Abstract
Importance: Poor outcomes after upper extremity peripheral nerve injury (PNI) may arise, in part, from the challenges and complexities of cortical plasticity. Occupational therapy practitioners need to understand how the brain changes after peripheral injury and how principles of cortical plasticity can be applied to improve rehabilitation for clients with PNI.
Objective: To identify the mechanisms of cortical plasticity after PNI and describe how cortical plasticity can contribute to rehabilitation.
Data Sources: PubMed and Embase (1900–2017) were searched for articles that addressed either (1) the relationship between PNI and cortical plasticity or (2) rehabilitative interventions based on cortical plastic changes after PNI.
Study Selection and Data Collection: PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. Articles were selected if they addressed all of the following concepts: human PNI, cortical plasticity, and rehabilitation. Phantom limb pain and sensation were excluded.
Findings: Sixty-three articles met the study criteria. The most common evidence level was Level V (46%). We identified four commonly studied mechanisms of cortical plasticity after PNI and the functional implications for each. We found seven rehabilitative interventions based on cortical plasticity: traditional sensory reeducation, activity-based sensory reeducation, selective deafferentation, cross-modal sensory substitution, mirror therapy, mental motor imagery, and action observation with simultaneous peripheral nerve stimulation.
Conclusion and Relevance: The seven interventions ranged from theoretically well justified (traditional and activity-based sensory reeducation) to unjustified (selective deafferentation). Overall, articles were heterogeneous and of low quality, and future research should prioritize randomized controlled trials for specific neuropathies, interventions, or cortical plasticity mechanisms.
What This Article Adds: This article reviews current knowledge about how the brain changes after PNI and how occupational therapy practitioners can take advantage of those changes for rehabilitation.
Peripheral nerve injury (PNI) in the upper extremity significantly affects functional performance in daily life (Bruyns et al., 2003). Because of the lost capability of the affected hand, clients with PNI often have trouble with activities of daily living, social interaction, self-expression, and interaction with the environment (Stonner et al., 2017). PNI can have a lifelong economic and emotional impact, including inability to work (Jaquet et al., 2001) and reduced quality of life (Lundborg & Rosén, 2007). Despite tremendous advances in neurosurgery, neuroscience, and rehabilitative treatment of PNI, clients seldom return to their full level of prior function. As many as 41% of people with upper extremity PNI fail to return to work within 2 yr, compared with 4% of people with traumatic hand injury (Bruyns et al., 2003; Opsteegh et al., 2009). Clients with PNI also score low on quality of life measures because of inability to complete activities of daily living and participate in other desired occupations (Jerosch-Herold, 2011).
Cortical plastic mechanisms may influence sensory and motor outcomes after PNI (Björkman et al., 2016). Altered or reduced afferent input leads to alteration of the corresponding body parts’ cortical representations, in a process called cortical plasticity (Kaas, 1991). Moreover, the loss of upper extremity function can interfere with healthy changes in cortical representations of body parts (Chen et al., 2002). Maladaptive plasticity refers to cortical plasticity that contributes to poor sensory and motor recovery outcomes (Flor et al., 2006). Knowledge of cortical plasticity mechanisms after PNI can enable clinicians to manipulate these alterations to clients’ benefit and presents new avenues for clinical research (Ferreri et al., 2014).
To better understand how researchers and rehabilitation clinicians are applying cortical plasticity principles for clients with upper extremity PNI, we completed a state-of-the-science scoping review to (1) identify and summarize the current body of evidence on cortical plastic changes after PNI and their relation to functional outcomes and recovery and (2) identify cortical plasticity–based interventions used in rehabilitative settings. In this review, we give clinicians a brief synopsis and reference guide to the basic principles of cortical plasticity after PNI and potential sensorimotor interventions based on these principles.
Method
We developed our scoping review with a four-stage approach: concept development, scope of search, study selection, and data extraction and analysis (Levac et al., 2010).
Development of Concepts
Concept 1: Upper Extremity Peripheral Nerve Injury and Cortical Plasticity.
The aim of Concept 1 was to identify the cortical plastic changes that occur after upper extremity PNI. Nerve injuries included in the scope of study were complete nerve avulsion, hand transplantation, hand replantation, nerve transfers, amputations, generalized PNI (lacerations or lesions), brachial plexus injury, nerve grafts, and any nerve compression or impingement condition. The search was not limited by nerve identity as long as other criteria were met (e.g., upper extremity).
Concept 2: Rehabilitative Interventions and Strategies Based on Cortical Plastic Changes After Peripheral Nerve Injury.
Concept 2 had two aims. The first was to identify how the mechanism of cortical plasticity affects sensory and motor function after PNI, and the second was to identify how the mechanism of cortical plasticity influences rehabilitative strategies for occupational therapy practitioners.
Scope of Search
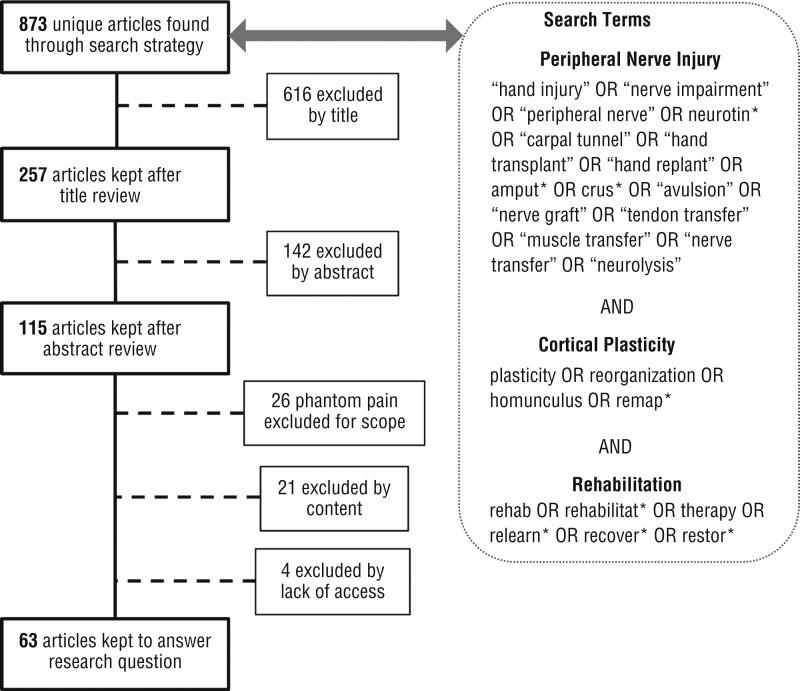
We searched for articles that included all three of the following constructs: PNI, cortical plasticity, and rehabilitation. The PubMed database was chosen for its coverage of biomedical research, and the Embase database was added for its additional focus on clinical and applied medicine. Duplicate articles were omitted. Search terms are listed in Figure 1. Phantom limb phenomena (pain and sensation) were considered to be outside the scope of this review.
Figure 1.
Flow diagram for inclusion and exclusion of studies for the scoping review, with search terms.
Note. Figure format from “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement,” by D. Moher, A. Liberati, J. Tetzlaff, and D. G. Altman; PRISMA Group, 2009, PLoS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
Study Selection
Studies needed to be written in English, involve human participants, not include pharmacological interventions, not include central nervous system injury, not include cranial nerve injury, examine people with upper extremity PNI exclusively, and be accessible. Studies in which pain was the primary outcome (rather than sensorimotor function or plasticity) were omitted because pain has been adequately reviewed elsewhere (Osborne et al., 2018).
All study designs were eligible. For an article to be included, the two reviewers (Zink and a graduate research assistant) had to identify it as meeting the study criteria at each stage. For articles they disagreed on (6.9%), the reviewers conferred and revised their criteria until they reached 100% agreement.
Our search strategy generated 873 unique articles between the PubMed (779) and Embase (94) databases. We reduced this total to 63 articles over the steps shown in Figure 1. We excluded 810 articles for the following reasons: animal or nonhuman study (204), no PNI (181), no cortical plasticity (127), pharmacological study (82), pain outcome study (43), lower extremity study (60), spinal cord injury study (42), phantom limb pain or sensation study (26), stroke study (12), optic nerve study (10), Parkinson’s or movement disorders study (6), facial nerve study (6), traumatic brain injury study (5), no access to article (4), and auditory study (2).
Data Extraction and Analysis
The two reviewers identified the evidence level and study design for the 64 included articles using the American Journal of Occupational Therapy’s guidelines for systematic reviews (American Occupational Therapy Association, 2017; based on Sackett et al., 1996).
Results
We found 63 articles pertaining to our research question (Table 1): 25 articles for Concept 1 only (39%), 3 articles for Concept 2 only (5%), and 35 articles (56%) for both concepts. Regarding evidence level, the articles described 2 Level I (3%), 13 Level II (21%), 9 Level III (14%), 10 Level IV (16%), and 29 Level V (46%) studies.
Table 1.
Evidence Table for the Scoping Review on Cortical Plasticity in Rehabilitation for Upper Extremity Peripheral Nerve Injury (N = 64)
| Citation | Evidence Level and Study Design | Concept 1: Cortical Plastic Changes | Concept 2: Rehabilitative Interventions |
| Abrams & Widenfalk (2005) | Level V Narrative review |
NA |
|
| Addas & Midha (2009) | Level V Narrative review |
|
|
| Anastakis et al. (2005) | Level II Two groups, nonrandomized |
|
NA |
| Anastakis et al. (2008) | Level V Narrative review |
|
|
| Baysefer et al. (2004) | Level III One group, nonrandomized |
|
NA |
| Beaulieu et al. (2006) | Level III One group, nonrandomized |
|
NA |
| Bhat et al. (2017) | Level II Two groups, nonrandomized |
|
NA |
| Bisio et al. (2015) | Level III One group, nonrandomized |
NA |
|
| Björkman et al. (2016) | Level III One group, nonrandomized |
|
|
| Bolognini et al. (2015) | Level V Narrative review |
|
|
| Brenneis et al. (2005) | Level IV Case series |
|
NA |
| Brown et al. (2009) | Level V Narrative review |
|
|
| Chemnitz et al. (2013) | Level II Two groups, nonrandomized |
|
NA |
| Chemnitz et al. (2015) | Level III One group, nonrandomized |
|
NA |
| Chen et al. (2002) | Level V Narrative review |
|
|
| Dahlin (2013) | Level V Narrative review |
|
|
| Dahlin et al. (2017) | Level V Case study |
|
NA |
| Duff (2005) | Level V Narrative review |
|
|
| Eickhoff et al. (2008) | Level II Two groups, nonrandomized |
|
|
| Elbert et al. (1994) | Level III One group, nonrandomized |
|
NA |
| Elbert & Rockstroh (2004) | Level V Narrative review |
|
|
| Ferreri et al. (2014) | Level V Narrative review |
|
NA |
| Flor (2003) | Level V Narrative review |
|
NA |
| Fornander et al. (2016) | Level II Two groups, nonrandomized |
|
|
| Fornander et al. (2010) | Level III One group, nonrandomized |
|
|
| Gao et al. (2005) | Level III One group, nonrandomized |
|
NA |
| Giraux et al. (2001) | Level V Case study |
|
NA |
| Grisold et al. (2007) | Level V Narrative review |
|
|
| Hallett (1999) | Level V Narrative review |
|
NA |
| Hernandez-Castillo et al. (2018) | Level V Case study |
|
NA |
| Hua et al. (2013) | Level II Two groups, nonrandomized |
|
NA |
| Jain et al. (1998) | Level V Narrative review |
|
NA |
| Jerosch-Herold (2011) | Level IV Descriptive study (survey) |
|
|
| Kaas (1991) | Level V Narrative review |
|
NA |
| Lanzetta et al. (2004) | Level IV Case series |
|
|
| Li et al. (2015) | Level V Case study |
|
NA |
| Lu, Liu, Hua, Shen, et al. (2016) | Level II Two groups, nonrandomized |
|
NA |
| Lu, Liu, Hua, Xu, Gu, & Shen (2016) | Level II Two groups, nonrandomized |
|
|
| Lu, Liu, Hua, Xu, Xu, & Gu (2016) | Level II Two groups, nonrandomized |
|
NA |
| Lundborg (2000a) | Level V Narrative review |
|
|
| Lundborg (2000b) | Level V Narrative review |
|
|
| Lundborg (2003) | Level V Narrative review |
|
|
| Lundborg & Rosén (2007) | Level V Narrative review |
|
|
| Maeda et al. (2014) | Level II Two groups, nonrandomized |
|
|
| Mano et al. (2003) | Level III One group, nonrandomized |
|
|
| Melzack et al. (2001) | Level V Narrative review |
|
|
| Mohanty et al. (2015) | Level V Narrative review |
|
|
| Moore & Novak (2014) | Level V Narrative review |
|
|
| Napadow et al. (2011) | Level V Narrative review |
|
NA |
| Navarro et al. (2007) | Level I Systematic review |
|
NA |
| Piza-Katzer & Estermann (2007) | Level IV Case series |
NA |
|
| Pourrier et al. (2010) | Level IV Case series |
|
|
| Priestley (2007) | Level V Narrative review |
|
NA |
| Rosén et al. (2015) | Level II Two groups, nonrandomized |
|
|
| Rossini & Pauri (2000) | Level V Narrative review |
|
NA |
| Schmidhammer et al. (2007) | Level II Two groups, nonrandomized |
|
|
| Socolovsky et al. (2017) | Level V Narrative review |
|
NA |
| Sokki et al. (2012) | Level II Two groups, nonrandomized |
|
NA |
| Sun et al. (2014) | Level V Narrative review |
|
|
| Takeuchi et al. (2012) | Level V Narrative review |
|
NA |
| Taylor et al. (2009) | Level V Narrative review |
|
NA |
| Udina et al. (2011) | Level I Systematic review |
|
|
| Walbruch & Kalliainen (2015) | Level IV Case series |
|
|
Note. adjacent map invasion = invasion and expansion of adjacent cortical maps of the affected nerve; AO–PNS = action observation with simultaneous peripheral nerve stimulation; axonal sprouting = axonal sprouting and misdirection of regenerating peripheral nerves; bilateral map alteration = alteration of bilateral somatosensory and supplementary motor cortex maps; cross-modal substitution = cross-modal sensory substitution strategies, including mirror visual feedback; mirror therapy = mirror visual feedback; NA = not applicable; reeducation = traditional and activity-based sensory reeducation (“activity-based” indicates articles that mentioned both forms but emphasized activity-based over traditional; no articles mentioned activity-based in isolation); selective deafferentation = cutaneous- and tourniquet-induced deafferentation; unmasking = unmasking or removal of inhibitory controls in the affected region.
Concept 1: Upper Extremity Peripheral Nerve Injury and Cortical Plasticity
For Concept 1, we identified four cortical plasticity mechanisms that occur after upper extremity PNI.
1. Unmasking or Removal of Inhibitory Controls in the Affected Region.
After deafferentation, cortical representations can change because of unmasking (removal) of inhibitory connections in the affected region. A reduction of inhibitory inputs allows originally subthreshold connections to become more active (Hallett, 1999, Level V). These unmasked secondary connections target the cortical representation of the affected body part, resulting in an alteration in cortical representation (Anastakis et al., 2008, Level V).
2. Axonal Sprouting and Misdirection of Regenerating Peripheral Nerves.
After reinnervation starts in the damaged peripheral nerve, newly regenerated axons can be misrouted to peripheral targets different from those of the original damaged nerve, resulting in cortical remodeling (Anastakis et al., 2005, Level II; Hallett, 1999, Level V). This remodeling changes the relationship between brain and body, altering the cortical representation (Mohanty et al., 2015, Level V).
3. Alteration of Bilateral Somatosensory and Supplementary Motor Cortex Maps.
PNI can lead to loss of sensory feedback and reduced or altered use of efferent motor signals, which together can significantly alter the cortical model of bilateral somatosensory and supplementary motor cortices (Lundborg & Rosén, 2007, Level V). A lost or damaged peripheral target alters the sensory and motor representations in the cortical map (Flor, 2003, Level V). These altered maps lead to dysfunctional movement (Lu, Liu, Hua, Xu, Xu, & Gu, 2016, Level II).
4. Invasion and Expansion of Adjacent Cortical Maps of the Affected Nerve.
After PNI, the adjacent cortical representations in the somatosensory representations (i.e., homunculi) may expand and invade the newly deafferented cortical zone (Elbert & Rockstroh, 2004, Level V; Mohanty et al., 2015, Level V; Socolovsky et al., 2017, Level V). However, evidence indicates that cortical map invasion may not occur at a functional scale in humans. Despite cellular-level evidence in nonhuman primates (Kaas, 1991, Level V), researchers have found no evidence that adjacent active zones invade deafferented cortex or that cortical remapping is related to any characteristic of injury or impairment (Makin et al., 2015, Level III; Valyear et al., 2019, Level II).
Concept 2: Rehabilitative Interventions and Strategies Based on Cortical Plastic Changes From Peripheral Nerve Injury
For Concept 2, we connected the mechanisms of cortical plasticity after PNI (as identified in Concept 1) with their functional impact and specific therapeutic interventions (Figure 2).
Figure 2.
Cortical plasticity mechanisms paired with functional implications and related interventions.
Note. AO–PNS = action observation with simultaneous peripheral nerve stimulation.
Functional Impact.
Each of the four cortical plasticity mechanisms has a distinct functional impact with implications for impairment and rehabilitation.
1. Unmasking or removal of inhibitory controls in the affected region.
After PNI, muscles innervated by nerves proximal to the lesion gain larger cortical representations and elevated motor evoked potentials, whereas muscles distal to the lesion experience afferent and efferent rerouting (Mohanty et al., 2015, Level V). This outcome causes unequal muscle recruitment and disarrayed sensory discrimination (Bhat et al., 2017, Level II).
2. Axonal sprouting and misdirection of regenerating peripheral nerves.
The regeneration of peripheral nerves creates novel cortical representations with a disorganized or inaccurate sensory map, resulting in diminished sensory discrimination and disarrayed afferent signals (Duff, 2005, Level V). Rehabilitation professionals should move the affected extremity through its passive range of motion and ensure that the movement stays within the mechanical range appropriate for nerve regeneration after surgery (Brown et al., 2009, Level V).
3. Alteration of bilateral somatosensory and supplementary motor cortex maps.
After PNI, the motor representation of the uninjured extremity appears in the hemisphere contralateral to injury (i.e., ipsilateral to the represented extremity). This new organization remains for years after nerve injury or repair and is believed to play a major role in motor recovery (Navarro et al., 2007, Level I), but it may also disrupt afferent signaling and cortical interpretation (Mohanty et al., 2015, Level V). During initial phases of recovery, reorganization of supplementary motor cortex may interfere with clients’ ability to prepare and initiate movements (Lu, Liu, Hua, Xu, Gu, & Shen, 2016, Level II). Massed practice (3–6 hr/day for 2–3 wk; e.g., Elbert & Rockstroh, 2004, Level V; Taub et al., 1999, Level V) and repetitive use may help by reinforcing newly formed cortical representations while also restoring those lost in PNI (Brown et al., 2009, Level V).
4. Invasion and expansion of adjacent cortical maps of the affected nerve.
As a result of maladaptive invasion from adjacent cortical maps, clients with PNI may experience referred sensations in unaffected body parts and distorted sensation perception in the affected extremity (Björkman et al., 2016, Level III). This distorted sensation may include sensory discrimination dysfunction, allodynia, and hyperalgesia (Pourrier et al., 2010, Level IV). Although cortical map invasion may not be a real phenomenon (see Concept 1, Item 4), some clients experience referred and distorted sensation (Pourrier et al., 2010, Level IV), and these functional implications must be addressed regardless of their neurophysiological cause.
Rehabilitative Interventions.
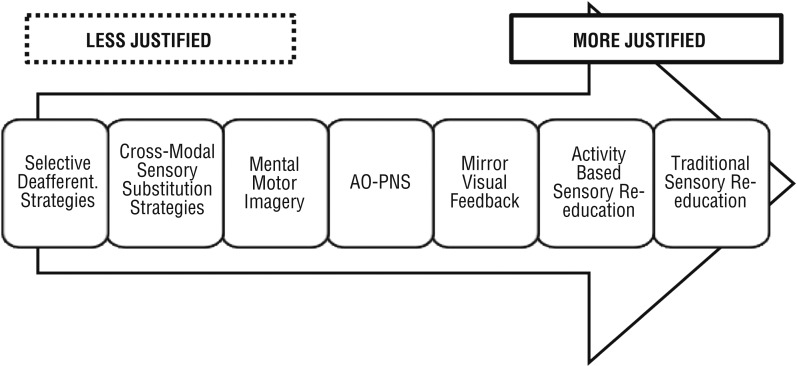
Our scoping review process identified seven rehabilitative interventions based on the mechanisms of cortical plasticity used for clients with PNI (Figure 3).
Figure 3.
Hierarchy of theoretical justification for the seven interventions.
Note. AO–PNS = action observation with simultaneous peripheral nerve stimulation; Deafferent. = deafferentation.
Traditional sensory reeducation.
Traditional sensory reeducation (Duff, 2005, Level V; Jerosch-Herold, 2011, Level IV; Stanley & Tribuzi, 1992, Level V) is based on reestablishing lost afferent sensory pathways through environmental interaction with progressively more discriminant sensory stimulation (Jerosch-Herold, 2011, Level IV). Sensory reeducation is believed to force adaptive cortical alterations in response to interaction with the environment and thereby restore the original contralateral cortical representations of the affected hand while simultaneously reducing overactivation in the compensating ipsilateral zone and reinforcing new adaptive plastic changes (Lundborg & Rosén, 2007, Level V).
Twenty-six articles mentioned traditional sensory reeducation (including articles that mentioned both forms of sensory reeducation; average evidence level = 4.08). Ten of these articles mentioned traditional sensory reeducation only (average evidence level = 4.40).
Activity-based sensory reeducation.
Activity-based sensory reeducation involves reestablishing lost cortical representations or creating alternative ones by forcing clients to use their affected extremity in meaningful sensory and motor experiences (Udina et al., 2011, Level I). Unlike traditional sensory reeducation, activity-based sensory reeducation uses simulated real-life activity rather than tactile stimuli. Activities should be selected collaboratively by the therapist and client on the basis of the client’s normal roles and routines (Duff, 2005, Level V; Jerosch-Herold, 2011, Level IV; Lundborg, 2000a, Level V).
Twenty-two articles mentioned activity-based sensory reeducation (including articles that mentioned both forms of sensory reeducation; average evidence level = 3.72). Six of these articles primarily emphasized activity-based sensory reeducation (average evidence level = 3.88).
Cutaneous- or tourniquet-induced deafferentation (selective deafferentation strategies).
Selective deafferentation involves temporarily deafferenting the intact cortical representations by temporarily cutting off sensory input from unaffected body parts via cutaneous anesthesia or a tourniquet nerve block. This procedure may limit enlargement of the intact representations and prevent them from invading the cortical zone deafferented by PNI (Mohanty et al., 2015, Level V; Walbruch & Kalliainen, 2015, Level IV). However, this intervention relies on the putative cortical plasticity mechanism of cortical map invasion and expansion. As noted in Concept 1, Item 4, evidence remains controversial for this mechanism. Interventions on its basis should be used with caution. Two articles were found involving cutaneous- and tourniquet-induced deafferentation (average evidence level = 4.50).
Cross-modal sensory substitution strategies.
Cross-modal sensory substitution involves using technology to convert information between modalities. For example, tactile information can be converted to audio, and cortical plasticity can allow the brain to learn to interpret this audio input as tactile information (Bolognini et al., 2015, Level V). With this approach, the cross-modal capacity of the brain can be used to create an illusion of activity from the deafferented body area, which may enhance the relearning process (Rosén et al., 2015, Level II), help maintain or restore healthy cortical plasticity (Lundborg & Rosén, 2007, Level V), or enhance the effectiveness of sensory compensation strategies (Chen et al., 2002, Level V).
Examples of cross-modal sensory substitution strategies include sensor gloves, 3-D audiovisual–kinesthetic learning, and mirror therapy. Sensor gloves convert tactile information on the hand into auditory information, which a user can receive and interpret without peripheral sensation (Dahlin, 2013, Level V; Lundborg, 2003, Level V). 3-D audiovisual–kinesthetic learning transforms tactile stimulation into 3-D optical and auditory signals, which participants learn to interpret as touch (Schmidhammer et al., 2007, Level II). Mirror therapy is technically a form of cross-modal sensory substitution, but it is a widely studied method that deserves its own section. Eleven articles were found involving cross-modal sensory substitution (not including mirror therapy; average evidence level = 3.73).
Mirror therapy.
Mirror visual feedback (MVF), or mirror therapy, is a cross-modal sensory substitution technique in which the client observes a mirror reflection of movement of the unaffected hand (Bolognini et al., 2015, Level V). MVF may be a way to stimulate deafferented cortical zones, and it may help regrowing nerves achieve well-organized and functional connections between the body and cortical maps. Through MVF, cerebral areas important for somatosensory processing become active through interaction among the visual, somatosensory, and motor networks (Rosén et al., 2015, Level II). Four articles were found involving mirror therapy (average evidence level = 3.25).
Mental motor imagery.
In mental motor imagery (MI), a client with PNI mentally simulates movement of the affected extremity in the absence of physical movement. MI is believed to catalyze the recovery process by activating or modulating cortical maps even when the injured extremity cannot physically move (Lu, Liu, Hua, Xu, Xu, & Gu, 2016, Level II; Macuga & Frey, 2012, Level III). By mentally simulating the lost movement, MI may help remodel altered cortical territories back to their original representations and maintain integrity of neighboring ones. MI may be beneficial for clients to practice preoperatively to help facilitate postoperation recovery (Mohanty et al., 2015, Level V). Four articles were found involving MI (average evidence level = 4.00).
Action observation with simultaneous peripheral nerve stimulation.
In action observation with simultaneous peripheral nerve stimulation (AO–PNS), clients observe a video or live demonstration of repetitive movement patterns that they can no longer perform while receiving simultaneous electrical stimulation of the injured peripheral nerve. The combination of visual observation and electrical stimulation can induce motor cortex plasticity up to 45 min after treatment, and it also activates sensory areas in the brain, which may allow the two cortical representations to reinforce each other (Bisio et al., 2015, Level III). One article was found involving AO–PNS (average evidence level = 3.00).
Discussion
This scoping review provides an overview of the relationship among cortical plasticity, function, and rehabilitation approaches after upper extremity PNI. In addition, it provides clinicians with the background to implement rehabilitative approaches for optimal recovery.
We found the strongest theoretical justification for traditional and activity-based sensory reeducation (28 articles; average evidence level = 3.86), which involves well-understood cortical changes resulting from axonal sprouting, misdirection of regenerating peripheral nerves, and alteration of the somatosensory maps. MVF (4 articles; average evidence level = 3.25) and AO–PNS (1 Level III article) also have a strong theoretical basis to their ability to induce cortical alterations but limited quality research in clients with PNI. For MI (4 articles; average evidence level = 4.00) and nonmirror cross-modal sensory substitution strategies (11 articles; average evidence level = 3.73), a consensus exists on the cortical plastic mechanisms involved but not on appropriate implementation and timing. Finally, cutaneous- and tourniquet-induced deafferentation (2 articles; average evidence level = 4.50) relies on a disputed mechanism of cortical plasticity (cortical map invasion) that may not be functionally meaningful or real (Makin et al., 2015).
Limitations
We did not critically appraise the methods or clinical outcomes of each study. Therefore, we cannot make claims about the efficacy of rehabilitative interventions or strategies. Our recommendations are based on the current theoretical and scientific justification for each intervention’s putative cortical plasticity basis, not on the interventions’ efficacy. Our study should be viewed as a map for future research and a reference for intervention ideas.
Implications for Research
The high frequency of Level V studies (narrative reviews) indicates that little original research on cortical plasticity has been performed in the PNI context. Therefore, there is significant need and opportunity for researchers to measure the impact of specific cortical plasticity–based therapies on upper extremity PNI. We also recommend that clinical researchers collaborate with basic and translational scientists to explore the validity of the underlying cortical plastic mechanisms on which these strategies are based.
Implications for Occupational Therapy Practice
On the basis of our review, we rank the seven cortical plasticity–based interventions in terms of their level of theoretical justification and established presence in occupational therapy practice as follows:
Highly recommended: traditional and activity-based sensory reeducation
Recommended for secondary use: MVF and AO–PNS
Weakly or situationally recommended: MI and cross-modal sensory substitution strategies other than MVF; MI preoperatively or in other situations in which the client has no motor capability
Not recommended: cutaneous- and tourniquet-induced deafferentation (selective deafferentation) because of its basis on a disputed form of cortical plasticity.
Conclusion
In this scoping review, we identified four common mechanisms of cortical plasticity and their functional implications after upper extremity PNI. We outlined seven commonly used or emergent rehabilitative interventions: traditional sensory reeducation, activity-based sensory reeducation, selective deafferentation, cross-modal sensory substitution, mirror therapy, mental motor imagery, and action observation with peripheral nerve stimulation. The theoretical basis for these interventions ranges from well justified (traditional and activity-based sensory reeducation) to unjustified (selective deafferentation). Future research should prioritize randomized controlled trials for specific neuropathies, interventions, or cortical plasticity mechanisms.
Acknowledgments
We thank Sarah Sherman for her assistance with manuscript review. This research was funded by the Program in Occupational Therapy at Washington University School of Medicine.
Footnotes
Indicates studies that were included in the scoping review.
References
- *Abrams M., & Widenfalk J. (2005). Emerging strategies to promote improved functional outcome after peripheral nerve injury. Restorative Neurology and Neuroscience, 23, 367–382. [PubMed] [Google Scholar]
- *Addas B. M., & Midha R. (2009). Nerve transfers for severe nerve injury. Neurosurgery Clinics of North America, 20, 27–38. 10.1016/j.nec.2008.07.018 [DOI] [PubMed] [Google Scholar]
- American Occupational Therapy Association. (2017). Guidelines for systematic reviews. Retrieved from https://ajot.submit2aota.org/journals/ajot/forms/systematic_reviews.pdf?_ga=2.178109606.2090326902.1568079453-2032253484.1533495792
- *Anastakis D. J., Chen R., Davis K. D., & Mikulis D. (2005). Cortical plasticity following upper extremity injury and reconstruction. Clinics in Plastic Surgery, 32, 617–634. 10.1016/j.cps.2005.05.008 [DOI] [PubMed] [Google Scholar]
- *Anastakis D. J., Malessy M. J., Chen R., Davis K. D., & Mikulis D. (2008). Cortical plasticity following nerve transfer in the upper extremity. Hand Clinics, 24, 425–444. 10.1016/j.hcl.2008.04.005 [DOI] [PubMed] [Google Scholar]
- *Baysefer A., Izci Y., Akay K. M., Kayali H., & Timurkaynak E. (2004). Surgical outcomes of ulnar nerve lesions in children: A retrospective clinical study. Pediatric Neurosurgery, 40, 107–111. 10.1159/000079851 [DOI] [PubMed] [Google Scholar]
- *Beaulieu J. Y., Blustajn J., Teboul F., Baud P., De Schonen S., Thiebaud J. B., & Oberlin C. (2006). Cerebral plasticity in crossed C7 grafts of the brachial plexus: An fMRI study. Microsurgery, 26, 303–310. 10.1002/micr.20243 [DOI] [PubMed] [Google Scholar]
- *Bhat D. I., Indira Devi B., Bharti K., & Panda R. (2017). Cortical plasticity after brachial plexus injury and repair: A resting-state functional MRI study. Neurosurgical Focus, 42, E14 10.3171/2016.12.FOCUS16430 [DOI] [PubMed] [Google Scholar]
- *Bisio A., Avanzino L., Gueugneau N., Pozzo T., Ruggeri P., & Bove M. (2015). Observing and perceiving: A combined approach to induce plasticity in human motor cortex. Clinical Neurophysiology, 126, 1212–1220. 10.1016/j.clinph.2014.08.024 [DOI] [PubMed] [Google Scholar]
- *Björkman A., Weibull A., Svensson H., & Dahlin L. (2016). Cerebral reorganization in patients with brachial plexus birth injury and residual shoulder problems. Frontiers in Neurology, 7, 240 10.3389/fneur.2016.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bolognini N., Russo C., & Vallar G. (2015). Crossmodal illusions in neurorehabilitation. Frontiers in Behavioral Neuroscience, 9, 212 10.3389/fnbeh.2015.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Brenneis C., Löscher W. N., Egger K. E., Benke T., Schocke M., Gabl M. F., . . . Poewe W. (2005). Cortical motor activation patterns following hand transplantation and replantation. Journal of Hand Surgery, 30, 530–533. 10.1016/j.jhsb.2005.05.012 [DOI] [PubMed] [Google Scholar]
- *Brown J. M., Shah M. N., & Mackinnon S. E. (2009). Distal nerve transfers: A biology-based rationale. Neurosurgical Focus, 26, E12 10.3171/FOC.2009.26.2.E12 [DOI] [PubMed] [Google Scholar]
- Bruyns C. N., Jaquet J.-B., Schreuders T. A., Kalmijn S., Kuypers P. D., & Hovius S. E. (2003). Predictors for return to work in patients with median and ulnar nerve injuries. Journal of Hand Surgery, 28, 28–34. 10.1053/jhsu.2003.50026 [DOI] [PubMed] [Google Scholar]
- *Chemnitz A., Andersson G., Rosén B., Dahlin L. B., & Björkman A. (2013). Poor electroneurography but excellent hand function 31 years after nerve repair in childhood. NeuroReport, 24, 6–9. 10.1097/WNR.0b013e32835b6efd [DOI] [PubMed] [Google Scholar]
- *Chemnitz A., Weibull A., Rosén B., Andersson G., Dahlin L. B., & Björkman A. (2015). Normalized activation in the somatosensory cortex 30 years following nerve repair in children: An fMRI study. European Journal of Neuroscience, 42, 2022–2027. 10.1111/ejn.12917 [DOI] [PubMed] [Google Scholar]
- *Chen R., Cohen L. G., & Hallett M. (2002). Nervous system reorganization following injury. Neuroscience, 111, 761–773. 10.1016/S0306-4522(02)00025-8 [DOI] [PubMed] [Google Scholar]
- *Dahlin L. B. (2013). The role of timing in nerve reconstruction. International Review of Neurobiology, 109, 151–164. 10.1016/B978-0-12-420045-6.00007-9 [DOI] [PubMed] [Google Scholar]
- *Dahlin L. B., Andersson G., Backman C., Svensson H., & Björkman A. (2017). Rehabilitation, using guided cerebral plasticity, of a brachial plexus injury treated with intercostal and phrenic nerve transfers. Frontiers in Neurology, 8, 72 10.3389/fneur.2017.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Duff S. V. (2005). Impact of peripheral nerve injury on sensorimotor control. Journal of Hand Therapy, 18, 277–291. 10.1197/j.jht.2005.02.007 [DOI] [PubMed] [Google Scholar]
- *Eickhoff S. B., Dafotakis M., Grefkes C., Shah N. J., Zilles K., & Piza-Katzer H. (2008). Central adaptation following heterotopic hand replantation probed by fMRI and effective connectivity analysis. Experimental Neurology, 212, 132–144. 10.1016/j.expneurol.2008.03.025 [DOI] [PubMed] [Google Scholar]
- *Elbert T., Flor H., Birbaumer N., Knecht S., Hampson S., Larbig W., & Taub E. (1994). Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. NeuroReport, 5, 2593–2597. 10.1097/00001756-199412000-00047 [DOI] [PubMed] [Google Scholar]
- *Elbert T., & Rockstroh B. (2004). Reorganization of human cerebral cortex: The range of changes following use and injury. Neuroscientist, 10, 129–141. 10.1177/1073858403262111 [DOI] [PubMed] [Google Scholar]
- *Ferreri F., Guerra A., & Rossini P. M. (2014). Neurophysiological markers of plastic brain reorganization following central and peripheral lesions. Archives Italiennes de Biologie, 152, 216–238. 10.12871/0003982920144 [DOI] [PubMed] [Google Scholar]
- *Flor H. (2003). Remapping somatosensory cortex after injury. Advances in Neurology, 93, 195–204. [PubMed] [Google Scholar]
- Flor H., Nikolajsen L., & Staehelin Jensen T. (2006). Phantom limb pain: A case of maladaptive CNS plasticity. Nature Reviews Neuroscience, 7, 873–881. 10.1038/nrn1991 [DOI] [PubMed] [Google Scholar]
- *Fornander L., Nyman T., Hansson T., Brismar T., & Engström M. (2016). Inter-hemispheric plasticity in patients with median nerve injury. Neuroscience Letters, 628, 59–66. 10.1016/j.neulet.2016.06.018 [DOI] [PubMed] [Google Scholar]
- *Fornander L., Nyman T., Hansson T., Ragnehed M., & Brismar T. (2010). Age- and time-dependent effects on functional outcome and cortical activation pattern in patients with median nerve injury: A functional magnetic resonance imaging study. Journal of Neurosurgery, 113, 122–128. 10.3171/2009.10.JNS09698 [DOI] [PubMed] [Google Scholar]
- *Gao G. J., Feng X. Y., Xu W. D., Gu Y. D., Tang W. J., Li K., . . . Geng D. Y. (2005). Regional modulation of primary motor cortex after peripheral nerve injury: A functional magnetic resonance imaging study. Zhonghua Yi Xue Za Zhi, 85, 1752–1756. [PubMed] [Google Scholar]
- *Giraux P., Sirigu A., Schneider F., & Dubernard J. M. (2001). Cortical reorganization in motor cortex after graft of both hands. Nature Neuroscience, 4, 691–692. 10.1038/89472 [DOI] [PubMed] [Google Scholar]
- *Grisold W., Vass A., Schmidhammer R., & Zifko U. (2007). Rehabilitation of neuropathies. Critical Reviews in Physical and Rehabilitation Medicine, 19, 19–53. 10.1615/CritRevPhysRehabilMed.v19.i1.20 [DOI] [Google Scholar]
- *Hallett M. (1999). Plasticity in the human motor system. Neuroscientist, 5, 324–332. 10.1177/107385849900500518 [DOI] [Google Scholar]
- *Hernandez-Castillo C. R., Diedrichsen J., Aguilar-Castaneda E., & Iglesias M. (2018). Decoupling between the hand territory and the default mode network after bilateral arm transplantation: Four-year follow-up case study. Brain Imaging and Behavior, 12, 296–302. 10.1007/s11682-017-9683-1 [DOI] [PubMed] [Google Scholar]
- *Hua X. Y., Liu B., Qiu Y. Q., Tang W. J., Xu W. D., Liu H. Q., . . . Gu Y. D. (2013). Long-term ongoing cortical remodeling after contralateral C-7 nerve transfer. Journal of Neurosurgery, 118, 725–729. 10.3171/2012.12.JNS12207 [DOI] [PubMed] [Google Scholar]
- *Jain N., Florence S. L., & Kaas J. H. (1998). Reorganization of somatosensory cortex after nerve and spinal cord injury. News in Physiological Sciences, 13, 143–149. 10.1152/physiologyonline.1998.13.3.143 [DOI] [PubMed] [Google Scholar]
- Jaquet J.-B., Luijsterburg A. J., Kalmijn S., Kuypers P. D., Hofman A., & Hovius S. E. (2001). Median, ulnar, and combined median-ulnar nerve injuries: Functional outcome and return to productivity. Journal of Trauma and Acute Care Surgery, 51, 687–692. 10.1097/00005373-200110000-00011 [DOI] [PubMed] [Google Scholar]
- *Jerosch-Herold C. (2011). Sensory relearning in peripheral nerve disorders of the hand: A web-based survey and Delphi consensus method. Journal of Hand Therapy, 24, 292–299. 10.1016/j.jht.2011.05.002 [DOI] [PubMed] [Google Scholar]
- *Kaas J. H. (1991). Plasticity of sensory and motor maps in adult mammals. Annual Review of Neuroscience, 14, 137–167. 10.1146/annurev.ne.14.030191.001033 [DOI] [PubMed] [Google Scholar]
- *Lanzetta M., Perani D., Anchisi D., Rosén B., Danna M., Scifo P., . . . Lundborg G. (2004). Early use of artificial sensibility in hand transplantation. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery, 38, 106–111. 10.1080/02844310310019860 [DOI] [PubMed] [Google Scholar]
- Levac D., Colquhoun H., & O’Brien K. K. (2010). Scoping studies: Advancing the methodology. Implementation Science, 5, 69 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Li T., Hua X. Y., Zheng M. X., Wang W. W., Xu J. G., Gu Y. D., & Xu W. D. (2015). Different cerebral plasticity of intrinsic and extrinsic hand muscles after peripheral neurotization in a patient with brachial plexus injury: A TMS and fMRI study. Neuroscience Letters, 604, 140–144. 10.1016/j.neulet.2015.07.015 [DOI] [PubMed] [Google Scholar]
- *Lu Y., Liu H., Hua X., Shen Y., Xu W. D., Xu J. G., & Gu Y. D. (2016). Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury. Neural Regeneration Research, 11, 670–675. 10.4103/1673-5374.180756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Lu Y., Liu H., Hua X., Xu J. G., Gu Y. D., & Shen Y. (2016). Attenuation of brain grey matter volume in brachial plexus injury patients. Neurological Sciences, 37, 51–56. 10.1007/s10072-015-2356-1 [DOI] [PubMed] [Google Scholar]
- *Lu Y., Liu H., Hua X., Xu W. D., Xu J. G., & Gu Y. D. (2016). Supplementary motor cortical changes explored by resting-state functional connectivity in brachial plexus injury. World Neurosurgery, 88, 300–305. 10.1016/j.wneu.2015.12.036 [DOI] [PubMed] [Google Scholar]
- *Lundborg G. (2000a). Brain plasticity and hand surgery: An overview. Journal of Hand Surgery, 25, 242–252. 10.1054/jhsb.1999.0339 [DOI] [PubMed] [Google Scholar]
- *Lundborg G. (2000b). A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. Journal of Hand Surgery, 25, 391–414. 10.1053/jhsu.2000.4165 [DOI] [PubMed] [Google Scholar]
- *Lundborg G. (2003). Richard P. Bunge memorial lecture. Nerve injury and repair—A challenge to the plastic brain. Journal of the Peripheral Nervous System, 8, 209–226. 10.1111/j.1085-9489.2003.03027.x [DOI] [PubMed] [Google Scholar]
- *Lundborg G., & Rosén B. (2007). Hand function after nerve repair. Acta Physiologica, 189, 207–217. 10.1111/j.1748-1716.2006.01653.x [DOI] [PubMed] [Google Scholar]
- Macuga K. L., & Frey S. H. (2012). Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. NeuroImage, 59, 2798–2807. 10.1016/j.neuroimage.2011.09.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Maeda Y., Kettner N., Holden J., Lee J., Kim J., Cina S., . . . Napadow V. (2014). Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain, 137, 1741–1752. 10.1093/brain/awu096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T. R., Scholz J., Henderson Slater D., Johansen-Berg H., & Tracey I. (2015). Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain, 138, 2140–2146. 10.1093/brain/awv161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Mano Y., Chuma T., & Watanabe I. (2003). Cortical reorganization in training. Journal of Electromyography and Kinesiology, 13, 57–62. 10.1016/S1050-6411(02)00086-X [DOI] [PubMed] [Google Scholar]
- *Melzack R., Coderre T. J., Katz J., & Vaccarino A. L. (2001). Central neuroplasticity and pathological pain. Annals of the New York Academy of Sciences, 933, 157–174. 10.1111/j.1749-6632.2001.tb05822.x [DOI] [PubMed] [Google Scholar]
- *Mohanty C. B., Bhat D., & Devi B. I. (2015). Role of central plasticity in the outcome of peripheral nerve regeneration. Neurosurgery, 77, 418–423. 10.1227/NEU.0000000000000851 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., & Altman D. G.; PRISMA Group. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Moore A. M., & Novak C. B. (2014). Advances in nerve transfer surgery. Journal of Hand Therapy, 27, 96–105. 10.1016/j.jht.2013.12.007 [DOI] [PubMed] [Google Scholar]
- *Napadow V., Maeda Y., Audette J., & Kettner N. (2011). Neuroplasticity in carpal tunnel syndrome. Journal of Pain Management, 4, 315–331. [Google Scholar]
- *Navarro X., Vivó M., & Valero-Cabré A. (2007). Neural plasticity after peripheral nerve injury and regeneration. Progress in Neurobiology, 82, 163–201. 10.1016/j.pneurobio.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Opsteegh L., Reinders-Messelink H. A., Schollier D., Groothoff J. W., Postema K., Dijkstra P. U., & van der Sluis C. K. (2009). Determinants of return to work in patients with hand disorders and hand injuries. Journal of Occupational Rehabilitation, 19, 245–255. 10.1007/s10926-009-9181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne N. R., Anastakis D. J., & Davis K. D. (2018). Peripheral nerve injuries, pain, and neuroplasticity. Journal of Hand Therapy, 31, 184–194. 10.1016/j.jht.2018.01.011 [DOI] [PubMed] [Google Scholar]
- *Piza-Katzer H., & Estermann D. (2007). Cognitive re-education and early functional mobilisation in hand therapy after bilateral hand transplantation and heterotopic hand replantation—Two case reports. Acta Neurochirurgica: Supplement, 100, 169–171. 10.1007/978-3-211-72958-8_35 [DOI] [PubMed] [Google Scholar]
- *Pourrier S. D., Nieuwstraten W., Van Cranenburgh B., Schreuders T. A., Stam H. J., & Selles R. W. (2010). Three cases of referred sensation in traumatic nerve injury of the hand: Implications for understanding central nervous system reorganization. Journal of Rehabilitation Medicine, 42, 357–361. 10.2340/16501977-0526 [DOI] [PubMed] [Google Scholar]
- *Priestley J. V. (2007). Promoting anatomical plasticity and recovery of function after traumatic injury to the central or peripheral nervous system. Brain, 130, 895–897. 10.1093/brain/awm041 [DOI] [PubMed] [Google Scholar]
- *Rosén B., Vikström P., Turner S., McGrouther D. A., Selles R. W., Schreuders T. A., & Björkman A. (2015). Enhanced early sensory outcome after nerve repair as a result of immediate post-operative re-learning: A randomized controlled trial. Journal of Hand Surgery, European Volume, 40, 598–606. 10.1177/1753193414553163 [DOI] [PubMed] [Google Scholar]
- *Rossini P. M., & Pauri F. (2000). Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas ‘plastic’ reorganisation. Brain Research Reviews, 33, 131–154. 10.1016/S0169-328X(00)00090-5 [DOI] [PubMed] [Google Scholar]
- Sackett D. L., Rosenberg W. M., Gray J. M., Haynes R. B., & Richardson W. S. (1996). Evidence based medicine: What it is and what it isn’t. BMJ, 312, 71 10.1136/bmj.312.7023.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schmidhammer R., Hausner T., Kröpfl A., Huber W., Hopf R., Leixnering M., . . . Redl H. (2007). Enhanced sensory re-learning after nerve repair using 3D audio-visual signals and kinaesthesia—Preliminary results. Acta Neurochirurgica: Supplement, 100, 127–129. 10.1007/978-3-211-72958-8_27 [DOI] [PubMed] [Google Scholar]
- *Socolovsky M., Malessy M., Lopez D., Guedes F., & Flores L. (2017). Current concepts in plasticity and nerve transfers: Relationship between surgical techniques and outcomes. Neurosurgical Focus, 42, E13 10.3171/2016.12.FOCUS16431 [DOI] [PubMed] [Google Scholar]
- *Sokki A. M., Bhat D. I., & Devi B. I. (2012). Cortical reorganization following neurotization: A diffusion tensor imaging and functional magnetic resonance imaging study. Neurosurgery, 70, 1305–1311. 10.1227/NEU.0b013e318241017d [DOI] [PubMed] [Google Scholar]
- Stanley B. G., & Tribuzi S. M. (1992). Concepts in hand rehabilitation. Philadelphia: F. A. Davis. [Google Scholar]
- Stonner M. M., Mackinnon S. E., & Kaskutas V. (2017). Predictors of disability and quality of life with an upper-extremity peripheral nerve disorder. American Journal of Occupational Therapy, 71, 7101190050 10.5014/ajot.2017.022988 [DOI] [PubMed] [Google Scholar]
- *Sun G., Wu Z., Wang X., Tan X., & Gu Y. (2014). Nerve transfer helps repair brachial plexus injury by increasing cerebral cortical plasticity. Neural Regeneration Research, 9, 2111–2114. 10.4103/1673-5374.147939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Takeuchi N., Oouchida Y., & Izumi S. (2012). Motor control and neural plasticity through interhemispheric interactions. Neural Plasticity, 2012, 823285 10.1155/2012/823285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E., Uswatte G., & Pidikiti R. (1999). Constraint-induced movement therapy: A new family of techniques with broad application to physical rehabilitation—A clinical review. Journal of Rehabilitation Research and Development, 36, 237–251. [PubMed] [Google Scholar]
- *Taylor K. S., Anastakis D. J., & Davis K. D. (2009). Cutting your nerve changes your brain. Brain, 132, 3122–3133. 10.1093/brain/awp231 [DOI] [PubMed] [Google Scholar]
- *Udina E., Cobianchi S., Allodi I., & Navarro X. (2011). Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Annals of Anatomy, 193, 347–353. 10.1016/j.aanat.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Valyear K. F., Philip B. A., Cirstea C. M., Chen P. W., Baune N., Marchal N., & Frey S. H. (2019). Interhemispheric transfer of post-amputation cortical plasticity within the human somatosensory cortex. NeuroImage, 116291 10.1016/j.neuroimage.2019.116291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Walbruch B., & Kalliainen L. (2015). The optimization of peripheral nerve recovery using cortical reorganization techniques: A retrospective study of wrist level nerve repairs. Journal of Hand Therapy, 28, 341–346. 10.1016/j.jht.2015.04.001 [DOI] [PubMed] [Google Scholar]