Abstract
Objective:
We aimed to assess whether endometrial cancer (EC) can be detected in shed DNA collected with vaginal tampon by analyzing copy number, methylation markers, and mutations.
Methods:
Tampons were collected prior to hysterectomy from 38 EC patients and 28 women with benign indications. Extracted tampon DNA underwent the following: 1) low-coverage whole genome sequencing (LC-WGS) to assess copy number, 2) pyrosequencing to measure percent promotor methylation of HOXA9, RASSF1, and CDH13 and 3) next generation sequencing (NGS) to identify mutations in 19 genes associated with EC identified through The Cancer Genome Atlas. Sensitivity and specificity for each test and test combinations were calculated.
Results:
Methylation analysis yielded the highest specificities but lowest sensitivities (37-40% sensitivity; 100% specificity for HOXA9, RASSF1 and HTR1B) while mutation analysis had improved sensitivity (50% sensitivity; 83% specificity). Only one “false positive” result for copy number variants was identified among women with benign surgical indications, which was based on detection of copy number changes, and associated with a leiomyosarcoma that was only recognized at hysterectomy. Considering any of the 3 biomarker classes as a positive, resulted in a sensitivity of 92% and specificity of 86%. Mutation analysis did not add sensitivity to the combination of analysis of copy number and methylation.
Conclusions:
This study demonstrates a proof-of-principle for non-invasive yet precise detection of endometrial cancer. We propose that with improved biomarker testing, it may be possible to develop a clinically useful test for detecting EC.
Keywords: endometrial cancer, methylation, mutation, copy number, tampon, cancer detection
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States. More than 60,000 new cases of EC are diagnosed each year; more than 13,000 women die from EC each year and the incidence is rising [1, 2]. By 2030, the incidence of EC is expected to surpass that of colorectal cancer [2] secondary to the increasing prevalence of EC risk factors, such as obesity and diabetes mellitus, as well as the aging population[3]. EC is highly curable in the early stages, with a 5-year overall survival of 95% for stage I disease. However, EC diagnosed at stage III and IV have 5-year overall survivals of 69% and 16%, respectively [4], suggesting that early detection could reduce mortality. Additionally, mortality from EC is disproportionately higher amongst African American women [4], possibly due to African American women more often being diagnosed with advanced stage, higher grade, and more aggressive histologic EC subtypes [5].
Presently, there is no early detection or screening method for EC. Early cancer detection, as evidenced through the success of the Pap test [6], has unequivocally decreased the morbidity and mortality associated with cervical cancer. Pap cytology testing requires a physician visit and has low sensitivity to detect EC [7]. Similar to HPV testing for cervical cancer, however, data suggest that testing for tumor mutations in cervical cytology specimens may be more sensitive for EC [7]. The current clinical evaluation for the presence of EC hinges on women presenting with symptoms, such as postmenopausal bleeding (PMB) or abnormal uterine bleeding (AUB) and these symptoms do not correlate with the stage of disease [8]. The detection of EC in asymptomatic women is more often coincidental based on abnormal uterine imaging or cellular abnormalities noted on Pap test [9]. The current workup for a woman with PMB or AUB includes pelvic imaging, endometrial biopsy, or occasionally hysterosonogram, hysteroscopy, or dilation and curettage, or all of these tests. Although only 5-10% of women with AUB or PMB have an underlying EC or an EC precursor (i.e. endometrial hyperplasia), 5% of all gynecologic visits are for PMB, suggesting that many women undergo intensive diagnostic work-up for benign disease [8, 10].
As previously mentioned, data suggest that testing for EC–associated mutations in liquidbased cervical Pap tests and analysis of vaginal fluid collected via tampon may be promising approaches for EC detection; however, analysis of multiple biomarker types in tampons has not been performed. [4, 11, 12]. Given that the tampon is a widely accepted hygiene product that women could use to self-collect a diagnostic vaginal fluid biospecimen [13], tampon testing offers the potential to increase access to care via at home collection with mailing to the clinic, analogous to the use of Cologuard as a self-collected stool test for colon cancer [14] and self-collected vaginal samples for HPV testing in cervical cancer [15].
In the current study, we examined the potential of mutation analysis and copy number testing to improve test accuracy beyond our prior results based on analysis of DNA methylation markers. We hypothesized that combining biomarker classes may improve discrimination of benign endometrium and EC.
Methods
Patient Identification
This study included 38 patients with EC and 28 women undergoing hysterectomy for other benign clinical indications. Briefly, women undergoing a clinically indicated hysterectomy at the Mayo Clinic in Rochester, Minnesota for EC and a comparison group age ≥45 years undergoing hysterectomy for benign indications were consented and prospectively enrolled to provide a preoperative intravaginal tampon and endometrial tissue from the hysterectomy specimen. Exclusion criteria included the presence of recurrent EC or a new diagnosis of cervical cancer, receipt of neoadjuvant chemotherapy or any prior pelvic radiotherapy, prior endometrial ablation or hysterectomy or presence of cervical stenosis and patients with pelvic organ prolapse for who tampon placement was not feasible. Women were excluded if tampon data were unsuccessfully collected. Vaginal fluid was collected using an intravaginal tampon self-placed by the patient in the preoperative area and removed just prior to surgery. Patients were instructed to refrain from placing anything per vagina for at least 24 hours prior to surgery. Final clinical pathology diagnosis of each patient’s endometrial findings was utilized as the diagnostic standard.
Vaginal Pool Biospecimen Processing
The intravaginal dwell duration for each tampon was recorded and PBS buffer was added to the tampon immediately upon removal. Samples were placed at 4° C until same-day processing. DNA was extracted from each tampon sample using the Roche High Pure Viral Nucleic acid kit (Roche Applied Science, Germany) using the manufacturer’s protocols as previously reported [11]. Then the DNA was eluted to 50μL and quantified using NanoDrop (mutation and copy number assays) from absorptions at 230 nm, 260 nm, and 280 nm [11].
Copy number assay
Tampon DNA underwent indexing and library preparation using the Tru-Seq Nano DNA library preparation kit v2 (Illumina). Samples were pooled and underwent low-pass whole genome sequencing on the Illumina HiSeq 2500 using a 51 base-pair single-end sequencing protocol in rapid-run mode (Illumina). The evaluation for copy number variants (CNV) was done using Wandy [16], an in-house pipeline developed to detect copy number changes from whole genome sequencing data. Wandy divided the input data into 10,000 base pair bins, reduced noise, and used a classification and regression tree (CART) algorithm to identify step-wise changes in the data. This was achieved by having the CART algorithm set to a certain cost-complexity parameter that achieved identification of changes.
Methylation assays
DNA from the tampon supernatant underwent bisulfite modification according to the manufacturer’s protocols using the EX-96 DNA Methylation Gold kit (Zymo Research, Irvine, CA). Pyrosequencing was performed on tampon samples using assays developed for 3 genes (RASSF1, HTR1B, and HOXA9) in which methylation has been previously described in EC [11, 17] using primers (Table 4) designed with the Pyrosequencing Assay Design Software (Qiagen). A region of up to 250bp was amplified using polymerase chain reaction (PCR) and included both methylated and unmethylated genes. Amplification was performed using TaqGold DNA polymerase (Applied Biosystems). Pyrosequencing was then performed on a Biotage PyroMark MD System (Qiagen) according to the manufacturer’s protocols. RASSF1, HTR1B, and HOXA9 were selected given their highest area under the curve (AUC) in our previous methylation-based pilot study [11]. Pyrosequencing primers spanned 16 CpGs within RASSF1, 2 within HTR1B, and 14 within HOXA9.
Table 4.
Primer sequences for 3 genes that underwent pyrosequencing in endometrial cancer and benign endometrium controls.
| HOXA9_F1 | GGGAATTTTGATTGTTAGTTGATGAGA |
| HOXA9_R1_biotin | ACCCTACCAAAACACTCC |
| HOXA9_Seq1 | GTAGTTGTGGGGATTTATA |
| RASSF1-F1 | GGGGGAGTTTGAGTTTATTGA |
| RASSF1-R1-biotin | CTACCCCTTAACTACCCCTTCC |
| RASSF1-Seq1f | GGGTYGTATTYGGTTGGAG |
| HTR1B_F1 | TTTGGGAGGGAGTAGAGGATAA |
| HTR1B_R1_biotin | ACTACCCCACCCATAACCTCTAATTTCAA |
| HTR1B_Seq1 | GATAGTAGGTTTGTTTTTAGTTGAT |
Mutation assays
Targeted coverage of eight genes (PIK3CA, KRAS, FGFR2, CTNNB1, NRAS, HRAS, AKT1, EGFR) and full exon coverage of eleven genes (PTEN, TP53, FBXW7, CDKN2A, ARID1A, PIK3R1, ARID5B, PPP2R1A, RPL22, POLE, POLD1) associated with EC were selected based on The Cancer Genome Atlas (TCGA) EC project[18]. Mutations were identified via NGS using custom-designed PCR amplification primers followed by MiSeq and Ion Torrent sequencing.[19] Using Leucippus software (https://github.com/abyzovlab/Leucippus) 250 bp MiSeq reads were merged using overlapping 3’-ends. Reads were aligned to the reference human genome with BWA. Then we searched for the presence of loss-of-function (LOF) or missense variants in COSMIC database. For each substitution type (like C to T) a per sample error profile was derived from amplified positions which were not in the list of sites from COSMIC or where a variant could not lead to LOF. Using the error profiles as a background we assigned statistical significance to putative LOF and missense variants. Additionally, the classification of each variant was manually reviewed.
Statistical analyses
Copy number status was reported in a binary (yes/no) fashion. If there was a pathogenic mutation detected in any of the 19 genes, the sample was considered positive for the presence of mutation. As pyrosequencing provides percent methylation as a continuous variable, methylation output was converted to a binary (positive/negative) variable. Percent methylation was averaged across all CpGs for each of the 3 genes and positive methylation was defined as mean methylation within a sample 3 standard deviations or greater above the mean for BE. Sensitivity and false positive rate (1-specificity) was calculated for each test, each combination of tests, and all three tests combined, based on the subset of patients evaluated. Ninety-five percent confidence intervals (95% CI) were constructed using an exact test for a binomial parameter.
Results
Patient and tumor characteristics
EC histologic types included 1 (2.6%) complex atypical hyperplasia (CAH), 29 (76.4 %) FIGO grade 1 or 2 endometrioid, 2 (5.3 %) FIGO grade 3 endometrioid, 3 (7.9%) clear cell and 3 (7.9%) and serous carcinomas. Stage distribution was: 1 (2.6%) CAH, 26 (68.4%) FIGO stage IA, 6 (15.8%) stage IB, 3 (7.9%) stage III, and 2 (5.3%) stage IV. Tumor characteristics are listed in Table 2. Benign indications for hysterectomy included: presumed fibroids (17, 63.0%), benign-appearing ovarian mass (5, 18.5%), dysmenorrhea (3, 11.1%), endometrial polyp (1, 3.7%), abnormal Pap (1, 3.7%), and endometriosis (1, 3.8%). One patient who underwent hysterectomy for presumed fibroids was diagnosed with leiomyosarcoma on final pathology; her endometrium was benign. Final pathology confirmed benign findings in the remaining control patients.
Table 2.
EC tumor characteristics.
| Tumor characteristic | Overall (N=38) |
|---|---|
| FIGO 2009 stage, n (%) | |
| CAH | 1 (2.6) |
| IA | 26 (68.4) |
| IB | 6 (15.8) |
| IIIC | 3 (7.9) |
| IVB | 2 (5.3) |
| FIGO Grade, n (%) | |
| CAH | 1 (2.6) |
| 1 or 2 | 29 (76.3) |
| 3 | 2 (5.2) |
| Histology, n (%) | |
| CAH | 1 (2.6) |
| Endometrioid | 31 (81.6) |
| Serous | 3 (7.9) |
| Clear cell | 3 (7.9) |
| MTD (cm), Median (IQR) | 3.4 (2.8, 4.8) |
| MI (%), Median (IQR) | 20.0 (5.0, 41.0) |
| LVSI, n (%) | 6 (15.8) |
Abbreviations: CAH, complex atypical hyperplasia; EC, endometrial cancer; IQR, interquartile range; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; MI, myometrial invasion; MTD, maximum tumor diameter.
Tampon biospecimen baseline data
Median intravaginal tampon dwell times were 111.5 (interquartile range (IQR) 91.25, 166.25) minutes and 98 (IQR 90.25, 115) minutes for EC and benign groups, respectively. HOXA9 methylation was performed on 37 samples from patients with EC and 25 BE, RASSF1 on 35 samples and 22 samples, respectively, HTR1B on 36 samples and 20 samples, respectively. Copy number analysis was performed on 14 samples with EC and 9 with BE. Mutation was performed on 20 EC samples and 18 BE samples (Table 3).
Table 3.
Sensitivity and specificity of each individual molecular test and combinations of tests. Number of evaluable cases and controls varies secondary to variable total DNA quantity and individual test quality control. Only specimens passing quality control measures included.
| Molecular test | Endometrial cancer cases (N=38) | Benign endometrium controls (N=27) | ||||
|---|---|---|---|---|---|---|
| No. evaluated |
Sensitivity | No. evaluated |
False Positive | |||
| No. (%) positive or present |
Exact 95% CI for sensitivity, % |
No. (%) positive or present |
Exact 95% CI for false positive rate, % |
|||
| HOXA9 methylation, positive vs. negative | 37 | 14 (37.8%) | (22.5, 55.2) | 25 | 0 (0.0%) | (0.0, 13.7) |
| RASSF1 methylation, positive vs. negative | 35 | 14 (40.0%) | (23.9, 57.9) | 22 | 0 (0.0%) | (0.0, 15.4) |
| HTR1B methylation, positive vs. negative | 36 | 14 (38.9%) | (23.1, 56.5) | 20 | 0 (0%) | (0.0, 16.8) |
| Copy number, present vs. absent | 14 | 5 (35.7%) | (12.8, 64.9) | 9 | 1 (11.1%) | (0.3, 48.3) |
| Mutation, present vs. absent | 20 | 10 (50.0%) | (27.2, 72.8) | 18 | 3 (16.7%) | (3.6, 41.4) |
| Methylation (positive for any gene) vs. all 3 tests negative | 35 | 21 (60.0%) | (42.1, 76.1) | 18 | 0 (0%) | (0, 18.5) |
| Methylation (positive for any gene) OR copy number change present vs. all 4 tests negative or absent | 26 | 23 (88.5%) | (69.9, 97.6) | 8 | 1 (12.5%) | (0.3, 52.7) |
| Methylation (positive for any gene) OR presence of any gene mutation vs. all 4 tests negative or absent | 30 | 25 (83.3%) | (65.3, 94.4) | 17 | 3 (17.7%) | (3.8, 43.4) |
| Copy number change present OR presence of any gene mutation vs. both tests negative or absent | 18 | 13 (72.2%) | (46.5, 90.3) | 10 | 4 (40.0%) | (12.2, 73.8) |
| Methylation (positive for any gene) OR copy number change present OR presence of any gene mutation vs. all 5 tests negative or absent | 28 | 27 (96.4%) | (81.7, 99.9) | 10 | 4 (40.0%) | (12.2, 73.8) |
| Any positive/present vs. all negative/absent, based on the patients tested for all 5 tests | 12 | 11 (91.7%) | (61.5, 99.8) | 7 | 1 (14.3%) | (0.4, 57.9) |
Sensitivity and specificity of individual and combined molecular platforms
The sensitivity and false positive rates of each individual molecular test and the combination of different tests to discriminate between EC and BE are summarized in Table 3. Copy number testing alone yielded 36% sensitivity and 89% specificity. Mutation testing alone yielded a 50% sensitivity and 83% specificity. Sensitivity and specificity of methylation analysis, respectively, was as follows: 1) RASSF1: 40%, 100%; 2) HTR1B: 39%, 100%; and 3) HOXA9: 38%, 100%. With all three tests combined, methylation sensitivity was 60% and specificity was 100%. The combination of methylation and copy number analysis demonstrated a sensitivity of 89% and specificity of 88% to identify the presence of EC. The combined sensitivity for all 3 molecular testing platforms (copy number, methylation, and mutation) was 92% with a combined specificity of 86%. Mutation testing was positive in single patients with leiomyoma (1), adenomyosis (1) and a mucinous cystadenoma (1). The one patient with complex atypical hyperplasia (CAH) was positive for RASSF1 and HTR1B methylation, but negative for HOXA9 methylation, copy number testing, and mutations.
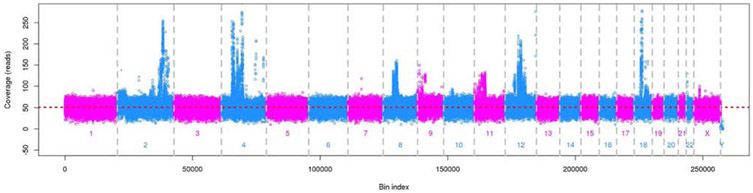
Copy number changes detected via tampon in a patient with unanticipated uterine leiomyosarcoma
One patient referred for hysterectomy for a leiomyoma tested positive for copy number changes (Figure 1). Final uterine pathology revealed a leiomyosarcoma and benign endometrium. This patient’s tampon sample was negative for the presence of methylation and mutations in the genes included in this study.
Figure 1.
Copy number changes detected on intravaginal tampon biospecimen from the BE control patient with uterine leiomyosarcoma identified on final hysterectomy pathology. Her endometrium was benign.
Discussion
The shedding of EC cells and/or cell free EC DNA into the lower genital tract has been recognized as an opportunity to leverage less invasive biospecimen sampling methods and ever more sensitive molecular testing for novel less invasive detection approaches [11, 12, 20, 21]. Optimizing sample collection methods and identifying the molecular markers with greatest sensitivity to detect the presence of EC and its precursor lesions are all critical in the development of both early detection and screening test development. In this pilot study testing multiple DNA-based molecular platforms in tampon-collected vaginal fluid, the combination of copy number, methylation, and mutation assays was complementary and yielded the highest sensitivity and specificity for the presence of EC at 92% and 86%, respectively. However, simplifying to a combination of only copy number and methylation achieved comparable high sensitivity and specificity at 89% and 87%, respectively. This suggests that a molecular test for EC using tampons to collect EC DNA may be achievable with improved DNA methylation alone or with the addition of a test for copy number.
While this study was a pilot to test various DNA-based platforms, methylation and mutation markers were selected based on prior published findings [21-24]. The TCGA project provided a thorough description of EC-associated mutations [18]; however, methylation assays in TCGA were limited in that microarray-based methylation assays are not as comprehensive as sequencing based assays and the comprehensive comparison to control benign endometrium was not part of the TCGA project. As such, sequencing-based approaches to identify the optimal methylation markers, as well as markers for each unique EC histology, are needed. The recent advances in technology that have led to the clinical application of non-invasive prenatal testing (NIPT) for fetal chromosome abnormalities have also suggested a role for circulating DNA analysis as a cancer detection method [25, 26]. While these findings have prompted further investigation into circulating cell-free copy number analysis as a marker of cancer presence[27], this current study suggests that the application of NIPT technology is promising for cancer detection using self-collected, less invasive, liquid biopsy biospecimens, such as vaginal fluid. In fact, the presence of CNV may be more indicative of an underlying carcinogenic event as not all mutations lead to gene dysfunction and cancer events. As such, mutation-based assays may yield a higher false-positive rate. For example, in this present study 3 patients with BE had single gene mutations detected despite histologically normal endometrium and benign gynecologic pathology. Plus, the presence of mutations did not appear to add to the sensitivity of CNV plus methylation in identifying an underlying EC. As such, molecular platforms that test for events more likely to represent underlying carcinogenesis have greater promise as diagnostic and screening tools.
The tampon represents a simple, cost effective and widely available tool for self-collection of vaginal fluid. Home self-collection of vaginal fluid for high-risk human papilloma virus (HR-HPV) testing has been shown to dramatically increase participation in cervical cancer and dysplasia screening [28] . In addition, self-collected vaginal fluid has been shown to be equivalent to provider-collected vaginal fluid in HR-HPV testing performance and women find self-sampling to be less invasive, faster, and preferable [15, 29, 30]. As such, a focus on patient-centered biospecimen collection has the potential to lead to the development of both diagnostic and screening tests for EC that can be initiated by women in the comfort of their own home. At present, assays using Pap test fluid for EC mutation detection thus far show promise with high sensitivity and specificity [20]; however, Pap sampling still requires a provider for collection and ease of access is an important factor in cancer screening. As such, a focus on patient-centered biospecimen collection has the potential to lead to the development of both diagnostic and screening tests for EC that can be initiated by women in the comfort of their own home[20].
Strengths of this study include the multidisciplinary expertise, independent performance of each molecular platform tested, prospective enrollment and controlled conditions in which the tampon and was collected, and inclusion of a variety of cancer stages and histologies. As this study includes consecutively consented women, over 80% of ECs were low grade and early stage endometrioid ECs. Given that time between diagnostic endometrial biopsy and tampon collection was unrelated to biomarker assay results (data not shown), we suspect that prior biopsy is unrelated to EC DNA detection. We have obtained similar DNA yields in tampons collected prior to biopsy. In future studies, enrichment of participants referred with a biopsy diagnosis of less common aggressive EC subtypes should be considered to achieve increased power to assess detection of these ECs separately, although theoretically patients with lower grade cancers may derive the most benefit from early detection. Additionally, methylation markers were identified through previously published literature in which endometrioid histologies also predominate. Our gene selections for this pilot study included RASSF1, a well-studied endometrial cancer tumor suppressor gene that is involved early in carcinogenesis [31]. Greater reach discovery and validation of novel methylated DNA markers unique to EC appear warranted and ongoing efforts include sequencing the common and rare EC histology methylomes to identify histology-specific methylation markers.
This study was designed as a pilot study and each test required different quantities of DNA. As such, with the small overall sample size, we were limited in the number of subjects that had all three test platforms completed. Additionally, given the different testing platforms, methylation, mutation, and copy number variant testing were not run concurrently and sample DNA quality over time may have impacted failure rates among the platforms. However, given the heterogeneity of drivers of carcinogenesis in EC [18, 32], complementary molecular markers of early cancer development that reflect the heterogeneity are important to consider in the early assessment of test feasibility. Additionally, in this pilot study, those with BE were enrolled given the clinical indication for their hysterectomy to be that of benign indications based on preoperative workup. Nevertheless, they all had symptoms or clinical indication which warranted a hysterectomy and this may have impacted the overall sensitivity. In addition, the impact of vaginal dwell time and impact of blood on the tampon was not assessed.
In future studies, we propose to include collection of multiple tampons to assess effects on sensitivity and specificity. The unexpected finding of spontaneously shed aneuploid DNA in the vaginal fluid of a patient ultimately diagnosed with leiomyosarcoma raises the possibility that our approach may occasionally detect occult cancers, and as such, pre-operative testing of hysterectomy patients may facilitate improved surgical planning, even when the clinical indications for surgery are benign.
In conclusion, testing even a relatively limited panel of markers in DNA from intravaginal tampons yielded sensitivity and specificity for EC detection exceeding 90%. Self-collected tampon specimens provide screening opportunities for EC using simple, less invasive methods, potentially improving access to healthcare among populations who might otherwise go unscreened. Leveraging refined molecular panels, complementary platforms, and self-collected vaginal fluid has the potential to revolutionize EC detection and outcomes.
Table 1.
Patient characteristics
| Characteristic | Endometrial cancer cases (N=38) |
Benign endometrium controls (N=27) |
|---|---|---|
| Age (years), Mean (SD) | 62.1(8.2) | 58.1 (9.9) |
| Body mass index (kg/m2), Mean (SD) | 33.8 (8.5) | 28.4 (7.6) |
| Use of hormone replacement therapy, n (%) | 11/35 (31) | 5/26 (19) |
Highlights:
A panel evaluating copy number, methylation, and point mutations is sensitive and specific in detecting endometrial cancer noninvasively.
This sensitivity and specificity is preserved with the combination of copy number and methylation alone.
One patient with a clinically unsuspected leiomyosarcoma diagnosed at hysterectomy yielded the only “false positive” test.
Footnotes
Conflicts of interest:
Dr. Kisiel and Dr. Bakkum have a pending patent filed on detection of endometrial cancer with Exact Sciences (Madison, WI). In addition, Dr. Kisiel receives royalties on an existing patent with Exact Sciences (Madison, WI).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in abstract form at the Western Association of Gynecologic Oncology (WAGO) Annual Meeting in Park City, Utah, on June 16, 2018.
References
- 1.Rahib L, et al. , Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Research, 2014. 74(11): p. 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 2018. 68(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Moore K and Brewer MA, Endometrial cancer: is this a new disease? American Society of Clinical Oncology Educational Book, 2017. 37: p. 435–442. [DOI] [PubMed] [Google Scholar]
- 4.Atlanta G, American Cancer Society: Cancer Facts and Figures. 2011.
- 5.Long B, Liu F, and Bristow R, Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecologic oncology, 2013. 130(3): p. 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson CC, et al. , Population screening for uterine cancer by vaginal cytology: Preliminary summary of results of first examination of 108,000 women and second testing of 33,000 women. Journal of the American Medical Association, 1956. 162(3): p. 167–173. [PubMed] [Google Scholar]
- 7.Serdy K, et al. , The value of Papanicolaou tests in the diagnosis of endometrial carcinoma: a large study cohort from an academic medical center. American journal of clinical pathology, 2016. 145(3): p. 350–354. [DOI] [PubMed] [Google Scholar]
- 8.Clarke MA, et al. , Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA internal medicine, 2018. 178(9): p. 1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu M, et al. , Pap smears in women with endometrial carcinoma. Acta cytologica, 2001. 45(4): p. 555–560. [DOI] [PubMed] [Google Scholar]
- 10.Moodley M and Roberts C, Clinical pathway for the evaluation of postmenopausal bleeding with an emphasis on endometrial cancer detection. Journal of Obstetrics and Gynaecology, 2004. 24(7): p. 736–741. [DOI] [PubMed] [Google Scholar]
- 11.Bakkum-Gamez JN, et al. , Detection of endometrial cancer via molecular analysis of DNA collected with vaginal tampons. Gynecologic oncology, 2015. 137(1): p. 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, et al. , Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Science translational medicine, 2018. 10(433): p. eaap8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woeller KE, et al. , Impact of Advertising on Tampon Wear-time Practices. Clinical Medicine Insights: Women’s Health, 2015. 8: p. CMWH. S25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imperiale TF, et al. , Multitarget stool DNA testing for colorectal-cancer screening. New England Journal of Medicine, 2014. 370(14): p. 1287–1297. [DOI] [PubMed] [Google Scholar]
- 15.Du H, et al. , A new PCR-based mass spectrometry system for high-risk HPV, part II: clinical trial. American journal of clinical pathology, 2011. 136(6): p. 920–923. [DOI] [PubMed] [Google Scholar]
- 16.Lin S, et al. , A data science approach for the classification of low-grade and high-grade ovarian serous carcinomas. BMC genomics, 2018. 19(1): p. 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiegl H, et al. , Methylated DNA Collected by Tampons—A New Tool to Detect Endometrial Cancer. Cancer Epidemiology Biomarkers & Prevention, 2004. 13(5): p. 882–888. [PubMed] [Google Scholar]
- 18.Levine DA and Network CGAR, Integrated genomic characterization of endometrial carcinoma. Nature, 2013. 497(7447): p. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abyzov A and Gerstein M, AGE: defining breakpoints of genomic structural variants at singlenucleotide resolution, through optimal alignments with gap excision. Bioinformatics, 2011. 275: p. 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinde I, et al. , Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Science translational medicine, 2013. 5(167): p. 167ra4–167ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiegl H, et al. , Methylated DNA collected by tampons—a new tool to detect endometrial cancer. Cancer Epidemiology and Prevention Biomarkers, 2004. 13(5): p. 882–888. [PubMed] [Google Scholar]
- 22.Pijnenborg J, et al. , RASSF1A methylation and K-ras and B-raf mutations and recurrent endometrial cancer. Annals of oncology, 2006. 18(3): p. 491–497. [DOI] [PubMed] [Google Scholar]
- 23.Suehiro Y, et al. , Aneuploidy predicts outcome in patients with endometrial carcinoma and is related to lack of CDH13 hypermethylation. Clinical Cancer Research, 2008. 14(11): p. 3354–3361. [DOI] [PubMed] [Google Scholar]
- 24.Arafa M, et al. , High frequency of RASSF1A and RARb2 gene promoter methylation In morphologically normal endometrium adjacent to endometrioid adenocarcinoma. Histopathology, 2008. 53(5): p. 525–532. [DOI] [PubMed] [Google Scholar]
- 25.Suzumori N, et al. , Classification of factors involved in nonreportable results of noninvasive prenatal testing (NIPT) and prediction of success rate of second NIPT. Prenatal diagnosis, 2019. 39(2): p. 100–106. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi DW, et al. , Noninvasive prenatal testing and Incidental detection of occult maternal malignancies. Jama, 2015. 314(2): p. 162–169. [DOI] [PubMed] [Google Scholar]
- 27.Arko-Boham B, et al. , Circulating cell-free DNA Integrity as a diagnostic and prognostic marker for breast and prostate cancers. Cancer Genetics, 2019. [DOI] [PubMed] [Google Scholar]
- 28.Sanner K, et al. , Self-sampling of the vaginal fluid at home combined with high-risk HPV testing. British journal of cancer, 2009. 101(5): p. 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilangovan K, et al. , Acceptability and feasibility of human papilloma virus self-SamplIng for cervical cancer screening. Journal of Women’s Health, 2016. 25(9): p. 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petignat P, et al. , Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecologic oncology, 2007. 105(2): p. 530–535. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y, et al. , Histone deacetylase Inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity In human endometrial cells. Cancer research, 2005. 65(7): p. 2684–2689. [DOI] [PubMed] [Google Scholar]
- 32.Fadare O and Zheng W, Insights Into endometrial serous carcinogenesis and progression. International journal of clinical and experimental pathology, 2009. 2(5): p. 411. [PMC free article] [PubMed] [Google Scholar]