Abstract
Cancer cells have diverse mechanisms for utilizing the vasculature; they can initiate the formation of new blood vessels from pre-existing ones (sprouting angiogenesis) or they can form cohesive interactions with the abluminal surface of pre-existing vasculature in the absence of sprouting (co-option). The later process has received renewed attention due to the suggested role of blood vessel co-option in resistance to anti-angiogenic therapies and the reported perivascular positioning and migratory patterns of cancer cells during tumor dormancy and invasion, respectively. However, only a few molecular mechanisms have been identified that contribute to the process of co-option and there has not been a formal survey of cell lines and laboratory models that can be used to study co-option in different organ microenvironments; thus, we have carried out a comprehensive literature review on this topic and have identified cell lines and described the laboratory models that are used to study blood vessel co-option in cancer. Put into practice, these models may help to shed new light on the molecular mechanisms that drive blood vessel co-option during tumor dormancy, invasion, and responses to different therapies.
Keywords: Tumor blood vessels, vessel co-option, angiotropism, tumor microenvironment, angiogenesis, pericytic mimicry
Introduction
Blood vessel co-option is a non-angiogenic process whereby tumor cells utilize the pre-existing vasculature to further their growth and spread [1]. Cancer cells migrate along the pre-existing vessels and infiltrate tissues between co-opted vessels to gain access to oxygen and nutrients [2]. Since seminal evidence of cancer cell vessel co-option was found in lung cancers with alveolar non-angiogenic growth patterns [3], co-option has been observed in multiple cancer types including liver, brain, skin, lymph node, and many others. In lung cancers, about 1/3 of small-cell lung cancer (SCLC) and up to 1/2 of non-small-cell lung cancers (NSCLC) display co-optive growth patterns [4, 5]. In patients with lung metastasis from different primary cancers, including highly angiogenic renal cell carcinoma, a large percentage of cancer cells grow by vessel co-option [6–8]. In liver cancers, small early hepatocellular carcinoma cells (HCC) co-opt sinusoidal vessels, leading to the incorporation of sinusoidal vessels within the tumor microenvironment [9]. Even in advanced-stages of HCC, some cancer cells may co-opt sinusoidal vessels [10, 11]. In liver metastasis patients, 90% of breast cancer liver metastasis grow via co-option while colorectal carcinoma (CRC) liver metastases show less evidence of co-optive growth [12–14]. In high-grade gliomas, cancer cells can co-opt pre-existing vessels by surrounding brain capillaries, or they directly attach to the abluminal surface, replacing astrocytes and pericytes [15–18]. In low-grade gliomas, cancer cells infiltrate the parenchyma via co-opted vessels [19]. In brain metastasis patients (melanoma, breast, lung and colorectal cancer), vessel co-option is also frequently observed [20–22]. In the skin, melanoma cells migrate via cohesive interactions with the abluminal surface of blood vessels [23–26]. In skin metastasis from breast cancer, > 50% of metastatic cancer cells display co-option with pre-existing skin vessels [27]. In the lymph nodes, node-metastatic cancer cells from head or neck cancer, breast cancer, or CRC, all show evidence of co-option with pre-existing lymph node vessels [28–30]. Collectively, these studies show that cancers of diverse cellular origins acquire the ability to engage in blood vessel co-option in different organ microenvionments.
Although many solid tumor types can utilize vessel co-option, the molecular mechanisms that coordinate this process are poorly understood. Studies from brain tumors suggest that soluble factors, such as bradykinin [31], CXC-chemokine receptor 4 (CXCR4) – binding cytokine [32] and Wnt 7a/b [33], are critical for cancer cell co-option. Furthermore, many cancer cells express adhesion molecules, including p1 integrin, a6 integrin, and L1CAM (cell adhesion molecule L1), to facilitate the attachment of cancer cells to the vascular surface [34–36]. Other studies in NSCLC co-option show that mitochondrial genes are upregulated during co-option, suggesting the metabolic signaling pathways may be activated [37]. A recent study using liver metastasis showed that Arp2/3 (actin-related protein 2/3 complex)-mediated cancer cell motility is required for vessel co-option in liver metastasis [13]. To better understand the molecular pathways that are important during co-option, various in vivo and in vitro models have been established. In this review, we have briefly summarized each model and provided details on some of the different cell lines used. Where known, the molecular mechanisms that appear to be necessary for mediating blood vessel co-option are described. A summary of these studies organized by tumor type is included in an accompanying table (Table I).
Table I.
Summary of tumor types and models demonstrating blood vessel co-option, angiotropism, or pericyte-like migratory patterns by cancer cells.
| Cancer type | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intracranial transplantation | Intracardiac transplantation | Intracarotid transplantation | Intravenous transplantation | Orthotopic transplantation | Genetically engineered mice | Zebra fish model | Chicken CAM & metastasis assay | Brain slice/tumor cell co-culture | Endothelial cell/tumor cell co-culture | Ear tissue invasion assay | A | |||
| Glioma | C6 murine (rat) | [39] | ||||||||||||
| Cdkn2a−/−;hEGFRvIII murine | [33] | |||||||||||||
| CNS-1 murine (rat) | [42] | |||||||||||||
| GL26 murine | √ | [17, 32] | ||||||||||||
| GL261 murine | [41, 42] | |||||||||||||
| Olig2+ (Olig2cre/+;Trp53fl/fl;hEGFRvIII) murine | [33] | |||||||||||||
| Olig2− (Olig2cre/cre;Trp53fl/fl;hEGFRvIII) murine | [33] | |||||||||||||
| D54 human GBM | [31, 33] | |||||||||||||
| G55 human GBM | [38, 40] | |||||||||||||
| HF2303 human | √ | [32] | ||||||||||||
| MGG8 human GBM | [33] | |||||||||||||
| SF10417 human oligodendroglioma | [33] | |||||||||||||
| U373 human GBM | [15] | |||||||||||||
| U87 human GBM | [15] | |||||||||||||
| Melanoma | B16F10 murine | √ | √ | √ | [17, 36, 47, 51, 53] | |||||||||
| D4M3A murine | This publication | |||||||||||||
| Hcmel12 murine | √ | √ | √ | [48] | ||||||||||
| Hcmel31 murine | √ | [48] | ||||||||||||
| HGF-CDK4(R24C) | √ | [48] | ||||||||||||
| K1735M2 murine | √ | [36] | ||||||||||||
| 530 human | √ | [24] | ||||||||||||
| A2058 human | √ | [26] | ||||||||||||
| A7 human | √ | [36] | ||||||||||||
| C8161 human | √ | √ | √ | √ | [25, 50, 52, 53, 54, 55] | |||||||||
| M14 human | √ | [24] | ||||||||||||
| MaMel15 human | √ | [48] | ||||||||||||
| MaMel48 human | √ | [48] | ||||||||||||
| MaMel65 human | √ | [48] | ||||||||||||
| MDA-MB-435 human | √ | [26] | ||||||||||||
| Mel57 human | √ | [24, 46] | ||||||||||||
| MZ7-MEL human | √ | [48] | ||||||||||||
| OMM 2.3 human uveal | √ | √ | [52] | |||||||||||
| OMM 2.5 human uveal | √ | [52] | ||||||||||||
| Breast | 4T1 murine adenocarcinoma | √ | √ | √ | √ | √ | [6, 36, 51] | |||||||
| MAT-B-III murine (rat) | √ | [47] | ||||||||||||
| RBA murine (rat) adenocarcinoma | [39] | |||||||||||||
| MDA-MB-231 human cells | √ | [36] | ||||||||||||
| MDA-MB-231-BrM human | √ | √ | [35, 36, 42, 44, 45] | |||||||||||
| MDA-MB-231-LM human | √ | √ | [6, 44] | |||||||||||
| HCC1954-LCC1 human | √ | [44] | ||||||||||||
| HMLE human | √ | √ | [51] | |||||||||||
| Lung | 393N1 murine adenocarcinoma | √ | [44] | |||||||||||
| Lewis murine | √ | [39] | ||||||||||||
| H2030-BrM human adenocarcinoma | √ | √ | [35, 44] | |||||||||||
| HTB177 human | √ | [26] | ||||||||||||
| Liver | Hep3B-hCG human | √ | [49] | |||||||||||
| Colon | C26 murine | √ | [6, 47] | |||||||||||
| HT25 human | √ | [47] | ||||||||||||
| HT29 human colorectal | √ | [13] | ||||||||||||
| Fibrosarcoma | HT1080 human | √ | [47] | |||||||||||
| Prostate | PC-3 human | √ | √ | [53] | ||||||||||
| Renal | RENCA murine | √ | [6] | |||||||||||
Summary of studies demonstrating vessel co-option by cancer cells
In vivo models
Multiple animal models using different cancer types are used to study the process of vessel co-option in vivo. This includes intracranial transplantation, intracardiac transplantation, intracarotid transplantation, intravenous transplantation, orthotopic transplantation, genetically engineered mice, zebrafish models, the chick chorioallantoic membrane (CAM) assay, and the chicken embryo cancer metastasis assay. The use of these models has provided important clues about the nature of blood vessel co-option by cancer cells in vivo.
Intracranial Transplantation
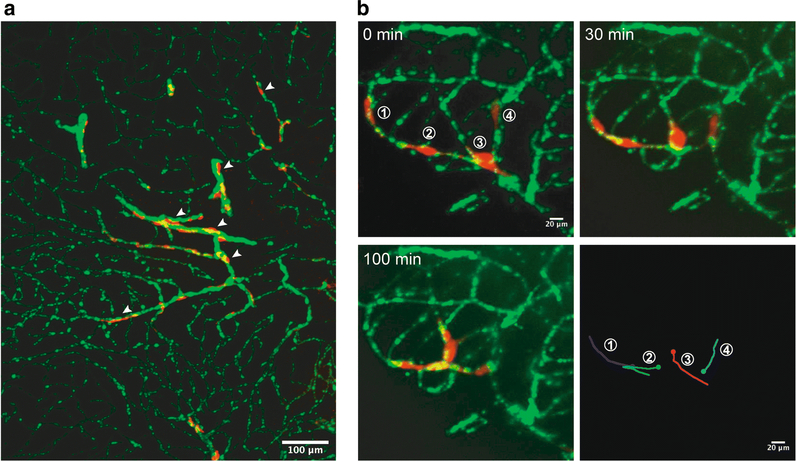
Intracranial transplantation is widely used to study primary brain tumors and brain metastases. Stereotaxic injection of tumor cells into the anesthetized rodent brain allows for spatiotemporal control and high reproducibility [38]. The injection site can easily be located according to the stereotaxic coordinates determined from a stereotaxic atlas such as “The Mouse Brain in Stereotaxic Coordinates” and “The Rat Brain in Stereotaxic Coordinates”. Tumor growth can be monitored by bioluminescence, imaging of brain slices, and intravital microscopy imaging. However, this model does present some disadvantages; for example, these “established” brain metastases do not recapitulate the different stages of the metastatic process. Moreover, injury to the brain tissue or vasculature caused by the injection itself may affect the growth of tumors and their co-option behaviors. Using this method, many studies have demonstrated various tumor cell lines to co-opt preexisting brain blood vessels. Holash et al. injected rat C6 glioma cells or RBA mammary adenocarcinoma cells into the Sprague-Dawley rat striatum, and suggested the term “vessel co-option” to describe the phenomenon of tumor cells utilizing existing brain vessels to support their initial growth in the absence of angiogenesis [39]. Anti-VEGF antibody treatment increased the fraction of co-opted vasculature in G55 human glioblastoma cells injected into nude rat striatum [38]. Systemic treatment with anti-VEGFR-2 antibody also inhibited G55 human glioblastoma angiogenesis and growth, but led to increased vessel co-option [40]. A series of studies from Lugassy, Barnhill, and colleagues demonstrated pericyte-like (pericytic mimicry) angiotropism of glioma and melanoma cells following intracranial injection of B16F10 murine melanoma cells, GL26 murine glioma cells, or C8161 human melanoma cells into mouse brain, prompting the term “extravascular migratory metastasis” as a distinct form of intravascular dissemination [17, 25]. Carbonell et al., demonstrated tumor cell growth with vascular co-option by injection of 4T1 murine mammary carcinoma, MDA-MB-231 human breast carcinoma, and B16F10 murine melanoma cells [36]. By injecting GL261 murine glioma cells into mouse brain, and later monitoring glioma cell invasion in real time using multiphoton laser scanning microscopy, Winkler et al. elegantly demonstrated co-option of existing brain vessels and that glioma cells invaded faster and more efficiently along brain microvessels [41]. The human glioblastoma multiforme (GBM) cell lines U87 and U37 also co-opt pre-existing blood vessels while employing Cdc42-dependent and actin-based cytoplasmic extensions to target and modify the contractility of pericytes [15]. By injecting HF2303 human glioma cells and GL26 murine glioma cells, Yadav et al. found that CXCR4 knockdown inhibits the growth and perivascular invasion of glioma cells [32]. Using intracranial transplantation of Olig2+ (Olig2cre/+;Trp53fl/fl;hEGFRvIII) and Olig2− (Olig2ore/cre;Trp53fl/fl;hEGFRvIII) glioma cells, Griveau et al. found Olig2+ glioma cells invade the brain by single-cell co-option, whereas Olig2” glioma cells promote angiogenesis. Mechanistically, they found Olig2 promotes Wnt7 expression, and Wnt7 signaling was essential for Olig2+ glioma single-cell vessel co-option. With patient-derived GBM tumor cells (MGG8 and D54 glioma cells) and intravital microscopy, they also showed that Wnt inhibition reduces vessel co-option [33]. By intravital microscopy imaging and mathematical modeling of glioblastomas (CNS-1 rat glioma cells and GL261 murine glioma cells) and breast cancer brain metastasis (MDA-231Br cells), the dynamics of tumor vessel co-option during tumor progression and responses to anti-angiogenic treatment were recently revealed [42]. Using the D4M3A murine melanoma cell line derived from Tyr::CreER;BrafCA;Ptenlox/lox mice [43], our lab recently found D4M3A cells efficiently co-opt the brain vasculature of immunocompetent mice indicated by a distinct, pericyte-like positioning of individual cancer cells along the surface of the brain endothelium (Fig. 1a).
Fig 1. Vessel co-option by D4M3A murine melanoma cells in the mouse brain and in ex vivo brain slice cultures.
(A) mCherry+ D4M3A cells were stereotaxically injected into the striatum of Cdh5-CreERT2;ZSGreenloxp/stop/loxp mice. Representative confocal image from a 100 μm-thick mouse brain slice showing mCherry+ D4M3A cells (red) co-opting ZSGreen+ brain vasculature (green) in the mouse cerebral cortex (arrowheads). (B) mCherry+ D4M3A cells were pipetted onto the surface of 250 μm-thick live brain slices from Cdh5-CreERT2;ZSGreenloxp/stop/loxp mice and time-lapse images were taken 24 hours later to monitor the interaction between the cancer cells and blood vessels. Representative images show D4M3A cells (red) spreading along brain capillaries (green) and the direction in which they have travelled over time.
Intracardiac Transplantation
Intracardiac transplantation into the left cardiac ventricle of anesthetized mice allows tumor cells to circulate throughout the body before arresting within the microvasculature of the lung and liver; this approach is most commonly used to model metastases to brain and bone. Intracardiac transplantation of tumor cells into the circulation is a surrogate for the later stages metastatic colonization. However, the metastatic efficiency of tumor cells injected this way is typically very low and largely cell line dependent. Tumor cell subpopulations with high metastatic abilities can be selected for by carrying out several round of intracardiac transplantation. But this approach may result in artifacts due to selection of clonal variants that propagate in vitro and do not represent the parental tumor cell population. Using this method, Massague and colleagues established various brain metastatic subpopulations (BrM) isolated from parental cancer cells and used them to study how metastatic cancer cells survive and grow in the brain microenvironment. Valiente et al. compared the transcriptomic signatures of BrM variants isolated from both lung and breast carcinoma cell lines, and found plasminogen activator-inhibitory Serpins promote cancer cell survival and L1CAM-mediated vessel co-option in brain metastasis [35]. Er et al. further investigated the mechanism and significance of L1 CAM-mediated metastatic colonization, and found disseminated cancer cells employ L1CAM to spread along capillaries and activate the mechanotransduction effectors Yes-associated protein (YAP) and myocardin-related transcription factor (MRTF) for metastatic outgrowth in multiple organs [44]. Many cancer cell lines injected via the heart utilize vascular basement membranes for their adhesion and invasion prior to angiogenesis, and this effect was dependent on the p1 integrin subunit [36]. To study the role of long noncoding RNAs in breast cancer brain metastases (BCBM), Wang et al. used intracardiac injection of MDA-MB-231-Br cells and demonstrated that Lnc-BM (a long noncoding RNA for brain metastasis) was required for brain blood vessel co-option [45].
Intracarotid transplantation
Intracarotid transplantation involves the direct injection of tumor cells into the internal carotid artery of anesthetized mice. This approach can generate more efficient and consistent experimental brain metastases. However, this model is only a surrogate for human brain metastasis because the carotid artery needs to be ligatured during the surgery, which may cause brain injury and affect the growth and vessel co-opting abilities of transplanted tumor cells. Using this method, Waal and colleagues found that brain metastases of human melanoma cell lines Mel57, M14, and 530 display infiltrative growth patterns suggestive of vascular co-option. They also found VEGF-A induces progression of melanoma brain metastases without induction of sprouting angiogenesis and it induced architectural and functional changes of co-opted, preexisting brain vasculature [24]. It was further shown that anti-angiogenic treatment of melanoma brain metastases sparked tumor progression via induction of vessel co-option [46]. By intracarotid artery injection of melanoma or lung cancer cells, together with in vivo real-time imaging by multiphoton laser scanning microscopy, the single steps required for brain metastases formation were demonstrated and included initial arrest at blood vessel branches, early extravasation, perpetuation of a perivascular position, and perivascular growth via vessel co-option (A2058 and MDA-MB-435 human melanoma cells) or induction of angiogenesis (lung cancer) [26].
Intravenous Transplantation
Intravenous transplantation of tumor cells via tail vein or jugular vein is a long-utilized model for studying lung metastasis. This model is representative of the later stages of human lung metastasis and is appropriate for studying metastatic outgrowth and tumor vessel co-option behavior in lung. Holash et al. injected Lewis lung carcinoma cells into the mouse jugular vein and found that lung metastases co-opted the lung microvasculature [39]. Using experimental lung metastases of various murine and human tumor cell lines (B16 murine melanoma, MAT-B-III rat mammary carcinoma, HT1080 human fibrosarcoma, HT25 human colon carcinoma, and C26 murine colon carcinoma), it was found that lung metastases can achieve vascularization by co-opting the pre-existing pulmonary vasculature [47]. Employing preclinical lung metastases models with 4T1 murine mammary cancer cells, C26 murine colon carcinoma cells, and RENCA murine renal cancer cells, is was shown that vessel co-option mediates resistance to anti-angiogenic therapy in lung metastasis [6]. Er et al. injected MDA-MB-231-LM cells (a lung metastatic subpopulation) into the circulation via the tail vein, and demonstrated L1CAM-mediated perivascular spreading of disseminated cancer cells in lung [44].
Orthotopic transplantation
Orthotopic transplantation is widely used to study primary tumor progression in the autochthonous tumor microenvironment. Spontaneous metastases from orthotopic sites recapitulate host-tumor interactions that mirror features of the complete metastatic cascade. However, compared to the other methods above, it usually requires a longer time period before mice develop detectable metastases. Carbonell et al. injected 4T1 cells into the mammary fat pad to generate spontaneous brain metastases and have verified perivascular growth patterns of brain micrometastases [36]. Interestingly, ultraviolet irradiation increases angiotropic growth and lung metastases of skin melanoma in a mouse model with intra-cutaneous injection of HCmel12 melanoma cells [48]. Studies using orthotopic injection of human hepatocellular carcinoma Hep3B-hCG cells into the livers of mice have recently shown that vessel co-option is a mechanism for acquired resistance to antiangiogenic therapy [49]. Using an advanced liver metastasis model by injecting HT29 human colorectal cancer cells directly into mouse liver, Frentza et al. found Arp2/3 (actin-related protein 2/3 complex)-mediated cancer cell motility is required for vessel co-option in liver metastasis and that combined inhibition of vessel co-option and angiogenesis is more effective than targeting angiogenesis alone [13]. MDA-MB-231LM2−4 cancer cells injected into the mammary fat pad were shown to co-opt pre-existing alveolar capillaries in spontaneous lung metastases [6]; similarly, Er et al., orthotopically injected MDA-MB-231-LM cells into the mammary fat pad and demonstrated L1CAM-mediated perivascular spreading of the disseminated cancer cells in lung and liver [44]. By intravital imaging following intradermal injection of C8161 human melanoma cells into the mouse ear skin, angiotropism of melanoma cells spreading along blood vessels was observed [50].
Genetically engineered mice
Genetically engineered mouse models can faithfully mimic human cancers, they avoid the side effects of experimental surgery, and they provide an opportunity to study the role of cell-mediated immunity in tumor progression in an immunocompetent host. However, genetically engineered mice typically take much longer to develop tumors compared to the other models described above. HGF-CDK4(R24C) mice, in which receptor tyrosine kinase signaling is deregulated by the overexpression of hepatocyte growth factor (HGF) and cell cycle control is impaired with an oncogenic CDK4(R24C) mutation, spontaneously develop invasive and metastatic melanoma. Using HGF-CDK4(R24C) mice with DMBA (carcinogen 7,12-dimethylbenzanthracene)-initiated primary melanoma, Bald et al. found ultraviolet-irradiation-induced inflammation promotes neutrophilmediated melanoma angiotropism and metastasis [48].
Zebrafish models
Taking advantage of transparent zebrafish embryos for in vivo imaging, zebrafish metastasis models are established by injecting tumor cells into the common cardinal vein of zebrafish embryos. Using embryos with brain-arrested cancer cells, the initial events of brain colonization such as extravasation and vessel co-option can be visualized. Using this model, Stoletov et al. showed that 4T1 murine mammary tumor cells and HMLE human mammary carcinoma cells co-opt brain blood vessels and that the connexin gap junction protein Cx43 is required for metastatic extravasation and brain colonization [51]. C8161 human cutaneous melanoma or OMM 2.3 human uveal melanoma cells also showed evidence of angiotropism when injected into the yolk sac of zebrafish embryos [52].
In vivo chick chorioallantoic membrane (CAM) assay and chicken embryo cancer metastasis assay
The chick chorioallantoic membrane (CAM) assay is an in vivo model that allows one to assess tumor cell growth and dissemination along blood vessels over time. A small hole is drilled on the top of a fertilized egg causing the CAM to detach from the shell membrane, and tumor cells mixed with Matrigel are inoculated onto the CAM. Tumor spreading and growth along blood vessels can be directly observed under the microscope. Using this method, Lugassy et al. found PC-3 human prostate carcinoma cells, B16F10 murine melanoma cells, and C8161 human melanoma cells spreading along the external surfaces of blood vessels in angiotropic patterns [53]. They also found that C16 laminin peptide increases angiotropic migration of C8161 human melanoma cells in a shell-less CAM assay [54]. The chicken embryo cancer metastasis assay involves the injection of tumor cells into the CAM vein, and after a few days of incubation, organs such as brain or liver are removed for imaging. Using this model, Stoletov et al. showed that 4T1 mammary tumor cells, HMLE mammary carcinoma cells, and B16 melanoma cells co-opt the vasculature, and they identified important roles for gap junction proteins (Cx43 and Cx26) during vessel co-option and brain colonization [51].
In vitro models
In vitro models are also suitable for studying the molecular and cellular mechanisms of blood vessel co-option by cancer cells. These models include ex vivo brain slice-tumor cell co-cultures, endothelial cell/tumor cell coculture, an ear tissue invasion assay, and the aortic ring assay.
Brain slice - tumor cell co-culture
Brain slice cultures have been widely used to model various cellular and molecular processes in the brain microenvironment. Brain slices are cut using a vibratome or tissue slicer, and then placed on top of cell culture inserts with porous membranes in a defined brain slice culture medium. After a short recovery incubation, cancer cells suspended in culture media are pipetted onto the surface of the brain slices. The dynamic interactions between cancer cells and brain blood vessels can be monitored in real-time using microscopy. The brain slice assay is an excellent model to study the mechanisms of perivascular localization and motility following metastatic seeding. Various tumor cell lines can be studied with this model, and it is straight-forward to evaluate vessel co-option following different drug treatments or genetic manipulations. However, the in vitro microenvironment and culture conditions in brain slices are vastly different from the brain microenvironment in situ, so any results found using this approach should be further verified using in vivo models. Carbonell et al. plated various murine and human tumor cells (4T1, MDA-MB-231, MDA231BR, A7, and K1735M2 cells) on mouse brain slices and found rapid spreading of cancer cells along the vasculature [36]. U373 and U87 human GBM cells seeded onto brain slices also rapidly co-opt pre-existing blood vessels [15]. Bradykinin appears to play an important role during the perivascular migration (and vessel enwrapping) of glioma cells as shown using D54 human GBM seeded onto brain slices [31]. To study the role of stroma-derived plasmin in the brain parenchyma during invasion, Valiente et al. plated lung adenocarcinoma (H2030 or H2030-BrM) and breast cancer (MDA231 or MDA231-BrM) cells on cultured brain slices and found that brain invasive BrM cells migrate towards and spread over brain capillaries, whereas low brain invasive parental cancer cells were killed by plasmin- and serpin-dependent mechanisms [35]. Lnc-BM (a long noncoding RNA for brain metastases) was shown to be important for vascular co-option in fresh brain slices as Lnc-BM knockdown cells could not co-opt vessels or invade into brain tissues [45]. To study the role of Wnt7 in glioma cell vessel co-option, Griveau et al. implanted Wnt7 wildtype or null murine glioma cells onto cultured brain slices, and found Olig2+ glioma cells with intact Wnt7 function showed single-cell co-option of blood vessels whereas Wnt7a/b null glioma cells failed to migrate along blood vessels. They also showed that inhibition of Wnt signaling prevents vessel co-option of murine Cdkn2a−/−;hEGFRvIII glioma progenitor cells and SF10417 human oligodendroglioma cells [33]. By placing D4M3A murine melanoma cells on live brain slices of Cdh5-CreERT2;ZSGreenloxp/stop/loxp immunocompetent mice, we found that D4M3A cells almost exclusively migrate along the pre-existing brain vasculature (Fig. 1b).
Endothelial cell - tumor cell co-cultures
The interaction between tumor cells and blood vessels can be studied in vitro by using cultured endothelial cells induced to form capillary-like structures. Time-lapse images can monitor the migration and localization of co-cultured tumor cells along the vasculature. This is a simple assay to establish, and it is straightforward to test the effect of different reagents or culture conditions on tumor cell spreading along endothelial cell-lined tubules. However, because these tubules are not perfused which differs from blood vessels in vivo, an in vivo model should be used to validate results. Using this in vitro model, studies showed that various tumor cells predominately spread along vascular tubes. For example, Lugassy et al. found B16F10 melanoma cells, GL26 glioma cells, and human melanoma cells occupying a pericyte-like location along the surface of cord-like structures formed by human microvascular endothelial cells (HMEC) [17]. Microarray analysis using C8161 human melanoma cells and HUVEC co-cultures showed that cancer cell-endothelial cell interactions induced differential expression of genes linked to cell migration, epithelial to mesenchymal transition, and pericyte recruitment including CCL2, ICAM1, IL6, and PDGFB [55]. By coculturing MDA-MB-231-Br cells with human brain endothelial cells (HBECs), human umbilical vein endothelial cells (HUVECs), or human dermal microvascular endothelial cells (HMECs), Wang et al. showed that tumor cells rapidly adhere to the endothelium with the strongest affinity for HBECs [45]. Fornabaio et al. co-cultured C8161 human cutaneous melanoma, OMM 2.3, and OMM 2.5 human uveal melanoma cells with HUVEC and demonstrated that each of these melanoma cell variants attached and spread along endothelial cell-lined tubules [52].
Ear tissue invasion assay
The ear tissue invasion assay is used to investigate melanoma-endothelial interactions in the skin ex vivo. In this model, mouse ventral ear sheets are prepared and placed in culture plates and melanoma cells are seeded in cloning rings on the ear dermal surface and allowed to migrate into the dermis. Using this assay, Bald et al. demonstrated that murine melanoma cells (HCmel12 and HCmel31) and human melanoma cells (MaMel15, MaMel65, MaMel48, and MZ7-MEL) migrate towards and closely associate with dermal blood vessels [48].
Aortic ring assay
Tumor-endothelial cell interactions can also be studied using co-cultures of tumor cells with mouse aortic ring explants. For example, Bald et al. showed that inflammation promotes murine melanoma cells (HCmel12) to migrate towards and spread along blood vessel surfaces using this assay [48].
Summary and perspective
Apart from angiogenesis-dependent growth, many cancer cells can co-opt the pre-existing vasculature to facilitate their growth and invasion. Moreover, blood vessel co-option (among other compensatory mechanisms) appears to underlie the poor performance of anti-angiogenic cancer therapies. Multiple studies have now demonstrated that many solid tumors can progress via vascular co-option and that blocking co-option alongside an anti-angiogenic therapy can more effectively suppress tumor growth [2, 13, 33]. In contrast to sprouting angiogenesis which has clear guidelines and methodologies for measurement, vessel co-option is more difficult to assess in vivo suggesting that blood vessel co-option by cancer cells could occur more frequently than has been considered [56]. Taken together, vessel co-option appears to be an important mechanism whereby cancer cells invade different organ microenvironments (surprisingly even in “liquid tumors”) [34], enter a state of dormancy, and evade anti-angiogenic strategies. Additional studies are therefore warranted to better understand the molecular mechanisms that guide the cohesive interactions between cancer cells and the vasculature.
Acknowledgements
ACD is supported by grants from the NIH (RO1-CA177874), the American Cancer Society (129755-RSG-16-176-01-DDC), and a pilot award from the Melanoma Research Alliance.
Footnotes
There are no conflicts of interest to disclose
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Donnem T, et al. , Non-angiogenic tumours and their influence on cancer biology. Nat Rev Cancer, 2018. 18(5): p. 323–336. [DOI] [PubMed] [Google Scholar]
- 2.Kuczynski EA, et al. , Vessel co-option in cancer. Nat Rev Clin Oncol, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Pezzella F, et al. , Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol, 1997. 151(5): p. 1417–23. [PMC free article] [PubMed] [Google Scholar]
- 4.Kadota K, et al. , Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol, 2015. 10(5): p. 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warth A, et al. , Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol, 2015. 39(6): p. 793–801. [DOI] [PubMed] [Google Scholar]
- 6.Bridgeman VL, et al. , Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol, 2017. 241(3): p. 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sardari Nia P, et al. , Distinct angiogenic and non-angiogenic growth patterns of lung metastases from renal cell carcinoma. Histopathology, 2007. 51(3): p. 354–61. [DOI] [PubMed] [Google Scholar]
- 8.Party BCPW, Evidence for novel non-angiogenic pathway in breast-cancer metastasis. Lancet, 2000. 355(9217): p. 1787–8. [PubMed] [Google Scholar]
- 9.Nakashima O, et al. , Pathomorphologic characteristics of small hepatocellular carcinoma: a special reference to small hepatocellular carcinoma with indistinct margins. Hepatology, 1995. 22(1): p. 101–5. [PubMed] [Google Scholar]
- 10.Nakashima T, et al. , Histologic growth pattern of hepatocellular carcinoma: relationship to orcein (hepatitis B surface antigen)-positive cells in cancer tissue. Hum Pathol, 1982. 13(6): p. 563–8. [DOI] [PubMed] [Google Scholar]
- 11.Kozaka K, et al. , A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: ‘bile ductular carcinoma’. Histopathology, 2007. 51(3): p. 390–400. [DOI] [PubMed] [Google Scholar]
- 12.Stessels F, et al. , Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer, 2004. 90(7): p. 1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frentzas S, et al. , Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med, 2016. 22(11): p. 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez Moro C, Bozoky B, and Gerling M, Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol, 2018. 5(1): p. e000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspani EM, et al. , Glioblastoma: a pathogenic crosstalk between tumor cells and pericytes. PLoS One, 2014. 9(7): p. e101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagano N, et al. , Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol, 1993. 86(2): p. 117–25. [DOI] [PubMed] [Google Scholar]
- 17.Lugassy C, et al. , Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol, 2002. 24(6): p. 473–8. [DOI] [PubMed] [Google Scholar]
- 18.Watkins S, et al. , Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun, 2014. 5: p. 4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernsen H, et al. , Gliomatosis cerebri: quantitative proof of vessel recruitment by cooptation instead of angiogenesis. J Neurosurg, 2005. 103(4): p. 702–6. [DOI] [PubMed] [Google Scholar]
- 20.Berghoff AS, et al. , Invasion patterns in brain metastases of solid cancers. Neuro Oncol, 2013. 15(12): p. 1664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung T, et al. , Angiotropism in primary cutaneous melanoma with brain metastasis: a study of 20 cases. Am J Dermatopathol, 2013. 35(6): p. 650–4. [DOI] [PubMed] [Google Scholar]
- 22.Siam L, et al. , The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget, 2015. 6(30): p. 29254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugassy C, et al. , Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron, 2014. 7(3): p. 139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusters B, et al. , Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res, 2002. 62(2): p. 341–5. [PubMed] [Google Scholar]
- 25.Bentolila LA, et al. , Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci Rep, 2016. 6: p. 23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kienast Y, et al. , Real-time imaging reveals the single steps of brain metastasis formation. Nat Med, 2010. 16(1): p. 116–22. [DOI] [PubMed] [Google Scholar]
- 27.Colpaert CG, et al. , Cutaneous breast cancer deposits show distinct growth patterns with different degrees of angiogenesis, hypoxia and fibrin deposition. Histopathology, 2003. 42(6): p. 530–40. [DOI] [PubMed] [Google Scholar]
- 28.Jeong HS, et al. , Investigation of the Lack of Angiogenesis in the Formation of Lymph Node Metastases. J Natl Cancer Inst, 2015. 107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naresh KN, Nerurkar AY, and Borges AM, Angiogenesis is redundant for tumour growth in lymph node metastases. Histopathology, 2001. 38(5): p. 466–70. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen PB, et al. , Lack of angiogenesis in lymph node metastases of carcinomas is growth pattern-dependent. Histopathology, 2002. 40(1): p. 105–7. [DOI] [PubMed] [Google Scholar]
- 31.Montana V and Sontheimer H, Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci, 2011. 31(13): p. 4858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav VN, et al. , CXCR4 increases in-vivo glioma perivascular invasion, and reduces radiation induced apoptosis: A genetic knockdown study. Oncotarget, 2016. 7(50): p. 83701–83719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griveau A, et al. , A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell, 2018. 33(5): p. 874–889 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao H, et al. , Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature, 2018. 560(7716): p. 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valiente M, et al. , Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 2014. 156(5): p. 1002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbonell WS, et al. , The vascular basement membrane as “soil” in brain metastasis. PLoS One, 2009. 4(6): p. e5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, et al. , Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene, 2005. 24(7): p. 1212–9. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein JL, et al. , Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia, 2000. 2(4): p. 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holash J, et al. , Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science, 1999. 284(5422): p. 1994–8. [DOI] [PubMed] [Google Scholar]
- 40.Kunkel P, et al. , Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res, 2001. 61(18): p. 6624–8. [PubMed] [Google Scholar]
- 41.Winkler F, et al. , Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia, 2009. 57(12): p. 1306–15. [DOI] [PubMed] [Google Scholar]
- 42.Voutouri C, et al. , Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc Natl Acad Sci U S A, 2019. 116(7): p. 2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins MH, et al. , Multiple murine BRaf(V600E) melanoma cell lines with sensitivity to PLX4032. Pigment Cell Melanoma Res, 2014. 27(3): p. 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Er EE, et al. , Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol, 2018. 20(8): p. 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, et al. , JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Invest, 2017. 127(12): p. 4498–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leenders WP, et al. , Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res, 2004. 10(18 Pt 1): p. 6222–30. [DOI] [PubMed] [Google Scholar]
- 47.Szabo V, et al. , Mechanism of tumour vascularization in experimental lung metastases. J Pathol, 2015. 235(3): p. 384–96. [DOI] [PubMed] [Google Scholar]
- 48.Bald T, et al. , Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature, 2014. 507(7490): p. 109–13. [DOI] [PubMed] [Google Scholar]
- 49.Kuczynski EA, et al. , Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst, 2016. 108(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bentolila NY, et al. , Intravital Imaging of Human Melanoma Cells in the Mouse Ear Skin by Two-Photon Excitation Microscopy. Methods Mol Biol, 2018. 1755: p. 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoletov K, et al. , Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci, 2013. 126(Pt 4): p. 904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fornabaio G, et al. , Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci Rep, 2018. 8(1): p. 10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lugassy C, et al. , Angiotropism of human melanoma: studies involving in transit and other cutaneous metastases and the chicken chorioallantoic membrane: implications for extra vascular melanoma invasion and metastasis. Am J Dermatopathol, 2006. 28(3): p. 187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lugassy C, et al. , C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br J Dermatol, 2007. 157(4): p. 780–2. [DOI] [PubMed] [Google Scholar]
- 55.Lugassy C, et al. , Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron, 2013. 6(1): p. 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowak-Sliwinska P, et al. , Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis, 2018. 21(3): p. 425–532. [DOI] [PMC free article] [PubMed] [Google Scholar]