Abstract
The modulation of the postsynaptic signaling machinery by protein phosphorylation has attracted much interest since it is key for the understanding of the regulation of a variety of synaptic functions. While advances in mass spectrometry have allowed us to begin performing large-scale analysis of protein phosphorylation in components of the PSD, the systematic collection of datasets and their functional significance within the context of regulatory signaling networks is in its infancy.
Here, we will focus on the composition of the PSD phosphoproteome describing kinase, phosphatase, and protein domain modules involved in the regulation of phosphorylation signaling. We will discuss the impact of synaptic plasticity mechanisms such as long-term potentiation (LTP) in mammalian kinomes and describe the general rules of signaling organization in the PSD phosphoproteome.
Introduction
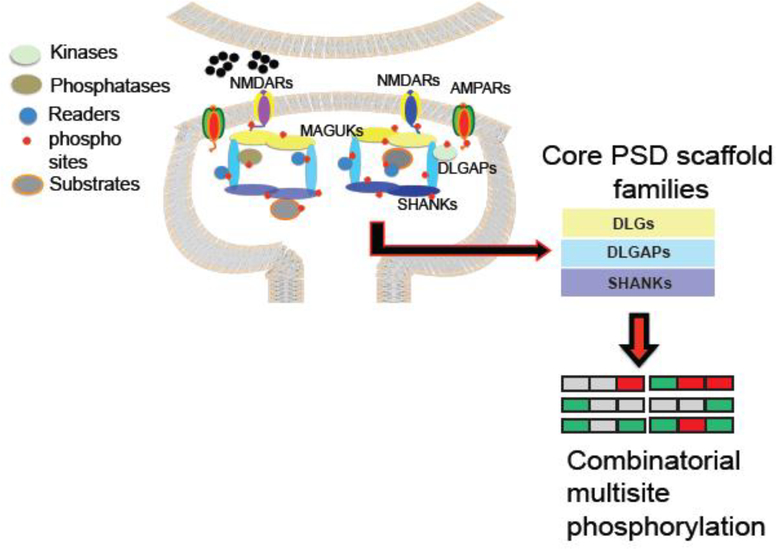
To begin to understand the organization and regulation of postsynaptic phosphorylation, we need to first review the basic structural organization where protein kinases, protein phosphatases, substrates and phosphorylation reader modules are embedded. The postsynaptic density (PSD) is a morphological and functional specialization of the postsynaptic membrane composed of 1,500–2,000 unique proteins [1,2] with a wide range of individual copy numbers [3,4] A characteristic signature of the structural organization of the PSD is the use of a variety of scaffold molecules containing protein domains specialized in protein-protein interactions [5–9] that help to assemble PSD components in protein interaction networks (PINs) at the right time and place. A schematic view of the core PSD-scaffolds can be described as three principal layers of scaffold proteins: a top layer composed of Disk Large Homolog (DLGs) family members that connect to neurotransmitter receptors, a bottom layer of SHANK family scaffolds, and a middle connecting layer composed of Disk Large Homolog Associated Proteins (DLGAPs) family members [5,10–13] (Figure 1). These three families are the most abundant protein-scaffolds of the PSD and can be considered as fundamental organizers of the core signaling machinery [10,12,14,15]. induced by changes in patterns of synaptic activity. At the PSD, like in many other signaling systems, protein localization, their activity and association to different partners can be modulated by post-translation modifications (PTMs) [16–19], and the most studied PTM is protein phosphorylation. The identification of individual phosphorylation sites relevant for postsynaptic function, the role of protein kinases and to a lesser degree, phosphatases, has been the focus of attention for many years. In particular, a number of protein kinases and phosphorylation sites have been implicated in diverse forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) [20–25]. It has been proposed that these two synaptic plasticity mechanisms might be regulated by opposite controls of protein phosphorylation (LTP) and protein dephosphorylation (LTD). This has been shown at the level of individual kinases and phosphatases. It has been proposed that, the opening of NMDARs during LTP produces a large and transient increase in calcium concentration within the dendritic spine. This increase in calcium concentration produces the autophosphorylation and activation of one the most abundant proteins at the PSD, the calcium/calmodulin-dependent kinase IIa (CAMKIIa), in a process that is absolutely required for the induction of LTP [26,27]. However it is proposed that the moderate increase in calcium concentration produced during NMDAR-dependent LTD, might trigger an opposite mechanisms, allowing a calcineurin-PP1 phosphatase cascade that can dephosphorylate and therefore, inactivate CAMKIIa, producing synaptic weakening and LTD [26]. These processes have been extensively reviewed and might be essential as a starting point or function as a signaling hub within PSD phosphorylation networks. Here as opposed to describing the role of individual kinases or phosphosites, we will discuss the organization of PSD kinases, phosphatases, substrates, and reader modules, what we know about their response to the induction of synaptic plasticity, and the general rules of organization within phosphorylation signaling networks.
Figure 1.
Schematic representation of PSD phosphorylation, showing kinase, phosphatase and phosphorylation readers. Cartoon shows core components of the PSD scaffold machinery. Top layer Disk Large associated (DLG) family members, also known as MAGUKs (see main text), middle scaffold layer composed of Disk Large Associated Guanylate Associated Proteins (DLGAPs) and a bottom layer of SHANK family scaffolds [5,10–13].
Large-scale analysis of PSD phosphorylation
The first large-scale analysis of synaptic proteins in 2005 identified 331 phosphorylation sites in 79 synaptic proteins [28]. While this analysis was performed in synaptosomes from mouse brain, and therefore contained a number of presynaptic proteins, it helped to identify and expand our knowledge on phophorylation events occurring in PSD proteins. By determining in vitro kinase/substrate pairs, it allowed the first map of synaptic protein phosphorylation to be drafted [28]. This was later followed by studies measuring protein phosphorylation in PSD fractions, thus determining PSD phosphorylation events in a more specific manner. These assays significantly increased the pool of phosphorylated proteins and sites at the PSD by describing more than 1500 phosphorylation sites in 2,000 PSD proteins [29–34]. Early reports consist of a collection of datasets describing phosphorylation of synaptic proteins under a variety of different methodological conditions and animal species. More importantly, very few assays were performed on purified PSD fractions and instead were performed using synaptosomes or total cell lysates from different brain regions. Thus, the number of reported phosphorylation sites reported has varied from a few hundred to several thousand in an equally variable number of phosphoproteins (Table 1A). This highly heterogeneous collection of sites and proteins confounds the analysis and determination of functional organization within PSD phosphorylation or protein interaction networks (PINs). Thus, it is extremely important that the analysis of PSD components should be performed in PSD fractions. Moreover, an appropriate interpretation of protein phosphorylation signaling requires the quantitation and understanding of protein phosphorylation ratios and therefore, the quantitation of total protein levels.
Table 1 A.
(top) Shows number of phosphorylation sites obtained in total brain lysates, synaptosomes and PSD preparations, describing phosphorylation sites (p-sites), total number of phosphorylated proteins detected (total proteins), ratio average of phosphorylation sites per protein, and sample preparation for each set of assays.
| Reference | Total p-sites | Total proteins | p-sites/protein average | sample type |
|---|---|---|---|---|
| Collins et al., 200524 | 331 | 79 | 4.2 | synaptosomes |
| Trinidad et al., 200625 | 723 | 287 | 2.5 | PSD |
| Munton et al., 200730 | 974 | 499 | 1.95 | PSD/synaptosomes |
| Trinidad et al., 200827 | 1564 | 831 | 1.88 | PSD |
| Li et al., 201632 | 1554 | 456 | 3.4 | PSD |
| Diering et al., 2017 31 | 10000 | 4500 | 2.2 | PSD/synaptosomes |
| Liu et al., 201835 | 60000 | 6700 | 8.9 | brain lysates |
| Wang et al., 201833 | 51821 | 7963 | 6.5 | brain lysates |
These sets of analysis were directed to address the extent of phosphorylation of synaptic and PSD proteins, and they were only mapping “basal” conditions. Recently, advances have been made to map specific states within the postsynaptic phosphoproteome by determining the composition of PSD phosphorylation during sleep [35] and after the induction of long-term potentiation [36]. Other studies have also allowed the identification of phosphorylation of synaptic proteins in total cell lysates under sleep conditions [37] phencyclidine treatment [38] and by modulation of kappa opioid receptors activity [39].
Phosphorylation stoichiometry enables an in-depth understanding of the regulatory mechanisms of cellular signaling networks. In the case of PSD signaling, it is also necessary to determine protein abundance within PSD fractions because changes in patterns of stimulation can promote the translocation of molecules in or out of the PSD in a time dependent manner. Unfortunately, this information is not always available. However, with more comprehensive datasets, we are able to collect larger number of PSD phosphorylation sites. Considering the total number of reported, 80 PSD kinases, they have the capacity to phosphorylate over 40,000 unique sites in 2,000/3,000 PSD proteins. Thus, we might expect highly heterogeneous ratios of phosphorylation sites per protein in reported datasets for proteins isolated from brain lysates (Table 1A). However, they are consistent with broad phosphorylation ratios observed in different tissues [40], with average phosphorylation ratios of 1.9–3.4 [29,35–37,39,40] (Table 1A). However, the stoichiometry seems to be higher for the core scaffold machinery of the PSD. Analysis of DLG (Dlg1–4), DLGAP (Dlgap1–4) and SHANK (Shank1–3) families shows a much larger number of phosphorylation sites per protein, ranging from 50 to more than 80 phosphorylation sites (Table 1B) on each individual scaffold protein. Certainly, a role in the large number of identified sites in scaffold proteins refers to their relative abundance and copy number in the PSD, since it is technically easier to initially identify phosphorylate sites from the more abundant proteins in a complex mixture. However, this does not seem to be the sole reason, since there is no apparent correlation between protein abundance, length, serine, threonine, tyrosine content and the number of phosphorylation sites per protein [8,31,36,39,41] (Table 1B). It is important to highlight that while PSD scaffolds are largely localized at the postsynaptic site, some of them are also not brain specific and a variable number of phosphorylation sites have been identified in non-neuronal tissue (https://www.phosphosite.org) [42], which implies a significantly lower relative stoichiometry for these sites in neuronal tissue. Recent studies support the notion that the high phosphorylation ratio of the PSD scaffold machinery may be functional relevant [36]. For example, it has been shown that the induction of LTP induces a large increase in protein phosphorylation in PSD scaffolds [36]. This is in contrast to balanced phosphorylation/dephosphorylation ratios observed for non-core PSD scaffolds [36]. It is important to highlight that early studies of postsynaptic phosphorylation suggested a more relevant role of phosphorylation in mechanisms of LTP, while a larger number of phosphatases were described in correlation with LTD [43–46]. However, recent studies suggest that the induction of LTP produces equal changes in PSD phosphorylation/dephosphorylation with no total gain or loss in global numbers of phosphorylation sites at the PSD. The relevance of protein kinase activity for LTP seems to be supported by an increase in the phosphorylation of the core PSD scaffold component and not a general increase in PSD phosphorylation.
Table 1 B.
shows total number of phosphorylation sites reported for PSD scaffold proteins, percentage of phosphorylation sites discovered in brain samples from the total reported sites, and number of aminoacids for each protein.
| Protein (PSD core) | Total p-sites reported | % present in brain | protein length (number of aminoacids) |
|---|---|---|---|
| Dig1 | 66 | 30% | 905 |
| Dlg2 | 46 | 80% | 852 |
| Dlg3 | 35 | 54% | 849 |
| Dlg4 | 50 | 96% | 724 |
| Dlgap1 | 61 | 82% | 992 |
| Dlgap2 | 76 | 83% | 1059 |
| Dlgap3 | 51 | 72% | 977 |
| Dlgap4 | 70 | 46% | 992 |
| Shank1 | 66 | 50% | 2167 |
| Shank2 | 82 | 40% | 1476 |
| Shank3 | 83 | 52% | 1730 |
| Homer1 | 11 | 81% | 366 |
| Homer2 | 8 | 60% | 354 |
| Homer3 | 18 | 11% | 356 |
| Syngap1 | 96 | 92% | 1340 |
The writer/reader/eraser toolkit of PSD phosphorylation
The regulatory machinery for protein phosphorylation at the PSD contains at least 81 protein kinases (PSD kinome), 20 protein phosphatases [19,31,36,47] (Figure 2A,B), and at least four classes of different phosphorylation reading modules (Figure 2C). These three components constitute the basic writer (kinases), eraser (phosphatases), and reader (phosphospecific binding domains) components of the PSD [19,36] (Figure 2). While we will not discuss the PSD phosphatome and phosphorylation reading modules in depth, we would like to point out that most of the phosphatases families are well represented at the PSD with the exception of dual-specifity phosphatases (DUSP). In addition, there is not a large collection of molecules containing phosphorylation reading modules, with proteins containing SH2 domains appearing to be particularly depleted from the PSD (Figure 2). This suggests that the reading of protein phosphorylation by SH2 domains is not a major signaling component in the adult PSD.
Figure 2.
A (top). Representation of PSD protein kinases present in the mouse kinome. Circles show kinases present in different families of the kinome tree. Main groups are indicated: AGC, CMCG, TK, STE, and CAMK. Color code for each kinase represent if kinases shows increase, decrease or not known changes in protein phosphorylation and activity after the induction of LTP. Bottom table shows kinase families in the mouse kinome, number of kinases presents in the mouse PSD for each family, number of phosphorylation sites reported for each kinase family and number of phosphorylation sites that increase or decrease after the induction of LTP.
B (top). Figure shows the composition of the mouse phosphatome and mapping of PSD phosphatases within the mouse phosphatome tree. Bottom table shows protein domains present in PSD proteins that have the capacity to recognize and bind to phosphorylated protein sequences (readers). Table shows domain ID (SMART database), domain name, number of PSD proteins containing each domain, if the domain is enriched in PSD fractions and phosphorylation specificity of each reader module.
PSD kinases are well distributed among all branches of the mouse kinome with larger representation in four families: AGC (including PKA, PKG, and PKC groups), STE (a family of the homologs of the yeast sterile kinases), CMGC (named after CDK, MAPK, GSK3, and CLK groups of kinases), and CAMK [36,38]. PSD phosphatases are enriched principally in serine/threonine-specific phosphatases, in particular members of the PPM and PPP subfamilies (Figure 2A).
The variety of kinases and kinases families gives the PSD the capacity to regulate phosphorylation sites within the whole range of kinase consensus motifs. However, it is difficult to analyze the representation of kinase-site relationships or if particular kinase families preferentially phosphorylate proteins with defined sets of functions. This is due to heterogeneity within assay conditions, with many being carried out in non-physiological conditions. For example, different patterns of phosphorylated motifs have been reported in total brain lysates versus PSD fractions. In total brain lysates, a strong signal for proline-directed and CK2 dependent phosphorylation sites has been found CK2-mediated phosphorylation of substrates were involved in the regulation of proteins that are part of the general cellular machinery, whereas proline-directed phosphorylation have been described predominantly in brain nuclear proteins including protein kinases and transcription factors [39,40]. However, these phosphorylation patterns might be indicative of non-PSD or soluble fractions since they were performed in conditions where the PSD cannot be solubilized.
Activity-dependent phosphorylation patterns have been reported for PSD fractions under non-physiological (NMDAR activation) and physiological (induction of LTP) conditions in the CA1 area of the mouse hippocampus. Although performed under different conditions, these assays show some common patterns of phosphorylation and activity-dependent kinase-substrate relationships at the PSD. The functional grouping of activated kinase-substrate pairs shows that after the induction of LTP, basophilic kinases such as AGC and CAMK families preferentially phosphorylate ion channels and glutamate receptors (upper PSD layers-Figure 1) whereas the CMCG (proline directed) kinases target downstream cytoskeletal components. Many of the basophilic kinases that exhibited phosphorylation-induced activation profiles mediate receptor-stimulated second-messenger cascades, such as PKA, PKC and calcium dependent pathways [36]. This suggests a close correlation between individual kinase classes and the types of binding interaction modules that they regulate. Chemical stimulation of NMDAR on the CA1 region also yielded a similar pattern of kinase family-substrate function [19]. While pharmacological NMDAR activation and electrophysiological LTP induction produced a similar pattern of phosphorylation within protein functional groups, both protocols yield different patterns of phosphorylation within individual proteins. Therefore, this suggests a model in which the PSD machinery uses a core “fixed” architecture of kinase-substrate pairs to modulate functional groups, while regulating the function of individual proteins using different patterns of protein phosphorylation through an input-specific regulation of kinase and phosphatase activity
PSD combinatorial phosphorylation
While protein phosphorylation can occur in conserved structured domains, clusters of multiphosphorylation can be frequently found in poorly conserved regions, with an apparent lack of structural detail [48,49]. These regions are generally not longer than 10aa in length and are the site of kinase multi-phosphorylation [19,28,50]. While it is believed that phosphorylation in structured domains helps modulate protein function in an allosteric manner, multiphosphorylation is often considered a mechanism of simple regulation such as electrostatic effects or the generation of phosphoepitopes that can be used for protein-protein associations [17,48,49,51]. These sites are usually poorly conserved and it has been proposed that multiphosphorylated regions can shift positions and provide simple ways to regulate protein-protein interactions [48]. These clusters might induce conformational changes, increase binding affinities, regulate protein degradation or phase separation among other functions [52–54]. This clustering of protein multiphosphorylation is also observed in PSD proteins with a large majority of phosphorylation occurring in non-structured regions. These sequences are no longer than 40aa [50] with a prevalence of multiphosphorylation in shorter sequences of no more than 10aa [19]. Therefore a large number of combinations of phosphorylation sites might be present in PSD proteins at any given physiological state. This raises a pressing question in the analysis of proteomes: how many proteoforms (in this case phosphoforms) exists for a set of proteins [55], what are their molecular composition and biological functions? Considering a binary system where an individual phopsphorylation site can be present or not, the number of possible phosphoforms is 2n, where n is the number of phosphorylation sites per protein. Even considering phosphorylation sites described only in brain fractions, this gives an astronomically high number of possible phohosphoforms. Moreover, since PSD scaffolds contains a larger number of multiphosphorylated sequences(Table 1B) it is expected that the number of possible phosphorylation combinations will be particularly high. While there are technological limitations for the detection of these putative protein variants, there are also natural constrains. For example, these large numbers of phosphoforms will exceed the total copy number of individual proteins in single cells [55]. While a complete discussion of an estimate of the number of PSD phosphoforms is out of the scope of this review, we can discuss their molecular composition, constrains, and putative functions.
An intriguing possibility is that PSD phosphoforms can be used as molecular switches, where the combinatorial properties of protein phosphorylation can be mapped to specific patterns of synaptic activity, activation of different neurotransmitter receptors, or function as synaptic “memory” of previous activity. In vitro studies have proposed a series of kinase-substrate relationships for multiphosphorylated proteins at the PSD (Figure 3A) where families of kinases can converge, diverge and produce patterns of multiphosphorylated sequences. These patterns of kinase/substrate phosphorylation showed a preference of kinase families to co-regulate multiphosphorylated sequences. These phosphorylation hubs are potentially key nodes of convergence between multiple signaling pathways. For example, second messengers and calcium regulated basophilic kinases such as PKA (I/II), PKC(α, β, γ, δ, ζ) and CAMK2A usually converge at different sites within multiphosphorylated sequences [19,28,36] (Figure 3A,B). This suggests that not only kinases from the same class converge on a similar set of substrates (scaffolds and receptors-see above), but they also converge onto adjacent sites within those substrates. This class modulation of protein function, can be used together with our knowledge of PSD kinome regulation following the induction of LTP [10,19,36]. For example, mapping substrates of Basophilic kinases within the PSD PIN shows that AGC/CAMK protein kinase families PKA, PKC, CAMK2A, CAMK2B are on average 2.5 interacting partners from the nearest glutamatergic receptor subunit. This is significantly closer than CMCG, Proline-directed, and STE Ser/Thr kinases, which are on average 3.6 steps from the receptor layer [10,19,36]. Thus, the sequential organization into upstream and downstream Ser/Thr kinases and their relationship with sets of functions appears to be supported and reinforced by the organization of protein-protein interactions within the PSD PIN [10]. Within this context, multiphosphorylated sequences regulated by 2nd messenger dependent kinases do not usually allow phosphorylation of proline directed kinase [19,36].
Figure 3.
Regulatory motifs in multiple-phosphorylated sequences, with one kinase (k) phosphorylating multiple sites (a), different kinases converging in multiple phosphorylated regions (b). These kinase interaction can prime (enhance) the phosphorylation of nearby sites (c) or impair the phosphorylation by a second kinase (d). Figure 3e and f shows convergence of AGC kinases such as PKC, PKA, PKG, AKT with basophilic kinases from CAMK family like CAMK2, TRIO, DAPK.
Overlapping multiphosphorylation sequences in glutamate receptors driven by activation of NMDA, mGlu and dopamine receptors (top 3 panels). Cartoon shows changes in phosphorylation observed in different subunits of NMDAR (GRIN1, GRIN2B) and AMPA (GRIA1) receptors. Lower 3 panels shows kinases involved in the phosphorylation of each site/sequence.
The spatial organization of kinase-substrate pairs at the PSD suggests that a mechanism of feedback control and modulation of ion channels and receptors is largely due to upstream basophilic kinases, whereas downstream kinases primarily coordinate output changes in cell-biological processes underlying plasticity such as cytoskeletal organization. This is in accordance with literature reporting a variety of basophilic or proline directed kinases regulating channel, receptor or cytoskeleton activities through the phosphorylation of individual sites in PSD proteins [21–23,25,43]. These sets of “rules” should have also an impact on the number of phosphoforms allowed to be present. It has been observed that the introduction of a phosphorylation site in a set of PSD proteins, have an effect on the incorporation of additional sites in multiphosphorylated sequences 66% of the time [19] (Figure 3A). Of these “primed” sites, 73% were inhibitory and 27% were enhancing, thus reducing the number of PSD phosphoforms available.
The priming seen within multiple phosphorylated sites might allow coincidence detection and pathway-switching. For example, NMDAR-mediated phosphorylation of site 1 in a pair of sites may interfere or enhance the ability of site 2 to be phosphorylated by a kinase driven by the dopamine receptor. In addition to modulating specific substrates, priming switches may control specific subnetworks of functional proteins (with different physiological outcomes). These mechanisms allow receptors individually, in combination, or in different temporal sequences to activate overlapping phosphorylation networks that orchestrate and differentially regulate a combination of effector substrates.
Future studies might be able to determine sets of multiphosphorylated sequences as barcodes that can be used to describe sets of functions within the PSD. It might be possible to determine what sets of neurotransmitters receptors were activated and what sets of kinases were up/downregulated by reading a short number of specific multiphosphorylated sequences (Figure 3B). A similar framework has been described for the profiling of phosphorylation sites in response to a variety of drug and chemical perturbations [56]. Thus, the “allowed” combinations of kinase/substrate pairs within short phosphorylation sequences might be a more general characteristic of PSD multi-phosphorylation. This might explain the differential phosphorylation of functional components of the PSD by families of protein kinases by phosphorylation sequence interactions and not dependent on the signaling input or the topology of the PSD PPI. For example the preferential phosphorylation of AGC/CAMK kinases of receptors and upper-layer components with respect to CMCG kinases might be due to the disfavored interaction of sites multi-phosphorylated by these kinase families (FIG 3) and not by protein kinases being targeted to their substrates via PPI.
Since the set of PSD proteins phosphorylated after the induction of LTP seems to contain the major risk for complex brain disorders within the PSD, it might be possible to correlate patterns of multiphosphorylation (barcodes) to defined perturbations within the PSD PIN. These barcodes can then be defined within the context of PSD phosphorylation networks. For these purpose we will need to acquire more complete datasets, defining PSD phosphorylation networks under a number of different physiological conditions in normal, and “disease” states. To be able to generate these networks we will need to combine and superimpose a variety of inputs, including PPI, phosphorylation and “disease” states. A number of mathematical approaches such as network propagation analysis (NPA) might be necessary to explore information flow through these networks under a variety of test conditions and define the network characteristic of phosphorylation barcodes. Hub barcodes might be further studied by low and medium throughput models including CRSIPR-Cas9 cell and animal models.
Advances in mass spectrometry have allowed us to determine large number of phosphorylation sites and will continue to provide us with increasingly larger datasets. While it will be easy to find variations within the PSD phosphoproteome, it will be a difficult task to determine functional roles. Concerted high and low throughput efforts will be needed to target phosphorylation events within the PSD PIN in a more systematic way that can allow us to determine the functional modulation of patterns of phosphorylation within the PSD signaling network.
Highlights.
Composition of the writer, reader, eraser toolkit of the PSD phosphoproteome
Modulation of the PSD phosphoproteome by synaptic plasticity - long-term potentiation (LTP)
Ratios of protein phosphorylation in the core scaffold machinery of the PSD
Combinatorial multisite phosphorylation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayes A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, Grant SG: Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci 2011, 14:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG: Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem 2006, 97 Suppl 1:16–23. [DOI] [PubMed] [Google Scholar]
- 3.Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, et al. : Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics 2006, 5:1158–1170. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS: Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci U S A 2005, 102:11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng M, Kim E: The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH: Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 1995, 269:1737–1740. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS: PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci 2011, 31:6329–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng M, Hoogenraad CC: The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem 2007, 76:823–847. [DOI] [PubMed] [Google Scholar]
- 9.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, et al. : Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 1999, 23:583–592. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhang W, Yang H, Howrigan DP, Wilkinson B, Souaiaia T, Evgrafov OV, Genovese G, Clementel VA, Tudor JC, et al. : Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders. Nat Neurosci 2017, 20:1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long JF, Tochio H, Wang P, Fan JS, Sala C, Niethammer M, Sheng M, Zhang M: Supramodular structure and synergistic target binding of the N-terminal tandem PDZ domains of PSD-95. J Mol Biol 2003, 327:203–214. [DOI] [PubMed] [Google Scholar]
- 12.Coba MP, Ramaker MJ, Ho EV, Thompson SL, Komiyama NH, Grant SGN, Knowles JA, Dulawa SC: Dlgap1 knockout mice exhibit alterations of the postsynaptic density and selective reductions in sociability. Sci Rep 2018, 8:2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M: Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999, 23:569–582. [DOI] [PubMed] [Google Scholar]
- 14.Coba MP, Valor LM, Kopanitsa MV, Afinowi NO, Grant SG: Kinase networks integrate profiles of N-methyl-D-aspartate receptor-mediated gene expression in hippocampus. J Biol Chem 2008, 283:34101–34107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson B, Li J, Coba MP: Synaptic GAP and GEF Complexes Cluster Proteins Essential for GTP Signaling. Sci Rep 2017, 7:5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold MG, Fowler DM, Means CK, Pawson CT, Stephany JJ, Langeberg LK, Fields S, Scott JD: Engineering A-kinase anchoring protein (AKAP)-selective regulatory subunits of protein kinase A (PKA) through structure-based phage selection. J Biol Chem 2013, 288:17111–17121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawson T, Gish GD, Nash P: SH2 domains, interaction modules and cellular wiring. Trends Cell Biol 2001, 11:504–511. [DOI] [PubMed] [Google Scholar]
- 18.Pawson T, Scott JD: Protein phosphorylation in signaling−−50 years and counting. Trends Biochem Sci 2005, 30:286–290. [DOI] [PubMed] [Google Scholar]
- 19.Coba MP, Pocklington AJ, Collins MO, Kopanitsa MV, Uren RT, Swamy S, Croning MD, Choudhary JS, Grant SG: Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signal 2009, 2:ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R: Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 1997, 88:615–626. [DOI] [PubMed] [Google Scholar]
- 21.Lee HK: Synaptic plasticity and phosphorylation. Pharmacol Ther 2006, 112:810–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huganir RL, Greengard P: Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron 1990, 5:555–567. [DOI] [PubMed] [Google Scholar]
- 23.Thomas GM, Huganir RL: MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 2004, 5:173–183. [DOI] [PubMed] [Google Scholar]
- 24.O’Dell TJ, Grant SG, Karl K, Soriano PM, Kandel ER: Pharmacological and genetic approaches to the analysis of tyrosine kinase function in long-term potentiation. Cold Spring Harb Symp Quant Biol 1992, 57:517–526. [DOI] [PubMed] [Google Scholar]
- 25.Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD: The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 1999, 19:4337–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisman JE, Zhabotinsky AM: A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 2001, 31:191–201. [DOI] [PubMed] [Google Scholar]
- 27.Lisman J: A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A 1989, 86:9574–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG: Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem 2005, 280:5972–5982. [DOI] [PubMed] [Google Scholar]
- 29.Trinidad JC, Specht CG, Thalhammer A, Schoepfer R, Burlingame AL: Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol Cell Proteomics 2006, 5:914–922. [DOI] [PubMed] [Google Scholar]
- 30.Trinidad JC, Thalhammer A, Burlingame AL, Schoepfer R: Activity-dependent protein dynamics define interconnected cores of co-regulated postsynaptic proteins. Mol Cell Proteomics 2013, 12:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinidad JC, Thalhammer A, Specht CG, Lynn AJ, Baker PR, Schoepfer R, Burlingame AL: Quantitative analysis of synaptic phosphorylation and protein expression. Mol Cell Proteomics 2008, 7:684–696. [DOI] [PubMed] [Google Scholar]
- 32.Trinidad JC, Thalhammer A, Specht CG, Schoepfer R, Burlingame AL: Phosphorylation state of postsynaptic density proteins. J Neurochem 2005, 92:1306–1316. [DOI] [PubMed] [Google Scholar]
- 33.Jaffe H, Vinade L, Dosemeci A: Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem Biophys Res Commun 2004, 321:210–218. [DOI] [PubMed] [Google Scholar]
- 34.Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, et al. : Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics 2007, 6:283–293. [DOI] [PubMed] [Google Scholar]
- 35.Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL: Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 2017, 355:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Wilkinson B, Clementel VA, Hou J, O’Dell TJ, Coba MP: Long-term potentiation modulates synaptic phosphorylation networks and reshapes the structure of the postsynaptic interactome. Sci Signal 2016, 9:rs8. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Ma J, Miyoshi C, Li Y, Sato M, Ogawa Y, Lou T, Ma C, Gao X, Lee C, et al. : Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature 2018, 558:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClatchy DB, Savas JN, Martinez-Bartolome S, Park SK, Maher P, Powell SB, Yates JR, 3rd: Global quantitative analysis of phosphorylation underlying phencyclidine signaling and sensorimotor gating in the prefrontal cortex. Mol Psychiatry 2016, 21:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JJ, Sharma K, Zangrandi L, Chen C, Humphrey SJ, Chiu YT, Spetea M, Liu-Chen LY, Schwarzer C, Mann M: In vivo brain GPCR signaling elucidated by phosphoproteomics. Science 2018, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, Olsen JV: Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat Commun 2012, 3:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M: Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem 2004, 279:21003–21011. [DOI] [PubMed] [Google Scholar]
- 42.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E: PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 2015, 43:D512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woolfrey KM, Dell’Acqua ML: Coordination of Protein Phosphorylation and Dephosphorylation in Synaptic Plasticity. J Biol Chem 2015, 290:28604–28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luscher C, Malenka RC: NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 2012, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pi HJ, Lisman JE: Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression. J Neurosci 2008, 28:13132–13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumgartel K, Mansuy IM: Neural functions of calcineurin in synaptic plasticity and memory. Learn Mem 2012, 19:375–384. [DOI] [PubMed] [Google Scholar]
- 47.Roy M, Sorokina O, McLean C, Tapia-Gonzalez S, DeFelipe J, Armstrong JD, Grant SGN: Regional Diversity in the Postsynaptic Proteome of the Mouse Brain. Proteomes 2018, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO: Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 2009, 325:1682–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moses AM, Heriche JK, Durbin R: Clustering of phosphorylation site recognition motifs can be exploited to predict the targets of cyclin-dependent kinase. Genome Biol 2007, 8:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins MO, Yu L, Campuzano I, Grant SG, Choudhary JS: Phosphoproteomic analysis of the mouse brain cytosol reveals a predominance of protein phosphorylation in regions of intrinsic sequence disorder. Mol Cell Proteomics 2008, 7:1331–1348. [DOI] [PubMed] [Google Scholar]
- 51.Serber Z, Ferrell JE Jr.: Tuning bulk electrostatics to regulate protein function. Cell 2007, 128:441–444. [DOI] [PubMed] [Google Scholar]
- 52.Manrai AK, Gunawardena J: The geometry of multisite phosphorylation. Biophys J 2008, 95:5533–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, Darzacq X, Zhou Q: Phase separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature 2018, 558:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salazar C, Hofer T: Versatile regulation of multisite protein phosphorylation by the order of phosphate processing and protein-protein interactions. FEBS J 2007, 274:1046–1061. [DOI] [PubMed] [Google Scholar]
- 55.Aebersold R, Agar JN, Amster IJ, Baker MS, Bertozzi CR, Boja ES, Costello CE, Cravatt BF, Fenselau C, Garcia BA, et al. : How many human proteoforms are there? Nat Chem Biol 2018, 14:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litichevskiy L, Peckner R, Abelin JG, Asiedu JK, Creech AL, Davis JF, Davison D, Dunning CM, Egertson JD, Egri S, et al. : A Library of Phosphoproteomic and Chromatin Signatures for Characterizing Cellular Responses to Drug Perturbations. Cell Syst 2018, 6:424–443 e427. [DOI] [PMC free article] [PubMed] [Google Scholar]