Abstract
To enhance infection, enveloped viruses exploit adhesion molecules expressed on the surface of host cells. Specifically, phosphatidylserine (PS) receptors—including members of the human T-cell immunoglobulin and mucin domain (TIM)-family—have gained attention for their ability to mediate the entry of many enveloped viruses. However, recent evidence that TIM-1 can restrict viral release reveals a new role for these PS receptors. Additionally, viral factors such as the HIV-1 accessory protein Nef can antagonize this antiviral activity of TIM-1 while host restriction factors such as SERINC5 can enhance it. In this review, we examine the various roles of PS receptors, specifically TIM-family proteins, and the intricate relationship between host and viral factors. Elucidating the multifunctional roles of PS receptors in virus-host interaction is important for understanding viral pathogenesis and developing novel antiviral therapeutics.
Keywords: phosphatidylserine receptor, viral entry, viral release, antagonism, HIV
Cellular Restriction Factors and TIM-Family Proteins
To disrupt viral replication, hosts have developed a myriad of proteins that interfere with the viral life-cycle including those dubbed “restriction factors.” These restriction factors typically share several characteristics. In addition to inhibiting viral replication, they are often induced by type I and III interferons, antagonized by viral factors, and undergo positive selection as hosts evolve to evade viral antagonism [1,2]. Such restriction factors can target any stage of the viral life-cycle. Examples of this are APOBEC, which causes hypermutation of the viral genome, SAMHD1, which depletes cellular dNTP levels to prevent complete viral genome processing, and tetherin, which inhibits the release of budding virions [1,3]. To evade these restriction factors, viruses have also evolved mechanisms to counteract the activity of many such restriction factors. For example, the human immunodeficiency virus (HIV) has several accessory proteins dedicated to disrupting cellular machinery to enhance viral replication [4]. These include Vif, Vpr, Vpu, and Nef, which are not required for viral replication in permissive cell lines but are needed for a productive infection in vivo, in part, because of their ability to antagonize host restriction factors [2,3,5]. Although efficient viral infections often overcome restriction factors, these factors do prevent the cross-species transmission of viruses, which typically have not evolved to antagonize the restriction factors of their non-native host [6–9]. As such, it is critical to investigate the functions of host restriction factors to further our understanding of viral cross-species transmission, particularly the circumstances for the introduction of new viruses into the human population, viral pathogenesis, and to inform the development of novel treatments to counteract this viral antagonism of host restriction factors.
The human T-cell immunoglobulin and mucin domain (TIM) proteins form a family of phosphatidylserine (PS) receptors that have varying roles in immunity [10]. TIM-1 is mainly expressed in Th2 cells and acts as a costimulatory molecule for T cell activation while TIM-3 is expressed in Th1 and Tc1 cells and acts as an apoptotic signal [10,11]. Additionally, TIM-3 and TIM-4 are expressed in dendritic cells and antigen-presenting cells, respectively, and both mediate the phagocytosis of apoptotic cells [11]. As a result, TIM proteins have also been linked to allergic and atopic diseases, including asthma [12–15]. Additionally, TIM-3 has recently received attention for its role as a possible target for antitumor immunotherapies [16]. One emerging theme is the complicated relationship of the TIM-family proteins with viral infections. On one hand, TIM-family proteins enhance viral entry into cells; on the other hand, TIM proteins block viral release from the plasma membrane. Of note, unlike typical restriction factors such as tetherin, TIM proteins are not induced by type I and type III IFN and therefore may not be classified as typical restriction factors [17]. This review will examine the multifaced functions of TIM-family proteins in viral infection, with particular focus on viral entry, release, as well as viral and cellular proteins that functionally interact with TIMs.
Role of TIM-family Proteins in Viral Entry
The TIM proteins first gained their attention in virology for their role in enhancing viral entry. In fact, TIM proteins were originally identified as “receptors” for the non-enveloped hepatitis A virus (HAV) [18,19], although several recent studies demonstrate a quasi-enveloped stage of HAV [20] and also disputed the claim of TIM-proteins as “receptors” [21,22]. Research in the last few years has clarified that the TIM family proteins, in particular TIM-1, function as entry co-factors for a number of enveloped viruses, which include Ebolavirus (EBOV), Marburgvirus, Dengue virus, Japanese encephalitis virus, and others (Table 1). This is thought to occur through the PS-binding activity of the TIM-family proteins (Figure 1) [23–25]. That is, PS is incorporated into viral particles upon budding from cells [26,27] and PS-TIM interaction in target cells facilitates the attachment of virions to the cell surface, thus enhancing viral entry (Figure 1) [23,28,29]. In fact, the expression of TIM-1 in target cells, although not absolutely required, is necessary for efficient EBOV entry [24,30]. EBOV binding to TIM-1 on the surface of a target cell leads to internalization of the virion; this is followed by binding of the EBOV GP to the viral receptor, the Niemann-Pick disease C1 (NPC1) receptor, leading to fusion of virions in endolysosomal compartments [30,32–35]. Thus, the expression of TIM-family proteins in target cells can enhance the infectivity of PS-containing virions.
Table 1:
PS receptors enhance viral infection (adapted and modified from reference 29).
| PS Receptor | Family | Virus | Ref. |
|---|---|---|---|
| TIM-1 | Filovirus | Ebola | [23,24,30,32,37] |
| Marburg | [23,24,37] | ||
| Flavivirus | Dengue | [23,36] | |
| Yellow Fever | [36] | ||
| Japanese Encephalitis | [72] | ||
| West Nile | [23,36] | ||
| Alphavirus | Sindbis | [23,25,37] | |
| Ross River | [23,25,37] | ||
| Chikungunya | [23,37] | ||
| Eastern Equine Encephalitis | [23,37] | ||
| Areanavirus | Lassa | [85] | |
| Ampari | [23] | ||
| Tacaribe | [23] | ||
| Junin | [23] | ||
| Machupo | [23] | ||
| Baculovirus | Autophrapha Californica Nucleopolyhedrovirus | [37] | |
| Rhabdovirus | Vesicular Stomatitis | [23,25] | |
| Picornovirus | Hepatitus A | [18–20] | |
| TIM-3 | Flavivirus | Dengue | [36] |
| West Nile | [23] | ||
| Areanavirus | Tacaribe | [23] | |
| TIM-4 | Filovirus | Ebola | [23,37] |
| Marburg | [23,37] | ||
| Flavivirus | Dengue | [36] | |
| Yellow Fever | [36] | ||
| West Nile | [23] | ||
| Alphavirus | Sindbis | [25] | |
| Ross River | [25] | ||
| Eastern Equine Encephalitis | [23] | ||
| Areanavirus | Tacaribe | [23] | |
| Machupo | [23] | ||
| Baculovirus | Autophrapha Californica Nucleopolyhedrovirus | [25] | |
| Rhabdovirus | Vesicular Stomatitis | [23,25] | |
| Axl/Gas6 | Filovirus | Ebola | [23,86] |
| Marburg | [23,86] | ||
| Flavivirus | Dengue | [36] | |
| West Nile | [23] | ||
| Alphavirus | Sindbis | [25,87] | |
| Ross River | [25,87] | ||
| Chikungunya | [23] | ||
| Eastern Equine Encephalitis | [23] | ||
| Areanavirus | Lassa | [88] | |
| Ampari | [23] | ||
| Tacaribe | [23] | ||
| Junin | [23] | ||
| Baculovirus | Autophrapha Californica Nucleopolyhedrovirus | [25,87] | |
| Rhabdovirus | Vesicular Stomatitis | [23,25] | |
| Pox Virus | Vaccinia | [87] | |
| CD300a | Flavivirus | Dengue | [89] |
| Alphavirus | Sindbis | [25] | |
| Integrin/MFG-E8 | Alphavirus | Sindbis | [25] |
| Ross River | [25] | ||
| Baculovirus | Autophrapha Californica Nucleopolyhedrovirus | [25] | |
| Stabilin | Not Known to Enhance Viral Infection (?) | [25] | |
| BAI1 | Not Known to Enhance Viral Infection (?) | [25] | |
| RAGE | Not Known to Enhance Viral Infection (?) | [37] | |
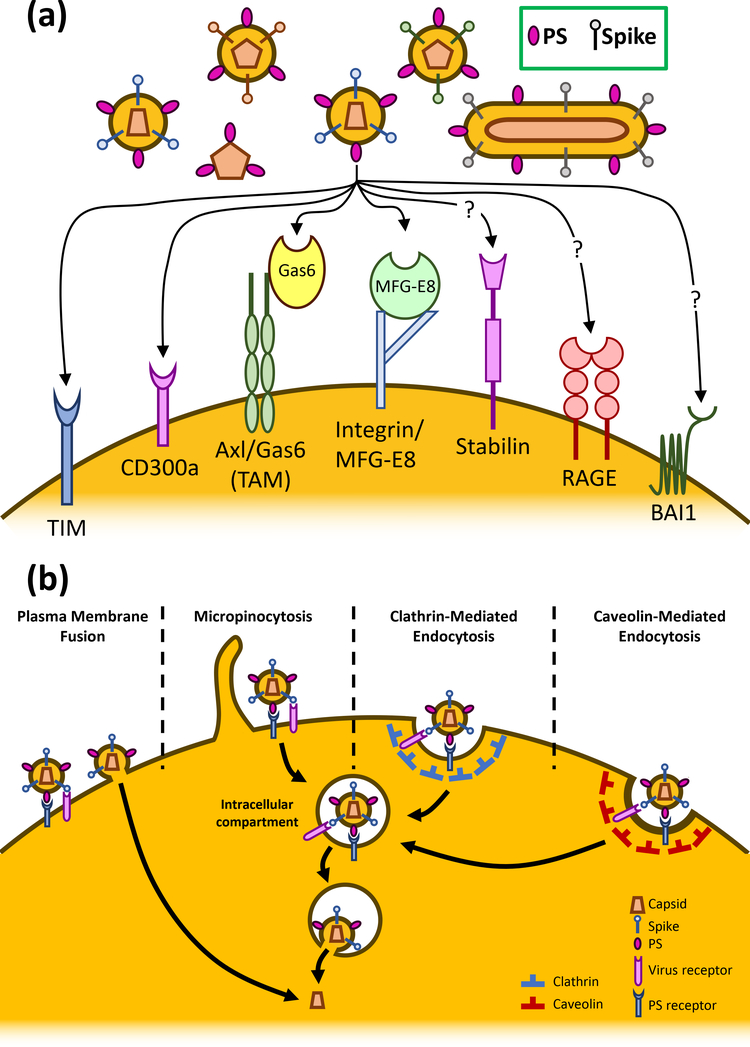
Figure 1:
PS receptors enhance viral entry. (a) A panel of PS receptors have been tested for their enhancing effect on viral entry. Among these, TIM proteins and CD300a are shown to directly promote viral entry. Gas6 and MFG-E8 bridge PS and the integral membrane proteins Axl and integrin, respectively, to enhance viral attachment. Stabilin, RAGE, and BAI1 are PS receptors that have not yet been shown to enhance the entry of any viruses. (b) PS receptors enhance viral entry through any four of the indicated viral entry pathways. PS receptors can augment the binding of a PS-containing virion on the plasma membrane thus enhancing viral fusion. PS receptors can also mediate the internalization of virions through micropinocytosis, clathrin-mediated endocytosis, and caveolin-mediated endocytosis, which allow viruses to be internalized for fusion in the endosome.
The capacity of TIM-1 to serve as a pan-viral entry co-factor may reflect a general role of PS receptors in facilitating entry. It has been shown that the extended stalk and PS-binding capacity of TIM-proteins are required to promote viral entry [28]. In fact, a GPI-anchored chimera of the PS-binding protein annexin V and the a-dystroglycan mucin-like domain is sufficient to promote viral entry, similarly to TIM-1 [28]. Several other PS-binding proteins have also been shown to enhance viral entry including TIM-3, TIM-4, CD300a, Gas6/Axl, and MFG-E8/Integrin (Table1, Figure 1). TIM-proteins and CD300a are both transmembrane PS receptors, with the transmembrane domain of TIM-1 being required for enhancement of viral entry [28]. However, the PS binding proteins Gas6 and MFG-E8 are not integral membrane proteins, but instead require binding to the transmembrane proteins Axl and integrin, respectively, to enhance viral entry (Figure 1) [25]. Although this may point to a common role for PS receptors in enhancing viral entry [29], it is important to note that this does not hold true for all PS receptors. For example, the PS receptors Stabilin-1/2, BAI1, and RAGE do not appear to enhance viral entry [25,36,37]. This may be because of differences in their binding affinities or the structure of these PS receptors that prohibit them from binding to the PS-containing virions [28,38,39]. The differences in the ability of some specific PS receptors to enhance entry of certain viruses require further investigation.
It is now generally accepted that PS receptors function as critical cellular cofactors for viral entry. However, we must make it clear that they are not authentic receptors for any viruses, because none of these PS receptors has been shown to directly interact with a viral protein that mediates entry and fusion-one of the gold standards defined for bona fide viral receptors.
Inhibitory effects of TIM-family Proteins in Virus Release
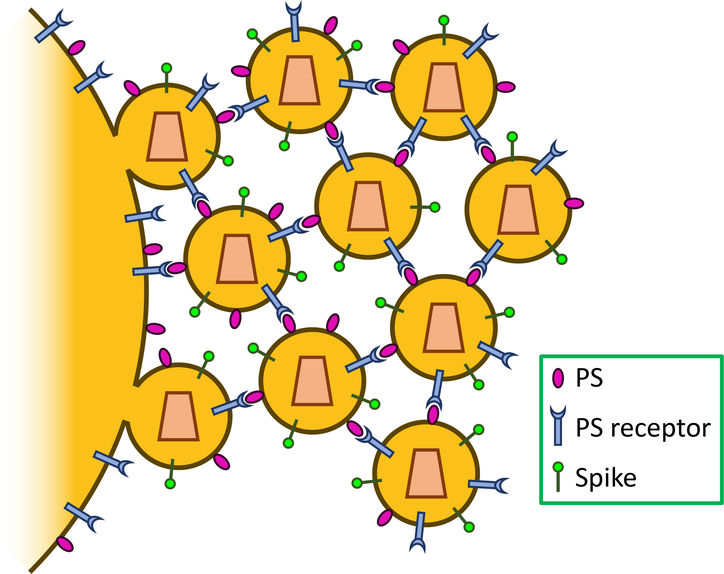
Recent evidence that TIM-family proteins can restrict enveloped virus release indicates another, inhibitory, role for these PS receptors. A study by our group demonstrates that exogenous expression of TIM-family proteins in viral producing cells reduces HIV-1 virion release [17,40]. Consistent with this finding, knockdown of the endogenous expression of TIM-1 and TIM-3 proteins in both CD4+ T cells and macrophages enhances HIV-1 release [17]. Interestingly, TIM-1 is incorporated into viral particles, similarly to PS, and TIM-1 restriction of HIV-1 is dependent on the PS-binding ability of TIM-1, because PS-binding deficient, mutant TIM-1 failed to block viral release [17]. Considering that both PS and TIM-1 are incorporated into viral particles [26,27,41], we propose a model where TIM-1 and PS incorporation into virions, as well as their physical interactions—occurring on the plasma membrane and between virions—can block viral release from the plasma membrane (Figure 2). This is consistent with the accumulation of mature HIV-1 particles observed by transmission electronic microscope at the surface of TIM-1 expressing producer cells [17].
Figure 2:
TIM-1 restricts viral release. Both PS and TIM-1 are incorporated into virions. The binding of TIM-1 to PS inhibits viral particle dissociation from the plasma membrane and from each other. Some other PS receptors also inhibit viral release, but in a virus-dependent and PS receptor-specific manner.
The ability of TIM-1 to restrict virion release does not appear unique to HIV-1, as TIM-1 is also able to restrict the release of virus like particles (VLP) of both murine leukemia virus (MLV) and EBOV [17]. This occurs despite overall EBOV replication being enhanced by TIM-1 [23,24,30,32], which differs from HIV-1 where, although TIM-1 expression in target cells enhances HIV-1 entry (by approximately 2–3 fold), the expression of TIM-1 in Jurkat cells reduced the prolonged HIV-1 replication [17,41]. This discrepancy may be explained by the relative degree of TIM-1 promotion of viral entry on one hand and impairment of viral release on the other. In other words, it is likely that the effect of TIM-1 to promote EBOV entry is greater than its inhibitory effect on release, yet for HIV, the inhibitory effect of TIM-1 on release supersedes its ability to enhance viral entry. It is now well established that EBOV utilizes an intracellular receptor NPC1 for binding and membrane fusion [31,34,35], yet TIM-1 is the most important cellular determinant, mediating the virus attachment to the target cell surface and facilitating endocytosis [24,30,32]. Thus, the effect of TIM-1 on EBOV entry is likely more pronounced than its effect on release.
This ability to inhibit viral release is not exclusive to TIM-1. Other PS receptors, such as TIM-3 and TIM-4, were also able to restrict VLP release for HIV-1, MLV, and EBOV. Interestingly, the PS receptor RAGE restricted only HIV-1 and EBOV, and Axl restricted only EBOV [17]. Hence, though broader, the effect of PS receptors on restricting enveloped virus release is ortholog specific and virus type dependent.
Antagonizing the inhibitory function of TIM by HIV Nef
To evade restriction from the host’s innate immune system, HIV has evolved an arsenal of accessory proteins, such as Nef, to disrupt cellular functions and host restriction factors [42,43]. Although not required for replication in permissive cell lines, evidence of selective pressure to maintain a functional Nef allele and evidence of delayed progression to AIDS in individuals infected with Nef-deficient HIV-1, highlight the importance of this accessory protein for maintaining a productive infection and viral pathogenesis [44–47]. Instructively, Nef has been shown to selectively downregulate the surface expression of several host proteins including CD4, MHC-I, CD8, CD28, and CD80 [48–50]. More broadly, Nef modulates the trafficking and internalization of cell membrane proteins in order to enhance viral replication [48–51]. One recent discovery is that Nef can effectively counteract the restriction of SERINC proteins that reduce HIV infectivity [52,53].
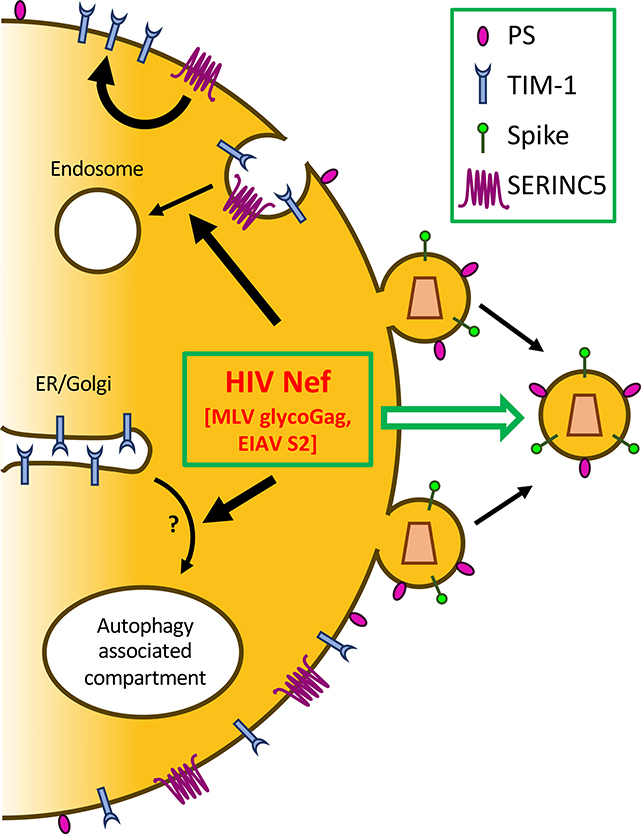
Nef also appears to antagonize TIM restriction of HIV-1 release. By using a series of biochemical and cell biological approaches, we have recently discovered that TIM-1 protein more potently restricts viral release of HIV-1 ΔNef than wild-type, and this restriction is relieved by co-expression of Nef [54]. Importantly, Nef from HIV-1 M, N, O, and P groups, as well as from the simian SIVgor, SIVcpz, SIVmac, and HIV-2, are all able to antagonize TIM-1 restriction of HIV-1 release, indicating that Nef antagonism of TIM-1 is conserved across primate lentiviruses [54]. Evidence suggests that HIV-1 Nef increases the rate of TIM-1 internalization, as well as causes re-localization of TIM-1 to an intracellular compartment positive for p62, an autophagy marker [54]. This suggests two independent and perhaps overlapping pathways—one is to increase internalization from the cell surface and the other is to disrupt its intracellular trafficking to the plasma membrane (Key Figure 3). Interestingly, TIM-1 does not appear to be degraded following the enhanced internalization or re-localization to an autophagy-related compartment [54], but rather accumulates within the viral producer cells—the exact mechanism of which still remains to be elucidated. Considering the latter effect of Nef, it is interesting to note that Nef has been previously shown to influence autophagy-related proteins including IRGM, BECLIN-1, and TFEB [55].
Figure 3:
HIV Nef, MLV glycoGag, and EIAV S2 antagonize TIM-mediated inhibition of HIV release, which is potentiated by SERINC. HIV Nef promotes the internalization of TIM-1, resulting in its decreased expression on the cell surface. Nef also sequesters TIM-1 into an autophagy-related, non-degradative compartment, which prevents TIM-1 trafficking to the plasma membrane. SERINC proteins (SERINC3 and SERINC5) can stabilize the expression of TIM-1 in viral producer cells, thus potentiating its inhibitory effect on viral release; both SERINCs and TIMs are counteracted by Nef, glycoGag, and S2, resulting in increased viral release.
Because the interaction between TIM-1 and PS is essential for TIM-mediated inhibition of HIV-1 release, we have hypothesized that PS may be affected by Nef, thus contributing to the Nef antagonism of TIM. However, expression of an infectious clones of HIV-1 WT or ΔNef, either in the absence or presence of TIM-1, showed no effect on the PS level on the surface of viral producer cells and in purified viral particles [54]. We also considered the possibility that Nef may disrupt TIM-1 and PS interaction. However, incubation of a soluble form of TIM-1 with purified Nef protein had no effect on PS-TIM-1 binding in vitro [54]. It should be emphasized that a direct interference of Nef with TIM-PS interaction is not topologically favorable, because Nef is an intracellular protein whereas functional TIM-PS interaction that blocks viral release occurs on the outer leaflet of the plasma membrane in the viral producer cells. Overall, we favor the model that Nef antagonizes TIM-mediated restriction of HIV-1 release by acting on the TIM protein (Figure 3).
Cooperation between TIM and SERINC: both antagonized by HIV Nef
TIM-mediated antagonism of viral replication is further complicated by its relationship with SERINC-family proteins [52,53]. Of the five SERINC family members, SERINC3 and SERINC5 were recently shown to inhibit viral replication [52,53]. Like TIM-1, SERINC3 and SERINC5 are incorporated into viral particles; but unlike TIM-1, SERINC5 inhibits virion infectivity, not virion release [52,53,56,57]. Intriguingly, Nef can efficiently counteract SERINC proteins by enhancing their endocytosis from the plasma membrane to endolysosomal compartments thus the reducing the SERINC incorporations into HIV virions. This mechanism of action by Nef is in line with the general function of Nef, which is to modulate trafficking and endocytosis of cellular transmembrane proteins [48,58]. However, it is currently not understood how SERINC proteins diminish HIV infectivity. Although several studies have shown effects of SERINC5 on HIV-1 Env [56,59], different Env proteins appear to be differentially inhibited by SERINC5, with Env that adopts a more open conformation being more sensitive to the action of SERINC5 [56,59–61].
We recently made a surprising observation that SERINC proteins are involved in TIM-mediated inhibition of HIV release [54]. We showed that knockdown of SERINC3 rescues HIV-1 ΔNef virion release that is restricted by TIM-1 expression, and co-expression of SERINC3 or SERINC5, along with TIM-1, enhances TIM-mediated restriction of HIV-1 release [54]. Importantly, knockdown of SERINC3 or SERINC5 in primary blood mononuclear cells, which express endogenous levels of TIMs, also enhances HIV-1 production, indicating that SERINC proteins do play a cooperative role with TIM-1 in inhibiting HIV-1 release in physiologically relevant cells [54]. Additional experiments revealed that SERINC proteins alone do not block HIV-1 release [54], similar to the original reports [52,53]. Rather, SERINC5 stabilizes the TIM-1 protein expression in viral producer cells, as exhibited by 3-fold increase in TIM-1 half-life [54]. While it is currently unclear exactly how SERINC proteins stabilize TIM-1, our flow cytometry data do suggest that the TIM-1 expression level on the cell surface is increased by SERINC5 [54]. Thus, there is a possibility that SERINC5 enhances restriction of viral release by stabilizing TIM-1 at the plasma membrane; however, more experiments are needed to directly visualize or detect TIM and SERINC interaction in the live cells. As SERINC proteins are initially identified as involved in the production of serine-incorporated lipids including PS [62], it has been tempting to speculate that this may contribute to the effect of SERINC proteins on TIM-1. However, several recent studies have refuted this scenario by showing that the SERINC proteins do not alter the lipid composition of host-cells or produced virions [63–65]; nor have we observed an effect of SERINC on PS incorporation into the HIV-1 virions in the presence or absence of TIM-1 or Nef [54]. Hence, a cellular function of SERINC-family proteins, outside the context of viral infection, remains unknown.
Similar to TIM-1, SERINC3 and SERINC5 are also antagonized by HIV-1 Nef. In fact, Nef expression in producer cells is known to enhance HIV-1 infectivity, and SERINC3 and SERINC5 have been identified as the restriction factors responsible for this Nef sensitivity [52,53]. Currently, although it is still unclear how Nef antagonizes SERINCs, the general consensus is that Nef promotes SERINC endocytosis similar to its effect on TIM-1. Specifically, Nef has been reported to target SERINC5 intracellular-loop 4 to cause AP2-mediated trafficking to endosomes [66,67]. However, Nef was able to restrict SERINC5 virion incorporation without appreciably reducing SERINC5 surface expression by an unknown mechanism [68].
In addition to Nef, SERINC5-mediated restriction of HIV-1 infectivity is also antagonized by the MLV glycoGag and equine infectious anemia virus (EIAV) S2 [69–72], two completely unrelated proteins from two different viruses. Amazingly, MLV glycoGag and EIAV S2 also counteract TIM-1 restriction of HIV-1 release, similar to that of HIV-1 Nef [54]. Thus, Nef, MLV glycoGag, and EIAV S2 all antagonize TIM-mediated restriction of viral release, likely in part by acting on SERINCs (Figure 3). However, we must emphasize that none of these proteins enhance HIV-1 release in cells not expressing TIM-1 or SERINCs [54]. Moreover, Nef can antagonize TIM-1 in a SERINC-independent manner [54]; however, this has not been tested for either MLV glycoGag or EIAV S2. Although SERINC stabilizes the expression of TIM-1 in viral producer cells—explaining its potentiating effect on TIM-1, whether or not there is a direct interaction between SERINC and TIM-1, and/or if Nef, MLV glycoGag, or EIAV S2 disrupts this potential SERINC-TIM interaction will need to be determined.
Evolutionary interplay between SERINC, TIM, and Nef
One important function of host restriction factors is to block cross-species transmission of viral infections. One well-known example of this is the restriction factor tetherin, which inhibits virion release similar to TIMs. Interestingly, human tetherin is resistant to antagonism by any SIV Nefs—which can successfully antagonize the tetherin of their native host—but human tetherin can be counteracted by HIV-1 Vpu [6,7,78,79]. Hence, the resistance of human tetherin to antagonism by SIV likely has prevented transmission of primate lentiviruses into humans until a novel mechanism of tetherin antagonism through Vpu evolved [6,7].
However, it is unlikely that SERINC5 alone represents such a cross-species barrier. Nef from HIV-1, HIV-2, or SIV can antagonize human, ape, monkey, and murine SERINC5 with similar efficiencies [76]. That is, Nef from a given primate lentivirus is expected to antagonize human SERINC5 with the same potency as it would antagonize SERINC5 from its native host. Hence, it is unlikely that human SERINC5 would act as a barrier to this transmission—the virus already being able to antagonize human SERINC5. However, SERINC-mediated potentiation of TIM-1 restriction of viral release would represent an unexplored barrier to cross species lentiviral transmission. One possible scenario would be that SIV Nefs disrupt human SERINC5-mediated potentiation of TIM-1, and in this sense, the relative ability of primate TIM-1 orthologs to block release of different primate lentiviruses will need to be characterized.
Host restriction factors also have the potential to inhibit the intraspecies spread of those lentiviruses that less efficiently antagonize the restriction factors of their own host. Again, the relationship between HIV-1 Vpu and tetherin serves as a great example. It is known that O-group HIV-1 Vpu, P-group HIV-1 Vpu, and HIV-2 Nef inefficiently antagonize human tetherin [7,80,81], while N-group HIV-1 Vpu can successfully antagonize tetherin but fails to cause the degradation of the HIV receptor, CD4 [7]. These viruses represent non-pandemic strains of HIV, which infect only a very small fraction of individuals worldwide [82]. In contrast, HIV-1 M-group, the only pandemic HIV strain, possesses a Vpu that is capable of efficiently antagonizing both CD4 and tetherin [7]. The ability of M-group HIV-1 Vpu to efficiently antagonize the host restriction factor tetherin likely has contributed to its rapid spread in the human population over other HIV strains and primate lentiviruses.
Similar evidence suggests that SERINC antagonism may represent such a determinant of lentiviral intraspecies spread. Specifically, there is a correlation between the potency of Nef antagonism of SERINC5 and the prevalence of a lentivirus within its native primate population [76]. Additionally, Nef from HIV-1 strains are more potent antagonists of SERINC5 than HIV-2 Nef [76]. This indicates that Nef antagonism of SERINC5 may be an important determinant of within-species spread. Given the stabilizing effect of SERINC5 on TIM-1 expression, it will be interesting and informative to explore how the ability of different lentiviral Nef proteins to disrupt the stabilizing effects of SERINC5 on TIM-1 could have influenced the intraspecies spread of primate lentiviruses and AIDS pandemic.
A hallmark of host restriction factors is a substantial degree of positive selection—this occurs as these restriction factors evolve to escape antagonism by viral proteins in an evolutionary arms race [73–75]. However, SERINC3 and SERINC5 both lack any evidence of this form of positive selection [75]. This is unusual because strong selective pressure appears to exist for viruses to evade SERINC given the wide range of SIV Nefs that antagonize SERINC—including SIVcol Nef which does so through an entirely unique proteasomal degradation mediated mechanism—and the convergent evolution of Nef, glycoGag, and S2 to antagonize SERINC [69,70,76,77]. It is thus interesting that the host protein does not show any evidence of positive selection during this evolutionary arms race. Perhaps, TIM proteins, or specific regions of TIMs that confer the antiviral effect, are under positive selection. As such, further investigation of the functional interplay between TIM-1 and SERINC5, in the context of Nef, will provide insights into the peculiar role of SERINC5 and TIMs in lentivirus-host co-evolution.
Concluding Remarks
TIM-family proteins, and PS receptors more broadly, have complicated effects on enveloped viruses. By incorporating PS into virions, these viruses are able to exploit a wide array of PS receptors to enhance entry [23,25,37]. This is consistent with the pattern that a large number of viruses exploit adhesion molecules, such as C-type lectin DC-SIGN, to enhance viral attachment [83,84]. For this reason, targeting the outer-leaflet PS on the plasma membrane has been proposed as a potential therapy for treating viral diseases [27]. However, recent evidence suggests that these PS receptors, including TIM-1/3/4, RAGE, and Axl, can also inhibit viral particle release [17,54]. In the case of HIV-1, the effect of this restriction by TIM-1 appears to supersede the enhancement of entry, thus reducing overall virus replication [17]. As such, caution is warranted in the development of any PS-targeting therapies as they may enhance replication for a subset of enveloped viruses. Further investigation of PS-mediated inhibition of virus release is needed to uncover the pervasiveness of this anti-viral strategy and its impact on disease pathogenesis.
The interplay of TIM-1, SERINC5, and Nef is of particular interest for understanding the strategies of HIV-1 evasion of host restriction factors. SERINC5, which restricts virion infectivity, can stabilize the expression of TIM-1 and potentiate TIM-mediated inhibition of virion release [17,52,53]. This two-pronged attack, inhibition of release and infectivity, is counteracted by the HIV-1 protein Nef, which downregulates surface expression of both TIM-1 and SERINC5 [17,32,33]. Understanding the evolutionary relationship and functional interplays between TIM and SERINC proteins, in the context of lentivirus Nef and other viral antagonists, will offer new insights into virus-host interaction and viral pathogenesis (see Outstanding Questions).
Outstanding Questions.
How do PS receptors promote viral entry but inhibit release in a PS receptor and virus-specific manner?
What other cellular co-factors participate in the PS receptor-mediated modulation of viral entry and release?
Do other viral proteins antagonize TIMs and SERINCs and how do they accomplish this task?
Do and how TIM and SERINC proteins physically interact with each other? If and how HIV Nef and other antagonists might disrupt these interactions in physiologically-relevant conditions?
What are the true biological roles of PS receptors in viral pathogenesis, including AIDS? Can therapeutics be developed by targeting these receptors?
Why are SERINC proteins not under positive selection during evolution? Are TIMs and other PS receptor positively selected and would this correlate with their antiviral functions?
Do the interplay between TIM, SERINC and Nef play a role in primate lentivirus cross-species transmissions leading to the AIDS pandemic?
Highlights.
Many PS receptors serve as cofactors for viral entry.
Some PS receptors, such as TIM-1, Axl, and RAGE, restrict viral release.
HIV Nef, MLV glycoGag, and EIAV S2 antagonize TIM-mediated inhibition of viral release, in part through SERINCs.
SERINCs potentiate TIM-mediated block of HIV release by stabilizing TIMs.
The functional interplay between TIM, SERINC, and Nef may play a role in HIV pathogenesis.
Acknowledgements
This work was supported by NIH Grants R01AI112381 and R01GM132069 to S.-L.L., and J. P.E. was supported by The Ohio State University, Distinguished University Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kluge SF et al. (2015) Snapshot: antiviral restriction factors. Cell. 163, 774. [DOI] [PubMed] [Google Scholar]
- 2.Harris RS et al. (2012) The restriction factors of human immunodeficiency virus. J. Biol. Chem 287, 40875–40883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle T et al. (2015) HIV-1 and interferons: who’s interfering with whom? Nat. Rev. Microbiol 13, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malim MH and Bieniasz PD (2012) HIV restriction factors and mechanisms of evasions. Cold Spring Harb. Perspect. Med 2, DOI: 10.1101/cshperspect.a006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoneham CA et al. (2017) Endocytic activity of HIV-1 Vpu: Phosphoserine-dependent interactions with clathrin adaptors. Traffic. 18, 545–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia B et al. (2009) Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5, DOI: 10.1371/journal.ppat.1000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauter D et al. (2009) Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and non-pandemic HIV strains. Cell Host Microbe. 6, 409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirmaier A et al. (2010) TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 8, DOI: 10.1371/journal.pbio.1000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauter D and Kirchhoff F (2019) Key viral adaptations preceding the AIDS pandemic. Cell Host Microbe. 25, 27–38 [DOI] [PubMed] [Google Scholar]
- 10.Kuchroo VK et al. (2003) The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol 3, 454–462 [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ et al. (2010) TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev 235, 172–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae SC et al. (2003) Molecular variations in the promoter and coding regions of human TIM-1 gene and their association in Koreans with asthma. Hum. Immunol 64, 117–1182 [DOI] [PubMed] [Google Scholar]
- 13.Umetsu DT et al. (2008) TIM gene family and their role in atopic diseases. Curr. Top. Microbiol. Immunol 321, 201–215 [DOI] [PubMed] [Google Scholar]
- 14.McIntire JJ (2003) Immunology: hepatitis A virus link to atopic diseases. Nature. 425, 576. [DOI] [PubMed] [Google Scholar]
- 15.Mclntire JJ et al. (2001) Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked TIM gene family. Nat. Immunol 2, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 16.Das M et al. (2017) TIM-3 and its role in regulating anti-tumor immunity. Immunol. Rev 276, 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M et al. (2014) TIM-family proteins inhibit HIV-1 release. Proc. Natl. Acad. Sci. U. S. A 111, E3699–E3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan G et al. (1996) Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 15, 4282–4296 [PMC free article] [PubMed] [Google Scholar]
- 19.Feigelstock D et al. (1998) The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol 72, 6621–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Z et al. (2013) A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 496, 367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das A et al. (2017) TIM1 (HAVCR1) is not essential for cellular entry of either quasi-enveloped or naked hepatitis A virions. MBio 8, DOI: 10.1128/mBio.00969-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das A et al. (2019) TIM1 (HAVCR1): an essential “receptor” or an “accessory attachment factor” for hepatitis A virus? J. Virol 93, DOI: 10.1128/JVI.01793-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jemielity S et al. (2013) TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 9, DOI: 10.1371/journal.ppat.1003232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondratowicz AS et al. (2011) T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. U. S. A 108, 8426–8431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morizono K and Chen IS (2014) Role of phosphatidylserine receptors in enveloped virus infection. J. Virol 88, 4275–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercer J and Helenius H (2008) Vaccinia virus uses micropinocytosis and apoptotic mimicry to enter host cells. Science. 320, 531–535 [DOI] [PubMed] [Google Scholar]
- 27.Soares MM et al. (2008) Targeting inside-out phosphatidylserine as a therapeutic strategy for viral disease. Nat. Med 14, 1357–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller-Tank S et al. (2014) Characterizing functional domains for TIM-mediated enveloped virus entry. J. Virol 88, 6702–6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moller-Tank S and Maury W (2014) Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology. 468–470, 565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda M et al. (2014) Interaction between TIM-1 and NPC1 is important for cellular entry of Ebola virus. J. Virol 89, 6481–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey RA et al. (2017) Mechanisms of filovirus entry. Curr. Top. Microbiol. Immunol 411, 323–352 [DOI] [PubMed] [Google Scholar]
- 32.Brunton B et al. (2019) TIM-1 serves as a receptor for Ebola virus in vivo, enhancing viremia and pathogenesis. PLoS Negl. Trop. Dis 13, DOI: 10.1371/journal.pntd.0006983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandran K et al. (2005) Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 308, 1643–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carette JE et al. (2011) Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 477, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Côté M et al. (2011) Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 477, 344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meertens L et al. (2012) The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 12, 544–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moller-Tank S et al. (2013) Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol 87, 8327–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dragovich MA et al. (2019) Biomechanical characterization of TIM protein-mediated Ebola virus-host cell adhesion. Sci. Rep 9, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhein BA et al. (2016) Characterization of human and murine T-cell immunoglobulin mucin domain 4 (TIM-4) IgV domain residues critical for Ebola virus entry. J. Virol 90, 6097–6111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlach J and Saad JS (2014) HIV: a victim. Trends Microbiol. 22, 603–604 [DOI] [PubMed] [Google Scholar]
- 41.Callahan MK et al. (2003) Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J. Immunol 170, 4840–4845 [DOI] [PubMed] [Google Scholar]
- 42.Malim MH and Emerman M (2008) HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe. 3, 388–398 [DOI] [PubMed] [Google Scholar]
- 43.Sauter D and Kirchoff F (2018) Multilayered and versatile inhibition of cellular antiviral factors by HIV and SIV accessory proteins. Cytokine Growth Factor Rev. 40, 3–12 [DOI] [PubMed] [Google Scholar]
- 44.Kestler HW et al. (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 65, 651–662 [DOI] [PubMed] [Google Scholar]
- 45.Gorry PR et al. (2007) Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology. 4, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirchhoff F et al. (1995) Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med 332, 228–232 [DOI] [PubMed] [Google Scholar]
- 47.Deacon NJ et al. (1995) Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 270, 988–991 [DOI] [PubMed] [Google Scholar]
- 48.Tokarev A and Guatelli J (2011) Misdirection of membrane trafficking by HIV-1 Vpu and Nef: keys to viral virulence and persistence. Cell. Logist 1, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia X et al. (2012) Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat. Struct. Mol. Biol 19, 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coleman SH et al. (2001) The HIV-1 Nef protein as a target for antiretroviral therapy. Expert Opin. Ther. Targets 5, 1–22 [DOI] [PubMed] [Google Scholar]
- 51.Roeth JF and Collins KL (2006) Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev 70, 548–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Usami Y et al. (2015) SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature. 526, 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosa A et al. (2015) HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature. 526, 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M et al. (2019) TIM-mediated inhibition of HIV-1 release is antagonized by Nef but potentiated by SERINC proteins. Proc. Natl. Acad. Sci. U. S. A 116, 5705–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nardacci R et al. (2017) Role of autophagy in HIV infection and pathogenesis. J. Intern. Med 281, 422–432 [DOI] [PubMed] [Google Scholar]
- 56.Sood C et al. (2017) SERINC5 protein inhibits HIV-1 fusion pore formation by promoting functional inactivation of envelope glycoproteins. J. Biol. Chem 292, 6014–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma S et al. (2018) An N-glycosylated form of SERINC5 is specifically incorporated into HIV virions. J. Virol 92, DOI: 10.1128/JVI.00753-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keppler OT et al. (2006) Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol 79, 616–627 [DOI] [PubMed] [Google Scholar]
- 59.Schulte B et al. (2018) Localization to detergent-resistant membranes and HIV-1 core entry inhibition correlate with HIV-1 restriction by SERINC5. Virology. 515, 52–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beitari S et al. (2017) Effect of HIV-1 Env on SERINC5 antagonism. J. Virol 91, DOI: 10.1128/JVI.02214-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X et al. (2019) CD4 expression and Env conformation are critical for HIV-1 restriction by SERINC5. J. Virol 93, DOI: 10.1128/JVI.00544-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inuzuka M et al. (2005) SERINC, an activity-regulated protein family, incorporates serine into membrane lipid synthesis. J. Biol. Chem 280, 35776–35783 [DOI] [PubMed] [Google Scholar]
- 63.Chu EP et al. (2017) Disruption of SERINC1 which facilitates serine-derived lipid synthesis, fails to alter macrophage functions, lymphocyte proliferation, or autoimmune disease susceptibility. Mol. Immunol 82, 19–33 [DOI] [PubMed] [Google Scholar]
- 64.Trautz B et al. (2017) The host-cell restriction factor SERINC5 restricts HIV-1 infectivity without altering the lipid composition and organization of viral particles. J. Biol. Chem 292, 13702–13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Firrito C et al. (2018) SERINC5 as a new restriction factor for human immunodeficiency virus and murine leukemia virus. Annu. Rev. Virol 5, 323–340 [DOI] [PubMed] [Google Scholar]
- 66.Shi J et al. (2018) HIV-1 Nef antagonizes SERINC5 restriction by downregulation of SERINC5 via the endosome/lysosome system. J. Virol 92, DOI: 10.1128/JVI.00196-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dia W et al. (2018) Long cytoplasmic loop governs the sensitivity of the anti-viral host protein SERINC5 to HIV-1 Nef. Cell Rep. 22, 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trautz B et al. (2016) The antagonism of HIV-1 Nef to SERINC5 particle infectivity restriction involves counteracting virion-associated pools of the restriction factor. J. Virol 90, 10915–10927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahi YS et al. (2016) Functional interplay between murine leukemia virus glycogag, SERINC5, and surface glycoprotein governs virus entry, with opposite effects on gammaretroviral and ebolavirus glycoproteins. MBio. 7, DOI: 10.1128/mBio.01985-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chande A et al. (2016) S2 from equine infectious anemia virus is an infectivity factor which counteracts the retroviral inhibitors SERINC5 and SERINC3. Proc. Natl. Acad. Sci. U. S. A 113, 13197–13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmad I et al. (2019) The retroviral accessory proteins S2, Nef, and glycoMA use similar mechanisms for antagonizing the host restriction factor SERINC5. J. Biol. Chem 294, 7013–7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez-Enriquez GV et al. (2017) SERINC as a restriction factor to inhibit viral infectivity and the interaction with HIV. J. Immunol. Res 2017, 1548905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lagguette N et al. (2012) Evolutionary and functional analyses of the interaction between the myeloid restriction factor SAMHD1 and the lentiviral Vpx protein. Cell Host Microbe. 11, 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim ES et al. (2010) Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses. J. Virol 84, 7124–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murrell B et al. (2016) The evolutionary histories of antiretroviral proteins SERINC3 and SERINC5 do not support an evolutionary arms race in primates. J. Virol 90, 8085–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heigele A et al. (2016) The potency of Nef-mediated SERINC5 antagonism correlates with the prevalence of primate lentiviruses in the wild. Cell Host Microbe. 20, 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kmiec D et al. (2018) SIVcol Nef counteracts SERINC5 by promoting its proteasomal degradation but does not efficiently enhance HIV-1 replication in human CD4+ T cells and lymphoid cells. PLoS Pathog. 14, DOI: 10.1371/journal.ppat.1007269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang F et al. (2009) Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 6, 54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma S et al. (2019) The C-terminal end of HIV-1 Vpu has a clade-specific determinant that antagonizes BST-2 and facilitates virion release. J. Virol 93, DOI: 10.1128/JVI.02315-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kluge SF et al. (2014) Nef proteins of epidemic HIV-1 group O strains antagonize human tetherin. Cell Host Microbe. 16, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sauter D et al. (2011) HIV-1 Group P is unable to antagonize human tetherin by Vpu, Env, or Nef. Retrovirology. 8, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leoz M et al. (2015) The two-phase emergence of non-pandemic HIV-1 group O in Cameroon. PLoS Pathog. 11, DOI: 10.1371/journal.ppat.1005029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhella D (2015) The role of cellular adhesion molecules in virus attachment and entry. Philos. Trans. R. Soc. Lond. B. Biol. Sci 370, 2140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jolly C et al. (2007) Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T-cell. J. Virol 81, 13916–13921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brouillette RB et al. (2018) TIM-1 mediates dystroglycan-independent entry of Lassa virus. J. Virol 92, DOI: 10.1128/JVI.00093-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimojima M et al. (2006) Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J. Virol 80, 10109–10116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morizono K et al. (2011) The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 9, 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fedeli C et al. (2018) Axl can serve as entry factor for Lassa virus depending on the functional glycosylation of dystroglycan. J. Virol 92, DOI: 10.1128/JVI.01613-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carnec X et al. (2016) The phosphatidylserine and phosphatidylethanolamine receptor CD300a binds dengue virus and enhances infection. J. Virol 90, 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]