Abstract
With better understanding of the role of type 2 inflammation in allergic asthma there has been progress made in the development of new biologic therapies targeting these specific pathways. This review will consider diagnostic criteria for using biologic therapies for pediatric asthma with special emphasis on populations that are likely to benefit the most from particular therapies. With the exception of the anti-IgE, omalizumab, very few studies have been published on efficacy and safety of biologic therapies in children, particularly anti-IL5 and anti-IL4/IL13 therapies. The review will highlight the scarcity of published data in pediatric specific populations. In addition, we will consider the cost effectiveness as well as potential long-term consequences of biologic therapies in pediatric asthma.
Keywords: monoclonal antibodies, childhood asthma, T-helper 2 asthma, anti-IgE therapy, anti-eosinophil therapy
Introduction
Over the last few decades there has been a paradigm shift in the approach to characterizing the multifaceted, chronic disorder known as asthma. Attempts have been made to classify asthma into phenotypes and endotypes in an effort to group individuals with common features, pathophysiology and treatment approaches1. Perhaps one of the most clearly delineated phenotypes of asthma is the allergic asthma phenotype that is often characterized by elevated T-helper 2 (TH2) cytokines and mediators2. In parallel there has also been an increase in therapeutic options for TH2 high asthma in the form of biologic therapies.
Biologic therapies are treatments that have been created from living animals, plants, or cells as opposed to chemical processes. Monoclonal antibodies are produced from an identical immune cell that is a clone of a parent cell. Monoclonal antibodies are derived from mouse, humans, or humanized antibodies that originate predominately from human sources with the exception of protein-binding regions. All current biologic therapies that are used for the treatment of asthma are either humanized monoclonal antibodies or full human monoclonal antibodies (Dupilumab), thus the terms biologic therapies and monoclonal antibodies are often used interchangeably when referring to this class of asthma medications.
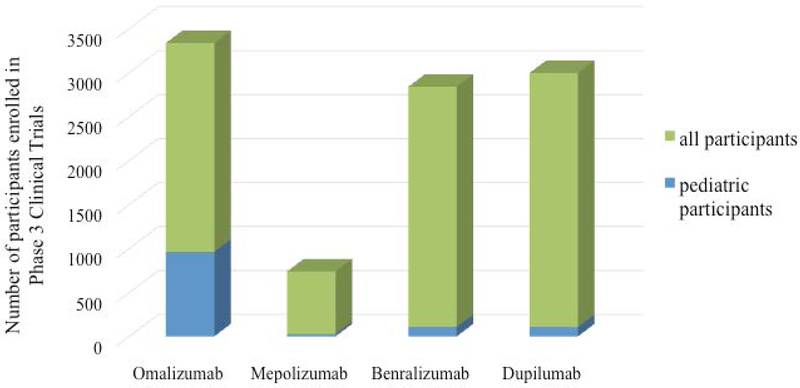
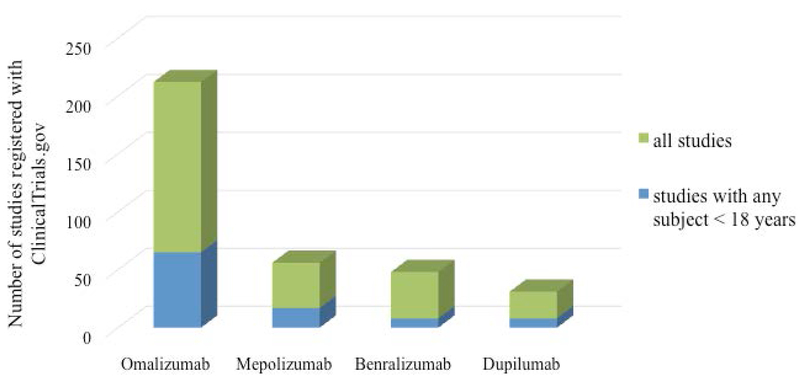
In this review we will discuss the biologic therapies that are currently available for the management of pediatric asthma (children less than 18 years), particularly difficult to treat asthma. It is important to note, that the evidence of safety and efficacy of biologic therapies in children is rather sparse. The number of pediatric patients enrolled in the phase 3 clinical trials of currently approved biologic therapies is quite low (Figure 1). Currently there are very few registered clinical trials of biologic asthma therapies that have enrolled participants younger than 18 years of age (Figure 2). Furthermore, studies that specifically target high-risk subpopulations of children with asthma including minority children and children from low-income families are rare. Thus, future studies are necessary to further understand the benefits and risks in a broad range of children with severe asthma.
Figure 1:
Evidence of safety and efficacy of biologic therapies in pediatric asthma is sparse. The green bars represent the total number of participants enrolled in Phase 3 clinical trials for each respective therapeutic and the blue bars represent the number of children age 12-17 years (age 6-12 years for Omalizumab) that were enrolled.
Figure 2:
Enrollment of children in studies of biologic therapies in pediatric asthma is sparse. Table represents the total number of clinical trials currently registered on ClinicalTrials.gov for each of the biologic asthma therapies that are currently approved for use in children. All studies are represented by green bars and studies that have proposed to enroll children < 18 years are in blue.
This review will consider diagnostic criteria for using biologic therapies for pediatric asthma with special emphasis on populations that are likely to benefit the most from particular therapies. In addition, we will consider the cost effectiveness as well as potential long-term consequences of biologic therapies in asthma. Immunologists have largely driven the management of monoclonal antibody therapy, particularly among children with asthma. However pulmonologist often have a large referral base for difficult to manage pediatric asthma. Thus, it is critical that we equip ourselves to manage the range of therapies available to our pediatric patients.
Overview of TH2 predominate asthma pathways
Allergic asthma is thought to be a disease of airway inflammation that is triggered by a variety of complex immunologic pathways stemming from the introduction of aeroallergens, viruses and pollutant particles that come in contact with antigen presenting cells, specifically, dendritic cells. Dendritic cells mobilize to local lymph nodes where they activate naïve T helper cells, stimulating them to differentiate into TH1 cells, TH17 cells or TH2 cells. Differentiation of naïve T cells into TH2 cells initiates the eosinophilic or type 2 inflammatory cascade. In lymph nodes TH2 cells secrete IL4 and mediate class-switching leading B cells to increase production of immunoglobulin E (IgE)3. IgE then binds to effector cells including mast cells, basophils and eosinophils that in the presence of allergens, trigger the release of histamine, leukotrienes, and prostaglandins which promote vascular permeability and smooth muscle contractility. In the airway epithelium TH2 cells secrete IL5 and IL13. IL5 promotes maturation and migration of eosinophils that trigger airway inflammation in response to allergens. IL13 induces secretion of mucin from goblet cells and alters airway smooth muscle leading to airway hyperreactivity3, 4. Deeper understanding of the mechanisms underlying these pathways is continually evolving; however, the current knowledge is the basis for recent biologic asthma therapy targets.
Anti IgE therapy
Omalizumab, an anti-IgE monoclonal antibody, was first approved by the United States Federal Drug Administration (US FDA) in 2003 and the European Medicines Agency (EMA) in 2005 for use in children 12 years and above. More recently in 2016, the US FDA approved the use of omalizumab in children as young as 6 years of age.
One of the mechanisms of action of omalizumab is the ability to cross-link with free IgE to decrease the quantity of IgE available to bind to cell surfaces5. In addition, omalizumab decreases the expression of the FCεRI or high affinity receptor on the surface of cells that normally bind IgE including mast cells, basophils, and eosinophils. As a result the inflammatory cells do not release mediators such as leukotrienes, prostaglandins, and histamines that are part of the allergic inflammatory cascade leading to downstream asthma symptoms.
Currently, omalizumab is indicated for use in children with moderate to severe persistent allergic asthma. Children should demonstrate inadequate control of asthma symptoms on inhaled glucocorticoids. Allergic asthma is demonstrated by sensitivity to perennial aeroallergens such as dust mite, animal dander, cockroaches or molds. Serum IgE should be in the range of 30 – 700 in children 12 or above.The upper limit of IgE level is expanded to 1,300 in US children age 6-11 and as high as 1,500 for children in Europe. A complex dosing algorithm has been developed based on total serum IgE level and the weight of the child6. However, currently, there is insufficient evidence to suggest a dose for younger overweight and obese children. In addition to injection site reactions, the most common side effect of omalizumab are included in Table 1.
Table 1:
Key points for a clinicians to consider in prescribing therapies to children
| Treatment | Target of Action | Age in Years | Indications for Treatment in Severe Asthma | Mode of Delivery | Loading Dose |
Maintenance Dose | Most Common Side Effects |
|---|---|---|---|---|---|---|---|
| Omalizumab | Ant-IgE | ≥6 | Perennial allergies and serum total IgE 30-1300 IU/mL1 | SC | none | 75 - 375 mg every 2-4 weeks2 | ≥12 years: arthralgia, general, leg and arm pain, fatigue, dizziness ≥6 years: nasopharyngitis, headache, pyrexia, upper abdominal pain, pharyngitis, otitis media, gastroenteritis, epistaxis |
| Mepolizumab | Anti-IL5 | ≥6 | Serum eosinophil >150/μL | SC | none | 100 mg every 4 weeks | Headache, injection site reaction, back pain, fatigue |
| Benralizumab | Anti-IL5rα | ≥12 | Serum eosinophil >300/µL | SC | 30 mg every 4 weeks for 1st 3 doses | 30 mg every 8 weeks | Headache, pharyngitis |
| Dupilumab | Anti IL4 and IL13 | ≥12 | Serum eosinophil >150/µL | SC | 400 mg (two 200 mg injections)3 | 200 mg every 2 weeks | Injection site reaction, oropharyngeal pain, and eosinophilia |
Total IgE 30–1500 in Europe.
Based on serum total IgE level and body weight.
For patients with oral corticosteroid-dependent asthma or with co-morbid moderate-severe atopic dermatitis start with initial dose of 600 mg (two 300mg injections) followed by 300 mg every other week.
SC: subcutaneous
To date the largest body of literature regarding efficacy and safety of biologic therapies in children exists for omalizumab. Several randomized double-blind placebo controlled trials have been conducted in children age 6-12 years7–15. The documented benefits of omalizumab include reduction in rate of exacerbation7, 9–13, 16 and inhaled corticosteroids dose7, 9, 10, 16, as well as improvement in symptoms8, 13, quality of life8, 15, forced expiratory volume in 1 sec (FEV1)15, 16 and fractional exhaled nitric oxide (FeNO)10.
Anti IL5 Therapies
The anti-IL5 therapies, mepolizumab and benralizumab, were approved for use in children as young as 12 years of age in 2015 and 2017 respectively. In 2019 the FDA approved mepolizumab for use in children as young as 6 years of age. Reslizumab is another anti-IL5 therapy that is FDA approved for the management of allergic asthma; however, currently it is only approved for patients 18 years and older. Therefore, a discussion of reslizumab is outside the scope of this review. Mepolizumab is an anti-IL5 antibody that binds directly to free IL5. In doing so, the mepolizumab antibody prevents interaction of IL5 with receptors leading to reduced production and survival of eosinophils. Benralizumab is an anti-IL5 receptor antibody that blocks IL5 from binding to its receptor and results in cytotoxic depletion of cells that express IL5 receptors. Dosing information for mepolizumab and benralizumab as well as the most common side effects can be found in Table 117, 18.
In a recent retrospective analysis of two large clinical trials of mepolizumab, Ortega and colleagues examined the predicted rate of clinically significant exacerbations per year against baseline blood eosinophil counts to determine the serum eosinophil level that corresponded with the most benefit from treatment19. In the DREAM study, participants that had higher baseline serum eosinophil counts had greater benefit with mepolizumab compared to controls (30% difference at 150 eosinophils/μL)20. Similarly in the MENSA study, there was greater divergence in the predicted rate of exacerbation between the treatment and placebo groups with higher baseline eosinophil counts (39% difference 150 eosinophils/μL)21. Therefore, the serum eosinophil count of 150/μL has been used as a threshold for treatment with mepolizumab with those at higher baseline blood eosinophil counts demonstrating the greatest benefits. Importantly, of the 616 participants enrolled in the DREAM study and 576 participants in the MENSA study only 26 total were children between ages 12-18 years (Figure 1).
In a secondary data analysis using pooled data from the SIROCCO22 and CALIMA23 trials, Bleeker and colleagues reviewed the baseline clinical and demographic characteristics that impacted clinical efficacy of benralizumab for severe asthma24. Overall there was a 36% rate reduction in annual exacerbation rate among participants treated with benralizumab compared to control. However, when data were stratified by various baseline clinical factors, a greater benefit of benralizumab compared to control was observed among individuals that were on oral corticosteroids, had nasal polyps, had lower pre bronchodilator forced vital capacity (FVC), had greater than 3 exacerbations per year and were older than 18 years at time of asthma diagnoses. Of the 2,510 participants across these two studies, 108 were children between 12-18 years of age (Figure 1).
Anti IL4 and IL13
Dupilumab is an anti-IL4 receptor alpha antibody. Both IL4 and IL13 express the IL4 receptor alpha subunit. Thus, dupilimab blocks both IL4 and IL13 from binding to their receptors, affecting B cell class-switching as well as IL13 mediated airway inflammation. Common side effects of dupilumab are included in Table 1.
In a randomized, double-blind, placebo-controlled trial, participants treated with dupilimab compared to placebo demonstrated significant reduction in rate of severe asthma exacerbation25. This association was only observed among those with serum eosinophil counts ≥ 150 cells/μL. Furthermore, individuals with higher eosinophil counts, ≥ 500 cells/μL, had even greater benefit compared to individuals with eosinophil counts of 150-300 cells/μL26. Similar findings were observed with forced expiratory volume in 1 sec (FEV1) such that individuals treated with dupilumab that had higher baseline eosinophil counts had greater improvement in FEV1 compared to individuals with lower baseline eosinophil counts. Of all the 1,338 participants enrolled in phase 3 clinical trials of dupilumab a total of 107 were children between the ages of 12-18 years (Figure 1).
Cost effectiveness
In order to fully understand the implications of management with biologic therapies it is necessary to consider the high cost of treatment. A 2018 report from the Institute for Clinical and Economic Review (ICER) indicated that the annual cost of biologic therapies for management of asthma, excluding administrative costs, ranged from $27,800 to $31,000 per year27. In a cost effective analysis, biologic therapies were compared to standard of care in asthma. The ICER cost effectiveness model used a commonly accepted threshold ratio of $100,000 - $150,000 for each quality-adjusted life-years (QALY) gained while on treatment. For each of the biologic therapies that were examined the cost effectiveness ratios far exceeded the threshold, ranging from $325,000 - $391,000. Thus, in order to be considered cost effective the prices of biologic therapies would have to be reduced by 62-80% of their current wholesale acquisition prices27, 28. In sensitivity analyses cost effectiveness ratios were improved when analyses were restricted to individuals treated chronically with oral corticosteroids (down to $174,000) and among long-term responders to therapy (down to $156,000).
Earlier studies of the cost-effectiveness of omalizumab revealed mixed results that vary based on the asthma outcomes that were assessed and the severity of the population under investigation29–32. Based on some of the earlier studies, omalizumab may be considered cost-effective in very high risk individuals with frequent exacerbations and poorly controlled symptoms despite maximum inhaled corticosteroid29, 30, 32. Therefore, in an environment of rising health care costs, careful selection of patients that will most likely benefit from biologic therapies is one critical aspect towards achieving cost effectives.
Determining the best candidate and setting for specific therapies
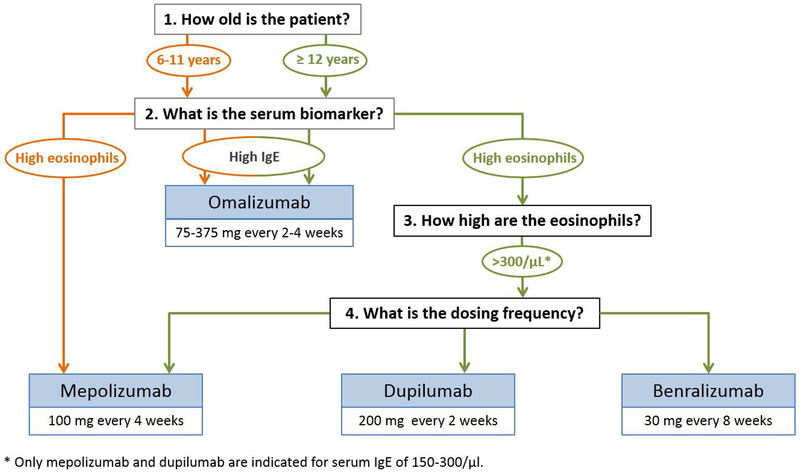
The Global Initiative for Asthma (GINA) recently published a pocket guide for difficult to treat asthma33. Relevant to this current review is the consideration of factors that may predict a good response to anti-IgE versus anti-IL5 therapies. In particular, although elevated IgE is the indicator for treatment with omalizumab, individuals with elevated blood eosinophils are more likely to respond to therapy compared to those with lower blood eosinophil counts. In addition, elevated FeNO (a biomarker of airway inflammation), allergen driven symptoms and childhood onset of asthma are all factors associated with likely improvement on treatment with omalizumab. Regarding anti-IL5 therapies, in addition to improved benefit among those with higher peripheral blood eosinophils, patients that have more frequent exacerbations, adult onset asthma, and nasal polyposis are more likely to benefit according to the GINA guidelines. Figure 3 offers considerations for selecting a biologic therapy in children younger than 18 years.
Figure 3:
Considerations for selecting a biologic therapy in children younger than 18 years of age. Orange lines represent options for children 6-11 years and green lines represent options for children ≥ 12 years.
Determining when to abort therapy is equally as important as determining the appropriate candidate for biologic asthma therapy. The GINA guidelines recommend switching to a different biologic therapy if there is no significant response after 4 months of treatment. Additionally, patients should be reevaluated every 3-6 months for improvement and oral corticosteroids should be discontinued first once adequate symptoms control has been achieved. Biologic therapies should be used as add on therapies, thus patients should be maintained on at least a moderate dose inhaled corticosteroid.33
Along with targeting treatment to patients that may benefit most from biologic therapies, limiting the timing of treatment to peak exacerbation seasons is another approach towards reducing overall costs. In a multi-center study within the Inner City Asthma Consortium, 419 children age 6-20 years with moderate to severe asthma were randomized to omalizumab versus placebo for 60 weeks.13 In addition to a significant reduction in number of days with asthma symptoms and frequency of exacerbations, investigators identified a greater benefit of omalizumab compared to placebo during the fall exacerbation months when viral triggered asthma exacerbations peak. This finding motivated a follow up study to investigate if short-term targeted therapy initiated 4-6 weeks prior to the start of school, for a total of 4 months, could reduce fall asthma exacerbations.14 Indeed, there was a significant improvement in rate of exacerbation among the children randomized to omalizumab compared to control. In a subset of patients that were treated with omalizumab, peripheral blood mononuclear cells and dendritic cells were isolated and stimulated ex vivo with rhinovirus in the presence or absence of IgE cross-linking34. Interestingly, decreased expression of the high affinity FCεRI, as seen with omalizumab treatment, was associated with increased secretion of interferon (INF) α from dendritic cells. Thus, one of the underlying mechanisms that explains a reduction in fall, viral induced exacerbations is the increased IFNα response to viruses.
Side effects and long-term consequences
The most common side effects of each of the biologic therapies have been listed in Table 1.. Anaphylaxis is one of the most concerning side effects observed with omalizumab and very rarely with the other biologics35–38. Lieberman and colleagues published the largest review to date of post-marketing report of anaphylaxis associated with omalizumab administration that included 132 cases39. Although late symptoms of anaphylaxis have been reported up to 24 hours following administration, the majority of events (64%) occurred within the first 60 minutes. Those patients that were most likely to develop anaphylaxis were female, mean age of 40 and had a prior episode of anaphylaxis. Ninety-five percent of patients that experienced anaphylaxis had respiratory symptoms. Thus, it is prudent to monitor for at least 60 minutes post-injection, particularly in pediatric patients that have had a history of anaphylaxis.
Regarding long-term side effects of biologics, there is a paucity of published data among the newer therapies, mepolizumab, benralizumab, and dupilumab. One of the greatest concerns has been the long-term risk of malignancy. The EXCELS trial was a prospective observational cohort study of patients 12 and above with moderate-to-severe allergic asthma that were treated with omalizumab40. Over the 5-year study period, there was no significant difference in the proportion of patients that developed primary malignancy comparing individuals treated with omalizumab (n=5,007) versus controls (n=2,829). Similarly, there was no significant difference in incidence of other organ system disease among omalizumab treated versus controls.
Conclusions
Data is sparse regarding the efficacy and safety of biologic therapies in pediatric patients younger than 18 years of age, specifically high-risk populations that include minority children and children from low income communities. Biologic therapies are also very expensive and at their current prices are not cost effective. Additionally, little is known about optimum duration for treatment in children and the potential long term side effects into adulthood, especially with newer biologic therapies. Therefore, careful consideration should be taken when deciding which patients should be initiated on biologic therapies and the potential for modifying the timing of treatment (e.g. fall exacerbation period). Nonetheless, the application into practice of currently approved biologics and future therapies that are in the pipeline is exciting. Forthcoming observational and experimental studies focused on children younger than 18 years of age will be valuable as we expand the use of these novel therapeutics in pediatric populations.
References
- 1.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 5:716–25. [DOI] [PubMed] [Google Scholar]
- 2.Schatz M and Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014; 6:645–8; quiz 49. [DOI] [PubMed] [Google Scholar]
- 3.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015; 1:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locksley RM. Asthma and allergic inflammation. Cell. 2010; 6:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holgate ST and Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008; 3:218–30. [DOI] [PubMed] [Google Scholar]
- 6.Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W and Lee IM. Dose Response Between Physical Activity and Risk of Coronary Heart Disease A Meta-Analysis. Circulation. 2011; 7:789–U84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF and Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001; 2:E36. [DOI] [PubMed] [Google Scholar]
- 8.Lemanske RF Jr., Nayak A, McAlary M, Everhard F, Fowler-Taylor A and Gupta N. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics. 2002; 5:e55. [DOI] [PubMed] [Google Scholar]
- 9.Berger W, Gupta N, McAlary M and Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003; 2:182–8. [DOI] [PubMed] [Google Scholar]
- 10.Silkoff PE, Romero FA, Gupta N, Townley RG and Milgrom H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics. 2004; 4:e308–12. [DOI] [PubMed] [Google Scholar]
- 11.Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I and Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009; 6:1210–6. [DOI] [PubMed] [Google Scholar]
- 12.Kulus M, Hebert J, Garcia E, Fowler Taylor A, Fernandez Vidaurre C and Blogg M. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Curr Med Res Opin. 2010; 6:1285–93. [DOI] [PubMed] [Google Scholar]
- 13.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011; 11:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr., Calatroni A, Wildfire JJ, Gergen PJ, Cohen RT, Pongracic JA, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015; 6:1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodlie M, McKean MC, Moss S and Spencer DA. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch Dis Child. 2012; 7:604–9. [DOI] [PubMed] [Google Scholar]
- 16.Deschildre A, Marguet C, Salleron J, Pin I, Rittie JL, Derelle J, Taam RA, Fayon M, Brouard J, Dubus JC, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013; 5:1224–33. [DOI] [PubMed] [Google Scholar]
- 17.Rivas I, Viana M, Moreno T, Pandolfi M, Amato F, Reche C, Bouso L, Alvarez-Pedrerol M, Alastuey A, Sunyer J, et al. Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environment international. 2014; 200–12. [DOI] [PubMed] [Google Scholar]
- 18.Chung MK, Lao TT, Ting YH, Wong TW and Leung TY. Seasonality of fetal trisomy 21--have ambient air pollutants played a role? J Matern Fetal Neonatal Med. 2015; 5:552–7. [DOI] [PubMed] [Google Scholar]
- 19.Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, Brightling CE and Pavord ID. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016; 7:549–56. [DOI] [PubMed] [Google Scholar]
- 20.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H and Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 9842:651–9. [DOI] [PubMed] [Google Scholar]
- 21.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID and Investigators S. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 13:1189–97. [DOI] [PubMed] [Google Scholar]
- 22.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, Sproule S, Gilmartin G, Aurivillius M, Werkstrom V, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016; 10056:2115–27. [DOI] [PubMed] [Google Scholar]
- 23.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016; 10056:2128–41. [DOI] [PubMed] [Google Scholar]
- 24.Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, Goldman M, Newbold P and Zangrilli JG. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018; 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018; 26:2486–96. [DOI] [PubMed] [Google Scholar]
- 26.Chung MK, Lao TT, Ting YH, Wong TW and Leung TY. Seasonality of fetal trisomy 21-have ambient air pollutants played a role? J Matern-Fetal Neo M. 2015; 5:552–57. [DOI] [PubMed] [Google Scholar]
- 27.James P, Jankowska M, Marx C, Hart JE, Berrigan D, Kerr J, Hurvitz PM, Hipp JA and Laden F. “Spatial Energetics” Integrating Data From GPS, Accelerometry, and GIS to Address Obesity and Inactivity. American Journal of Preventive Medicine. 2016; 5:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson WC 3rd and Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: To biologic or not to biologic? Ann Allergy Asthma Immunol. 2019; 4:367–72. [DOI] [PubMed] [Google Scholar]
- 29.Brown R, Turk F, Dale P and Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007; 2:149–53. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan SD and Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008; 6:670–84. [DOI] [PubMed] [Google Scholar]
- 31.Wu AC, Paltiel AD, Kuntz KM, Weiss ST and Fuhlbrigge AL. Cost-effectiveness of omalizumab in adults with severe asthma: Results from the asthma policy model. J Allergy Clin Immun. 2007; 5:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oba Y and Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immun. 2004; 2:265–69. [DOI] [PubMed] [Google Scholar]
- 33.Ross Z, Ito K, Johnson S, Yee M, Pezeshki G, Clougherty JE, Savitz D and Matte T. Spatial and temporal estimation of air pollutants in New York City: exposure assignment for use in a birth outcomes study. Environ Health-Glob. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, Gern JE, Togias A and Busse WW. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018; 5:1735–43 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genentec. Omalizumab (xolair) [package insert]. website. https://www.gene.com/download/pdf/xolair_prescribing.pdf. Revised May 2019 Accessed November 6, 2019.
- 36.GlaksoSmithKline LLC. Mepolizumab (nucala) [package insert]. website. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF. Revised September 2019 Accessed November 6, 2019.
- 37.Regereron Pharmaceuticals, Inc. Dupilumab (dupixent). website. https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf. Revised June 2019 Accessed November 6, 2019.
- 38.AstraZeneca Pharmaceuticals LP. Benralizumab (fasenra) [package insert]. website. https://www.azpicentral.com/fasenra/fasenra.pdf. Revised October 2019 Accessed November 6, 2019.
- 39.Lieberman PL, Jones I, Rajwanshi R, Rosen K and Umetsu DT. Anaphylaxis associated with omalizumab administration: Risk factors and patient characteristics. J Allergy Clin Immunol. 2017; 6:1734–36 e4. [DOI] [PubMed] [Google Scholar]
- 40.Long A, Rahmaoui A, Rothman KJ, Guinan E, Eisner M, Bradley MS, Iribarren C, Chen H, Carrigan G, Rosen K, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014; 3:560–67 e4. [DOI] [PubMed] [Google Scholar]