Abstract
Background
Hepatitis B virus infection is a serious health problem worldwide. Traditional Chinese medicinal herbs have been widely used to treat chronic liver diseases, and many controlled trials have been done to investigate their efficacy.
Objectives
To assess the efficacy and safety of traditional Chinese medicinal herbs for chronic hepatitis B infection.
Search methods
Searches were applied to the following electronic databases: the Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Complementary Medicine Field Trials Register, The Cochrane Library (Issue 2, 2000), MEDLINE, EMBASE, and BIOSIS (October 2000). Five Chinese journals and conference proceedings were handsearched. No language restriction was used.
Selection criteria
Randomised or quasi‐randomised trials with at least three months follow‐up. Trials of Chinese medicinal herbs (single or compound) compared with placebo, no intervention, general non‐specific treatment or interferon treatment were included. Trials of Chinese medicinal herbs plus interferon versus interferon alone were also included. Trials could be double‐blind, single‐blind, or unblinded.
Data collection and analysis
Data were extracted independently by two reviewers. The methodological quality of trials was evaluated using the Jadad‐scale plus allocation concealment. Intention‐to‐treat analyses were performed.
Main results
Nine randomised trials, including 936 patients, met the inclusion criteria. Methodological quality was considered adequate in only one trial. There was a significant funnel plot asymmetry (regression coefficient=3.37, standard error 1.40, P=0.047).
Ten different medicinal herbs were tested in the nine trials. Compared to non‐specific treatment or placebo, Fuzheng Jiedu Tang (compound of herbs) showed significantly positive effects on clearance of serum HBsAg, HBeAg, and HBV DNA; Polyporus umbellatus polysaccharide on serum HBeAg and HBV DNA; Phyllanthus amarus on serum HBeAg. Phyllanthus compound and kurorinone showed no significant effect on clearance of serum HBeAg and HBV DNA and on alanine aminotransferase normalisation compared to interferon treatment. There were no significant effects of the other examined herbs.
Authors' conclusions
Some Chinese medicinal herbs may work in chronic hepatitis B. However, the evidence is too weak to recommend any single herb. Rigorously designed, randomised, double‐blind, placebo‐controlled trials are required.
Plain language summary
Still awaiting evidence on Chinese medicinal herbs for chronic hepatitis B
Chronic hepatitis B is an infectious disease of the liver caused by hepatitis B virus. Around 350 million people worldwide are chronic infected carriers of the virus. Traditional Chinese medicinal herbs have for long been used for treating chronic liver diseases both in China and in Southeast Asia. This systematic review evaluates the effect of Chinese medicinal herbs (single or compound) for treating chronic hepatitis B infection.
The review of trials found that some of the Chinese medicinal herbs may have a positive effect on the clearance of hepatitis B virus and on the diseased liver. However, the methodological quality of the trials evaluating these herbs was generally poor. Analysis of the identified trials also indicated that trials with positive findings are more likely to be published than trials without significant findings. Further, medicinal herbs may be associated with side effects. Therefore, Chinese medicinal herbs should not be used outside new trials. Testing the herbs in larger, well‐designed trials is needed in order to establish the necessary evidence for their use.
Background
Hepatitis B is an infectious disease of the liver caused by hepatitis B virus (HBV). More than two billion people alive today have been infected worldwide (WHO 1998) and approximately 350 million people are chronically infected carriers of the virus (Purcell 1993; WHO 1998). These carriers are at high risk of getting serious illness and die from cirrhosis of the liver and liver cancer. Each year more than one million carriers worldwide die from these diseases (WHO 1998). China bears a heavy burden of this disease, with an estimated 120 million chronically infected carriers; up to 12 million people suffer from chronic hepatitis B disease, and 300 thousand people die each year (CMH 1999). Hepatitis B is, therefore, an important public health problem in China where treatment costs amount to 30 to 50 billion RMB Yuan (3.57˜5.95 billion US $) per year (CMH 1999).
The hepatitis B virus is transmitted by body fluids, such as blood and serum, and can be passed from mother to child. The virus belongs to the family hepadnaviridae (Gitlin 1997). The virus is a round structure (42 nm diameter), called the Dane particle, which has an outer coat and an inner core. A protein on the surface, hepatitis B surface antigen (HBsAg) is a standard marker of infection when found in a patient's serum. The inner core is made of nucleocapsid protein (HBcAg) which encloses the virus DNA (HBV DNA). There is another core protein called hepatitis B 'e' antigen (HBeAg) which is used in diagnosis as a marker of infection and virus replication.
The incidence of new HBV carriers in children has decreased in high endemic areas thanks to the availability of a safe and effective vaccine, but for the areas of low endemicity, high risk group immunization strategy is not leading to a significant reduction of HBV infection on a national or international scale (WHO 1998; WHO 1999). Treating adults who have chronic HBV infection with alpha‐2b‐interferon (IFN) results in viral, biochemical and histological remission in about 30 per cent of the cases. Interferon therapy, which is presently considered 'standard therapy' for hepatitis B, however, even in combination with a nucleoside analogue, is associated with a high incidence of recurrence, serious side effects, and is very expensive; hence it is not widely used in developing countries.
Traditional Chinese medicine (TCM) has been used widely for more than 2000 years to treat chronic liver disease, including chronic hepatitis B infection. Many controlled trials have been completed investigating treatment with TCM. The quality of these trials, however, has not been assessed systematically. Furthermore, the spontaneous evolution of chronic hepatitis B disease is difficult to predict and a definitive assessment of the effects of treatment requires long‐term follow‐up. TCM has its unique theories for concepts of etiology, systems of diagnosis, and treatment which are vital to their practice. TCM drug treatment consists typically in complex prescriptions of a combination of several components. The combination based on the Chinese diagnostic patterns (i.e., inspection, listening, smelling, inquiry, and palpation) follows a completely different rationale than many western drug treatments. Beside, for hepatitis B, the diagnostics includes laboratory evidence through liver function indices and viral markers.
Treatment with alternative medicines may have great potential as viral hepatitis B disease therapy (Wang 2000). However, the effectiveness of such treatment needs to be reviewed systematically and appraised critically to inform the current practice and direct the continued search for new treatment regimens. Chinese medicinal herbs for HBV infection are considered collectively in this review as a special experimental treatment.
Objectives
The primary objective of this review was to assess the efficacy of traditional Chinese medicinal herbs as a special experimental treatment for chronic hepatitis B compared with placebo, no intervention, non‐specific treatment, or IFN treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials regardless of whether they were single‐blind, double‐blind, or unblinded. Only trials with a minimum follow‐up of three months were to be included.
Types of participants
Male or female patients, of any age or ethnic origin, who had chronic hepatitis B, defined as serum HBsAg positive persisting six months or more accompanied by elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) or with recurrent fluctuation, whether symptomatic or symptomless, and with or without liver biopsy findings compatible with chronic hepatitis (Dusheiko 1999). Trials in populations with superinfection or coinfection of chronic hepatitis B with another hepatitis virus were excluded.
Types of interventions
Chinese medicinal herbs (a single herb or compound of herbs) were compared with placebo or no intervention, or general non‐specific treatments such as vitamins, or interferon (IFN) treatment (no limitation regarding IFN type or IFN treatment regimen). Trials of Chinese medicinal herbs plus IFN treatment versus IFN alone were also included.
In this review, Chinese medicinal herbs are defined as products derived from plants or parts of plants that grow and have been widely used in China for medicinal purpose. This definition does not exclude some herbal compounds that contain nonplant materials as supplements, like minerals or animal parts (Ergil 1996).
Types of outcome measures
The following primary outcome measures were sought at three, six, and 12 months after completion of the treatment:
1. Mortality
2. Hepatocellular carcinoma
3. Viral response: ‐ Loss of serum HBeAg. ‐ Seroconversion of HBeAg to HBeAb (antibody against HBeAg). ‐ Loss of serum HBV DNA. ‐ Loss of serum HBsAg.
Viral response was measured by validated methods as follows: HBeAg, HBeAb, and HBsAg were measured by either enzyme immunoassay or radial immunoassay, and HBV DNA‐by molecular hybridisation or polymerase chain reaction.
4. Biochemical response: Normalisation of ALT and/or AST levels (less than 40 IU/L) or decrease of ALT and/or AST levels.
5. Liver histological recovery: Decrease of liver inflammatory disease activity by comparison of pre‐ and post‐trial inflammatory severity through liver biopsy. The Knodell histologic activity index (HAI) reflects the inflammatory activity. The score can be 0‐18 and, when comparing before and after treatment, a decrease greater than or equal to two is called improvement (Ishak 1995).
The following secondary outcome measures were sought at three, six, and 12 months after completion of the treatment:
6. Quality of life.
7. Adverse effects: fever, chills, fatigue, malaise, urticaria, headache, gastrointestinal reaction, myalgia, decreased white blood cell count.
Search methods for identification of studies
Electronic searches The Trials Registers of The Cochrane Hepato‐Biliary Group, The Cochrane Library (Issue 2, 2000), and The Cochrane Complementary Medicine Field were searched. MEDLINE (March 1966 to October 2000), EMBASE (January 1980 to October 2000) and BIOSIS (January 1969 to October 2000) were searched from their date of inception onwards. The MeSH terms included hepatitis‐B, medicine‐Chinese‐traditional, plants‐medicinal, drugs‐Chinese‐herbal, plants extracts, and herbs. No language restrictions were applied.
Handsearches Chinese Journal of Infectious Diseases, Chinese Journal of Hepatology, Chinese Journal of Clinical Hepatology, Chinese Journal of Integrated Traditional and Western Medicine, and Journal of Integrated Traditional and Western Medicine on Liver Diseases were handsearched from the first publication date onwards (October 2000) by the contact reviewer. Conference proceedings in Chinese were also handsearched. Results of handsearching were submitted to the Cochrane Hepato‐Biliary Group. The bibliographic references of identified RCTs were checked in order to find RCTs not identified by the handsearched journals.
Data collection and analysis
Selection of trials for inclusion Two authors independently selected the trials to be included in the review according to the prespecified selection criteria. Disagreement was resolved by discussion.
Assessment of methodological quality The methodological quality of included studies was assessed using the instrument developed by Jadad et al. (Jadad 1996). The scale awards one to five points to a RCT. RCTs with one or two points would be considered as low quality, and three to five as high quality (Moher 1998; Kjaergard 1999). An unblinded quasi‐randomised trials with alternate allocation which does not report on drop‐outs would score zero. In addition, concealment of the allocation sequence was scored A (adequate), B (unclear) or C (inadequate), following criteria adopted from The Cochrane Handbook and Schulz et al. (Schulz 1995) as follows: A ‐ Adequate measures to conceal allocations such as central randomisation; serially numbered, opaque, sealed envelopes; or other description that contained convincing elements of concealment. B ‐ Unclearly concealed trials, in which the authors either did not report an allocation concealment approach at all, or reported an approach that did not fall into one of the categories in (A). C ‐ Inadequately concealed trials, in which method of allocation was not concealed, such as alteration methods or use of case record numbers.
Data extraction Data were extracted independently by two reviewers using a self‐developed data extraction form. Disagreement was resolved by discussion. Papers not in English or Chinese were translated with the help of the Cochrane Hepato‐Biliary Group.
Data on the number of patients with each outcome event and by allocated treatment group, irrespective of compliance or follow‐up, were sought to allow an intention‐to‐treat analysis. If the above data were not available in the trial reports, additional information was sought by correspondence with the principal author. If the information was still lacking after the author was contacted, this was recorded.
Data synthesis Dichotomous data were presented as relative risk (RR) and continuous outcomes as weighted mean difference (WMD), both with 95% confidence intervals (CI). Analyses were performed on intention‐to‐treat basis where possible. For dichotomous outcomes, patients with incomplete or missing data were included in sensitivity analyses by counting them as treatment failures ('worst‐case' scenario analysis). Counting these patients in the treatment group as treatment failures and those in the control group as responders was considered as a 'worst‐worst case' scenario analysis.
We intended to display studies as comparisons of traditional Chinese medicinal herbs with: ‐ placebo or no treatment ‐ general non‐specific treatments (such as vitamins) ‐ IFN treatment.
Trials of Chinese medicinal herbs plus IFN treatment versus IFN treatment alone were presented as a separate comparison.
Meta‐analysis was performed within the comparisons where individual trials compared similar treatment and control interventions.
We pre‐specified the following subgroup analyses for each outcome: ‐ ethnic origin ‐ age at time of infection. Age is a major factor in determining the outcome of HBV infection as children and adults exhibit different patterns of disease outcome. Young children rarely develop symptomatic HBV infection, but about 25 per cent of the children infected under the age of seven become HBsAg carriers. After the age of seven, children exhibit an adult pattern of disease outcome with about 5 to 10% becoming HBsAg carriers (WHO 1998). ‐ a single herb or a compound of herbs.
Sensitivity analysis If a sufficient number of trials was found, sensitivity analyses were performed as follows: ‐ excluding quasi‐randomised trials (such as alternate allocation or systematic allocation). ‐ excluding studies with inadequate concealment of allocation (Schulz 1995). ‐ excluding studies in which outcome evaluation was not blinded.
When we evaluated trials for inclusion in this review, a high proportion was found to have followed patients only to the end of treatment, and not for a minimum of three months as we had pre‐specified for inclusion. In order not to lose these data altogether, we performed a separate analysis of these trials according to the duration of follow‐up.
Potential publication bias (Vickers 1998) was investigated by a funnel plot where possible (Egger 1997).
Results
Description of studies
Our initial searches (October 2000) identified 521 articles; 312 from the electronic searches and 209 from the handsearching. After reading titles and abstracts, 439 of these articles were excluded because they were duplicates, non‐clinical studies, or had study objectives different from this review. A total of 82 articles published in five languages (Chinese, English, German, Japanese, and Polish) were retrieved for further assessment. Of these, 50 articles were excluded because they did not meet our inclusion criteria. The reasons for exclusion were listed under 'Characteristics of excluded studies'.
The remaining 32 articles consisted of one quasi‐RCT and 31 RCTs that reported random allocation of patients (n=3509) with chronic hepatitis B to Chinese medicinal herbs versus control (placebo in four trials, non‐specific treatment in 15 trials, no treatment in one trial, and IFN treatment in four trials) or to Chinese medicinal herbs plus IFN versus IFN alone (eight trials). However, of these 32 trials, 23 had follow‐up duration less than three months after the end of intervention with Chinese medicinal herbs. According to our inclusion criteria, each RCT had to report a minimum of three months follow‐up. As this criterion would lead to exclusion of the majority of the RCTs, we decided, before embarking on data analysis, to include the 23 RCTs in sensitivity analyses to investigate immediate therapeutic effects. These 23 trials are described in Additional tables (Table 6: Trials with less than three months follow‐up).
1. Trials with less than three months follow‐up.
| Study ID | Methods | Participants | Interventions | Outcomes | Quality |
| Chang 1994 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unclear. | Ethnic: Chinese. 82 patients (73 males and nine females, average age 31 yrs, range from 2˜60 yrs). Setting: unclear. Inclusion criteria: the National Conference Criteria of China. Exclusion criteria: severe heart or kidney disease, and pregnancy. | Experimental: Tentinan (an extracted ingredient of herb) tablet, 25 mg, tid, orally, for three months. Control: Hypoxanthosine, 0.4 g, plus vitamin C, 0.2 g, tid, for three months. | Viral response: HBsAg, HBeAg, anti‐HBe Ab, anti‐HBc Ab. Biochemical response: ALT, AST, Bilirubin, IgG, albumin/globulin (A/G). Symptoms and signs. Adverse effects. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Chen 1990 | Randomised double‐ blind trial. Generation of allocation sequence : central pharmacy control, adequate. Allocation concealment: adequate. Blinding: patients and clinicians. Loss to follow up: yes, three cases in treatment group. | Ethnic : Chinese. 96 patients (51 in treatment group, 45 in placebo group). Setting: outpatients. Inclusion criteria: chronic hepatitis B with damaged liver function, HBsAg positive for more than one yr, HBeAg and/or HBV DNA positive. Exclusion criteria: unstated. | Experimental : Jiedu Yanggan Gao (compound of herbs), syrup, 20 ml, tid, orally, for three months. Control: Placebo (Jiao San Xian Gao) , syrup, 20 ml, tid, orally, for three months. | Viral response: HBsAg, HBeAg, DNAP, HBV DNA. Biochemical response: ALT, AST. Outcomes assessed at the end of treatment. | Jadad score=3 |
| Cui 1994 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 60 patients (30 in treatment group, 24 males and six females, mean age: 49 yrs, ranging 29˜74 yrs; 30 in control group, 26 males and four females, mean age: 43 yrs, ranging 24˜56 yrs). Setting: inpatients. Inclusion criteria: chronic active hepatitis with HBsAg positive, symptoms and signs and abnormal liver function. Exclusion criteria: unstated. | Experimental: Panax pseudo‐ginseng (a herb), 1.5 g, plus Yiganling (herbal compound), 4 tablet, orally, tid, for six week. Control: Yiganling, 4 tablet, orally, tid, for six weeks. | Symptoms and signs. Liver function: ALT, serum bilirubin, A/G. Adverse effects. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Fang 1994 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 95 patients (30 in group I, 16 males and 14 females, mean age: 42 yrs, ranging 11˜65 yrs; 31 in group II, 20 males and 11 females, mean age: 41 yrs, ranging 15˜63 yrs; 34 in group III, 22 males and 12 females, mean age: 42 yrs, ranging from 14˜61 yrs). Setting: unstated. Inclusion criteria: chronic active hepatitis and chronic persistent hepatitis with positive HBsAg, HBeAg and anti‐HBc, and abnormal liver function. Exclusion criteria: unstated. | Experimental: Group I: IFN, 100,000 U, i.m., two times a week, for three months. Group II Gankangling (compound of more than 11 herbs): one dose orally, divided into two times, for three months. Group III IFN plus Gankangling: dosage and usage were the same as above; for three months. | Symptoms and signs. Liver function: ALT, serum bilirubin, A/G. HBV markers: HBsAg, HBeAg, anti‐HBe. Outcomes assessed at the end of treatment. Follow‐up of six months after the end of treatment described, but no data given on the outcomes. | Jadad score=1 |
| Hirayama 1989 | Randomised double‐ blind multicenter clinical trial. Generation of allocation sequence : adequate. Allocation concealment: adequate. Blinding: patients and doctors. Withdrawal during treatment: 12 cases in treatment group, six cases in placebo group. | Ethnic: Japanese. 222 patients (116 in treatment group, 85 males and 31 females; 106 in placebo group, 78 males and 28 females). Setting: outpatients from 42 hospitals. Diagnosis confirmed by liver biopsies. Exclusion criteria: corticosteroids, azathioprine, IFN, Ara‐A and glycyrrhizin were discontinued three months before the study; patients with renal disease, diabetes, alcohol abuse and pregnant. | Experimental: Shosai‐ko‐to (SST, herbal compound) granules, 5.4 g, orally, daily, for 12 weeks, followed by the same dose for further 12 weeks. Control: Placebo(containing 0.5 g of SST), for 12 weeks, followed by a cross‐over to SST for a further 12 weeks. Only the data of the first 12 weeks were used in this review. | Symptoms. HBV markers: HBsAg, HBeAg, anti‐HBe Ab. ALT, AST. Adverse effects. (We got further data of some outcomes from Hirayama). Outcomes assessed at the end of treatment. | Jadad score=5 |
| Huang 1999a | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 70 patients (40 in treatment group, 28 males and 12 females, mean age: 32 yrs, ranging 15˜55 yrs; 30 in control group, 22 males and eight females, mean age: 30 yrs, ranging 16˜52 yrs). Setting: unstated. Inclusion criteria: chronic hepatitis B with HBsAg positive, and abnormal liver function. Exclusion criteria: unstated. | Experimental: Phyllanthus compound (composed of mainly Phyllanthus and Panax pseudo‐ginseng) capsule, 2.6 g, orally, tid; plus IFN 1 MU, im, every other day; both for three months except eight for six months. Control: IFN 1 MU, im, every other day, for three months. | Symptoms and signs. Liver function: ALT, AST, serum bilirubin, A/G. HBV markers: HBsAg, HBeAg, HBV DNA. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Huang 1999b | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 70 patients (38 in treatment group, 30 males and eight females, age ranging 20˜48 yrs; 32 in control group, 26 males and six females, age ranging 18˜47 yrs). Setting: outpatients and inpatients. Inclusion criteria: chronic hepatitis B with HBsAg positive, and abnormal liver function. Exclusion criteria: unstated. | Experimental: Phyllanthus herb (grown in Guangdong, China) 40 g, decocted, orally, daily; for three months. Control: hypoxanthosine 0.4 g, plus vitamin C 4.0 g in 250 ml of 10% glucose, intravenous, daily, for three months. | Liver function: ALT, AST, serum bilirubin. HBV markers: HBsAg, HBeAg, HBV DNA. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Huang 2000 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: none. | Ethnic: Chinese. 104 patients (50 males, 10 females, age 17˜48 yrs, in experimental group; 38 males and six females, age 19˜49 yrs in control group). Setting: in and outpatients. Inclusion criteria: chronic hepatitis B with abnormal liver function, and HBsAg positive. Exclusion criteria: unstated. | Experimental: Ganyanling injection (a herbal extract), 2 ml, i.m., bid, plus Salviae miltiorrhizae injection 16 mg in 250 ml of 10% glucose, intravenous infusion, daily, for three months. Control: Hypoxanthosine, 0.4 g, plus vitamin C 3.0 g in 250 ml of 10% glucose, intravenous infusion, daily, for three months. | Symptoms and signs. Liver function: ALT, AST, serum bilirubin, A/G. HBV markers: HBeAg, HBV DNA. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Jiao 1994 | Quasi‐randomised clinical trial, alternative allocation by admission order. Blinding: no. Withdrawal/drop‐out: none. | Ethnic: Chinese. 250 patients (212 males and 38 females, age 19˜60 yrs, average 39 yrs). Setting: inpatients. Inclusion criteria: chronic active hepatitis B with HBsAg positive. Exclusion criteria: unstated. | Experimental: Group I: IFN, 1 MU, i.m., every other day. Group II (herbal decoction): 250 ml, bid, orally. Group III (IFN plus herbal decoction): IFN, 1 Mega Unit (MU), i.m., every other day; decoction, 250 ml, orally. Group IV: thymosin, 8 mg, i.m., daily. Group V: Polyporus umbellatus polysaccharide injection, i.m., daily, no dosage reported, for 20 days, stop 10 days, then repeat; plus hepatitis B vaccination, 30 ug, subcutaneous injection, two times a month. Group VI (self‐LAK cells infusion): 100 ml, once a week. All six groups were treated for two months. Only data of group I, II and III were used in this review. | HBV markers: HBsAg, HBeAg, anti‐HBe Ab, HBV DNA. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Li 1998 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 55 patients (30 in treatment group, 23 males and seven females, mean age: 31 yrs, ranging 15˜55 yrs; 25 in control group, 20 males and five females, mean age 33 yrs, ranging 18˜50 yrs). Setting: unstated. Inclusion criteria: chronic hepatitis B with HBsAg positive, and elevated serum ALT level over one time above the normal value; age between 15 and 55 yrs; with no severe complication. Exclusion criteria: patients allergic to IFN; or having remarkable hematological disease; or women with pregnancy or breast feeding. | Experimental: Phyllanthus compound (composed of mainly Phyllanthus and Panax pseudo‐ginseng) capsule, 2.6 g, orally, tid; for three months. Control: IFN 3 MU, i.m., every other day, for three months. | Liver function: ALT, AST, serum bilirubin, A/G. HBV markers: HBsAg, HBeAg, HBV DNA. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Lin 1996 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 32 patients (10 males and six females, age 19˜33 yrs, in experimental group; eight males and eight females, age 17˜35 yrs, in control group). Setting: outpatients. Inclusion criteria: chronic hepatitis B with HBsAg, HBeAg, and anti‐HBc Ab positive. Exclusion criteria: unstated. | Experimental: Huatan Jiedu Fuzheng recipe (a prescribed compound of herbs), orally, no data on dosage, plus IFN, 3 MU, i.m., thrice a week, both for three months. Control: IFN, 3 MU, i.m., thrice a week, for three months. | Liver function: ALT. HBV markers: HBsAg, HBeAg. Adverse effects. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Lv 1991 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no report. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 150 patients (102 males and 48 females, mean age 32 yrs, ranging 9˜70 yrs; randomised into Chinese medicine plus IFN group 80 and IFN alone group 70). Setting: inpatients. Inclusion criteria: chronic hepatitis with HBsAg, HBeAg, anti‐HBc Ab positive and damaged liver function, including 83 chronic persistent hepatitis and 67 chronic active hepatitis. Exclusion criteria: unstated. | Experimental: Self‐prescibed medicinal compound of herbs, one dose, daily, plus IFN, 40,000 U, i.m., daily, for three months. Control: IFN, 40,000 U, i.m., daily, for three months. | HBV markers: HBsAg, HBeAg, HBV DNA, anti‐HBc antibody. Adverse effects. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Miao 1993 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 277 patients (84 in experimental group I, 64 males and 17 females, average age 29 yrs; 72 in experimental group II, 59 males and 13 females, average age 30 yrs; 62 in experimental group III, 43 males and 19 females, average age 32 yrs; 62 in control group, 42 males and 20 females, average age 31 yrs). Setting: unstated. Inclusion criteria: unstated. Exclusion criteria: unstated. | Experimental: group I: Jianganling (extract of three herbs) capsules, 3 tab, tid, orally, for two months. group II: Potenlini injection (an herbal extract), 60˜100 ml in 250 ml of 10% glucose, intravenous infusion, daily, for two months. group III: Huganpian (an extract from Chinese magnoliavine fruit), 4 tab, tid, orally, for two months. control: vitamin C and B, plus alcamin, no information on usage and dosage, for two months. | ALT. HBV markers and adverse effects. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Miao 1994 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 150 patients (30 cases in IFN group; 64 in experimental group, 56 in thymosin group). No data on sex and age. Setting: inpatients. Inclusion criteria: chronic hepatitis B diagnosed by the Conference Criteria. Exclusion criteria: no use of steroids, antiviral drugs and bioimmune formulations within six months before trial. | Experimental: group I: Polyporus umbellatus polysaccharide injection (an herbal extract), 40 mg, i.m., daily, for 20 days, then stop for 10 days, repeated till three months. group II: thymosin, 10 mg, i.m., daily, for three months. Control: IFN, 1 MU, i.m., daily, for three months. Only data of group I and control were used. | Liver function: ALT, serum bilirubin, A/G. HBV markers: HBsAg, HBeAg, conversion of HBeAg to HBeAb, HBV DNA. Adverse effects. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Wang 1995a | Randomised clinical trial. Generation of allocation sequence: no report. Allocation concealment: no information. Blinding: no blinding. Exclusion during trial: unstated. | Ethnic: Chinese. 123 patients (11 in group I, nine males and two females, average age 24 yrs; 35 in group II, 30 males and five females, average age 35 yrs; 42 in group III, 29 males and 13 females, average age 35 yrs; 35 in control group, 30 males and five females, average age 35 yrs). Setting: unstated. Inclusion criteria: The Symposium Criteria of China. Exclusion criteria: unstated. | Experimental: group I: Phyllanthus amarus (a herb from India) capsule, orally, tid, for three months. group II: Phyllanthus urinaria (from Henan, China), orally, tid, three months. group III: Phyllanthus niruri (from Hainan, China), orally, tid, three months. Control: no herbal treatment. | HBV markers: HBeAg, anti‐HBe Ab, HBsAg. ALT. adverse effect. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Wang 1995b | Randomised trial. Generation of allocation sequence: yes, by drawing. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: none. | Ethnic: Chinese. 218 patients including 126 chronic active hepatitis B (72 males and 54 females, mean age 40 yrs), 60 post‐hepatitis liver cirrhosis (48 males and 12 females, mean age 49 yrs) and 32 alcoholic liver disease (all males, mean age 38 yrs). Only data from 126 of chronic active hepatitis B were used. Setting: unstated. Inclusion criteria: The National Conference Criteria of China. Exclusion criteria: unstated. | Experimental: Shenhuang granule (herbal compound), 10 g, bid, orally, plus hypoxanthosine, 0.2 g, in 500 ml of 10% glucose, intravenous infusion, daily, total 15 times, for two months. Control: vitamin E, 0.1 g, tid, orally, plus vitamin C, in 500 ml of 10% glucose, intravenous infusion, daily, total 15 times, no data on dosage, for two months. | HBV markers: HBsAg, HBeAg. Biochemical indicators: ALT, bilirubin. Adverse effects. Outcomes assessed at the end of treatment. | Jadad score=2 |
| Wang 1999b | Randomised controlled trial. Generation of random allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 70 patients (40 in experimental group, 28 males and 12 females, average age 32 yrs, range 15˜55 yrs; 30 in control group, 22 males and eight females, average age 30 yrs, from 16˜52 yrs). Setting: inpatients. Inclusion criteria: chronic hepatitis B with HBsAg positive and abnormal liver function, no serious complications. Exclusion criteria: serious hematological disease, allergy to IFN, pregnancy or in maternal feeding. | Experimental: Genus Phyllanthus (a herb) capsule, 4 cap, tid, orally, plus IFN 1 MU, i.m., every other day, for three months. Control: IFN, 1 MU, i.m., every other day, three months. | Symptoms and signs. Liver function: ALT, AST, A/G, serum bilirubin. HBV markers: HBsAg, HBeAg, HBV DNA. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Wen 1999 | Randomised clinical trial. Generation of allocation sequence: no report. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 36 patients (average age 32 yrs, range from 23˜46 yrs. 19 in experimental group, 13 males and six females; 17 in control group, 12 males and five females). Setting: inpatients. Inclusion criteria: chronic hepatitis B with HBsAg positive. Exclusion criteria: unstated. | Experimental: Youguiying (herbal compound), one dose, daily, orally, for two month. Control: Hypoxanthosine, covitamins, usage and dosage unstated, for two months. No other immunoregulatory agents used before and during treatment. | Symptoms and signs. Liver function: ALT. Peripheral blood T lymphocyte counts. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Wu 1996 | Randomised clinical trial. Generation of allocation sequence: no report. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 71 patients (43 cases in experimental group, 23 were chronic active hepatitis, 20 chronic persistent hepatitis, 28 in control group, 15 were chronic active hepatitis, 13 chronic persistent hepatitis). Setting: inpatients. Inclusion criteria: chronic hepatitis B with symptoms and damaged liver function. Exclusion criteria: unstated. | Experimental: Lentinan (ingredient extracted from a herb) injection, 8 mg, in saline, i.m., daily, for three months. Control: hypoxanthosine injection, 0.4 g, in 500 ml of 10% glucose, intravenous infusion, daily, for three months. | ALT, endotoxin and tumour necrotic factor. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Yu 1999b | Randomised trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: unblinded. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 72 patients (60 males and 12 females, age: 23˜51 yrs). Setting: inpatients. Inclusion criteria: National Conference Criteria of China. Exclusion criteria: unstated. | Experimental: group I (n=20): Potenlini, 80 ml, plus Salviae miltiorhhizae, 20 ml, in 250 ml of 10% glucose, intravenous infusion, daily, for two to three months. group II (n=18): Salviae miltiorhhizae, 20 ml, in 250 ml of 10% glucose, intravenous infusion, daily, for two to three months. group III (n=19): Potenlini, same as group I. Control group (n=15): non‐specific treatment, for two to three months, no information on drugs, usage, dosage. | Liver function. HBV marker. Biochemical parameters of fibrosis. Adverse effect. Outcomes assessed at the end of treatment. | Jadad score=1 |
| Zhang 1998 | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: unblinded. Withdrawal/drop‐out: none. | Ethnic: Chinese. 38 patients (20 in treatment group, 14 males and six females, mean age 34 yrs; 18 in control group, 11 males and seven females, mean age 36 yrs). Setting: inpatients. Inclusion criteria: based on National Conference Criteria of China, all patients diagnosed by liver biopsy. Exclusion criteria: unstated. | Experimental: glycyrrhizin (herbal extract), 150 mg, in 500 ml of 5% glucose, intravenous infusion, daily; plus hypoxanthosine and vitamins, orally, dosage unstated; all for three months. Control: potassium magnesium aspartate injection, 20 ml, in 500 ml of 5% glucose, intravenous infusion, daily, plus hypoxanthosine and vitamins, oral; for three months. | Liver tissue pathology: assessed by grade of inflammatory activity, graded as 0˜4 score, at the end of treatment of three months. Adverse effect. | Jadad score=1 |
| Zhang 1999 | Randomised trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: unblinded. Withdrawal/drop‐out: unstated. | Ethnic: Chinese. 135 patients (45 in treatment group, 30 males and 15 females, age 20˜50 yrs; 45 in control group I and 45 in control group II). Setting: in and outpatients. Inclusion criteria: accordance with IFN usage indications. Exclusion criteria: unclear. | Experimental: IFN, 3 MU, i.m., thrice a week, plus Yigansan (herbal compound), one bag, tid, orally, for three months. Control: group I: IFN, 3 MU, i.m., thrice a week, for three months. group II: Yigansan, one bag, tid, orally, for three months. | Liver function. HBV markers: HBeAg, HBV DNA. Both were assessed at the end of treatment. | Jadad score=1 |
| Zhou 1999 | Randomised trial. Generation of allocation sequence: no report. Allocation concealment. no report. Blinding: unblinded. Withdrawal during trial: seven cases in control group. | Ethnic: Chinese. 97 patients (60 in treatment group, 32 males and 28 females, average age 36 yrs; 37 in control group, 24 males and six females, average age 35 yrs). Setting: inpatients. Inclusion criteria: accordance with the Symposium Criteira of China. Exclusion criteria: unstated. | Experimental: IFN, 3 MU, subcutaneous injection, thrice a week, plus Yigan No.3 granules (herbal compound), 10 g, orally, tid, for six months. Control: IFN, 3 MU, subcutaneous injection, thrice a week, for six months. | Symptoms. Liver function: ALT, AST. HBV markers: HBV DNA, HBeAg. Adverse effects. All assessed at the end of treatment. | Jadad score=1 |
Nine RCTs (including a total of 936 Chinese participants) met all our pre‐specified inclusion criteria, of which one trial was published in English (Chen 2000) and the remaining in Chinese. Duration of follow‐up after end of interventions was three to 12 months. Six trials included inpatients and one trial included outpatients. The other two did not specify the status of the patients. Inclusion criteria were explicit chronic hepatitis B diagnosed by clinical manifestations, biochemical responses, and virological markers. The diagnostic criteria were based on different consensus by the Chinese associations for the diagnosis of chronic hepatitis B depending on different period of times when the trials were conducted (CMA 1984; CMA 1990; CMA 1995). The diagnosis was confirmed by liver biopsy in three trials. From the available information, it was not possible to conduct subgroup analysis on ethnic origin or of age at infection.
All nine RCTs included adults; mean age was 32 years in eight trials. One trial (Huang 1993) did not describe the sex or age of participants (n=122), another trial (Zhang 1993a) did not report sex (n=67), and the rest reported a male to female ratio of three to one (564:183). The average size of the trials was 106 patients, ranging from 40 to 252.
Medicinal herbs According to the category of Chinese medicinal herbs, four trials studied a single herb or ingredient of a single herb (Phyllanthus amarus, Potenlini, kurorinone, and Polyporus umbellatus polysaccharide), four trials studied compounds of herbs (Kangdu Wan, Fuzheng Jiedu Tang, Phyllanthus compound, and Anisodamine plus Salviae miltiorrhizae), and one trial studied a combination of a single and a compound of herbs (Polyporus umbellatus polysacharide plus a decoction called Buqi Jiedu Tang). The constituents and dosage of medicinal herbs varied (Table 7). The large heterogeneity of the intervention prevented us from doing a meaningful subgroup analysis on herbs either single or compound. The median duration of treatment was three months (range one to six months). Two trials used placebo (Yan 1988; Yang 1986), five used non‐specific drugs (Huang 1993; Wang 1991a; Wang 1994a; Xiao 1994; Zhang 1993a), and two used IFN (Chen 2000; Zheng 1999) in their control groups. The non‐specific drugs were vitamins, hypoxanthosine, alcamin (orazamide), potassium magnesium aspartate, adenosine triphosphate, and coenzyme A pantothenic acid.
2. Interventions of Chinese medicinal herbs and outcome measures.
| Study ID | CMH interventions | Control intervention | Loss of HBsAg | Loss of HBeAg | Loss of HBV DNA | ALT normalisation |
| Chen 2000 | Kurorinone | interferon‐alfa2a | ‐ | NS | NS | NS |
| Huang 1993 | Phyllanthus amarus | non‐specific drugs | NS | + | ‐ | ‐ |
| Wang 1991a | Potenlini | non‐specific drugs | NS | NS | ‐ | NS |
| Wang 1994a | Polyporus umbellatus polysaccharide | non‐specific drugs | NS | + | + | ‐ |
| Wang 1994a | Buqi Jiedu Tang | non‐specific drugs | NS | NS | NS | ‐ |
| Xiao 1994 | Fuzheng Jiedu Tang | non‐specific drugs | + | + | + | ‐ |
| Yan 1988 | Polyporus umbellatus polysaccharide | placebo | NS | + | ‐ | NS |
| Yang 1986 | Kangdu Wan | placebo | NS | NS | NS | NS |
| Zhang 1993a | Anisodamine plus Salvia miltiorrhiza | non‐specific drugs | NS | NS | NS | + |
| Zheng 1999 | HB‐Granule‐3 (Phyllanthus compound) | interferon‐gamma | NS | NS | NS | ‐ |
| +: positive ‐: not estimated NS: not significant |
Outcomes None of the nine trials reported mortality, quality of life, or incidence of liver cirrhosis and/or hepatocellular carcinoma. The common outcomes reported were viral responses (including serum HBsAg, HBeAg, HBeAb, HBV DNA), biochemical responses (serum ALT, AST, bilirubin), symptoms and signs, as well as adverse effects. One trial (Yan 1988) described liver histology. One RCT awaiting assessment reported incidence of hepatocellular carcinoma and survival rate in 260 patients with liver cirrhosis, but only 37 patients of them were HBsAg positive and no data of the outcomes were reported in this group of patients (Oka 1995a). This trial is listed in 'Studies awaiting assessment' until more data become available from the authors.
Risk of bias in included studies
Of the nine included RCTs, one was assessed as high quality (a Jadad‐score of three for Yan 1988) and the remaining as low quality (a Jadad‐score of two for Chen 2000; Huang 1993; Xiao 1994; Yang 1986, and a Jadad‐score of one for Wang 1991a; Wang 1994a; Zhang 1993a; Zheng 1999). Allocation concealment was described and considered to be adequate in one trial (Yan 1988). Only two of the nine trials reported a double‐blind design (Yan 1988; Yang 1986), and seven gave no information on how the allocation sequence was generated.
We decided that an insufficient number of trials were found to perform meaningful sensitivity analysis excluding studies with inadequate concealment of allocation. None of the trials reported a sample size calculation. Four RCTs reported the numbers lost‐to‐follow‐up (Chen 2000; Wang 1991a; Xiao 1994; Zhang 1993a). No RCT stated that they used an intention‐to‐treat analysis to evaluate their data.
Among the 23 randomised trials with a follow‐up of less than three months, one was quasi‐randomised. Only two were double‐blind and reported adequate allocation concealment (Chen 1990; Hirayama 1989). Applying the Jadad‐score, three RCTs were assessed as high quality trials (a score of five for Hirayama 1989, and a score of three for Chen 1990 and Wang 1995b) and the rest as low quality trials (a score of one).
Effects of interventions
MORTALITY No mortality data were reported.
HEPATOCELLULAR CARCINOMA No data on hepatocellular carcinoma were reported.
ANTIVIRAL RESPONSE Phyllanthus herb Phyllanthus amarus (a single herb) showed a significantly better effect on clearance of serum HBeAg (RR 3.35, 95% CI 1.49 to 7.56) (Analysis 1.2) compared to non‐specific drugs (vitamin plus hypoxanthosine plus orazamide), but did not show a significant effect on clearance of serum HBsAg (Analysis 1.1) and seroconversion of HBeAg to HBeAb (Analysis 1.3) at three months follow‐up after the end of treatment. A compound in which Phyllanthus herb was the principal drug showed no significant difference compared to gamma‐IFN (5 mega unit/two days, for three months) on clearance of serum HBeAg and HBV DNA (Analysis 2.2 and Analysis 2.3).
1.2. Analysis.
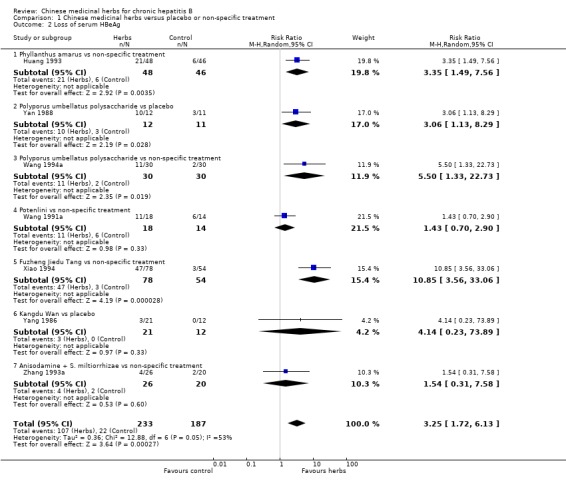
Comparison 1 Chinese medicinal herbs versus placebo or non‐specific treatment, Outcome 2 Loss of serum HBeAg.
1.1. Analysis.
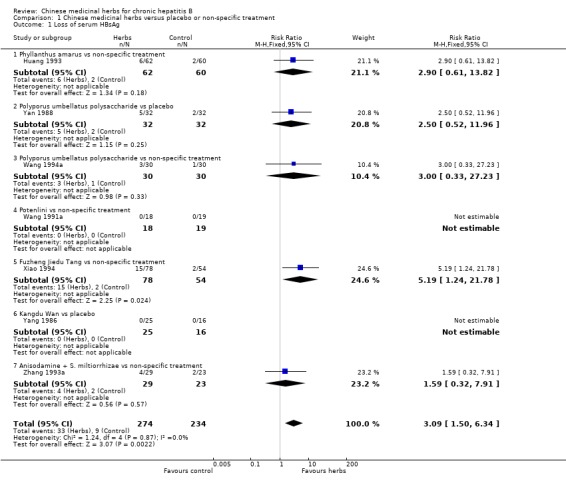
Comparison 1 Chinese medicinal herbs versus placebo or non‐specific treatment, Outcome 1 Loss of serum HBsAg.
1.3. Analysis.
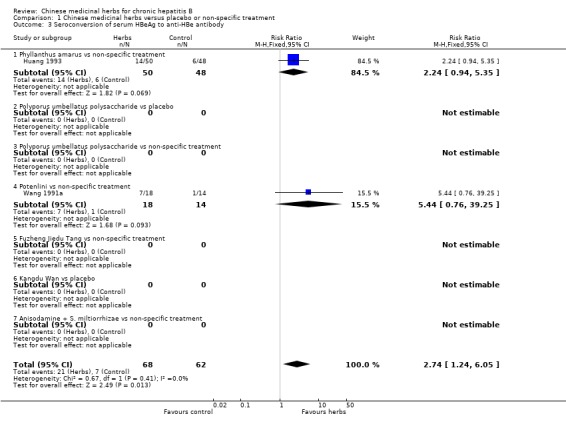
Comparison 1 Chinese medicinal herbs versus placebo or non‐specific treatment, Outcome 3 Seroconversion of serum HBeAg to anti‐HBe antibody.
2.2. Analysis.
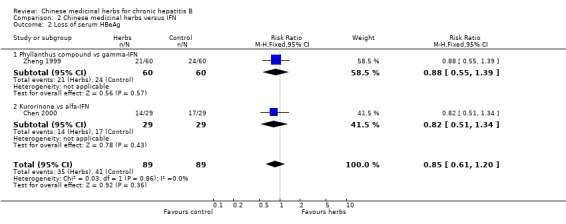
Comparison 2 Chinese medicinal herbs versus IFN, Outcome 2 Loss of serum HBeAg.
2.3. Analysis.
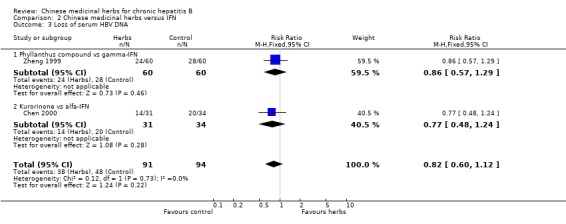
Comparison 2 Chinese medicinal herbs versus IFN, Outcome 3 Loss of serum HBV DNA.
Polyporus umbellatus polysaccharide Polyporus umbellatus polysaccharide (extract of a herb Polyporus) was tested in two trials in the same dosage and treatment duration. Compared to placebo, Polyporus umbellatus polysaccharide showed a positive effect on clearance of serum HBeAg (RR 3.06, 95% CI 1.13 to 8.29) (Analysis 1.2), but did not show a significant effect on clearance of serum HBsAg (Analysis 1.1). Compared to non‐specific treatment (vitamin C plus hypoxanthosine), Polyporus umbellatus polysaccharide showed positive effects on clearance of serum HBeAg (RR 5.50, 95% CI 1.33 to 22.73) (Analysis 1.2) and HBV DNA (RR 4.14, 95% CI 1.00 to 17.19) (Analysis 1.4).
1.4. Analysis.
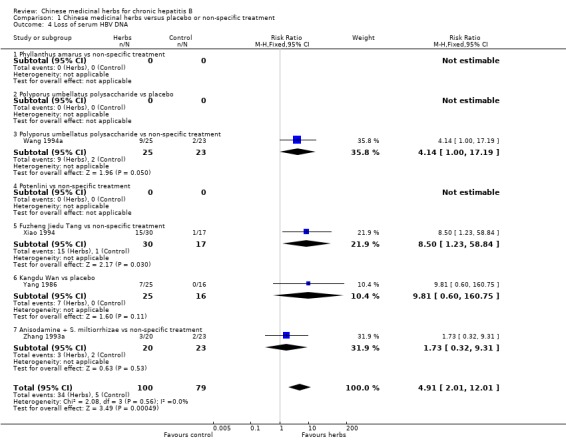
Comparison 1 Chinese medicinal herbs versus placebo or non‐specific treatment, Outcome 4 Loss of serum HBV DNA.
Potenlini Potenlini (a formulation of extracted ingredient of liquorice root plus cysteine and glycine) showed no significant effect on viral response compared to non‐specific treatment (potassium magnesium aspartate plus vitamins) (Analysis 1.1, Analysis 1.2, and Analysis 1.3).
Fuzheng Jiedu Tang Compared to non‐specific treatment (hypoxanthosine plus vitamin), Fuzheng Jiedu Tang (a compound of herbs) showed significant antiviral activity (RR 5.19, 95% CI 1.24 to 21.79) for clearance of serum HBsAg (comparison 01‐01); RR 10.85, 95% CI 3.56 to 33.06 for clearance of serum HBeAg (Analysis 1.2); and RR 8.50, 95% CI 1.23 to 58.85 for clearance of serum HBV DNA (Analysis 1.4).
Kangdu Wan Compared to placebo, Kangdu Wan (a compound of herbs) was not effective in terms of clearance of serum HBsAg, HBeAg and HBV DNA at three months follow‐up after the end of treatment (Analysis 1.1, Analysis 1.2, and Analysis 1.4).
Anisodamine plus Salviae miltiorrhizae Anisodamine (an extract from herb Anisodus tangutica) combined with Salviae miltiorrhizae (extract from herb Salvia miltiorrhiza) showed no significant effect on clearance of serum HBsAg, HBeAg, and HBV DNA when tested against non‐specific treatment (Analysis 1.1, Analysis 1.2, and Analysis 1.4).
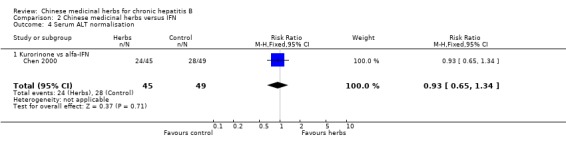
Kurorinone Kurorinone (matrine, extract from Sophora flavescens Ait) showed no significant effects on clearance of serum HBeAg and HBV DNA when tested against alfa‐IFN (Analysis 2.2, Analysis 2.3).
'Worst‐case scenario' analysis Based on numbers lost‐to‐follow‐up reported in four trials, a 'worst‐case scenario' analysis for these outcomes including all patients lost‐to‐follow‐up as treatment failure was performed (Analysis 5.1, Analysis 5.2, Analysis 5.3). Fuzheng Jiedu Tang still kept the effect size and direction. Kurorinone, Potenlini, and Anisodamine plus Salviae miltiorrhizae showed no significant effects on clearance of serum HBeAg and HBV DNA. A 'worst‐worst case' scenario showed no effect from these herbs (P=0.08).
5.1. Analysis.
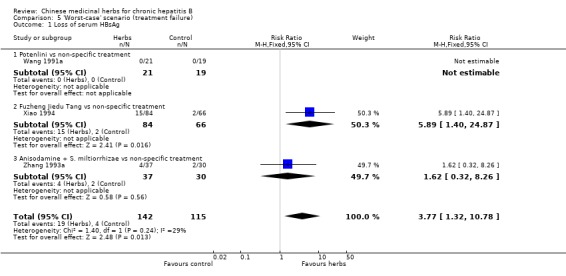
Comparison 5 'Worst‐case' scenario (treatment failure), Outcome 1 Loss of serum HBsAg.
5.2. Analysis.
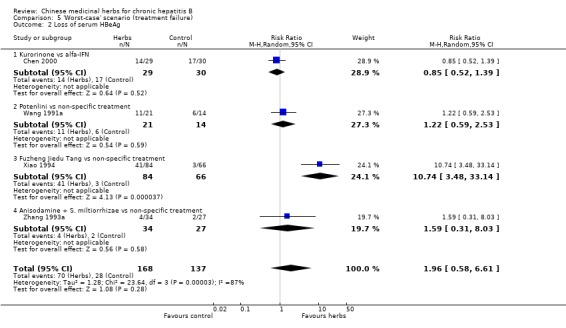
Comparison 5 'Worst‐case' scenario (treatment failure), Outcome 2 Loss of serum HBeAg.
5.3. Analysis.
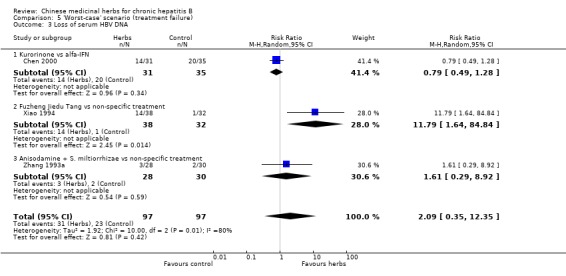
Comparison 5 'Worst‐case' scenario (treatment failure), Outcome 3 Loss of serum HBV DNA.
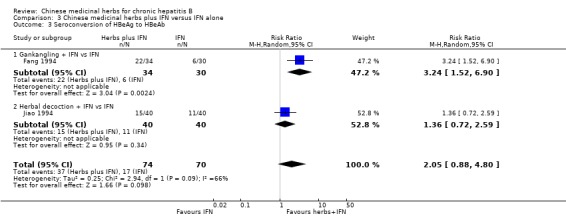
Medicinal herbs plus IFN versus IFN alone Chinese medicinal herbs plus IFN versus IFN alone were compared in eight trials, all with a follow‐up of less than three months. The average dosage of IFN in both arms was 1.84 mega unit/day, and the median duration of treatment was four months. Eight trials (n=628 patients) reported the clearance of serum HBeAg, six (n=466) the clearance of serum HBsAg, and six (n=524) the clearance of serum HBV DNA. Chinese medicinal herbs plus IFN showed a significantly better effect than IFN alone on clearance of serum HBeAg (RR 2.02, 95% CI 1.63 to 2.51), HBsAg (random effects model RR 2.61, 95%CI 1.10 to 6.21), and HBV DNA (RR 1.90, 95% CI 1.50 to 2.41) (Analysis 3.1, Analysis 3.2, Analysis 3.4). No significant difference was shown for seroconversion from HBeAg to HBeAb (two trials, n=144, random effects model RR 2.05, 95% CI 0.88 to 4.80).
3.1. Analysis.
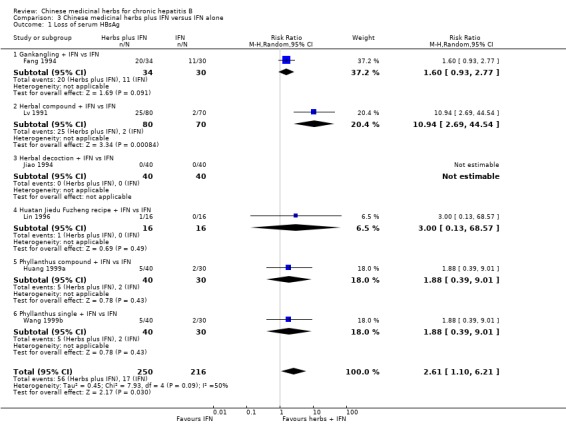
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 1 Loss of serum HBsAg.
3.2. Analysis.
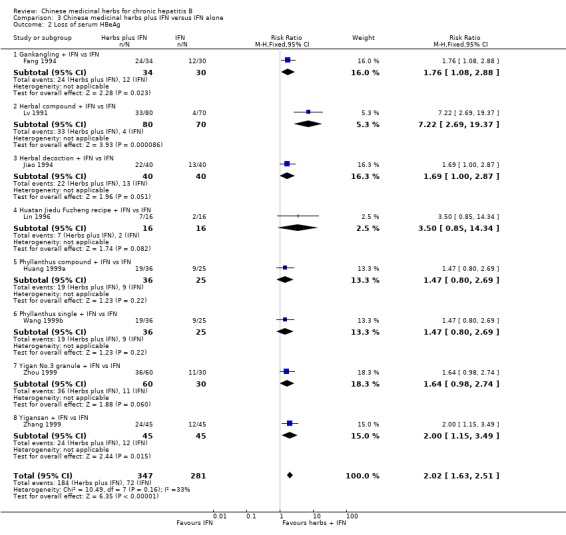
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 2 Loss of serum HBeAg.
3.4. Analysis.
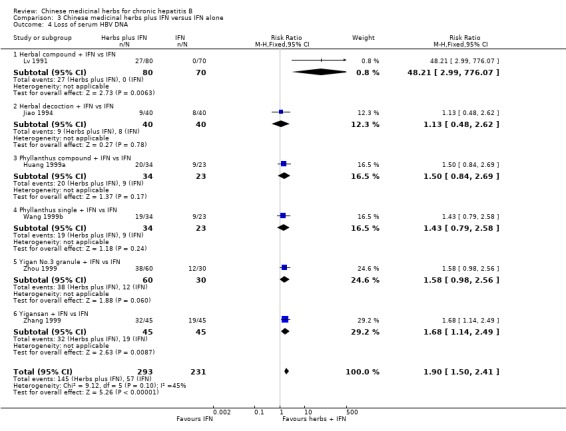
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 4 Loss of serum HBV DNA.
Duration of follow‐up RCTs with less than three months follow‐up after the end of treatment were included in analyses to examine how their findings compared with those from trials that reported a minimum of three months follow‐up after the end of intervention. Most data were available on clearance of serum HBsAg, HBeAg and HBV DNA. Because of the huge heterogeneity, this comparison was limited to trials comparing Chinese medicinal herbs versus placebo or non‐specific treatment (excluding trials comparing herbs versus IFN). The effect size was almost the same comparing the results of trials with shorter follow‐up with the results of trials with longer follow‐up (Analysis 4.2, Analysis 4.4, Analysis 4.8).
4.2. Analysis.
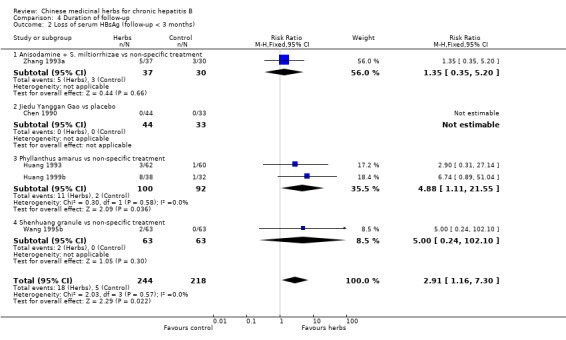
Comparison 4 Duration of follow‐up, Outcome 2 Loss of serum HBsAg (follow‐up < 3 months).
4.4. Analysis.
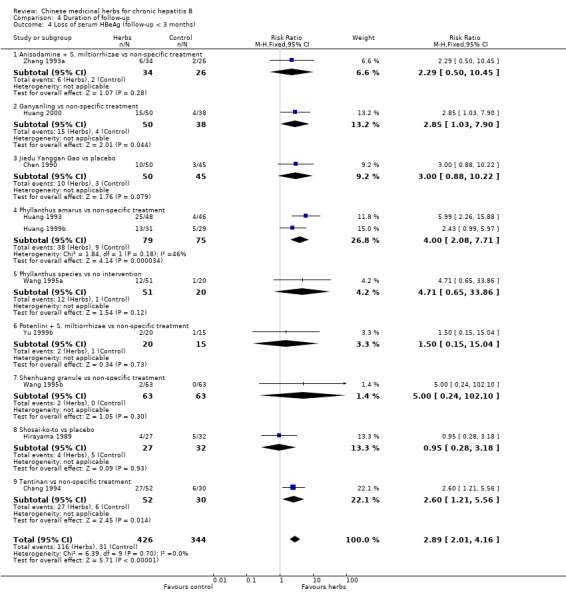
Comparison 4 Duration of follow‐up, Outcome 4 Loss of serum HBeAg (follow‐up < 3 months).
4.8. Analysis.
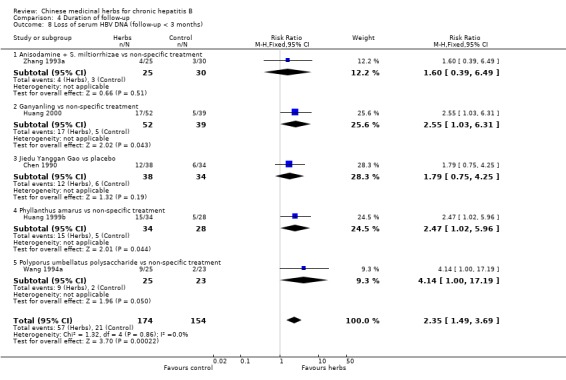
Comparison 4 Duration of follow‐up, Outcome 8 Loss of serum HBV DNA (follow‐up < 3 months).
BIOCHEMICAL RESPONSE Four trials involving 189 patients assessed biochemical response. Only serum ALT normalisation data were available for analysis. In trials with over three months follow‐up, the combined result of Chinese medicinal herbs was better than placebo or non‐specific drugs in reducing elevated serum ALT to normal (RR 1.41, 95% CI 1.14 to 1.75) (Analysis 1.5). Individually, Anisodamine plus Salvia miltiorrhiza was better than non‐specific treatment in normalising ALT (RR 1.29, 95% CI 1.03 to 1.62) (Analysis 1.5).
1.5. Analysis.
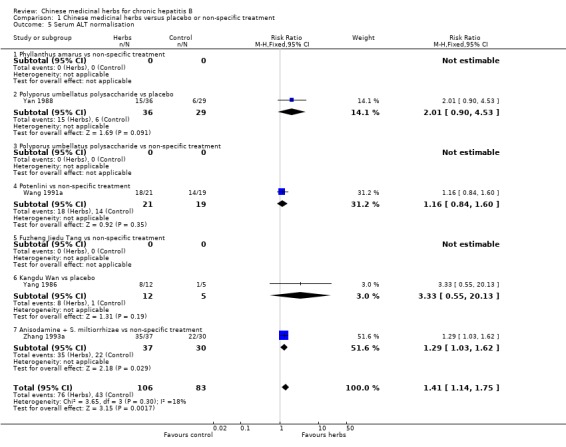
Comparison 1 Chinese medicinal herbs versus placebo or non‐specific treatment, Outcome 5 Serum ALT normalisation.
Chinese medicinal herbs plus IFN showed a better effect of the combination than of IFN treatment alone (for ALT normalisation: n=204, RR 1.66, 95% CI 1.30 to 2.13; for AST normalisation: n=122, RR 1.83, 95% CI 1.31 to 2.55; for serum bilirubin normalisation: n=54, RR 2.15, 95% CI 1.22 to 3.78) in trials with follow‐up less than three months (Analysis 3.5, Analysis 3.6, Analysis 3.7).
3.5. Analysis.
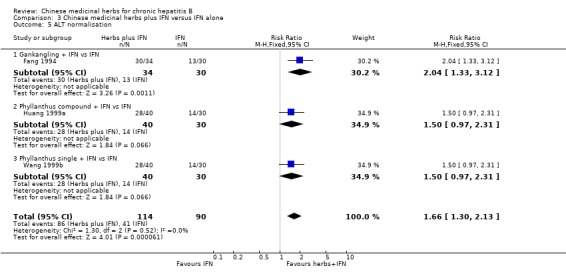
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 5 ALT normalisation.
3.6. Analysis.
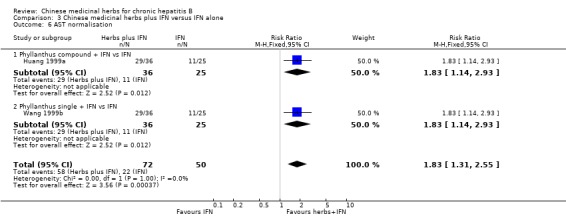
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 6 AST normalisation.
3.7. Analysis.
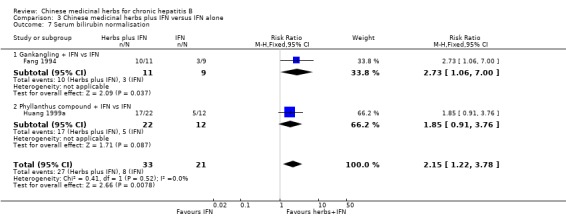
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 7 Serum bilirubin normalisation.
Analysis including trials with less than three months follow‐up (n=606) showed a similar effect on ALT normalisation (random effects model RR 1.72, 95% CI 1.22 to 2.42) (Analysis 4.10).
4.10. Analysis.
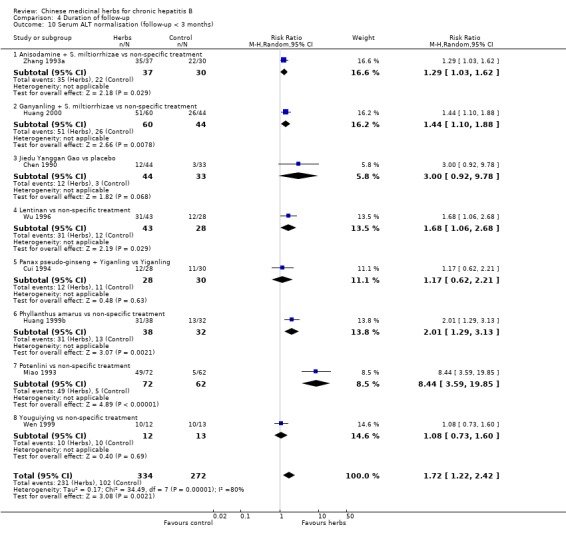
Comparison 4 Duration of follow‐up, Outcome 10 Serum ALT normalisation (follow‐up < 3 months).
LIVER HISTOLOGY One trial (Yan 1988) described liver histological improvement in six patients among 40 using Polyporus umbellatus polysaccharide treatment, but it did not report the result from the control group. Another trial (Zhang 1998), with follow‐up of less than three months, reported outcome of liver histopathology in both the experimental group (20 patients) and control group (18 patients) after three months treatment with glycyrrhisin. They found no statistically significant difference between the two groups in grading of pathological changes (WMD ‐0.55, 95%CI ‐1.29 to 0.19, P=0.15).
SYMPTOMS AND SIGNS Only three of the nine included trials reported improvement of patients' symptoms and signs. One trial (Yan 1988) reported 20/22 patients with improvement in Polyporus umbellatus polysaccharide treatment group, and 8/19 in the placebo group, a statistically significant difference (Chi‐square=9.07, P=0.002). The other trial (Yang 1986) reported improvement of fatigue in 13/16 in Kangdu Wan treatment group, and in 1/11 in the placebo group, a statistically significant difference (Chi‐square=10.19, P=0.01). Similarly, nine patients out of nine with abdominal distention improved in the Kangdu Wan group compared to two of eight in the placebo group, a statistically significant difference (Chi‐square=8.39, P=0.001) (Yang 1986). Eighty per cent of patients treated with Yigan No. 3 granule was reported to have disappearance or improvement of symptoms, but no data were reported in control arm of IFN treatment (Zheng 1999).
QUALITY OF LIFE No trials evaluated quality of life.
ADVERSE EVENTS Adverse events were reported in eight trials. These reports, however, did not indicate whether the effects were ascertained through standardised monitoring or voluntary self‐report. One trial described an occurrence of ascites and lower limb edema in one case with early‐stage liver cirrhosis during treatment with Potenlini (Wang 1991a). In another trial 8/60 patients given Polyporus umbellatus polysaccharide injection developed enlarged groin lymph nodes after one week of treatment and persisted for one more week (Wang 1994a).
A few patients were found to have symptoms of dry throat from the herbal compound formula Kang Du Wan (Yang 1986), and thirst and rapid heart rate from Anisodamine and Salviae miltiorrhizae (Zhang 1993a).
No serious adverse events were reported.
FUNNEL PLOT The outcome measure loss of serum HBeAg in the nine included trials was used for a funnel plot analysis. A linear regression analysis, in which the standard normal deviate, defined as the RR divided by its standard error, was regressed against the estimate's precision, defined as the inverse of the standard error, showed a significant funnel plot asymmetry (intercept 3.37, standard error 1.40; t=2.40; P=0.047).
Discussion
The present systematic review suggests that some Chinese medicinal herbs may have positive effects on the clearance of HBV markers and on normalisation of liver biochemistry in patients with chronic hepatitis B. However, at present there is no strong evidence to recommend any of these medicinal herbs for treatment of chronic hepatitis B due to the significant funnel plot asymmetry as well as the general low methodological quality of the trials.
However, it seems that Fuzheng Jiedu Tang (a compound of more than 10 herbs such as raw Astragalus membranaceus, scorched large head Atractylodes rhizome, Tangshen, Rhizoma polygoni cuspidati, Salviae miltiorrhizae, and Medlar) works on clearance of serum HBsAg, HBeAg and HBV DNA; Polyporus umbellatus polysaccharide works on clearance of serum HBeAg and HBV DNA; and Phyllanthus amarus works on clearance of serum HBeAg. The antiviral effect of Phyllanthus compound and kurorinone appears to be the same as IFN in terms of clearance of serum HBeAg and HBV DNA. These positive effects are supported by eight RCTs with less than three months of follow‐up after the end of the interventions examining the combination of Chinese medicinal herbs plus IFN versus IFN alone. These findings must be interpreted conservatively due to the following facts:
Methodological quality The majority of the RCTs included in this review had poor methodological quality. They provided only limited descriptions of study design, randomisation, and allocation concealment. Most trials state only that random assignment was used, but do not give sufficient information to allow a judgment of whether or not it was conducted properly. Methodologically less rigorous trials show larger differences between experimental and control groups than do those conducted with better rigor (Schulz 1995; Moher 1998; Kjaergard 1999). The insufficient number of trials prohibited us from performing meaningful sensitivity analysis to illuminate how robust the results of the review are to the exclusion of the trials with inadequate methodology. No multi‐centre, large scale RCTs were identified.
Funnel plot asymmetry An asymmetry in a funnel plot can originate from several sources such as publication bias, location bias, heterogeneity of trials, chance, and poor methodological design of small trials (Egger 1997). As our literature searches covered both electronic databases and handsearches, English language and any other languages, published and unpublished studies, location bias is not considered as a likely explanation of the funnel plot asymmetry in this review. Vickers and colleagues (Vickers 1998) found that some countries including China publish unusually high proportions of positive results; publication bias being a possible explanation. All of the included trials in this review were conducted in China and eight out of the nine trials were published in Chinese (the remaining one in English). The presence of publication bias might explain the funnel plot asymmetry. Although we undertook extensive searches for unpublished material, few trials of the identified qualified for inclusion, but at the same time we cannot disregard the fact that trials with negative findings remain unpublished. Secondly, the heterogeneity of medicinal herbs and control interventions may contribute to the asymmetry. Thirdly, the general low methodological quality of the trials and small trials may also contribute to the asymmetry.
'Worst‐case' scenario From reports of four trials on loss‐to‐follow‐up patients, the effect size of Anisodamine plus Salviae miltiorrhizae, Fuzheng Jiedu Tang, kurorinone, and Potenlini did not change significantly by counting those lost as treatment failure. But a 'worst‐worst case' scenario, performed by counting those lost in the treatment group as treatment failures and those in the control group as responders, made the beneficial effect disappear.
Five trials used non‐specific treatment as control intervention. A search of the Cochrane Library and PubMed did not provide any evidence on efficacy of these non‐specific drugs for chronic hepatitis B. On the other hand, we are not able to exclude the possibility that these non‐specific drugs may have had a negative effect on the course of the HBV infection. This could lead to an inflated positive effect of Chinese medicinal herbs. However, Chinese medicinal herbs versus placebo showed similar effects as Chinese medicinal herbs versus non‐specific interventions.
Surrogate outcomes The long‐term goal of treatment for chronic hepatitis B is to prevent progression to liver cirrhosis and/or hepatocellular carcinoma and ultimately to prolong survival. Available outcomes from the included trials are mainly laboratory tests, i.e., surrogate outcomes. There is lack of data from RCTs on clinically relevant outcomes from long‐term follow‐up such as mortality, incidence of liver cirrhosis or cancer and, therefore, we are not able to draw conclusions about these important outcomes.
Liver biopsy diagnosis Only three trials had the clinical diagnosis of chronic hepatitis B confirmed by liver biopsy. Therefore, we were unable to obtain direct evidence on effects of the Chinese medicinal herbs on liver pathology.
Nevertheless, Chinese medicinal herbs have been used for treating chronic liver diseases for more than 2000 years and are still being widely used for chronic hepatitis B in China. It is estimated that more than 300 Chinese herbal medicines have been officially registered and approved for use in treatment of viral hepatitis in China during the past two decades (CMH 1999). Every year there are at least dozens of clinical trials testing Chinese medicinal herbs for viral hepatitis published in Chinese medical literature and most of them reported a positive effect (Liu 1998). In this review some Chinese medicinal herbs seem to have an effect on antiviral activity and decrease hepatic inflammatory activity. Two double blind RCTs demonstrate an effect of Chinese medicinal herbs on improvement of symptoms and signs. The suggested benefit of some Chinese medicinal herbs combined with IFN for treatment of hepatitis B should also encourage further trials to verify this efficacy. As IFN treatment even in combination with nucleoside analogues is unsatisfactory, the long‐term therapy for persistent HBV infections leads to the risk of cumulative toxicity and development of viral resistence. Therefore, it is necessary to explore which drug combinations or sequences of drug combinations are likely to be most useful (Fontana 1997; Shaw 2000). The combination of 'western' conventional therapy with 'eastern' classical herbal therapy may reveal itself as an attractive option.
A definite conclusion on adverse events associated with Chinese medicinal herbs cannot be drawn from this review due to the limited number of trials identified, the limited duration of treatment and follow‐up, and inadequate recording and reporting of adverse events. In China, only about 50% of clinical trials of Chinese medicinal herbs report adverse effects, and the reason for the insufficient report is possibly that Chinese practitioners perceive Chinese medicinal herbs as free of side effects (Liu 1999b). However, there are reports of liver toxicity and other serious adverse events associated with using Chinese herbal medicines (Ishizaki 1996; Melchart 1999; Gottieb 2000; Tomlinson 2000). Among them, Sho‐saiko‐to (Xiao Chai Hu Tang), a widely used Chinese herbal compound for chronic liver diseases, was found to be associated with interstitial pneumonitis when used either alone or combined with IFN in Japan (Ishizaki 1996). For this reason, Sho‐saiko‐to is no longer allowed to be used in Japan (Hirayama 2000). Safety of complementary medicines needs to be monitored (Vautier 1995). In clinical trials efficacy and safety should receive equal attention and occasional and severe adverse events identified from large scale epidemiological studies need to be investigated.
Authors' conclusions
Implications for practice.
Based on this systematic review, it appears that some Chinese medicinal herbs may have positive effects on viral activity and liver biochemistry in chronic hepatitis B. However, due to the low methodological quality of the RCTs and the risk of publication bias, there is no strong evidence for treating chronic hepatitis B with Chinese medicinal herbs.
Implications for research.
The methodological quality of clinical trials of treatment with Chinese medicinal herbs for chronic hepatitis B needs to be improved (Liu 1999). The following aspects should be attended: (i) detailed reporting of the generation of allocation sequence and the allocation concealment; (ii) application of blinding and placebo control; (iii) clear description of withdrawal/dropout during the trial; (iv) reporting of clinically important outcome measures from long‐term follow‐up.
Rigorously designed, multicentre, large scale RCTs are required to evaluate Chinese medicinal herbs versus placebo. From the results of this systematic review it seems worthwhile to evaluate these interventions in patients treated with IFN, and possibly with nucleoside analogues like lamivudine. The outcome measures should include virological and biochemical outcomes as well as liver pathology, such as hepatic fibrosis, cirrhosis and/or liver cancer, quality of life, and mortality. Adverse events should be critically assessed by standardised monitoring or an effective self‐report system. Attention should also be paid to long‐term adverse events of Chinese medicinal herbs.
What's new
| Date | Event | Description |
|---|---|---|
| 9 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 30 June 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Klaus Linde for his advice and constructive comments on our protocol. We also appreciate the helpful comments and suggestions from Chris Silagy, Elmer Villanueva, Sally Green, and Steve McDonald. We are grateful to Christian Gluud for expert suggestions and corrections and to Lise Lotte Kjaergard and Dimitrinka Nikolova for their helpful advice and assistance. We thank Chisato Hirayama, who kindly gave us further information on their trial.
Data and analyses
Comparison 1. Chinese medicinal herbs versus placebo or non‐specific treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg | 7 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.50, 6.34] |
| 1.1 Phyllanthus amarus vs non‐specific treatment | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.61, 13.82] |
| 1.2 Polyporus umbellatus polysaccharide vs placebo | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.52, 11.96] |
| 1.3 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 1.4 Potenlini vs non‐specific treatment | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.19 [1.24, 21.78] |
| 1.6 Kangdu Wan vs placebo | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.32, 7.91] |
| 2 Loss of serum HBeAg | 7 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [1.72, 6.13] |
| 2.1 Phyllanthus amarus vs non‐specific treatment | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [1.49, 7.56] |
| 2.2 Polyporus umbellatus polysaccharide vs placebo | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [1.13, 8.29] |
| 2.3 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 5.5 [1.33, 22.73] |
| 2.4 Potenlini vs non‐specific treatment | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.70, 2.90] |
| 2.5 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 10.85 [3.56, 33.06] |
| 2.6 Kangdu Wan vs placebo | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [0.23, 73.89] |
| 2.7 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.31, 7.58] |
| 3 Seroconversion of serum HBeAg to anti‐HBe antibody | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.24, 6.05] |
| 3.1 Phyllanthus amarus vs non‐specific treatment | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.94, 5.35] |
| 3.2 Polyporus umbellatus polysaccharide vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Polyporus umbellatus polysaccharide vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Potenlini vs non‐specific treatment | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.44 [0.76, 39.25] |
| 3.5 Fuzheng Jiedu Tang vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Kangdu Wan vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Loss of serum HBV DNA | 4 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [2.01, 12.01] |
| 4.1 Phyllanthus amarus vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Polyporus umbellatus polysaccharide vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.00, 17.19] |
| 4.4 Potenlini vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.5 [1.23, 58.84] |
| 4.6 Kangdu Wan vs placebo | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.81 [0.60, 160.75] |
| 4.7 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.32, 9.31] |
| 5 Serum ALT normalisation | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.14, 1.75] |
| 5.1 Phyllanthus amarus vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Polyporus umbellatus polysaccharide vs placebo | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.90, 4.53] |
| 5.3 Polyporus umbellatus polysaccharide vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Potenlini vs non‐specific treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 5.5 Fuzheng Jiedu Tang vs non‐specific treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Kangdu Wan vs placebo | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.55, 20.13] |
| 5.7 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.03, 1.62] |
Comparison 2. Chinese medicinal herbs versus IFN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Phyllanthus compound vs gamma‐IFN | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Kurorinone vs alfa‐IFN | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Loss of serum HBeAg | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.20] |
| 2.1 Phyllanthus compound vs gamma‐IFN | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.39] |
| 2.2 Kurorinone vs alfa‐IFN | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.51, 1.34] |
| 3 Loss of serum HBV DNA | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.12] |
| 3.1 Phyllanthus compound vs gamma‐IFN | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.29] |
| 3.2 Kurorinone vs alfa‐IFN | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.48, 1.24] |
| 4 Serum ALT normalisation | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.34] |
| 4.1 Kurorinone vs alfa‐IFN | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.34] |
2.1. Analysis.
Comparison 2 Chinese medicinal herbs versus IFN, Outcome 1 Loss of serum HBsAg.
2.4. Analysis.
Comparison 2 Chinese medicinal herbs versus IFN, Outcome 4 Serum ALT normalisation.
Comparison 3. Chinese medicinal herbs plus IFN versus IFN alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg | 6 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.10, 6.21] |
| 1.1 Gankangling + IFN vs IFN | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.93, 2.77] |
| 1.2 Herbal compound + IFN vs IFN | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 10.94 [2.69, 44.54] |
| 1.3 Herbal decoction + IFN vs IFN | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Huatan Jiedu Fuzheng recipe + IFN vs IFN | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.57] |
| 1.5 Phyllanthus compound + IFN vs IFN | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.39, 9.01] |
| 1.6 Phyllanthus single + IFN vs IFN | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.39, 9.01] |
| 2 Loss of serum HBeAg | 8 | 628 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.63, 2.51] |
| 2.1 Gankangling + IFN vs IFN | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.08, 2.88] |
| 2.2 Herbal compound + IFN vs IFN | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.22 [2.69, 19.37] |
| 2.3 Herbal decoction + IFN vs IFN | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.00, 2.87] |
| 2.4 Huatan Jiedu Fuzheng recipe + IFN vs IFN | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.85, 14.34] |
| 2.5 Phyllanthus compound + IFN vs IFN | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.69] |
| 2.6 Phyllanthus single + IFN vs IFN | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.69] |
| 2.7 Yigan No.3 granule + IFN vs IFN | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.98, 2.74] |
| 2.8 Yigansan + IFN vs IFN | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.15, 3.49] |
| 3 Seroconversion of HBeAg to HBeAb | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.88, 4.80] |
| 3.1 Gankangling + IFN vs IFN | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.52, 6.90] |
| 3.2 Herbal decoction + IFN vs IFN | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.72, 2.59] |
| 4 Loss of serum HBV DNA | 6 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.50, 2.41] |
| 4.1 Herbal compound + IFN vs IFN | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 48.21 [2.99, 776.07] |
| 4.2 Herbal decoction + IFN vs IFN | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.48, 2.62] |
| 4.3 Phyllanthus compound + IFN vs IFN | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.84, 2.69] |
| 4.4 Phyllanthus single + IFN vs IFN | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.79, 2.58] |
| 4.5 Yigan No.3 granule + IFN vs IFN | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.98, 2.56] |
| 4.6 Yigansan + IFN vs IFN | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.14, 2.49] |
| 5 ALT normalisation | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.30, 2.13] |
| 5.1 Gankangling + IFN vs IFN | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.33, 3.12] |
| 5.2 Phyllanthus compound + IFN vs IFN | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.97, 2.31] |
| 5.3 Phyllanthus single + IFN vs IFN | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.97, 2.31] |
| 6 AST normalisation | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.31, 2.55] |
| 6.1 Phyllanthus compound + IFN vs IFN | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.14, 2.93] |
| 6.2 Phyllanthus single + IFN vs IFN | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.14, 2.93] |
| 7 Serum bilirubin normalisation | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.22, 3.78] |
| 7.1 Gankangling + IFN vs IFN | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.06, 7.00] |
| 7.2 Phyllanthus compound + IFN vs IFN | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.91, 3.76] |
3.3. Analysis.
Comparison 3 Chinese medicinal herbs plus IFN versus IFN alone, Outcome 3 Seroconversion of HBeAg to HBeAb.
Comparison 4. Duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg (follow‐up >/= 3 months) | 7 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.50, 6.34] |
| 1.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.32, 7.91] |
| 1.2 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.19 [1.24, 21.78] |
| 1.3 Kangdu Wan vs placebo | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Phyllanthus amarus vs non‐specific treatment | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.61, 13.82] |
| 1.5 Polyporus umbellatus polysaccharide vs placebo | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.52, 11.96] |
| 1.6 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 1.7 Potenlini vs non‐specific treatment | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Loss of serum HBsAg (follow‐up < 3 months) | 5 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [1.16, 7.30] |
| 2.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.35, 5.20] |
| 2.2 Jiedu Yanggan Gao vs placebo | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Phyllanthus amarus vs non‐specific treatment | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [1.11, 21.55] |
| 2.4 Shenhuang granule vs non‐specific treatment | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.10] |
| 3 Loss of serum HBeAg (follow‐up >/= 3 months) | 7 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [1.72, 6.13] |
| 3.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.31, 7.58] |
| 3.2 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 10.85 [3.56, 33.06] |
| 3.3 Kangdu Wan vs placebo | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [0.23, 73.89] |
| 3.4 Phyllanthus amarus vs non‐specific treatment | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [1.49, 7.56] |
| 3.5 Polyporus umbellatus polysaccharide vs placebo | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [1.13, 8.29] |
| 3.6 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 5.5 [1.33, 22.73] |
| 3.7 Potenlini vs non‐specific treatment | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.70, 2.90] |
| 4 Loss of serum HBeAg (follow‐up < 3 months) | 10 | 770 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [2.01, 4.16] |
| 4.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.50, 10.45] |
| 4.2 Ganyanling vs non‐specific treatment | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.03, 7.90] |
| 4.3 Jiedu Yanggan Gao vs placebo | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.88, 10.22] |
| 4.4 Phyllanthus amarus vs non‐specific treatment | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [2.08, 7.71] |
| 4.5 Phyllanthus species vs no intervention | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.71 [0.65, 33.86] |
| 4.6 Potenlini + S. miltiorrhizae vs non‐specific treatment | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.15, 15.04] |
| 4.7 Shenhuang granule vs non‐specific treatment | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.10] |
| 4.8 Shosai‐ko‐to vs placebo | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.18] |
| 4.9 Tentinan vs non‐specific treatment | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.21, 5.56] |
| 5 Seroconversion of serum HBeAg to HBeAb (follow‐up >/= 3 months) | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.24, 6.05] |
| 5.1 Phyllanthus amarus vs non‐specific treatment | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.94, 5.35] |
| 5.2 Potenlini vs non‐specific treatment | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.44 [0.76, 39.25] |
| 6 Seroconversion of serum HBeAg to HBeAb (follow‐up < 3 months) | 5 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.52, 5.54] |
| 6.1 Phyllanthus amarus vs non‐specific treatment | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [0.90, 7.72] |
| 6.2 Phyllanthus species vs no intervention | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.02 [1.02, 48.35] |
| 6.3 Potenlini + S. miltiorrhizae vs non‐specific treatment | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.15, 15.04] |
| 6.4 Shosai‐ko‐to vs placebo | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.34, 10.57] |
| 6.5 Tentinan vs non‐specific treatment | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.77, 8.07] |
| 7 Loss of serum HBV DNA (follow‐up >/= 3 months) | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.16 [2.59, 14.69] |
| 7.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.32, 9.31] |
| 7.2 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.5 [1.23, 58.84] |
| 7.3 Kangdu Wan vs placebo | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.81 [0.60, 160.75] |
| 7.4 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [1.98, 29.70] |
| 8 Loss of serum HBV DNA (follow‐up < 3 months) | 5 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.49, 3.69] |
| 8.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.39, 6.49] |
| 8.2 Ganyanling vs non‐specific treatment | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.03, 6.31] |
| 8.3 Jiedu Yanggan Gao vs placebo | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.75, 4.25] |
| 8.4 Phyllanthus amarus vs non‐specific treatment | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.02, 5.96] |
| 8.5 Polyporus umbellatus polysaccharide vs non‐specific treatment | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.00, 17.19] |
| 9 Serum ALT normalisation (follow‐up >/= 3 months) | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.08, 2.22] |
| 9.1 Kangdu Wan vs placebo | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.55, 20.13] |
| 9.2 Polyporus umbellatus polysaccharide vs placebo | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.90, 4.53] |
| 9.3 Potenlini vs non‐specific treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 10 Serum ALT normalisation (follow‐up < 3 months) | 8 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.22, 2.42] |
| 10.1 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.03, 1.62] |
| 10.2 Ganyanling + S. miltiorrhizae vs non‐specific treatment | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.10, 1.88] |
| 10.3 Jiedu Yanggan Gao vs placebo | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.92, 9.78] |
| 10.4 Lentinan vs non‐specific treatment | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.06, 2.68] |
| 10.5 Panax pseudo‐ginseng + Yiganling vs Yiganling | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.62, 2.21] |
| 10.6 Phyllanthus amarus vs non‐specific treatment | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.29, 3.13] |
| 10.7 Potenlini vs non‐specific treatment | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [3.59, 19.85] |
| 10.8 Youguiying vs non‐specific treatment | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.60] |
4.1. Analysis.
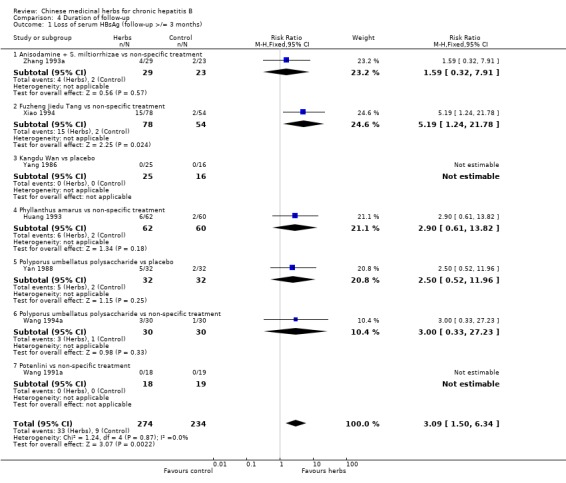
Comparison 4 Duration of follow‐up, Outcome 1 Loss of serum HBsAg (follow‐up >/= 3 months).
4.3. Analysis.
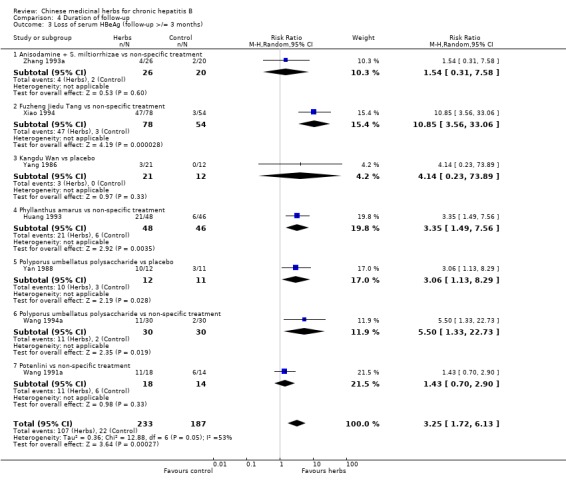
Comparison 4 Duration of follow‐up, Outcome 3 Loss of serum HBeAg (follow‐up >/= 3 months).
4.5. Analysis.
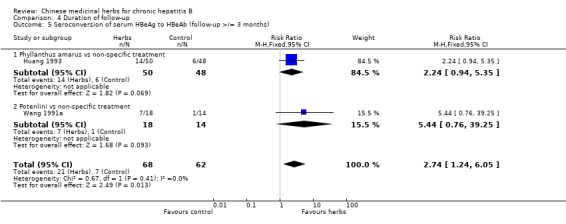
Comparison 4 Duration of follow‐up, Outcome 5 Seroconversion of serum HBeAg to HBeAb (follow‐up >/= 3 months).
4.6. Analysis.
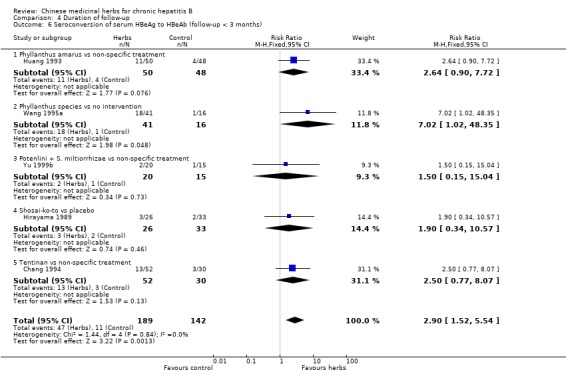
Comparison 4 Duration of follow‐up, Outcome 6 Seroconversion of serum HBeAg to HBeAb (follow‐up < 3 months).
4.7. Analysis.
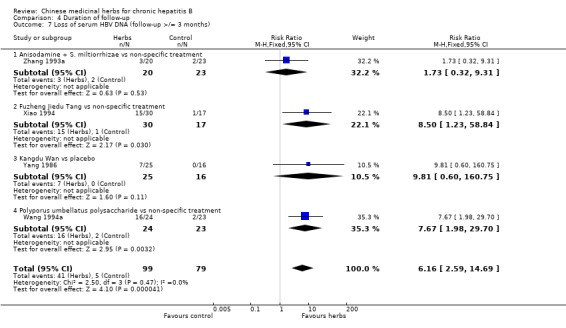
Comparison 4 Duration of follow‐up, Outcome 7 Loss of serum HBV DNA (follow‐up >/= 3 months).
4.9. Analysis.
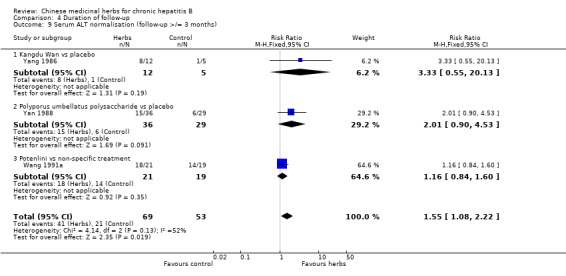
Comparison 4 Duration of follow‐up, Outcome 9 Serum ALT normalisation (follow‐up >/= 3 months).
Comparison 5. 'Worst‐case' scenario (treatment failure).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of serum HBsAg | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.77 [1.32, 10.78] |
| 1.1 Potenlini vs non‐specific treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [1.40, 24.87] |
| 1.3 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.32, 8.26] |
| 2 Loss of serum HBeAg | 4 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.58, 6.61] |
| 2.1 Kurorinone vs alfa‐IFN | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.39] |
| 2.2 Potenlini vs non‐specific treatment | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.53] |
| 2.3 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 10.74 [3.48, 33.14] |
| 2.4 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.31, 8.03] |
| 3 Loss of serum HBV DNA | 3 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.35, 12.35] |
| 3.1 Kurorinone vs alfa‐IFN | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.49, 1.28] |
| 3.2 Fuzheng Jiedu Tang vs non‐specific treatment | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 11.79 [1.64, 84.84] |
| 3.3 Anisodamine + S. miltiorrhizae vs non‐specific treatment | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.29, 8.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chang 1994.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen 1990.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chen 2000.
| Methods | Randomised clinical trial. Generation of allocation sequence: computer‐generated program. Allocation concealment: no information. Blinding: no blinding. Loss to follow‐up: one patient of control group failed to be followed after six months follow‐up. Jadad score=2. | |
| Participants | Ethnic: Chinese. 94 patients (66 males and 28 females; age range from 18 to 50 yrs; 45 in kurorinone group and 49 in IFN control group). Setting: inpatients. Inclusion criteria: chronic hepatitis B, age between 18 and 50 yrs, with serum positivity for HBsAg, HBeAg and/or HBV DNA, and elevated ALT levels for at least six months prior to inclusion. Exclusion criteria: patients with decompensated liver disease, a history of alcohol consumption > 80 g/day, a seropositive test for hepatitis C virus or hepatitis D virus; other causes of liver disease; chronic renal failure; malignancy, and pregnancy, as well as no previous immunosuppressive or antiviral therapy. | |
| Interventions | Experimental: kurorinone (matrine extracted from Sophora flavescens Ait), 400 mg, i.m, daily, for three months. Control: IFN alfa2a, 3 mega unit (MU), subcutaneous injection, daily, for one month; then every other day for two months. | |
| Outcomes | Liver function, serum HBeAg, HBV DNA, blood count, and adverse effects. Maximum follow‐up: 12 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cui 1994.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fang 1994.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hirayama 1989.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Huang 1993.
| Methods | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Loss to follow‐up: none. Jadad score=2. | |
| Participants | Ethnic: Chinese. 122 patients, no data on sex and age. Setting: unstated. Inclusion criteria: chronic hepatitis B with HBsAg positive for more than six months, diagnosed according to the criteria of the National Conference on Viral Hepatitis (CMA 1990). Exclusion criteria: unstated. | |
| Interventions | Experimental: Phyllanthus amarus (single herb) syrup, 20 ml (5 g) orally, t.i.d, for 30 days. Control: Vitamins, plus hypoxanthosine and alcamin, for 30 days; but no data on dosage and route. | |
| Outcomes | HBV markers, liver function and adverse effects. Follow‐up: six months. | |
| Notes | Sent letter (7/6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Huang 1999a.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Huang 1999b.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Huang 2000.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jiao 1994.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Li 1998.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lin 1996.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lv 1991.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miao 1993.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miao 1994.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1991a.
| Methods | Randomised clinical trial. Generation of allocation sequence: no information. Allocation concealment: no information. Blinding: no blinding. Loss to follow‐up: three cases in Potenlini group, none in control group. Jadad score=1. | |
| Participants | Ethnic: Chinese. 40 patients (19 males and two females, average age 33 yrs in Potenlini group; 19 males, average age 35 yrs in control group). Setting: inpatients. Inclusion criteria: chronic active hepatitis with HBsAg positive and ALT abnormal for at least six months, complicated with or without cirrhosis, no secondary infection, no use of steroids or Potenlini within six months. All diagnosed by liver biopsies. Exclusion criteria: unstated. | |
| Interventions | Experimental: Parenterol infusion of Potenlini (extract of herb), 80˜100 ml in 500 ml of 5% glucose, daily, during the first month; during the second and third months, the infusion was given every other day; during the fourth and fifth months, the infusion was given twice a week and during the sixth month, once a week. Control: Parenterol infusion of potassium magnesium aspartate, 20 ml in 500 ml 5% glucose, daily, for four weeks; then changed to vitamin compounds, orally, till six months. No data on dosage. | |
| Outcomes | Viral responses: HBeAg, HBeAb; Biochemical responses: ALT, serum bilirubin. Adverse effects. Outcomes assessed at six months. Follow‐up: six months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1994a.
| Methods | Randomised clinical trial. Generation of allocation sequence: no report. Allocation concealment: no information. Blinding: no blinding. Withdrawal/drop‐out: unstated. Jadad score=1. | |
| Participants | Ethnic: Chinese. 120 patients (group A, 18 males and 12 females, average age 33 yrs; group B, 17 males and 13 females, average age 29 yrs; group C, 20 males and 10 females, average age 30 yrs; group D, 17 males and 13 females, average age 31 yrs). Setting: outpatients. Inclusion criteria: chronic persistent hepatitis with positive HBV markers. Exclusion criteria: unstated. | |
| Interventions | Experimental: group A: Polyporus umbellatus polysaccharide, 40 mg, i.m., daily, 20 days, stop 10 days, repeated for 90 days. group B: Buqi Jiedu Tang, a compound of herbs, one dose, daily, orally, for 90 days. group C: Polyporus umbellatus polysaccharide plus Buqi Jiedu Tang, administered as the same as group A and B. Control: group D: vitamin C plus hypoxanthosine, orally, for 90 days. No data for dosage. | |
| Outcomes | Viral responses: HBsAg, HBeAg, anti‐HBeAb, HBV DNA. Biochemical responses: ALT. Adverse effects. Outcomes assessed at the follow‐up of three months. Follow up: three months. | |
| Notes | Sent letter (7/6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1995a.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1995b.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1999b.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Wen 1999.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wu 1996.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Xiao 1994.
| Methods | Randomised clinical trial. Generation of allocation sequence: no report. Allocation concealment: no information. Blinding: unblinded. Loss to follow‐up: yes, 11 cases in group A, six cases in group B, 12 cases in group C. Jadad score=2. | |
| Participants | Ethnic: Chinese. 252 patients (208 males and 44 females, average age 30 yrs; 102 in group A, 84 in group B, 66 in control group). Setting: unstated. Inclusion criteria: criteria set by the National Conference on Viral Hepatitis (CMA 1990). Exclusion criteria: unstated. | |
| Interventions | Experimental: Group A: Fuzheng Jiedu Tang (compound of herbs), one dose, daily, orally, for six months; plus Hepatitis B vaccine, 30 ug, i.m., once two weeks, for four months. Group B: Fuzheng Jiedu Tang, administered as above. Control: Group C: hypoxanthosine, plus vitamin, orally, for six months. No data on dosage. As group A received co‐intervention (hepatitis B vaccination), data of group A were not used in this review. | |
| Outcomes | Viral responses: HBsAg, HBeAg, HBV DNA, anti‐HBc IgM. Liver function. Adverse effects. Follow up: six months. | |
| Notes | The distribution of patients in the three arms of the trial showed a ratio of 1.5:1.3:1.0, and the trial report did not explain how this happened. We have sent letter requesting an explanation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yan 1988.
| Methods | Randomised double‐blind paired trial. Generation of allocation sequence: unspecified. Allocation concealment: described as adequate. Blinding: provider and patients. Loss of follow‐up: none. Jadad score=3. | |
| Participants | Ethnic: Chinese. 80 patients(54 males and 26 females; age: 17‐50 yrs). Setting:inpatients. Inclusion criteria: chronic hepatitis with HBsAg positive, diagnosis confirmed by liver biopsy; and no immune regulatory drugs were used for one year prior to the trial. Exclusion during trial: unstated. | |
| Interventions | Experimental: Polyporus umbellatus polysaccharide (extract of herb) injection, 40 mg, i.m., daily, over 20 days, rest for 10 days, repeating for three months. Control: Placebo (1/80 compound Salviae miltiorrhizae), with the same appearance, usage, dosage and treatment duration as the intervention drug. | |
| Outcomes | Symptoms and signs. Viral responses: HBsAg, HBeAg. ALT. Histology of liver. Adverse effects. Follow‐up: three months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yang 1986.
| Methods | Randomised double‐blind trial. Generation of allocation sequence: no data. Allocation concealment: no information. Blinding: provider and patients. Loss to follow‐up: none. Jadad score=2. | |
| Participants | Ethnic: Chinese. 41 patients (25 in treatment group,16 males and nine females, average age 34 yrs, range 21˜58 yrs; 16 in control group, 12 males and four females, average age:34 yrs, range 22˜54). Setting: unstated. Inclusion criteria: chronic hepatitis B, diagnosed according to the criteria of the National Conference on Viral Hepatitis (CMA 1983). Exclusion criteria: unstated. | |
| Interventions | Experimental: Kangdu Wan ( a compound of herbs): 4˜6 pills, daily, orally, for three to four months. Control: Placebo (Qi Pi Wan, made from two edible vegetables), 2˜4 pills, daily, orally, for three to four months. | |
| Outcomes | Symptoms and signs.Viral response: HBsAg, HBeAg, HBeAb, HBV DNA, DNA‐P. Liver function. Adverse effects. Follow‐up: three, six months. | |
| Notes | Sent letter (7/6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yu 1999b.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang 1993a.
| Methods | Randomised trial. Generation of allocation sequence and allocation concealment: no details. Blinding: unblinded. Loss to follow‐up: yes, eight patients in treatment group, seven in control group. Jadad score=1. | |
| Participants | Ethnic: Chinese. 67 patients (37 in treatment group, average age 33 yrs, range 14˜57 yrs; 30 in control group, average age 35 yrs, range 12˜63yrs). Setting: inpatients. Inclusion criteria: chronic active hepatitis B, diagnosed according to the criteria set by the National Conference on Viral Hepatitis (CMA 1983); the diagnosis confirmed by liver biopsy. Exclusion criteria: unstated. | |
| Interventions | Experimental: Anisodamine (a herbal extract), 20 ml + Salviae miltiorrhizae ( another herbal extract), 20 ml, in 500 ml of 10% glucose, intravenous infusion, daily, for three months. Control: vitamin C 500 mg plus adenosine triphosphate (ATP) 40 mg and coenzyme‐A 200 u, in 500 ml of 10% glucose, intravenous infusion, daily, for three months. | |
| Outcomes | Biochemical responses: ALT, r‐GT, albumin/globulin. Viral responses: HBsAg, HBeAg, HBV DNA. Adverse effect. Follow‐up max: six months. | |
| Notes | Letter sent for details(2/6). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang 1998.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhang 1999.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zheng 1999.
| Methods | Randomised trial. Generation of allocation sequence and allocation concealment: no details. Blinding: unblinded. Loss to follow‐up: unstated. Jadad score=1. | |
| Participants | Ethnic: Chinese. 120 patients (60 in treatment group, 48 males and 12 females, age range 13˜60 yrs; 60 in control group, 50 males and 10 females, age range 14˜59 yrs). Setting: inpatients. Inclusion criteria: chronic hepatitis B with mediate and severe disease, and positive serum HBeAg and HBV DNA, diagnosed according to the criteria set by the National Conference on Viral Hepatitis in China (CMA 1995). Exclusion criteria: unstated. | |
| Interventions | Experimental: HB‐Granule‐3 (compound of 11 herbs, major of genus Phyllanthus), 10 g orally, t.i.d, for 90 days. Control: gamma‐IFN 5 MU, subcutaneous injection, thrice weekly, for 90 days. | |
| Outcomes | Symptoms and signs. Liver function, and hepatofibrotic indices. Viral responses: HBsAg, HBeAg, HBV DNA. Maximum follow‐up: three months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhou 1999.
| Methods | See Additional tables: trials with less than three months follow‐up. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berk 1991 | Randomised, double‐blind, cross‐over trial. Ten patients were allocated to Phyllanthus amarus group or placebo group; patients received Phyllanthus amarus treatment for 28 days followed by placebo for 28 days, or received placebo for 28 days followed by Phyllanthus amarus for 28 days; the results of the first treatment period were not reported. |
| Blumberg 1989 | Description of data from another study in this systematic review. |
| Chen 1986 | Randomised clinical trial comparing Fuganning (herbal compound) versus non‐specific treatment, but using bifendate when ALT level was above 400 U/L in patients of control group. |
| Chen 1992 | Randomised trial comparing different formula of the same herb, i.e., lipid‐coated Cordycops polysaccharide versus Cordycops polysaccharide alone. |
| Chen 1997 | Randomised trial comparing two different herbal medicines, Zishen Rougan granule versus Yigan Yangyin Huoxue granule. |
| Ding 1995 | Randomised trial comparing two different herbal medicines, Hong Bao versus Ganbifu. |
| Feng 1992 | Randomised trial comparing two different herbal prescriptions, Compound Yigan No. 1 versus Mieaoling. |
| Fu 1992 | Randomised trial comparing ara‐A dauricine plus Chinese herbs versus ara‐A dauricine alone. |
| Fu 1994 | Randomised trial comparing two different herbal ingredients, Potenlini plus Cinobufacin versus Caryophyllin plus Yiganling and vitamin C. |
| Hu 1992 | Randomised trial comparing two different medicinal herbs, Shenqi Yigan granule versus Ganbifu tablet. |
| Hu 2000 | Randomised trial comparing a mixture of herbs versus another herbal medicine plus non‐specific treatment. |
| Li 1995 | Randomised trial comparing Ginkgo Biloba composita (a herbal mixture) versus control interventions that included another formula, Mythic fungus syrup, plus thymosin and vitamins. |
| Li 1997 | Randomised trial comparing Potenlini Injection (a herbal extract) versus Yiganling and Hulusu tablets (another two herbal formula). |
| Liu 1996 | Randomised trial comparing Astragali composita versus three different herbal medicines (control interventions). |
| Liu 1997 | Quasi‐randomised trial comparing Chaoyang Wan (herbal pills) versus another herbal medicine (Yiganyiqi granule). |
| Lou 1997 | Prospective controlled study without random allocation comparing Erzhi pill (compound of herbs) versus vitamin C and vitamin B co in 158 patients with chronic hepatitis B. |
| Lv 1996 | Randomised double‐blind trial, comparing two herbal medicinal prescriptions: Composita No 319 capsule versus Dahuang Zhecong Wan capsule. |
| Ma 1997 | Randomised trial comparing Chinese herbs '9250' (compound of 13 herbs) versus Potenlini (extract of herb) in 120 patients with chronic active hepatitis B and posthepatitis cirrhosis. |
| Miao 1998 | Randomised trial. Experimental interventions included thymosin plus Phyllanthus amarus (herbal medicine) and acyclovir; control intervention was comprehensive treatment. However, the use of co‐interventions in experimental group led to exclusion. |
| Milne 1994 | Randomised, placebo‐controlled trial comparing Phyllanthus amarus versus placebo in "healthy carriers" of HBV infection. |
| Nurul 1998 | The study reports an observational study and a randomised trial. The participants in the randomised trial were 20 cases of chronic hepatitis, but no virological diagnosis was given. |
| Oka 1995 | An observational study. |
| Qiu 1993 | Randomised, double‐blind, placebo‐controlled trial comparing Gan Ling Wan (herbal compound) versus placebo in 93 patients with viral hepatitis and 76 asymptomatic HBV carriers. The 93 patients with viral hepatitis included acute and chronic active hepatitis with no virologic diagnosis and the results were not reported separately. |
| Tajiri 1991 | Controlled clinical trial without randomisation. |
| Thamlikitkul 1991 | Randomised trial using asymptomatic HBsAg carriers with normal liver enzymes as participants. |
| Thyagarajan 1988 | Randomised double‐blind, placebo‐controlled trial comparing Phyllanthus amarus versus placebo. The participants included 28 symptom‐free carriers of hepatitis B virus, 14 carriers with symptoms and 18 carriers with glomerular nephritis, but the outcomes were not reported separately. We have written a letter to the authors and if we get useful information, it will be included in this review. |
| Thyagarajan 1990 | Observational study without control group. |
| Tian 1998 | Randomised trial comparing Chai Hu Huoxue Jiedu decoction (compound of 13 herbs) versus Yiganling (compound of herbs) plus acyclovir in 194 patients with chronic hepatitis B. |
| Wang 1991b | A total of 154 patients with chronic active hepatitis B were randomised into three group. Syrup tonifying kidney (experimental group) versus another syrup made of three herbs plus bifendate plus Stringy stonecrop herb plus Yiganling, and versus Bianzheng recipe. |
| Wang 1992a | Randomised trial comparing two herbal compounds: Huoxue Jiedu Fuzheng recipe versus Qingre Jiedu recipe. |
| Wang 1994b | A total of 300 patients with chronic hepatitis B were randomised into three group: poly‐inosinic and cytidylic acid (Poly I:C) plus hepatitis B vaccination (HBvac) versus Potenlini plus HBvac versus Polyporus umbellatus polysaccharide plus HBvac. |
| Wang 1996 | Randomised trial comparing Polyporus umbellatus polysaccharide (a herbal extract) plus anti‐hepatitis B immune ribonucleic acid versus IFN. |
| Wang 1999a | Randomised trial comparing two herbal medicines: Gankang versus Yiganning. |
| Wen 1998 | Randomised trial comparing Chinese herbs (a compound of 11 herbs) versus Xiaoyaosan (compound of herbs) in 84 patients with chronic hepatitis B. |
| Wu 1991 | Randomised trial comparing matrine injection (an ingredient from herbal extraction) versus Yinzhihuang injection and Salviae miltiorrhizae injection (two other herb extracts). |
| Wu 1994 | Quasi‐randomised trial comparing Chaoyang Wan (a herbal compound) versus Stringy stonecrop herb granule. |
| Xiong 1993 | Randomised trial comparing two medicinal herbs: Salviae miltiorrhizae versus Polyporus umbellatus polysaccharide. |
| Yamamoto 1983 | Non‐randomised clinical study. |
| Yu 1991 | Randomised trial comparing Angelic acid plus phetolamine versus non‐specific treatment. However, the use of co‐intervention in the experimental group (phetolamine) led to exclusion. |
| Yu 1999a | Randomised trial comparing Jianpi Shugan syrup (a herbal compound) versus control interventions including anti‐hepatitis B immune ribonucleic acid plus bifendate and vitamin C in 60 children with chronic hepatitis B. |
| Zhang 1992 | Randomised trial comparing Phyllanthus amarus plus basic treatment versus Indigowood root plus basic treatment. |
| Zhang 1993b | Randomised trial comparing two herbal medicines: Yiganning granule versus oleanolic acid. |
| Zhang 1993c | Randomised trial comparing diammonium glycyrrhizinate (active ingredient of herb) versus a similar drug, Potenlini (herbal extracts). |
| Zhang 1996 | Randomised trial comparing two Chinese herbal compounds: Yigan Kangte capsule versus Mieaoling capsule. |
| Zhang 1997 | Randomised trial comparing two Chinese herbs: Keganling tablet versus Mieaoling tablet. |
| Zheng 1992 | Randomised trial studying herbal treatment of chronic liver diseases including chronic hepatitis and liver cirrhosis, but no virological diagnosis was given. |
| Zhou 1993 | Randomised trial comparing two similar herbal extracts: diammonium glycyrrizinate (active ingredient of herb) capsule versus glycyrrhizin tablet. |
| Zhu 1992 | Randomised trial comparing two different formulations of Phyllanthus urinaria (decoction and granule) versus poly I:C. |
| Zhuo 1985 | Controlled clinical trial without random allocation, comparing compound Chinese medicine pill (848) with no treatment in chronic asymptomatic HBV carriers with normal liver function. |
Contributions of authors
Jianping Liu drafted and revised the protocol, developed search strategy, handsearched journals, coordinated the identification of studies, selected trials for inclusion, performed data extraction, and wrote the review. Heather McIntosh developed search strategy for the protocol, provided methodological perspective, and checked and revised all these processes. Hui Lin handsearched journals, retrieved papers, and extracted data.
Sources of support
Internal sources
The Copenhagen Trial Unit, Denmark.
External sources
The 1991 Pharmacy Foundation, Denmark.
The Danish Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
Copenhagen Hospital Corporation's Research Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g., employment, consultancy, stock ownership, honoraria, expert testimony).
Edited (no change to conclusions)
References
References to studies included in this review
Chang 1994 {published data only}
- Chang QH, Liu XP, Chang MX. Lentinan tablet for treatment of 52 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1994;4(3):36‐7. [Google Scholar]
Chen 1990 {published data only}
- Chen ZT, Zhao BZ, Gao YH, Xie FZ, Chen Y, Su JG, et al. Clinical study of 96 cases with chronic hepatitis B treated with Jiedu Yanggan Gao by a double‐blind method. Chinese Journal of Integrated Traditional & Western Medicine 1990;10(2):71‐4. [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen C, Guo SM, Liu B. A randomised controlled trial of kurorinone versus interferon‐alfa2a treatment in patients with chronic hepatitis B. Journal of Viral Hepatitis 2000;7:225‐9. [DOI] [PubMed] [Google Scholar]
Cui 1994 {published data only}
- Cui XD, Wang BP. Panax pseudo‐ginseng powder for the treatment of 30 patients with chronic active hepatitis. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1994;4(2):37‐8. [Google Scholar]
Fang 1994 {published data only}
- Fang XD. [Therapeutic observation on Gan Kang Ling combined with interferon for treatment of chronic hepatitis B]. Journal of Zhejiang College of Traditional Chinese Medicine 1994;18(4):16‐7. [Google Scholar]
Hirayama 1989 {published data only}
- Hirayama C, Okumura M, Tanikawa K, Yano M, Mizuta M, Ogawa N. A multicenter randomized controlled clinical trial of Shosaiko‐to in chronic active hepatitis. Gastroenterol Jpn 1989;24(6):715‐9. [DOI] [PubMed] [Google Scholar]
Huang 1993 {published data only}
- Huang ZR, Zhong JP, Zhu GL, Chen YR, Wang GQ. Therapeutic observation on Phyllanthus amarus for hepatitis B. Chinese Journal of Clinical Hepatology 1993;9(2):108‐10. [Google Scholar]
Huang 1999a {published data only}
- Huang L, Zhang FX, Li CQ. [Composita Phyllanthus combined with interferon for treatment of 40 patients with chronic hepatitis B]. Shaanxi Journal of Traditional Chinese Medicine 1999;20(4):146‐7. [Google Scholar]
Huang 1999b {published data only}
- Huang KM. [Herb Phyllanthus for treatment of 38 patients with chronic hepatitis B]. Information on Traditional Chinese Medicine 1999;16(6):32. [Google Scholar]
Huang 2000 {published data only}
- Huang KM. Ganyanling combined with Danshen injection for treatment of 60 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 2000;10(1):41‐2. [Google Scholar]
Jiao 1994 {published data only}
- Jiao WJ, Zhang P, Liu CB, Fu QP, Dai W, Xiao JL. Comparison of therapeutic effects of six therapies for chronic active hepatitis B. Chinese Journal of Infectious Diseases 1994;12(2):114‐5. [Google Scholar]
Li 1998 {published data only}
- Li CQ, Wang XH, Li GQ, Fang HX. [Clinical observation of composita Phyllanthus for treatment of chronic hepatitis B]. Journal of New Traditional Chinese Medicine 1998;30(6):45‐6. [Google Scholar]
Lin 1996 {published data only}
- Lin F, Wang JH, Ji YZ. Interferon combined with Huatan Jiedu Fuzheng Fang for treatment of 16 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1996;6(3):20. [Google Scholar]
Lv 1991 {published data only}
- Lv XL. A preliminary observation on Chinese herbs combined with interferon for treatment of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine 1991;1(2):35‐6. [Google Scholar]
Miao 1993 {published data only}
- Miao HZ, Qian MB, Lu DR. Comparative observation on therapeutic effects of Jianganling, Potenlini, Hugan tablet in patients with chronic viral hepatitis. Chinese Journal of Hepatology 1993;1(2):110‐1. [Google Scholar]
Miao 1994 {published data only}
- Miao ZQ, Tao BG, Ye JM. Observation on short‐term therapeutic effect of interferon, Polyporus umbellatus polysaccharide and thymosin D in treatment of chronic hepatitis B. Chinese Journal of Clinical Hepatology 1994;10(1):49‐51. [Google Scholar]
Wang 1991a {published data only}
- Wang JY, Liu HY, Hu DC, Xia DQ. A randomized controlled trial of Potenlini on chronic active hepatitis B. Chinese Journal of Infectious Diseases 1991;9(4):208‐11. [Google Scholar]
Wang 1994a {published data only}
- Wang JH, Lu LX, Zuo JH. Effect observation on Polyporus umbellatus polysaccharide and Buqi Jiedu Tang for treatment of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1994;4(2):35‐6. [Google Scholar]
Wang 1995a {published data only}
- Wang MX, Cheng HW, Li YJ, Meng LM, Zhao GL, Mai K. Herbs of the genus Phyllanthus in the treatment of chronic hepatitis B: observations with three preparations from different geographic sites. Journal of Laboratory and Clinical Medicine 1995;126(4):350‐2. [PubMed] [Google Scholar]
- Wang MX, Cheng HW, Li YJ, Meng LM, Zhao GL, Mai K. Observations on the efficacy of Phyllanthus spp. in the treating patients with chronic hepatitis B. China Journal of Chinese Materia Medica [Zhongguo Zhongyao Zazhi] 1994;19(12):750‐2, 764. [PubMed] [Google Scholar]
Wang 1995b {published data only}
- Wang QT, Ding SS, Cheng PF, Zhang YR, Liu LQ. Antilipidperoxidation effect of Shenhuang granule to the patients with chronic liver diseases. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1995;5(3):12‐4. [Google Scholar]
Wang 1999b {published data only}
- Wang XH, Li CQ, Guo XB, Li H, Lao SX. Clinical observation on 40 cases of chronic hepatitis B treated by Phyllanthus compound combined with interferon. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1999;9(1):12‐3. [Google Scholar]
Wen 1999 {published data only}
- Wen SC, Yang SJ, Su GY. Effect of Youguiyin on subpopulations of peripheral T lymphocytes in patients with chronic hepatitis B. Chinese Journal of Infectious Diseases 1999;17(3):205‐6. [Google Scholar]
Wu 1996 {published data only}
- Wu LK, Yu DH, Wang HL, Zhou JP, Li JL, Lv ZG. Effect of Lentinan injection on peripheral blood endoxin and TNF in patients with chronic hepatitis B. Chinese Journal of Hepatology 1996;4(1):49‐50. [Google Scholar]
Xiao 1994 {published data only}
- Xiao HJ, Yang PM, Peng LK, Liu X, Shi CG, Wang ZQ, et al. Study on anti‐viral effect of combination of Fuzheng Jiedu Tang and HB vaccine for chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1994;4(4):28‐9. [Google Scholar]
Yan 1988 {published data only}
- Yan SC, Cao WF, Zhang YH, Li LF, Yu CY, Zhu ZS, et al. Clinical and experimental research on Polyporus umbellatus polysaccharide in treating chronic viral hepatitis. Chinese Journal of Integrated Traditional & Western Medicine 1988;8(3):141‐3. [PubMed] [Google Scholar]
Yang 1986 {published data only}
- Yang JY, Guan MH, Wang JH. A preliminary clinical observation on the effect of Kangdu Wan on chronic HBV infection. Chinese Journal of Integrated Traditional & Western Medicine 1986;6(9):530‐2. [PubMed] [Google Scholar]
Yu 1999b {published data only}
- Yu ZS, Wang YG, Wang RJ, Xia SZ, Chang XH, Zhao XL. The clinical effect of Potenlini (glycyrrhizic acid) and Salviae miltiorrhizae for antifibrosis. Chinese Journal of Clinical Hepatology 1999;15(2):116‐7. [Google Scholar]
Zhang 1993a {published data only}
- Zhang SL, Zhong FJ, Zhang CF, Feng XY, Tao LZ. Combined use of Anisodamine and Salviae miltiorrhizae for treatment of chronic active hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1993;3(1):37‐8. [Google Scholar]
Zhang 1998 {published data only}
- Zhang LC, Wang WL, Xie XY. Effect of glycyrrhizin on liver pathology of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1998;8(1):54. [Google Scholar]
Zhang 1999 {published data only}
- Zhang JR. Interferon combined with Yigan San for treatment of 45 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1999;9(2):48. [Google Scholar]
Zheng 1999 {published data only}
- Zheng XY, Zhou DQ, Gao H, Huang B, Zhou XZ. The clinical study of chronic hepatitis B treated with HB‐Granule‐3. Chinese Journal of Integrated Traditional and Western Medicine on Gastro‐Spleen 1999;7(1):22‐4. [Google Scholar]
Zhou 1999 {published data only}
- Zhou DQ, Zheng XY, Gao H, Zhou XZ, Peng LS, Qiu M, et al. Clinical study on chronic hepatitis B treated by IFN‐alpha combined with Hepatitis B Granule No.3. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1999;9(1):5‐7. [Google Scholar]
References to studies excluded from this review
Berk 1991 {published data only}
- Berk L, Man RA, Schalm SW, Labadie RP, Heijtink RA. Beneficial effects of Phyllanthus amarus for chronic hepatitis B, not confirmed. Journal of Hepatology 1991;12(3):405‐6. [DOI] [PubMed] [Google Scholar]
Blumberg 1989 {published data only}
- Blumberg BS, Millman I, Venkateswaran PS, Thyagarajan SP. Hepatitis B virus and hepatocellular carcinoma ‐ treatment of HBV carriers with Phyllanthus amarus. Cancer Detection and Prevention 1989;14(2):195‐201. [PubMed] [Google Scholar]
Chen 1986 {unpublished data only}
- Chen YQ, Guo LF, Xu FG, Wu YL. Clinical observation on 103 cases of children with HBsAg positive viral hepatitis using Fuganning. National Conference on Viral Hepatitis. 1986:handed out paper.
Chen 1992 {published data only}
- Chen BL, Tan YJ, Qiu DK, Zhang Y, Yang LK. Clinical observation on Cordycops polysaccharide liposomes in the treatment of chronic active hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1992;2(3):1‐3. [Google Scholar]
Chen 1997 {published data only}
- Chen ZT, Zhao BZ, Han CX, Xu CJ, Liu M, Tian MP, et al. The clinical study of treating chronic hepatitis B and hepatofibrosis with Zishen Rougan granule. Chinese Journal of Integrated Traditional Chinese and Western Medicine on Liver Diseases 1997;7(3):131‐4. [Google Scholar]
Ding 1995 {published data only}
- Ding MM, Wu XN, Shao L, Wang YF. Late result of cleaning serum HBV DNA and e antigen of patients with chronic hepatitis B by Hongbao. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1995;5(1):18‐20. [Google Scholar]
Feng 1992 {published data only}
- Feng YK, Qin SX, Wan WQ, Chen MX, Zhao CT, Tan WF. Clinical observation on oral liquid of Fufang Yigan Yihao in treatment of 208 patients with chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1992;2(4):8‐9. [Google Scholar]
Fu 1992 {published data only}
- Fu XX. Therapeutic effect of combined treatment with ara‐A dauricine and Chinese herbs in chronic hepatitis B infection. Chinese Medical Journal 1992;105(4):348‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Fu 1994 {published data only}
- Fu CY, Liu PL, Shi YX, Xu XY. Observation on therapeutic effects of combined treatment of Potenlini and Cinobufacin for chronic active hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1994;4(3):34‐5. [Google Scholar]
Hu 1992 {published data only}
- Hu JH, Wu JH, Wang XJ, Huang W, Li F. Infusion Hepatitis B Shen Qi for treating chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1992;2(1):11‐2. [Google Scholar]
Hu 2000 {published data only}
- Hu YX, Li XD, Xia JY. Shugan Jianpi Huoxue Jiedu therapy for treating 37 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 2000;10(1):43. [Google Scholar]
Li 1995 {published data only}
- Li W, Dai QT, Liu ZE, Lu FX, An P. Preliminary study on early fibrosis of chronic hepatitis B treated with Ginkgo Biloba Composita. Chinese Journal of Integrated Traditional & Western Medicine 1995;15(10):593‐5. [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li CP, Liu HY, Hu DC, Hou J, Zhou K. Effects of Potenlini on levels of IL‐6 and TNF‐alpha in patients with chronic hepatitis. Chinese Journal of Infectious Diseases 1997;15(3):156‐7. [Google Scholar]
Liu 1996 {published data only}
- Liu KZ, Xu CH, Zhang MT, Chen Z, Gu YX, Cai PR, et al. Clinical and experimental studies on effects of chronic hepatitis B treated with Astragali Composita. Chinese Journal of Integrated Traditional & Western Medicine 1996;16(7):394‐7. [PubMed] [Google Scholar]
Liu 1997 {published data only}
- Liu ZC, Zhao HT. Treatment of 126 cases with chronic hepatitis B using Chaoyang Wan. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1997;7(2):109‐10. [Google Scholar]
Lou 1997 {published data only}
- Lou XH. Treatment of 80 cases of chronic hepatitis B with deficiency of Liver‐Yin and Kidney‐Yin with modified Erzhi pill. Journal of Zhejiang College of Traditional Chinese Medicine 1997;21(3):35. [Google Scholar]
Lv 1996 {published data only}
- Lv P, Yang JL, Liu C, Liu P. Compound 319 capsule for treatment of 51 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1996;6(1):32‐3. [Google Scholar]
Ma 1997 {published data only}
- Ma YF, Li GP, Tang BZ, Li M. Clinical studies of patients with chronic active hepatitis B and posthepatitis cirrhosis were treated by 9250 Chinese herb tablets. Chinese Journal of Integrated Traditional and Western Medicine on Gastro‐Spleen 1997;5(4):208‐10. [Google Scholar]
Miao 1998 {published data only}
- Miao XQ, Lu SW, Luo GL, Kong QF, Lei YP, Zeng RK, et al. Combined treatment of thymosin, Phyllanthus amarus, and acyclovir for chronic hepatitis B. Chinese Journal of Hepatology 1998;6(1):7. [Google Scholar]
Milne 1994 {published data only}
- Milne A, Hopkirk N, Lucas CR, Waldon J, Foo Y. Failure of New Zealand hepatitis B carriers to respond to Phyllanthus amarus. New Zealand Medical Journal 1994;107(980):243. [PubMed] [Google Scholar]
Nurul 1998 {published data only}
- Nurul A, Rino A, Gani T, Widayat D, Santoso S, Sumaryono HM, et al. Effectiveness of the analogue of natural Schisandrin C (HpPro) in treatment of liver diseases: an experience in Indonesian patients. Chinese Medical Journal 1998;111(3):248‐51. [PubMed] [Google Scholar]
Oka 1995 {published data only}
- Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Kim SR, et al. Prospective study of chemoprevention of hepatocellular carcinoma with Sho‐saiko‐to (TJ‐9). Cancer 1995;76(5):743‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qiu 1993 {published data only}
- Qiu YQ, Liu HR, Lei BJ, Yi SF, Xi YM. [A randomised, double‐blind, controlled trial of Gan Ling Wan in treating viral hepatitis and chronic hepatitis B virus carriers]. West China Medical Journal 1993;8(4):380‐1. [Google Scholar]
Tajiri 1991 {published data only}
- Tajiri H, Ozaki Y, Miki K, Shimuzu K, Okada S. Effect of Sho‐saiko‐to(Xiao‐Chai‐Hu‐Tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. American Journal of Chinese Medicine 1991;19(2):121‐9. [DOI] [PubMed] [Google Scholar]
Thamlikitkul 1991 {published data only}
- Thamlikitkul V, Wasuwat S, Kanchanapee P. Efficacy of Phyllanthus amarus for eradication of hepatitis B virus in chronic carriers. J Med Assoc Thai 1991;74(9):381‐5. [PubMed] [Google Scholar]
Thyagarajan 1988 {published data only}
- Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet 1988;2(8614):764‐6. [DOI] [PubMed] [Google Scholar]
Thyagarajan 1990 {published data only}
- Thyagarajan SP, Jayaram S, Valliammai T, Madanagopalan N, Pal VG, Jyaraman K. Phyllanthus amarus and hepatitis B. Lancet 1990;336(8720):949‐50. [DOI] [PubMed] [Google Scholar]
Tian 1998 {published data only}
- Tian ZC. Bupteurum decoction for detoxicating and promoting blood circulation to treat chronic hepatitis B. Journal of Practical Traditional Chinese Medicine 1998;14(6):33‐4. [Google Scholar]
Wang 1991b {published data only}
- Wang LT, Chen JJ, Zhang HX, Ren JW, Xia DY. Clinical study on treatment of chronic active hepatitis B with Syrup Tonifying Kidney. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1991;1(3):4‐6. [Google Scholar]
Wang 1992a {published data only}
- Wang CB. Treatment of severe chronic hepatitis B by combination of traditional Chinese medicine and Western medicine therapy‐‐with an analysis of 122 cases. Chinese Journal of Integrated Traditional & Western Medicine 1992;12(4):203‐6, 195. [MEDLINE: ] [PubMed] [Google Scholar]
Wang 1994b {published data only}
- Wang BL, Xie SL, Wu YX. Polyporus umbellatus polysaccharide combined with hepatitis B vaccine for treatment of 120 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1994;4(4):30. [Google Scholar]
Wang 1996 {published data only}
- Wang YJ, Sheng YM, Huang S. Comparison of therapeutic effect of combining Polyporus umbellatus polysaccharide with anti‐hepatitis B immune ribonucleic acid and interferon in chronic hepatitis B. Chinese Journal of Hepatology 1996;4(1):39. [Google Scholar]
Wang 1999a {published data only}
- Wang L, Luo SW, Zhang JC, Dong J, Zhang Y. Study on anti‐HBV effect of Gankang for the treatment of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1999;9(4):14‐5. [Google Scholar]
Wen 1998 {published data only}
- Wen CS, Zhao QX. Clinical effect of Chinese herbs to treat 84 cases with chronic hepatitis B. Hainan Medicine 1998;15(3):205‐6. [Google Scholar]
Wu 1991 {published data only}
- Wu SM, Jiang J, Wu JJ, Ma ZQ, He MH. Clinical observation on matrine injection in treating chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1991;1(2):1‐2. [Google Scholar]
Wu 1994 {published data only}
- Wu SM, Jiang J. A preliminary report on clinical effects of Chaoyang Wan for treatment of chronic hepatitis. Chinese Journal of Hepatology 1994;2(2):84. [Google Scholar]
Xiong 1993 {published data only}
- Xiong LL, Cao GM, Wu LH. Therapeutic effect of combined therapy of Salvia miltiorrhiza and Polyporus umbellatus polysaccharide in treating chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine 1993;13(9):533‐5. [PubMed] [Google Scholar]
Yamamoto 1983 {published data only}
- Yamamoto M, Uemura T, Nakama S, Uemiya M, Kasayama S, Kishida Y, et al. A fundamental study of chronic hepatitis treated with saiko agents. Proc Symp WakanYaku 1983;16:245‐8. [Google Scholar]
Yu 1991 {published data only}
- Yu SB, Li DX. Combination of phentolamine and angelicini in cholestasis and severe type of chronic active hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine 1991;11(3):135‐7, 131. [PubMed] [Google Scholar]
Yu 1999a {published data only}
- Yu P, Fu PF, Zhou YP. Jianpi Shugan Syrup for treatment of 60 children with chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1999;9(2):44‐5. [Google Scholar]
Zhang 1992 {published data only}
- Zhang JL, He WN, Ye P. Clinical observation on Phyllanthus amarus for treating chronic hepatitis B virus infection. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1992;2(1):8‐10. [Google Scholar]
Zhang 1993b {published data only}
- Zhang BZ, Ding F, Tan LW. Clinical and experimental study on Yiganning granule in treating chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine 1993;13(10):597‐9, 580. [MEDLINE: ] [PubMed] [Google Scholar]
Zhang 1993c {published data only}
- Zhang BG, Zhou YS, Zhang DF, Chen YG, Cao T, Zhou HX, et al. Therapeutic effects of diammonium glycyrrhizinate injection for chronic hepatitis. Chinese Journal of Hepatology 1993;1(2):112‐4. [Google Scholar]
Zhang 1996 {published data only}
- Zhang JJ, Sun WQ, Wang BX. Yigan Kang Te capsula for treatment of 69 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1996;6(4):33‐4. [Google Scholar]
Zhang 1997 {published data only}
- Zhang AG, Zhou SQ. Keganling tablet for treatment of 58 cases of chronic hepatitis B. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1997;7(1):47‐8. [Google Scholar]
Zheng 1992 {published data only}
- Zheng XY, Xia JY, Yang PM. Tetramethylpy razine for treating 41 cases of chronic viral hepatitis. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1992;2(1):42, 27. [Google Scholar]
Zhou 1993 {published data only}
- Zhou YS, Zhang DF, Zhang BG, Chen YG, Cao T, Zhou HX, et al. Therapeutic effects of diammonium glycyrrhizinate capsule for chronic viral hepatitis. Chinese Journal of Hepatology 1993;1(3):173‐5. [Google Scholar]
Zhu 1992 {published data only}
- Zhu FM, Zhang JQ, Zhang XZ, Zhang XM. Observation on the effect of Fujian's Phyllanthus amarus in treatment of HBV infection. Chinese Journal of Integrated Traditional & Western Medicine on Liver Diseases 1992;2(2):10‐1. [Google Scholar]
Zhuo 1985 {published data only}
- Zhuo HC, Wang BE, Yin WY, Wang HZ, Hu ZH, Song ZZ, et al. A preliminary clinical study of the effect of complex TCM pills (848) on chronic HBV carriers. Chinese Journal of Integrated Traditional & Western Medicine 1985;5(10):606‐8. [PubMed] [Google Scholar]
Additional references
CMA 1984
- Academic Committee of Infectious Diseases and Parasitic Diseases of Chinese Medical Association. Consensus on Prevention and Management of Viral Hepatitis (first version). Chinese Journal of Infectious Diseases 1984;3(3):194. [Google Scholar]
CMA 1990
- Academic Committee of Infectious Diseases and Parasitic Diseases of Chinese Medical Association. Consensus on Prevention and Management of Viral Hepatitis (second version in 1990). Chinese Journal of Infectious Diseases 1991;9(1):52. [Google Scholar]
CMA 1995
- Academic Committee of Infectious Diseases and Parasitic Diseases of Chinese Medical Association. Consensus on Prevention and Management of Viral Hepatitis (third version). Chinese Journal of Internal Medicine 1995;34:788. [Google Scholar]
CMH 1999
- Chinese Ministry of Health. Health News. Newspaper of Health News 22/01/1999:4th Edition.
Dusheiko 1999
- Dusheiko G. Hepatitis B. In: Bircher J, Benhamon JP, McIntyre N, Rizzetto M, Rodes J editor(s). Oxford Textbook of Clinical Hepatology. Vol. 1, Oxford: Oxford University Press, 1999:891. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ergil 1996
- Ergil KV. Chinese Medicine. In: Micozzi MS editor(s). Fundamentals of Complementary and Alternative Medicine. New York: Churchill Livingstone Inc, 1996. [Google Scholar]
Fontana 1997
- Fontana RJ, Lok AS. Combination therapy for chronic hepatitis B. Hepatology 1997;26:234‐7. [DOI] [PubMed] [Google Scholar]
Gitlin 1997
- Gitlin N. Hepatitis B: diagnosis, prevention, and treatment. Clinical Chemistry 1997;43(8 Pt 2):1500‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Gottieb 2000
- Gottieb S. Chinese herb may cause cancer (news). BMJ 2000;320:1623. [PMC free article] [PubMed] [Google Scholar]
Hirayama 2000
- Hirayama C. Xiao Chai Hu Tang for chronic hepatitis B. Personal communication, 2000.
Ishak 1995
- Ishak K, Baptista A, Bianchi L, Callea F, Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. Journal of Hepatology 1995;22(6):696‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ishizaki 1996
- Ishizaki T, Sasaki F, Ameshima S, Shiozaki K, Takahashi H, Abe Y, et al. Pneumonitis during interferon and/or herbal drug therapy in patients with chronic active hepatitis. European Respiratory Journal 1996;9(12):2691‐6. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Kjaergard 1999
- Kjaergard LL, Villumsen J, Gluud C. Quality of randomised clinical trials affects estimates of intervention efficacy (abstract). VII Cochrane Colloquium. Rome, 1999:57.
Liu 1998
- Liu JP, Lin H, Wang SX. Randomized controlled trials relevant to viral hepatitis in the Chinese literature (abstract). VI Cochrane Colloquium. Baltimore, 1998:83.
Liu 1999
- Liu JP, Lin H, Liu LL, Yang S. Methodological evaluation on randomized controlled trials for viral hepatitis in Chinese medical literature. West China Medical Journal 1999;14(2):126‐8. [Google Scholar]
Liu 1999b
- Liu JP, Liu C, Leng TJ. Evaluation of clinical trials for viral hepatitis in the past nine years in Chinese medical literatures. West China Medical Journal 1999;14(3):264‐5. [Google Scholar]
Melchart 1999
- Melchart D, Linde K, Weidenhammer W, Hager S, Shaw D, Bayer R. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA 1999;282(1):28‐9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Purcell 1993
- Purcell RH. The discovery of the hepatitis virus. Gastroenterology 1993;104(4):955‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaw 2000
- Shaw T, Locarnini S. Combination chemotherapy for hepatitis B virus: the final solution?. Hepatology 2000;32(2):430‐2. [DOI] [PubMed] [Google Scholar]
Tomlinson 2000
- Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. J Clin Pharmacol 2000;40(5):451‐6. [DOI] [PubMed] [Google Scholar]
Vautier 1995
- Vautier G, Spiller RC. Safety of complementary medicines should be monitored. BMJ 1995;311:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19:159‐66. [DOI] [PubMed] [Google Scholar]
Wang 2000
- Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol and Hepatol 2000;15(Suppl):E67‐E70. [DOI] [PubMed] [Google Scholar]
WHO 1998
- WHO 1998. Hepatitis B. Fact Sheet WHO/204 1998, issue November.
WHO 1999
- WHO 1999. Diseases and Vaccines. Hepatitis B. www.who.int/gpv‐dvacc/diseases/hepatitis_b.htm 1999, issue March.
References to other published versions of this review
Liu 2001
- Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B: a systematic review. Liver 2001;21(4):280‐286. [DOI] [PubMed] [Google Scholar]
Liu 2001b
- Liu J, Lin H, McIntosh H. Genus Phyllanthus for chronic hepatitis B virus infection: a systematic review. Journal of viral hepatitis 2001;8(5):358‐366. [DOI] [PubMed] [Google Scholar]


