significantly smaller degree of organ and tissue damage compared with transfusion with conventionally stored RBCs to arrive to the same clinically relevant hemodynamic goal.
RBCs stored under anaerobic and anaerobic/hypercapnia conditions were different in terms of blood pH, but no differences in pH were observed after transfusion. Conventionally stored RBCs did not fully achieve the resuscitation goals in all cases, whereas anaerobically stored RBCs met the resuscitation goals in all animals. Anaerobically stored RBCs reduced the volume necessary to resuscitate from HS compared with conventionally stored RBCs. Anaerobically stored RBCs restored MAP and vascular resistance compared with conventionally stored RBCs. In addition, anaerobically stored RBCs decreased lactate levels, reduced the extent of the organ hypoxia, preserved organ function, and prevented vital organ injury relative to conventionally stored RBCs. Lastly, results indicate that the lack of O2 in during storage of anaerobically stored RBCs did not have a negative impact in oxygen delivery and that preoxygenation of anaerobically stored RBCs before transfusion was not necessary.
In this study's protocol, the decision to administer additional volume was not based on transfusion triggers (e.g., Hb concentration), but on a hemodynamic goal (blood pressure). Thus, the volume transfused was defined by extent of injury due to the HS, and the response of the cardiovascular system to the transfusion. Systemic hemodynamic parameters, such as MAP and HR, reflect the adherence to the experimental protocol. However, conventionally stored RBCs did not effectively restore MAP, suggesting that these RBCs produced a negative cardiovascular response compared with anaerobically stored RBCs because shock was identical for all groups. Indices of cardiac function primarily dependent on BV (e.g., SW, CO, and dp/dtmax) improved after resuscitation independent of the storage condition. However, cardiac parameters dependent on active pressure generation, such as SVR and IEU, were corrected only with anaerobically stored RBCs. The inability of conventionally stored RBCs to recover MAP after resuscitation is likely a consequence of differences in myocardial oxygen delivery and/or the low arterial O2 saturation compared with transfusion of anaerobically stored RBCs. Although the recovery in MAP with anaerobically stored RBCs was due to an increase in SVR, no obvious vasoactive stimulus (such as hemolysis or increased blood viscosity) was associated with anaerobically stored RBCs (as hemolysis and blood viscosity were lower for anaerobically stored RBCs compared to conventionally stored RBCs).
During shock, lactate accumulated due to low perfusion, reduced oxygen delivery, and increased anaerobic cellular metabolism. Using blood lactate as a marker of the efficacy of transfusion, animals receiving conventionally stored RBCs showed a reduced recovery of O2 delivery relative compared with anaerobically stored RBCs. Similarly, other important metabolic markers of hypoxia, such as succinate and carboxylic acids, were elevated in animals given conventionally stored RBCs. Conventionally stored RBCs did not restore aerobic metabolism after HS, either due to inappropriate O2 delivery to tissues or the inability to restore microcirculatory perfusion (34). The observed lactate levels after transfusion of conventionally stored RBCs allude to poor cardiac microvascular perfusion because the myocardium preferentially uses lactate as an energy source (35, 36). Improper cardiac O2 release in the conventionally stored RBCs relative to the anaerobically stored RBCs was also evidenced by the decreased LV IEU (29). As the LV consumes nearly all O2 delivered, IEU is an excellent indicator of appropriate O2 delivery/consumption to/by the LV (37). Inability to preserve MAP after transfusion of conventionally stored RBCs can also be attributed to limited IEU, as elevated SV is less energetically costly than high end systolic left ventricular pressure (37). Suppressed dp/dtmin in the conventionally stored RBCs group is another indicator of cardiac ischemia (38). However, this suppression is also likely due to the high transfusion volumes and the increase in blood viscosity posttransfusion of conventionally stored blood.
The larger (nearly double) transfusion volume given to the conventional storage group significantly increased hematocrit and consequently blood viscosity, which impaired the left ventricular ejection of blood. In addition, results obtained with conventionally stored RBCs suggest poor coronary perfusion resulting from higher blood viscosity and reduced RBC deformability (24), which reduces ventricular O2 extraction by limiting coronary microcirculatory function (34). The conventionally stored RBCs may have acted more as nitric oxide (NO) sinks than anaerobically stored RBCs, due to oxidative stress during storage, resulting in vasoconstriction and further exacerbating poor coronary perfusion posttransfusion (39, 40). Despite only receiving approximately double the RBC volume, animals transfused with conventionally stored RBCs experienced more than 4 times the levels of plasma Hb. This indicates a higher degree of intravascular hemolysis, overwhelming the free Hb and heme scavenging system, and further impacting NO bioavailability, in animals transfused with conventionally stored RBCs compared with anaerobically stored RBCs (39). Higher hematocrit achieved after transfusion of conventionally stored RBCs increased DO2 to levels exceeding those of animals transfused with anaerobically stored RBCs. When normalized to the Hb, all three groups had very similar DO2. As the experimental design of the study had all animals breathing room air (21% O2), it is possible to speculate that animals receiving conventionally stored RBCs could have benefited from a higher FiO2 to increase posttransfusion arterial O2 saturation and to prevent systemic vasorelaxation resulting from low arterial O2 saturation (41). However, other evidence suggests that the transfused conventionally stored RBCs were less effective releasing O2 to tissues, as confirmed by the increased lactate and other metabolic markers of anaerobic metabolism, and the detected vital organ hypoxia with pimonidazole staining (Fig. 7K).
Conventionally stored RBCs resulted in additional sequelae in the form of organ damage and elevated levels of organ and systemic hypoxia, as also shown by plasma levels of lactate, succinate, and other carboxylic acids. Organs with elevated levels of hypoxia also showed elevated inflammatory markers, but organ damage was not always associated with hypoxia. Conventionally stored RBCs resulted in elevated markers of kidney injury and impaired function, but no hypoxia was detected in the kidneys. However, hemolysis is known to affect kidneys and other vital organs (42). Systemic hemodynamic parameters do not reflect the increased vital organ hypoxia, organ injury, or increased inflammation. While conventionally stored RBCs passed the 24-h recovery metric set in place to ensure safety of RBC units, they were only able to partially restore cardiac function, and their infusion resulted in additional sequelae.
The 24-h posttransfusion recovery is the FDA approved metric for determining the life span of stored RBCs in additive solutions (15). The rationale underpinning this parameter is that RBCs removed from circulation do not transport and deliver O2, so at least 75% of the transfused RBCs must circulate for at least 24-h posttransfusion. This concept has been recently brought into question by the FDA, (15) which is working to determine better parameters to assess the efficacy of transfusions. Posttransfusion 24-h recovery studies were performed by infusing a small volume of radiolabeled cells and measuring the number of surviving labeled RBCs after 24 h. The 24-h recovery studies do not seem to correlate with the storage condition's ability to resuscitate from HS. Both anaerobically and conventionally stored RBCs had 24-h posttransfusion recovery over 75%, but conventionally stored RBCs had obvious functional limitations in resuscitation from HS. The 24-h posttransfusion recovery studies tend to overestimate the real posttransfusion recovery, as the RBC labeling processes mechanically and biochemically challenge the cells and eliminate the most fragile and weak RBCs before infusion. In addition, posttransfusion recovery studies do not take into account the other materials accumulated in the blood during storage (25). From a functional standpoint, defining “expiration dates” for stored RBCs in additive solutions based on 24-h posttransfusion recovery is inappropriate, especially as RBC units age at different rates (21, 22) and 24-h recovery does not correlate with other functional markers.
Storage under hypoxic conditions has been shown to prevent and reduce some irreversible storage lesions induced by oxidative stress (43). However, one of the challenges of transfusing anaerobically stored RBCs is the risk of depleting O2 from circulating blood by transfusing deoxygenated RBCs. However, our results indicate that transfused deoxygenated RBCs rapidly become oxygenated as they circulate, with no effect on arterial blood gasses or arterial O2 saturation. One-hour posttransfusion, the arterial O2 saturation was significantly higher in animals transfused with anaerobically stored RBCs compared with conventionally stored RBCs. Anaerobically stored RBCs have been shown to have improved preservation of membrane deformability and decreased metHb formation, which can reduce damage of organs in the reticuloendothelial system (24, 25). Microvesiculation of the RBC membrane, which is known to cause adverse reactions (8–10), is also reduced during anaerobic storage (26).
Limitations
Studies in rats are not directly translatable to clinical scenarios, but the results of the study indicate that anaerobically storage of RBCs preserves cell viability and functionality compared with conventional storage. Rat RBCs likely have some metabolic differences from human RBCs during storage, but the ATP, 2,3-DPG, and lactate at the end of storage are similar to those in humans, albeit the changes observed occur on a faster time scale (44). Studies have recently pointed out the advantages and caveats of focusing on circulating levels of lactate and carboxylates alone in rat models of shock and resuscitation. Furthermore, the results of the study are comparable with other animal models phylogenetically closer to humans (e.g., porcine and nonhuman primate models) as well as critically injured patients (45, 46). As the study's resuscitation goal was aimed to replicate clinical scenarios, where transfusion is given until a defined hemodynamic goal is achieved, the differences in cardiac function among groups were not expected to be great, which limits the capability to discern functional differences between resuscitation groups. However, other primary outcomes of the study, like the volume infused during resuscitation, organ damage, and organ hypoxia, showed significant differences. To ensure there are no sequalae that come from transfusing anaerobically stored RBCs, primary outcomes would need to be observed over longer time periods and functional outcomes, such as neurological function, should be measured.
In summary, the results presented here show that anaerobically stored RBCs improved outcomes during resuscitation from HS. Anaerobically stored RBCs required significantly lower volume and resulted in fewer sequela than conventionally stored RBCs. Anaerobically stored RBCs clearly improved systemic metabolic parameters associated with HS and ameliorated/prevented the generation of proinflammatory cytokines, molecular mediators of some of the most common sequelae observed after trauma (e.g., acute lung injury) (47). Functionally, there are no discernable differences between storing blood anaerobically or anaerobically with CO2, despite the metabolic differences of the two storage conditions (26). Anaerobically stored RBCs seem to maintain viability for an extended duration, both from a 24-h recovery, as well as from a functional point of view. These data suggest that storing RBCs anaerobically could result in better transfusion recipient outcomes. Additional prospective clinical trials for trauma patients may be needed to better understand the association between RBC storage conditions and outcomes.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Cynthia Walser (UC San Diego) for surgical preparation of the animals, and Joyce B. Li (UC San Diego) for assistance with illustrations.
Footnotes
Authors’ contributions: ATW and PC designed the experimental studies; ATW performed experiments and acquired data. ATW, VPJ, and AL analyzed data. TN and ADA acquired and analyzed metabolomics data. TY and AD generated technology for ex vivo oxygen-controlled preservation of RBCs and provided reagents. ATW, VPJ, ADA, and PC wrote the manuscript.
Reagents were provided by Hemanext for completion of the study. Hemanext did not participate in implementation of the study. Additional funding was provided by NIH grants from the Heart Lung and Blood Institute, T32-HL105373, R01-HL126945, and R01 HL138116.
The authors would like to disclose that TY and AD are a part of Hemanext. ADA is a consultant for Hemanext.
REFERENCES
- 1.Karch DL, Lubell KM, Friday J, Patel N, Williams DD. Centers for Disease Control and Prevention (CDC): Surveillance for violent deaths-National Violent Death Reporting System, 16 states, 2005. MMWR Surveill Summ 57 (3):1–45, 2008. [PubMed] [Google Scholar]
- 2.Norton R, Kobusingye O. Injuries. N Engl J Med 368 (18):1723–1730, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 123 (5):615–624, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg 32 (11):925–1002, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Horton JW. Hemorrhagic shock depresses myocardial contractile function in the guinea pig. Circ Shock 28 (1):23–35, 1989. [PubMed] [Google Scholar]
- 6.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med 125 (8):680–687, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Colwell C. Initial management of moderate to severe hemorrhage in the adult trauma patient. In: Moreira ME, Grayzel J, editors. Waltham, MA: UpToDate Inc. 2017. Available at: https://www.uptodate.com/contents/initial-management-of-moderate-to-severe-hemorrhage-in-the-adult-traumapatient. Accessed May 10, 2019. [Google Scholar]
- 8.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 118 (25):6675–6682, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hod EA, Spitalnik SL. Stored red blood cell transfusions: Iron, inflammation, immunity, and infection. Transfus Clin Biol 19 (3):84–89, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115 (21):4284–4292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones AR, Patel RP, Marques MB, Donnelly JP, Griffin RL, Pittet J-F, Kerby JD, Stephens SW, DeSantis SM, Hess JR, et al. Older blood is associated with increased mortality and adverse events in massively transfused trauma patients: secondary analysis of the PROPPR trial. Ann Emerg Med 73 (6):650–661, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe LC. The membrane and the lesions of storage in preserved red cells. Transfusion 25 (3):185–203, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfus Med Rev 15 (2):91–107, 2001. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, Zolla L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 55 (1):205–219, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Vostal JG, Buehler PW, Gelderman MP, Alayash AI, Doctor A, Zimring JC, Glynn SA, Hess JR, Klein H, Acker JP, et al. Proceedings of the Food and Drug Administration's public workshop on new red blood cell product regulatory science. Transfusion 58 (1):255–266, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol 34: 3 Suppl. 2: 34–40, 1997. [PubMed] [Google Scholar]
- 17.Hofmann A, Ozawa S, Farrugia A, Farmer SL, Fellow R, Shander A, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol 27:59–68, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Jani VP, Yalcin O, Williams AT, Popovsky MA, Cabrales P. Rat red blood cell storage lesions in various additive solutions. Clin Hemorheol Microcirc 67 (1):45–57, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raval JS, Fontes J, Banerjee U, Yazer MH, Mank E, Palmer AF. Ascorbic acid improves membrane fragility and decreases haemolysis during red blood cell storage. Transfus Med 23 (2):87–93, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Hess JR. An update on solutions for red cell storage. Vox Sang 91 (1):13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Jani VP, Mailo S, Athar A, Lucas A, Williams AT, Cabrales P. Blood quality diagnostic device detects storage differences between donors. IEEE Trans Biomed Circuits Syst 11 (6):1400–1405, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzounakas VL, Georgatzakou HT, Kriebardis AG, Voulgaridou AI, Stamoulis KE, Foudoulaki-Paparizos LE, Antonelou MH, Papassideri IS. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion 56 (6):1274–1286, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Blair A, D’alessandro A, Nemkov T, Dioguardi M, Silliman CC, Dunham A. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus 15 (2):172–181, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardi G, Banfi G, Lippi G, Sanchis-gomar F, Burns JM, Yoshida T, Dumont LJ, Yang X, Piety NZ, Shevkoplyas SS. Deterioration of red blood cell mechanical properties is reduced in anaerobic storage. Blood Transfus 14 (1):80–88, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reisz JA, Wither MJ, Dzieciatkowska M, Nemkov T, Issaian A, Yoshida T, Dunham AJ, Hill RC, Hansen KC, D’Alessandro A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 128 (12):e32–42, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, Yoshida T. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion 56 (2):392–403, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Surgeons Committee on Trauma. Encyclopedia of Trauma Care. 2012; Berlin Heidelberg: Springer, p. 1342015ss. [Google Scholar]
- 28.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3 (9):1422–1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle M, Weinberg N, Pohost GM, Merz CNB, Shaw LJ, Sopko G, Fuisz A, Rogers WJ, Walsh EG, Johnson BD, et al. Left ventricular energy model predicts adverse events in women with suspected myocardial ischemia: results from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. Cardiovasc Diagn Ther 3 (2):64–72, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zink SI, Ohki SK, Stein B, Zambuto DA, Rosenberg RJ, Choi JJ, Tubbs DS. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99m Tc-labeled RBC scintigraphy. Am J Roentgenol 191 (4):1107–1114, 2008. [DOI] [PubMed] [Google Scholar]
- 31.D’Alessandro A, Moore HB, Moore EE, Wither M, Nemkov T, Gonzalez E, Slaughter A, Fragoso M, Hansen KC, Silliman CC, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol 308 (12):R1034–R1044, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom 31 (8):663–673, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’alessandro A, Nemkov T, Moore HB, Moore EE, Wither M, Nydam T, Slaughter A, Silliman CC, Banerjee A, Hansen KC. Metabolomics of trauma-associated death: shared and fluid-specific features of human plasma vs lymph. Blood Transfus 14 (2):185–194, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. Am J Physiol Heart Circ Physiol 293 (2):H1206–H1215, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Drake AJ, Haines JR, Noble MI. Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc Res 14 (2):65–72, 1980. [DOI] [PubMed] [Google Scholar]
- 36.Chatham JC, Gao ZP, Forder JR. Impact of 1 wk of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation. Am J Physiol 277 (2 Pt. 1):E342–E351, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Britman NA, Levine HJ. Contractile element work: a major determinant of myocardial oxygen consumption. J Clin Invest 43 (7):1397–1408, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaurin LP, Rolett EL, Grossman W. Impaired left ventricular relaxation during pacing-induced ischemia. Am J Cardiol 32 (6):751–757, 1973. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci 104 (43):17058–17062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner SJ, Glynn SA, Welniak LA. NHLBI working group on strategies to optimize red blood cell products. Research opportunities in optimizing storage of red blood cell products. Transfusion 54 (2):483–494, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulandavelu S, Balkan W, Hare JM. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Natl Acad Sci USA 112 (20):6254–6255, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 18 (2):414–420, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Nemkov T, Sun K, Reisz JA, Song A, Yoshida T, Dunham A, Wither MJ, Francis RO, Roach RC, Dzieciatkowska M, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 103 (2):361–372, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA 104 (43):17063–17068, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reisz JA, Wither MJ, Moore EE, Slaughter AL, Moore HB, Ghasabyan A, Chandler J, Schaub LJ, Fragoso M, Nunns G, et al. All animals are equal but some animals are more equal than others: plasma lactate and succinate in hemorrhagic shock-A comparison in rodents, swine, nonhuman primates, and injured patients. J Trauma Acute Care Surg 84 (3):537–541, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DʼAlessandro A, Moore HB, Moore EE, Reisz JA, Wither MJ, Ghasasbyan A, Chandler J, Silliman CC, Hansen KC, Banerjee A. Plasma succinate is a predictor of mortality in critically injured patients. J Trauma Acute Care Surg 83 (3):491–495, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunns GR, Banerjee A, Moore EE, Kelher M, D’Alessandro A, Burke TA, Peltz ED, Sauaia A, Silliman CC. Post-traumatic acute lung injury: the role of succinate. J Am Coll Surg 225 (4):e183–e184, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


