Abstract
Purpose
The purpose of this study was to investigate the long-term outcomes 1 year after undertaking an audiologist-guided Internet-based cognitive behavioral therapy (iCBT) intervention for tinnitus. Secondary aims were to identify any predictors of outcome and whether there were any unwanted events related to undertaking iCBT for tinnitus.
Method
Participants who had previously undertaken a randomized iCBT efficacy trial for tinnitus were invited to participate. Of the 146 who were initially randomized for the efficacy trial, 104 participants completed the 1-year postintervention assessment measures.
The primary outcome was a change in tinnitus distress as assessed by the Tinnitus Functional Index. Secondary assessment measures were included for insomnia, anxiety, depression, hearing handicap, hyperacusis, cognitive failures, and satisfaction with life. An intention-to-treat analysis using repeated-measures analysis of variance and hierarchical multiple regression was used for statistical analysis. Unwanted effects were categorized according to the unwanted events checklist.
Results
Undertaking iCBT for tinnitus led to significant improvements 1 year postintervention for tinnitus and related difficulties, for example, insomnia, anxiety, depression, hearing handicap, hyperacusis, and life satisfaction. The best predictors of improving tinnitus severity at 1-year postintervention were greater baseline tinnitus severity scores, reading more of the modules, and higher satisfaction with the intervention. Unwanted events were reported by 11% of the participants and were more likely to be reported by women than men. These events were related to worsening of symptoms, the emergence of new symptoms, negative well-being, and prolongation of treatment.
Conclusions
The clinical benefits of audiologist-guided iCBT for tinnitus and tinnitus-related difficulties were sustained 1 year postintervention. Predictors of outcome indicated that the intervention is applicable to a wide range of participants regardless of their demographic backgrounds. Attempts should be made to minimize unwanted events in subsequent trials.
Innovative ways of providing sustainable cost and clinically effective ways of managing chronic health care conditions are required (West, 2012). One such chronic condition is tinnitus, defined as the conscious perception of unwanted subjective auditory sensations in the absence of a related external stimulus (Baguley, McFerran, & Hall, 2013). It is one of the most distressing and debilitating audiologic symptoms (Cima, Vlaeyen, Maes, Joore, & Anteunis, 2011). It is a prevalent complaint, with 10%–30% of the adult population reporting tinnitus across the globe, for example, Korea (Kim et al., 2015), New Zealand (Wu, Searchfield, Exeter, & Lee, 2015), Nigeria (Lasisi, Abiona, & Gureje, 2010), the United Kingdom (Davis & Rafaie, 2000; Dawes et al., 2014), and the United States (Bhatt, Lin, & Bhattacharyya, 2016; Shargorodsky, Curhan, & Farwell, 2010).
As no cure has been identified to eliminate tinnitus, interventions are directed toward alleviating or managing the accompanying symptoms, making the tinnitus less intrusive or distressing (Langguth, Kreuzer, Kleinjung, & De Ridder, 2013). Although various management strategies have evolved, many lack empirical support (Martinez-Devesa, Perera, Theodoulou, & Waddell, 2010). Psychological interventions, such as cognitive behavioral therapy (CBT), currently have the most evidence of efficacy in reducing tinnitus distress (Cima, Andersson, Schmidt, & Henry, 2014; Hesser, Weise, Westin, & Andersson, 2011; Martinez-Devesa et al., 2010). Despite the known efficacy of CBT in reducing tinnitus-related distress and the fact that it is one of the most researched tinnitus management interventions, it is rarely offered in clinical practice (Gander, Hoare, Collins, Smith, & Hall, 2011; Hall et al., 2011; Hoare, Broomhead, Stockdale, & Kennedy, 2015). This is largely due to the associated costs and a shortage of suitably trained psychologists and psychotherapists (Andersson, 2015; Hall et al., 2011; McFerran & Baguley, 2009). Tinnitus services are also not consistently available and are particularly sparse in remote geographical regions (Hoare et al., 2015). In addition, they are costly. An economic evaluation of the health care cost of tinnitus management in the United Kingdom in 2017 indicated that the annual cost of tinnitus interventions was £750 million in total or £717 per patient with tinnitus (Stockdale et al., 2017). This is equivalent to 0.6% of the annual U.K. national health care spending. It is not only health care costs that need to be considered. The annual societal costs related to tinnitus were estimated to be £2.7 billion per year in the United Kingdom (Stockdale et al., 2017), although higher costs have been quoted, for example, €6.8 billion in the Netherlands (Maes, Cima, Vlaeyen, Anteunis, & Joore, 2013). Moreover, the prevalence of tinnitus is predicted to increase because of factors such as an increase in life expectancy and recreational noise exposure, which is a known risk factor for developing tinnitus (Martinez, Wallenhorst, McFerran, & Hall, 2015). This will place further financial constraints on already pressurized health care systems (Smith, McKeon, Blunt, & Edwards, 2014). Innovative planning is required to meet these additional demands and address existing challenges faced with regard to the provision of tinnitus services.
Technological advances can assist innovations in health care. One example is the use of telehealth for patient diagnosis, treatment, and prevention of health-related conditions (Michie, Yardley, West, Patrick, & Greaves, 2017). It has the potential to improve access to care, reduce costs, and improve the patient experience for numerous health-related conditions (Polisena, Coyle, Coyle, & McGill, 2009). Considering the difficulties accessing CBT for tinnitus together with the potential of telehealth, an Internet-delivered CBT (iCBT) intervention for tinnitus was developed (Andersson, Strömgren, Ström, & Lyttkens, 2002). The addition of iCBT for tinnitus distress could complement existing tinnitus pathways by providing a more cost-effective, evidence-based, accessible, comprehensive, and standardized intervention. Efficacy of iCBT for tinnitus provided has been indicated (Hedges' g = 0.60), largely evaluated in Sweden and Germany (Andersson, 2015). Outcomes have been maintained up to 1 year after completing guided iCBT for tinnitus (Hesser et al., 2012; Kaldo et al., 2008; Weise, Kleinstauber, & Andersson, 2016).
Because of the limited provision of CBT for tinnitus within the United Kingdom, a comprehensive, user-friendly iCBT intervention tailored for a U.K. population was designed (Beukes et al., 2016). Better outcomes are reported for guided mental health interventions (Baumeister, Reichler, Munzinger, & Lin, 2014; Richards & Richardson, 2012). For Internet-based tinnitus interventions, the evidence for the benefit of guidance is inconclusive. A systematic review and meta-analysis on the efficacy of self-help interventions in tinnitus found that tinnitus distress and depressiveness were not influenced by the presence of therapists (Nyenhuis, Golm, & Kröner-Herwig, 2013). For this study, a guided intervention was selected to obtain further information regarding outcomes obtainable with such a guided intervention.
Guidance in previous iCBT for tinnitus studies was provided by clinical psychologists, because of their expertise in the provision of CBT. As guidance from psychologists would not be feasible in a U.K. context where tinnitus is largely treated within the audiology community (McFerran & Baguley, 2009), an audiologist was selected to guide the intervention. Feasibility of audiology-guided iCBT in the United Kingdom was indicated (Beukes, Allen, Manchaiah, Baguley, & Andersson, 2017), and efficacy was established when compared with weekly monitoring (Beukes, Baguley, Allen, Manchaiah, & Andersson, 2018; Beukes, Manchaiah, Baguley, Allen, & Andersson, 2017). Before such an intervention is accepted as credible, further evaluation of its efficacy and effectiveness is required. The long-term outcomes of audiologist-guided iCBT are not known. Therefore, investigating whether intervention effects are maintained 1 year postintervention for audiologist-guided iCBT for a U.K. population is important. The results will hopefully influence future evidence-based management of tinnitus.
Moreover, to date, there are no established predictors of outcomes for guided iCBT interventions (Andersson, 2016). Continued searches for moderators and mediators of outcome should be undertaken as these may help to triage participants to the most appropriate intervention route. There is also the possibility of unwanted events from such an intervention. Unwanted events are defined as all events of negative quality occurring alongside interventions but not intended by the intervention (Linden, 2013). The incidence of these events does not imply a causal relationship between the intervention and does not necessarily influence intervention outcomes. Circumstances unrelated to treatment such as personal or vocational issues may contribute.
As information to date on iCBT for tinnitus has been primarily focused on examining effectiveness, little is known about the occurrence or characteristics of unwanted events in these trials. It is important to establish whether tinnitus may worsen in some participants or if participants encounter adverse events when undertaking such an Internet-based intervention to address these in future interventions (Boettcher, Rozental, Andersson, & Carlbring, 2014). Unwanted effects may include a deterioration instead of an improvement in outcomes after undertaking an intervention. An individual patient data meta-analysis of 29 clinical trials of iCBT (n = 2,866) indicated that 6% of participants in intervention groups and 17% of those in control conditions showed a deterioration in outcomes after receiving iCBT (Rozental, Magnusson, Boettcher, Andersson, & Carlbring, 2017).
The purpose of this study was to investigate the long-term outcomes 1 year after undertaking an audiologist-guided iCBT intervention for tinnitus. The hypothesis was that the reduction of tinnitus distress and tinnitus-related difficulties established would be sustained 1 year postintervention. Further aims were to identify any predictors of outcome and to establish whether there were any unwanted events related to undertaking iCBT for tinnitus.
Method
Study Design
An efficacy randomized controlled trial with a delayed intervention group preceded this study investigating the long-term effects of this intervention. The iCBT experimental group received the iCBT intervention for 8 weeks (n = 73), whereas the weekly check-in group was monitored weekly (n = 73). This monitoring involved the weekly completion of 10 questions from the Tinnitus Handicap Inventory–Screening Version online questionnaire (Newman, Jacobson, & Spitzer, 1996).
Once the experimental group completed the intervention, the control group underwent the same iCBT intervention. As both groups undertook the same intervention, a repeated-measures single-group analysis was conducted for this study.
This study was registered on the Clinical Trials Database: NCT02370810 on May 3, 2015. To ensure best practice was followed, the Transparent Reporting of Evaluations with Nonrandomized Designs checklist (Des Jarlais, Lyles, Crepaz, & TREND Group, 2004) was used to report this trial. For the full study protocol, see Beukes, Manchaiah, Allen, Baguley, and Andersson (2015). There were no changes to the methods or assessment measures used after the trial commenced.
Ethical Considerations
The central electronic online data capturing system was held at Linköping University (Sweden) and complied with a high level of data security to safeguard confidentiality (Vlaescu, Carlbring, Lunner, & Andersson, 2015). Ethical approval was granted by the Faculty Research Ethics Panel of Anglia Ruskin University (FST/FREP/14/478). The trial was conducted in accordance with good clinical practice together with the ethical principles of the Declaration of Helsinki.
Study Population
Sample size estimation for the original efficacy trial indicated that 58 participants were required for each group (1:1 allocation ratio) to achieve a clinically relevant change using the main outcome measure with a two-sided significance level of .05, an effect size of 0.5, and an 80% power (G*Power Version 3.1.6; Faul, Erdfelder, Lang, & Buchner, 2007). To account for possible dropouts, 73 participants were recruited for each group using a range of strategies such as newspaper and magazine articles, social media, and tinnitus support forums and groups.
Participants therefore represent a research instead of a clinical population with tinnitus. To undertake the intervention, participants had to meet the original eligibility criteria for the randomized controlled trial (Beukes et al., 2015) of being 18 years or older, living in the United Kingdom, and having experienced tinnitus for a minimum of 3 months. Their tinnitus severity, assessed by the Tinnitus Functional Index (TFI; Meikle et al., 2012), had to indicate the need for intervention (score > 25), and no major mental or medical disorder could be present.
All participants assigned to either the experimental group or the control group in the efficacy trial, except for those who withdrew during the study, were invited to partake in this study (n = 139).
Intervention
The study intervention was Internet based to provide a standardized intervention that could be easily accessible. It was created on the Iterapi (http://www.iterapi.se/) purpose-built web-based platform (Vlaescu, Alasjö, Miloff, Carlbring, & Andersson, 2016; Vlaescu et al., 2015). To access the intervention, a link with instructions and log-in information was e-mailed to the participants. Those who had not accessed the link were contacted to offer assistance. To ensure the intervention encouraged engagement (such as reading the materials and completing quizzes and worksheets), the design was visually stimulating and interactive (Beukes et al., 2016). Because of the efficacy of CBT for tinnitus (Hesser et al., 2011), CBT principles based on a self-help program originally developed by Andersson and Kaldo (2004) were incorporated. There were 16 recommended modules and five optional modules, which were delivered over 8 weeks. Each week, two recommended modules were released. During weeks 2–6, an additional optional module was released. A message was sent to introduce the new modules on their release. If participants were unable to complete the modules, they were able to request additional time before receiving the next set of modules.
Recommended modules included CBT content such as applied relaxation, thought analysis, cognitive restructuring, imagery, and exposure techniques. Optional modules were available to add an element of tailoring, and participants could choose whether or not to do these modules. They included strategies for insomnia, hearing difficulties, hyperacusis, concentration, and the use of sound enrichment.
Intervention Guidance
Asynchronous audiologist guidance using an encrypted two-way messaging system was provided during the intervention. Guidance included monitoring progress, providing feedback on worksheets completed, sending encouraging messages to those who have not accessed the intervention for a few days, and answering queries participants had. A minimum of 10 min of guidance per week per participant was provided, with additional time given if required. There were no restrictions on the number of messages that participants could send to the audiologist. Some participants who were not engaged made limited use of the messaging system. The audiologist was trained to master's level in audiology, was registered with the Health and Care Professions Council, and had experience in managing patients with tinnitus together with a suitable understanding of CBT principles but no formal CBT training. Supervision was provided by a clinical psychologist who was specialized in providing tinnitus interventions.
Assessment Measures
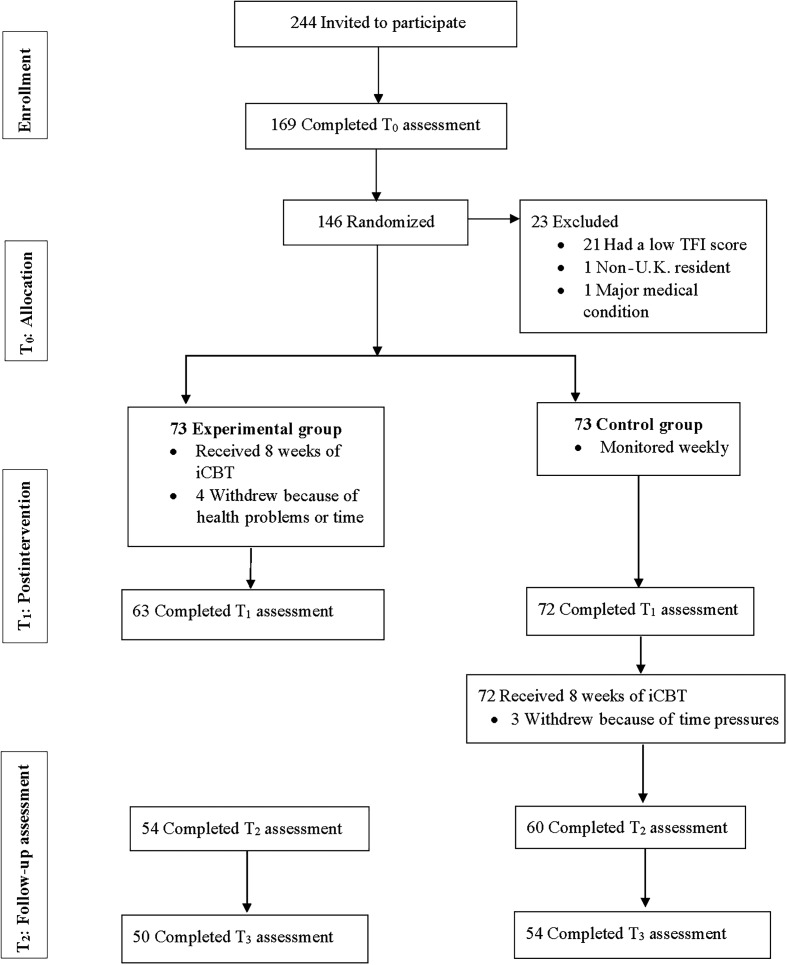
Data collection was online throughout the trial. Assessment measures were integrated into the intervention platform, and participants were sent a message when they were required to complete them. The assessment timeline was as follows: T0, baseline; T1, postintervention assessment; T2, follow-up assessment; and T3, at 1-year postintervention follow-up (see Figure 1). The T3 assessment measures were collected at different time points for each group to ensure that 12 months had passed postintervention for each group (initially taken for the experimental group and taken for the control group 2 months later). To minimize attrition, encouraging reminders were provided throughout for participants who had not completed questionnaires or worksheets on time. Three reminders were automatically and electronically sent on the 3 consecutive days after the release of the questionnaire. A further reminder was sent out 1 and 2 weeks later.
Figure 1.
The study profile. TFI = Tinnitus Functional Index; iCBT = Internet-based cognitive behavioral therapy; T0 = baseline; T1 = postintervention; T2 = after intervention follow-up; T3 = 1 year postintervention.
The assessment measures that were selected are discussed hereinafter.
Demographical Information
A demographic questionnaire was used to obtain information related to gender, age, tinnitus duration, previous tinnitus treatments (such as audiologic, complementary approaches, medical), and hearing aid use.
Primary Assessment Measure
The TFI (Meikle et al., 2012) was selected as the primary assessment measure to measure tinnitus distress because of its validation for assessing intervention responsiveness. The TFI has acceptable psychometric properties with an internal consistency of .80 and an intraclass reliability of .91 for a U.K. research population (Fackrell, Hall, Barry, & Hoare, 2016). It is a 25-item questionnaire, scored on a scale of 0–100. Scores less than 25 indicate mild tinnitus, with no need for intervention, whereas scores ranging from 25 to 50 signify significant tinnitus and the possible need for intervention. A score of 50 or greater demonstrates more severe tinnitus and indicates the need for more intensive intervention. A reduction in TFI scores shows improvement in tinnitus distress. Meikle et al. (2012) reported that meaningful changes occur when scores are reduced by 13 points or more, whereas the smallest detectable change score of 22.4 is proposed by Fackrell et al. (2016) for a U.K. research population.
Secondary Assessment Measures
To assess intervention effects on tinnitus-related difficulties, the following secondary assessment measures were included:
i. The Insomnia Severity Index (ISI; Bastien, Vallières, & Morin, 2001) assessed the presence of insomnia, as sleep difficulties are prevalent among those with tinnitus (Crönlein et al., 2016). This seven-item questionnaire is scored between 0 and 28 and has an acceptable internal consistency of .74 (Bastien et al., 2001).
ii. The Generalized Anxiety Disorder-7 (GAD-7; Spitzer, Kroenke, Williams, & Löwe, 2006) quantified the level of anxiety, as the prevalence of anxiety is high in those with severe tinnitus (Pinto et al., 2014). This seven-item questionnaire is scored between 0 and 21 and has an internal consistency of .89 (Löwe et al., 2008).
iii The Patient Health Questionnaire-9 (PHQ-9; Spitzer, Kroenke, Williams, & Patient Health Questionnaire Primary Care Study Group, 1999) indicated symptoms of depression, as depression among those with severe tinnitus is often reported (Pinto et al., 2014). Scoring is between 0 and 28 on this nine-item questionnaire, with an internal consistency of .83 (Spitzer et al., 1999).
iv. The screening version of the Hearing Handicap Inventory for Adults (HHIA-S; Newman, Weinstein, Jacobson, & Hug, 1991) assessed difficulty hearing, which in this context may be related to the penetrating nature of tinnitus or the presence of hearing loss, commonly found in those with tinnitus (Langguth et al., 2017). This measure consists of 10 items, scored between 0 and 40 with an internal consistency of .93 (Newman et al., 1991).
v. The Hyperacusis Questionnaire (HQ; Khalfa et al., 2002) was administered to assess the presence of reduced tolerance of everyday sounds, otherwise known as hyperacusis, as there is a large overlap in the prevalence of tinnitus and hyperacusis (Schecklmann, Landgrebe, Langguth, & TRI Database Study Group, 2014). This 14-item questionnaire is scored between 0 and 42 and has an internal consistency of .88 (Fackrell, Fearnley, Hoare, & Sereda, 2015).
vi. The Cognitive Failures Questionnaire (CFQ; Broadbent, Cooper, FitzGerald, & Parkes, 1982) was administered to assess cognitive functions, as tinnitus may impact the control of attention leading to cognitive slips and errors in task completion (Tegg-Quinn, Bennett, Eikelboom, & Baguley, 2016). This 25-item questionnaire is scored between 0 and 100, with an internal consistency of .89 (Broadbent et al., 1982).
vii. The Satisfaction With Life Scale (SWLS; Diener, Emmons, Larsen, & Griffin, 1985) was administered as a quality-of-life measure assessing global life satisfaction as opposed to quality-of-life measures often related to self-care and mobility. Scoring is between 0 and 35 for five items and has an internal consistency of .87 (Diener et al., 1985).
Assessment measures were used with permission of the copyright holders, and agreements were established for those that are not freely available to use, such as the TFI and ISI. A low score signifies fewer problems than a high score, and a reduction in score indicates improvement for all these measures except for the SWLS. For the SWLS, a higher score indicates more life satisfaction than a lower score and an increase in score reveals improved life satisfaction.
Intervention Variables
To assess intervention variables, data logging was recorded of the number of log-ins, the number of modules read, and the number of messages sent during the intervention. As assessing intervention satisfaction was important, a standardized satisfaction questionnaire was sought. As an appropriate measure was not found, one was designed. Although it was not standardized, it provided the opportunity to collect information regarding participants' views on the presentation, content, usability, and information provided on a 1- to 5-point Likert scale (see Appendix). The overall score for the 15 questions asked was used to determine intervention satisfaction (with higher scores indicating more satisfaction). This questionnaire was piloted during the feasibility study (Beukes, Allen, et al., 2017).
Unwanted Events
Recommendations from leading experts in the field of Internet interventions for measuring unwanted events (Rozental et al., 2014) were followed. These included using both quantitative and qualitative methods. Preintervention and postintervention data were compared to identify no response or deterioration in outcomes, and dropout rates were recorded. As recommended, probing for unwanted effects was undertaken by asking an open-ended question. The following additional follow-up questions deemed to provide important information were included:
Did you experience any unwanted effects/events associated with the Internet intervention you undertook? (yes/no)
If yes, please list all the unwanted affects you experienced associated with undertaking this intervention. (open question)
What was the negative impact of the event/s at the time of the event? (select on a 5-point Likert scale from a range of minimal to very severe)
What is the negative impact of the event/s at present? (i.e., 1 year postintervention; select on a 5-point Likert scale from a range of minimal to very severe) 1
Data Analysis
Version 23.0 of IBM SPSS Statistics was used for statistical analysis. For all analyses, a two-tailed significance level of < .05 was considered statistically significant. For purposes of data analysis, results at T1 were not used, as not all the participants (the original control group) had undertaken the intervention at this point. To evaluate the long-term outcomes, the pooled results from T0, T2, and T3 were used for data analysis.
The primary study outcome was a change in TFI score at 1-year postintervention (T3). Secondary study outcomes were changes in the scores of secondary assessment measures at T3. A difference in scores between T2 and T3 was used to assess long-term stability of intervention effects.
Missing Data Analysis
An intention-to-treat (ITT) paradigm was used, as this analysis is less susceptible to bias than complete case analysis techniques. Missing value analysis was conducted to determine how to account for missing data. Little's missing completely at random test (Little, 1988) indicated that data were likely to be missing completely at random, χ2(67) = 77.73, p = .17. This suggested that missing values were likely to be randomly distributed across all observations and there was no systematic pattern to the missing data. Missing data could thus be imputed through the multiple imputation procedure offered by SPSS using the Markov chain Monte Carlo method, which uses five imputation runs (Asendorpf, van de Schoot, Denissen, & Hutteman, 2014). All preintervention assessment measure results were used as predictors. Results obtained by averaging the five imputation runs (pooled results) were used where available. For some of the statistics, a pooling algorithm was not available. When this was the case, the first imputed set of results was reported.
Sample Characteristics
Descriptive statistics including gender, age, tinnitus duration, hearing aid use, previous treatment, tinnitus severity, and intervention engagement (number of log-ins, worksheets completed, and modules read) were used to describe the sample characteristics for the participants completing the 1-year postintervention outcomes and the original trial cohort.
Significance Testing
Repeated-measures analysis of variance with the independent variable of time (T0, T2 [after both groups completed the intervention], and T3) was carried out to compare the assessment measure results across the three time points. The main effects were followed up by Bonferroni-corrected post hoc testing.
Effect Sizes
Effect sizes at postintervention were calculated by dividing the mean in preintervention and 1-year postintervention means by the pooled standard deviations. Effect sizes of d = 0.20 represent small effect sizes; those of d = 0.50, medium effect sizes; and those equal or greater than d = 0.80, large effect sizes (Cohen, 1992).
Clinically Significant Change
A statistical significance of differences in group means is the standard analysis of clinical trials. Supplementing these results with an evaluation to determine whether the change in score is clinically meaningful is an indicator of the value of the intervention. The Reliable Change Index (RCI; Jacobson & Truax, 1991) was used to determine clinical significance.
For the primary outcome measure, the RCI was calculated using the baseline standard deviation and means, 1-year postintervention means, and a test–retest reliability coefficient of .78 for the TFI, as reported in the TFI validation study (Meikle et al., 2012). For the secondary assessment measures, the Cronbach's alpha was used where test–retest reliability coefficient was not available. Individual's mean difference scores between T0 and T3 were also evaluated against the RCI criterion for each assessment measure.
Outcome Predictors
Hierarchical multiple regression analysis was performed to investigate the ability of baseline clinical, intervention, and demographic variables to predict improvement in FTI scores 1 year postintervention (T0–T3 difference scores). The dependent variable was the TFI difference score (continuous variable). For the sample size (n = 146), the model could accommodate the most likely 10 predicators of outcome. The independent variables selected were three blocks of variables: baseline clinical (baseline scores for the TFI, GAD-7, and PHQ-9), intervention (satisfaction with the intervention and modules read), and demographic (age, tinnitus duration, previous tinnitus treatment received, and hearing aid use). The assumptions of homogeneity of variance and linearity were tested, and the distribution of the data was assessed.
Unwanted Events
Unwanted events, reported in an open-format question, were coded according to the checklist for unwanted events and adverse treatment reactions (Linden, 2013). Two raters independently coded the events (E. B. and G. A.). Unwanted events were categorized as either a lack of clear treatment results, prolongation of treatment, noncompliance, emergence of new symptoms, negative well-being, strains in relationships, or stigmatization. Both raters judged how related these events were to the intervention using the checklist for unwanted events categories of either unrelated, probably unrelated, possibly related, probably related, or related. The interrater reliability for the categorization was calculated using Cohen's kappa (Cohen, 1960). The kappa coefficient indicated substantial agreement (100%) between the two raters (κ = 1.0).
To assess if there were any group differences between those reporting unwanted events and those not reporting unwanted events, independent-samples t tests for continuous variables and chi-square tests for categorical variables were used. Levene's test for equality of variances was performed to assess for equality of variances.
Results
Participant Characteristics
All participants who undertook the iCBT intervention, except seven who withdrew, were invited to complete the 1-year postassessment intervention questionnaire (n =139). Of these, 104 (76%) completed the questionnaire. They consisted of 50 from the original experimental group and 54 from the control group, as seen in Figure 1. Completion rates were not significantly different between these groups, with 68% from the experimental group and 74% from the control group completing the 1-year assessment, χ2(85) = 89.31, p = .35.
From the cohort completing the long-term outcomes, the mean age was 58.30 (SD = 12.48) years. As found at baseline (see Table 1), a higher proportion of the participants were male (56%), whereas 44% were female, χ2(85) = 93.19, p = .26. No significant baseline differences in terms of age, gender, employment status, level of education, tinnitus severity, insomnia, anxiety, or depression were found between those who completed the assessment measures and those who chose not to complete them.
Table 1.
Baseline demographic and clinical characteristics of the participants.
| Category | Description | Original trial cohort at T0 (n = 146) | Participants completing outcomes at T3 (n = 104) | Participants reporting unwanted effects (n = 11) | Differences between those reporting and not reporting unwanted effects |
|---|---|---|---|---|---|
| Gender | Male | 83 (57%) | 58 (56%) | 2 (18%) | χ2(1) = 6.88, p = .011* |
| Female | 63 (43%) | 46 (44%) | 9 (82%) | ||
| Age (years) | M (SD) | 55.6 (12.9) | 58.3 (12.5) | 60.4 (5.1) | t(29.71) = −1.18, p = .25 |
| Range | 22–83 | 23–84 | 53–67 | ||
| Tinnitus duration | M (SD), years | 11.7 (11.9) | 12.0 (10.7) | 7.3 (5.9) | t(102) = 2.09, p = .55 |
| Range | 4 months–56 years | 4 months–50 years | 4 months–20 years | ||
| Using hearing aids | No | 92 (63%) | 67 (64%) | 10 (91%) | t(102) = 1.92, p = .58 |
| Yes | 54 (37%) | 37 (36%) | 1 (9%) | ||
| Previous tinnitus treatment at baseline (1 year previously) | No | 112 (77%) | 83 (80%) | 2 (18%) | χ2(1) = 0.04, p = .85 |
| Yes | 34 (23%) | 21 (20%) | 9 (82%) | ||
| TFI score at baseline (1 year previously) | 59.49 (SD = 18.4) | 59.29 (SD = 17.43) | 54.87 (SD = 19.87) | t(102) = 0.90, p = .37 | |
| Satisfaction with the intervention rating | Rating out of 100 | 84.97 (SD = 15.75) | 86.67 (SD = 16.95) | t(102) = −0.37, p = .71 | |
| Number of modules read during the intervention | Read out of 21 | 15.47 (SD = 6.15) | 18.36 (SD = 3.04) | t(102) = −1.64, p = .11 | |
| Number of log-ins | M (SD) | 27.92 (20.54) | 33.09 (17.52) | t(102) = −0.85, p = .40 |
Note. T0 = preintervention; T3 = 1-year postintervention follow-up; TFI = Tinnitus Functional Index.
Significance at p < .05.
Long-Term Effects for Tinnitus Distress
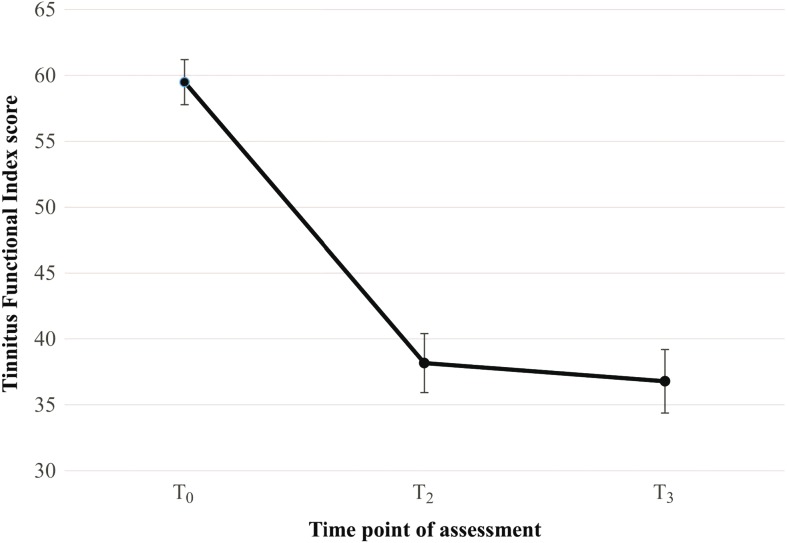
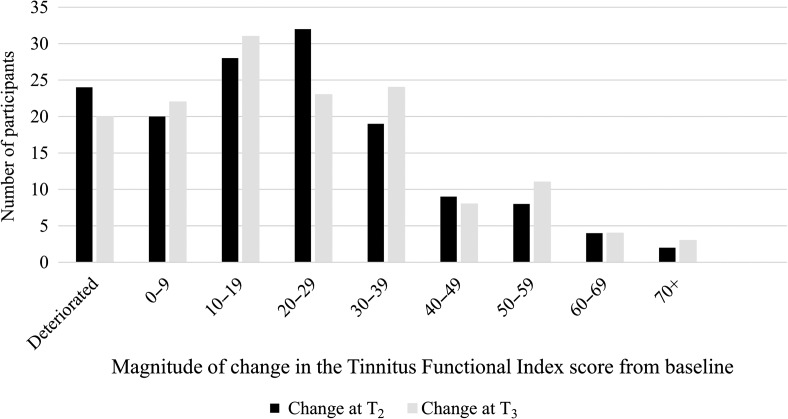
Differences between the TFI means were not constant over time. The T3 mean improved by 22.70 points (SD = 22.85 points) when compared with the preintervention mean (T0). This difference was statistically significant (Cohen's d = 1.04), as seen in Table 2. This was a clinically significant change for 46% of the ITT sample (n = 146), using the reliable change criterion of 22.66 in TFI score. There were no significant differences in the scores between T2 and T3 indicating that scores had been maintained 1 year postintervention, as seen in Figure 2. There was one participant who had no change in score and there were 20 (14%) of the ITT sample who had a deterioration in score (M = 8.37 points, SD = 6.70 points). Comparison of the magnitude of change between T0–T2 and T0–T3 is shown in Figure 3.
Table 2.
Within-group comparisons of the assessment measures over time.
| Measure |
M (SD) score at each time point |
F statistic repeated-measures ANOVA |
Bonferroni post hoc testing, M difference ± SE, p value |
Cohen's d [95% CI] |
||||
|---|---|---|---|---|---|---|---|---|
| T0 | T2 | T3 | T0–T2–T3 | T0–T2 | T0–T3 | T2–T3 | T0–T3 | |
| TFI | 59.49 (18.40) | 38.17 (24.58) | 36.79 (24.84) | F = 589.81, p = .001* | 21.29 ± 0.77, p = .001* | 22.07 ± 0.79, p = .001* | 1.38 ± 0.49, p = 1.00 | 1.04 [0.69, 1.38] |
| ISI | 12.94 (7.03) | 9.01 (6.93) | 9.05 (6.99) | F = 182.55, p = .001* | 3.93 ± 0.19, p = .001* | 3.89 ± 0.23, p = .001* | −0.04 ± 0.17, p = .47 | 0.55 [0.22, 0.88] |
| GAD-7 | 7.42 (5.52) | 5.55 (4.90) | 6.00 (5.53) | F = 55.75, p = .001* | 1.87 ± 0.19, p = .001* | 1.42 ± 0.22, p = .001* | −0.45 ± 0.13, p = .002* | 0.32 [0.01, 0.65] |
| PHQ-9 | 7.99 (5.66) | 5.88 (5.23) | 6.74 (6.08) | F = 79.52, p = .001* | 2.09 ± 0.17, p = .001* | 1.24 ± 0.20, p = .001* | −0.86 ± 0.13, p = .001* | 0.21 [−0.11, 0.54] |
| HHIA-S | 17.84 (11.41) | 14.62 (10.52) | 16.83 (10.85) | F = 58.29, p = .006* | 3.22 ± 0.29, p = .001* | 1.02 ± 0.35, p = .013* | 2.21 ± −0.26, p = .001* | 0.09 [−0.23, 0.41] |
| HQ | 19.22 (8.48) | 16.92 (9.04) | 18.19 (9.67) | F = 41.63, p = .001* | 2.30 ± 0.24, p = .001* | 1.03 ± 0.30, p = .002* | −1.26 ± 0.19, p = .001* | 0.11 [−0.21, 0.44] |
| CFQ | 40.63 (15.92) | 39.96 (16.97) | 42.36 (18.43) | F = 15.69, p = .001* | 0.67 ± 0.45, p = .411* | −1.73 ± 0.54, p = .004* | −2.40 ± 0.31, p = .001* | −0.10 [−0.42, 0.22] |
| SWLS | 16.54 (6.14) | 18.42 (6.19) | 21.46 (8.46) | F = 319.18, p = .001* | 1.88 ± 0.18, p = .001* | 4.93 ± 0.23, p = .001* | 3.05 ± 0.18, p = .001* | 0.67 [0.33, 1.00] |
Note. T0 = preintervention; T2 = follow-up; T3 = at 1-year postintervention follow-up; TFI = Tinnitus Functional Index; ISI = Insomnia Severity Index; GAD-7 = Generalized Anxiety Disorder-7; PHQ-9 = Patient Health Questionnaire-9; HHIA-S = Hearing Handicap Inventory for Adults–Screening Version; HQ = Hyperacusis Questionnaire; CFQ = Cognitive Failures Questionnaire; SWLS = Satisfaction With Life Scale.
Significance at p < .05.
Figure 2.
Change in tinnitus distress over time as measured by the Tinnitus Functional Index at baseline (T0), after intervention (T2), and at 1-year postintervention (T3). T1 was not included, as the control group had not received the intervention at this time point. Error bars represent standard error of the mean.
Figure 3.
Distribution of Tinnitus Functional Index change at T0–T2 and T0–T3. T2 = after intervention follow-up; T3 = 1 year postintervention.
Long-Term Effects for Tinnitus-Related Difficulties
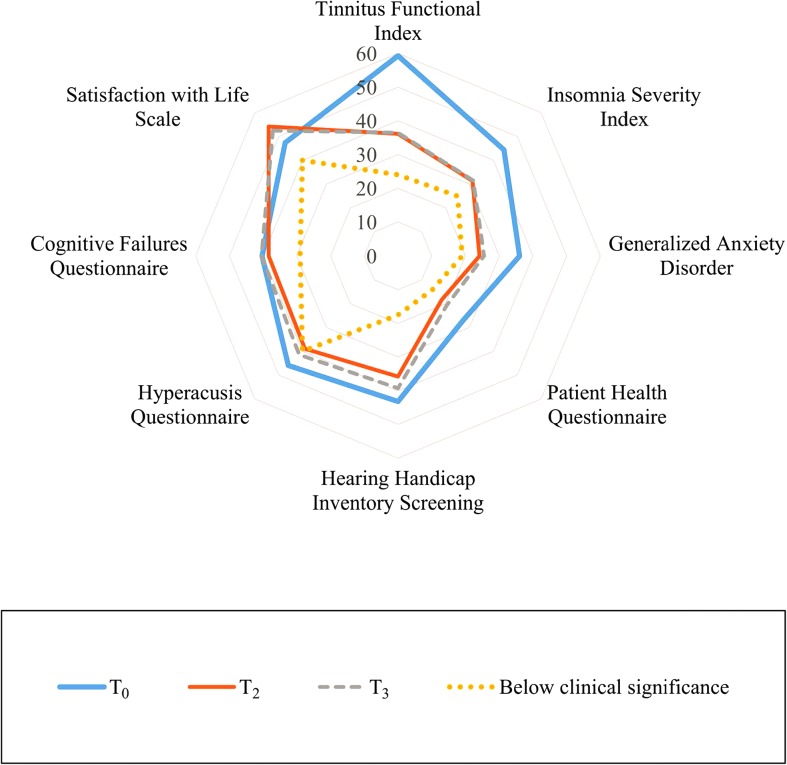
Differences between the secondary assessment measures were not constant. These had all improved significantly over time, except for the CFQ, in which scores were significantly worse at T3 (scores increased). Figure 4 shows the magnitude of change from baseline (T0) to postintervention (T2) and 1 year postintervention (T3) for the various assessment measures. The magnitude of T0–T3 change was greatest in assessment measures associated with life satisfaction, insomnia, and anxiety, with less change for the other variables. The T2–T3 were maintained for the ISI, improved for the SWLS, and had deteriorated for the other secondary measures.
Figure 4.
Change in the assessment measures over time. The average scores presented as percentages at baseline (T0) in a thick blue line, postintervention (T2) in a thin orange line, and 1 year postintervention (T3) in a broken gray. The inner ring (yellow dots) is provided as a reference point and represents scores that would be considered not clinically significant for each assessment measure.
Clinical significance for the secondary assessment measures using the ITT data was not reached by many participants, as expected with the small effect sizes seen in Table 2. Clinical significance (score change > 9.63) was reached by 14% for the ISI. For the GAD-7, it was attained by 22% (score change of > 5.07). Clinical significance for the PHQ-9 was reached by 14% (score change of > 6.02). It was attained by 20% for the Hearing Handicap Inventory for Adults and 4% for the Hyperacusis Questionnaire (score changes of > 8.83 and > 14.83, respectively). Clinical significance was 8% for the CFQ and 14% for the SWLS (score changes of > 15.03 and > 6.13, respectively).
Predictor Variables
Hierarchical multiple regression analysis was carried out to investigate the ability of demographic, clinical, and intervention variables to predict improvements in TFI score 1 year postintervention (see Table 3). The data met the assumptions of homogeneity of variance, and the residuals were approximately normally distributed. There was no risk of multicollinearity, as indicated by the tolerance and variance inflation factor values. The model significantly improved the ability to predict the outcome variables, F(6, 140) = 4.43, p = .001, and explained 28% of the variance in T0–T3 difference scores. The best predictors of greater improved TFI scores were baseline TFI scores (β = .31, p = .005), followed by intervention satisfaction (β = .27, p = .001) and then the number of modules read (β = .22, p = .01). There was a positive relationship between these variables and the difference in the T0–T3 TFI scores (increases in these variables increased the chance of greater TFI improvements).
Table 3.
Hierarchical multiple regression results.
| Regression step | Variable | b | SE b | β | p | r | R 2 | Variance | F | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Constant | −13.28 | 12.01 | .27 | .45 | .13 | 13% | 4.76 | .001* | |
| Baseline TFI | 0.41 | 0.14 | .31 | .005* | ||||||
| Baseline ISI | 0.52 | 0.35 | .16 | .12 | ||||||
| Baseline GAD-7 | 0.71 | 0.50 | .17 | .15 | ||||||
| Baseline PHQ-9 | −0.95 | 0.61 | −.22 | .12 | ||||||
| Step 2 | Constant | −28.43 | 7.97 | .001* | .50 | .25 | 25% | 7.07 | .001* | |
| Satisfaction | 0.29 | 0.09 | .27 | .001* | ||||||
| Modules read | 0.70 | 0.27 | .22 | .01* | ||||||
| Step 3 | Constant | −13.28 | 12.01 | .27 | .53 | .28 | 28% | 4.43 | .001* | |
| Age | −0.30 | 0.16 | −.16 | .06 | ||||||
| Tinnitus duration | 0.13 | 0.17 | .06 | .44 | ||||||
| Past tinnitus treatments received | −7.92 | 4.70 | −.14 | .09 | ||||||
| Wearing hearing aids | −0.48 | 1.47 | −.03 | .75 |
Note. Durbin–Watson statistic = 1.95. TFI = Tinnitus Functional Index; ISI = Insomnia Severity Index; GAD-7 = Generalized Anxiety Disorder-7; PHQ-9 = Patient Health Questionnaire-9.
Significance at p < .05.
Unwanted Events
There were 11 (11%) of the 104 participants who reported unwanted events during the intervention period. There were 12 events in total, as one participant mentioned two unwanted events. These events were categorized to be “related to the intervention” 82% of the time and “probably related” to the intervention 18% of the time. The events were classified according to the checklist for unwanted events (Linden, 2013) into the following four categories: worsening of symptoms, emergence of new symptoms, negative well-being, and prolongation of treatment, as shown in Table 4.There were no significant differences in clinical or demographical characteristics between those reporting unwanted events and those not reporting them (see Table 1), except that women were more likely than men to report unwanted events, χ2(1) = 6.88, p = .011.
Table 4.
Unwanted events reported.
| Classification | Examples of reported unwanted effects | Number of meaning units | Severity during the intervention | Severity 1 year postintervention |
|---|---|---|---|---|
| Worsening of symptoms | To begin with, the process made me more aware of my tinnitus until I became better at controlling its impact. | 4 | Severe | Mild |
| Emergence of new symptoms | I found the exercise where I had to tune into my tinnitus really difficult. It made me extremely anxious and panicky. | 3 | Severe | Moderate |
| Negative well-being | I looked at the tinnitus in greater detail and became more aware of the limiting effect it has on me. | 3 | Moderate | Moderate |
| Prolongation of treatment | It went on too long. | 2 | Moderate | Moderate |
Discussion
The primary objective of this study was to evaluate the efficacy of audiologist-guided iCBT for tinnitus distress and tinnitus-associated difficulties up to 1 year postintervention. Additional objectives were to identify predictors of outcome and to investigate the occurrence of unwanted events during the intervention period. This discussion considers the results obtained for each objective.
Long-Term Efficacy of iCBT
The benefit of audiologist-guided iCBT was sustained 1 year postintervention for tinnitus and all related difficulties except for cognitive functioning. This could be attributed to concentration tips targeting cognitive functioning being an optional module and not read by all participants (read by 57%). It may also be that the CFQ was not an optimum assessment measure of the ability to concentrate and focus on mental activities as its focus is on cognitive failure in areas of perception, memory, and motor function (Broadbent et al., 1982).
These findings are in line with previous iCBT for tinnitus studies also reporting stability of intervention effects up to 1 year postintervention. Jasper et al. (2014) indicated stability of effects 6 months after completing iCBT for tinnitus severity, anxiety, depression, and insomnia in a German population. Kaldo et al. (2008) and Hesser et al. (2012), both using a Swedish population, and Weise et al. (2016), using a German population, reported stability (and improvements) of results 1 year after undertaking iCBT for tinnitus severity, anxiety, and depression, but not for insomnia. Kaldo et al. (2008) compared 6 weeks of iCBT (n = 26) with those doing seven sessions of group-based CBT. They also found no significant changes from postintervention to 1-year follow-up. In contrast to these studies and this study, Nyenhuis, Zastrutzki, Weise, Jäger, and Kröner-Herwig (2013) reported a deterioration of results at 6-month follow-up (d = 1.04 at T1 to d = 0.66 at T2 when using ITT analysis). This result may have been related to the difference in the program selected, as the CBT-oriented tinnitus coping training (Kröner-Herwig, Frenzel, Fritsche, Schilkowsky, & Esser, 2003) was used during this study, whereas the other studies have been based on the CBT self-help program for tinnitus developed by Andersson and Kaldo (2004).
More information is still required regarding the long-term efficacy of iCBT for tinnitus beyond 1 year postintervention. Enduring effects up to 3 years post-iCBT have been indicated for conditions such as anxiety, depression, stress, and fatigue (Andersson, Rozental, Shafran, & Carlbring, 2018).
Predictors of Outcome
Certain patients with tinnitus may benefit more or less from iCBT (Kaldo-Sandström, Larsen, & Andersson, 2004). Identifying if specific patient variables can predict who may benefit from iCBT is therefore of importance. Demographic, clinical, and intervention variables were investigated to aid in identifying who was best suited for iCBT. Demographic variables did not predict outcomes, indicating that iCBT is applicable to a wide range of participants, regardless of their demographic characteristics.
The best predictor of improvement in tinnitus severity was a higher baseline TFI score. It is possible that the relationship between TFI score and improvement in tinnitus indicates a regression to the mean phenomenon, in that variables at extremes tend to be closer to the mean during follow-up measurements, letting natural data variation appear to be a real change (Barnett, Van Der Pols, Jolieke, & Dobson, 2004).
The next best predictors of improvement in tinnitus severity were higher intervention satisfaction and a higher number of modules read. Similar results were reported by Kaldo-Sandström et al. (2004), who reported that intervention compliance, how intensely participants worked at the intervention, and the number of messages sent were associated with outcomes. Further identified trends were that patients referred from external routes and those undertaking previous treatments had better outcomes, which was not identified as a predictor by this study. Kaldo-Sandström et al. used a clinical population, as opposed to a research population used in this study, which could contribute to the difference in findings. Results from both Kaldo-Sandström et al.'s study and this study suggest that positive intervention engagement contributes to improved outcomes. Identifying traits that promote engagement may therefore be important. It has been reported that personality traits such as openness and conscientiousness may suggest greater suitability for iCBT for tinnitus (Kleinstäuber, Weise, Andersson, & Probst, 2018). Moreover, higher scores for helplessness and lower scores for actively changing behaviors and attitudes and maintaining these behaviors and attitudes using the Tinnitus Stages of Change Questionnaire were associated with better outcomes for both group and Internet-based CBT for tinnitus (Kaldo, Richards, & Andersson, 2006). Furthermore, Langguth et al. (2007) found that low agreeableness (competitive, self-centred, and more susceptible to anger) was correlated with greater tinnitus distress. On the other hand, neuroticism (higher emotional responses such as anxiety, fear, anger, and frustration) positively correlated with depressiveness. It may be that other factors, not investigated in this study, may also predict outcomes.
Unwanted Events During the Intervention Period
Unwanted events after undertaking iCBT for tinnitus were investigated, as empirical studies on the nature and frequency of unwanted events are scarce in iCBT trials and have not been investigated for iCBT for tinnitus (Boettcher et al., 2014). Unwanted events were reported by 11% of the participants. This frequency is consistent with the 10% reported by a meta-analysis of previous nontinnitus iCBT trials (Barak, Hen, Boniel-Nissim, & Shapira, 2008). The reported events were generally related, or probably related, to the intervention, and the severity thereof was described as moderate to severe. The most commonly mentioned unwanted event was that symptoms worsened (n = 4). One potential reason for this is that some participants may have become more aware of their tinnitus during the initial parts of the intervention. Three participants also mentioned the emergence of new symptoms as the exposure techniques caused anxiety. By doing the intervention, three participants came to fully realize the impact their tinnitus was having on them, and this led to negative well-being. Two participants mentioned that the intervention was too prolonged. During a process evaluation of the trial, it was, however, found that the intervention period was not long enough to complete all the information for around 17% of the participants (Beukes, Manchaiah, et al., 2017). Identifying an optimal intervention period to suit all participants is one challenge surrounding such an intervention. As these particular unwanted events were only mentioned by a very small percentage of participants, these findings only provide indications of possible unwanted events. Further investigations are required to reach more concrete conclusions.
There may also be specific moderators associated with the reporting of unwanted events while undertaking such an intervention. In this trial, a significantly higher proportion (82%) reporting unwanted events were female (p = .01). It is possible that demographic characteristics not investigated in this study may be associated with unwanted events. The possible unwanted events associated with this intervention, such as an initial deterioration of symptoms, negative well-being, or emergence of new symptoms, should be disclosed in future trials. Moreover, providing some flexibility in the timings to complete the intervention should be provided.
Study Limitations
This study is not without limitations, which have implications for result interpretation. Because of the nature of the study design, randomization was not obtainable to assess long-term outcomes. Furthermore, not all participants completed the postintervention assessment measures, which could have resulted in treatment bias. The assessment measures selected may not have been optimal to identify intervention effects, and this may have affected the results obtained.
Further Research
Further longitudinal studies would be of benefit to monitor outcomes to at least 3 years postintervention for audiologist-guided iCBT. As identifying outcome variables will be useful for triaging participants, wider demographic and clinical variables should be searched for moderators and mediators of outcome. This may include factors such as helplessness, behavior and/or attitude change, and ability to maintain these behaviors. These factors were indicated to be predictors of outcome by Kaldo et al. (2006). Because of the importance of effective (i.e., sufficient) engagement in achieving the intended outcomes, ways of promoting such engagement are required (Yardley et al., 2016). Implementing qualitative research methods using semistructured interviews to provide a more in-depth understanding of users' experiences with the intervention will provide further insights into wanted and unwanted intervention effects (Yardley et al., 2016). Further insights regarding unwanted events that need to be addressed or disclosed in future iCBT trials for tinnitus trials are required.
Conclusion
This study has demonstrated that the benefits of audiologist-guided iCBT are maintained 1 year postintervention. Few predictors of outcome could be identified, indicating the applicability of this study regardless of demographic and clinical profiles. This was the first study investigating unwanted events from iCBT for tinnitus, and knowledge of these effects can assist in improving future iCBT for tinnitus studies.
Acknowledgments
Anglia Ruskin, Lamar, and Linköping Universities and the National Institute for Health Research, Nottingham, supported the undertaking of this study, but the views expressed are those of the authors and not of these institutions. Portions of this article were presented at the 3rd International Internet & Audiology Meeting, Louisville, KY, July 2017, which was funded by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant 1R13DC016547 and the Oticon Foundation. The conference committee is thanked for providing a travel bursary for the first author to present this work at the conference. The authors wish to thank all participants and organizations that promoted and supported this study. They would also like to thank Linköping University for hosting the web portal and their webmaster, George Vlaescu, for the technical assistance provided.
Appendix
Intervention Satisfaction Evaluation
Please state the extent to which you agree or disagree with the following statements, where 1 is “strongly disagree” and 5 is “strongly agree” (choose one per statement).
-
ABOUT THE USABILITY
It was straightforward to use the Internet platform.
It was easy to navigate through the materials.
The length of the modules was appropriate.
-
ABOUT THE CONTENT
The level of information was at a suitable level.
The materials were informative.
The subject matter was interesting.
-
ABOUT THE PRESENTATION
The content was presented in a well-structured manner.
The use of presentation of materials was suitable, i.e., the use of diagrams, text, pictures, videos.
The text was easy to read.
-
ABOUT THE SUITABILITY
The intervention is suitable for those suffering with tinnitus.
The range of modules were appropriate.
The topics covered were beneficial.
-
ABOUT THE EXERCISES GIVEN
The worksheets and quizzes asked appropriate questions.
I clearly understood how to practice the various techniques.
I was motivated to do the exercises.
ABOUT THE INTERVENTION AS A WHOLE
Open-ended questions:
How long did it take you take to read each module's information on average?
What was the best aspect of the intervention?
What needs improving?
Any further suggestions?
The evaluation presented in this Appendix appears courtesy of the authors.
Funding Statement
Anglia Ruskin, Lamar, and Linköping Universities and the National Institute for Health Research, Nottingham, supported the undertaking of this study, but the views expressed are those of the authors and not of these institutions. Portions of this article were presented at the 3rd International Internet & Audiology Meeting, Louisville, KY, July 2017, which was funded by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant 1R13DC016547 and the Oticon Foundation.
Footnotes
The questions presented here appear courtesy of the authors.
References
- Andersson G. (2015). Clinician-supported Internet-delivered psychological treatment of tinnitus. American Journal of Audiology, 24(3), 299–301. [DOI] [PubMed] [Google Scholar]
- Andersson G. (2016). Internet-delivered psychological treatments. Annual Review of Clinical Psychology, 12, 157–179. [DOI] [PubMed] [Google Scholar]
- Andersson G., & Kaldo V. (2004). Internet-based cognitive behavioral therapy for tinnitus. Journal of Clinical Psychology, 60(2), 171–178. [DOI] [PubMed] [Google Scholar]
- Andersson G., Rozental A., Shafran R., & Carlbring P. (2018). Long-term effects of Internet-supported cognitive behaviour therapy. Expert Review of Neurotherapeutics, 18, 21–28. [DOI] [PubMed] [Google Scholar]
- Andersson G., Strömgren T., Ström L., & Lyttkens L. (2002). Randomized controlled trial of Internet-based cognitive behavior therapy for distress associated with tinnitus. Psychosomatic Medicine, 64(5), 810–816. [DOI] [PubMed] [Google Scholar]
- Asendorpf J. B., van de Schoot R., Denissen J. J. A., & Hutteman R. (2014). Reducing bias due to systematic attrition in longitudinal studies: The benefits of multiple imputation. International Journal of Behavioral Development, 38(5), 453–460. [Google Scholar]
- Baguley D., McFerran D., & Hall D. (2013). Tinnitus. The Lancet, 382(9904), 1600–1607. [DOI] [PubMed] [Google Scholar]
- Barak A., Hen L., Boniel-Nissim M., & Shapira N. (2008). A comprehensive review and a meta-analysis of the effectiveness of Internet-based psychotherapeutic interventions. Journal of Technology in Human Services, 26(2–4), 109–160. [Google Scholar]
- Barnett A. G., Van Der Pols J. C., Jolieke C., & Dobson A. J. (2004). Regression to the mean: What it is and how to deal with it. International Journal of Epidemiology, 34(1), 215–220. [DOI] [PubMed] [Google Scholar]
- Bastien C. H., Vallières A., & Morin C. M. (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. [DOI] [PubMed] [Google Scholar]
- Baumeister H., Reichler L., Munzinger M., & Lin J. (2014). The impact of guidance on Internet-based mental health interventions—A systematic review. Internet Interventions, 1(4), 205–215. [Google Scholar]
- Beukes E. W., Allen P. M., Manchaiah V., Baguley D. M., & Andersson G. (2017). Internet-based intervention for tinnitus: Outcome of a single-group open trial. Journal of the American Academy of Audiology, 28(4), 340–351. [DOI] [PubMed] [Google Scholar]
- Beukes E. W., Baguley D. M., Allen P. M., Manchaiah V., & Andersson G. (2018). Audiologist-guided Internet-based cognitive behavior therapy for adults with tinnitus in the United Kingdom: A randomized controlled trial. Ear and Hearing, 39, 423–433. [DOI] [PubMed] [Google Scholar]
- Beukes E. W., Manchaiah V., Allen P. M., Baguley D. M., & Andersson G. (2015). Internet-based cognitive behavioural therapy for adults with tinnitus in the UK: Study protocol for a randomised controlled trial. BMJ Open, 5(9), e008241 https://doi.org/10.1136/bmjopen-2015-008241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes E. W., Manchaiah V., Baguley D. M., Allen P. M., & Andersson G. (2017). Process evaluation of Internet-based cognitive behavioural therapy for adults with tinnitus in the context of a randomised control trial. International Journal of Audiology, 57, 1–12. [DOI] [PubMed] [Google Scholar]
- Beukes E. W., Vlaescu G., Manchaiah V., Baguley D. M., Allen P. M., Kaldo V., & Andersson G. (2016). Development and technical functionality of an Internet-based intervention for tinnitus in the UK. Internet Interventions, 6, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt J. M., Lin H. W., & Bhattacharyya N. (2016). Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngology—Head & Neck Surgery, 142(10), 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher J., Rozental A., Andersson G., & Carlbring P. (2014). Side effects in Internet-based interventions for social anxiety disorder. Internet Interventions, 1(1), 3–11. [Google Scholar]
- Broadbent D. E., Cooper P. F., FitzGerald P., & Parkes K. R. (1982). The Cognitive Failures Questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology, 21(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Cima R. F., Andersson G., Schmidt C. J., & Henry J. A. (2014). Cognitive–behavioral treatments for tinnitus: A review of the literature. Journal of the American Academy of Audiology, 25(1), 29–61. [DOI] [PubMed] [Google Scholar]
- Cima R. F., Vlaeyen J. W., Maes I. H., Joore M. A., & Anteunis L. J. (2011). Tinnitus interferes with daily life activities: A psychometric examination of the Tinnitus Disability Index. Ear and Hearing, 32(5), 623–633. https://doi.org/10.1097/AUD.0b013e31820dd411 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20(1), 37–46. [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Crönlein T., Langguth B., Pregler M., Kreuzer P. M., Wetter T. C., & Schecklmann M. (2016). Insomnia in patients with chronic tinnitus: Cognitive and emotional distress as moderator variables. Journal of Psychosomatic Research, 83, 65–68. [DOI] [PubMed] [Google Scholar]
- Davis A., & Rafaie E. A. (2000). Epidemiology of tinnitus. In Tyler R. S. (Ed.), Tinnitus handbook (pp. 1–23). San Diego, CA: Singular. [Google Scholar]
- Dawes P., Fortnum H., Moore D. R., Emsley R., Norman P., Cruickshanks K., … Munro K. (2014). Hearing in middle age: A population snapshot of 40- to 69-year olds in the United Kingdom. Ear and Hearing, 35(3), e44–e51. https://doi.org/10.1097/AUD.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E., Emmons R. A., Larsen R. J., & Griffin S. (1985). The Satisfaction With Life Scale. Journal of Personality Assessment, 49(1), 71–75. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D. C., Lyles C., Crepaz N., & TREND Group. (2004). Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. American Journal of Public Health, 94(3), 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackrell K., Fearnley C., Hoare D. J., & Sereda M. (2015). Hyperacusis Questionnaire as a tool for measuring hypersensitivity to sound in a tinnitus research population. BioMed Research International, 2015, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackrell K., Hall D. A., Barry J. G., & Hoare D. J. (2016). Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hearing Research, 335, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., & Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Gander P. E., Hoare D. J., Collins L., Smith S., & Hall D. A. (2011). Tinnitus referral pathways within the National Health Service in England: A survey of their perceived effectiveness among audiology staff. BMC Health Services Research, 11, 162 https://doi.org/10.1186/1472-6963-11-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. A., Láinez M. J., Newman C. W., Sanchez T. G., Egler M., Tennigkeit F., … Langguth B. (2011). Treatment options for subjective tinnitus: Self reports from a sample of general practitioners and ENT physicians within Europe and the USA. BMC Health Services Research, 11(1), 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesser H., Gustafsson T., Lunden C., Henrikson O., Fattahi K., Johnsson E., … Andersson G. (2012). A randomized controlled trial of Internet-delivered cognitive behavior therapy and acceptance and commitment therapy in the treatment of tinnitus. Journal of Consulting and Clinical Psychology, 80(4), 649–661. https://doi.org/10.1037/a0027021 [DOI] [PubMed] [Google Scholar]
- Hesser H., Weise C., Westin V. Z., & Andersson G. (2011). A systematic review and meta-analysis of randomized controlled trials of cognitive–behavioral therapy for tinnitus distress. Clinical Psychology Review, 31(4), 545–553. [DOI] [PubMed] [Google Scholar]
- Hoare D. J., Broomhead E., Stockdale D., & Kennedy V. (2015). Equity and person-centeredness in provision of tinnitus services in UK National Health Service audiology departments. European Journal for Person Centered Healthcare, 3(3), 318–326. [Google Scholar]
- Jacobson N. S., & Truax P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12–19. [DOI] [PubMed] [Google Scholar]
- Jasper K., Weise C., Conrad I., Andersson G., Hiller W., & Kleinstäuber M. (2014). Internet-based guided self-help versus group cognitive behavioral therapy for chronic tinnitus: A randomized controlled trial. Psychotherapy and Psychosomatics, 83(4), 234–246. [DOI] [PubMed] [Google Scholar]
- Kaldo V., Levin S., Widarsson J., Buhrman M., Larsen H., & Andersson G. (2008). Internet versus group cognitive–behavioral treatment of distress associated with tinnitus: A randomized controlled trial. Behavior Therapy, 39(4), 348–359. [DOI] [PubMed] [Google Scholar]
- Kaldo V., Richards J., & Andersson G. (2006). Tinnitus Stages of Change Questionnaire: Psychometric development and validation. Psychology, Health & Medicine, 11(4), 483–497. [DOI] [PubMed] [Google Scholar]
- Kaldo-Sandström V., Larsen H. C., & Andersson G. (2004). Internet-based cognitive–behavioral self-help treatment of tinnitus: Clinical effectiveness and predictors of outcome. American Journal of Audiology, 13(2), 185–192. [DOI] [PubMed] [Google Scholar]
- Khalfa S., Dubal S., Veuillet E., Perez-Diaz F., Jouvent R., & Collet L. (2002). Psychometric normalization of a hyperacusis questionnaire. ORL, 64(6), 436–442. [DOI] [PubMed] [Google Scholar]
- Kim H., Lee H., An S., Sim S., Park B., Kim S. W., … Choi H. G. (2015). Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One, 10(5), e0127578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstäuber M., Weise C., Andersson G., & Probst T. (2018). Personality traits predict and moderate the outcome of Internet-based cognitive behavioural therapy for chronic tinnitus. International Journal of Audiology, 31, 1–7. [DOI] [PubMed] [Google Scholar]
- Kröner-Herwig B., Frenzel A., Fritsche G., Schilkowsky G., & Esser G. (2003). The management of chronic tinnitus: Comparison of an outpatient cognitive–behavioral group training to minimal-contact interventions. Journal of Psychosomatic Research, 54(4), 381–389. [DOI] [PubMed] [Google Scholar]
- Langguth B., Kleinjung T., Fischer B., Hajak G., Eichhammer P., & Sand P. (2007). Tinnitus severity, depression, and the big five personality traits. Progress in Brain Research, 166, 221–225. [DOI] [PubMed] [Google Scholar]
- Langguth B., Kreuzer P. M., Kleinjung T., & De Ridder D. (2013). Tinnitus: Causes and clinical management. The Lancet Neurology, 12(9), 920–930. [DOI] [PubMed] [Google Scholar]
- Langguth B., Landgrebe M., Schlee W., Schecklmann M., Vielsmeier V., Steffens T., … Frick U. (2017). Different patterns of hearing loss among tinnitus patients: A latent class analysis of a large sample. Frontiers in Neurology, 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasisi A. O., Abiona T., & Gureje O. (2010). Tinnitus in the elderly: Profile, correlates, and impact in the Nigerian study of ageing. Otolaryngology—Head and Neck Surgery, 143(4), 510–515. [DOI] [PubMed] [Google Scholar]
- Linden M. (2013). How to define, find and classify side effects in psychotherapy: From unwanted events to adverse treatment reactions. Clinical Psychology & Psychotherapy, 20(4), 286–296. [DOI] [PubMed] [Google Scholar]
- Little R. J. A. (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404), 1198–1202. [Google Scholar]
- Löwe B., Decker O., Müller S., Brähler E., Schellberg D., Herzog W., & Herzberg P. Y. (2008). Validation and standardization of the Generalized Anxiety Disorder Screener (GAD–7) in the general population. Medical Care, 46(3), 266–274. [DOI] [PubMed] [Google Scholar]
- Maes I. H. L., Cima R. F. F., Vlaeyen J. W., Anteunis L. J. C., & Joore M. A. (2013). Tinnitus: A cost study. Ear and Hearing, 34(4), 508–514. [DOI] [PubMed] [Google Scholar]
- Martinez C., Wallenhorst C., McFerran D., & Hall D. A. (2015). Incidence rates of clinically significant tinnitus: 10-year trend from a cohort study in England. Ear and Hearing, 36(3), e69–e75. https://doi.org/10.1097/AUD.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Devesa P., Perera R., Theodoulou M., & Waddell A. (2010). Cognitive behavioural therapy for tinnitus. The Cochrane Database of Systematic Reviews, (9), CD005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerran D., & Baguley D. (2009). Is psychology really the best treatment for tinnitus? Clinical Otolaryngology, 34(2), 99–101. [DOI] [PubMed] [Google Scholar]
- Meikle M. B., Henry J. A., Griest S. E., Stewart B. J., Abrams H. B., McArdle R., … Vernon J. A. (2012). The Tinnitus Functional Index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear and Hearing, 33(2), 153–176. https://doi.org/10.1097/AUD.0b013e31822f67c0 [DOI] [PubMed] [Google Scholar]
- Michie S., Yardley L., West R., Patrick K., & Greaves F. (2017). Developing and evaluating digital interventions to promote behavior change in health and health care: Recommendations resulting from an international workshop. Journal of Medical Internet Research, 19(6), e232 https://doi.org/10.2196/jmir.7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. W., Jacobson G. P., & Spitzer J. B. (1996). Development of the Tinnitus Handicap Inventory. Archives of Otolaryngology—Head & Neck Surgery, 122(2), 143–148. [DOI] [PubMed] [Google Scholar]
- Newman C. W., Weinstein B. E., Jacobson G. P., & Hug G. A. (1991). Test–retest reliability of the Hearing Handicap Inventory for Adults. Ear and Hearing, 12(5), 355–357. [DOI] [PubMed] [Google Scholar]
- Nyenhuis N., Golm D., & Kröner-Herwig B. (2013). A systematic review and meta-analysis on the efficacy of self-help interventions in tinnitus. Cognitive Behaviour Therapy, 42(2), 159–169. [DOI] [PubMed] [Google Scholar]
- Nyenhuis N., Zastrutzki S., Weise C., Jäger B., & Kröner-Herwig B. (2013). The efficacy of minimal contact interventions for acute tinnitus: A randomised controlled study. Cognitive Behaviour Therapy, 42(2), 127–138. [DOI] [PubMed] [Google Scholar]
- Pinto P. C. L., Marcelos C. M., Mezzasalma M. A., Osterne F. J. V., de Melo Tavares de Lima M. A., & Nardi A. E. (2014). Tinnitus and its association with psychiatric disorders: Systematic review. The Journal of Laryngology & Otology, 128(8), 660–664. [DOI] [PubMed] [Google Scholar]
- Polisena J., Coyle D., Coyle K., & McGill S. (2009). Home telehealth for chronic disease management: A systematic review and an analysis of economic evaluations. International Journal of Technology Assessment in Health Care, 25(3), 339–349. [DOI] [PubMed] [Google Scholar]
- Richards D., & Richardson T. (2012). Computer-based psychological treatments for depression: A systematic review and meta-analysis. Clinical Psychology Review, 32(4), 329–342. [DOI] [PubMed] [Google Scholar]
- Rozental A., Andersson G., Boettcher J., Ebert D. D., Cuijpers P., Knaevelsrud C., … Carlbring P. (2014). Consensus statement on defining and measuring negative effects of Internet interventions. Internet Interventions, 1(1), 12–19. [Google Scholar]
- Rozental A., Magnusson K., Boettcher J., Andersson G., & Carlbring P. (2017). For better or worse: An individual patient data meta-analysis of deterioration among participants receiving Internet-based cognitive behavior therapy. Journal of Consulting and Clinical Psychology, 85(2), 160–177. [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Landgrebe M., Langguth B., & TRI Database Study Group. (2014). Phenotypic characteristics of hyperacusis in tinnitus. PLoS One, 9(1), e86944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J., Curhan G. C., & Farwell W. R. (2010). Prevalence and characteristics of tinnitus among US adults. The American Journal of Medicine, 123(8), 711–718. [DOI] [PubMed] [Google Scholar]
- Smith P., McKeon A., Blunt I., & Edwards N. (2014). NHS hospitals under pressure: Trends in acute activity up to 2022. London, United Kingdom: Nuffield Trust. [Google Scholar]
- Spitzer R. L., Kroenke K., Williams J. B. W., & Löwe B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Spitzer R. L., Kroenke K., Williams J. B., & Patient Health Questionnaire Primary Care Study Group. (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA, 282(18), 1737–1744. [DOI] [PubMed] [Google Scholar]
- Stockdale D., McFerran D., Brazier P., Pritchard C., Kay T., Dowrick C., & Hoare D. J. (2017). An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Services Research, 17(1), 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegg-Quinn S., Bennett R. J., Eikelboom R. H., & Baguley D. M. (2016). The impact of tinnitus upon cognition in adults: A systematic review. International Journal of Audiology, 55(10), 533–540. [DOI] [PubMed] [Google Scholar]
- Vlaescu G., Alasjö A., Miloff A., Carlbring P., & Andersson G. (2016). Features and functionality of the Iterapi platform for Internet-based psychological treatment. Internet Interventions, 6, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaescu G., Carlbring P., Lunner T., & Andersson G. (2015). An e-platform for rehabilitation of persons with hearing problems. American Journal of Audiology, 24(3), 271–275. [DOI] [PubMed] [Google Scholar]
- Weise C., Kleinstauber M., & Andersson G. (2016). Internet-delivered cognitive–behavior therapy for tinnitus: A randomized controlled trial. Psychosomatic Medicine, 78(4), 501–510. https://doi.org/10.1097/PSY.0000000000000310 [DOI] [PubMed] [Google Scholar]
- West D. (2012). How mobile devices are transforming healthcare. Issues in Technology Innovation, 18(1), 1–11. [Google Scholar]
- Wu B., Searchfield G., Exeter D., & Lee A. (2015). Tinnitus prevalence in New Zealand. The New Zealand Medical Journal, 128, 24–34. [PubMed] [Google Scholar]
- Yardley L., Spring B. J., Riper H., Morrison L. G., Crane D. H., Curtis K., … Blandford A. (2016). Understanding and promoting effective engagement with digital behavior change interventions. American Journal of Preventive Medicine, 51(5), 833–842. [DOI] [PubMed] [Google Scholar]