Abstract
We examined the relationship between intrauterine dichlorodiphenyltrichloroethane (DDT) exposure (o,p’-DDT, p,p’-DDT, and p,p’-DDE) and mammographic breast density (MBD) in midlife, one of the strongest risk factors for breast cancer. We focused our analyses on o,p’-DDT exposure given our previous report of a positive association between intrauterine o,p’-DDT exposure and daughter’s breast cancer (BC) risk. Here we estimated associations of intrauterine serum DDTs with MBD in 224 daughters of women in the Child Health and Development Studies pregnancy cohort whose mothers did not develop BC (MBCa−) and 156 daughters whose mothers did develop BC (MBCa+). In MBCa+ daughters, highest relative to lowest quartile of o,p’-DDT exposure was associated with a 17-unit higher dense area (95% CI=2.6-31.2; Ptrend=0.01). We did not observe an association between o,p’-DDT and density measures in MBCa− daughters. MBD, an intermediate marker of BC risk, may be affected by intrauterine DDT exposures; MBCa status may modify the association.
Keywords: DDT organochlorines; in utero exposure; mammographic breast density; percent density; dense area, nondense area; child health and development studies
Introduction
Studies examining intrauterine environmental exposures and breast cancer risk are challenged by the long induction period between exposure and breast cancer development. One strategy to address this challenge is to examine the relationship between exposures and intermediate markers of breast cancer risk. Given our previous finding that intrauterine DDT exposure was associated with breast cancer risk in daughters,1 here we examine if intrauterine DDT exposure is associated with mammographic breast density (MBD), an intermediate marker of breast cancer.
Despite organochlorines being labeled as a probable carcinogen2 and studied as a possible endocrine disruptor in animal studies,3,4 evidence from epidemiological studies have largely been inconsistent. A recent comprehensive review by the Silent Spring Institute suggest that the inconsistency may be attributed to the majority of studies collecting exposure information outside of biologically relevant exposure periods.5 Organochlorine exposure during key periods of breast development, when the breast tissue is more susceptible to carcinogenesis, has been associated with breast cancer risk.1,6–8 Given that DDT exposure may affect breast tissue development and that MBD is an intermediate marker that captures breast tissue composition; in this study we include MBD as an alternative strategy to examine early life exposures and breast cancer risk. We also examine if there are differences by underlying familial risk inferred by maternal breast cancer family history.
MBD is considered an intermediate marker for breast cancer - having mostly dense breasts is associated with nearly a 4-fold increase in breast cancer as compared with having mostly fatty breasts.9 MBD measures breast composition, providing the amount of dense (fibroglandular) breast tissue as visualized on a mammogram. This issue of Reproductive Toxicology provides the first set of studies to examine the role of intrauterine toxic exposures and MBD. For the present study, we examined intrauterine exposure to dichlorodiphenyltrichloroethane (DDT) compounds (o,p’-DDT, p,p’-DDT, and p,p’-DDE) and daughters’ MBD using the Child Health and Development Studies (CHDS) pregnancy cohort. The CHDS enrolled women who were pregnant between 1959-1967 to examine the association between prenatal exposures and health and development in mothers and offspring.10–12 The CHDS is one of the only long-term cohorts with blood samples drawn at the time when DDT was heavily used within the United States.
Materials & Methods
Study Population
Subjects were recruited from the Child Health and Development Studies (CHDS), a longitudinal pregnancy cohort designed to examine the associations between the prenatal environment and health over the lifecourse in mothers and daughters. Pregnant women were recruited from Oakland, CA and were members of the Kaiser Permanente Health Plan seeking obstetric care between 1959 and 1967.13 The time period that CHDS enrolled pregnant women coincides with a period of time when DDT was most heavily used.14 Participation in CHDS was voluntary and recruited women provided oral informed consent for an in-person interview, blood specimen collection throughout the pregnancy, including early postpartum (1-3 days), and permission for access to their own and their children’s medical record. Daughters born into the CHDS are the subjects of this study.
Beginning in 2010, CHDS daughters who had participated in the “Three Generations of Breast Cancer (3Gs)” or the Health Disparities (DISPAR) study, in adulthood at ages 44-54, were recruited for the Prenatal Environmental Determinants of Intergenerational Risk (PEDIGREE) study (Supplementary Figure 1). Details of the 3Gs and DISPAR study recruitment strategies and response rates are described elsewhere.10,12,15 Both prior studies achieved a response rate of 60% or higher among eligible, phone-locatable subjects. In order to be eligible for the current PEDIGREE study, women had to have participated in the telephone interview phase of the 3Gs or DISPAR study, and had to have completed a home visit or provided a biospecimen, and had to have had a mammogram, or be scheduled for one within a year of the study interview. Among the eligible women who met these criteria, in order to accommodate stratification of density associations by first degree family history, daughters were targeted for PEDIGREE recruitment according to maternal breast cancer history in approximately equal proportion: daughters whose mothers had been diagnosed with breast cancer, n=231; and, daughters whose mothers were not known to have been diagnosed with breast cancer at the time of recruitment, n=281. Recruitment targets were bounded by budget required for collection and digitizing of mammograms. From the daughters targeted for recruitment (n=512), authorization to collect mammography was received from 491 (96%) and mammograms were successfully collected for 397 (81%). For our analysis, we used the mammogram that was closest to the interview date. The institutional review boards at Columbia University and the Public Health Institute approved PEDIGREE, a nested cohort study. This study is in compliance with all federal guidelines governing studies of human participants.
Data Collection
At the CHDS baseline, pregnant women were interviewed in-person to collect information on age at the time of pregnancy and pre-pregnancy body mass index (BMI). BMI at the first prenatal visit and pregnancy weight gain were recorded from mothers’ medical records. Levels of intrauterine DDTs, triglycerides, and cholesterol were measured in 1-3 days postpartum or in earlier trimester samples. We collected information from the adult daughters who were the offspring of the CHDS pregnancies, during telephone interviews between 2010 and 2013.10,12 Daughters reported on height, current weight, race/ethnicity, and reproductive characteristics including age at menarche, age at first birth, parity, and exogenous hormone use. History of maternal breast cancer was captured from linkage to the California Cancer Registry (CCR), which has been conducted on an annual basis for the last six decades for all CHDS cohort members. California hospitals and health care facilities that provide treatment to cancer patients are required by law to report cancer diagnoses to the CCR.16 The CCR has established that its cancer coverage is more than 99% complete after a lag time of about 2 years.17 Life table analyses using SEER rates to estimate expected numbers of breast cancer diagnoses in CHDS mothers show close comparability with observed cases identified through CCR linkage (unpublished). CHDS surveillance efforts routinely identify >90% of all CHDS mothers.18
Mammographic Breast Density
The PEDIGREE MBD study was nested within the larger cohort study. If the adult daughters responded in the telephone interview that they had or were planning on having a mammogram, we then asked them to report on the facility where they had or planned to have their mammogram. We collected consents to procure mammograms for density assessments from 494 women. We were unable to retrieve mammograms for 19 participants, 9 mammograms were of poor quality, and 69 participants had no mammograms available. Of the remaining 80% of participants (N=397), 17 women were missing organochlorine exposure data; thus, the analyses were restricted to 380 daughter participants. Of these 380, there were 34 sister sets: 33 sets consisted of two sisters and one set included three sisters, and the remainder (N=311) were unrelated.
We assessed mammographic density using a computer assisted method with Cumulus, a thresholding program.19 We measured total breast area, total dense area (cm2), total non-dense area, and percent density (dense area divided by breast area multiplied by 100). All cranio-caudal (CC) films that were available for a participant were read by a trained technician (co-author NE) in one batch and all sibling-sets were read within the same batch. We read mammograms in batches of approximately 50 and 10% of the films had repeated readings from the same batch. We repeated an additional 10% of films in every batch to estimate batch-to-batch variability. The overall within-batch correlation coefficient was 0.93 for percent density and the intraclass correlation coefficient for between-batch reliability was 0.92.
Laboratory Assays
Our study included exposure data for the following organochlorines: p,p’-dichlorodiphenyltrichloroethane (p,p’-DDT), o,p’-DDT, and p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE). Details on the laboratory assays for organochlorine DDT-related compounds (p,p’-DDT, o,p’-DDT, p,p’-DDE) and serum lipids can be found in a previous publication.1 In brief, in 2014 we measured DDTs and serum lipids in perinatal maternal non-fasting blood that was collected between 1959 and 1967. We preferred to use postpartum blood samples that were taken 1 to 3 days after delivery; however, when unavailable, we used prior trimester samples.1 Studies have shown that the concentrations of organochlorines throughout pregnancy and soon after delivery are consistent.20 Frozen serum samples were shipped to the laboratory of the California Department of Toxic Substances Control where they were assayed for DDTs using previously developed methods. Moreover, given we observed a full range of concentration values for the DDT compounds, as opposed to narrow low ranges that would be expected for denatured samples, we do not expect long term storage impacted the measurement of these compounds nor our final analysis. Details of this protocol can be found in prior publications.1,21
Serum lipids, total cholesterol and triglycerides, were measured enzymatically on a Roche P Modular system using reagents and calibrations from Roche Diagnostics at the Clinical and Epidemiologic Research Laboratory at Boston’s Children’s Hospital, which is certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program. Detailed methods for the measurement of cholesterol and triglycerides are in previous publications.1
Statistical Analyses
We examined the distribution of intrauterine measures and daughter variables by mother and daughter characteristics by mother breast cancer status (MBCa) (Table 1). We performed spearman correlations between the DDT compounds and found strong correlations ranging from 0.63-0.71 (P<0.0001).
Table 1.
Intrauterine exposures and daughter variables by maternal breast cancer status, PEDIGREE
| Maternal Breast Cancer Negative (N=224) | Maternal Breast Cancer Positive (N=156) | |||||||
|---|---|---|---|---|---|---|---|---|
| Percentiles | Missing Values | Percentiles | Missing Values | |||||
| 25% | 50% | 75% | N (%) | 25% | 50% | 75% | N (%) | |
| Intrauterine Exposures1 | ||||||||
| p,p’-DDT (ng/ml) | 6.51 | 10.2 | 15.66 | n/a | 7.66 | 11.51 | 18.21 | n/a |
| o,p’-DDT (ng/ml)* | 0.21 | 0.39 | .62 | n/a | 0.29 | 0.50 | 0.76 | n/a |
| p,p’-DDE (ng/ml) | 30.00 | 45.00 | 56.72 | n/a | 33.3 | 48.51 | 60.45 | n/a |
| Age at birth (years) | 23 | 27 | 31 | n/a | 23 | 28 | 34 | 1 (0.45) |
| BMI Before Pregnancy (kg/m2) | 20.66 | 22.42 | 24.19 | 13 (5.80) | 20.09 | 21.90 | 24.59 | 9 (4.02) |
| Pregnancy Weight Gain (kg) | 16.90 | 21.05 | 25.10 | 6 (2.68) | 15.68 | 20.15 | 24.54 | 5 (2.23) |
| Triglycerides (mg/dL)* | 154 | 190 | 245 | 1 (0.45) | 138 | 173 | 228 | n/a |
| Cholesterol (mg/dL) | 202 | 240 | 293 | 1 (0.45) | 201.5 | 248 | 290 | n/a |
| Daughter Variables2 | ||||||||
| Percent Density | 20.87 | 30.29 | 41.93 | n/a | 22.89 | 34.08 | 47.86 | n/a |
| Dense Area (cm2) | 26.35 | 37.42 | 57.85 | n/a | 29.19 | 41.03 | 54.07 | n/a |
| Nondense Area (cm2) | 50.75 | 91.10 | 143.47 | n/a | 43.60 | 83.94 | 135.72 | n/a |
| Age at Mammogram (years) | 46.74 | 48.83 | 50.52 | n/a | 46.63 | 48.37 | 49.95 | n/a |
| BMI (kg/m2) | 22.86 | 26.30 | 31.17 | 10 (4.46) | 23.03 | 25.84 | 31.46 | 13 (5.80) |
| Menopausal Status, N (%) | 16 (7.14) | 14(8.97) | ||||||
| Transition/Postmenopause | 109 (48.66) | 78 (50.00) | ||||||
| Premenopause | 99 (44.20) | 64 (41.03) | ||||||
BMI; body mass index
For serum related measures, we used early postpartum serum samples when available (1-3 days post-delivery), and prior trimester serum samples when unavailable.
Daughter variables are assessed at the time of interview (menopausal status, age at mammogram, and BMI) or closest to the time of the study interview (mammographic density measures).
Variables were significantly different by the mother’s breast cancer status with statistical P<0.05.
Each DDT variable was categorized into two sets of quartiles. The first set of quartiles used cut-points defined in our prior publication that demonstrated a significant, positive association between o,p’-DDT exposure and daughters’ breast cancer risk.1 In our prior publication, we defined DDT cut points based on the control population of mothers’ who did not develop breast cancer; we refer to these cut points as ‘a priori quartiles’. The second set of quartiles used the current study population specific quartiles that we refer to as ‘population quartiles’. Supplementary Table 1 provides the values for the cut points. When modeling DDT, each DDT variable was categorized into quartiles and represented by 3 nominal variables, where quartile 2 (Q2), quartile 3 (Q3), and quartile 4 (Q4) were compared to reference category quartile 1 (Q1) representing the lowest exposure level. When assessing linear trend, for all DDT exposure measures within the model, the quartiles were modeled as a continuous variable. We also generated splines (3 knots) for each DDT variable based on the overall population and the results of these models were similar to the quartile models (data not shown).
We focused our primary analyses on modeling o,p’-DDT exposures when mutually adjusted for p,p’-DDE because our prior publication demonstrated a positive association between intrauterine o,p’-DDT exposure and daughter’s breast cancer risk when adjusted for p,p’-DDE.1 We assessed the association between the DDT compounds and continuous measures of MBD (percent density, total dense area, total non-dense area) using generalized estimating equation (GEE) models to account for the correlated nature of the outcome among sibling sets (Table 2). Our starting models were fully adjusted models that included daughters’ age at mammogram, daughters’ BMI, and a priori confounders. A priori confounders included mother variables referred to as intrauterine exposures (age at birth, pre-pregnancy BMI, pregnancy weight gain, triglycerides, cholesterol), and daughter variables (age at mammogram, BMI, race/ethnicity, menopausal status). Using backward selection, we generated the most parsimonious model for o,p’-DDT when mutually adjusted for p,p’-DDE when modeled in MBCa+ daughters. The associations for DDT were of similar magnitude in fully adjusted and parsimonious models (data not shown). We tested for an interaction between o,p’-DDT and maternal breast cancer history and Figure 1 presents our parsimonious models stratified by mothers’ breast cancer status. We conducted a secondary analysis that included adjustment for all DDT compounds. We also conducted a sensitivity analysis examining if the use of hormone replacement therapy impacts the observed associations between o,p’-DDT and any of the density measures. We used STATA software for all analyses (STATA IC/14 for Windows).
Table 2.
Regression analysis between intrauterine o,p’-DDT exposure, across two sets of quartiles, and daughters’ MBD, PEDIGREE
| MODELS | A Priori Quartiles | Population Quartiles | ||||||
|---|---|---|---|---|---|---|---|---|
| Age and BMI Adjusted | Parsimonious Adjusteda | Age and BMI Adjusted | Parsimonious Adjusteda | |||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| PERCENT DENSITY | ||||||||
| o,p’- DDT | ||||||||
| Quartile 2 | 1.33 | −3.30, 5.97 | 1.80 | −3.03, 6.62 | 1.31 | −3.46, 6.08 | 1.20 | −3.83, 6.24 |
| Quartile 3 | 2.34 | −3.37, 8.96 | 1.55 | −4.29, 7.40 | 0.96 | −4.44, 6.35 | 0.19 | −5.37, 5.74 |
| Quartile 4 | 4.91 | −1.58, 11.39 | 5.98 | −0.77, 12.73 | 3.82 | −2.16, 9.78 | 4.07 | −2.16, 10.30 |
| p,p’-DDE | ||||||||
| Quartile 2 | 1.20 | −3.61, 6.02 | 1.78 | −3.17, 6.73 | −1.10 | −5.70, 3.49 | −1.11 | −5.90, 3.68 |
| Quartile 3 | 5.57 | −0.06, 11.21 | 5.54 | −0.33, 11.41 | 4.72 | −0.96, 10.40 | 4.40 | −1.63, 10.41 |
| Quartile 4 | −2.32 | −8.23, 3.59 | −2.86 | −8.98, 3.26 | −3.18 | −8.73, 2.37 | −4.01 | −9.86, 1.84 |
| DENSE AREA | ||||||||
| o,p’-DDT | ||||||||
| Quartile 2 | 2.01 | −4.91, 8.92 | 2.77 | −4.49, 10.03 | 3.09 | −4.51, 10.69 | 2.55 | −5.53, 10.63 |
| Quartile 3 | 5.29 | −2.92, 13.50 | 4.54 | −3.96, 13.03 | 2.17 | −5.48, 9.82 | 1.41 | −6.65, 9.47 |
| Quartile 4 | 6.00 | −5.50, 17.50 | 6.70 | −5.16, 18.57 | 6.78 | −3.40, 16.95 | 5.81 | −4.77, 16.40 |
| p,p’-DDE | ||||||||
| Quartile 2 | −0.68 | −8.28, 6.93 | −0.26 | −8.14, 7.63 | −1.54 | −9.00, 5.92 | −1.62 | −9.44, 6.20 |
| Quartile 3 | 1.50 | −7.01, 10.01 | 2.50 | −6.49, 11.49 | −0.11 | −8.30, 8.08 | 0.66 | −8.13, 9.46 |
| Quartile 4 | −6.92 | −16.23, 2.39 | −7.36 | −17.42, 2.70 | −6.88 | −15.31, 1.55 | −7.23 | −16.51, 2.05 |
| NONDENSE AREA | ||||||||
| o,p’- DDT | ||||||||
| Quartile 2 | −5.89 | −20.64, 8.86 | −5.34 | −20.89, 10.22 | −13.52 | −29.04, 1.99 | −13.92 | −30.26, 2.43 |
| Quartile 3 | 5.51 | −14.52, 25.54 | 7.48 | −13.42, 28.38 | 4.03 | −15.73, 23.79 | 5.25 | −15.33, 25.83 |
| Quartile 4 | −13.64 | −35.80, 8.52 | −15.60 | −39.08, 7.89 | −8.37 | −30.13, 13.40 | −10.39 | −33.20, 12.41 |
| p,p’-DDE | ||||||||
| Quartile 2 | −7.94 | −25.46, 9.57 | −8.65 | −26.80, 9.49 | 0.96 | −15.73, 17.65 | 2.01 | −15.34, 19.36 |
| Quartile 3 | −16.90 | −35.81, 2.00 | −15.82 | −35.53, 3.88 | −17.26 | −34.98, 0.46 | −14.96 | −33.27, 3.50 |
| Quartile 4 | 4.73 | −20.28, 29.73 | 7.97 | −18.43, 34.37 | 8.92 | −14.70, 32.54 | 13.88 | −11.10, 38.86 |
Abbreviations: BMI, Body Index Mass; BC, Breast Cancer; CI, Confidence interval
Parsimonious models are adjusted for p,p’- DDE, daughters age at mammogram and BMI and maternal cholesterol and pre-pregnancy BMI. Quartile 1 is the reference for all models.
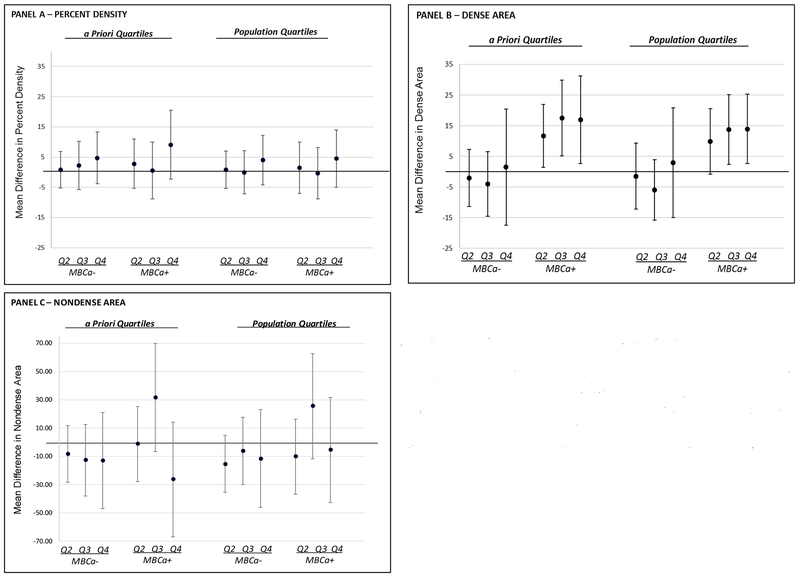
Figure 1. Parsimonious regression model for the association between intrauterine o,p’-DDT exposure and daughters’ MBD by maternal breast cancer status (MBCa), PEDIGREE.
Abbreviations: MBCa, Maternal Breast Cancer; Quartile, Q
Parsimonious models display the point estimates (β, dots) and the 95% confidence intervals (line with bar) for the association between intrauterine o,p’-DDT exposure and daughters’ percent density (Panel A), dense area (Panel B), and nondense area (Panel C) adjusted for p,p’-DDE, daughter’s age at mammogram and BMI and maternal cholesterol and pre-pregnancy BMI. Quartile 1 is the reference for all models.
RESULTS
Table 1 summarizes the descriptive statistics for mother and daughter variables by the mothers’ breast cancer status (MBCa). The average age of daughters at time of the mammogram was 48.7 years (Standard Deviation (SD) 3.0) and the average BMI was 26.2 kg/m2 (SD 7.3) and the distribution of these variables did not significantly differ by MBCa. We also observed no significant difference by MBCa in our outcome MBD measures, percent density, dense area, and non-dense area (Table 1). However, MBCa+ daughters were observed to have significantly higher o,p’-DDT levels (p=0.02) and significantly lower triglyceride levels (p=0.03) compared to MBCa− daughters.
Table 2 presents the regression models for our primary analyses modeling o,p’-DDT exposure when mutually adjusted for p,p’-DDE exposure. We observed no association between o,p’-DDT and any of the MBD outcomes when o,p’-DDT was modeled using the a priori quartiles nor the population quartiles (Table 2). We then examined if there were differences by underlying familial risk inferred by MBCa status. Only in MBCa+ daughters did we observe intrauterine exposure to o,p’-DDT was significantly associated with an approximate 12-17 unit higher dense area when the exposure was modeled using a priori quartiles (Ptrend=0.01) and an approximate 10-14 unit higher dense area when the exposure was modeled using population quartiles (Ptrend=0.02) (Figure 1, Panel B). The positive association between o,p’-DDT and dense area in MBCa+ daughters remained when additionally adjusted for p,p’-DDT (Q4 to Q1 β=22.9, 95% Confidence Interval 6.2, 39.6; ptrend=0.002) (Table 3). The interaction between o,p’-DDT (dichotomized as quartiles 2,3,4 vs 1) and maternal breast cancer history in the dense area models was statistically significant (p=0.03 using a priori quartiles and p<0.01 using population quartiles).
Table 3.
Parsimonious regression models for mutually adjusted intrauterine DDT compounds, using a priori quartiles, and daughters’ MBD by maternal breast cancer status, PEDIGREE
| Maternal Breast Cancer Negative | Maternal Breast Cancer Positive | |||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| PERCENT DENSITY | ||||
| p,p’-DDT | ||||
| Quartile 2 | 3.19 | −2.84, 9.22 | −0.51 | −8.52, 7.49 |
| Quartile 3 | 4.01 | −3.44, 11.46 | −1.74 | −11.49, 8.01 |
| Quartile 4 | 10.00 | 1.73, 18.28 | −5.57 | −17.34, 6.20 |
| o,p’-DDT | ||||
| Quartile 2 | −0.17 | −6.20, 5.85 | 2.89 | −5.22, 10.99 |
| Quartile 3 | 0.02 | −7.88, 7.92 | 1.29 | −8.44, 11.01 |
| Quartile 4 | −0.99 | −10.41, 8.43 | 11.51 | −1.50, 24.52 |
| p,p’-DDE | ||||
| Quartile 2 | −3.17 | −9.41, 3.08 | 5.41 | −3.42, 14.24 |
| Quartile 3 | 1.13 | −6.84, 9.09 | 8.68 | −1.20, 18.55 |
| Quartile 4 | −6.88 | −15.32, 1.57 | 0.81 | −8.99, 10.61 |
| DENSE AREA | ||||
| p,p’-DDT | ||||
| Quartile 2 | −1.73 | −11.09, 7.62 | −7.72 | −18.61, 3.18 |
| Quartile 3 | −3.80 | −15.56, 7.96 | −6.19 | −19.97, 7.58 |
| Quartile 4 | 1.32 | −11.78, 14.41 | −16.38 | −33.55, 0.79 |
| o,p’-DDT | ||||
| Quartile 2 | −1.82 | −10.88, 7.25 | 12.55 | 2.25, 22.85 |
| Quartile 3 | −3.18 | −14.03, 7.66 | 20.29 | 7.99, 32.58 |
| Quartile 4 | 0.53 | −18.44, 19.50 | 22.90 | 6.20, 39.61 |
| p,p’-DDE | ||||
| Quartile 2 | −3.40 | −14.04, 7.23 | 3.02 | −7.78, 13.82 |
| Quartile 3 | 0.40 | −12.44, 13.24 | 6.96 | −5.49, 19.41 |
| Quartile 4 | −4.54 | −20.74, 11.66 | −4.85 | −16.49, 6.78 |
| NONDENSE AREA | ||||
| p,p’-DDT | ||||
| Quartile 2 | −21.13 | −40.73, −1.53 | 9.98 | −20.21, 40.18 |
| Quartile 3 | −40.18 | −68.11, −12.25 | −9.28 | −39.11, 20.54 |
| Quartile 4 | −34.99 | −69.94, −0.04 | −19.37 | −66.64, 27.90 |
| o,p’-DDT | ||||
| Quartile 2 | −3.51 | −24.52, 17.51 | −1.70 | −29.18, 25.78 |
| Quartile 3 | 1.93 | −25.79, 29.66 | 33.67 | −4.49, 71.83 |
| Quartile 4 | 5.92 | −36.16, 48.00 | −14.73 | −55.90, 26.44 |
| p,p’-DDE | ||||
| Quartile 2 | −2.92 | −23.62, 17.78 | 3.56 | −32.79, 39.92 |
| Quartile 3 | 1.37 | −22.02, 24.77 | −14.91 | −56.56, 26.75 |
| Quartile 4 | 26.41 | −6.96, 59.77 | 15.32 | −33.31, 63.96 |
Parsimonious models examined the association between intrauterine o,p’-DDT exposure and daughters’ percent density, dense area, and nondense area when adjusted for p,p’-DDT, p,p’-DDE, daughter’s age at mammogram and BMI and maternal cholesterol and pre-pregnancy BMI. Quartile 1 is the reference for all models.
We observed no statistically significant association between intrauterine o,p’-DDT exposure and percent density or with nondense area, among women with or without MBCa history (Figure 1, Table 3). We did however observe that in models that were mutually adjusted for all compounds a significant positive association between p,p’-DDT and percent density for the highest quartile of p,p’-DDT exposure; and, an inverse association between p,p’-DDT and non-dense area (Table 3).
The overall inference and magnitude of the associations did not change with the exclusion of the 19 daughters with personal history of breast cancer. About 16% of daughters indicated they were taking hormone replacement therapy (HRT) at the time of the study interview. Taking HRT was itself not associated with density and adjustment for HRT status had no impact on o,p’-DDT associations with any of the density measures (data not shown).
DISCUSSION
DDT compounds are organochlorines synthetically designed to disrupt physiological function; bioaccumulative, they are characterized by stable chemical properties, low biodegradability, lipophilicity, and a long half-life. This special edition includes the first studies to quantify intrauterine exposure to DDT and MBD. We have previously shown that intrauterine o,p’-DDT exposure was associated with an almost 4-fold increase in breast cancer risk in daughters when comparing the lowest quartile to the highest quartiles of exposure.1 Using those previously published cut points,1 we observed that when mutually adjusted for p,p’-DDE, intrauterine exposure to o,p’-DDT was associated with a 10-17 unit higher dense area in daughters whose mothers were diagnosed with breast cancer. Our findings are robust as the positive association was also observed when models included population-based and spline-based cut-point and when additionally adjusted for p,p’-DDT.
We only observed an association between o,p’-DDT and MBD in women with an underlying susceptibility as proxied by first degree family history information. Even when population sizes are large, without sufficient numbers of women at the higher breast cancer risk spectrum, statistical power is limited to test for interactions between DDT exposure and underlying susceptibility risk. For example, we previously reported that the association between polycyclic aromatic hydrocarbons, an environmental carcinogen released during combustion processes, and breast cancer risk was stronger based on absolute risk as predicted by breast cancer family history.22 Our findings support that the signal of intrauterine environmental exposures may be stronger in women with a greater underlying familial risk for developing breast cancer, inferred from MBCa status.
These findings are consistent with the heterogeneity in risk observed for many established breast cancer risk factors.23–27 For example, obesity associations differ radically by age at diagnosis24, 28 and it is known that genetic risk modifies reproductive and environmental risk factors for breast cancer.22,23,25,29 In our cohort we have recently reported on heterogeneity of DDT associations with breast cancer by timing of exposure and also by induction time.6, 30 The premise of the present paper was to directly test the hypothesis that breast density could explain our previously reported association of in utero o,p’-DDT with breast cancer.1 Building on that earlier finding, here we report that breast density may contribute to o,p’-DDT-associated risk for women with a family history, but not for women without a family history. These results suggest that for CHDS daughters without a maternal breast cancer history, not all o,p’-DDT-associated breast cancer is through breast density.
Our findings suggests that environmental exposures may be deleterious to breast tissue during sensitive windows of development. In the CHDS pregnancy cohort we observed that mothers exposed to DDT when they were under age 14 in 1945, which is the youngest age at which a woman could have been exposed to DDT compound, was associated with maternal breast cancer risk.6 Using proxy measures, the Long Island Breast Cancer Study Project (LIBCSP) and the Sister Study confirmed an association between childhood and adolescent exposure to pesticides and breast cancer risk.7,8 We have also observed, when mutually adjusted for p,p’-DDE, a positive association between intrauterine o,p’-DDT exposure and daughters’ breast cancer risk (Q4 compared to Q1 OR=3.7, 95% CI 1.5, 9.0).1 Our findings presented here corroborate these results using mammographic density as a marker of breast cancer risk. Using the exact same cut-points we found o,p’-DDT exposure, when mutually adjusted for p,p’-DDE, was associated with higher total dense area in the women most susceptible to breast cancer (MBCa+ daughters).
In our previous study showing that higher levels of in utero o,p’-DDT exposure were associated with increased breast cancer risk,1 daughters were under age 55 years. Therefore, those who developed breast cancer were more likely to develop pre-menopausal breast cancer which is associated with dense breast tissue. Our group has also observed a positive association between maternal p,p’-DDT exposure before 14 years of age and breast cancer risk.6 In contrast, our current study observed that when we look in MBCa− daughters and models are mutually adjusted for all three DDT compounds, p,p’-DDT was significantly associated with higher percent density. However, this association was primarily driven by a lower nondense area, and not a higher dense area. Together these findings suggest that the timing of exposure (intrauterine and early life through puberty), the source of the exposure, and the functional activities may differ across the DDT compounds, resulting in differing outcomes.31 Different effects of exposure may also be due to different biological activities of the various DDT compounds: o,p’-DDT is most estrogenic of the three compounds32, 33 and p,p’-DDE is an anti-androgen33,34.
There are several strengths to our study that should be highlighted. Given the long latency period, our analysis provides a proof of concept that intermediate markers may be used to identify early life exposures and breast cancer risk. Second, we assessed the association between DDT exposure and MBD during a window of susceptibility, where the breast tissue is suggested to be more vulnerable to carcinogenic exposures. Third, we quantified DDT exposure during 1959-1967 which maps with the peak years of DDT use in the United States.35, 36 Fourth, in contrast to self-reported proxy measures, we measured DDT exposure using state of the art laboratory methods which assessed all three DDT compounds, o,p’-DDT, p,p’-DDT and p,p’-DDE. Fifth, our analyses were able to assess associations in women with a greater underlying familial risk for developing breast cancer as proxied by MBCa. Despite the strengths of the study, it is not without limitations. We had limited overall power which resulted in larger confidence intervals and greater imprecision in our estimates. Moreover, given we are missing paternal history of breast cancer as well as a full maternal history of breast cancer, we introduce possible misclassification of the daughters underlying familial risk for breast cancer. As with any observational study, we cannot rule out unmeasured confounders. However, for an unmeasured confounder to explain our observed findings, it would have to be distributed in the same dose dependent way with breast density, just as DDT is distributed in daughters with a maternal breast cancer family history only.
In a companion paper in this special edition of Reproductive Toxicology Krigbaum et al (Krigbaum, et.al.) we analyzed a larger sample of women without a family history of breast cancer. Findings in this sample are consistent with the findings reported in the companion paper for women without maternal family history. In both samples, o,p’-DDT was not associated with breast density measures for women without family history.
Conclusion
Our findings support that studies enriched with women who have above average breast cancer risk are needed to understand the association between early life environmental exposures and MBD. Moreover, given the long induction period between exposure and breast cancer diagnosis, our study supports examining the association between exposure and an intermediate marker for risk. Once widely used as a pesticide before its eventual ban in the United States in 1972, the evidence of widespread DDT use can still be found in blood, urine, and environmental samples.37,38 In addition, DDT use is still recommended for malaria control by global agencies.37, 38 The two studies presented in this special edition are the first to directly measure environmental chemical exposures during pregnancy in relation to MBD. These findings suggest that MBD, an important intermediate marker of breast cancer risk, may be affected by intrauterine environmental exposures in women with an underlying susceptibility as proxied by maternal breast cancer history.
Supplementary Material
Highlights.
Intermediate marker, breast density, addresses long induction period challenges
In utero o,p’-DDT associated with higher dense breast area in maternal BC+ daughters.
MBD is affected by intrauterine DDT and maternal BC status.
Acknowledgments:
We thank the CHDS families for their participation in this study. We acknowledge the late Jacob Yerushalmy who had the foresight to design and implement the CHDS; the late Barbara van den Berg, the second Director of the CHDS, whose steadfast efforts were responsible for preserving the data and serum archive, thus granting the CHDS longevity.
Funding:
This work was supported by the Breast Cancer and the Environment Research Program (BCERP) grant U01 ES019457 from the National Institute of Environmental Health Sciences (NIEHS) and the National Cancer Institute (NCI), NIH, DHHS; by funding from the California Breast Cancer Research Program through the Special Research Initiative under Grant 15ZB-0186 and the Breast Cancer Research Foundation; and, by funding from the NCI grant K01 CA186943.
Abbreviations:
- CHDS
Child Health and Development Studies
- MBD
mammographic breast density
- MBCa
mother breast cancer status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jasmine A. McDonald, Department of Epidemiology, Mailman School of Public Health, Columbia University Medical Center, 722 West 168th Street, 8th Floor; Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY 10032
Piera M. Cirillo, Center for Research on Women’s and Children’s Health, Public Health Institute, Berkeley, California
Parisa Tehranifar, Department of Epidemiology, Mailman School of Public Health, Columbia University Medical Center 722 West 168th Street, 8th Floor; Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY 10032.
Nickilou Y. Krigbaum, Center for Research on Women’s and Children’s Health, Public Health Institute, Berkeley, California
Natalie Engman, Department of Epidemiology, Mailman School of Public Health, Columbia University Medical Center.
Barbara A. Cohn, Center for Research on Women’s and Children’s Health, Public Health Institute, Berkeley, California
Mary Beth Terry, Department of Epidemiology, Mailman School of Public Health, Columbia University Medical Center, 722 West 168th Street, 16th Floor; Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY 10032.
REFERENCES
- 1.Cohn BA, La Merrill M, Krigbaum NY, et al. DDT Exposure in Utero and Breast Cancer. J Clin Endocrinol Metab. 2015;100(8):2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomis D, Guyton K, Grosse Y, et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. The Lancet Oncology. 2015;16(8):891–892. [DOI] [PubMed] [Google Scholar]
- 3.Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110(2):125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101(5):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ Res. 2018;160:152–182. [DOI] [PubMed] [Google Scholar]
- 6.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White AJ, Teitelbaum SL, Wolff MS, Stellman SD, Neugut AI, Gammon MD. Exposure to fogger trucks and breast cancer incidence in the Long Island Breast Cancer Study Project: a case-control study. Environ Health. 2013;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niehoff NM, Nichols HB, White AJ, Parks CG, D’Aloisio AA, Sandler DP. Childhood and Adolescent Pesticide Exposure and Breast Cancer Risk. Epidemiology. 2016;27(3):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald JA, Goyal A, Terry MB. Alcohol Intake and Breast Cancer Risk: Weighing the Overall Evidence. Curr Breast Cancer Rep. 2013;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link BG, Susser ES, Factor-Litvak P, et al. Disparities in self-rated health across generations and through the life course. Soc Sci Med. 2017;174:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Susser E, Buka S, Schaefer CA, et al. The Early Determinants of Adult Health Study. J Dev Orig Health Dis. 2011;2(6):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Merrill MA, Cirillo PM, Krigbaum NY, Cohn BA. The impact of prenatal parental tobacco smoking on risk of diabetes mellitus in middle-aged women. J Dev Orig Health Dis. 2015;6(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2(3):265–282. [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Occupational Safety and Health. NIOSH Special Occupational Hazard Review: DDT. 1978; 1–181. https://www.cdc.gov/niosh/docs/78-200/ Accessed July 30, 2018.
- 15.La Merrill M, Cirillo PM, Terry MB, Krigbaum NY, Flom JD, Cohn BA. Prenatal Exposure to the Pesticide DDT and Hypertension Diagnosed in Women before Age 50: A Longitudinal Birth Cohort Study. Environmental Health Perspectives. 2013;121(5):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer reporting in California: abstracting and coding procedures for hospitals California Cancer Reporting System standards. Vol I Sacramento, CA: California Department of Health Services, Cancer Surveillance Section, 2012. [Google Scholar]
- 17.Kwong SLPC, Morris CR, Cohen R, Allen M, Wright WE. Cancer in California: 1988-1999. Sacramento, CA: California Department of Health Services, Cancer Surveillance Section; December 2001. [Google Scholar]
- 18.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation. 2015; 132(13): 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The Quantitative-Analysis of Mammographic Densities. Phys Med Biol. 1994;39(10): 1629–1638. [DOI] [PubMed] [Google Scholar]
- 20.Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. Serial Levels of Serum Organochlorines During Pregnancy and Postpartum. Archives of Environmental Health: An International Journal. 1999;54(2):110–114. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ Res. 2015;136:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Liao Y, Hopper JL, Goldberg M, Santella RM, Terry MB. Dependence of cancer risk from environmental exposures on underlying genetic susceptibility: an illustration with polycyclic aromatic hydrocarbons and breast cancer. Br J Cancer. 2017; 116(9): 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotsopoulos J, Gronwald J, Lynch HT, et al. Age at first full-term birth and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2018;171(2):421–426. [DOI] [PubMed] [Google Scholar]
- 24.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014;106(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96(3):218–228. [DOI] [PubMed] [Google Scholar]
- 27.Gaudet MM, Gierach GL, Carter BD, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res. 2018;78(20):6011–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol. 2011;174(8):909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terry MB, Liao Y, Kast K, et al. The Influence of Number and Timing of Pregnancies on Breast Cancer Risk for Women With BRCA1 or BRCA2 Mutations. JNCI Cancer Spectrum. 2019;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohn BA, Cirillo PM, Terry MB. DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohn BA, Cirillo PM, Sholtz RI, Wolff MS. DDT and Breast Cancer: Cohn et al. Respond. Environmental Health Perspectives. 2008;116(4):A153–A154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapiero H, Ba GN, Tew KD. Estrogens and environmental estrogens. Biomed Pharmacother. 2002;56(1):36–44. [DOI] [PubMed] [Google Scholar]
- 33.Safe SH, Zacharewski T. Organochlorine exposure and risk for breast cancer. Prog Clin Biol Res. 1997;396:133–145. [PubMed] [Google Scholar]
- 34.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature. 1995;375(6532):581–585. [DOI] [PubMed] [Google Scholar]
- 35.Ritter R, Scheringer M, MacLeod M, Hungerbuhler K. Assessment of nonoccupational exposure to DDT in the tropics and the north: relevance of uptake via inhalation from indoor residual spraying. Environ Health Perspect. 2011;119(5):707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol. 1991;120:1–82. [DOI] [PubMed] [Google Scholar]
- 37.Center for Disease Control. Parasites - Lice - Head Lice - Treatment. https://www.cdc.gov/parasites/lice/head/treatment.html Published 2016. Accessed May 25, 2018.
- 38.WHO (World Health Organization). The use of DDT in malaria vector control: WHO position statement 2011. http://www.who.int/malaria/publications/atoz/who_htm_gmp_2011/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.