Abstract
Polymorphonuclear neutrophils (PMN) play a critical role in the innate immune response to invading pathogens. However, dysregulated mucosal trafficking of PMN and associated epithelial tissue damage is a pathological hallmark of numerous inflammatory conditions including inflammatory bowel disease. The glycoprotein CD11b/CD18 plays a well described role in regulating PMN transepithelial migration and PMN inflammatory functions. Previous studies have demonstrated that targeting of the N-linked glycan Lewis X on CD11b blocks PMN transepithelial migration (TEpM). Given evidence of glycosylation dependent regulation of CD11b/CD18 function, we performed MALDI TOF Mass Spectrometry (MS) analyses on CD11b/CD18 purified from human PMN. Unusual glycan epitopes identified on CD11b/CD18 included high Mannose oligosaccharides recognized by the Galanthus Nivalis (GNA) lectin and biantennary galactosylated N-glycans recognized by the Phaseolus Vulgaris erythroagglutinin (PHA-E) lectin. Importantly, we show that selective targeting of glycans on CD11b with such lectins results in altered intracellular signaling events that inhibit TEpM and differentially affect key PMN inflammatory functions including phagocytosis, superoxide release and apoptosis. Taken together, these data demonstrate that discrete glycan motifs expressed on CD11b/CD18 such as biantennary galactose could represent novel targets for selective manipulation of CD11b function and reduction of PMN associated tissue damage in chronic inflammatory diseases.
Keywords: Neutrophil, Glycans, Inflammation, Colitis
Introduction
Polymorphonuclear neutrophils (PMN) are the first innate immune cells to respond to infection or tissue injury and they are essential for successful elimination of invading pathogens (1). In addition to their role as professional phagocytes, PMN contribute to wound healing and restitution of mucosal homeostasis at later stages of an inflammatory response (2). However, under conditions of non-resolving inflammation, ongoing PMN migration and release of toxic anti-microbial agents directly contributes to host tissue damage. As such, dysregulated influx of activated PMN into mucosal tissues is a pathological hallmark of a number of chronic inflammatory diseases including inflammatory bowel disease (IBD) and chronic obstructive pulmonary disease COPD (3–5).
Trafficking of PMN to sites of infection or mucosal inflammation begins with migration out of the microcirculation. Initial PMN rolling on vascular endothelium is mediated by reversible adhesion interactions between Selectins and their glycosylated ligands including P Selectin glycoprotein ligand 1 (6, 7). PMN arrest during rolling is triggered by chemokines/cytokines and is mediated by binding of PMN CD11a/CD18 β2-integrins to endothelial expressed immunoglobulin superfamily members such as ICAM-1 and VCAM-1. The final step of PMN transendothelial migration involves passage to the interstitium through paracellular or less common transcellular routes (8, 9). In the case of epithelial lined organs, including the lungs, intestine and urinary tract, following extravasation PMN trafficking to sites of infection/inflammation requires migration across a polarized epithelial barrier. In contrast to PMN transendothelial migration, the steps that mediate PMN transepithelial migration (TEpM) have not been nearly as well characterized. However, previous studies have identified several epithelial expressed glycoproteins that are important for PMN TEpM including Intracellular adhesion molecule 1 (ICAM-1), CD55, CD47 and CD44v6 (10–13). Furthermore, there is evidence for selective involvement of multiple Lewis family glycans including Sialyl Lewis A (14), Lewis A (15) and Lewis X (16) in regulation of TEpM and other PMN effector functions in the intestine. In terms of leukocyte expressed adhesion molecules, multiple lines of evidence have shown that a major PMN receptor that regulates TEpM is the highly glycosylated β2-integrin CD11b/CD18 (17–21).
In addition to being critical for PMN TEpM, CD11b/CD18 also regulates other important PMN inflammatory functions including phagocytosis, respiratory burst activity, degranulation and apoptosis (22, 23). Interestingly, regulation of these key PMN functions via glycan binding protein (lectin) activity has also been previously described. Specifically, studies have demonstrated that concavalin A binding to mannose and wheat germ agglutinin (WGA) binding to N-Acetylglucosamine activates human PMN, inducing phagocytosis and respiratory burst responses (24, 25). The importance of glycan mediated binding events in stimulating PMN immune function is further highlighted by leukocyte adhesion deficiency II, a genetic disorder resulting in defective glycosylation of Selectin ligands. Individuals with this fucosylation deficiency have recurrent bacterial infections, persistent peripheral blood leukocytosis as well as severe mental and growth retardation (26). Furthermore altered expression of glycans has been implicated in disease pathogenesis during murine colitis and in the inflamed mucosa of humans with active inflammatory bowel disease (14, 27–29).
While CD11b/CD18 is known to be a key regulator of PMN inflammatory effector functions, the role of specific CD11b/CD18 glycans in mediating PMN inflammatory activity is poorly understood. In this study, we performed tandem MALDI-TOF Mass Spectrometry (MS) to generate, for the first time, a complete profile of the N-linked glycans on human PMN CD11b/CD18. N-glycan analysis of CD11b/CD18 revealed a surprising lack of sialylation. Unusual glycan epitopes identified on CD11b/CD18 included high Mannose oligosaccharides recognized by the Galanthus Nivalis (GNA) lectin and the Macrophage Mannose Receptor (MMR) as well as biantennary galactosylated glycans recognized by the Phaseolus Vulgaris erythroagglutinin (PHA-E) lectin. Furthermore, we show that selective targeting of these specific CD11b expressed glycans with lectins results in intracellular signaling events that differentially regulate key PMN inflammatory functions including chemotaxis, TEpM, phagocytosis, superoxide generation and apoptosis. These data highlight the functional significance of CD11b glycans as targets to regulate PMN inflammatory effector functions and ameliorate PMN mediated mucosal tissue damage.
Materials and Methods
Antibodies, Lectins and Reagents
Galanthus Nivalis (GNA), Phaseolus Vulgaris erythroagglutinin (PHA-E), Wheat Germ Agglutinin (WGA) Lectins and protein free blocking solution were purchased from Vector Labs (Burlingame, CA). 1μm FITC-conjugated carboxylate FluoSpheres (505/5150) were purchased from Molecular Probes (Carlsbad, CA). N-Formyl-L-methionyl-Leucyl-L-phenylalanine (fMLF), Leukotriene B4 (LTB4), Latrunculin B, Cytochrome c from bovine heart and Superoxide dismutase (SOD) were purchased from Sigma-Aldrich (St. Louis, MO). BCECF, AM (2’,7’-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester) and fluorescent counting beads were purchased from ThermoFisher Scientific (Waltham, MA). The functionally inhibitory anti-CD11b antibody CBRM1/29 has been characterized elsewhere (30). Antibodies against CD18 were purchased from Abcam (Cambridge, UK). PNGaseF was purchased from New England Biolabs (Ipswich, MA). Recombinant human Mannose Macrophage Receptor (CD206) was purchased from R&D Systems (Minneapolis, MN). Mouse Fc block (CD16/CD32), anti-CD45 PerCP (Clone30-F11) and FITC conjugated human anti-CD11a mAbs were purchased from BD Bioscience (San Jose, CA). Anti-CD11b PE (clone M1/70) was purchased from eBioscience (Waltham, MA). Anti-Ly6G Alexa Fluor 647 (clone 1A8) was purchased from Biolegend (San Diego, CA). Signaling antibodies for immunoblotting were published from Cell Signaling Technologies (Danvers, MA).
Cell culture and PMN isolation
Cultures of T84 (19) and SKCO15 IECs (31) were grown as previously described. PMN were isolated from whole blood obtained from normal human volunteers, with approval from the University of Michigan Institutional Review Board on human subjects, using a previously described Polymorphprep density gradient centrifugation technique (11). PMN isolated in this way were 97% pure and >95% viable and were used for all assays within 2 hours of blood draw. Colonoids were generated from biopsies of normal human colonic mucosa as previously described (32, 33). All human colon sample collection was performed in accordance with the University of Michigan Institutional Review Board regulations. Isolated colonoids were resuspended in matrigel and cultured in growth media containing Wnt3a, R-Spondin and Noggin which was replaced every other day. Colonoid cultures were maintained via passaging once per week. To generate 2D monolayers, colonoids grown as described above were spun out of matrigel and dissociated into a single cell suspension using Trypsin/EDTA as described previously (34). Following one day of culture in complete growth media, cells were switched to differentiation media (growth media minus Wnt3a, R-Spondin and with a 50% reduction in Noggin) for 4–5 days of differentiation into monolayers of differentiated colonic epithelium.
Immunoblotting and Protein Purification
PMN cell lysates for immunoblotting were prepared with the following lysis buffer (20mM Tris pH 7.5, 150mM NaCl, 1mM EDTA, 1% TX-100, 1mM Na3VO4, and 1mM PMSF) supplemented with 10% mammalian tissue protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). PMN cell lysates were boiled in sample buffer under reducing conditions, and then subjected to SDS-PAGE followed by transfer to PVDF under standard conditions. Binding of biotinylated lectins was detected using HRP conjugated streptavidin. Data are representative of PMN isolated from 5 independent healthy donors. N-glycans were removed from CD11b/CD18 using PNGaseF according to manufacturer’s instructions. Functionally active CD11b/CD18 was purified from human PMNs by LM2/1 immunoaffinity chromatography as described previously (35, 36).
Preparation of N-glycans for mass spectrometry
10μg of protein (CD11b/CD18 immunopurified from human PMN) was resolved by SDS Page gel electrophoresis. Upon staining with CBB, the CD11b/CD18-corresponding bands were excised out of the gel, washed with ammonium bicarbonate (AMBIC) and 50% acetonitrile (ACN) prior to incubation with 1,4-dithiothreitol (DTT) and iodoacetamide (IAA). The DTT/IAA solutions were removed, and the samples were then incubated overnight with trypsin. The peptides were recovered from the gel by sequential washes with AMBIC and ACN buffers. The N-glycans were subsequently removed with PNGaseF (New England Biolabs) and recovered using a C18 Sep-Pak (50 mg) column (Waters). Flow thru and wash fractions containing the N-glycans, were pooled and lyophilized
Permethylation of N-glycans
N-glycans were permethylated by incubation with iodomethane in a NaOH/DMSO solution under vigorous shaking. Permethylated N-glycans were then recovered in chloroform and washed with MilliQ water. The chloroform fraction, containing permethylated N-glycans was evaporated, the dried N-glycans re-suspended in a 50% methanol solution and loaded onto a C18 Sep-Pak (50 mg). Finally, N-glycans were eluted with 50% ACN and lyophilized
MALDI-TOF data acquisition
Dried permethylated N-glycans were re-suspended in a 50% methanol solution and spotted on a MALDI target plate (polished steel, Bruker Daltonics) with 2,5-dihydroxybenzoic acid as matrix. MS data was acquired on a Bruker UltraFlex II MALDI-TOF Mass Spectrometer instrument. MS signals matching an N-glycan composition were considered for further analysis. Subsequent MS post-data acquisition analysis were made using mMass (37).
PMN Transmigration and Chemotaxis Assay
For transmigration experiments, IECs were grown on collagen-coated, permeable 0.33-cm2 polycarbonate filters (3μm pore size; Costar Corp.) and effects of 10μg/ml indicated lectins or glycan binding proteins on PMN TEpM in the physiologically relevant basolateral to apical direction quantified as described previously (11, 14, 19). For migration assays PMN were incubated with indicated lectins after application to intestinal epithelial cells. PMNs remaining adherent to IEC monolayers following basolateral to apical migration were quantified as follows. Following completion of migration T84 monolayers were removed and transferred to new 24 well tissue culture plates containing 500μl HBSS+. Plates were spun at low speed (50 x g) for 5 minutes and detachment of PMN from the apical surface of epithelial cells was quantified by MPO assay as described above. For PMN chemotaxis assays, PMN were incubated with 10μg/ml indicated antibodies/lectins before migration across collagen-coated, permeable 0.33-cm2 polycarbonate filters to 100nm fMLF was assessed by measurement of MPO as described above.
CD11b/CD18 T84 IEC Adhesion Assay
Functionally active human CD11b/CD18 (purified to homogeneity as described above) was added to Nunc maxisorp flat bottomed 96 well plates at a concentration of 5μg/ml for protein immobilization as previously described (30, 38).
Immunofluorescence detection of glycan expression.
Immunofluorescent labeling of IECs was achieved as follows. Confluent T84 monolayers were fixed and permeabilized in absolute ethanol and subsequently blocked with protein free blocking buffer. Following blocking, monolayers were incubated with 5μg/ml Alexa Fluor 555 conjugated anti-ZO-1 mAb (ZO1–1A12), FITC conjugated lectins and Hoechst stain for nuclear detection. All images were captured using a Leica SP8 confocal microscope system.
Flow Cytometry and Phagocytosis assay
For flow cytometry analyses, non-stimulated PMN or PMN stimulated with 10nM fMLF(human) or 1 μM fMLF (mouse) were incubated with 10μg/ml of FITC conjugated lectins or antibodies. Flow cytometric analysis was carried out using an ACEA NovoCyte Flow Cytometer. For phagocytosis assays, PMN were incubated with 10μg/ml relevant lectins (unconjugated) and FITC conjugated FluoSpheres at a ratio of 1:100 (PMN:FluoSpheres) in the presence of 10nM fMLF for 30 minutes at 37oC. Uptake of FluoSpheres by PMN was assessed by flow cytometry as described previously (16).
PMN degranulation assay
PMNs were isolated as described above and incubated with relevant antibodies at a concentration of 10μg/ml for 30 mins at 37oC. To induce degranulation PMNs were treated with 1.25μM Latrunculin B (La-B) for 5 mins followed by stimulation with 5μM fMLF for 10 mins. As a positive control for inhibition of degranulation, PMN were incubated with the anti-Lex mAb H198. Following indicated incubations, PMNs were washed with PBS-EDTA and stained with antibodies against CD63 and CD66b (as indicators of primary and specific granule contents), and then fixed overnight using BD LyseFix PhosFlow before data acquisition using an LSRII. Data are representative of PMN isolated from 3–5 independent donors.
Assay of PMN superoxide generation
For measurement of oxidative burst 0.5 × 106 human PMN were treated with 10μg/ml indicated lectin or vehicle control for 15 minutes at 37oC. Following lectin incubations, cells were spun down, washed and re-suspended in HBSS+ containing 100μM cytochrome C as described previously (39). As a control for each sample a duplicate well containing 250U/ml superoxide dismutase (SOD) was also prepared. Reduction of cytochrome c following addition of 500nM fMLF was assessed by measuring changes in absorbance at 550nm at indicated timepoints up to 20 minutes using a Cytation 5 imaging reader (Biotek, Winooski, VT).
PMN aggregation assay
To determine the direct effect of lectin binding human PMN were incubated for 15 minutes at 37oC with 10μg/ml GNA, PHA-E or WGA in HBSS+ before levels of aggregation were captured using a Zeiss Axiovert 200M microscope at 10 x magnification.
PMN Apoptosis assay
For PMN apoptosis assays, PMN were incubated with 10μg/ml indicated glycan binding protein (or HBSS+ only as a control) for 15 minutes at 37oC. Following treatments PMN were stained for markers of apoptosis according to manufacturer’s instructions using an apoptosis detection kit (BD Bioscience, Franklin Lakes, NJ). Briefly PMN were washed and resuspended in Annexin V buffer containing 7AAD and incubated for 30 mins at 37oC before flow cytometric analysis was carried out using an ACEA NovoCyte Flow Cytometer.
Isolation and purification of murine neutrophils.
Neutrophils were isolated from bone marrow extracted from the femur and tibias of WT C57BL6 mice using an EasySep kit from Stem Cell Technologies as previously described (40, 41). For isolation of circulating murine PMN, WT C57BL6 mice were anesthetized with isoflurane before peripheral blood removal by cardiac puncture followed by cervical dislocation. Red blood cells were lysed using Ack buffer and PMN were then isolated by density centrifugation using a histopaque gradient (1:1 mix of histopaque 1119 and histopaque 1077).
Quantification of effect of CD11b/CD18 glycan targeting on PMN TEpM in vivo.
In vivo experiments were performed using C57BL/6 WT mice (8–12 weeks, both sexes) which were maintained under standard conditions with 12-hour day/night cycles and ad libitium access to food and water. To quantify PMN TEpM in vivo a recently published proximal colon protocol was used (17). Briefly, mice were pretreated with proinflammatory cytokines before a 2cm loop of vascularized proximal colon was injected with 1nM LTB4 and migration or epithelial association of PMN quantified by flow cytometry.
Immunoblot analysis of signaling pathways in human neutrophils
For human PMN signaling western blotting, PMN were isolated as described above and stimulated with 10ug/ml indicated lectin for 5,15,30,45 and 60 minutes at 37oC. Following stimulation PMN were lysed by the addition of an equal volume of 2X loading buffer and boiled. Western blotting was performed as described above using primary antibodies from Cell Signaling Technologies (Total Syk #131985, p-Syk Tyr323 #2715S, Total Erk1/2 #4695S, p-Erk1/2 Thr202/Tyr204 #4307T, Total P38 MAPK #86905, p-P38 MAPK Thr180/Try182 #4511).
Statistics
Statistical differences were determined by one way ANOVA with Tukey post-hoc testing using PRISM 8, Graphpad software, Inc. Values are expressed as the mean and SEM from at least three separate experiments.
Study approval
All experimental procedures involving animals were conducted in accordance with NIH guidelines and protocols approved by the University Committee on Use and Care of Animals at University of Michigan. All experiments using human blood were conducted in accordance with NIH guidelines approved by the Institutional Review Board of the University of Michigan Medical School. Written informed consent was received from participants prior to inclusion in the study.
Results
Purification and MALDI-TOF Mass Spectrometry (MS) analysis of human CD11b/CD18
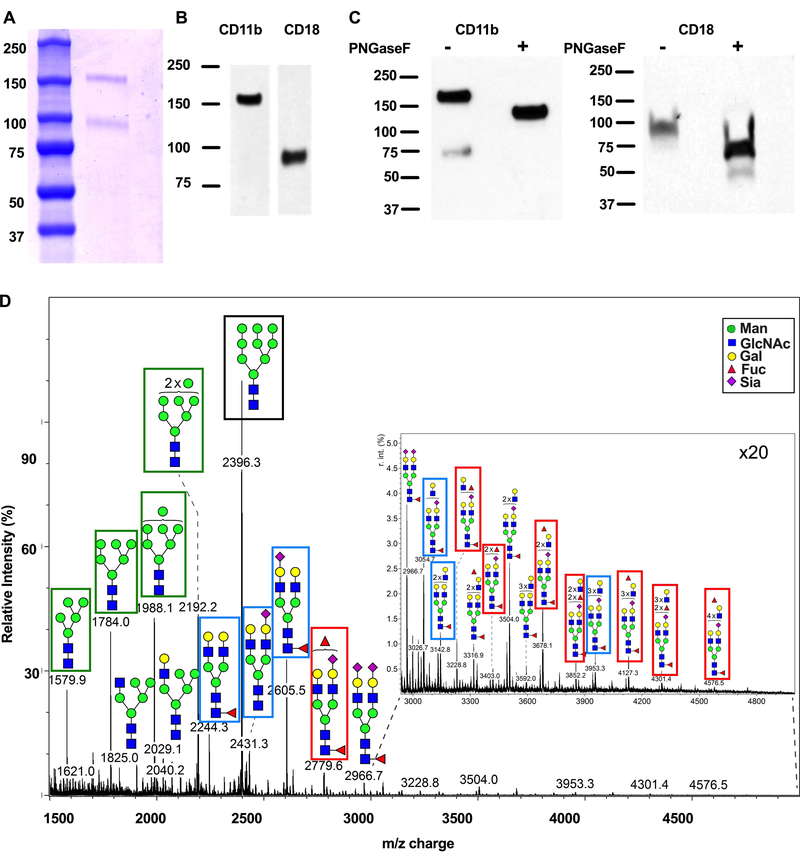
In order to determine its glycosylation profile and study consequences of CD11b glycan targeting, CD11b/CD18 was purified from human PMN. SDS PAGE and Coomassie staining of isolated CD11b/CD18 revealed purification to homogeneity with two protein staining bands consistent in size with CD11b (Mw~170 kDa) and CD18 (Mw~85 kDa, Fig. 1A). Western blotting with anti-CD11b and anti-CD18 mAbs confirmed the presence of both integrin subunits in the purified material (Fig. 1B). Treatment of CD11b/CD18 with PNGaseF resulted in enhanced electrophoretic mobility, consistent with N- linked glycosylation of both β2-integrin subunits (Fig. 1C).
Figure 1. Identification of N-linked glycans on human CD11b/CD18.
CD11b/CD18 purified from human PMNs was run on a gel and stained with Coomassie Blue (A) or immunoblotted with antibodies against CD11b or CD18 (B). (C) Purified human CD11b/CD18 was treated with PNGaseF before immunoblotting for CD11b or CD18. D) N-glycan MALDI-TOF profile for human neutrophil CD11b.
Given the observed presence of N-linked glycosylation on CD11b/CD18, we performed glycometric analyses of human CD11b and CD18 using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) Mass Spectrometry (MS). As CD18 is a subunit common to multiple integrins including CD11b/CD18, CD11a/CD18 and CD11c/CD18, we initially focused on N-linked glycans of the unique CD11b subunit. MS analysis of CD11b revealed a surprising lack of sialylation (Fig. 1D). This lack of glycans terminating in sialic acid was in stark contrast to the highly sialylated nature of the N-linked glycans identified by MS analysis of human neutrophil total cell lysates (http://www.functionalglycomics.org/glycomics/common/jsp/samples/searchSample.jsp?templateKey=2&12=CellType&operation=refine&2=205). The most abundantly expressed N-linked glycan on CD11b, representing approximately 22% of total N-glycans (blue shading, supplemental Table 1), was predicted to be an uncommon terminal high mannose type oligosaccharide (Man9GlcNAc2, m/z 2396.3, black box Fig. 1D). These complex glycans with non-reducing end mannose residues represent ligands for Galanthus Nivalis lectin (GNA) (42) and mannose macrophage receptor (MMR)(43, 44). In addition, more common mannose containing glycans were detected on CD11b (Fig. 1D, green boxes). Several biantennary galactosylated N-glycans (blue boxes, Fig. 1D) accounting for approximately 18% of all predicted N-glycans on CD11b (yellow shading, Supplemental table 1) were also identified. These galactosylated N-glycans represent high affinity ligands for the legume lectin phytohemagglutinin from Phaseolus vulgaris (PHA-E) (45).
In addition, Lewis family glycans including Sialyl Lewis X and Lewis A/X (Fig. 1D, red boxes) were also identified. In contrast to the glycan profile for CD11b, analysis of the N-linked glycans expressed on CD18 revealed a less diverse glycosylation profile (Supplemental Fig. 1). However, the glycan ligands for PHA-E (Fig. 1D, blue box) as well as Lex were also detected on CD18. In addition, the high mannose glycan recognized by GNA (the most abundant N-glycan detected on CD11b) was also predicted to be present (Supplemental Fig. 1, black box). However, this glycan represented only 1.8% of the total N-glycans predicted for CD18 compared to a relative abundance of 22% of the N-glycans on CD11b (Supplemental table 1).
Increase in surface expression of N-Glycans recognized by PHA-E and GNA in stimulated human PMN
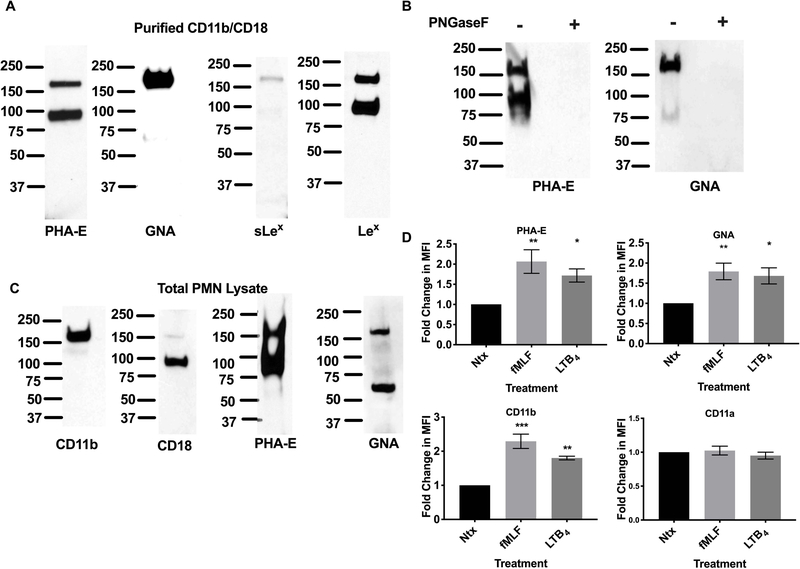
Western blotting of purified CD11b/CD18 with biotinylated PHA-E lectin confirmed the expression of biantennary galactosylated glycans on both CD11b and CD18 (Fig. 2A). Immunoblotting with a biotinylated lectin against high mannose glycans (biotinylated GNA) confirmed abundant expression of these glycans on CD11b (Mw~ 170 kDa, Fig. 2A). In contrast there was little to no detection of these oligosaccharides on the CD18 subunit (band absent at 85kDa, Fig. 2A) consistent with the low relative abundance predicted for these glycans on CD18 (1.8%, supplemental table 1). Expression of Lex on both CD11b and CD18 from human PMN was also confirmed by western blotting. Furthermore, consistent with the Mass Spec data, sLex expression was restricted to CD11b (Fig. 2A).
Figure 2. N-glycans recognized by PHA-E and GNA expressed on human CD11b.
(A) Purified CD11b/CD18 was immunoblotted using biotinylated PHA-E, GNA lectins or antibodies against sLex and Lex. (B) Immunoblots showing the CD11b/CD18 glycans recognized by PHA-E and GNA are N-glycan linked. (C) Total cell lysates from human PMN were immunoblotted with antibodies against CD11b and CD18 or biotinylated PHA-E and GNA lectins. (D) Changes in surface expression of CD11b/CD18, CD11a/CD18 and glycans recognized by PHA-E and GNA on human PMNs were analyzed by flow cytometry in non-stimulated (Ntx) PMNs and PMNs exposed to 100nM FMLF or 10nM LTB4 using FITC conjugated mAbs or lectins. Data are mean fluorescent intensities ± SEM (n=5). *, p<0.05, **, p<0.01, ***, p<0.001.
Treatment of CD11b/CD18 with PNGaseF to remove N-glycans completely ablated binding of GNA and PHA-E demonstrating that the glycans recognized by these lectins are exclusively N-glycan-linked on human PMN CD11b/CD18 (Fig. 2B). Similar to immunoblotting of purified CD11b/CD18, western blotting of total PMN lysates with biotinylated PHA-E revealed two heavily glycosylated bands consistent in size with CD11b and CD18 (Fig. 2C), suggesting restricted expression of these galactosylated biantennary glycans to CD11b/CD18 in human PMN. Immunoblotting of human PMN cell lysates with biotinylated GNA revealed a protein consistent in size with CD11b (Mw~170 kDa) and confirmed the lack of expression of this high mannose oligosaccharide on CD18 (no band observed at 85 kDa). An additional band at approximately 65 kDa was also observed following western blotting of human PMN lysates with biotinylated GNA.
CD11b/CD18 is a known activation marker for human neutrophils with increased surface expression of this integrin observed following stimulation with the bacterial N-formylated tripeptide N-Formyl-Met-Leu-Phe (fMLF). Consistent with CD11b glycan expression, flow cytometry analysis of human neutrophils revealed a significant increase in surface expression of glycan ligands for GNA and PHA-E following stimulation with fMLF (**, p < 0.01) or LTB4 (*, p<0.05). This increase in surface expression was paralleled by the expected increase in surface expression of CD11b following stimulation with fMLF (***, p < 0.001) or LTB4 (**, p < 0.01, Fig. 2D). In contrast there was no increase in surface expression of CD11a downstream of PMN stimulation with fMLF or LTB4, consistent with GNA and PHA-E selectively recognizing glycans expressed on CD11b but not CD11a.
Targeting of CD11b glycans with PHA-E blocks PMN TEpM and PMN detachment from the apical epithelial surface.
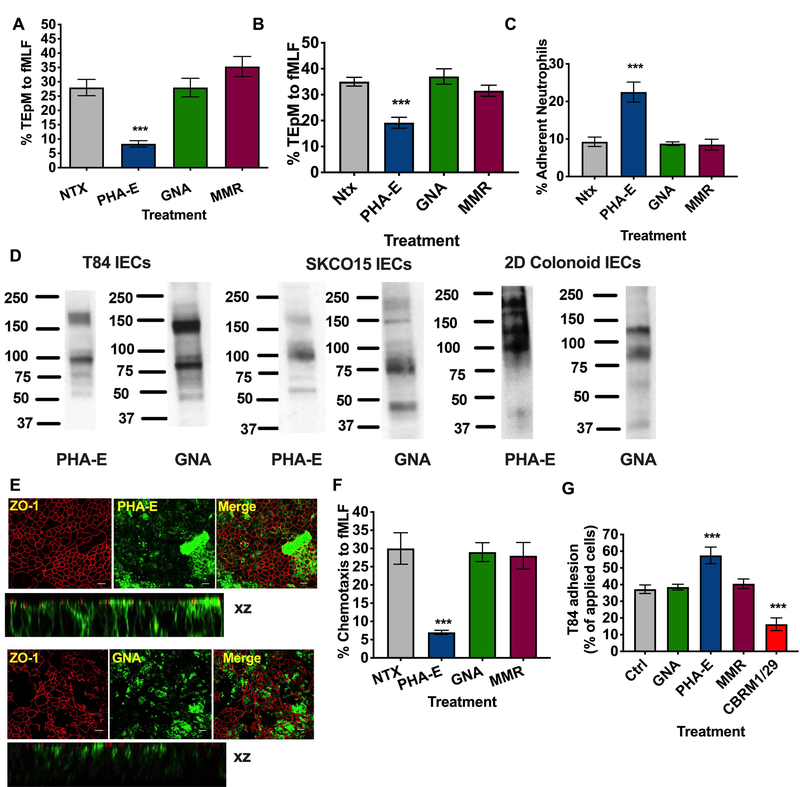
Having confirmed expression of specific N-linked glycans on CD11b, we then determined the effect of targeting these glycans on key PMN inflammatory functions including PMN trafficking across intestinal epithelium. As can be seen in Fig. 3A targeting of biantennary galactosylated glycans with 10μg/ml PHA-E significantly inhibited PMN TEpM in the physiologically relevant basolateral to apical direction (***, p >0.001). In contrast, treatment of PMN with 10μg/ml GNA or Macrophage Mannose Receptor (MMR), to target high mannose oligosaccharides, had no effect on PMN TEpM to fMLF (Fig. 3A). For initial migration assays, PMN were incubated with lectins after application to the basolateral epithelial surface (top chamber of transwell). The effect of lectins on PMN binding to the apical epithelial surface (bottom chamber of transwell) was also determined. As shown in Fig. 3B, apical application of 10μg/ml PHA-E significantly inhibited PMN TEpM (***, p>0.001). To determine if the observed decrease in transmigration was due to inhibition of PMN detachment from the apical epithelial surface, numbers of PMN that had reached but not detached from the apical epithelial surface following treatment with lectins were quantified. As shown in Fig. 3C, apical PHA-E treatment increased the number of apically adherent PMN by about 50% (***, p>0.001). As was shown for basolateral PMN incubation, apical application of GNA or MMR had no effect on PMN TEpM or PMN apical epithelial adhesion.
Figure 3. Targeting of CD11b glycans blocks PMN chemotaxis and TEpM and increases PMN adhesive interactions.
(A) T84 Intestinal epithelial cells were cultured to confluency on porous polycarbonate filters (Transwell). Human PMNs were applied to the upper chamber of transwell filters, incubated with 10μg/ml PHA-E or 10μg/ml GNA or 10μg/ml MMR and induced to migrate into the bottom chamber in response to 100nm fMLF. Migration was quantified by MPO assay (n=6 independent donors. ***, p<0.001). Effect of addition of 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR to the apical epithelial surface on PMN TEpM (B) and PMN adherence to the apical epithelial surface (C). (D) Expression of glycans recognized by PHA-E and GNA by immunoblotting of whole cell lysates from T84 IECs, SKCO15 IECs and differentiated human colonoids. (E) Fixed and permeabilized T84 IECs were stained with 10μg/ml anti- ZO-1 mAb (in red) and or 10μg/ml PHA-E/GNA (in green) and analyzed by confocal microscopy. Original magnification x 60, scale bar = 20μm. Representative images from n=3 experiments are shown en face or in the xz plane of section. (F) PMN chemotaxis was quantified by MPO following incubation of human PMN with 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR. Data depict means ± SEM (A-C, G). n=4 independent donors ***, p<0.001. (G) CD11b/CD18 was added to Nunc maxisorp plates at a concentration of 5μg/ml before addition of 2.5 × 105 BCECF labeled T84 IECs treated with 10μg/ml indicated lectins (PHA-E, GNA, MMR) or blocking CD11b mAb (CBRM1/29). Following washing CD11b/CD18-IEC adhesion was quantified by measuring fluorescence at 485nm. Data shown are % adhesion of applied cells and depict means ± SEM (n=4) ***, p<0.001.
Taken together, these data suggest that targeting of glycans on CD11b with PHA-E blocks PMN TEpM though alteration of PMN binding interactions with both basolateral and apical epithelial ligands. Immunoblotting of T84 IEC lysates with biotinylated lectins was performed in order to determine if the glycans recognized by PHA-E, GNA and MMR were also expressed by human intestinal epithelial cells. Fig. 3D demonstrates expression of a high molecular weight (Mr~ 170 kDa) as well as lower molecular weight proteins (Mr ~ 100 kDa) recognized by biotinylated PHA-E. Epithelial proteins (Mr ~ 80kDa and 130kDa) were also detected by GNA. Glycoproteins of similar size were also detected by PHA-E and GNA in SKCO15 cells (a second IEC line) as well as in differentiated 2D colonoid monolayers (non-transformed human colonic epithelial cells, Fig. 3D). Having demonstrated epithelial expression of glycans recognized by PHA-E and GNA in IECs, we then identified the cellular localization of these glycans by immunofluorescence staining (IF). IF analysis of T84 monolayers (Fig. 3E) revealed apical and basolateral expression of biantennary galactosylated glycans recognized by PHA-E in addition to some co-localization with the junctional protein ZO-1. In contrast expression of high mannose glycans recognized by GNA was restricted to the apical epithelial surface.
Given the epithelial expression of glycans recognized by PHA-E and GNA in human intestinal epithelial cell lines and primary human colon derived cells, we next determined the effect of CD11b glycan targeting on PMN migration in a system with no intestinal epithelial cells. For these assays PMN chemotaxis to 100nm fMLF across collagen coated transwells was assessed. Similar to effects on TEpM, incubation of PMN with PHA-E significantly inhibited PMN chemotaxis (Fig. 3F, ***, p > 0.001), while treatment with GNA and MMR had no effect on transfilter migration of PMN. To determine if inhibition of PMN TEpM and chemotaxis by PHA-E was the result of lectin induced PMN aggregation, PMN were treated with 10μg/ml PHA-E and GNA and visualized by light microscopy. Incubation of PMN with PHA-E or GNA did not result in significant levels of aggregation compared to cells incubated with a known agglutinating lectin WGA (Supplemental Fig. 2) demonstrating that PHA-E induced inhibition of PMN migration is not the result of PMN aggregation. Next, the effect of glycan targeting on T84 IEC adhesion to immobilized CD11b/CD18 was measured. Fig. 3G demonstrates that incubation with PHA-E increased the level of T84 adherence to CD11b/CD18. As can be seen, addition of 10μg/ml PHA-E resulted in a 25% increase in adherent epithelial cells when compared with non-treated, GNA treated or MMR treated cells (***, p > 0.001). As expected, addition of a functionally inhibitory anti-CD11b mAb (CBRM1/29) significantly reduced adhesion of T84 IECs to CD11b (***, p<0.001).
Targeting of CD11b/CD18 glycans regulates PMN phagocytosis, ROS production and apoptosis but not degranulation.
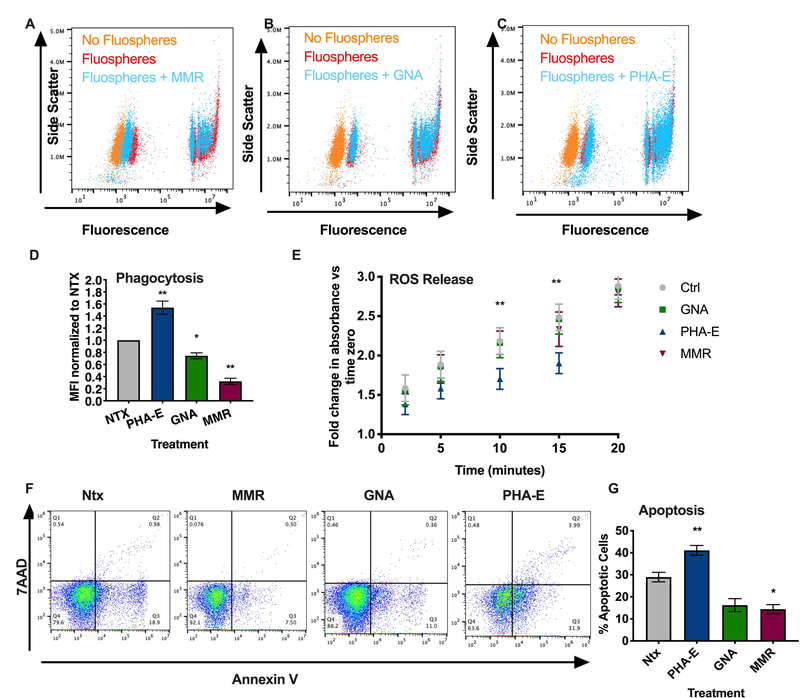
Given the effects of CD11b glycan targeting on PMN TEpM, the potential role of CD11b glycans in regulating other important PMN inflammatory functions was assessed. Flow cytometric analyses demonstrated that engagement of the most abundantly expressed CD11b high mannose glycans by 10μg/ml GNA or MMR significantly decreased the uptake of fluorescent microspheres (fluospheres) by PMN (*, p > 0.05, **, p > 0.01, Fig. 4A, B, D). By contrast, targeting CD11b glycans with 10μg/ml PHA-E significantly increased PMN fluosphere phagocytosis (**, p > 0.01, Fig. 4C, D). Taken together, these data suggest differential regulation of PMN phagocytosis upon ligation of specific CD11b glycans. Effects of CD11b glycan targeting on PMN degranulation in response to Latrunculin B and fMLF stimulation was also assessed. As a positive control for inhibition of degranulation, PMN were incubated with antibodies against Lex (16). As can be seen in Supplemental figure 3 incubation of PMN with 10μg/ml PHA-E, GNA or MMR did not reduce La-B and fMLF induced increases in surface expression of markers of specific (CD66b) and azurophilic granules (CD63). In contrast ligation of Lex glycans by mAb H198 significantly reduced upregulation of surface expression of degranulation markers (CD66b and CD63). Taken together these data suggest that targeting of discrete CD11b glycans differentially regulates PMN phagocytosis while having little effect on mobilization of granules to the PMN surface.
Figure. 4. Engagement of CD11b N-linked glycans regulates PMN phagocytosis, ROS release and apoptosis.
Human PMN were incubated with 10μg/ml MMR (A), 10μg/ml GNA (B) or 10μg/ml PHA-E (C) for 60 minutes at 37oC before fluorescent microsphere phagocytosis/uptake was quantified by measuring changes in fluorescence by flow cytometry. (D) Data is mean fluorescence intensity normalized to non-treated PMN and is expressed as mean ± SEM. n = 3 independent donors *, p<0.05, **, p<0.01. (E) Human PMN were incubated with 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR before treatment with 100μM cytochrome C. Reduction of cytochrome C in response to 500nM fMLF was measured by quantifying changes in absorbance at 550nm at 2,5,10,15 and 20 minutes. Data is fold change in absorbance relative to time zero and is expressed as ± SEM. n = 3 independent donors **, p<0.01, ***, p<0.001. (F) For apoptosis assays, human PMN were incubated with 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR before assessment of surface expression of Annexin V and 7AAD by flow cytometry. Cells negative for Annexin IV and 7AAD were considered non-apoptotic. Representative flow plots show percentage apoptotic PMN under the defined conditions. (G) Quantification of data from flow plots. All Annexin-V positive cells were considered early apoptotic neutrophils. Data is expressed as mean ± SEM. n = 4 independent donors *, p<0.05, **, p<0.01.
In addition to migration and phagocytosis, PMN oxidative burst generation is crucial for host defense against invading microbes. Therefore, we examined the effects of CD11b glycan engagement on PMN respiratory burst responses. As can be seen from Fig. 4E, incubation of human PMN with PHA-E significantly reduced reactive oxygen mediated reduction of cytochrome c at 10- and 15-minutes post fMLF stimulation relative to non-lectin treated and GNA treated samples (**, p>0.01). However, after 20 minutes of fMLF stimulation there was no difference in levels of superoxide production/reduction of cytochrome c in PHA-E treated neutrophils compared to controls, suggesting a delay rather than a total block in superoxide production downstream of PHA-E targeting of CD11b glycans. In contrast to effects observed with PHA-E, targeting of high mannose glycans on CD11b with GNA or MMR had no effect on superoxide mediated cytochrome c reduction at any time points measured.
While PMN are traditionally considered relatively short-lived cells, several studies have reported delayed PMN apoptosis downstream of CD11b activation. As shown in Fig. 4F,G targeting of high Mannose glycans on CD11b with 10μg/ml MMR resulted in significantly delayed spontaneous PMN apoptosis as measured by quantification of Annexin V positive cells (*, p>0.05, Fig. 4F.G). By contrast, targeting of CD11b glycans with 10μg/ml PHA-E significantly increased the number of Annexin V positive/early apoptotic PMNs (**, p>0.01). These results therefore demonstrate differential regulation of PMN apoptosis following targeting of high mannose versus biantennary galactosylated glycans on CD11b.
Targeting glycan ligands of PHA-E, GNA expressed by murine PMN in vivo blocks TEpM in the proximal colon.
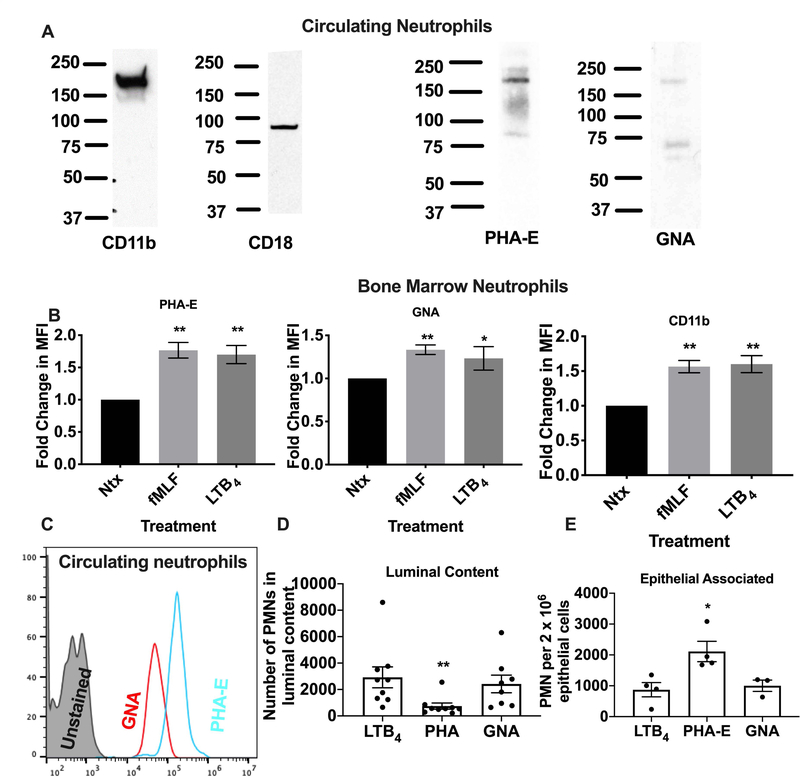
Having demonstrated the expression and functional consequences of targeting human CD11b/CD18 glycans, we compared expression of CD11b glycans in human PMN with those on murine PMN. Western blotting of lysates from circulating murine PMN revealed a protein consistent in size with CD11b (Mw~170kDa) detected by PHA-E and GNA suggesting conservation of glycosylation between human and mouse CD11b. Consistent with glycan expression on CD11b, flow cytometry analysis of bone marrow mouse neutrophils revealed a significant increase in surface expression of glycan ligands for GNA and PHA-E following stimulation with fMLF (**, p < 0.01) or LTB4 (*, p<0.05). This increase in surface expression was paralleled by the expected increase in surface expression of CD11b following stimulation with fMLF (**, p < 0.01) or LTB4 (**, p < 0.01, Fig. 5B). Expression of glycans recognized by GNA and PHA-E was also confirmed by flow cytometry of circulating murine PMN (Fig. 5C). Given the similar western blotting profiles observed for human and murine PMN, we tested the effect of glycan targeting by GNA and PHA-E on PMN trafficking to the proximal colon in vivo using a recently described model (17). Analysis of PMN migration into the lumen of the colon in response to a solution of luminally applied LTB4 (1nm) revealed a significant reduction in transmigrated PMN in PHA-E treated colon loops compared to non-treated (Ntx) or GNA-treated loops after 60 minutes (Fig. 5D, **, p<0.001). In addition, examination of PMN retention in the epithelial enriched fraction revealed significantly increased numbers of epithelial associated PMN following treatment with PHA-E (Fig. 5E, *, p>0.05) consistent with increased CD11b/CD18 mediated PMN epithelial adhesion downstream of PHA-E glycan targeting in vivo.
Figure 5. Glycan ligands of PHA-E, GNA expressed by murine PMN and can be targeted to block PMN TEpM into the proximal colon.
(A) Lysates from circulating murine PMN were immunoblotted with anti-CD11b, anti-CD18 mAbs or biotinylated PHA-E, GNA lectins. (B) Changes in surface expression of CD11b/CD18 and glycans recognized by PHA-E and GNA on murine PMNs were analyzed by flow cytometry in non-stimulated (Ntx) PMNs and PMNs exposed to 1μM FMLF, or 10nM LTB4 using FITC conjugated mAbs or lectins. Data are mean fluorescent intensities ± SEM (n=3, *, p<0.05, **, p<0.01). (C) PMN in whole blood isolated from WT C57BL/6 mice were stained with FITC conjugated GNA and PHA-E and analyzed by flow cytometry. (D) Number of PMN recruited into the lumen of proximal colon loops following luminal injection of PHA-E or GNA. Data are mean ± SEM (n=3 independent experiments, **, p<0.01). (E) Number of PMN in epithelial enriched fractions after luminal injection of PHA-E or GNA. Data are means ± SEM (n=2 independent experiments. *, p<0.05).
Targeting of CD11b/CD18 glycans regulates downstream integrin signaling in human PMN
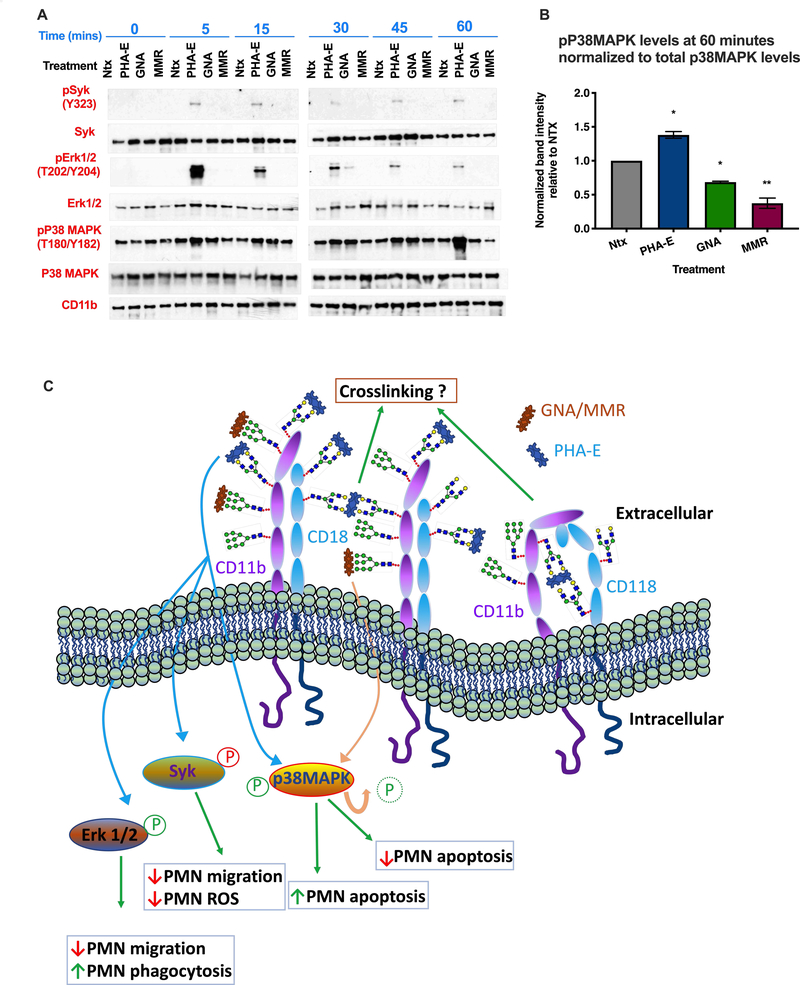
It is well appreciated that CD11b/CD18 mediates PMN functional effects (including adhesion, chemotaxis, migration, phagocytosis and superoxide production) through signaling via several intracellular kinases including Syk, Erk1/2 and p38MAPK (12, 46, 47). We thus investigated the effect of CD11b/CD18 glycan targeting on regulation of these signaling pathways in human PMN. As can be seen in Fig. 6A, targeting biantennary galactosylated glycans on CD11b/CD18 with PHA-E resulted in robust phosphorylation of Syk [which has been shown to positively regulate neutrophil migration (48)] at all time points measured between 5 and 60 minutes post treatment. By contrast, targeting high mannose glycans on CD11b/CD18 with MMR or GNA did not result in enhanced phosphorylation of Syk on Tyr323. Since phosphorylation at Tyr323 has been reported to decrease Syk kinase activity (49), our finding suggests that ligation of CD11b glycans with PHA-E results in negative regulation of Syk mediated integrin signaling. In addition to phosphorylation of Syk kinase, robust phosphorylation of Erk1/2 was observed downstream of PHA-E treatment. Activation of Erk1/2 downstream of PHA-E glycan targeting strongly peaked at 5 minutes and gradually diminished over the 60 minute time-course. By contrast, treatment of PMN with GNA and MMR did not result in Erk1/2 activation. Finally, increased activation of p38MAPK was observed 60 minutes after targeting CD11b/CD18 glycans with PHA-E (Fig. 6A,B). In contrast, decreased phosphorylation of p38MAPK was observed 60 minutes after treatment with MMR and GNA (Fig. 6A, B). Taken together these results suggest that selective regulation of β2-integrin intracellular signaling may underlie different PMN functional responses observed downstream of CD11b glycan targeting (Fig. 6C).
Figure 6.
(A) Human neutrophils were stimulated with 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR for indicated time points before lysis and immunoblotting for Syk, Erk1/2 and p38MAPK. (B) Densitometry analysis of p38MAPK phosphorylation at 60 minutes of stimulation normalized to total p38MAPK levels at the same time point. Values shown are relative to Ntx (*, p<0.05, **, p>0.01). (C) Model showing how differential modulation of intracellular signaling cascades downstream of CD11b glycan engagement may regulate different patterns of PMN functional responses.
Discussion
Mucosal recruitment of PMN represents a double-edged sword in that while neutrophil tissue migration is necessary for defense against invading microbes, dysregulated influx of PMN is a pathological hallmark of numerous inflammatory diseases. Though the complex mechanisms that regulate neutrophil transepithelial migration remain to be fully elucidated, it is well established that the β2 integrin CD11b/CD18 is a key regulator of neutrophil TEpM as well as PMN inflammatory functions including phagocytosis, superoxide release and degranulation. While both subunits of the CD11b/CD18 heterodimer are extensively modified by post translational glycosylation, the role of CD11b glycans in regulating PMN function have not been well defined. Here we demonstrate that the majority of glycans on neutrophil CD11b are N-glycan linked consistent with previous studies on human macrophage CD11b (50). Mass Spectrometry analysis of N-glycans on neutrophil CD11b revealed a dramatic lack of sialylation. This is in stark contrast to the high levels of sialylation identified by MS analysis of all human neutrophil glycoproteins (http://www.functionalglycomics.org/glycomics/common/jsp/samples/searchSample.jsp?templateKey=2&12=CellType&operation=refine&2=205) suggesting unusual post translational glycosylation of human neutrophil CD11b. Given that sialic acid residues frequently mask subterminal galactose moieties (51), the observed lack of sialylation is consistent with the abundance of glycans terminating with Galactose identified on CD11b. In addition, as terminal sialylation and fucosylation are competing processes (52), the low abundance of terminal sialyation also corresponds with the high levels of terminally fucosylated glycan structures including Lex identified on CD11b.
Expression of unusual glycan signatures including high mannose glycans and biantennary galactosylated N-glycans on CD11b was verified using glycan binding proteins/lectins PHA-E, GNA and MMR that have complex glycan binding specificities. Previous studies showing altered electrophoretic mobility of CD11b after treatment with Endo H are supportive of the observed predominance of high mannose N-linked glycans on human CD11b (50). Importantly, data suggest differential glycosylation between neutrophil expressed β2 integrins. In support of this, previous studies have shown that CD11a is modified by glycans that are resistant to N-glycanase treatment. This therefore suggests a predominance of O-linked (rather than N-linked) glycosylation on this member of the β2 integrin family. Furthermore, here we report increased surface expression of CD11b (but not CD11a) as well as the glycans recognized by PHA-E and GNA in activated PMN. This is consistent with previous studies that show no change in CD11a localization following neutrophil stimulation (53, 54) and, is strongly suggestive of the existence of glycan determinants on CD11b/CD18 that are not found on other PMN expressed β2 integrins.
It is well accepted that mammalian lectins including selectins and galectins play important roles in regulating neutrophil extravasation (55). In keeping with this, we show that lectin mediated targeting of biantennary galactosylated glycans on CD11b/CD18 blocks PMN chemotaxis and PMN TEpM. This is consistent with previous reports demonstrating that inhibitory antibodies against CD11b block PMN TEpM in vitro and in vivo (17–19). Furthermore, lectin binding to CD11b glycans increased adhesive interactions between T84 intestinal epithelial cells and PMN CD11b/CD18. Such carbohydrate specific regulation of CD11b mediated PMN epithelial adhesion is supported by previous work demonstrating reduced adhesion of T84 IECs to CD11b/CD18 mediated by fucose-containing polysaccharides (38). Of interest the majority of predicated N-glycosylation sites on CD11b are outside of the I domain suggesting that glycan mediated functional effects are I domain independent. In support of CD11b glycan mediated binding events not being mediated by the I domain, previous studies have shown that the β-glucan-binding-lectin-like domain of CD11b [which is important for PMN trafficking (56)] is also located outside of the I domain (57). Such CD11b glycan mediated regulation of PMN trafficking is in keeping with previous studies which demonstrate that targeting Lex glycans on PMN CD11b blocks PMN chemotaxis, TEpM and increases PMN adhesive interactions with intestinal epithelial cells (16). A number of other studies also detail important lectin mediated binding interactions during PMN trafficking. For example, Macrophage galactose type lectin 1 deficiency is associated with increased neutrophilia and hyperinflammation during gram negative pneumonia (58), while the Galactose binding Bauhinia bauhinioides lectin inhibits PMN peritoneal adhesion and migration in response to cytokines including TNF-α (59). In addition, it has been reported that other lectins including Concavalin A and peanut agglutinin lectin stimulate a chemotactic response in human neutrophils (60). However, these studies did not identify the specific neutrophil glycoproteins that are the binding targets for these lectins.
In addition to mediating neutrophil adhesion, migration and tissue recruitment, CD11b participates in many pro-inflammatory PMN functions that are associated with controlling infection including phagocytosis and superoxide production (61). Such CD11b/CD18 mediated functions are typically triggered by direct integrin ligation. Consistent with CD11b/CD18 being the major integrin contributing to phagocytosis in human PMN (62), here we report that targeting of PMN CD11b/CD18 sugars with glycan binding proteins differentially regulates PMN phagocytosis. Interestingly, specific engagement of biantennary galactosylated N-glycans increased the rate of bead uptake by PMN while targeting of high mannose glycans on CD11b/CD18 decreased phagocytosis. These findings highlight fine tuning of PMN functional responses downstream of selective binding interactions with glycans expressed on CD11b/CD18 and are analogous to previous studies implicating C-type lectins including Dectin-1, DC-SIGN and surfactant proteins A and D in the control of pathogen phagocytosis by neutrophils and macrophages (63). Similarly, it has been reported that a Galactose specific plant lectin viscum album agglutinin (VAA-I) induces phagocytosis by rabbit PMN (64).
In addition to effects on phagocytosis, we also observed increased superoxide production in PMN after ligation of biantennary galactosylated glycans on CD11b/CD18. These effects were transient suggesting a delay rather than a total block in superoxide release and are consistent with previous reports demonstrating that induction of ROS by PMN requires signaling by CD11b (65). However, despite being the most abundant N-linked glycans on CD11b, targeting of high mannose glycans by GNA or MMR had no effect on stimulation of PMN superoxide production suggesting glycan specific regulation of PMN ROS release mediated by bisected galactose but not mannose glycans on CD11b/CD18.
Following pathogen destruction mediated by neutrophil antimicrobial effector functions, including phagocytosis and ROS production, apoptosis of activated neutrophils is required for effective resolution of infection/inflammation. Here we show that targeting of biantennary galactosylated glycans on CD11b/CD18 with PHA-E increases spontaneous PMN apoptosis. By contrast, targeting of high mannose glycans on CD11b resulted in decreased levels of PMN apoptosis. These observations add to previous reports implicating CD11b/CD18-dependent signaling in regulating PMN apoptosis (66). Consistent with the observed inhibitory effects downstream of targeting high mannose glycans on CD11b, others have shown that the macrophage inducible C type lectin (Mincle) reduces PMN apoptosis in a murine model of fungal keratitis (67). Other studies have shown that treatment with a galactoside specific plant lectin VAA-I can induce spontaneous apoptosis in human neutrophils (64).
Similar to findings in human PMN, we identified glycans recognized by PHA-E and GNA on CD11b/CD18 from murine PMN. This is consistent with published work demonstrating expression of high mannose glycans on murine CD11b (68). In addition, consistent with effects on human PMN trafficking, luminal application of the CD11b/CD18 binding lectin PHA-E inhibited PMN trafficking to the lumen in vivo. Taken together these results highlight the broad biological relevance of CD11b/CD18 glycan targeting as a strategy for reducing PMN mucosal influx during inflammation. Multiple lines of evidence have shown that ligand binding to CD11b/CD18 induces outside-in signaling mediated by specific kinases to regulate key PMN functions including migration, phagocytosis and superoxide production (46, 47, 69). Here we show that targeting of biantennary galactosylated glycans on CD11b decreases activation of Syk, consistent with PHA-E-mediated decreases both in PMN migration and superoxide production (49, 70, 71). Robust phosphorylation of Erk1/2 was also observed downstream of targeting bisected biantennary galactosylated glycans on CD11b, consistent with PHA-E-mediated upregulation of PMN phagocytosis and downregulation (72). Previous studies have demonstrated that p38MAPK signaling positively regulates PMN phagocytosis and apoptosis responses (47, 73, 74). Therefore, differential activation of this kinase is likely implicated in the differing phagocytosis and apoptosis responses observed in PMN downstream of CD11b glycan targeting. These findings therefore suggest that the type and extent of glycosylation of CD11b could greatly influence PMN function downstream of CD11b/CD18 ligation.
While PMN have been traditionally thought of as being poorly transcriptionally active more recent studies have demonstrated robust de novo synthesis (including upregulation of CD11b transcription) in response to inflammatory stimuli (75–77). Therefore, differential PMN glycosyltransferase expression patterns during an immune or inflammatory response could result in variations in CD11b glycosylation status. As such, there is a need for more systems biology analyses to determine how PMN regulation of glycosylation gene expression and enzyme activity directly influences integrin glycosylation and downstream function. CD11b/CD18 is the most promiscuous integrin with more than 30 reported ligands including members of the ICAM family (35, 78), extracellular matrix components including fibrinogen (79), complement C3 fragment (80) as well as microbial ligands (81). Given the multitude of reported ligands for CD11b/CD18 it is likely that multiple mechanisms (including glycan mediated mechanisms) exist by which CD11b recognizes its endogenous binding partners. Furthermore, the identification here of high mannose glycans on CD11b suggests that mannose binding proteins such as MMR could be endogenous glycan binding ligands for PMN CD11b/CD18.
Interestingly, the data presented here also suggests an expansion of the potential of plant lectins beyond their traditional use as tools to delineate biological processes resulting from glycan dependent cell binding. These observations support the biological relevance of plant lectins that is further highlighted by studies demonstrating that dietary plant lectins can translocate across the intestinal epithelium and act as effective mucosal antigens in the gut (82, 83). Taken together, these results suggest that highly specific glycosylation modifications present on CD11b/CD18 can be selectively targeted to modify different signaling pathways that underlie distinct PMN effector functional responses that exacerbate chronic mucosal inflammatory states.
Supplementary Material
Acknowledgements:
The authors would like to thank the National Center for Functional Glycomics (NCFG) at Beth Israel Deaconess Medical Center, Harvard Medical School (supporting grant P41 GM103694). This work was supported by Crohn’s and colitis foundation (544596) funding to JCB, NIH DK59888 and DK55679 to AN, and DK072564, DK079392 and DK061379 to CAP.
Non-Standard Abbreviations:
- CD
Crohn’s disease
- COPD
Chronic Obstructive Pulmonary Disease
- fMLF
N-Formyl-Met-Leu-Phe
- GNA
Galanthus Nivalis Lectin
- IEC
intestinal epithelial cell
- IBD
Inflammatory Bowel Disease
- La-B
Latrunculin B
- Lex
Lewis X
- MMR
Macrophage Mannose Receptor
- PHA-E
Phaseolus Vulgaris erythroagglutinin
- PMN
Polymorphonuclear neutrophils
- sLea
sialyl Lewis A
- sLec
sialyl Lewis c
- TEpM
Transepithelial Migration
- (UC)
Ulcerative colitis
- WGA
wheat germ agglutinin
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
References
- 1.Mayadas TN, Cullere X, and Lowell CA (2014) The multifaceted functions of neutrophils. Annu Rev Pathol 9, 181–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier BM, and Parkos CA (2012) The role of neutrophils during intestinal inflammation. Mucosal Immunol 5, 354–366 [DOI] [PubMed] [Google Scholar]
- 3.Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, and Peyrin-Biroulet L (2015) Comparing histological activity indexes in UC. Gut 64, 1412–1418 [DOI] [PubMed] [Google Scholar]
- 4.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, and Lofberg R (2000) A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 47, 404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson JL, Phipps S, and Gibson PG (2009) Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol Ther 124, 86–95 [DOI] [PubMed] [Google Scholar]
- 6.Kansas GS (1996) Selectins and their ligands: current concepts and controversies. Blood 88, 3259–3287 [PubMed] [Google Scholar]
- 7.McEver RP, and Cummings RD (1997) Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 100, S97–103 [PubMed] [Google Scholar]
- 8.Ley K, Laudanna C, Cybulsky MI, and Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 9.Rosen SD, and Bertozzi CR (1994) The selectins and their ligands. Curr Opin Cell Biol 6, 663–673 [DOI] [PubMed] [Google Scholar]
- 10.Louis NA, Hamilton KE, Kong T, and Colgan SP (2005) HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J 19, 950–959 [DOI] [PubMed] [Google Scholar]
- 11.Brazil JC, Lee WY, Kolegraff KN, Nusrat A, Parkos CA, and Louis NA (2010) Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J Immunol 185, 7026–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, O’Connor MB, Mandell KJ, Zen K, Ullrich A, Buhring HJ, and Parkos CA (2004) Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha. J Immunol 172, 2578–2585 [DOI] [PubMed] [Google Scholar]
- 13.Sumagin R, Robin AZ, Nusrat A, and Parkos CA (2014) Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunol 7, 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazil JC, Liu R, Sumagin R, Kolegraff KN, Nusrat A, Cummings RD, Parkos CA, and Louis NA (2013) alpha3/4 Fucosyltransferase 3-dependent synthesis of Sialyl Lewis A on CD44 variant containing exon 6 mediates polymorphonuclear leukocyte detachment from intestinal epithelium during transepithelial migration. J Immunol 191, 4804–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brazil JC, Sumagin R, Stowell SR, Lee G, Louis NA, Cummings RD, and Parkos CA (2017) Expression of Lewis-a glycans on polymorphonuclear leukocytes augments function by increasing transmigration. J Leukoc Biol 102, 753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brazil JC, Sumagin R, Cummings RD, Louis NA, and Parkos CA (2016) Targeting of Neutrophil Lewis X Blocks Transepithelial Migration and Increases Phagocytosis and Degranulation. Am J Pathol 186, 297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemming S, Luissint AC, Nusrat A, and Parkos CA (2018) Analysis of leukocyte transepithelial migration using an in vivo murine colonic loop model. JCI Insight 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkos CA, Colgan SP, Bacarra AE, Nusrat A, Delp-Archer C, Carlson S, Su DH, and Madara JL (1995) Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am J Physiol 268, C472–479 [DOI] [PubMed] [Google Scholar]
- 19.Parkos CA, Delp C, Arnaout MA, and Madara JL (1991) Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 88, 1605–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnaout MA, Dana N, Pitt J, and Todd RF 3rd. (1985) Deficiency of two human leukocyte surface membrane glycoproteins (Mo1 and LFA-1). Fed Proc 44, 2664–2670 [PubMed] [Google Scholar]
- 21.Springer TA, Teplow DB, and Dreyer WJ (1985) Sequence homology of the LFA-1 and Mac-1 leukocyte adhesion glycoproteins and unexpected relation to leukocyte interferon. Nature 314, 540–542 [DOI] [PubMed] [Google Scholar]
- 22.Arnaout MA (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75, 1037–1050 [PubMed] [Google Scholar]
- 23.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, and Mayadas TN (1996) A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5, 653–666 [DOI] [PubMed] [Google Scholar]
- 24.Karlsson A (1999) Wheat germ agglutinin induces NADPH-oxidase activity in human neutrophils by interaction with mobilizable receptors. Infect Immun 67, 3461–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lock R, Johansson A, Orselius K, and Dahlgren C (1988) Analysis of horseradish peroxidase-amplified chemiluminescence produced by human neutrophils reveals a role for the superoxide anion in the light emitting reaction. Anal Biochem 173, 450–455 [DOI] [PubMed] [Google Scholar]
- 26.Becker DJ, and Lowe JB (1999) Leukocyte adhesion deficiency type II. Biochim Biophys Acta 1455, 193–204 [DOI] [PubMed] [Google Scholar]
- 27.Rhodes JM (1996) Unifying hypothesis for inflammatory bowel disease and associated colon cancer: sticking the pieces together with sugar. Lancet 347, 40–44 [DOI] [PubMed] [Google Scholar]
- 28.Ryder SD, Parker N, Ecclestone D, Haqqani MT, and Rhodes JM (1994) Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology 106, 117–124 [DOI] [PubMed] [Google Scholar]
- 29.Werner L, Sturm A, Roggenbuck D, Yahav L, Zion T, Meirowithz E, Ofer A, Guzner-Gur H, Tulchinsky H, and Dotan I (2013) Antibodies against glycoprotein 2 are novel markers of intestinal inflammation in patients with an ileal pouch. J Crohns Colitis 7, e522–532 [DOI] [PubMed] [Google Scholar]
- 30.Balsam LB, Liang TW, and Parkos CA (1998) Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J Immunol 160, 5058–5065 [PubMed] [Google Scholar]
- 31.Mandell KJ, McCall IC, and Parkos CA (2004) Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem 279, 16254–16262 [DOI] [PubMed] [Google Scholar]
- 32.Sato T, and Clevers H (2013) Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, and Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 34.Zou WY, Blutt SE, Crawford SE, Ettayebi K, Zeng XL, Saxena K, Ramani S, Karandikar UC, Zachos NC, and Estes MK (2017) Human Intestinal Enteroids: New Models to Study Gastrointestinal Virus Infections. Methods Mol Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, and Springer TA (1990) ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol 111, 3129–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller LJ, Wiebe M, and Springer TA (1987) Purification and alpha subunit N-terminal sequences of human Mac-1 and p150,95 leukocyte adhesion proteins. J Immunol 138, 2381–2383 [PubMed] [Google Scholar]
- 37.Strohalm M, Kavan D, Novak P, Volny M, and Havlicek V (2010) mMass 3: a cross-platform software environment for precise analysis of mass spectrometric data. Anal Chem 82, 4648–4651 [DOI] [PubMed] [Google Scholar]
- 38.Zen K, Liu Y, Cairo D, and Parkos CA (2002) CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol 169, 5270–5278 [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, and Junger WG (2012) Measurement of oxidative burst in neutrophils. Methods Mol Biol 844, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, and Lichtman AH (2012) IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188, 6287–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swamydas M, and Lionakis MS (2013) Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp, e50586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibuya N, Goldstein IJ, Van Damme EJ, and Peumans WJ (1988) Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem 263, 728–734 [PubMed] [Google Scholar]
- 43.Morishima S, Morita I, Tokushima T, Kawashima H, Miyasaka M, Omura K, and Murota S (2003) Expression and role of mannose receptor/terminal high-mannose type oligosaccharide on osteoclast precursors during osteoclast formation. J Endocrinol 176, 285–292 [DOI] [PubMed] [Google Scholar]
- 44.Oki T, Yamazaki Y, Nomura N, Furumai T, and Igarashi Y (1999) High-mannose type oligosaccharide-dependent apoptosis in U937 cells induced by pradimicin, a mannose-binding antibiotic. J Antibiot (Tokyo) 52, 449–454 [DOI] [PubMed] [Google Scholar]
- 45.Nagae M, Soga K, Morita-Matsumoto K, Hanashima S, Ikeda A, Yamamoto K, and Yamaguchi Y (2014) Phytohemagglutinin from Phaseolus vulgaris (PHA-E) displays a novel glycan recognition mode using a common legume lectin fold. Glycobiology 24, 368–378 [DOI] [PubMed] [Google Scholar]
- 46.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, and Lowell CA (2006) Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol 7, 1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheth K, Friel J, Nolan B, and Bankey P (2001) Inhibition of p38 mitogen activated protein kinase increases lipopolysaccharide induced inhibition of apoptosis in neutrophils by activating extracellular signal-regulated kinase. Surgery 130, 242–248 [DOI] [PubMed] [Google Scholar]
- 48.Schymeinsky J, Sindrilaru A, Frommhold D, Sperandio M, Gerstl R, Then C, Mocsai A, Scharffetter-Kochanek K, and Walzog B (2006) The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for beta2 integrin (CD11/CD18)-mediated neutrophil migration. Blood 108, 3919–3927 [DOI] [PubMed] [Google Scholar]
- 49.Lupher ML Jr., Rao N, Lill NL, Andoniou CE, Miyake S, Clark EA, Druker B, and Band H (1998) Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem 273, 35273–35281 [DOI] [PubMed] [Google Scholar]
- 50.Miller LJ, and Springer TA (1987) Biosynthesis and glycosylation of p150,95 and related leukocyte adhesion proteins. J Immunol 139, 842–847 [PubMed] [Google Scholar]
- 51.Varki A (2007) Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 52.Zerfaoui M, Fukuda M, Sbarra V, Lombardo D, and El-Battari A (2000) alpha(1,2)-fucosylation prevents sialyl Lewis x expression and E-selectin-mediated adhesion of fucosyltransferase VII-transfected cells. Eur J Biochem 267, 53–61 [DOI] [PubMed] [Google Scholar]
- 53.Bateman J, Parida SK, and Nash GB (1993) Neutrophil integrin assay for clinical studies. Cell Biochem Funct 11, 87–91 [DOI] [PubMed] [Google Scholar]
- 54.Jutila MA, Rott L, Berg EL, and Butcher EC (1989) Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol 143, 3318–3324 [PubMed] [Google Scholar]
- 55.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, and Bergeron MG (2002) Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol 168, 1813–1822 [DOI] [PubMed] [Google Scholar]
- 56.Tsikitis VL, Albina JE, and Reichner JS (2004) Beta-glucan affects leukocyte navigation in a complex chemotactic gradient. Surgery 136, 384–389 [DOI] [PubMed] [Google Scholar]
- 57.Thornton BP, Vetvicka V, Pitman M, Goldman RC, and Ross GD (1996) Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol 156, 1235–1246 [PubMed] [Google Scholar]
- 58.Jondle CN, Sharma A, Simonson TJ, Larson B, Mishra BB, and Sharma J (2016) Macrophage Galactose-Type Lectin-1 Deficiency Is Associated with Increased Neutrophilia and Hyperinflammation in Gram-Negative Pneumonia. J Immunol 196, 3088–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girao DK, Cavada BS, de Freitas Pires A, Martins TV, Franco AX, Morais CM, Santiago do Nascimento K, Delatorre P, da Silva HC, Nagano CS, Assreuy AM, and Soares PM (2015) The galactose-binding lectin isolated from Bauhinia bauhinioides Mart seeds inhibits neutrophil rolling and adhesion via primary cytokines. J Mol Recognit 28, 285–292 [DOI] [PubMed] [Google Scholar]
- 60.Kuehn C, and Van Epps DE (1980) Lectin-mediated induction of human neutrophil chemotaxis, chemokinesis, and cap formation. Infect Immun 29, 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dupuy AG, and Caron E (2008) Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci 121, 1773–1783 [DOI] [PubMed] [Google Scholar]
- 62.Graham IL, Gresham HD, and Brown EJ (1989) An immobile subset of plasma membrane CD11b/CD18 (Mac-1) is involved in phagocytosis of targets recognized by multiple receptors. J Immunol 142, 2352–2358 [PubMed] [Google Scholar]
- 63.Kerrigan AM, and Brown GD (2009) C-type lectins and phagocytosis. Immunobiology 214, 562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajto T, Hostanska K, and Gabius HJ (1989) Modulatory potency of the beta-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res 49, 4803–4808 [PubMed] [Google Scholar]
- 65.Conejeros I, Patterson R, Burgos RA, Hermosilla C, and Werling D (2011) Induction of reactive oxygen species in bovine neutrophils is CD11b, but not dectin-1-dependent. Vet Immunol Immunopathol 139, 308–312 [DOI] [PubMed] [Google Scholar]
- 66.El Kebir D, Jozsef L, Pan W, and Filep JG (2008) Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res 103, 352–359 [DOI] [PubMed] [Google Scholar]
- 67.Lin J, He K, Zhao G, Li C, Hu L, Zhu G, Niu Y, and Hao G (2017) Mincle inhibits neutrophils and macrophages apoptosis in A. fumigatus keratitis. Int Immunopharmacol 52, 101–109 [DOI] [PubMed] [Google Scholar]
- 68.Sastre L, Kishimoto TK, Gee C, Roberts T, and Springer TA (1986) The mouse leukocyte adhesion proteins Mac-1 and LFA-1: studies on mRNA translation and protein glycosylation with emphasis on Mac-1. J Immunol 137, 1060–1065 [PubMed] [Google Scholar]
- 69.Mocsai A, Zhou M, Meng F, Tybulewicz VL, and Lowell CA (2002) Syk is required for integrin signaling in neutrophils. Immunity 16, 547–558 [DOI] [PubMed] [Google Scholar]
- 70.Futosi K, and Mocsai A (2016) Tyrosine kinase signaling pathways in neutrophils. Immunol Rev 273, 121–139 [DOI] [PubMed] [Google Scholar]
- 71.Schymeinsky J, Then C, Sindrilaru A, Gerstl R, Jakus Z, Tybulewicz VL, Scharffetter-Kochanek K, and Walzog B (2007) Syk-mediated translocation of PI3Kdelta to the leading edge controls lamellipodium formation and migration of leukocytes. PLoS One 2, e1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, Wang G, Minshall RD, Li Y, Zhao Y, Ye RD, and Xu J (2012) Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol 13, 457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu MJ, Lee SS, Lee ST, and Lin WW (2003) Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum. Br J Pharmacol 139, 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Qin W, Xu X, Xiong Y, Zhang Y, Zhang H, and Sun B (2017) Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc Natl Acad Sci U S A 114, 4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X, Gao XP, Fan J, Liu Q, Anwar KN, Frey RS, and Malik AB (2005) LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am J Physiol Lung Cell Mol Physiol 288, L655–662 [DOI] [PubMed] [Google Scholar]
- 76.Watanabe K, Blew B, Scherer M, Burke J, Koh G, Block C, Ramakrishnan V, and Frommel TO (1997) CD11b mRNA expression in neutrophils isolated from peripheral blood and gingival crevicular fluid. J Clin Periodontol 24, 814–822 [DOI] [PubMed] [Google Scholar]
- 77.Dong G, Song L, Tian C, Wang Y, Miao F, Zheng J, Lu C, Alsadun S, and Graves DT (2017) FOXO1 Regulates Bacteria-Induced Neutrophil Activity. Front Immunol 8, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gahmberg CG (1997) Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol 9, 643–650 [DOI] [PubMed] [Google Scholar]
- 79.Altieri DC, Bader R, Mannucci PM, and Edgington TS (1988) Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J Cell Biol 107, 1893–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beller DI, Springer TA, and Schreiber RD (1982) Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med 156, 1000–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ehlers MR (2000) CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect 2, 289–294 [DOI] [PubMed] [Google Scholar]
- 82.Clark MA, Jepson MA, Simmons NL, and Hirst BH (1995) Selective binding and transcytosis of Ulex europaeus 1 lectin by mouse Peyer’s patch M-cells in vivo. Cell Tissue Res 282, 455–461 [DOI] [PubMed] [Google Scholar]
- 83.Wang Q, Yu LG, Campbell BJ, Milton JD, and Rhodes JM (1998) Identification of intact peanut lectin in peripheral venous blood. Lancet 352, 1831–1832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.