Abstract
Background:
S1400B is a biomarker-driven Lung-MAP sub-study evaluating the phosphatidylinositide 3-kinase (PI3K) inhibitor taselisib (GDC-0032) in patients with PI3K pathway-activated squamous NSCLC (SqNSCLC).
Methods:
Eligible patients had tumoral PIK3CA alterations by next generation sequencing and disease progression after at least one line of platinum-based therapy. Patients received 4 mg taselisib orally daily. The primary analysis population (PAP) was a subset of patients having substitution mutations believed to be associated with clinical benefit of PI3K inhibitors. Primary endpoint was response by RECIST 1.1; secondary endpoints included progression-free survival (PFS), overall survival (OS) and duration of response (DoR).
Results:
Twenty-six patients treated with taselisib comprised the full eligible population (FEP); 21 patients comprised the PAP. Median age in FEP was 68 y (53–83), 19 were male (73%). The study was closed for futility at interim analysis with one responder in the PAP (5% RR, 95% CI 0%−24%). Two possibly treatment-related deaths (1 respiratory failure, 1 cardiac arrest) were observed; 1 patient had Grade 4 and 11 had Grade 3 adverse events. Median PFS and OS in the PAP were 2.9 months (95% CI, 1.8–4.0 mos) and 5.9 months (95% CI, 4.2–7.8 mos), respectively. These numbers were nearly the same in the FEP.
Conclusions:
Study S1400B evaluating taselisib in PIK3CA altered SqNSCLC failed to meet its primary endpoint and was closed after an interim futility analysis. The trial is unique in cataloguing the diversity of PIK3CA mutations in SqNSCLC.
Introduction
The Lung Master Protocol (Lung-MAP, SWOG 1400) is an umbrella protocol with a screening component and multiple independently conducted and analyzed treatment sub-studies.[1] Herein we report on the results of SWOG S1400B, a phase II Lung-MAP sub-study evaluating taselisib, a phosphoinositide-3 kinase (PI3K) inhibitor in patients with chemo-refractory SqNSCLC tumors harboring alterations in PI3KCA.
PI3Ks are a family of lipid kinases involved in tumor cell proliferation, survival, and migration upon activation by growth factor receptors and integrins. PI3Ks catalyze phosphorylation of phosphatidylinositol-4, 5 bisphosphate to generate phosphatidylinositol-3, 4, 5 trisphosphate, a second messenger involved in phosphorylation of AKT and associated proteins in the AKT/mTOR pathway. [2, 3] Activating and transforming mutations, as well as amplification, in the p110 alpha isoforms of PI3K are commonly found in solid and hematological tumors. [4] Additionally, the PI3K/AKT pathway is activated in numerous types of cancer by receptor tyrosine kinase signaling, loss of phosphatase and tensin homolog (PTEN), or RAS mutations. [2, 5–8] PI3K alterations, including PI3K mutations and PTEN loss or mutations, are observed in 30%−50% of squamous lung cancers. PI3K mutations are observed in 2–5% of non-squamous and in 8–10% of sqNSCLC. [7], and human tumors carrying mutated PIK3CA or deleted PTEN have responded favorably to PI3K inhibition. [6]
Taselisib is a potent, selective small molecule inhibitor of Class 1 PI3Ks developed by Genentech as an anticancer therapeutic agent that is a potent growth inhibitor in nonclinical models of PI3K mutant tumors.[8, 9]
Methods
Patients with previously treated advanced SqNSCLC were eligible for the S1400 screening study. Briefly, eligibility for S1400B stipulated age ≥ 18 years, Zubrod PS of 0–2 (modified to 0–1 during the study), measurable disease by RECIST, and adequate hematologic, hepatic, and cardiac function with no supplemental oxygen requirement. Calcium and phosphate levels had to be within institutional limits. Patients had to be able to take oral medications with no impairment of gastrointestinal function or gastrointestinal disease that could significantly alter the absorption of taselisib. Patients with leptomeningeal disease; symptomatic, untreated brain metastases; and chemotherapy within 21 days prior to registration were excluded.
Eligibility for treatment with taselisib required base substitutions in PIK3CA (see Table 1). The primary analysis population (PAP) was defined by the presence of alterations expected to derive the greatest benefit from PI3K inhibition. Mutational analysis was performed on archival formalin-fixed paraffinembedded (FFPE) tumor specimens using FoundationOne® (Foundation Medicine, Cambridge, MA). Tumor mutational burden (TMB) was calculated as the number of somatic, coding, short variants, excluding known driver mutations, per megabase of genome interrogated.
Table 1.
PI3KCA Mutations in Patients Eligible for Treatment with Taselisib
| Eligible Populations | Number of Patients | Base Substitution Mutations Allowed* |
|---|---|---|
| Full Evaluable Population (FEP) | 26 | R38C, R38H, E81K, R88Q, R93Q, R93W, P104L, P104R, G106V, G106R, P104_G106>R, R108H, E110del, E110K, K111E, K111N, K111del, G118D, V344G, V344M, N345K, N345I, E365K, C378F, E418K, C420R, E453K, E453Q, P539R, E542K, E542A, E542V, E542G, E542Q, E545A, E545G, E545K, E545Q, E545D, Q546E, Q546H, Q546K, Q546L, Q546P, Q546R, E726K, G1007R, D1017H, Y1021C, Y1021H, T1025A, A1035V, A1035T, M1043I, M1043L, M1043V, H1047L, H1047R, H1047Y, H1047N, H1047Q, G1049R, G1049S, I1058L |
| Primary Analysis Population (PAP) | 21 | E542K, E545A, E545G, E545K, E545Q, H1047L, H1047R, or H1047Y |
Base substitutions, small insertions and deletions, focal copy number amplifications, homozygous gene deletions, and genomic rearrangements were analyzed. Patients with disease characterized by PI3KCA gene amplifications and fusions were not eligible
Taselisib was administered orally at 4 mg qd on an empty stomach in 21 day treatment cycles. Disease assessment occurred every two cycles, and treatment was continued until disease progression or untoward toxicity. Dose reductions and adjustments were discussed with the study chair and were followed as specified in the protocol (Appendix).
This study was originally designed as a randomized trial of taselisib versus docetaxel in the second line setting post progression on platinum-based treatment. However, upon approval of immunotherapy in the second line setting [10–13], in December 2015, the S1400B trial was redesigned and became a single arm phase II study; the docetaxel arm permanently closed to accrual, and eligibility criteria were modified to allow second and later lines of therapy and only allow PS 0–1. Patients on the docetaxel arm were not included in the analyses presented in this paper.
Statistical Considerations
The primary objective was evaluation of the RECIST 1.1 response rate (RR; confirmed and unconfirmed, complete and partial) in patients in the PAP. The accrual goal was 40 response-evaluable PAP patients. The observation of 10/40 (25% RR) responses in the PAP was considered evidence to rule out the null RR and to pursue a randomized phase III trial. If at least three responses were observed on interim analysis of 20 evaluable patients, the trial would continue accrual to 40 patients. Other objectives included assessment of response in the full evaluable population (FEP), PFS and OS in the PAP and FEP, duration of response (DoR) among all responders, and evaluation of the frequency and severity of toxicities in the FEP. A key secondary objective was investigator assessment of median PFS (mPFS) in the PAP. An RR rate <25% with mPFS ≥4.5 months, would have been considered sufficient evidence to continue to a followon Phase III.
Results
Between June 16, 2014 and December 12, 2016, 55 patients (5% of those screened on S1400 while S1400B was actively accruing) were assigned to S1400B; 39 were enrolled, and of these, 31 were registered to receive taselisib.
Five of those registered were deemed ineligible (2 with inadequate baseline disease assessment; 1 with chemotherapy within 21 days and 1 with last radiotherapy within 14 days of registration; and 1 death prior to treatment). Twenty-one of the 26 (81%) FEP had at least one of the PAP alterations. Baseline patient characteristics are enumerated in Table 2 and mutations for FEP and PAP are listed in Tables 1 and 3.
Table 2.
Patient Demographics
| Primary Analysis Population | ||
|---|---|---|
| (n=26) | (n=21) | |
| Age Median (Range) | 68.1 (52.9–82.9) | 70.5 (52.9–82.9) |
| Male Gender | 19(73%) | 16(76%) |
| Performance Status | ||
| 0 | 7(27%) | 6(29%) |
| 1 | 18(69%) | 14(67%) |
| 2 | 1(4%) | 1(5%) |
| Race/Ethnicity | ||
| White | 19(73%) | 15(71%) |
| Black | 4(15%) | 4(19%) |
| Asian | 1(4%) | 1(5%) |
| Native American | 1(4%) | 0(0%) |
| Unknown race | 1(4%) | 1(5%) |
| Hispanic ethnicity | 1(4%) | 1(5%) |
| Number of Prior Lines of Therapy For Stage IV Disease | ||
| 0 | 3(12%) | 1(5%) |
| 1 | 14(54%) | 12(57%) |
| 2 or more | 9(35%) | 8(38%) |
| Smoking Status | ||
| Current Smoker | 8(31%) | 5(24%) |
| Former Smoker | 17(65%) | 15(71%) |
| Never Smoker | 1(4%) | 1(5%) |
| In primary analysis population | 21(81%) | 21(100%) |
Table 3.
Gene Alterations Detected on FoundationOne® Screening in Eligible Taselisib Patients
| Taselisib (n=26) | |
|---|---|
| PI3K Study Gene Alterations | |
| E545K* | 11(42%) |
| E542K* | 6(23%) |
| H1047R* | 4(15%) |
| N345K | 2(8%) |
| E453K | 1(4%) |
| G1049R | 1(4%) |
| M1043I | 1(4%) |
| *Included in PAP | |
| Number of PI3K Gene Alterations | |
| 1 | 26(100%) |
| Tumor Mutation Burden | |
| Median | 9.67 |
| Range | 2.42–41.11 |
| Interquartile range | 6.05–16.93 |
| <10 | 15(58%) |
| >=10 | 11(42%) |
| Other Concomitant Gene Alterations | |
| Short Variants | |
| TP53 | 23(88%) |
| MLL2 | 8(31%) |
| NOTCH1 | 5(19%) |
| CDKN2A, NF1 | 4(15%) |
| BRAF, LRP1B, NFE2L2 | 3(12%) |
| FBXW7, PMS2, RB1, STK11 | 2(8%) |
| APC, ARID1A, ASXL1, ATR, ATRX, BRCA1, BRCA2, BRIP1, CDK12, CREBBP, EGFR, EP300, ERBB2, FANCC, FANCD2, FGFR3, GRIN2A, KDM6A, MUTYH, NOTCH3, PBRM1, PIK3C2G, PIK3CG, RUNX1T1, SETD2, SMARCA4, SPEN, STAG2, TGFBR2 | 1(4%) |
| Copy Number Alterations | |
| CDKN2A | 6(23%) |
| CDKN2B | 5(19%) |
| CCND1, FGF12, FGF19, FGF3, FGF4, SOX2 | 4(15%) |
| MYC, RICTOR | 3(12%) |
| AKT2, FGF10, FGFR1, MDM2, PIK3CA, ZNF703 | 2(8%) |
| AXL, BRCA2, CCNE1, CDK4, EGFR, EPHB1, ERBB2, FGFR4, FLT4, KDM5A, KDM6A, KRAS, NFKBIA, NKX2-1, RET, TOP1 | 1(4%) |
| Rearrangements | |
| MAP3K13, PBRM1 | 1(4%) |
The most common concomitant gene alterations included mutations in TP53, MLL2, and NOTCH1, and copy number alterations in CDKN2A and CDKN2B (see Table 3). The median and range TMB scores were 9.62 (2.42–41.11), with 11 (42%) patients with TMB scores ≥10.
Two on-study deaths possibly related to treatment occurred, one due to respiratory failure and one due to cardiac arrest. In addition, one patient experienced multiple grade 4 AEs (dyspnea, thrombocytopenia, and pneumonitis). Eleven additional patients experienced Grade 3 AEs including five patients each with hyperglycemia or diarrhea, and three with lymphopenia. Patients received a median of 3.5 cycles (range = 2–13, interquartile range (IQR)=2–4) of taselisib. Five patients were removed from treatment due to toxicity, 18 due to progression/relapse, 2 due to death, and 1 patient for other reasons. No patients remain on treatment. See Table 3 for a full listing of adverse events.
One patient in the PAP with an E545K gene alteration responded (5% RR, 95% Confidence Interval [CI] 0%−24%). This patient was removed from treatment due to toxicity and subsequently exhibited disease progression (DoR = 4.4 months). There were no additional responses in the FEP resulting in a study-wide RR of 4% (95% CI, 0%−20%), but 16 patients had stable disease for a disease control rate of 65% (95% CI: 47–84%). Figure 1 depicts the waterfall plot for individual responses by mutational status with no obvious pattern in terms of magnitude of change in tumor measurements or PI3K alteration type. In the PAP, mPFS was 2.9 months (95% CI, 1.8–4.0 mos) and median OS was 5.9 months (95% CI, 4.2–7.8 mos). These figures were virtually identical in the FEP (see Figure 2). The 1- and 2-year OS estimates were 23.8% and 17.9% in the PAP; and 30.8% and 22.4% in the FEP. The analysis evaluating the association between patient characteristics and PFS and OS in the FEP yielded limited results. Current versus former or never smokers were associated with worse prognosis (OS HR = 2.85, p=0.03) and number of lines of previous therapy for stage IV (0 versus 1 or more) was associated with shorter time to progression (HR PFS = 7.88, p = 0.006); however, this observation was based on only 3 patients with no prior lines of therapy for stage IV NSCLC, and these results should be interpreted with caution. Age, gender, performance status, type of PI3K alteration, and tumor mutation burden were not associated with PFS or OS.
Figure 1: Waterfall Plot with Annotations for Individual Alterations.
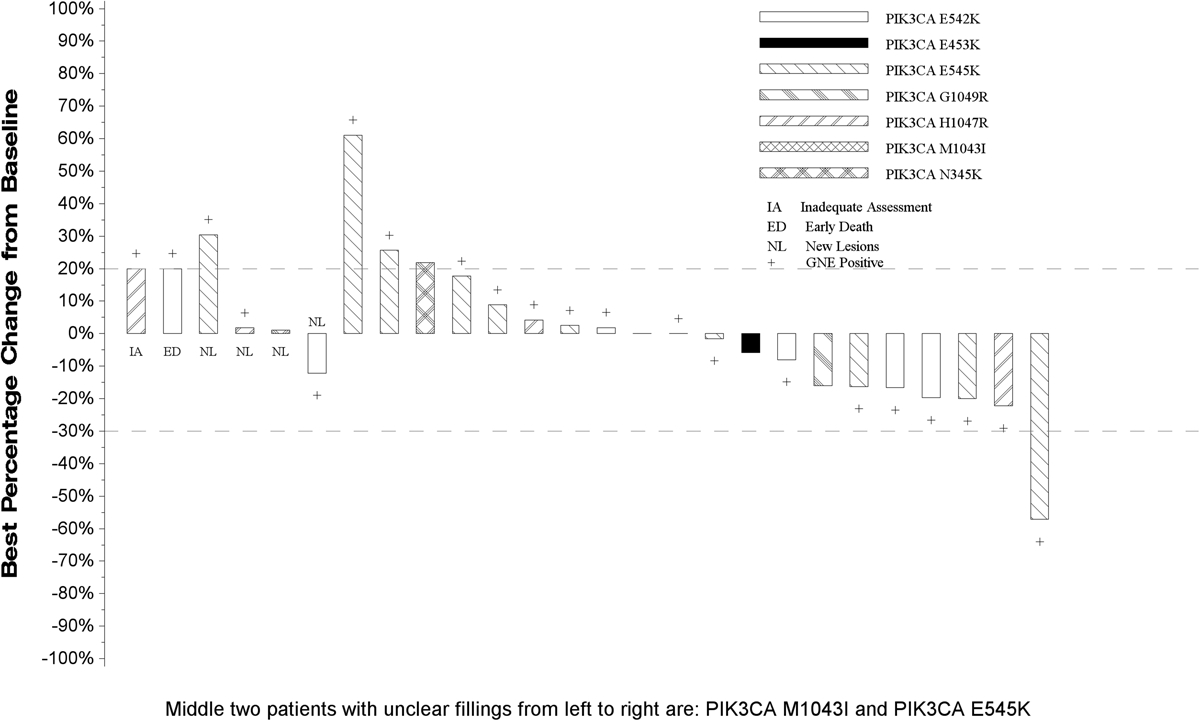
This plot depicts the response magnitude/status for all patients in the full eligible population. Patients who did not have a follow-up tumor disease assessment are presented at the very left of the plot marked with ‘inadequate response assessment’. In addition, patients who expired due to causes other than disease progression prior to their first disease assessment were coded as an ‘early death’ and are also presented at the left of the plot. Patients who had new lesions appear at their first follow-up assessment or who expired due to disease progression prior to the first scheduled the disease assessment are represented graphically as a 100% increase in tumor burden. For the remaining patients with follow-up disease assessments, the vertical bars represent the best percent decrease in tumor burden when compared to baseline as defined by RECIST 1.1. Negative numbers represent decrease in tumor burden from baseline while positive numbers represent increase in tumor burden from baseline. ‘+’ indicates a patient was in the primary analysis population (PAP).
Figure 2. Kaplan-Meier Plots of PFS and OS in the Full Eligible Population (FEP).
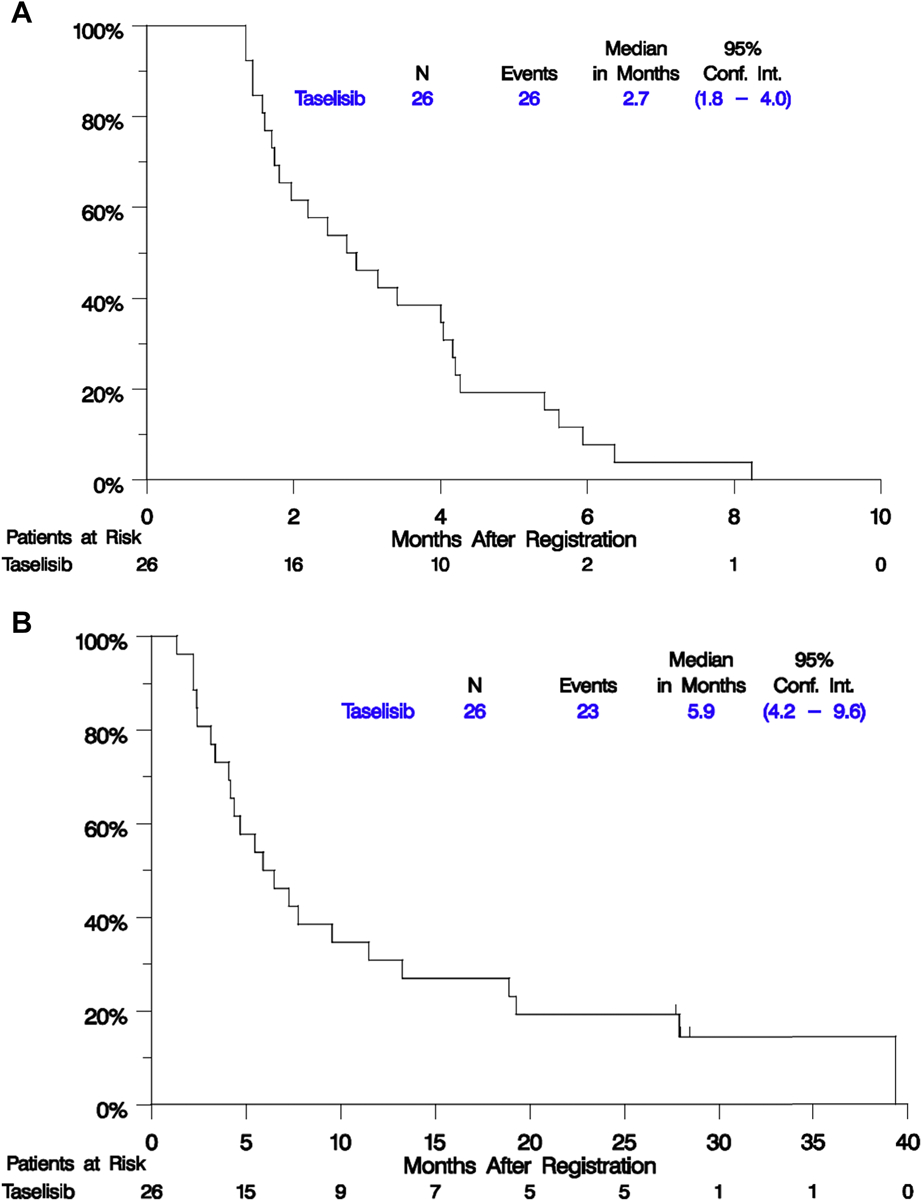
Survival distributions were estimated using the Kaplan-Meier method and the Brookmeyer-Crowley method was used to estimate confidence intervals.
Discussion
The paradigm for second-line therapy for SqNSCLC has changed significantly since 2015 with the approval of checkpoint inhibitors of programmed death receptor pathway based on Phase III studies showing superior OS compared with the erstwhile standard, docetaxel. [10–13] Recently, molecular genotyping has become the “norm” in the evaluation of patients with advanced non-squamous NSCLC and has led to major interest in applying targeted agents for mutations and other genetic aberrations prevalent in sqNSCLC. Genetic alterations within lung adenocarcinomas and SqNSCLC are generally distinct. SqNSCLC tends to be genetically more complex and is usually characterized by a high overall mutational burden. Due to the genetic diversity and lack of clear oncogenic drivers in this disease, we recognized the need to develop clinical trials solely focused on SqNSCLC that could evaluate single agent as well as combination targeted therapies along with newer immunotherapeutic approaches.
Lung-MAP sub-study S1400B was one such effort. Unfortunately, this study failed to meet its primary endpoint and was closed after an interim analysis for futility. The lone response observed on taselisib was brief; both the median PFS of 2.9 months and the median OS of 5.9 months in the targeted population proved disappointing. Although single agent taselisib resulted in a fair amount of hyperglycemia and fatigue, toxicities were manageable. The trial, though unsuccessful, proved unique in cataloguing the diversity of mutations in the PI3K pathway in SqNSCLC, some of which may prove “targetable” in the future if a more active agent emerges. It is unclear why this agent failed. It is conceivable that PI3KA is not a true driver of tumor growth in squamous NSCLC, or that bypass pathways circumvented the potential benefit of taselisib. Another PI3K inhibitor, buparlisib was negative in a broader NSCLC population with a wider range of PI3K activating mutations. [14] In contrast, taselisib in combination with fulvestrant yielded a modest PFS benefit of two months compared with fulvestrant alone in patients with estrogen receptor–positive PIK3CA mutant locally advanced or metastatic breast cancer. [15] Based on the experience in breast, it is conceivable that taselisib may work better in combination with other agents in advanced NSCLC, but this is speculative; there are no preclinical data to suggest this might be the case.
Also, the heterogeneity of molecular aberrations in advanced sqNSCLC suggests that targeting a single pathway may be insufficient. In this regard, it should be noted that additional genetic alterations detailed in Table 3 were present in the majority of patients enrolled on this sub-study. It is also posited that PI3K alterations may simply not be as powerful oncogenic drivers as we have observed with EGFR mutations and ALK rearrangements in advanced non-sqNSCLC.
Though S1400B failed to identify a promising agent targeting PI3K, the study design of Lung-MAP has proven quite promising. Under a single umbrella protocol, in a single disease venue, with a single IRB approval, we are now able to separately investigate multiple different pathways of interest, quickly discarding agents that prove inactive and focusing resources on new agents that may prove efficacious. This model of protocol design, under the aegis of the cooperative group system in the US, may be the most efficient means of investigating less common, as well as newly identified, oncogenic drivers.
Figure 3. Forest Plot Comparison of Patient Characteristics and PFS and OS in the Full Eligible Population (FEP).
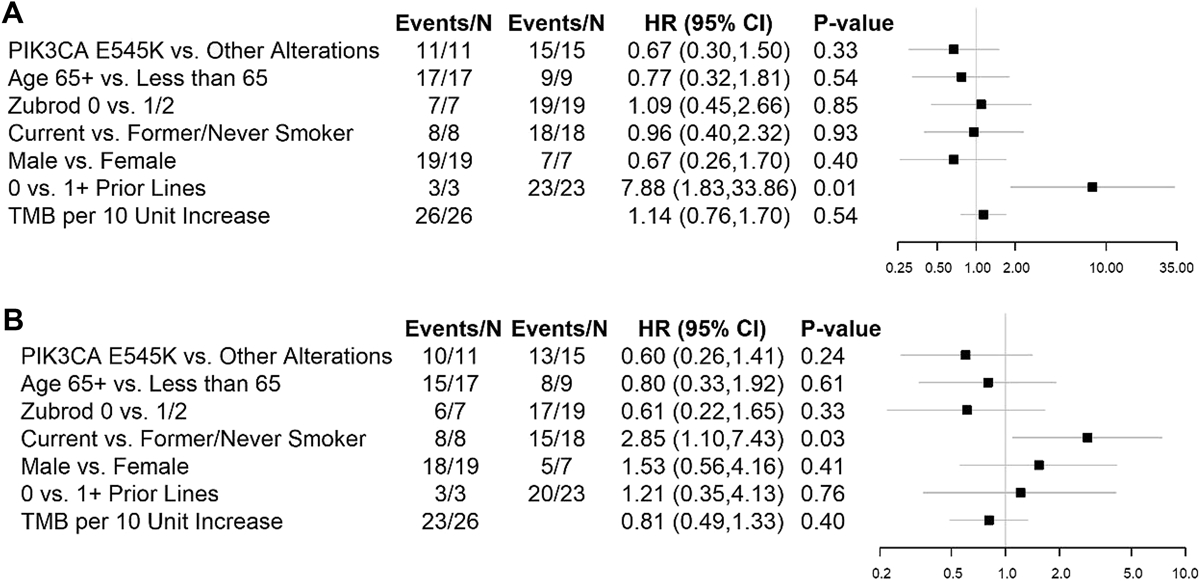
The Brookmeyer-Crowley method was used to estimate confidence intervals.
Table 4.
Adverse Events Attributable to Treatment
| Grade of AE | |||
|---|---|---|---|
| N = 26 | |||
| 3 | 4 | 5 | |
| Cardiac arrest | 1(4%) | ||
| Dehydration | 1(4%) | ||
| Diarrhea | 5(19%) | ||
| Dyspnea | 2(8%) | 1(4%) | |
| Fatigue | 3(12%) | ||
| Hyperglycemia | 5(19%) | ||
| Hypertension | 1(4%) | ||
| Hyponatremia | 1(4%) | ||
| Hypoxia | 1(4%) | ||
| Lung infection | 1(4%) | ||
| Lymphocyte count decreased | 3(12%) | ||
| Nausea | 1(4%) | ||
| Platelet count decreased | 1(4%) | ||
| Pneumonitis | 1(4%) | 1(4%) | |
| Pneumothorax | 1(4%) | ||
| Rash maculo-papular | 1(4%) | ||
| Respiratory failure | 1(4%) | ||
| Vomiting | 1(4%) | ||
| Maximum Grade of any AE | 11(42%) | 1(4%) | 2(8%) |
Funding:
this research supported in part by NIH/NCI grants CA180888, CA180819, CA180820, CA180821, CA180868, CA189954, CA189971, CA189809, CA180828, CA189872, CA189972, CA189953, CA189858, CA180801; and by Amgen, AstraZeneca, Bristol-Myers Squibb Company, Genentech and Pfizer through the Foundation for the National Institutes of Health, in partnership with Friends of Cancer Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: CJL reports other from Genentech/Roche, AstraZeneca, Bristol Myers Squibb, grants and other from Merck, Takeda, Advantagene, Inovio, and Eli Lilly, outside the submitted work; JDB reports personal fees and non-financial support from AstraZeneca, Inc, grants, personal fees and other from Mevion Medical Systems, Inc, personal fees and other from ViewRay, Inc, outside the submitted work; JC reports grants from AstraZeneca, Bayer, Helsinn, Beyond Spring, Celgene, G1 Therapeutics, Janssen, Merrimack, Mulan, Roche, outside the submitted work; RSH reports personal fees from Abbvie Pharmaceuticals, Biodesix, Bristol-Myers Squibb, EMD Serrano, Genentech/Roche, Heat Biologics, Jun Shi Pharmaceuticals, Loxo Oncology, Nektar, NextCure, Novartis, Pfizer, Sanofi, Seattle Genetics, Shire PLC, Spectrum Pharmaceuticals, Symphogen, TESARO, Neon Therapeutics, Infinity Pharmaceuticals, grants and personal fees from Merck and Company, AstraZeneca, Eli Lilly and Company, outside the submitted work; KK reports grants and personal fees from Genentech, outside of the submitted work; DRG reports grants and other from Roche Genentech during the conduct of the study; VAP reports advisory Boards for Nektar Therapeutics, Astra Zeneca Pharmaceuticals, Arrys Therapeutics, Merck&Co, LOXO Oncology, Araxes Pharma, F.Hoffman-LaRoche Ltd, Janssen Research Foundation, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly &Co, Novartis Pharmaceuticals Corp. Takeda Pharmaceuticals, Abbvie, TRM Oncology, Exelixis, Abbvie, Tsars Research/Grants from: Eli Lilly &Co, Novartis, Merck, Astra Zeneca Pharmaceuticals, F Hoffman-La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte.
No Conflicts of Interest: MWR, JLW, CA, PJS, JM, KM, MHK
References
- 1.Herbst RS, et al. , Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res, 2015. 21(7): p. 1514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantley LC, The phosphoinositide 3-kinase pathway. Science, 2002. 296(5573): p. 1655–7. [DOI] [PubMed] [Google Scholar]
- 3.Guertin DA and Sabatini DM, Defining the role of mTOR in cancer. Cancer Cell, 2007. 12(1): p.9–22. [DOI] [PubMed] [Google Scholar]
- 4.Karakas B, Bachman KE, and Park BH, Mutation of the PIK3CA oncogene in human cancers. Br J Cancer, 2006. 94(4): p. 455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massion PP, et al. , Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med, 2004. 170(10): p. 1088–94. [DOI] [PubMed] [Google Scholar]
- 6.Engelman JA, et al. , Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med, 2008. 14(12): p. 1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoerke JM, et al. , Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res, 2012. 18(24): p. 6771–83. [DOI] [PubMed] [Google Scholar]
- 8.Jia S, et al. , Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature, 2008. 454(7205): p. 776–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward S, et al. , Therapeutic potential of phosphoinositide 3-kinase inhibitors. Chem Biol, 2003. 10(3): p. 207–13. [DOI] [PubMed] [Google Scholar]
- 10.Herbst RS, et al. , Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet, 2016. 387(10027): p. 1540–50. [DOI] [PubMed] [Google Scholar]
- 11.Borghaei H, et al. , Nivolumab versus Docetaxel in advanced nonsquamous non-amall-cell lung Cancer. N Engl J Med, 2015. 373(17): p. 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J, et al. , Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung Cancer. N Engl J Med, 2015. 373(2): p. 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rittmeyer A, et al. , Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet, 2017. 389(10066): p. 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adjei AA, et al. , Safety and efficacy of buparlisib (BKM120) and chemotherapy in advanced, squamous non-small cell lung cancer (sqNSCLC): Results from the phase Ib/II BASALT-2 and BASALT-3 studies. J Clin Oncol, 2016. 34(15_suppl): p. e20522–e20522. [Google Scholar]
- 15.Baselga J, et al. , Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J Clin Oncol, 2018. 36(18 suppl): p. LBA1006–LBA1006. [Google Scholar]


