Abstract
Background
Nosocomial, late onset, sepsis occurs in up to 50% of infants of less than 1000gm at birth. The most frequent organism isolated is coagulase negative staphylococcus (CoNS). A number of studies have evaluated the efficacy of prophylactic low dose vancomycin given either as a continuous infusion added to the infant's hyperalimentation fluid or by intermittent intravenous administration. These studies in very low birth weight infants are the subject of this review.
Objectives
To evaluate the safety and efficacy of vancomycin prophylaxis for the prevention of late‐onset sepsis, coagulase negative staphylococcal sepsis, mortality, and effects on length of stay, total vancomycin exposure, evidence of vancomycin toxicity, and the development of vancomycin resistant organisms in the preterm neonate.
Search methods
Searches were made of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2003), MEDLINE, (MeSH terms: Vancomycin and Sepsis; limits: age groups, newborn infants), HealthStar and EMBase, electronic abstracts, personal files and conference proceedings up to October 2003.
Selection criteria
Randomized controlled trials which compared the incidence of sepsis and mortality in preterm neonates receiving vancomycin prophylaxis versus a control group receiving no prophylaxis.
Data collection and analysis
Data regarding clinical outcomes including the overall incidence of sepsis, the incidence of coagulase negative staphylococcal sepsis, mortality, length of stay, total vancomycin exposure, evidence of vancomycin toxicity, and the development of vancomycin resistant organisms were excerpted from previous clinical trials. Data analysis was done in accordance with the standards of the Cochrane Neonatal Review Group.
Main results
The administration of prophylactic vancomycin reduced the incidence of both total neonatal nosocomial sepsis and coagulase negative staphylococcal sepsis in eligible preterm infants. Mortality, length of stay, and evidence of vancomycin toxicity were not significantly different between the two groups. There was insufficient evidence to ascertain the risks of development of vancomycin resistant organisms in the nurseries involved in these trials.
Authors' conclusions
The use of prophylactic vancomycin in low doses reduces the incidence of nosocomial sepsis in the neonate. The methodologies of these studies may have contributed to the low rate of sepsis in the treated groups, as the blood cultures drawn from central lines may have failed to grow due to the low levels of vancomycin in the infusate. Although there is a theoretical concern regarding the development of resistant organisms with the administration of prophylactic antibiotic, there is insufficient evidence to ascertain the risks of development of vancomycin resistant organisms. Few clinically important benefits have been demonstrated for very low birth weight infants treated with prophylactic vancomycin. It therefore appears that routine prophylaxis with vancomycin should not be undertaken at present.
Plain language summary
Vancomycin for prophylaxis against sepsis in preterm neonates
Premature babies have immature immune systems and frequently pick up harmful infections in the hospital. This means they are at high risk of sepsis (life‐threatening bacterial infection). The most common bacteria causing sepsis in neonatal intensive care are coagulase negative staphylococci (CoNS). One way of trying to prevent CoNS infection is by infusing low doses of the antibiotic vancomycin (giving the drug by intermittent infusion or continuous drip). The review of trials found that low dose continuous infusions, or low dose intermittent administration, of vancomycin reduce the risk of a baby getting sepsis in the neonatal intensive care unit. There is not enough evidence to show if this approach increases antibiotic resistance in nurseries.
Background
Nosocomial infections are a frequent and significant cause of morbidity in the preterm infant. Infections diagnosed after the first 72 hours of life are arbitrarily deemed to be "nosocomial", and are frequently associated with clinical deterioration including increased apnea, temperature instability, abdominal distension, lethargy, septic shock, necrotising enterocolitis, meningitis and death. Preterm infants are at higher risk of infection than term infants because of immature immune responses, which include reduced phagocytosis, opsonisation and intracellular killing (Kallman 1998; Drossou 1997), decreased immunoglobulin concentrations (Ballow 1986), and poor skin (Rutter 1996) and gut barrier function (Insoft 1996). The risk of sepsis is further increased by the use of central venous catheters to administer parenteral nutrition (Johnson‐Robbins 1996) and lipids (Nataro 1994).
Coagulase negative staphylococci (CoNS) have emerged as the most common cause of bacteremia in the neonatal intensive care unit (Patrick 1990). There are difficulties in the diagnosis of CoNS septicemia as the organism is a frequent skin commensal, which often results in contamination of blood cultures, either at the time of blood draw or during the blood handling process. Common diagnostic definitions (such as that of the National Nosocomial Infections Surveillance System (Gaynes 1995)) require the presence of clinical signs of sepsis and a positive blood culture to confirm the diagnosis. However, clinical signs are often subtle and blood cultures are rarely drawn in the absence of clinical signs. These definitions do not adequately differentiate between true CoNS sepsis, catheters colonized with CoNS, and contaminated blood cultures. These unresolved distinctions significantly impact upon this review.
The use of low dose vancomycin infusions to prevent CoNS contamination of intravenous nutrition solutions, at a concentration above the minimal inhibitory concentration (MIC) of the organism in the hyperalimentation solution, but well below therapeutic dosing levels, is one suggested way of preventing CoNS sepsis during intravenous hyperalimentation. To date there have been five randomized controlled trials evaluating vancomycin for prophylaxis against sepsis in the preterm neonate. Nosocomial sepsis has been associated with morbidity, mortality, increased length of stay, and increased hospital costs (Stoll 1996). Therefore, a reduction in the incidence of sepsis carries significant potential benefit.
The relevant and important outcomes that will be reviewed include the incidence of overall sepsis, as well as CoNS sepsis, as defined by standard clinical criteria plus positive blood cultures, CoNS associated morbidity (such as necrotising enterocolitis), mortality (although death is rarely attributed to CoNS sepsis), length of hospital stay (as this appears to be increased by CoNS sepsis), total exposure to vancomycin (as this may be reduced by prophylaxis if fewer therapeutic courses are required), vancomycin toxicity, particularly ototoxicity (as nephrotoxicity appears to be rare (Bhatt‐Mehta 1999), and the development of resistant organisms.
The groups who are most at risk are the very low birth weight infant (less than 1500 grams) and the extremely low birth weight infant (less than 1000 grams); these groups will be analyzed separately where possible. A continuous infusion of vancomycin may have a different efficacy to intermittent dosing, would likely require different total doses, and will create very different serum concentration profiles; therefore, these two modes of prophylaxis will be analyzed independently.
Objectives
To evaluate the effects of vancomycin prophylaxis on the incidence of late‐onset sepsis, coagulase negative staphylococcal (CoNS) sepsis and mortality, as well as the effects on length of stay, total vancomycin exposure, evidence of vancomycin toxicity, and the development of vancomycin resistant organisms in the preterm neonate. Planned subgroup analyses will include stratification by birthweight, where possible, and the use of continuous versus intermittent vancomycin dosing.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials comparing the incidence of sepsis in preterm neonates receiving prophylactic vancomycin versus a control group receiving no prophylaxis were evaluated.
Types of participants
Preterm infants, with additional risk factors for infection such as a birth weight less than 1500 grams, use of central venous catheters, and administration of intravenous hyperalimentation.
Types of interventions
Prophylactic vancomycin as intermittent intravenous doses or by continuous infusion.
Types of outcome measures
Data for the following clinical outcomes were sought: 1. Sepsis, all pathogens (number of patients with one or more episodes). 2. Sepsis, coagulase negative staphylococci (number of patients with one or more episodes). 3. Mortality before hospital discharge. 4. Length of stay in the NICU (days). 5. Total vancomycin exposure (milligrams per patient during the NICU stay). 6. Complications, hearing impairment (results of auditory brainstem evoked response prior to hospital discharge). 7. Post‐treatment culture to determine the presence of vancomycin resistant organisms (number of infants with any post‐treatment culture positive for vancomycin resistant organisms).
Search methods for identification of studies
A search was conducted using the standard methods of the Cochrane Neonatal Review Group. In addition, searches were made of MEDLINE using the PubMed search engine, from the years 1966 ‐ 1999, and the search terms "vancomycin and sepsis or infection or septicemia" and "infant, newborn". This strategy, performed in May 1999, retrieved 163 articles; further filtering using "randomized" or "controlled" reduced this to 14 and 9 reports, respectively. The HealthStar database was also searched using 'Internet Grateful Med' and the search terms vancomycin and premature or preterm. This retrieved 28 articles and no new randomized controlled trials. Also in May 1999 the EMBase paediatric and 'drugs and pharmacology' databases were searched from 'Healthgate' using the terms vancomycin and preterm or premature; 19 and 22 references were retrieved, respectively, and no further randomized controlled trials. In addition, we searched electronic abstracts of the Academic Pediatric Societies annual meetings from 1996‐1999, and personal files.
Updated searches were made of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2003), MEDLINE, (MeSH terms: Vancomycin and Sepsis; limits: age groups, newborn infants), HealthStar and EMBase, electronic abstracts, personal files and conference proceedings up to October 2003. No additional studies were identified from these sources.
Data collection and analysis
Studies were assessed for inclusion in the review by each reviewer individually. All five prospective randomized trials that were identified were included in this review. Only the prospective randomized phase of the trial by Moller 1993 was included in the statistical analyses. The included studies were judged to be of acceptable quality for inclusion in the review by all evaluators.
Data regarding clinical outcomes including the overall incidence of sepsis, the incidence of coagulase negative staphylococcal (CoNS) sepsis, mortality, length of stay, exposure to vancomycin, evidence of vancomycin toxicity, and the development of vancomycin resistant organisms were excerpted from the studies. Data analysis was done in accordance with the standards of the Cochrane Neonatal Review Group.
Statistics: For categorical outcomes, typical estimates for relative risk and risk difference were calculated. For outcomes measured on a continuous scale, typical estimates for weighted mean difference were calculated. 95% confidence intervals were used. A fixed effect model was assumed.
Pre‐specified subgroup analyses were: 1. Continuous vancomycin infusion compared with intermittent dosing. 2. Extremely low birth weight infants (<1000 g).
Results
Description of studies
Studies included in this review are: Baier 1998 n=38, Cooke 1997 n=72, Kacica 1994 n=150, Moller 1993 n=41, and Spafford 1994 n=70. Details of each study are given in the "Characteristics of Included Studies" table and references.
Three of the five studies administered prophylactic vancomycin as a continuous infusion in the hyperalimentation. Cooke (1997) and Moller (1993) administered vancomycin at a dose of 5 mg/kg over one hour twice daily. Only Spafford (1994) appears to have exclusively enrolled infants < 1000 gm at birth, and this study included only infants with a central venous catheter; the other studies included infants with a birthweight less than 1500 grams, with or without central catheters. All studied infants were receiving parenteral nutrition.
Baier (1998) enrolled infants within two weeks of birth, with normal renal function, who were not receiving vancomycin treatment. Infants were randomized by birthweight stratified blocks of 250 g, to receive either vancomycin 25 microg/ml or placebo added to their hyperalimentation for the duration of such therapy. Surveillance for infection involved sepsis evaluations at the discretion of the attending physician, and required at least two positive blood cultures for the diagnosis of sepsis. Empiric therapy for sepsis 'often' included vancomycin. Upon removal, the catheter tips were rolled and cultured with colony counts. Bacteremia was defined as a positive culture from 2 sites.
Cooke (1997) enrolled infants of less than two weeks with normal renal function. The allocation was blinded but not the treatment which was 5 mg/kg of vancomycin over one hour twice daily in a peripheral vein. Treatment continued during hyperalimentation or until four weeks of age. They performed daily complete blood counts and C‐reactive proteins, and did not specify the use of paired cultures. Weekly surveillance cultures were performed from groin, rectum and throat. They defined two groups with positive cultures, those with and without C‐reactive protein elevation. Routine therapy for suspected sepsis was co‐amoxyclav and gentamicin.
Kacica (1994) enrolled infants within two weeks of birth, not on antibiotics with normal renal function. No stratification was indicated. Vancomycin was added to the hyperalimentation fluid at a dose of 25 microg/ml. Therapy was for the duration of hyperalimentation or a maximum of one month. Surveillance for infection involved sepsis evaluations at the discretion of the attending physician, and required at least one blood culture. At the time of culture, an additional aliquot of blood was placed in an aerobic culture bottle containing a resin to remove antibacterial activity. Empiric therapy for suspected sepsis included amikacin and ampicillin. Routine surveillance cultures of skin and rectum were performed at initiation and termination. Hearing screening was performed at hospital discharge. Data for the number of infants who had a culture positive for CoNS are not given, although the large majority of the Gram positive cultures(37/43) were CoNS.
Moller (1993) administered vancomycin for prophylaxis as the second phase of a three phase trial involving different infants in each phase in an attempt to reduce the incidence of nosocomial sepsis. The second phase included 41 infants less than 1500 g. Infants were enrolled after ruling out or treating "early onset sepsis" and vancomycin prophylaxis continued until intravenous access was no longer needed, the infant weighed greater than 1500 g, or the infant required antibiotic therapy for an intercurrent infection. Infants were randomly assigned to receive 5 mg/kg twice daily of vancomycin; no details of the randomization were provided, and nursing staff but not physicians were blinded. The diagnostic criteria for sepsis and the usual treatment of suspected sepsis were not specified.
Spafford (1994) did not specify postnatal age, but it would appear that all the enrolled infants were less than 1000g at birth, with most infants being enrolled on day three or four of life. Infants had normal renal function, and a negative culture at the time of enrollment. There was no stratification. The treatment dose of vancomycin was 25 microg/ml of hyperalimentation fluid .There were no routine surveillance cultures and catheters were cultured on removal. Paired cultures were performed for suspected sepsis, but there is no description for sepsis apart from catheter related sepsis, which was defined as a colony count that was ten times greater from the catheter specimen than the peripheral specimen. Ampicillin and gentamicin were used for empiric therapy. Hearing screening was performed at hospital discharge.
Study outcomes include the incidence of sepsis, as well as the incidence of CONS sepsis. Mortality was assessed for each group, although most deaths were attributed to noninfectious etiologies.
Risk of bias in included studies
Randomization: In four of the five studies, sealed envelopes with randomly allocated group assignments were utilized for determining treatment vs. control group. We assume therefore that the allocation was adequately masked for Baier (1998), Cooke (1997), Kacica (1994), and Spafford (1994). Moller (1993) gave no details of the randomization procedure.
Blinding to Treatment: Two of the five studies were blinded to treatment assignment (Baier (1998), Spafford (1994)). The study by Kacica (1994) was not blinded, nor was the study by Cooke (1997) which required intermittent administration of vancomycin. The study by Moller (1993) was apparently blinded to nursing and housestaff but not to attending physicians.
Exclusion After Randomization: No infants were excluded following randomization from the studies by Baier (1998), Moller (1993), or Spafford (1994). In the Cooke (1997) trial, 72 infants were enrolled; data were included on all infants although three infants from each group died within the first 16 days of life. Nine of the original 150 infants enrolled in the study by Kacica (1994) were excluded from analysis for a variety of reasons.
Blinding of the Outcome Assessment: It appears that only the two trials blinded to intervention (Baier (1998), Spafford (1994)) were blinded to outcome assessment as well, although this information was difficult to ascertain.
Effects of interventions
All five studies noted a decrease in the incidence of both overall sepsis events and coagulase negative staphylococcal (CoNS) sepsis events in neonates receiving prophylactic vancomycin.
Overall Sepsis Each of the five trials reported the effect of prophylactic vancomycin on the incidence of sepsis (all pathogens). In four of the five trials, there was a significant reduction in sepsis in the treatment group. The study by Cooke evaluated for number of positive blood cultures as opposed to number of infants with sepsis and is therefore not included in the meta‐analysis. The study did however demonstrate a statistically significant decrease in number of positive blood cultures in the treatment group (18 vs. 35). The meta‐analysis of the results demonstrates that overall sepsis incidence was reduced significantly, RR 0.11, 95% CI 0.05, 0.24.
CoNS Sepsis Each of the five trials reported the effect of prophylactic vancomycin on the incidence of CoNS sepsis. All five trials demonstrated a statistically significant reduction in the incidence of CoNS sepsis. Kacica, however, evaluated the number of positive blood cultures as opposed to the number of infants with a positive blood culture for CoNS. For this reason, the data have not been included in the meta‐analysis although results from that study demonstrated only one positive blood culture for CoNS in the treatment group as compared to 37 positive blood cultures for CoNS in the control group. The meta‐analysis shows that the incidence of CoNS sepsis was reduced, RR 0.33, 95% CI 0.19, 0.59.
The intermittent vancomycin regime appears to be less effective in reducing the number of infants with an episode of CoNS sepsis than the continuous infusion, RR 0.49, 95% CI 0.27, 0.90 for intermittent dosing and RR 0.07, 95% CI 0.01, 0.52 for continuous infusion.
Mortality Prior to Hospital Discharge Four of the five studies reported on mortality prior to hospital discharge. Individually, none of these studies demonstrated that mortality was affected by vancomycin prophylaxis. The meta‐analysis also reveals no evidence that mortality was affected by vancomycin prophylaxis, 13/168 experimental patients and 16/162 controls, RR 0.79, 95% CI 0.4, 1.58.
Length of NICU Stay There was no evidence that length of NICU stay was affected as recorded in two studies (Spafford 1994; Moller 1993). The length of stay was measured from hospital admission and was measured in all trial participants. Spafford (1994) found length of stay to be 5 days longer in the vancomycin treated infants, but the difference was not significant. Moller reported only mean values for length of stay, 86 days in the vancomycin group and 73 days in the control group; but without any reported measure of variance, the significance of this difference cannot be assessed.
Total Vancomycin Exposure The total vancomycin dose received was reported only by Moller (1993). The dose received was 98.00 +/‐ 81.9 mg/patient for the experimental population (n=21) and 114.18 +/‐ 60.9 mg/patient for the control population (n=20) ). These results were not statistically different. This finding may be due to more frequent administration of vancomycin as therapy for suspected or proven sepsis in the control population.
Hearing Impairment Kacica (1994) and Spafford (1994) performed audiology screening prior to discharge in all infants using auditory brainstem response systems. Kacica (1994) noted that one infant in each group had "severe hearing impairment" which remains undefined. Spafford (1994) noted that no treatment infants but three controls had "significant hearing impairment", which is also undefined. If it is assumed that these outcomes are comparable, there is no evidence of a significant effect on the development of hearing impairment, RR 0.33, 95% CI 0.05, 2.07.
Serum Creatinine Two studies reported serum creatinine during therapy. Spafford (1994) stated that creatinine concentrations did not differ between the two groups (80 micromol/L for the vancomycin group and 88 micromol/L for the control group, no standard deviations reported). It was not stated when the measurements were taken. Moller (1993) reported serum creatinine on day three of the study, which was 57 micromol/L in each group; no standard deviation was noted. Kacica (1994) estimated serum creatinine weekly during therapy and states there were no differences between groups although no data were reported. Baier (1998) states there were no changes in serum creatinine associated with the use of vancomycin, but no methodology or results were given.
Development of Vancomycin Resistant Organisms Kacica obtained skin and rectal specimens for surveillance cultures at the time of enrollment and at termination of the protocol. The isolates were screened for the presence of vancomycin resistant gram positive organisms. The vancomycin susceptibility of gram positive blood culture isolates did not vary during the study period: mean MIC 1.5 microg/mL, range 0.5 to 2.0 microg/mL. No vancomycin resistant isolates were detected in study patients, although the data are not presented. There is insufficient evidence to ascertain the risks of development of vancomycin resistant organisms at present.
Extremely Low Birth Weight Infants Despite a planned analysis restricted to infants < 1000 g, there are insufficient data available to determine either the number of infants less than 1000 grams in these studies or the benefits of vancomycin prophylaxis restricted to this particular patient population.
Discussion
Five randomized trials were identified which compared prophylactic administration of vancomycin to no prophylaxis in reducing the incidence of neonatal nosocomial sepsis. Entry criteria were similar between the studies with four of five studies evaluating infants less than 1500 grams birth weight. Methodology also was similar; three of five studies administered vancomycin at a dose of 25 mcg/mL as a continuous infusion in the hyperalimentation for infants in the first two weeks of life. The remaining two studies administered 5 mg/kg twice daily. There were some differences in the definition of sepsis used, which is a recurring problem in studies of neonatal sepsis.
As many as 20% of infants weighing less than 1500 g have at least one episode of sepsis with a coagulase negative staphylococcus (as defined by a positive blood culture in the presence of clinical signs of infection) during their hospitalization, with rates reaching 54% during the first 37 days of life in infants weighing less than 1000 g (Johnson‐Robbins 1996).
The majority of these infants are treated with vancomycin, as the sensitivity pattern usually shows resistance to other agents. Specifically, many hospital acquired CoNS are methicillin resistant. Thus many preterm infants experience substantial exposure to vancomycin. Newer alternatives such as teicoplanin (Moller 1997) are not currently widely used. The occurrence of CoNS sepsis in the neonate has also been associated with the need for possible removal of central venous catheters, and increased length of hospital stay (Stoll 1996).
Good outcome from very restricted use of vancomycin, where many infants with cultured CoNS sensitive only to vancomycin demonstrated clinical improvement without vancomycin therapy, has been reported (Matrai‐Kovalskis 1998). This suggests that some infants with positive blood cultures may not have infection requiring therapy.
This review provides reasonably strong evidence for a significant decrease in the incidence of neonatal nosocomial sepsis and CoNS sepsis, using current clinical definitions of sepsis, following vancomycin prophylaxis. However, before such an approach is adopted as routine practice, vancomycin prophylaxis must be evaluated for its impact on vancomycin resistance for CoNS and other organisms (Barefield 1994) and for potential toxicity, as well as morbidity from CoNS sepsis. Vancomycin has a fairly broad spectrum of activity against Gram positive organisms. Exposing large numbers of infants to prolonged infusions of the drug, with excretion in the urine and stool, presumably may select for vancomycin resistant organisms. The NICU may become colonized with resistant organisms, such as vancomycin resistant enterococci, which may be very difficult to treat, and are associated with greater morbidity than CoNS.
Based on the studies reviewed, administration of prophylactic vancomycin has been found to reduce the incidence of sepsis, particularly CoNS sepsis. Current neonatal practice is to treat CoNS sepsis with antibiotics of variable duration and to consider removal of any indwelling central catheters. There is a suggestion that CoNS sepsis may not require specific therapy in all situations and thus the overall clinical benefit of preventing such episodes may include the decreased usage of potentially toxic drugs. However, further studies to evaluate the overall benefit, safety and cost effectiveness of vancomycin prophylaxis, including studies which evaluate the clinical significance of CoNS bacteremia, are warranted.
Since the original review was completed, there have been no additional randomized controlled trials of vancomycin prophylaxis in neonates. However, the United States Center for Disease Control commented on the use of vancomycin prophylaxis in their Guidelines for the Prevention of Intravascular Catheter Related Infections, 2002. While we are all in agreement that the randomized controlled trials of vancomycin prophylaxis demonstrated a reduction in catheter related bloodstream infection, the prophylactic use of vancomycin is a risk factor for the development of vancomycin resistant organisms. The risk of acquiring a resistant organism likely outweighs any benefit of prophylactic vancomycin (CDC 2002).
Authors' conclusions
Implications for practice.
The use of prophylactic vancomycin in low doses reduces the incidence of nosocomial sepsis in the neonate, using currently accepted clinical definitions of sepsis. A continuous infusion of vancomycin appears to be more effective than intermittent dosing, although this may reflect methodology whereby blood cultures drawn from infants receiving continuous vancomycin contain enough of the drug to limit growth of vancomycin sensitive organisms. Although there is a concern regarding the theoretical development of resistant organisms with the administration of prophylactic antibiotic, there is insufficient evidence to ascertain the risks of development of resistant organisms.
Implications for research.
We believe that a prospective trial to further evaluate the role of prophylactic vancomycin or other agent may be helpful. Such a study should have adequate power combined with a rigorous study design. Attempts should be made to enroll infants less than 1000g, with indwelling catheters, which will increase the risk of nosocomial sepsis in the control population. Using a conservative estimate of nosocomial sepsis of 40% for this high risk population, with a power of 80%, and potential reductions in the incidence of sepsis in the treated group to 15%, 10% and 5%, the trial would require minimal sample sizes of 50, 35 and 22 infants per group. This trial should also evaluate safety with respect to potential oto‐ and nephrotoxicities.
There is a pressing need to determine the risk of the development of resistant organisms with low dose vancomycin prophylaxis. However, randomized trials to assess this outcome would require very much larger sample sizes and, indeed, may never achieve adequate power to evaluate this potential adverse effect; therefore, other research designs may be needed.
The true pathogenicity of CoNS in preterm infants with and without indwelling central catheters needs to be further evaluated in order to assess the true risk/benefit ratio of any intervention. There is a need to develop an acceptable clinical definition of CoNS sepsis.
Additional studies can address the use, safety, and efficacy of alternative antibiotics, such as teicoplanin (Moller 1997). Another approach that warrants further evaluation is the use of an antibiotic lock technique utilized at the time of tubing changes to the stopcock and catheter, an approach which avoids exposing the patient to any significant antibiotic concentrations (Carratala 1999). Perhaps most promising are studies in adults that have demonstrated that catheters impregnated with antibiotics and/or antibacterials may reduce nosocomial catheter related infections. These approaches may hold the greatest promise for reducing nosocomial infection in the preterm neonate and merit further study.
What's new
| Date | Event | Description |
|---|---|---|
| 23 October 2008 | Amended | Converted to new review format. |
Acknowledgements
None
Data and analyses
Comparison 1. Vancomycin versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of sepsis (all pathogens); number of patients with one or more episodes | 4 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.24] |
| 1.1 Continuous vancomycin infusion | 3 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.23] |
| 1.2 Intermittent vancomycin treatment | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.84] |
| 2 Incidence of CoNS sepsis; number of patients with one or more episodes | 4 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.19, 0.59] |
| 2.1 Continuous vancomycin infusion | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 2.2 Intermittent vancomycin treatment | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.90] |
| 3 Mortality prior to hospital discharge | 4 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.58] |
| 3.1 Continuous vancomycin infusion | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.63] |
| 3.2 Intermittent vancomycin treatment | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.20, 4.38] |
| 4 Length of NICU stay (days) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐24.65, 34.65] |
| 4.1 Continuous vancomycin infusion | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐24.65, 34.65] |
| 5 Hearing impairment (auditory brainstem evoked response) | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.07] |
1.1. Analysis.
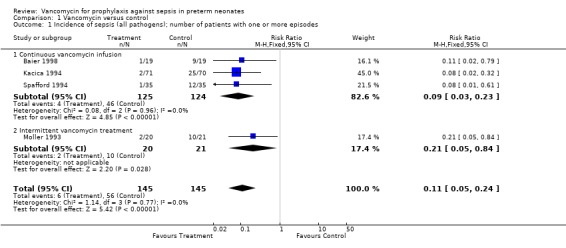
Comparison 1 Vancomycin versus control, Outcome 1 Incidence of sepsis (all pathogens); number of patients with one or more episodes.
1.2. Analysis.
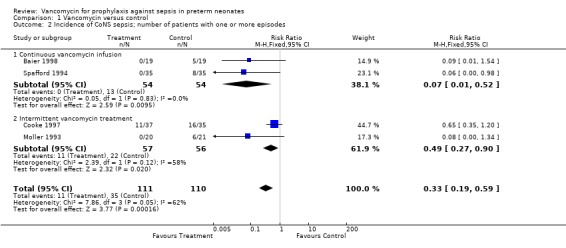
Comparison 1 Vancomycin versus control, Outcome 2 Incidence of CoNS sepsis; number of patients with one or more episodes.
1.3. Analysis.
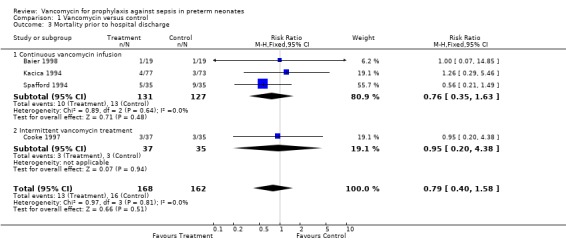
Comparison 1 Vancomycin versus control, Outcome 3 Mortality prior to hospital discharge.
1.4. Analysis.
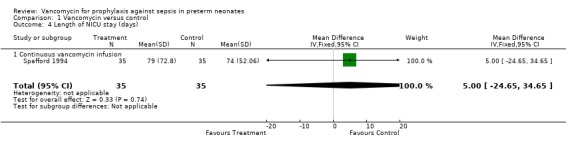
Comparison 1 Vancomycin versus control, Outcome 4 Length of NICU stay (days).
1.5. Analysis.
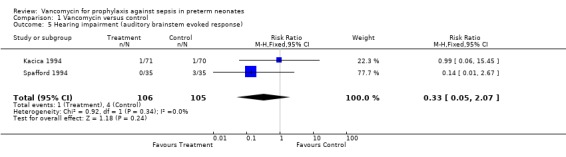
Comparison 1 Vancomycin versus control, Outcome 5 Hearing impairment (auditory brainstem evoked response).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baier 1998.
| Methods | Masking of allocation;yes. Masking of intervention; yes. Completeness of follow‐up; yes. Masking of outcome assessment; yes. | |
| Participants | 38 infants less than 1500g birth weight and less than 2 weeks of age requiring hyperalimentation. | |
| Interventions | Continuous infusion of hyperalimentation with either 25 microg/ml vancomycin or placebo | |
| Outcomes | Bacteremia, defined as growth of an organism from 2 sites. Secondary outcomes included numbers of sepsis evaluations, mortality and bacteremia during hyperalimentation. | |
| Notes | n= 38 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cooke 1997.
| Methods | Masking of allocation; yes. Masking of intervention; no. Completeness of follow‐up; yes. Masking of outcome assessment; no. | |
| Participants | 72 infants < 1500g, < 2 weeks, normal renal function receiving hyperalimentation | |
| Interventions | Twice daily vancomycin 5 mg/kg iv | |
| Outcomes | CoNS bacteremia with and without elevated CRP, total sepsis, mortality | |
| Notes | n=72 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kacica 1994.
| Methods | Masking of allocation; unclear. Masking of intervention; no. Completeness of follow‐up; 9 infants not evaluated. Masking of outcome assessment; no. | |
| Participants | 150 infants less than 1500g birth weight and less than 2 weeks of age requiring hyperalimentation. | |
| Interventions | Continuous infusion of hyperalimentation with either 25 microg/ml vancomycin or placebo | |
| Outcomes | CoNS bacteremia. Secondary outcomes were total sepsis, mortality, hearing impairment | |
| Notes | n=150 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Moller 1993.
| Methods | Single center randomized controlled trial. Masking of allocation unclear; masking of intervention to nursing and housestaff but not to attending physicians. Completeness of follow up: yes. Masking of outcome assessment: no. | |
| Participants | 41 preterm infants less than 1500 g | |
| Interventions | Vancomycin i.v. at 10 mg/kgday in two doses. | |
| Outcomes | CoNS sepsis | |
| Notes | A 3 phase "study", the middle phase of the study is a report of a randomized controlled trial n=41 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Spafford 1994.
| Methods | Masking of allocation; yes. Masking of intervention; yes. Completeness of follow‐up; yes. Masking of outcome assessment; yes. | |
| Participants | 70 infants of < 1000g birthweight with central venous catheters less than 2 weeks of age. | |
| Interventions | Continuous infusion of hyperalimentation with either 25 microg/ml vancomycin or placebo | |
| Outcomes | Colonization rate of central catheters Death, hospital stay, sepsis evaluations, brainstem auditory evoked responses | |
| Notes | No specification of birthweight or gestation n=70 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Marchand 1990 | Randomized study which investigated pharyngeal instillation rather than systemic prophylaxis with vancomycin, and did not report results of CoNS sepsis episodes. Reduced airway colonization rates with CoNS were demonstrated. |
| Ocete 1998 | Comparison of sepsis rates after introducing routine low dose vancomycin prophylaxis, with rates in historical controls. Not restricted to very low birth weight infants. |
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Baier 1998 {published data only}
- Baier RJ, Bocchini JA, Brown EG. Selective use of vancomycin to prevent coagulase negative staphylococcal nosocomial bacteremia in high risk very low birth weight infants. Pediatric Infectious Disease Journal 1998;17:179‐83. [DOI] [PubMed] [Google Scholar]
Cooke 1997 {published data only}
- Cooke RWI, Nycyk JA, Okuonghuae H, Shah V, Damjanovic V, Hart CA. Low dose vancomycin prophylaxis reduces coagulase negative staphylococcal bacteraemia in very low birthweight infants. Journal of Hospital Infection 1997;37:297‐303. [DOI] [PubMed] [Google Scholar]
Kacica 1994 {published data only}
- Kacica MA, Horgan MJ, Ochoa L, Sandler R, Lepow ML, Venezia RA. Prevention of gram positive sepsis in neonates weighing less than 1500 grams. Journal of Pediatrics 1994;125:253‐8. [DOI] [PubMed] [Google Scholar]
Moller 1993 {published data only}
- Moller JC, Rossa M, Nachtrodt G, Richter A, Tegtmeyer FK. Praventive antibiotikagabe zur verhinderung nosokomialer septikamien bei sehr kleinen fruhgeborenen (VLBW Infants).. Klinische Padiatrie 1993;205:140‐4. [DOI] [PubMed] [Google Scholar]
Spafford 1994 {published data only}
- Spafford PS, Sinkin RA, Cox C, Reubens L, Powell KR. Prevention of central venous catheter related coagulase negative staphylococcal sepsis in neonates. Journal of Pediatrics 1994;125:259‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Marchand 1990 {published data only}
- Marchand S, Poisson D, Borderon JC, Gold F, Chantepie A, Saliba E, Laugier J. Randomized study of vancomycin pharyngeal instillation as a prophylaxis of bronchopulmonary infection in intubated neonates. Biology of the Neonate 1990;58:521‐6. [DOI] [PubMed] [Google Scholar]
Ocete 1998 {published data only}
- Ocete E, Ruiz‐Extremera A, Goicoechea A, Lozano E, Robles C, Rey ML, et al. Low‐dosage prophylactic vancomycin in central‐venous catheters for neonates. Early Human Development 1998;53:S181‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Ballow 1986
- Ballow M, Cates KL, et al. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatric Research 1986;20:899‐904. [DOI] [PubMed] [Google Scholar]
Barefield 1994
- Barefield ES, Philips JB. Vancomycin prophylaxis for coagulase‐negative staphylococcal bacteremia. Journal of Pediatrics 1994;125:230‐2. [DOI] [PubMed] [Google Scholar]
Bhatt‐Mehta 1999
- Bhatt‐Mehta V, Schumacher RE, Faix RG, Leady M, Brenner T. Lack of vancomycin‐associated nephrotoxicity in newborn infants: A case‐ control study. Pediatrics 1999;103:e48. [DOI] [PubMed] [Google Scholar]
Carratala 1999
- Carratala J, Niubo J, Fernandez‐Sevilla A, Juve E, et al. Randomized, double‐blind trial of an antibiotic‐lock technique for prevention of gram‐positive central venous catheter‐related infection in neutropenic patients with cancer. Antimicrob Agents Chemother 1999;43:2200‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2002
- Center for Disease Control. Guidelines for the prevention of intravascular catheter related infections 2002. MMWR Recommendations and Reports 2002;51(RR‐10):10. [PubMed] [Google Scholar]
Drossou 1997
- Drossou V, Kanakoudi F, Tzimouli V, Sarafidis K, Taparkou A, Bougiouklis D, et al. Impact of prematurity, stress and sepsis on the neutrophil respiratory burst activity of neonates. Biology of the Neonate 1997;72:201‐9. [DOI] [PubMed] [Google Scholar]
Gaynes 1995
- Gaynes RP, Horan TC. Definitions of nosocomial infections. In: Mayhall CG editor(s). Hospital Epidemiology and Infection Control. Baltimore, MD: Williams and Wilkins, 1995:Chap 77, Appendix A1. [Google Scholar]
Insoft 1996
- Insoft RM, Sanderson IR, Walker WA. Development of immune function in the intestine and its role in neonatal diseases. Pediatric Clinics of North America 1996;43:551‐71. [DOI] [PubMed] [Google Scholar]
Johnson‐Robbins 1996
- Johnson‐Robbins LA, El‐Mohandes AE, Simmens SJ. Staphylococcus epidermidis sepsis in the intensive care nursery: a characterization of risk associations in infants <1,000 g. Biology of the Neonate 1996;69:249‐56. [DOI] [PubMed] [Google Scholar]
Kallman 1998
- Kallman J, Schollin J, Schalen C, Erlandsson A, Kihlstrom E. Impaired phagocytosis and opsonisation towards group B streptococci in preterm neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;78:F46‐F50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matrai‐Kovalskis '98
- Matrai‐Kovalskis Y, Greenberg D, Shinwell ES, Fraser D, Dagan R. Positive blood cultures for coagulase‐negative staphylococci in neonates: does highly selective vancomycin usage affect outcome?. Infection 1998;26:85‐92. [DOI] [PubMed] [Google Scholar]
Moller 1997
- Moller JC, Nelskamp I, Jensen R, Reiss I, Kohl M, Gatermann S, et al. Comparison of vancomycin and teicoplanin for prophylaxis of sepsis with coagulase negative staphylococci (CONS) in very low birth weight infants. Journal of Perinatal Medicine 1997;25:361‐7. [DOI] [PubMed] [Google Scholar]
Nataro 1994
- Nataro JP, Corcoran L, Zirin S, et al. Prospective analysis of coagulase‐negative staphylococcal infection in hospitalized infants. Journal of Pediatrics 1994;125:798‐804. [DOI] [PubMed] [Google Scholar]
Patrick 1990
- Patrick CC. Coagulase‐negative staphylococci: Pathogens with increasing clinical significance. Journal of Pediatrics 1990;116:497‐507. [DOI] [PubMed] [Google Scholar]
Rutter 1996
- Rutter N. The immature skin. European Journal of Pediatrics 1996;155(2 Suppl):S18‐20. [DOI] [PubMed] [Google Scholar]
Stoll 1996
- Stoll BJ, Gordon T, Korones SB, et al. Late onset sepsis in very low birth weight neonates: A report from the National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1996;129:63‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Craft 2000
- Craft AP, Finer NN, Barrington KJ. Vancomycin for prophylaxis against sepsis in preterm neonates. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001971] [DOI] [PMC free article] [PubMed] [Google Scholar]


