Abstract
Objectives:
HIV-associated neurocognitive disorder (HAND) is characterized by chronic immune activation. We aimed to identify biomarkers associated with HAND and to investigate their association with cognitive function and sex, in a homogenous cohort of HIV infected (HIV+) young adults, parenterally infected during early childhood.
Methods:
144 HIV+ Romanian participants (51% women) without major confounders underwent standardized neurocognitive and medical evaluation in a cross-sectional study. IFN-γ, IL-1β, IL-6, CCL2, CXCL8, CXCL10, and TNF-α were measured in plasma in all participants and in cerebrospinal fluid (CSF) in a subgroup of 56 study participants. Biomarkers were compared to neurocognitive outcomes, and the influence of sex and HIV disease biomarkers were assessed.
Results:
In this cohort of young adults (median age of 24 years), the rate of neurocognitive impairment (NCI) was 36.1%. Median current CD4+ count was 479 cells/mm3 and 36.8% had detectable plasma viral load. Women had better HIV-associated overall status. In plasma, controlling for sex, higher levels of IL-6 and TNF-α were associated with NCI (p<0.05). Plasma CXCL10 showed a significant interaction with sex (p=0.02); higher values were associated with NCI in women only (p=0.02). Individuals with undetectable viral load had significantly lower plasma CXCL10 (p<0.001) and CCL2 (p=0.02) levels, and CSF CXCL10 (p=0.01), IL-6 (p=0.04), and TNF-α (p=0.04) levels.
Conclusions:
NCI in young men and women living with HIV was associated with higher IL-6 and TNF-α in plasma, but not in the CSF. CXCL10 was identified as a biomarker of NCI specifically in women with chronic HIV infection.
Keywords: HIV women, CXCL-10, neurocognitive, HIV inflammation, young adults
INTRODUCTION
HIV-associated central nervous system (CNS) dysfunction is attributed in a large part to inflammatory responses that damage neurons (Wiley et al., 1986) (González-Scarano and Martín-García, 2005). Increased inflammation in the CNS during chronic HIV infection was associated with neurocognitive impairment (NCI) (Cinque et al., 1998), (Burdo et al., 2013) leading to HIV-associated neurocognitive disorders (HAND), despite efficient suppressive antiretroviral treatment (ART) (Burdo et al., 2013). Since HAND can also occur in clinically asymptomatic persons infected with HIV (HIV+) (Heaton et al., 2011), (Gott et al., 2017), diagnostic tests in easily accessible body fluids are currently main research goals.
Lower neurocognitive performances in HIV infected (HIV+) women compared to HIV+ men have been reported in several cohorts (Morlat et al., 1992) (Robertson et al., 1996), (Kabuba et al., 2016), (Royal et al., 2016), (Bacon et al., 2005), (Holguin et al., 2011), (Manly et al., 2011), (Hestad et al., 2012) despite better HIV indicators (Kabuba et al., 2016), (Royal et al., 2016). Also, HIV+ women have a higher prevalence of NCI compared to HIV negative (HIV-) women regardless of symptom status and AIDS diagnosis (Richardson et al., 2002) (Stern et al., 1998).
Different patterns of cognitive impairment have been described in HIV+ men and women (Faílde-Garrido et al., 2008), (Kabuba et al., 2016), (Royal et al., 2016), (Royal et al., 2016) with impairment in psychomotor tasks being particularly noted (Richardson et al., 2002), although other publications don’t report specific differences (Robertson et al., 2004). Our team has previously reported a specific negative effect of HIV on motor functioning in HIV+ females only (Burlacu et al., 2018).
It has been speculated that, in spite of better HIV surrogate markers in women, immunogenic responses, which might be influenced by hormonal patterns, impact on the size of viral reservoir (Hagen and Altfeld, 2016) and that there may be specific hormonal influences associated with higher levels of inflammatory biomarkers in response to HIV, in women (Owen et al., 2010).
Infiltration of HIV-infected macrophages, monocytes and T cells into the CNS determines a cascade of inflammation that leads to the activation of microglia, astrocytes, and perivascular macrophages and persistent neuroinflammation (Heaton et al., 2011) (Heaton et al., 2004) (Canestri et al., 2010) (Peluso et al., 2012). Chemokine ligand 10 (CXCL10) is chemoattractant and a potent proinflammatory cytokine. Macrophages produce interferon gamma (IFN-γ) in self-amplifying loop and release CXCL10 when interacting with T cells. Transmigration of CD8 T cells into the CNS resulting in elevated cerebrospinal fluid (CSF) IFN-γ has specifically been correlated with increased risk of HIV-associated NCI (Schrier et al., 2015). In presence of HIV-1 proteins, CXCL10 is neurotoxic and leads to the production of further pro-inflammatory cytokine as tumor necrosis factor alpha (TNF-α) and IFN-γ (Williams et al., 2009) (Luster and Ravetch, 1987; van Marle et al., 2004; Williams et al., 2009).
We aimed to identify immune activation biomarkers associated with NCI and the relation to viral characteristics and sex in a highly homogenous young cohort, with chronic HIV infection.
METHODS
Study population
144 HIV+ adults were evaluated at “Dr. Victor Babes” Hospital (VBH), in Bucharest, Romania. Participants are part of the Romanian cohort of children infected in their first years of life, in the late 1980s, with HIV clade F (Patrascu and Dumitrescu, 1993), (Apetrei et al., 1998) The study was approved by the institutional review boards of VBH and the University of California at San Diego. All participants provided written informed consent. The inclusion and exclusion criteria were previously described (Ene et al., 2014).
Neurocognitive assessment
Seven ability domains (Verbal Fluency, Speed of Information Processing, Attention/Working Memory, Executive Function, Learning, Delayed Recall, and Motor) were tested using an internationally validated battery (Ene et al., 2014). Analyses for individual test raw scores were conducted using independent samples t test. In order to examine performance within and across cognitive domains, each of the tests was transformed into Z scores based on the mean and standard deviation (SD) of the HIV– group on which norms were developed (Ene et al., 2014). The domain-specific Z scores were then averaged and independent samples t test was used to examine if mean group differences exist. In order to estimate impairment rates, we utilized a global deficit-type approach (Carey et al., 2004b) (Carey et al., 2004a) by assigning a score from 0 to 5 based on number of Z score SD from normal. Worse cognitive performance results in a higher Z score deficit score. A Z score of >0.50 was the cut point for classification as neuropsychological impaired.
Current and past alcohol and substance use were determined using a specific questionnaire and MINI-International Neuropsychiatric Interview. Depression was evaluated with Beck Depression Inventory II. The Patient’s Assessment of Own Functioning Inventory (PAOFI) was administered to evaluate the impact of NCI on daily functioning.
Neuromedical evaluation
The neuromedical examination included a review of medical files, any current or past ART medications and a brief medical and neurological examination. Current CD4+ count and HIV viral load were measured with a detection limit for HIV RNA of 50 copies/ml. Lumbar puncture was proposed to all participants and performed in those who consented.
Biomarker assays
The following biomarkers were measured in plasma the day or the following day of the neurocognitive assessment: (N=144) and CSF (N=56): interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (CXCL8), tumor necrosis factor alpha (TNF-α), interferon gamma-induced protein 10 (CXCL10), monocyte chemo-attractant protein-1 (CCL2) and interferon gamma (IFN-γ).
Cytokine and chemokine levels in plasma and CSF samples were measured using a Meso-Scale Discovery-MSD (Rockville, Maryland, USA) MULTI-SPOT® Assay System. The data were acquired on a SECTOR Imager-2400 instrument (Rockville, MD, USA) and analyzed using MSD Discovery Workbench® analysis software. All assays were performed according to the manufacturer’s instructions.
All samples were assayed in duplicate and blind to the subject’s diagnostic record; mean intra-assay coefficients of variability (CVs) for all analyses were <25%. Cytokine concentrations below the lower limits of detection were reported as undetectable.
Statistical analysis
Demographic and clinical characteristics were summarized by mean and standard deviation or median and interquartile range for numeric variables and by count (N) and percent for categorical variables. The number of measurements differed between the participants, thus the sample sizes differ between analyses of each biomarker. Number of participants with complete data is listed for each analysis in the results section. Raw values of the biomarkers (in pg/mL) were log10 transformed and all necessary assumptions for parametric analyses were checked with appropriate methods prior to analyses. One extreme outlier in CSF IL-6 was excluded from analysis. Outliers were detected by visual inspection of the biomarkers distributions as well as diagnostic methods for detecting outliers in linear regression (Cook’s distance and externally studentized residuals), when regressing biomarker measured in CSF on values measured in plasma.
The two-sample t-test method was used to compare biomarkers between sexes and, separately, between individuals with undetectable and detectable HIV plasma RNA. Effect sizes were estimated with the Cohen’s d. We also compared biomarkers between normal and impaired subjects using non-parametric analysis, for each of the 4 groups (Female/detectable and undetectable HIV RNA; Male/detectable and undetectable HIV RNA) in plasma and CSF.
Multivariable analyses were conducted to investigate the association of biomarkers (plasma and CSF) and sex. Separate multivariable logistic regressions modelled NCI on biomarkers, sex and HIV RNA. Their interactions were investigated at α=0.05 significance level. A significant interaction would indicate that the association of a biomarker with the NCI differs between sexes. Non-significant interactions were not kept in the models. The effect size was measured by odds ratio (OR).
For the 56 patients with CSF samples, we compared biomarkers levels and HIV RNA in CSF and plasma using Spearman’s rho method. The nonparametric method was chosen to accommodate potential non-linear associations.
RESULTS
Cohort description
Demographic and clinical characteristics of the participants are summarized in Table 1. Participants were young adults (age range = 19–26 years) and half were women. The average duration of HIV infection was 22.9 years. The majority had controlled HIV infection: 63.2% (91 of 144 participants) had undetectable plasma HIV RNA. Only 12.3% had detectable CSF HIV RNA (7 of the 57 patients for whom data were available). Plasma and CSF HIV RNA were strongly associated with each other (Spearman rho = 0.72, p< 0.001). All but 7 male and 5 female participants (8.3%) were on ART. The median current CD4+ count was 479 cells/mm3. 37% of men (26 of 71) and 36% of women (49 of 73) had NCI; the difference was not statistically significant (p=0.31). Among the 53 (36.8%) participants with detectable HIV RNA, 19 (35.8%) had NCI. Among the 91 persons with undetectable HIV RNA, 33 (36.3%) had NCI. There was no significant association between NCI and detectable plasma viral load (Chi-squared test: χ2 = 0.002, df = 1, p = 0.96).
Table 1.
Demographic and clinical characteristics of the cohort (N=144).
| Demographic Characteristic | Male (N=71) | Female (N=73) | P-valuea | Total |
|---|---|---|---|---|
| Age (years), Mean (SD) | 23.7 (1.21) | 23.7 (1.16) | 0.81 | 23.7 (1.18) |
| Education (years), Mean (SD) | 11.6 (2.44) | 11.7 (2.87) | 0.83 | 11.6 (2.66) |
| Employed or in school | 36.6% | 34.2% | 0.90 | 35.4% |
| Beck Depression Inventory > 13 | 14.1% | 12.3% | 0.95 | 13.2% |
| PAOFI Total Score: > 3 | 15.5% | 13.7% | 0.95 | 14.6% |
| GDS, Median (IQR) | 0.30 [0.09, 0.61] | 0.39 [0.17, 0.68] | 0.31 | 0.39 [0.13, 0.65] |
| HIV Characteristic | ||||
| AIDS | 43.7% | 45.2% | 0.99 | 44.4% |
| Estimated years with HIV from the date of infection, Mean (SD) | 23.0 (2.66), N=68 | 22.7 (2.76), N=68 | 0.55 | 22.9 (2.70) |
| Nadir CD4+ Count, Median (IQR) | 61 (15.5, 171) | 105 (35,168) 134 (138) | 0.20 | 83 (22,170) |
| Current CD4+ Count, Median (IQR) | 420 (162, 662) | 516 (347, 771) | 0.006** | 479 (259, 698) |
| CD4/CD8, Mean (SD) | 0.51 (0.40) | 0.83 (0.50) | <0.001*** | 0.67 (0.48) |
| Currently taking ART | 90.1% | 93.2% | 0.73 | 91.7% |
| Months on current ART regimen, Mean (SD) | 35.5 (31.5), N=64 | 31.2 (23.4), N=68 | 0.38 | 33.3 (27.6) |
| Detectable HIV viral load in plasma | 47.9% | 26.0% | 0.011* | 36.8% |
| Detectable HIV viral load in CSF | 13.3%, N=30 | 11.1%, N=27 | >0.99 | 12.3% |
| Years with detectable HIV RNA, Median (IQR) Mean (SD) | 5.6 (2.2,8.7) 5.7 (4.0) | 2.8 (0.7,5.9) 4.0 (3.6) | p=0.007** | 4.2 (1.4, 7.6) 4.8 (3.9) |
| Months of exposure to ARV medications, Mean (SD) | 119 (61.3) | 122 (51.8) | 0.72 | 121 (56.5) |
| Biomarker | ||||
| Plasma IFN-γ (log10) | 1.78 (0.70) N=44 | 1.73 (0.72) N=37 | 0.77 | 1.76 (0.71) |
| Plasma CXCL10 (log10) | 2.76 (0.37) N=47 | 2.65 (0.31) N=52 | 0.11 | 2.71 (0.34) |
| Plasma IL-6 (log10) | 0.37 (0.55) N=68 | 0.31 (0.53) N=62 | 0.55 | 0.34 (0.54) |
| Plasma TNF-α (log10) | 1.06 (0.68) N=64 | 0.93 (0.69) N=63 | 0.28 | 1.00 (0.68) |
| Plasma CCL2 (log10) | 1.96 (0.18) N=49 | 1.89 (0.21) N=51 | 0.07 | 1.92 (0.19) |
| Plasma CXCL8 (log10) | 0.57 (0.20) N=69 | 0.54 (0.26) N=73 | 0.52 | 0.56 (0.23) |
| CSF CXCL10 (log10) | 2.87 (0.48) N=24 | 2.71 (0.43) N=20 | 0.25 | 2.80 (0.46) |
| CSF IL-6 (log10) | 0.18 (0.16) N=21 | 0.25 (0.19) N=20 | 0.21 | 0.24 (0.27) |
| CSFTNF-α (log10)b | −0.33 (0.32) N=25 | −0.43 (0.26) N=21 | 0.27 | −0.37 (0.30) |
| CSF CCL2 (log10) | 2.53 (0.12) N=20 | 2.54 (0.16) N=21 | 0.86 | 2.54 (0.14) |
| CSF CXCL8 (log10) | 1.53 (0.17) N=29 | 1.56 (0.21) N=27 | 0.56 | 1.55 (0.19) |
Notes:
P-values are obtained using the two-sample t-test for numeric variables, chi-squared tests for categorical variables, and Fisher’s exact test for detectable HIV in CSF.
Negative log10 values reflect the mean raw biomarker values between 0 and 1.
P<0.05
P<0.01
P<0.001.
AIDS = acquired immunodeficiency syndrome; GDS = global deficit score; IQR = interquartile range; PAOFI = Patient Assessment of Own Functioning Inventory; SD = standard deviation.
13.2% of participants (9 of 144) had evidence of depression (BDI>13), but none had major depression. The subgroup of study participants with available biomarkers in CSF and plasma had similar demographic and clinical characteristics (data not shown). None of the study participants had increased blood-brain barrier (BBB) permeability as measured by the albumin ratio index (data not shown).
Associations between cognition and biomarkers, controlling for sex and for viral load
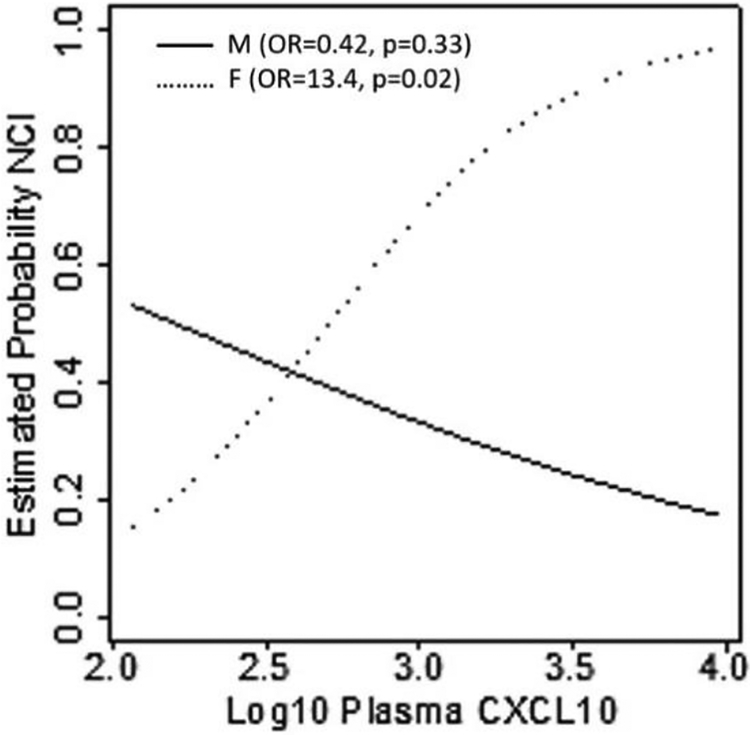
Table 2 shows the results of multivariable logistic regression analyses: in plasma, higher levels of two biomarkers were associated with NCI (ps<0.05), controlling for sex and HIV RNA (IL-6: OR = 2.85; TNF-α: OR = 1.9). One biomarker, CXCL10, showed a significant interaction with sex (p=0.02), such that higher level was associated with NCI only in women (OR=13.4 per 1 log10 increase, p=0.02), but not in men (p=0.33). For each biomarker, the odds ratio demonstrate effect size per 1 unit increase on the log10 scale. Figure 1 shows this interaction effect. No CSF biomarkers were associated with NCI in the smaller sample of participants who underwent lumbar puncture (n=56).
Table 2.
Results of logistic regression models of NCI on biomarkers and sex, controlling for HIV viral load.
| Predictors | N | OR (95% Cl)a | P-value |
|---|---|---|---|
| Plasma IFN-γ | 81 | 1.71 (0.88, 3.33) | 0.12 |
| Plasma CXCL10 | 99 | 13.4(1.23,145.52) | 0.021* |
| Plasma IL-6 | 130 | 2.85 (1.37, 5.91) | 0.005** |
| Plasma TNF-α | 127 | 1.90 (1.09,3.33) | 0.025* |
| Plasma CCL2 | 100 | 0.45 (0.05, 4.25) | 0.49 |
| Plasma CXCL8 | 142 | 1.82 (0.40, 8.19) | 0.44 |
| CSF CXCL10 | 44 | 0.88 (0.16, 4.70) | 0.88 |
| CSF IL-6 | 41 | 0.13 (0.001, 13.4) | 0.39 |
| CSF TNF-α | 46 | 0.45 (0.04, 5.77) | 0.54 |
| CSF CCL2 | 41 | 0.38 (0.004, 39.3) | 0.68 |
| CSF CXCL8 | 56 | 0.32 (0.01, 8.61) | 0.50 |
Notes:
The odds ratio > 1 indicates higher odds of cognitive impairment, and the odds ratio < 1 indicates lower odds of cognitive impairment. For biomarkers, the odds ratio is measured per 1 unit increase on log10 scale. For sex, the odds ratio compares men to women (reference group). For HIV viral load, the odds ratio compares detectable to undetectable (reference group).
For the interaction, the provided estimate are a ratio of odds ratios.
P<0.05
P<0.01.
OR = odds ratio; CI = confidence interval.
Figure 1.
Results from a logistic regression model of NCI on log10 plasma CXCL10, sex, VL and their interaction. The figure shows how the estimated probability of NCI changes with the changes in the log10 plasma CXCL10 separately for men (M) and women (F). The shown odds ratios (OR) and p-values (p) measure effect of log10 plasma CXCL10 on NCI, separately, for men and women. The 95% CI for the OR are M: (0.07, 2.46); F: (1.23, 145.52).
Associations of biomarkers with HIV disease characteristics
In plasma, persons with undetectable HIV RNA had lower CXCL10 (d=−1.38, p<0.001) and CCL2 (d=−0.54, p=0.02) than persons with detectable HIV RNA. Comparisons of other plasma biomarkers showed medium effects sizes: IFN-γ (d=0.36), IL-6 (d=0.31), TNF-α (d=0.34), and CXCL8 (d=−0.30).
For the CSF biomarkers, persons with undetectable HIV RNA had significantly lower levels of CXCL10 (d=−1.44, p=0.01), IL-6 (d=−0.72, p=0.04), and TNF-α (d=−0.85, p=0.04), compared to the persons with detectable HIV RNA. Results of these analyses are summarized in Table 3.
Table 3.
Biomarkers by HIV viral load in plasma.
| Biomarker (log10) | Undetectable (N=91) | Detectable (N=53) | Cohen’s d (p-valuea) |
|---|---|---|---|
| Plasma IFN-γ | 1.83 (0.72), N=57 | 1.58 (0.64), N=24 | 0.36 (0.15) |
| Plasma CXCL10 | 2.59 (0.25), N=71 | 2.99 (0.36), N=28 | −1.38 (<0.001***) |
| Plasma IL-6 | 0.40 (0.58), N=82 | 0.24 (0.45), N=48 | 0.31 (0.09) |
| Plasma TNF-α | 1.08 (0.73), N=81 | 0.85 (0.57), N=46 | 0.34 (0.07) |
| Plasma CCL2 | 1.89 (0.20), N=72 | 1.99 (0.17), N=28 | −0.54 (0.018*) |
| Plasma CXCL8 | 0.53 (0.23), N=89 | 0.60 (0.23), N=53 | −0.30 (0.08) |
| CSF CXCL10 | 2.64 (0.24), N=32 | 3.20 (0.64), N=12 | −1.44 (0.013*) |
| CSF IL-6 | 0.17 (0.16), N=27 | 0.29 (0.19), N=14 | −0.72 (0.036*) |
| CSF TNF-αb | −0.45 (0.22), N=32 | −0.21 (0.38), N=14 | −0.85 (0.045*) |
| CSF CCL2 | 2.54 (0.13), N=29 | 2.53 (0.17), N=12 | 0.10 (0.77) |
| CSF CXCL8 | 1.53 (0.19), N=41 | 1.59 (0.19), N=15 | −0.32 (0.29) |
Values represent means (standard deviations). For biomarkers with missing values, sample size (N) with the available data are given.
Notes: Cohen’s d for the difference (undetectable - detectable); P-values are obtained from a two-sample t-test.
Negative log10 values reflect the mean raw biomarker values between 0 and 1.
P<0.05
P<0.01
P<0.001
Correlations between plasma and CSF biomarkers
Correlation analyses for plasma and CSF pairs showed that CXCL10 values were significantly correlated with each other (Spearman rho = 0.50, p=0.002, N=37). The other four correlations were not significant (ps>0.05).
Comparison of biomarkers by sex
Men had marginally higher values of plasma CCL2 (d=0.37, p=0.07) compared to women. Effect sizes of similar magnitude (absolute value), but without the marginal significance (ps>0.05) were also observed for plasma CXCL10 (d=0.32), CSF CXCL10 (d=0.35), CSF IL-6 (d=−0.40), and CSF TNF-α (d=0.33). Other comparisons did not yield large effect sizes.
Comparison of biomarkers between impaired and unimpaired groups stratified by sex and HIV RNA
For plasma CXCL10, IL-6, TNF-α and plasma CXCL8 the impaired female group had higher median (and mean) than unimpaired in both detectable and undetectable HIV RNA groups. The results were significant only for the detectable group (data not shown). Because of small sample sizes, we were unable to investigate 3-way interactions. No significant results for males or CSF biomarkers were found (data not shown).
DISCUSSION
In this analysis of young adults infected with HIV as children, NCI was associated with high plasma levels of IL-6 and TNF-α. Biomarker levels did not differ between women and men on global cognition with the exception of CXCL10, for which higher plasma levels had a negative association with cognitive functioning, only in women.
These findings are particularly interesting since HIV+ women had better HIV-associated characteristics such as higher levels of current CD4 count, higher CD4/CD8 ratio, lower proportion with detectable HIV RNA and shorter cumulative estimated time spent with detectable HIV RNA.
We also showed that patients who had controlled HIV replication had lower levels of plasma and CSF CXCL10, lower levels of plasma CCL2, as well as lower CSF levels of TNF-α and CCL2.
Our study population was a highly homogenous group of young adults, with approximately 23 years of chronic HIV infection, a balanced sex distribution and no significant medical, psychiatric or behavioral confounding conditions. Indeed, they are issued of the major HIV epidemic in children that occurred between 1987 and 1990, in Romania, when the majority acquired the infection via transfusions of unscreened blood or therapeutic injections with improper reuse of nonsterile needles and syringes (Hersh et al., 1993).
They were treated for a median period of 11 years, had good current immunological status, but a relatively high proportion (36.8%) of detectable HIV RNA while on ART. The overall rate of NCI was 36.1% which is similar to the rate in other studies (Heaton et al., 2010), (Robertson et al., 2007). Impaired motor skills have particularly been described in this cohort and a possible neurotropic effect of HIV clade F has been proposed (Ene et al., 2014). Impaired myelination processes during childhood and adolescence reported previously by other pediatric studies ((Abubakar et al., 2008; Blanchette et al., 2001) may be responsible for these findings.
We found evidence of chronic, sustained immune activation in the CNS as illustrated by elevated cytokines (CXCL10, IL-6, TNF-α) in the CSF, even in participants with controlled HIV replication. However, there was no association between CSF biomarkers and NCI, possibly due to the limited number of CSF study samples. Nonetheless, NCI was correlated with elevated plasma levels of IL-6 and TNF-α.
Plasma CXCL10 levels were positively correlated with CSF CXCL10 levels and appeared to be the most reliable marker and a close correlate of NCI, especially in women.
We hypothesize that NCI is the consequence of exposure of the developing brain to HIV, before treatment was started, and of persistent inflammation and that ART failed to prevent or reverse neurological damage.
CXCL10, IL-6 and TNF-α versus cognition and sex
In our study, plasma CXCL10 showed a significant interaction with sex: increased values were associated with greater odds of NCI in women, but not in men. To our knowledge, this is the first report showing that there was a preferential negative association of plasma CXCL10 with neurocognition, in women only. Moreover, our team previously described, in the same cohort, that after controlling for age and education, HIV+ females had worse Motor skills than HIV- females, but there was no difference in mean Motor scaled scores between HIV+ and HIV- males suggesting a specific negative effect of HIV on motor functioning in HIV+ females only (Burlacu et al., 2018).
These specific differences between sexes may be due to specific hormonal influences on plasmacytoid dendritic cells which were already associated with higher levels of inflammatory biomarkers in response to HIV, in women (Owen et al., 2010). Other reports, however, did not find an interaction effect when looking at the association between soluble factor levels and sex adjusting for HIV RNA, CD4 counts, and/or severity of neurocognitive disease (Krebs et al., 2016).
Higher plasma IL-6 and TNF-α levels were associated with increased risk of NCI with no differences between sexes, in our study. Higher IL-6 levels were associated with worse neuropsychological test scores, in predominantly male populations, suggesting the role of chronic inflammation in NCI (Lake et al., 2015). Conversely, in some uncontrolled HIV infected women populations no elevations were found (Keating et al., 2011). Also, elevation of plasma TNF-α was associated with time to the development of HIV associated dementia (Sevigny et al., 2004) and impaired women had elevated levels of TNF-α and its receptor compared to unimpaired women in both plasma and CSF. (Nolting et al., 2009).
Biomarkers and HIV replication
In our cohort, detectable HIV plasma RNA was associated with higher plasma levels of CXCL10 and CCL2 and higher CSF CXCL10 respectively. Specifically, we found that both plasma and CSF CXCL10 levels were significantly higher in HIV patients with detectable viral RNA. Previous reports show that plasma CXCL10 is produced in response to HIV and positively associated with HIV RNA (Keating et al., 2011), (Simmons et al., 2013). Studies also show a correlation between CXCL10 and its CXCR3 receptor expression and neurological dysfunction and progression of the HIV-1-induced CNS disease (Sanders et al., 1998) (Williams et al., 2009). CXCR3 expression concomitantly increases in the brain of HIV-1 infected patients and associate with severity of HAND (Juompan et al., 2008).
In the WIHS, untreated and viremic participants with HIV had significantly higher serum levels of CXCL10 as compared to HIV negative women, and treated and undetectable participants had significantly lower CXCL10 levels compared to untreated women, underlining the importance of this chemokine in the response to HIV (Keating et al., 2011).
Higher plasma levels of CCL2 were associated with detectable plasma HIV RNA in our cohort consistent with other publications (Chang et al., 2004), (Ansari et al., 2006). Elevated CCL2 plasma levels were found during neuroinflammation (Yang et al., 2009) and were predictive of HAD (Sevigny et al., 2004). Conversely, CCL2 levels in the CSF were not significantly different between treated and untreated patients in other reports, which might suggest that ART has limited effect on local inflammation in the CNS during HIV infection (Yuan et al., 2013).
Correlations between CSF and plasma biomarkers
A previous study found elevated levels of CXCL10 in both plasma and CSF of HIV-infected individuals with NCI, and a correlation between plasma and CSF CXCL10 levels in the cognitively impaired group only. The authors suggested that increased permeability of the BBB leading to infiltration of activated T lymphocytes from periphery and an overexpression of CXCL10 in the CNS was the underlying mechanism for NCI. (Yuan et al., 2015). This cohort included older patients, with more advanced HIV disease (including study participants with opportunistic infections at the time of neurocognitive evaluation) and individuals with limited exposure to ART, which could explain higher levels of inflammation. Although in our study, we found a correlation between paired CSF and plasma concentrations for CXCL10, CSF CXCL10 levels were not associated with NCI probably due to smaller sample size.
Also, we found no correlations between plasma and CSF IL-6 and TNF-α levels in our study and this is consistent with previous reports (Yuan et al., 2015).
Limitations of our study include the relatively small number of CSF samples and its cross-sectional design. Future studies involving a longitudinal evaluation are necessary in order to determine if the cognitive pattern that we found is influenced by treatment adaptation or further development of these young adults.
We report for the first time, a negative correlation between CXCL10 plasma levels and neurocognitive function in HIV infected women. Our study suggests that plasma CXCL10 levels may be a valuable immune activation biomarker for NCI.
ACKNOWLEDGEMENTS:
The authors would like to thank all participants in the study; Terence Hendrix from the HIV Neurobehavioral Research Center in San Diego for neuropsychological training and study coordination, Anca Luca and Adrian Luca, psychologists for their help with neuropsychological testing, Roxana Radoi, MD, for neuromedical evaluation and Gratiela Tardei, MD, PhD for the clinical laboratory assessments.
FUNDING: This work was supported by 1R01MH094159 and P30MH62512 from National Institute of Mental Health (NIMH).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICT OF INTEREST: R.B., A.U., A.B.T., A.T., S.M.R. and C. C. D. report grants from National Institute of Mental Health (NIMH), during the conduct of the study. L.E. reports grants from National Institute of Mental Health (NIMH), during the conduct of the study; personal fees and non-financial support from Abbvie, personal fees from Johnson & Johnson, personal fees from Merck Sharp & Dohme and personal fees from Bristol-Myers Squibb, outside the submitted work. C. L. A., T.D.M., B.S. and S. L. have nothing to disclose.
References:
- Abubakar A, Van Baar A, Van de Vijver FJR, Holding P, Newton CRJC, 2008. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop. Med. Int. Health 13, 880–887. 10.1111/j.1365-3156.2008.02079.x [DOI] [PubMed] [Google Scholar]
- Ansari AW, Bhatnagar N, Dittrich-Breiholz O, Kracht M, Schmidt RE, Heiken H, 2006. Host chemokine (C-C motif) ligand-2 (CCL2) is differentially regulated in HIV type 1 (HIV-1)-infected individuals. Int. Immunol 18, 1443–1451. 10.1093/intimm/dxl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Necula A, Holm-Hansen C, Loussert-Ajaka I, Pandrea I, Cozmei C, Streinu-Cercel A, Pascu FR, Negut E, Molnar G, Duca M, Pecec M, Brun-Vézinet F, Simon F, 1998. HIV-1 diversity in Romania. AIDS Lond. Engl 12, 1079–1085. [PubMed] [Google Scholar]
- Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA, 2005. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin. Diagn. Lab. Immunol 12, 1013–1019. 10.1128/CDLI.12.9.1013-1019.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette N, Smith ML, Fernandes-Penney A, King S, Read S, 2001. Cognitive and motor development in children with vertically transmitted HIV infection. Brain Cogn 46, 50–53. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC, 2013. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS Lond. Engl 27, 1387–1395. 10.1097/QAD.0b013e32836010bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlacu R, Umlauf A, Luca A, Gianella S, Radoi R, Ruta SM, Marcotte TD, Ene L, Achim CL, 2018. Sex-based differences in neurocognitive functioning in HIV-infected young adults. AIDS Lond. Engl 32, 217–225. 10.1097/QAD.0000000000001687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canestri A, Lescure F-X, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C, 2010. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 50, 773–778. 10.1086/650538 [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK, HNRC Group, 2004a. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J. Clin. Exp. Neuropsychol 26, 307–319. 10.1080/13803390490510031 [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Heaton RK, HNRC Group, 2004b. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin. Neuropsychol 18, 234–248. 10.1080/13854040490501448 [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, St Hillaire C, Conant K, 2004. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir. Ther 9, 431–440. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G, 1998. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS Lond. Engl 12, 1327–1332. [DOI] [PubMed] [Google Scholar]
- Ene L, Franklin DR, Burlacu R, Luca AE, Blaglosov AG, Ellis RJ, Alexander TJ, Umlauf A, Grant I, Duiculescu DC, Achim CL, Marcotte TD, 2014. Neurocognitive functioning in a Romanian cohort of young adults with parenterally-acquired HIV-infection during childhood. J. Neurovirol 10.1007/s13365-014-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faílde-Garrido JM, Alvarez MR, Simón-López MA, 2008. Neuropsychological impairment and gender differences in HIV-1 infection. Psychiatry Clin. Neurosci 62, 494–502. 10.1111/j.1440-1819.2008.01841.x [DOI] [PubMed] [Google Scholar]
- González-Scarano F, Martín-García J, 2005. The neuropathogenesis of AIDS. Nat. Rev. Immunol 5, 69–81. 10.1038/nri1527 [DOI] [PubMed] [Google Scholar]
- Gott C, Gates T, Dermody N, Brew BJ, Cysique LA, 2017. Cognitive change trajectories in virally suppressed HIV-infected individuals indicate high prevalence of disease activity. PloS One 12, e0171887 10.1371/journal.pone.0171887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen S, Altfeld M, 2016. The X awakens: multifactorial ramifications of sex-specific differences in HIV-1 infection. J. Virus Erad 2, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group, 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group, HNRC Group, 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol 17, 3–16. 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, HNRC Group, 2004. The impact of HIV-associated neuropsychological impairment on everyday functioning. J. Int. Neuropsychol. Soc. JINS 10, 317–331. 10.1017/S1355617704102130 [DOI] [PubMed] [Google Scholar]
- Hersh BS, Popovici F, Jezek Z, Satten GA, Apetrei RC, Beldescu N, George JR, Shapiro CN, Gayle HD, Heymann DL, 1993. Risk factors for HIV infection among abandoned Romanian children. AIDS Lond. Engl 7, 1617–1624. [DOI] [PubMed] [Google Scholar]
- Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR, Imasiku ML, Kalima K, Heaton RK, 2012. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. J. Nerv. Ment. Dis 200, 336–342. 10.1097/NMD.0b013e31824cc225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin A, Banda M, Willen EJ, Malama C, Chiyenu KO, Mudenda VC, Wood C, 2011. HIV-1 effects on neuropsychological performance in a resource-limited country, Zambia. AIDS Behav 15, 1895–1901. 10.1007/s10461-011-9988-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juompan LY, Hutchinson K, Montefiori DC, Nidtha S, Villinger F, Novembre FJ, 2008. Analysis of the immune responses in chimpanzees infected with HIV type 1 isolates. AIDS Res. Hum. Retroviruses 24, 573–586. 10.1089/aid.2007.0182 [DOI] [PubMed] [Google Scholar]
- Kabuba N, Menon JA, Franklin DR, Heaton RK, Hestad KA, 2016. HIV- and AIDS-associated neurocognitive functioning in Zambia - a perspective based on differences between the genders. Neuropsychiatr. Dis. Treat 12, 2021–2028. 10.2147/NDT.S105481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, Cohen MH, Zhang J, Greenblatt RM, Desai S, Wu S, Landay AL, Gange SJ, Norris PJ, Women’s Interagency HIV Study, 2011. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS Lond. Engl 25, 1823–1832. 10.1097/QAD.0b013e3283489d1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs SJ, Slike BM, Sithinamsuwan P, Allen IE, Chalermchai T, Tipsuk S, Phanuphak N, Jagodzinski L, Kim JH, Ananworanich J, Marovich MA, Valcour VG, SEARCH 011 study team, 2016. Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS Lond. Engl 30, 1533–1542. 10.1097/QAD.0000000000001096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JE, Vo QT, Jacobson LP, Sacktor N, Miller EN, Post WS, Becker JT, Palella FJ, Ragin A, Martin E, Munro CA, Brown TT, 2015. Adiponectin and interleukin-6, but not adipose tissue, are associated with worse neurocognitive function in HIV-infected men. Antivir. Ther 20, 235–244. 10.3851/IMP2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Ravetch JV, 1987. Genomic characterization of a gamma-interferon-inducible gene (IP-10) and identification of an interferon-inducible hypersensitive site. Mol. Cell. Biol 7, 3723–3731. 10.1128/mcb.7.10.3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Robison E, Martin EM, Young M, 2011. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV- women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. J. Clin. Exp. Neuropsychol 33, 853–863. 10.1080/13803395.2010.547662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlat P, Parneix P, Douard D, Lacoste D, Dupon M, Chêne G, Pellegrin JL, Ragnaud JM, Dabis F, 1992. Women and HIV infection: a cohort study of 483 HIV-infected women in Bordeaux, France, 1985–1991. The Groupe d’Epidémiologie Clinique du SIDA en Aquitaine. AIDS Lond. Engl 6, 1187–1193. [PubMed] [Google Scholar]
- Nolting T, Lindecke A, Koutsilieri E, Maschke M, Husstedt I-W, Sopper S, Stüve O, Hartung H-P, Arendt G, Competence Network HIV/AIDS, 2009. Measurement of soluble inflammatory mediators in cerebrospinal fluid of human immunodeficiency virus-positive patients at distinct stages of infection by solid-phase protein array. J. Neurovirol 15, 390–400. 10.3109/13550280903350192 [DOI] [PubMed] [Google Scholar]
- Owen RE, Heitman JW, Hirschkorn DF, Lanteri MC, Biswas HH, Martin JN, Krone MR, Deeks SG, Norris PJ, 2010. HIV+ elite controllers have low HIV-specific T cell activation yet maintain strong, polyfunctional T cell responses. AIDS Lond. Engl 24, 1095–1105. 10.1097/QAD.0b013e3283377a1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrascu IV, Dumitrescu O, 1993. The epidemic of human immunodeficiency virus infection in Romanian children. AIDS Res. Hum. Retroviruses 9, 99–104. 10.1089/aid.1993.9.99 [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, Gisslén M, Angoff N, Price RW, Cinque P, Spudich S, 2012. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS Lond. Engl 26, 1765–1774. 10.1097/QAD.0b013e328355e6b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JL, Martin EM, Jimenez N, Danley K, Cohen M, Carson VL, Sinclair B, Racenstein JM, Reed RA, Levine AM, 2002. Neuropsychological functioning in a cohort of HIV infected women: importance of antiretroviral therapy. J. Int. Neuropsychol. Soc. JINS 8, 781–793. [DOI] [PubMed] [Google Scholar]
- Robertson K, Fiscus S, Wilkins J, van der Horst C, Hall C, 1996. Viral Load and Neuropsychological Functioning in HIV Seropositive Individuals:A Preliminary Descriptive Study. J. Neuro-AIDS 1, 7–15. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Kapoor C, Robertson WT, Fiscus S, Ford S, Hall CD, 2004. No gender differences in the progression of nervous system disease in HIV infection. J. Acquir. Immune Defic. Syndr 1999 36, 817–822. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ, 2007. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS Lond. Engl 21, 1915–1921. 10.1097/QAD.0b013e32828e4e27 [DOI] [PubMed] [Google Scholar]
- Royal W, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA, 2016. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PloS One 11, e0147182 10.1371/journal.pone.0147182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL, 1998. Chemokines and receptors in HIV encephalitis. AIDS Lond. Engl 12, 1021–1026. [PubMed] [Google Scholar]
- Schrier RD, Hong S, Crescini M, Ellis R, Pérez-Santiago J, Spina C, Letendre S, HNRP Group, 2015. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PloS One 10, e0116526 10.1371/journal.pone.0116526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K, 2004. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 63, 2084–2090. [DOI] [PubMed] [Google Scholar]
- Simmons RP, Scully EP, Groden EE, Arnold KB, Chang JJ, Lane K, Lifson J, Rosenberg E, Lauffenburger DA, Altfeld M, 2013. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS Lond. Engl 27, 2505–2517. 10.1097/01.aids.0000432455.06476.bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Arruda JE, Somerville JA, Cohen RA, Boland RJ, Stein MD, Martin EM, 1998. Neurobehavioral functioning in asymptomatic HIV-1 infected women. J. Int. Neuropsychol. Soc. JINS 4, 172–178. [DOI] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C, 2004. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology 329, 302–318. 10.1016/j.virol.2004.08.024 [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB, 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. U. S. A 83, 7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ, 2009. Pro-inflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia 57, 734–743. 10.1002/glia.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Akhter S, Chaudhuri A, Kanmogne GD, 2009. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc. Res 77, 212–219. 10.1016/j.mvr.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D, 2015. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. BioMed Res. Int 2015, 506872 10.1155/2015/506872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, Smith D, Li N, Chen D, 2013. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J. Neurovirol 19, 144–149. 10.1007/s13365-013-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]