Abstract
Background
Alcohol Use Disorder (AUD) increases brain stress systems while suppressing reward system functioning. One expression of stress system recruitment is elevated GABAergic activity in the central amygdala (CeA), which is involved in the excessive drinking seen with AUD. The sulfonic amino acid taurine, a glycine receptor partial agonist, modulates GABAergic activity in the rewarding effects of alcohol. Despite taurine abundance in the amygdala, its role in the dysregulation of GABAergic activity associated with AUD has not been studied. Thus, here, we evaluated the effects of taurine on locally stimulated GABAergic neurotransmission in the central amygdala of naïve and alcohol dependent rats.
Methods
We recorded intracellularly from CeA neurons of naïve and alcohol dependent rats, quantifying locally evoked GABAA receptor mediated inhibitory postsynaptic potentials (eIPSP). We examined the effects of taurine and alcohol on CeA eIPSP to characterize potential alcohol dependence induced changes in the effects of taurine.
Results
We found that taurine decreased amplitudes of eIPSP in CeA neurons of naïve rats, without affecting the acute alcohol-induced facilitation of GABAergic responses. In CeA neurons from dependent rats, taurine no longer had an effect on eIPSP, but now blocked the ethanol-induced increase in eIPSP amplitude normally seen. Additionally, pre-application of the glycine receptor specific antagonist strychnine blocked the ethanol induced increase in eIPSP amplitude in neurons from naïve rats.
Conclusions
These data suggest taurine may act to oppose the effects of acute alcohol via the glycine receptor in the CeA of naïve rats, and this modulatory system is altered in the CeA of dependent rats.
Keywords: GABA, taurine, glycine, central amygdala, alcohol
INTRODUCTION
Alcohol Use Disorder (AUD) is a major public health concern, with 5.3% of all deaths worldwide attributable to the harmful use of alcohol (World Health Organization, 2018). Continued episodes of alcohol abuse lead to recruitment of brain stress systems and suppression of brain reward systems, resulting in the chronic relapse of AUD, where compulsive alcohol intake is driven by the elimination of negative withdrawal symptoms (Koob, 2013). However, ethanol (alcohol) is a non-selective drug, affecting many different neurotransmitter systems, receptors, enzymes, etc., in many brain regions and the complex mechanism by which ethanol misuse results in the dependent phenotype is not yet fully understood. The inhibitory GABAergic neuronal circuitry in the central amygdala (CeA), the major output nucleus of the larger amygdala complex, contributes to the development of dependence and withdrawal (Koob and Le Moal, 2008). Specifically, acute ethanol increases GABA release into the CeA, and chronic ethanol results in a sustained increase in GABAergic activity there (Roberto et al., 2003, Roberto et al., 2004). Additionally, ethanol is known to potentiate inhibitory neurotransmission through binding directly to chloride conductive ligand-gated ion channels (LGICs) (Harris et al., 1995, Kirson et al., 2018b, Lobo and Harris, 2005, Lobo and Harris, 2008, Lynch, 2004, Mihic, 1999, Mihic et al., 1997, Welsh et al., 2009, Welsh et al., 2010). Traditionally, fast inhibitory neurotransmission via LGICs in the majority of the brain was thought to primarily involve GABAA receptors, while inhibition in the brain stem and spinal cord involved the chloride conductive glycine receptors (GlyR). However, GlyR have now been found throughout the brain (Baer et al., 2009, Dudeck et al., 2003, Waldvogel et al., 2007, Waldvogel et al., 2009), including the CeA (Delaney et al., 2010), and are a promising target for therapeutics as some behavioral effects of alcohol may be mediated by GlyR (Adermark et al., 2011, Ericson et al., 2011).
The sulfonic amino acid taurine is the second most abundant amino acid in the brain, and acts as a partial agonist at the GlyR and GABAA receptor, with varying efficacy depending on the receptor composition (Albrecht and Schousboe, 2005, Dahchour et al., 1996, De Saint Jan et al., 2001, Kletke et al., 2013, Mori et al., 2002). GlyR can be composed of heteromeric α and β subunits or homomeric α subunits alone, and while the GlyR β subunit is expressed throughout the brain, GlyR α subunit expression varies. The CeA contains the α1, α2, α3, and β subunits, with α2β containing GlyR likely the predominant form (Delaney et al., 2010, McCracken et al., 2017). Primarily released by astrocytes, taurine is a major avenue of glia-neuron communication that can modulate synaptic function (Adermark et al., 2011, Choe et al., 2012, Danober and Pape, 1998, Ericson et al., 2011, McCool and Botting, 2000, McCool and Chappell, 2007), and taurine may act as the endogenous ligand of the GlyR in some brain regions such as the hippocampus and nucleus accumbens (Choe et al., 2012, Ericson et al., 2006, Jonsson et al., 2009, Molander et al., 2007, Molander et al., 2005, Mori et al., 2002, Shibanoki et al., 1993). Although taurine is canonically released via volume-regulated anion channels (VRAC) due to osmotic changes in the extracellular environment (Choe et al., 2012, Deleuze et al., 1998), drugs of abuse such as alcohol, nicotine, and cocaine also induce increases in taurine concentrations in brain specific regions (Dahchour et al., 1996, Kashkin and De Witte, 2004, Kashkin and De Witte, 2005, Li et al., 2012, Ulenius et al., 2017). While the exact mechanism for the drug induced release of taurine is not fully known, in the nucleus accumbens of rodents, ethanol induction of taurine release is blocked by the Na-K-Cl cotransporter (NKCC1) blocker furosemide, and ethanol induced astrocyte swelling in primary cultures is blocked by furosemide, suggesting ethanol induces astrocyte swelling via NKCC1, leading to release of taurine from astrocyte VRAC (Adermark et al., 2011). In the nucleus accumbens, this increase in extracellular taurine concentration increases the likelihood of binding and activating GlyR, thus inhibiting GABAergic medium spiny neurons and disinhibiting cholinergic terminals in the ventral tegmental area, allowing cholinergic activation of dopaminergic neurons in the ventral tegmental area, finally resulting in the ethanol-induced dopamine release seen in the nucleus accumbens (Adermark et al., 2011, Ericson et al., 2011).
Ethanol also increases taurine concentration in the CeA of rodents (Quertemont et al., 1999), and dysregulation of inhibitory signaling in the CeA has been linked to the development of AUD (Gilpin et al., 2015, Roberto et al., 2003, Roberto et al., 2004). Interestingly, activation of GlyR in the human amygdala increases release of acetylcholine (Dudeck et al., 2003), similar to the effect in the ventral tegmental area, and taurine can alter membrane properties of basolateral amygdala neurons via GlyR (McCool and Botting, 2000). Thus, here we hypothesized that taurine may inhibit GABAergic neurons of the amygdala, decreasing GABA release in the CeA, and interfering with the actions of ethanol in potentiating CeA GABAergic signaling. Therefore, taurine would essentially be acting as a brake on excessive GABA in the CeA, and this modulation may be disrupted in AUD resulting in the increased GABAergic signaling phenotype. In this study, we used intracellular sharp electrode electrophysiology to investigate taurine and ethanol effects on GABAergic signaling in the CeA of naïve and alcohol dependent rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (n=41, average weight: 382.9±12.1 g) were obtained from Charles River (Raleigh, NC) and housed 3 animals per cage in a temperature- and humidity-controlled room on a 12-h light/dark cycle (lights off at 10 AM) with food and water available ad libitum. All procedures and care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of The Scripps Research Institute.
Chronic Intermittent Ethanol Exposure
Chronic intermittent ethanol rats (n=20) underwent 5–7 weeks of daily ethanol vapor exposure (14 h vapor/10 h air) to produce physical ethanol dependence. Previous studies from our lab and others have demonstrated that this protocol results in increased ethanol-drinking behavior, anxiety-like behavior, and reward deficits in the dependent rats (Gilpin et al., 2008, O’Dell et al., 2004, Roberto et al., 2010). Blood alcohol levels (BALs) were measured weekly by tail-bleeding and upon sacrifice, and the mean BAL was 166.4±14.9 mg/dL. Dependent rats were sacrificed 15–30 min prior to the end of their final ethanol vapor exposure and slice preparation occurred in ethanol-free solutions. As a result, the electrophysiology recordings were obtained from neurons undergoing acute ethanol withdrawal (2–8 hours).
Electrophysiology
Slice Preparation
Briefly, rats were anesthetized with isoflurane (3–5%) followed by rapid decapitation and immediate removal of the brain into an ice-cold high sucrose cutting solution of the following composition (in mM): Sucrose 206; KCl 2.5; CaCl2 0.5; MgCl2 7; NaH2PO4 1.2; NaHCO3 26; glucose 5; HEPES 5; pH 7.4. Coronal slices containing the CeA (400 μm) were cut on a Leica 1200S vibratome (Buffalo Grove, IL), incubated in an interface configuration for 5 – 17 min, and then submerged and continuously superfused (flow rate of 2–4 ml/min) with 95% O2/5% CO2 equilibrated room temperature artificial cerebrospinal fluid (aCSF) of the following composition (in mM): NaCl 130; KCl 3.5; NaH2PO4 1.25; MgSO4·7H2O 1.5; CaCl2 2.0; NaHCO, 24; glucose 10; pH 7.4. All recordings were performed 1 – 8 hours after slice preparation in neurons from the medial subdivision of the CeA, and each experimental group contained neurons from at least 3 different animals. Inhibitory neurotransmission was pharmacologically isolated with 20 μM DNQX (to block AMPA receptors), 30 μM DL-AP5 (to block NMDA receptors), and 1 μM CGP 55845A (to block GABAB receptors). All drugs were constituted in aCSF and applied by bath superfusion.
Intracellular Recordings of Evoked IPSPs
We blindly advanced sharp micropipettes filled with 3M KCl into the medial CeA in 2 μm steps until a neuron was identified. Recordings were made using discontinuous current-clamp mode, and neurons were held near their resting membrane potential (−80.8±0.7 mV). Data were acquired with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA) and stored for later analysis using pClamp 10 software (Molecular Devices, Sunnyvale, CA). We evoked GABAergic inhibitory postsynaptic potentials (eIPSPs) by stimulating locally within the CeA through a bipolar stimulating electrode. We performed an input–output (I/O) protocol consisting of a range of five current stimulations, starting at the threshold current required to elicit an eIPSP, up to the strength required to elicit the maximum subthreshold amplitude. These stimulus strengths were maintained throughout the duration of the experiment. We examined paired pulse ratio (PPR), whereby two stimuli of the same intensity are applied at varied interstimulus intervals (Andreasen and Hablitz, 1994, Kirson et al., 2018a, Logrip et al., 2016, Manabe et al., 1993). PPR is calculated as the amplitude of the second eIPSP over that of the first eIPSP. The stimulus strength was adjusted such that the amplitude of the first eIPSP was ~50 percent of the maximal amplitude determined by the I/O protocol. A drug-induced change in PPR reflects presynaptic effects such that an increase in PPR suggests a decrease in neurotransmitter (GABA) release. We used strychnine, a competitive antagonist of GlyR that binds non-covalently in an overlapping but not identical binding pocket as ligands such as glycine or taurine (Lynch, 2004), to block any potential activation of postsynaptic and extrasynaptic GlyR (Babaev et al., 2018). Strychnine binding was used to first identify GlyR, and canonically strychnine is thought of as selective for the GlyR, particularly when compared to other anionic conductive LGICs (Lynch, 2004). All measures were performed prior to (baseline) and during drug application.
Drugs
Taurine (Tau) and strychnine (Strych) were purchased from Sigma (St. Louis, MO, USA). CGP 55845A, DL-2-amino-5-phosphonovalerate (DL-AP5), and 6,7-dinitroquinoxaline-2,3-dione (DNQX) were obtained from Tocris (Ellisville, MO). Ethanol (EtOH) was purchased from Remet (La Mirada, CA, USA). All drugs were added to aCSF from stock solutions to obtain known concentrations in the superfusate, and only applied once per CeA slice.
Data Analysis
Local CeA eIPSP amplitudes were analyzed with Clampfit 10 (Molecular Devices, Sunnyvale, CA). Electrophysiology data were analyzed with t-tests for individual drug effects compared to baseline and for comparison of a single drug effect between alcohol treatment groups. For comparison of alcohol treatment groups with either multiple drug effects or stimulation amplitudes, two way ANOVA with Bonferroni post hoc analyses was used. All data are presented as mean +/− SEM. All statistics were performed in Prism 6 (GraphPad Software, Inc, La Jolla, CA). In all cases, p<0.05 was the criterion for statistical significance. Graphs were generated with Prism and figures created in Adobe Illustrator CS6 (Adobe Systems Inc, San Jose, CA).
RESULTS
We recorded from neurons (n=59) intracellularly with sharp pipettes and evoked inhibitory postsynaptic potentials (eIPSP) locally within the CeA. Average neuronal membrane resistance was 156±9.4 MΩ for naïve animals and 146.7±10.9 MΩ for dependent animals. We first assessed potential differences in baseline evoked GABAergic transmission in CeA neurons from naïve rats compared to ethanol dependent rats. Baseline eIPSP input–output (I/O) curves generated by five equivalent normalized stimulus intensities exhibited generally increased amplitudes in dependent (3.8±0.5, 8.8±0.8, 11.7±1.1, 15.5±1.3, and 18.4±1.1 mV) compared to naïve (4.3±0.5, 6.8±0.5, 8.8±0.8, 11.6±1.0, and 12.8±1.1 mV) rats (Fig. 1A,B). A repeated measures two-way ANOVA of ethanol treatment and stimulus intensity found significant main effects of treatment (F1,41=6.86; p<0.05) and intensity (F4,164=127.5; p<0.0001) and an interaction effect (F4,164=7.94; p<0.0001). A Bonferroni multiple comparison post-hoc test found a significant difference between eIPSP amplitudes from naïve and dependent rats at the two highest stimulation intensities (t205=3.0, p<0.05; t205=4.39, p<0.0001, respectively). This suggests neurons from dependent rats have elevated evoked GABAergic release into the CeA compared to naïve rats, as shown previously in our studies (Roberto et al., 2010, Roberto et al., 2004, Roberto and Siggins, 2006).
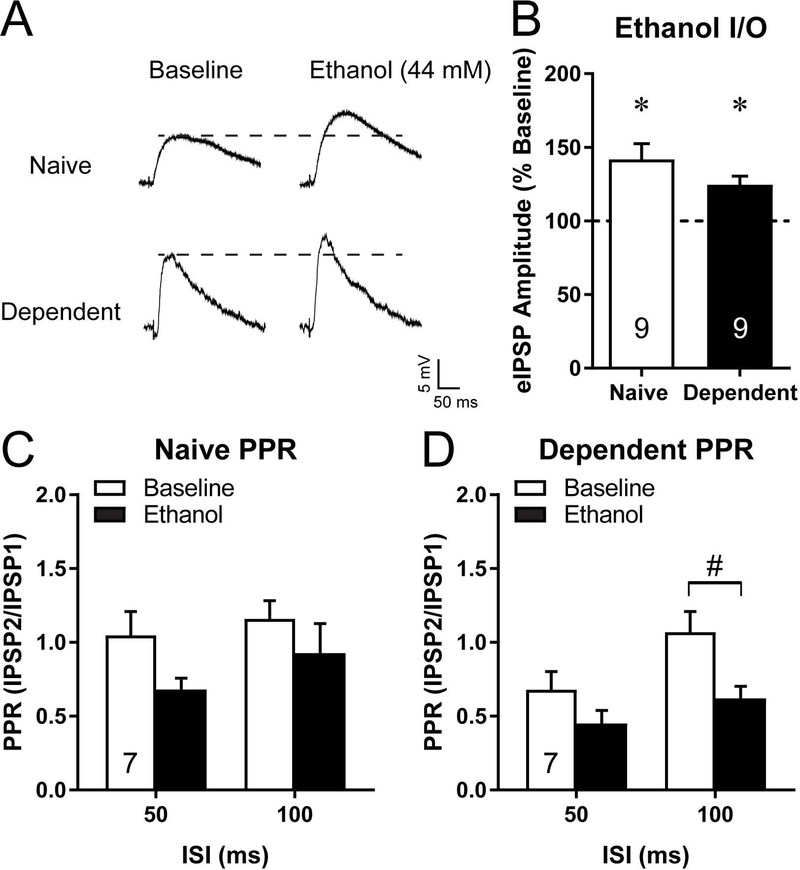
Figure 1:
Dependent rats display elevated GABAergic signaling in the central amygdala (CeA). (A) Representative evoked inhibitory postsynaptic potentials (eIPSP) showing the five step input/output (I/O) evoked response for a CeA neuron from a naïve and a dependent rat. (B) Average eIPSP amplitude at each I/O step for naïve (n=23 neurons) and dependent (n=20 neurons) rats. (C) Representative paired-pulse eIPSPs (stimulation at 50 and 100 ms) for a neuron from a naïve and a dependent rat. (D) Average paired-pulse ratios (PPR) at 50 and 100 ms for neurons from naïve and dependent rats. Number in each bar is n number of neurons per group. #p<0.05 between groups. *p<0.05 by Bonferroni posthoc.
We evoked paired IPSPs using interstimulus intervals (ISI) of 50 and 100 ms and calculated the paired pulse ratio (PPR) as the amplitude of the second eIPSP over the amplitude of the first. We found that in CeA of dependent rats (Fig. 1C), PPR of eIPSPs (50 ms: 0.81±0.09; 100 ms: 1.04±0.06) were significantly lower than those observed in naïve rats (50 ms: 1.1±0.09; 100 ms: 1.31±0.09), at both the 50 ms (t39=2.35, p<0.05) and the 100 ms ISI (t39=2.34, p<0.05), suggesting increased GABA release in dependent rats compared to naïve rats. The magnitude of the ratio is a measure of probability of release for stimulated neurons, such that PPR values above 1 indicate a relative low probability of release whereas PPR below 1 indicate a relative high probability of release. Additionally, a drug-induced change in PPR reflects presynaptic effects such that an increase in PPR suggests a decrease in neurotransmitter (GABA) release (Andreasen and Hablitz, 1994, Kirson et al., 2018a, Logrip et al., 2016, Manabe et al., 1993).
Next, we recapitulated that acute ethanol (10–12 minutes, 44 mM; (Roberto et al., 2003)) significantly (t8=3.94, p<0.01) increased eIPSP amplitude to 141.8±10.6% of baseline in CeA neurons from naïve rats (Fig. 2A,B). Similarly, in CeA neurons from dependent rats (Fig. 2A,B), ethanol significantly (t8=4.11; p<0.01) increased eIPSP amplitudes to 124.5±6.0% of baseline. In accordance to our previous work (Roberto et al., 2004), the increase in eIPSP amplitude with acute ethanol was to the same extent in naive and dependent neurons, indicating a lack of tolerance for the acute effect of ethanol on evoked GABAergic signaling despite the higher baseline release in dependent neurons. However, although PPR appears to decrease with ethanol in both the naïve and dependent neurons (Fig. 2C,D), the ethanol induced decrease was only significant (t6=2.65; p<0.05) at the 100 ms ISI in dependent neurons (Fig. 2D).
Figure 2:
Acute ethanol increases evoked GABA release into the central amygdala (CeA) of naïve and dependent rats. (A) Representative evoked inhibitory postsynaptic potentials (eIPSP) at baseline and in acute ethanol (44 mM) for a CeA neuron from a naïve and a dependent rat. (B) Average normalized eIPSP amplitude response to acute ethanol for CeA neurons from naïve and dependent rats. (C,D) Average paired-pulse ratios (PPR) at 50 and 100 ms at baseline and in acute ethanol for neurons from naïve (C) and dependent (D) rats. Number in each bar is n number of neurons per group. *p<0.05 drug effect compared to baseline. #p<0.05 between drug effects.
In separate experiments, we applied acutely the sulfonic amino acid taurine (10–15 min, 100 μM) and observed a significant (t9=5.06, p<0.001) decrease (to 72.3±5.5% of baseline) in the amplitude of eIPSPs in CeA from naive animals (Fig. 3A,B). Subsequent co-application of 44 mM ethanol in the presence of taurine increased eIPSP amplitudes back to approximately baseline levels (94.0±10.2%). The effect of these drugs on eIPSP amplitudes were significantly different from each other (t9=2.70, p<0.05). In fact, ethanol potentiation of eIPSP amplitudes in the absence of taurine is not different from ethanol potentiation in the presence of taurine (Fig. 3C), indicating no apparent interaction between the taurine and ethanol with an essentially additive effect during co-application. Taurine increased PPR at the 50 ms ISI from 1.07±0.1 to 1.46±0.2 and subsequent ethanol slightly lowered the PPR back to 1.35±0.12 (Fig. 3D), but neither these changes nor drug effects at the 100 ms ISI were significantly different, suggesting some of the effects of taurine on GABA signaling may be postsynaptic.
Figure 3:
Taurine decreases evoked GABA release into the central amygdala (CeA) of naïve rats. (A) Representative evoked inhibitory postsynaptic potentials (eIPSP) at baseline, in taurine (100 μM) and in taurine co-applied with ethanol (EtOH; 44 mM) for a CeA neuron from a naïve rat. (B) Average normalized eIPSP amplitudes in taurine and subsequent co-applied taurine and ethanol in neurons from naïve rats. (C) Comparison of the potentiating ethanol effect on eIPSP amplitudes in the absence of (from Fig. 2B) and presence of taurine for neurons from naïve rats. (D) Average paired-pulse ratios (PPR) at 50 and 100 ms at baseline, in taurine, and in co-applied taurine and ethanol for neurons from naïve rats. Number in each bar is n number of neurons per group. *p<0.05 drug effect compared to baseline. #p<0.05 between drug effects.
Notably, in dependent CeA neurons (Fig. 4A,B), application of taurine had no effect on eIPSP amplitude (100.6±8.5% of baseline), but taurine blocked the acute ethanol-induced increase in amplitude of eIPSPs (106.9±8.0% of baseline). Although eIPSP amplitudes of ethanol are not changed in the presence of taurine, the effect is not significantly different from the ethanol enhancement of GABA signaling seen in the absence of taurine (Fig. 4C). Additionally, neither taurine nor subsequent ethanol had any effect on PPR (Fig. 4D). Overall, this data suggests dependence induced dysregulation of the receptor that binds taurine in the CeA GABAergic network, but such that taurine binding interferes with the normally consistent effect of ethanol to potentiate GABAergic signaling.
Figure 4:
Taurine (Tau) has no per se effect but blocks ethanol (EtOH) potentiation of evoked GABA release in the central amygdala (CeA) of dependent rats. (A) Representative evoked inhibitory postsynaptic potentials (eIPSP) at baseline, in taurine (100 μM) and in taurine co-applied with ethanol (44 mM) for a CeA neuron from a dependent rat. (B) Average normalized eIPSP amplitude responses to taurine and co-applied taurine and ethanol for neurons from dependent rats. (C) Comparison of the ethanol effect on eIPSP amplitudes in the absence of (from Fig. 2B) and presence of taurine for neurons from dependent rats. (D) Average paired-pulse ratios (PPR) at 50 and 100 ms at baseline, in taurine, and in co-applied taurine and ethanol for neurons from dependent rats. Number in each bar is n number of neurons per group. *p<0.05 drug effect compared to baseline.
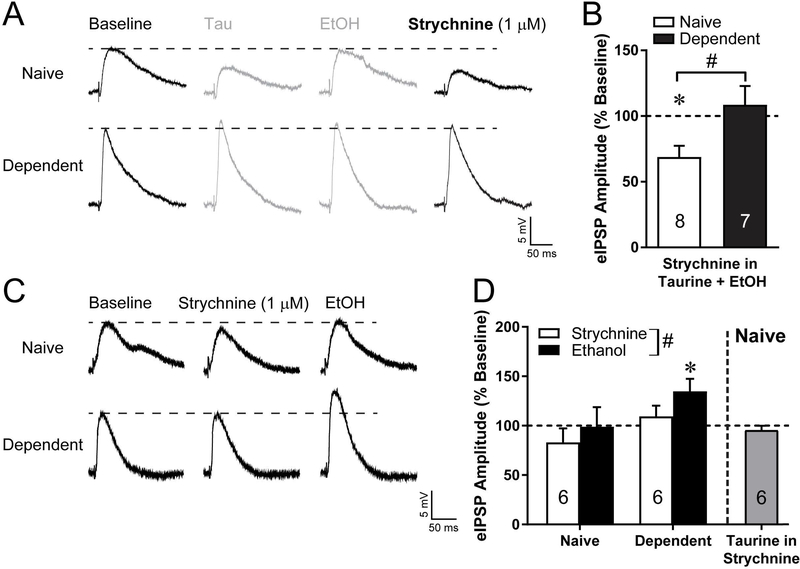
Next, we investigated the likelihood of the GlyR being responsible for the taurine induced effects. In a subset of the neurons that received taurine and ethanol, we subsequently applied the selective GlyR competitive antagonist strychnine (10–12 min, 1 μM; Fig. 5A). In CeA neurons from naïve rats, strychnine significantly (t7=3.65, p<0.01) reduced eIPSP amplitudes to 68.9±8.5% of baseline (Fig. 5B), similar to the reduction seen with taurine alone, suggesting that the ethanol potentiation of eIPSP amplitudes seen with the co-application (taurine and ethanol) may involve GlyR. In contrast, in dependent CeA neurons strychnine applied in taurine and ethanol had no effect on eIPSPs (108.5±14.4% of baseline, Fig. 5B). This is similar to the lack of taurine and ethanol effect in dependent neurons, suggesting that alcohol dependence induces some dysregulation of GlyR in the CeA.
Figure 5:
Selective glycine receptor antagonist strychnine blocks ethanol (EtOH) potentiation of evoked GABA release in the central amygdala (CeA) of naive rats. (A) Representative evoked inhibitory postsynaptic potentials (eIPSP) from Figures 3A and 4A (gray) and response to addition of strychnine (1 μM) to the taurine (Tau) and ethanol. (B) Average of effects of strychnine on eIPSP amplitudes in the presence of co-applied taurine and ethanol for neurons from naïve and dependent rats. (C) Representative eIPSPs at baseline, in strychnine (1 μM), and ethanol applied in strychnine for a CeA neuron from a naïve and a dependent rat. (D) Average normalized eIPSP amplitude effects of strychnine and ethanol in strychnine for neurons from naïve and dependent rats, and of taurine in strychnine for neurons from naïve rats. Number in each bar is n number of neurons per group. *p<0.05 drug effect compared to baseline. #p<0.05 between groups.
In order to test whether the ethanol induced potentiation of eIPSP amplitude in the CeA of naïve animals is an effect at the GlyR, we pre-applied strychnine before application of acute ethanol in the aCSF with strychnine. Strychnine alone had no significant effects on eIPSP amplitudes in CeA neurons from naïve or dependent rats (Fig. 5C,D). Subsequent acute application of ethanol in the presence of strychnine had no effect on eIPSP amplitudes of naïve CeA neurons (98.9±19.8% of baseline), confirming the ethanol effects may be mediated by GlyR. However, in dependent CeA neurons, acute ethanol in strychnine does significantly (t5=2.71, p<0.05) increase eIPSP amplitudes to 134.7±12.8% of baseline, and a repeated measures two-way ANOVA of drug and ethanol treatment found a main effect of drug (F1,10=5.45, p<0.05) such that eIPSP amplitudes were higher in ethanol than strychnine alone. This recovery of the acute ethanol effect in dependent neurons further suggests ethanol dependence induced changes in GlyR abundance or function. Finally, we tested whether pre-applied strychnine would also block the inhibitory effect of taurine seen in CeA neurons from naïve animals. Taurine in the presence of strychnine had no effect on eIPSP amplitudes (Fig. 5D), indicating the inhibitory effect of taurine is through action at GlyR.
DISCUSSION
Here, we report that the endogenous GlyR and GABAA receptor partial agonist taurine inhibits stimulated GABAergic neurotransmission in the ethanol naïve CeA, effectively suppressing the potentiating effects of acute ethanol, such that the combined taurine and ethanol effects are indistinguishable from baseline. The mechanism of action of this effect of taurine is altered in alcohol dependence, such that taurine no longer has an effect. Thus, the ability of taurine to act as a brake on excessive GABAergic signaling is removed, suggesting at least part of the elevated GABAergic neurotransmission seen in dependent rats compared to naïve rats may be due to this removal. Importantly, the concentration of taurine we used (100 μM) is biologically relevant. Taurine concentrations can be in the millimolar range within neurons and glia (Olson and Li, 2000), and although basal extracellular levels may be too low for GlyR activation (Choe et al., 2012), release of taurine by glia can increase the local concentration to the micromolar (Dopico et al., 2006) or millimolar (Olson and Li, 2000) range dependent on brain region.
In the CeA of naive and dependent rats, we recapitulated that acute ethanol increases the amplitude of eIPSPs. In this study, the GlyR selective competitive antagonist strychnine blocked both the taurine-induced decrease and the ethanol-induced increase in naïve CeA eIPSP amplitude, suggesting the effects of acute application of taurine or ethanol on GABAergic transmission involves action at GlyR. GlyR are cys-loop receptors and share some homology with this family of LGICs, the prototypical member of which is the nicotinic acetylcholine receptor (nAChR). Strychnine acts by stabilizing the GlyR in the closed state (Du et al., 2015). There is evidence for strychnine binding the α7-nAChR, but with an affinity three orders of magnitude lower than GlyR (Albuquerque et al., 1998, Lynch, 2004). Thus, strychnine is the appropriate antagonist for these experiments due to its high selectivity for GlyR and no other receptors known to be present in the CeA. However, strychnine did not block the combined effects of taurine and ethanol. Although strychnine is a competitive antagonist of the GlyR, its acute application in already present taurine and ethanol could result in a mix of taurine and strychnine bound GlyR with enough taurine bound to activate some GlyR, but enough strychnine bound to inhibit allosteric modulation of GlyR by ethanol. An important consideration is that microcircuitry of the CeA is not fully described, and it is unclear what cell types may express GlyR. Thus, our synaptic results may be a composite of multiple effects on different GABAergic neurons of the CeA.
In the CeA of dependent rats, taurine had no effect on its own, suggesting a few possible mechanisms of alcohol dependence induced alterations in CeA functioning. One possibility is that GlyR may be downregulated in alcohol dependence, as this would explain the lack of effect of taurine and ethanol on dependent CeA eIPSPs. However, in the dependent CeA, ethanol alone increases eIPSP amplitudes, which would not occur if the ethanol site of action is the GlyR and the receptors are not expressed. Another possibility is that ethanol actions may have shifted to a new target in alcohol dependence away from GlyR. In a separate form of GABAergic transmission incorporating both spontaneous action potential dependent release and independent vesicular release, ethanol effects on CeA GABAergic activity occur through voltage-gated calcium channels in naïve rats but shifts to CRF1 receptors in dependent rats (Varodayan et al., 2017). A similar shift may be occurring here for stimulated GABAergic activity, and one potential candidate would be GABAA receptors. Ethanol is known to potentiate GABAA-mediated currents just as in GlyR (Lobo and Harris, 2008), and while taurine is a weak partial agonist at gamma subunit containing GABAA receptors, it is a much better agonist at delta containing GABAA receptors (Kletke et al., 2013). Both gamma containing and delta containing GABAA receptors are often found extrasynaptically where they are responsible for tonic inhibitory currents (Borghese and Harris, 2007, Herman and Roberto, 2014, Marowsky and Vogt, 2014), and in some brain regions taurine has been found to affect tonic currents via delta containing receptors (Jia et al., 2008). Additionally, there is evidence that GlyR in the forebrain can be located in the extrasynaptic space (McCracken et al., 2017), and taurine may be the endogenous ligand for these extrasynaptic GlyR (Muller et al., 2008). Both GlyR and GABAA receptors may aggregate near each other on membranes, and shuttle between synaptic and extrasynaptic membranes, suggesting dynamic regulation of receptor types is possible (Muller et al., 2008).
Thus, alcohol dependence may downregulate extrasynaptic GlyR, and any delta containing GABAA receptors, while upregulating extrasynaptic GABAA receptors, presumably containing the gamma subunit. This change could explain taurine, ethanol, and strychnine effects on naïve CeA eIPSPs, as well as the lack of taurine and strychnine effects on dependent CeA eIPSPs. As ethanol potentiates GABA-activated gamma containing receptors, strychnine would have no effect on ethanol potentiation in dependents, but when extrasynaptic gamma containing GABAA receptors are bound by taurine, the weak partial agonism could buffer the actions of ethanol to where the net effect is neutral. More research into the expression of these receptors and any changes in alcohol dependence is needed to verify if this hypothesized mechanism is correct.
Although taurine derivatives (acamprosate) have been used to treat AUD (Olive, 2003, Whitworth et al., 1996), they have not found widespread efficacy for all sufferers (Leggio et al., 2019). Despite this, taurine activity at GlyR and perhaps GABAA receptors can dramatically oppose the effects of alcohol at both the behavioral and synaptic levels and warrants further research into possible treatments using this system. Increasing the understanding of the interaction between these very important inhibitory circuits and alcohol will allow the development of treatments to help sufferers of AUD worldwide.
ACKNOWLEDGMENTS
The authors declare no conflict of interest. This is manuscript number 29890 from The Scripps Research Institute. This work was supported by NIH/NIAAA grants K99 AA026638, F32 AA025262, T32 AA007456, AA021491, AA017447, AA015566, AA006420, AA027700, and AA013498, and The Pearson Center for Alcoholism and Addiction Research.
Financial Support: NIAAA/NIH grants K99 AA026638, F32 AA025262, T32 AA007456, AA021491, AA017447, AA015566, AA006420, AA027700, and AA013498, and The Pearson Center for Alcoholism and Addiction Research.
REFERENCES
- Adermark L, Clarke RB, Olsson T, Hansson E, Soderpalm B, Ericson M (2011) Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol 16:43–54. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Schousboe A (2005) Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30:1615–1621. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Braga MF, Matsubayashi H, Alkondon M (1998) Neuronal nicotinic receptors modulate synaptic function in the hippocampus and are sensitive to blockade by the convulsant strychnine and by the anti-Parkinson drug amantadine. Toxicol Lett 102–103:211–218. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ (1994) Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J Neurophysiol 72:326–336. [DOI] [PubMed] [Google Scholar]
- Babaev O, Piletti Chatain C, Krueger-Burg D (2018) Inhibition in the amygdala anxiety circuitry. Exp Mol Med 50:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Waldvogel HJ, Faull RL, Rees MI (2009) Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Frontiers in molecular neuroscience 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA (2007) Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol 41:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KY, Olson JE, Bourque CW (2012) Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J Neurosci 32:12518–12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, De Witte P (1996) Taurine increases in the nucleus accumbens microdialysate after acute ethanol administration to naive and chronically alcoholised rats. Brain Res 735:9–19. [DOI] [PubMed] [Google Scholar]
- Danober L, Pape HC (1998) Strychnine-sensitive glycine responses in neurons of the lateral amygdala: an electrophysiological and immunocytochemical characterization. Neuroscience 85:427–441. [DOI] [PubMed] [Google Scholar]
- De Saint Jan D, David-Watine B, Korn H, Bregestovski P (2001) Activation of human alpha1 and alpha2 homomeric glycine receptors by taurine and GABA. J Physiol 535:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Esmaeili A, Sedlak PL, Lynch JW, Sah P (2010) Differential expression of glycine receptor subunits in the rat basolateral and central amygdala. Neurosci Lett 469:237–242. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N (1998) Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol 507 ( Pt 2):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico JG, Gonzalez-Hernandez T, Perez IM, Garcia IG, Abril AM, Inchausti JO, Rodriguez Diaz M (2006) Glycine release in the substantia nigra: Interaction with glutamate and GABA. Neuropharmacology 50:548–557. [DOI] [PubMed] [Google Scholar]
- Du J, Lu W, Wu S, Cheng Y, Gouaux E (2015) Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck O, Lubben S, Eipper S, Knorle R, Kirsch M, Honegger J, Zentner J, Feuerstein TJ (2003) Evidence for strychnine-sensitive glycine receptors in human amygdala. Naunyn Schmiedebergs Arch Pharmacol 368:181–187. [DOI] [PubMed] [Google Scholar]
- Ericson M, Chau P, Clarke RB, Adermark L, Soderpalm B (2011) Rising taurine and ethanol concentrations in nucleus accumbens interact to produce dopamine release after ethanol administration. Addict Biol 16:377–385. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Stomberg R, Soderpalm B (2006) Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. Eur J Neurosci 23:3225–3229. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M (2015) The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF (2008) Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9:Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Proctor WR, McQuilkin SJ, Klein RL, Mascia MP, Whatley V, Whiting PJ, Dunwiddie TV (1995) Ethanol increases GABAA responses in cells stably transfected with receptor subunits. Alcohol Clin Exp Res 19:226–232. [DOI] [PubMed] [Google Scholar]
- Herman MA, Roberto M (2014) Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL (2008) Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci 28:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytia P, Ericson M, Soderpalm B (2009) Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res 1305 Suppl:S27–36. [DOI] [PubMed] [Google Scholar]
- Kashkin VA, De Witte P (2004) Ethanol but not acetaldehyde induced changes in brain taurine: a microdialysis study. Amino acids 26:117–124. [DOI] [PubMed] [Google Scholar]
- Kashkin VA, De Witte P (2005) Nicotine increases microdialysate brain amino acid concentrations and induces conditioned place preference. Eur Neuropsychopharmacol 15:625–632. [DOI] [PubMed] [Google Scholar]
- Kirson D, Oleata CS, Parsons LH, Ciccocioppo R, Roberto M (2018a) CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and Wistar rats. Addict Biol 23:676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Todorovic J, Mihic SJ (2018b) Single Channel Analysis of Isoflurane and Ethanol Enhancement of Taurine-Activated Glycine Receptors. J Pharmacol Exp Ther 364:70–76. [DOI] [PubMed] [Google Scholar]
- Kletke O, Gisselmann G, May A, Hatt H, Sergeeva OA (2013) Partial agonism of taurine at gamma-containing native and recombinant GABAA receptors. PloS one 8:e61733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013) Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008) Addiction and the brain antireward system. Annu Rev Psychol 59:29–53. [DOI] [PubMed] [Google Scholar]
- Leggio L, Falk DE, Ryan ML, Fertig J, Litten RZ (2019) Medication Development for Alcohol Use Disorder: A Focus on Clinical Studies. Handb Exp Pharmacol. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan GY, Zhou JQ, Bu Q, Deng PC, Yang YZ, Lv L, Deng Y, Zhao JX, Shao X, Zhu RM, Huang YN, Zhao YL, Cen XB (2012) (1)H NMR-based metabonomics in brain nucleus accumbens and striatum following repeated cocaine treatment in rats. Neuroscience 218:196–205. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA (2005) Sites of alcohol and volatile anesthetic action on glycine receptors. Int Rev Neurobiol 65:53–87. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA (2008) GABA(A) receptors and alcohol. Pharmacol Biochem Behav 90:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M (2016) Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84:1051–1095. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie D, Perkel DJ, Nicoll RA (1993) Modulation of synaptic transmission and long-term potentiation in the CA1 region of the hippocampus. J Neurophysiol 70:1451–1459. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Vogt KE (2014) Delta-subunit-containing GABAA-receptors mediate tonic inhibition in paracapsular cells of the mouse amygdala. Frontiers in neural circuits 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Botting SK (2000) Characterization of strychnine-sensitive glycine receptors in acutely isolated adult rat basolateral amygdala neurons. Brain Res 859:341–351. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell A (2007) Strychnine and taurine modulation of amygdala-associated anxiety-like behavior is ‘state’ dependent. Behav Brain Res 178:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Lowes DC, Salling MC, Carreau-Vollmer C, Odean NN, Blednov YA, Betz H, Harris RA, Harrison NL (2017) Glycine receptor alpha3 and alpha2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proc Natl Acad Sci U S A 114:E7179–e7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ (1999) Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int 35:115–123. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389. [DOI] [PubMed] [Google Scholar]
- Molander A, Lido HH, Lof E, Ericson M, Soderpalm B (2007) The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol 42:11–18. [DOI] [PubMed] [Google Scholar]
- Molander A, Lof E, Stomberg R, Ericson M, Soderpalm B (2005) Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res 29:38–45. [DOI] [PubMed] [Google Scholar]
- Mori M, Gahwiler BH, Gerber U (2002) Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol 539:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E, Le-Corronc H, Legendre P (2008) Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Frontiers in molecular neuroscience 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28:1676–1682. [DOI] [PubMed] [Google Scholar]
- Olive MF (2003) The anti-craving taurine derivative acamprosate: failure to extinguish morphine conditioned place preference. Adv Exp Med Biol 526:481–484. [PubMed] [Google Scholar]
- Olson JE, Li GZ (2000) Osmotic sensitivity of taurine release from hippocampal neuronal and glial cells. Adv Exp Med Biol 483:213–218. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Dahchour A, Ward RJ, Witte P (1999) Ethanol induces taurine release in the amygdala: an in vivo microdialysis study. Addict Biol 4:47–54. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH (2010) Corticotropin Releasing Factor-Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol Psychiatry:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A 100:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004) Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24:10159–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Siggins GR (2006) Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A 103:9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibanoki S, Kogure M, Sugahara M, Ishikawa K (1993) Effect of systemic administration of N-methyl-D-aspartic acid on extracellular taurine level measured by microdialysis in the hippocampal CA1 field and striatum of rats. J Neurochem 61:1698–1704. [DOI] [PubMed] [Google Scholar]
- Ulenius L, Adermark L, Soderpalm B, Ericson M (2017) Ethanol-Induced Taurine Elevation in the Rat Dorsal Striatum. Adv Exp Med Biol 975 Pt 1:173–181. [DOI] [PubMed] [Google Scholar]
- Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M (2017) Alcohol Dependence Disrupts Amygdalar L-Type Voltage-Gated Calcium Channel Mechanisms. J Neurosci 37:4593–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel HJ, Baer K, Allen KL, Rees MI, Faull RL (2007) Glycine receptors in the striatum, globus pallidus, and substantia nigra of the human brain: an immunohistochemical study. J Comp Neurol 502:1012–1029. [DOI] [PubMed] [Google Scholar]
- Waldvogel HJ, Baer K, Faull RL (2009) The localization of inhibitory neurotransmitter receptors on dopaminergic neurons of the human substantia nigra. J Neural Transm Suppl:59–70. [DOI] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ (2009) Single-channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther 330:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh BT, Kirson D, Allen HM, Mihic SJ (2010) Ethanol enhances taurine-activated glycine receptor function. Alcohol Clin Exp Res 34:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, Walter H, Fleischhacker WW (1996) Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet 347:1438–1442. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2018) Global status report on alcohol and health 2018, in Series Global status report on alcohol and health; 2018, Switzerland. [Google Scholar]