Abstract
Background
Intravesical therapy with Bacillus Calmette‐Guérin (BCG) aims to reduce the incidence of tumour recurrence following transurethral resection (TUR) for patients with superficial bladder cancer.
Objectives
The objective of this review was to compare the incidence of tumour recurrence after the standard therapy of transurethral resection versus transurethral resection plus intravesical Bacillus Calmette‐Guérin.
Search methods
We searched the Cochrane Controlled Trials Register (March 2000), Medline (February, 2000), EMBASE (February, 2000), Cancerlit (February, 2000), Healthstar (February, 2000), Database of Abstracts of Reviews of Effectiveness (February, 2000) and the Bath Information Data Service. The Proceedings of the American Society Clinical Oncology was hand searched (1996 to 1999).
Selection criteria
Randomised or quasi‐randomised trials of transurethral resection alone versus transurethral resection plus intravesical Bacillus Calmette‐Guérin. Patients with Ta and T1 bladder cancer of medium or high risk of tumour recurrence, were eligible for inclusion.
Data collection and analysis
Four reviewers assessed trial quality and two abstracted the data independently. The Peto odds ratios and log hazard ratios were determined to compare the number of patients with disease recurrence at 12 months and the rate of recurrence, respectively.
Main results
Six randomised trials were included involving 585 eligible patients. There were significantly fewer patients with disease recurrence at 12 months in the BCG plus TUR group compared to those that received TUR alone (odds ratio 0.30, CI 0.21 to 0.43). The overall log hazard ratio for recurrence (‐0.83, variance 0.02) indicated a significant benefit of BCG treatment in reducing tumour recurrence. Toxicities associated with BCG consisted mainly of cystitis (67%), haematuria (23%), fever (25%) and urinary frequency (71%). No BCG‐induced deaths were reported.
Authors' conclusions
In patients with medium/high risk Ta or T1 bladder cancer, immunotherapy with intravesical BCG following TUR appears to provide a significant advantage over TUR alone in delaying tumour recurrence.
Plain language summary
Local treatment with Bacillus Calmette‐Guérin following surgery for superficial bladder cancer reduces the risk of the cancer returning.
Worldwide, bladder cancer is common in both men and women. In most cases, the cancer occurs in the superficial layers of the bladder and can be surgically removed. However, in many people the cancer returns. Drugs placed directly into the bladder tissue following surgery are therefore often used to try to prevent the cancer recurring. Bacillus Calmette‐Guérin (BCG) is a live attenuated bacterium used for immunization against tuberculosis, and is safe and effective for that purpose; it has also been licensed by the US FDA and other national regulatory agencies for use in superficial bladder‐cancer treatment. The review found that BCG treatment was effective in preventing cancer recurrence following surgery. Further studies into making treatment more effective are needed.
Background
Cancer of the urinary bladder accounts for approximately 2% of all malignant disease and is the fourth most common cancer in men and the ninth in women. The mortality rate and incidence of new cases of bladder cancer increases with age, and worldwide continues to rise by 5% to 10% every 5 years (Wingo 1995). Approximately 80% of all new bladder cancers present as superficial tumours confined to the epithelium or lamina propria (Young 1996). Superficial disease may consist of non‐invasive tumours (Ta), tumours invading the lamina propria (T1) and carcinoma in situ (Tis). Each of these tumour types differ in prognosis. Transurethral resection (TUR) is considered to be the standard treatment for single low grade Ta tumours which have a low potential to recur (Coptcoat 1998). TUR involves endoscopic visualization via the urethra, with endoscopic resection and/or cystodiathermy to destroy the tumour. TUR is also the initial treatment for multifocal higher grade Ta tumours, T1 and Tis with the aim of preventing tumour recurrence and disease progression. However, new tumours (recurrence) will develop in 50% to 70% of these patients and, when recurrences are numerous, it becomes difficult to ablate all the tumours by this procedure. In about 10% to 30% of these cases, the tumours recur with invasion into the muscle layer of the bladder (tumour progression) resulting in a poorer prognosis because of the potential to metastasize (Raghavan 1990).
Direct instillation of selected chemotherapeutic agents into the bladder (intravesical therapy) is often used as an adjunct to TUR to improve the clinical outcome in superficial disease as defined by a reduction in tumour recurrence. Among these agents is the non‐specific immune stimulant Bacillus Calmette‐Guérin (BCG) first used by Morales in 1976 (Morales 1976) to treat superficial bladder cancer. Whilst the precise mechanism is unknown, intravesical BCG elicits a local host immune response against tumour cells accompanied by the release of interleukin‐1, interleukin‐2 and tumour necrosis factor (Bohle 1990). Intravesical BCG has been reported to reduce the number of recurrences and tumour progression in Ta and T1 disease, and induce complete remission in Tis (Lamm 1995). Side effects may occur with intravesical BCG, both locally (cystitis) and centrally (fever, malaise, nausea). They can be severe in about 5% of patients and a few fatalities have been reported. A number of different strains of BCG have been used for intravesical therapy and although some comparisons have been made in clinical trials (Fellows 1994), there are no clear guidelines as to what strain to use. A number of different doses and schedules have been used (Hudson 1987), including concomitant percutaneous administration of BCG (Lamm 1991) and questions remain as regards optimization of these parameters. Intravesical BCG appears to be the preferred agent in the USA and Canada for prophylaxis against recurrence in Ta and T1, and treatment of Tis. The recently published clinical guidelines convened by the American Urological Association (Smith 1999), recommends the use of either intravesical BCG or mitomycin‐C for Ta, T1 and Tis bladder cancer. However, studies by the European Organisation for Research and Treatment of Cancer comparing intravesical BCG against mitomycin‐C in superficial bladder cancer have been inconclusive (Debruyne 1989).
Our goal was to conduct a systematic review and, where possible, a meta‐analysis to evaluate and quantify the efficacy and safety of intravesical BCG following TUR compared with TUR alone. A subsequent review will be concerned with comparing intravesical BCG with other intravesically administered agents in superficial bladder cancer.
Objectives
The primary aim was to assess the efficacy of intravesically administered BCG following TUR in patients with Ta and T1 superficial bladder cancer compared to TUR alone. Efficacy is defined as tumour recurrence, as measured by the number of patients developing recurrence at 12 months and the hazard ratio. The secondary objective was to evaluate and compare adverse events in the two treatment groups. A tertiary aim was to undertake a subgroup analysis to assess the effects of BCG strain, dose and schedule on the above clinical outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled clinical trials in superficial bladder cancer, comparing intravesical BCG plus TUR with TUR alone. All randomised studies in which other intravesical chemotherapeutic agents have been used but have a BCG plus TUR arm and a TUR arm were included.
Types of participants
Studies on adults with histologically confirmed Ta and T1 superficial bladder cancer. Eligible patients were those that had medium or high risk of recurrence according the criteria of Hall 1994. These criteria were as follows: Medium risk are those patients with solitary tumour at presentation and tumour recurrence at 3 months or multiple tumours at presentation and no tumour at 3 months. High risk patients are those with multiple tumours at presentation and recurrence at 3 months. To assess for the effects of TUR and intravesical BCG on tumour progression, patients should be at risk of progression based on the criteria of Kurth 1995, which were high grade, recurrent tumours greater than 3 centimetres in diameter.
Types of interventions
All randomised or quasi‐randomised studies comparing intravesically administered BCG plus TUR with TUR alone. BCG of any strain, dose and schedule would be considered appropriate.
Types of outcome measures
The main outcome measure in this review was treatment efficacy, as measured by the time to recurrence after treatment, and the number of patients that recur at 12 months post‐TUR. Local toxicities such as cystitis, haematuria and urinary frequency, and systemic toxicities including fever, malaise and nausea were assessed.
Search methods for identification of studies
An electronic search of Medline was undertaken to identify all relevant randomised clinical trials comparing intravesical BCG plus TUR against TUR alone in superficial bladder cancer before October 1999. The search strategy was as follows:
randomised controlled trial.pt
controlled clinical trial.pt
randomised controlled trials/
random allocation/
double blind method/
single‐blind method/
or/1‐6
(animal not human).sh.
7 not 8
clinical trial.pt.
exp clinical trials/
(clin$adj25 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
placebos/
placebo$.tw.
random$.tw.
research design/
or/10‐17
18 not 8
19 not 9
comparative study/
exp evaluation studies/
follow up studies/
prospective studies/
(control$ or prospectiv$ or volunteer$).tw
or/2‐25
26 not 8
26 not (9 or 20)
9 or 20 or 28
exp bladder neoplasm/
exp BCG vaccine/
intravesic$.tw.
install$.tw.
region$.tw
or/31‐34
29 and 30 and 35
Additional electronic searches of the following databases were conducted: EMBASE (Excerpta Medica Database), Cancerlit, Database of Abstracts of Reviews of Effectiveness and the Cochrane Library. Hand searching of recent Proceedings of the American Society for Clinical Oncology was also undertaken (1996 to 1999). The reference list contained within each primary reference was scrutinised for additional randomised trials. Reports of randomised trials in any language were eligible for assessment.
Data collection and analysis
Data were extracted independently by two reviewers. Discrepancies were resolved by discussion. All randomised controlled clinical trials were included for assessment. Criteria to assess concealment (randomisation) was scored according to the Cochrane guidelines: A: adequate, B: unclear, C: concealment not used. All studies that met the inclusion criteria were evaluated and analysed according to treatment allocation concealment.
Our major outcome measure in this review is tumour recurrence. A common method for expressing published time‐to‐event data, i.e. time to first tumour recurrence, is to plot the proportion of patients remaining free of tumour recurrence per month for the complete study period ( Kaplan‐Meier plot). A useful measure for summarizing this type of curve is to determine the hazard rate which gives the overall rate of tumour recurrence. In this review we are concerned with two groups of patients, namely those that have TUR plus BCG and those receiving TUR alone, and therefore need to compare the differences between the respective survival curves. One way to do this is to calculate the hazard ratio (hr) defined as the ratio of the two hazard rates. A ratio of 1 indicates that both groups have the same incidence of tumour recurrence per unit time, whereas a ratio of less than 1 indicates that the rate of recurrence is less in the treatment group (TUR plus BCG). The hazard ratio can vary from zero to infinity and the value of 1 is not the central value. Therefore to normalise the scale, the hazard ratio is log transformed (ln(hr)).
In the case of published data based on the time to first recurrence, or when the recurrence frequency was reported at specific times, an attempt was made to calculate the log hazard ratio (ln(hr)) and its variance using Cox regression. Where a Kaplan‐Meier plot of time to first recurrence was given, the method of Parmar (Parmar 1998) was used to estimate ln(hr). The relative risk (Parmar 1998) was used to approximate the ln(hr) when only the number of recurrences at 12 and 18 months was presented. The ln(hr) for all the included trials were combined as a weighted mean using the reciprocal of the variance (Parmar 1998).
The odds ratio for patients who have recurrent tumours at 12 months was also calculated for both the TUR plus BCG group and the TUR alone group.
Results
Description of studies
The search identified 26 published trials comparing TUR plus intravesical BCG versus TUR alone. Six trials were included for analysis and were as follows: Krege 1996, Lamm 1985, Pagano 1990, Pinsky 1985, Melekos 1990 and Yamamoto 1990. Two trials (Chopin 1990 and Somogyi 1993) were non‐randomised and excluded on this basis. The trial by Badalament 1987 was excluded because randomisation took place after all participants had received intravesical BCG. The randomised trial by Ibrahiem 1988 was also excluded because there was insufficient data to determine the patient's risk category. The remaining reports were excluded as they were duplicate publications of the included trials.
Risk of bias in included studies
Four trials specified the randomisation procedure used and were accepted to be truly randomised. The two other included studies stated that the trials were randomised but the methods were not reported. Based on the participant inclusion criteria, four studies were considered as including medium/high risk patients (Krege 1996;Lamm 1985;Pagano 1990;Pinsky 1985 = group 01). However, in the former two only the patients with recurrent bladder cancer were included for analysis. The remaining two trials (Melekos 1990;Yamamoto 1990) were included (group 02), although it was unclear if there was a proportion of low risk patients as part of the study populations. A sensitivity analysis was performed to ascertain the influence of the latter two trials on the overall outcome.
Effects of interventions
The six trials included for analysis represented 585 eligible patients, 304 in the BCG plus TUR arm and 281 in the TUR alone arm. The mean age was 65 and 64 in the TUR alone group and TUR plus BCG group, respectively, with the corresponding male to female ratios of 3.9 and 3.5. The mean percentage of patients with Ta and T1 tumours were 49% and 51% (TUR) and 41% and 59% (TUR plus BCG). The maximum follow up duration for the 6 studies ranged from 14 to 36 months.
The calculated log hazard ratios and variance (BCG + TUR/TUR) for tumour recurrence are tabulated in 'Table 3'. The trials were initially divided into two groups. Group 01 trials were considered to include only medium or high risk patients for recurrence, and in group 02 trials, it was unclear if a proportion of low risk patients were included. The log hazard ratio and variance for group 01 was ‐0.78 with a variance of 0.02. This converts into a hazard ratio of 0.46 and indicates that intravesical BCG following TUR reduces the hazard of recurrence by 54% compared to TUR alone. The log hazard ratio and variance for group 02 was ‐0.98 and 0.08 which translates into a 62% reduction in the hazard with BCG. Both subgroup meta‐analyses are in the same direction i.e. favouring the BCG plus TUR arm. A combined meta‐analysis of all the trials produced an overall log hazard ratio of ‐0.83 (variance 0.02) which corresponds to a hazard ratio of 0.44. This indicates that a 56% reduction in the probability of tumour recurrence per unit time was associated with the BCG plus TUR group compared to TUR alone.
1. The log hazard ratios with variances.
| Trial | ratio BCG/Control | variance |
| (Medium/High risk Patients) | ||
| Krege 1996 | ‐ 0.482 | 0.058 |
| Lamm 1985 | ‐ 0.613 | 0.462 |
| Pagano 1990 | ‐ 1.740 | 0.150 |
| Pinsky 1985 | ‐ 0.742 | 0.049 |
| Subgroup Meta‐analysis | ‐ 0.7827 | 0.0215 |
| Medium/high risk but unclear if some low risk patients included | ||
| Melekos 1990 | ‐ 0.818 | 0.099 |
| Yamamoto 1990 | ‐ 1.520 | 0.323 |
| Subgroup Meta‐analysis | ‐ 0.9827 | 0.0758 |
| Overall Meta ‐analysis: Weighted Sum of ln(HR) | ‐ 0.8269 | 0.0168 |
The total number of patients presenting with tumour recurrence at 12 months was 79 (26%) in the BCG plus TUR group and 144 (51%) in the TUR alone group. Odds ratio for tumour recurrence at 12 months was calculated using the numbers at risk either stated explicitly (Pagano 1990;Melekos 1990;Lamm 1985) or estimated from the maximum and minimum follow‐up times assuming uniform censoring [Yamamoto 1990]. In the case of Pinsky (Pinsky 1985) and Krege (Krege 1996) the number of recurrences and cases at risk at 12 months were estimated from the Kaplan‐Meier plot (Parmar 1998).
The Peto odds ratios determined for the number of patients recurring at 12 months, are illustrated graphically using a Forest plot (figure 1). The odds ratio for each trial is shown by a solid square and the horizontal line through it represents the 95% confidence intervals. The vertical central line represents an odds ratio of 1 and results falling on this line indicate no difference between the patients treated with TUR plus BCG and those treated with TUR alone. The six trials were divided into two groups as described above for the meta‐analysis of the log hazard ratios.
The odds ratio and confidence intervals for group 01 (definitely medium/high risk patients) was 0.33 (95% CI 0.22,0.50) and that for 02 group (unclear if low risk patients were included) was 0.22 (95% CI 0.10 to 0.46). Both these values indicate a beneficial effect of intravesical BCG after TUR in reducing the number of tumour recurrences at 12 months. The overall Peto odds ratio for all the trials combined was 0.33 (95% CI 0.23 to 0.47) indicating that there was a 67% reduction in the incidence of tumour recurrence at 12 months in the BCG plus TUR group compared to the TUR alone group. There was no evidence of heterogeneity on combining all the trials (heterogeneity statistic, Q = 9.34, df = 5, P = 0.096), which suggests that the trials were statistically similar and that combining them for a meta‐analysis was justified.
The strains of BCG, doses and schedules are illustrated in 'Table 4'. Four trials used Pasteur BCG, one Connaught and one Tokyo. Five trials used an initial 6 week treatment, whereas one (Melekos 1990) used an eight‐week treatment. Krege 1996 followed the initial BCG therapy with monthly treatment for 4 months, and Yamamoto continued every 2 weeks for 6 weeks then monthly for 20 months. Pagano followed their initial BCG treatment with monthly BCG for 12 months then 3 monthly for 3 months if no tumour was evident, or a repeat of the initial 6 weeks of BCG if tumour was found. Doses of BCG varied from 75 mg (n = 1), 80 mg (n = 1), 120 mg (n = 3) and 150 mg (n = 1). However, the more important value for the colony forming unit (CFU), which represents the biological activity of the intravesically administered BCG rather than the dry weight in milligrams, was only reported in two studies (Melekos 1990;Pagano 1990). The duration of instillation was either for one hour (Krege 1996) or two hours and three trials gave concomitant intradermal BCG with doses of 0.5 mg (Krege 1996), 5 mg (Lamm 1985) and 5 x 10(7) CFU (Pinsky 1985). The wide range of doses, schedules and the different strains of BCG used in such a small number of studies, precludes any informative analysis as to the association of these variables with outcome.
2. Strain, Dose and Schedule of BCG administration.
| Krege 1996 | Lamm 1985 | Melekos 1990 | Pagano 1990 | Pinsky 1985 | Yamamoto 1990 | |
| Strain | Connaught | Pasteur | Pasteur | Pasteur | Pasteur (Armand Frappier | Tokyo |
| weekly dose (mg) | 120 | 120 | 150 | 75 | 120 | 80 |
| CFU | not given | not given | 6 x 10 (8) | 4‐5 x 10 (8) | not given | not given |
| Duration of instillation (h) | 1 | 2 | 2 | 2 | 2 | 2 |
| Schedule | weekly x 6, mnthly x 4 | weekly x 6 | weekly x 8 | weekly x 6, (if no tumour = mthly x 12 then 3 mthly x 3, if tumour present = additional weekly x 6) | weekly x 6 | weekly x 6, every 2 weeks x 6, mthly x 20 |
| Concomitant intradermal or percutaneous BCG | 0.5mg | 5mg | no | no | 5 X 10 (7) | no |
Only the side effects induced with intravesical BCG were reported in the included trials ('Table 5') although Pagano 1990 states a 2% incidence of orchidoepididymitis with TUR alone. The main toxicities associated with BCG were cystitis (67%), haematuria (23%), fever (25%) and urinary frequency (71%). No BCG sepsis or deaths were reported.
3. BCG‐Induced Toxicities (%).
| Krege 1990 | Lamm 1985 | Melekos 1990 | Pagano 1990 | Pinsky 1985 | Yamamoto 1990 | Mean (%) | |
| cystitis | 34 | 93 | 84 | 27 | 88 | 76 | 67 |
| Haematuria | 6 | 34 | 21 | 3 | 58 | 14 | 23 |
| Fever | 18 | 28 | 27 | 16 | 44 | 14 | 25 |
| Frequency | ‐ | 90 | ‐ | ‐ | 51 | ‐ | 71 |
| Flu‐like | ‐ | 7 | 10 | ‐ | 28 | ‐ | 15 |
| Nausea | ‐ | 11 | 7 | ‐ | 5 | ‐ | 8 |
| Malaise | ‐ | 10 | 7 | ‐ | 26 | ‐ | 14 |
| Prostatitis | 5 | 1 | 1 | 2 | 2 | ‐ | 3 |
| Epididymitis | 10 | 1 | ‐ | 2 | ‐ | ‐ | 6 |
| allergic | 3 | ‐ | ‐ | ‐ | 19 | ‐ | 10 |
| Contracted bladder | ‐ | 0 | ‐ | 1 | 0 | 10 | 2 |
| BCG‐Sepsis | ‐ | 0 | ‐ | 0 | 0 | ‐ | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
Superficial transitional cell carcinoma of the bladder has the propensity to recur in 50% to 70% of patients. This means that frequent follow‐up with cystoscopy is required to monitor patients and remove any recurrent tumour if present. This not only decreases the quality of life for patients with this disease, but also has considerable cost implications for health care. Interventions that can reduce tumour recurrence would be of enormous benefit. The results of this present meta‐analysis of available published randomised trials, indicates that immunotherapy with intravesical BCG, as an adjunct to TUR, is significantly more effective in reducing the number of patients with tumour recurrence at 12 months and delaying the time to recurrence, compared to TUR alone.
It was originally intended to evaluate the effect of intravesical BCG on disease progression and survival in patients with superficial bladder cancer. However, in order to assess disease progression, it is appropriate that patients included in the selected trials should be at high risk of progression. The inclusion criterion for the six trials in this review was based on medium/high risk for tumour recurrence (Hall 1994) which does not necessarily indicate these patients were at high risk of progression. Kurth 1995 evaluated prognostic factors affecting progression in 576 patients with superficial bladder cancer and found that high tumour grade, tumour size (> 3 cm) and previous recurrence were powerful predictors. Only two trials in the present report stated tumour size. Yamamoto 1990 reported 14% and 74% of patients with < 1 cm and < 5 cm tumours, respectively, and Krege 1996 reported only 6% of patients with tumours > 3 cm. Four of the six studies reported tumour grade. In the studies by Krege 1996, Melekos 1990 and Yamamoto 1990, 36%, 35% and 23% of patients presented with G1, respectively. Although all trials, except for that of Lamm 1985, reported data for disease progression, it was considered that this review should concentrate on tumour recurrence due to the poor or inconclusive data for progression risk.
There are two reports on the effect of intravesical BCG plus TUR on survival compared with TUR alone and both are extensions of the Pinsky 1985 trial. Herr 1995 details the results on a ten‐year follow up and Cookson 1997 reports after 15 years. In the former paper, it is stated that control patients (TUR alone) with recurring tumours were eligible to receive BCG, which makes interpretation of progression and survival data difficult. This was re‐iterated in the later paper by Cookson 1997.
The American Urological Association recently reported guidelines for the treatment of Ta and T1 bladder cancer (Smith 1999). The data for this report were derived from English articles identified in a MEDLINE search from 1966 to 1998. The use of intravesical BCG or mitomycin C were recommended for prevention of tumour recurrence, although no conclusive statement could be made regarding the delay of tumour progression. Guidance on how to decide whether to use BCG or mitomycin C was not given. It was also stated that most of the studies they reviewed, combined subjects with low grade stage Ta tumours, that are of low risk for recurrence, with T1 lesions and higher grade cancers, confounding data extraction. In the present review, no language restrictions were made, and seven medical and scientific databases were searched, including MEDLINE from 1966 to 2000, and hand searching of the recent proceedings of the American Society of Clinical Oncology was undertaken. Our review represents the total available evidence from properly conducted randomised controlled trials comparing TUR alone versus TUR plus intravesical BCG in Ta and T1 bladder cancer. Our conclusion, that intravesical BCG following TUR is more effective in preventing tumour recurrence compared to TUR alone, is in accordance with that of Smith 1999. We will address the relative clinical efficacy and morbidity of intravesical BCG compared to mitomycin C in a subsequent review.
Authors' conclusions
Implications for practice.
The evidence from the RCTs clearly indicates that intravesical BCG following TUR is effective for the prophylaxis of tumour recurrence in Ta and T1 bladder cancers. This intervention is associated with local and systemic side effects, but the majority are manageable. We would therefore advocate that intravesical BCG should be routinely considered following TUR for the prophylaxis of tumour recurrence in patients with medium to high risk Ta and T1 bladder cancer.
Implications for research.
We suggest that RCTs of sufficient power are undertaken to establish the optimal strain, dose and schedule for intravesical BCG administration. This may allow an efficacious dose to be given with fewer side‐effects. Uniformity should be given to the CFU dose, which reflects the 'active ' component, rather than the weight (mg) of BCG since there appears to be a poor correlation between the two variables (van der Meijden APM 1999). The relative efficacy of intravesical BCG compared with other intravesical agents in the treatment of superficial bladder cancer needs to be determined. Our group will focus on this aspect in another review.
What's new
| Date | Event | Description |
|---|---|---|
| 4 February 2010 | Amended | We changed a factual error in the 'plain language summary' that BCG caused tuberculosis. The corrected sentence reads "Bacillus Calmette‐Guérin (BCG) is a live attenuated bacterium used for immunization against tuberculosis, and is safe and effective for that purpose; it has also been licensed by the US FDA and other national regulatory agencies for use in superficial bladder‐cancer treatment." |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 30 May 2008 | Amended | Converted to new review format. |
| 4 July 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Mrs Benadette Coles M.Sc. for her superb librarian skills and guidance with electronic searching. We are extremely grateful to the following for their time and effort in translations: Claire Lipetz (French), Takeo Nakayama, Yuri Kajioka and Seong‐Yu Cho (Japanese) Lorenzo Manti (Spanish) and Andras Szentkuti (Hungarian). We would also like to thank Donald Gerson, who alerted us to a factual error: ""Bacillus Calmette‐Guérin (BCG), a bacterium responsible for tuberculosis, can be used in the bladder to try to prevent the cancer returning." This has been changed to "Bacillus Calmette‐Guérin (BCG) is a live attenuated bacterium used for immunization against tuberculosis, and is safe and effective for that purpose; it has also been licensed by the US FDA and other national regulatory agencies for use in superficial bladder‐cancer treatment."
Data and analyses
Comparison 1. BCG + TUR versus TUR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 number of patients recurring at 12 months | 6 | 532 | Peto Odds Ratio (Peto, Fixed, 99% CI) | 0.30 [0.18, 0.49] |
| 1.1 medium/high risk patients | 4 | 392 | Peto Odds Ratio (Peto, Fixed, 99% CI) | 0.33 [0.19, 0.58] |
| 1.2 medium/high but possibly some low risk patients | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 99% CI) | 0.22 [0.08, 0.59] |
1.1. Analysis.
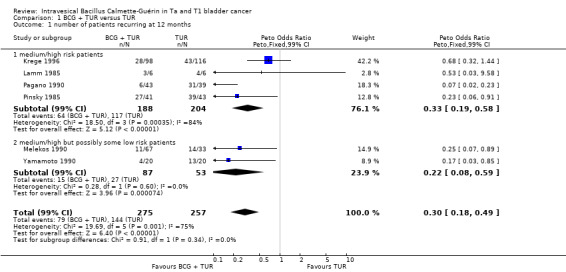
Comparison 1 BCG + TUR versus TUR, Outcome 1 number of patients recurring at 12 months.
Comparison 2. recurrence‐free survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ln(HR) | 6 | 12 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 recurrence‐free survival, Outcome 1 ln(HR).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Krege 1996.
| Methods | randomized by permuted block | |
| Participants | 224 Ta, T1, G1 ‐3 (no pTa G1 tumours) | |
| Interventions | TUR vs TUR + BCG 122: 102 Connaught intraves. 120mg 1 hr weekly X 6 then monthly x 4 plus 0.5mg subcut. | |
| Outcomes | recurrence Progression rate/number BCG toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lamm 1985.
| Methods | randomized by closed envelope | |
| Participants | 57 mean stage 1.4 and 1.8, mean No. previous recurrences 1.3 | |
| Interventions | TUR vs TUR + BCG 27: 30 Pasteur intraves. 120mg weekly X 6 plus 5mg percut. | |
| Outcomes | recurrence rate/number /time BCG toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Melekos 1990.
| Methods | randomized method not stated | |
| Participants | 100 primary or recurrent with either single or multiple Ta, T1 GI ‐III | |
| Interventions | TUR vs TUR + BCG 33: 67 Pasteur intraves. 150mg 2 hr weekly X 8 | |
| Outcomes | tumour response recurrence‐ rate/number/time progression ‐ number BCG toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pagano 1990.
| Methods | randomized method not stated | |
| Participants | 133 multiple (> 3) papillary TA, T1 | |
| Interventions | TUR vs TUR + BCG 33: 67 Pasteur intraves. 75mg 2 hr weekly X 6 | |
| Outcomes | tumour response recurrence‐ rate/number progression‐ number/rate BCG toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pinsky 1985.
| Methods | randomized by permuted block | |
| Participants | 86 ‐ multiple tumours papilloma, T1, some with Tis | |
| Interventions | TUR vs TUR + BCG 43: 43 Armand Frappier intraves. 120mg 2 hr weekly X 6 plus pecut. | |
| Outcomes | recurrence No./mnth and time progression‐ time BCG toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yamamoto 1990.
| Methods | randomized by closed envelope method | |
| Participants | 44 ‐‐ 22 pTA, 22 pT1, G 1 ‐ 3, single tumour 30, multiple 14 | |
| Interventions | TUR vs TUR + BCG 21: 23 Tokyo intraves. 80mg weekly X 6, 2 weekly x 12, mnthly x 20 | |
| Outcomes | recurrence‐ rate progression number BCG toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Badalament 1987 | Both treatment and control groups received BCG before randomisation |
| Bassi 1990 | Repeat report of trial (Pagano 1990) |
| Camacho 1990 | Repeat report of trial (Pinsky 1985) |
| Chopin 1990 | Non‐randomised |
| Cookson 1997 | Repeat report of trial (Pinsky 1985) |
| Herr 1983 | Repeat report of trial (Pinsky 1985) |
| Herr 1985 | Repeat report of trial (Pinsky 1985) |
| Herr 1986 | Repeat report of trial (Pinsky 1985) |
| Herr 1988 | Repeat report of trial (Pinsky 1985) |
| Herr 1995 | Repeat report of trial (Pinsky 1985) |
| Ibrahiem 1988 | Insufficient data to classify risk for recurrence |
| Lamm 1980 | Repeat report of trial (Lamm 1985) |
| Lamm 1981 | Repeat report of trial (Lamm 1985) |
| Lamm 1982a | Repeat report of trial (Lamm 1985) |
| Lamm 1982b | Repeat report of trial (Lamm 1985) |
| Pagano 1989 | Repeat report of Pagano 1990 |
| Pagano 1991 | Repeat report of Pagano 1990 |
| Pinsky 1982 | Repeat report of trial (Pinsky 1985) |
| Rubben 1990 | Repeat report of Krege 1996 |
| Somogyi 1993 | Non‐randomised trial. |
Contributions of authors
Mike Shelley: primary contact, concept, protocol, literature search, data extraction and translations, quality assessment, exploratory analysis, draft manuscript
Jon Court: data extraction, manuscript review, statistical analysis
Howard Kynaston: protocol review, quality assessment, manuscript review, analysis review
Timothy Wilt: protocol review, translations, review analysis, manuscript review
Reg Fish: concept, literature search
Malcolm Mason: alternative contact, protocol review, translations, quality assessment, review analysis, review manuscript
Sources of support
Internal sources
Velindre NHS Trust, UK.
Veterans Affairs Health Services Research and Development Office, USA.
External sources
No sources of support supplied
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Krege 1996 {published data only}
- Krege S, Giani R, Meyer R, Otto T, Rubben H, Participating Clinics. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: transurethral resection only versus transurethral resection plus mitomycin C versus transurethral resection plus bacillus Calmette‐Guérin. Journal of Urology 1996;156:962‐6. [DOI] [PubMed] [Google Scholar]
Lamm 1985 {published data only}
- Lamm DL. Bacillus Calmette‐Guérin immunotherapy for bladder cancer. Journal of Urology 1985;134:40‐7. [DOI] [PubMed] [Google Scholar]
Melekos 1990 {published data only}
- Melekos MD. Intravesical Bacillus Clamette‐Guérin prophylactic treatment for superficial bladder tumors: Results of a controlled prospective study. Urology International 1990;45:137‐41. [DOI] [PubMed] [Google Scholar]
Pagano 1990 {published data only}
- Pagano F, Bassi P, Milani C, Meneghini A, Artibani W, Maruzzi D, Garbeglio A. Low dose BCG therapy in superficial bladder cancer: a clinicopathological prospective study. In: deKernion JB editor(s). Immnunotherapy of Urological Tumours: International Society of Urological Reports. New York: Churchill Livingstone, 1990:69‐81. [Google Scholar]
Pinsky 1985 {published data only}
- Pinsky CM, Camacho FJ, Kerr D, Geller NL, Klein FA, Herr H, Whitmore WF, Oettegen HF. Intravesical administration of bacillus Calmette‐Guérin in patients with recurrent superficial carcinoma of the urinary bladder: Report of a prospective randomized trial. Cancer Treatment Reports 1985;69:47‐53. [PubMed] [Google Scholar]
Yamamoto 1990 {published data only}
- Yamamoto T, Hagiwara M, Nakazono M, Yamamoto H. Intravesical bacillus Calmette‐Guérin (BCG) in the treatment of superficial bladder cancer. Japanese Journal of Urology 1990;81(7):99‐1001. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Badalament 1987 {published data only}
- Badalament RA, Herr H, Wong G, Gnecco C, Pinsky C, Whitmore W, Fair W, Oettgen H. A prospective randomised trial of maintenance versus nonmaintenance intravesical BCG therapy for superficial bladder cancer. Journal of Clinical Oncology 1987;5:441‐9. [DOI] [PubMed] [Google Scholar]
Bassi 1990 {published data only}
- Bassi PF, Milani C, Meneghini A, Maruzzi D, Garbeglio A, Aragona F, Tejerizo JC, Pagano F. Low dose BCG regimen in the treatment of superficial bladder cancer [BCG a baja dosis en la terapia de las neoplasias superficiales vesicales]. Arch. Esp. de Urol. 1990;43(5):503‐7. [PubMed] [Google Scholar]
Camacho 1990 {published data only}
- Camacho C, Pinsky C, Kerr D, Whitmore W, Oettgen H. Treatment of superficial bladder cancer with intravesical BCG. American Society of Clinical Oncology. 1980:359.
Chopin 1990 {published data only}
- Chopin D, Hoznek A, Colombel M, Surig‐Biscarat M, Antiphon P, Herve JM, Bellot J, Auvert J, Abbou CC. Results of the treatment of superficial bladder tumours by transurethral resection alone or transurethral resection followed by endovesical instillation of Calmette‐Guérin bacillus [Resultat du traitement des tumeurs superficielles de la vessie par resection transurethrale seule et resection transurethrale suivie d'instillation endovesicale de bacille Calmette‐Guérin]. Annales D'Urolgie 1990;24(5):435‐40. [PubMed] [Google Scholar]
Cookson 1997 {published data only}
- Cookson M, Herr H, Zhang Z‐F, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15 year outcome. Journal of Urology 1997;158:62‐7. [DOI] [PubMed] [Google Scholar]
Herr 1983 {published data only}
- Herr H, Pinsky C, Willet F Whitmore WF, Oettgen HF, Melamed MR. Effect of intravesical bacillus Calmette‐Guérin (BCG) on carcinoma in situ of the bladder. Cancer 1983;51:1323‐6. [DOI] [PubMed] [Google Scholar]
Herr 1985 {published data only}
- Herr H, Pinsky C, Whitmore W, Sogani PG, Oettgen HF, Melamed MR. Experience with intravesical bacillus Calmette‐Guérin therapy of superficial bladder tumours. Urology 1985;25(2):119‐23. [DOI] [PubMed] [Google Scholar]
Herr 1986 {published data only}
- Herr H, Pinsky CM, Whitmore WF, Sogani PC, Oettgen HF, Melamed MR. Long‐term effect of intravesical bacillus Calmette‐Guérin on flat carcinoma in situ of the bladder. Journal of Urology 1986;135:265‐7. [DOI] [PubMed] [Google Scholar]
Herr 1988 {published data only}
- Herr H, Laudone VP, Badalament RA, Oettgen HF, Sogani PC, Freedmen BD, Melamed MR, Whitmore WF. Bacillus Calmette‐Guérin therapy alters the progression of superficial bladder cancer. Journal of Clinical Oncology 1988;6:1450‐5. [DOI] [PubMed] [Google Scholar]
Herr 1995 {published data only}
- Herr H, Schwalb DM, Zhang Z‐F, Sogani PC, Fair WR, Whitmore WF, Oettgen HF. Intravesical bacillus Calmette‐Guérin therapy prevents tumour progression and death from superficial bladder cancer: ten‐year follow‐up of a prospective randomized trial. Journal of Clinical Oncology 1995;13(6 June):1404‐8. [DOI] [PubMed] [Google Scholar]
Ibrahiem 1988 {published data only}
- Ibrahiem E‐LI, Ghoneim MA, Nigam V, Brailovsky C, Elhilali MM. Prophylactic maltose tetrapalmitate and bacillus Calmette‐Guérin immunotherapy of recurrent superficial bladder tumours: preliminary report. The Journal of Urology 1988;140:498‐500. [DOI] [PubMed] [Google Scholar]
Lamm 1980 {published data only}
- Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette‐Guérin immunotherapy of superficial bladder cancer. The Journal of Urology 1980;124:38‐42. [DOI] [PubMed] [Google Scholar]
Lamm 1981 {published data only}
- Lamm DL, Thor DE, Winters WD, Stogdill VD, Radwin HM. BCG immunotherapy of bladder cancer: inhibition of tumour recurrence and associated immune responses. Cancer 1981;48:82‐8. [DOI] [PubMed] [Google Scholar]
Lamm 1982a {published data only}
- Lamm DL, Thor DE, Harris SC, Stogdill VD, Radwin HM. Intravesical and percutaneous BCG immunotherapy of recurrent superficial bladder cancer. In: Terry WD, Rosenberg SA editor(s). Immunotherapy of Human Cancer. New York: Elsevier North Holland, 1982:315‐22. [Google Scholar]
Lamm 1982b {published data only}
- Lamm DL, Thor DE, Stogdill VD, Radwin HM. Bladder cancer immunotherapy. The Journal of Urology 1982;128:931‐5. [DOI] [PubMed] [Google Scholar]
Pagano 1989 {published data only}
- Pagano F, Bassi P, Milani C, Meneghini A, Tuccitto G, Garbeglio A, Guazzieri S. Low‐dose BCG‐Pasteur strain in the treatment of superficial bladder cancer: preliminary results. In: Debruyne FMJ, Denis L, Meijden APM editor(s). EORTC Genitourinary Group Monograph 6: BCG in Superifial Bladder Cancer. New York: Alan R Liss Inc., 1989:253‐61. [PubMed] [Google Scholar]
Pagano 1991 {published data only}
- Pagano F, Bassi P, Milani C, Meneghini A, Maruzzi D, Garbeglio A. A low dose bacillus Calmette‐Guérin regimen in superficial bladder cancer therapy: is it effective?. The Journal of Urology 1991;146(July):32‐5. [DOI] [PubMed] [Google Scholar]
Pinsky 1982 {published data only}
- Pinsky CM, Camacho FJ, Kerr D, Braun DW, Whitmore WF, Oettgen HF. Treatment of superficial bladder cancer with intravesical BCG. In: Terry WD, Rosenberg SA editor(s). Immunotherapy of Human Cancer. New York: Elsevier North Holland, 1982:309‐13. [Google Scholar]
Rubben 1990 {published data only}
- Rubben H, Graf‐Dobberstein C, Ostwald R, Stauffenberg A, Jaeger N, Deutz FJ, Steffens L, Giani C. Prospective randomized study of adjuvant therapy after complete resection of superficial bladder cancer; mitomycin C vs BCG Connaught vs TUR alone. In: deKernion JB editor(s). International Society of Urological Reports: Immunotherapy of urological Tumours. 1990:27‐36. [Google Scholar]
Somogyi 1993 {published data only}
- Somogyi L, Szanto A, Polyak L, Baranyay F, Drinoczy M. Adjuvant BCG immunotherapy in the treatment of superficial bladder tumours [BCG immunterapia a felutes holyagtumorok adjuvans kezeleseben]. Orvosi Hetilap 1993;134(34):1851‐1856. [PubMed] [Google Scholar]
Additional references
Bohle 1990
- Bohle A, Nowc C, Ulmer AJ, et al. Detection of urinary TNF, IL1, and IL2 after local BCG immunotherapy for bladder carcinoma. Cytokine 1990;2:175‐81. [DOI] [PubMed] [Google Scholar]
Coptcoat 1998
- Coptcoat MJ, Oliver RTD. The role of surgery in the multimodality treatment of bladder cancer. In: Oliver RTD, Coptcoat MJ editor(s). Cancer Surveys: Bladder Cancer. Vol. 31, New York: Cold Spring Harbor Press, 1998:129‐47. [PubMed] [Google Scholar]
Debruyne 1989
- Debruyne FM, Meijden AP, Franssen MPH. BCG‐(RIVM) versus mitomycin intravesical therapy in patients with superficial bladder cancer. Therapeutic Progress in Urologic Cancers 1989;Alan R Liss Inc:435‐46. [PubMed] [Google Scholar]
Fellows 1994
- Fellows GJ, Parmar MKB, Grigor KM, et al. Marker tumour response to Evans and Pasteur bacille Calmette‐Guérin in multiple recurrent pTa/pT1 bladder tumours: report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). British Journal of Urology 1994;73:639‐644. [DOI] [PubMed] [Google Scholar]
Hall 1994
- Hall RR, Parmar MKB, Richards AB, Smith PH. Proposal for changes in cystoscopic follow up of patients with bladder cancer and adjuvant intravesical chemotherapy. British Medical Journal 1994;308(22nd January):257‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hudson 1987
- Hudson M, Ratcliff TL, Gillen DP, et al. Single course versus maintenance Bacillus Calmette‐Guérin therapy for superficial bladder tumours: a prospective, randomized trial. J Urol 1987;138:295‐8. [DOI] [PubMed] [Google Scholar]
Kurth 1995
- Kurth KH, Denis L, Bouffioux Ch, Sylvester R, Debruyne FMJ, Pavone‐Macaluso M, Oosterlinck W. Factors affecting recurrence and progression in superficial bladder tumours. European Journal of Cancer 1995;31A(11):1840‐6. [DOI] [PubMed] [Google Scholar]
Lamm 1991
- Lamm DL, DeHaven JI, Shriver J, et al. Propective randomized comparison of intravesical with percutaneous bacillus Calmette‐Guerin versus intravesical bacillus Calmette‐Guérin in superficial bladder cancer. Journal of Urology 1991;145:738‐40. [DOI] [PubMed] [Google Scholar]
Lamm 1995
- Lamm DL. BCG immunotherapy for carcinoma in situ of the bladder. Oncology 1995;9:947‐56. [PubMed] [Google Scholar]
Morales 1976
- Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette Guérin in the treatment of superficial bladder tumours. Journal of Urology 1976;137:180‐3. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine. Vol. 17, John Wiley & Sons Ltd., 1998:2815‐34. [DOI] [PubMed] [Google Scholar]
Raghavan 1990
- Raghavan D, Shipley WU, Garnick MB, Russel PJ, Richie JP. Biology and management of bladder cancer. New England Journal of Medicine 1990;322(16):1129‐38. [DOI] [PubMed] [Google Scholar]
Smith 1999
- Smith JA, Labasky RF, Cockett AT, Fracchia JA, Montie JE, Rowland RG. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS). Journal of Urology 1999;162:1697‐1701. [PubMed] [Google Scholar]
van der Meijden APM
- Meijden APM. Bacillus Calmette‐Guérin (BCG) for transitional cell carcinoma of the bladder. In: Reginald R Hall editor(s). Clinical Management of Bladder Cancer. London: Arnold, 1999:125‐47. [Google Scholar]
Wingo 1995
- Wingo WJ, Tong T, Bolden S. Cancer Statistics, 1995. CA 1995;45:8‐30. [DOI] [PubMed] [Google Scholar]
Young 1996
- Young RH. Pathology of Bladder Cancer. In: Vogelzang N, Scardino P, Shipley W, Coffey D editor(s). Comprehensive Textbook of Genitourinary Oncology. Baltimore Maryland: Williams & Wilkins, 1996. [Google Scholar]


