Abstract
Background/aim
Losartan, an antihypertensive drug, is highly preferred in patients with diabetes mellitus (DM) and hypertension because of its retarding effect on diabetic nephropathy. In this study, we investigated the potential therapeutic effect of different doses of losartan on hepatic damage in a streptozotocin (STZ, 50 mg/kg)-induced DM model in rats.
Materials and methods
In this study, five different groups were formed: control, DM, low-dose losartan (5 mg/kg), mid-dose losartan (20 mg/kg), and high-dose losartan (80 mg/kg). Liver tissues of experimental groups were evaluated immunohistochemically for TUNEL, iNOS, eNOS, VEGF, and NF-κB pathways. In addition to immunohistochemical analysis, analyses of SOD and MDA, which are oxidative stress markers, were also performed and the results were evaluated together.
Results
When biochemical and immunohistochemical findings were evaluated together, it was found that the results obtained from the mid-dose losartan group were closer to those of the control than the other groups.
Conclusion
This study indicated that mid-dose losartan administration may have a therapeutic effect by inhibiting apoptosis and regulating iNOS, eNOS, VEGF, and NF-κB protein expressions in DM-induced hepatic damage.
Keywords: Hepatic damage, angiotensin II receptor blocker, iNOS, eNOS, VEGF, NF-κB
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by increased blood glucose level, hyperglycemia, and glucosuria, which are usually caused by deficiency in insulin secretion and/or insulin hormone activity in the pancreatic beta cells [1,2]. Chronic hyperglycemia arising from untreated DM can cause damage to several organs such as the eyes, kidneys, heart, and liver [3]. For example, many studies have shown that DM may be a trigger in different liver diseases such as nonalcoholic fatty liver (NAFLD), nonalcoholic steatohepatitis, fibrosis, cirrhosis, and later hepatocellular carcinoma (HCC) [4].
Nitric oxide (NO) is an important signaling molecule produced by three different nitric oxide synthase (NOS) isoforms: neuronal NOS (nNOS; NOS1), inducible NOS (iNOS; NOS2) and endothelial NOS [5]. Nitric oxide derivatives are involved in biological processes such as immune activation, cellular communication, metabolism, and blood pressure regulation and play a paradoxical role in the regulation of liver physiology and pathophysiology [6,7]. For example, NO expressed by eNOS in the liver sinusoidal endothelial cells (LSECs), portal vein, central vein, and lymphatic vessels protects the homeostasis of the liver and inhibits the formation of pathological conditions in the liver [8]. In contrast, NO expressed by iNOS in various liver cells, including LSECs, hepatocytes, and Kupffer cells (hepatic stellate cells), plays a role in the emergence of liver pathologies by triggering the formation of reactive nitrogen species (RNS) [9]. In addition, studies have shown that hyperglycemia activates iNOS expression, which can cause inflammation, apoptosis, and other diabetic complications in the liver [10]. On the other hand, oxidative stress induced by hyperglycemia causes an increase in the expression of vascular endothelial growth factor (VEGF), which is considered to be the key regulator of both physiological and pathological angiogenesis [11]. It has been demonstrated by studies that VEGF has a role in the pathogenesis of diabetic complications by inducing NOS expressions, resulting in an increase in NO production [12]. It is also known that VEGF has a fibrinogenic effect on the liver via the triggering of inflammation and the stimulation of endothelial and stellate cells [13].
Nuclear factor κB (NF-κB), an important factor in the regulation of processes such as inflammation and apoptosis, is another molecule that changes the activation pattern in DM. Activation of NF-κB due to hyperglycemia caused by DM induces the expression of RNS and reactive nitrogen species (ROS) directly or indirectly and paves the way for DM-induced hepatic damage. It has been suggested that inappropriate activation of NF-κB may lead to hepatocellular carcinomas by increasing insulin resistance in diabetic mice and may increase the likelihood of hepatic steatosis in cirrhosis, thus causing more hepatic damage [14,15]. In addition, increased ROS due to hyperglycemia activates many different gene expressions, including iNOS, via the NF-κB pathway [16].
Losartan, an angiotensin II type I receptor antagonist, is an antihypertensive drug that has a delaying effect on diabetic nephropathy, especially in patients with DM and hypertension [17]. On the other hand, in a study conducted with chronic hepatitis C patients in 2005, it was found that 6-week losartan treatment reduced subendothelial fibrosis in the liver [18].
In this study, we investigated the therapeutic effect of different doses of losartan on hepatic damage in a streptozotocin (STZ)-induced diabetic rat model. The liver tissues of the experimental groups were evaluated immunohistochemically for iNOS, eNOS, VEGF, and NF-κB pathways.
2. Materials and methods
2.1. Animals
Thirty-five adult male Wistar albino rats (weighing between 200 and 250 g) were used in this study. Rats caged in controlled rooms with 22 ± 3 °C temperature and 12-h light/dark cycles were fed with standard rat feed and water ad libitum. Experimental procedures used in this study were approved by the Ege University Local Ethics Committee for Animal Experiments. All procedures were carried out in strict compliance with the animal experiment guidelines prepared for the care and use of laboratory animals.
2.2. Experimental design
DM was induced in 28 rats by intraperitoneal injection of STZ (Sigma-Aldrich, St. Louis, MO, USA) (50 mg/kg in 0.1 M citrate buffer, pH 4.5). No drug was administered to the other 7 rats that all had blood glucose levels of <120 mg/dL (control group, n = 7). DM was verified after 24 h by evaluating the blood glucose levels. Rats with blood glucose levels of >250 mg/dL were included in the study as the diabetic rat group (n = 28) [19]. The 28 diabetic rats were then randomly separated into 4 groups. For a period of 4 weeks, the DM group (n = 7) was given no medication. The DM + low-dose losartan group (n = 7) was administered 5 mg/kg/day oral losartan (Cozaar, 50 mg; Merck Sharp & Dohme, Kenilworth, NJ, USA) for a 4-week period. The DM + mid-dose losartan group (n = 7) was administered 20 mg/kg/day oral losartan for a 4-week period. The DM + high-dose losartan group (n = 7) was administered 80 mg/kg/day oral losartan for a 4-week period.
At the end of the study, blood samples of 1 mL were taken into heparinized tubes for biochemical analysis from the rats, which were sedated under anesthesia. After bloodletting, liver tissues of euthanized rats were rapidly dissected for histopathological and immunohistochemical examinations.
2.3. Analysis of lipid peroxidation
Blood samples collected by cardiac puncture under sterile conditions were centrifuged at 4 °C and 1000 × g for 15 min so that blood plasmas were obtained. Plasma samples frozen rapidly on dry ice were stored at –80 °C until they were used. Lipid peroxidation was determined by measuring malondialdehyde (MDA) levels in plasma samples. In this respect, MDA levels were determined in accordance with the instructions of a commercially available lipid peroxidation (MDA) colorimetric/fluorometric assay kit (BioVision, Milpitas, CA, USA). The absorbance of each sample was measured at 532 nm with an ELISA plate reader (PolarSTAR Omega, BMG LABTECH, Germany) and results were obtained.
2.4. Analysis of serum superoxide dismutase (SOD) activity
Blood samples collected by cardiac puncture under sterile conditions were centrifuged at 4 °C and 1000 × g for 15 min so that blood plasmas were obtained. Plasma samples frozen rapidly on dry ice were stored at –80 °C until they were used. The SOD levels of the groups were determined according to a commercially available SOD activity assay kit (BioVision). The absorbance of each sample was measured at 450 nm with an ELISA plate reader (PolarSTAR Omega, BMG LABTECH) and results were obtained.
2.5. Histological analysis of liver tissues
The rats were euthanized under combined ketamine (60 mg/kg, Ege Vet, Alfamine, Alfasan International B.V., Woerden, the Netherlands) and xylazine (10 mg/kg, Ege Vet, Alfazyne, Alfasan International B.V.) anesthesia. Liver tissues were fixed for 48 h in 4% paraformaldehyde. Afterwards, 5-µm sections were taken from the tissues embedded in paraffin blocks using routine protocols. Sections were stained with hematoxylin and eosin (H&E) after deparaffinization and dehydration. The tissues were photographed with a digital camera (C-5050, Olympus, Tokyo, Japan) mounted on a microscope (BX5, Olympus) after staining.
2.6. Immunoexpressions of iNOS, eNOS, VEGF, and NF-κB
Sections were incubated with 10% H2O2 (Sigma-Aldrich) for 10 min for endogenous peroxidase blockade. To prevent nonspecific antibody–antigen binding, sections were incubated with Super Block (ScyTec Inc., Greenwood Village, CO, USA) for 1 h at room temperature and washed with PBS. After this step, sections were incubated with 1:200 diluted primary antibodies (iNOS, eNOS, VEGF, and NF-κB, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 24 h at 4 °C. At the end of this time, the sections were respectively incubated with biotinylated secondary antibody (ScyTec Inc.) and horseradish peroxidase conjugated streptavidin (ScyTec Inc.). Finally, the contrast staining of the sections incubated with diaminobenzidine (DAB) was performed with Mayer’s hematoxylin (Merck, Germany). Sections were cleaned with xylol and then closed with Entellan (Merck) [20].
2.7. Analysis of terminal-deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
TUNEL analysis was performed to determine apoptosis in liver tissues belonging to groups. After TUNEL staining was performed according to the procedure of the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Merck), the sections were photographed and the percentages of TUNEL-positive cells between all experimental groups were compared.
2.8. Statistical analysis
Data analysis was performed with SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Comparisons were then made between the control and treatment groups using one-way analysis of variance followed by a Tukey post hoc test. Values were presented with mean standard errors and P < 0.05 was considered statistically significant.
3. Results
3.1. SOD and MDA findings
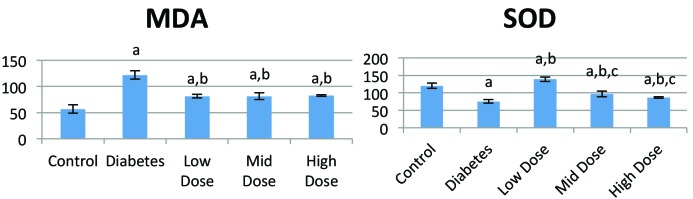
In the SOD analysis it was found that SOD activation was higher in the losartan-treated groups than in the DM group (P < 0.05). On the other hand, MDA levels were significantly lower in losartan-treated groups than in the diabetic group (P < 0.05) (Figure 1).
Figure 1.
MDA levels (nmol/mL) and SOD activities (% inhibition rate) of rat blood plasmas. a: Statistically significant compared to control group (P < 0.05), b: statistically significant compared to diabetes group (P < 0.05), c: statistically significant compared to high-dose losartan group (P < 0.05).
3.2. Histological findings
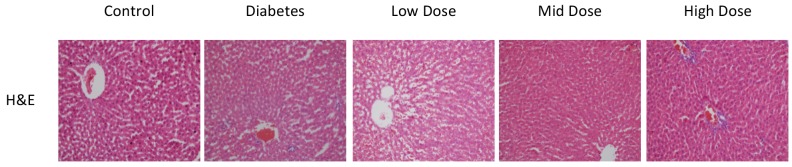
There were regular hepatocytes extending radially around the central vein in the sections of the control group. Sinusoids and Kupffer cells were normal and there were no signs of hemorrhage and infiltration in this group.
The number of hepatocytes in the DM group decreased and there were losses in the radial configuration of hepatocytes. Hepatocyte deformation and vacuolization were noted. Hemorrhage and infiltration were present.
The findings for the mid-dose losartan group were found to be closer to those of the control group compared to other groups. In the high-dose group, there was no difference in the number of hepatocytes and their regulation and sinusoids compared to the medium dose, whereas an increase in the number of Kupffer cells and infiltration were noted (Figure 2).
Figure 2.
H&E staining of sections from all experimental groups. Control group livers showed normal radially extending hepatocytes. DM group showed decreased radial configuration of hepatocytes and vacuolation. Low-dose losartan group showed little improvement in contrast with diabetes group. Mid-dose losartan group showed similar configuration to control group. High-dose losartan showed increased number of Kupffer cells and infiltration compared to other losartan-treated groups (20× magnification).
3.3. Immunohistochemical findings
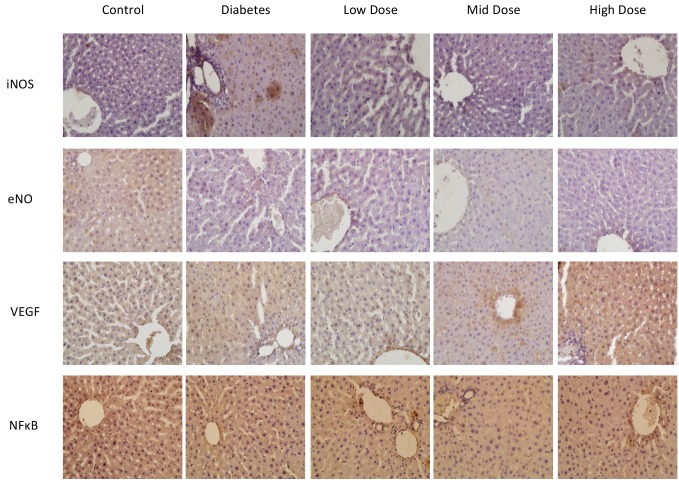
In histochemical analyses, it was found that iNOS protein expression increased and eNOS protein expression decreased in the DM group compared to the control and losartan-treated groups (P < 0.05). However, a level of iNOS protein expression close to that of the control group was determined in the mid-dose losartan group, and in the group with high-dose losartan, an increase in iNOS expression was observed.
We observed increased VEGF protein expression in the livers of diabetic rats (P < 0.05). It is noteworthy that in groups treated with losartan, there was a decrease in VEGF protein expression, just as in iNOS expressions. We also observed that VEGF expression was more similar to the control group in the mid-dose losartan treatment group compared to the other groups.
NF-κB immunoexpression levels were observed to be higher in the DM group than in the control group. When losartan-treated groups were evaluated among themselves, it was found that NF-κB expression was significantly lower in the mid-dose losartan group compared to the other groups (P < 0.05) (Figure 3). The immunoexpression levels and the P-values of the groups are shown in the Table.
Figure 3.
iNOS, eNOS, VEGF, and NF-κB immunostaining of sections from all groups (40× magnification).
Table.
Immunoexpression levels and TUNEL scores of control and other experimental groups
| Control | DM | Low-dose | Mid-dose | High-dose | P value | |
| Immunoexpression levels | ||||||
| iNOS | 1 ± 0.258 | 3 ± 0.378 | 2 ± 0.32 | 1,5 ± 0.32 | 2 ± 0.258 | 0.000 |
| eNOS | 3 ± 0.378 | 1 ± 0.258 | 1.5 ± 0.353 | 2 ± 0.378 | 1.5 ± 0.176 | 0.000 |
| VEGF | 1 ± 0.231 | 3 ± 0.231 | 1.5 ± 0.258 | 2.5 ± 0.258 | 3 ± 0.378 | 0.000 |
| NF-ᴋB | 1 ± 0.231 | 2.75 ± 0.267 | 2.5 ± 0.258 | 1.5 ± 0.231 | 2 ± 0.231 | 0.000 |
| TUNEL scores (%) | ||||||
| TUNEL-positive cells | 2 ± 0.175% | 4 ± 0.375% | 3 ± 0.312% | 2 ± 0.212% | 3 ± 0.262% | 0.000 |
| Groups | SOD activation(% inhibition rate) | MDA levels(nmol/mL) |
| Control | 120.15 ± 7.39 | 56.98 ± 8.22 |
| DM | 75.37 ± 5.28a | 122.09 ± 8.22a |
| Low-dose | 138.81 ± 6.33a,b | 81.4 ± 3.29a,b |
| Mid-dose | 97.01 ± 8.44a,b,c | 81.4 ± 6.58a,b |
| High-dose | 86.57 ± 2.11a,b | 82.56 ± 1.64a,b |
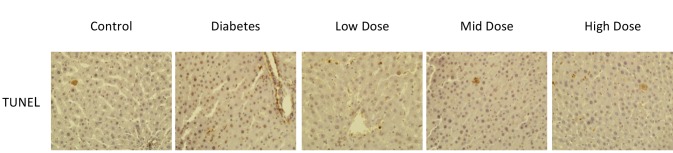
3.4. TUNEL staining findings
In TUNEL staining analysis, there was an increase in the number of apoptotic cells in the diabetic group compared to the control group. In addition, when the diabetic groups were evaluated among themselves, the number of TUNEL-positive cells decreased in the mid-dose losartan group compared to the other groups (P < 0.05) (Figure 4). TUNEL scores and the P-values of the groups are shown in the Table.
Figure 4.
TUNEL staining of sections from control and other groups (40× magnification).
4. Discussion
DM is a common metabolic disorder characterized by hyperglycemia, which occurs due to impaired insulin secretion in the pancreas [21]. This disease, which causes disruptions in the metabolism of carbohydrate, lipids, and proteins in the body, prepares the ground for serious functional and structural pathologies in many organs such as the heart, kidneys, eyes, and liver in the long term [22]. For example, previous studies suggested that DM is associated with liver diseases such as cirrhosis, NAFLD, fibrosis, and HCC [23]. The main mechanisms involved in the development of DM-induced liver diseases are ROS production and oxidative stress induced by hyperglycemia [16]. ROS production and oxidative stress induced by hyperglycemia enhance NO formation in diabetic liver damage. Interacting with ROS, NO induces many cellular signaling pathways, causing lipid peroxidation and protein nitration and thereby leading to DM-induced tissue damage [24,25]. NO, which has a significant role in diabetic liver damage, is produced by three different NOS isoforms: neuronal NOS (nNOS; NOS1), inducible NOS (iNOS; NOS2), and endothelial NOS (eNOS; NOS3) [6]. The role of NO in liver physiology is paradoxical [26]. For example, NO expressed by eNOS in the LSECs, portal vein, central vein, and lymphatic vessels protect hepatocytes and endothelial cells against ischemic reperfusion injury [27]. On the other hand, it has been found that NOS produced by iNOS might exacerbate liver damage after the inflammatory response [28]. In addition, Ingaramo et al. showed that iNOS expression and NO level increased in diabetic liver injury and this increase could cause significant impairment of liver function [29]. Jeddi et al. found a decrease in eNOS expression and an increase in iNOS expression in the hearts of diabetic rats. They argued that this might play an important role in the pathophysiology of DM-associated cardiac problems [30]. In our study, increased iNOS protein expression and decreased eNOS protein expression were determined in the livers of diabetic rats. However, the level of iNOS protein expression closest to the control group was determined in the mid-dose losartan group. Studies on renal fibrogenesis [31] and left ventricular remodeling [32] by different groups have shown that mid-dose losartan suppresses iNOS mRNA and protein expression. In addition, Matsuhisa et al. reported that losartan triggered iNOS-to-eNOS dependence [32]. When the findings of this study are evaluated together with the literature, the present study clearly suggests that mid-dose losartan may be a candidate for modulation of NOS derivatives that play different roles in liver injury.
Hyperglycemia-induced oxidative stress causes an increase in the expression of some enzymes and growth factors [33]. For example, VEGF, which is considered to be the key regulator of both physiological and pathological angiogenesis, is one of the growth factors that have increased expression in hyperglycemia-induced oxidative stress [11]. Also, studies have shown that VEGF, which is known to cause an increase in NO production by inducing eNOS and iNOS mRNA expressions, plays a role in the pathogenesis of diabetic complications and especially in retinopathy [12]. In their study of diabetic retinopathy, Djordjevic et al. observed an increase in the concentration of VEGF, which is thought to be very important for the destruction of the blood–retinal barrier and for neovascularization, with an increase in oxidative damage markers and iNOS concentration [33]. In another study Liu et al. showed increased expression of iNOS and VEGF in the cochlea of diabetic rats [34]. In our study, we observed increased expressions of iNOS and VEGF protein in the livers of diabetic rats. However, in groups treated with losartan, there was a decrease in VEGF protein expression, just like the iNOS expressions. In addition, we found that VEGF expression was more similar to the control group in the mid-dose losartan treatment group. Similar to our findings in this study, there is evidence that losartan suppresses VEGF mRNA and protein expression in different studies [35,36]. Also, Kamper et al. suggested that losartan administration in diabetic rats suppresses the production of VEGF and excess NO in the pancreas; thus, losartan can exert an antioxidant effect by suppressing oxidative and nitrosative stress [37].
NF-κB is a transcription factor that is required for the expression of most proinflammatory molecules, including enzymes, cytokines, and chemokines [38]. In vitro and in vivo experiments show that NF-κB activation plays a key role in the pathobiology of DM [39]. In this context, studies have shown that increased ROS levels associated with DM cause NF-κB activation, while this activation induces transcription of inflammatory genes such as cyclooxygenase-2 (COX-2), tumor necrosis factor-alpha (TNF-α), interleukin 1 (IL-1), and iNOS [40]. In addition to these studies, Pan et al. showed that the activation of NF-κB in diabetic cardiomyocyte cells leads to apoptosis, and inhibition of this pathway corrects cardiac dysfunction in diabetic mice [41]. These studies showed that NF-κB has an important role in DM-induced apoptosis. In our study, we determined that NF-κB immunoexpression intensity was higher in the diabetic groups than in the control group. When losartan-treated groups were evaluated among themselves, it was found that NF-κB expression was lower in the mid-dose group than the other groups. Also, our TUNEL staining analysis showed that there was an increase in the number of apoptotic cells (TUNEL-positive cells) in the diabetic group compared to the control group. In addition, when the diabetic groups were evaluated among themselves, the number of TUNEL-positive cells was decreased in the mid-dose group compared to the other groups. A number of past and present studies suggest that lipid peroxidation is one of the major causes of apoptotic injury associated with DM [42]. For example, Oyenihi et al. found increases in lipid peroxidation products such as MDA in the livers of diabetic rats but a decrease in the activity of antioxidant enzymes such as SOD [43]. The same group also revealed that TUNEL-positive cells were increased in diabetic rats, correlated with lipid peroxidation [43]. However, there are reports that losartan protects podocytes from apoptosis [44], cleans lipid peroxidation products in retinal [45] and pancreatic cells [37], and inhibits NF-κB activation [46]. The results of the present study support these reports.
In conclusion, these results suggested that mid-dose losartan administration may have a therapeutic effect by inhibiting apoptosis and regulating iNOS, eNOS, VEGF, and NF-κB protein expressions, which have been shown to play a role in the pathogenesis of DM-induced hepatic damage. Investigating the effects of the drugs such as losartan used in clinics for many years on the comorbidities observed in diabetic individuals may be effective in improving the quality of life of diabetic individuals and the emergence of new approaches in the treatment of this disease.
Acknowledgement/Disclaimers/Conflict of interest
This study was supported by the Ege University Scientific Research Projects Coordination Unit [grant number: 17-ILAM-002 (to Altuğ Yavaşoğlu)]. The experimental procedures on the animals in this study were performed at Ege University, Drug Research and Development and Pharmacokinetic Applications (ARGEFAR).
References
- Shi GJ Li ZM Zheng J Chen J Han XX Diabetes associated with male reproductive system damages: onset of presentation, pathophysiological mechanisms and drug intervention. Biomedicine & Pharmacotherapy. 2017;90:562. doi: 10.1016/j.biopha.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Current Medical Diagnosis & Treatment. 2017.
- Ziamajidi N Khaghani S Hassanzadeh G Vardasbi S Ahmadian S Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1. Food and Chemical Toxicology. 2013;58:198. doi: 10.1016/j.fct.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Mukherjee P Cinelli MA Kang S Silverman RB Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chemical Society Reviews. 2014;43:6814. doi: 10.1039/c3cs60467e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri Y Kim MY Nitric oxide in liver diseases. Trends in Pharmacological Sciences. 2015;36:524. doi: 10.1016/j.tips.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG Moncada S. Nitric oxide synthases in mammals. Biochemical Journal. 1994;298:249. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amara M Yang SY Seifalian A Davidson B Fuller B. The nitric oxide pathway - evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver International. 2012;32:531. doi: 10.1111/j.1478-3231.2012.02755.x. [DOI] [PubMed] [Google Scholar]
- Abdelmegeed MA Song BJ Functional roles of protein nitration in acute and chronic liver diseases. Oxidative Medicine and Cellular Longevity. 2014;10 doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar Z Kalet-Litman S Stark AH Inducible nitric oxide synthase activity and expression in liver and hepatocytes of diabetic rats. Pharmacology. 2005;73:106. doi: 10.1159/000081952. [DOI] [PubMed] [Google Scholar]
- Marazita MC Dugour A Marquioni-Ramella MD Figueroa JM Suburo AM Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biology. 2016;7:78. doi: 10.1016/j.redox.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J Waltenberger J VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR) Biochemical and Biophysical Research Communications. 1998;252:743. doi: 10.1006/bbrc.1998.9719. [DOI] [PubMed] [Google Scholar]
- Yoshiji H Kuriyama S Yoshii J Ikenaka Y Noguchi R Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC Hevener AL Greten FR Maeda S Li ZW #946; links inflammation to obesity-induced insulin resistance. Nature Medicine. 2005;11:191. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Mohamed J Nazratun Nafizah AH Zariyantey AH Budin SB Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Universty Medical Journal. 2016;16:132. doi: 10.18295/squmj.2016.16.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- extract on iNOS gene expression. Indian Journal of Clinical Biochemistry. 2018;33:147. doi: 10.1007/s12291-017-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JN Role of the renin-angiotensin system in cardiovascular disease. Cardiovascular Drugs and Therapy. 2010;24:341. doi: 10.1007/s10557-010-6230-3. [DOI] [PubMed] [Google Scholar]
- doi: 10.1007/s10557-010-6230-3.
- Sookoian S ndez MA o G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World Journal of Gastroenterology. 2005;225:7560. doi: 10.3748/wjg.v11.i48.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigitturk G Acara AC Erbas O Oltulu F Yavasoglu NUK The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomedicine & Pharmacotherapy. 2017;87:240. doi: 10.1016/j.biopha.2016.12.102. [DOI] [PubMed] [Google Scholar]
- Acikgoz E Aktug H Yigitturk G Demir K Guven U Repression of the Notch pathway prevents liver damage in streptozotocin-induced diabetic mice. Folia Histochemica et Cytobiologica. 2017;55:140. doi: 10.5603/FHC.a2017.0014. [DOI] [PubMed] [Google Scholar]
- Xu Q Wang L Luo J Shi D Luo J The hot and potential targets of type 2 diabetes mellitus treatment in recent decade. Current Drug Targets. 2017;19:55. doi: 10.2174/1389450118666170307111714. [DOI] [PubMed] [Google Scholar]
- ) increases SIRT1 and SIRT2 gene expressions in the kidney and liver tissues of STZ- and STZ+niacinamide-induced diabetic rats. Journal of Basic and Clinical Physiology and Pharmacology. 2018;29:463. doi: 10.1515/jbcpp-2017-0079. [DOI] [PubMed] [Google Scholar]
- Chen Z Wang C Pan Y Gao X Chen H. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food and Function. 2018;9:426. doi: 10.1039/c7fo00983f. [DOI] [PubMed] [Google Scholar]
- Griendling KK FitzGerald GA Oxidative stress and cardiovascular injury Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- Spitaler MM Graier WF Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- Lin HI Wang D Leu FJ Chen CF Chen HI Ischemia and reperfusion of liver induces eNOS and iNOS expression: effects of a NO donor and NOS inhibitor. Chinese Journal of Physiology. 2004;47:121. [PubMed] [Google Scholar]
- Cottart CH Do L Blanc MC Vaubourdolle M Descamps G Hepatoprotective effect of endogenous nitric oxide during ischemia-reperfusion in the rat. Hepatology. 1999;29:809. doi: 10.1002/hep.510290317. [DOI] [PubMed] [Google Scholar]
- Hines IN Kawachi S Harada H Pavlick KP Hoffman JM Role of nitric oxide in liver ischemia and reperfusion injury. Molecular and Cellular Biochemistry. 2002;234:229. [PubMed] [Google Scholar]
- Ingaramo PI Ronco MT s DEAA Monti JA Pisani GB Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Molecular Immunology. 2011;233:12–12. doi: 10.1016/j.molimm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Jeddi S Khalifi S Ghanbari M Bageripour F Ghasemi A. Effects of nitrate intake on myocardial ischemia-reperfusion injury in diabetic rats. Arquivos Brasileiros de Cardiologia. 2016;107:339. doi: 10.5935/abc.20160137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manucha W Oliveros L Carrizo L Seltzer A Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney International. 2004;233:2091. doi: 10.1111/j.1523-1755.2004.00643.x. [DOI] [PubMed] [Google Scholar]
- Matsuhisa S Otani H Okazaki T Yamashita K Akita Y N-Acetylcysteine abolishes the protective effect of losartan against left ventricular remodeling in cardiomyopathy hamster. Antioxidant & Redox Signaling. 2008;10:1999. doi: 10.1089/ars.2008.2069. [DOI] [PubMed] [Google Scholar]
- Djordjevic B Cvetkovic T Stoimenov TJ Despotovic M Zivanovic S Oral supplementation with melatonin reduces oxidative damage and concentrations of inducible nitric oxide synthase, VEGF and matrix metalloproteinase 9 in the retina of rats with streptozotocin/nicotinamide induced pre-diabetes. European Journal of Pharmacology. 2018;833:290. doi: 10.1016/j.ejphar.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Liu F Xia M Xu A Expression of VEGF eNOS is increased in cochlea of diabetic rat. Acta Oto-laryngologica. 2008;128:1178. doi: 10.1080/00016480801901774. [DOI] [PubMed] [Google Scholar]
- Effects of spironolactone and losartan on diabetic nephropathy in a type 2 diabetic rat model. Diabetes & Meta. [DOI] [PMC free article] [PubMed]
- bolism Journal. 2011;35:130. [Google Scholar]
- Kim NH Oh JH Seo JA Lee KW Kim SG Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney International. 2005;67:167. doi: 10.1111/j.1523-1755.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- Kamper M Tsimpoukidi O Chatzigeorgiou A Lymberi M Kamper EF The antioxidant effect of angiotensin II receptor blocker, losartan, in streptozotocin-induced diabetic rats. Translational Research. 2010;156:26. doi: 10.1016/j.trsl.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Liu T Zhang L Joo D Sun SC B signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2:17023–17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacological Reports. 2009;61:595. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- rez S rrez-Fern ndez B S nchez-Campos S lez-Gallego J n MJ Quercetin attenuates nuclear factor-κB activation and nitric oxide production in interleukin-1. Journal of Nutrition. 2005;237:nez–nez. doi: 10.1093/jn/135.6.1359. [DOI] [PubMed] [Google Scholar]
- pp. 1359–1359.
- Nasiri A Ziamajidi N Abbasalipourkabir R Goodarzi MT Saidijam M Beneficial effect of aqueous garlic extract on inflammation and oxidative stress status in the kidneys of type 1 diabetic rats. Indian Journal of Clinical Biochemistry. 2017;32:329. doi: 10.1007/s12291-016-0621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- s DE Ronco MT Monti JA Ingaramo PI Pisani GB Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: new insights into the insulin effect. Journal of Endocrinology. 2010;233:187. doi: 10.1677/JOE-09-0462. [DOI] [PubMed] [Google Scholar]
- Oyenihi OR Brooks NL Oguntibeju OO Effects of kolaviron on hepatic oxidative stress in streptozotocin induced diabetes. BMC Complementary and Alternative Medicine. 2015;15:236–236. doi: 10.1186/s12906-015-0760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SY Qi R Zhao H Losartan reverses glomerular podocytes injury induced by AngII via stabilizing the expression of GLUT1. Molecular Biology Reports. 2013;40:6295. doi: 10.1007/s11033-013-2742-9. [DOI] [PubMed] [Google Scholar]
- Silva KC Rosales MAB Biswas SK Lopes de Faria JB Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes. 2009;58:1382. doi: 10.2337/db09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G Cheng X Fu R Losartan inhibits nuclear factor-κB activation induced by small, dense LDL cholesterol particles in human umbilical vein endothelial cells. Current Therapeutic Research, Clinical and Experimental. 2014;76:17. doi: 10.1016/j.curtheres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]