Abstract
Background:
Rodent models of high alcohol drinking offer opportunities to better understand factors for Alcohol Use Disorders (AUD) and test potential treatments. Selective breeding was carried out to create two unique High Drinking in the Dark (HDID-1, HDID-2) mouse lines that represent models of genetic risk for binge-like drinking. A number of studies have indicated that neuroimmune genes are important for regulation of alcohol drinking. We tested whether compounds shown to reduce drinking in other models also reduce alcohol intake in these unique genetic lines.
Methods:
We report tests of gabapentin, tesaglitazar, fenofibrate, caffeic acid phenethyl ester (CAPE), ibrutinib, and rolipram. Although these compounds have different mechanisms of action, they have all been shown to reduce inflammatory responses. We evaluated effects of these compounds on alcohol intake. In order to facilitate comparison with previously published findings for some compounds, we employed similar schedules that were previously used for that compound.
Results:
Gabapentin increased ethanol binge-like alcohol drinking in female HDID-1 and HS/NPT mice. Tesaglitazar and fenofibrate did not alter two bottle choice (2BC) drinking in male HDID-1 or HS/NPT mice. However, tesaglitazar had no effect on DID ethanol intake but reduced blood alcohol levels (BAL), and fenofibrate increased DID intake with no effects on BAL. CAPE had no effect on ethanol intake. Ibrutinib reduced intake in female HDID-1 in initial testing, but did not reduce intake in a second week of testing. Rolipram reduced DID intake and BALs in male and female HDID-1, HDID-2, and HS/NPT mice.
Conclusions:
A number of compounds shown to reduce ethanol drinking in other models and genotypes are not effective in HDID mice, or their genetically heterogeneous founders, HS/NPT. The most promising compound was the PDE4 inhibitor, rolipram. These results highlight the importance of assessing generalizability when rigorously testing compounds for therapeutic development.
Keywords: Alcohol drinking, genetic models, pharmacological testing, drinking in the dark
1. Introduction
Pre-clinical studies using rodent models of high alcohol drinking offer opportunities to better understand genetic and environmental risk factors for Alcohol Use Disorders (AUD), as well as molecular and physiological consequences of chronic alcohol experience (Crabbe, 2014, Ozburn et al., 2015). A major goal moving forward is to use the knowledge gained from these studies to improve treatment for individuals with AUD. There are currently three compounds approved for AUD treatment (disulfiram, naltrexone, and acamprosate). However, many individuals with AUD receive no treatment, and for those that do, the side effects and low compliance associated with these compounds are major limitations (Testino et al., 2014). Many compounds have shown significant promise in pre-clinical studies, but were not effective when tested in clinical trials. We suggest it is important to test promising compounds in multiple drinking paradigms, as well as different species and strains (including both male and female subjects), in order to improve the translatability of pre-clinical studies.
For initial compound screening, we focus here on paradigms that model continuous access voluntary alcohol preference drinking via two bottle choice (2BC) and binge-like drinking seen during limited access sessions via drinking in the dark (DID). In the 2BC paradigm, animals are offered access to two bottles for 22-24-hrs per day, one containing water and the other containing an ethanol solution. This paradigm allows assessment of daily alcohol intake and preference (often for increasing concentrations of ethanol solutions over periods of several days). After intense selective breeding, some genotypes of mice achieve pharmacologically relevant blood alcohol levels (BAL > 80mg%) in this paradigm (Matson and Grahame, 2013, Matson et al., 2014). However, a disadvantage of 2BC for compound screening is the relative lack of temporal precision of this measure. The half-life of investigational compounds can vary widely, which could lead to false negative results if a compound has a short half-life. We model binge drinking in mice using the limited access drinking in the dark (DID) paradigm (Rhodes et al., 2005, Crabbe et al., 2012c). In the DID paradigm, mice are offered a single bottle containing a 20% ethanol solution for two (or four) hrs early in their active cycle (three hrs after lights off). Like 2BC, DID intake also depends on strain (Rhodes et al., 2007, Yoneyama et al., 2008, Crabbe et al., 2012c, Wahlsten et al., 2006). Importantly, a few strains of inbred, hybrid, and selectively bred mice have been shown to consume enough alcohol to achieve pharmacologically relevant BALs (Rhodes et al., 2007, Rhodes et al., 2005, Blednov et al., 2005, Crabbe et al., 2009, Crabbe et al., 2014, Crabbe et al., 2012c, Matson and Grahame, 2013). Additionally, a modified DID method that includes the element of choice drinking has been developed [2BC-DID; (Blednov et al., 2015)]. In 2BC-DID, mice are offered a bottle containing water and another containing a 15% ethanol solution (three hrs after lights off, for 3 hrs).
Starting with a genetically heterogeneous progenitor stock (HS/NPT), selective breeding was carried out to create two unique High Drinking in the Dark (HDID-1, HDID-2) mouse lines that represent models of genetic risk for binge-like drinking (Crabbe et al., 2009, Crabbe et al., 2014). HDID and HS/NPT mice have been extensively characterized (Barkley-Levenson and Crabbe, 2015a, Barkley-Levenson and Crabbe, 2015b, Barkley-Levenson and Crabbe, 2014, Barkley-Levenson and Crabbe, 2012, Barkley-Levenson et al., 2015, Barkley-Levenson et al., 2016, Crabbe et al., 2012a, Crabbe et al., 2012b, Crabbe et al., 2014, Crabbe et al., 2012c, Crabbe et al., 2019, Crabbe et al., 2017, Crabbe et al., 2011, Ferguson et al., 2018a, Ferguson et al., 2018b, Fritz et al., 2014, Iancu et al., 2018, Iancu et al., 2013, Metten et al., 2011). Of particular relevance to the current studies are the findings that HDID mice exhibit behavioral impairment after DID (Crabbe et al 2009), withdrawal after a single binge-drinking session (Crabbe et al 2014), and do not exhibit altered preference for saccharin or avoidance of quinine solutions (Crabbe et al, 2011) or alcohol clearance rates (Crabbe et al, 2014). We have recently focused on attempting to reduce binge-like drinking pharmacologically in HDID mice. We first tested whether drugs of known clinical relevance could modulate binge-like ethanol drinking in HDID mice and found that HDID mice were sensitive to the effects of acamprosate and baclofen, but not naltrexone (Crabbe et al., 2017). These findings suggested that genetic differences between HDID and C57BL/6 mice may result in different responses to therapeutic compounds. We also employed transcriptome-based drug discovery to identify and test novel compounds for efficacy in reducing binge drinking in HDID mice. We predicted two compounds, terreic acid and pergolide, that reduced binge-like drinking and BALs (Ferguson et al., 2018a). Moving forward, we take advantage of the genetic diversity of HDID mice by continuing to use informatics guided approaches to identify and test novel compounds, and to collaborate with other Integrative Neuroscience Initiative on Alcoholism (INIA)-Neuroimmune consortium laboratories to perform testing in various models, strains, and species. We think this approach has the potential to improve translation to clinical work. Our findings thus far suggest HDID mice may represent a useful model for screening novel compounds.
This paper focuses on testing compounds that target pathways relevant for our INIA-Neuroimmune consortium efforts. INIA-Neuroimmune is a multi-disciplinary, multi-site, highly collaborative consortium funded by the National Institute on Alcohol Abuse and Alcoholism that combines genomic and systems-level analyses to identify potential drug treatments for AUD. A number of studies have indicated that neuroimmune pathways (e.g. NFKB, TNFalpha, PPAR, and PDE; discussed further below) are important for regulation of alcohol drinking (Harris and Blednov, 2013, Mayfield et al., 2013, and others reviewed in Erickson et al., 2019). The overarching framework for this body of work is that neuroimmune dysregulation may play a role in regulation of alcohol consumption and agents that target neuroimmune pathways may reduce alcohol drinking (Mayfield et al., 2013, Ray et al., 2014).
Two INIA-Neuroimmune laboratories have focused intensively on mouse models of alcohol drinking. The Blednov-Harris labs (UT-Austin, TX) have studied C57BL/6J male mice in 2BC and 2BC-DID paradigms. Our laboratories (Crabbe and Ozburn, Portland, OR) have generally focused on studies of HDID mice using DID paradigms. Progress and results have been freely and frequently shared between sites. For many of the neuroimmune-related drugs, Drs. Blednov and Harris performed important early drinking studies that established effective dose ranges, vehicles, routes and temporal factors surrounding administration, and they detected positive results for several drugs. Our studies were designed to see whether positive results in C57BL/6J mice could also be seen in HDID mice, which reach intoxicating blood alcohol levels during DID. While we often modeled our experimental designs on the Texas methods and findings, we did not attempt strict replication of the Texas studies. For example, the Texas group customarily uses a variant of the 2BC-DID method (15% EtOH vs water) they have refined for C57BL/6J male mice, while we customarily use the original single-bottle (20% EtOH) DID test because it yields intoxicating BALs in HDID mice. Given the natures of the various drugs tested, the specific procedures used differed from drug to drug. Because our results differed from theirs quite a bit (see Results), we present Table S1, which outlines the procedural details we used in these experiments.
We discuss below the compounds we tested, their mechanism of action, and published effects on alcohol drinking. Gabapentin (900 mg and particularly 1800 mg/day) has shown efficacy for the treatment of AUD (Mason et al., 2014). Gabapentin inhibits voltage sensitive calcium channels, acts as an antiepileptic agent, and is FDA-approved for use to control focal seizures. Tesaglitazar is an investigational compound for treatment of type 2 diabetes and fenofibrate is FDA-approved for treating hypercholesterolemia or lipid abnormalities. Both these PPAR (peroxisome proliferator-activated receptor) agonists reduced ethanol intake and preference in a continuous access 2BC paradigm performed with male C57BL/6J and in 129S4 x C57BL/6J hybrid mice; fenofibrate was ineffective in C57BL/6J females (Blednov et al., 2015, Blednov et al., 2016a). PPAR has known anti-inflammatory actions, and can antagonize NFkB resulting in suppression of pro-inflammatory gene expression. Caffeic acid phenethyl ester (CAPE) is a naturally occurring compound found in a variety of plants that acts as an NFKB inhibitor with multiple anti-inflammatory actions. Harris and Blednov (2013) reported that 40 mg/kg CAPE reduced 2BC ethanol intake and preference in C57BL/6J x 129S4 female mice. In a previous study we found that a single administration of terreic acid, an investigational Bruton’s tyrosine kinase (BTK) inhibitor, reduced DID intake and BALs in HDID-1 mice (Ferguson and Ozburn et al., 2018a). We pursued this finding with the BTK inhibitor ibrutinib, an anti-inflammatory compound, FDA approved for treating B cell cancers. Finally, the phosphodiesterase-4 PDE4 and TNFα inhibitor rolipram is an investigational anti-inflammatory compound. Reductions in 2BC ethanol preference drinking by systemic rolipram administration has been seen in C57BL/6J mice (Blednov et al., 2014, Hu et al., 2011) and in three different rat lines genetically predisposed to high preference drinking (Wen et al., 2012, Franklin et al., 2015). Although these compounds have different mechanisms of action, they have all been shown to reduce inflammatory responses (Abdel-Salam and Sleem, 2009, Ji et al., 2014, Daynes and Jones, 2002, Belfort et al., 2010, Tambuwala et al., 2018, Zhu et al., 2001) (and see Table S1). We evaluated the effects of these compounds on alcohol intake in HDID mice and/or their non-selected controls (HS/NPT).
2. Materials and methods
2.1. Animals
Mice of the High Drinking in the Dark (HDID) replicate 1 line (HDID-1) from selected generations S26-S29.G30r were included in all experiments. For some experiments, HDID-2 (S20-21) or the HS/NPT (non-selected generation G74-77) were available and included. We used adult (53-90 days old) mice of both sexes, when available.
All experiments were conducted with the approval of the VA Portland Health Care System’s Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed in standard plastic cages on Bed-o’cobs® bedding (Andersons, Maumee, OH, USA). Rodent chow (Purina 5LOD; PMI Nutrition International, Brentwood, MO, USA) and water were provided ad libitum. Colonies and testing rooms were maintained on a 12-hr:12-hr reverse light:dark cycle (2130 lights on: 0930 lights out) at a temperature of 21±1°C.
Experimentally naïve adult mice were moved to a procedure room and individually housed for 6-8 days prior to the start of all experiments. Cages were changed every 7-8 days and animals were weighed 1 hr before lights off 2-4 times before and during testing for drinking in the dark (DID), or every 4-8 days before and during two-bottle choice (2BC) drinking. Schematic indications of features of our more complex studies are included in the Figures.
2.2. Drugs
Drug or vehicle was administered prior to the drinking tests as described below. Drugs were administered either intraperitoneally (i.p.; 10mL/kg), subcutaneously (s.c.; 2mL/kg), or per os (p.o.; 5mL/kg; via FTP-20-30 20 gauge, 30 mm length plastic feeding tubes from Instech Laboratories, Inc., Plymouth Meeting, PA, USA). Table S1 specifies doses and routes of administration for each experiment.
Grain ethanol (200 proof, Decon Laboratories, Inc.) was used in all experiments. Drugs administered during ethanol drinking experiments were gabapentin (Sigma-Aldrich, Milwaukee, WI, USA), tesaglitazar (Tocris, Minneapolis, MN, USA), fenofibrate (Sigma-Aldrich, Milwaukee, WI, USA), caffeic acid phenethyl ester (CAPE; Tocris, Minneapolis, MN, USA), ibrutinib (Toronto Research Chemicals, Inc., Toronto, ON, Canda) and rolipram (Sigma-Aldrich, Milwaukee, WI, USA). The vehicle used for gabapentin and CAPE was saline (Baxter Healthcare Corporation, Deerfield, IL, USA), while for tesaglitazar, fenofibrate, and rolipram, the vehicle was saline and 1.5-2% Tween-80 (Sigma-Aldrich, Milwaukee, WI, USA). The vehicle for ibrutinib was saline, 1.75% Tween-80, and 5% DMSO in week 1, and 100% DMSO in week 2. Table S1 provides a list of drugs, doses, and mechanisms of action for each experiment.
2.3. General Ethanol Drinking Methods
2.3.1. Drinking in the dark (DID) and blood collection methods
DID was carried out as previously published (Rhodes et al., 2005, Crabbe et al., 2009, Crabbe et al., 2014). Three hrs after lights out, 20% ethanol (v/v in tap water) was offered for 2 or 4 hrs in experiments 1, 3, 4, 6, and 7. In experiment 5, two tubes, one containing water and the other containing a 15% ethanol solution, were offered during DID (i.e., 2BC-DID) which began at lights out to enable direct comparison of intake with the 4-hr measurement in the standard 2BC group. After the last drinking session, a 20 μl periorbital blood sample was collected to determine blood alcohol concentration [BAL; analyzed by gas chromatography using methods described previously (Crabbe et al., 2014)].
2.3.2. Two-bottle choice (2BC) 24-hr access methods
The 24hr access 2BC procedure was used for experiments 2 and 5. Details have been published (Crabbe et al., 2011, Blednov et al., 2016a, Blednov et al., 2015). At the onset of the dark cycle, water bottles were removed and replaced with two tubes (one containing water and another containing an ethanol solution). To obtain a measure of consumption during the first 4 (Expt 5) or 6 hrs (Expt 2) of the dark phase, reading times were also added for some days.
2.4. Experiment 1. Effect of Gabapentin on Ethanol DID
We tested whether DID intake in female HDID-1 and HS/NPT mice was altered by gabapentin (n=13-15 mice/Line/Treatment). Due to its cost, we chose a single dose estimated for its near equivalence to a human dose of 900mg (Mason et al., 2014). Each day for 4 days, saline or gabapentin (120 mg/kg; i.p.) was administered 30 minutes prior to ethanol and on day 4 at the end of the 4-hr drinking session, blood was obtained from the periorbital sinus to determine whether gabapentin affected BAL.
2.5. Effects of Peroxisome Proliferator-Activated Receptor (PPAR) Agonists on ethanol drinking (Experiments 2-4)
2.5.1. Experiment 2. Effects of Tesaglitazar and Fenofibrate on 24-hr 2BC Drinking
Experiments in HDID-1 and HS/NPT male mice were conducted simultaneously and a common vehicle group was used to compare with two dose groups for each drug (n=9-12 mice/Line/Drug/Dose). On days 1-2, two bottles, one containing water and the other 3% ethanol (v/v in tap water), were offered to mice. On days 3-4, mice were offered 6% ethanol and water. On days 5-7, 10% ethanol and water were provided and, on days 8-31, mice were given 15% ethanol and water. Tube positions were alternated at each concentration change and every Tuesday and Thursday thereafter. On day 18 and daily beginning on day 22, fluid volumes were recorded at 6 hrs, as well as 24-hrs. Mice were habituated to p.o. saline administration 1-2-hrs before lights out on days 22-23. Tubes were placed on cages at lights out and fluid volumes at 6 hrs and 24-hrs were recorded. At the same time each day (1-2-hr prior to lights off) from days 24-31, mice were treated with vehicle, 0.75 or 1.5 mg/kg tesaglitazar, or 75 or 150 mg/kg fenofibrate (administered p.o. in a volume of 5 ml/kg) prior to measuring consumption of ethanol and water.
2.5.2. Experiment 3. Effect of Tesaglitazar on Ethanol DID in HDID-1 mice
HDID-1 mice (n=12/Sex/Treatment) were treated with tesaglitazar (1.5 mg/kg, p.o.; volume 5 ml/kg) or vehicle 45 minutes prior to 3 hrs into the dark cycle for two days prior to commencing the 4 day DID test (one bottle of 20% ethanol). Injections were given at the same time each day prior to the DID test (intakes assessed at 2-hrs on days 1-4 and 4-hrs on day 4). A periorbital sinus blood sample was obtained after DID on day 4 to determine whether tesaglitazar affected BAL.
2.5.3. Experiment 4. Effect of Fenofibrate on Ethanol DID in HDID-1 mice
HDID-1 (n=11-12 mice/Sex/Treatment) were given either fenofibrate (150 mg/kg, p.o.; volume 5 ml/kg) or vehicle prior to DID as described in Section 2.5.2. Methods were identical to Experiment 3.
2.6. Experiment 5. Effect of CAPE on Ethanol 2BC and DID after stable 2BC Drinking
HDID-1 and HDID-2 female mice were available to test whether CAPE would alter 2BC drinking (continuous or limited access) in HDID mice. There were 8 experimental groups [n=15 mice/Line (HDID-1, HDID-2)/Treatment (vehicle or drug)/Drinking procedure (2BC or 2BC-DID)]. Mice were offered two bottles (water and 15% ethanol). Volumes consumed were measured at 4-hrs and 24-hrs on days 1, 3, 5, 7, 9, and 10. Data were analyzed to determine whether mice were drinking stable amounts for both the 4-hr period and in 24-hrs by the 10th day (see Results Section 3.3). On day 11, treatment groups were formed to match average consumption on days 9 and 10. On days 11-18, half of the animals began the DID procedure with 2 bottles (2BC-DID; 15% ethanol vs water) for 4-hrs each day and the other half were maintained on the 2BC 24-hr access procedure, measuring daily at 4-hrs and 24-hrs [note that there were 5 days of 24-hr (days 13-17), but 6 days of 4-hr (days 13-18), consumption with drug treatment]. On days 11 and 12, all mice were habituated to i.p. injections of saline at 1 hr before lights out, and began drinking at lights out according to their group assignments. On day 13 and daily for 6 days total, mice were administered either vehicle or 20 mg/kg CAPE 1 hr before lights out, and then continued with their assigned drinking procedure at lights out (see Table S1).
2.7. Experiment 6. Effect of Ibrutinib on Ethanol DID
Mice were subjected to a 3 day DID procedure with access to 20% ethanol solution for 2-hrs on day 1, and for 4-hrs on day 2. Ibrutinib was administered (0, 6.25, 12.5, and 25 mg/kg i.p. in a 5% DMSO, 1.75% Tween 80 in saline vehicle) 30 minutes prior to DID on day 2. A periorbital sinus blood sample was obtained to determine whether ibrutinib affected BAL. There appeared to be effects of the 6.25 and 25 mg/kg doses in female mice on day 2. Ibrutinib covalently binds to BTK, so to determine whether the drug effect lasted 24-hrs, we next measured 4-hr DID intake on day 3; when no injections were given. To determine whether the 25 mg/kg dose effect could be observed again, and to test additional higher doses, mice were then re-randomized into new dosing groups (0, 25, 50, 100mg/kg) and tested the following week in another 3 day DID. The low solubility of higher concentrations of ibrutinib required a change in the vehicle and subsequently, a new route of administration for further testing [subcutaneous administration of vehicle (100% DMSO) or ibrutinib in 2 mL/kg volume; again, treatment was administered only on day 2].
2.8. Experiment 7. Effect of Rolipram on Ethanol DID
Methods were identical to Experiment 1. HDID-1, HDID-2, and HS/NPT mice of both sexes were injected p.o. with rolipram (5 mg/kg; volume 5 mL/kg) or vehicle 30 minutes prior to each day of DID testing. (n=8-9 mice/Line/Sex/Treatment).
2.9. Statistical Analyses
2.9.1. DID data
For DID, the volume consumed was converted to g ethanol / kg body weight prior to data analysis. Data were first analyzed in a mixed-factor repeated measures (RPM) analysis of variance (ANOVA) over the first 2-hr block of each day with drug Treatment, Line, and Sex, where appropriate, as between groups factors (Systat version 13). We next analyzed the two, 2-hr blocks on day 4 similarly. Finally, one-way ANOVAs on day 4 total g/kg consumed in 4-hrs and BAL at 4-hrs were conducted. Significant interactions were pursued by simpler ANOVAs and/or followed by Tukey’s honestly significant different (HSD) test. Within subjects significant effects were evaluated for differences using paired t-tests. Significance was set at p < 0.05.
2.9.2. 24-hr 2BC data
For 2BC, the volume of ethanol intake was converted to g ethanol / kg body weight for each ethanol concentration. Water and total fluid consumption were converted to mL intake / kg body weight. Ethanol preference was calculated as mL ethanol / mL total fluid. Data were analyzed in a manner similar to DID using separate mixed factor repeated measures ANOVAs over baseline drinking days or injection habituation days. In the absence of treatment differences within pre-drug phases, data were averaged over days within each phase prior to further analyses. During the drug treatment phase, data were similarly analyzed to determine whether the drug affected consumption of ethanol. In the absence of interactions with Day, data for each animal were averaged over the drug treatment days.
3. Results
3.1. Experiment 1. Effect of Gabapentin on Ethanol DID
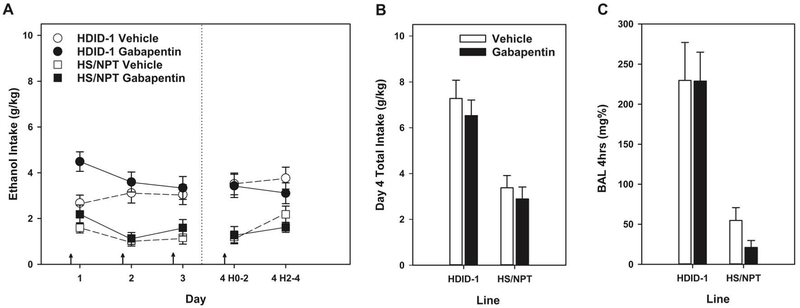
Ethanol intake data in g/kg consumed and BALs after day 4 DID are shown in Figure 1. Overall, we observed differences in intake for the different mouse lines (as expected), and unexpectedly, we observed an increase in DID intake with gabapentin treatment on the first day of DID. Data from the 2-hr blocks each day showed a main effect of Line (F[1,50] = 38.1, p < 0.0001; HDID-1 > HS/NPT). The main effect of Line (on day 4, total intake for HDID-1 mice was 6.89 ± 0.52 g/kg/2-hr vs HS/NPT = 3.14 ± 0.37) was expected, because HDID-1 mice were selectively bred for high BALs and exhibit significantly greater ethanol intake as compared to HS/NPT (Crabbe et al., 2009). The 2-hr data showed a trend for an effect of Treatment (F[1,50] = 3.46, p < 0.07), a significant effect of Day (F[3,150] = 3.89, p < 0.05) and a Day x Treatment interaction (F[3,150] = 3.03, p < 0.05). Individual two-way ANOVAs of each day’s 2-hr data showed significant main effects of Line each day (all Fs[1,51-53] ≥ 19.7, p values ≤ 0.0001) and a main effect of Treatment only on Day 1 (F[1,51] = 11.09, p < 0.01; all other Fs < 1). Gabapentin significantly increased intake on Day 1. We next analyzed Day 4 total consumption. There was a significant main effect of Line (F[1,52] = 33.5, p < 0.0001), but neither treatment nor their interaction were significant (both Fs < 1). Similarly, BAL at the end of the 4-hr drinking period on day 4 showed the same pattern, Line (F[1,53] = 36.9, p < 0.0001), but no other effects (Fs < 1).
Figure 1.
Gabapentin increased ethanol intake on day 1 of Drinking in the Dark (DID), but did not alter ethanol intake or achieved blood alcohol levels (BALs) on subsequent treatment days (as compared with vehicle) in female HDID-1 or HS/NPT mice. Arrows indicate drug or vehicle injections. Means ± SEM shown for n=13-15 mice/line/treatment dose.
3.2. Peroxisome Proliferator-Activated Receptor (PPAR) Agonists
3.2.1. Experiment 2. Effect of Tesaglitazar or Fenofibrate on 24 and 6 hr 2BC Drinking
Our results indicate overall that neither tesaglitazar or fenofibrate reduce ethanol 2BC drinking in HDID-1 or HS/Npt mice. Data from the first 18 days of this experiment are not shown [analyses of the 2BC 15% ethanol intake across Days 8-18 showed no significant effects of drug (which had not yet been given) or dose (data not shown)]. Pre-saline injection baseline data for the 24-hr 2BC intake were calculated as the average ethanol intake over days 19 – 21, while the effect of saline habituation on ethanol intake was indexed as the average of days 22 – 23. Data from one high drinking, vehicle group HDID-1 male mouse were removed to better equate baseline differences among the 5 dose groups. Data (mean +/− SEM) for each period are presented in Table 1. 24-hr data and results are presented first, followed by 6 hr data.
Table 1.
Effects of PPAR Antagonists, Tesaglitazar and Fenofibrate, on 24-hr and 6 hr 2BC Drinking
| HDID-1 | HS/NPT | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Habituation | Treatment | Baseline | Habituation | Treatment | ||
| 24-hr intake (g/kg) | |||||||
| mg/kg | |||||||
| Vehicle | 0 | 1.96 ± 0.59 | 3.12 ± 1.13 | 2.82 ± 1.19 | 1.22 ± 0.17 | 2.28 ± 0.68 | 1.46 ± 0.19 |
| Tesaglitazar | 0.75 | 3.53 ± 1.04 | 3.35 ± 0.92 | 3.57 ± 0.96 | 1.60 ± 0.32 | 1.28 ± 0.32 | 1.12 ± 0.14 |
| Tesaglitazar | 1.5 | 2.05 ± 0.42 | 3.44 ± 1.28 | 2.47 ± 0.34 | 1.22 ± 0.21 | 1.83 ± 0.41 | 1.11 ± 0.13 |
| Fenofibrate | 75 | 1.73 ± 0.47 | 2.89 ± 1.02 | 2.91 ± 0.93 | 2.17 ± 0.91 | 2.92 ± 1.28 | 1.98 ± 0.54 |
| Fenofibrate | 150 | 3.87 ± 1.46 | 2.83 ± 1.11 | 3.28 ± 1.07 | 2.01 ± 0.87 | 1.35 ± 0.31 | 1.35 ± 0.22 |
| 6-hr intake (g/kg) | |||||||
| mg/kg | |||||||
| Vehicle | 0 | 0.61 ± 0.20 | 1.24 ± 0.51 | 1.19 ± 0.52 | 1.18 ± 0.52 | 1.06 ± 0.52 | 0.75 ± 0.15 |
| Tesaglitazar | 0.75 | 0.46 ± 0.24 | 1.35 ± 0.40 | 1.76 ± 0.48 | 0.44 ± 0.26 | 0.66 ± 0.15 | 0.50 ± 0.10 |
| Tesaglitazar | 1.5 | 0.62 ± 0.26 | 0.75 ± 0.23 | 1.38 ± 0.32 | 0.35 ± 0.19 | 0.77 ± 0.26 | 0.43 ± 0.08 |
| Fenofibrate | 75 | 1.30 ± 0.54 | 1.33 ± 0.45 | 1.25 ± 0.42 | 1.13 ± 0.73 | 1.45 ± 0.78 | 0.73 ± 0.24 |
| Fenofibrate | 150 | 2.35 ± 1.05 | 1.23 ± 0.51 | 1.52 ± 0.55 | 1.03 ± 0.24 | 0.56 ± 0.22 | 0.65 ± 0.14 |
Means ± SEM shown
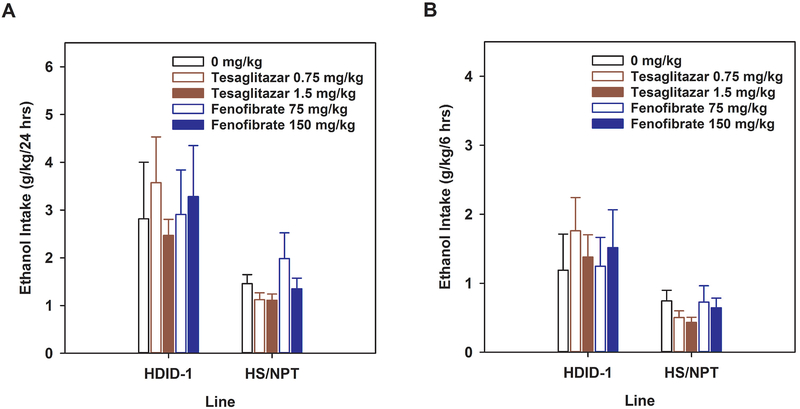
Repeated measures ANOVA performed on the Baseline and Habituation phases of the experiment for the 24-hr data showed the expected significant main effect of Line (F[1,85] = 4.97, p < 0.05) with HDID-1 mice consuming more ethanol than HS/NPT, but Line did not interact significantly with any other factor, and no significant effects of Drug, Dose, Treatment phase, or their interactions were observed (Fs[1-4,85] < 1.76, p values > 0.14). Response to treatment with drugs or vehicle was indexed as the average of days 24 – 31. Analysis of the treatment phase (Figure 2A) showed a significant main effect of Line (HDID-1 > HS/NPT; F[1,86] = 14.56, p < 0.001), but neither the effect of Dose nor the interaction of Line x Dose were significant (Fs[4, 86] ≤ 1.26, ps > 0.29). Results were similar when analyzed separately by Drug.
Figure 2.
PPAR agonists, tesaglitazar and fenofibrate, did not alter ethanol intake in a two bottle choice (2BC) assay with male HDID-1and HS/NPT mice. 2BC testing offered 3-10% ethanol (Days 1-7) and 15% ethanol on Days 8-31. Drugs were administered daily on Days 24-31; average daily intakes from Days 24-31 are shown (Mean ± SEM). A) 24-hr ethanol intake. B) 6 hr intake. n=9-12 mice/line/treatment dose.
The first measurement of intake at 6 hrs occurred on day 18, and this served as the baseline. Habituation to saline injections over the first 6 hrs were indexed as the average of days 22 – 23. For the 6-hr baseline and habituation data, there were no significant effects of Line, Phase, or Dose, nor their interactions (Fs[1-4,81] < 1.27, p > 0.28). Response to treatment with drugs or vehicle was indexed as the average of days 24 – 31. During the Treatment phase, we observed the expected main effect of Line (F[1,86] = 14.44, p < 0.001), but no main effect of Dose or Dose x Line interaction (Fs[4,86] ≤ 1.63, ps > 0.17; Figure 2B). Results were similar when we analyzed the drug vs vehicle comparisons separately. For tesaglitazar, a main effect of Line and the Line x Dose interaction were significant (Fs[1-2,53] ≥ 4.50, ps < 0.05), while the main effect of Dose was not (F[2,53] = 1.61, p = 0.21). Analysis of the interaction showed that the lines differed at the 0.75 mg/kg dose, with HDID-1 > HS/NPT, while only a trend for an effect of Dose was observed in the HDID-1 line (F[2,26] = 3.13, p = 0.06), but not in HS/NPT mice (F[2,27] = 1.86, p = 0.17). For fenofibrate, the main effect of Line was not significant (F[1,54] = 3.44, p < 0.068); neither were the effect of Dose nor the Line x Dose interaction (Fs[2,54] ≤ 1.40, ps > 0.25).
3.2.2. Experiment 3. Effect of Tesaglitazar on Ethanol DID in HDID-1 mice
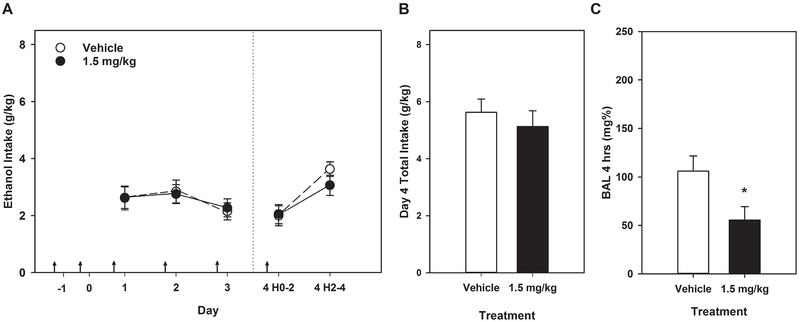
Data from this experiment are shown in Figure 3. No significant effects of or interactions with Sex were seen (all Fs ≤ 1.15), so data are shown collapsed across Sex. Daily 2-hr ethanol consumption in combination with tesaglitazar treatment showed a main effect of Day (F[3,132] = 3.08, p < 0.05) with 2-hr consumption decreasing or tending to decrease (p ≤ 0.055) on Days 3 and 4 relative to Days 1 and 2, paired t-tests). No effects of Treatment or any interactions were significant (all Fs < 1). Total consumption on day 4 also showed no significant effects (all Fs < 1). Surprisingly, daily tesaglitazar treatment significantly lowered BAL relative to vehicle Treatment (F[1,44] = 5.70, p < 0.05), with no effect of Sex or their interaction.
Figure 3.
Tesaglitazar did not alter ethanol intake in HDID-1 mice, but reduced the BAL achieved after a 4-hr DID on day 4. * p < 0.05. Arrows indicate drug or vehicle injections. Means ± SEM shown for n=12 mice/sex/treatment dose. Data are collapsed on sex.
3.2.3. Experiment 4. Effect of Fenofibrate on Ethanol DID in HDID-1 mice
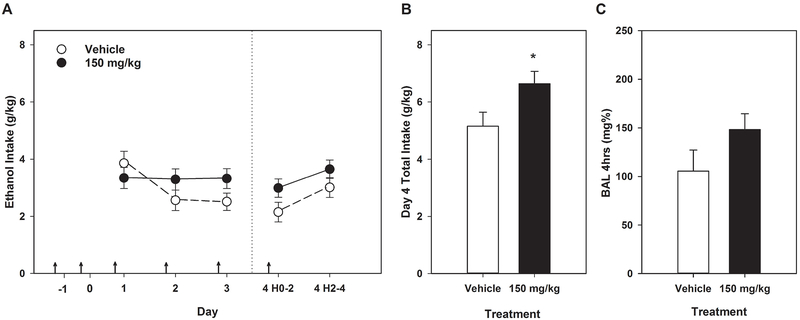
Data from this experiment are shown in Figure 4. Results with fenofibrate differed from those with the other PPAR agonist, tesaglitazar. Analysis of daily 2-hr ethanol consumption revealed significant effects of Day (F[3,117] = 5.55, p < 0.01) and interactions of Day x Treatment (F[3,117] = 2.95, p < 0.05) and Day x Sex (F[3,117] = 2.74, p < 0.05). The Day x Sex interaction was investigated using 1-way ANOVAs for each day separately; females consumed more ethanol only on Day 3 than male mice did (F[1,41] = 7.33, p < 0.01). In the absence of a significant 3-way interaction (F[3,117] = 1.94, p > 0.12), as well as the lack of main effects of Sex, Treatment, or their interaction (all Fs [1,39] < 3.37, p values > 0.07), we pursued only the interaction of Day x Treatment with 1-way ANOVAs by day. Fenofibrate did not significantly affect 2-hr intake on any day (all Fs[1,41-42] ≤ 3.2, ps > 0.08). Fenofibrate treatment significantly increased day 4 total ethanol consumption relative to vehicle treatment (F[1,42] = 5.14, p < 0.05), but BAL was not affected (F[1,42] = 2.52, p = 0.12).
Figure 4.
Fenofibrate increased ethanol intake on day 4 of DID in HDID-1 mice, but did not alter BAL. * = p < 0.05. Arrows indicate drug or vehicle injections. Means ± SEM shown for n=12 mice/sex/treatment dose. Data are collapsed on sex.
3.3. Experiment 5. Effect of CAPE on Ethanol 2BC and DID after stable 2BC Drinking
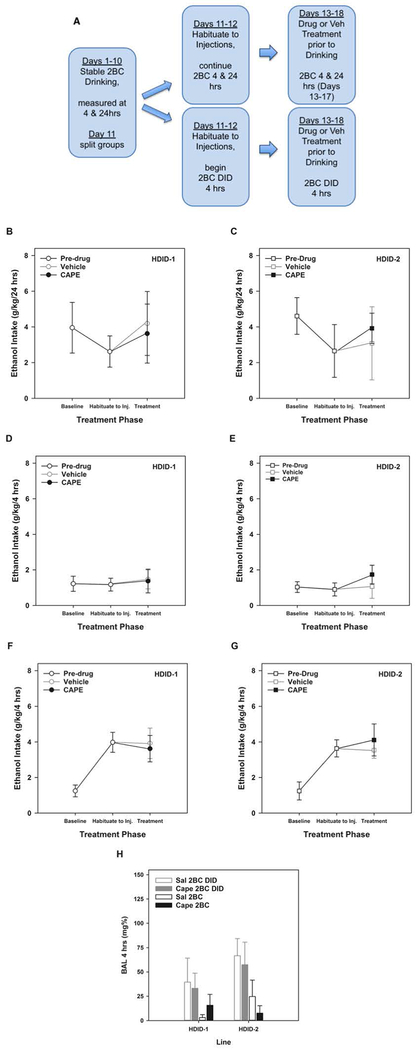
Figure 5A shows the experimental schedule for testing the effects of CAPE on ethanol drinking in HDID-1 and −2 female mice.
Figure 5.
CAPE did not alter ethanol intake in HDID-1 or HDID-2 mice. (A) Schematic timeline for experimental design. (B) 24-hr 2BC intake for each experimental period in HDID-1 and (C) HDID-2 mice. 4-hr intake for mice in the 24-hr 2BC groups for each experimental period in HDID-1 (D) and HDID-2 (E) mice. 4-hr intake for mice in the 2BC DID groups for each experimental period in HDID-1 (F) and HDID-2 (G) mice. H) BALs achieved after 4 hours of ethanol intake for mice in the 2BC and the 2BC DID groups. Baseline = average of Days 9 & 10. Habituation = Days 11-12. Treatment = average of Days 13-17 for 24-hr 2BC (panels B-C) and 13-18 for 4-hr 2BC DID (panels D-G) with drug or vehicle injections each day. Means ± SEM shown for n=15 mice/line/dose/drinking procedure.
3.3.1. Effect of CAPE on 24-hr 2BC
Figures 5B (HDID-1 mice) and 5C (HDID-2 mice) show the 24-hr ethanol g/kg intake for each experimental period (baseline, habituation, and treatment) in mice that maintained the 2BC procedure throughout the experiment. The average intake for days 9-10 represent the pre-treatment baseline and the average of days 11-12 represent the response to saline injection (habituation), neither of which differed by Line, (future) Treatment, or their interaction (all Fs[1,23] < 1.67, p > 0.20). Daily treatment with CAPE did not affect 24hr consumption on days 13-17 in either HDID-1 or HDID-2 mice (Fs[1,22] < 1 for Line and Drug and their interaction; F[4,88] < 1.81, p > 0.13 for Day and the 3-way interaction of Day, Line and Drug; and F[4,88] < 1 for all other interactions).
3.3.2. Effect of CAPE on the first 4hrs of 2BC each day
Similarly, Figures 5D (HDID-1 mice) and 5E (HDID-2 mice) show the first 4-hr of ethanol intake for each experimental period (baseline, habituation, and treatment) in mice that maintained the 2BC procedure throughout the experiment. Prior to drug treatment, there were no significant main effects of Line or future Treatment x Drinking Procedure groups, or their interaction (all Fs[1-3,53] < 1) on average 4-hr consumption on days 9-10. Neither the day 9-10 average nor the day 11-12 average differed by Line, (future) Treatment or their interaction (all Fs[1,23] < 1). Similar to the 24-hr intakes, daily treatment with CAPE did not affect 4-hr consumption (between subjects effects: all Fs[1,23] < 1; within subjects effects of Day and interactions: all Fs[5,115] < 1.37, p values > 0.24). Overall, BALs were very low and, after the last 4-hr consumption period, were not affected by CAPE treatment or Line, or their interaction (all Fs[1,23] < 1.84, p values > 0.18; see Figure 5H).
3.3.3. Effect of CAPE on 4hr DID
Due to the experimental design, we were able to analyze the 2BC DID group both separately (discussed first) and together (discussed in paragraph below) with the 4-hr consumption data from the 24-hr access groups, allowing a direct comparison between limited (Figures 5F–G) and continuous access periods (Figures 5D–E) for HDID-1 and HDID-2 mice, respectively. Note that Baseline intake in Figures 5F–G represents 4-hr consumption data during the period where mice have 24-hr access via 2BC procedure, and as such, is similar to Baseline intake in Figures 5D–E. Within the groups that went through the DID procedures after stable 24-hr access, there were no effects of Line or (future) drug treatment on average consumption on days 11 and 12 (all Fs[1,30] < 1). Repeated daily CAPE treatment did not affect DID consumption on days 13-18 (all between subjects effects: Fs[1,30] < 1; all within subjects effects Fs[5,150] < 2.12, p values > 0.067). Similarly, BAL was not affected by CAPE treatment (Figure 5H; all Fs[1,30] < 1.53, p values > 0.22).
Since no Line or Drug effects were significant in any comparison, we next compared 4-hr consumption between the two drinking procedures (2BC DID vs 24-hr 2BC). For this analysis, we averaged consumption across Treatment days 13-18. Consumption by HDID mice was greater during the 4-hr limited access DID procedure compared with the 4-hr period during the continuous access procedure (F[1,59] = 20.7, p < 0.0001; compare Treatment data in Figures 5F and 5G with Figures 5D and 5E).
3.4. Experiment 6. Effect of Ibrutinib on Ethanol DID
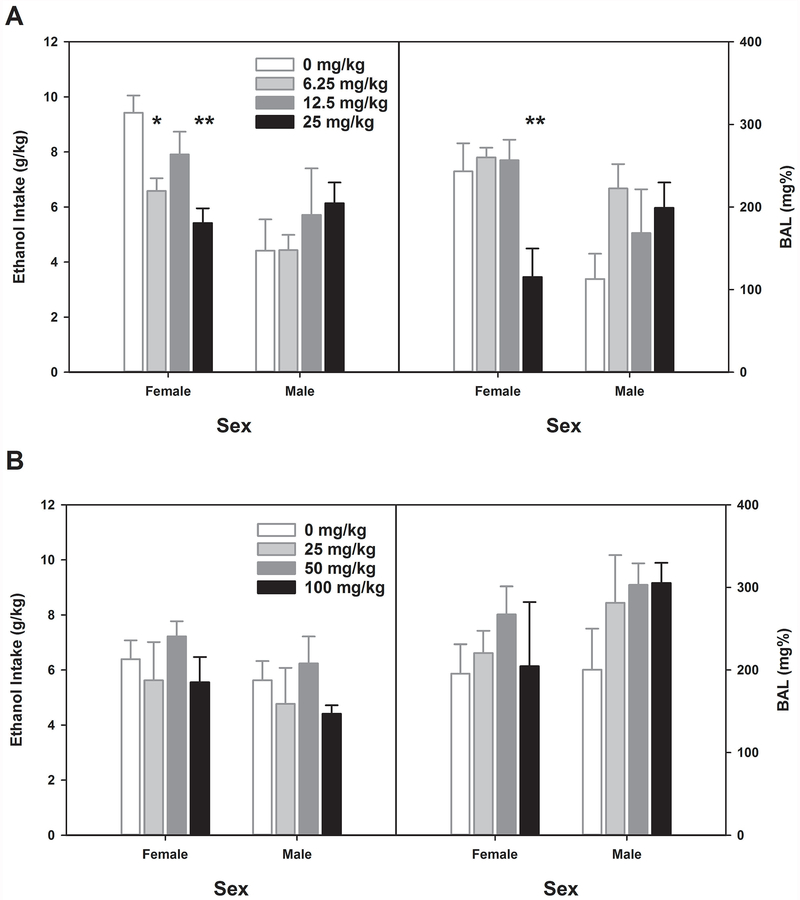
Data for this experiment are presented in Figure 6 and Table 2; data were analyzed generally in the manner of Experiment 1, except that only Day 2 was a treatment day. We first sought confirmation that there were no differences among future Treatment groups in Day 1 drinking of Week 1 (baseline). A two-way ANOVA with future Treatment assignment and Sex as between groups factors showed no differences on this day (all Fs < 1; see Table 2). There was a significant Treatment (on Day 2 of week 1) x Sex interaction for ethanol intake (F[3,39] = 3.13, p < 0.05). The main effect of Sex was also significant (F[1,39] = 10.9, p < 0.01), but not the main effect of Treatment ([F3,39] = 1.21, p > 0.31). We followed up this interaction by analyzing the sexes separately. Female HDID-1 mice showed a significant main effect of ibrutinib treatment (F[3,19] = 7.08, p < 0.01), while male HDID-1 mice did not (F[3,20] < 1). Tukey’s HSD test showed that female mice showed reduced drinking at the 6.25 mg/kg and 25 mg/kg doses relative to vehicle [t = 2.84 (p < 0.05) and t = 4.01 (p < 0.01), respectively (see Figure 6A)]. Similar results were found for Day 2 BALs: Treatment interacted with Sex (F[3,39] = 3.80, p < 0.05, but main effects did not reach significance (Fs[1–3, 39] ≤ 3.38, ps > 0.07). Female mice showed significant reduction in BAL on Day 2 after ibrutinib (F[3,19] = 5.84, p < 0.01). Post-hoc testing showed that this effect was entirely due to lower BALs in the 25 mg/kg dose group, which differed significantly from vehicle and both other dose groups (Tukey’s HSD, ps < 0.05). As predicted by the lack of effect of ibrutinib on Day 2 intake by male mice, no differences among dose groups were observed in BAL (F[3,20] = 1.61, p = 0.21; see Figure 7). When we analyzed Day 3 data, we found that there were no significant effects (Fs[1-3, 39] < 1.65, ps > 0.20), indicating that the effects of ibrutinib treatment did not either last or accrue at these doses (see Table 2).
Figure 6.
Effects of Ibrutinib on DID ethanol intake on Day 2 each week. A) 6.25 and 25 mg/kg Ibrutinib reduced ethanol intake in female HDID-1 mice. 25 mg/kg Ibrutinib reduced achieved BALs in female HDID-1 mice. B) No effect of Ibrutinib was observed upon re-testing re-randomized groups in week 2. * p<0.05, ** p<0.01. Means ± SEM shown for n=6 mice/sex/treatment dose.
Table 2.
Effects of BTK Inhibitor Ibrutinib on DID Drinking on Non-Treatment Days
| HDID-1 | |||
|---|---|---|---|
| Week 1 | g/kg Intake | ||
| Ibrutinib dose (mg/kg) | Day 1 | Day 3 | |
| 0 | 3.02 ± 0.51 | 2.88 ± 0.47 | |
| 6.25 | 2.87 ± 0.45 | 2.33 ± 0.50 | |
| 12.5 | 2.89 ± 0.37 | 2.98 ± 0.39 | |
| 25 | 3.04 ± 0.42 | 2.58 ± 0.50 | |
| Week 2 | |||
| Ibrutinib dose (mg/kg) | Day 1 | Day 3 | |
| 0 | 2.36 ± 0.48 | 3.53 ± 0.57 | |
| 25 | 2.50 ± 0.61 | 2.60 ± 0.53 | |
| 50 | 2.79 ± 0.68 | 3.51 ± 0.47 | |
| 100 | 2.71 ± 0.83 | 2.32 ± 0.43 | |
Means ± SEM shown
Figure 7.
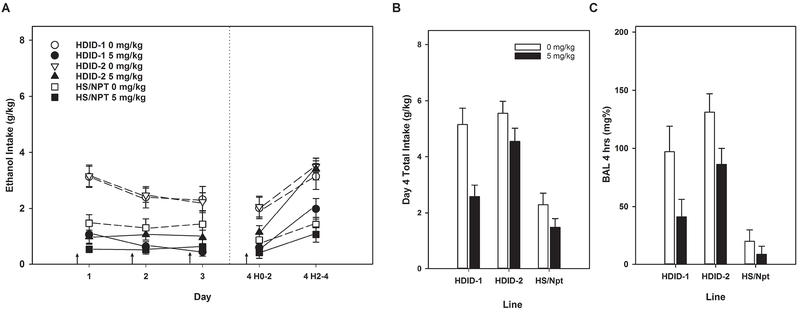
Rolipram reduced DID ethanol intake and achieved BALs in HDID-1, HDID-2, and HS/NPT mice. Arrows indicate drug or vehicle injections. Means ± SEM shown for n=8-9 mice/line/sex/treatment dose. Data are collapsed on sex.
Similar analyses were performed on Week 2 data after re-randomization into higher dose groups. On Day 1, no differences in ethanol intake were observed prior to drug treatment (Fs[1-3,39] < 1; Table 2). Additionally, no significant effects of ibrutinib dosing on either intake (Fs[1-3, 39] ≤ 2.17, ps > 0.14) or BAL (Fs[1-3, 39] ≤ 2.42, ps > 0.12) were observed in this week. Day 3 results also showed no effects of ibrutinib at these doses (Fs[1-3, 39] ≤ 1.89, ps > 0.17; see Table 2).
3.5. Experiment 7. Effect of Rolipram on Ethanol DID
Data for this experiment are presented in Figure 7, and were analyzed similarly to Experiment 1, where drug was also administered every day. Although significant main effects of Sex were found in ethanol consumption ANOVAs, with females > males, no significant interactions of Sex were found with Line, Treatment, or Day, or 2-hr Block. Therefore, all analyses were collapsed on Sex.
Daily 2-hr intake showed significant main effects of Line (F[2,92] = 8.49, p < 0.001; HDID-1 > HDID-2 and HS/NPT) and Treatment (F[1,92] = 52.36, p < 0.0001; vehicle > rolipram), and a trend for their interaction (F[2,92] = 2.87, p < 0.062). Day (F[3,276] = 6.38, p < 0.001) and its interaction with Treatment (F[3,276] = 3.26, p < 0.05) were significant, but Day did not interact with either Line or Line x Treatment (both Fs[6,276] < 1.10, all p > 0.36). Analysis of the two 2-hr blocks on day 4 showed that Lines differed significantly in consumption (F[2,92] = 25.9, p < 0.0001). Rolipram treatment significantly reduced ethanol intake on day 4 (F[1,92] = 16.37, p ≤ 0.0001), but Line and Treatment did not interact significantly (F[2,92] = 2.37, p = 0.099). As we have observed in most DID experiments, animals consumed more ethanol in the 2nd 2-hr block than they did in the 1st (F[1,92] = 50.4, p < 0.0001) and this interacted significantly with Line (F[2,92] = 4.24, p < 0.05). Neither the Block x Treatment nor the 3-way interaction was significant (Fs[1-2,92] < 1.30, p values > 0.25), indicating that rolipram’s effects were equivalent in reduction across Blocks and similar across Lines. The analysis of total day 4 g/kg intake showed similar results. Rolipram also significantly reduced BAL (F[2,89] = 13.37, p < 0.001). Lines also differed significantly in BAL (F[2,92] = 26.3, p < 0.0001; HDID-2 > HDID-1 >> HS), but Line x Treatment did not interact significantly(F[2,89] = 1.54, p > 0.22).
4. Discussion
We report here the results of seven experiments, each designed to assess the potential of an experimental compound to reduce ethanol drinking in mice. Each compound was nominated for testing by the INIA-Neuroimmune consortium for various reasons. For gabapentin (Experiment 1), we were guided by 3 studies (one preclinical, and two clinical) that evaluated the effects of gabapentin on alcohol-related behaviors (Besheer et al., 2016, Mason et al., 2014, Falk et al., 2019). For the PPAR agonists tesaglitazar and fenofibrate (Experiments 2-4), the NFkB inhibitor CAPE (Experiment 5), and rolipram (Experiment 7) we modeled our tests on earlier reports of efficacy conducted in C57BL/6J inbred mice (Blednov et al., 2016b, Blednov et al., 2015, Ferguson et al., 2014, Blednov et al., 2014, Cui et al., 2013). For ibrutinib (Experiment 6), there were no extant preclinical data regarding such effects. A novel feature of our work is that we employed mice with high genetic risk for binge-like drinking (High Drinking in the Dark mice) and their genetically heterogeneous founder stock, HS/NPT. The high levels of ethanol consumption HDID mice display during limited access binge-like sessions allowed us to test the effects of these compounds on animals that drink to intoxicating blood alcohol levels.
The most promising results we report here are the findings in Experiment 7 showing that 5 mg/kg rolipram significantly reduced intake and reduced BALs to well below the level of behavioral intoxication. We saw this consistently in mice of both sexes of two independently-derived (and therefore genetically distinct) HDID mouse lines, as well as in the low-drinking genetically heterogeneous founder stock (HS/NPT mice). Ongoing work is focused on PDE4 inhibitors with high translational value for drug repurposing. PDE4 inhibitors can directly and indirectly exert effects (e.g. increase cAMP signaling, alter cell signaling and neuronal activity, reduce pro-inflammatory immune signaling, increase anti-inflammatory signaling). Thus, we are currently following up on this work to delineate mechanisms by which PDE4 reduces binge-like drinking. Moreover, we are collaborating with several INIA-Neuroimmune labs to test PDE4 inhibitors using multiple models, both sexes, and multiple genotypes.
Although positive findings have been reported for each of the other drugs we tested (except ibrutinib), we consider our results for those drugs to be essentially negative (we discuss ibrutinib below). Why would this be? We considered several possible alternatives. First, although we modeled our studies after others, there could be procedural differences between ours and the previous experiments could have led to different results (See Table S1). Second, genetic differences between our subjects and others’ subjects may have led to our animals’ lack of sensitivity. We consider these explanations the most likely.
For gabapentin (Experiment 1), we used a variant of our standard DID design with injections of drug or vehicle every day. Gabapentin transiently increased ethanol intake (Days 1 and 2), but this effect did not persist during Days 3 and 4; nor was BAL affected on Day 4 (Fig 2). We were only able to find one other study that evaluated the effects of gabapentin on alcohol drinking in rodents. Although different paradigms (as well as different species and sexes) were employed, they reported similar findings for the acute effects of gabapentin on drinking. Besheer et al. (2016) found that 60mg/kg gabapentin increased alcohol self-administration in male rats. Moreover, Besheer et al. (2016) further characterized the interoceptive effects of gabapentin and found that 120 mg/kg had alcohol-like effects, and was able to potentiate the discriminative stimulus effects of alcohol (Besheer et al., 2016). In contrast, gabapentin dose-dependently (900, 1800 mg/d) decreased alcohol drinking in a 12 week, double-blind, placebo-controlled randomized dose-ranging trial of adult men and women with current alcohol dependence (Mason et al., 2014). Mason reported that the rate of abstinence was four times greater and the rate of no heavy drinking doubled for 1800 mg/d gabapentin vs placebo. It is possible that the dose used in our study was too low. The dose we administered to mice (120 mg/kg) translates to a human equivalent dose range of 576-768 mg/d (for 60-80kg body weight, respectively) and is therefore not equivalent to the 1800 mg/d dose used by Mason et al. (2014). For reference, the doses Besheer administered to rats (60 and 120mg/kg) translate to the following human equivalent dose ranges: 576-768 mg/d (for 60-80kg body weight, respectively) for the 60 mg/kg rat dose, and 1152-1536 mg/d (for 60-80kg body weight, respectively) for the 120 mg/kg rat dose. Further, we had only female HDID-1 and HS/NPT mice available to test. We have no explanation for the transient, unexpected increase in drinking. It is possible that administering higher doses in a more chronic manner might also have revealed an effect. A recent publication reported that gabapentin (600mg 2x per day) had no effects on alcohol consumption or craving in a randomized, double-blind, placebo-controlled, multi-site trial (Falk et al., 2019). This suggests that high doses of gabapentin, higher than those recommended for treating other conditions, may be required to observe reductions in consumption and craving. Such high doses are often accompanied by significant side effects, which likely reduces the clinical translational value of gabapentin for AUD.
For the PPAR agonists tesaglitazar and fenofibrate, we find an explanation for our negative finding based on procedural differences less convincing. We tested the ability of tesaglitazar and fenofibrate to alter 2BC continuous access preference drinking, as well as limited access DID (Experiments 2-4). Each drug had been tested using very similar continuous 2BC paradigms. Previous work found that tesaglitizar (1.5mg/kg) reduced 2BC drinking in male and female C57BL/6J mice (Blednov et al., 2015, Blednov et al., 2016a), while we found no effect of that dose on 2BC intake of either HDID-1 or HS/NPT male mice (Figure 3). For fenofibrate, Blednov et al. (2015, 2016a) found dose-dependent reductions in drinking in male, but not female, C57BL/6J mice. We found no effects of fenofibrate on 2BC drinking in either genotype we tested (Figure 3). Blednov et al. (2016a) also tested fenofibrate (150 mg/kg dose) using a continuous 24-hr access paradigm where ethanol was offered every other day and found that it reduced intake of both sexes of C57BL/6J mice. This indicates that testing paradigms indeed can make a difference in outcomes even when compared with very similar protocols. We next assessed the effects of these compounds on DID in male and female HDID-1 mice and found that our results also differ from the effects observed in C57BL/6J mice by the Blednov group. We saw no effect of tesaglitazar at 1.5 mg/kg on either sex of HDID-1 mice (Figure 4), but we did see an unexplained reduction in BALs. The highest dose of fenofibrate tested (150 mg/kg) in HDID-1 resulted in a small increase in drinking during days 2-4 of DID that was not accompanied by an increase in BALs. Both sexes in our study were equivalently affected (Figure 5). Blednov et al. (2015) assessed both drugs in male C57BL/6J mice using a 3 hr limited access 2BC DID test where mice had been drinking 15% ethanol for 3 or more weeks under continuous access 2BC prior to testing and noted that both drugs reduced DID intake. Because we consider the procedural differences between these and prior studies to be modest, together, these experiments suggest that genotype can have a large influence on response to compounds targeting PPAR.
To further pursue compounds targeting neuroimmune pathways, we tested the effects of CAPE and ibrutinib on ethanol drinking. However, we did not observe any change in ethanol intake or preference by female HDID-1 or HDID-2 mice after treatment with 20 mg/kg CAPE. Thus, additional testing with higher doses could be required to observe reductions in ethanol drinking.
Ibrutinib is a small molecule permanent BTK inhibitor currently FDA approved for a number of indications (including mantle cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and chronic graft versus host disease). Ferguson and Ozburn et al. (2018) utilized the Library of Integrated Network-Based Cellular Signatures (LINCS-L1000; http://lincsproject.org/; http://clue.io) database to identify a similar, but less well characterized (and not FDA approved), BTK inhibitor, terreic acid and demonstrated that treatment with terreic acid led to a significant reduction in both DID ethanol intake and BALs following treatment in male and female HDID-1 mice. With consideration of the National Institutes of Health’s (NIH) drug repurposing initiative, here we used a similar approach to test whether the FDA approved and more-well characterized BTK inhibitor, ibrutinib, would also reduce ethanol intake and BALs. Moreover, given ibrutinib’s irreversible binding to BTK, as well as considerably long half-life at relatively intermediate doses [T1/2 = 87.4 hr for a single dose of 24 mg/kg (Goldwirt et al., 2018)], we sought to investigate the lasting effects of ibrutinib utilizing a 3-day DID procedure. Although we hypothesized a reduction in ethanol intake and BALs with ibrutinib treatment, reduction in intake and BAL were only observed in female mice during week 1 on the day of treatment (no lasting effects were observed the day after treatment). Notably, we randomized treatment groups and tested mice a second time (including same and higher doses) during week 2, and saw no effects of ibrutinib on DID. It is possible that the lack of effect in week 2 could be due to differences in route of administration. Given the modest effects observed only in female mice, and only at one time, and an undesirable side effects profile associated with higher doses, we decided not to further pursue this compound. We think the difference between the terreic acid and ibrutinib effects on DID are due to non-overlapping mechanisms of action for ibrutinib and terreic acid. Both compounds have an array of targets, although these are less well defined for terreic acid.
In summary, we report that a number of compounds shown to reduce ethanol drinking in other models and genotypes are not effective in HDID mice, or their genetically heterogeneous founders, HS/NPT. It is difficult to speculate further on what exactly this tells us about the role of neuroimmune signaling in alcohol use disorders. We don’t know why certain anti-inflammatory compound may reduce alcohol intake (rolipram), others may increase alcohol intake / seeking (gabapentin, fenofibrate), and others may be ineffective at reducing alcohol drinking in a line selectively bred for high, binge-like alcohol intake. One take away lesson from this body of work is that genetic differences may contribute a great deal of variability in effectiveness for targeting a particular pathway, or perhaps we learn that more than one pathway needs to be targeted in heterogeneous populations in order to reduce harmful drinking. Given that rolipram, a PDE4 inhibitor, reduced binge-like drinking in multiple lines of mice, it appears to be the most promising target we identified. Inhibition of PDE4 affects a number of cellular processes (as does alcohol!). However, rolipram’s side effect profile is not desirable due to nausea and emesis; both are thought to be due to its high affinity for PDE4 subtype D. Notably, rolipram at 1 or 5 mg/kg did not reduce total fluid intake in C57BL/6J mice, although 5 mg/kg reduced total fluid intake in the DID test (Blednov et al, 2014). Newer compounds, with different PDE4 subtype selectivity profiles, have been developed and are currently being tested. Based on the results of these studies, as well as our previous work, we highlight the importance of rigorously testing compounds (in both sexes, using multiple doses, paradigms, and genotypes) when determining whether compounds are suitable for drug repurposing.
Supplementary Material
Acknowledgements
We gratefully acknowledge the following individuals for their expert technical assistance in performance of these experiments: Jason P. Schlumbohm, Stephanie E. Spence, and Lawrence C. Huang.
Source of Funding and Conflict of Interest: Supported by NIAAA [Integrative Neuroscience Initiative on Alcoholism (INIA-Neuroimmune) grants AA013519 and AA013520; NIAAA Center grant AA10760; NIAAA R24 AA020245; the US Department of Veterans Affairs Grants BX000313 and CDA2 BX002488; and the John R. Andrews Family. Authors have full control of all primary data and agree to allow the journal and any subsequent readers of the published work to review the data if requested. All authors report no conflicts of interest.
References
- ABDEL-SALAM OM & SLEEM AA 2009. Study of the analgesic, anti-inflammatory, and gastric effects of gabapentin. Drug Discov Ther, 3, 18–26. [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM & CRABBE JC 2012. Ethanol drinking microstructure of a high drinking in the dark selected mouse line. Alcohol Clin Exp Res, 36, 1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM & CRABBE JC 2014. High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol, 48, 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM & CRABBE JC 2015a. Distinct ethanol drinking microstructures in two replicate lines of mice selected for drinking to intoxication. Genes Brain Behav, 14, 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM & CRABBE JC 2015b. Genotypic and sex differences in anxiety-like behavior and alcohol-induced anxiolysis in High Drinking in the Dark selected mice. Alcohol, 49, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM, CUNNINGHAM CL, SMITASIN PJ & CRABBE JC 2015. Rewarding and aversive effects of ethanol in High Drinking in the Dark selectively bred mice. Addict Biol, 20, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM, RYABININ AE & CRABBE JC 2016. Neuropeptide Y response to alcohol is altered in nucleus accumbens of mice selectively bred for drinking to intoxication. Behav Brain Res, 302, 160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELFORT R, BERRIA R, CORNELL J & CUSI K 2010. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J Clin Endocrinol Metab, 95, 829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESHEER J, FRISBEE S, RANDALL PA, JARAMILLO AA & MASCIELLO M 2016. Gabapentin potentiates sensitivity to the interoceptive effects of alcohol and increases alcohol self-administration in rats. Neuropharmacology, 101, 216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, BENAVIDEZ JM, BLACK M, FERGUSON LB, SCHOENHARD GL, GOATE AM, EDENBERG HJ, WETHERILL L, HESSELBROCK V, FOROUD T & HARRIS RA 2015. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res, 39, 136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, BENAVIDEZ JM, BLACK M & HARRIS RA 2014. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci, 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, BLACK M, BENAVIDEZ JM, STAMATAKIS EE & HARRIS RA 2016a. PPAR Agonists: I. Role of Receptor Subunits in Alcohol Consumption in Male and Female Mice. Alcohol Clin Exp Res, 40, 553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, BLACK M, BENAVIDEZ JM, STAMATAKIS EE & HARRIS RA 2016b. PPAR Agonists: II. Fenofibrate and Tesaglitazar Alter Behaviors Related to Voluntary Alcohol Consumption. Alcohol Clin Exp Res, 40, 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, METTEN P, FINN DA, RHODES JS, BERGESON SE, HARRIS RA & CRABBE JC 2005. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res, 29, 1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC 2014. Use of animal models of alcohol-related behavior. Handb Clin Neurol, 125, 71–86. [DOI] [PubMed] [Google Scholar]
- CRABBE JC, COLVILLE AM, KRUSE LC, CAMERON AJ, SPENCE SE, SCHLUMBOHM JP, HUANG LC & METTEN P 2012a. Ethanol tolerance and withdrawal severity in high drinking in the dark selectively bred mice. Alcohol Clin Exp Res, 36, 1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, KRUSE LC, COLVILLE AM, CAMERON AJ, SPENCE SE, SCHLUMBOHM JP, HUANG LC & METTEN P 2012b. Ethanol sensitivity in high drinking in the dark selectively bred mice. Alcohol Clin Exp Res, 36, 1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, METTEN P, BELKNAP JK, SPENCE SE, CAMERON AJ, SCHLUMBOHM JP, HUANG LC, BARKLEY-LEVENSON AM, FORD MM & PHILLIPS TJ 2014. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes Brain Behav, 13, 236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, METTEN P, HUANG LC, SCHLUMBOHM JP, SPENCE SE, BARKLEY-LEVENSON AM, FINN DA, RHODES JS & CAMERON AJ 2012c. Ethanol Withdrawal-Associated Drinking and Drinking in the Dark: Common and Discrete Genetic Contributions. Addict Genet, 1, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, METTEN P, RHODES JS, YU CH, BROWN LL, PHILLIPS TJ & FINN DA 2009. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry, 65, 662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, METTEN P, SAVARESE AM, OZBURN AR, SCHLUMBOHM JP, SPENCE SE & HACK WR 2019. Ethanol Conditioned Taste Aversion in High Drinking in the Dark Mice. Brain Sci, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, OZBURN AR, METTEN P, BARKLEY-LEVENSON A, SCHLUMBOHM JP, SPENCE SE, HACK WR & HUANG LC 2017. High Drinking in the Dark (HDID) mice are sensitive to the effects of some clinically relevant drugs to reduce binge-like drinking. Pharmacol Biochem Behav, 160, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE JC, SPENCE SE, BROWN LL & METTEN P 2011. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol, 45, 427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI C, GRANDISON L & NORONHA A 2013. Neural-immune interactions in brain function and alcohol related disorders, New York, Springer. [Google Scholar]
- DAYNES RA & JONES DC 2002. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol, 2, 748–59. [DOI] [PubMed] [Google Scholar]
- ERICKSON EK, GRANTHAM EK, WARDEN AS & HARRIS RA 2019. Neuroimmune signaling in alcohol use disorder. Pharmacol Biochem Behav, 177, 34–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK DE, RYAN ML, FERTIG JB, DEVINE EG, CRUZ R, BROWN ES, BURNS H, SALLOUM IM, NEWPORT DJ, MENDELSON J, GALLOWAY G, KAMPMAN K, BROOKS C, GREEN AI, BRUNETTE MF, ROSENTHAL RN, DUNN KE, STRAIN EC, RAY L, SHOPTAW S, AIT-DAOUD TIOURIRINE N, GUNDERSON EW, RANSOM J, SCOTT C, LEGGIO L, CARAS S, MASON BJ, LITTEN RZ, NATIONAL INSTITUTE ON ALCOHOL, A. & ALCOHOLISM CLINICAL INVESTIGATIONS GROUP STUDY, G. 2019. Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety. Alcohol Clin Exp Res, 43, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON LB, MOST D, BLEDNOV YA & HARRIS RA 2014. PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology, 86, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON LB, OZBURN AR, PONOMAREV I, METTEN P, REILLY M, CRABBE JC, HARRIS RA & MAYFIELD RD 2018a. Genome-Wide Expression Profiles Drive Discovery of Novel Compounds that Reduce Binge Drinking in Mice. Neuropsychopharmacology, 43, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON LB, ZHANG L, KIRCHER D, WANG S, MAYFIELD RD, CRABBE JC, MORRISETT RA, HARRIS RA & PONOMAREV I 2018b. Dissecting Brain Networks Underlying Alcohol Binge Drinking Using a Systems Genomics Approach. Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ BM, CORDERO KA, BARKLEY-LEVENSON AM, METTEN P, CRABBE JC & BOEHM SL 2ND 2014. Genetic relationship between predisposition for binge alcohol consumption and blunted sensitivity to adverse effects of alcohol in mice. Alcohol Clin Exp Res, 38, 1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDWIRT L, BECCARIA K, PLE A, SAUVAGEON H & MOURAH S 2018. Ibrutinib brain distribution: a preclinical study. Cancer Chemother Pharmacol, 81, 783–789. [DOI] [PubMed] [Google Scholar]
- IANCU OD, COLVILLE AM, WILMOT B, SEARLES R, DARAKJIAN P, ZHENG C, MCWEENEY S, KAWANE S, CRABBE JC, METTEN P, OBERBECK D & HITZEMANN R 2018. Gender-Specific Effects of Selection for Drinking in the Dark on the Network Roles of Coding and Noncoding RNAs. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IANCU OD, OBERBECK D, DARAKJIAN P, METTEN P, MCWEENEY S, CRABBE JC & HITZEMANN R 2013. Selection for drinking in the dark alters brain gene coexpression networks. Alcohol Clin Exp Res, 37, 1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JI RR, XU ZZ & GAO YJ 2014. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov, 13, 533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON BJ, QUELLO S, GOODELL V, SHADAN F, KYLE M & BEGOVIC A 2014. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med, 174, 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSON LM & GRAHAME NJ 2013. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol, 18, 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSON LM, KASTEN CR, BOEHM SL 2ND & GRAHAME NJ. 2014. Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res, 38, 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METTEN P, BROWN LL & CRABBE JC 2011. Limited access ethanol drinking in the dark in adolescent and adult mice. Pharmacol Biochem Behav, 98, 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZBURN AR, JANOWSKY AJ & CRABBE JC 2015. Commonalities and Distinctions Among Mechanisms of Addiction to Alcohol and Other Drugs. Alcohol Clin Exp Res, 39, 1863–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES JS, BEST K, BELKNAP JK, FINN DA & CRABBE JC 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav, 84, 53–63. [DOI] [PubMed] [Google Scholar]
- RHODES JS, FORD MM, YU CH, BROWN LL, FINN DA, GARLAND T JR. & CRABBE JC 2007. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav, 6, 1–18. [DOI] [PubMed] [Google Scholar]
- TAMBUWALA MM, KESHARWANI P, SHUKLA R, THOMPSON PD & MCCARRON PA 2018. Caffeic acid phenethyl ester (CAPE) reverses fibrosis caused by chronic colon inflammation in murine model of colitis. Pathol Res Pract, 214, 1909–1911. [DOI] [PubMed] [Google Scholar]
- TESTINO G, LEONE S & BORRO P 2014. Treatment of alcohol dependence: recent progress and reduction of consumption. Minerva Med, 105, 447–466. [PubMed] [Google Scholar]
- WAHLSTEN D, BACHMANOV A, FINN DA & CRABBE JC 2006. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A, 103, 16364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEYAMA N, CRABBE JC, FORD MM, MURILLO A & FINN DA 2008. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol, 42, 149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU J, MIX E & WINBLAD B 2001. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev, 7, 387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.