Abstract
BACKGROUND
Degenerative cervical myelopathy (DCM) involves spinal cord compression, which causes neurological decline. Neurological impairment in DCM is variable and can involve complex upper limb dysfunction including loss of manual dexterity, hyperreflexia, focal weakness, and sensory impairment. The modified Japanese Orthopaedic Association (mJOA) score relies on the patients’ subjective perceptions, whereas existing objective measures such as strength and sensory testing do not capture subtle changes in dexterity and function.
OBJECTIVE
1) To characterize arm and hand function in DCM; and 2) To develop and validate Graded Redefined Assessment of Strength, Sensibility, and Prehension Version-Myelopathy (GRASSP-M), a clinical assessment that quantifies upper limb impairment.
METHODS
A total of 148 DCM patients (categorized into mild, moderate, and severe based on mJOA grade) and 21 healthy subjects were enrolled. A complete neurological exam, the mJOA, the QuickDASH, grip dynamometry, and the GRASSP-M were administered.
RESULTS
Strength, sensation, and manual dexterity significantly declined with increasing DCM severity (P ≤ .05). Impairment in hand dexterity showed better discrimination between mild, moderate, and severe DCM categories than strength or sensation. The GRASSP-M was found to be both a reliable (intraclass correlation coefficient >0.75 for intra- and inter-rater reliability) and valid (with both concurrent and construct validity) tool.
CONCLUSION
These results demonstrate that patients’ subjective reporting of functional status, especially in the mild DCM category, may underrepresent the extent of functional impairment. The GRASSP-M is an objective tool designed to characterize patients’ functional impairment related to the upper limb, which proves useful to diagnose and quantify mild dysfunction, monitor patients for deterioration, and help determine when patients should be treated surgically.
Keywords: Degenerative cervical myelopathy, Upper limb, Impairment, Function, Disability, Hand function, Outcome measure
ABBREVIATIONS
- CI
confidence interval
- DCM
degenerative cervical myelopathy
- GRASSP
Graded Redefined Assessment of Strength, Sensibility, and Prehension
- ICC
intraclass correlation coefficient
- MRI
magnetic resonance imaging
- mJOA
modified Japanese Orthopaedic Association
Degenerative cervical myelopathy (DCM) is an overarching term used to describe an array of degenerative changes that narrow the spinal canal and compress the spinal cord.1-3 Patients with DCM usually present with at least one of the following symptoms: weakness and/or numbness of the upper limbs, reduced manual dexterity, unstable gait, neuropathic pain, and bladder dysfunction. In addition, they can present with at least one of the following neurological signs: hyperreflexia, positive Hoffman's sign, positive plantar response, long tract signs, intrinsic hand muscle weakness, and lower limb spasticity. Functional impairment in these patients can significantly reduce independence and quality of life.3-6 Recent guidelines have recommended surgical decompression as the preferred treatment strategy for patients with moderate to severe DCM.7-13 Patients with mild myelopathy, however, may benefit from a trial of non-operative intervention provided their disease is stable.10
Cardinal symptoms of DCM include impaired manual dexterity and weakness of the intrinsic hand muscles.4,8 As such, characterizing hand function is essential for understanding the natural history of the disease and defining optimal management strategies, especially in patients with mild DCM. Several studies have indicated that the refined components of hand function are not always responsive to decompressive surgery and that manual dexterity may not completely recover to baseline following treatment.14-16 Dexterity is a complex function that is difficult to objectively quantify. Although the modified Japanese Orthopaedic Association (mJOA) score includes an upper limb motor and sensory subscore, it is a coarse self-reported measure that does not fully define a patient's functional impairment or hand deficits.17
A barrier in defining DCM management is the lack of an assessment tool that can characterize the motor, sensory, and dexterity changes that occur in the hand following cord compression. To fill this critical knowledge gap, we conducted an observational cross-sectional study to characterize upper limb function and hand impairment in patients with mild, moderate, and severe DCM, and develop and validate a sensitive and specific assessment tool to better quantify upper limb impairment in these patients. The sensitivity and psychometric properties of the Graded Redefined Assessment of Strength, Sensibility, and Prehension – Version Myelopathy (GRASSP-M) were assessed.
METHODS
Study Design
A single-center, observational, cross-sectional study was conducted at Toronto Western Hospital between May 2013 and December 2016. Research ethics board approval was obtained and informed consent was provided by participating patients.
Patients were enrolled in this study if they satisfied the following criteria: 1 or more signs of myelopathy (corticospinal motor deficits, hand atrophy, hyperreflexia, a positive Hoffman sign, upgoing plantar reflexes, lower limb spasticity, and/or gait ataxia); 1 or more symptoms of myelopathy (numb hands, clumsy hands, gait impairment, bilateral hand paresthesia, L’Hermitte's phenomena, and/or weakness); and evidence of compression of the spinal cord on magnetic resonance imaging (MRI). Patients were excluded if their upper limb deficit could not be entirely explained by cervical cord compression (focal neurological comorbidities such as stroke, multiple sclerosis, rheumatoid arthritis, brachial plexus injury, and peripheral neuropathies); they were unable to speak English; or they were medically unstable. Furthermore, individuals with a diagnosis of diabetes and no clinical evidence of neuropathy were included as well as individuals with hypertension. Healthy controls were recruited through the clinical research team's networks, patients who presented to the spine program with no cervical or upper limb deficits, and clinicians at the hospital that were above the age of 45. There was an emphasis on recruiting control subjects that would serve as an age-matched control group.
Severity of functional impairment was classified using the mJOA scale. Patients were stratified into mild (mJOA = 15-17), moderate (mJOA = 12-14), and severe (mJOA < 12) groups.10,11 Data were collected from each patient, including demographic information, findings from a neurological exam, mJOA, QuickDASH, grip dynamometry, and GRASSP-M scores. Grip strength was measured 3 times and results were averaged across trials. Videotaped GRASSP-M prehension/dexterity tasks were reviewed and independently scored by 2 assessors (blinded to DCM severity), and inter-rater reliability was computed (n = 44). A subset of patients were asked to perform the prehension/dexterity task 2 times in 1 day; a single assessor scored both performances, and intra-rater reliability data were evaluated (n = 31). Reliability of the strength and sensation subtests was previously assessed.18
Scales/Outcomes
The mJOA scale consists of 4 categories: motor dysfunction of the upper limbs (5 points) and lower limbs (7 points), sensory impairment of the upper limbs (3 points), and bladder dysfunction (3 points). A score of 18 represents no functional deficit. The QuickDASH is a measure of self-perceived disability (patient reported outcome), which evaluates symptoms associated with upper limb, neck, and musculoskeletal disorders; the higher the score, the greater the disability.19 Grip dynamometry measures handgrip force; greater force equates to greater strength.20,21 GRASSP-M evaluates hand impairment through strength, sensation, and prehension/dexterity subtests; scores closer to zero demonstrate greater deficit.
Modifications to the GRASSP Version 1.0
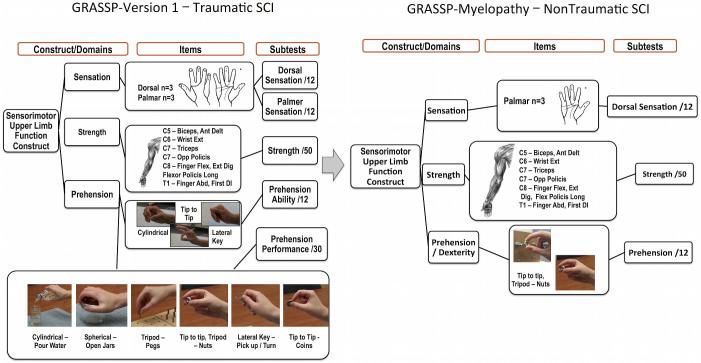
The GRASSP Version 1.0 (GRASSP-V1; Neural Outcomes, Toronto, Canada) is a reliable and valid outcome measure originally developed to measure upper limb impairment in patients with traumatic tetraplegia. This tool is sensitive in detecting the sensory, motor, and functional changes that occur within 1 yr following injury18,22; however, it is not sensitive enough to capture the upper limb deficits in DCM patients. As a result, the following modifications were made to the GRASSP-V1 to increase its applicability in patients with DCM (Figure 1):
Sensation: Measurement of upper limb sensation was reduced from palmar and dorsal sensation to palmar sensation only.
Prehension: The GRASSP-M was developed to specifically analyze manual dexterity. The prehension portion of GRASSP-V1 consists of 6 prehension tasks scored on a 5-point scale; this section was reduced to a single task and the scoring was modified to objectively define dexterous function. These changes give the scale greater sensitivity to better discriminate dexterity differences among patients with mild, moderate, and severe DCM.
Strength testing was not modified.
FIGURE 1.
Development of the GRASSP-M. This figure defines the domains in the original GRASSP and the reduced domains in the GRASSP-myelopathy.
Analysis
Statistical analysis was performed using SPSS Version 21.0 (IBM, Armonk, New York). Continuous variables were described using means and standard deviations, and categorical variables were summarized using frequencies. The significance level was set at P ≤ .05. Differences in manual dexterity scores between the dominant and non-dominant hand were evaluated using paired t-tests. Differences in age and gender distribution between healthy controls and DCM patients were assessed using an unpaired 2-way t-test (unequal variance) and chi square test, respectively. Analysis of variance and/or unpaired 2-way t-tests (unequal variance) were used to evaluate differences in strength, sensation, and dexterity between controls and DCM patients, as well as among patients with mild, moderate, and severe myelopathy.
Inter- and intra-rater reliabilities were calculated using intraclass correlation coefficient (ICC) under the 2-way random effects model; an ICC of 0.75 was considered acceptable.23
To assess concurrent validity, the following hypothesis was first generated: the GRASSP-M can discriminate among patients with mild, moderate, and severe myelopathy but does not perfectly correlate with the mJOA. Pearson correlation coefficients were computed to evaluate the association between the GRASSP-M strength, sensation, and prehension/dexterity subtests and the subscores of the mJOA that measure upper limb function. A moderate, positive correlation would demonstrate concurrent validity. Known-groups validity was used to establish construct validity. Unpaired 2-way t-tests (unequal variance) with a Bonferroni correction (P ≤ .016) were used to examine whether patients with mild, moderate, and severe DCM (based on the mJOA) could be differentiated by their GRASSP-M strength, sensation, and prehension/dexterity subtest scores.
RESULTS
Sample
A total of 148 study participants and 21 control subjects were enrolled. The sample consisted of 84 males and 64 females, with a mean age of 56.89 ± 10.67 yr. Mean duration of symptoms was 45.5 ± 60.4 mo. The control sample consisted of 11 males and 10 females, with a mean age of 53.67 ± 16.81 yr. No significant differences in the age (P = .403) and gender (P = .705) distribution between healthy controls and DCM patients were present. The sample consisted of 75 mild (mJOA 15.85 ± 0.748), 41 moderate (mJOA 13.20 ± 0.782), and 32 severe (mJOA 9.94 ± 1.110) patients.
Scores
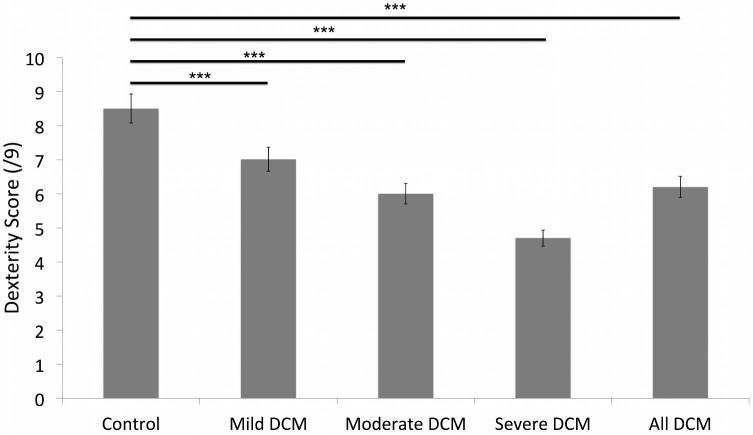
Table 1 reports baseline mJOA, QuickDASH, grip strength, and GRASSP-M subtest (strength, sensation, and prehension/dexterity) scores for DCM patients and controls. Figure 2 illustrates the dexterity scores of patients with DCM and controls. GRASSP-M strength scores in the dominant hand were weaker in patients with moderate (vs mild) and severe (vs mild and moderate) myelopathy. GRASSP-M sensation scores were also reduced in patients with severe myelopathy compared to those with mild and moderate myelopathy in both the dominant and non-dominant hand. GRASSP-M sensation scores, however, were not significantly different between patients with mild and moderate DCM. Finally, GRASSP-M prehension/dexterity scores were different between controls and patients with DCM, as well as among patients with mild, moderate, and severe myelopathy in both the dominant and non-dominant hands. The mean values in Table 1 were used to set GRASSP-M severity thresholds.
TABLE 1.
Sample Data Stratified by mJOA
| Variable | Control (n = 21) mean (SD) | Mild (n = 75) mean (SD) | Moderate (n = 41) mean (SD) | Severe (n = 32) mean (SD) | |
|---|---|---|---|---|---|
| Duration of symptoms (months) | 0 | 40.16 (±58.25) | 57.62 (±63.36) | 41.46 (±61.01) | |
| mJOA (/18) | 18 | 15.85 (±0.748) | 13.20 (±0.782) | 9.94 (±1.11) | |
| UL mJOA (/8) | 8 | 6.43 (±0.701) | 5.29 (±0.901) | 4.19 (±0.780) | |
| UL motor mJOA (/5) | 5 | 4.47 (±0.502) | 3.59 (±0.631) | 2.81 (±0.738) | |
| Sensation mJOA (/3) | 3 | 1.96 (±0.478) | 1.71 (±0.512) | 1.38 (±0.554) | |
| QuickDASH (%) | 0 | 27.59 (±17.26) | 45.07 (±19.83) | 62.12 (±22.41) | |
| Grip strength | Dominant (Kg) | 35.92† | 31.00 (±13.34) | 27.05 (±19.83) | 24.92 (±9.71) |
| Non-dominant (Kg) | 29.60† | 30.25 (±12.60) | 26.13 (±13.24) | 24.38 (±9.05) | |
| GRASSP-M strength | Dominant (/50) | 50‡ | 47.95a,c (±3.31) | 46.34a,b (±3.53) | 43.52b,c (±6.33) |
| Non-dominant (/50) | 50‡ | 48.17a,c (±2.586) | 46.27a (±5.05) | 43.77c (±6.55) | |
| GRASSP-M sensation | Dominant (/12) | 12‡ | 10.73c (±1.87) | 10.69b (±1.47) | 8.56b,c (±3.26) |
| Non-dominant (/12) | 12‡ | 11.03c (±1.65) | 10.85b (±1.49) | 8.34b,c (±3.28) | |
| GRASSP-M prehension/dexterity | Dominant (/9) | 8.57d,e,f (±0.676) | 7.05a,c,d (±1.22) | 5.95a,b,e (±1.55) | 4.66b,c,f (±2.03) |
| Dominant time (sec) | 47.05 (±22.41) | 52.71a,c (±18.17) | 66.93a (±25.39) | 84.06c (±45.53) | |
| Non-dominant (/9) | 8.62d,e,f (±0.669) | 6.58a,c,d (±1.31) | 5.54a,b,e (±1.55) | 4.23b,c,f (±1.82) | |
| Non-dominant time (sec) | 50.52d,e,f (±14.97) | 60.21a,c,d (±18.91) | 72.61a,b,e (±23.18) | 94.13b,c,f (±44.73) | |
aIndicates statistical significance between mild and moderate groups (P ≤ .05).
bIndicates statistical significance between moderate and severe groups (P ≤ .05).
cIndicates statistical significance between mild and severe groups (P ≤ .05).
dIndicates statistical significance between control and mild groups (P ≤ .05).
eIndicates statistical significance between control and moderate groups (P ≤ .05).
fIndicates statistical significance between control and severe groups (P ≤ .05).
†Indicates normative value taken from the literature21.
‡Indicates values expected of healthy controls.
FIGURE 2.
Dexterity scores for healthy controls and patients. This figure demonstrates the discrimination of severity of DCM compared with healthy controls. The dexterity score defines even the subtle deficit in the mild DCM patient.
In a sub-analysis of mild DCM patients, the opponens pollicis, abductor digiti minimi, first dorsal interossi, and anterior deltoid muscles consistently demonstrated mild weakness (grade 3 and 4) (P = 1.69 E-07).
Imaging
All study participants did have a conventional MRI, and we used signal change and number of levels to describe the sample. In all, 41% to 48% of each severity group presented with T2 weighted signal change, (48%-mild, 41%-moderate, and 46%-severe). In all, 3% to 8% of each severity group presented with T1 weighted signal change, (5%-mild, 3%-moderate, and 8%-severe). In all, 5% to 17% of each severity group presented with both T1 and T2 weighted signal change, (5%-mild, 10%-moderate, and 17%-severe). Within each severity group, 29% to 42% of the sample had no signal change (42%-mild, 46%-moderate, and 29%-severe). There is not a large difference between the mild and moderate groups, whereas severe patients clearly have greater prevalence of signal change. There was an increase in the number of levels compressed as patients became more severe. The prevalence of 1 to 2 level compression for each severity group ranged from 62% to 30% (62%-mild, 54%-moderate, and 30%-severe), 3 levels of compression for each severity group ranged between 11% and 26% (18%-mild, 26%-moderate, and 11%-severe), and 4 to 5 levels of compression ranged from 19% to 37% (19%-mild, 20%-moderate, and 37%-severe). Again, the number of levels compressed was found to have some relationship to severity and some discriminative qualities. In this report of imaging, 22% to 29% of the imaging was not available for the signal change summary; however, it was complete for the number of levels compressed summary.
Reliability
Table 2 reports the ICC and CI for inter-rater (n = 31) and intra-rater reliabilities (n = 44). The ICC for inter-rater reliability was 0.869 (95% CI: 0.759-0.928) in the dominant hand and 0.862 (95% CI: 0.748-0.925) in the non-dominant hand. The ICC for intra-rater reliability was 0.868 (95% CI: 0.727-0.936) in the dominant hand and 0.790 (95% CI: 0.565-0.899) in the non-dominant hand.
TABLE 2.
Inter- and Intra-rater Reliability for GRASSP-M Prehension/Dexterity Subtest
| Inter-rater reliability | Intra-rater reliability | |||
|---|---|---|---|---|
| Variable | ICC – Cronbach's alpha | CI | ICC – Cronbach's alpha | CI |
| Dominant hand | 0.869* | 0.759-0.928 | 0.868* | 0.727-0.936 |
| Non-dominant hand | 0.862* | 0.748-0.925 | 0.790* | 0.565-0.899 |
*Indicates statistical significance (P ≤ .05).
Validity
Table 3 shows the Pearson correlation coefficients between baseline GRASSP-M and mJOA. All GRASSP-M subtests showed a positive, moderate correlation with the upper limb motor, upper limb sensory, and total upper limb mJOA scores. Dexterity time showed a negative, moderate correlation with these scores. All correlations were significant except for the correlation between dexterity time in the dominant hand and the mJOA upper limb sensation score. Known groups’ validity is illustrated in Table 4. The prehension/dexterity score was the most discriminative subtest of the GRASSP-M, followed by the strength subscore. Finally, Table 5 defines the thresholds set for each severity specific to the hand with the GRASSP-M.
TABLE 3.
Concurrent Validity
| Subtest | UL mJOA | UL motor mJOA | Sensation mJOA | |
|---|---|---|---|---|
| GRASSP-M strength | Dominant | 0.431* | 0.373* | 0.323* |
| Non-dominant | 0.406* | 0.384* | 0.251* | |
| GRASSP-M sensation | Dominant | 0.412* | 0.374* | 0.280* |
| Non-dominant | 0.488* | 0.448* | 0.323* | |
| GRASSP-M prehension/dexterity | Dominant | 0.502* | 0.511* | 0.250* |
| Non-dominant | 0.533* | 0.524* | 0.301* | |
| GRASSP-M prehension/dexterity time | Dominant | −0.407* | −0.455* | −0.138 |
| Non-dominant | −0.439* | −0.469* | −0.186* | |
*Indicates statistical significance (P ≤ .05).
TABLE 4.
Discriminative Qualities of GRASSP-M Domains
| P-value | ||||
|---|---|---|---|---|
| Subtest | Mild vs moderate | Moderate vs severe | Mild vs severe | |
| GRASSP-M strength | Dominant | 0.019 | 0.028 | 0.001 |
| Non-dominant | 0.028 | 0.079 | 0.001 | |
| GRASSP-M sensation | Dominant | 0.883 | 0.001 | 0.001 |
| Non-dominant | 0.567 | 0.000 | 0.000 | |
| GRASSP-M prehension/dexterity | Dominant | 0.000 | 0.004 | 0.000 |
| Non-dominant | 0.001 | 0.002 | 0.000 | |
| GRASSP-M prehension/dexterity time | Dominant | 0.002 | 0.062 | 0.001 |
| Non-dominant | 0.005 | 0.019 | 0.000 | |
(P ≤ .016) refers to a significant difference between groups and the P-value reflects a Bonferroni Correction.
TABLE 5.
GRASSP-M Discriminative Values for Assessment of DCM
| GRASSP-M | Control | Mild | Moderate | Severe |
|---|---|---|---|---|
| GRASSP-Str (0-50) | 50 | 48-46 note which muscles | 45-40 | Less than 40 |
| GRASSP-Sens (0-12) | 11-12 | 10 | 9-8 | Less than 8 |
| GRASSP-PD (0-9) | 8-9 | 8-7 | 6-5 | Less than 5 |
DISCUSSION
Although impairment of manual dexterity and weakness of intrinsic hand muscles are some of the earliest symptoms of DCM,4,8 upper limb dysfunction has not been adequately characterized. This study aims to fill this knowledge gap by developing an assessment tool to quantify impairment in hand function. It is crucial to understand patients’ upper limb deficits at the time of diagnosis to appropriately determine their clinical plan.11 Proper patient management requires using gold standard measures, such as the mJOA, and objective, sensitive clinical tools to define the different DCM severities. We have presented the characteristics of upper limb impairment and introduced a new upper limb tool to the DCM field. We conclude that there are elements of dysfunction that are currently not captured with standard DCM assessments, indicating clinical decisions have not always been based on patients’ true deficits. For example, the traditional myotomal upper limb exam does not assess the intrinsic hand muscles,24 which we have shown are impacted early in the disease process.
The majority of DCM patients in this study reported upper limb dysfunction, including those with mild DCM. This finding is consistent with previous studies, which reveal that upper limb numbness and impaired dexterity are experienced early in the disease process.2,8,25,26 There was a significant difference in the prehension/dexterity subtest scores and time for completion between the patients’ 2 hands; thus, the average grip dynamometry and GRASSP-M subtest scores for dominant and non-dominant hands were reported separately. We noted that strength (GRASSP-M strength), sensation, and dexterity decreased as severity increased, which is consistent with the literature.26-28 However, grip strength did not significantly decrease as severity increased in our sample. We emphasize the importance of an objective clinical tool that can quantify all aspects of upper limb impairment, such as the GRASSP-M, in guiding clinical decision-making.
Reliability and Validity
Inter- and intra-rater reliabilities for all GRASSP-M subtests were high and above our predefined threshold of 0.75. These results indicate that the GRASSP-M is a reliable tool.
Concurrent validity was evaluated by assessing the correlation between the GRASSP-M subsets and the mJOA upper extremity subscores. The GRASSP-M measures subtle changes in hand function that may not be fully captured by the mJOA. As a result, we did not expect a strong correlation between all GRASSP-M subtests and the mJOA subscores. Based on our results, the majority of GRASSP-M subtests were moderately and significantly correlated with the mJOA upper extremity subscores. The exception to this was the correlation between dexterity time and mJOA sensation score, which did not reach statistical significance. These results demonstrate concurrent validity and indicate that the GRASSP-M captures aspects of hand function that the mJOA may not. Furthermore, the GRASSP-M strength and sensation subtests, as well as the time required to complete the prehension/dexterity subtest, were able to discriminate among patients with mild, moderate, and severe DCM. These results support the use of the GRASSP-M as an adjuvant tool for the assessment of dexterity function and disease progression.
GRASSP-M
Through this study we characterized hand impairment in DCM and also set discriminative thresholds related to impairment. The initial step was to define discriminative qualities of the new measures in relation to the mild, moderate, and severe sub-groupings defined by the mJOA. The mean was used as the primary value to define the thresholds for mild, moderate, and severe (Table 1). The thresholds in Table 5 can be used to define severity specific to the hand and upper limb. The dexterity score is the most useful subtest; it can be used to confirm the mJOA severity or determine if there is more loss of function specific to the hand than a mild patient should present with.
Insights Into Mild DCM
The opponens pollicis, abductor digiti minimi, first dorsal interossei, and anterior deltoid muscles demonstrated significant weakness early in the disease process. These findings are not surprising, as weakness of the intrinsic hand muscles is one of the first symptoms of DCM.14,15 Furthermore, the anterior deltoid muscle often activates during movement of intrinsic hand muscles29,30; as a result, concomitant impairment of the anterior deltoid and intrinsic hand muscles was observed. These results suggest that assessing intrinsic hand muscles is as important as evaluating the larger muscle groups, especially in patients with mild DCM. Therefore, in addition to the traditional C5-T1 myotomal testing, these 4 muscles should be tested to elucidate the level of impairment in patients with mild DCM.
This study also demonstrated that a proportion of patients classified as mild on the mJOA in fact have moderate impairment of dexterity. With the GRASSP-M, patients with moderate DCM scored, on average, a 5.95/9 and 5.54/9 on their dominant and non-dominant hands, respectively. As such, a score ≤5 is likely representative of moderate dexterity impairment. In the mild cohort, 28% of patients scored a ≤5/9 on the prehension/dexterity subtest with at least 1 hand; these mild patients actually presented with moderately impaired dexterity. Recent guidelines have recommended surgical treatment for mild DCM patients with progressive myelopathy to prevent further neurologic deterioration and optimize outcomes.10 Given these guidelines, it is important to differentiate between patients who are likely to progress and those who may benefit from a trial of nonoperative therapy. Patients who present with a greater degree of functional impairment are likely at a higher risk of neurological decline and may be candidates for surgery.31 Assessment using the GRASSP-M could help to determine which mild patients have moderate levels of functional hand impairment and should be counseled for surgical intervention.
Limitations
We excluded patients with focal neurological comorbidities such as stroke, multiple sclerosis, rheumatoid arthritis, brachial plexus injury, and peripheral neuropathies, as these comorbidities may cause similar deficits as DCM. However, we did not exclude patients with diabetes, hypertension, and osteoarthritis; these diseases may impact a patient's total mJOA score. However, by excluding individuals with pathologies affecting the upper limbs, we reduced the impact or influence of any other disease on this sample. Another limitation is that although a control sample was recruited and tested, some comparative variables were defined by the existing medical literature. In particular, grip strength for the sample was compared to normative values defined in the medical literature. Published normative values are typically based on a sample specifically collected to reflect normal values, and we felt our control sample was not superior to this study. With respect to GRASSP domains of strength and sensation, we compare the impaired group to expected normal values, which is represented by the highest score. The GRASSP is designed to score with respect to normal, which is the maximum score on each scale.18
It is also important to note that hand dysfunction and upper extremity deficits are only one aspect of the impairments that individuals with DCM present with. Gait deficits also play a significant role in their global disability, and while we do not report on gait in this paper, many of the mild DCM study participants and all of the moderate and severe individuals did present with some gait impairment. The gait impairment findings will be reported in a separate study. Finally, it is important to mention that this study was conducted at a single center, which may introduce selection bias.
CONCLUSION
The GRASSP-M can objectively quantify hand impairment and can assess DCM severity more accurately than the widely used subjective measures. This information allows for earlier diagnosis of DCM, improved patient monitoring, and treatment planning. This study has demonstrated that the GRASSP-M is a valid and reliable tool for quantifying fine motor skill dysfunction in a clinical setting. As a result, we recommend that clinicians use the GRASSP-M to assess and monitor patients with DCM, regardless of whether they are candidates for surgery. The GRASSP-M can be administered by any clinician with good assessment skills, and the test can be performed in 10 to 15 min, providing useful diagnostic information for the clinical team. Unification of outcome assessment will allow the field to accurately quantify the progression of DCM and prognosticate treatment outcomes.
Disclosures
This work was funded by the Cervical Spine Research Society and AOSpine Foundation. Dr Kalsi-Ryan and Dr Fehlings are two of the original inventors of GRASSP and receive a royalty through licensing and distribution of GRASSP products. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors wish to acknowledge the GRASSP Development and Research Group, the Toronto Western Hospital Spine Program, the Cervical Spine Research Society, AO Spine North America, the Craig Neilsen Foundation, and the Gerry and Tootsie Halbert Chair in Neural Repair and Regeneration.
REFERENCES
- 1. Nouri A, Tetreault L, Singh A et al.. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40(12):E675-E693. [DOI] [PubMed] [Google Scholar]
- 2. Tetreault L, Goldstein CL, Arnold P et al.. Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging spine. Neurosurgery. 2015;77(4):S51-S67. [DOI] [PubMed] [Google Scholar]
- 3. Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62(5):1064-1070. [PubMed] [Google Scholar]
- 4. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421. [DOI] [PubMed] [Google Scholar]
- 5. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187. [DOI] [PubMed] [Google Scholar]
- 6. King JTJ, McGinnis KA, Roberts MS. Quality of life assessment with the medical outcomes study short form-36 among patients with cervical spondylotic myelopathy. Neurosurgery. 2003;52(1):113-120. [DOI] [PubMed] [Google Scholar]
- 7. Kinoshita M, Matsui R, Kato S et al.. Genetic dissection of the circuit for hand dexterity in primates. Nature. 2012;487(7406):235-238. [DOI] [PubMed] [Google Scholar]
- 8. Mattei TA, Goulart CR, Milano JB et al.. Cervical spondylotic myelopathy: pathophysiology, diagnosis, and surgical techniques. ISRN Neurology. 2011;2011:463729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ono K, Ebara S, Fuji T et al.. Myelopathy hand. New clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69(2):215-219. [DOI] [PubMed] [Google Scholar]
- 10. Tetreault L, Kopjar B, Nouri A et al.. The modified Japanese Orthopaedic Association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26(1):78-84. [DOI] [PubMed] [Google Scholar]
- 11. Fehlings MG, Wilson JR, Kopjar B et al.. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95(18):1651-1658. [DOI] [PubMed] [Google Scholar]
- 12. Kadanka Z, Mares M, Bednarik J et al.. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine (Phila Pa 1976). 2002;27(20):2205-2210. [DOI] [PubMed] [Google Scholar]
- 13. Fehlings MG, Tetreault L, Riew KD et al.. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3_Suppl):70S-83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mummaneni PV, Kaiser MG, Matz PG et al.. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):130-141. [DOI] [PubMed] [Google Scholar]
- 15. Hirai T, Okawa A, Arai Y et al.. Middle-term results of a prospective comparative study of anterior decompression with fusion and posterior decompression with laminoplasty for the treatment of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2011;36(23):1940-1947. [DOI] [PubMed] [Google Scholar]
- 16. Satomi K, Nishu Y, Kohno T et al.. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994;19(5):507-510. [DOI] [PubMed] [Google Scholar]
- 17. Igarashi K, Shibuya S, Sano H et al.. Functional assessment of proximal arm muscles by target-reaching movements in patients with cervical myelopathy. Spine J. 2011;11(4):270-280. [DOI] [PubMed] [Google Scholar]
- 18. Kalsi-Ryan S, Beaton D, Curt A et al.. The Graded Redefined Assessment of Strength Sensibility and Prehension: reliability and validity. J Neurotrauma. 2012;29(5):905-914. [DOI] [PubMed] [Google Scholar]
- 19. Beaton DE, Wright JG, Katz JN; Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038-1046. [DOI] [PubMed] [Google Scholar]
- 20. Kalsi-Ryan S, Singh A, Massicotte E et al.. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(22):S111-S122. [DOI] [PubMed] [Google Scholar]
- 21. Mathiowetz V, Kashman N, Volland G et al.. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69-74. [PubMed] [Google Scholar]
- 22. Kalsi-Ryan S, Curt A, Verrier M et al.. Development of the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine. 2012;17(Suppl 1):65-76. [DOI] [PubMed] [Google Scholar]
- 23. Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford University Press, Oxford, United Kingdom; 2015. [Google Scholar]
- 24. Cates JR, Soriano MM. Cervical spondylotic myelopathy. J Manipulative Physiol Ther. 1995;18(7):471-475. [PubMed] [Google Scholar]
- 25. Wilson JR, Tetreault L, Kim J et al.. State of the art in degenerative cervical myelopathy: an update on current clinical evidence. Neurosurgery. 2017;80(3S):S33-S45. [DOI] [PubMed] [Google Scholar]
- 26. Clarke E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79(3):483-510. [DOI] [PubMed] [Google Scholar]
- 27. Karadimas SK, Erwin WM, Ely CG et al.. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(22):S21-S36. [DOI] [PubMed] [Google Scholar]
- 28. Lemon RN, Johansson RS, Westling G. Modulation of corticospinal influence over hand muscles during gripping tasks in man and monkey. Can J Physiol Pharmacol. 1996;74(4):547-558. [PubMed] [Google Scholar]
- 29. Lemon RN, Johansson RS, Westling G. Corticospinal control during reach, grasp, and precision lift in man. J Neurosci. 1995;15(9):6145-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kadanka Z, Bednarik J, Novotny O et al.. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20(9):1533-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harrop JS, Naroji S, Maltenfort M et al.. Cervical myelopathy: a clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2010;35(6):620-624. [DOI] [PubMed] [Google Scholar]