Abstract
Importance: When a neonate’s sucking, swallowing, and breathing are disorganized, oropharyngeal aspiration often occurs and results in illness, developmental problems, and even death. Occupational therapists who work in the neonatal intensive care unit (NICU) need to identify neonates who are at risk for aspirating so they can provide appropriate treatment.
Objective: To ascertain whether client factors and performance skills of infants ages 0–6 mo during occupational therapy feeding evaluations are related to results of videofluoroscopic swallowing studies (VFSSs).
Design: Retrospective chart reviews.
Setting: 187-bed NICU in a nonprofit teaching hospital.
Participants: A purposive sample of 334 infants ages 0–6 mo, ≥33 wk gestational age at birth, admitted to a Level II, III, or IV NICU as defined by the American Academy of Pediatrics.
Outcomes and Measures: Neonates were administered a feeding evaluation by an occupational therapist and a VFSS by a speech-language pathologist, which yielded information about client factors and performance skills.
Results: Signs and symptoms of aspiration on the evaluations were significantly associated with VFSS results. Of 310 patients, 79 had silent aspiration. Of 55 infants who demonstrated no aspiration symptoms during the feeding evaluation, 45% demonstrated aspiration symptoms on the VFSS, and 55% aspirated on the VFSS but demonstrated no symptoms of aspiration.
Conclusions and Relevance: Aspiration among infants occurs inconsistently and depends on client factors, contexts, and environments. Occupational therapists are encouraged to assess an infant’s feeding over several sessions to obtain a more accurate picture of the infant’s feeding status.
What This Article Adds: This study provides information that helps occupational therapists identify infants at risk for aspiration and make optimal recommendations regarding safe feeding practices and appropriate referrals for a VFSS. Making appropriate referrals for VFSS is also important in preventing unnecessary exposure to radiation for preterm infants.
Oral nipple feeding is a significant occupation of infants, and attainment of nutritive sucking is often the primary criterion determining an infant’s readiness for discharge from the hospital (American Academy of Pediatrics, 2008; Bertoncelli et al., 2012; Lau & Smith, 2011). Integrating sucking, swallowing, and breathing while in an appropriate behavioral state is an infant’s most highly organized performance skill (Bertoncelli et al., 2012; Tamilia et al., 2014). Swallowing safely is essential for growth and life (Rommel et al., 2014; Ulualp et al., 2013), and coordinated feeding mechanisms are essential for speech (Selley et al., 1990) as well as for an infant’s social interaction and cultural identity (AOTA, 2017). When sucking, swallowing, and breathing are disorganized, numerous difficulties can arise, including aspiration. Aspiration occurs in two ways: (1) ingestion of food, liquid, or foreign objects into the airway (oropharyngeal aspiration) and (2) movement of gastric contents into the lungs (Thach, 2007, 2008; Tutor & Gosa, 2012). In this article, we address oropharyngeal aspiration during oral nipple feeding of infants ages 0–6 mo in the neonatal intensive care unit (NICU).
Consequences and Symptoms of Aspiration
Infants who aspirate are at risk for many problems, including minor respiratory problems, chronic lung disease, pneumonia, asthma, malnutrition, neurodevelopmental problems, brain damage and other injuries, and even death (Altkorn et al., 2008; Altmann & Ozanne-Smith, 1997; Beligere & Rao, 2008; Lefton-Greif et al., 2006; Oğuzkaya et al., 1998; Prasse & Kikano, 2009; Thach, 2008; Tutor & Gosa, 2012). The presence of the laryngeal chemoreflex (LCR) in younger infants is a symptom of oropharyngeal aspiration (Thach 2007, 2008). The LCR clears the airway of younger infants and protects them from aspirating. As the LCR matures, apnea decreases, and coughing occurs or increases (Thach, 2007, 2008). Other symptoms of aspiration are gagging, hiccups, cyanosis, dyspnea, choking, apnea, bradycardia, wheezing, wet breathing, wet voice, nasal regurgitation, and noisy breathing (Rommel et al., 2014; Saki et al., 2009; Shany et al., 2002; Sheikh et al., 2001).
Not all infants cough, choke, or exhibit respiratory symptoms, however, when they aspirate (silent aspiration; Arvedson, 2008; Ayoob & Barresi, 2007; Weir et al., 2011). Silent aspiration may occur as a result of numerous client factors, including sensory and feedback deficits. Without symptoms, aspiration often goes undetected or is misdiagnosed and may eventually cause morbidity and even mortality (Shany et al., 2002). Infants with silent aspiration are at high risk for developing pneumonia and other respiratory disorders (Oğuzkaya et al., 1998; Saki et al., 2009).
Risks for Aspiration
Many risks for oropharyngeal aspiration among infants have been identified, including anatomical differences between infants and older children (John & Swischuk, 1992), deficient cough reflex in infants (Thach, 2007, 2008), and difficulty coordinating swallowing and breathing (Altmann & Ozanne-Smith, 1997; Tamilia et al., 2014). Premature infants are often intubated because of respiratory distress, pneumonia, bronchopulmonary dysplasia, or apnea, which increases their risk of aspirating (Goodwin et al., 1985; Uhm et al., 2013). Goodwin et al. (1985) reported that 80% of premature infants who have been intubated aspirate. Dysphagia often leads to aspiration (Arvedson et al., 1994; Lefton-Greif et al., 2006). Kim et al. (2014) reported supraglottic penetration and subglottic aspiration in 33% of children with dysphagia.
Disorders linked to aspiration among infants include laryngomalacia (Midulla et al., 2004), cleft palate, and craniofacial syndromes (Miller, 2011). Children with Down syndrome are reported to be prone to aspiration, and O’Neill and Richter (2013) recommended that all children with Down syndrome receive a videofluoroscopic swallowing study (VFSS). Aspiration also occurs in typically developing full-term infants, who sometimes silently aspirate without any known diagnosis (Rommel et al., 2014). According to Sheikh et al. (2001), typical infants who silently aspirate may present with unexplained and isolated respiratory problems.
Tools to Assess Aspiration
Several tools are used to diagnose aspiration, including auscultation (Frakking et al., 2013; Vice et al., 1995), radiography (Tutor & Gosa, 2012), fiberoptic endoscopic evaluation of swallow (Ulualp et al., 2013), automated impedance manometry (Rommel et al., 2014), ultrasound (Arvedson, 2008), the Blue Dye Test (Altkorn et al., 2008; John & Swischuk, 1992), and VFSS or modified barium swallow (Kim et al., 2014; López et al., 2014; Noll et al., 2011; Silva-Munhoz et al. 2015).
VFSS is the gold standard for assessing swallowing disorders (Arvedson, 2008). Information from a VFSS includes whether food enters the airway rather than the stomach, which oropharyngeal components function incorrectly, which foods are safe to swallow, and which strategies facilitate swallowing (American Speech-Language-Hearing Association, n.d.). A VFSS helps occupational therapists determine the most effective way to feed an infant. Despite the benefits of VFSS, its use is controversial because it is invasive (Rommel et al., 2014) and can cause harm. VFSS involves exposure to radiation, and the possible long-term effects of radiation on infants are a concern. Hall (2002, 2006) reported that children are more sensitive to radiation-induced cancer than adults, with reported exposure resulting in leukemia, breast cancer, and developmental delay. Infants are often not referred for VFSS until they present with a serious respiratory illness (Noll et al., 2011). Occupational therapists need evidence to identify infants ages 0–6 mo in the NICU who would benefit from having a VFSS. Therefore, the relation of VFSS results to client factors and performance skills assessed during oral feeding evaluations by occupational therapists needs to be determined.
Purpose
The purpose of this study was to ascertain whether clinical indictors of aspiration observed by occupational therapists during oral nipple feeding evaluations of infants ages 0–6 mo admitted to a Level II, III, or IV NICU after birth were significantly associated with aspiration during a VFSS. With better identification of the client factors associated with the results of a VFSS, occupational therapists can help prevent infants who do not need a VFSS from being exposed to radiation. The research question was “Which factors in occupational therapy oral feeding evaluations are associated with a diagnosis of aspiration on the VFSS?”
Method
We conducted a retrospective chart review of 334 infants who met the following inclusion criteria: ages 0–6 mo; admitted after birth to a Level II, III, or IV NICU as described and defined by the American Academy of Pediatrics (2012); had a gestational age (GA) ≥33 wk; and had a feeding evaluation by an occupational therapist and a VFSS by a speech-language pathologist. GA was defined as age at birth from last known menstrual cycle calculated in days. No early preterm (<32 wk) infants were included because oral feeds are initiated later. Only data obtained from the infant’s first feed with an occupational therapist were collected and analyzed. Data from additional feeding sessions with an occupational therapist were excluded.
We used International Classification of Diseases, 10th Version (World Health Organization [WHO], 2016) codes to obtain the purposive sample. Client factors and performance skills examined included demographics; medical diagnosis; behavioral state; muscle tone; gag reflex; coordination of suck, swallow, and breathing; tongue function; aspiration signs and symptoms (apnea, bradycardia, choking, coughing, gagging, increased nasopharyngeal congestion, tachycardia, tachypnea, wet breath sounds); wet chest after feeds; wet voice after feeds; signs and symptoms of reflux (apnea, arching, avoidance behaviors, bradycardia, choking, congestion, emesis, frequent pulls away from nipple, gagging, increased nasopharyngeal congestion, increased irritability, oral aversion, poor weight gain, wet burps, wet hiccups, other); and changes in vital signs during feeding. A copy of the occupational therapy (OT) feeding evaluation may be obtained from O. Jayne Bowman. Three data collectors (graduate occupational therapy students) were trained to record data from medical records and to code and enter the data into the database. Raters obtained 85% agreement with the primary trainer (Rose Toruno) before initiating data collection. Agreement between data collectors and the trainer ranged from 87% to 95%. Approval was obtained from the appropriate institutional review boards.
Results
We used the χ2 test to examine bivariate associations of categorical variables with aspiration and Wilcoxon rank-sum and Mann–Whitney U tests to investigate associations with quantitative variables. Logistic regression was used to investigate multivariate associations of risk factors with presence of aspiration. All candidate predictors exhibiting a statistically significant (α = .05) bivariate association with aspiration were included in the multivariate logistic regression model. We used SAS (Version 9.4; SAS Institute Inc., Cary, NC) for all data analyses except the receiver operating characteristic (ROC) curve analysis, which was performed using IBM SPSS Statistics (Version 23; IBM Corporation, Armonk, New York). A power analysis using the Fleiss et al. (1980) method indicated that 117 patients with aspiration present and 172 patients without aspiration would provide 81% statistical power to detect a true odds ratio of 2.00.
Three hundred thirty-four infants met the inclusion criteria, with 136 (41%) diagnosed with aspiration and 195 (59%) not diagnosed with aspiration (aspiration data were missing for 3). The number of preterm infants who met the inclusion criteria was 167 (50%; Table 1). The bivariate odds ratio indicated no statistical differences between middle preterm infants (GA 32 wk, 0 days–33 wk, 6 days) and late preterm infants (GA 34 wk, 0 days–36 wk, 6 days). Silent aspiration data were available for 310 (93%) of the 334 patients, of whom 79 (25%) had silent aspiration. The majority of patients had respiratory disorders (72%) and congenital heart anomalies of the sternum (69%).
Table 1.
Client Factors (N = 334)
| Client Factor | n (%) |
| Gender (N = 333) | |
| Male | 189 (57) |
| Female | 144 (43) |
| Ethnicity (N = 313) | |
| White/non-Hispanic | 111 (35) |
| White/Hispanic | 52 (17) |
| Black | 129 (41) |
| Other | 21 (7) |
| Gestational age at birth (N = 334) | |
| <37 wk | 167 (50) |
| ≥37 wk | 167 (50) |
| Aspiration (N = 331) | |
| Present | 136 (41) |
| Absent | 195 (59) |
| History of intubation | 112 (34) |
| Diagnosesa (N = 334) | |
| Intraventricular hemorrhage | 53 (16) |
| Hydrocephalous | 32 (10) |
| Hypoxi-ischemic encephalopathy | 13 (4) |
| Genetic disorder | 80 (24) |
| Metabolic mitochondrial disorder | 14 (4) |
| Sternum congenital heart anomaly | 230 (69) |
| Thoracic congenital heart anomaly | 64 (19) |
| Respiratory disorder | 242 (72) |
| GI disorder | 162 (49) |
| Transesophageal echocardiogram | 4 (1) |
| Stridor (high-pitched breathing) | 55 (16) |
| Tracheomalacia | 15 (5) |
| Vocal cord paralysis or paresis | 48 (14) |
| Craniofacial anomalies | 42 (13) |
| Pneumonia or atelectasis | 81 (24) |
Note. Ns for each client factor differ because the number of missing responses for each differed. GI = gastrointestinal.
Patients could have multiple diagnoses.
In the bivariate analysis, aspiration signs and symptoms (χ2 = 6.1, p = .009), other diagnoses (χ2 = 8.3, p = .004), genetic disorders (χ2 = 6.2, p = .013), vocal cord paralysis or paresis (χ2 = 5.5, p = .019), history of intubation (χ2 = 11.5, p < .001), thoracic congenital heart anomalies (χ2 = 7.0, p = .008), and pneumonia or atelectasis (χ2 = 12.4, p < .001) were all significantly associated with increased risk of aspiration. However, in the multivariate analysis, only aspiration signs and symptoms (χ2 = 5.6, p = .018), history of intubation (χ2 = 5.8, p = .016), and pneumonia or atelectasis (χ2 = 5.1, p = .024) significantly increased the risk of aspiration after adjusting for the other covariates in the logistic regression model (Table 2).
Table 2.
Bivariate and Multivariate Associations With Aspiration
| Risk Factor | Unadjusted | Adjusted | ||
| OR [95% CI] | p | OR [95% CI] | p | |
| Aspiration signs & symptoms | 1.76 [1.10, 2.83] | .020* | 1.84 [1.11, 3.04] | .018* |
| Other diagnoses | 1.94 [1.12, 3.37] | .018* | 1.78 [0.99, 3.21] | .055 |
| Genetic disorder | 1.88 [1.10, 3.22] | .021* | 1.48 [0.81, 2.72] | .207 |
| Vocal cord paralysis or paresis | 2.08 [1.08, 4.000] | .029* | 1.94 [0.97, 3.86] | .060 |
| History of intubation | 2.35 [1.38, 3.99] | .002* | 2.00 [1.14, 3.52] | .016* |
| Thoracic congenital heart anomaly | 2.03 [1.11, 3.71] | .021* | 1.47 [0.74, 2.91] | .268 |
| Pneumonia or atelectasis | 2.27 [1.33, 3.87] | .003* | 1.92 [1.09, 3.39] | .024* |
Note. CI = confidence interval; OR = odds ratio.
Statistically significant association at α = .05.
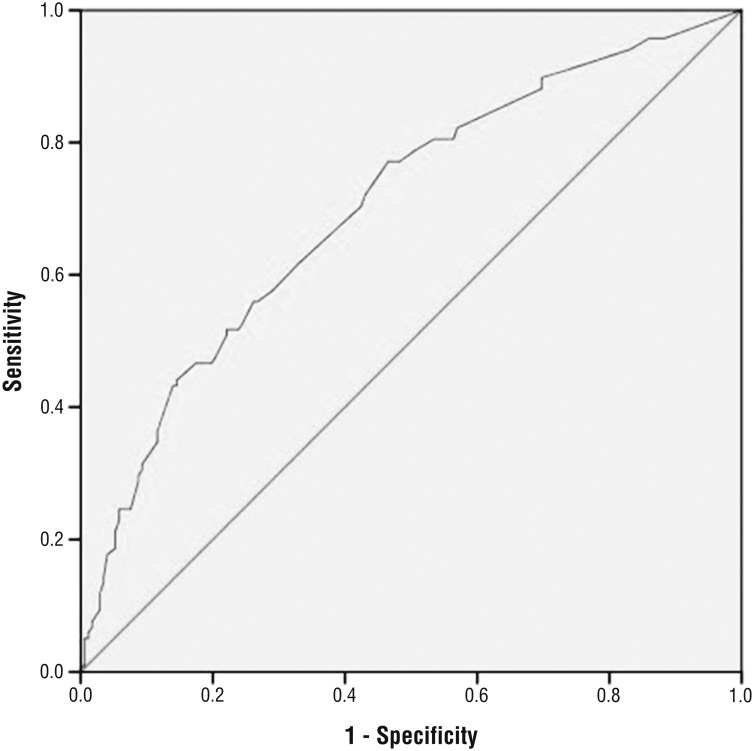
The fitted multivariate logistic regression model contributed significantly to prediction of the presence of aspiration (χ2 = 35.6, p < .001), with 70% of the area under the ROC curve (Figure 1). The Hosmer–Lemeshow goodness-of-fit test did not indicate a significant lack of fit for the multivariate logistic regression model (χ2 = 4.3, p = .746). The highest overall accuracy (66%) of the fitted logistic regression model in predicting the presence of aspiration was obtained by using a cutoff that considered any patient with a predicted probability of aspiration higher than 46% as positive and any patient with a predicted probability of aspiration of 46% or lower as negative. This cutoff yielded a sensitivity of 52%, specificity of 76%, a false-positive rate of 41%, and a false-negative rate of 31%.
Figure 1.
Receiver operating characteristic curve showing sensitivity and specificity when using estimated probabilities from the multivariable regression model to predict aspiration.
Fifty-eight infants who did not demonstrate overt signs and symptoms of aspiration during the OT evaluation aspirated during the VFSS. Of those 58 infants, 55 had data available on signs and symptoms of aspiration during the VFSS. Of these 55 infants who did not demonstrate overt signs and symptoms of aspiration on the OT evaluation, 25 (45%) demonstrated positive signs and symptoms of aspiration during the VFSS, and 30 (55%) consistently demonstrated no signs and symptoms of aspiration despite the presence of aspiration on the VFSS. Moreover, in the total data set, 79 infants (24%) demonstrated silent aspiration during the VFSS.
Discussion
In the bivariate analysis, aspiration signs and symptoms from the OT feeding evaluations were significantly associated with increased risk of aspiration and were also significant in the multivariate logistic regression model, thus answering the research question. However, the sensitivity (52%), specificity (76%), positive predictive value (48%), and negative predictive value (66%) were fairly low, with an overall accuracy of 57%.
Much literature on aspiration in infants is available; however, we were unable to locate any that examined associations between performance of infants ages 0–6 mo in the NICU on occupational therapy feeding evaluations and on VFSS. This study helps fill that void. Numerous methods to identify feeding problems are available, but the clinician must select the instrument that is most useful (Martin-Harris & Jones, 2008). The VFSS is the instrument of choice of most clinicians who treat swallowing disorders because it provides information that helps to confirm a diagnosis of aspiration and helps develop treatment plans. VFSS results can also help document improvement in swallowing. The importance of feeding problems, including aspiration, as a topic for occupational therapists to investigate cannot be overemphasized. Arvedson (2008) reported that the incidence of feeding disorders is between 25% and 45% among typically developing children and as many as 80% in children with developmental disabilities. WHO’s (2012) report revealed that feeding disorders are a worldwide problem.
Intubation was statistically significant in the multivariate analysis. It is difficult to determine whether post-extubation played a role in causing aspiration because of issues such as subglottic edema or vocal cord injury or whether the acuity of illness requiring intubation led to fragility and weakness in those infants, resulting in aspiration. Although the multivariate analysis found that pneumonia and atelectasis were significant factors, we cannot determine whether pneumonia occurred before the feeding evaluation and VFSS or whether the severity of illness in those infants led to aspiration.
Regarding infants who aspirated during the VFSS but who demonstrated no overt signs and symptoms of aspiration, the signs and symptoms manifested by many infants differed during the OT evaluation compared with the VFSS. More than half (55%) of infants who demonstrated no overt signs and symptoms of aspiration during the OT evaluation or the VFSS aspirated during the VFSS. Of the 334 infants in the data set, 79 (24%) demonstrated silent aspiration during the VFSS. In their study of infants diagnosed with dysphagia, Newman et al. (2001) reported that 81% exhibited silent aspiration on a VFSS. In addition to having a larger sample size, the current study included infants with clinical signs of aspiration and those without, perhaps accounting for the difference.
This study’s results are consistent with the literature that aspiration in infants varies from feed to feed, depending on the interaction of client factors (such as the infant’s behavioral state, degree of fatigue, extent of hunger, feeding position, gastrointestinal conditions), textures of food and drink, temporal context, and environments. The results are also consistent with occupational therapists’ emphasis that performance skills depend on the dynamic interaction among client, context, and activity (AOTA, 2014). Although the patients’ OT evaluations and the VFSS were conducted during the same hospitalization, the physical and social environments of a VFSS and a bedside feeding evaluation by an occupational therapist differ. Those differences can affect the outcomes of VFSS and occupational therapy feeding evaluations. Because aspiration in infants ages 0–6 mo in the NICU is inconsistent in nature, more precise comparison of occupational therapists’ findings with those of VFSS requires that occupational therapy evaluations occur concurrently with VFSS to control for context and environment.
Limitations
Limitations of this study include that the analyses included only data from infants 0–6 mo who had a GA of ≥33 wk; who were admitted to a Level II, III, or IV NICU after birth; and who received a feeding evaluation by an occupational therapist and a VFSS from a speech-language pathologist during the same hospitalization and from an infant’s first OT feeding evaluation.
The broad diagnostic categories used in the analyses may have diluted the risk of aspiration for a specific diagnosis. For example, all infants with intraventricular hemorrhage were included in a single diagnostic category; therefore, we cannot determine whether an infant with a Grade 4 intraventricular hemorrhage has a higher risk for aspiration than an infant with a Grade 1 intraventricular hemorrhage. Moreover, the signs and symptoms of aspiration on the feeding evaluation were grouped for the analysis; therefore, we cannot ascertain whether a specific sign or symptom was associated with aspiration during a VFSS.
Implications for Occupational Therapy Practice
Feeding is the most significant occupation in which infants 0–6 mo engage, and oropharyngeal aspiration is a frequent problem encountered when feeding infants in the NICU. The gold standard for diagnosing aspiration is the VFSS, which is invasive and may have long-term negative effects on infants because of their sensitivity to radiation. The results of this study have the following implications for occupational therapy practice:
Feeding evaluations of infants in the NICU must be conducted over several sessions, and occupational therapists must be vigilant in identifying the presence of aspiration over time.
Occupational therapists must educate parents of at-risk infants in the signs and symptoms of aspiration and in safe feeding practices so caregivers can help ensure the safety of their children after discharge.
Signs and symptoms of aspiration from OT feeding evaluations were significantly related to VFSS results. This information can help occupational therapists make optimal recommendations regarding safe feeding practices and appropriate referrals for a VFSS.
When conducting feeding evaluations, occupational therapists may need to give special attention to infants who have a history of intubation, pneumonia, or atelectasis, regardless of the child’s diagnosis.
Conclusion
Aspiration in infants in the NICU may not occur with every feed because occupational performance depends on the dynamic interaction of the client’s factors, textures ingested, temporal context, and social and physical environments of the feeding session. Current practice is to promote early achievement of feeding and quick discharge of infants to minimize costs. This may place infants who aspirate inconsistently at risk and lead to hospital readmissions. Ultimately, this practice may be more costly and leads to greater morbidity among infants.
It is recommended that occupational therapists who evaluate feeding of infants in NICUs obtain information over several feeding sessions. Moreover, in addition to teaching caregivers of high-risk infants appropriate feeding techniques, occupational therapists must educate them to recognize signs and symptoms of aspiration. Roper and David (1987) stated that the sharp decline in deaths of infants younger than age 3 mo from choking in England and Wales resulted from educating caregivers in infant feeding practices. That decline suggests that a reduction in the morbidity and mortality of infants in the United States from aspiration may also be possible if occupational therapists educate caregivers about best feeding practices.
Because of their unique education and training, occupational therapists who work in the NICU are in an excellent position to take a health promotion and risk prevention approach by identifying and treating aspiration in at-risk infants and by educating caregivers. That approach may help reduce the morbidity and mortality of infants not only during hospital stays but also after discharge. Helping infants improve their feeding skills and patterns will also facilitate their engagement in the occupations of play participation and play exploration, which are requisite for their development.
Acknowledgments
We thank Marilyn Goff, certified medical librarian, at Texas Woman's University in Houston for her assistance with the references; Amy Ciatto, OTR, for her assistance with initial pilot work related to the project; graduate occupational therapy students from Texas Woman’s University for their assistance in chart review for data collection; and Texas Children's Hospital Physical Medicine and Rehabilitation leadership for providing support in scheduling to conduct the study.
References
- Altkorn R., Chen X., Milkovich S., Stool D., Rider G., Bailey C. M., . . . Reilly J. S. (2008). Fatal and non-fatal food injuries among children (aged 0-14 years). International Journal of Pediatric Otorhinolaryngology, 72, 1041–1046. 10.1016/j.ijporl.2008.03.010 [DOI] [PubMed] [Google Scholar]
- Altmann A., & Ozanne-Smith J. (1997). Non-fatal asphyxiation and foreign body ingestion in children 0–14 years. Injury Prevention, 3, 176–182. 10.1136/ip.3.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Fetus and Newborn. (2008). Hospital discharge of the high-risk neonate. Pediatrics, 122, 1119–1126. 10.1542/peds.2008-2174 [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Fetus and Newborn. (2012). Levels of neonatal care. Pediatrics, 130, 587–597. 10.1542/peds.2012-1999 [DOI] [PubMed] [Google Scholar]
- American Occupational Therapy Association. (2014). Occupational therapy practice framework: Domain and process (3rd ed). American Journal of Occupational Therapy, 68(Suppl. 1), S1–S48. 10.5014/ajot.2014.682006 [DOI] [PubMed] [Google Scholar]
- American Occupational Therapy Association. (2017). The practice of occupational therapy in feeding, eating, and swallowing. American Journal of Occupational Therapy, 71(Suppl. 2), 7112410015 10.5014/ajot.2017.716S04 [DOI] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association. (n.d).Videofluoroscopic swallowing study (VFSS): Do you have problems swallowing? Retrieved from http://www.asha.org/public/speech/swallowing/Videofluoroscopic-Swallowing-Study/
- Arvedson J. C. (2008). Assessment of pediatric dysphagia and feeding disorders: Clinical and instrumental approaches. Developmental Disabilities Research Reviews, 14, 118–127. 10.1002/ddrr.17 [DOI] [PubMed] [Google Scholar]
- Arvedson J., Rogers B., Buck G., Smart P., & Msall M. (1994). Silent aspiration prominent in children with dysphagia. International Journal of Pediatric Otorhinolaryngology, 28, 173–181. 10.1016/0165-5876(94)90009-4 [DOI] [PubMed] [Google Scholar]
- Ayoob K. T., & Barresi I. (2007). Feeding disorders in children: Taking an interdisciplinary approach. Pediatric Annals, 36, 478–483. 10.3928/0090-4481-20070801-09 [DOI] [PubMed] [Google Scholar]
- Beligere N., & Rao R. (2008). Neurodevelopmental outcome of infants with meconium aspiration syndrome: Report of a study and literature review. Journal of Perinatology, 28(Suppl. 3), S93–S101. 10.1038/jp.2008.154 [DOI] [PubMed] [Google Scholar]
- Bertoncelli N., Cuomo G., Cattani S., Mazzi C., Pugliese M., Coccolini E., . . . Ferrari F. (2012). Oral feeding competences of healthy preterm infants: A review. International Journal of Pediatrics, 2012, 896257 10.1155/2012/896257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss J. L., Tytun A., & Ury H. K. (1980). A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics, 36, 343–346. 10.2307/2529990 [DOI] [PubMed] [Google Scholar]
- Frakking T. T., Chang A. B., O’Grady K. A., Walker-Smith K., & Weir K. A. (2013). Cervical auscultation in the diagnosis of oropharyngeal aspiration in children: A study protocol for a randomised controlled trial. Trials, 14, 377 10.1186/1745-6215-14-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S. R., Graves S. A., & Haberkern C. M. (1985). Aspiration in intubated premature infants. Pediatrics, 75, 85–88. [PubMed] [Google Scholar]
- Hall E. J. (2002). Lessons we have learned from our children: Cancer risks from diagnostic radiology. Pediatric Radiology, 32, 700–706. 10.1007/s00247-002-0774-8 [DOI] [PubMed] [Google Scholar]
- Hall E. J. (2006). Intensity-modulated radiation therapy, protons, and the risk of second cancers. International Journal of Radiation Oncology, Biology, Physics, 65, 1–7. 10.1016/j.ijrobp.2006.01.027 [DOI] [PubMed] [Google Scholar]
- John S. D., & Swischuk L. E. (1992). Stridor and upper airway obstruction in infants and children. Radiographics, 12, 625–643, discussion 644. 10.1148/radiographics.12.4.1636030 [DOI] [PubMed] [Google Scholar]
- Kim B. R., Sung I. Y., Choi K. H., Kim L. S., & Ryu J. S. (2014). Long-term outcomes in children with swallowing dysfunction. Developmental Neurorehabilitation, 17, 298–305. 10.3109/17518423.2013.770102 [DOI] [PubMed] [Google Scholar]
- Lau C., & Smith E. O. (2011). A novel approach to assess oral feeding skills of preterm infants. Neonatology, 100, 64–70. 10.1159/000321987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefton-Greif M. A., Carroll J. L., & Loughlin G. M. (2006). Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatric Pulmonology, 41, 1040–1048. 10.1002/ppul.20488 [DOI] [PubMed] [Google Scholar]
- López C. P., Chiari B. M., Goulart A. L., Furkim A. M., & Guedes Z. C. (2014). Assessment of swallowing in preterm newborns fed by bottle and cup. CoDAS, 26, 81–86. 10.1590/s2317-17822014000100012 [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., & Jones B. (2008). The videofluorographic swallowing study. Physical Medicine and Rehabilitation Clinics of North America, 19, 769–785,viii. 10.1016/j.pmr.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midulla F., Guidi R., Tancredi G., Quattrucci S., Ratjen F., Bottero S., . . . Cutrera R. (2004). Microaspiration in infants with laryngomalacia. Laryngoscope, 114, 1592–1596. 10.1097/00005537-200409000-00017 [DOI] [PubMed] [Google Scholar]
- Miller C. K. (2011). Feeding issues and interventions in infants and children with clefts and craniofacial syndromes. Seminars in Speech and Language, 32, 115–126. 10.1055/s-0031-1277714 [DOI] [PubMed] [Google Scholar]
- Newman L. A., Keckley C., Petersen M. C., & Hamner A. (2001). Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatrics, 108, E106 10.1542/peds.108.6.e106 [DOI] [PubMed] [Google Scholar]
- Noll L., Rommel N., Davidson G. P., & Omari T. I. (2011). Pharyngeal flow interval: A novel impedance-based parameter correlating with aspiration. Neurogastroenterology and Motility, 23, 551–e206. 10.1111/j.1365-2982.2010.01634.x [DOI] [PubMed] [Google Scholar]
- Oğuzkaya F., Akçali Y., Kahraman C., Bilgin M., & Sahin A. (1998). Tracheobronchial foreign body aspirations in childhood: A 10-year experience. European Journal of Cardio-Thoracic Surgery, 14, 388–392. 10.1016/S1010-7940(98)00205-X [DOI] [PubMed] [Google Scholar]
- O’Neill A. C., & Richter G. T. (2013). Pharyngeal dysphagia in children with Down syndrome. Otolaryngology—Head and Neck Surgery, 149, 146–150. 10.1177/0194599813483445 [DOI] [PubMed] [Google Scholar]
- Prasse J. E., & Kikano G. E. (2009). An overview of pediatric dysphagia. Clinical Pediatrics, 48, 247–251. 10.1177/0009922808327323 [DOI] [PubMed] [Google Scholar]
- Rommel N., Selleslagh M., Hoffman I., Smet M. H., Davidson G., Tack J., & Omari T. I. (2014). Objective assessment of swallow function in children with suspected aspiration using pharyngeal automated impedance manometry. Journal of Pediatric Gastroenterology and Nutrition, 58, 789–794. 10.1097/MPG.0000000000000337 [DOI] [PubMed] [Google Scholar]
- Roper H. P., & David T. J. (1987). Decline in deaths from choking on food in infancy: An association with change in feeding practice? Journal of the Royal Society of Medicine, 80, 2–3. 10.1177/014107688708000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki N., Nikakhlagh S., Rahim F., & Abshirini H. (2009). Foreign body aspirations in infancy: A 20-year experience. International Journal of Medical Sciences, 6, 322–328. 10.7150/ijms.6.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley W. G., Ellis R. E., Flack F. C., & Brooks W. A. (1990). Coordination of sucking, swallowing and breathing in the newborn: Its relationship to infant feeding and normal development. British Journal of Disorders of Communication, 25, 311–327. 10.3109/13682829009011980 [DOI] [PubMed] [Google Scholar]
- Shany E., Greenberg D., & Shahak E. (2002). Infant choking or the “A” of resuscitation. Israel Medical Association Journal, 4, 136–137. [PubMed] [Google Scholar]
- Sheikh S., Allen E., Shell R., Hruschak J., Iram D., Castile R., & McCoy K. (2001). Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest, 120, 1190–1195. 10.1378/chest.120.4.1190 [DOI] [PubMed] [Google Scholar]
- Silva-Munhoz L. F., Bühler K. E., & Limongi S. C. (2015). Comparison between clinical and videofluoroscopic evaluation of swallowing in children with suspected dysphagia. CoDAS, 27, 186–192. 10.1590/2317-1782/20152014149 [DOI] [PubMed] [Google Scholar]
- Tamilia E., Taffoni F., Formica D., Ricci L., Schena E., Keller F., & Guglielmelli E. (2014). Technological solutions and main indices for the assessment of newborns’ nutritive sucking: A review. Sensors, 14, 634–658. 10.3390/s140100634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach B. T. (2007). Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulmonary Pharmacology and Therapeutics, 20, 365–370. 10.1016/j.pupt.2006.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach B. T. (2008). Some aspects of clinical relevance in the maturation of respiratory control in infants. Journal of Applied Physiology, 104, 1828–1834. 10.1152/japplphysiol.01288.2007 [DOI] [PubMed] [Google Scholar]
- Tutor J. D., & Gosa M. M. (2012). Dysphagia and aspiration in children. Pediatric Pulmonology, 47, 321–337. 10.1002/ppul.21576 [DOI] [PubMed] [Google Scholar]
- Uhm K. E., Yi S. H., Chang H. J., Cheon H. J., & Kwon J. Y. (2013). Videofluoroscopic swallowing study findings in full-term and preterm infants with dysphagia. Annals of Rehabilitation Medicine, 37, 175–182. 10.5535/arm.2013.37.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulualp S., Brown A., Sanghavi R., & Rivera-Sanchez Y. (2013). Assessment of laryngopharyngeal sensation in children with dysphagia. Laryngoscope, 123, 2291–2295. 10.1002/lary.24024 [DOI] [PubMed] [Google Scholar]
- Vice F. L., Bamford O., Heinz J. M., & Bosma J. F. (1995). Correlation of cervical auscultation with physiological recording during suckle-feeding in newborn infants. Developmental Medicine and Child Neurology, 37, 167–179. 10.1111/j.1469-8749.1995.tb11986.x [DOI] [PubMed] [Google Scholar]
- Weir K. A., McMahon S., Taylor S., & Chang A. B. (2011). Oropharyngeal aspiration and silent aspiration in children. Chest, 140, 589–597. 10.1378/chest.10-1618 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2012). Born too soon: The global action report on preterm birth. Geneva: Author. Retrieved from http://www.who.int/maternal_child_adolescent/documents/born_too_soon/en/ [Google Scholar]
- World Health Organization. (2016). International classification of diseases, 10th version. Geneva: Author. [Google Scholar]