Abstract
A risk mitigation strategy was implemented to determine if a higher prophylactic voriconazole dosage in CYP2C19 rapid metabolizer neutropenic AML patients reduces the incidence of subtherapeutic trough concentrations. AML patients (n=263) were preemptively genotyped for CYP2C19*2, *3, and *17 alleles as part of a single-center prospective, interventional, quality improvement study. CYP2C19 rapid metabolizers (CYP2C19*1/*17) were recommended to receive interventional voriconazole 300 mg twice daily, ultrarapid metabolizers (CYP2C19*17/*17) were recommended to avoid voriconazole, and all others received the standard prophylactic dosage of 200 mg twice daily. In this real-world setting, 202 patients (76.8%) were prescribed prophylactic voriconazole and of these patients 176 (87.1%) received CYP2C19-guided prophylactic dosing. Voriconazole trough concentrations were obtained for 41 of the 58 (70.7%) CYP2C19 rapid metabolizers prescribed prophylactic voriconazole. Interventional voriconazole resulted in higher plasma trough concentrations (median 2.7 μg/mL) compared to the standard prophylactic dosage (median 0.6 μg/mL, P=0.001). Subtherapeutic concentrations were avoided in 83.8% of CYP2C19 rapid metabolizers receiving interventional dosage compared to 46.2% receiving standard dosage (P=0.02). CYP2C19 genotyping to preemptively guide prophylactic voriconazole dosing is feasible and may be a potential strategy for reducing the risk of subtherapeutic trough concentrations that potentiate breakthrough fungal infections.
Keywords: Precision Medicine, Voriconazole, CYP2C19, AML, fungal prophylaxis, pharmacogenomics
INTRODUCTION
Neutropenic patients with acute myeloid leukemia (AML) have among the highest risk for fungal infections when compared to other hematologic malignancies.(1) The proven or probable fungal infection rate in AML immunocompromised patients ranges from 8–12%, though incidences over 20% have been observed.(1–3) For those who develop an invasive fungal infection, mortality rates are 30–80% dependent on the specific fungal species.(2, 3) Epidemiology studies have demonstrated that mold infections (e.g., Aspergillus species) are more prevalent in neutropenic hematologic malignancy populations.(1, 2) Prophylactic use of antifungals with a broader spectrum of activity such as voriconazole reduce the incidence of invasive fungal disease, though breakthrough infections are commonly observed.(3–8)
Voriconazole is an effective antifungal prophylactic and is recommended as primary treatment for invasive aspergillosis in immunocompromised patients.(2, 5, 9, 10) Voriconazole displays highly variable non-liner pharmacokinetics, and there is a strong correlation between voriconazole plasma trough concentrations and clinical outcomes in those with invasive fungal infections.(10, 11) Trough concentrations less than 1–2 μg/mL are predictive of treatment failure, whereas concentrations greater than 5–6 μg/mL are associated with reversible neurotoxicity.(12) Optimal voriconazole concentrations in the prophylactic setting are not well defined, though studies have suggested trough concentrations < 1 μg/mL are a risk factor for breakthrough fungal infections.(6, 8)
Voriconazole is metabolized by the polymorphic CYP2C19 enzyme to compounds that have minimal antifungal activity. A variation in the promoter region of CYP2C19, referred to as CYP2C19*17, results in upregulation of protein transcription and thus increased metabolic capacity. Approximately 25% of individuals are predicted to be CYP2C19 rapid (CYP2C19*17 heterozygotes) or ultrarapid (CYP2C19*17 homozygotes) metabolizers, though among those of Asian ancestry the CYP2C19*17 allele is less common.(13) There is robust evidence demonstrating that CYP2C19 rapid and ultrarapid metabolizers are likely to have subtherapeutic trough concentrations when receiving the standard voriconazole prophylactic dosage of 200 mg twice daily, defined as a trough concentration < 1 μg/mL.(6, 8, 12–15) Given a substantial portion of patients carry the CYP2C19*17 allele, utilizing CYP2C19 genotype to optimize prophylactic voriconazole dosing may be a potential risk mitigation strategy for reducing morbidity, morality, and health care costs associated with progressive fungal infections caused by subtherapeutic voriconazole concentrations.(16) Herein, we describe the implementation of a prospective quality improvement study to determine if a higher prophylactic voriconazole dosage of 300 mg twice daily for CYP2C19 rapid metabolizers reduces the incidence of subtherapeutic trough concentrations without increasing voriconazole-induced toxicities.
RESULTS
A total of 263 AML patients who were CYP2C19 genotyped to guide prophylactic voriconazole dosing were analyzed with demographic characteristics summarized in Table 1. There were no significant differences in the distribution of weight among CYP2C19 diplotypes (P=0.36), but there was a difference in age (P =0.04) and sex (P =0.004, Supplemental Table 1). The observed allele frequencies for CYP2C19*1 (63.3%), *2 (16.4%), and *17 (20.3%) are representative of expected allele frequencies in our patient population.(13) The median time to return for CYP2C19 results was 3 days, ranging from results returned on the same day to as long as 12 days later. Ordering of CYP2C19 in the ambulatory setting was a contributor to longer turnaround times as blood collection for genotyping did not always occur on the same day as test ordering.
Table 1.
Patient Characteristics (n=263)
| Age | |
| Median (years) | 64 |
| Range (years) | 19–86 |
| Sex | No. (%) |
| Female | 124 (47.1) |
| Male | 139 (52.9) |
| Weight | |
| Median (kg) | 80.4 |
| Range (kg) | 38–165.8 |
| Self-declared race | No. (%) |
| Native American | 1 (0.4) |
| Asian | 8 (3) |
| Other | 13 (5) |
| Black | 15 (5.7) |
| Unknown | 26 (9.9) |
| White | 200 (76) |
| Reason for CYP2C19 genotyping | No. (%) |
| Admitted to hospital for diagnosis workup | 3 (1.1) |
| Ambulatory management | 17 (6.5) |
| Admitted to hospital for febrile neutropenia | 20 (7.6) |
| Admitted to hospital to receive chemotherapy | 223 (84.8) |
| CYP2C19 genotyping turnaround time | |
| Median (days) | 3 |
| Range (days) | 0–12 |
| CYP2C19 genotypes/phenotypes | No. (%) |
| CYP2C19*17/*17‒ultrarapid metabolizer | 5 (1.9) |
| CYP2C19*1/*17‒rapid metabolizer | 74 (28.1) |
| CYP2C19*1/*1‒normal metabolizer | 105 (39.9) |
| CYP2C19*2/*17‒intermediate metabolizer | 23 (8.7) |
| CYP2C19*1/*2‒intermediate metabolizer | 49 (18.6) |
| CYP2C19*2/*2‒poor metabolizer | 7 (2.7) |
| CYP2C19 allele frequencies | No. (%) |
| CYP2C19*1 | 333 (63.3) |
| CYP2C19*17 | 107 (20.3) |
| CYP2C19*2 | 86 (16.4) |
Forty-four patients (16.7%) who were CYP2C19 genotyped did not receive voriconazole. Of the 219 patients receiving voriconazole, 17 (7.8%) had voriconazole prescribed for a suspected fungal infection while 202 patients (92.2%) received prophylactic voriconazole (Table 2). Among the 202 patients receiving prophylactic voriconazole (Supplemental Table 2 & 3), CYP2C19-guided dosage recommendations were followed for 176 patients (87.1%), with a slightly lower rate (79.3%) for CYP2C19 rapid metabolizers receiving interventional dosing. The most common reason for not following CYP2C19-guided recommendations was discharge from the hospital before CYP2C19 results were returned. Fewer days of hospitalization for those admitted for febrile neutropenia, and delays in CYP2C19 ordering along with laboratory processing contributed to results not returned before discharge from the hospital. Other reasons for not following CYP2C19-guided voriconazole dosage recommendations included dose adjustments based on weight, elevated liver enzymes, and prior voriconazole pharmacokinetic data.
Table 2.
Percent of patients prescribed voriconazole (VCZ) per the CYP2C19-guided prophylaxis protocol
| Patients CYP2C19 genotyped receiving/not receiving VCZ (n=263) | No. (%) |
| Did not receive VCZ | 44 (16.7) |
| Received VCZ | 219 (83.3) |
| Reason for VCZ prescribing (n=219) | No. (%) |
| Treatment | 17 (7.8) |
| Prophylaxis | 202 (92.2) |
| Followed CYP2C19-guided prophylaxis protocol | No. (%) |
| All genotype groups (n=202) | 176 (87.1) |
| CYP2C19*1/*17 group (n=58) | 46 (79.3) |
| CYP2C19*17/*17 group (n=5) | 4 (80) |
| Reason for not following the protocol | No. (%) |
| Weight‒obese dosing | 1 (3.9) |
| Prior VCZ pharmacokinetics | 1 (3.9) |
| Patient died before CYP2C19 returned | 1 (3.9) |
| Elevated liver enzymes | 3 (11.5) |
| Unknown | 6 (23.1) |
| Patient discharged from hospital before CYP2C19 returned | 14 (53.8) |
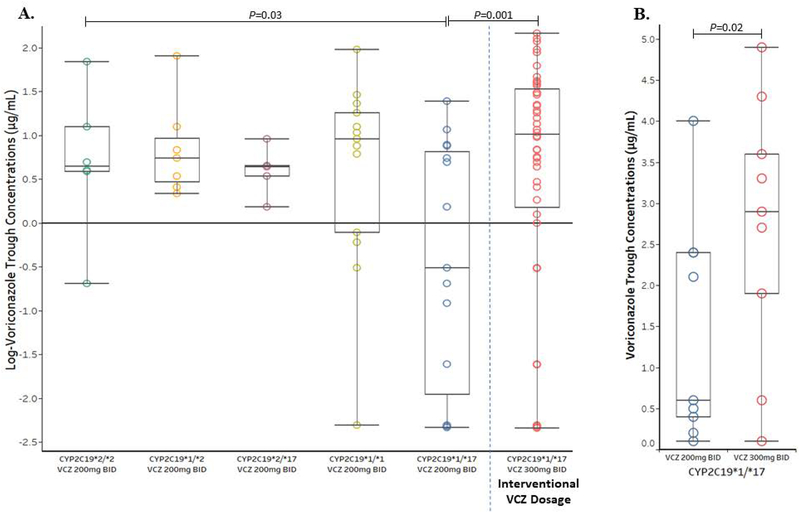
A total of 90 trough concentrations among 70 patients were obtained and stratified by CYP2C19 diplotypes along with dosage (Supplemental Table 4 & 5). Voriconazole trough concentrations were obtained for 41 of the 58 CYP2C19 rapid metabolizers (70.7%) prescribed prophylactic voriconazole. The median time for specimen processing and return of voriconazole concentrations was six days (range 2–15 days). Among the stratified groups, CYP2C19 rapid metabolizers (CYP2C19*1/*17) receiving the standard prophylactic voriconazole dosage had significantly lower trough concentrations (median 0.6 μg/mL, P =0.03, Figure 1A, Supplemental Figure 6), which is consist with historical data.(12–15, 17–20) CYP2C19 rapid metabolizers receiving interventional voriconazole 300 mg twice daily attained higher trough concentrations (median 2.7 μg/mL) when compared to those receiving 200 mg twice daily (P =0.001, Figure 1A, Supplemental Figure 6).
Figure 1.
A) Voriconazole (VCZ) trough concentrations stratified by CYP2C19 diplotype and dosage were compared using a liner-mixed-effects model fitted to log-transformed concentrations. VCZ concentrations were also compared between CYP2C19 rapid metabolizers receiving the standard VCZ dosage (200 mg twice daily [BID]) and the interventional dosage (300 mg BID). B) Wilcoxon signed-rank test compared trough concentrations between nine CYP2C19 rapid metabolizers who receive both VCZ 200 mg and 300 mg BID. Box plots represent the median and interquartile range, and data points with darker outlines represent >1 trough concentration of the same value.
Voriconazole concentrations were determined for nine CYP2C19 rapid metabolizers who initially received voriconazole 200 mg twice daily then switched to the interventional 300 mg twice daily dosage after return of CYP2C19 results. The median voriconazole trough was significantly higher for the interventional dosage (median 2.9 μg/mL) when compared to the standard voriconazole 200 mg twice daily dosage (median 0.6 μg/mL, P=0.02, Figure 1B). There were no differences in voriconazole concentrations among patients who were prescribed a proton pump inhibitor (P=0.92) or steroid (P=0.28) when compared to those not prescribed a proton pump inhibitor or steroid (Supplemental Figure 7).(14)
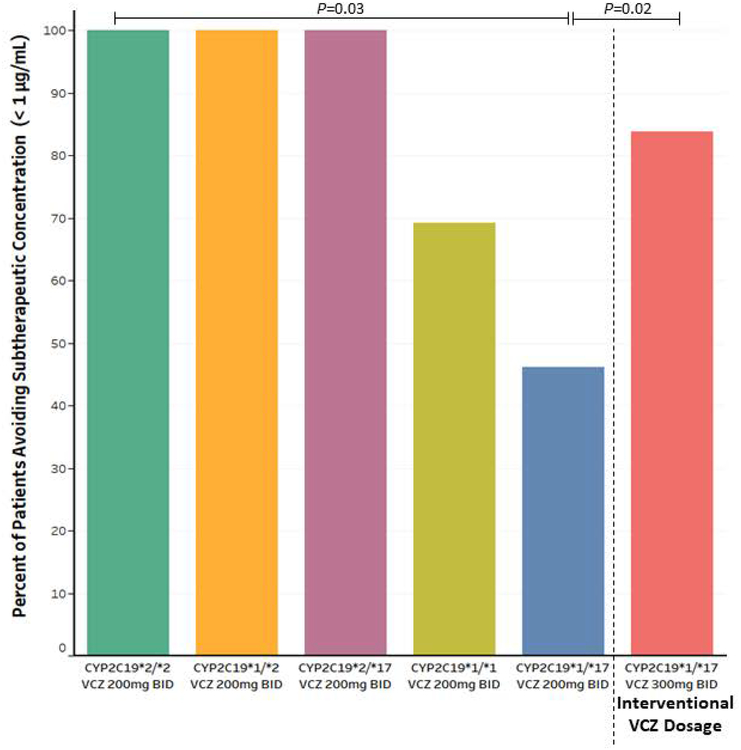
Only 46.2% (n=6/13) of CYP2C19 rapid metabolizers receiving voriconazole 200 mg twice daily avoided subtherapeutic concentrations (< 1 μg/mL), whereas 83.8% (n=31/37) of those receiving voriconazole 300 mg twice daily avoided subtherapeutic concentrations (P =0.02, Figure 2). Every CYP2C19 intermediate and poor metabolizer receiving the standard prophylactic voriconazole dosage avoided subtherapeutic concentrations, with 69% (n=9/13) of normal metabolizers avoiding subtherapeutic concentrations. The hospital acquired nodular pneumonia rate was slightly lower after implementation of CYP2C19-guided voriconazole dosing (2.1 nodular pneumonia cases per 1000 neutropenic days) when compared to a historical control (2.2 nodular pneumonia cases per 1000 neutropenic days, P=0.46), though a larger patient cohort is needed to be powered for statistical analysis. No breakthrough fungal infections were observed among the four CYP2C19 ultrarapid metabolizers prescribed isavuconazonium instead of voriconazole.
Figure 2.
Percent of unique patients avoiding subtherapeutic voriconazole (VCZ) concentrations, defined as a trough less than 1 μg/mL. The Fisher’s exact test compared groups stratified by CYP2C19 diplotype and dosage. Unique patients avoiding subtherapeutic VCZ concentrations were also compared between CYP2C19 rapid metabolizers receiving the standard VCZ dosage (200 mg twice daily [BID]) and the interventional dosage (300 mg BID).
Voriconazole discontinuation due to neurotoxicity or elevated liver enzymes was determined among the 176 patients (Supplemental Table 6 & 7) dosed per CYP2C19 recommendations. Among CYP2C19 rapid metabolizers receiving voriconazole 300 mg twice daily, 8.7% (n=4/46) of patients experienced neurotoxicity and 6.5% (n=3/46) of patients developed elevated liver transaminases that required drug discontinuation. There were no significant differences in voriconazole discontinuation due to neurotoxicity (P =0.18) or liver toxicity (P =0.83) when compared among groups stratified by CYP2C19 diplotypes and dosage (Table 3).
Table 3.
Percent of patients with neurotoxicity or elevated liver transaminases prescribed voriconazole per the CYP2C19-guided prophylaxis protocol
| Neurotoxicity | |||
|---|---|---|---|
| CYP2C19 genotype | Yes, No. (%) | No, No. (%) | P |
| CYP2C19*1/*17 | 4 (8.7) | 42 (91.3) | 0.18 |
| CYP2C19*1/*1 | 14 (21.9) | 50 (78.1) | |
| CYP2C19*2/*17 | 3 (18.7) | 13 (81.3) | |
| CYP2C19*1/*2 | 3 (7.5) | 37 (92.5) | |
| CYP2C19*2/*2 | 1 (14.3) | 6 (85.7) | |
| Elevated liver transaminases | |||
| CYP2C19 genotype | Yes, No. (%) | No, No. (%) | P |
| CYP2C19*1/*17 | 3 (6.5) | 43 (93.5) | 0.83 |
| CYP2C19*1/*1 | 7 (10.9) | 57 (89.1) | |
| CYP2C19*2/*17 | 1 (6.3) | 15 (93.7) | |
| CYP2C19*1/*2 | 3 (7.5) | 37 (92.5) | |
| CYP2C19*2/*2 | 1 (14.3) | 6 (85.7) | |
DISCUSSION
Breakthrough fungal infection rates in neutropenic patients receiving prophylactic voriconazole are commonly observed, with trough concentrations < 1 μg/mL associated with progressive fungal disease.(6, 8, 16, 21, 22) Epidemiology studies have shown that drug resistant Aspergillus and non-Aspergillus molds are commonly observed among those with breakthrough infections.(21, 22) Prolonged exposure to suboptimal voriconazole concentrations may mechanistically promote acquired resistance and selection of subpopulations with primary resistance.(23–25) The CYP2C19*17 allele is a risk factor for low voriconazole concentrations, and can affect approximately 25% of patients. There is limited clinical guidance for utilization of prophylactic voriconazole in CYP2C19 rapid metabolizers, but Clinical Pharmacogenetic Implementation Consortium guidelines have recommended avoidance of voriconazole for CYP2C19 rapid metabolizers with an invasive fungal infection.(13) Voriconazole avoidance would result in a significant proportion of patients not being eligible for an effective antifungal with a limited number of other drug options currently available for prophylaxis against, or treatment of, fungal infections. Therefore, other risk mitigation strategies beyond avoidance of voriconazole are needed for CYP2C19*17 carriers.
Based on numerous investigations supporting the clinical utility of CYP2C19 to guide voriconazole therapy, CYP2C19 genotyping was implemented to identify patients at risk of subtherapeutic concentrations and preemptively increasing the voriconazole prophylactic dosage (for CYP2C19 rapid metabolizers) or selecting another antifungal agent (for CYP2C19 ultrarapid metabolizers). We demonstrated that systematic implementation of CYP2C19 genotyping in a neutropenic AML population to prospectively guide prophylactic voriconazole dosage is feasible. Interventional voriconazole dosing almost doubled the percent of CYP2C19 rapid metabolizers avoiding subtherapeutic concentrations, increasing from 46% avoidance for those receiving the standard voriconazole prophylactic dosage to 84% avoidance for those receiving the interventional dosage. Compared to CYP2C19 normal metabolizers receiving the standard prophylactic voriconazole dosage, a greater number of CYP2C19 rapid metabolizers receiving the interventional voriconazole dosage avoided subtherapeutic concentrations. Fewer CYP2C19 normal metabolizers having voriconazole concentrations obtained could influence results, along with the possibility of not detecting a rare non-functional CYP2C19 allele among normal metabolizers. Notably, higher doses for CYP2C19 rapid metabolizers did not increase the discontinuation of voriconazole therapy due to toxicities. To our knowledge, this is the first report demonstrating that preemptively increasing the prophylactic voriconazole dosage in neutropenic patients with AML rescues the CYP2C19 rapid metabolizer phenotype.
Therapeutic drug monitoring can be utilized to adjust voriconazole doses after steady-state plasma concentrations are attained, which is typically 5 to 7 days after initiating therapy.(12) Voriconazole loading doses can reduce time to steady-state concentrations, though this practice is less common in the prophylactic setting. Specimen processing and return of voriconazole concentrations in this study was a median of 6 days, therefore determining if a patient has a subtherapeutic voriconazole concentration could take two weeks or longer after initiation of therapy. The median incubation time for invasive aspergillosis in neutropenic AML patients is estimated to be 14 days, thus a breakthrough fungal infection could occur before adjustment of voriconazole dosage based on plasma concentrations.(26) Furthermore, several studies have suggested that unfavorable clinical outcomes, including mortality, associated with initial low voriconazole concentrations are not completely overcome by subsequently prescribing higher doses based on therapeutic drug monitoring.(6, 8, 27, 28) Our results demonstrate that CYP2C19 genotyping to preemptively guide voriconazole therapy greatly decreases the incidence of initial low steady-state concentrations. Combining preemptive CYP2C19 genotyping with therapeutic drug monitoring to further refine dosage is a potential strategy for avoidance of initial and prolonged subtherapeutic concentrations.
A trough concentration of ≤ 0.5 μg/mL has been proposed to define suboptimal prophylactic voriconazole concentrations.(12) Immunocompromised patients with voriconazole trough concentrations < 1 μg/mL may be at an increased risk of breakthrough fungal infections, with recent studies suggesting higher minimal concentrations are associated with reduced treatment failure rates.(6, 8, 12) Only 53.8% (n=7/13) of CYP2C19 rapid metabolizers prescribed the standard voriconazole prophylactic dosage had a trough concentration greater than 0.5 μg/mL. Therefore, utilization of < 1 μg/mL, instead of ≤ 0.5 μg/mL, to define subtherapeutic prophylactic voriconazole concentrations did not greatly influence outcomes.
Voriconazole trough concentrations were utilized as a biomarker for outcomes, founded on robust correlations between low voriconazole concentrations and increased likelihood of morbidity along with mortality.(6, 8, 10–12) Ultimately, the goal is to investigate the impact CYP2C19-guided voriconazole dosing has on occurrence of breakthrough fungal infections. Nodular pneumonia rates were determined, with a slight decrease observed after implementation of CYP2C19-guided voriconazole dosing. Baseline and sequential CT scans of the chest have been demonstrated to have clinical utility for early detection of progressive fungal disease.(29, 30) Other diagnostic assays such as bronchoalveolar lavage fluid aspergillus culture or galactomannan are less sensitive for detecting early progressive disease for those receiving antifungal prophylaxis.(31)
A prior budget impact analysis conservatively predicted that CYP2C19-guided voriconazole dosing would prevent approximately two breakthrough fungal infections per 100 neutropenic patients with AML resulting in modest cost savings of $415 dollars per patient while also improving outcomes.(16) Validation of the budget impact analysis in a larger patient cohort is warranted to determine if the risk mitigation strategy of CYP2C19-guided voriconazole dosing to prevent breakthrough fungal infections is cost-effective. Furthermore, prospective studies are needed that explore the use of CYP2C19 genotyping to guide voriconazole dosing in those initially diagnosed with an invasive fungal infection. All CYP2C19 intermediate and poor metabolizers prescribed voriconazole 200 mg twice daily avoided subtherapeutic concentrations; which brings into question whether these patients are at an elevated risk of toxicities if prescribed higher voriconazole treatment doses.
Limitations of this study included the underrepresentation of minority and ethnic groups. A prior unpublished principle component analysis suggests that approximately 10% of Moffitt patients who self-declare race as white are genetically of Hispanic ethnicity. Irrespective, caution should be given to extrapolating our findings to minority populations that may carry allelic variants not tested for in this study or have other characteristics that may influence voriconazole exposure. Although therapeutic drug monitoring was not performed for every CYP2C19 genotyped patient prescribed voriconazole, the majority of CYP2C19 rapid metabolizers (41 of 58, 70.7%) did have trough concentrations collected which was the primary focus for this investigation. By study design a limited number of CYP2C19 rapid metabolizers received the standard voriconazole prophylactic dosage of 200 mg twice daily, but observed trough concentrations were consistent with prior published studies.(15, 20) Greater therapeutic drug monitoring among other CYP2C19 diplotypes may have further elucidated the application of CYP2C19-guided voriconazole dosing.
Another limitation is that the sample size was not adequately powered to determine if CYP2C19 genotyping reduced breakthrough fungal infections in a cost-effective manner. Our quality improvement study is ongoing, and based on CYP2C19 allele frequencies along with estimated breakthrough fungal infection rates we will be fully powered for outcomes analysis within two years. However, this does not weaken our finding that interventional voriconazole dosing for CYP2C19 rapid metabolizers resulted in avoidance of subtherapeutic concentrations.
CONCLUSION
Implementation of CYP2C19 genotyping in a neutropenic population with AML to preemptively guide prophylactic voriconazole dosage is feasible. CYP2C19 genotyping to optimize voriconazole dosing may be a potential strategy for reducing the risk of subtherapeutic trough concentrations that potentiate breakthrough fungal infections.
MATERIALS AND METHODS
Implementation Design & Patient Population
CYP2C19-guided prophylactic voriconazole dosing for AML patients hospitalized to receive chemotherapy and/or supportive care and anticipated to have prolonged neutropenia (absolute neutrophil count < 500 cells/mm3) was implemented as a single-center, prospective, interventional, quality improvement study at Moffitt Cancer Center. CYP2C19 normal, intermediate and poor metabolizers were recommended to receive the standard voriconazole prophylactic dosage of 200 mg twice daily. An interventional voriconazole dosage of 300 mg twice daily was recommended for rapid metabolizers, whereas ultrarapid metabolizers were recommended to receive a different antifungal such as isavuconazonium (Supplemental Figure 1). Therapeutic drug monitoring to further optimize voriconazole dosing was at the discretion of the primary medical team, with emphasis on obtaining voriconazole trough concentrations for CYP2C19 rapid metabolizers. Voriconazole plasma concentration analysis was performed by a reference laboratory (Quest Diagnostics, Valencia, CA) with a goal trough concentration of 1–5.5 μg/mL.
Automated processes for CYP2C19 genotyping and dissemination of dosing recommendations were deployed to the electronic health record (EHR). CYP2C19 test ordering was integrated into inpatient and ambulatory AML order sets (i.e., Cerner PowerPlans™). In this workflow design, CYP2C19 results are available prior to completion of induction/re-induction chemotherapy when voriconazole is initiated. CYP2C19 can also be manually ordered as part of an infectious disease consultation. To avoid host versus donor genomics, those with a history of liver transplant are ineligible for CYP2C19 genotyping and patients with a history of allogenic stem cell transplant must have a pre-transplant germline DNA sample available for CYP2C19 genotyping. CYP2C19 results are entered into the EHR with clinical decision support to assist with CYP2C19-guided voriconazole dosing recommendations as previously described by Hicks et al. (Supplemental Figures 2–5).(32, 33)
CYP2C19 Genotyping & Phenotype Assignment
CYP2C19 genotyping was performed in a College of American Pathologists/Clinical Laboratory Improvement Amendment-certified laboratory at Moffitt Cancer Center using the Luminex xTAG® CYP2C19 Kit v3. The CYP2C19 test interrogates for *2 (no function), *3 (no function), and *17 (increase function) alleles. CYP2C19*1 (normal function allele) is assigned by default when no genetic variants are detected. Phenotypes are assigned per Clinical Pharmacogenetics Implementation Consortium guidelines as follows: ultrarapid metabolizer (CYP2C19*17/*17), rapid metabolizer (CYP2C19*1/*17), normal metabolizer (CYP2C19*1/*1), intermediate metabolizer (CYP2C19*1/*2, *1/*3, *2/*17), and poor metabolizer (CYP2C19*2/*2, *2/*3, *3/*3).(13)
Data Collection
Patients CYP2C19 genotyped from September 1, 2016 - May 31, 2018 were eligible for chart review. Patient demographics were collected along with voriconazole-induced toxicity, plasma trough concentrations, and concomitantly prescribed drugs during the hospitalization CYP2C19 was ordered, or if genotyping was performed in the ambulatory setting, the hospitalization immediately after CYP2C19 testing. Voriconazole-induced toxicity is defined as the discontinuation of voriconazole due to neurotoxicity (including visual disturbances) or liver toxicity per the primary medical team’s judgment. Voriconazole oral administration was scheduled 12 hours apart. Blood samples for determining voriconazole trough concentrations must have been collected at steady-state (defined as consistently taking voriconazole for a minimum of 5 days(10, 12)) and within four hours of the scheduled 12 hour trough and before receiving the next scheduled dose. Voriconazole concentrations below the level of assay detection were reported as either < 0.5 μg/mL or < 0.1 μg/mL. For purposes of analysis, all voriconazole concentrations below the level of detection were defaulted to 0.1 μg/mL.
Hospital acquired nodular pneumonia, defined as any new pulmonary nodule larger than 1cm that was not present on baseline computed tomographic (CT) chest scans, was determined as a surrogate for breakthrough fungal infections.(29, 30) Hospital acquired nodular pneumonia rate per 1000 neutropenic days was calculated from quarterly summarized reports obtained from Moffitt’s Infection Prevention program. Data from April 1, 2017–September 30, 2017 was omitted due to construction, which is known to increase the risk of fungal infections.(9) Hospital acquired nodular pneumonia rate was also determined for a historical control one year prior to implementation. All data collection and analysis was approved by an institutional review board.
Statistical Analysis
Patient characteristics along with CYP2C19 allele and diplotypes frequencies are presented as median and range for continuous variables, and frequencies and percentages for categorical variables. Categorical variables were assessed using Fisher’s exact test, and continuous variables were assessed using the Kruskal-Wallis rank sum test. To account for some patients having multiple voriconazole plasma trough concentrations, a linear mixed-effects model evaluated log-transformed voriconazole trough concentrations among CYP2C19 diplotypes stratified by voriconazole dosage, concomitantly prescribed proton pump inhibitors or steroids. The Wilcoxon signed-rank test compared voriconazole plasma concentrations between CYP2C19 rapid metabolizers who receive both the 200 mg and 300 mg voriconazole dosage. Patients avoiding subtherapeutic voriconazole trough concentrations were compared between CYP2C19 diplotypes stratified by voriconazole dosage using Fisher’s exact test. P-values less than 0.05 were considered statistically significant. Data analysis was conducted using R.3.5.0.
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Neutropenic AML patients have among the highest risk for invasive fungal infections with mortality rates reaching 80%. Although voriconazole is an effective antifungal prophylactic, breakthrough fungal infections are commonly observed with trough concentrations < 1 μg/mL a risk factor for progressive disease. Voriconazole is metabolized by CYP2C19, with a substantial portion of patients carrying a genetic variant that upregulates CYP2C19 activity. Prior evidence demonstrated that CYP2C19 rapid metabolizers, when receiving the standard voriconazole prophylactic dosage of 200 mg twice daily, are likely to have concentrations < 1 μg/mL. There is limited clinical guidance for dosing prophylactic voriconazole in CYP2C19 rapid metabolizers to prevent subtherapeutic concentrations.
What question did this study address?
We implemented a risk mitigation strategy that utilizes CYP2C19 genotype to preemptively guide voriconazole dosing in neutropenic patients with AML. CYP2C19 rapid metabolizers receiving an interventional voriconazole prophylactic dosage of 300 mg twice daily avoided subtherapeutic concentrations without increasing voriconazole-induced toxicities. This is one of the first and largest studies to prospectively use CYP2C19 genotype to prevent the occurrence of subtherapeutic voriconazole concentrations, thus providing real-world clinical evidence for prophylactic voriconazole dosing in CYP2C19 rapid metabolizers.
What does this study add to our knowledge?
CYP2C19 rapid metabolizers are at an increased risk of subtherapeutic voriconazole concentrations which can potentiate poor pharmacotherapy outcomes. The results from our study suggests that a prophylactic voriconazole dosage of 300 mg twice daily in adults rescues the CYP2C19 rapid metabolizer phenotype.
How might this change clinical pharmacology or translational science?
Implementation of CYP2C19 genotyping in neutropenic patient populations to prospectively guide prophylactic voriconazole dosage is feasible and may be a potential strategy for reducing the risk of subtherapeutic trough concentrations that potentiate breakthrough fungal infections.
Funding:
This work has been supported in part by Pinellas Partners, the Collins Family Trust, the DeBartolo Family Personalized Medicine Institute, a grant from the American Society of Health Systems Pharmacy (ASHP), and the H. Lee Moffitt Cancer Center & Research Institute (NIH P30-CA076292).
Footnotes
Conflict of Interest: The authors declared no competing interests for this work.
REFERENCES
- (1).Pagano L et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91, 1068–75 (2006). [PubMed] [Google Scholar]
- (2).Hachem R et al. Comparing the safety and efficacy of voriconazole versus posaconazole in the prevention of invasive fungal infections in high-risk patients with hematological malignancies. International journal of antimicrobial agents 50, 384–8 (2017). [DOI] [PubMed] [Google Scholar]
- (3).Cornely OA et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356, 348–59 (2007). [DOI] [PubMed] [Google Scholar]
- (4).Pagano L et al. Evaluation of the practice of antifungal prophylaxis use in patients with newly diagnosed acute myeloid leukemia: results from the SEIFEM 2010-B registry. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 55, 1515–21 (2012). [DOI] [PubMed] [Google Scholar]
- (5).Maertens JA et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother 73, 3221–30 (2018). [DOI] [PubMed] [Google Scholar]
- (6).Trifilio S et al. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant 40, 451–6 (2007). [DOI] [PubMed] [Google Scholar]
- (7).Marks DI, Liu Q & Slavin M Voriconazole for prophylaxis of invasive fungal infections after allogeneic hematopoietic stem cell transplantation. Expert Rev Anti Infect Ther 15, 493–502 (2017). [DOI] [PubMed] [Google Scholar]
- (8).Mitsani D et al. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrobial agents and chemotherapy 56, 2371–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Patterson TF et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 63, e1–e60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG & McLachlan AJ Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrobial agents and chemotherapy 56, 4793–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Pascual A, Calandra T, Bolay S, Buclin T, Bille J & Marchetti O Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 46, 201–11 (2008). [DOI] [PubMed] [Google Scholar]
- (12).Owusu Obeng A, Egelund EF, Alsultan A, Peloquin CA & Johnson JA CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy 34, 703–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Moriyama B et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clinical pharmacology and therapeutics 102, 45–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hicks JK et al. Voriconazole plasma concentrations in immunocompromised pediatric patients vary by CYP2C19 diplotypes. Pharmacogenomics 15, 1065–78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lamoureux F et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. International journal of antimicrobial agents 47, 124–31 (2016). [DOI] [PubMed] [Google Scholar]
- (16).Mason NT, Bell GC, Quilitz RE, Greene JN & McLeod HL Budget impact analysis of CYP2C19-guided voriconazole prophylaxis in AML. J Antimicrob Chemother 70, 3124–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wang G et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. European journal of clinical pharmacology 65, 281–5 (2009). [DOI] [PubMed] [Google Scholar]
- (18).Berge M et al. Effect of cytochrome P450 2C19 genotype on voriconazole exposure in cystic fibrosis lung transplant patients. European journal of clinical pharmacology 67, 253–60 (2011). [DOI] [PubMed] [Google Scholar]
- (19).Dolton MJ & McLachlan AJ Voriconazole pharmacokinetics and exposure-response relationships: Assessing the links between exposure, efficacy and toxicity. International journal of antimicrobial agents 44, 183–93 (2014). [DOI] [PubMed] [Google Scholar]
- (20).Hamadeh IS et al. Impact of the CYP2C19 genotype on voriconazole exposure in adults with invasive fungal infections. Pharmacogenetics and genomics 27, 190–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lionakis MS, Lewis RE & Kontoyiannis DP Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 67, 1621–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lamoth F, Chung SJ, Damonti L & Alexander BD Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 64, 1619–21 (2017). [DOI] [PubMed] [Google Scholar]
- (23).Revie NM, Iyer KR, Robbins N & Cowen LE Antifungal drug resistance: evolution, mechanisms and impact. Curr Opin Microbiol 45, 70–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kanafani ZA & Perfect JR Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 46, 120–8 (2008). [DOI] [PubMed] [Google Scholar]
- (25).Perlin DS, Rautemaa-Richardson R & Alastruey-Izquierdo A The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17, e383–e92 (2017). [DOI] [PubMed] [Google Scholar]
- (26).Benet T, Voirin N, Nicolle MC, Picot S, Michallet M & Vanhems P Estimation of the incubation period of invasive aspergillosis by survival models in acute myeloid leukemia patients. Med Mycol 51, 214–8 (2013). [DOI] [PubMed] [Google Scholar]
- (27).Miyakis S, van Hal SJ, Ray J & Marriott D Voriconazole concentrations and outcome of invasive fungal infections. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 16, 927–33 (2010). [DOI] [PubMed] [Google Scholar]
- (28).Lestrade PP et al. Voriconazole Resistance and Mortality in Invasive Aspergillosis: A Multicenter Retrospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, (2018). [DOI] [PubMed] [Google Scholar]
- (29).Patsios D et al. Chest low-dose computed tomography in neutropenic acute myeloid leukaemia patients. Respir Med 104, 600–5 (2010). [DOI] [PubMed] [Google Scholar]
- (30).Mendoza D et al. Baseline and Bimonthly High-Resolution Computed Tomographic Imaging of the Chest in the Early Detection and Treatment of Pulmonary Mold Infections in Patients With Leukemia With Prolonged Neutropenia. Infectious Diseases in Clinical Practice 22, 210–5 (2014). [Google Scholar]
- (31).Marr KA, Laverdiere M, Gugel A & Leisenring W Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 40, 1762–9 (2005). [DOI] [PubMed] [Google Scholar]
- (32).Hicks JK et al. A clinician-driven automated system for integration of pharmacogenetic interpretations into an electronic medical record. Clinical pharmacology and therapeutics 92, 563–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hicks JK et al. Implementation of Clinical Pharmacogenomics within a Large Health System: From Electronic Health Record Decision Support to Consultation Services. Pharmacotherapy 36, 940–8 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.