Herpes simplex virus 1 (HSV-1) can be responsible for life-threatening HSV encephalitis (HSE). The mortality rate of patients with HSE who do not receive antiviral treatment is 70%, with most survivors suffering from permanent neurological sequelae. The use of intravenous acyclovir together with improved diagnostic technologies such as PCR and magnetic resonance imaging has resulted in a reduction in the mortality rate to close to 20%. However, 70% of surviving patients still do not recover complete neurological functions.
KEYWORDS: encephalitis, herpes simplex virus, immune response, immunomodulatory drugs
SUMMARY
Herpes simplex virus 1 (HSV-1) can be responsible for life-threatening HSV encephalitis (HSE). The mortality rate of patients with HSE who do not receive antiviral treatment is 70%, with most survivors suffering from permanent neurological sequelae. The use of intravenous acyclovir together with improved diagnostic technologies such as PCR and magnetic resonance imaging has resulted in a reduction in the mortality rate to close to 20%. However, 70% of surviving patients still do not recover complete neurological functions. Thus, there is an urgent need to develop more effective treatments for a better clinical outcome. It is well recognized that cerebral damage resulting from HSE is caused by viral replication together with an overzealous inflammatory response. Both of these processes constitute potential targets for the development of innovative therapies against HSE. In this review, we discuss recent progress in therapy that may be used to ameliorate the outcome of patients with HSE, with a particular emphasis on immunomodulatory agents. Ideally, the administration of adjunctive immunomodulatory drugs should be initiated during the rise of the inflammatory response, and its duration should be limited in time to reduce undesired effects. This critical time frame should be optimized by the identification of reliable biomarkers of inflammation.
INTRODUCTION
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) belong to the Alphaherpesvirinae subfamily in the Herpesviridae family (1). These enveloped viruses contained double-stranded DNA (dsDNA) located in an icosahedral capsid surrounded by a tegument. Alphaherpesviruses are characterized by a short replicative cycle leading to host cell lysis. After a primary infection, these viruses migrate to the sensory ganglia, where they enter into a latent state for the host’s lifetime. During the latency period, the transcription of viral genes is largely suppressed. These viruses can reactivate periodically to induce recurrent infections. HSVs typically cause mucocutaneous infections that are usually self-limiting in immunocompetent hosts but can be serious in immunocompromised patients. HSVs can also be responsible for stromal keratitis, which represents the most frequent cause of blindness in the United States. More rarely, HSVs can invade the central nervous system (CNS) and cause life-threatening encephalitis in adults and children. Furthermore, in the newborn, HSV infections can be localized and affect the skin, eyes, and mouth or be disseminated with or without encephalitis.
HERPES SIMPLEX VIRUS ENCEPHALITIS
Epidemiology
In the United States, it is estimated that 20% to 50% of encephalitis cases result from a viral infection (2, 3). HSVs account for 50% to 75% of viral encephalitis, whereas varicella-zoster virus, enterovirus, and arbovirus are responsible for most of the remaining cases. The annual incidence of herpes simplex virus encephalitis (HSE) is estimated to be 2 to 4 individuals per million population (4–6). In adults and children, over 90% of HSE cases result from HSV-1 infection (7). Encephalitis caused by HSV-2 usually occurs in the newborn or in immunocompromised patients. About 30% of HSE cases are due to a primary HSV-1 infection, whereas the remaining cases are attributed to viral reactivation or reinfection. In contrast to arboviruses and enteroviruses, the incidence of HSE does not vary with seasons or geographic locations (2, 3). The incidence of the disease shows a bimodal age distribution, with a first small peak in the pediatric population (age range from 6 months to 3 years) and a second greater peak in adult patients (over 50 years of age) (2, 4). The occurrence of HSE in immunocompromised patients seems to be similar to that observed in immunocompetent individuals (8), but epidemiologic data are still lacking in this population.
Clinical Manifestations
Encephalitis can result from infectious, postinfectious, or noninfectious etiologies and consists of brain parenchyma inflammation associated with clinical evidences of neurologic dysfunctions. Brain inflammation leads to an altered mental status that manifests in the form of reduced consciousness and altered cognitive functions, as well as changes in personality and/or behavior. Gnann and Whitley (9) compiled the clinical signs for 388 patients diagnosed with HSE that were extracted from several studies. The most common clinical manifestations related to HSE include fever (80%), confusion/disorientation (72%), changes in personality/abnormal behavior (59%), headache (58%), impaired mental status/altered consciousness (58%), seizures (54%), focal neurological disabilities (41%), nausea and vomiting (40%), aphasia/altered speech (40%), coma (33%), and meningismus (28%). The state of consciousness of a patient can be assessed by the Glasgow coma score (GCS), which is based on eye, verbal, and motor responses. However, the GCS is a crude test for the detection of subtle alterations in behavior. Clinical features that may be more specifically related to HSE include prodromal symptoms such as headache and fever, which mimic upper respiratory tract or other systemic infections, as well as neurologic abnormalities associated with dysfunctions of the fronto-temporal lobes. The lack of a specific clinical picture makes the diagnosis of HSE difficult. The unusual clinical and neurologic presentations of HSE in immunocompromised patients, exhibiting fewer prodromal symptoms (29% versus 80%) or focal neurologic deficits (29% versus 73%) than immunocompetent individuals, make the diagnosis even more challenging in this population (10).
DIAGNOSTIC PROCEDURES
Brain biopsy used to be the gold standard diagnostic test for HSE, but nowadays, this neuroinvasive procedure is performed only on rare occasions. Today, the gold standard method for the diagnosis of HSE consists of nucleic acid amplification by PCR for the detection of HSV-1 and HSV-2 genes in the cerebrospinal fluid (CSF) (11, 12). Brain inflammation can be demonstrated by surrogate markers such as inflammatory changes in the CSF and/or inflammation of the brain parenchyma on neuroimaging (13). Cerebral dysfunction leading to abnormal behavior or subtle motor or nonconvulsive seizures can be evidenced by electroencephalography (EEG) (14). Thus, current diagnostic tests are based on CSF analysis (including differential cell count, glucose and protein levels, amplification of HSV-1/-2 DNA by PCR, and intrathecal HSV-specific antibody production), neuroimaging, and EEG.
Histopathological Analysis
HSE causes acute inflammation, hemorrhage, and congestion in one or both temporal lobes and in adjacent areas of the limbic system in adults, whereas brain involvement is more diffuse in the newborn. A congestion of the meninges overlying the temporal lobes may also be observed. In early stages of HSE, a shrinkage of the cytoplasm of neuronal cells located in infected regions of the brain suggests an acute ischemia that is associated with marked congestion, dilatation of capillaries, and hemorrhage. Several cells contain Cowdry type A inclusions, consisting of large, eosinophilic intranuclear inclusions that support the diagnosis of HSE. Viral antigens are detected mainly in the medial and inferior temporal lobes, amygdaloid nuclei, hippocampus, insula, and cingulate gyrus as well as in the olfactory cortex (15). An infiltration of neutrophils in infected areas of the brain usually occurs in the first 2 to 3 days after onset of the disease, whereas macrophages and lymphocytes become the predominant populations after 10 to 15 days (16). After 3 weeks, the necrosis progresses to frank necrosis, with the presence of inflammation and gliosis, although the detection of viral antigens is reduced at this stage (15).
In immunocompromised patients, brain involvement is more extensive, with lesions in the brainstem and the cerebellum as well as in atypical regions (e.g., diffuse lesions in the cerebrum) without necessarily affecting the temporal lobes. The histopathologic features are atypical, with a lack of necrosis and hemorrhage, the presence of noninflammatory lesions, and abundant viral antigens that persist for more than 3 weeks after disease onset (10, 17). The wider distribution of infected areas and the presence of noninflammatory lesions may result from an ineffective host immune response that is unable to control the viral spread while inducing less tissue damage.
Cerebrospinal Fluid Analysis
All patients with clinically suspected encephalitis should be subjected to a lumbar puncture as early as possible (except if this is contraindicated by elevated intracranial pressure due to brain shift or herniation). The opening pressure is generally normal or slightly elevated. Most of the patients suffering from HSE typically exhibit lymphocytic pleocytosis, elevated protein levels, and unchanged glucose levels in CSF specimens. In more than 95% of these patients, the CSF presents a modest pleocytosis (5 to 500 cells/mm3) with a predominance of lymphocytes (60 to 98%). However, normal leukocyte counts in CSF (<5 cells/mm3) may rarely occur, especially in early stages of the disease as well as in the neonate. CSF specimens obtained from immunocompromised patients with HSE often present with a lack of pleocytosis and, in some cases, a predominance of polymorphonuclear cells. The CSF also commonly contains slightly elevated numbers of erythrocytes which result from hemorrhage. A mild increase in the protein level in CSF (50 to 200 mg/dl) occurs in more than 80% of cases. The glucose level is usually normal (60 to 75 mg/dl), and it is estimated that less than 5% of patients with biopsy-confirmed HSE have hypoglycorrhachia (18). Nucleic acid amplification in CSF specimens by PCR allows detection of HSV-1 and HSV-2 genes. The sensitivity and specificity values of this technique are high in adults (96% and 99%, respectively) and more variable in neonates and children (19, 20). The timing of testing with respect to onset of disease and antiviral therapy should be taken into account when interpreting PCR results. Indeed, PCR testing in CSF performed very early in the course of the disease (i.e., during the first 3 days) may be negative (21–23), and testing should be repeated if the clinical presentation strongly suggests HSE (22). PCR is positive during the first week of treatment with acyclovir and usually becomes undetectable after 10 to 14 days of therapy (11). A false-negative PCR can be due to a suboptimized method or to the presence of hemoglobin or other PCR inhibitors in the CSF. False-positive results may occur in the case of cross-contamination of samples. If the results of the first lumbar puncture are inconclusive, a second one should be performed if HSE is strongly suspected. Furthermore, as the blood-brain barrier (BBB) cannot be crossed by IgM antibodies, the detection of intrathecal HSV-specific IgM by ELISA at 10 to 14 days after onset of symptoms is suggestive of a neuroinvasive infection and can help to confirm the diagnosis of HSE in selected cases.
Multiplexed molecular diagnostic tests for the simultaneous, rapid (less than 1 h) detection of bacteria, viruses, and yeast directly from CSF specimens have been recently developed. The FilmArray meningitis/encephalitis (ME) panel (BioFire Diagnostics LLC) is an FDA-cleared diagnostic test designed to identify 14 pathogens (7 viral targets, 6 bacterial targets, and 1 yeast target) involved in community-acquired meningitis/encephalitis. The FilmArray ME panel is simple to use, allows more rapid results, and has good performance (although is slightly less sensitive) in comparison to culture and molecular reference diagnostic methods (24, 25). However, a systematic review and meta-analysis reported that high numbers of false-negative results were obtained for the detection of HSV-1 and HSV-2 targets (26). Therefore, in cases of neonates or immunocompromised patients with clinically suspected HSE, it is recommended to confirm a negative multiplex result by running a singleplex PCR assay.
Neuroimaging
Brain swelling is a severe complication of HSE that results in intracranial hypertension. The swelling is generally asymmetric and may cause brain tissue shift and herniation. Computed tomographic (CT) imaging can be used for a rapid assessment of patients with suspected elevated intracranial pressure that may contraindicate a lumbar puncture. Abnormal CT scans related to HSE are characterized by hypodense lesions (typically in the temporal lobe), edema, or contrast enhancement. However, the CT imaging method (with or without administration of an intravenous [i.v.] contrast agent) lacks sensitivity, especially in early stages of the disease (27).
In contrast, magnetic resonance imaging (MRI), including T1- or T2-weighted, diffusion-weighted imaging (DWI) or fluid-attenuated inversion recovery (FLAIR) sequences, is the most sensitive and specific neuroimaging method for the diagnosis of HSE in the early stages of the disease (28). Abnormalities on MRI scans have been demonstrated in more than 90% of patients with PCR-confirmed HSE (29). Findings on MRI include asymmetric hypodense lesions on T1-weighted images and hyperintense lesions on T2-weighted and FLAIR images. Neuroimaging abnormalities correspond to edematous changes and hemorrhage that are typically localized in the medial temporal lobes with a unilateral or bilateral involvement and that distribute along the limbic system to reach the inferior frontal lobes as well as the insular cortex. DWI has been shown to be superior to FLAIR sequences when MRI is performed within 2 weeks after the onset of symptoms (30). However, high-resolution FLAIR allows the detection of thalamus involvement in HSE that cannot be visualized with DWI. Bilateral temporal lobe involvement or more extensive brain involvement at admission is associated with a poor prognosis (31). In immunocompromised patients, brain involvement is broader and regions other than the temporal lobes, such as the brainstem and the cerebellum, are affected.
Electroencephalography
Damage caused by HSE is located mainly in the highly epileptogenic mesial temporal lobe and the hippocampus. It has been reported that 40% to 60% of patients had epileptic seizures at early stages of HSE (32). EEG patterns in patients with HSE usually reveal spike and slow-wave activity as well as periodic lateralized epileptiform discharges (PLEDs) in the fronto-temporal and occipital regions, which are typically recorded 2 to 14 days after the onset of symptoms (14).
TREATMENT
Antiviral Therapy
Before the availability of antiviral therapy, the mortality rate of patients suffering from HSE was 70% and most of the survivors were left with severe neurological deficits, seizures, and/or neuropsychological dysfunctions (33). The nucleoside analogue vidarabine was the first antiviral agent used for the treatment of HSE (33). The benefit of acyclovir in the treatment of HSE was then demonstrated in two randomized, controlled clinical trials (34, 35). Both studies compared acyclovir (10 mg/kg three times daily) to vidarabine (15 mg/kg once daily) administered intravenously to patients with suspected HSE for 10 days. The 6-month mortality rate was significantly reduced in the acyclovir arm compared to the vidarabine arm (28% versus 54% and 19% versus 50% in the first and second studies, respectively). Intravenous acyclovir is now the drug of choice for the treatment of HSE. Acyclovir, which is a nucleoside analogue, needs to be phosphorylated once by the viral thymidine kinase and twice by cellular kinases to be converted into its active form (36). The triphosphorylated form competes with deoxynucleotide analogues for incorporation into replicating DNA by the viral DNA polymerase. In addition, acyclovir triphosphate acts by terminating viral DNA chain elongation. Current guidelines recommend extending the duration of acyclovir treatment from 10 days to 14 to 21 days to reduce the incidence of HSE relapses (37). The recommended dosage of acyclovir is 10 mg/kg given intravenously every 8 h for 14 days in immunocompetent individuals and for 21 days in immunocompromised patients and children in the 3-month to 12-year age range. A higher dose of 20 mg/kg three times daily for 21 days is suggested in neonates (38). The dose of acyclovir should be adjusted in patients with an altered renal function. Antiviral therapy must be started as early as possible after the onset of symptoms for a better clinical outcome (39–41). A multivariate analysis showed that a delay of more than 2 days from the time of admission to initiation of acyclovir therapy is an independent predictor for an increased risk of severe neurological sequelae or death at 6 months with an odds ratio of 3.1 (39). The atypical clinical, laboratory, or neurologic features in immunocompromised patients suffering from HSE may result in a delay in diagnosis, leading to significantly worse outcomes and mortality (10).
Management
The Infectious Diseases Society of America and the Association of British Neurologists and British Infection Association National Guidelines have proposed recommendations for the management of patients with suspected encephalitis (42, 43). All patients with clinically suspected HSE should be subjected as soon as possible to CSF analysis and MRI scans. Empiric antiviral therapy with intravenous acyclovir at a dose of 10 mg/kg every 8 h should be initiated promptly, pending results of laboratory tests of CSF and MRI. The diagnosis is typically established by the detection of viral DNA in the CSF by PCR, and results should be as follows. (i) If the initial PCR is positive, U.S. guidelines recommend that acyclovir treatment be administered for 14 to 21 days without the need of a systematic second lumbar puncture. In contrast, the British recommendation is to repeat the viral DNA PCR in CSF at the end of acyclovir course and, if positive, to continue antiviral treatment for 7 additional days and until a negative PCR is obtained. (ii) If the initial PCR is negative, U.S. guidelines recommend that the PCR should be repeated 3 to 7 days later in patients who exhibit clinical features of HSE or temporal lobe lesions on MRI scans, whereas the British guidelines recommend repeating the PCR after 24 to 48 h. In both cases, acyclovir therapy can be stopped if the second PCR is negative. Of note, it is also suggested that an absence of HSE may be confirmed by a negative initial PCR together with negative intrathecal HSV-specific IgM antibodies at 10 to 14 days after the onset of symptoms (44).
Attempts To Improve Antiviral Therapy
Recent studies evaluated the long-term outcome of patients with proven HSE following acyclovir therapy and showed that the mortality rate by day 180 after onset of symptoms was 15%. In the remaining population, 14% completely recovered, 23% had minimal impairment, 28% had moderate neurological disorders, and 20% had severe neurological sequelae (39, 45). The incomplete recovery of neurological functions constitutes an enormous burden for the health care system (3). There is thus an urgent need for improved treatment to reduce the occurrence of long-term sequelae resulting from HSE. It was suggested that persistent viral replication at a low level in the brain may contribute to the neurological disabilities of patients surviving from HSE. However, the administration of higher doses of acyclovir (15 mg/kg every 8 h for 14 or 21 days) did not improve the outcome in adult patients (27). Furthermore, a randomized, placebo-controlled clinical study evaluated the benefit of long-term therapy with valacyclovir (an l-valyl ester prodrug of acyclovir with higher oral bioavailability than the parent drug) in PCR-confirmed HSE patients who completed a standard treatment with intravenous acyclovir (46). Results showed that an additional 3-month administration of oral valacyclovir (2 g three times daily) did not provide added benefit compared to standard acyclovir therapy, as determined by the Mini-Mental State Examination or the Mattis Dementia Rating Scale tests after 12 months.
COMPLICATIONS AFTER ANTIVIRAL THERAPY
Viral Resistance to Acyclovir
The emergence of HSV strains resistant to acyclovir has been very rarely observed in patients treated for HSE (47–54). Antiviral drug resistance may be suspected in patients who do not respond to treatment or who develop neurological deterioration despite a full course of acyclovir. A lumbar puncture should be performed. The CSF should be tested for the detection of viral DNA by PCR, and the presence of resistance-associated mutations should be investigated by genotypic testing. The mechanisms of acyclovir resistance in HSV involved mutations in viral genes encoding thymidine kinase and/or DNA polymerase (55). In the case of viral resistance, the pyrophosphate analogue foscarnet constitutes the second-line drug. The dose and schedule of administration of intravenous foscarnet for the treatment of HSE are 90 mg/kg every 12 h or 60 mg/kg every 8 h (13). The dose of foscarnet should be adjusted for patients with altered renal function. A combination of foscarnet and acyclovir has also been successfully used to treat patients who develop HSE caused by an acyclovir-resistant strain (47) or in whom an acyclovir-resistant strain was selected during therapy (48, 54). Cidofovir, an acyclic nucleoside analogue, is not indicated for the treatment of HSE. The orally bioavailable lipid conjugate of cidofovir, CMX001 or brincidofovir, has been developed to limit the toxicity of the parent drug (56), but it is not approved for the treatment of infections caused by HSV. Brincidofovir has been shown to diffuse readily across the BBB and to be superior to acyclovir for the treatment of HSE in a murine model (57). Furthermore, a combination of brincidofovir and acyclovir acts synergistically to decrease the mortality rate of mice infected by the intranasal route with HSV-1 (58). Thus, the use of brincidofovir alone or combined with acyclovir in the treatment of HSE warrants further developments.
Viral and Autoimmune Relapses
The incidence of relapses that occur after a first episode of HSE is estimated to be between 5% and 27% (59–61). Most relapses affect children and develop within 3 months after completion of a full course of antiviral therapy, but HSE relapses have been also reported in adults. Relapses can manifest in two different ways. In a first subset of patients, the detection of viral DNA is positive in the CSF, suggesting a persistent infection or a viral reactivation, and represents true relapses of HSE. The presence of new necrotic and hemorrhagic lesions can also be detected at a distance from the primary site of infection on brain imaging (62). The severity of relapses is generally lower than that of the initial episode of HSE.
In a second subset of patients, the HSV PCR in the CSF is negative. However, IgG antibodies against a restricted epitope region of the N-methyl-d-aspartate receptor (NMDAR) (63) expressed on many excitatory glutamate synapses and other neuronal surface proteins such as the dopamine-2 receptor (64) are detected in the serum or CSF. The mechanisms involved in the synthesis of autoimmune antibodies are unknown. It is suggested that neuronal autoimmunity results from an exposition of antigens following virus-induced neuronal cell lysis in a severely inflamed environment, a nonspecific B-cell activation as reported in other neurologic disorders such as multiple sclerosis, or a molecular mimicry between NMDAR and viral proteins. This secondary immune-mediated neurological deterioration triggered by HSV infection of the CNS is called HSE-induced autoimmune encephalitis. The clinical manifestations of HSE-induced autoimmune encephalitis include choreoathetosis, impaired consciousness, and refractory seizures in children under 4 years of age, whereas cognitive deficits and psychiatric symptoms are observed in older children and adults. Brain examination by MRI does not reveal the presence of new necrotic lesions. The lack of markers of destruction of neural (i.e., neuron-specific enolase) and glial (i.e., glial fibrillary acidic protein and S-100B) cells in the CSF suggests that viral cell lysis does not occur during HSE-induced autoimmune encephalitis (65). In contrast, the levels of soluble CD8 (a marker of cytotoxicity mediated by T cells) and of several cytokines/chemokines produced by Th1 (such as CXC motif ligand 9 [CXCL9] and CXCL10) and B cells (such as CXCL13, CC motif ligand 19 [CCL19], and a proliferation-inducing ligand [APRIL]) were increased during post-HSE anti-NMDAR encephalitis (66). Furthermore, HSE-induced anti-NMDAR autoimmunity has been shown to affect cognitive performance in the Mattis Dementia Rating Scale and Mini-Mental State Examination tests (67). A lack of efficacy of antiviral therapy in improving choreoathetosis has been reported in children (60). In contrast, older children and adult patients with HSE-induced autoimmune encephalitis receiving aggressive immunotherapy had a favorable outcome, with a reduction in serum NMDAR antibody titers (64, 68–73). First-line therapies for HSE-induced autoimmune encephalitis consist of steroids, immunoglobulins, and plasma exchange, which can be escalated to more aggressive combinations with second-line drugs such as rituximab and cyclophosphamide, if needed (74).
Thus, after initial clinical improvement of HSE, patients who present with new or recurrent neurologic symptoms and with a negative HSV PCR in CSF should be tested for the presence of anti-NMDAR IgG antibodies in serum and CSF. Immunotherapy may be effective in these patients and should be initiated even if a clinical trial is still lacking to establish formal recommendations. Anti-NMDAR antibodies are not detected during the acute phase of HSE but appear 1 to 4 weeks later (75). It is thus suggested that adjunctive anti-inflammatory drugs or immunotherapy may be effective for the treatment of HSE during the acute phase as well as for the prevention of the subsequent development of anti-NMDAR encephalitis.
Long-Term Persistent Immune Activation after HSE
Several studies have reported that the HSV DNA load in CSF is not a reliable prognostic marker of the clinical outcome of patients with HSE treated with a full course of acyclovir (76, 77). This suggests not only that the pathogenesis of HSE is associated with viral replication in brain tissue but that the host inflammatory response also contributes indirectly to brain injury. In the acute phase of HSE, patients exhibit an intrathecal inflammatory response, as indicated by the increased production of interleukin-6 (IL-6) and gamma interferon (IFN-γ). This is followed by the late appearance and long-term persistence of markers of T cell activation, such as soluble CD8 and virus-specific antibody synthesis in the CSF that suggest a persistent immune activation (78). Furthermore, two markers of intrathecal immune activation, neopterin and β2-microglobulin, reach high levels in the CSF of patients during the acute phase of HSE and may persist for several years, suggesting long-lasting inflammatory activity (79). Progressive cranial MRI abnormalities that still persisted at 6 months after onset of the disease have been reported in some patients despite early acyclovir therapy (80). Positron emission tomography (PET) scans performed in two patients with HSE confined to one temporal lobe using a microglial and brain macrophage marker {i.e., [11C](R)-PK11195} revealed a persistent microglial activation more than 12 months after a successful antiviral course (81). The persistent elevated binding of PK11195 spread along the initially affected neuronal circuitry indicates a continuing neurodegenerative process. Brain biopsy samples obtained from three children who recovered from acute HSE and developed secondary focal epilepsy also showed a marked inflammatory process characterized by abundant CD3+ and CD8+ T lymphocytes as well as activated microglial cells and macrophages that persisted for several years after onset of the disease (82). Histopathological analysis of the brains of patients who survived between 4 months and 17 years after acute encephalitis revealed neuronal loss and gliosis in temporal, frontal, and insular regions, whereas a persistent inflammatory infiltrate was also seen in the cerebrum and the brainstem and correlated with the distribution of viral DNA detected by PCR on paraffin sections (83).
Some reports have also described children and adult patients who developed transient or chronic lesions in the white matter after HSE, as evidenced by MRI scans (84–86). Patients are asymptomatic or present with secondary neurological deteriorations that are acute or chronic and associated or not with extrapyramidal movement disorder. The mechanisms implicated in the delayed white matter involvement are not yet clearly understood. Although viral DNA has been detected in the brain of some patients and suggests a direct viral invasion, it has also been suggested that delayed white matter lesions may be the result of a chronic inflammatory process (such as edema or demyelination) complicating the first episode of HSE (87). An overlap between demyelinating lesions and anti-NMDAR encephalitis has been described in a cohort of patients (88). Indeed, patients with lesions in the white matter and atypical symptoms may also have anti-NMDAR encephalitis. Furthermore, patients with anti-NMDAR encephalitis may present either simultaneously or sequentially with demyelinating disorders. Patients with demyelinating disorders generally require more aggressive immunotherapies and are left with more severe neurological sequelae than those with anti-NMDAR encephalitis.
Overall, the mechanisms involved in the long-term immune activation that was reported in some patients who survived acute HSE should be investigated in more detail. This may allow the identification of potential targets for novel immunomodulatory strategies with the aim to improve the treatment of this disease.
IMMUNE RESPONSE TO HERPES SIMPLEX VIRUS
Innate Immune Response
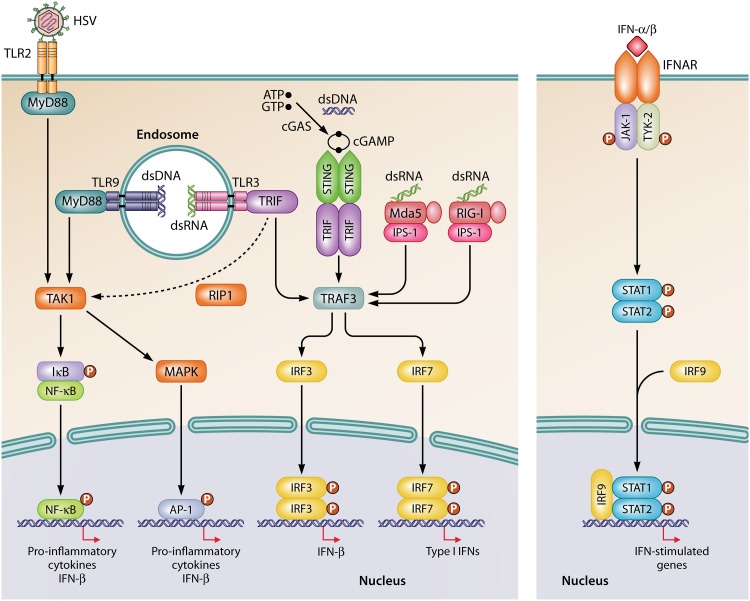
The innate immune system represents the first-line of host defense that restricts viral spread and mediates the activation of the ensuing adaptive response. The innate immune response is initiated by pathogen recognition receptors (PRRs), which detect conserved pathogen-associated molecular patterns (PAMPs) (89–91). Type I interferons (IFNs) are key players in the control of viral infection, and their expressions are induced mainly through viral nucleic acids. The signaling pathways implicated in the sensing of HSV are shown in Fig. 1. Toll-like receptors (TLRs) 2, 3, and 9 are involved in the recognition of HSV (92–94). Host cell surface TLR2 senses viral glycoproteins present in the envelope of HSV particles (95, 96). After entry into cells, endosomal TLR9 detects unmethylated CpG motifs contained in the genomic DNA of HSV (97–99). Viral dsDNA is also sensed by cyclic GMP-AMP (cGAMP) synthase (cGAS) in the cytosol (100). Once activated by dsDNA, cGAS catalyzes the synthesis of the cyclic dinucleotide cGAMP from ATP and GTP. The second messenger cGAMP binds to and mediates the activation of the adaptor protein stimulator of IFN genes (STING) (101, 102). Other cytosolic dsDNA sensors, such as interferon-inducible protein 16 (IFI16), absent in melanoma 2 (AIM2), and RNA polymerase III, have been suggested to contribute to the recognition of HSV (103). During its replication, HSV produces intermediate viral dsRNAs (104) that are sensed by endosomal TLR3 and cytosolic RNA helicases such as melanoma differentiation-associated gene 5 (Mda5) and retinoic acid-inducible gene I (RIG-I) (105–108). These PRRs signal through their respective adaptor proteins, namely, Toll-interleukin-1 receptor domain-containing adaptor inducing IFN-β (TRIF for TLR3) and IFN-β promoter stimulator 1 (IPS-1 for both Mda5 and RIG-I) (89). In addition to TLR3, the adaptor protein TRIF also interacts with STING to trigger an innate immune response to pathogens and thereby to allow a redundancy between these two signaling pathways (109). After recognition of viral components by these PRRs, the associated signaling pathways trigger the activation of the nuclear factor kappa light chain enhancer of activated B cells (NF-κB), IFN regulatory factor (IRF) family members, and activating protein 1 (AP-1). These transcription factors modulate the expression of cytokines, chemokines, and type I IFNs (92–94), which consist of one IFN-β molecule and 13 subtypes of IFN-α in humans (110). The initial type I IFNs produced bind to the IFN-α/β receptor (IFNAR) and act through autocrine and paracrine manners to activate components of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways (111) to induce the expression of IFN-stimulated genes encoding antiviral effectors that restrict viral spread and create an antiviral state (112).
FIG 1.
Signaling pathways involved in the recognition of HSV. Viral glycoproteins are sensed by TLR2 located at the cell surface, whereas the abundant CpG motifs present in the viral dsDNA are recognized by endosomal TLR9. Both TLR2 and TLR9 signal through the MyD88/TAK1 pathway to activate NF-κB and AP-1. In the cytosol, viral dsDNA also induces the synthesis of cGAMP by cGAS, which activates the STING/TRIF/TRAF3 signaling pathway. Intermediate dsRNA produced during viral replication is recognized by endosomal TLR3 or cytosolic RIG-I and Mda5 sensors that signal, respectively, through TRIF/TRAF3 or IPS-1/TRAF3 pathways to activate IRF3 and IRF7. Activated NF-κB, AP-1, or dimers of IRF3 and IRF7 induce the production of type I IFNs (IFN-α/β) and proinflammatory cytokines. IFN-α/β bind to the IFNAR receptor, and the signal is transduced to JAK-1 and TYK-2, which phosphorylate STAT1 and STAT2, respectively. The complex of phosphorylated STAT1 and STAT2 heterodimerizes with IRF9 and translocates to the nucleus to induce the transcription of interferon-stimulated genes.
In humans, the route by which HSV reaches the CNS most likely includes the olfactory nerve (113). The virus spreads from the olfactory mucosa through the cribriform plate of the ethmoid bone to the orbital side of the frontal lobe and the medial side of the temporal lobe. The trigeminal nerve is another suggested portal of entry for the virus to the brain, but its role remains unclear (114). The virus then infects and replicates in both neurons and glial cells. The innate immune system is essential to mount an initial and robust immune response to control the dissemination of the virus in the brain. Both nonhematopoietic and hematopoietic resident cells of the CNS participate in the innate immune response to HSV. For instance, TLR2 and TLR9 are detected in microglia and astrocytes, whereas TLR3 is expressed by microglia, neurons, astrocytes, and oligodendrocytes (115). The cGAS-STING signaling pathway is found in microglia and astrocytes (116). In vitro studies have shown that cytosolic Mda5 and RIG-I sensors are present in microglia, neurons, and astrocytes (117, 118). In the CNS, cGAS-STING and TLR3-TRIF are the main signaling pathways involved in the synthesis of type I IFNs in response to HSV-1 infection. Indeed, microglia initiate the production of type I IFNs through a cGAS-STING signaling pathway and orchestrate the innate antiviral response (116). Type I IFNs secreted by microglia then act in a paracrine manner to inhibit the replication of HSV-1 in highly permissive neurons. Furthermore, microglia induce the production of type I IFNs through the TLR3-TRIF pathway in astrocytes. The cross talk between microglia, neurons, and astrocytes through IFNAR signaling is proposed to constitute an innate immune barrier against viral infection of the CNS (119). It is also suggested that, during HSV-1 infection, activated TLR3 in neurons recruits the metabolic kinase complex mTORC2 (mammalian target of rapamycin complex 2), leading to the trafficking of TLR3 at the cell periphery. Peripheral TLR3 then interacts with tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3) and mTORC1 to induce the synthesis of IFN-β (120).
Microglia represent 5% to 20% of all glial cells in the different regions of the brain. Microglia exert a role of surveillance through their ramifications and maintain immune homeostasis in the brain (121). In cases of injury or invasion of pathogens in the CNS, microglia are the first line of defense and constitute the major phagocytic cells. Neurons infected with HSV-1 release purinergic mediators such as ATP, which are strong chemotactic signals for microglia (122, 123). The purinergic receptor P2Y12 at the surface of microglia senses purine nucleotides and induces the recruitment of microglia around infected neurons to contribute to viral clearance through its phagocytic activity (124). In response to viral infection, there is an increase in the number of P2Y12 receptors expressed at the surface of microglial cells. Then, the activation of microglia induces their proliferation and increases their phagocytic activity. In the temporal lobe of patients with HSE, P2Y12-positive microglia are located in proximity of HSV-1-positive cells, and it is estimated that one to three activated microglia are present around each infected neuron (124).
The CNS constitutes an immune-privileged site protected by the BBB, which is formed by microvascular endothelial cells sealed with tight junctions, pericytes, a basement membrane shared by pericytes and endothelial cells, and astrocyte end-feet (125). The BBB is involved in the control of the trafficking of leukocytes from the blood to the brain. During HSE, the BBB is disrupted, leading to vascular brain edema and hemorrhage. HSV-1 infection has been shown to induce structural and functional changes to all constituents of the BBB (126). Activated microglia also express mediators of inflammation such as chemokines (e.g., CCL2, CCL3, and CCL5), which induce a marked mobilization of peripheral immune cells to affected regions of the brain (124). Brain resident cells and infiltrating immune cells release cytokines (such as IL-1β, IL-6, IL-8, IL-10, tumor necrosis factor-α [TNF-α], and IFN-γ) that are implicated in the control of viral spread in the CNS. Furthermore, microglia, macrophages, and neutrophils increase the respiratory burst of reactive oxygen species (ROS) and express inducible nitric oxide synthase (iNOS), which leads to the production of nitric oxide (NO) (127). The production of both ROS and NO contributes to the control of viral spread. However, the inflammatory response that develops during HSE may become exaggerated and further increase cerebral damage.
Adaptive Immune Response
Following the spread of HSV to the brain, effector CD4+ T cells and especially CD8+ T cells infiltrate the CNS and, once activated, play a pivotal role in the control of the infection (15, 16). The function of CD8+ T cells involves the synthesis of cytokines and chemokines and the rapid and robust production of IFN-γ (127), a potent antiviral mediator (128). For instance, IFN-γ inhibits neuronal apoptosis by increasing the expression of the proto-oncogene bcl-2 and thereby protects neurons from massive destruction during HSE (129). CD8+ T cells also participate in the clearance of cells infected with HSV-1 through recognition of virus-derived peptides displayed at the surface of infected cells in the context of major histocompatibility complex class I (MHC-1) by the T-cell receptor. Immunohistochemistry analysis of the hippocampus from patients with HSE showed viral antigens in the nuclei and cytoplasm of astrocytes, cortical neurons, and oligodendrocytes (130). Cytotoxic CD8+ T cells are recruited to the CNS and interact with HSV-1-infected cells. The cytoplasm and granzyme B granules demonstrate polarization toward infected cells, suggesting their active killing. Analysis of apoptotic pathways suggests that virus-infected cells die by caspase-mediated apoptosis most likely induced by granzyme B released by cytotoxic T cells. Furthermore, another mechanism of apoptosis could involve the binding of Fas ligand (FasL) expressed at the surface of CD8+ cells to Fas (a member of the TNF-α receptor family) that is present at the surface of virus-infected cells (131).
Following retrograde axonal transport from the primary site of infection, HSV-1 migrates to the trigeminal ganglia, where it establishes lifelong latency. HSV-1 genomic sequences are detected in trigeminal ganglia of 65% of immunocompetent individuals as well as in the brain of 35% of them (132). Expanded virus-specific CD8+ T cells infiltrate the trigeminal ganglia and surround neurons infected with HSV-1 to prevent reactivation by maintaining the virus in a latent state (133). CD8+ T cells specific to HSV can persist in trigeminal ganglia for life without being replenished by the circulating CD8+ T cell pool. The rate of HSV reactivation depends on the number of latently infected neurons and the number of CD8+ T cells that infiltrated the trigeminal ganglia (134).
GENETIC PREDISPOSITION TO DEVELOP HSE
Children with primary immunodeficiencies affecting the responses of B or T lymphocytes do not present an increased risk for developing HSE (135). In contrast, deficiencies that affect the number or function of natural killer cells have been suggested to predispose children to severe HSE (136). Several genetic etiologies related to the intrinsic immunity to HSV-1 have also been reported to predispose children and adult patients to develop HSE, suggesting a key role of the innate immune response to limit viral spread in the CNS. An autosomal recessive deficiency in UNC93B, a protein involved in the translocation of TLRs 3, 7, 8, and 9 from the endoplasmic reticulum to the endosomes (137), has been reported in children with HSE (138). However, patients with mutations in IL-1 receptor-associated kinase-4 (IRAK-4), which is implicated in the signaling pathways of all TLRs except TLR3, were not more susceptible to viral infections, suggesting that sensing through TLRs 7, 8, and 9 may be redundant for protection against these infections (139). The implication of TLR3 in the susceptibility to HSE was definitely highlighted by the identification of several autosomal dominant or autosomal recessive mutations in the TLR3 gene in children (140–143) and adults (142, 144, 145). Several reports describing a series of deficiencies in the TLR3-dependent signaling pathway further confirmed the importance of this axis in protective immunity against HSE. For instance, mutations in the TLR3 adapter protein TRIF and in the transcription factor IRF3 have been described in children and adults with HSE (144, 146, 147). Furthermore, an autosomal dominant mutation in TRAF3, which is implicated in the production of type I and III IFNs downstream of several TLRs, RIG-I, and Mda5, was detected in a child with HSE (148). Mutations in TANK-binding kinase 1 (TBK1), which plays a role at the crossroads in the sensing of dsRNA and dsDNA, have also been identified in children and adults with HSE (144, 149). It is estimated that 10% of children with HSE have inborn errors in the TLR3 signaling pathway, and approximately 66% of these patients develop relapses (142). Mutations in NF-κB essential modulator (NEMO), STAT1, and tyrosine kinase 2 (TYK-2) affect the production of type I and III IFNs as well as that of other cytokines. These mutations are thus associated with an increased host susceptibility to a broad range of infections, including HSE (144, 150, 151).
All of these mutations result in altered expression of a functional protein (such as premature termination or lack of cleavage) or a functional loss of the protein. This leads to impaired synthesis of IFN-β and -λ in patients’ fibroblasts after infection with HSV-1 or stimulation with poly(I·C) (which mimics dsRNA), which can be rescued with exogenous IFN-α2b.
The clinical penetrance of deficiencies in the TLR3 signaling pathway is incomplete, as relatives of HSE patients harboring the same mutation and infected with HSV-1 have not developed the disease, suggesting that host (age, etc.)- and pathogen (viral load and inoculum)-related factors may also be involved. Children with deficiencies in the TLR3 signaling pathway suffering from HSE do not present an increased susceptibility to other viral infections. This suggests that the IFN responses to other viral components mediated by signaling pathways independent from TLR3 may confer protection against infections caused by a broad range of viruses in these patients. Furthermore, these children do not have an increased susceptibility to HSV-1 infections at peripheral sites. This suggests that the function of TLR3 may be nonredundant for protection against a primary infection with HSV-1 in the CNS, whereas it may be widely redundant as a host defense mechanism outside the CNS (140, 141). It is proposed that the restriction of HSE susceptibility to the CNS results from impaired production of type I IFNs dependent on TLR3 by resident nonhematopoietic cells infected with HSV-1. Indeed, induced pluripotent stem cells (iPSCs) derived from patients deficient for TLR3 and UNC93B that were differentiated into neurons and oligodendrocytes (but not astrocytes) were more permissive to HSV-1 infection than those derived from control individuals (152). Viral infection might be inhibited by pretreating these cells with exogenous IFN-α or IFN-β. Thus, intrinsic immunity mediated by nonhematopoietic cells of the CNS may exert a more important role in protection against primary infection with HSV-1 than the immune response induced by hematopoietic cells.
Recently, two cases of HSE have been reported in adult patients harboring mutations in mannan-binding lectin serine protease 2 (MASP-2), the central activator of the lectin pathway of the complement system (153). The lectin pathway is involved in the recognition of mannan and carbohydrate structures present on the surface of pathogens by PRRs such as mannose-binding lectin, collectins, and ficolins (154). MASP-2 has the ability to form complexes with these PRRs and initiates the cleavage of C4 and C2, which are involved in opsonization, inflammation, and lysis of infected cells. The first identified mutation was shown to reduce the secretion of the protein, whereas the second one resulted in an abolished protein secretion associated with an inability to cleave MASP-2 precursor into its active form, which led to decreased antiviral activity (153).
OCCURRENCE OF HSE AFTER IMMUNOMODULATORY THERAPY
An increased risk of viral encephalitis was reported after the administration of OKT-3 or alemtuzumab for T-cell depletion in allogeneic stem cell transplant recipients (155). Viruses that were identified in the CSF included human herpesvirus 6 (28%), Epstein-Barr virus (19%), HSV (13%), JC virus (9%), cytomegalovirus (6%), varicella-zoster virus (6%), and adenovirus (3%). Two viruses or more were detected in the CSF of 16% of these patients. A retrospective study of cancer patients with PCR-proven HSE as well as several case reports has indicated that immunosuppressive treatments for metastatic brain diseases by concomitant brain radiation and use of corticosteroids may increase the risk of developing HSE (17, 51, 156–163). The presentation of HSE may be atypical in this population of immunosuppressed patients. An increased risk level may be associated with each separate intervention, as patients receiving whole-brain radiation therapy (164, 165) or high-dose dexamethasone (162) alone have been shown to develop HSE. The administration of both dexamethasone and cyclophosphamide has been used to induce viral reactivation in rabbits infected intranasally with HSV-1 (166). However, it is difficult to attribute this effect to dexamethasone, as cyclophosphamide was concomitantly administered. A study suggested that long-term treatment with high doses of corticosteroids may reduce CD4+ T cell counts and further increase the risk of infections during radiation therapy (167).
Furthermore, the use of several immunomodulatory drugs for the treatment of immune disorders has been reported to predispose patients to develop HSE, although the associated risk level is not defined. Azathioprine is indicated for the treatment of inflammatory bowel diseases. It has been suggested that azathioprine and its metabolites induce apoptosis of T lymphocytes, which could predispose patients to viral infections and, in particular, to HSE. A first patient treated with azathioprine and prednisolone for acute exacerbation of ulcerative colitis developed severe HSE with brain herniation (168). A second patient who received azathioprine for Crohn’s disease was diagnosed with HSE involving both temporal lobes (169). Both patients were treated with acyclovir and recovered normal neurologic functions. TNF-α inhibitors are indicated for the treatment of rheumatologic disorders, inflammatory bowel diseases, and psoriasis.
The multifunctional cytokine TNF-α induces and regulates host innate and adaptive immune responses. Mice deficient in TNF-α were more susceptible to HSE than wild-type animals due to increased viral replication in the brain (170, 171). A series of three patients treated with anti-TNF-α monoclonal antibodies were reported to develop HSE (172). The first patient under infliximab treatment presented with focal inflammatory changes in the right temporal lobe and edema. After acyclovir therapy, the patient was left with mild neuropsychiatric alterations that disappeared after 1 year. The other two patients received corticosteroids and adalimumab and presented with edema and inflammation in the right temporal lobe and involvement in both temporal lobes, respectively. Both patients were left with neuropsychiatric disabilities after acyclovir therapy. Another patient who received adalimumab developed acyclovir-resistant HSE although the patient was naive for this antiviral (47). Lesions were localized in the right medial temporal lobe, right insular cortex, and subcortical white matter and then progressed in both hemispheres. The patient was successfully treated with a combination of acyclovir and foscarnet. A patient receiving etanercept (i.e., a fusion protein made of the extracellular domain of human TNF receptor 2 and the Fc end of human IgG1) developed HSE (173). MRI scans showed lesions in the right fronto-temporoparietal region with the presence of edema. The patient was treated with acyclovir combined with dexamethasone to decrease edema and inflammation.
Fingolimod is indicated for the treatment of multiple sclerosis. It is an antagonist of sphingosine-1-phosphate receptor, which induces receptor internalization and makes T and B cells unable to egress from lymph nodes, leading to reduced numbers of peripheral lymphocytes. It has been suggested that fingolimod may compromise the immune response against latent HSV in the CNS. During a phase 3 study, one patient treated with fingolimod for relapsing-remitting multiple sclerosis was diagnosed with HSE (174). Acyclovir was initiated 1 week after presentation, but the patient died 2 months later. Another patient had severe HSE while he was treated with fingolimod (175). MRI scans demonstrated signs of nonhemorrhagic encephalitis of the archeocortical areas of both hemispheres. The patient was treated with acyclovir but presented with severe neurological sequelae at the 9-month follow-up.
HSE was also diagnosed in a patient treated with dimethyl fumarate for relapsing-remitting multiple sclerosis and who presented with a marked and rapid decrease in lymphocyte count (176). The clinical picture was similar to that observed in immunocompromised patients with a lower CSF pleocytosis, cortical lesions distributed bilaterally, and less extensive tissue necrosis. After acyclovir treatment, the improvement was considered incomplete, with a mild short-term memory deficit and emotional disorder.
Natalizumab is used in the treatment of multiple sclerosis and Crohn’s disease. It is a monoclonal antibody targeting α-4 integrin, a cell adhesion molecule which interferes with the migration of peripheral cells to the CNS, resulting in a decreased CD4+/CD8+ T cell ratio in the CSF compared to that in the blood, similar to what has been reported in HIV-infected individuals (177). This leads to impaired immune surveillance in the CNS. A case series of 20 patients treated with natalizumab for multiple sclerosis and who developed laboratory-confirmed herpesvirus infections, with half of the cases presenting HSE, has been reported (178). Among the 10 HSE cases, 5 were caused by HSV-1, two unusual cases were due to HSV-2, and the last three cases remained nontyped. Furthermore, it has been reported that natalizumab therapy in a patient with multiple sclerosis induced HSE with lesions in both temporal lobes (179). The patient was treated with acyclovir but was left with persistent and severe memory defects, anosognosia, and mild aphasia. Another case presented with atypical lesions restricted to the parietal lobe, which are characteristic in immunocompromised patients (180). The patient was successfully treated with acyclovir and steroid (initiated 1 day later). The last patient under natalizumab treatment had more conventional HSE, with lesions in the right medial temporal lobe and insular cortex extending to the lentiform nucleus, and responded well to acyclovir therapy (181).
Atypical clinical and radiological features that may occur in these immunosuppressed patients could eventually lead to misdiagnosis or a delay in diagnosis, with a negative impact on the prognosis of HSE, since acyclovir therapy should be initiated early.
Most of the immunomodulatory drugs reported to increase the risk of patients to develop HSE act through a reduction in the number of lymphocytes that are crucial for the maintenance of HSV in a latent state. HSE generally occurs after long-term treatment of patients with these immunomodulatory agents, and in most cases, more than one drug or whole-brain radiation was used concomitantly, which may further enhance the risk to induce viral reactivation.
IMMUNOMODULATORY STRATEGIES
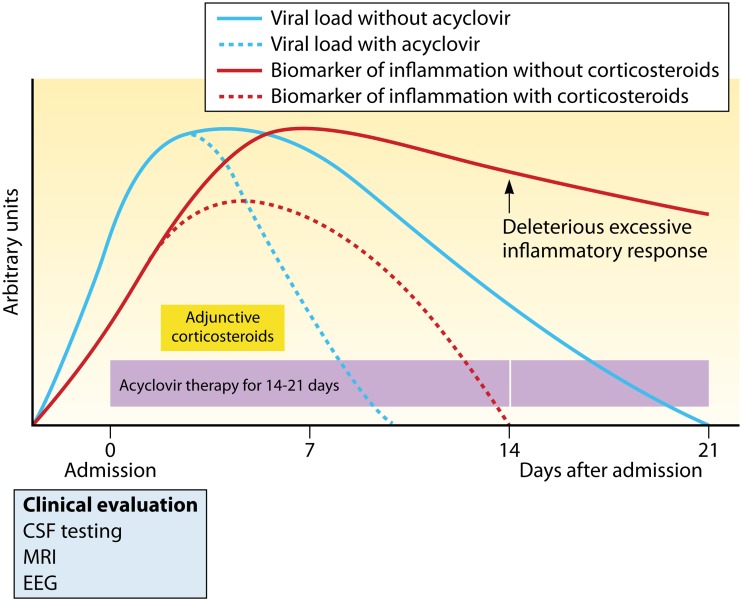
The immune response that is induced in the brain during HSE should limit viral replication early after infection and should be controlled thereafter to prevent the development of an exaggerated inflammatory response that may be detrimental for a sensitive organ such as the brain. The host immune response that develops during HSE is thus considered a “double-edged sword” and must be finely balanced. Immunomodulatory drugs should be administered at a critical time during HSE to reduce the late-onset excessive inflammation of the CNS and thereby limit the risk of developing neurological sequelae. Such immunomodulatory strategies should be combined with antiviral therapy to reduce both viral replication and the inflammatory response.
Clinical and Animal Studies with Corticosteroids
Glucocorticoids are broad-spectrum anti-inflammatory agents which act via genomic and nongenomic mechanisms (182). The genomic mechanism of action of glucocorticoids involves the interaction of the glucocorticoid receptor with transcription factors such as AP-1 and NF-κB, which leads to the repression of proinflammatory genes. In a nongenomic mechanism, glucocorticoids can modulate signal transduction pathways through interaction of the glucocorticoid receptor with several kinases, such as phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and AKT. Conversely, glucocorticoids can also exert proinflammatory effects in response to injury or pathogen invasion. Rats depleted in circulating glucocorticoids by adrenalectomy, hypophysectomy, or pharmacological blocking of its receptor were inoculated in the cerebral ventricle with HSV-1 1 day later (183). The brains of rats depleted in circulating glucocorticoids exhibited similar viral titers and decreased synthesis of IL-1β and prostaglandin E2 in comparison to control animals. Of note, prostaglandin E2 is implicated in the downregulation of microglial activation and cytokine production (184). Interestingly, this was associated with less fever, motor activity, and aggressive behavior, suggesting a role of the host response in behavioral deterioration during HSE.
Although we do not advocate the use of corticosteroids in the absence of a full course of acyclovir therapy, we reviewed case reports and series that describe the use of corticosteroids alone to decrease cerebral edema, intracranial pressure, and brain shift in children (185) and adults (186–188) suffering from HSE. These reports were published before the availability of acyclovir and demonstrated that corticosteroids may improve the prognosis of this disease in some cases (Table 1). A retrospective analysis of patients with HSE together with a review of published cases also indicated that treatment with adenocorticotropic hormones or corticosteroids improves the outcome in some patients (189). Thereafter, several case reports and series described the outcome of patients with HSE treated with acyclovir and adjunctive corticosteroid therapy. A 4-year-old boy who developed atypical acute encephalopathy with bilateral striatal necrosis was successfully treated with prednisolone, followed 5 days later with acyclovir (190). A 16-month-old girl with clinical and radiologic deterioration of HSE was treated with acyclovir and high-dose methylprednisolone and exhibited a favorable outcome (191). A pregnant woman with deteriorating HSE despite acyclovir therapy was successfully treated with adjunctive dexamethasone (192). In a first case series of five patients with HSE, three were successfully treated with acyclovir combined with pulse steroid therapy started 5 days after onset of the disease whereas two other patients who received pulse steroid therapy after 3 weeks did not improve and died from infection (193). In another case series of six children suffering from HSE, three patients who received adjunctive steroid therapy had improved cognition, motor function, and control of seizures compared to the three other children who were treated with acyclovir alone, but the radiologic abnormalities were similar in both groups (194). A nonrandomized retrospective study evaluated the efficacy of steroids on the outcome of HSE in 45 adult patients (195). This is the largest study showing that administration of adjunctive dexamethasone or prednisolone (22 patients) results in a better neurological outcome than treatment with acyclovir alone (23 patients) whereas older age and a low GCS at admission resulted in a worse prognosis of the disease. Further analysis showed that the better outcome of these patients may be due to a lower production of proinflammatory cytokines such as IL-6 (196).
TABLE 1.
Case reports and series of patients with HSE treated with corticosteroids alone or in combination with acyclovira
| Reference | Brain involvement, clinical sign(s), or patient group | Age | Single or combined therapy and dose(s) | Clinical outcome | Neurological disability or outcome |
|---|---|---|---|---|---|
| 185 | Both hemispheres | 35 mo | DEX at 8 mg i.v. and then at 4 mg i.v. 4× daily for 17 days | Improved | Mild |
| Meningoencephalitis | 20 mo | DEX at 2 mg i.v. and then at 2 mg i.m. 4× daily for 3 days | Improved | Moderate | |
| 186 | Right temporal lobe | 35 yr | Hydrocortisone at 200 mg i.v. for 10 days | Improved slowly | Mild |
| Right temporal lobe | 70 yr | Hydrocortisone for 30 days | Improved | Severe | |
| 187 | Left temporal lobe | 20 yr | DEX at 5 mg 4× daily for 7 days | Improved | Mild |
| Left temporal lobe | 32 yr | DEX at 5 mg 4× daily for 7 days | Improved slowly | Severe | |
| 188 | Right temporal lobe | 47 yr | Adrenal corticosteroids, high dose i.v. | Improved | Recovered |
| Right temporal lobe | 50 yr | Adrenal corticosteroids, high dose, + IDU | Improved | Recovered | |
| 189 | 23 patients with HSE from the literature and personal cases | 5 mo to 77 yr | ACTH at 40–80 U daily or cortisone at 75 mg daily or hydrocortisone at 300–400 mg daily or prednisolone at 40 mg daily or DEX at 16–20 mg daily | 7 improved, 7 improved with IDU, 2 improved slowly, 7 no change | 12 recovered, 1 severe, 10 died |
| 190 | Acute encephalopathy with bilateral striatal necrosis | 4 yr | Prednisolone at 30 mg i.v. 1× daily for 30 days; 5 days after initiation of prednisolone, ACV at 10 mg/kg i.v. 3× daily for 14 days | Improved | Recovered |
| 191 | Focal edema in bilateral parieto-frontal lobes, cerebellum, and left thalamus | 16 mo | ACV at 1,500 mg/m2 i.v. for 21 days; 9 days later, methylprednisolone pulse therapy i.v. at 1 g/1.7 m2 for 3 days | Improved | Mild |
| 192 | Right temporal lobe | 30 yr | ACV at 750 mg i.v. 3× daily for 21 days; DEX at 10 mg 4× daily for 4 days | Improved | Recovered |
| 193 | Five patients with altered consciousness, tremor, rigidity, and seizure | ACV at 1,500 mg/day i.v. plus methylprednisolone at 1,000 mg/day i.v. for 3 days, started 5 days after onset of disease (3/5 patients) | 3 improved | 3 recovered | |
| ACV at 1,500 mg/day i.v. plus methylprednisolone at 1,000 mg/day i.v. for 3 days, started 3 weeks after onset of disease (2/5 patients) | 2 no change | 2 died | |||
| 194 | Case series of 6 patients with HSE | 4 mo to 10 yr | ACV at 15 mg/kg 3× daily for 21–28 days (3 patients) | 3 improved | 2 severe, 1 mild |
| ACV at 15 mg/kg 3× daily for 21–28 days (3 patients) plus pulse steroid methylprednisolone at 30 mg/kg/day for 1–3 days, followed by methylprednisolone orally for 2 weeks (2/6 patients), or prednisolone at 2 mg/kg/day i.v. (1/6 patients) | 3 improved | 3 mild | |||
| 195 | Case series of 45 patients with HSE | 17 yr to 77 yr | ACV at 10 mg/kg 3× daily for 14 days (23/45 patients) | 13 poor outcome, 10 good outcome | 8 recovered, 2 mild, 2 moderate, 6 severe, 5 died |
| ACV at 10 mg/kg 3× daily for 14 days plus DEX or prednisolone at 40–96 mg/day for 2 to 42 days (22/45 patients) | 6 poor outcome, 16 good outcome | 6 recovered, 10 mild, 6 moderate |
ACTH, adenocorticotropic hormone; ACV, acyclovir; DEX, dexamethasone; IDU, idoxuridine; i.m., intramuscular; i.v., intravenous; HSE, herpes simplex virus encephalitis; ×, times. Mild, moderate, and severe describe neurological disabilities.
Different animal models that mimic the pathogenesis of HSE in humans have been established (197). After infection, the virus invades the CNS, and its replication is associated with an important inflammatory response. These animal models are thus convenient to study the efficacy of corticosteroids or other immunomodulatory drugs to ameliorate the outcome of HSE, as reported in Table 2. The first study evaluated the effect of subcutaneous injections of methylprednisolone started 1 day prior to inoculation of HSV-1 on the scarified cornea of rabbits and continued until their sacrifice (198). Treatment with steroids did not markedly affect viral replication. Previous studies from our laboratory showed that the delayed administration of glucocorticoids initiated at the onset of symptoms (i.e., on day 3 postinfection) in mice infected intranasally with HSV-1 decreased the viral burden and cytokine production in the brain and was associated with an increased survival rate (199). In contrast, early glucocorticoid treatment started the day of infection (day 0) increased the mortality rate. It is generally accepted that treatment with steroids should be initiated as early as possible in CNS infections such as bacterial meningitis. In contrast, our study demonstrated that administration of immunomodulatory agents should be delayed after the onset of symptoms to ameliorate the outcome of HSE.
TABLE 2.
Animal studies evaluating the effects of corticosteroids or experimental immunomodulatory agents alone or in combination with acyclovir for HSEb
| Reference | Animal model | Single or combined therapy and dose(s) | Outcome |
|---|---|---|---|
| 198 | Rabbits inoculated on scarified cornea with HSV-1 | Methylprednisolone at 10 mg/kg s.c. 1× daily one day before infection and until sacrifice | Slightly delayed brain viral clearance in treated rabbits vs untreated rabbits |
| 199 | BALB/c mice infected i.n. with HSV-1 | Corticosterone at 0.2 mg/ml ad libitum from day 0 to 14 p.i. | Increased mortality rate in treated mice vs untreated mice |
| Corticosterone at 0.2 mg/ml ad libitum from day 3 to 17 p.i. | Decreased mortality rate in treated mice vs untreated mice | ||
| DEX at 10 mg/kg i.p. 1× daily from day 3 to 17 p.i. | Decreased mortality rate, viral replication, and cytokine synthesis in treated mice vs untreated mice | ||
| 200 | Rats inoculated in cervical vagus nerve with HSV-1 | ACV at 30 mg/kg i.p. 2× daily from day 0 to 3 p.i.; DEX at 5 mg/kg i.p. 2× daily from day 0 to 3 p.i. | Reduced viral antigen staining in rats treated with ACV alone or combined with DEX vs untreated rats |
| 201 | SJL mice infected i.n. with HSV-1 | Cortisone at 40 mg/kg i.p. 1× daily from day 1 to 15 p.i.; ACV at 50 μg/g i.p. 1× daily from day 1 to 15 p.i. | Decrease in brain viral load and iNOS mRNAs in mice treated with ACV alone or combined with cortisone vs untreated mice |
| 202 | SJL mice infected i.n. with HSV-1 | ACV at 25 mg/kg i.p. 2× daily on day 0 for 14 days alone or with methylprednisolone at 20 mg/kg i.p. 2x daily for 7 days | Decrease in brain viral load in mice treated with ACV alone or combined with methylprednisolone vs untreated mice; reduction of long-term brain MRI abnormalities in ACV- and methylprednisolone-treated group |
| 203 | BALB/c mice infected i.n. with HSV-1 | Agonist of TLR3, poly(I·C), at 50 μg/mouse i.p. 1× one day before infection | Increased survival rate in poly(I·C) group vs untreated group; increased early expression of several immune genes and reduced brain viral load in poly(I:C) group vs untreated group |
| Agonist of TLR9, ODN, at 50 μg/mouse i.n. 1× one day before infection | Increased survival rate in ODN-treated group vs untreated group | ||
| 120 | BALB/c mice infected i.c. with HSV-1 | Agonistic antibody to TLR3 at 20 μg/mouse i.c. 1× the day of infection | Increased survival rate, decreased brain viral titers, and increased CCL5 and IFN-β mRNAs in treated mice vs untreated mice |
| 204 | BALB/c mice infected i.c. with HSV-1 | Corilagin at 0.4 mg/mouse intragastrically 1× at 1 hpi | In infected mice, corilagin suppresses mRNAs of TLR2 and its downstream mediators and reduces the synthesis of TNF-α and IL-6 vs untreated mice |
| 205 | BALB/c mice infected i.c. with HSV-1 | Corilagin at 40 mg/kg intragastrically 1× at 1 hpi | In infected mice, corilagin suppresses mRNAs of TLR3 and its downstream mediators and reduces the synthesis of TNF-α, IL-6, and IFN-β vs untreated mice |
| 206 | BALB/c mice infected i.n. with HSV-1 | Agonist of TLR9, ODN 2395, at 50 μg/mouse i.n. 1× one day before infection | Reduced mortality rate, viral load, and production of IL-6, CCL2, and CCL5 in brains of mice treated with ODN 2395 vs those of untreated mice |
| Antagonist of TLR9, ODN 2088, at 50 μg/mouse i.n. 1× on day 3 p.i. | Reduced mortality rate and production of cytokines in mice treated with ODN 2088 vs untreated mice | ||
| 207 | C57BL/6J mice inoculated on scarified cornea with HSV-1 | STING agonist, DMXAA, at 25 mg/kg i.p. 1× one day before infection and on days 1, 3, and 5 p.i. | Increased survival rate and reduced brain viral burden and neurological symptoms in DMXAA-treated group vs untreated group |
| 210 | BALB/c mice infected i.n. with HSV-1 | VACV at 1 mg/ml in drinking water ad libitum from day 3 to 21 p.i. alone or with artesunate at 30 mg/kg i.p. 1× daily from day 4 to 13 p.i. or with the mTOR inhibitor rapamycin at 20 mg/kg i.p. 1× daily from day 4 to 13 p.i. | Increased survival rate in groups treated with VACV combined with artesunate or rapamycin vs group treated with VACV alone; brain viral titers were similar between treated groups; reduced production of cytokines/chemokines in group treated with VACV and artesunate vs group treated with antiviral alone; increased production of IL-1β, IL-6, and IFN-γ at peak of infection in group treated with VACV and rapamycin vs group treated with VACV alone |
| 212 | BALB/c mice infected i.n. with HSV-1 | Anti-TNF-α inhibitor, etanercept, at 400 μg/mouse i.p. 1× on day 3 p.i.; VACV at 1 mg/ml of drinking water ad libitum from day 3 to 21 p.i. | Increased survival rate in group treated with etanercept and VACV vs group treated with antiviral therapy alone; no difference in brain viral titers between mice treated with VACV alone and those treated with VACV combined with etanercept |
| 213 | BALB/c mice infected i.n. with HSV-1 | Antioxidant inducer, sulforaphane, at 50 mg/kg i.p. 1× daily from day 3 to 6 p.i. | No difference in brain viral titers between mice treated with sulforaphane and untreated animals; reduced microglial activation, infiltration of macrophages and neutrophils in the brain, and ROS production in treated group vs untreated group |
| 201 | SJL mice infected i.n. with HSV-1 | iNOS inhibitor, N-nitro-l-arginine, at 100 mg/kg i.p. 1× daily from day 1 to 15 p.i.; ACV at 50 μg/g i.p. 1× daily from day 1 to 15 p.i. | Reduced brain viral load and expression of iNOS mRNAs in mice treated with N-nitro-l-arginine and ACV vs untreated mice |
| 217 | BALB/c mice infected i.c. with HSV-1 | MMP-9 siRNA at 5 μg/mouse i.c. 1× on day 1 p.i.; ACV at 100 mg/kg i.p. 2× daily from day 1 to 8 p.i. | Reduced clinical signs and mortality in mice treated with siRNA and ACV vs antiviral alone; no difference between HSV-1 gD gene expression in siRNA-treated mice vs untreated; decreased Evans blue uptake, brain water content, and perivascular aquaporin 4 level in siRNA-treated mice vs untreated; decreased TNF-α and IL-6 in siRNA group vs untreated mice |
| 218 | BALB/c mice infected i.c. with HSV-1 | Inhibitor of angiotensin-converting enzyme, captopril, at 50 mg/kg 1× on day 1 p.i. | Reduced HSV-1-induced ROS release and brain water content in treated group vs untreated group; increased neurological function in treated mice vs untreated mice |
| 219 | BALB/c mice infected i.n. with HSV-1 | IVIG at 40 to 50 mg/ml in 500 μl i.p. 1× one day before or after infection | Increased survival rate in IVIG group vs untreated group; no difference in brain viral titers, although IVIG blocked the production of neutralizing antibody |
| 220 | 129S6 mice infected with HSV-1 after corneal scarification | IVIG at 3.75 mg/mouse i.v. 1× on day 1 p.i. | Increased survival rate in IVIG group vs untreated group; minor role of neutralizing antibodies; reduced infiltration of inflammatory and anti-inflammatory monocytes in the brain; increased IL-10 synthesis by CD4+ T cells in the brain |
| 221 | C57BL/6J mice infected with HSV-1 after corneal scarification | IVIG at 25 mg/mouse i.p. 1× daily from day 4 to 11 p.i.; ACV at 50 mg/kg i.p. 1× daily from day 4 to 11 p.i. | Increased survival rates in mice treated with ACV alone or in combination with IVIG vs untreated mice; antagonistic effect of IVIG and ACV in learning and memory tests |
| 230 | Swiss Webster mice infected i.n. with HSV-1 | Recombinant human IFN-α at 100,000 IU i.p. 2× daily from day 1 to 6 p.i.; ACV at 7.5 mg/kg i.p. 1× daily from day 1 to 6 p.i. | Increased survival rate in mice treated with ACV and recombinant human IFN-α vs those treated with ACV alone |
ACV, acyclovir; DEX, dexamethasone; DMXAA, 5,6-dimethylxanthenone-4-acetic acid; gD, glycoprotein D; HSV-1, herpes simplex virus 1; i.c., intracerebral; i.n., intranasal; iNOS, inducible nitric oxide synthase; i.p., intraperitoneal; IVIG, intravenous immunoglobulins; MMP-9, matrix metalloproteinase 9; MRI, magnetic resonance imaging; mTOR, mammalian target of rapamycin; ODN, oligodeoxynucleotide; p.i., postinfection; poly(I·C), poly(I):poly(C); ROS, reactive oxygen species; s.c., subcutaneous; siRNA, silencing RNA; STING, stimulator of interferon genes; TLR, Toll-like receptor; VACV, valacyclovir; hpi, hours postinfection; ×, times.
The effects of combinations of acyclovir and corticosteroids were also evaluated during experimental HSE. Intraperitoneal administration of dexamethasone alone or combined with acyclovir in rats infected with HSV-1 in the cervical vagus nerve resulted in reduced staining of viral antigens in the brain by immunohistochemistry on day 3 postinfection (200). Combinations of acyclovir and cortisone administered intraperitoneally to mice 1 day after intranasal infection with HSV-1 for 14 days decreased the viral load and the expression of iNOS transcripts in the brain (201). In both of these studies, the brain viral loads were not significantly different between the groups treated with acyclovir alone or acyclovir combined with corticosteroids, indicating that the immunomodulatory drug does not increase viral replication or inhibit the effect of the antiviral. More interestingly, the severity of long-term MRI abnormalities was significantly reduced in the brains of mice that received a combination of acyclovir and methylprednisolone compared with those receiving antiviral therapy alone (202).
However, clinical trials evaluating the benefit of adjunctive corticosteroids are still needed before this approach can be translated into clinical practices. A multinational, randomized, controlled trial (DexEnceph; ClinicalTrials.gov identifier NCT03084783) evaluating the benefit of administering intravenous dexamethasone (10 mg every 6 h for 4 days) and a standard full course of intravenous acyclovir (10 mg/kg three times daily for at least 14 days) compared to that of standard antiviral therapy alone to adult patients suffering from HSE is ongoing. The administration of dexamethasone should be initiated within 24 h postrandomization. The primary outcome will be a verbal memory score assessed using the Wechsler Memory Scale (WMS-IV) Auditory Memory Index at 6 months after randomization. However, results are not expected until 2021.
Other Experimental Immunomodulatory Strategies
The efficacy of a series of experimental immunomodulatory strategies to ameliorate the prognosis of HSE has been evaluated in animal models, as described in Table 2. The administration of agonists or antagonists of TLRs before or after infection may modulate the immune response and result in a better HSE outcome. For instance, administration of poly(I·C) to stimulate the TLR3 signaling pathway before intranasal challenge of mice with HSV-1 decreased the brain viral load and increased the survival rate (203). An agonistic antibody to TLR3 administered intracerebrally together with HSV-1 also increased the survival rate of mice by decreasing brain viral titers and increasing the expression of CCL5 and IFN-β mRNAs on day 3 postinfection (120). The immunomodulatory drug corilagin administered by the gastric route to mice infected with HSV-1 prevents brain histopathological changes induced by the virus. The mechanisms may involve a reduction in the expression of TLR2, TLR3, and other protein mediators involved in the associated signaling pathways, resulting in decreased synthesis of IL-6, TNF-α, and IFN-β and thereby less inflammation in the CNS (204, 205). The administration of agonists of TLR9 (oligodeoxynucleotides [ODNs] that contain unmethylated CpG motifs) before intranasal challenge of mice with HSV-1 increased the survival rate (203) by reducing the viral load and cytokine levels in the brain (206). More interestingly, the administration of an antagonist of TLR9 at the onset of symptoms (i.e., on day 3 postinfection) increased the mouse survival rate likely by reducing the inflammatory response in the brain (206). The majority of cytoplasmic sensing pathways signal through STING to induce the synthesis of IFN-β. Pretreatment of mice with a murine STING agonist, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), before corneal challenge with HSV-1 resulted in a decreased brain viral burden, delayed and reduced neurological symptoms, and improved mouse survival (207).
Apart from compounds acting on pathways involved in the recognition of HSV, other immunomodulatory strategies aimed at reducing the inflammatory response during HSE were also evaluated in animal models. Artesunate and the mTOR inhibitor rapamycin are immunomodulatory drugs occasionally used for the treatment of infections caused by multidrug-resistant herpesviruses (cytomegalovirus and HSV) in immunocompromised patients (208, 209). Our group evaluated the efficacy of combining valacyclovir with artesunate and rapamycin to improve the outcome of HSE in mice (210). Valacyclovir treatment was started on day 3 postinfection for 21 days, whereas the administration of artesunate or rapamycin was initiated 1 day later for 9 days. Drug combinations did not affect the viral load compared to antiviral therapy alone. On the other hand, the synthesis of several cytokines/chemokines was decreased with artesunate, whereas the levels of inflammatory cytokines and IFN-γ were increased with rapamycin at the peak of infection.
In the CNS, resident microglial cells are progressively activated following an injury or an invasion of pathogens, leading to the synthesis of TNF-α (211). This cytokine then acts in autocrine and paracrine manners to regulate the innate and adaptive immune responses in the brain parenchyma. During the acute phase of HSE, the level of TNF-α increased and remained sustained thereafter (78, 196) and may cause brain injury. Our group showed that treatment of mice with a combination of valacyclovir and etanercept (an anti-TNF-α antibody) started at the onset of symptoms (i.e., on day 3 postinfection) significantly increased the survival rate compared to that of the antiviral drug alone without decreasing the brain viral titers (212).
Resident microglia and infiltrating macrophages and neutrophils are involved in the control of viral spread through a rapid and robust production of ROS (127). However, ROS production is also associated with oxidative tissue damage. Systemic administration of sulforaphane, a potent inducer of cellular antioxidants, between day 3 and 6 postinfection has been shown to reduce the recruitment of macrophages and neutrophils to the CNS, microglial activation, and ROS production during experimental HSE, but the brain viral load remained unchanged (213). Thus, antioxidant stimulation is a potential strategy for modulating brain inflammation and neurotoxicity. The expression of iNOS, which is involved in the production of NO, is upregulated during HSE in mice (214). However, excessive production of NO mediates neuronal cell death (215). The effect of a selective iNOS inhibitor, N-nitro-l-arginine, has been evaluated during experimental HSE (201). N-Nitro-l-arginine alone or combined with acyclovir was administered intraperitoneally 1 day after intranasal challenge with HSV-1 for 14 days. Combination therapies were shown to reduce the viral burden as well as the expression of iNOS transcripts in the brain.
The level of matrix metalloproteinase-9 (MMP-9) has been shown to increase during HSE and to induce degradation of collagen type IV, the principal constituent of the neurovascular matrix. This may lead to cerebrovascular complications, such as cerebral edema, elevated intracranial pressure, and hemorrhage (216). Local injection of MMP-9 silencing RNA (siRNA) into mice after intracerebral injection of HSV-1 has been shown to reduce brain edema and proinflammatory cytokine synthesis (IL-6 and TNF-α) but not the expression of HSV-1 glycoprotein D gene in the brain (217). When combined with acyclovir, MMP-9 siRNA improved neurological symptoms and survival rates compared to the antiviral alone. Angiotensin-converting enzyme inhibitors such as captopril have been shown to inhibit the activity of MMP-9. Administration of captopril to mice infected intracerebrally with HSV-1 showed that the drug reduced the expression of MMP-9 by reducing ROS production through NADPH oxidase (218). This intervention reduces cerebral edema and improves neurological symptoms after HSE.
Pooled human immunoglobulins for intravenous administration (IVIG) contain a broad repertoire of neutralizing antibodies developed against a series of infectious agents. However, single intraperitoneal injection of IVIG 24 h before or after intranasal challenge with HSV-1 increased mouse survival by a mechanism independent of direct viral neutralization (219). When administered intravenously 24 h postinfection, IVIG protected susceptible mice from fatal HSE by reducing brain inflammation (220). Indeed, IVIG increase the infiltration of CD4+ IL-10-producing T lymphocytes in the brain and reduce the recruitment of inflammatory and anti-inflammatory monocytes. Furthermore, IVIG also induce the expansion of regulatory T cells in peripheral lymphoid organs. However, when combined with acyclovir, IVIG antagonized the beneficial effect of the antiviral on the behavioral deficits induced by HSV (221).
Overall, these data show that several pathways involved in the immune response to HSV may be potential targets for the development of immune-based therapy that could eventually be combined with antiviral agents to ameliorate the prognosis of patients suffering from HSE. However, the kinetics of reliable biomarkers of inflammation should be determined in the blood or CSF to define an appropriate time window for administration of immunomodulatory therapy.
BIOMARKERS OF INFLAMMATION IN THE CSF OR SERUM
Attempts have been made to identify soluble biomarker signatures in the CSF or serum that could be used as reliable surrogate markers reflecting the inflammatory response in the brain of patients with HSE. Kothur et al. reviewed a series of published studies that evaluated cytokines and chemokines as biomarkers of the inflammatory response in patients with HSE (222) and suggested that the levels of IFN-γ, TNF-α, IL-6, IL-2 receptor, and CCL2 are the most affected during the disease. Their levels increased in the CSF during the acute phase of HSE and were sustained thereafter, suggesting a persistent immune activation in the CNS (78, 196, 223). The cytokines IL-1α and IL-1β bind the IL-1 receptor (IL-1R), and this interaction can be blocked by the IL-1R antagonist (IL-1RA) and IL-10. In patients with HSE, the IL-1β/IL-1RA ratio in the CSF is increased in cases with a poor prognosis of the disease (224). An IL-1β/IL-1RA ratio greater than 0.55 had high specificity (100%) and sensitivity (83%) for a poor outcome. Furthermore, an elevated level of IL-10 in serum is associated with a high GCS score.
Neopterin and β2-microglobulin are used as markers of intrathecal immune activation in infectious and noninfectious inflammatory disorders of the CNS. Monocytes and macrophages produce neopterin, an intermediate in the metabolism of pterin, in response to IFN-γ, whereas β2-microglobulin is the small component of class I MHC. The levels of both neopterin and β2-microglobulin markedly increased in the CSF of patients with HSE and persisted for a long period of time (>13 years) (79). Another study reported that the levels of IL-6 and neopterin in the CSF but not in serum correlated with the clinical course of HSE in patients (225).
Markers of neuronal and glial cell lysis were also evaluated in the CSF of patients with HSE (226). Neuron-specific enolase is a soluble glycolytic enzyme of neurons that is reported to be a CSF marker of neuronal damage in cases of stroke and encephalitis. The neurofilament constitutes one of the principal structural components of axons, and its level in the CSF has been shown to increase in neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis. The glial fibrillary acidic protein is a main structural constituent of astrocytes and is a reliable astroglial marker in patients with CNS injuries. The S-100B protein is an acidic calcium-binding protein present in the cytoplasm of astrocytes, and its level increases in the CSF after structural damage of the CNS during stroke and encephalitis. Furthermore, a small proportion of S-100B leaks into the circulation. The neuron-specific enolase, glial fibrillary acidic protein, and S-100B protein increase to very high levels in the CSF during the acute phase of HSE, whereas the level of neurofilament increases later and persists in the CSF for 3 to 10 months (226). Furthermore, the serum levels of S-100B protein is markedly increased in the acute stage of the disease in patients with HSE in comparison to control individuals (227).
Finally, soluble Fas is a marker of the Fas/FasL system that regulates apoptosis through alternative splicing of full-length Fas mRNA. Higher levels of soluble Fas have been reported in the CSF of patients with various neurological diseases. Patients with HSE presenting with severe neurological deficits have been reported to exhibit a higher level of soluble Fas than those with moderate and mild neurological disabilities (131).
Overall, published data are quite heterogeneous with respect to the timing of sample collection and patient characteristics, and a prognostic use of these biomarkers could not be formally established. Therefore, the identification of reliable biomarkers of HSE is still required.
CONSIDERATIONS FOR THE USE OF IMMUNOMODULATORY DRUGS
Management of Patients with Adjunctive Corticosteroids
Figure 2 shows the hypothetical management of patients suffering from HSE by the administration of acyclovir and adjunctive corticosteroid therapy. Intravenous acyclovir (10 mg/kg every 8 h) should be initiated empirically as early as possible pending the results of CSF and MRI testing and continued for 14 days (in immunocompetent individuals) or 21 days (in children aged 3 months to 12 years) if PCR in the CSF is positive for HSV. Adjunctive corticosteroid therapy (e.g., dexamethasone 10 mg i.v. every 6 h for 4 days) should be started later, during the rise of the inflammatory response, as evidenced by the measurement of a surrogate biomarker(s) in the CSF or serum. The administration of corticosteroids should be limited in time to prevent the occurrence of undesired effects. In this way, treatment with corticosteroids should prevent the development of an exaggerated inflammatory response during HSE that could be harmful for the host while maintaining a beneficial immune response that limits viral dissemination in the brain.
FIG 2.
Hypothetical management of patients suffering from HSE with acyclovir and adjunctive corticosteroid therapy. At admission, a clinical evaluation of the patients should be performed together with laboratory testing of the cerebrospinal fluid (CSF), magnetic resonance imaging (MRI), and electroencephalography (EEG). The level of production of a reliable biomarker of inflammation in the CSF or serum should be determined. Empirical intravenous acyclovir should be initiated pending results of CSF and MRI testing and continued for 14 to 21 days if the PCR is positive. Adjunctive corticosteroid therapy should be started later, during the rise of the inflammatory response, and should be limited in time to prevent undesired effects. This combined therapy should reduce both the viral load (dotted blue line) and the excessive inflammatory response (dotted red line) and result in less damage to the brain.
Management of Children with Inborn Errors in Innate Immunity
The management of children with inborn errors in innate immunity presenting with clinically suspected or proven HSE requires that antiviral therapy be initiated as soon as possible (41). Because of their immature immune system, the age of children with inherited deficiencies at primary infection with HSV-1 may be a risk factor for developing HSE (228). Therefore, the status of children who belong to at-risk families should be identified by prenatal or neonatal genetic testing. These children should benefit from an appropriate follow-up until adolescence or until HSV-1 seroconversion occurs. Furthermore, children with inherited deficiencies in the TLR3 signaling pathway who have a first episode of HSE should be carefully followed, because the risk of relapses is higher in these patients (142). The administration of antiviral prophylaxis may be envisaged in some cases. In this population, the administration of adjunctive immunomodulatory therapy should enhance the innate immune response rather than reducing an excessive inflammatory response. For instance, children with a genetic defect affecting the production of IFN-α or the response to IFN-α who present with a first episode or a recurrence of HSE would benefit from a combination of acyclovir and recombinant IFN-α2b (229). As an example, the administration of a combination of acyclovir and IFN-α to mice on day 1 or 2 after intranasal challenge with HSV-1 resulted in a 30% increase in survival rate compared to that of treatment with acyclovir alone (Table 2) (230).
CONCLUDING REMARKS
In spite of a full course of acyclovir therapy, the mortality of patients suffering from HSE is still 20%, with 70% of surviving individuals left with moderate or severe neurological dysfunctions. The diagnosis of HSE has been markedly improved by PCR testing in the CSF and magnetic resonance imaging. However, a reduction in delay between the time of admission and the initiation of acyclovir treatment is the only intervention that has been demonstrated to improve the prognosis of this disease. Since acyclovir became available, there has been no further development toward a more effective treatment of HSE. It is well recognized that the cerebral damage that occurs during HSE is caused by both the viral replication and the excessive inflammatory response. However, the use of corticosteroids in the management of HSE is currently recommended only in cases of cerebral edema, elevated intracranial pressure, and brain shift and depends on the physician. Animal studies, case reports, and small case series suggest a benefit for adjunctive corticosteroids in the treatment of HSE, but clinical trial validation is still lacking to translate this approach to patients. To be safe and effective, immunomodulatory drugs should be administered within an optimal time window, which requires the identification of reliable biomarkers of inflammation in the CSF or serum. Thus, a short course of adjunctive immunomodulatory drugs should dampen the inflammatory response and might reduce the neurological sequelae, persistent immune activation, and autoimmune encephalitis that could develop after HSE.
ACKNOWLEDGMENTS
This study was supported by a Foundation Grant from the Canadian Institutes of Health Research (grant no. 148361 to G.B.). G.B. is the holder of the Canada research chair on emerging viruses and antiviral resistance.
We have no conflict of interests to declare.
Biographies

Jocelyne Piret obtained her Ph.D., in biological sciences, at the Catholic University of Louvain (Belgium) in 1993. She performed two 3-year postdoctoral fellowships at the same university and at Laval University (Canada). She was appointed a project leader at the Research Center in Infectious Diseases of Laval University in 1999. Her main areas of interest include the prevention and treatment of herpesvirus infections. She participated in the development of microbicides for the prevention of sexually transmitted infections and of topical formulations containing antiviral agents for the treatment of cutaneous herpetic infections. Thereafter, her work has focused on the study of the innate immune response during herpes simplex virus encephalitis and the evaluation of immunomodulatory strategies that may improve the treatment of this disease. She also has a particular interest in the study of the mechanisms involved in the resistance of herpes simplex virus and cytomegalovirus to antiviral drugs.

Guy Boivin is a medical virologist and infectious disease specialist working at the Québec City University Hospital Center (CHU of Quebec) in Canada since 1994. He is also a professor of microbiology-immunology at Laval University and a senior researcher in virology at the Research Center in Infectious Diseases of the same university. He holds an M.D. from Laval University, a master’s (M.Sc.) degree in virology from the University of Montréal, and a 3-year specialized research training (fellowship) in molecular virology from the University of Minnesota. His main research interests concern the diagnosis, pathogenesis, prevention, and treatment of respiratory viral diseases as well as herpesvirus infections. One of his areas of research relates to the study of the cerebral innate immune response during herpes simplex virus encephalitis and the evaluation of immunomodulatory strategies. He is also highly recognized for his work on the mechanisms of drug resistance of influenza viruses and cytomegalovirus.
REFERENCES
- 1.Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 2.George BP, Schneider EB, Venkatesan A. 2014. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000-2010. PLoS One 9:e104169. doi: 10.1371/journal.pone.0104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. 2014. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology 82:443–451. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 4.Hjalmarsson A, Blomqvist P, Skoldenberg B. 2007. Herpes simplex encephalitis in Sweden, 1990–2001: incidence, morbidity, and mortality. Clin Infect Dis 45:875–880. doi: 10.1086/521262. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen LK, Dalgaard LS, Ostergaard LJ, Norgaard M, Mogensen TH. 2017. Incidence and mortality of herpes simplex encephalitis in Denmark: a nationwide registry-based cohort study. J Infect 74:42–49. doi: 10.1016/j.jinf.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Dagsdottir HM, Sigurethardottir B, Gottfreethsson M, Kristjansson M, Love A, Baldvinsdottir GE, Guethmundsson S. 2014. Herpes simplex encephalitis in Iceland 1987-2011. Springerplus 3:524. doi: 10.1186/2193-1801-3-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aurelius E, Johansson B, Skoldenberg B, Forsgren M. 1993. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J Med Virol 39:179–186. doi: 10.1002/jmv.1890390302. [DOI] [PubMed] [Google Scholar]
- 8.Whitley RJ, Kimberlin DW. 2005. Herpes simplex encephalitis: children and adolescents. Semin Pediatr Infect Dis 16:17–23. doi: 10.1053/j.spid.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Gnann JW Jr, Whitley RJ. 2017. Herpes simplex encephalitis: an update. Curr Infect Dis Rep 19:13. doi: 10.1007/s11908-017-0568-7. [DOI] [PubMed] [Google Scholar]
- 10.Tan IL, McArthur JC, Venkatesan A, Nath A. 2012. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology 79:2125–2132. doi: 10.1212/WNL.0b013e3182752ceb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakeman FD, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 1995. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis 171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 12.Steiner I, Schmutzhard E, Sellner J, Chaudhuri A, Kennedy PG, European Federation of Neurological Sciences, European Neurologic Society. 2012. EFNS-ENS guidelines for the use of PCR technology for the diagnosis of infections of the nervous system. Eur J Neurol 19:1278–1291. doi: 10.1111/j.1468-1331.2012.03808.x. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw MJ, Venkatesan A. 2016. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 13:493–508. doi: 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutter R, Kaplan PW, Cervenka MC, Thakur KT, Asemota AO, Venkatesan A, Geocadin RG. 2015. Electroencephalography for diagnosis and prognosis of acute encephalitis. Clin Neurophysiol 126:1524–1531. doi: 10.1016/j.clinph.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Esiri MM. 1982. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci 54:209–226. doi: 10.1016/0022-510x(82)90183-6. [DOI] [PubMed] [Google Scholar]
- 16.Sobel RA, Collins AB, Colvin RB, Bhan AK. 1986. The in situ cellular immune response in acute herpes simplex encephalitis. Am J Pathol 125:332–338. [PMC free article] [PubMed] [Google Scholar]
- 17.Schiff D, Rosenblum MK. 1998. Herpes simplex encephalitis (HSE) and the immunocompromised: a clinical and autopsy study of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection. Hum Pathol 29:215–222. doi: 10.1016/s0046-8177(98)90038-7. [DOI] [PubMed] [Google Scholar]
- 18.Davis R, Jeffery K, Atkins BL. 2004. Hypoglycorrhachia in herpes simplex encephalitis. Clin Infect Dis 38:1506–1507. doi: 10.1086/383579. [DOI] [PubMed] [Google Scholar]
- 19.Tebas P, Nease RF, Storch GA. 1998. Use of the polymerase chain reaction in the diagnosis of herpes simplex encephalitis: a decision analysis model. Am J Med 105:287–295. doi: 10.1016/S0002-9343(98)00259-9. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Lakeman FD, Arvin AM, Prober CG, Corey L, Powell DA, Burchett SK, Jacobs RF, Starr SE, Whitley RJ. 1996. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 174:1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- 21.Guffond T, Dewilde A, Lobert PE, Caparros-Lefebvre D, Hober D, Wattre P. 1994. Significance and clinical relevance of the detection of herpes simplex virus DNA by the polymerase chain reaction in cerebrospinal fluid from patients with presumed encephalitis. Clin Infect Dis 18:744–749. doi: 10.1093/clinids/18.5.744. [DOI] [PubMed] [Google Scholar]
- 22.Weil AA, Glaser CA, Amad Z, Forghani B. 2002. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis 34:1154–1157. doi: 10.1086/339550. [DOI] [PubMed] [Google Scholar]
- 23.De Tiege X, Heron B, Lebon P, Ponsot G, Rozenberg F. 2003. Limits of early diagnosis of herpes simplex encephalitis in children: a retrospective study of 38 cases. Clin Infect Dis 36:1335–1339. doi: 10.1086/374839. [DOI] [PubMed] [Google Scholar]
- 24.Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S, Lephart P, Salimnia H, Schreckenberger PC, DesJarlais S, Reed SL, Chapin KC, LeBlanc L, Johnson JK, Soliven NL, Carroll KC, Miller JA, Dien Bard J, Mestas J, Bankowski M, Enomoto T, Hemmert AC, Bourzac KM. 2016. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol 54:2251–2261. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liesman RM, Strasburg AP, Heitman AK, Theel ES, Patel R, Binnicker MJ. 2018. Evaluation of a commercial multiplex molecular panel for diagnosis of infectious meningitis and encephalitis. J Clin Microbiol 56:e01927-17. doi: 10.1128/JCM.01927-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tansarli GS, Chapin KC. 2019. Diagnostic test accuracy of the BioFire(R) FilmArray(R) meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect doi: 10.1016/j.cmi.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Stahl JP, Mailles A, De Broucker T, Steering Committee and Investigators Group. 2012. Herpes simplex encephalitis and management of acyclovir in encephalitis patients in France. Epidemiol Infect 140:372–381. doi: 10.1017/S0950268811000483. [DOI] [PubMed] [Google Scholar]
- 28.Misra UK, Kalita J, Phadke RV, Wadwekar V, Boruah DK, Srivastava A, Maurya PK, Bhattacharyya A. 2010. Usefulness of various MRI sequences in the diagnosis of viral encephalitis. Acta Trop 116:206–211. doi: 10.1016/j.actatropica.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Domingues RB, Fink MC, Tsanaclis AM, de Castro CC, Cerri GG, Mayo MS, Lakeman FD. 1998. Diagnosis of herpes simplex encephalitis by magnetic resonance imaging and polymerase chain reaction assay of cerebrospinal fluid. J Neurol Sci 157:148–153. doi: 10.1016/s0022-510x(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 30.Renard D, Nerrant E, Lechiche C. 2015. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol 262:2101–2105. doi: 10.1007/s00415-015-7818-0. [DOI] [PubMed] [Google Scholar]
- 31.Sili U, Kaya A, Mert A, HSV Encephalitis Study Group. 2014. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol 60:112–118. doi: 10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Misra UK, Tan CT, Kalita J. 2008. Viral encephalitis and epilepsy. Epilepsia 49(Suppl 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 33.Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch'ien LT, Alford CA. 1977. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study. N Engl J Med 297:289–294. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]
- 34.Whitley RJ, Alford CA, Hirsch MS, Schooley RT, Luby JP, Aoki FY, Hanley D, Nahmias AJ, Soong SJ. 1986. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 35.Skoldenberg B, Forsgren M, Alestig K, Bergstrom T, Burman L, Dahlqvist E, Forkman A, Fryden A, Lovgren K, Norlin K. 1984. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet 2:707–711. doi: 10.1016/s0140-6736(84)92623-0. [DOI] [PubMed] [Google Scholar]
- 36.Andrei G, de Clercq E, Snoeck R. 2009. Viral DNA polymerase inhibitors, p 481–526. In Cameron CE, Gotte M, Raney K (ed), Viral genome replication. Springer, New York, NY. [Google Scholar]
- 37.VanLandingham KE, Marsteller HB, Ross GW, Hayden FG. 1988. Relapse of herpes simplex encephalitis after conventional acyclovir therapy. JAMA 259:1051–1053. doi: 10.1001/jama.1988.03720070051034. [DOI] [PubMed] [Google Scholar]
- 38.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, Arvin AM, Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Weller S, Soong SJ, Kiell J, Lakeman FD, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2001. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 108:230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 39.Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. 2002. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- 40.Poissy J, Wolff M, Dewilde A, Rozenberg F, Raschilas F, Blas M, Georges H, Chaffaut C, Yazdanpanah Y. 2009. Factors associated with delay to acyclovir administration in 184 patients with herpes simplex virus encephalitis. Clin Microbiol Infect 15:560–564. doi: 10.1111/j.1469-0691.2009.02735.x. [DOI] [PubMed] [Google Scholar]
- 41.Erdem H, Cag Y, Ozturk-Engin D, Defres S, Kaya S, Larsen L, Poljak M, Barsic B, Argemi X, Sørensen SM, Bohr AL, Tattevin P, Gunst JD, Baštáková L, Jereb M, Johansen IS, Karabay O, Pekok AU, Sipahi OR, Chehri M, Beraud G, Shehata G, Del Vecchio RF, Maresca M, Karsen H, Sengoz G, Sunbul M, Yilmaz G, Yilmaz H, Sharif-Yakan A, Kanj SS, Parlak E, Pehlivanoglu F, Korkmaz F, Komur S, Kose S, Ulug M, Bolukcu S, Coskuner SA, Ince N, Akkoyunlu Y, Halac G, Sahin-Horasan E, Tireli H, Kilicoglu G, Al-Mahdawi A, Nemli SA, Inan A, Senbayrak S, Stahl JP, Vahaboglu H. 2015. Results of a multinational study suggest the need for rapid diagnosis and early antiviral treatment at the onset of herpetic meningoencephalitis. Antimicrob Agents Chemother 59:3084–3089. doi: 10.1128/AAC.05016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ, Infectious Diseases Society of America. 2008. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 47:303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 43.Solomon T, Michael BD, Smith PE, Sanderson F, Davies NW, Hart IJ, Holland M, Easton A, Buckley C, Kneen R, Beeching NJ, National Encephalitis Guidelines Development and Stakeholder Group. 2012. Management of suspected viral encephalitis in adults—Association of British Neurologists and British Infection Association National Guidelines. J Infect 64:347–373. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Cinque P, Cleator GM, Weber T, Monteyne P, Sindic CJ, van Loon AM. 1996. The role of laboratory investigation in the diagnosis and management of patients with suspected herpes simplex encephalitis: a consensus report. The EU Concerted Action on Virus Meningitis and Encephalitis. J Neurol Neurosurg Psychiatry 61:339–345. doi: 10.1136/jnnp.61.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, François P, Guillon A. 2015. Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Crit Care 19:345. doi: 10.1186/s13054-015-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gnann JW Jr, Skoldenberg B, Hart J, Aurelius E, Schliamser S, Studahl M, Eriksson BM, Hanley D, Aoki F, Jackson AC, Griffiths P, Miedzinski L, Hanfelt-Goade D, Hinthorn D, Ahlm C, Aksamit A, Cruz-Flores S, Dale I, Cloud G, Jester P, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2015. Herpes simplex encephalitis: lack of clinical benefit of long-term valacyclovir therapy. Clin Infect Dis 61:683–691. doi: 10.1093/cid/civ369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schepers K, Hernandez A, Andrei G, Gillemot S, Fiten P, Opdenakker G, Bier JC, David P, Delforge ML, Jacobs F, Snoeck R. 2014. Acyclovir-resistant herpes simplex encephalitis in a patient treated with anti-tumor necrosis factor-alpha monoclonal antibodies. J Clin Virol 59:67–70. doi: 10.1016/j.jcv.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Schulte EC, Sauerbrei A, Hoffmann D, Zimmer C, Hemmer B, Muhlau M. 2010. Acyclovir resistance in herpes simplex encephalitis. Ann Neurol 67:830–833. doi: 10.1002/ana.21979. [DOI] [PubMed] [Google Scholar]
- 49.Gateley A, Gander RM, Johnson PC, Kit S, Otsuka H, Kohl S. 1990. Herpes simplex virus type 2 meningoencephalitis resistant to acyclovir in a patient with AIDS. J Infect Dis 161:711–715. doi: 10.1093/infdis/161.4.711. [DOI] [PubMed] [Google Scholar]
- 50.Cakmak Celik F, Dağdemir A, Eroğlu C, Aydin OF, Incesu L, Yilmaz H. 2007. A herpes simplex virus encephalitis case with no clinical response to acyclovir treatment. Mikrobiyol Bul 41:613–619. doi: 10.5578/mb.66921. [DOI] [PubMed] [Google Scholar]
- 51.Koudriavtseva T, Onesti E, Tonachella R, Pelagalli L, Vidiri A, Jandolo B. 2010. Fatal herpetic encephalitis during brain radiotherapy in a cerebral metastasized breast cancer patient. J Neurooncol 100:137–140. doi: 10.1007/s11060-010-0134-8. [DOI] [PubMed] [Google Scholar]
- 52.Bache M, Andrei G, Bindl L, Bofferding L, Bottu J, Geron C, Neuhauser C, Gillemot S, Fiten P, Opdenakker G, Snoeck R. 2014. Antiviral drug-resistance typing reveals compartmentalization and dynamics of acyclovir-resistant herpes simplex virus type-2 (HSV-2) in a case of neonatal herpes. J Pediatric Infect Dis Soc 3:e24–e27. doi: 10.1093/jpids/pit045. [DOI] [PubMed] [Google Scholar]
- 53.Kakiuchi S, Nonoyama S, Wakamatsu H, Kogawa K, Wang L, Kinoshita-Yamaguchi H, Takayama-Ito M, Lim CK, Inoue N, Mizuguchi M, Igarashi T, Saijo M. 2013. Neonatal herpes encephalitis caused by a virologically confirmed acyclovir-resistant herpes simplex virus 1 strain. J Clin Microbiol 51:356–359. doi: 10.1128/JCM.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergmann M, Beer R, Kofler M, Helbok R, Pfausler B, Schmutzhard E. 2017. Acyclovir resistance in herpes simplex virus type I encephalitis: a case report. J Neurovirol 23:335–337. doi: 10.1007/s13365-016-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piret J, Boivin G. 2011. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hostetler KY. 2010. Synthesis and early development of hexadecyloxypropylcidofovir: an oral antipoxvirus nucleoside phosphonate. Viruses 2:2213–2225. doi: 10.3390/v2102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. 2010. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis 202:1492–1499. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prichard MN, Kern ER, Hartline CB, Lanier ER, Quenelle DC. 2011. CMX001 potentiates the efficacy of acyclovir in herpes simplex virus infections. Antimicrob Agents Chemother 55:4728–4734. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito Y, Kimura H, Yabuta Y, Ando Y, Murakami T, Shiomi M, Morishima T. 2000. Exacerbation of herpes simplex encephalitis after successful treatment with acyclovir. Clin Infect Dis 30:185–187. doi: 10.1086/313618. [DOI] [PubMed] [Google Scholar]
- 60.De Tiège X, Rozenberg F, Des Portes V, Lobut JB, Lebon P, Ponsot G, Héron B. 2003. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 61:241–243. doi: 10.1212/01.wnl.0000073985.71759.7c. [DOI] [PubMed] [Google Scholar]
- 61.Pruss H, Finke C, Holtje M, Hofmann J, Klingbeil C, Probst C, Borowski K, Ahnert-Hilger G, Harms L, Schwab JM, Ploner CJ, Komorowski L, Stoecker W, Dalmau J, Wandinger KP. 2012. N-methyl-d-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 72:902–911. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Tiège X, Rozenberg F, Héron B. 2008. The spectrum of herpes simplex encephalitis in children. Eur J Paediatr Neurol 12:72–81. doi: 10.1016/j.ejpn.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. 2012. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci 32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohammad SS, Sinclair K, Pillai S, Merheb V, Aumann TD, Gill D, Dale RC, Brilot F. 2014. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-d-aspartate receptor or dopamine-2 receptor. Mov Disord 29:117–122. doi: 10.1002/mds.25623. [DOI] [PubMed] [Google Scholar]
- 65.Skoldenberg B, Aurelius E, Hjalmarsson A, Sabri F, Forsgren M, Andersson B, Linde A, Strannegard O, Studahl M, Hagberg L, Rosengren L. 2006. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 253:163–170. doi: 10.1007/s00415-005-0941-6. [DOI] [PubMed] [Google Scholar]
- 66.Kothur K, Gill D, Wong M, Mohammad SS, Bandodkar S, Arbunckle S, Wienholt L, Dale RC. 2017. Cerebrospinal fluid cyto-/chemokine profile during acute herpes simplex virus induced anti-N-methyl-d-aspartate receptor encephalitis and in chronic neurological sequelae. Dev Med Child Neurol 59:806–814. doi: 10.1111/dmcn.13431. [DOI] [PubMed] [Google Scholar]
- 67.Westman G, Studahl M, Ahlm C, Eriksson BM, Persson B, Ronnelid J, Schliamser S, Aurelius E. 2016. N-methyl-d-aspartate receptor autoimmunity affects cognitive performance in herpes simplex encephalitis. Clin Microbiol Infect 22:934–940. doi: 10.1016/j.cmi.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 68.Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, Llufriu S, Muchart J, Erro ME, Abraira L, Moris G, Monros-Giménez L, Corral-Corral Í, Montejo C, Toledo M, Bataller L, Secondi G, Ariño H, Martínez-Hernández E, Juan M, Marcos MA, Alsina L, Saiz A, Rosenfeld MR, Graus F, Dalmau J, Spanish Herpes Simplex Encephalitis Study Group. 2018. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris NA, Kaplan TB, Linnoila J, Cho T. 2016. HSV encephalitis-induced anti-NMDAR encephalitis in a 67-year-old woman: report of a case and review of the literature. J Neurovirol 22:33–37. doi: 10.1007/s13365-015-0364-9. [DOI] [PubMed] [Google Scholar]
- 70.Sutcu M, Akturk H, Somer A, Tatli B, Torun SH, Yıldız EP, Şık G, Citak A, Agacfidan A, Salman N. 2016. Role of autoantibodies to N-methyl-d-aspartate (NMDA) receptor in relapsing herpes simplex encephalitis: a retrospective, one-center experience. J Child Neurol 31:345–350. doi: 10.1177/0883073815595079. [DOI] [PubMed] [Google Scholar]
- 71.Bamford A, Crowe BH, Hacohen Y, Lin JP, Clarke A, Tudor-Williams G, Sancho-Shimizu V, Vincent A, Lim M, Pullaperuma SP. 2015. Pediatric herpes simplex virus encephalitis complicated by N-methyl-d-aspartate receptor antibody encephalitis. J Pediatric Infect Dis Soc 4:e17–e21. doi: 10.1093/jpids/piu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Armangue T, Titulaer MJ, Malaga I, Bataller L, Gabilondo I, Graus F, Dalmau J, Spanish Anti-N-Methyl-D-Aspartate Receptor (NMDAR) Encephalitis Work Group. 2013. Pediatric anti-N-methyl-d-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 162:850–856. doi: 10.1016/j.jpeds.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armangue T, Moris G, Cantarín-Extremera V, Conde CE, Rostasy K, Erro ME, Portilla-Cuenca JC, Turón-Viñas E, Málaga I, Muñoz-Cabello B, Torres-Torres C, Llufriu S, González-Gutiérrez-Solana L, González G, Casado-Naranjo I, Rosenfeld M, Graus F, Dalmau J, Spanish Prospective Multicentric Study of Autoimmunity in Herpes Simplex Encephalitis. 2015. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 85:1736–1743. doi: 10.1212/WNL.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalmau J, Armangue T, Planaguma J, Radosevic M, Mannara F, Leypoldt F, Geis C, Lancaster E, Titulaer MJ, Rosenfeld MR, Graus F. 2019. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol 18:1045–1057. doi: 10.1016/S1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 75.Armangue T, Leypoldt F, Málaga I, Raspall-Chaure M, Marti I, Nichter C, Pugh J, Vicente-Rasoamalala M, Lafuente-Hidalgo M, Macaya A, Ke M, Titulaer MJ, Höftberger R, Sheriff H, Glaser C, Dalmau J. 2014. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 75:317–323. doi: 10.1002/ana.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poissy J, Champenois K, Dewilde A, Melliez H, Georges H, Senneville E, Yazdanpanah Y. 2012. Impact of herpes simplex virus load and red blood cells in cerebrospinal fluid upon herpes simplex meningo-encephalitis outcome. BMC Infect Dis 12:356. doi: 10.1186/1471-2334-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hjalmarsson A, Granath F, Forsgren M, Brytting M, Blomqvist P, Skoldenberg B. 2009. Prognostic value of intrathecal antibody production and DNA viral load in cerebrospinal fluid of patients with herpes simplex encephalitis. J Neurol 256:1243–1251. doi: 10.1007/s00415-009-5106-6. [DOI] [PubMed] [Google Scholar]
- 78.Aurelius E, Andersson B, Forsgren M, Skoldenberg B, Strannegard O. 1994. Cytokines and other markers of intrathecal immune response in patients with herpes simplex encephalitis. J Infect Dis 170:678–681. doi: 10.1093/infdis/170.3.678. [DOI] [PubMed] [Google Scholar]
- 79.Aurelius E, Forsgren M, Skoldenberg B, Strannegard O. 1993. Persistent intrathecal immune activation in patients with herpes simplex encephalitis. J Infect Dis 168:1248–1252. doi: 10.1093/infdis/168.5.1248. [DOI] [PubMed] [Google Scholar]
- 80.Meyding-Lamade UK, Lamade WR, Wildemann BT, Sartor K, Hacke W. 1999. Herpes simplex virus encephalitis: chronic progressive cerebral magnetic resonance imaging abnormalities in patients despite good clinical recovery. Clin Infect Dis 28:148–149. doi: 10.1086/517183. [DOI] [PubMed] [Google Scholar]
- 81.Cagnin A, Myers R, Gunn RN, Lawrence AD, Stevens T, Kreutzberg GW, Jones T, Banati RB. 2001. In vivo visualization of activated glia by [11C] (R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain 124:2014–2027. doi: 10.1093/brain/124.10.2014. [DOI] [PubMed] [Google Scholar]
- 82.Lellouch-Tubiana A, Fohlen M, Robain O, Rozenberg F. 2000. Immunocytochemical characterization of long-term persistent immune activation in human brain after herpes simplex encephalitis. Neuropathol Appl Neurobiol 26:285–294. doi: 10.1046/j.1365-2990.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- 83.Nicoll JA, Love S, Kinrade E. 1993. Distribution of herpes simplex virus DNA in the brains of human long-term survivors of encephalitis. Neurosci Lett 157:215–218. doi: 10.1016/0304-3940(93)90740-c. [DOI] [PubMed] [Google Scholar]
- 84.Tamura T, Morikawa A, Kikuchi K. 1996. Diffuse white matter lesions associated with herpes simplex encephalitis as observed on magnetic resonance imaging. Brain Dev 18:150–152. doi: 10.1016/0387-7604(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 85.Kataoka H, Tanizawa E, Ueno S. 2007. Herpes simplex virus encephalitis with progressive severe white-matter lesions. Eur J Neurol 14:e18–e19. doi: 10.1111/j.1468-1331.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 86.Ueda N, Miyasaki H, Kuroiwa Y. 2003. Diffuse white matter lesions in a case of herpes simplex encephalitis. J Neurol 250:867–868. doi: 10.1007/s00415-003-1093-1. [DOI] [PubMed] [Google Scholar]
- 87.Ono Y, Manabe Y, Nishimura H, Kono S, Narai H, Omori N, Nanba Y, Abe K. 2009. Unusual progression of herpes simplex encephalitis with basal ganglia and extensive white matter involvement. Neurol Int 1:e9. doi: 10.4081/ni.2009.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Titulaer MJ, Hoftberger R, Iizuka T, Leypoldt F, McCracken L, Cellucci T, Benson LA, Shu H, Irioka T, Hirano M, Singh G, Cobo Calvo A, Kaida K, Morales PS, Wirtz PW, Yamamoto T, Reindl M, Rosenfeld MR, Graus F, Saiz A, Dalmau J. 2014. Overlapping demyelinating syndromes and anti-N-methyl-d-aspartate receptor encephalitis. Ann Neurol 75:411–428. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 90.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 92.Melchjorsen J. 2012. Sensing herpes: more than toll. Rev Med Virol 22:106–121. doi: 10.1002/rmv.716. [DOI] [PubMed] [Google Scholar]
- 93.Vandevenne P, Sadzot-Delvaux C, Piette J. 2010. Innate immune response and viral interference strategies developed by human herpesviruses. Biochem Pharmacol 80:1955–1972. doi: 10.1016/j.bcp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 94.Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. 2011. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 11:143–154. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. 2005. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol 175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 96.Aravalli RN, Hu S, Lokensgard JR. 2007. Toll-like receptor 2 signaling is a mediator of apoptosis in herpes simplex virus-infected microglia. J Neuroinflammation 4:11. doi: 10.1186/1742-2094-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 99.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A 101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shu C, Li X, Li P. 2014. The mechanism of double-stranded DNA sensing through the cGAS-STING pathway. Cytokine Growth Factor Rev 25:641–648. doi: 10.1016/j.cytogfr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai X, Chiu YH, Chen ZJ. 2014. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 103.Luecke S, Paludan SR. 2015. Innate recognition of alphaherpesvirus DNA. Adv Virus Res 92:63–100. doi: 10.1016/bs.aivir.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Kozak M, Roizman B. 1975. RNA synthesis in cells infected with herpes simplex virus. IX. Evidence for accumulation of abundant symmetric transcripts in nuclei. J Virol 15:36–40. doi: 10.1128/JVI.15.1.36-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol 80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol 81:13315–13324. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rasmussen SB, Jensen SB, Nielsen C, Quartin E, Kato H, Chen ZJ, Silverman RH, Akira S, Paludan SR. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J Gen Virol 90:74–78. doi: 10.1099/vir.0.005389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Melchjorsen J, Rintahaka J, Soby S, Horan KA, Poltajainen A, Ostergaard L, Paludan SR, Matikainen S. 2010. Early innate recognition of herpes simplex virus in human primary macrophages is mediated via the MDA5/MAVS-dependent and MDA5/MAVS/RNA polymerase III-independent pathways. J Virol 84:11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X, Majumdar T, Kessler P, Ozhegov E, Zhang Y, Chattopadhyay S, Barik S, Sen GC. 2016. STING requires the adaptor TRIF to trigger innate immune responses to microbial infection. Cell Host Microbe 20:329–341. doi: 10.1016/j.chom.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gibbert K, Schlaak JF, Yang D, Dittmer U. 2013. IFN-alpha subtypes: distinct biological activities in anti-viral therapy. Br J Pharmacol 168:1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schindler C, Levy DE, Decker T. 2007. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 112.Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dinn JJ. 1980. Transolfactory spread of virus in herpes simplex encephalitis. Br Med J 281:1392. doi: 10.1136/bmj.281.6252.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davis LE, Johnson RT. 1979. An explanation for the localization of herpes simplex encephalitis? Ann Neurol 5:2–5. doi: 10.1002/ana.410050103. [DOI] [PubMed] [Google Scholar]
- 115.Kielian T. 2009. Overview of toll-like receptors in the CNS. Curr Top Microbiol Immunol 336:1–14. doi: 10.1007/978-3-642-00549-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reinert LS, Lopušná K, Winther H, Sun C, Thomsen MK, Nandakumar R, Mogensen TH, Meyer M, Vægter C, Nyengaard JR, Fitzgerald KA, Paludan SR. 2016. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat Commun 7:13348. doi: 10.1038/ncomms13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Furr SR, Chauhan VS, Sterka D Jr, Grdzelishvili V, Marriott I. 2008. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J Neurovirol 14:503–513. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peltier DC, Simms A, Farmer JR, Miller DJ. 2010. Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J Immunol 184:7010–7021. doi: 10.4049/jimmunol.0904133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chhatbar C, Detje CN, Grabski E, Borst K, Spanier J, Ghita L, Elliott DA, Jordao MJC, Mueller N, Sutton J, Prajeeth CK, Gudi V, Klein MA, Prinz M, Bradke F, Stangel M, Kalinke U. 2018. Type I interferon receptor signaling of neurons and astrocytes regulates microglia activation during viral encephalitis. Cell Rep 25:118–129. doi: 10.1016/j.celrep.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sato R, Kato A, Chimura T, Saitoh SI, Shibata T, Murakami Y, Fukui R, Liu K, Zhang Y, Arii J, Sun-Wada GH, Wada Y, Ikenoue T, Barber GN, Manabe T, Kawaguchi Y, Miyake K. 2018. Combating herpesvirus encephalitis by potentiating a TLR3-mTORC2 axis. Nat Immunol 19:1071–1082. doi: 10.1038/s41590-018-0203-2. [DOI] [PubMed] [Google Scholar]
- 121.Li Q, Barres BA. 2018. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- 122.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 123.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. 2006. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 124.Fekete R, Cserép C, Lénárt N, Tóth K, Orsolits B, Martinecz B, Méhes E, Szabó B, Németh V, Gönci B, Sperlágh B, Boldogkői Z, Kittel Á, Baranyi M, Ferenczi S, Kovács K, Szalay G, Rózsa B, Webb C, Kovacs GG, Hortobágyi T, West BL, Környei Z, Dénes Á. 2018. Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol 136:461–482. doi: 10.1007/s00401-018-1885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. 2010. Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 126.Liu H, Qiu K, He Q, Lei Q, Lu W. 2019. Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J Neuroimmune Pharmacol 14:157–172. doi: 10.1007/s11481-018-9821-6. [DOI] [PubMed] [Google Scholar]
- 127.Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. 2008. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol 181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 129.Geiger KD, Nash TC, Sawyer S, Krahl T, Patstone G, Reed JC, Krajewski S, Dalton D, Buchmeier MJ, Sarvetnick N. 1997. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology 238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 130.Laukoter S, Rauschka H, Troscher AR, Kock U, Saji E, Jellinger K, Lassmann H, Bauer J. 2017. Differences in T cell cytotoxicity and cell death mechanisms between progressive multifocal leukoencephalopathy, herpes simplex virus encephalitis and cytomegalovirus encephalitis. Acta Neuropathol 133:613–627. doi: 10.1007/s00401-016-1642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sabri F, Granath F, Hjalmarsson A, Aurelius E, Skoldenberg B. 2006. Modulation of sFas indicates apoptosis in human herpes simplex encephalitis. J Neuroimmunol 171:171–176. doi: 10.1016/j.jneuroim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 132.Baringer JR, Pisani P. 1994. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann Neurol 36:823–829. doi: 10.1002/ana.410360605. [DOI] [PubMed] [Google Scholar]
- 133.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. 2007. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol 81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Casanova JL, Sullivan KE. 2018. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol 38:129–143. doi: 10.1007/s10875-017-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Almerigogna F, Fassio F, Giudizi MG, Biagiotti R, Manuelli C, Chiappini E, Galli L, Romagnani S, De Martino M. 2011. Natural killer cell deficiencies in a consecutive series of children with herpetic encephalitis. Int J Immunopathol Pharmacol 24:231–238. doi: 10.1177/039463201102400128. [DOI] [PubMed] [Google Scholar]
- 137.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. 2008. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 138.Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Senechal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Heron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 139.Sancho-Shimizu V, Zhang SY, Abel L, Tardieu M, Rozenberg F, Jouanguy E, Casanova JL. 2007. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol 7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- 140.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 141.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Perez de Diego R, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Valerie D, Jouanguy E, Chaussabel D, Geissmann F, Abel L, Casanova JL, Zhang SY. 2011. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med 208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lim HK, Seppanen M, Hautala T, Ciancanelli MJ, Itan Y, Lafaille FG, Dell W, Lorenzo L, Byun M, Pauwels E, Ronnelid Y, Cai X, Boucherit S, Jouanguy E, Paetau A, Lebon P, Rozenberg F, Tardieu M, Abel L, Yildiran A, Vergison A, Roivainen R, Etzioni A, Tienari PJ, Casanova JL, Zhang SY. 2014. TLR3 deficiency in herpes simplex encephalitis: high allelic heterogeneity and recurrence risk. Neurology 83:1888–1897. doi: 10.1212/WNL.0000000000000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Armangue T, Baucells BJ, Vlagea A, Petit-Pedrol M, Esteve-Solé A, Deyà-Martínez A, Ruiz-García R, Juan M, Pérez de Diego R, Dalmau J, Alsina L, Armangue T, Baucells BJ, Vlagea A, Petit-Pedrol M, Esteve-Solé A, Deyà-Martínez A, Ruiz-García R, Juan M, Pérez de Diego R, Dalmau J, Alsina L. 2019. Toll-like receptor 3 deficiency in autoimmune encephalitis post-herpes simplex encephalitis. Neurol Neuroimmunol Neuroinflamm 6:e611. doi: 10.1212/NXI.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mork N, Kofod-Olsen E, Sorensen KB, Bach E, Orntoft TF, Ostergaard L, Paludan SR, Christiansen M, Mogensen TH. 2015. Mutations in the TLR3 signaling pathway and beyond in adult patients with herpes simplex encephalitis. Genes Immun 16:552–566. doi: 10.1038/gene.2015.46. [DOI] [PubMed] [Google Scholar]
- 145.Sironi M, Peri AM, Cagliani R, Forni D, Riva S, Biasin M, Clerici M, Gori A. 2017. TLR3 Mutations in adult patients with herpes simplex virus and varicella-zoster virus encephalitis. J Infect Dis 215:1430–1434. doi: 10.1093/infdis/jix166. [DOI] [PubMed] [Google Scholar]
- 146.Sancho-Shimizu V, Perez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, Fabrega S, Cardon A, Maluenda J, Tatematsu M, Mahvelati F, Herman M, Ciancanelli M, Guo Y, AlSum Z, Alkhamis N, Al-Makadma AS, Ghadiri A, Boucherit S, Plancoulaine S, Picard C, Rozenberg F, Tardieu M, Lebon P, Jouanguy E, Rezaei N, Seya T, Matsumoto M, Chaussabel D, Puel A, Zhang SY, Abel L, Al-Muhsen S, Casanova JL. 2011. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J Clin Invest 121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Andersen LL, Mork N, Reinert LS, Kofod-Olsen E, Narita R, Jorgensen SE, Skipper KA, Honing K, Gad HH, Ostergaard L, Orntoft TF, Hornung V, Paludan SR, Mikkelsen JG, Fujita T, Christiansen M, Hartmann R, Mogensen TH. 2015. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J Exp Med 212:1371–1379. doi: 10.1084/jem.20142274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, Herman M, Cardon A, Durandy A, Bustamante J, Vallabhapurapu S, Bravo J, Warnatz K, Chaix Y, Cascarrigny F, Lebon P, Rozenberg F, Karin M, Tardieu M, Al-Muhsen S, Jouanguy E, Zhang SY, Abel L, Casanova JL. 2010. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Herman M, Ciancanelli M, Ou YH, Lorenzo L, Klaudel-Dreszler M, Pauwels E, Sancho-Shimizu V, Perez de Diego R, Abhyankar A, Israelsson E, Guo Y, Cardon A, Rozenberg F, Lebon P, Tardieu M, Heropolitanska-Pliszka E, Chaussabel D, White MA, Abel L, Zhang SY, Casanova JL. 2012. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J Exp Med 209:1567–1582. doi: 10.1084/jem.20111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Al Ghonaium A, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova JL. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet 33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 151.Audry M, Ciancanelli M, Yang K, Cobat A, Chang HH, Sancho-Shimizu V, Lorenzo L, Niehues T, Reichenbach J, Li XX, Israel A, Abel L, Casanova JL, Zhang SY, Jouanguy E, Puel A. 2011. NEMO is a key component of NF-kappaB- and IRF-3-dependent TLR3-mediated immunity to herpes simplex virus. J Allergy Clin Immunol 128:610–617.e4. doi: 10.1016/j.jaci.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A, Ying SW, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova JL, Studer L, Notarangelo LD. 2012. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bibert S, Piret J, Quinodoz M, Collinet E, Zoete V, Michielin O, Menasria R, Meylan P, Bihl T, Erard V, Fellman F, Rivolta C, Boivin G, Bochud PY. 2019. Herpes simplex encephalitis in adult patients with MASP-2 deficiency. PLoS Pathog 15:e1008168. doi: 10.1371/journal.ppat.1008168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mason CP, Tarr AW. 2015. Human lectins and their roles in viral infections. Molecules 20:2229–2271. doi: 10.3390/molecules20022229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schmidt-Hieber M, Schwender J, Heinz WJ, Zabelina T, Kuhl JS, Mousset S, Schuttrumpf S, Junghanss C, Silling G, Basara N, Neuburger S, Thiel E, Blau IW. 2011. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica 96:142–149. doi: 10.3324/haematol.2010.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dragoje S, Tolnay M, Dalquen P, Probst A. 1995. Brain irradiation and antioedematous dexamethasone treatment–risk factors for herpes simplex encephalitis? Schweiz Arch Neurol Psychiatr (1985) 146:277–280. [PubMed] [Google Scholar]
- 157.Silvano G, Lazzari G, Resta F, Buccoliero G, Pezzella G, Pisconti S. 2007. A herpes simplex virus-1 fatal encephalitis following chemo-radiotherapy, steroids and prophylactic cranial irradiation in a small cell lung cancer patient. Lung Cancer 57:243–246. doi: 10.1016/j.lungcan.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 158.Jacobs DH. 1999. Herpes simplex virus encephalitis following corticosteroids and cranial irradiation. Neurology 52:1108–1109. doi: 10.1212/wnl.52.5.1106-c. [DOI] [PubMed] [Google Scholar]
- 159.Lizarraga KJ, Alexandre LC, Ramos-Estebanez C, Merenda A. 2013. Are steroids a beneficial adjunctive therapy in the immunosuppressed patient with herpes simplex virus encephalitis? Case Rep Neurol 5:52–55. doi: 10.1159/000350572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sermer DJ, Woodley JL, Thomas CA, Hedlund JA. 2014. Herpes simplex encephalitis as a complication of whole-brain radiotherapy: a case report and review of the literature. Case Rep Oncol 7:774–779. doi: 10.1159/000369527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bloch KC. 2012. Atypical herpes simplex encephalitis after total cranial irradiation: casting a wider net? Crit Care Med 40:1385–1386. doi: 10.1097/CCM.0b013e31823b96b2. [DOI] [PubMed] [Google Scholar]
- 162.Graber JJ, Rosenblum MK, DeAngelis LM. 2011. Herpes simplex encephalitis in patients with cancer. J Neurooncol 105:415–421. doi: 10.1007/s11060-011-0609-2. [DOI] [PubMed] [Google Scholar]
- 163.Jakob NJ, Lenhard T, Schnitzler P, Rohde S, Ringleb PA, Steiner T, Wildemann B. 2012. Herpes simplex virus encephalitis despite normal cell count in the cerebrospinal fluid. Crit Care Med 40:1304–1308. doi: 10.1097/CCM.0b013e3182374a34. [DOI] [PubMed] [Google Scholar]
- 164.Matikas A, Kontopodis E, Nintos G, Bilidas T, Kofteridis DP, Papadaki EZ, Lyraraki E, Kanatsouli K, Mavroudis D. 2014. A case of herpes simplex-associated encephalitis after brain irradiation for lung cancer metastases. Anticancer Res 34:4411–4414. [PubMed] [Google Scholar]
- 165.Chakraborty S, Donner M, Colan D. 2013. Fatal herpes encephalitis in a patient with small cell lung cancer following prophylactic cranial radiation—a case report with review of literature. Anticancer Res 33:3263–3268. [PubMed] [Google Scholar]
- 166.Stroop WG, Schaefer DC. 1986. Production of encephalitis restricted to the temporal lobes by experimental reactivation of herpes simplex virus. J Infect Dis 153:721–731. doi: 10.1093/infdis/153.4.721. [DOI] [PubMed] [Google Scholar]
- 167.Hughes MA, Parisi M, Grossman S, Kleinberg L. 2005. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys 62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 168.Alimohamadi SM, Malekzadeh R, Mirmadjless SH, Mohamadnejad M, Zamani F. 2004. Herpes simplex virus encephalitis during immunosuppressive treatment of ulcerative colitis. MedGenMed 6:7. [PMC free article] [PubMed] [Google Scholar]
- 169.Robineau O, Enrico J, Lemaire X, Poissy J, Legout L, Senneville E, Colombel JF, Yazdanpanah Y. 2010. Herpes simplex virus meningoencephalitis in a patient with Crohn’s disease on azathioprine therapy. Am J Gastroenterol 105:240–241. doi: 10.1038/ajg.2009.544. [DOI] [PubMed] [Google Scholar]
- 170.Sergerie Y, Rivest S, Boivin G. 2007. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis 196:853–860. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- 171.Lundberg P, Welander PV, Edwards CK III, van Rooijen N, Cantin E. 2007. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J Virol 81:1451–1460. doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bradford RD, Pettit AC, Wright PW, Mulligan MJ, Moreland LW, McLain DA, Gnann JW, Bloch KC. 2009. Herpes simplex encephalitis during treatment with tumor necrosis factor-alpha inhibitors. Clin Infect Dis 49:924–927. doi: 10.1086/605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Crusio RH, Singson SV, Haroun F, Mehta HH, Parenti DM. 2014. Herpes simplex virus encephalitis during treatment with etanercept. Scand J Infect Dis 46:152–154. doi: 10.3109/00365548.2013.849816. [DOI] [PubMed] [Google Scholar]
- 174.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L, TRANSFORMS Study Group. 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 175.Pfender N, Jelcic I, Linnebank M, Schwarz U, Martin R. 2015. Reactivation of herpesvirus under fingolimod: a case of severe herpes simplex encephalitis. Neurology 84:2377–2378. doi: 10.1212/WNL.0000000000001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perini P, Rinaldi F, Puthenparampil M, Marcon M, Perini F, Gallo P. 2018. Herpes simplex virus encephalitis temporally associated with dimethyl fumarate-induced lymphopenia in a multiple sclerosis patient. Mult Scler Relat Disord 26:68–70. doi: 10.1016/j.msard.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 177.Stüve O, Marra CM, Bar-Or A, Niino M, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Jerome KR, Cook L, Grand'Maison F, Hemmer B, Monson NL, Racke MK. 2006. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 63:1383–1387. doi: 10.1001/archneur.63.10.1383. [DOI] [PubMed] [Google Scholar]
- 178.Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. 2013. Central nervous system herpes simplex and varicella zoster virus infections in natalizumab-treated patients. Clin Infect Dis 57:849–852. doi: 10.1093/cid/cit376. [DOI] [PubMed] [Google Scholar]
- 179.Kwiatkowski A, Gallois J, Bilbault N, Calais G, Mackowiak A, Hautecoeur P. 2012. Herpes encephalitis during natalizumab treatment in multiple sclerosis. Mult Scler 18:909–911. doi: 10.1177/1352458511428082. [DOI] [PubMed] [Google Scholar]
- 180.Haggiag S, Prosperini L, Galgani S, Pozzilli C, Pinnetti C. 2016. Extratemporal herpes encephalitis during natalizumab treatment: a case report. Mult Scler Relat Disord 10:134–136. doi: 10.1016/j.msard.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 181.Sharma K, Ballham SA, Inglis KE, Renowden S, Cottrell DA. 2013. Does natalizumab treatment increase the risk of herpes simplex encephalitis in multiple sclerosis? Case and discussion. Mult Scler Relat Disord 2:385–387. doi: 10.1016/j.msard.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 182.Cruz-Topete D, Cidlowski JA. 2015. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ben-Hur T, Cialic R, Itzik A, Barak O, Yirmiya R, Weidenfeld J. 2001. A novel permissive role for glucocorticoids in induction of febrile and behavioral signs of experimental herpes simplex virus encephalitis. Neuroscience 108:119–127. doi: 10.1016/s0306-4522(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 184.Levi G, Minghetti L, Aloisi F. 1998. Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie 80:899–904. doi: 10.1016/s0300-9084(00)88886-0. [DOI] [PubMed] [Google Scholar]
- 185.Habel AH, Brown JK. 1972. Dexamethasone in herpes-simplex encephalitis. Lancet 1:695. doi: 10.1016/s0140-6736(72)90505-3. [DOI] [PubMed] [Google Scholar]
- 186.Carmon A, Behar A, Beller AJ. 1965. Acute necrotizing haemorrhagic encephalitis presenting clinically as a space-occupying lesion. A clinico-pathological study of six cases. J Neurol Sci 2:328–343. doi: 10.1016/0022-510X(65)90017-1. [DOI] [PubMed] [Google Scholar]
- 187.Upton AR, Barwick DD, Foster JB. 1971. Dexamethasone treatment in herpes-simplex encephalitis. Lancet 1:290–291. doi: 10.1016/s0140-6736(71)91019-1. [DOI] [PubMed] [Google Scholar]
- 188.Johnson KP, Rosenthal MS, Lerner PI. 1972. Herpes simplex encephalitis. The course in five virologically proven cases. Arch Neurol 27:103–108. doi: 10.1001/archneur.1972.00490140007003. [DOI] [PubMed] [Google Scholar]
- 189.Illis LS, Merry RT. 1972. Treatment of herpes simplex encephalitis. J R Coll Physicians Lond 7:34–44. [PMC free article] [PubMed] [Google Scholar]
- 190.Yamamoto K, Chiba HO, Ishitobi M, Nakagawa H, Ogawa T, Ishii K. 1997. Acute encephalopathy with bilateral striatal necrosis: favourable response to corticosteroid therapy. Eur J Paediatr Neurol 1:41–45. doi: 10.1016/s1090-3798(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 191.Musallam B, Matoth I, Wolf DG, Engelhard D, Averbuch D. 2007. Steroids for deteriorating herpes simplex virus encephalitis. Pediatr Neurol 37:229–232. doi: 10.1016/j.pediatrneurol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 192.Mesker AJ, Bon GG, de Gans J, de Kruijk JR. 2011. Case report: a pregnant woman with herpes simplex encephalitis successfully treated with dexamethasone. Eur J Obstet Gynecol Reprod Biol 154:231–232. doi: 10.1016/j.ejogrb.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 193.Nakano A, Yamasaki R, Miyazaki S, Horiuchi N, Kunishige M, Mitsui T. 2003. Beneficial effect of steroid pulse therapy on acute viral encephalitis. Eur Neurol 50:225–229. doi: 10.1159/000073864. [DOI] [PubMed] [Google Scholar]
- 194.Maraş Genç H, Uyur Yalçın E, Sayan M, Bayhan A, Öncel S, Arısoy ES, Kara B. 2016. Clinical outcomes in children with herpes simplex encephalitis receiving steroid therapy. J Clin Virol 80:87–92. doi: 10.1016/j.jcv.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 195.Kamei S, Sekizawa T, Shiota H, Mizutani T, Itoyama Y, Takasu T, Morishima T, Hirayanagi K. 2005. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry 76:1544–1549. doi: 10.1136/jnnp.2004.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kamei S, Taira N, Ishihara M, Sekizawa T, Morita A, Miki K, Shiota H, Kanno A, Suzuki Y, Mizutani T, Itoyama Y, Morishima T, Hirayanagi K. 2009. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine 46:187–193. doi: 10.1016/j.cyto.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 197.Mancini M, Vidal SM. 2018. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mamm Genome 29:425–445. doi: 10.1007/s00335-018-9772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baringer JR, Klassen T, Grumm F. 1976. Experimental herpes simplex virus encephalitis. Effect of corticosteroids and pyrimidine nucleoside. Arch Neurol 33:442–446. doi: 10.1001/archneur.1976.00500060048010. [DOI] [PubMed] [Google Scholar]
- 199.Sergerie Y, Boivin G, Gosselin D, Rivest S. 2007. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J Infect Dis 195:817–825. doi: 10.1086/511987. [DOI] [PubMed] [Google Scholar]
- 200.Thompson KA, Blessing WW, Wesselingh SL. 2000. Herpes simplex replication and dissemination is not increased by corticosteroid treatment in a rat model of focal herpes encephalitis. J Neurovirol 6:25–32. doi: 10.3109/13550280009006379. [DOI] [PubMed] [Google Scholar]
- 201.Meyding-Lamade U, Seyfer S, Haas J, Dvorak F, Kehm R, Lamade W, Hacke W, Wildemann B. 2002. Experimental herpes simplex virus encephalitis: inhibition of the expression of inducible nitric oxide synthase in mouse brain tissue. Neurosci Lett 318:21–24. doi: 10.1016/s0304-3940(01)02469-7. [DOI] [PubMed] [Google Scholar]
- 202.Meyding-Lamadé UK, Oberlinner C, Rau PR, Seyfer S, Heiland S, Sellner J, Wildemann BT, Lamadé WR. 2003. Experimental herpes simplex virus encephalitis: a combination therapy of acyclovir and glucocorticoids reduces long-term magnetic resonance imaging abnormalities. J Neurovirol 9:118–125. doi: 10.1080/13550280390173373. [DOI] [PubMed] [Google Scholar]
- 203.Boivin N, Sergerie Y, Rivest S, Boivin G. 2008. Effect of pretreatment with toll-like receptor agonists in a mouse model of herpes simplex virus type 1 encephalitis. J Infect Dis 198:664–672. doi: 10.1086/590671. [DOI] [PubMed] [Google Scholar]
- 204.Guo YJ, Luo T, Wu F, Liu H, Li HR, Mei YW, Zhang SL, Tao JY, Dong JH, Fang Y, Zhao L. 2015. Corilagin protects against HSV1 encephalitis through inhibiting the TLR2 signaling pathways in vivo and in vitro. Mol Neurobiol 52:1547–1560. doi: 10.1007/s12035-014-8947-7. [DOI] [PubMed] [Google Scholar]
- 205.Li LJ, Zhang SJ, Liu P, Wang YQ, Chen ZL, Wang YJ, Zhou JB, Guo YJ, Zhao L. 2019. Corilagin interferes with Toll-like receptor 3-mediated immune response in herpes simplex encephalitis. Front Mol Neurosci 12:83. doi: 10.3389/fnmol.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Boivin N, Menasria R, Piret J, Boivin G. 2012. Modulation of TLR9 response in a mouse model of herpes simplex virus encephalitis. Antiviral Res 96:414–421. doi: 10.1016/j.antiviral.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 207.Ceron S, North BJ, Taylor SA, Leib DA. 2019. The STING agonist 5,6-dimethylxanthenone-4-acetic acid (DMXAA) stimulates an antiviral state and protects mice against herpes simplex virus-induced neurological disease. Virology 529:23–28. doi: 10.1016/j.virol.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ozaki KS, Camara NO, Nogueira E, Pereira MG, Granato C, Melaragno C, Camargo LF, Pacheco-Silva A. 2007. The use of sirolimus in ganciclovir-resistant cytomegalovirus infections in renal transplant recipients. Clin Transplant 21:675–680. doi: 10.1111/j.1399-0012.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- 209.Sellar RS, Ward KN, Thomson KJ, Peggs KS. 2012. Evidence for clinical activity of artesunate in multidrug-resistant herpes simplex infection following HSCT. Bone Marrow Transplant 47:1482–1483. doi: 10.1038/bmt.2012.46. [DOI] [PubMed] [Google Scholar]
- 210.Canivet C, Menasria R, Rheaume C, Piret J, Boivin G. 2015. Valacyclovir combined with artesunate or rapamycin improves the outcome of herpes simplex virus encephalitis in mice compared to antiviral therapy alone. Antiviral Res 123:105–113. doi: 10.1016/j.antiviral.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 211.Nadeau S, Rivest S. 1999. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol 58:61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 212.Boivin N, Menasria R, Piret J, Rivest S, Boivin G. 2013. The combination of valacyclovir with an anti-TNF alpha antibody increases survival rate compared to antiviral therapy alone in a murine model of herpes simplex virus encephalitis. Antiviral Res 100:649–653. doi: 10.1016/j.antiviral.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 213.Schachtele SJ, Hu S, Lokensgard JR. 2012. Modulation of experimental herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLoS One 7:e36216. doi: 10.1371/journal.pone.0036216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Meyding-Lamade U, Haas J, Lamade W, Stingele K, Kehm R, Fath A, Heinrich K, Storch Hagenlocher B, Wildemann B. 1998. Herpes simplex virus encephalitis: long-term comparative study of viral load and the expression of immunologic nitric oxide synthase in mouse brain tissue. Neurosci Lett 244:9–12. doi: 10.1016/s0304-3940(98)00115-3. [DOI] [PubMed] [Google Scholar]
- 215.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. 1992. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149:2736–2741. [PubMed] [Google Scholar]
- 216.Sellner J, Simon F, Meyding-Lamade U, Leib SL. 2006. Herpes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res 1125:155–162. doi: 10.1016/j.brainres.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 217.Zhou Y, Lu ZN, Guo YJ, Mei YW. 2010. Favorable effects of MMP-9 knockdown in murine herpes simplex encephalitis using small interfering RNA. Neurol Res 32:801–809. doi: 10.1179/016164110X12644252260556. [DOI] [PubMed] [Google Scholar]
- 218.Zhou Y, Zeng YP, Zhou Q, Guan JX, Lu ZN. 2016. The effect of captopril on the expression of MMP-9 and the prognosis of neurological function in herpes simplex encephalitis mice. Neurol Res 38:733–739. doi: 10.1080/01616412.2016.1202462. [DOI] [PubMed] [Google Scholar]
- 219.Erlich KS, Dix RD, Mills J. 1987. Prevention and treatment of experimental herpes simplex virus encephalitis with human immune serum globulin. Antimicrob Agents Chemother 31:1006–1009. doi: 10.1128/aac.31.7.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ramakrishna C, Newo AN, Shen YW, Cantin E. 2011. Passively administered pooled human immunoglobulins exert IL-10 dependent anti-inflammatory effects that protect against fatal HSV encephalitis. PLoS Pathog 7:e1002071. doi: 10.1371/journal.ppat.1002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ramakrishna C, Golub MS, Chiang A, Hong T, Kalkum M, Cantin EM. 2017. Effects of acyclovir and IVIG on behavioral outcomes after HSV1 CNS infection. Behav Neurol 2017:5238402. doi: 10.1155/2017/5238402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kothur K, Wienholt L, Brilot F, Dale RC. 2016. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 77:227–237. doi: 10.1016/j.cyto.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 223.Rosler A, Pohl M, Braune HJ, Oertel WH, Gemsa D, Sprenger H. 1998. Time course of chemokines in the cerebrospinal fluid and serum during herpes simplex type 1 encephalitis. J Neurol Sci 157:82–89. doi: 10.1016/s0022-510x(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 224.Michael BD, Griffiths MJ, Granerod J, Brown D, Keir G, Wnęk M, Cox DJ, Vidyasagar R, Borrow R, Parkes LM, Solomon T. 2016. The interleukin-1 balance during encephalitis is associated with clinical severity, blood-brain barrier permeability, neuroimaging changes, and disease outcome. J Infect Dis 213:1651–1660. doi: 10.1093/infdis/jiv771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bociąga-Jasik M, Cieśla A, Kalinowska-Nowak A, Skwara P, Garlicki A, Mach T. 2011. Role of IL-6 and neopterin in the pathogenesis of herpetic encephalitis. Pharmacol Rep 63:1203–1209. doi: 10.1016/s1734-1140(11)70640-5. [DOI] [PubMed] [Google Scholar]
- 226.Studahl M, Rosengren L, Gunther G, Hagberg L. 2000. Difference in pathogenesis between herpes simplex virus type 1 encephalitis and tick-borne encephalitis demonstrated by means of cerebrospinal fluid markers of glial and neuronal destruction. J Neurol 247:636–642. doi: 10.1007/s004150070134. [DOI] [PubMed] [Google Scholar]
- 227.Studahl M, Gunther G, Rosengren L. 2009. Serum S-100B protein levels in patients with herpes simplex encephalitis and tick-borne encephalitis—a marker of CNS damage during the initial stage of disease. J Neurol 256:586–590. doi: 10.1007/s00415-009-0124-y. [DOI] [PubMed] [Google Scholar]
- 228.Abel L, Plancoulaine S, Jouanguy E, Zhang SY, Mahfoufi N, Nicolas N, Sancho-Shimizu V, Alcais A, Guo Y, Cardon A, Boucherit S, Obach D, Clozel T, Lorenzo L, Amsallem D, Berquin P, Blanc T, Bost-Bru C, Chabrier S, Chabrol B, Cheuret E, Dulac O, Evrard P, Heron B, Lazaro L, Mancini J, Pedespan JM, Rivier F, Vallee L, Lebon P, Rozenberg F, Casanova JL, Tardieu M. 2010. Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J Pediatr 157:623–629. doi: 10.1016/j.jpeds.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 229.Jouanguy E, Zhang SY, Chapgier A, Sancho-Shimizu V, Puel A, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. 2007. Human primary immunodeficiencies of type I interferons. Biochimie 89:878–883. doi: 10.1016/j.biochi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 230.Wintergerst U, Gangemi JD, Whitley RJ, Chatterjee S, Kern ER. 1999. Effect of recombinant human interferon alpha B/D (rHu-IFN-alpha B/D) in combination with acyclovir in experimental HSV-1 encephalitis. Antiviral Res 44:75–78. doi: 10.1016/s0166-3542(99)00055-8. [DOI] [PubMed] [Google Scholar]