Abstract
Purpose
The introduction of connectivity technologies in hearing implants allows new ways to support cochlear implant (CI) users remotely. Some functionalities and services that are traditionally only available in an in-clinic care model can now also be accessed at home. This study explores the feasibility of a prototype of a tablet computer application (MyHearingApp [MHA]) in a group of senior experienced CI users at home, evaluating usability and user motivation.
Method
Based on user feedback, a tablet computer application (MHA) for the Cochlear Nucleus 6 CP910 sound processor was designed implementing six different functionalities: (a) My Hearing Tests, (b) My Environment, (c) My Hearing Journey, (d) Tip of the Day, (e) Recipient Portal, and (f) Program Use and Events. The clinical evaluation design was a prospective study of the MHA in 16 senior experienced CI users. During 4 weeks, participants could freely explore the functionalities. At the end, the usability and their motivation for uptake and adherence were measured using a baseline and follow-up questionnaire.
Results
Based on the System Usability Score (as part of the follow-up questionnaire), a good level of usability was indicated (M = 75.6, range: 62.5–92.5, SD = 8.6). The ability to perform hearing tests at home is ranked as the most relevant functionality within the MHA. According to the Intrinsic Motivation Inventory (Deci, Eghrari, Patrick, & Leone, 1994) questionnaire (as part of the follow-up questionnaire), participants reported high levels of interest and enjoyment, found themselves competent, and did not experience pressure while working with the app.
Conclusions
This study evaluated a tablet computer application (MHA) for experienced senior CI users by means of a prospective design, which provided novel insights into delivering CI care into the home of the CI user. The user feedback from this small-scale study suggests that the participants are open to take more responsibility for and to become a more active actor in their own hearing care, if only this is facilitated with the right tools. This may foster the evolution from a clinic-led to a more patient-centered care model, where CI users feel more empowered in the self-management of their hearing implant device.
The World Health Organization states that over 5% of the world's population—360 million people—has a disabling hearing loss and that approximately one third of people over 65 years of age are affected by disabling hearing loss (World Health Organization, 2017). Currently, only 6% to 15% of potential adult cochlear implant (CI) candidates receive a CI (De Raeve & van Hardeveld, 2014). As the number of implant users will grow, the current model of intensive clinic-centric CI programs will become unsustainable, with implant centers questioning their specialized methods of service delivery as an effective means of provision (H. Cullington et al., 2016). Currently, CIs are typically still only provided in specialized centers requiring a multidisciplinary team consisting of clinical audiologists; ear, nose, and throat surgeons; radiologists; CI audiologists; psychologists; and speech and language therapists.
Recently, Athalye, Archbold, Mulla, Lutman, and Nikolopoulous (2015) explored the perspectives of CI users, parents of pediatric CI users, and professionals in the United Kingdom. They used a questionnaire with close- and open-ended responses to explore the views of the current CI service delivery and the potential issues in the long term. Seven hundred forty-eight responses were obtained, the majority of which (69%) were from CI professionals. The remaining respondents were parents (19%) and CI users (12%). The study showed that current services are perceived to be predominantly led by CI centers where decisions related to appointments, provision of standard care, treatment, accessories, management, and long-term maintenance are made by the team at the CI center. In the future, participants (whether user, parent, or professional) in Athalye et al.'s study would like these decisions to be predominantly led by the users themselves. Both qualitative and quantitative study results revealed that the majority of participants opted for care to take place closer to home, avoiding travel, and for local audiology services in which educational and other support services are integrated into CI provision. In addition, other researchers (Archbold & O'Donoghue 2007; Punch & Hyde, 2011) have emphasized the importance of a close liaison between CI centers and local educational services to ensure the best possible management and the continuous use of the CI in school-age children. Furthermore, Grenness, Hickson, Laplante-Lévesque, and Davidson (2014) defined patient-centered care in the context of audiologic rehabilitation. They concluded that individualized care was the overarching theme and highlighted the importance of flexibility in rehabilitation.
Hence, there appear to be arguments that suggest that future care models should be much more centered on the patient and closer to the home environment. In such a model, it is crucial to reconcile the desire for more autonomy from the patient perspective with the clinician perspective to provide a highly effective care. This can be enabled by a digital platform, where both professionals and patients have access to relevant information. There is a growing interest internationally in the power of eHealth technology to deliver improved health care services and information to more people and more effectively. The use of eHealth in hearing health care is relatively new. Initially, these applications mainly included delivering diagnostic services and fitting of hearing aids (HAs) or CIs for those in rural and underserved areas (Swanepoel & Hall, 2010). Currently, eHealth can also be applied as a more innovative way to look at audiologic rehabilitation and long-term self-management of hearing problems and HAs (Ferguson, Brandreth, Brassington, Leighton, & Wharrad, 2016; Greenwell, Featherstone, & Hoare, 2015; Henshaw & Ferguson, 2013; Hesser et al., 2012; Molander et al., 2015; Thorén, Oberg, Wänström, Andersson, & Lunner, 2014).
Prompted by the realization that insights in Internet-based self-management tools for CI users are scarce, the Supported Hearing in Elderly Citizens project (funded by the Active and Assisted Living Programme, Project Number 2013-6-065) was set up. This project aimed at developing an eHealth tool to empower senior CI recipients in increasing the self-management of their hearing implant device and using this device more effectively. In the first phase of the project, focus groups were held to identify the biggest user needs. Based on the outcomes of these sessions, six functionalities were adopted and implemented on a tablet computer application called MyHearingApp (MHA).The aim of the study reported here was to conduct an evaluation of the MHA in a group of senior experienced CI users. As we wanted to understand the barriers and facilitators of the use of and adherence to the MHA, we have opted to focus on assessing the usability of and motivation for uptake and adherence to the MHA. In parallel, a study was conducted with adult newly implanted CI users (De Graaff et al., 2018). The main goal in this study was to evaluate the use and feasibility of the self-test functionality (i.e., one of the six functionalities of the MHA) within the first months after cochlear implantation.
Materials and Methods
Description of the MHA
As the involvement of key stakeholders in the development of eHealth interventions is being increasingly recognized as a means to embed the patients' perspective in the intervention, focus groups with CI users were held prior to the development of the MHA (Ferguson, Brandreth, Brassington, & Wharrad, 2015; Ferguson et al., 2016). Insights gathered during three focus groups (eight adult CI users per group) guided the development of the MHA. In short, the MHA is a tablet computer application that stores all information in a secure data repository in the cloud. The application can be used by a hearing implant user or by a clinician. The clinician has full access to all the data sets of the users. An individual user can only access his or her own data. Currently, the application only runs with the Cochlear Nucleus 6 CP910 sound processor. It includes six functionalities: (a) My Hearing Tests, (b) My Environment, (c) My Hearing Journey, (d) Tip of the Day, (e) Recipient Portal, and (f) Program Use and Events.
My Hearing Tests
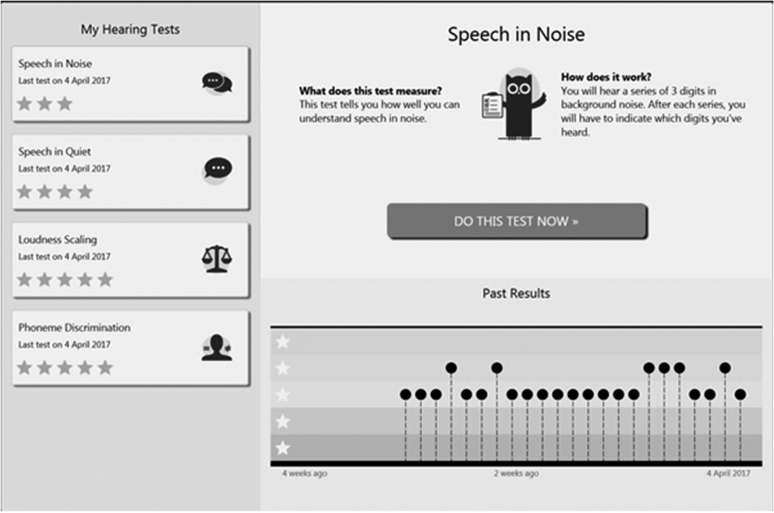
The hearing assessment module in the MHA includes self-test versions of tests that are normally conducted in a clinic environment: phoneme discrimination and the categorical loudness scaling test of the auditory speech sounds evaluation (A§E) test battery (Govaerts et al., 2006), a common Dutch monosyllabic consonant–vowel–consonant (CVC) words-in-quiet test (Bosman & Smoorenburg, 1995), and a digits-in-noise test (Smits, Goverts, & Festen, 2013). Each hearing test could only be administered once a day by each participant, in order to overcome a possible learning effect. Details about connectivity and calibration can be found in the Participants section. Figure 1 shows a screen shot of the My Hearing Tests functionality of one participant.
Figure 1.
Screen shot of the My Hearing Tests functionality of one participant. The detailed view (right hand pane) shows the results obtained for the Speech-in-Noise Tests during the study. Each dot represents a Speech-in-Noise Test result, performed on a specific day.
Phoneme discrimination. This test is a self-test version of the A§E Phoneme Discrimination Test. It is a discrimination test in which one speech sound is repeatedly presented and, at a certain moment, a deviating speech sound is presented. The CI user has to react as soon as he or she hears the deviating speech sound. Eight pairs are tested and presented in quiet at 70 dB SPL. The score is the total number of correctly discriminated phoneme pairs, expressed as a percentage.
Categorical loudness scaling. This test is a self-test version of the A§E Loudness Scaling Test. Loudness scaling is particularly useful to ascertain the fitting of CI processors for different frequencies (Vaerenberg, Govaerts, De Ceulaer, Daemers, & Schauwers, 2011). The test assesses the normality of the implant user's intensity perception. The user has to rate the loudness (seven categories, from inaudible dB SPL to too loud) of a narrow band noise centered at 1000 Hz at different intensity levels, ranging from 30 to 80 dB HL. Each intensity is presented twice. The score is the absolute difference from the user's loudness rating to the reference curve, consisting of the loudness ratings of young normal listeners, averaged over all intensity levels.
Monosyllabic words in quiet. Speech recognition in quiet is assessed using monosyllabic words with a CVC structure, presented by a female Dutch speaker (Bosman & Smoorenburg, 1995). Two lists of 12 CVC words are chosen at random and presented in quiet at 65 dB SPL. The participants are instructed to enter everything they understand, even if it is a single phoneme or a nonsense word. The response to the first word in each list is not included in the calculation of the test score. In the clinic, a trained audiologist scores the test by counting phoneme errors in the verbal user responses. In the MHA version, a scoring version was developed to calculate the phoneme error rate based on the typed user responses. This algorithm was validated prior to the study (De Graaff et al., 2016).
Digits in noise. This test is based on the standard digits-in-noise test (Smits et al., 2013). In the MHA implementation, digit triplets (e.g., 5-8-6) are presented in speech-shaped noise at an overall presentation level of 65 dB A, with the signal-to-noise ratio varied adaptively in 2-dB up/down steps depending on the correctness of each preceding response (all three digits to be identified for a correct response). A total of 27 triplets are presented, and the responses to triplets seven to 27 are used to calculate the speech reception threshold, that is, the signal-to-noise ratio which corresponds to a score of 50%.
My Environment
The Nucleus 6 CP910 sound processor constantly analyzes the sound environment and classifies the sound in six categories (speech in quiet, speech in noise, music, quiet, noise, and wind noise; Mauger, Warren, Knight, Goorevich, & Nel, 2014) with the primary purpose of automatically controlling the sound enhancement features (SmartSound iQ) and the secondary purpose of providing an overview of the sound environment (Nucleus 6 data logging). The MHA extends the data logging functionality in the clinician software (Cochlear Custom Sound Suite) by providing the CI user for the first time, without going to the clinic, an overview of personal CI use over the last 30 days in 1-day intervals. This functionality requires downloading the data logs from the Nucleus 6 CP910 sound processor to the MHA by using the Cochlear Nucleus CR230 Remote Assistant and can be downloaded via a USB cable for display in the MHA. For each day, the user can see the proportion of time spent in conversations, in listening to music, or in environmental sounds.
My Hearing Journey
In keeping with the MHA objective of motivating the user through personal empowerment, a screen is provided to track a user's progress on a set of hearing milestones, known as My Hearing Journey. In this version of the application, the goals were predetermined by an expert clinician. In a future version, it would be desirable for a user to personalize the hearing goals. During the rehabilitation process, the user collects “badges” to represent milestones in performance as they are reached. In total, six badges can be earned, of which four relate to a certain environment the participant has to spend a specific amount of time in, based on data logging (e.g., Badge 1 is earned when the participant uses his or her sound processor for a total of 25 hr). The remaining badges (Badges 2 and 5) are earned when the participant carries out a specified hearing test (e.g., Badge 2 is earned after performing the phoneme discrimination test). In order not to discourage the participants, they were not informed regarding the total number of badges that can be earned.
Tip of the Day
In order to build up competences in the optimal use of the implant system in daily life, the MHA offers a tip of the day. These tips have been derived from various sources, for example, the Recipient Portal (described in the next section) and guidelines, by expert clinicians. Some can help participants to reach their milestones (from the My Hearing Journey functionality). For example, users are reminded that they can make use of disposable batteries if they forget to charge their reusable ones.
Recipient Portal
The Recipient Portal is a secure portal delivering personalized content and services to support the optimization of CI use in senior CI recipients. For example, information regarding the use of the Nucleus 6 CP910 sound processor (e.g., how to use rechargeable batteries, how to change microphone covers), CR230 Remote Assistant, and accessories is readily available. Tips regarding talking on the phone, having conversations, and listening to music are also accessible.
Program Use and Events
This module helps users to identify which programs are used at home. It also keeps track of device-related events, for example, how often the battery runs flat or the sound processor coil falls off.
Participants
Eighteen participants were recruited through the Dutch CI user society “Onafhankelijk Platform voor Cochleaire Implantatie.” An invitation to participate was posted on the organization's website and in their newsletter. Inclusion criteria were having a Nucleus 6 system and being older than age 60 years. No inclusion criteria were set in terms of the participants' performance or their computer literacy. The study was approved by the medical ethics committee of the VU University Medical Center. The participants enrolled into the study voluntarily and provided informed consent. They received a voucher and travel expenses as compensation for participation. Two participants were not able to explore the MHA functionalities due to technical issues. The first participant had to use a loaner Nucleus 6 CP910 sound processor because of a technical issue with his own processor. No data logs with the loaner processor could be obtained. The second participant was not able to connect the tablet computer to the Wi-Fi network at home.
The data of the remaining 16 participants (12 male, four female) were statistically analyzed (SPSS; IBM, 2011). The mean age was 68 years (range: 61–80 years, SD = 5). The participants had between 13 months and 15 years of implant experience and indicated that they used their CI on average 15.9 h per day (range: 10–24 h). Ten were unilateral CI users, and six were bimodal users (i.e., wearing a contralateral HA). Two participants stated having visual problems, and four reported having arthritis in their fingers. The majority (87%) lived together with a partner (and children), and the remaining participants lived alone (13%).
Study Protocol
Participants took part in an initial group (up to five persons) counseling session (90 min). The investigator explained the study protocol, and informed consent was obtained. Each participant received a loaner (Windows) tablet computer (ACER Aspire Switch 10 V) with the MHA preinstalled and a 10-min hands-on training prior to taking the tablet computer home, mimicking a typical counseling session possible in a busy clinic setting. A baseline questionnaire was completed by the participants at the end of this session. During a 4-week take-home period, the participants could freely explore the functionalities of the MHA, after which they returned the tablet computer at the evaluation session. During this session, a follow-up questionnaire was completed.
For speech recognition testing, a personal audio cable was used to directly inject audio signals from the tablet computer to the sound processor. The setup was calibrated such that the signals presented via the audio cable were delivered at predefined levels for phoneme discrimination, loudness scaling, words in quiet and digits-in-noise tests (De Graaff et al., 2016). The accessory-mixing ratio of the sound processor was set to “accessory only”; this was to ensure that the participants only received sound via the audio cable and were not distracted by ambient sound received via the microphones. Bimodal users were instructed to switch off their HA during speech tests. The results of all four hearing tests were shown to the participants in a uniform manner, an ordinal scale consisting of a five-star rating, ranging from poor to perfect performance. For each hearing test, an expert clinician determined appropriate boundaries between the categories based on known statistics of these test outcomes in a clinic setting (see Table 1).
Table 1.
Star ratings used to provide feedback of the hearing tests to the participants.
| Hearing test | 1 star | 2 stars | 3 stars | 4 stars | 5 stars | |
|---|---|---|---|---|---|---|
| Phoneme discrimination | %correct | [0–85] | [86–90] | [91–95] | [96–99] | p = 100 |
| Loudness scaling | Loudness scale units (7) | RMS ≥ 1.53 | RMS in [1.33, 1.53[ | RMS in [1.12, 1.33[ | RMS in [0.92, 1.12[ | RMS in [0, 0.92[ |
| Words in quiet | Phoneme score %correct | [0–60] | [61–70] | [71–80] | [81–90] | [91–100] |
| Digits in noise | SRT [dB SNR] | > 4.50 | ]−1.50, 4.50] | ]−4.50, −1.50] | ]−7.50, −4.50] | ≤ −7.50 |
Note. Boundaries have been set based on the following references: phoneme discrimination and loudness scaling (Vaerenberg et al., 2014), words in quiet (Meeuws et al., 2017), digits in noise (Kaandorp et al., 2015). % = percentage; RMS = root-mean-square; SRT = speech reception threshold; SNR = speech-to-noise ratio.
Questionnaires
To evaluate the experience of CI users with the MHA and to understand the motivation of the participants for uptake and adherence of the app, two questionnaires (baseline and follow-up) were developed.
Literature from chronic health domains suggest that an individual's motivation plays a significant role in treatment compliance (Vermeire, Hearnshaw, van Royen, & Denekens, 2001). The self-determination theory (SDT; Deci & Ryan, 1985) explains motivation from people's natural tendencies to behave in effective and healthy ways. The SDT distinguishes between different types of motivation based on the different reasons or goals that give rise to an action. The basic distinction is between intrinsic motivation, which refers to doing something because it is inherently interesting or enjoyable, and extrinsic motivation, which refers to doing something because it leads to a separable outcome (Ryan & Deci, 2000). This theory highlights three basic human psychological needs, which, when satisfied, yield enhanced motivation and well-being (Ryan & Deci, 2000): autonomy: the feeling of psychological freedom or choice; competence: perceived self-efficacy (i.e., one's belief in one's ability to succeed in a particular domain); and relatedness: the need to belong somewhere and to feel connected with others.
In audiology, the SDT has previously been employed to examine first-time hearing help seekers' motivations for HA adoption (Ridgeway, Hickson, & Lind, 2013, 2015). Based on the responses of 253 adults, a multivariate logistic regression was used to examine associations between autonomous (or self-determined) and controlled (or externally determined) motivation, sociodemographic and audiometric variables, and HA adoption. Their results showed that three factors were significantly associated with increased HA adoption: autonomous motivation, perceived hearing difficulty, and poorer hearing. Hence, the SDT model is potentially useful in understanding how HA adoption decisions are made and how hearing health behavior is internalized and maintained over time. Furthermore, it may provide a useful framework to better understand individuals' motivations for engagement and adherence to other hearing interventions, such as CIs. Therefore, the baseline and follow-up questionnaire included SDT-based questionnaires.
Baseline Questionnaire
The baseline questionnaire is composed of two parts. The first part consisted of sociodemographic questions, questions on the experience with Internet and tablet computer use, and hearing characteristics. The second part was based on the Treatment Self-Regulation Questionnaire (TSRQ; Ryan, & Connell, 1989). The TSRQ addresses why people engage in some healthy behavior, enter treatment for a medical condition, try to change unhealthy behaviors, follow a treatment regimen, or engage in some other health-relevant behavior. It assesses the degree to which a person's motivation for health behaviors is relatively autonomous (e.g., “I'd like to use the MyHearingApp because I find it a personal challenge to do so”) or controlled (e.g., “I'd like to use the MyHearingApp because other people would be mad at me if I did not”). The responses on the autonomous and controlled items were averaged separately in order to calculate the autonomous and controlled regulation scores for the target behavior. The scoring is performed using a 1 to 7 Likert scale from not at all true to very true. From previous TSRQ research, it is known that the autonomous style represents the most self-determined form of motivation and has consistently been associated with maintained behavior change and positive health care outcomes.
Follow-Up Questionnaire
The follow-up questionnaire consisted of three parts. First, participants were required to fill in the System Usability Scale (SUS; Brooke, 1996). This scale is a simple, 10-item scale giving a global view of subjective assessments of usability. It uses a 5-point Likert scale ranging from 1 = strongly disagree to 5 = strongly agree. To gain more insight in the evaluation of the MHA, the term system in the SUS was replaced by MyHearingApp. The SUS yields a single score (range: 0–100) representing a composite measure of the overall usability of the MHA, with a higher score indicating higher usability.
Second, the Intrinsic Motivation Inventory (IMI; Deci, Eghrari, Patrick, & Leone, 1994) was used. This is a multidimensional measurement device designed to assess participants' subjective experience related to a target activity in laboratory experiments. It has been used in several studies related to intrinsic motivation and self-regulation. The instrument assesses participants' interest/enjoyment, perceived competence, effort, value/usefulness, perceived pressure and tension, perceived choice while performing a given activity, and relatedness, each on a scale from 1 (not at all true) to 7 (very true), thus yielding seven subscale scores. The Relatedness subscale is used to gain insight in interpersonal interactions and was used to understand how the MHA possibly affected the role of the participants' family/friends regarding their CI (e.g., “Working with the MyHearingApp increased the involvement of my family/friends in the care for my CI.”).
Finally, participants' feedbacks were gathered regarding the MHA with respect to current functionalities and future use of the MHA. This was done by
Ranking exercise. Participants ranked the six functionalities from most useful (Score 6) to least useful (Score 1).
Descriptor words (based on Benedek & Milner, 2002). Participants were presented 30 descriptor words, of which 15 were categorized as positive (e.g., “stimulating, motivating”) and the remaining 15 as negative (e.g., “too technical, boring”). Each participant was asked to identify five words that best described their experience with the MHA.
Yes/no questions. Participants were asked three yes/no questions to assess the future use of the MHA, whether they would recommend the MHA to others, and finally, whether they thought the MHA improved the quality of their CI care. If needed, they could refine their answers (space was available for any comments on the yes/no questions).
Results
Baseline Questionnaire
Participants' Internet and Tablet Computer Experience
The majority of the participants (87%) reported going online on a daily basis, whereas the remaining participants used the Internet a couple of days per week (13%). Half of the participants rated themselves as having average Internet skills, whereas 31% stated being an experienced Internet user. The remaining participants rated their own Internet skills as “beginner.” Ten participants possessed a tablet computer themselves, with the majority of them (60%) having over a year of experience with such a device. Two rated their own tablet computer skills as beginner, five as average user, and the remaining three as having advanced skills.
Subjects' Motivation to Participate in the Study (TSRQ)
Responses on the TSRQ questions (scale range: 1–7) were provided by 15 out of the 16 participants (one participant forgot to answer the questions relating to the TSRQ). Results showed that all subjects were highly autonomously motivated (average: 5.9, SD = 0.8, range: 3.5–7.0) with a variable amount of controlled regulation (average: 2.8, SD = 1.6, range: 1.0–6.6), which is in line with the expectation for a clinical study with volunteers. The autonomous style represents the most self-determined form of motivation and has consistently been associated with maintained behavior change and positive health care outcomes.
Follow-Up Questionnaire
SUS
After the 4-week take-home trial with the MHA, the participants were asked to rate the usability of the app at the follow-up session. The averaged SUS score was 75.6 (range: 62.5–92.5, SD = 8.6), corresponding to a good general usability rating (Bangor, Kortum, & Miller, 2008).
IMI
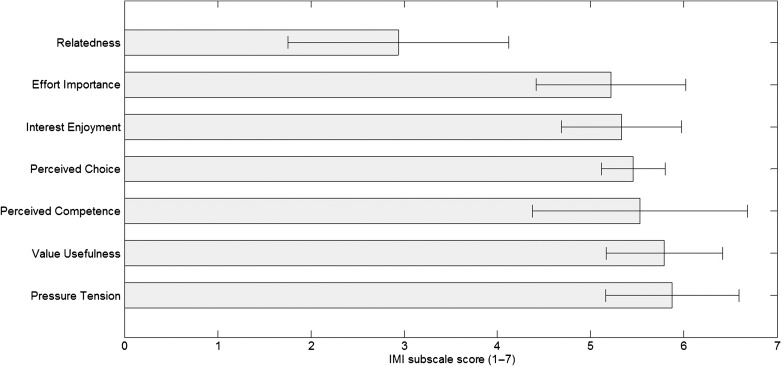
Seven subscales (ratings ranging from 1 to 7) of the IMI were taken into account, and results are shown in Figure 2, with high scores representing a positive outcome. Participants did not experience a lot of pressure while using the app (5.9, SD = 0.7), valued it greatly (5.8, SD = 0.6), and found themselves competent to work with the app (5.5, SD = 1.2). Moreover, they felt they had choice and autonomy (5.5, SD = 0.3) while working with the app, and they reported high levels of interest and enjoyment (5.3, SD = 0.6). They have also put quite some effort in working with the app during the evaluation trial (5.2, SD = 0.8). As the app does not contain any features yet to support communication functions, the rating on the relatedness with family and friends was low (2.9, SD = 1.2).
Figure 2.
Averaged results (mean) of the Intrinsic Motivation Inventory (N = 16). Bars represent standard deviations. The Pressure Tension subscale has been reversed; a high value represents less pressure/tension. IMI = Intrinsic Motivation Inventory.
Ranking Exercise
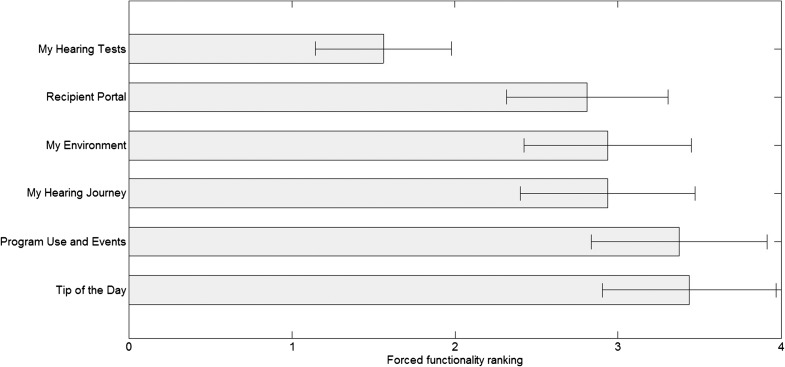
The 16 participants had access to six different functionalities in the MHA, and they were asked to rank these from most useful (Score 1) to least useful (Score 6). Figure 3 shows the results of this ranking exercise averaged over all participants. The functionality clearly standing out in terms of participant usefulness was the possibility to perform hearing tests in the home environment. The other functionalities received more average ratings.
Figure 3.
Averaged ranking results (mean) for the six different functionalities of the MyHearingApp (N = 16). Bars represent standard errors. A low score (left side on the x-axis) implies a high ranking and is seen as most useful by the participants. A high score (right side of the x-axis) implies a lower ranking score and is seen as least useful by the participants.
Descriptor Words
The frequency of selection for participants' descriptor words to describe their experience with the MHA is illustrated in a word cloud in Figure 4, where words with the greatest frequency of selection appear larger than those words that were less frequently selected. Accessible, motivating, and stimulating were the most frequently selected (by nine out of 16 participants). No words with a negative connotation were selected by any of the participants.
Figure 4.
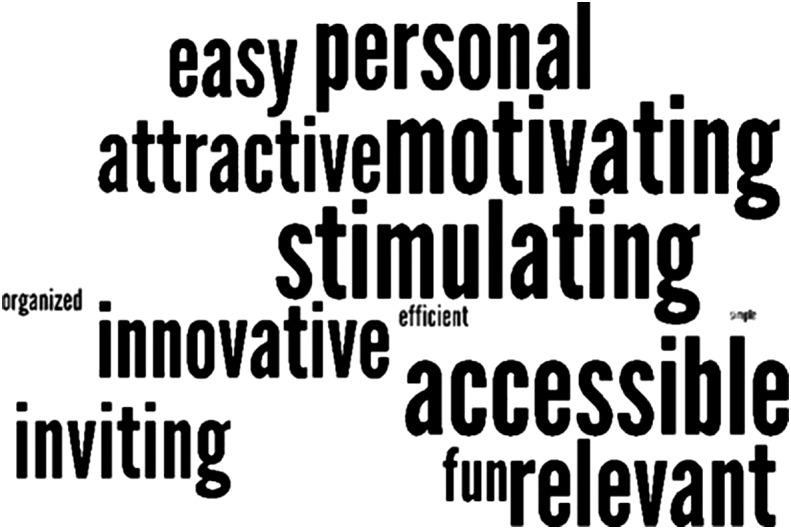
Word cloud based on the selection of participants' descriptor words.
Future Use
Overall, the participants' feedback was overwhelmingly positive; the majority (93%) would like to use the MHA in the future and stated that having access to the app increased the quality of their CI care (93%). Only one participant was not keen on using MHA in the future. This participant commented that she felt she was already sufficiently proficient at managing her CI and that this tool did not provide her with additional useful information. All the participants indicated that they would recommend other CI users to use the MHA, however.
Tablet and Internet Experience
On average, participants having a personal tablet (besides the tablet computer they used during the study) indicated a lower SUS score (M = 73.8, SD = 8.8) compared with participants not possessing a tablet (M = 79.5, SD = 7.8). This difference was not significant, t(13) = −1.24, p > .05. In addition, self-rated tablet and Internet competency (beginner, average, or experienced user) were taken into account, and no significant effect was found in the self-rated tablet competency, F(2, 11) = 2,94, p > .05, nor self-rated Internet competency, F(2, 12) = 1,13, p > .05, on the SUS score.
Hearing Test Frequency
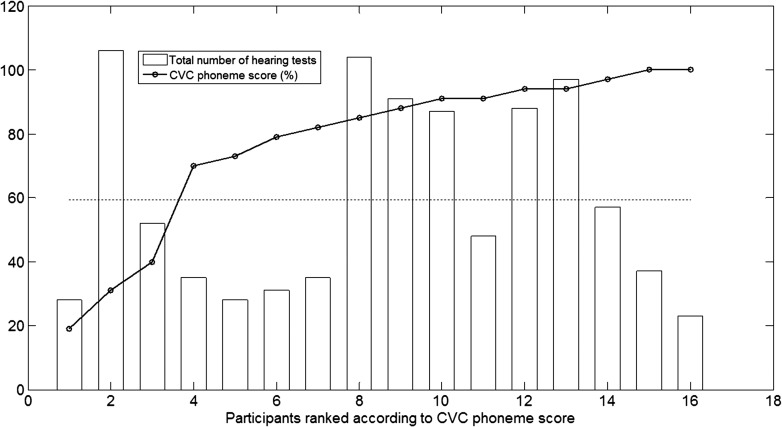
Participants could only perform each of the four hearing tests once per day to overcome possible learning effects. They performed the hearing tests at their own will. Figure 5 shows the total number of hearing tests, summed over all four tests and performed by each participant, during the study. The participants are rank ordered based on their final CVC phoneme score. It was found that they conducted many hearing tests during the trial period. On average, each participant performed 59.2 hearing tests (range: 23–106) during the 4-week study period. No significant correlation was obtained between the number of hearing tests and the final CVC phoneme score (r = .062, p = .82), indicating that participants obtaining a high CVC score did not significantly perform more hearing tests compared with participants obtaining a lower CVC score.
Figure 5.
Total number of hearing tests performed during the study, per participant. Participants are rank ordered from lowest to highest final consonant–vowel–consonant (CVC) score, obtained at their final CVC test. The dashed line indicates the average number of tests performed.
Analysis of the Milestones
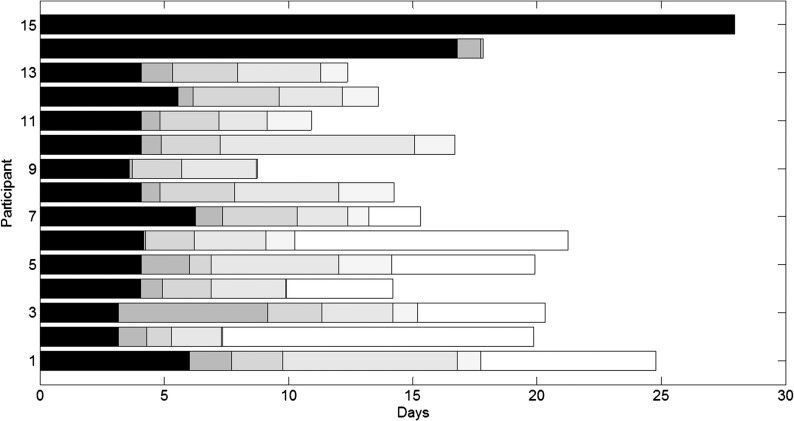
Figure 6 shows which milestone was finally reached by each participant over time and how long it took to reach each milestone. The participants are rank ordered from the highest to lowest final milestone reached (e.g., Participant 1 reached Milestone 6, Participant 15 only reached Milestone 1). Note that the criterion to order the participants is different than the one used in Figure 5. Only achieved milestones are shown in the figure, indicated by the different shading levels (e.g., Participant 3 reached six milestones, whereas Participant 15 only reached one milestone). Seven (out of 15) participants reached the sixth and final milestone. Hence, eight participants were still trying to reach a milestone prior to the end of the study, but the time spent while still trying to reach this milestone is not shown. The amount of time needed to reach a certain milestone is indicated by the length of each block. The time needed to reach Milestones 2 and 5, which requested the participant to perform a hearing test, is shorter than the time needed to reach the remaining milestones. In order to reach these latter milestones, the participants were requested to spend a certain amount of time in a particular environment (e.g., use his or her sound processor for a total of 25 hr).
Figure 6.
Milestones reached per participant (N = 15). Participants are rank ordered from lowest to highest final milestone reached. Per participant, each shaded block indicates the amount of time needed to reach a certain milestone. The different levels of shading stand for the six milestones (e.g., black indicates Milestone 1 is reached).
Discussion
Because CI users are requesting a more proactive and patient-centered role in their CI care (Athalye et al., 2015) and the number of individuals receiving a CI will increase in the coming years (a tripling of numbers was seen in the last 7 years; Peters, Wyss, & Manrique, 2010), changes are needed to maintain the scalability of CI services. In this study, we evaluated the MHA on a tablet computer provided to senior experienced CI users in their home environment. More specifically, we evaluated the usability of the MHA in senior experienced CI users and explored participants' motivation for uptake of and adherence to the MHA.
Regarding the usability of the MHA, the average SUS score of 75.6 indicates good usability (Bangor et al., 2008). One participant was not able to connect the tablet computer with the Wi-Fi connection at home, and as a consequence, the MHA could not be launched. Moore, Rothpletz, and Preminger (2015) gained insight into the effect of chronological age on the acceptance of Internet-based hearing health care. They suggested that, in order to increase the likelihood of acceptance, discrepancies in computer self-efficacy and computer literacy should be given consideration. Moreover, Internet-based hearing health care programs should be easy to navigate (Schneider, van Osch, & de Vries, 2012) and should incorporate video and audio fragments with text-based information (Hou, 2012).
With respect to the individual functionalities, currently available in the MHA, it is clear that our participants were in favor of being able to perform hearing tests in their home environment. Not only does the MHA provide them with more insight into their listening performance with their current sound processor but it also enables them with the autonomy to perform these tests when and where they wish to do them, as opposed to when they are tested during an audiologic appointment in their CI clinic in a sound booth. Recently, H. E. Cullington and Agyemang-Prempeh (2017) evaluated the feasibility of an online speech recognition test (Digit Triplet Test, DTT) and questionnaire in the home environment of 17 adult CI users. Fifteen of the participants (88%) were able to obtain a valid score on the DTT, and the majority felt positive about testing their own hearing using the DTT at home. It should be noted, however, that no training for the test was given; a simple link was sent by e-mail. The authors suggested that improved compliance may be obtained after one training session in the clinic to introduce the test. In our study, a short training session was indeed given, and no major issues with performing the hearing tests were found.
From the patient's perspective, participants were very keen on having access to the Recipient Portal. Research has shown that having access to personalized information is more useful compared with having access to general information of patients with chronic conditions (e.g., Urowitz et al., 2012). In the current study, most participants found it very useful having personalized information (e.g., warranty information) at a single location, accessible at any time. Kessels (2003) reported that between 40% and 80% of information communicated in a health care appointment is immediately forgotten. For the majority of CI users, most information on CI use, maintenance, warranty information, limitations, and communication strategies is provided within the first fitting session(s). At this first fitting session, CI users have very little speech understanding (Lenarz, Sönmez, Joseph, Büchner, & Lenarz, 2012). Their hearing is still poor, creating a risk that most information provided during the first fitting session might not even be understood by the CI user, who might therefore become dependent on his or her significant others regarding CI knowledge and problem solving. Kelly, Tolson, Day, McColgan, Kroll, and Maclaren (2013) explored what older people believed would enable them to adjust to and gain maximum benefit from wearing an HA. One of their key findings was that the informational needs of older HA users were not met because only 52% of HA users reported receiving enough practical help postfitting and only 41% reported receiving enough support. Furthermore, approximately 40% stated not feeling confident in the use of their HAs or controls. These findings on unmet informational support needs were already suggested in previous HA studies (Gatehouse, 2003; Jennings, 2009). As a CI is a more complex device than an HA, the unmet need of information certainly holds for CI users. Besides the lack of information, the way information is currently being delivered might also be adapted. Caposecco, Hickson, and Meyer (2014) analyzed the content, design, and readability of 36 printed HA manuals to determine if they are suitable for older adults. Their results showed that HA manuals are not optimal for this population, which is a concern because poorly designed manuals may impact on self-efficacy, outcomes, and success. Major perceived weaknesses of HA manuals concerned the inclusion of too many HA models in each user guide, frequent use of unfamiliar vocabulary, small text size and graphics, excessive amounts of technical information, and problems with layout. In a follow-up study, which evaluated a modified user guide for HA management, Caposecco, Hickson, Meyer, and Khan (2016) suggested that HA management information could also be provided through a smartphone/tablet app, which uses diagrams and videos to supplement text. These findings are in line with our results, as having the Recipient Portal with short texts, videos, tips and tricks, and testimonials from peers as information source was well received by the participants.
Thirdly, participants appreciated having access to their data logs via the My Environment functionality. With the commercially available Nucleus 6 CP900 system, long-term averaged data logs (from the previous fitting until the current fitting) are available to the CI professional, not to the CI user. By giving the CI users insight into their own listening environment, they might better understand in what kind of environments they mainly use their CI and might use this knowledge to initiate new behaviors. They can also share this information with other caregivers, such as speech and language therapists, which may improve the quality of their counseling. For example, someone who spends most of his or her time in a quiet (unchallenging) environment might consider changing this by going out more often or trying to have conversations in more challenging listening environments.
Fourthly, having access to “My Hearing Journey” received an equal ranking score as the “My Environment” functionality. In our study, each participant could earn the same six milestones. These milestones were not particularly ambitious (e.g., use your sound processor for 25 hr) but were set up to motivate the participants to keep using the MHA during the 4-week study period. Previous research has taken into account gamification for seniors in order to increase adherence to eHealth applications (de Vette, Tabak, Dekker-van Weering, & Vollenbroek-Hutten, 2015). According to de Vette et al. (2015), gamification is the application of game elements to the nongame fields to motivate and increase user activity and retention. Recently, Allam, Kostova, Nakamoto, and Schulz (2015) have already provided evidence of the potential positive effect of gamification on health and behavioral outcomes.
As it is known that patients who are active and involved in their own care have improved health outcomes (Hibbard, Mahoney, Stockard, & Tusler, 2005; Mosen et al., 2007), we explored our participants' motivation for uptake of and adherence to the MHA. The positive results of the IMI on a group level indicated that participants perceived the MHA to be interesting and that they enjoyed engagement with it (“interest/enjoyment” was high). As the interest/enjoyment subscale is considered the self-report measure of the intrinsic motivation, the participants' intrinsic motivation to make use of the MHA was high. Intrinsic motivation refers to doing something because it is inherently interesting or enjoyable (Ryan & Deci, 2000). Hence, intrinsic motivation is important for completing a task (in this case, the MHA). The participants also indicated possessing the right competences to use the MHA. Although they did their best during the evaluation trial (“effort” was high), they did not perceive mental stress during the evaluation (“pressure/tension” was low). Furthermore, they valued the MHA greatly (“value” was high). Henshaw, McCormack, and Ferguson (2015) explored intrinsic and extrinsic motivations in uptake and engagement with and adherence to computer-based auditory training (CBAT). Participants found the CBAT intervention easy to use, interesting, and enjoyable. Initial participation in the study was associated with extrinsic motivation (e.g., hearing difficulties). However, engagement and adherence with CBAT was influenced by intrinsic (e.g., desire to achieve higher scores) and extrinsic (e.g., to help others with hearing loss) motivations. Furthermore, Ferguson et al. (2016) evaluated the benefits of a multimedia educational program for first-time HA users. They developed a series of seven short interactive videos that covered a broad range of practical and psychosocial issues relevant to their target group. These videos were rated as highly useful, and the majority of users agreed that the videos were enjoyable. One of the interesting findings of this study was that reusing the videos was common, with at least half of the participants watching the videos two or more times, and some using them up to six or seven times. Ferguson et al. considered that this reusability suggests that their participants were using the videos as a means to self-manage their hearing loss, HA, and communication. As such, reusability was seen as an indicator of motivation. Reusability can also be seen as an indicator for motivation and adherence in our study. Indeed, although participants were not instructed to perform hearing tests on a regular basis and they could only perform each hearing test once per day, we found that participants performed on average 59 hearing tests over a 4-week period. As such, the intervention was used throughout the entire trial period, and these data serve as a behavioral measure of adherence. Moreover, the number of hearing tests performed was not correlated with speech perception outcomes with the CI, indicating that not only “star performers” appreciate the opportunity to check their hearing at home.
Finally, descriptors of their experience with the MHA from the postquestionnaire were highly positive in nature, with participants selecting only positive descriptor words, such as accessible, motivating, and stimulating.
Limitations of the Study
The current study is not without limitations. First, the small participant group included in this study may limit generalizability of the results to all CI users. Moreover, the participants who took part in this study were a volunteer sample and, therefore, may have different motivations from the group of CI users who choose not to take part. Thirdly, the participants were recruited from the Dutch CI user society, and it is likely that individuals who are more engaged with their health care and generally more intrinsically motivated joined the study. Therefore, the participant sample source is likely biased. In addition, these participants were aware that the study explored the usability of an eHealth application on a tablet computer, introducing the possibility of reporting bias. A recent study by Maidment, Brassington, Wharrad, and Ferguson (2016) showed that greater Internet competency predicts superior practical HA knowledge and handling skills in first-time HA users. In our study, no difference in the SUS score could be attributed to the self-rated tablet and Internet competency. Possibly, the participants from our study who indicated their tablet and Internet competencies as beginner would in fact have greater tablet and computer skills compared with the cohort of senior experienced CI users. As such, it is unlikely that all senior experienced CI users would be willing or would have the skills to make use of an eHealth application with their CI. Another limitation is that, currently, the MHA is only compatible with the Nucleus 6 CP910 sound processor. In the future, gaining insight in the added value of the app outside of a study concept would provide information into the real-world perspective of all users.
In this study, participants were asked to use a direct connection between their speech processor and the tablet computer (personal audio cable). Hence, background noise at home was eliminated, and there was no dependence on computer speaker quality. Some participants indicated that making use of the personal audio cable was not convenient. Also the My Environment functionality could only be used if data from the CR230 Remote Assistant were uploaded to the tablet computer by means of a USB cable. Usability for less technically able CI users could be increased by reducing the technical requirements of the MHA.
Future Directions
Apart from senior experienced CI users, other target groups might also benefit from an eHealth tool. One particular group is parents of CI children. By means of an eHealth tool, parents might be able to gain more insight in the current CI use of their child and be more involved in the care for their child's CI. Because it has been suggested that the timing of the delivery of educational support is most beneficial early on in the HA users' journey (Kramer, Allessie, Dondorp, Zekveld, & Kapteyn, 2005), offering the MHA to adult users with newly implanted CIs is of interest. De Graaff et al. (2018) have performed a study with this target group to investigate the possible benefits of the MHA during the first months of their CI hearing journey. Finally, significant others (family, spouse, professional care workers) could also benefit from such an eHealth tool. As such, significant others could be effectively engaged in the rehabilitation process. Recently, Grenness, Meyer, Scarinci, Ekberg, and Hickson (2016) pointed out the importance of recognizing the needs of significant others and considering both the person with hearing impairment and the significant other in any clinical exchange, which might result in a family-centered care.
Currently, no auditory training functionality was included in the MHA. Henshaw et al. (2015) offered a 4-week CBAT intervention to 44 adults with mild sensorineural hearing loss who did not have an HA. The participants experienced a benefit of the intervention in terms of improved concentration and attention, and they reported being more aware of their hearing difficulties. Possibly, CI users' hearing journey might be facilitated if they have easy access to an individualized auditory training functionality within the MHA.
Another functionality that might be included in the app in the future is the possibility to self-adjust the upper electrical stimulation levels of the user programs. For cochlear CI recipients, mySmartSound can be activated by the audiologist, which allows CI recipients to fine tune their hearing in real-world listening environments by allowing them to adjust the bass, the treble, and the master volume. The ability to self-adjust the upper electrical stimulation levels by CI recipients in the home environment has already been explored by Vroegop, Dingemanse, van der Schroeff, Metselaar, and Goedegebure (2017). They concluded that self-adjustment is a useful and clinically applicable tool that may help CI recipients to improve perceived sound quality in their daily lives.
Furthermore, the Recipient Portal could be divided into “chunks” of information instead of the current mode of delivery (“one-size-fits-all”). As such, these chunks of information could be ordered and delivered based on the hearing journey CI users are going through. Ferguson et al. (2016) developed a series of seven short interactive videos for first-time HA users and established the level of accessibility, take-up, acceptability, and adherence. The interactive videos were rated as highly useful and enjoyable by the majority of the participants. By individualized tailoring of information and hearing tests, to maximize the relevance to the users of the MHA, usage of the app could be increased. Hence, users could perform hearing tests whenever and wherever they want, and they have a reliable information source regarding CI issues, which allows them to have a more proactive role in their CI care than is currently available. Moreover, a shift from a clinic-led schedule to a patient-centered schedule will become feasible. The current CI pathway typically involves annual visits to the CI center for the duration of the patients' life. These visits are scheduled by the clinic, and as such, some of these review visits may provide little benefit to the CI user. Making the CI care pathway more patient centered instead, implying that the CI user attends the clinic only as needed rather than for routine appointments, may provide a more efficient service (H. Cullington et al., 2016). As such, making use of such a tool as the MHA could be a real game changer to current CI service delivery.
Conclusion
This study evaluated a tablet computer application (MHA) for experienced senior CI users by means of a prospective design, which provided novel insights into delivering CI care into the home of the CI user. The results showed that being able to perform hearing tests at home is ranked as the most relevant functionality within the MHA. According to the IMI, participants reported high levels of interest and enjoyment and found themselves competent but not stressed while working with the app. To conclude, we can suggest that such an eHealth tool is of value and can facilitate the transition from a clinic-led CI care to a patient-centered CI care for experienced senior CI users, where they feel more empowered in the self-management of their hearing implant device.
Acknowledgments
Portions of this article were presented at the 3rd International Internet & Audiology Meeting, Louisville, KY, July 2017, which was funded by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant 1R13DC016547 and the Oticon Foundation. This research was part of the Supported Hearing in Elderly Citizens project, funded by AAL JP (project number 2013-6-065). The authors would like to thank Feike de Graaff for her contribution, Ann De Bolster and Stefan Lievens for developing the MyHearingApp, Tobias Busch for developing the MyHearingApp GUI, and all study participants for their involvement in the research.
Funding Statement
Portions of this article were presented at the 3rd International Internet & Audiology Meeting, Louisville, KY, July 2017, which was funded by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant 1R13DC016547 and the Oticon Foundation. This research was part of the Supported Hearing in Elderly Citizens project, funded by AAL JP (project number 2013-6-065).
References
- Allam A., Kostova Z., Nakamoto K., & Schulz P. J. (2015). The effect of social support features and gamification on a web-based intervention for rheumatoid arthritis patients: Randomized controlled trial. Journal of Medical Internet Research, 17(1), e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold S., & O'Donoghue G. M. (2007). Ensuring the long-term use of cochlear implants in children: The importance of engaging local resources and expertise. Ear and Hearing, 28(Suppl. 2), 3S–6S. [DOI] [PubMed] [Google Scholar]
- Athalye S., Archbold S., Mulla I., Lutman M., & Nikolopoulous T. (2015). Exploring views on current and future cochlear implant service delivery: The perspectives of users, parents and professionals at cochlear implant centres and in the community. Cochlear Implants International, 16(5), 241–253. [DOI] [PubMed] [Google Scholar]
- Bangor A., Kortum P. T., & Miller J. T. (2008). An empirical evaluation of the System Usability Scale. International Journal of Human Computer Interaction, 24(6), 574–594. [Google Scholar]
- Benedek J., & Milner T. (2002). Measuring desirability: New methods for evaluating desirability in a usability lab setting. In Proceedings of the Usability Professional Association Conference, Orlando, FL. [Google Scholar]
- Bosman A. J., & Smoorenburg G. F. (1995). Intelligibility of Dutch CVC syllables and sentences for listeners with normal hearing and with three types of hearing impairment. Audiology, 34(5), 260–284. [DOI] [PubMed] [Google Scholar]
- Brooke J. (1996). SUS: A quick and dirty usability scale. In Jordan P. W., Thomas B., Weerdmeester B. A., & McClelland A. L. (Eds.), Usability evaluation in industry. London, United Kingdom: Taylor & Francis. [Google Scholar]
- Caposecco A., Hickson L., & Meyer C. (2014). Hearing aid user guides: Suitability for older adults. International Journal of Audiology, 53(Suppl. 1), S43–S51. [DOI] [PubMed] [Google Scholar]
- Caposecco A., Hickson L., Meyer C., & Khan A. (2016). Evaluation of a modified user guide for hearing aid management. Ear and Hearing, 37(1), 27–37. [DOI] [PubMed] [Google Scholar]
- Cullington H., Kitterick P., DeBold L., Weal M., Clarke N., Newberry E., & Aubert L. (2016). Personalised long-term follow-up of cochlear implant patients using remote care, compared with those on the standard care pathway: Study protocol for a feasibility randomised controlled trial. BMJ Open, 13(5), e011342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullington H. E., & Agyemang-Prempeh A. (2017). Person-centred cochlear implant care: Assessing the need for clinic intervention in adults with cochlear implants using a dual approach of an online speech recognition test and a questionnaire. Cochlear Implants International, 18(2), 76–88. [DOI] [PubMed] [Google Scholar]
- Deci E. L., Eghrari H., Patrick B. C., & Leone D. R. (1994). Facilitating internalization: The self-determination theory perspective. Journal of Personality, 62(1), 119–142. [DOI] [PubMed] [Google Scholar]
- Deci E. L., & Ryan R. M. (1985). Intrinsic motivation and self-determination in human behavior. New York, NY: Plenum Publishing Co. [Google Scholar]
- De Graaff F., Huysmans E., Qazi O. U., Vanpoucke F. J., Merkus P., Goverts S. T., & Smits C. (2016). The development of remote speech recognition tests for adult cochlear implant users: The effect of presentation mode of the noise and a reliable method to deliver sound in home environments. Audiology and Neurotology, 21(Suppl. 1), 48–54. [DOI] [PubMed] [Google Scholar]
- De Graaff F., Huysmans E., Philips B., Merkus P., Goverts S. T., Kramer S. E., & Smits C. (2018). Home self-assessment of speech recognition in the care pathway for newly-implanted adult cochlear implant users. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raeve L., & van Hardeveld R. (2014). Prevalence of cochlear implants in Europe: What do we know and what can we expect. Journal of Hearing Science, 3(4), 9–19. [Google Scholar]
- de Vette F., Tabak M., Dekker-van Weering M., & Vollenbroek-Hutten M. (2015). Engaging elderly people in telemedicine through gamification. JMIR Serious Games, 18(2), e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M., Brandreth M., Brassington W., Leighton P., & Wharrad H. (2016). A randomized controlled trial to evaluate the benefits of a multimedia educational program for first-time hearing aid users. Ear and Hearing, 37(2), 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M., Brandreth M., Brassington W., & Wharrad H. (2015). Information retention and overload in first-time hearing aid users: An interactive multimedia educational solution. American Journal of Audiology, 24(3), 329–332. [DOI] [PubMed] [Google Scholar]
- Gatehouse S. (2003). Rehabilitation: Identification of needs, priorities and expectations, and the evaluation of benefit. International Journal of Audiology, 42(Suppl. 2), S77–S83. [PubMed] [Google Scholar]
- Govaerts P. J., Daemers K., Yperman M., De Beukelaer C., De Saegher G., & De Ceulaer G. (2006). Auditory speech sounds evaluation (A§E®): A new test to assess detection, discrimination and identification in hearing impairment. Cochlear Implants International, 7(2), 97–106. [DOI] [PubMed] [Google Scholar]
- Greenwell K., Featherstone D., & Hoare D. J. (2015). The application of intervention coding methodology to describe the tinnitus E-programme, an Internet-delivered self-help intervention for tinnitus. American Journal of Audiology, 24(3), 311–315. [DOI] [PubMed] [Google Scholar]
- Grenness C., Hickson L., Laplante-Lévesque A., & Davidson B. (2014). Patient-centred care: A review for rehabilitative audiologists. International Journal of Audiology, 53(Suppl. 1), S60–S67. [DOI] [PubMed] [Google Scholar]
- Grenness C., Meyer C., Scarinci N., Ekberg K., & Hickson L. (2016). The international classification of functioning, disability and health as a framework for providing patient- and family-centered audiological care for older adults and their significant others. Seminars in Hearing, 37(3), 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw H., & Ferguson M. A. (2013). Efficacy of individual computer-based auditory training for people with hearing loss: A systematic review of the evidence. PLoS One, 10(5), e62836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw H., McCormack A., & Ferguson M. A. (2015). Intrinsic and extrinsic motivation is associated with computer-based auditory training uptake, engagement, and adherence for people with hearing loss. Frontiers in Psychology, 6(6), 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesser H., Gustafsson T., Lundén C., Henrikson O., Fattahi K., Johnsson E., … Andersson G. (2012). A randomized controlled trial of Internet-delivered cognitive behavior therapy and acceptance and commitment therapy in the treatment of tinnitus. Journal of Consulting and Clinical Psychology, 80(4), 649–661. [DOI] [PubMed] [Google Scholar]
- Hibbard J. H., Mahoney E. R., Stockard J., & Tusler M. (2005). Development and testing of a short form of the patient activation measure. Health Services Research, 40(6 Pt. 1), 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S. I. (2012). Health literacy online: A guide to writing and designing easy-to-use health web sites. Health Promotion Practice, 13, 577–580. [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2011). IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jennings M. B. (2009). Clinical report: Evaluating the efficacy of a group audiologic rehabilitation program for adults with hearing loss using a goal attainment scaling approach. Canadian Journal of Speech-Language Pathology and Audiology, 33(3), 146–153. [Google Scholar]
- Kaandorp M. W., Smits C., Merkus P., Goverts S. T., & Festen J. M. (2015). Assessing speech recognition abilities with digits in noise in cochlear implant and hearing aid users. International Journal of Audiology, 54(1), 48–57. [DOI] [PubMed] [Google Scholar]
- Kelly T. B., Tolson D., Day T., McColgan G., Kroll T., & Maclaren W. (2013). Older people's views on what they need to successfully adjust to life with a hearing aid. Health & Social Care in the Community, 21(3), 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels R. P. (2003). Patients' memory for medical information. Journal of the Royal Society of Medicine, 96(5), 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S. E., Allessie G. H., Dondorp A. W., Zekveld A. A., & Kapteyn T. S. (2005). A home education program for older adults with hearing impairment and their significant others: A randomized trial evaluating short- and long-term effects. International Journal of Audiology, 44(5), 255–264. [DOI] [PubMed] [Google Scholar]
- Lenarz M., Sönmez H., Joseph G., Büchner A., & Lenarz T. (2012). Cochlear implant performance in geriatric patients. Laryngoscope, 122(6), 1361–1365. [DOI] [PubMed] [Google Scholar]
- Maidment D., Brassington W., Wharrad H., & Ferguson M. (2016). Internet competency predicts practical hearing aid knowledge and skills in first-time hearing aid users. American Journal of Audiology, 25(3S), 303–307. [DOI] [PubMed] [Google Scholar]
- Mauger S. J., Warren C. D., Knight M. R., Goorevich M., & Nel E. (2014). Clinical evaluation of the Nucleus® 6 cochlear implant system: Performance improvements with SmartSoundiQ. International Journal of Audiology, 53, 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuws M., Pascoal D., Bermejo I., Artaso M., De Ceulaer G., & Govaerts P. J. (2017). Computer-assisted CI fitting: Is the learning capacity of the intelligent agent FOX beneficial for speech understanding? Cochlear Implants International, 18(4), 198–206. [DOI] [PubMed] [Google Scholar]
- Molander P., Hesser H., Weineland S., Bergwall K., Buck S., Hansson-Malmlöf J., … Andersson G. (2015). Internet-based acceptance and commitment therapy for psychological distress experienced by people with hearing problems: Study protocol for a randomized controlled trial. American Journal of Audiology, 24(3), 307–310. [DOI] [PubMed] [Google Scholar]
- Moore A. N., Rothpletz A. M., & Preminger J. E. (2015). The effect of chronological age on the acceptance of Internet-based hearing health care. American Journal of Audiology, 24(3), 280–283. [DOI] [PubMed] [Google Scholar]
- Mosen D. M., Schmittdiel J., Hibbard J., Sobel D., Remmers C., & Bellows J. (2007). Is patient activation associated with outcomes of care for adults with chronic conditions? The Journal of Ambulatory Care Management, 30(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Peters B. R., Wyss J., & Manrique M. (2010). Worldwide trends in bilateral cochlear implantation. Laryngoscope, 120(Suppl. 2), S17–S44. [DOI] [PubMed] [Google Scholar]
- Punch R., & Hyde M. B. (2011). Communication, psychosocial, and educational outcomes of children with cochlear implants and challenges remaining for professionals and parents. International Journal of Otolaryngology, 2011, 573820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway J., Hickson L., & Lind C. (2013). Self-determination theory: Motivation and hearing aid adoption. Journal of the Academy of Rehabilitative Audiology, 46, 11–37. [Google Scholar]
- Ridgeway J., Hickson L., & Lind C. (2015). Autonomous motivation is associated with hearing aid adoption. International Journal of Audiology, 54, 478–484. [DOI] [PubMed] [Google Scholar]
- Ryan R. M., & Connell J. P. (1989). Perceived locus of causality and internalization: Examining reasons for acting in two domains. Journal of Personality and Social Psychology, 57, 749–761. [DOI] [PubMed] [Google Scholar]
- Ryan R. M., & Deci E. L. (2000). Intrinsic and extrinsic motivations: Classic definitions and new directions. Contemporary Educational Psychology, 25, 54–67. [DOI] [PubMed] [Google Scholar]
- Schneider F., van Osch L., & de Vries H. (2012). Identifying factors for optimal development of health-related websites: A Delphi study among experts and potential future users. Journal of Medical Internet Research, 14(1), e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits C., Goverts S. T., & Festen J. M. (2013). The digits-in-noise test: Assessing auditory speech recognition abilities in noise. The Journal of the Acoustical Society of America, 133(3), 1693–1706. [DOI] [PubMed] [Google Scholar]
- Swanepoel de W., & Hall J. W. III. (2010). A systematic review of telehealth applications in audiology. Telemedicine Journal and E-Health, 16(2), 181–200. [DOI] [PubMed] [Google Scholar]
- Thorén E. S., Oberg M., Wänström G., Andersson G., & Lunner T. (2014). A randomized controlled trial evaluating the effects of online rehabilitative intervention for adult hearing-aid users. International Journal of Audiology, 53(7), 452–461. [DOI] [PubMed] [Google Scholar]
- Urowitz S., Smith K., Alkazaz N., Apatu E., Quartey N. K., & Wiljer D. (2012). Patient accessible electronic heath records for the chronically ill: A review of the literature. Journal of Hospital Administration, 1(2), 64–72. [Google Scholar]
- Vaerenberg B., De Ceulaer G., Szlávik Z., Mancini P., Buechner A., & Govaerts P. J. (2014). Setting and reaching targets with computer-assisted cochlear implant fitting. Scientific World Journal, 16, 646590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerenberg B., Govaerts P. J., De Ceulaer G., Daemers K., & Schauwers K. (2011). Experiences of the use of FOX, an intelligent agent, for programming cochlear implant sound processors in new users. International Journal of Audiology, 50, 50–58. [DOI] [PubMed] [Google Scholar]
- Vermeire E., Hearnshaw H., van Royen P., & Denekens J. (2001). Patient adherence to treatment: Three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics, 26, 331–342. [DOI] [PubMed] [Google Scholar]
- Vroegop J. L., Dingemanse J. G., van der Schroeff M. P., Metselaar R. M., & Goedegebure A. (2017). Self-adjustment of upper electrical stimulation levels in CI programming and the effect on auditory functioning. Ear and Hearing, 38(4), e232–e240. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2017). Deafness and hearing loss. Retrieved from http://www.who.int/mediacentre/factsheets/fs300/en/