Abstract
Alcohol use disorder (AUD) is a chronic, relapsing disorder that is characterized by the compulsive use of alcohol despite numerous health, social, and economic consequences. Initially, the use of alcohol is driven by positive reinforcement. Over time, however, alcohol use can take on a compulsive quality that is driven by the desire to avoid the negative consequences of abstinence, including negative affect and heightened stress/anxiety. This transition from positive- to negative-reinforcement-driven consumption involves the corticotropin-releasing-factor (CRF) system, although mounting evidence now suggests that the CRF system interacts with other neural systems to ultimately produce behaviors that are symptomatic of compulsive alcohol use, such as the hypocretin (Hcrt) system. Hypocretins are produced exclusively in the hypothalamus, but Hcrt neurons project widely throughout the brain and reach regions that perform regulatory functions for numerous behavioral and physiological responses – including the infralimbic cortex (IL) of the medial prefrontal cortex (mPFC). Although the entire mPFC undergoes neuroadaptive changes following prolonged alcohol exposure, the IL appears to undergo more robust changes compared with other mPFC substructures. Evidence to date suggests that the IL is likely involved in EtOH-seeking behavior, but ambiguities with respect to the specific role of the IL in this regard make it difficult to draw definitive conclusions. Furthermore, the manner in which CRF interacts with Hcrt in this region as it pertains to alcohol-seeking behavior is largely unknown, although immunohistochemical and electrophysiological experiments have shown that CRF and Hcrt directly interact in the mPFC, suggesting that the interaction between CRF and Hcrt in the IL may be critically important for the development and subsequent maintenance of, compulsive alcohol seeking. This review aims to consolidate recent literature regarding the role of the IL in alcohol-seeking behavior and to discuss evidence that supports a functional interaction between Hcrt and CRF in the IL.
Keywords: Ethanol, alcohol, addiction, corticotropin-releasing-factor (CRF), hypocretin (Hcrt), infralimbic cortex (IL), medial prefrontal cortex (mPFC)
Introduction
Alcohol use disorder (AUD) is a chronic, relapsing disorder that is accompanied by significant neuroplastic changes in brain regions that are involved in reward seeking and processing. Findings strongly suggest that these changes are responsible for the maladaptive and compulsive behavior that is indicative of the pathology (Aston-Jones & Harris, 2004; Kalivas & O’Brien, 2008; Kelley & Berridge, 2002; Wanat et al., 2009). Such brain regions as the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), ventral tegmental area (VTA), nucleus accumbens (NAC), hippocampus, and dorsal striatum (Carnicella et al., 2008; Chen et al., 2011; Dayas et al., 2007; Janak & Chaudhri, 2010; Kalivas & Volkow, 2005; Steketee & Kalivas, 2011; Topple et al., 1998; Zhao et al., 2006) are major components of interconnected cortical and limbic brain regions that are responsible for addiction-related behaviors, such as drug cue-, drug prime-, and stress-induced reinstatement of drug-seeking (Daglish & Nutt, 2003; Dayas et al., 2007; Goldstein & Volkow, 2002; Heinz et al., 2010; Heinz et al., 2005; Miller & Goldsmith, 2001; Zhao et al., 2006). The chronic administration of alcohol is well known to significantly dysregulate brain stress responses that are mediated by corticotropin- releasing factor (CRF), including the hypothalamic-pituitary-adrenal (HPA) axis and extrahypothalamic stress systems (Quadros et al., 2016; Stephens & Wand, 2012). Specifically, repeated and chronic exposure to drugs with a potential for dependence, including alcohol, appears to increase extrahypothalamic CRF activity in several brain structures that have a role in withdrawal-related anxiety and dysphoria (Merlo-Pich et al., 1995; Olive et al., 2002; Sommer et al., 2008; see Becker et al., 2012 for review) and blunt the responsivity of the HPA axis (Koob & Kreek, 2007; Koob, 2008). The dysregulation in these stress systems contributes to the transition from controlled alcohol consumption, which is largely motivated by positive reinforcement and reward-seeking, to the escalation of consumption that occurs in tandem with the user becoming increasingly tolerant to the effects of alcohol. At the latter stage, alcohol consumption is largely driven by dysphoria and negative reinforcement. This “dark side” of addiction (Koob & Le Moal, 1997) is characterized by long-term, persistent plasticity in the activity of neural circuitry that is involved in mediating motivational systems (Koob, 2009). These neuroplastic changes are hypothesized to be significantly involved in compulsive alcohol- seeking and taking behavior that is indicative of AUD.
Stress often precipitates relapse of drug-seeking behavior in previously abstinent individuals. Several neuropeptides have been proposed to interact with CRF to promote or control reinstatement of drug-seeking behavior, which is a commonly used procedure to model stress-induced relapse in animals (see Shalev et al., 2010 for a review). In squirrel monkeys, for example, it has been shown that kappa opioid receptor agonist-induced reinstatement of cocaine-seeking was attenuated by the CRF1 antagonist CP-154, 526 (Valdez et al., 2007), suggesting a possible CRF-dynorphin interaction. Other neuropeptides such as nociceptin and leptin have also been suggested to interact with CRF. Evidence suggests that nociceptin can ameliorate the anorectic and anxiogenic effects of CRF receptor stimulation in the BNST, a region critical for footshock-stress induced reinstatement (Ciccocioppo et al., 2003; Rodi et al., 2008). It has also been shown that the administration of leptin and a non-selective CRF antagonist α-helical CRF both decrease food-deprivation-induced reinstatement (Shalev et al., 2001; Shalev et al., 2006) which suggests the possibility that CRF-leptin interactions contributes to this form of reinstatement, although leptin administration has no effect on footshock-induced reinstatement, which requires VTA dopamine transmission (Hahn et al., 2009; Hommel et al., 2006; Wang et al., 2005). It is possible that food deprivation-induced reinstatement of drug-seeking involves CRF-leptin interactions in different brain region, and more research is required to elucidate this possibility.
The hypocretinergic/orexinergic (Hcrt/Orx) neural system also appears to interact with CRF and has an integral role in driving uncontrolled alcohol use. The Hcrt system has been repeatedly shown to play a significant role in stress- and motivation-related behaviors and has thus been a prime target for studies of its involvement in addiction since its near-simultaneous discovery by two separate research groups in 1998 (de Lecea et al., 1998; Sakurai et al., 1998). Furthermore, neuroanatomical findings show that the Hcrt and CRF systems interact. Corticotropin-releasing factor terminals have been reported to directly contact Hcrt neurons that express both CRF receptor subtypes (CRF1 and CRF2; Winskey-Sommer et al., 2004), and CRF neurons are recipients of Hcrt inputs. Interestingly, evidence supports the hypothesis that these two systems interact in brain systems that are related to stress and drug-seeking behaviors. For example, the mPFC is a target of Hcrt projections (see Fig. 1) and CRF immunoreactive cells are distributed throughout the cortex, particularly in limbic regions such as the prefrontal cortex (Swanson et al., 1983) The possibility that the CRF and Hcrt systems interact is intriguing when one considers the roles they both have in regulating stress responses. However, the resultant behavioral outcomes of such interactions may differ depending on which functional subdivision of the mPFC is the recipient of Hcrt projections, as the dorsal mPFC (prelimbic cortex) and ventral mPFC (infralimbic cortex) have been shown to be dissociable with regard to their anatomical connectivity and functionality (Vertes, 2004, 2006) . The present review summarizes findings that (1) implicate the IL in alcohol-seeking behavior and (2) suggest a functional interaction between CRF and Hcrt in the IL, and that this interaction has important consequences for dysregulated alcohol-seeking behavior.
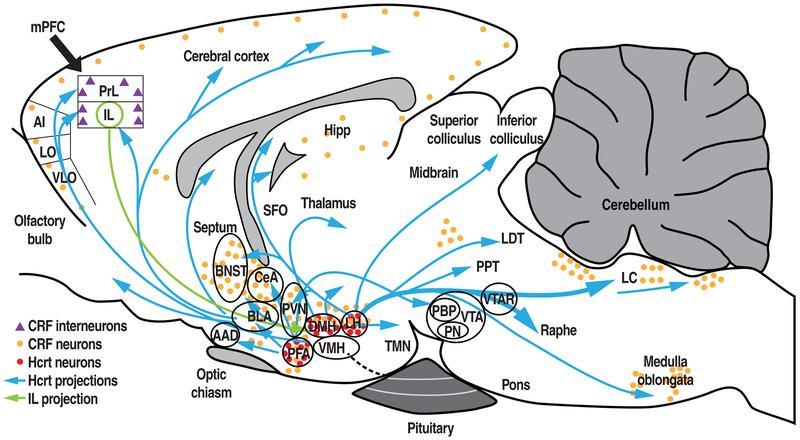
Figure 1.
Schematic diagram of various Hcrt projections that originate from the DMH, LH, and PFA (blue arrows), IL projection to the HYP (green arrow), and locations of Hcrt neurons (red circles). Locations of CRF-expressing neurons and putative CRF-expressing interneurons are shown as orange circles and purple triangles, respectively. mPFC, medial prefrontal cortex; AI, aganular insular cortex; LO, lateral orbitocortex; VLO, ventrolateral orbitocortex; PrL, prelimbic cortex; IL, infralimbic cortex; BNST, bed nucleus of the stria terminalis; Hcrt, hypocretin; Hipp, hippocampus; SFO, subfornial organ; CRF, corticotropin-releasing factor; CeA, central nucleus of the amygdala; BLA, basolateral amygdala, AAD, anterior amygdaloid area; PFA, perifornical area; PVN, paraventricular nucleus of the hypothalamus; VMH, ventromedial hypothalamus; DMH, dorsomedial hypothalamus, LH, lateral hypothalamus; TMN, tuberomammillary nucleus; PPT, pendunculopontine nucleus; LDT, laterodorsal tegmental nucleus; LC, locus coeruleus; VTA, ventral tegmental area; PBP, parabrachial pigmented nucleus of the ventral tegmental area; PN, paranigral nucleus of the ventral tegmental area; VTAR, ventral tegmental area, rostral.
Modified from Baimel et al., 2015; Moorman et al., 2015; Paxinos & Watson, 1986; Swanson et al., 1983; Tsujino & Sakurai, 2013; Vertes, 2004.
Role of Hypocretin in Drug/Alcohol-Motivated Behaviors
The Hcrt system plays an important role in modulating reward function and drug-directed behavior (Harris et al., 2005). Hypocretin-1 (Hcrt-1) and hypocretin-2 (Hcrt-2) are secondary products that are obtained by the proteolytic cleavage of a common precursor, prepro-Hcrt (de Lecea et al., 1998; Gatfield et al., 2010; Tsujino & Sakurai, 2009). Hcrt-1 and Hcrt-2 are neuropeptides that regulate a wide variety of physiological functions, including feeding, energy metabolism, and arousal (Edwards et al., 1999; Haynes et al., 2000; Haynes et al., 2002; Sakurai et al, 1998; Sutcliffe & de Lecea, 2002; Teske et al., 2010; Taheri et al., 2002; Willie et al., 2011). Two Hcrt receptors have been identified: Hcrt-r1 and Hcrt-r2 (Ammoun et al., 2003; Sakurai et al., 1998; Scammell & Winrow, 2011). Hcrt-r1 has higher affinity for Hcrt-1 (~20-30 nM) than Hcrt-2 (10- to 1000-fold lower), whereas Hcrt-r2 has similar affinity for both Hcrt-1 and Hcrt-2 (~40 nM; Ammoun et al., 2003; Sakurai et al., 1998; Scammell & Winrow, 2011). Hcrt neurons are exclusively located in the hypothalamus (see Fig. 1), predominately in the lateral hypothalamus (LH), dorsomedial hypothalamus (DMH), and perifornical area (PFA; Baldo et al., 2003; DiLeone et al., 2003; Winsky-Sommerer et al., 2004). Hcrt neurons are activated by a wide variety of biologically significant factors, including the onset of stimuli that signal the availability of drugs of abuse, such as morphine, cocaine, and alcohol (Dayas et al., 2008; Harris et al., 2005; Jupp et al., 2011; Martin-Fardon et al., 2010; Martin-Fardon et al., 2016). Consistent with these observations, SB334867 also blocked the acquisition of cocaine-induced behavioral sensitization and the cocaine-induced potentiation of excitatory currents in dopamine neurons in the VTA (Borgland et al., 2006). Additionally, the intra-VTA administration of Hcrt-1 increased the motivation to self-administer cocaine both in discrete trials procedures and under a progressive-ratio (PR) schedule of reinforcement and enhanced the effects of cocaine on both tonic and phasic dopamine signaling (Espana et al., 2011). The intra-VTA administration of SB334867 reduced cocaine self-administration and attenuated the cocaine-induced enhancement of dopamine signaling (Espana et al., 2010).
Evidence of the role of Hcrt neurons in specifically alcohol-related behavior can be traced back to early animal experiments that used intracranial self-stimulation (ICSS). In these studies (Lestang et al., 1985; Wayner et al., 1971) rats that received electrode implants in the LH would respond robustly for electrical stimulation, and electrical stimulation of the LH subsequently led the rats to drink alcohol to the point of intoxication (Wayner et al., 1971). This latter finding raised the intriguing possibility that the LH is involved in modulating the reinforcing effects of alcohol. Supporting this hypothesis, Lestang et al. (1985) found that ibotenic acid-induced lesions of the LH diminished the rate at which rats self-stimulated, suggesting that the LH indeed plays a significant role in reinforcement-seeking, possibly including behaviors that are maintained by the reinforcing effects of alcohol. Evidence of the involvement of LH Hcrt neurons in drug-seeking behavior came from subsequent observations that these neurons innervate brain structures that were previously shown to be involved in modulating responses to drugs of abuse and arousal (Peyron et al., 1998; Fadel & Deutch, 2002; Winsky-Sommerer et al., 2003). Importantly, Lawrence et al. (2006) found that the Hcrt system, specifically the Hcrt-r1, is involved in expression of the cue-induced reinstatement of alcohol-seeking behavior. Lawrence et al. (2006) found that SB334867 reversed alcohol-conditioned reinstatement in alcohol-preferring iP rats and decreased alcohol self-administration under a fixed-ratio (FR) schedule (Table 1). Operant responses for water remained unaffected, demonstrating that the effect of SB334867 was specific to alcohol self-administration and not attributable to untoward side effects, thus lending further credence to the notion that the Hcrt system is involved in alcohol-seeking. Additional findings corroborated these results, indicating that the Hcrt-r1 antagonism has a greater impact on alcohol-related behaviors compared with behaviors that are motivated by natural rewards (e.g., food and sucrose). Richards et al. (2008) found that peripheral injections of SB334867 (up to 20 mg/kg) did not interfere with sucrose self-administration. Similarly, Cason and Aston-Jones (2013) reported that SB334867 did not decrease sucrose intake in rats that were maintained on varying feeding regimens (i.e., ad libitum vs. food-restricted) when administered at a dose of 10 mg/kg; however, these authors observed a reduction of sucrose self-administration when the rats received 20 or 30 mg/kg SB334867. Additional evidence that Hcrt-r1 antagonism differentially affects alcohol self-administration compared with the self-administration of natural reinforcers comes from the results of Jupp et al. (2011) in alcohol-preferring iP rats. These authors tested the effects of 5 mg/kg SB334867 on the self-administration of either alcohol or sucrose under both fixed- and progressive-ratio schedules. SB334867 reduced responding for both alcohol and sucrose under an FR3 schedule of reinforcement (Table 1). However, under a PR schedule, the same dose of SB334867 reduced responding for alcohol and breakpoint for alcohol, only. These findings partially support the hypothesis that the Hcrt system affects self-administration in a reinforcer type-dependent manner. Importantly, these findings also imply that the involvement of the Hcrt system is somewhat specific to tasks that require a high level of motivation, such as a PR schedule of reinforcement. Anderson et al. (2014) failed to observe similar results in female alcohol-preferring P rats that were treated with 10 and 30 mg/kg SB334867, however, suggesting possible sex-differences in the efficacy of SB334867 in attenuating alcohol-seeking behaviors.
Table 1.
Summary of the effect of Hcrt-r1 blockade on alcohol-related behaviors
| Measure | Ligand (dose) | Findings | References | |
|---|---|---|---|---|
| Int. | SB334867 (20 mg/kg) | FR Alcohol SA | ↓ | Lawrence et al., 2006 |
| SB334867 (20 mg/kg) | FR Water SA | – | ||
| SB334867 (10 & 20 mg/kg) | FR Alcohol SA | ↓ | Richards et al., 2008 | |
| SB334867 (10 & 20 mg/kg) | FR Sucrose SA | – | ||
| SB334867 (10 mg/kg) | FR Sucrose SA | – | Cason & Aston-Jones, 2013 | |
| SB334867 (20 & 30 mg/kg) | FR Sucrose SA | ↓ | ||
| SB334867 (5 mg/kg) | FR Alcohol SA | ↓ | Jupp et al., 2011 | |
| SB334867 (5 mg/kg) | FR Sucrose SA | ↓ | ||
| SB334867 (5 mg/kg) | PR Alcohol SA | ↓ | ||
| SB334867 (5 mg/kg) | PR Sucrose SA | – | ||
| SB334867 (10 & 30 mg/kg) | 2BC Alcohol SA | – | Anderson et al., 2014 | |
| SB334867 (10 & 30 mg/kg) | PR Alcohol SA | – | ||
| SB408124 (1, 10, & 30 mg/kg) | FR Alcohol SA | – | Shoblock et al., 2011 | |
| SB334867 (30 mg/kg) | 2BC Alcohol SA | ↓ | Moorman & Aston-Jones, 2009 | |
| Reinst. | SB334867 (20 mg/kg) | Alcohol cue | ↓ | Lawrence et al., 2006 |
| SB334867 (5 & 10 mg/kg) | Stress | ↓ | Richards et al., 2008 | |
| Pref. | SB334867 (30 mg/kg) | 2BC | ↓ | Moorman & Aston-Jones, 2009 |
Int.: Intake
Reinst.: Reinstatement
Pref.: Preference
2BC: 2-bottle choice
FR: Fixed Ratio
PR: Progressive Ratio
SA: Self-administration
: no effect
: decrease
Importantly, although the previously discussed study by Lawrence et al. (2006) is compelling and has also been replicated in outbred male Long-Evans rats using SB334867 (Richards et al., 2008; see Table 1), a different Hcrt-r1 antagonist, SB408124, did not alter alcohol self-administration in male Wistar rats at any of the doses tested (1, 10, and 30 mg/kg; Shoblock et al., 2011). Notably, however, the authors noted that the rats self-administered relatively low amounts of alcohol. When considered within the context of the findings of Moorman and Aston-Jones (2009), who showed that SB334867 (30 mg/kg, i.p ) only reduced alcohol consumption and preference in rats that expressed a high preference for alcohol in the two-bottle choice procedure, the discrepant results of Shoblock et al. (2011; see Table 1) may be partially attributable to the possibility that Hcrt-r1 is recruited only under conditions of high alcohol consumption or heightened motivation to consume alcohol. Given the procedural differences between studies in the routes of administration and the fact that SB334867 and SB408124 differ with regard to their bioavailability and selectivity for Hcrt-r1 (Langmead et al., 2009), the specific manner in which Hcrt-r1 is involved in alcohol-related behaviors remains to be fully characterized.
Hypocretin and Stress
Hypocretin neurons have been reported to be activated by various stressful events (Gerashchenko et al., 2011; Ida et al., 2000). Hypocretin promotes bodily reactions that accompany states of stress such as elevations of blood pressure, heart rate, oxygen consumption, body temperature, energy metabolism, and respiratory rate (Lubkin & Stricker-Krongrad, 1998; Samson et al., 1999; Shahid et al., 2011; Tupone et al., 2011; Yoshimichi et al., 2001). Although Hcrt is exclusively produced in the LH, DMH, and PFA (Baldo et al., 2003; DiLeone et al., 2003; Winsky-Sommerer et al., 2004), Hcrt neurons project widely throughout the brain (Peyron et al., 1998) and densely innervate such brain regions as the paraventricular nucleus of the thalamus (PVT), nucleus accumbens shell (NACsh), ventral pallidum (VP), VTA, CeA, BNST, and mPFC (Fig. 1), all of which are associated with arousal, motivation, and responses to stress and anxiety (e.g., Baldo et al., 2003; Peyron et al., 1998; and for an extensive review, see Grafe & Bhatnagar, 2018). The anxiolytic effects of Hcrt-r1 blockade, for example, were associated with a decrease in neural activity in both the BNST and CeA (Johnson et al., 2012). Furthermore, the Hcrt system has been shown to interact with brain stress systems, such as the norepinephrine system and extended amygdala (Berridge et al., 2010; Kilduff & Peyron, 2000; Li et al., 2014). Hypocretin-expressing neurons are predominantly located in a part of the hypothalamus that has been associated with fight-or-flight responses (Johnson et al., 2012). Additional behavioral evidence shows that acute stressors, such as footshocks, novel contexts, short-term forced swim stress, restraint stress, food restriction, panic-like states, and social stress all engage the Hcrt system in rodents and increase Hcrt signaling, possibly through inputs from the CRF system to the Hcrt system (Carrive, 2013; Yeoh et al., 2014; Johnson et al., 2012; Winsky-Sommerer, et al., 2004). In contrast, chronic, unavoidable stressors, such as those that are used in various animal models of depression (e.g., social defeat, chronic unpredictable mild stress, and tail suspension; Nocjar et al., 2012; Nollet et al., 2011) generally yield the downregulation of Hcrt transmission and cessation of coping behaviors. Similarly, Hcrt hypoactivity has been associated with depression symptoms in humans, although somewhat inconsistent results have been reported (Bayard & Dauvilliers, 2013; Johnson et al., 2012; Salomon et al., 2003; Yeoh et al., 2014). Overall, the findings that are reviewed above suggest that during stressful situations, the Hcrt system may initially respond by motivating adaptive coping behaviors when stressors are perceived as temporary. Once the stressor becomes unavoidable, chronic, and predictable, the consequent downregulation of the Hcrt system may yield behavioral traits that are reminiscent of depression or learned helplessness (Yeoh et al., 2014; Johnson et al., 2012; James et al., 2014).
Corticotrophin-Releasing Factor, Stress, and the “Dark Side” of Addiction
Corticotropin-releasing factor plays a prominent role in mediating brain stress responses. Given the role of stress in the development of dysregulated, compulsive drug use, CRF has received much research attention to uncover its role in the development and persistence of AUD (for a review, see Quadros et al., 2016). Corticotropin-releasing factor is a 41-amino-acid neuropeptide that interacts with two G-protein-coupled CRF receptors, CRF1 and CRF2, that are positively coupled to adenylate cyclase via Gs protein (Bale & Vale, 2004; Zorrilla & Koob, 2004; Zorrilla & Koob, 2004). Corticotropin-releasing factor is the primary activator of the HPA stress axis via anterior pituitary CRF1 receptor activation, which leads to an increase in adrenocorticotropic hormone (ACTH) secretion that ultimately stimulates glucocorticoid production and release from the adrenal gland (Vale et al., 1981). CRF1 receptors are widely distributed in stress-responsive brain regions (see Fig. 1), including the neocortex, the CeA, the medial septum, the hippocampus, the thalamus, the cerebellum, and autonomic midbrain and hindbrain nuclei (Grigoriadis et al., 1996; Primus et al., 1997; Sanchez et al., 1999; Van Pett et al., 2000).
Severe anxiety has been postulated to be caused by excessive alcohol consumption through neuroadaptive changes that heavily involve the CRF system (e.g., Heilig & Koob, 2007). These neuroadaptive changes appear to counter the hedonic effects of alcohol (i.e., the overexcitation of brain reward systems) and eventually exert a powerful influence on the motivation to use alcohol or other drugs of abuse. The behavioral process that is associated with this neuroadaptive or homeostatic change is well-characterized by Solomon’s opponent-process theory of motivation (Solomon & Corbit, 1974), which fundamentally coalesced the concept of motivation and the various emotional, affective, and hedonic states that are experienced during various stages of addiction. Briefly, these stages consist of the preoccupation/anticipation stage, binge/intoxication stage, and withdrawal/negative affect stage. Impulsivity in drug-seeking/taking may dominate at earlier stages, whereas later stages are characterized by compulsive drug- seeking/taking, in which the motivation for drug use shifts from positive reinforcement to negative reinforcement (Koob, 2009).
The transition from alcohol use to abuse and ultimately AUD is indeed mediated at least partially by changes in brain stress pathways (Lu & Richardson, 2014). These changes are forged through repeated cycles of intoxication and withdrawal (Breese et al., 2011; Heilig & Koob, 2007), and AUD eventually comes to be characterized by HPA axis impairments that contribute to psychological and behavioral symptoms, such as dysphoria, craving, and the propensity to relapse early in abstinence (Li et al., 2011; Lovallo, 2006; Sinha et al., 2011; Stephens & Wand, 2012). Importantly, alcohol is an acute stressor that activates the HPA axis, an effect that has been observed in studies featuring experimenter-administered alcohol Pruett et al., 1998; Rivier, 1993; Rivier et al., 1984; Selvage, 2012) as well as voluntary alcohol consumption (Richardson et al., 2008). In fact, a strong correlation exists between blood alcohol levels (BALs) in rats following acute dosing of alcohol and stress hormonal response measures (Ellis, 1966; Ogilvie et al., 1997a). Using a range of alcohol doses (0.5 - 4 g/kg), Ellis (1966) found that increases in plasma corticosterone levels strongly paralleled the dose of alcohol that elicited the response. Ogilvie et al. (1997a) expanded these findings by testing the potentially distinct effects of various routes of alcohol administration on HPA axis activity. These authors found that intraperitoneal (i.p) and intragastric (i.g) alcohol administration resulted in strong correlations between increases in ACTH levels and BALs. While it should be noted that the aforementioned methods of experimenter-administration can be in and of themselves stressful to rodents (e.g., due to forced injections, gavage, or restraint), similar effects of alcohol on stress responses have been observed with voluntary drinking in humans (Ekman et al., 1994; King et al., 2006; Schuckit et al., 1987; Lex et al., 1991). King et al. (2006) showed that a relatively high dose of alcohol (0.8 g/kg, or a four-drink equivalent) increased cortisol levels in subjects, although an interaction was found with the subjects’ reported drinking habits, such that heavy social drinkers exhibited an attenuated cortisol response following alcohol consumption compared with light social drinkers.
The differential response to the alcohol challenge that was reported by King et al. (2006) fits with other findings that demonstrated that chronic alcohol exposure suppresses the function of the neuroendocrine stress system, and this dysregulation has been suggested to contribute to symptoms of AUD. In rats, when neuroendocrine tolerance is challenged by an experimenter-administered dose of alcohol (e.g., 1 g/kg, i.v), the HPA response differs greatly, and depends on the individual’s prior experience with alcohol in an inversely proportional manner (Richardson et al., 2008). For example, in postdependent rats with an extensive history of alcohol consumption, the alcohol challenge dose (1 g/kg, i.v) resulted in a blunted endocrine response, whereas low-drinking nondependent rats that were given the same challenge dose exhibited a robust ACTH and corticosterone response. Similar findings have been reported in humans. Blunted basal levels of stress hormones were shown to predict alcohol craving in human alcoholics (Kiefer et al., 2002). A strong relationship was found between the suppression of HPA hormone levels, an increase in heavy drinking, and a tendency to relapse early during abstinence (Gianoulakis, 1998; Kiefer et al., 2002; Junghanns et al., 2003; Adinoff et al., 2005a,b,c; Sinha et al., 2011).
Alcohol administration stimulates the HPA axis to release ACTH and corticosterone. This is thought to be one possible mechanism by which alcohol-driven activity of the HPA axis results in neuroadaptive changes in the CRF system. High levels of corticosterone are known to increase CRF mRNA in the CEA and lateral BNST and decrease the presence of CRF mRNA in the PVN (Makino et al., 1994; Albeck et al., 1997; Schulkin et al., 1998; Shepard et al., 2000). The relationship among these structures is complex. Briefly, the CeA has a stimulatory effect on the CRF system in the PVN, whereas the lateral BNST plays both a stimulatory and inhibitory role. Importantly, this relationship is susceptible to “allostatic overload” under conditions of chronic high stress (Cullinan et al., 1993; Gray et al., 1993; Herman et al., 1994, 1996; Herman & Cuilliman, 1997; Schulkin et al., 1998; Schulkin, 1999), such as chronic repeated alcohol exposure. Ultimately, chronic alcohol exposure is thought to sensitize CRF activation in the extended amygdala and decrease HPA axis function via neuroadaptive changes that occur because of the constant stimulation of CRF expression in the CeA and lateral BNST. A decrease in HPA axis function is associated with a hypothesized shift in the ability to regulate stress-system responses to external stressors, thus contributing to a shift in the allostatic set point (Koob, 2003), the negative affective state that characterizes alcohol dependence and a shift to the “dark side” of addiction (Koob, 2010).
Involvement of the Medial Prefrontal Cortex in Drug Seeking
The mPFC is a key component of the mesocorticolimbic system and has been implicated in the regulation of drug-taking behavior (Kalivas, 2008, 2009). The mPFC receives inputs from both limbic and sensory structures, including the hippocampus and amygdala (Hoover & Vertes, 2007)-subsequently, the motivational significance and salience of drug-associated contexts and stimuli is processed through the mPFC, which allows the organism to take action accordingly (Kalivas, 2009; Lasseter et al., 2010). The mPFC is also critical for the ability of the organism to exert executive control over various drug-related behaviors, such as the selection and initiation of drug-seeking behavior – this is accomplished by virtue of mPFC projections to the NAC. This eventually results in the engagement of motor-related brain regions such as the dorsal striatum and ventral pallidum (Kalivas, 2009; Lasseter et al., 2010; Vertes, 2004).
The mPFC is divided neuroanatomically and functionally along a dorsal-ventral gradient. The dorsal region consists of the precentral cortex (PrC) and anterior cingulate cortex (ACC). The ventral region consists of the Prl, IL, and ventral orbital cortex (VO). Additional frontal structures such as the dorsal agranular insular cortex (AID) and dorsolateral orbitofrontal cortex share connections with the PrL and the IL and constitute portions of an extended prefrontal network (Conde et al., 1995; Kesner & Churchwell, 2011; Vertes, 2004). The dorsal-ventral division of the mPFC is commonly demarcated at the PrL-IL border, however. This is especially the case in behavioral studies because these two subregions of the mPFC are distinct and dissociable with regard to their anatomical connectivity and functionality (Vertes, 2004, 2006). For example, while the PFC generally projects to the BNST and CeA, IL projections to the BNST and CeA are far denser than PrL projections to those same regions (Vertes, 2004). This is an important distinction when considering compulsive drug-seeking behaviors that result from chronic alcohol exposure, as the IL was shown to undergo more robust changes than the PrL following chronic intermittent alcohol vapor exposure (Pleil et al., 2015), and these changes extended beyond overall, general changes in mPFC functionality that were observed following chronic alcohol exposure observed in earlier studies (Holmes et al., 2012; Hu et al., 2015; Kroener et al., 2012; Pava & Woodward, 2014).
Considering the observed differences in connectivity as well as alcohol-induced plasticity, it is unsurprising that the PrL and IL are also quite distinct from one another with respect to their roles in PFC-mediated behaviors (Sierra-Mercado et al., 2011; Holmes et al., 2012). Inactivation of the IL for example, was found to have no effect on fear expression in rats, whereas inactivation of the PrL impaired fear expression (Sierra-Mercado et al., 2011). Additionally, Sierra-Mercado et al. (2011) demonstrated that inactivation of the IL impaired the within-session acquisition of extinction and extinction memory; in contrast, PrL inactivation had no effect on extinction memory. Similarly, chronic intermittent alcohol exposure reduced the N-methyl-D-aspartate (NMDA) receptor-dependent burst firing of IL neurons but not PrL neurons, and this reduction was shown to mediate the extinction of fear-related behavior in mice (Holmes et al., 2012).
Though findings regarding fear-related behaviors seem to be consistent with the commonly proposed role of the PrL in response initiation (“go”) and the IL in response inhibition (“stop”), the exact manner in which the IL is involved in alcohol-seeking behavior specifically is currently the subject of some ambiguity. Meinhardt et al. (2013) for example reported a deficit in the expression of the metabotropic glutamate receptor 2 (mGluR2) autoreceptors after a history of alcohol dependence. Behaviorally, this deficit manifested as an increase in alcohol seeking and was subsequently reversed by local mGluR2 overexpression in the IL, suggesting an inhibitory role for the IL in cue-induced alcohol-seeking behavior. Knowing that mGluR2 is a presynaptic autoreceptor that negatively modulates glutamatergic transmission (e.g., Pin & Duvoisin, 1995; Schoepp, 2001), one interpretation of these findings is that the downregulation of IL mGluR2 after chronic alcohol exposure translates into increased glutamate release, and consequently, an increase in IL glutamatergic signaling into the NAc that in turn manifests as enhanced alcohol-seeking behavior. Similarly, Pfarr et al. (2015) used the Daun02 inactivation method in rats to demonstrate that the IL was specifically implicated in the inhibition of excessive alcohol seeking, in which inactivation of a neuronal ensemble in the IL but not PrL resulted in uncontrolled alcohol-seeking behavior. However, Pfarr et al. (2015) noted that the targeted neuronal ensemble was specific for cue-induced responses, because footshock stress-induced alcohol-seeking behavior was unaffected by the Daun02 manipulation. Since reinstatement of alcohol-seeking was measured using the same approach (i.e., lever pressing) across reinstatement experiments, it seems plausible that separate neuronal ensembles in the IL are each responsive to distinctive stimuli, but eventually act on the same behavior output through another functional ensemble which may be located elsewhere. It remains to be determined whether the putative stress-responsive neuronal ensemble exerts an excitatory or inhibitory influence on alcohol seeking, therefore necessitating further research.
Other sources suggest that the IL may fulfill a more general role in forming cue-reward associations, as it has been noted that cue-induced seeking of either alcohol or a non-drug reinforcer (saccharin) activated largely overlapping neuronal ensembles in the IL which were of similar size and organization (Pfarr et al., 2018). Further complicating the notion that the Prl and IL serve generally opposing “go-stop” functions with respect to behavior, Moorman & Aston-Jones (2015) found cue-evoked activity in both the PrL and the IL during reward-seeking and extinction, such that neuronal activity in both mPFC subregions appeared be contextually driven – that is, both PrL and IL encoded behavioral initiation during reward-seeking and behavioral inhibition during extinction. Dayas et al. (2007) had previously reported similar results, wherein the presence of a previously alcohol-associated olfactory cue increased c-fos expression in both the PrL and IL. Other investigators presented similarly equivocal findings. Baclofen/muscimol inactivation of the PrL for instance, attenuated ABA renewal of alcoholic beer seeking but augmented reacquisition, whereas baclofen/muscimol-induced inactivation of the IL had no effect on reinstatement or reacquisition of alcoholic beer seeking. Interestingly, IL inactivation increased the latencies with which rats responded when placed in the extinction context (Willcocks & McNally, 2013). In light of the findings reported by Moorman & Aston-Jones (2015), these findings may suggest a role for the IL driving contextually appropriate responses to relevant external stimuli, though more research is clearly needed to disambiguate the exact role of the IL in alcohol-seeking behavior.
Evidence of Hypocretin and Corticotropin-Releasing Factor Interaction (Fig. 1)
Corticotropin-releasing factor immunoreactive cells have been found in the mPFC (Swanson et al., 1983), a region that is known to be impaired following chronic exposure to drugs of abuse, including alcohol (Kalivas & Volkow, 2005; Kalivas et al., 2005). Evidence suggests that these cells are CRF-expressing interneurons, although this determination was made primarily according to cell morphology (Swanson et al., 1983). As such, the role of these putative CRF-expressing interneurons in compulsive drug-seeking behavior remains to be fully elucidated. Nevertheless, converging evidence suggests a link between impairments in mPFC-related cognitive function and CRF in the mPFC in the development of compulsive drug seeking (Koob, 2008). For example, rats that were given chronic intermittent access to two-bottle choice alcohol drinking presented an increase in the activation of GABA and CRF neurons in the mPFC during abstinence, and working memory deficits were correlated directly with greater alcohol consumption during acute abstinence (George et al., 2012).
Similarly, mounting evidence suggests that there Hcrt-CRF interactions take place in the brain, especially in regions that are responsible for mediating brain stress responses. Neuroanatomically, such structures as the hypothalamus, extended amygdala, and the paraventricular nucleus of the hypothalamus (PVN) are all targets of Hcrt-containing projections (Baldo et al., 2003; Ciriello et al., 2003; Schmitt, et al., 2012). The immunostaining for CRF1 and CRF2 receptors in the LH showed that a large proportion of Hcrt- immunoreactive neurons (~60%) also expressed CRF receptors, and CRF terminals made direct contact with Hcrt neurons that expressed both CRF1 and CRF2 receptors (Winsky-Sommer et al., 2004). Compellingly, Winsky-Sommerer et al. (2004) showed that the activity of hypothalamic Hcrt neurons ex vivo is directly and dose-dependently stimulated by CRF, thus providing confirmatory evidence that these two systems interact directly with one another in this region.
Behavioral evidence of an interaction between Hcrt and CRF has also been described. For example, intracerebroventricular (i.c.v) Hcrt administration was shown to enhance anxiety-like behavior in mice in the light-dark exploration test and in both mice and rats in the elevated plus maze (Suzuki et al., 2005) and lower brain reward function, reflected by elevations of ICSS reward thresholds (Boutrel et al., 2005). The latter effect was subsequently shown to be mediated by CRF (Hata et al., 2011). Intracerebroventricular Hcrt administration was also found to activate PVN CRF neurons (Sakamoto et al., 2004) and elevate HPA hormone levels (Kuru et al., 2000), suggesting the direct modulation of CRF-mediated neuroendocrine output by Hcrt. Furthermore, the anxiolytic-like effects of Hcrt blockade were associated with lower neural activation in the BNST and CeA (Johnson et al., 2012). Altogether, these data suggest that Hcrt interactions with CRF neurons in the PVN, BNST, and CeA are associated with anxiogenic and anhedonic states (Hata et al., 2011).
Hypocretin-CRF interactions have been shown to be reciprocal, as corticotropin-releasing factor provides excitatory inputs to Hcrt neurons and Hcrt neurons undergo CRF-dependent transcriptional activation following exposure to various stressors (Winsky-Sommerer et al., 2004). Acute withdrawal following chronic drug exposure induces a stress-like state of hyperarousal. Withdrawal from morphine and nicotine increased the transcriptional activity of Hcrt neurons in the LH and CRF neurons in the PVN and CeA (Georgesu et al., 2003; Laorden et al., 2012). Morphine withdrawal-induced activation of the PVN, BNST, and CeA was decreased by systemic Hcrt-r1 blockade (Laorden et al., 2012). Local Hcrt-r1 blockade in the PVN reduced the behavioral expression of nicotine withdrawal (Plaza-Zabala et al., 2012). These findings raise the possibility that the Hcrt modulation of CRF neurons participates in the chronically relapsing, negative affective state that characterizes drug addiction (i.e., the “dark side” of addiction). The findings by Boutrel et al. (2005) that central administration of Hcrt-1 reinstated cocaine-seeking behavior in a CRF-dependent manner supports this possibility. Boutrel et al. also found that the blockade of noradrenergic and CRF systems prevented the Hcrt-induced reinstatement of cocaine seeking, suggesting that Hcrt-1 reinstated cocaine-seeking behavior through the induction of a stress-like state via the noradrenergic and CRF systems. This was further corroborated by the finding that SB334867 blocked the footshock-induced reinstatement of previously extinguished cocaine seeking.
Disruptions of neuronal transmission and excitability in the IL that are caused by chronic alcohol exposure are much more pronounced than the observed disruptions of the PrL following similar alcohol treatment (Pliel et al., 2015), which may suggest that the IL has a disproportionate role relative to the PrL in alcohol-related behaviors. Promisingly, recent evidence seems to indicate that this disruption of IL function is likely a critical factor in the development of dysregulated alcohol-seeking behavior (Meinhardt et al., 2013; Pfarr et al., 2015), and evidence seems to suggest that CRF may play an important role in this regard. The presence of CRF-stained cells in limbic PFC regions such as the infralimbic cortex (Swanson et al., 1983) is also a promising indicator, although it must be stressed that cortical CRF neurons have only been sparsely studied, and the exact role of these putative CRF-expressing interneurons is unknown. However, it has been reported that rats given chronic intermittent access to two-bottle choice alcohol drinking presented an increase in the mPFC CRF neurons (in addition to GABAergic interneurons) during abstinence, and the working memory deficits observed during abstinence were correlated directly with elevated alcohol consumption during acute abstinence (George et al., 2012). The upregulation of these putative CRF-expressing interneurons in tandem with the observed upregulation of GABAergic interneurons may eventually result in a decrease in glutamatergic activity in the mPFC, consistent with earlier reports (Meinhardt et al., 2013; Kufahl et al., 2011; Sidhpura et al., 2010; Zhao et al., 2006). The glutamatergic dysregulation observed by Meinhardt et al. (2013) may be due to an upregulation of GABAergic interneurons, leading to general mPFC hypofunction which may manifest as, for example, a deficit in working memory as demonstrated by George et al. (2012). Additionally, George et al. (2012) report that although there was an increase in the number of mPFC CRF neurons during alcohol withdrawal, those CRF neurons did not show Fos activation 24 h into the withdrawal period, which was markedly different from what they observed in GABAergic neurons. However, given that CRF has been shown to facilitate GABAergic signaling (Roberto et al., 2010) and the number of CRF neurons was increased, it seems quite plausible that CRF was indeed involved in the working-memory deficits reported by George et al (2012).
There is also evidence to suggest that mPFC CRF neurons interact with Hcrt in the IL. The IL receives Hcrt projections from the LH (Date et al., 1999; see Fig. 1), and Hcrt receptor mRNA has been detected in the IL (Marcus et al., 2001). Conversely, Hcrt neurons in the hypothalamus have been found to express both CRF receptor subtypes, and the application of CRF to hypothalamic slices ex vivo was shown to depolarize the membrane potential of these cells (Winsky-Sommerer et al., 2004). Intriguingly, it has also been reported that the IL projections to the LH are significant (Floyd et al., 2001; Vertes, 2004; and see Fig. 1), raising the possibility that chronic alcohol-induced dysregulation of IL function (e.g., Pfarr et al., 2015; Pliel et al., 2015) may influence the activity of hypothalamic Hcrt neurons in return. Considering the reciprocal nature of Hcrt-CRF interactions (Winsky-Sommerer et al., 2004) and the dysregulation of CRF that is observed following chronic alcohol exposure, it may be the case that IL dysregulation feeds back to LH to influence Hcrt expression in the IL.
Finally, additional evidence of Hcrt-CRF interactions came from Richards et al. (2008), who examined the role of Hcrt-r1 during the reinstatement of alcohol-seeking behavior that was induced by the pharmacological stressor yohimbine. Yohimbine activates c-fos and CRF mRNA in brain regions that also express c-fos and CRF mRNA following footshock-induced stress (Funk et al., 2006). Behaviorally, yohimbine induces a stress-like response in both humans and laboratory animals (Bremner et al., 1996a, b; Vythilingam et al., 2000). Importantly, yohimbine is known to upregulate CRF expression in the CeA (Funk et al., 2006), an effect that is analogous to the long-term upregulation of CRF1 receptors during withdrawal following a history of alcohol dependence (Merlo-Pich et al., 1995). Richards et al. (2008) showed that SB334867 (5 and 10 mg/kg) significantly decreased the yohimbine-induced reinstatement of alcohol-seeking behavior (see Table 1), suggesting that Hcrt-r1 blockade can prevent the CRF-mediated stress response, thus supporting the possibility of a Hcrt-CRF functional interaction.
Concluding Statements
Although a burgeoning number of studies demonstrate interactions between Hcrt and CRF (e.g., Boutrel et al., 2005; Hata et al., 2011; Johnson et al., 2012; Kuru et al., 2000; Laorden et al., 2012; Sakamoto et al., 2004; Winsky- Sommerer et al., 2004), the clearest findings that demonstrate that Hcrt-CRF interactions are a critical contributor to the maintenance of compulsive drug-taking/seeking behavior still come from an earlier study by Boutrel et al. (2005), who convincingly demonstrated that the central administration of Hcrt reinstated cocaine-seeking behavior in a CRF-dependent manner, providing significant support for the hypothesis that the Hcrt modulation of CRF neurons participates in the maintenance and persistence of the negative affective state that characterizes drug addiction and predisposes addicted individuals to relapse. Unclear from the results of Boutrel et al. (2005) is whether this form of Hcrt modulation of CRF involves CRF neurons in the PVN, BNST, CeA, or other CRF-rich regions such as the mPFC. The specific role of the IL with regard to Hcrt-CRF interactions is also currently unknown, and more work is required to elucidate the purported role of the IL in this process. It is also unclear whether CRF neurons found in the mPFC are interneurons as proposed by Swanson et al. (1983). Interestingly, although Pfarr et al. (2015) confirmed that the alcohol-cue responsive functional neuronal ensemble does contain interneurons, only <10% of the functional ensemble were identified as such. Furthermore, it is uncertain whether those interneurons expressed CRF, and importantly, Pfarr et al. (2015) note that the majority of neurons (~70%) found in the functional ensemble are principally glutamatergic neurons. This latter finding further supports the notion that glutamatergic transmission is critically important in dysregulated alcohol-seeking behaviors – especially in light of findings that utilized various reinstatement procedures to demonstrate a role for group II metabotropic glutamate receptors (mGluR2/3) in dysregulated alcohol-related behaviors (Kufahl et al., 2011; Sidhpura et al., 2010; Zhao et al., 2006). Future studies should confirm the exact nature of CRF-expressing neurons in the mPFC, and the IL specifically, as this may have potentially important consequences for how Hcrt-CRF interactions in this region.
Nevertheless, as has been discussed, several lines of evidence suggest that the IL is an excellent candidate for further investigations. Given the interactions between Hcrt and CRF in regions that mediate stress responses (Winskey-Sommerer et al., 2004), the presence of putative CRF-expressing interneurons in the mPFC and the IL (Swanson et al., 1983; Fig. 1), the reciprocal interactions between Hcrt and CRF (Winskey-Sommerer et al., 2004), and the observation that the IL sends robust projections back to the LH (Floyd et al., 2001; Vertes, 2004; Fig. 1) this suggests that disruptions to Hcrt-CRF interactions in the IL may have downstream effects on the hypothalamic Hcrt neurons, and it seems highly plausible that these relationships between Hcrt and CRF will have important ramifications in determining the driver of dysregulated alcohol-seeking behavior that is indicative of AUD.
Acknowledgements
This is publication number 29853 from Scripps Research. The authors thank Michael Arends for assistance with manuscript preparation. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant no. AA024146, AA006420, AA022249, AA026999, and T32 AA007456).
Footnotes
Conflicts of Interest
None
References
- Adinoff B, Junghanns K, Kiefer F, & Frishnan-Sarin S 2005a. Suppression of the HPA axis stress-response: implications for relapse. Alcoholism: Clinical and Experimental Research, 29, 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B Krebaum SR, Chandler PA, Ye W, Brown MB, & Williams MJ 2005b. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcoholism: Clinical and Experimental Research, 29, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B Krebaum SR, Chandler PA, Ye W, Brown MB, & Williams MJ 2005c. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcoholism: Clinical and Experimental Research, 29, 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I & Maroun M 2007. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007, 30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nijulina J, McEwen BS, & Sakai RR 1997. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. The Journal of Neuroscience, 17, 4895–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, & Kukkonen JP 2003. Distinct recognition of ox1 and ox2 receptors by orexin peptides. Journal of Pharmacology and Experimental Therapeutics, 305, 507–14. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, Adams BL, Jesudason CD, & Rorick-Kehn LM 2014. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G & Harris GC 2004. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology, 47, 167–179. [DOI] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou L-C, Lawrence AJ, Muschamp JW, Patkar O, Tung L-W, & Borgland SL 2015. Orexin/hypocretin role in reward: implications for opioid and other addictions. British Journal of Pharmacology, 172, 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, & Kelley AE 2003. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. Journal of Comparative Neurology, 464, 220–237. [DOI] [PubMed] [Google Scholar]
- Bale TL & Vale WW 2004. Crf and Crf receptors: Role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology, 44, 525–557. [DOI] [PubMed] [Google Scholar]
- Bayard S & Dauvilliers YA 2013. Reward-based behaviors and emotional processing in human with narcolepsy-cataplexy. Frontiers in Behavioral Neuroscience, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC 2012. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research, 34, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, & Vittoz NM 2010. Hypocretin/orexin in arousal and stress. Brain Research, 1314, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, & Everitt BJ 2008. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 57, 432–441. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, & Bonci A 2006. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron, 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, & de Lecea L 2005. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. PNAS, 102, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, & Heilig M 2011. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & Therapeutics, 129, 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS 1996a. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies. Synapse, 23, 28–38. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS 1996b. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse, 23, 39–51. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, & Ron D 2008. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. PNAS, 105, 8114–8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P 2013. Orexin, orexin receptor antagonists and central cardiovascular control. Frontiers in Neuroscience, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM & Aston-Jones G 2013. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology, 226, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, & Buck KJ 2011. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcoholism: Clinical and Experimental Research, 35, 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, & Massi M 2003. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. Journal of Neuroscience, 23, 9445–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Rosas-Arellano MP, Solano-Flores LP, & de Oliveira CV 2003. Identification of neurons containing orexin-b (hypocretin-2) immunoreactivity in limbic structures. Brain Research, 967, 123–31. [DOI] [PubMed] [Google Scholar]
- Conde F, Marie-Lepoivre E, Audinat E, & Crepel F 1995. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. Journal of Comparative Neurology, 352, 567–593. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, & Watson SJ 1993. Ventral subicular interaction with the hypothalamic paraventicular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. Journal of Comparative Neurology, 332, 1–20. [DOI] [PubMed] [Google Scholar]
- Daglish MR & Nutt DJ 2003. Brain imaging studies in human addicts. European Neuropsychopharmacology, 13, 453–458. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, & Weiss F 2007. Distinct patterns of neural activation associated with ethanol seeking: Effects of naltrexone. Biological Psychiatry, 61, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F 2008. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biological Psychiatry, 63, 152–157. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd , Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, & Sutcliffe JG (1998). The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 95, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, & Nestler EJ 2003. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sciences, 73, 759–768. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, & Bloom SR 1999. The effect of the orexins on food intake: Comparison with neuropeptide y, melanin-concentrating hormone and galanin. Journal of Endocrinology. 160, R7–R12. [DOI] [PubMed] [Google Scholar]
- Ekman AC, Vakkuri O, Vuolteenaho O, & Lepopaluoto J 1994. Delayed pro-opiomelanocortin activation after ethanol intake in man. Alcohol: Clinical & Experimental Research, 18, 1226–1229. [DOI] [PubMed] [Google Scholar]
- Ellis FW 1966. Effect of ethanol on plasma corticosterone levels. Journal of Pharmacology and Experimental Therapeutics, 153, 121–127. [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DCS, & Jones SR 2011. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology, 214, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, & Jones SR 2010. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. European Journal of Neuroscience, 31, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, & Robbins TW 2001. The neuropsychological basis of addictive behaviour. Brain Research: Brain Research Reviews, 36, 129–38. [DOI] [PubMed] [Google Scholar]
- Fadel J & Deutch A 2002. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience, 111, 379–387. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, & Bandler R 2001. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. The Journal of Comparative Neurology, 432, 307–328. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, & Koob GF 2006. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rat. Journal of Neuroscience, 26, 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield J, Brisbare-Roch C, Jenck F, & Boss C 2010. Orexin receptor antagonists: A new concept in cns disorders? ChemMedChem. 5, 1197–1214. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Horvath TL, & Xie XS 2011. Direct inhibition of hypocretin/orexin neurons in the lateral hypothalamus by nociceptin/orphanin fq blocks stress-induced analgesia in rats. Neuropharmacology. 60, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, & Koob GF 2012. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 109, 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, & DiLeone RJ 2003. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. Journal of Neuroscience. 23, 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C 1998. Alcohol-seeking behavior: the roles of the hypothalamic-pituitary-adrenal axis and the endogenous opioid system. Alcohol Heath & Research World, 22, 202–210. [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ & Volkow ND 2002. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the prefrontal cortex. American Journal of Psychiatry, 159, 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA & Bhatnagar S 2018. Orexins and stress. Frontiers in Neuroendocrinology, 51, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, & Van de Kar LD 1993. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH, and corticosterone. Neuroendocrinology, 57, 517–524. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu SJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N & De Souza EB 1996. 125I-Tyro-sauvagine: A novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Molecular Pharmacology, 50, 679–686. [PubMed] [Google Scholar]
- Hahn J, Hopf FW, & Bonci A 2009. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. Journal of Neuroscience, 29, 6335–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437, 556–559. [DOI] [PubMed] [Google Scholar]
- Hata T, Chen J, Ebihara K, Date Y, Ishida Y, & Nakahara D 2011. Intra-ventral tegmental area or intracerebroventricular orexin-a increases the intra-cranial self-stimulation threshold via activation of the corticotropin-releasing factor system in rats. European Journal of Neuroscience. 34, 816–826. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, & Arch JR 2002. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regulatory Peptides, 104, 153–159. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, & Arch JR 2000. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regulatory Peptides, 96, 45–51. [DOI] [PubMed] [Google Scholar]
- Heilig M, & Koob GF 2007. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neuroscience, 30, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Mir J, Grusser SM, Grace AA, & Wrase J 2010. Alcohol craving and relapse prediction: Imaging studies in Kuhn CM & Koob GF (eds.), Advances in the Neuroscience of Addiction, 2nd edition Boca Raton (FL):CRC Press/Taylor & Francis, chapter 4. [PubMed] [Google Scholar]
- Heinz A, Siessmier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, & Bartenstein P 2005. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: A combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. American Journal of Psychiatry, 162, 1515–1520. [DOI] [PubMed] [Google Scholar]
- Herman JP & Cullinan WE 1997. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience, 20, 78–84. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, & Watson SJ 1994. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. The Journal of Neuroendocrinology, 6, 433–442. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, & Cullinan WE 1996. Neuronal circuit regulation of the hypothalamic-pituitary-adrenocortical stress axis. Critical Reviews in Neurobiology, 10, 371–394. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, & Kenny PJ 2008. Insular hypocretin transmission regulates nicotine reward. PNAS, 105, 19480–19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pliel KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-CInar O, & Camp M 2012. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience, 15, 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu Z-w., Gao XB, Thurmon JJ, Marinelli M, & DiLeone RJ 2006. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron, 51, 801–810. [DOI] [PubMed] [Google Scholar]
- Hoover WB & Vertes RP 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function, 212, 149–179. [DOI] [PubMed] [Google Scholar]
- Hu W, Morris B, Carrasco A, & Kroener S 2015. Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice. Alcohol Clin Exp Res. 39, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, & Murakami N 2000. Possible involvement of orexin in the stress reaction in rats. Biochemical and Biophysical Research Communications, 270, 318–323. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ 2002. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. Journal of Neuroscience, 22, 6247–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, & Dayas CV 2014. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Frontiers in Behavioral Neuroscience, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH & Chaudhri N 2010. The potent effect of environmental context on relapse to alcohol-seeking after extinction Open Addiction Journal, 3, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, & Shekhar A 2012. Orexin, stress, and anxiety/panic states. Progress in Brain Research, 198, 133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, & Shekhar A 2012. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiology & Behavior. 107, 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, & Driessen M 2003. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and Alcoholism. 38, 189–193. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, & Lawrence AJ 2011. The orexin1 receptor antagonist SB 334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Research, 1391, 54–59. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, & Lawrence AJ 2011. Discrete cue-conditioned alcohol-seeking after protracted abstinence: Pattern of neural activation and involvement of orexin1 receptors. The British Journal of Pharmacology, 162, 880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW 2008. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotoxicity Research, 14, 185–189. [DOI] [PubMed] [Google Scholar]
- Kalivas PW 2009. The glutamate homeostasis hypothesis of addiction. Nature Reviews Neuroscience, 10, 561–572. [DOI] [PubMed] [Google Scholar]
- Kalivas PW & O’Brien C 2008. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology, 33, 166–180. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Volkow ND 2005. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry, 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND, & Seamans J 2005. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron, 45, 647–650. [DOI] [PubMed] [Google Scholar]
- Kelley AE & Berridge KC 2002. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience, 22, 3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP & Churchwell JC 2011. An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of Learning and Memory, 96, 417–431. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, & Wiedemann K 2002. Alcohol self-administration, craving, and HPA-axis activity: an intriguing relationship. Psychopharmacology (Berl), 164, 239–240. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, & Peyron C 2000. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends in Neuroscience, 23, 359–365. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, & Lin S 2006. Attenuated cortisol response to alcohol in heavy social drinkers. International Journal of Psychophysiology, 59, 203–209. [DOI] [PubMed] [Google Scholar]
- Koob GF 2008. A role for brain stress systems in addiction. Neuron. 59, 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF 2009. Brain stress systems in the amygdala and addiction. Brain Research, 1293, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF 2010. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Research, 1314, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF & Kreek MJ 2007. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. American Journal of Psychiatry, 164, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF & Le Moal M 1997. Drug abuse: Hedonic homeostatic dysregulation. Science, 278, 52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF & Le Moal M 2001. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24, 97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M 2005. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience, 8, 1442–1444. [DOI] [PubMed] [Google Scholar]
- Koob GF & Le Moal M 2008. Addiction and the brain antireward system. Annual Review of Psychology, 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, & Chandler LJ 2012. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One, 7, e37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, & Yamashita H 2000. Centrally administered orexin/hypocretin activates hpa axis in rats. Neuroreport. 11, 1977–1980. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, & Weiss F 2011. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR2/3 agonist LY379268 and increased functional activity of mGluR2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology, 36, 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, & Herdon HJ 2009. Characterization of the binding of [3H]-SB674042, a novel nonpeptide antagonist to the human orexin-1 receptor. British Journal of Pharmacology, 141, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laorden ML, Ferenczi S, Pinter-Kubler B, Gonzalez-Martin LL, Lasheras MC, Kovacs KJ, Milanes MV, & Nunez C 2012. Hypothalamic orexin--a neurons are involved in the response of the brain stress system to morphine withdrawal. PLoS One. 7, e36871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, & Fuchs RA 2010. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Current Topics in Behavioral Neurosciences, 3, 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, & Oldfield B 2006. The orexin system regulates alcohol-seeking in rats. British Journal of Pharmacology, 148, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestang I, Cardo B, Roy MT, & Velley L 1985. Electrical self-stimulation deficits in the anterior and posterior parts of the medial forebrain bundle after ibotenic acid lesion of the middle lateral hypothalamus. Neuroscience, 15, 379–388. [DOI] [PubMed] [Google Scholar]
- Lex BW, Ellingboe JE, Teoh SK, Mendelson JH, & Rhoades E 1991. Prolactin and cortisol levels following acute alcohol challenges in women with and without a family history of alcoholism. Alcohol, 8, 383–387. [DOI] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, & Ye J–H . 2011. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addiction Biology, 16, 600–614. [DOI] [PubMed] [Google Scholar]
- Li J, Hu Z, & de Lecea L 2014. The hypocretins/orexins: integrators of multiple physiological functions. The British Journal of Pharmacology, 171, 332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR 2006. Cortisol secretion patterns in addiction and addiction risk. International Journal of Psychophysiology, 59, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-L & Richardson AN 2014. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience, 277, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkin M, & Stricker-Krongrad A 1998. Independent feeding and metabolic actions of orexins in mice. Biochemical and Biophysical Research Communications, 253, 241–245. [DOI] [PubMed] [Google Scholar]
- Makino S Gold PW, & Shulkin J 1994. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis: comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Research, 657, 141–149. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK 2001. Journal of Comparative Neurology, 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Cauvi G, Kerr TM, & Weiss F 2016. Differential role of hypothalamic orexin/hypocretin neurons in reward seeking motivated by cocaine versus palatable food. Addiction Biology, 23, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, & Weiss F 2014a. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: Comparison with natural reward seeking. Neuroreport, 25, 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F 2014b. N-(2-methyl-6-benzoxazolyl)-N’-1,5-napthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: Comparison with natural reward seeking. Addiction Biology, 19, 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, & Weiss F 2010. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociception/orphanin FQ, and orexin/hypocretin. Brain Research, 145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, & Kalivas PW 2001. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience, 21, 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, & Sommer WH 2013. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. Journal of Neuroscience, 33, 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo-Pich EM, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, & Weiss F 1995. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. Journal of Neuroscience, 15, 5439–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS & Goldsmith J 2001. Craving for alcohol and drugs in animals and humans. Journal of Addictive Diseases, 20, 87–104. [DOI] [PubMed] [Google Scholar]
- Moorman DE & Aston-Jones G 2009. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol-preferring Spraue-Dawley rats. Alcohol, 43, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE & Aston-Jones G 2015. Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci U S A. 112, 9472–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, & Aston-Jones G 2015. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res, 1628(Pt A), 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, & Panksepp J 2012. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience, 218, 138–153. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, & Leman S 2011. Activation of orexin neurons in doromedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology, 61, 336–346. [DOI] [PubMed] [Google Scholar]
- Oglivie K, Lee S, & Rivier C 1997a. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol: Clinical and Experimental Research, 21, 467–476. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koening HN, Nannini MA, & Hodge CW 2002. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology, Biochemistry, & Behavior, 72, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ & Woodward JJ 2014. Chronic ethanol alters network activity and endocannabinoid signaling in the prefrontal cortex. Front Integr Neurosci. 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Watson C 1986. The rat brain in stereotaxic coordinates, Academic Press, San Diego. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, & Kilduff TS 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience, 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, & Sommer WH 2015. Losing control: Excessive alcohol seeking after selective inactivation of cue-responsive neurons in the infralimbic cortex. Journal of Neuroscience, 35, 10750–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Schaff L, Reinert JK, Paul E, Herrmannsdorfer F, Robmanith M, Kuner T, Hansson AC, Spanagel R, Korber C, & Sommer WH 2018. Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. The Journal of Neuroscience, 38, 14, 3507–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP & Duvosin R 1995. The metabotropic glutamate receptors: structure and functions. Neuropharmacology, 34, 1–26. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Maldonado R, & Berrendero F 2012. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biological Psychiatry, 71, 214–223. [DOI] [PubMed] [Google Scholar]
- Pliel KE, Lowery-Gionta E, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL 2015. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology, 99, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, & Gallager DW 1997. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology, 17, 308–316 [DOI] [PubMed] [Google Scholar]
- Pruett SB, Collier SD, & Wu WJ 1998. Ethanol-induced activation of the hypothalamic-pituitary-adrenal axis in a mouse model for binge-drinking: role of Ro 15-4513-sensitive gamma aminobutyric acid receptors, tolerance, and relevance to humans. Life Sciences, 63, 1137–1146 [DOI] [PubMed] [Google Scholar]
- Quadros IMH, Macedo GC, Domingues LP, & Favoretto CA 2016. An update on CRF mechanisms underlying alcohol use disorders and dependence. Frontiers in Endocrinology, 7, 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, & Bartlett SE 2008. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology, 199, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL 2008. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Behavioral Neuroscience, 28, 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C 1993. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol: Clinical and Experimental Research, 17, 127–131. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, & Vale W 1984. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). Journal of Pharmacology and Experimental Therapeutics, 229, 127–131. [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorilla EP, Koob GF, Siggins GR, & Parsons LH 2010. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biological Psychiatry, 67, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, & Polidori C 2008. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: Evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl), 196, 523–531. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, & Ueta Y 2004. Centrally administered orexin-a activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: Possible involvement of central orexins on stress-activated central crf neurons. Regulatory Peptides. 118, 183–191. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H,…Yanagisawa M 1998. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, & Mignot E 2003. Diurnal variation of CSF hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biological Psychiatry, 54, 96–104. [DOI] [PubMed] [Google Scholar]
- Samson WK, Gosnell B, Chang JK, Resch ZT, & Murphy TC 1999. Cardiovascular regulatory actions of the hypocretins in brain. Brain Research. 831, 248–253. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM & Insel TR 1999. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. Journal of Comparative Neurology, 308, 365–377. [PubMed] [Google Scholar]
- Scammell TE, & Winrow CJ 2011. Orexin receptors: Pharmacology and therapeutic opportunities. Annual Review of Pharmacology and Toxicology, 51, 243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, & Were A 2012. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain structure & function, 217, 233–256. [DOI] [PubMed] [Google Scholar]
- Schoepp DD 2001. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. Journal of Pharmacology and Experimental Therapeutics, 299, 12–20. [PubMed] [Google Scholar]
- Schuckit MA, Gold E, & Risch C 1987. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Archives of General Psychiatry, 44, 942–945. [DOI] [PubMed] [Google Scholar]
- Schulkin J 1999. Corticotropin-releasing hormone signals adversity in both the placenta and the brain: regulation by glucocorticoids and allostatic overload. The Journal of Endocrinology, 161, 349–356. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, & McEwen BS 1998. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology, 23, 219–243. [DOI] [PubMed] [Google Scholar]
- Selvage D 2012. Roles of the locus coreulus and adrenergic receptors in brain-mediated hypothalamic-pituitary-adrenal axis responses to intracerebroventricular alcohol. Alcohol: Clinical and Experimental Research, 36, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, & Pilowsky PM 2011. Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. British Journal of Pharmacology, 162, 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, & Shaham Y 2010. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Research, 1314, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Finnie PS, Quinn T, Tobin S, & Wahi P 2006. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Pharmacology, 187, 376–384. [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, & Shaham Y 2001. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. The Journal of Neuroscience, 21, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, & Myers DA 2000. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Research, 861, 288–295. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, & Kannan H 1999. Sympathetic and cardiovascular actions of orexins in conscious rats. American Journal of Physiology. 277(6 Pt 2), R1780–R1785. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, & Galici R 2011. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology, 215, 191–203. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, & Martin-Fardon R 2010. Effects of the mGluR2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol-seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biological Psychiatry, 69, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coeano N, & Quirk GJ 2011. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong K-IA, Hansen J, Tuit K, & Kreek MJ 2011. Effects of adrenal sensitivity, stress- and cue-induced craving, an anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry, 68, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, & Aston-Jones G 2010. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology, 58, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD 1974. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review, 81, 119–145. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, & Heilig MA 2008. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biological Psychiatry, 63, 139–145. [DOI] [PubMed] [Google Scholar]
- Steketee JD & Kalivas PW 2011. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Review, 63, 348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MAC & Wand G 2012. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Research: Current Reviews, 34, 468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG & de Lecea L 2002. The hypocretins: Setting the arousal threshold. Nature Reviews Neuroscience, 3, 339–349. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, & Sawai T 2005. Orexin-a (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Research. 1044, 116–121. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, & Vale W 1983. The organization of ovine corticotropin-releasing factor (crf) immunohistochemical study. Neuroendocrinology. 36, 165–186. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, & Mignot E 2002. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annual Review of Neuroscience, 25, 283–313. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, & Kotz CM 2010. Hypocretin/orexin and energy expenditure. Acta Physiologica (Oxf). 198, 303–312. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, & McGregor IS 1998. Possible neural substrates of beer-craving in rats. Neuroscience Letters, 252, 99–102 [DOI] [PubMed] [Google Scholar]
- Tsujino N & Sakurai T 2009. Orexin/hypocretin: A neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacological Review. 61, 162–176. [DOI] [PubMed] [Google Scholar]
- Tsujino N & Sakurai T 2013. Role of orexin in modulating arousal, feeding, and motivation. Frontiers in Behavioral Neuroscience, 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, & Morrison SF 2011. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. Journal of Neuroscience. 31, 15944–15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Platt D, Rowlett J, Ruedi-Bettschen D, & Spealman R 2007. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: A role for opioid and stress-related mechanisms. Journal of Pharmacology and Experimental Therapeutics, 323, 525–533. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C & Rivier J 1981. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, & Sawchenko PE 2000. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. Journal of Comparative Neurology, 428, 191–212. [DOI] [PubMed] [Google Scholar]
- Vertes RP 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Vertes RP 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience, 142, 1–20. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson GM, Owens MJ., Halaszynski TM, Bremner JD, Carpenter LL, Heninger GR, Neeroff CB, & Charney DS 2000. Cerebrospinal fluid corticotropin-releasing hormone in healthy humans: Effects of yohimbine and naloxone. The Journal of Clinical Endocrinology & Metabolism, 85, 4138–4145. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PEM 2009. Phasic dopamine release in appetitive behaviors and drug addiction. Current Drug Abuse Issues, 2, 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise R, & You Z 2005. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: A role in stress-induced relapse to drug seeking. Journal of Neuroscience, 25, 5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Carey RJ, & Nolley D 1971. Ethanol drinking elicited during electrical stimulation of the lateral hypothalamus. Physiol Behav, 7, 793–795. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, & McNally GP 2013. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Behavioral Neuroscience, 37, 259–268. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, & Yanagisawa M 2001. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annual Review of Neuroscience, 24, 429–458. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, & De Lecea L 2003. The role of the hypocretinergic system in the integration of networks that dictate the states of arousal. Drug News Perspect, 16, 504–512. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, & de Lecea L 2004. Interaction between the corticotropin- releasing factor system and hypocretins (orexins): A novel circuit mediating stress response. Journal of Neuroscience, 24, 11439–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, & Dayas CV 2014. Orexin antagonist for neuropsychiatric disease: Progress and potential pitfalls. Frontiers in Neuroscience, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimichi G, Yoshimatsu H, Masaki T, & Sakata T 2001. Orexin-a regulates body temperature in coordination with arousal status. Experimental Biology and Medicine (Maywood), 226, 468–476. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, & Weiss F 2006. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. Journal of Neuroscience, 26, 9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP & Koob GF 2004. The therapeutic potential of crf1 antagonists for anxiety. Expert Opinion on Investigational Drugs, 13, 799–828 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP & Koob GF 2004. The roles of urocorins 1, 2 and 3 in the brain, In: Steckler T, Kalin NH, ReuL JMHM (eds). Handbook of Stress, Immunology and Behaviour. New York: Elsevier Science. [Google Scholar]