Abstract
Background
This study aims to discuss the early-term postoperative thoracic complications in videothoracoscopic anterior vertebral body tethering surgery.
Methods
The study included 56 patients (3 males, 53 females; mean age 12.6 years; range, 10 to 16 years) operated with a total of 65 videothoracoscopic anterior vertebral body tethering surgeries between April 2014 and November 2018. Surgical indications were adolescents with different growth potentials, who had thoracic, thoracolumbar or double curves less than 70°. Surgical details and postoperative thoracic complications were recorded.
Results
Forty-two patients were administered thoracic tether, whereas five and nine patients were administered thoracolumbar tether and both approaches concomitantly, respectively. Two patients developed ipsilateral total atelectasis, one patient contralateral lobar atelectasis, one patient chylothorax, one patient pleural effusion, and one patient pneumothorax after chest drain removal. Overall thoracic complication rate was 9.2% and 30-day readmission rate was 1.8%. All patients achieved their rehabilitation goals.
Conclusion
Videothoracoscopy-assisted anterior vertebral body tethering is a safe and efficient technique that yields low complication rates. Early postoperative functional results are promising with high patient satisfaction. Pre- and postoperative respiratory rehabilitation may decrease thoracic complication rates.
Keywords: Adolescent idiopathic scoliosis, vertebral body tethering, videothoracoscopy
Introduction
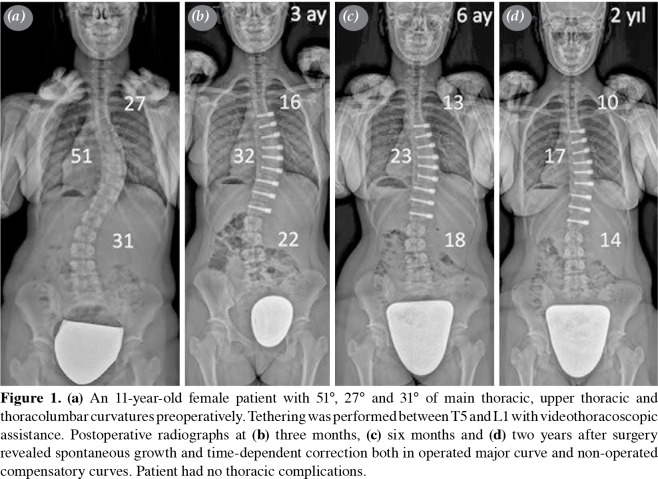
In early childhood and adolescence, the standard approach to idiopathic scoliosis is as follows: observation and physical therapy for curves <20°, brace and physical therapy for 20-40° curves and surgery for curves ≥40°.[1] Even though instrumented fusion remains the gold standard in surgical treatment, it prevents motion within the fused segments and halts longitudinal spinal. Spinal fusion further affects thoracic and lung growth due to the close anatomical and physiological relationship between the thoracic cage and spinal column.[2] As adverse effects of fusion have been better understood, pursuit of non-fusion surgical methods increased in order to maintain, direct, and modulate the spinal and thoracic growth.[3] Anterior vertebral body tethering (VBT) is a relatively new technique in the treatment of adolescent idiopathic scoliosis.[4] The operation can be performed via open thoracotomy or video-assisted thoracoscopy. Vertebral body screws are placed on the convexity after which a tether is attached and tightened. The pressure applied on the convexity, as stated by the Hueter-Volkmann law, reduces the growth on the convex side while accelerating the concave growth.[5] Therefore, as the patient continues to grow after the surgery, the growth results in gradual curve correction instead of progression as indicated by natural history (Figure 1).[6]
Figure 1. (a) An 11-year-old female patient with 51°, 27° and 31° of main thoracic, upper thoracic and thoracolumbar curvatures preoperatively. Tethering was performed between T5 and L1 with videothoracoscopic assistance. Postoperative radiographs at (b) three months, (c) six months and (d) two years after surgery revealed spontaneous growth and time-dependent correction both in operated major curve and non-operated compensatory curves. Patient had no thoracic complications.
After the demonstration of the effects of tethering in biomechanical and animal studies,[7-9] the first human case was performed with encouraging outcomes.[10] This was followed by two published clinical series reporting on early clinical and orthopedic findings.[4,11] Although currently performed in a limited number of medical centers, there is an increasing demand from families and patients. In this study, we aimed to discuss the early-term postoperative thoracic complications in videothoracoscopic anterior VBT surgery.
Patients and Methods
The current study is a retrospective analysis of prospectively collected data where early postoperative thoracic complications were evaluated. The study was conducted at Acıbadem Mehmet Ali Aydınlar University Maslak Hospital and included 56 patients (3 males, 53 females; mean age 12.6 years; range, 10 to 16 years) operated with a total of 65 videothoracoscopic anterior VBT surgeries between April 2014 and November 2018. Surgical indications were limited to patients with significant growth potential and less than 70° of thoracic curves for the first couple of cases. As physicians gained experience, indications were expended to adolescents with no or limited growth potential who had flexible curves, and to cases of thoracolumbar and double curves. The study protocol was approved by the Acıbadem Mehmet Ali Aydınlar University Medical Research Ethics Committee. A written informed consent was obtained from each patient and/or their legal guardians. The study was conducted in accordance with the principles of the Declaration of Helsinki.
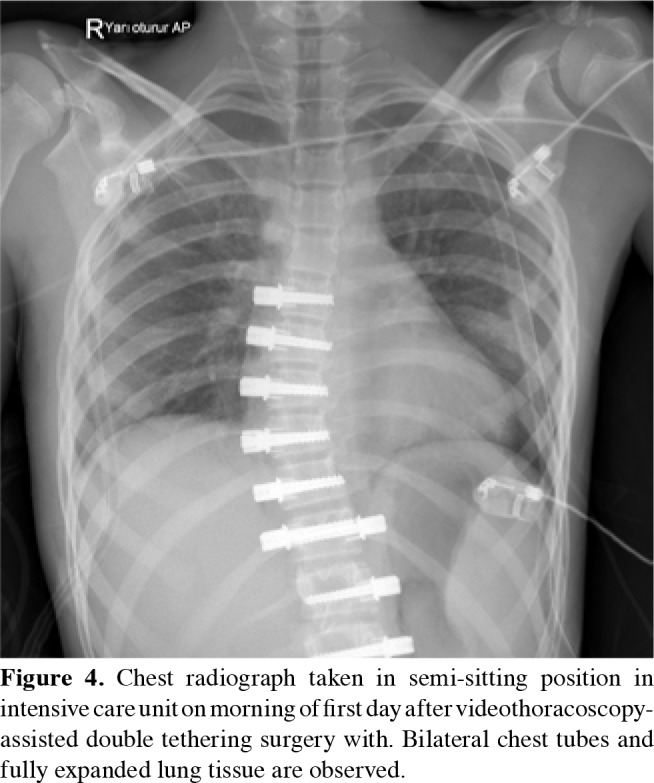
The patients were intubated with double-lumen tubes and positioned in lateral decubitus. Six-port thoracoscopy was used for instrumentation between T5 and L1. Three 5 mm ports were inserted in the anterior axillary line through 1 cm incisions for visualization and exposure of the vertebral bodies (Figure 2). Three 15 mm ports were inserted in the posterior axillary line through 2.5 cm incisions. Instrumentation of L1 was possible via the dissection of the diaphragm from posterior costavertebral junction to the vertebral bodies. The four-port technique, two of which were placed in the anterior and two in the posterior axillary line, was used for thoracolumbar curves which was accompanied by a retroperitoneal mini-open (3-4 cm) approach for the instrumentation of L2 and L3 (Figure 3). Vertebral levels were confirmed via fluoroscopy. Parietal pleura on the vertebral bodies were incised and segmental vessels were ligated using an ultrasonic scalpel to allow screw placement. Vertebral bodies were instrumented with hydroxyapatite-coated screws and pronged staples. Screws were placed bicortically under fluoroscopic assistance. Patients with both thoracic and lumbar curves underwent thoracic VBT on one side and thoracolumbar VBT on the other side in a single session (Figure 4). After placing the screws, a tether was placed across the tulips of the screws. Screw inserters were used for corrective translation and derotation maneuvers while applying compression to tighten the tether via a tensioning device. The correction rate was set according to the remaining potential of growth of the child. A greater correction was performed for the patients with little potential. In contrast, a bigger residual curve was desired for patients with significant remaining growth to ensure spontaneous follow-up correction. The integrity of the tether was examined and the ones damaged during insertion and tightening were replaced. A chest drain was inserted through the lowest port incision in the anterior axillary line.
Figure 2. Preparation of vertebral body for instrumentation through three anterior ports.
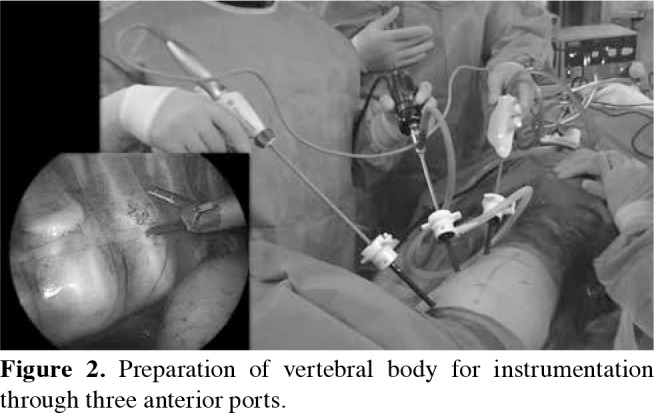
Figure 3. Four-port technique and mini flank incision for thoracolumbar tethering applications.

Figure 4. Chest radiograph taken in semi-sitting position in intensive care unit on morning of first day after videothoracoscopy- assisted double tethering surgery with. Bilateral chest tubes and fully expanded lung tissue are observed.
Results
A total of 65 videothoracoscopic interventions were performed. A mean of 7.9 (range, 5-11) were tethered in the whole cohort. The mean duration of surgery was 290 min (range, 120-660 min). The mean estimated blood loss was 76 mL (range, 10-200 mL). Thoracic-only tethering was applied for 42 patients. T5 or T6 was selected as the upper-instrumented vertebra. In 38 patients, the lower-instrumented vertebra was T11, T12 or L1. However, in four patients with long thoracic curvature, a mini open retroperitoneal approach was used for the instrumentation of L2 and L3. A mean of 7.5 levels (range, 6-9 levels) were tethered. The mean duration of surgery was 237 min (range, 120-360 min). The mean estimated blood loss was 63 mL (range, 10-150 mL). In five patients with thoracolumbar curves, the instrumentation was performed between T9, T10 or T11 to L2 or L3 with video-assisted thoracoscopy and retroperitoneal mini open approach. A mean of 5.8 levels (range, 5-6 levels) were tethered. The mean duration of surgery was 330 min (range, 240-450 min). The mean estimated blood loss was 112 mL (range, 10-200 mL). Bilateral approach was performed on the remaining nine patients as they had both thoracic and lumbar curves. The instrumentation was performed between T5, T6 or T7 to L3 or L4. A mean of 10.8 levels (range, 10-11 levels) were tethered. The mean duration of surgery was 540 min (range, 420-660 min). The mean estimated blood loss was 128 mL (50-200 mL).
Respiratory physiotherapy was started on postoperative day one. The mean duration to remove the chest drain was two days (range, one-four days). The patients were discharged after 4.5 days on average (range, 3-7 days). The patients returned to school within 10 to 18 days. All patients were allowed to return to their daily activities six weeks after the operation.
Early thoracic complications occurred in six (9.2%) of the 65 interventions for 56 patients. Two patients had ipsilateral atelectasis, one patient had contralateral lobar atelectasis, one patient had chylothorax, one patient had pleural effusion, and one patient had pneumothorax after removal of the chest tube.
Patients developing atelectasis had acetylcysteine, inhaled bronchodilator therapy, and cold steam application in addition to intensive respiratory physiotherapy. Atelectasis was resolved within 24 hours in all three patients.
Pleural effusion was detected in one patient whose chest drain was removed on the third day and who was discharged on the fourth day following the surgery. Three days after discharge, the patient returned with subfebrile fever. The patient was hospitalized and tube thoracostomy was performed. Approximately 350 mL and 50 mL of serous drainage were accumulated in the first and second days, respectively. The patient was discharged after the chest radiograph revealed that the lung was expanded following chest drain removal. Overall, the rate of 30-day readmission was 1.8% in this case series. In one patient who underwent VBT between T6 and L3, chylothorax developed on the second postoperative day. Approximately 300 mL of chylous drainage was accumulated during that day. Oral feeding was stopped and total parenteral nutrition was started. Approximately 200 mL was accumulated and gradually turned into serous fluid on the second day of treatment. On the third day, the drainage was 100 mL and completely serous. The patient started oral feeding on the fourth day with a fat-free diet. After observing less than 50 mL of serous drainage on day five, the chest drain was removed. On the seventh postoperative day, she was recommended a fat-free diet and discharged. The chest radiographs showed no complications in the following week. The chest tube and the lumbar drain of one patient, who had thoracolumbar operation, were consecutively pulled out. Subsequently, the patient was detected to develop ipsilateral pneumothorax. Tube thoracostomy was inserted under sedoanalgesia. The chest radiograph showed that the lung was expanded. The thoracic drain was removed one day later. All patients reached their rehabilitation goals.
Discussion
Spinal fusion surgery, the gold standard for adolescent idiopathic scoliosis treatment, is known to have various adverse effects such as limited spinal motion, growth inhibition in the operated segments, and adjacent segment degeneration.[12,13] Non-fusion surgical techniques have been developed to overcome these disadvantages. In VBT, convexity compression is applied also leading to concavity distraction. Thus, the curves gradually decrease with the remaining growth potential of the patient according to the Hueter-Volkmann law that states that an epiphyseal growth plate under pressure will have reduced growth compared to one that is not.[5]
The use of video-assisted thoracoscopy in thoracic spinal deformities is not a new approach. Thoracoscopic approach was used for anterior fusion in 1990s and 2000s.[14,15] Later on, as posterior fusion gained popularity, open and thoracoscopic anterior surgeries were performed rarely. Video-assisted thoracoscopy is regaining popularity with the use of the tethering technique, which presents a fusionless alternative in spinal surgery. This surgical technique performed together with orthopedic surgeons emerges as a promising field open to improvement.
Tethering was first reported by Crawford and Lenke[10] in a case report. Later on, Samdani et al.[4,11] published two different case series with one and two year follow-up discussing its safety and efficacy. Overcorrection, persistent atelectasis, and worsening of the deformity were among reported complications.[4,11] Infection, nerve or vessel injury, tether breakage, implant related complications, and chylothorax are also among potential complications which, to our knowledge, have not yet been reported.
Anatomically, ductus thoracicus enters the thorax through the aortic hiatus as a continuation of the cisterna chyli, and proceeds upwards in the anterior of the vertebral bodies. At the level of T5, it moves to the left of the aorta and travels up on the left side of the esophagus ending up in the venous system at the junction of the subclavian and internal jugular vein. Dissections at T12 and lumbar levels require attention as cisterna chyli is located closer to the surgical field in these levels.
The current findings indicate that duration of surgery and estimated blood loss are quite low compared to reported thoracoscopic fusions,[16,17] and are compatible with other thoracoscopic VBT series.[4,11] The current study also demonstrates that VBT technique provides a quick recovery period with low average length of hospitalization and early return to school. The rate of early thoracic complications was 9.2% and all recovered without sequelae. The rate of 30-day hospital readmission was 1.8%. None of the thoracic complications required reoperation.
In conclusion, videothoracoscopy-assisted application of vertebral body tethering is a safe and effective method in terms of thoracic complications for adolescent idiopathic scoliosis with thoracic, thoracolumbar, and double curves. Early postoperative functional results are promising with high patient satisfaction. Careful planning of pre- and postoperative respiratory rehabilitation may decrease thoracic complication rates. Longer-term follow-up with wider case series is warranted on this subject.
Footnotes
Conflict of Interest: The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The author received no financial support for the research and/or authorship of this article.
References
- 1.Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical Management of Adolescent Idiopathic Scoliosis. J Am Acad Orthop Surg. 2016;24:555–564. doi: 10.5435/JAAOS-D-14-00416. [DOI] [PubMed] [Google Scholar]
- 2.Akbarnia BA. Management themes in early onset scoliosis. J Bone Joint Surg [Am] 2007;89:42–54. doi: 10.2106/JBJS.F.01256. [DOI] [PubMed] [Google Scholar]
- 3.Yilgor C, Alanay A. Novel non-fusion growth-modulation techniques for pediatric scoliosis. In: Berven S, de Kleuver M, editors. AOSpine Master Series. Pediatric Spinal Deformity. Vol. 9. 1. New York: Thieme; 2017. pp. 53–62. [Google Scholar]
- 4.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, et al. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J. 2015;24:1533–1539. doi: 10.1007/s00586-014-3706-z. [DOI] [PubMed] [Google Scholar]
- 5.Mehlman CT, Araghi A, Roy DR. Hyphenated history: the Hueter-Volkmann law. Am J Orthop (Belle Mead NJ) 1997;26:798–800. [PubMed] [Google Scholar]
- 6.Guille JT, D'Andrea LP, Betz RR. Fusionless treatment of scoliosis. Orthop Clin North Am. 2007;38:541–545. doi: 10.1016/j.ocl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll M, Aubin CE, Moreau A, Parent S. Biomechanical comparison of fusionless growth modulation corrective techniques in pediatric scoliosis. Med Biol Eng Comput. 2011;49:1437–1445. doi: 10.1007/s11517-011-0801-8. [DOI] [PubMed] [Google Scholar]
- 8.Braun JT, Ogilvie JW, Akyuz E, Brodke DS, Bachus KN. Creation of an experimental idiopathic-type scoliosis in an immature goat model using a flexible posterior asymmetric tether. Spine (Phila Pa 1976) 2006;31:1410–1414. doi: 10.1097/01.brs.0000219869.01599.6b. [DOI] [PubMed] [Google Scholar]
- 9.Newton PO, Farnsworth CL, Upasani VV, Chambers RC, Varley E, Tsutsui S. Effects of intraoperative tensioning of an anterolateral spinal tether on spinal growth modulation in a porcine model. Spine (Phila Pa 1976) 2011;36:109–117. doi: 10.1097/BRS.0b013e3181cc8fce. [DOI] [PubMed] [Google Scholar]
- 10.Crawford CH, Lenke LG. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: a case report. J Bone Joint Surg [Am] 2010;92:202–209. doi: 10.2106/JBJS.H.01728. [DOI] [PubMed] [Google Scholar]
- 11.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, et al. Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine (Phila Pa 1976) 2014;39:1688–1693. doi: 10.1097/BRS.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 12.Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case-control study. Spine (Phila Pa 1976) 2006;31:275–283. doi: 10.1097/01.brs.0000197652.52890.71. [DOI] [PubMed] [Google Scholar]
- 13.Kepler CK, Meredith DS, Green DW, Widmann RF. Longterm outcomes after posterior spine fusion for adolescent idiopathic scoliosis. Curr Opin Pediatr. 2012;24:68–75. doi: 10.1097/MOP.0b013e32834ec982. [DOI] [PubMed] [Google Scholar]
- 14.Pehrsson K, Danielsson A, Nachemson A. Pulmonary function in adolescent idiopathic scoliosis: a 25 year follow up after surgery or start of brace treatment. Thorax. 2001;56:388–393. doi: 10.1136/thorax.56.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonner BS, Scharf C, Antonacci D, Goldstein Y, Panagopoulos G. The learning curve associated with thoracoscopic spinal instrumentation. Spine (Phila Pa 1976) 2005;30:2835–2840. doi: 10.1097/01.brs.0000192241.29644.6e. [DOI] [PubMed] [Google Scholar]
- 16.Grewal H, Betz RR, D Andrea LP, Clements DH, Porter ST. A prospective comparison of thoracoscopic vs open anterior instrumentation and spinal fusion for idiopathic thoracic scoliosis in children. J Pediatr Surg. 2005;40:153–156. doi: 10.1016/j.jpedsurg.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, Wang B, Zhu F. Comparison of the curative effects of video assisted thoracoscopic anterior correction and small incision, thoracotomic anterior correction for idiopathic thoracic scoliosis. Chin Med J (Engl) 2008;121:1369–1373. [PubMed] [Google Scholar]