Abstract
Background
In this article, we report mid-term follow-up results of the Solysafe® septal occluder for percutaneous closure of secundum atrial septal defects.
Methods
A total of 25 patients (8 males, 17 females; mean age 8.4±3.6 years; range 5 to 12 years) who underwent percutaneous closure of secundum atrial septal defect between July 2008 and June 2010 were included in this study.
Results
The mean follow-up was 6.1±0.5 (range, 5.2 to 7.2) years. The device was successfully implanted in 22 of 25 patients. The mean stretched diameter of the atrial septal defect as assessed by balloon sizing was 13.6±4.4 (range, 8 to 26) mm. Nine 15-mm devices, eight 20-mm devices, six 25-mm devices, and two 35-mm devices were used. A 20-mm and two 35-mm devices were used in three patients and the procedure failed in these patients. Among the remaining 22 patients, no pericardial effusion, endocarditis, hemolysis, electrocardiographic changes, valvular problems, or suspicious echocardiographic findings were observed during or after the procedure. Only in one patient, a wire fraction was seen at six years, while another patient had a residual shunt during a six-year follow-up. Device embolization (n=1) and hemiparesis (n=1) were the early major complications related to the procedure.
Conclusion
Although percutaneous closure of secundum atrial septal defects is successful, it would be wiser to check the device regularly, at least once a year, as the manufacturing of the device has been discontinued due to wire fractions.
Keywords: Atrial septal defect, complication, self-centering device, transcatheter closure
Introduction
Transcatheter device closure of secundum atrial septal defects (ASDs) or valvular incompetent patent foramen ovale (PFO) has become a well-accepted alternative to surgical therapy for more than two decades.[1-6] Solysafe® septal occluder (SSO) (Swissimplant AG, Solothurn, Switzerland) was a self-centering device and was first introduced for transcatheter closure of secundum ASDs and PFOs.[7-9] The CE mark approval was obtained for both smaller (15, 20, and 25 mm) and larger (30- and 35-mm) devices in 2007 and 2009, respectively.[7-9] There were promising advantages of this device with the flat design and the reduced amount of foreign metal material. An additional possible advantage of this occluder is that it does not require a long sheath, which is also beneficial for young children.[7,9] After unpublished cases of device fracture and embolization, however, the manufacturer (Swissimplant AG, Solothurn, Switzerland) issued an "urgent field safety notice" on August 4th, 2010, asking for an immediate discontinuation of implantation and distribution of device with diameters of 30 and 35 mm, and prompting all healthcare providers to check all patients with SSO. On August 13th, 2010 this warning was extended to cover all models including implants of diameters 15 mm, 20 mm, and 25 mm.
Follow-up data are critically important for the evaluation of complications previously mentioned. Limited data exist on the use of Solysafe® device and its outcomes in children. In this study, we report our mid-term results of transcatheter closure of secundum ASDs using the Solysafe® device in children.
Patients and Methods
A total of 25 patients (8 males, 17 females; mean age 8.4±3.6 years; range 5 to 12 years) who underwent percutaneous closure of secundum ASD at two centers including Izmir Behcet Uz Children's Hospital Department of Pediatric Cardiology and Adana Baskent University, Pediatric Cardiology Unit between July 2008 and June 2010 were included in this study. Transthoracic echocardiography (TTE) was performed routinely before ASD closure to assess the right ventricular size and function. Detailed anatomy of the defect and interatrial septum was examined using transesophageal echocardiography (TEE) at least one week before the closure operation. The study protocol was approved by the Adana Baskent University Ethics Committee. A written informed consent was obtained from each patient. The study was conducted in accordance with the principles of the Declaration of Helsinki.
We selected patients who had secundum ASD from 7 to 8 mm or larger with significant left-to-right shunt (Qp/Qs >1.5) and evidence of right heart enlargement for interventional closure. We excluded patients who had defects with deficient rims (inferior and superior rims smaller than 5 mm), Qp/Qs ratio less than 1.5, and pulmonary vascular resistance exceeding 8 Woods units/m² and very large defects with atrial septal length to ASD ratio <1.5/1.
Solysafe® septal occluder and intervention protocol The Solysafe® septal occluder (Swissimplant AG, Solothurn, Switzerland) is a self-centering device which consists of eight metal wires made of phynox (special cobalt-based alloy), two foldable polyester patches, and two wire holders.[7,10,11] It is often recommended that the nominal diameter of the device should exceed the stretched defect size by at least 3 mm.[10] Balloon sizing was performed with a balloon catheter to determine the stretched diameter of the ASD. The device size was chosen based on the manufacturer's recommendations: 15 mm device for defects between 4 and 12 mm, 20 mm device for defects between 13 and 17 mm, 25 mm device for defects between 18 and 22 mm, 30 mm device for defects between 23 and 26 mm, and 35 mm device for defects between 27 and 30 mm.[3,4] Before implantation, the occluder was stretched by two coaxial control catheters and could be easily configured due to the special arrangement of the metal wires to take the typical shape of bananas. The stretched device was introduced through a short 10 French sheath into the femoral vein over a stiff 0.018-inch guide-wire already positioned in the left atrium or left upper pulmonary vein. The implantation was successfully completed without the assistance of the long sheath. We controlled the device configuration under fluoroscopy (45 LAO and 15 cranial angulation). After the placement of the streched device in the defect, by pulling the distal and pushing the proximal position control catheter, the two discs were fixed by the locking mechanism.[10,11]
All interventions were performed under general anaesthesia with the TEE guidance. All patients received a bolus of 50 U/kg heparin at the beginning of procedure, followed by a second bolus of 50 U/kg during the placement of a stiff guide-wire. An antibiotic prophylaxis with cefazolin (100 mg/kg) was given before the procedure and repeated after eight hours with half of the initial dose.
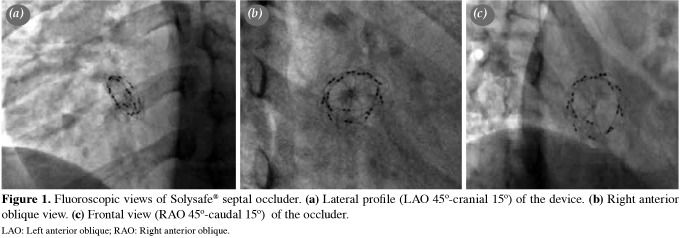
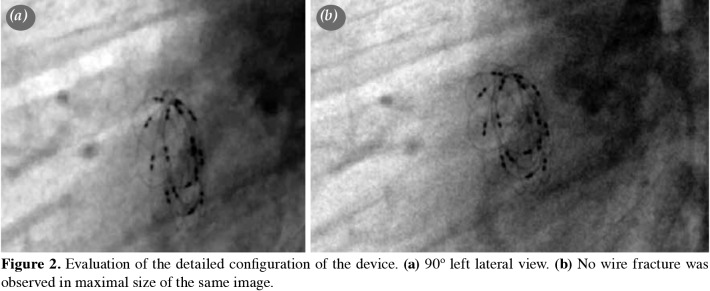
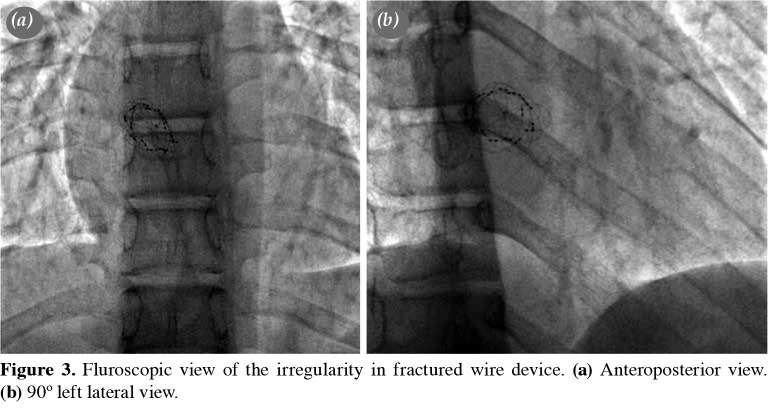
Follow-up The patients were discharged the day after the procedure after the careful assessment of the device position by chest X-ray and TTE. Low-dose acetylsalicylic acid (3-5 mg/kg) therapy was initiated for six months following the defect occlusion. Infective endocarditis prophylaxis was continued for at least six months. Follow-up included clinical assessment, telecardiography, TTE, and electrocardiography (ECG) at one, three, six, and 12 months after the procedure and annually thereafter, as a policy of our practice. After the announcement of the withdrawal of the device when the mean follow-up duration was 1.1±0.6 years, we called back all patients to evaluate with telecardiography, ECG, TTE, and fluoroscopy. All patients had at least one fluoroscopic examination during the total follow-up period. In order to evaluate possible wire-fractures, we examined all patients with fluoroscopy according to the positions shown in Figure 1. In cases of suspected irregularity on TEE, detailed fluoroscopy was reperformed to check for wire fracture (Figures 2 and 3). In one patient who had a residual shunt at the end of six years after the device implantation, TEE was performed. A Holter ECG was performed in all patients after one year of intervention.
Figure 1. Fluoroscopic views of Solysafe® septal occluder. (a) Lateral profile (LAO 45o-cranial 15o) of the device. (b) Right anterior oblique view. (c) Frontal view (RAO 45o-caudal 15o) of the occluder. LAO: Left anterior oblique; RAO: Right anterior oblique.
Figure 2. Evaluation of the detailed configuration of the device. (a) 90º left lateral view. (b) No wire fracture was observed in maximal size of the same image.
Figure 3. Fluroscopic view of the irregularity in fractured wire device. (a) Anteroposterior view. (b) 90º left lateral view.
Results
Demographic, clinical, and angiographic data are presented in Table 1. It was attempted to close secundum ASDs with the Solysafe® device in 25 patients in two centers (15 patients at the first center, 10 patients at the second center). The mean size of the defects measured by TTE was 11.0±3.8 mm (range, 6 to 21-mm) and measured by TEE was 11.5±4.0 mm (range, 7 to 23 mm). The mean stretched diameter of ASD assessed by balloon sizing was 13.6±4.4 mm (range, 8 to 26 mm). As a result, TEE and angiographic measurements of nine of 15-mm devices, eight of 20-mm devices, six of 25-mm devices, and two of 35-mm devices were used.
Table 1. Demographic and clinical data of the patients (n=25).
| n | % | Mean±SD | Range | |
| Procedural success | 22 | 88 | ||
| Age at the time of intervention (year) | 8.4±3.6 | |||
| Gender | ||||
| Female | 17 | |||
| Male | 8 | |||
| Atrial septal defect size by TTE (mm) | 11.0±3.8 | 6-21 | ||
| Atrial septal defect size by TEE (mm) | 11.5±4.0 | 7-23 | ||
| Atrial septal defect size by balloon stretched (mm) | 13.6±4.4 | 8-26 | ||
| Follow-up duration (year) | 6.1±0.5 | 5.2-7.2 | ||
| TEE: Transesophageal echocardiography; TTE: Transthoracic echocardiography. | ||||
Procedural success and complications The procedure was completed successfully in 22 of 25 patients (14 of 15 patients at the first center and eight of 10 patients at the second center). The overall success rate was 88% (22/25). In 22 patients, successful ASD closure was achieved using single SSO device. In one patient from the first center, complete implantation of a 25-mm SSO was unable to be achieved due to aneurysmatic floppy septum. The device was unclicked, straightened, and totally retrieved through the guidewire. Therefore, elective surgery was planned for this patient. In two patients from the second center, the attempts of ASD closure (35 mm in both patients) failed. In the first patient, floopy rims prevented the secure placement of a 35-mm SSO device. Each attempt of device implantation resulted with a significant shunt as shown by TEE. Therefore, we decided to refer the patient to surgery. The device was implanted successfully in the second patient. However, the day after the intervention, TTE showed that device was not at the previous location of the atrial septum and adopted the shape of the loading position by opening the locking mechanism and embolized into the main pulmonary artery. There was no detection of clinical symptoms or deterioration related to embolization. The patient was referred to surgery immediately and the device was removed and ASD was closed surgically.
There were no mortality or pericardial effusions. However, there were two major complications (8%). The first one was an embolization into the pulmonary artery in a 17-year-old girl, as discussed above. The other case was an 11 year-old girl from the first center who had implantation of 15-mm SSO device into an 8-mm defect. Right hemiparesis developed after the intervention. Left hemispheric infarct was noted using cranial magnetic resonance imaging (MRI). Physical therapy was initiated in this patient. There were two minor complications (8%) related to the procedure. One patient developed junctional rhythm during the procedure in the first center, which improved spontaneously without any medication. Other patient developed partial occlusion of the right femoral vein in the second center, which resolved with heparin infusion in a short time. Additionally, one patient had a residual shunt immediately after the procedure (4%) in the first center.
Mid-term follow-up data At least six control visits were done during the first year of the implantation (on Day 1, Week 1, Month 1, Month 3, Month 6, and Year 1). Follow-up examinations were scheduled annually starting by the end of the first year following the procedure. The complete ASD closure rate immediately after the procedure was 95% (21/22). All patients had mid-term follow-up at a mean of 6.1±0.5 years (range, 5.2 to 7.2 years). During six-year follow-up, a residual shunt was persistent in one patient. The rate of successful implementation without the occurrence of a residual shunt was 95% after six years of follow-up. There were no cases of mortality or major adverse events during follow-up. Echocardiographic follow-up data were available for all patients. We did not observe clinical symptoms related to the procedure during follow-up. All patients were in sinus rhythm; none developed significant arrhythmia in Holter ECG monitorizations. Initially, the patients were evaluated with conventional left anterior oblique (LAO) cranial and lateral views in angiogram for the wire fractures. None had wire fracture and during follow-up, we repeated fluoroscopy in eight patients with suspected irregularity shown in TEE. We also used fluoroscopic views suggested by Gielen et al.[12] after the announcement of urgent safety notice. The patient with right hemiparesis had improved motor control and strength with physical therapy and botox injections into her right extremities. However, she did not recover fully, and she is still on physical therapy. In our patient group, we observed wire fracture only in one patient without major or minor health problems at the end of the six years of follow-up.
Discussion
There are few reports regarding short- and mid-term follow-up data of ASD closure with the SSO.[13] In the present study, we report the results from two centers regarding ASD closure with SSO in children. During the mean six years of follow-up period, we found only one wire fraction; however, we observed no embolization of wire fragments and other complications including pericardial effusion, valvular problems, endocarditis or thrombus formation, hemolysis, and dysrhythmias in our patients, except for periprocedural complications. Preliminary reports showing wire fractures in the short-term follow-up warned the manufacturer and urged the company to make a decision on the fate of SSO device.[13] On August 4th, 2010, the manufacturer immediately stopped implanting, marketing, selling, and the distribution of devices with big sizes (30- and 35-mm devices). The notice was, then, extended for all sizes on August 13th, 2010. The first wire fracture was observed in early 2010 in Turkey (at the Department of Pediatric Cardiology, Istanbul University, Turkey). Following these case reports, other centers continued to report wire fractures and embolizations.[12,14] Gielen et al.[12] showed that the overall probability of freedom from wire fractures was 82.3% at five years. In this most large-scale study on SSO, the authors suggested clinical and fluoroscopic examinations in all patients with SSO device occlusion to control possible device fractures. The manufacturer's urgent safety notice did not contain information about the fluoroscopy views and timing of evaluation of patients. During the follow-up period, we evaluated patients with chest X-ray and TEE. We preferred 45° of LAO and 15° of cranial angulation during the device implementation. After urgent safety field notice, we used additional views to see the device from as many angles as possible, until the suggestions of Gielen et al.[12] were reported. In our study, therefore, all patients were evaluated after five years of implementation and underwent fluoroscopy as suggested by Gielen et al.[12]
In our series, we observed wire fractures only in one case in the mid-term follow-up. Each patient had at least one fluoroscopic evaluation with TEE and ECG. In the current study, TEE showed only mild regurgitation in a patient with a residual shunt. Gielen et al.[12] showed that device fractures developed only in adult patients with PFO. A possible explanation suggested by the aforementioned authors is that extraseptal tissue, which was only present in PFO, but not in secundum type atrial septal defect, might have caused increased stress on the wires of the selfcentering device. Moreover, Knirsch et al.[13] reported wire fractures in two boys (a five-year-old and an eight-year-old children) with a 30-mm and 25-mm SSO, respectively. Complications were reported more frequently in patients with PFO and in patients with the larger sizes of SSO. We attempted to use 35-mm devices in two patients. We were unable to achieve stabilization of the device in the septum in one patient. Another attempt resulted in the embolization of the device, due to the unexpected opening of the locking system. Low-profile feature of the device may be related to this complication in which the wires were unable to show resistance at large defects. However, devices with locking systems may have the probability of opening in a steady beating heart. Although the pressure would not open the locker, it may produce enough force to break the wire. Excluding these two cases, we chose particular patients with smaller size defects and appropriate rims for this device. Wire fractures were usually observed the first few years after implantation in the reported series. Our study provides the longest follow-up period and we observed a wire fracture in one case for whom we used a 20-mm device for 12-mm ASD at six years of follow-up. As there is no guarantee that any wire fractures would not occur in the long-term, close monitoring is required in these patients and our plan is to check all intact devices annually using fluoroscopy.
In the present study, one of our patients had right hemiparesis due to cerebral embolization. Cranial MRI revealed the cerebral infarct area. We routinely use heparin 100 U/kg in all patients during the procedure. Our patient had normal coagulation studies. Probably, thrombogenicity of the device and the delivery system may have given rise to thrombus formation, despite the concurrent use of heparin.
The SSO was previously used in several tertiary care centers. In the present study, we summarized data from two centers, which have mutual cooperation between each other and patients with similar indications for closure. Two centers did not present data separately due to relatively small number of patients. However, we believe that study population of the present study is adequate to draw conclusion from these findings of the device.
One of our patients had a residual shunt that persisted after the implantation of the device without showing any regression. During the last visit, we decided to check the residual shunt with TEE due to the suspicions of an increasing shunt size at the TTE imaging which revealed that the shunt did not increase and the device had no abnormality. The SSO has a lower profile than other products in the market.[7-9] Based on this unique character, it was initially thought that it could be used to close defects with aneurysmatic interatrial septums. However, aneurysmatic nature, thin thickness, and flexibility of the interatrial septum, when combined with this device, potentiate the risk of residual shunts and embolizations. Among our patients, one had an aneurysmatic interatrial septum, and the device mostly attached to the upper rim. This patient had a trivial shunt which was considered to improve over time. Unfortunately, in this case, residual shunt persisted; however, the device did not mobilize, nor had a wire fracture.
Although the SSO is no longer available in the market, it has been emerged as a promising low-profile device. Basically, we can make two statements based on our experience with this device. First, low-profile devices are not sufficient in closing large defects. Therefore, we pay more attention in clinical practice. For larger defects, we use Amplatzer occluders or devices with similar configuration. Second, we believe that devices such as SSO with the locking mechanism are not the most optimal choices in the beating heart, as one cannot predict when the locking mechanism would be released.
In conclusion, although this study was conducted with a relatively small group of patients, we believe that our report contributed to the current literature owing to its longest follow-up results. In our study, we did not observe any significant complications during the mid-term, except for periprocedural complications including device embolization, thrombus formation, and residual shunt. The main reason of low number of wire fractures in this population may be related to the selection of patients with smaller size defects at baseline. As a result, we continue to regularly follow our patient cohort and we are willing to report any complications related to this device. Our plan is to check all intact devices annually using fluoroscopy, as recommended by the manufacturer.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Masura J, Gavora P, Formanek A, Hijazi ZM. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn. 1997;42:388–393. doi: 10.1002/(sici)1097-0304(199712)42:4<388::aid-ccd7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Meier B. Percutaneous Interventions for Congenital Heart Disease, 1st ed. Patent foramen ovale-amplatzer PFO occluders; pp. 289–309. [Google Scholar]
- 3.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976;235:2506–2509. [PubMed] [Google Scholar]
- 4.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–1844. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 5.Salehian O, Horlick E, Schwerzmann M, Haberer K, McLaughlin P, Siu SC, et al. Improvements in cardiac form and function after transcatheter closure of secundum atrial septal defects. J Am Coll Cardiol. 2005;45:499–504. doi: 10.1016/j.jacc.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 6.Yılmazer MM, Güven B, Vupa-Çilengiroğlu Ö, Öner T, Demirpençe S, Meşe T, et al. Improvement in cardiac structure and functions early after transcatheter closure of secundum atrial septal defect in children and adolescents. Turk J Pediatr. 2013;55:401–410. [PubMed] [Google Scholar]
- 7.Ewert P, Söderberg B, Dähnert I, Hess O, Schuler G, Bussmann C, et al. ASD and PFO closure with the Solysafe septal occluder - results of a prospective multicenter pilot study. Catheter Cardiovasc Interv. 2008;71:398–402. doi: 10.1002/ccd.21360. [DOI] [PubMed] [Google Scholar]
- 8.Nişli K, Oner N, Aydoğan U, Ertugrul T. Atrial septal defect closure with Solysafe device: first experience in Turkey. Transcatheter Derg. 2007;7:451–452. [PubMed] [Google Scholar]
- 9.Kretschmar O, Sglimbea A, Daehnert I, Riede FT, Weiss M, Knirsch W. Interventional closure of atrial septal defects with the Solysafe Septal Occluder--preliminary results in children. Int J Cardiol. 2010;143:373–377. doi: 10.1016/j.ijcard.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 10.Ewert P. The Solysafe septal occluder for the closure of ASDs and PFOs. In: Hijazi ZM, Feldman T, Al-Qbandi MHA, Sievert H, editors. Transcatheter Closure of ASDs and PFOs. A comprehensive Assessment. 1. Minneapolis: Cardiotext Publishing; 2010. pp. 417–413. [Google Scholar]
- 11.Hein R, Bayard Y, Taaffe M, Büscheck F, Ostermayer S, Billinger K, et al. Patent foramen ovale and left atrial appendage: new devices and methods for closure. Pediatr Cardiol. 2005;26:234–240. doi: 10.1007/s00246-005-1011-7. [DOI] [PubMed] [Google Scholar]
- 12.Gielen S, Riede FT, Schuler G, Dähnert I. Wire fractures in Solysafe septal occluders: a single center experience. Catheter Cardiovasc Interv. 2012;79:1161–1168. doi: 10.1002/ccd.23399. [DOI] [PubMed] [Google Scholar]
- 13.Knirsch W, Quandt D, Dave H, Prêtre R, Kretschmar O. Mid-term follow-up of interventional closure of atrial septal defect using Solysafe™ Septal Occluder - impact of standardized fluoroscopy for complication detection. Int J Cardiol. 2011;152:127–128. doi: 10.1016/j.ijcard.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Cabrera M, Contreras A, Peirone A. Late cardiac perforation following percutaneous atrial septal defect closure using the Solysafe device. E139-41J Invasive Cardiol. 2011;23 [PubMed] [Google Scholar]