Abstract
The advent of direct-acting antiviral (DAA) therapies has dramatically transformed HCV treatment, with most recent trials demonstrating high efficacy rates (>90%) across all genotypes and special populations, including patients with HIV/HCV coinfection. The efficacy rates of HCV treatment are nearly identical between patients with HCV monofection and patients with HIV/HCV coinfection; however, there are limited studies to compare real-world efficacy with efficacy observed in clinical trials. Using a database from HIV clinics across the United States (US), we identified 432 patients with HIV/HCV coinfection who completed DAA therapy from January 1, 2014 to March 31, 2017 and were assessed for efficacy. Efficacy was evaluated as sustained virologic response (SVR) 12 weeks after DAA completion; furthermore, factors associated with achieving SVR12 were identified. In this analysis, we found DAA therapies to be effective, with 94% of the patients achieving SVR12 and 6% experiencing virologic failure. Baseline variables, including older age, HCV viral load <800K IU/ML, FIB-4 score <1.45, absence of depression, diabetes, substance abuse, and use of DAA regimens without ribavirin were significant predictors of achieving SVR12. Patients with fewer comorbidities, better liver health, and lower HCV viral loads at baseline were more likely to achieve treatment success. Our results were consistent with other real-world studies, supporting the use of HCV therapy in HIV/HCV coinfected patients.
Introduction
It is estimated that 2.27 to 2.75 million people around the world have hepatitis C virus (HCV) and human immunodeficiency virus (HIV) coinfection.[1–3] In the United States (US), of the people living with HIV (PLWH), about 25% are estimated to have HCV infection, which consequently triples the risk for liver disease, liver failure, and liver related death.[4] Nearly 75% to 82% of PLWH who inject drugs are also infected with HCV.[2,4,5] Prior to potent combination antiretroviral therapy (ART), early studies suggested that progression to fibrosis is accelerated in patients with HIV/HCV coinfection. Factors affecting disease progression in this population include increased alcohol use, older age, longer duration of HCV infection, and immunosuppression (CD4 < 200 cells/mm3). More recent data, however, show a similar course of progression in HIV positive patients primarily due to sufficient immune reconstitution in the modern ART era.[6–9] Notwithstanding, HIV/HCV coinfection has been associated with increased risk of death in large cohort studies compared to HIV monoinfection.[9–12] Advanced liver disease accounted for 13% of all deaths among PLWH prior to treatment with direct-acting antivirals (DAAs), and in the US chronic HCV infection has been shown to be the leading cause of liver disease and related mortality in coinfected individuals.[13–17]
In the past, persons with HIV/HCV coinfection achieved lower rates of sustained virologic response (SVR) after use of interferon and ribavirin HCV treatment compared with those with HCV mono-infection.[5] In addition to poor efficacy, older HCV treatment regimens were associated with many adverse effects such as depression, anemia, flu-like symptoms, and were often contraindicated due to additional comorbidities within the PLWH.[18–20] With the development of interferon-free DAA therapies, the treatment platform for PLWH has been revolutionized and efficacy in patients with HIV/HCV coinfection has improved. There are recent published clinical trials that reported high rates of SVR (>90%) with DAA therapy in patients with HIV/HCV coinfection.[21] However, the rigid inclusion criteria in these clinical trials may not be an accurate representation of patients with HIV/HCV coinfection in clinical care. [22–27] Many co-infected patients, especially those in urban clinics, struggle with psychosocial issues such as drug and alcohol abuse, unstable housing, and mental health disparities. Access to medications due to individual state restrictions as well as drug interactions with antiretrovirals remain a challenge in this patient population. Substance abuse remains a common restriction on many state Medicaid plans.[20, 28–31]
HCV treatment can be complicated with the number of drug-drug interactions associated with antiretroviral therapies; however, the Department of Health and Human Services (DHHS) recommends first-line ART regimens with minimal drug interactions and that are compatible with most DAA therapies. There has been concern that SVR results obtained in clinical trials may not be generalizable due to the selection of patients or stringent inclusion criteria; however a number of real-world studies in the US have been completed and reported high rates of both adherence and SVR in the HIV/HCV coinfected population.[32–38] Some of these studies have evaluated efficacy of specific DAA therapies in one clinic/hospital setting with small patient sample sizes (range 30–109 patients) using US data.[32,33,35,37,38] One study by Del Bello et al evaluated predictors of treatment failure, which included fibrosis-4 (FIB-4) score ≥3.25, low AST levels, and use of simeprevir/sofosbuvir/ribavirin (SMV/SOF/RBV); however, the sample consisted of 89 patients with genotype 1 treated in a hospital setting in New York, USA.[34] These studies have added valuable real-world evidence by reporting high SVR rates comparable to those seen in clinical trials, but had some limitations such as small patient numbers, use of data prior to 2018, and single sites and thus are not easily generalizable to other institutions and geographic regions.
There are limited data from multi-site HIV clinics across the US, including patients with multiple HCV genotypes and all DAA therapies, and identifying factors associated with treatment response. Our objective for this analysis was to evaluate the SVR rates assessed at 12 weeks after completion of DAA therapy and to identify predictors of SVR12 achievement among patients with HIV/HCV coinfection using a multi-site HIV database.
Materials and methods
Study design and data
This was an observational retrospective cohort study using real-world data with linked electronic medical records (EMR) and pharmacy dispense data from a proprietary longitudinal database established by Trio Health. The database contains records of over 38,000 HIV patients from 50 US states and Puerto Rico. The data generated through the EMR are available for research purposes and include information on patient clinical and demographic characteristics, prescriptions and dispenses, coded diagnoses, and laboratory test results collected at 11 large HIV-treating US clinics since 2014. This study was approved as an Institutional Review Board (IRB) exemption under category 45 CFR 46 that waived the requirement for informed consent, since this was a retrospective database study without direct patient contact using deidentified data.
Study population
Patients coinfected with HCV/HIV, aged ≥18 years old, who initiated DAA +/- ribavirin therapies (HCV regimen) from January 1, 2014 to March 31, 2017 were identified. The index date was defined as the date of HCV regimen initiation using pharmacy dispensing records. Patients were followed until their SVR was assessed at 12 weeks after completion of their HCV regimen. Patients were further categorized as achieving SVR12 and not achieving SVR12. Baseline characteristics included, age, gender, ethnicity, geographic region, body mass index (BMI), fibrosis burden (FIB-4 score), transplant status, and comorbidities (cancer, cirrhosis, depression, diabetes, hepatitis B infection, heart disorders [cardiovascular disease, coronary artery disease, aortic aneurysm], chronic kidney disease, substance abuse). Collected laboratory values included HCV genotype, initial viral load, alanine aminotransferase (ALT) levels, aspartate aminotransferase (AST) levels, total bilirubin levels, absolute CD4+ T-cell counts (CD4 count), and HIV viral load.
Study outcomes
The outcomes of the study were to evaluate the rates of SVR12 and to identify factors associated with SVR12 among HCV/HIV coinfected patients. SVR12 was defined as an undetectable plasma HCV RNA at 12 weeks after completion of HCV regimen.
Statistical analysis
Unadjusted descriptive statistics summarized patient characteristics of the two study cohorts (achieving SVR12 versus not achieving SVR12). Differences between these patient groups were tested using Student’s t test or Mann-Whitney test for continuous variables and chi squared statistic or Fisher’s exact tests for categorical variables. A multivariable logistic regression model was generated with SVR12 success as the dependent variable. Some of the potential risk factors for the model included demographic information (age, gender, ethnicity, geographic region); indices of liver disease severity (cirrhosis, FIB-4 score>3.25, and liver transplant); HCV genotype; laboratory results such as HCV viral load, absolute CD4 cell count, ALT, AST levels; comorbidities (depression, diabetes, chronic kidney disease, substance abuse, etc.). A backwards stepwise selection process was used to include factors with the significance level of P ≤ 0.2 for the final model. Odds ratio (OR) and 95% confidence intervals (95% CI) for predictors of achieving SVR from the logistic regression model were reported. All analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC). A two-sided P value <0.05 was considered statistically significant.
Results
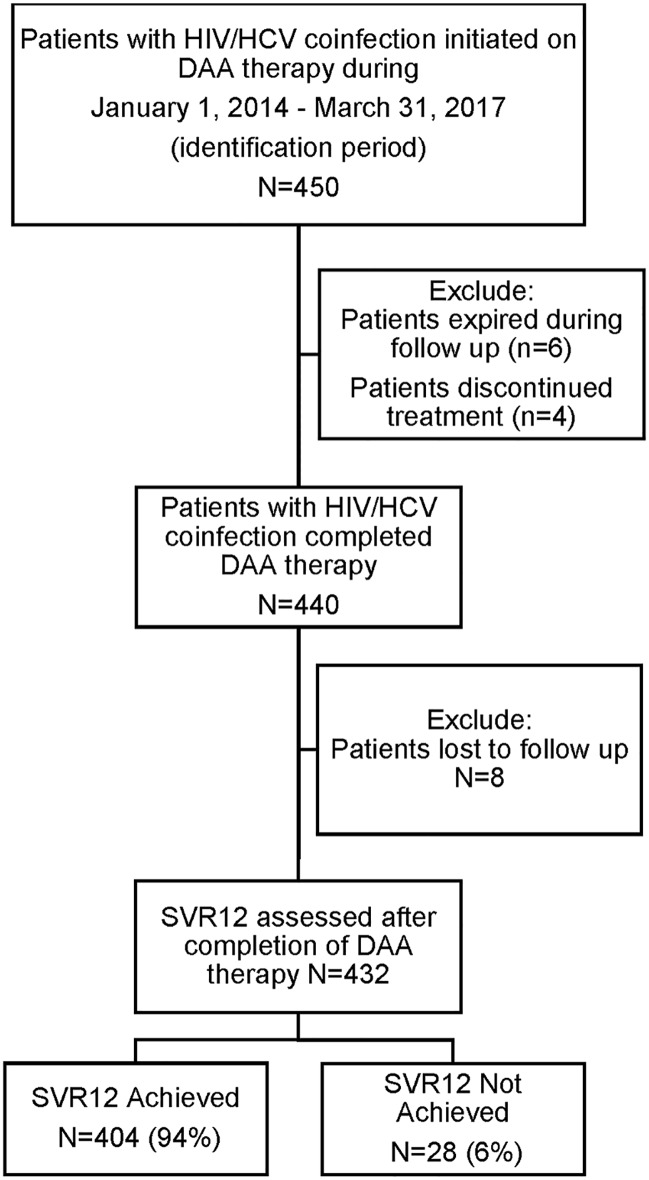
A total of 450 HCV/HIV coinfected patients were identified on HCV regimens from January 1, 2014 to March 31, 2017. Of them, 6 patients died during the follow-up period, 4 discontinued treatment, and 8 were lost to follow-up. The final study cohort included 432 patients who completed DAA therapy and were evaluated for SVR12 (Fig 1).
Fig 1. Patient cohort flow diagram.
DAA = direct acting antiviral; HIV = human immunodeficiency virus; HCV = hepatitis C virus; SVR12 = sustained virologic response assessed 12 weeks after completion of DAA therapy.
Of the patients who completed therapy and were assessed for SVR12, 404 patients (94%) achieved SVR12 and 28 patients (6%) experienced virologic failure.
Patient characteristics
The baseline clinical and demographic patient characteristics are described in Table 1. Patients who achieved SVR12 (SVR12 group) were older than those who did not achieve SVR12 (non-SVR12 group) with mean age 55.3 years vs 51.7 years as shown in Table 1.
Table 1. Baseline clinical and demographic characteristics.
| Total Population N = 432 |
SVR12 N = 404 |
No SVR12 N = 28 |
P-valuea | |
|---|---|---|---|---|
| Age (years), mean ± SD | 55.7±9.7 | 55.3±9.2 | 51.7±9.4 | 0.039 |
| Gender, n (%) | 0.561 | |||
| Male | 335 (77.5%) | 312 (77.2%) | 23 (82.1%) | |
| Female | 96 (22.2%) | 91 (22.5%) | 5 (17.8%) | |
| Unknown | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) | |
| Ethnicity, n (%) | 0.101 | |||
| Black | 236 (54.6%) | 225 (55.7%) | 11 (39.3%) | |
| White | 101 (23.4%) | 90 (22.3%) | 11 (39.3%) | |
| Hispanic | 73 (16.9%) | 67 (16.6%) | 6 (21.4%) | |
| Unknown | 22 (5.1%) | 22 (5.5%) | 0 (0.0%) | |
| Insurance Type, n (%) | 0.587 | |||
| Commercial | 54 (12.5%) | 51 (12.6%) | 3 (10.7%) | |
| Medicaid | 148 (34.3%) | 136 (33.7%) | 12 (42.9%) | |
| Medicare | 81 (18.7%) | 78 (19.3%) | 3 (10.7%) | |
| Self-pay | 13 (3.0%) | 13 (3.2%) | 0 (0.0%) | |
| Other or unknown | 136 (31.4%) | 126 (31.2%) | 10 (35.7%) | |
| Geographic Region, n (%) | 0.819 | |||
| Northeast | 193 (44.7%) | 179 (44.3%) | 14 (50.0%) | |
| Midwest | 150 (34.7%) | 142 (35.2%) | 8 (28.6%) | |
| South | 84 (19.4%) | 78 (19.3%) | 6 (21.4%) | |
| West | 5 (1.2%) | 5 (1.2%) | 0 (0.0%) | |
| BMIb, n (%) | 0.893 | |||
| Underweight: <18.5 | 8 (1.8%) | 8 (2.4%) | 0 (0.0%) | |
| Normal weight: 18.5 to 24.9 | 168 (28.9%) | 156 (46.0%) | 12 (48.0%) | |
| Overweight: > 25.0 to 29.9 | 103 (23.8%) | 96 (28.3%) | 7 (28.0%) | |
| Obese: ≥30 | 85 (19.7%) | 79 (23.3%) | 6 (24.0%) | |
| Unknown | 68 (15.7%) | 65 (16.1%) | 3 (10.7%) | |
| Genotype, n (%) | ||||
| Not genotyped | 2 (0.5%) | 2 (0.5%) | 0 (0.0%) | 0.713 |
| Genotyped | ||||
| GT1Ac | 275 (63.7%) | 256 (63.4%) | 19 (67.9%) | 0.936 |
| GT1B | 92 (21.2%) | 87 (21.5%) | 5 (17.9%) | |
| GT1X | 7 (1.6%) | 6 (1.5%) | 1 (3.6%) | |
| GT2 | 25 (5.8%) | 23 (5.7%) | 2 (7.1%) | |
| GT3 | 27 (6.3%) | 26 (6.4%) | 1 (3.6%) | |
| GT4 | 4 (0.9%) | 4 (1.0%) | 0 (0.0%) | |
| Baseline Labs | ||||
| CD4 count, n (%) | 325 (75.2%) | 303 (75.0%) | 22 (78.6%) | 0.826 |
| CD4 count <200 cells/ mm3, n (%) | 27 (6.3%) | 24 (7.9%) | 3 (13.6%) | 0.035 |
| Initial HCV VLd, n (%) | 358 (82.9%) | 334 (82.7%) | 24 (85.7%) | 0.128 |
| HCV VL <800K IU/ML | 92 (21.3%) | 90 (27.0%) | 2 (8.3%) | 0.021 |
| HCV VL 800K-6MM IU/ML | 210 (48.6%) | 193 (57.8%) | 17 (70.8%) | |
| HCV VL >6MM IU/ML | 56 (12.9%) | 51 (15.3%) | 5 (20.8%) | |
| HIV VL, n (%) | 357 (82.6%) | 334 (82.7%) | 23 (82.1%) | 0.701 |
| HIV VL <200 copies/ML, n (%) | 336 (77.8%) | 315 (94.3%) | 21 (91.3%) | 0.553 |
| ALTe, n (%) | 349 (80.8%) | 324 (80.2%) | 25 (89.2%) | 0.611 |
| ALT (IU/L), mean ± SD | 62.1±62.8 | 59.5±59.2 | 83.9±77.6 | 0.012 |
| ALT >55 IU/L, n (%) | 118 (27.3%) | 106 (26.2%) | 12(42.9%) | 0.001 |
| ASTf, n (%) | 342 (79.2%) | 319 (78.9%) | 23 (82.1%) | 0.419 |
| AST (IU/L), mean ± SD | 60.5±53.4 | 54.9±37.0 | 89.5±78.9 | 0.019 |
| AST >48 IU/L, n (%) | 144 (33.3%) | 129 (31.9%) | 15 (53.6%) | 0.037 |
| Total Bilirubin, n (%) | 349 (80.8%) | 324 (80.2%) | 25 (89.2%) | 0.621 |
| Total Bilirubin (mg/DL), mean ± SD | 0.9±1.1 | 0.7±0.6 | 1.1±1.4 | 0.512 |
| Total Bilirubin >1.2 mg/DL, n (%) | 45 (10.4%) | 40 (9.9%) | 5 (17.8%) | 0.022 |
| FIB-4g n, (%) | 334 (11.1%) | 312 (77.2%) | 22 (78.5%) | 0.712 |
| FIB-4 < 1.45 | 92 (21.3%) | 89 (28.5%) | 3 (13.6%) | 0.012 |
| FIB-4 1.45to ≤ 3.25 | 161 (37.3%) | 151 (48.4%) | 10 (45.5%) | |
| FIB-4 > 3.25 | 81 (18.8%) | 72 (23.1%) | 9 (40.9%) | |
| Comorbidities, n (%) | ||||
| Asthma | 62 (14.4%) | 59 (14.6%) | 3 (10.7%) | 0.570 |
| Cancer | 32 (7.4%) | 28 (6.9%) | 4 (14.3%) | 0.016 |
| Cirrhosis | 100 (23.1%) | 89 (22.0%) | 11 (40.7%) | 0.026 |
| Depression | 148 (34.3%) | 134 (33.1%) | 14 (50.0%) | 0.039 |
| Diabetes | 59 (13.6%) | 53 (13.1%) | 6 (21.4%) | 0.022 |
| Heart Disordersh | 68 (15.7%) | 67 (16.6%) | 1 (3.6%) | 0.016 |
| Hepatitis B Infection | 11 (2.5%) | 9 (2.2%) | 2 (7.1%) | 0.031 |
| Hypertension | 186 (43.1%) | 173 (42.8%) | 13 (46.4%) | 0.709 |
| Hyperlipidemia | 76 (17.6%) | 72 (17.8%) | 4 (14.3%) | 0.635 |
| Chronic kidney disease | 53 (12.2%) | 48 (11.9%) | 5 (17.9%) | 0.035 |
| Liver transplant recipient | 1 (0.2%) | 0 (0.0%) | 1 (3.6%) | 0.065 |
| Psychiatric Disorders | 87 (20.1%) | 81 (20.1%) | 6 (21.4%) | 0.860 |
| Substance abuse | 157 (36.3%) | 141 (34.9%) | 16 (57.1%) | 0.018 |
| Tobacco use | 199 (46.1%) | 183 (45.3%) | 16 (57.1%) | 0.022 |
| DAA Therapies | 0.001 | |||
| + RBVi, n (%) | 70 (16.2%) | 59 (14.6%) | 11 (39.3%) | |
| - RBV, n (%) | 362 (83.8%) | 345 (85.4% | 17 (60.7%) |
a P-value <0.05 was considered statistically significant.
b BMI = body mass index.
c GT = genotype.
d VL = viral load.
e ALT = alanine aminotransferase.
f AST = aspartate aminotransferase.
g FIB-4 = fibrosis-4 score for liver.
h Heart disorders = cardiovascular disease, coronary artery disease, aortic aneurysm.
i RBV = ribavirin.
There were no statistically significant differences between the two study groups by gender, ethnicity, insurance type, geographic region, BMI categories, or HCV genotype. Most of the patients (>70%) had genotype 1 infection (GT1A, GT1B, and GT1X) within both groups. Compared to the SVR12 group, the non-SVR12 group had significantly higher proportion of patients with CD4 counts <200 cells/mm3, HCV viral load >800K IU/ML, and FIB-4 score >3.25 as well as higher mean ALT, AST, and total bilirubin. The SVR12 group had fewer comorbidities than the non-SVR12 group, with lower rates of cancer (6.9% vs 14.3%, p = 0.016), cirrhosis (22.0% vs 40.7%, p = 0.026), depression (33.1% vs 50.0%, p = 0.039), diabetes (13.1% vs 21.4%, p = 0.022), hepatitis B infection (2.2 vs 7.1%, p = 0.031), chronic kidney disease (11.9 vs 17.9%, p = 0.035), substance abuse (34.9% vs 57.1%, p = 0.018), and tobacco use (45.3% vs 57.1%, p = 0.022).
HCV regimens
Patients in the SVR12 group were mostly on DAA therapies without concomitant ribavirin (RBV) therapy compared to the non-SVR12 group (85.4% [n = 345] vs 60.7% [n = 17]). The majority of SVR12 patients (67.3% [n = 272]) received ledipasvir-sofosbuvir (LDV+SOF) compared to 46.4% (n = 13) of the patients in the non-SVR12 group. Patients in the non-SVR12 group were more likely to be on DAA with concomitant RBV therapy (39.3% [n = 11]) compared to SVR12 group (14.6% [n = 59]). Patients who did not receive ribavirin therapy achieved treatment success 95.3% of the time, while those who received ribavirin achieved SVR12 84.3% of the time. Table 2 lists treatment success rates by regimen.
Table 2. HCV therapies and treatment success.
| Total Population N = 432 |
SVR12 N = 404 |
No SVR12 N = 28 |
|
|---|---|---|---|
| HCV Therapies | N (%) | n (% SVR12) | n (% No SVR12) |
| DAA +RBVa | 70 (16.2%) | 59 (84.3%) | 11 (15.7%) |
| SOFb + RBV | 39 (9.0%) | 32 (82.1%) | 7 (17.9%) |
| PRODc + RBV | 13 (3.0%) | 10 (76.9%) | 3 (23.1%) |
| SOF + PEGd + RBV | 6 (1.4%) | 6 (100.0%) | 0 (0.0%) |
| LDVe-SOF + RBV | 5 (1.2%) | 5 (100.0%) | 0 (0.0%) |
| SMVf + SOF + RBV | 4 (0.9%) | 3 (75.0%) | 1 (25.0%) |
| SOF-VELg + RBV | 2 (0.5%) | 2 (100.0%) | 0 (0.0%) |
| DCVh + SOF + RBV | 1 (0.2%) | 1 (100.0%) | 0 (0.0%) |
| DAA—RBV | 362 (83.8%) | 345 (95.3%) | 17 (4.7%) |
| LDV-SOF | 285 (66.0%) | 272 (95.4%) | 13 (4.6%) |
| SMV + SOF | 29 (6.7%) | 27 (93.1%) | 2 (6.9%) |
| EBR-GZRi | 17 (3.9%) | 16 (94.1%) | 1 (5.9%) |
| DCV + SOF | 15 (3.5%) | 15 (100.0%) | 0 (0.0%) |
| SOF + VEL | 11 (2.5%) | 10 (90.9%) | 1 (9.1%) |
| PROD | 5 (1.2%) | 5 (100.0%) | 0 (0.0%) |
a RBV = ribavirin.
b SOF = sofosbuvir.
c PROD = paritaprevir/ritonavir-ombitasvir and dasabuvir.
d PEG = peginterferon.
e LDV = ledipasvir.
f SMV = simeprevir.
g VEL = velpatasvir.
h DCV = daclatasvir.
i EBR-GZR = elbasvir-grazoprevir.
Predictive factors associated with SVR12 success
In the final multivariable logistic regression model, several factors were associated with achieving SVR12 Table 3.
Table 3. Factors associated with SVR12 achievement.
| Patient Characteristics | Odds Ratio (95% CI) | P-valuea |
|---|---|---|
| Age | 1.14 (1.03–1.27) | 0.011 |
| Cirrhosis | 0.34 (0.09–1.34) | 0.126 |
| Depression | 0.82 (0.02–0.96) | 0.040 |
| Diabetes | 0.68 (0.13–0.94) | 0.028 |
| Substance abuse | 0.48 (0.07–0.97) | 0.039 |
| Baseline Initial Viral Load | ||
| HCV VLb <800K IU/ML | 13.77 (5.37–18.82) | 0.026 |
| HCV VL 800K to 6MM IU/ML | 1.00 | - |
| HCV VL >6MM IU/ML | 0.57 (0.16–2.08) | 0.398 |
| Baseline FIB-4c | ||
| FIB-4 <1.45 | 5.18 (1.12–8.28) | 0.008 |
| FIB-4 1.45 to ≤3.25 | 1.00 | - |
| FIB-4 >3.25 | 0.54 (0.16–1.84) | 0.327 |
| Baseline CD4 count | ||
| ≥200 cells/mm3 | 1.00 | - |
| <200 cells/mm3 | 0.42 (0.11–1.92) | 0.265 |
| DAA Therapies | ||
| HCV regimen—RBVd | 1.00 | - |
| HCV regimen + RBV | 0.13 (0.03–0.60) | 0.009 |
a C-statistic (model fit) = 0.899; p-value <0.05 was considered statistically significant.
b VL = viral load.
c FIB-4 = fibrosis-4 score for liver.
d RBV = ribavirin.
Older patients were more likely to achieve SVR12 (OR 1.14, 95 CI: 1.03–1.27, p = 0.011). Patients with baseline depression (OR 0.82, 95 CI: 0.02–0.96, p = 0.040), diabetes (OR 0.68, 95 CI: 0.13–0.94, p = 0.028), substance abuse (OR 0.48, 95 CI: 0.07–0.97, p = 0.039), and on ribavirin therapy (OR 0.13, CI: 0.03–0.60, p = 0.009) were less likely to achieve SVR12. Baseline HCV viral load <800K IU/ML was associated with 13 times greater likelihood of achieving SVR12 versus having an HCV viral load between 800K to 6MM IU/ML (OR 13.77, CI: 5.37–18.82, p = 0.026). Patients with FIB-4 score <1.45 were 5 times more likely to achieve SVR12 (OR 5.18, 95% CI: 1.12–8.28, p = 0.008) compared to those with FIB-4 1.45 to ≤3.25.
Discussion
To our knowledge, this is one of the largest US cohort studies of patients with HIV/HCV coinfection who initiated DAA therapies in a real-world setting. In this analysis, we observed SVR12 rates of 94%, consistent with prior real-world studies showing >90% SVR rates. [32,35,38] Current guidelines[39] recommend that patients with HIV/HCV coinfection be treated using the approach followed for non-HIV infected patients, because efficacy of current DAA regimens does not differ between HCV-mono-infected and coinfected patients, while PLWH represent an especially vulnerable population. In HIV-infected men having sex with men (MSM) HCV transmission continues to rise; therefore, HCV elimination efforts directed toward this population will be important to decrease the HCV reservoir to prevent further transmission. [40–42] Treatment of HCV as prevention in this high risk population is critical to decrease the overall HCV incidence in PLWH, particularly with the expansion of the HIV U = U (undetectable = untransmissible) message in clinics across the US.[43,44] Although an empowering message for PLWH, the U = U message has the potential to increase the incidence of acute HCV in patients engaging in condomless sex.[45,46]
Possible barriers to a successful HCV response in PLWH include access to medical care, timely diagnosis of HIV/HCV and initiation of DAA therapy, costs associated with therapy, underinsured status, longer time needed for screening HIV/HCV and time required for counseling.[5,47] Achieving SVR12 has also been shown to affect quality of life (QoL) in patients with HIV/HCV coinfection. Prior to DAA therapy availability, QoL reported by patients with HIV/HCV coinfection was significantly lower than in HIV mono-infected patients.[16,48] However, after completion of DAA therapy, QoL scores were comparable between the two study groups, and DAA therapy demonstrated improvement in general health and emotional well-being of patients with HIV/HCV coinfection.[48] Overall, the advent of DAA therapy has had a positive impact on this population.
High SVR rates achieved with DAA therapy and the small number of patients included in the registration trials have made it difficult to identify factors associated with treatment failure in both monoinfected and coinfected patients.[49] Therefore, we evaluated predictors of treatment success. Based on baseline characteristics, patients with SVR12 had lower rates of comorbidities, lower HCV viral loads and ALT, AST levels, and FIB-4 score compared to the non-SVR group. The descriptive findings were consistent with the multivariable model, where patients with older age, less fibrotic liver (FIB-4 score <1.45) and lower levels of HCV initial viral load (<800K IU/ML) were more likely to achieve treatment success (SVR12). Factors such as depression, diabetes, substance abuse, HCV viral load >6MM IU/ML, FIB-4 score >3.25, CD4 count <200 cells/mm3, and DAA plus ribavirin therapy were independently associated with not achieving SVR12. However, these findings should not be interpreted as lack of DAA efficacy in these groups as SVR12 rates were still high in patients with CD4 <200 cells/mm3 (88.8% [n = 27]), depression (90.5% [n = 148]), and substance abuse (89.8% [n = 157]).
Prior studies supported our findings as related to factors associated with not achieving SVR12. Berenguer et al found male sex, CD4 count <200 cells/mm3, HCV RNA viral load ≥800K IU/mL, compensated cirrhosis, and the use of sofosbuvir plus ribavirin were associated with treatment failure based on the analysis of patients in Madrid, Spain.[49] Del Bello et al found that patients receiving simeprevir/sofosbuvir/ribavirin therapy were more likely to have treatment failure.[34] Tapper et al found that presence of cirrhosis, prior treatment experience, treatment at an academic center, and treatment outside of the FDA recommendations were each associated with lower odds of achieving SVR.[50]
In the few patients who experienced treatment failure, active drug and alcohol use and depression were the greatest predictors of not achieving SVR. Based on prior studies, drug use within 6 months of initiating HCV therapy is not associated with decline in response to treatment; however, more frequent drug use correlates with decreased efficacy of treatment.[51–55] Social functioning, including unstable housing, transportation difficulties, stigmatization, and clinic attendance are better indicators of treatment outcome, independently associated with SVR after adjusting for drug use.[56] International guidelines support prioritizing persons at risk for transmitting HCV, including active injection drug users, to reduce the duration that the person is infectious.[57] Colocation of HIV care, HCV testing and treatment, medically assisted treatment for illicit drug use, and mental health services, as offered in the majority of the clinical settings in our study, can increase success rates on HCV therapy in this population.[58,59]
Limitations of this multicenter retrospective study include lack of uniformity of staging modalities other than FIB-4 calculations to determine severity of fibrosis, which may have generated variability in center-specific HCV treatment decisions. In addition, many coinfected patients with active drug or alcohol abuse within these HIV centers may have been excluded from DAA eligibility due to strict individual state Medicaid restrictions denying access to these medications, which may limit the generalizability of these results to these subpopulations.[60] In the Canadian Coinfection Cohort (CCC), a representative HIV/HCV population in an industrialized country was used to evaluate the percentage of current cohort participants who would be eligible to participate in all oral DAA clinical trials. Exclusivity to specific antiretroviral therapies as well as illicit drug use excluded greater than 50% of the HIV co-infected population from receiving HCV treatment.[61] Individual state level Medicaid restrictions in several jurisdictions in the US also require rigid criteria as to who has access to DAA therapy, potentially limiting these curative therapies among PLWH.[30]
Additionally, due to the heterogeneity of HIV regimens in the sample, they were not included in the model. Lastly, the database platform does not record DAA adherence patterns, a behavioral factor central to successful DAA treatment. However, shorter courses of treatment and the inclusion of the dispensing records in this database provides some reassurance that the medications were taken properly.
Conclusions
Currently, there are limited real-world data on HCV treatment outcomes for patients with HIV/HCV coinfection. This large, real-world, multi-site, US study evaluating rates of SVR12 achievement and factors associated with treatment success significantly contributes to evidence in the coinfected population. In this analysis of patients with HIV/HCV coinfection, we found DAA therapies to be effective, with 94% of the patients achieving SVR12, consistent with rates seen in monoinfected HCV patients. Baseline variables, including older age, HCV viral load <800K IU/ML, FIB-4 score <1.45, absence of depression, diabetes, substance abuse, and use of DAA regimens without ribavirin were significant predictors of achieving SVR12. However, even in patients with characteristics negatively associated with achieving SVR12, success rates were high compared to success rates prior to DAA regimen availability. Patients with fewer comorbidities, better liver health, and lower HCV viral loads at baseline were more likely to achieve treatment success. Our results were consistent with other real-world studies, supporting the use of HCV therapy in HIV/HCV coinfected patients.
Acknowledgments
The authors thank Matthew Budday (Trio Health) for assisting with data collection on this project and Nazia Rashid (Trio Health) for medical writing support.
Data Availability
The data underlying the results presented in the study are available from Trio Health and can be requested at Trio Health 7777 Fay Avenue, suite 310 La Jolla, CA, 92037 phone: (858)-771-2500 email: info@triohealth.com Based on our contracts with the study funder, the data may not be deposited into a public repository to maintain patient confidentiality.
Funding Statement
Trio Health provided support in the form of salaries to authors JR and RE. Gilead Sciences provided funds for this study to Trio Health, however the authors were independent from Gilead Sciences in terms of the study design, data collection, data analysis, writing or editing the manuscript, decision to publish, and target journal choice. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Abutaleb A, Sherman K. A changing paradigm: management and treatment of the HCV/HIV-coinfected patient. Hepatology International. 2018;12: 500–509. 10.1007/s12072-018-9896-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7): 797–808 10.1016/S1473-3099(15)00485-5 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. HIV and hepatitis coinfections. https://www.who.int/hiv/topics/hepatitis/en/ Cited December 20 2018.
- 4.Centers for Disease Control and Prevention. Epidemiology and Prevention of HIV and Viral Hepatitis Co-infections. https://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf. Cited December 20 2018.
- 5.Meissner EG. Update in HIV/HCV Co-Infection in the Direct Acting Antiviral Era Curr Opin Gastroenterol. 2017;33(3): 120–127. 10.1097/MOG.0000000000000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo Re V 3rd, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160: 369–379. 10.7326/M13-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konerman MA, Mehta SH, Sutcliffe CG, Vu T, Higgins Y, Torbenson MS, et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014;59(3): 767–75. 10.1002/hep.26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterling RK, Wegelin JA, Smith PG, Stravitz RT, Luketic VA, Fuchs M, et al. Similar progression of fibrosis between HIV/HCV-infected and HCV-infected patients: Analysis of paired liver biopsy samples. Clin Gastroenterol Hepatol. 2010;8(12):1070–1076. 10.1016/j.cgh.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Helm J, Geskus R, Sabin C, Meyer L, del Amo J, Chêneet G, et al. Effect of HCV Infection on Cause-Specific Mortality After HIV Seroconversion, Before and After 1997. Gastroenterology. 2013;144(4): 751–760. 10.1053/j.gastro.2012.12.026 [DOI] [PubMed] [Google Scholar]
- 10.Thornton AC, Jose S, Bhegani S, Chadwick D, Dunn D, Gilson R, et al. Hepatitis B, Hepatitis C, and mortality among HIV-positive individuals. AIDS. 2017;31(18): 2525–2532. 10.1097/QAD.0000000000001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: The D:A:D study. Arch Intern Med. 2006;166: 1632–1641. 10.1001/archinte.166.15.1632 [DOI] [PubMed] [Google Scholar]
- 12.Chen TY, Ding EL, Seage-Iii GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009;49(10): 1605–1615. 10.1086/644771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44(1): 47–55. 10.1016/j.jhep.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 14.Klein MB, Althoff KN, Jing Y, Lau B, Kitahata M, Lo Re V III, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis. 2016;63(9): 1160–1167. 10.1093/cid/ciw531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mena A, Meijide H, Rodriguez-Osorio I, Castro A, Poveda E. Liver-related mortality and hospitalizations attributable to chronic hepatitis C virus coinfection in persons living with HIV. HIV Med. 2017;18(9): 685–689. 10.1111/hiv.12502 [DOI] [PubMed] [Google Scholar]
- 16.Sikavi C, Chen P, Lee A, Saab EG, Choi G, Saab S, et al. Hepatitis C and Human Immunodeficiency Virus coinfection in the Era of Direct-Acting Antiviral Agents: No Longer A Difficult-to-Treat Population. Hepatology 2018;67 (3): 847–857. 10.1002/hep.29642 [DOI] [PubMed] [Google Scholar]
- 17.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011: a multicohort collaboration. Lancet 2014;384(9939): 241–248. 10.1016/S0140-6736(14)60604-8 [DOI] [PubMed] [Google Scholar]
- 18.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Shermanet KE, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351(5): 451–459. 10.1056/NEJMoa032653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006. November 28;20(18): 2361–2369. 10.1097/QAD.0b013e32801086da [DOI] [PubMed] [Google Scholar]
- 20.Thomas DL. The challenge of hepatitis C in the HIV-infected person. Annu. Rev. Med. 2008;59: 473–485. 10.1146/annurev.med.59.081906.081110 [DOI] [PubMed] [Google Scholar]
- 21.Wyles DL, Sulkowski MS, Dieterich D. Management of Hepatitis C/HIV Coinfection in the Era of Highly Effective Hepatitis C Virus Direct-Acting Antiviral Therapy. Clin Infect Dis. 2016;63(Suppl 1): S3–S11. Recent comprehensive review of clinical trials design, results, and outcomes, as well as discussion of clinical management of HIV/HCV co-infected persons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya D, Belperio PS, Shahoumian TA, Loomis TP, Goetz MB, Mole LA, et al. Effectiveness of All-Oral Antiviral Regimens in 996 Human Immunodeficiency Virus/Hepatitis C Virus Genotype 1-Coinfected Patients Treated in Routine Practice. Clin Infect Dis. 2017;64(12): 1711–1720. 10.1093/cid/cix111 [DOI] [PubMed] [Google Scholar]
- 23.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8): 705–713. 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sulkowski MS, Eron JJ, Wyles DL, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313(12): 1223–1231. 10.1001/jama.2015.1328 [DOI] [PubMed] [Google Scholar]
- 25.Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8): 714–725. 10.1056/NEJMoa1503153 [DOI] [PubMed] [Google Scholar]
- 26.Wyles D, Bräu N, Kottilil S, Daar ES, Ruane P, Workowski K, et al. Sofosbuvir and Velpatasvir for the Treatment of HCV in Patients Coinfected with HIV-1: An Open-Label, Phase 3 Study. Clin Infect Dis. 2017;65(1): 6–12. 10.1093/cid/cix260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312(4): 353–361. 10.1001/jama.2014.7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oramasionwu CU, Moore HN, Toliver JC. Barriers to hepatitis C antiviral therapy in HIV/HCV co-infected patients in the United States: a review. AIDS Patient Care STDS. 2014;28(5): 228–39. 10.1089/apc.2014.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. http://aidsinfo.nih.gov Cited December 7 2018.
- 30.National Hepatitis Roundtable. Hepatitis C: State of Medicaid Access. https://stateofhepc.org/wp-content/uploads/2017/10/State-of-Access-Infographic.pdf Cited December 7 2018.
- 31.Barua S, Greenwald R, Grebely J., Dore GJ, Swan T, Taylor LE, et al. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015;163: 215–223. 10.7326/M15-0406 [DOI] [PubMed] [Google Scholar]
- 32.Hawkins C, Grant J, Ammerman LR, Palella F, Mclaughlin M, Green R, et al. High rates of hepatitis C virus (HCV) cure using direct-acting antivirals in HIV/HCV-coinfected patients: a real-world perspective. J Antimicrob Chemother. 2016;71: 2642–2645. 10.1093/jac/dkw203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cachay ER, Wyles D, Hill L, Ballard C, Torriani F, Colwell B, et al. The Impact of Direct-Acting Antivirals in the Hepatitis C-Sustained Viral Response in Human Immunodeficiency Virus-Infected Patients with Ongoing Barriers to Care. Open Forum Infect Dis. 2015;2(4): ofv168 10.1093/ofid/ofv168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Bello D, Cha A, Sorbera M, Bichoupan K, Levine C, Doyle E, et al. Real-World Sustained Virologic Response Rates of Sofosbuvir-Containing Regimens in Patients Coinfected With Hepatitis C and HIV. Clin Infect Dis. 2016;62: 1497–1504. 10.1093/cid/ciw119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milazzo L, Lai A, Calvi E, Ronzi P, Micheli V, Binda F, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med. 2017;18(4): 284–291. 10.1111/hiv.12429 [DOI] [PubMed] [Google Scholar]
- 36.Sogni P, Gilbert C, Lacombe K, Piroth L, Rosenthal E, Miailhes P, et al. All-oral Direct-acting Antiviral Regimens in HIV/Hepatitis C Virus-coinfected Patients with Cirrhosis Are Efficient and Safe: Real-life Results from the Prospective ANRS CO13-HEPAVIH Cohort. Clin Infect Dis. 2016;63: 763–770. 10.1093/cid/ciw379 [DOI] [PubMed] [Google Scholar]
- 37.Townsend K, Petersen T, Gordon LA, Kohli A, Nelson A, Seamon C, et al. Effect of HIV co-infection on adherence to a 12-week regimen of hepatitis C virus therapy with ledipasvir and sofosbuvir. Aids. 2016;30: 261–266. 10.1097/QAD.0000000000000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowan SE, Rogers M, Bayer J, Smith L, Gardner EM, Johnson S, et al. Treatment of Hepatitis C Virus in HIV-Coinfected Individuals in Real-world Clinical Settings: Results From 2 Large HIV Care Clinics. Clin Infect Dis. 2016;63: 994–995. 10.1093/cid/ciw447 [DOI] [PubMed] [Google Scholar]
- 39.American Association for the Study of Liver Diseases, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C http://www.hcvguidelines.org Cited December 21 2018.
- 40.Witt MD, Seaberg EC, Darilay A, Young S, Badri S, Rinaldo CR, et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis. 2013;57: 77–84. 10.1093/cid/cit197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Helm JJ, Prins M, del Amo J, Bucher HC, Chêne G, Dorrucci M, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25: 1083–1091. 10.1097/QAD.0b013e3283471cce [DOI] [PubMed] [Google Scholar]
- 42.CDC. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men—New York City, 2005–2010. MMWR. Morbidity and mortality weekly report. 2011;60(28): 945–950. [PubMed] [Google Scholar]
- 43.Braun D, Hampel B. Nguyen H, Flepp M, Stoeckle M, Béguelinet C, et al. A Treatment as Prevention Trial to Eliminate HCV in HIV+ MSM: The SWISS HCVREE Trial. Conference on Retroviruses and Opportunistic Infections (CROI) 2018 abstract 81LB. http://www.croiconference.org/sessions/treatment-prevention-trial-eliminate-hcv-hiv-msm-swiss-hcvree-trial Cited December 7 2018.
- 44.Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Current Opinion in HIV and AIDS. 2015;10(5): 374–380. 10.1097/COH.0000000000000179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, Davidovich U, Hogewoning A, de Vries HJC, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection: AIDS. 2017;31(11): 1603–1610. 10.1097/QAD.0000000000001522 [DOI] [PubMed] [Google Scholar]
- 46.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS. 2015; 29: 2335–2345. 10.1097/QAD.0000000000000834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer KH, Crawford P, Dant L, Gillespie S, Singal R, Vandermeer M, et al. HIV and Hepatitis C Virus Screening Practices in a Geographically Diverse Sample of American Community Health Centers. AIDS Patient Care STDS. 2016;30: 237–246. 10.1089/apc.2015.0314 [DOI] [PubMed] [Google Scholar]
- 48.Pereira M, Fialho R. Assessment of factors associated with the quality of life of patients living with HIV/HCV co-infection. J Behav Med. 2016;39: 767–781. 10.1007/s10865-016-9778-y [DOI] [PubMed] [Google Scholar]
- 49.Berenguer J, Gil-Martin A, Jarrin I, Moreno A, Dominguez L, Montes M, et al. All-Oral Direct-Acting Antiviral Therapy Against Hepatitis C Virus (HCV) in Human Immunodeficiency Virus/HCV–Coinfected Subjects in Real-World Practice: Madrid Coinfection Registry Findings. Hepatology. 2018;68(1): 32–47. 10.1002/hep.29814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapper EB, Bacon BR, Curry MP, Dieterich DT, Flamm SL, Guest LE, et al. Real-world effectiveness for 12 weeks of ledipasvir-sofosbuvir for genotype 1 hepatitis C: The Trip Health Study. J Viral Hepat. 2017;24: 22–27. 10.1111/jvh.12611 [DOI] [PubMed] [Google Scholar]
- 51.Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. The Lancet Gastroenterology & Hepatology. 2018;3(3): 153–161. [DOI] [PubMed] [Google Scholar]
- 52.Kattakuzhy S, Mathur P, Gross C, Silk R, Akoth E, Hill K, et al. High SVR in PWID with HCV Despite Imperfect Medication Adherence: Data from the Anchor Study. Hepatology. 2018;68(suppl 1). AASLD Meeting abstract 18. [Google Scholar]
- 53.Dore G, Grebely J, Altice F, Litwin AH, Dalgard O, Gain E, et al. HCV Reinfection and Injecting Risk Behavior Following Elbasvir/Grazoprevir Treatment in Patients on Opioid Agonist Therapy: C-Edge Co-STAR Part B. Hepatology. 2018;68(suppl 1). AASLD Meeting abstract 52. [Google Scholar]
- 54.Dore G, Hellard M, Matthews G, Grebely J, Haber PS, Petoumenos K, et al. Effective Treatment of Injecting Drug Users with Recently Acquired Hepatitis C Virus Infection. Gastroenterology. 2010; 138(1): 123–135. 10.1053/j.gastro.2009.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grebely J, Dore GJ, Zeuzem S, Aspinall RJ, Fox R, Han L, et al. Efficacy and Safety of Sofosbuvir/Velpatasvir in Patients with Chronic Hepatitis C Virus Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ASTRAL Trials. Clinical Infectious Diseases. 2016;63(11): 1479–1481. 10.1093/cid/ciw579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doyle JS, Grebely J, Spelman T, Alavi M, Matthews GV, Thompson AJ, et al. Quality of Life and Social Functioning during Treatment of Recent Hepatitis C Infection: A Multi-Centre Prospective Cohort. PLoS One. 2016;11(6): e0150655 Published 2016 Jun 29. 10.1371/journal.pone.0150655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.EASL Recommendations on Treatment of Hepatitis C 2018. Journal of Hepatology. 2018;69(2): 461–511. 10.1016/j.jhep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 58.Litwin AH, Agyemang L, Akiyama M, Norton B, Ning Y, Heo M, et al. The PREVAIL Study: Intensive Models of HCV Care for People Who Inject Drugs. Journal of Hepatology. 2017; 66(1): S72. [Google Scholar]
- 59.Ward JW. Strategies for expanding access to HBV and HCV testing and care in the United States: The CDC Hepatitis Testing and Linkage to Care Initiative, 2012–2014 Public Health Rep. 2016. May-Jun;131(Suppl 2): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jayaweera D, Althoff K, Eron J, Huhn G, Milligan S, Mills A, et al. Untreated HCV in HIV/HCV Co-Infection: Data from the TRIO Network. abstract THU-294. Journal of Hepatology. 2018;68: S105–364. [Google Scholar]
- 61.Saeed S, Strumpf E, Walmsley S, Rollet-Kurhajec K, Pick N, Martel-Laferrière V, et al. How Generalizable Are the Results from Trials of Direct Antiviral Agents to People Co-infected with HIV/HCV in the Real World? Clin Infect Dis. 2016;62: 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the results presented in the study are available from Trio Health and can be requested at Trio Health 7777 Fay Avenue, suite 310 La Jolla, CA, 92037 phone: (858)-771-2500 email: info@triohealth.com Based on our contracts with the study funder, the data may not be deposited into a public repository to maintain patient confidentiality.