Abstract
Background
This study aims to investigate whether preconditioning with alpha-lipoic acid has any protective effect in neuronal damage in an experimental spinal cord ischemia-reperfusion injury model.
Methods
Eighteen adult male New Zealand rabbits (2.4-3.5 kg) were equally divided into sham, control and treatment groups. The abdominal aorta was occluded for 30 min proximally 1 cm below the renal artery and distally 1 cm above the bifurcation using aneurysm clips in control and treatment groups. Treatment group received intraperitoneal 100 mg/kg lipoic acid 20 min before aortic cross-clamping. The animals were sacrificed 48 hours after the operation and spinal cord segments between L2 and L5 were removed for biochemical and histopathological analysis. Levels of glutathione, malondialdehyde, total nitrate/nitrite, advanced oxidation protein products, catalase, superoxide dismutase, and glutathione peroxidase were examined in spinal cord.
Results
Preconditioning with alpha-lipoic acid demonstrated significantly favorable effects in all measured parameters of oxidative stress. Histopathological evaluation of the tissues also demonstrated significantly decreased neuronal degeneration, axonal damage, and microglial and astrocytic infiltration in the treatment group compared to the control group.
Conclusion
The results of this study indicate that alpha-lipoic acid administration before aortic cross-clamping has significant neuroprotective effect on spinal cord injury in rabbits.
Keywords: Alpha-lipoic acid, spinal cord injury, thoraco-abdominal aneurysm
Introduction
Spinal cord injury (SCI) due to ischemia is one of the most detrimental complications after thoracic and thoraco-abdominal aortic repairs with a reported incidence of up to 40%.[1] Contemporary methods for perioperative spinal cord protection include systemic hypothermia, partial cardiopulmonary bypass, drainage of cerebrospinal fluid, reattachment of critical segmental arteries during surgery and pharmacologic approaches.[2,3] Despite various strategies to reduce the risk of SCI, its occurrence is still high.[1,4,5]
Studies regarding the pathophysiologic mechanism of ischemic SCI are going on as it has not been fully explained yet. Mechanism of injury can be divided into two: (i) incidents during ischemia (primary injury) and (ii) incidents occurring during reperfusion (secondary damage), which are aggravated by activation of various endogenous substances, formation of inflammation and free radicals. The latter is often the target of pharmacologic agents.[6-8] Various pharmacologic agents (e.g. methylprednisolone, naloxone, melatonin, magnesium, mexiletine, N-acetylcysteine, gabapentin and rosuvastatin) have been used in experimental studies to mitigate secondary injury, with only methylprednisolone providing clinical benefits.[9-14]
Alpha-lipoic acid (ALA) is an antioxidant with neuroprotective effects which has also been described as a therapeutic agent in animal models of multiple sclerosis, autoimmune encephalomyelitis, ischemic heart diseases, diabetic neuropathy and some liver diseases.[15-17] Alpha-lipoic acid affects some cellular processes through regeneration of endogenous antioxidants, modulation of transcription factors and accelerates glutathione synthesis; also regulates the expression of several antioxidant and anti-inflammatory genes.[18,19]
Therefore, in this study, we aimed to investigate whether preconditioning with ALA has any protective effect in neuronal damage in an experimental spinal cord ischemia-reperfusion injury model.
Patients and Methods
This study was conducted at Gazi University Faculty of Medicine between March 2016 and April 2016. Eighteen adult male New Zealand rabbits (2.4-3.5 kg) were divided into sham (n=6), control (n=6), and treatment groups (n=6). A standard diet was applied to the rabbits at optimal room temperature (18°C-21°C). Ketamine (70 mg/kg, Ketalar, Parke- Davis, Eczacibasi, Istanbul, Turkey) and xylazine (5 mg/kg, Rompun, Bayer, Istanbul, Turkey) were given to anesthetize the animals. All animals were continuously monitored and body temperatures were maintained at 37°C throughout the procedure. The study was approved by the Ethics Review Committee of Gazi University Faculty of Medicine. The study was conducted in accordance with the principles of the Declaration of Helsinki. Animals received humane care and treatment in accordance with the "Principles of Laboratory Animal Care" formulated by the National Society for Medical Research and the "Guide for the Care and Use of Laboratory Animals" prepared by the Institute of Laboratory Animal Resources and published by the National Institute of Health.
Following sterile preparation, a 10 cm abdominal midline incision was performed and the abdominal aorta was exposed through a transperitoneal approach. Intravenous heparin (150 IU/kg) was used for anticoagulation 5 min before aortic cross-clamping. The aorta was occluded for 30 min with two clips below the renal artery and above the bifurcation. The efficiency of the occlusion was established by an immediate and persistent loss of detectable pulse distally. Following 30 min of ischemia, reperfusion was initiated by removal of the clips. The incision was closed in layers. Aortic occlusion was not performed to the sham-operated animals. Twenty minutes before aortic cross-clamping, control and treatment group animals received 25 mL of normal saline and intraperitoneal 100 mg/kg lipoic acid (Thioctacid, Gen İlaç, Ankara, Turkey), respectively.
In the postoperative period, animals recovered in their cages where they were allowed to feed after 2 h. Animals were sacrificed with pentobarbital (200 mg/kg) at postoperative 48th hour. Following laminectomies between T12 to S1 segments, spinal cord segments between L2 and L5 were removed for analysis. Glutathione (GSH), malondialdehyde (MDA), total nitrate/nitrite (NOx), advanced oxidation protein products (AOPP), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) levels were studied in spinal cord.
Spinal cord tissue (100 mg) was homogenized by 10% trichloroacetic acid (1 mL), then the homogenate was centrifuged and the supernatant MDA levels assayed by the thiobarbituric acid reactive substances formation. Modified Ellman method was used to measure GSH levels. The lipid peroxidation level was expressed in terms of the MDA equivalent by using an extinction coefficient of 1.56x105 M-1 cm-1. The GSH levels were calculated using an extinction coefficient of 13.600 M-1 cm-1.
Spinal cord tissue (100-200 mg) was diluted, homogenized (Virtis-Virtishear), centrifuged and analyzed in the supernatant fraction. Spectrophotometry method was used to measure AOPP levels and the results were expressed as nmol/mg of protein in liver tissue.
Bradford method (bovine serum albumin as the standard) was used to measure Tissue protein levels. Tissue was diluted, homogenized (1/5 [w/v]) and centrifuged. Cayman SOD assay kits were used to measure SOD activities and the results were presented as U/mg protein. The sample was diluted, homogenized (1/5 [w/v]) and centrifuged. Cayman CAT assay kits were used to measure CAT activities and the results were presented as nmol/min/mg protein. The sample was diluted, homogenized (1/5 [w/v]) and centrifuged. Cayman GPx assay kits were used to measure GPx activities and the results were presented as nmol/min/mg protein. The sample was diluted, homogenized (1/5 [w/v]) and centrifuged. Cayman nitrate/nitrite assay kits were used to measure NOx levels and the results were presented as micromol.
Following fixation in 10% buffered formalin for 10 days, five-micrometer-thick sections were cut for hematoxylin & eosin staining. Specimens were assessed with a light microscope (DMI 4000 B Leica) by the same histologist, who was blinded to the groups.
Histopathological changes including neuronal degeneration, axonal damage, astrocyte and microglia infiltration were analyzed. Axonal injury was scaled as: G-0 (normal), G-1 (mild swelling and vacuolization in axons), and G-2 (severe swelling and vacuolization in axons). Each field was observed for the amount of degenerated neurons, microglia and astrocyte infiltration and graded as +1, +2, +3, and +4 due to their intensity.
Statistical analysis The analyses were performed using the IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). The continuous data are presented as the mean±standard deviation, median (minimum-maximum) values and box-plot graphics; the categorical data are presented with numbers (n) and percentages. The comparisons between groups were performed with chi-square test for categorical variables. Kruskal-Wallis test was used to compare the groups for the continuous variables. To determine the groups creating the difference; Bonferroni adjusted Mann-Whitney U test was performed. P values under 0.05 were considered statistically significant (p<0.016; for Bonferroni adjusted tests).
Results
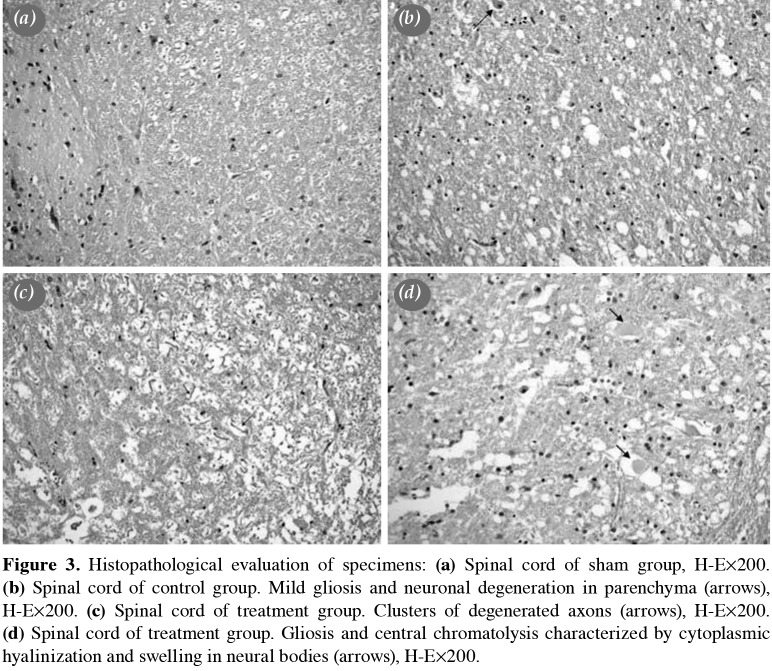
Tissue GSH level was 0.75±0.3 in the sham group, and ischemia-reperfusion injury through aortic clamping declined this level to 0.29±0.1 in the control group (p=0.05). In the treatment group, preconditioning with ALA significantly raised the tissue GSH level to 0.6±0.2 (p=0.016). Tissue MDA level was 3.4±0.6 in the sham group. In the control group, this level significantly increased to 7.6±0.7 (p=0.004). Preconditioning with ALA significantly dropped this level to 4.2±1.5 (p=0.004). Tissue GPx level was 3.5±1.0 in the sham group. In the control group, this level significantly decreased to 1.7±0.1 (p=0.006). In the treatment group, tissue level of GPx was 2.3±0.6 (p=0.065).
Tissue NOx level was 32.5±1.6 in the sham group, and ischemia-reperfusion injury significantly increased this level to 68.9±10.6 (p=0.004) in the control group. In the treatment group, preconditioning with ALA significantly decreased the tissue NOx level to 36.4±6.4 (p=0.004). Tissue AOPP level was 10.8±1.1 in the sham group. In the control group, this level significantly increased to 23.1±5.4 (p=0.004). Preconditioning with ALA significantly dropped this level to 13.7±1.4 (p=0.004). Tissue CAT level was 29.7±3.5 in the sham group, and ischemia-reperfusion significantly decreased this level to 17.0±2.9 in the control group (p=0.004). In the treatment group, preconditioning with ALA significantly raised this level to 24.7±5.1 (p=0.016). Tissue SOD level was 30.7±4.0 in the sham group, and ischemia-reperfusion significantly decreased this level to 14.0±2.1 in the control group (p=0.004). In the treatment group, preconditioning with ALA significantly raised this level to 25.4±1.9 (p=0.004). Results of all parameters were summarized in Figures 1 and 2.
Figure 1. Comparison of treatment and control groups. GSH: Glutathione; MDA: Malondialdehyde: GPx: Glutathione peroxidase.
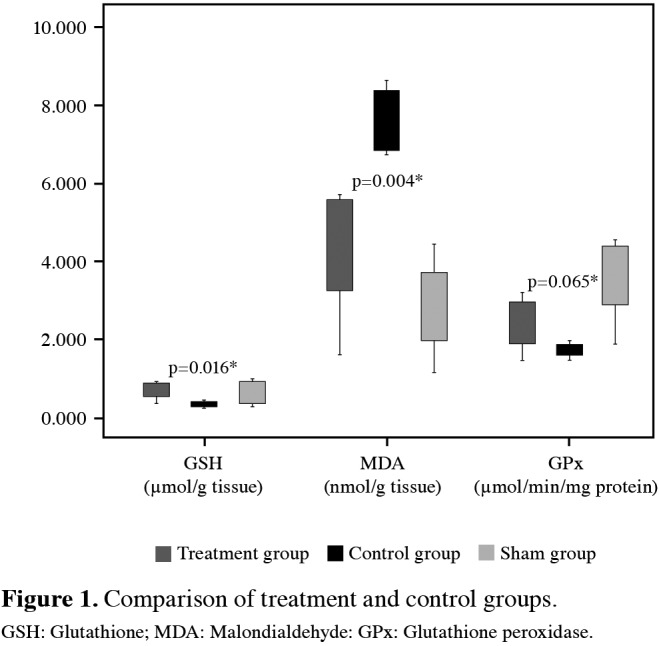
Figure 2. Comparison of treatment and control groups. MDA: Malondialdehyde: AOPP: Advanced oxidation protein products; CAT: Catalase; SOD: Superoxide dismutase.
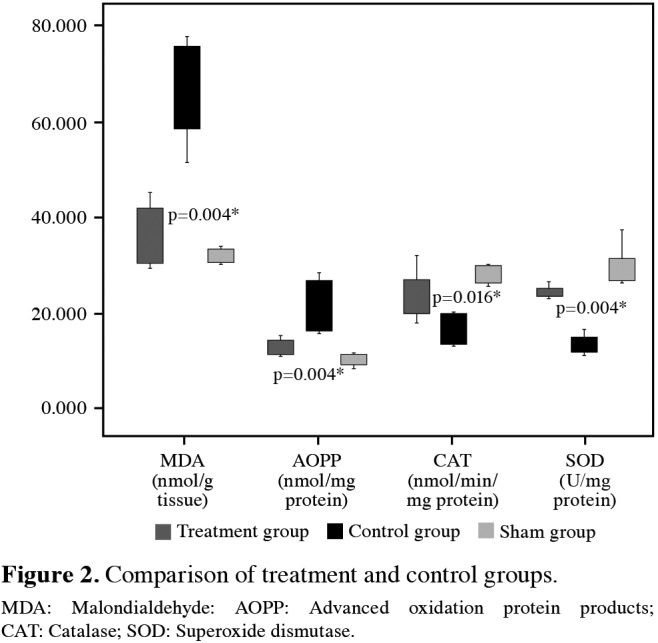
The histopathological evaluation of the specimens from the sham group showed normal structural changes. Ischemia-reperfusion injury, however, initiated significant tissue destruction (Figure 3). Neuronal degeneration, axonal damage, and microglial and astrocytic infiltration were significantly lesser in the treatment group compared to the control group (p=0.001, p=0.017, and p=0.001, respectively). The results of histopathologic evaluation are shown in Table 1.
Figure 3. Histopathological evaluation of specimens: (a) Spinal cord of sham group, H-Ex200. (b) Spinal cord of control group. Mild gliosis and neuronal degeneration in parenchyma (arrows), H-Ex200. (c) Spinal cord of treatment group. Clusters of degenerated axons (arrows), H-Ex200. (d) Spinal cord of treatment group. Gliosis and central chromatolysis characterized by cytoplasmic hyalinization and swelling in neural bodies (arrows), H-Ex200.
Table 1. Results of histopathologic evaluation.
| Subject | Neuronal degeneration | Axonal damage | Microglial and | |
| Grade | Grade | astrocytic infiltration | ||
| Sham | 1 | 0/10 | G-0 | + |
| 2 | 1/10 | G-0 | + | |
| 3 | 0/10 | G-0 | + | |
| 4 | 0/10 | G-0 | + | |
| 5 | 0/10 | G-0 | + | |
| 6 | 0/10 | G-0 | + | |
| Control | 1 | 7/10 | G-2 | ++++ |
| 2 | 7/10 | G-1 | +++ | |
| 3 | 8/10 | G-2 | ++++ | |
| 4 | 9/10 | G-2 | ++++ | |
| 5 | 7/10 | G-2 | +++ | |
| 6 | 7/10 | G-1 | +++ | |
| Treatment | 1 | 2/10 | G-2 | ++ |
| 2 | 3/10 | G-2 | + | |
| 3 | 2/10 | G-1 | + | |
| 4 | 2/10 | G-0 | ++ | |
| 5 | 1/10 | G-0 | + | |
| 6 | 2/10 | G-0 | + |
Discussion
Spinal cord injury is a catastrophic event with profound impacts on early mortality, longevity and healthcare cost, and finally a significant socioeconomic problem.[20] Currently, influences that lead to hypoxic ischemic damage in the spinal medulla and the methods to eliminate SCI have not been reached up to date. Based on experimental evidence, drugs that reduce oxygen demand (barbiturates), decrease inflammation (corticosteroids), and scavenge free radicals (mannitol) reduce reperfusion injury in animal models of spinal cord ischemia and are used during thoraco-abdominal aortic aneurysm surgery.[21]
The most critical step triggering irreversible cellular damage during SCI is believed to be the degeneration in calcium ion homeostasis. During ischemia, intracellular calcium raises and initiates several actions such as mitochondrial dysfunction, activation of nitric oxide (NO) synthase, and stimulation of phospholipase A2. Triggered phospholipase A2 then initiates arachidonic acid metabolism which is responsible from the axonal damage in SCI.[22,23]
Moreover, during early days of injury, oxidative stress damages highly vulnerable cell membranes by triggering lipid peroxidation pathways. During the reperfusion phase, the lipid peroxidation augments the intracellular calcium and further damages neuronal cells, which then leads to apoptosis or necrosis and neurological dysfunction.[24-26]
Lipid peroxidation is a key step in oxidative stress and has damaging effects on free radicals following SCI.[27] Alpha-lipoic acid is a potent biological antioxidant and can scavenge hydroxyl radicals and singlet oxygen.[28] Alpha-lipoic acid also raises intracellular GSH and keeps GSH levels constant throughout oxidative stress.[29] Thus, in the present study, we aimed to carry out an animal model of ischemia-reperfusion to examine the protective effects of ALA on spinal cord. For this purpose, both levels of biochemical markers such as GSH, MDA, GPx, NOx, AOPP, CAT and SOD, and histological changes were investigated. Our study demonstrated that preconditioning with ALA protects the spinal cord from the consequences of ischemia-reperfusion.
Glutathione, GPx, CAT, and SOD protect cells from free radicals and reactive oxygen metabolites.[30] Superoxide dismutase converts superoxide radicals to hydrogen peroxide. Either GPx or CAT enzymes convert hydrogen peroxide to water molecules. As activity and level of CAT enzymes are low in the brain tissue, GPx is the prior enzyme that detoxificates hydrogen peroxide in the neural tissue.[31,32] Senoglu et al.[33] showed in their study that the decline in the tissue SOD activity after sciatic nerve damage was reversed with ALA treatment. Similar experimental studies based on oxidative stress models also demonstrated decreased SOD level with ALA treatment.[34,35] Turamanlar et al.[31] also constituted ischemia-reperfusion damage on rat sciatic nerve and showed increased levels of GPx with addition of ALA in treatment group. Consistent with the literature, our study validated that levels of all above mentioned antioxidant enzymes decreased with ischemia-reperfusion and significantly increased with preconditioning with ALA in the treatment group, with the exception of GPx levels, in which the increase was not statistically significant.
The central nervous system is also prone to the effects of free radical induced lipid peroxidation. Malondialdehyde, which is formed from the interruption of polyunsaturated fatty acids, gives reliable information about the degree of the peroxidation reaction and thus, can be used as a marker to determine oxidative damage.[36] Cosar et al.[37] demonstrated in their study that both plasma and tissue MDA levels in ischemia group were decreased with ALA administration. Similarly, in our study, administration of ALA significantly decreased MDA levels in the neural tissue.
Nitric oxide is a free radical which is synthesized from L-arginine with NO synthase. It has been demonstrated that low NO concentrations are essential in physiological processes. However, high amounts boost oxidative stress in various ways such as linking to the transition metals, producing peroxynitrite, and creating N-nitroso compounds.[38] Such high levels of NO are cytotoxic, induce neuronal apoptosis, and cause neural degeneration.[39,40] Yavuz et al.[41] investigated the levels of NOx during mesenteric ischemia durations in their experimental study and demonstrated that NOx levels can be a decisive biomarker for prediction of the critical ischemia period. Abdel-Zaher et al.[42] composed potassium cyanide-induced stroke in rats and showed that high NO levels decreased with administration of ALA. Likewise, in our study, administration of ALA significantly reduced the levels of NOx which were increased following ischemia-reperfusion.
Advanced oxidation protein products are markers of protein oxidation and not associated with markers that express lipid peroxidation. Accordingly, they are both markers of oxidative stress and inflammatory mediators.[43,44] Kurt et al.[45] and Guven et al.[46] demonstrated that the spinal cord AOPP levels of ischemia-reperfusion group were significantly increased compared to the sham group. In our study, tissue levels of AOPP increased following reperfusion and were significantly reduced to near normal with ALA administration.
Histopathological examination included neuronal degeneration, axonal damage and microglial and astrocytic infiltration. Significant tissue destruction induced by ischemia-reperfusion improved with administration of ALA in the treatment group. We obtained significantly better results for all of the histopathologic examination parameters. These results suggest that preconditioning with ALA also has favorable effects on keeping the spinal cord morphologic structure by diminishing the oxidative insult.
Recently, Emmez et al.[29] investigated the protective effects of ALA on spinal cord ischemiareperfusion injury with an experimental model. They examined both the histopathologic changes and the biochemical changes in plasma and neural tissue with ALA administration following ischemia and demonstrated favorable outcomes. In the present study, we administered ALA before aortic ischemia. We aimed to increase the efficacy of the treatment by distributing the ALA to the tissues before reperfusion occurs and demonstrated similar results with their study.
Our study has some limitations. The lack of functional outcome assessment and immunohistochemistry evaluation of the spinal cord are the major pitfalls of this study. Functional outcome assessment may be analyzed with different dose regimens of ALA in further studies. In addition, number of animals in each group was limited to six as the ethical committee allowed using six animals per group.
In conclusion, the results of this study demonstrated that alpha-lipoic acid administration before aortic cross-clamping has a considerable neuroprotective effect on spinal cord injury in rabbits. These data may contribute to the potential use of alpha-lipoic acid for neuroprotection in open or endovascular surgical treatment of thoracic or thoraco-abdominal aortic pathologies. Thus, preconditioning with alphalipoic acid may be used as a pharmacologic approach to reduce the risk of spinal cord injury in aortic surgical practice. However, further experimental and/ or human studies should be performed before any recommendations can be made.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Etz CD, Weigang E, Hartert M, Lonn L, Mestres CA, Di Bartolomeo R, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery†. Eur J Cardiothorac Surg. 2015;47:943–957. doi: 10.1093/ejcts/ezv142. [DOI] [PubMed] [Google Scholar]
- 2.Acher CW, Heisey DM. Regarding "Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair". J Vasc Surg. 1998;28:570–571. doi: 10.1016/s0741-5214(98)70148-x. [DOI] [PubMed] [Google Scholar]
- 3.Etz CD, Luehr M, Kari FA, Bodian CA, Smego D, Plestis KA, et al. Paraplegia after extensive thoracic and thoracoabdominal aortic aneurysm repair: does critical spinal cord ischemia occur postoperatively. J Thorac Cardiovasc Surg. 2008;135:324–330. doi: 10.1016/j.jtcvs.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Safi HJ, Miller CC, Huynh TT, Estrera AL, Porat EE, Winnerkvist AN, et al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection. Ann Surg. 2003;238:372–380. doi: 10.1097/01.sla.0000086664.90571.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svensson LG. Paralysis after aortic surgery: in search of lost cord function. Surgeon. 2005;3:396–405. doi: 10.1016/s1479-666x(05)80050-2. [DOI] [PubMed] [Google Scholar]
- 6.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Genovese T, Cuzzocrea S. Role of free radicals and poly(ADP-ribose)polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. Curr Med Chem. 2008;15:477–487. doi: 10.2174/092986708783503177. [DOI] [PubMed] [Google Scholar]
- 8.Oruckaptan HH, Ozisik P, Atilla P, Tuncel M, Kilinc K, Geyik PO, et al. Systemic administration of interleukin-10 attenuates early ischemic response following spinal cord ischemia reperfusion injury in rats. J Surg Res. 2009;155:345–356. doi: 10.1016/j.jss.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Rothstein JD, Kuncl RW. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem. 1995;65:643–651. doi: 10.1046/j.1471-4159.1995.65020643.x. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman S, Düz B, Kayali H, Korkmaz A, Oter S, Aydin A, et al. Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: a comparative study in rats. Neurochem Res. 2007;32:1547–1551. doi: 10.1007/s11064-007-9354-5. [DOI] [PubMed] [Google Scholar]
- 11.Kahraman S, Düz B, Kayali H, Korkmaz A, Oter S, Aydin A, et al. Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: a comparative study in rats. Neurochem Res. 2007;32:1547–1551. doi: 10.1007/s11064-007-9354-5. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Tsuchida M, Umehara S, Kohno T, Yamamoto H, Hayashi J. Reduction of spinal cord ischemia/reperfusion injury with simvastatin in rats. Anesth Analg. 2011;113:565–571. doi: 10.1213/ANE.0b013e318224ac35. [DOI] [PubMed] [Google Scholar]
- 13.Bardakci H, Kaplan S, Karadeniz U, Ozer C, Bardakci Y, Ozogul C, et al. N-acetylcysteine reduces ischemia/ reperfusion induced spinal cord injury: an experimental study. Turk Gogus Kalp Dama. 2013;21:114–121. [Google Scholar]
- 14.Yavuz C, Demirtas S, Guclu O, Karahan O, Caliskan A, Yazici S, et al. Rosuvastatin may have neuroprotective effect on spinal cord ischemia reperfusion injury. CNS Neurol Disord Drug Targets. 2013;12:1011–1016. doi: 10.2174/18715273113129990085. [DOI] [PubMed] [Google Scholar]
- 15.Cameron NE, Cotter MA, Horrobin DH, Tritschler HJ. Effects of alpha-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia. 1998;41:390–399. doi: 10.1007/s001250050921. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175:87–96. doi: 10.1016/j.jneuroim.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Yu Y, Ji L, Liang X, Zhang T, Hai CX. Alpha-lipoic acid protects against myocardial ischemia/reperfusion injury via multiple target effects. Food Chem Toxicol. 2011;49:2750–2757. doi: 10.1016/j.fct.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 18.Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 19.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 20.Bicknell CD, Riga CV, Wolfe JH. Prevention of paraplegia during thoracoabdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2009;37:654–660. doi: 10.1016/j.ejvs.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Wynn MM, Acher CW. A modern theory of spinal cord ischemia/injury in thoracoabdominal aortic surgery and its implications for prevention of paralysis. J Cardiothorac Vasc Anesth. 2014;28:1088–1099. doi: 10.1053/j.jvca.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 24.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 25.Kale A, Börcek AÖ, Emmez H, Yildirim Z, Durdağ E, Lortlar N, et al. Neuroprotective effects of gabapentin on spinal cord ischemia-reperfusion injury in rabbits. J Neurosurg Spine. 2011;15:228–237. doi: 10.3171/2011.4.SPINE10583. [DOI] [PubMed] [Google Scholar]
- 26.Xu M, Yip GW, Gan LT, Ng YK. Distinct roles of oxidative stress and antioxidants in the nucleus dorsalis and red nucleus following spinal cord hemisection. Brain Res. 2005;1055:137–142. doi: 10.1016/j.brainres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Hall ED. The role of oxygen radicals in traumatic injury: clinical implications. J Emerg Med. 1993;11:31–36. [PubMed] [Google Scholar]
- 28.Mitsui Y, Schmelzer JD, Zollman PJ, Mitsui M, Tritschler HJ, Low PA. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J Neurol Sci. 1999;163:11–16. doi: 10.1016/s0022-510x(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 29.Emmez H, Yildirim Z, Kale A, Tönge M, Durdağ E, Börcek AO, et al. Anti-apoptotic and neuroprotective effects of a-lipoic acid on spinal cord ischemia-reperfusion injury in rabbits. Acta Neurochir (Wien) 2010;152:1591–1600. doi: 10.1007/s00701-010-0703-9. [DOI] [PubMed] [Google Scholar]
- 30.Varija D, Kumar KP, Reddy KP, Reddy VK. Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J Med Res. 2009;129:587–592. [PubMed] [Google Scholar]
- 31.Turamanlar O, Özen OA, Songur A, Yağmurca M, Akçer S, Mollaoğlu H, et al. Protective Effect of Alpha Lipoic Acid on Rat Sciatic Nerve Ischemia Reperfusion Damage. Balkan Med J. 2015;32:196–202. doi: 10.5152/balkanmedj.2015.15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 33.Senoglu M, Nacitarhan V, Kurutas EB, Senoglu N, Altun I, Atli Y, et al. Intraperitoneal Alpha-Lipoic Acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj. 2009;4:22–22. doi: 10.1186/1749-7221-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Militão GC, Ferreira PM, de Freitas RM. Effects of lipoic acid on oxidative stress in rat striatum after pilocarpineinduced seizures. Neurochem Int. 2010;56:16–20. doi: 10.1016/j.neuint.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res. 2006;84:647–654. doi: 10.1002/jnr.20899. [DOI] [PubMed] [Google Scholar]
- 36.Schmidley JW. Free radicals in central nervous system ischemia. Stroke. 1990;21:1086–1090. doi: 10.1161/01.str.21.7.1086. [DOI] [PubMed] [Google Scholar]
- 37.Cosar E, Sahin FK, Köken G, Toy H, Basarali K, Büyükbas S. The protective effect of alpha-lipoic acid in experimental ovarian ischaemia-reperfusion injury. Aust N Z J Obstet Gynaecol. 2007;47:499–503. doi: 10.1111/j.1479-828X.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 38.Cosar E, Sahin FK, Köken G, Toy H, Basarali K, Büyükbas S. The protective effect of alpha-lipoic acid in experimental ovarian ischaemia-reperfusion injury. Aust N Z J Obstet Gynaecol. 2007;47:499–503. doi: 10.1111/j.1479-828X.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, et al. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Kimura S, Hosaka N, Yuge I, Yamazaki A, Suda K, Taneichi H, et al. Cerebrospinal fluid concentrations of nitric oxide metabolites in spinal cord injury. Spine (Phila Pa 1976) 2009;34:645–652. doi: 10.1097/BRS.0b013e3181abda1d. [DOI] [PubMed] [Google Scholar]
- 41.Yavuz C, Yazici S, Karahan O, Demirtas S, Caliskan A, Guclu O, et al. Serum nitric oxide level could be a predictive biomarker for detection of critical ischaemia duration. Biomarkers. 2013;18:116–120. doi: 10.3109/1354750X.2012.745165. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Zaher AO, Abdel-Hady RH, Abdel Moneim WM, Salim SY. Alpha-lipoic acid protects against potassium cyanide-induced seizures and mortality. Exp Toxicol Pathol. 2011;63:161–165. doi: 10.1016/j.etp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 44.Alderman CJ, Shah S, Foreman JC, Chain BM, Katz DR. The role of advanced oxidation protein products in regulation of dendritic cell function. Free Radic Biol Med. 2002;32:377–385. doi: 10.1016/s0891-5849(01)00735-3. [DOI] [PubMed] [Google Scholar]
- 45.Kurt G, Gokce EC, Cemil B, Yildirim Z, Kaptanoglu G, Durdag E, et al. Neuroprotective effects of rosuvastatin in spinal cord ischemia-reperfusion injury in rabbits. Neurosurg Q. 2015;25:189–196. [Google Scholar]
- 46.Guven C, Borcek AO, Cemil B, Kurt G, Yildirim Z, Ucankus NL, et al. Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci. 2010;17:1563–1567. doi: 10.1016/j.jocn.2010.04.027. [DOI] [PubMed] [Google Scholar]