Abstract
Objectives:
To evaluate the incidence and risk factors for lymphedema associated with surgery for gynecologic malignancies on GOG study 244.
Methods:
Women undergoing a lymph node dissection for endometrial, cervical, or vulvar cancer were eligible for enrollment. Leg volume was calculated from measurements at 10-cm intervals starting 10 cm above the bottom of the heel to the inguinal crease. Measurements were obtained preoperatively and postoperatively at 4–6 weeks, and at 3-, 6-, 9-, 12-, 18-, and 24- months. Lymphedema was defined as a limb volume change (LVC) ≥10% from baseline and categorized as mild: 10–19% LVC; moderate: 20–40% LVC; or severe: >40% LVC. Risk factors associated with lymphedema were also analyzed.
Results:
Of 1054 women enrolled on study, 140 were inevaluable due to inadequate measurements or eligibility criteria. This left 734 endometrial, 138 cervical, and 42 vulvar patients evaluable for LVC assessment. Median age was 61 years (range, 28–91) in the endometrial, 44 years (range, 25–83) in the cervical, and 58 years (range, 35–88) in the vulvar group. The incidence of LVC ≥10% was 34% (n=247), 35% (n=48), and 43% (n=18), respectively. The peak incidence of lymphedema was at the 4–6 week assessment. Logistic regression analysis showed a decreased risk with advanced age (p=0.0467). An exploratory analysis in the endometrial cohort showed an increased risk with a node count >8 (p=0.033).
Conclusions:
For a gynecologic cancer, LVC decreased with age greater than 65, but increased with a lymph node count greater than 8 in the endometrial cohort. There was no association with radiation or other risk factors.
Keywords: endometrial cancer, vulvar cancer, cervical cancer, lymphedema, lymphadenectomy, staging
INTRODUCTION
In 2012, when GOG 244-The Lymphedema and Gynecologic Cancer study was opened, a lymph node assessment was considered to be integral part of staging for endometrial, cervical and vulvar cancer patients and it remains so today [1–3]. These staging procedures are associated with lymphedema of the lower extremity (LLE), one of the most challenging complications associated with the diagnosis and treatment of a gynecologic cancer. It is commonly reported that an estimated 20% to 60% of gynecologic cancer patients will struggle with lymphedema [4–6]. The true incidence of lymphedema in the general population, as well as in gynecologic cancer patients, is difficult to determine in part because there are many way to measure it and therefore define it [7]. Most of the previous lymphedema analyses on patients with a gynecologic malignancy have been limited, largely retrospective, and frequently via a survey questionnaire [6, 8, 9]. In recent reports, LLE has been objectively measured as a change in bioimpedance [10, 11] or a change in limb volume as compared to the opposite leg [10]. The various assessment and diagnostic methods in these published reports have been inconsistent, which contributes to the wide range in reported incidence.
Gynecologic Oncology Group (GOG) study 195, a randomized trial evaluating the use of a fibrin sealant in the inguinal incisions of vulvar cancer patients, was one of the initial efforts to prospectively evaluate for lymphedema in a gynecologic oncology population [12]. In that study, circumferential measurements were used to assess limb volume change (LVC), which was then used as a surrogate for lymphedema. Patients were evaluated for LVC as determined by three measurements in the lower, middle and upper leg, which were compared to preoperative baseline measurements. Although the study was negative for the use of a fibrin sealant to impact the incidence of lymphedema, GOG 195 identified that the incidence of lymphedema was 60–67% in the study and control arm, respectively. Concurrent with this high incidence of lymphedema was a concern that medical professional awareness of lower extremity lymphedema was less than awareness for upper extremity lymphedema as evidence by being less likely to receive an early referral to a lymphedema specialist [13]. These factors initiated a broader GOG interest in LLE and an intent to investigate lymphedema across a larger number of endometrial, cervical and vulvar cancer patients. Submersion with water displacement has been considered the gold standard for evaluating LVC, a surrogate for lymphedema [14]. The technique, however, is labor-intensive, complicated, and not available in many communities. More recently, sequential circumference measurements in the upper extremity have demonstrated excellent intra- and inter-observer reliability, and have yielded results statistically indistinguishable from those of the water displacement method[15–17]. The reported upper extremity correlation between the volumes measured with circumferential measurements and water displacement measurement is 0.99 [18]. The current study expanded on the experience from GOG-195 and increased the number of leg measurements from three locations along the limb to every 10 cm to improve the ability to detect a change in limb volume in the lower extremity. This study expanded on the clinical variables collected during GOG-195 to allow for any confounding or exploratory relationships to LLE. This study also incorporated patient self-reported symptoms associated with the development of LLE so that more than one method was used to assess patients. These self-reported symptoms have been well documented in the upper extremity lymphedema literature [19, 20]. The Gynecologic Cancer Lymphedema Questionnaire (GCLQ) has an internal consistency reliability of 0.95 [21] and underwent additional adaptation and validation for inclusion in this trial.
The primary management of endometrial, cervical and vulvar cancer has included some type of regional nodal assessment that is commonly associated with increasing the risk of lymphedema[22]. It is believed that this surgical disruption of normal lymphatic channel causes a pooling of extracellular fluid distal to the dissection that is further complicated by the dependent position of the lower extremities. At the time it opened, this study incorporated gynecologic cancers where there was consensus in the nodal assessment such as the pelvic lymphadenectomy for cervical and endometrial cancer, and the inguinal lymphadenectomy for vulvar cancer [2, 3, 23]. Ovarian and other peritoneal malignancies were not included in this study as the role of primary retroperitoneal nodal assessment was less clear.
The primary objective of this study was to prospectively evaluate the incidence of LLE in patients undergoing primary surgery with a concurrent lymphadenectomy for a gynecologic malignancy of the cervix, endometrium, or vulva. Multiple variables were collected from the surgery and any adjuvant therapy to be analyzed for their relationship to lymphedema of the lower extremity.
METHODS
The LEG Study (GOG-244) was a multi-institutional, prospective study of women with newly diagnosed endometrial, cervical, or vulvar cancer who underwent a surgery that included a lymphadenectomy as primary intervention, with the intent of 2 years of follow-up. Eligible patients had to satisfy the following criteria: 1) planned for hysterectomy/bilateral salpingo-oophorectomy (BSO) and pelvic lymphadenectomy +/− para-aortic node sampling via open or laparoscopic technique for clinical stage I-II uterine carcinoma; 2) planned for radical hysterectomy or trachelectomy and pelvic lymphadenectomy +/− para-aortic node sampling via open or laparoscopic technique for clinical stage IA-IIA cervical carcinoma; or 3) planned for definitive surgery for primary stage I-IV vulvar cancer, consisting of radical vulvectomy or radical local excision with concurrent unilateral or bilateral inguinal or inguinal-femoral lymphadenectomy. Participants were able to receive therapy (radiation and/or chemotherapy) after primary surgical treatment.
All study participants signed written informed consent and were enrolled between June 4, 2012 and November 17, 2014. Patients underwent lower limb volume measurements at baseline (within 14 days prior to surgery), at 4–6 weeks, and 3-, 6-, 9-, 12-, 18-, and 24- months after surgery. Cohort variables such as medical co-morbidities and cancer treatments were collected, as well as known, suspected, and possible risk factors for the development of LLE. In addition, participants completed patient-reported outcomes (PRO) assessments of LLE symptoms (GCLQ), quality of life (QoL), and psychological adjustment and function during the same time points. The PRO and GCLQ data are reported in the accompanying manuscript by Carter et al. The other assessments will be in a future publication.
Measurements
Standard measurements of limb volume involved taking bilateral circumferential measurements at 10-cm intervals starting 10 cm above the bottom of the patient’s heel (with the heel flexed at 90 degrees to the leg) and continued to the inferior aspect of the inguinal crease of the groin [24]. The last measurement was taken at the last 10-cm interval below the inguinal crease. Measurements were obtained twice at each level and verified, allowing for a variance of 1.0 cm, for human error. The hands-on training programs for participating research associates were coordinated and performed during semi-annual group meetings and was required prior to an institution enrolling any patients. The training continued until the trainee could perform all measurements to within 1 cm of the trainer. Leg volumes were calculated from the circumferential measurements based on the formula for a truncated cone: V = (h)(C2 + Cc + c2)/12(π) (where h = height of the segment; C = circumference at top of segment; c = circumference at bottom of segment) [18, 25]. The leg volume was determined by the summation of each truncated cone volume. To be classified as lymphedema, a LVC of at least 10% was required [26]. Additionally, lymphedema was further categorized as mild: 10–19% excess limb volume; moderate: 20–40% excess limb volume; or severe: >40% excess limb volume [27]. To be considered evaluable for general analysis, the patient needed preoperative measurements and at least one postoperative measurement. Treatment for lymphedema was allowed during the follow-up. The patient-reported diagnosis of lymphedema and types of lymphedema treatment were collected as part of the PRO data (see Carter et al. in the accompanying manuscript).
As yet another method of lymphedema surveillance, the Stemmer’s sign was assessed during each of the data collection points concurrent with the measurements. The Stemmer’s sign was assessed by pinching a fold of skin at the base of the second toe on each foot. Stemmer’s sign was present and indicative of lymphedema when a skin fold could not be raised.
Statistical Analysis
The incidence of lymphedema was calculated as the number of patients demonstrating at least a 10% increase in limb volume at any of the time points for which they had a measurement taken over 24 months. For a patient to be evaluable for the risk factor analysis, the per-protocol criteria required that at least 5 of the 8 measurements were obtained (preoperative measurement and at least 4 postoperative measurements). The protocol power analysis was designed in anticipation that some patients would be lost to follow-up over time. Protocol eligibility criteria for risk analysis required a preoperative measurement and at least 4 postoperative measurements, not necessarily in sequence. The power analysis was performed based on the predicted incidence of LLE. Logistic regression was used for the comparison of the potential risk factors, with the incidence of lymphedema as the primary outcome variable. The Mantel-Haenszel test was used to evaluate the Stemmer’s sign. A p-value less than 0.05 was considered significant.
RESULTS
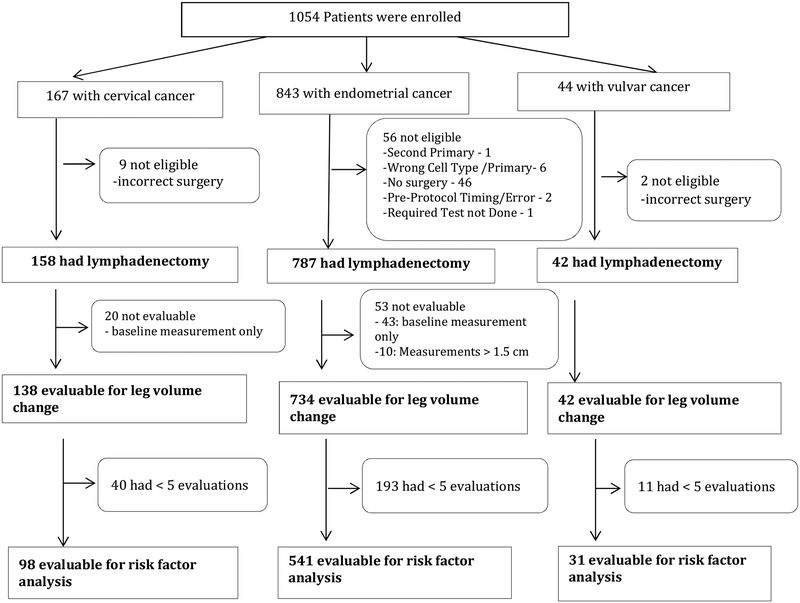
Of 1054 women enrolled on study, 54 were excluded for not meeting various eligibility criteria, the majority involving the omission of lymphadenectomy during the surgical procedure (n=44). Of the 1000 remaining patients, 86 were inevaluable due to inadequate or missing measurements. This left 734 endometrial, 138 cervical, and 42 vulvar cancer patients evaluable for LVC assessment (Figure 1). Median age was 61 years (range, 28–91) in the endometrial cohort, 44 years (range, 25–83) in the cervical cohort, and 58 years (range, 35–88) in the vulvar cohort. Overall patient characteristics are presented in Table 1 with additional characteristics for the endometrial cohort presented in Table 2 (online only). Clinical comorbidities identified during follow-up are presented in Table 3, with postoperative infection in the vulvar cohort being the most commonly reported at 26%. The per-protocol definition of lymphedema was an LVC ≥10%. The incidence of LVC ≥10% was 34% (n=247) in the endometrial, 35% (n=48) in the cervical, and 43% (n=18) in the vulvar cohorts (Table 4). For the endometrial cancer patients with LVC ≥10%, LVC severity was considered mild in 22.8% (n=167), moderate in 9.5% (n=70), and severe in 1.4% (n=10). Similarly, severity was considered mild in 22.5% (n= 31), moderate in 10.9% (n=15), and severe in 1.5% (n=2) of the cervical cancer patients. LVC severity was mild in 28.6% (n=12), moderate in 11.9% (n=5), and severe in 2.4% (n=1) of the vulvar cancer patients. The peak incidence of LVC increase was at the 4–6 week assessment point. (Table 5 online only), but new patients with a LVC ≥ 10% were identified at each point of follow-up through two years. However, there was a fairly persistent loss of patients to follow-up during the protocol; only 54% of the endometrial cancer patients, 48% of the cervical cancer patients, and 40% of the vulvar cancer patients completed the 24 months of follow-up (Table 6).
Figure 1. Flow Chart of Enrolled Patients.
To be eligible for risk factor analysis, patients were required to have a baseline measurement and at least four follow-up measurements.
Table 1.
Characteristics of Enrolled Patients
| Cancer Site | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Endometrial | Cervical | Vulvar | Total | ||||
| N | % | N | % | N | % | N | % | |
| Age Group | ||||||||
| 20–29 | 1 | 0.1 | 9 | 6.5 | 0 | 0 | 10 | 1.1 |
| 30–39 | 18 | 2.5 | 40 | 29.0 | 1 | 2.4 | 59 | 6.5 |
| 40–49 | 49 | 6.7 | 47 | 34.1 | 6 | 14.3 | 102 | 11.2 |
| 50–59 | 266 | 36.2 | 20 | 14.5 | 17 | 40.5 | 303 | 33.2 |
| 60–69 | 274 | 37.3 | 16 | 11.6 | 8 | 19.0 | 298 | 32.6 |
| 70–79 | 113 | 15.4 | 5 | 3.6 | 7 | 16.7 | 125 | 13.7 |
| 80–89 | 12 | 1.6 | 1 | 0.7 | 3 | 7.1 | 16 | 1.8 |
| ≥90 | 1 | 0.1 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| Ethnicity | ||||||||
| Hispanic or Latino | 38 | 5.2 | 20 | 14.5 | 2 | 4.8 | 60 | 6.6 |
| Non-Hispanic | 685 | 93.3 | 114 | 82.6 | 39 | 92.9 | 838 | 91.7 |
| Refused to answer | 6 | 0.8 | 2 | 1.4 | 0 | 0 | 8 | 0.9 |
| Unknown/Not specified | 5 | 0.7 | 2 | 1.4 | 1 | 2.4 | 8 | 0.9 |
| Race | ||||||||
| Missing/Unknown | 27 | 3.7 | 14 | 10.1 | 0 | 0 | 41 | 4.5 |
| Asian | 19 | 2.6 | 11 | 8.0 | 0 | 0 | 30 | 3.3 |
| Black | 68 | 9.3 | 6 | 4.3 | 4 | 9.5 | 78 | 8.5 |
| Am. Indian/Alaskan Native | 14 | 1.9 | 4 | 2.9 | 1 | 2.4 | 19 | 2.1 |
| Native Hawaiian/PI | 0 | 0 | 2 | 1.4 | 0 | 0 | 2 | 0.2 |
| White | 604 | 82.3 | 100 | 72.5 | 35 | 83.3 | 739 | 80.9 |
| White/Asian | 1 | 0.1 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| White/Indian | 1 | 0.1 | 0 | 0 | 2 | 4.8 | 3 | 0.3 |
| White/Native Hawaiian | 0 | 0 | 1 | 0.7 | 0 | 0 | 1 | 0.1 |
| Total | 734 | 80.3 | 138 | 15.1 | 42 | 4.6 | 914 | 100.0 |
PI, Pacific Islander
Table 3.
Comorbidities Identified in Evaluable Patients
| Cancer Type | Vascular Insufficiency | Infection | Infection + Vascular Insufficiency | VTE | VTE + Infection |
|---|---|---|---|---|---|
| Endometrial (n=734) | 3 (0.4%) | 22 (3.0%) | 1 (0.1%) | 4 (0.5% | 1 (0.1%) |
| Cervical (n=138) | 9 (6.5%) | 1 (0.7%) | 1 (0.7%) | ||
| Vulvar (n=42) | 1 (2.4%) | 11 (26.2%) | 2 (4.8%) |
VTE, venous thromboembolism
Table 4.
Lymphedema Diagnosis by Cancer Site
| LVC | Cancer Site | |||
|---|---|---|---|---|
| Endometrial (%) | Cervical (%) | Vulvar (%) | Total | |
| <10% Increase | 487 (66.4) | 90 (65.2) | 24 (57.1) | 601 |
| ≥10% Increase | 247 (33.7) | 48 (34.8) | 18 (42.9) | 313 |
| Total | 734 | 138 | 42 | 914 |
LVC, limb volume change
Lymphedema was defined as a LVC ≥10% from baseline.
Table 6.
Patient Compliance Over Time
| Endometrial | Cervical | Vulvar | |
|---|---|---|---|
| Baseline | 734 | 138 | 42 |
| Postop | 669 | 124 | 38 |
| 3 months | 576 | 103 | 30 |
| 6 months | 543 | 104 | 34 |
| 9 months | 504 | 91 | 31 |
| 12 months | 512 | 88 | 29 |
| 18 months | 448 | 83 | 21 |
| 24 months | 400 (54%) | 66 (48%) | 17 (40%) |
This table shows the number of patients who successfully completed planned follow-up at each assessment time point.
The Stemmer’s sign was used as secondary method to evaluate for potential lymphedema. A comparison between assessment with Stemmer’s sign and LVC ≥10 showed no significant correlation between the Stemmer’s sign and lymphedema in this study (Graph 1 Online only).
Risk Factor Analysis
A total of 541 endometrial, 98 cervical, and 31 vulvar cancer patients had the preoperative and at least 4 postoperative measurements. Because of the small numbers in the cervical and vulvar cohorts, only the endometrial cohort was analyzed for different risk factors. The logistic regression analysis for these risk factors in the endometrial cohort is shown in Table 7. An increasing age was the only risk factor identified to be significantly associated with lymphedema in this cohort. The analysis showed that the risk of lymphedema decreased with advancing age, with an odds ratio of 0.816 (95% CI, 0.670–0.994). All other risk factors, including a comparison of surgical approach (open, laparoscopic or robotic), were not significant for the endometrial cohort, or the cervical cohort, when it was evaluated in an exploratory fashion. Radiation was further evaluated, as it is commonly associated with lymphedema. The timing of the identification of the LVC in the radiated cohort was important. If the LVC preceded the onset of radiation, the patient was excluded from that risk factor evaluation. In this analysis, external beam radiation was also not associated with lymphedema. The transitory nature of LVC was identified in the endometrial cohort with more than 5 measurements. In this group, 59% (n=320) did not have a LVC ≥10% and 19% (n=100) had only one transition in that they developed and maintained a LVC ≥10% for the remainder of the study. But, 15% (n=79) of this group had two transitions where they manifested an LVC ≥10%, but then had an episode of LVC < 10% before they had a future measurement with LVC ≥10%. Of these patients, only 9 were documented to be “in treatment”. Approximately 8% (n=42) of this endometrial cohort had more than two transitions (range 3–6) where there they had episodes of LVC < 10% followed by a LVC ≥10% (Table 8 Online only). Venous insufficiency and orthopedic procedures were monitored, but occurred so infrequently, that an odds ratio could not be reliably established.
Table 7.
Variables analyzed for association with a 10%, or greater, change in limb volume.
| Effect | Estimate | 95% Confidence | Limits | p value |
|---|---|---|---|---|
| Age* | 0.816 | 0.670 | 0.994 | 0.0437 |
| Surgical approach | 0.875 | 0.537 | 1.426 | 0.5915 |
| Blood Loss | 1.002 | 0.978 | 1.027 | 0.8666 |
| Number of Nodes | 1.040 | 0.890 | 1.215 | 0.6237 |
| Stage 2 vs 1 | 0.885 | 0.382 | 2.050 | |
| Stage 3 vs 1 | 1.421 | 0.662 | 3.050 | 0.7742 |
| Stage 4 vs 1 | 1.703 | 0.289 | 10.028 | |
| Race Black vs White | 0.940 | 0.500 | 1.766 | |
| Race Other vs White | 0.574 | 0.280 | 1.180 | 0.3189 |
| Lymphocyst Formation | 0.820 | 0.357 | 0.1.884 | 0.6399 |
| Performance Status | 1.096 | 0.546 | 2.201 | 0.7960 |
| BMI 26–29 vs normal | 0.846 | 0.487 | 1.471 | 0.3331 |
| BMI >30 vs normal | 0.707 | 0.439 | 1.139 | |
| Presence of metastatic nodes | 1.193 | 0.517 | 2.753 | 0.6795 |
| External Beam Radiation | 0.651 | 0.367 | 1.157 | 0.1435 |
| Chemotherapy | 1.010 | 0.621 | 1.642 | 0.9692 |
| Post Surgical Infection | 0.996 | 0.374 | 2.648 | 0.9932 |
| VTE | 1.211 | 0.170 | 8.655 | 0.8484 |
| Use of Heparin | 1.304 | 0.865 | 1.966 | 0.2043 |
| Use of Compression Stockings | 0.920 | 0.621 | 1.364 | 0.6794 |
This anlaysis is of the endocmtrial cohort who had 5 or more measurements obtained. Surgical approach was analyzed as both a comparison of open verses laparoscopic verses robotic and again as open verses minimally invasive techniques (laparoscopic and robotic combine). There wer no associations between surgical approach and an increased LVC identified in this discriptive analysis. In the logistic regression analysis in those endometrial patients who had at least 5 measurements, there was a reduction in risk of lymphedema associated with advancing age (p<0.04)
BMI, body mass index; VTE, venous thromboembolism
Lymph node count was evaluated in relation to an LVC ≥10% in the endometrial group, the largest cohort. For this descriptive analysis of node count, a dichotomy of ≤ 8 nodes compared to >8 nodes was explored. This number was selected based on the bilateral removal of at least one node from each of the four critical node basins (external and common iliac, obturator, and periaortic). The variables that were included for this exploratory analysis were endometrial cancer, surgical approach, stage, race, age, performance status, serum albumin, nodes count (over/under 8), presence of metastatic nodes, radiation, chemotherapy, use of heparin, and use of compression stockings. Patients that had a post-surgical infection, vascular insufficiency, or VTE were excluded from the exploratory analysis. In this endometrial cohort, only a node count >8 was significantly related to increased severity of lymphedema, with an odds ratio of 2.031 (95% CI, 1.058–3.901; p=0.0333). Of the endometrial cancer patients who had ≤8 nodes removed, 23.2% (19/82) developed lymphedema, as defined by a ≥10% increase in limb volume. This compared to 35.0% (228/652) in those patients who had more than 8 nodes removed. A similar analysis with the smaller cervical cohort did not show a significant relationship.
There were too few vulvar cancer patients enrolled for risk factor analysis. To be eligible for this protocol, the vulvar cancer patients needed a clinical indication for a full inguinal lymphadenectomy. This protocol was initiated after the adaptation of the vulvar sentinel lymph node approach, which contributed to the low enrollment among these patients. The protocol was later amended to exclude any additional vulvar patients in an attempt to increase the enrollments of the endometrial and cervical patients.
Measurements
The study was conducted at 52 enrolling parent institutions with 72 affiliates as well as NCORP sites. There was a median of 4 research associates involved in performing assessments (range 1–26). The protocol defined the method of leg measurement to be utilized. As described in the methods section above, extensive training was implemented prior to initiating enrollment. Yet, 32% of the patients had some variance in leg length reported during the study. For statistical analysis, the leg length in these patients was truncated to the longest length where there were consistent measurements across all of the intervals of follow up in these circumstances. The protocol also defined that each circumferential measurement would be obtained twice and verified, allowing for up to a 1.0 cm variance in each of the paired measurements. Yet, of the 1054 patients in this study, 11.3% had greater than a 1.0 cm difference in the paired values reported at some point in the study. Of these, 10 patients had measurements that exceeded > 1.5 cm in the paired measurements and these measurement assessments were excluded from the analysis.
DISCUSSION
Lymphedema is defined as a chronic, dynamic condition in which protein-rich fluid accumulates in the superficial tissues. Lymphedema can be problematic causing discomfort, or heaviness and reduced mobility. It has the potential to be progressive and extremely disfiguring and disabling for some patients. There is evidence that early intervention can reduce the severity of lymphedema in breast cancer patients [28]. The diagnosis, best interventions and awareness is still evolving for gynecologic cancers. The objectives of this protocol were to estimate the incidence of lymphedema following surgery for endometrial, cervical, or vulvar malignancies with sequential measures over time. Per the NCCN guidelines, the surgery for endometrial and early cervical cancer patients allowed for the removal of lymph nodes from similar anatomic locations. It was reassuring to identify the incidence of lymphedema was also similar at 34% and 35%, for endometrial and cervical cancer patients, respectively. Vulvar cancer patients have some of the highest reported incidence of lymphedema [10–12], consistent with the higher 43% incidence in this study. There are historic difficulties associated with lymphedema assessment concerning how it is defined and measured [7, 29]. For gynecologic patients, the primary sites of lymphedema are the lower extremities, and to a lesser extent, the vulva and mons areas, which are difficult to assess with objective measurements [11] but may be detected by PRO questionnaires. Using volumetric calculations to define lymphedema precludes conclusions gained from the PROs linked to the secondary objectives of this protocol (Carter et al. in the accompanying manuscript).
Lymphedema is commonly associated with the surgical disruption of the lymphatic channels during a staging procedure [30]. Using bioimpedance techniques, lymphedema has been documented in gynecologic cancer patients prior to surgery, which further complicates our understanding of this disease [11]. The transitory nature of lymphedema was confirmed in this study as 23% of the endometrial cohort had two or more transitions in their LVC during their follow-up. Lymphedema severity changes over time and with treatment or activity [10] and can be difficult to assess even in a prospective fashion In this study, there were PROs declaring a diagnosis of lymphedema in some patients undergoing treatment who did not have an LVC ≥10%. Relying solely on objective measurements may over-report the true incidence of this dynamic process. In general, the incorporation of PROs into gynecologic oncology research and clinical care has become widely accepted [31] and may be extremely pertinent for lymphedema assessments [21, 32, 33].
The risk factors associated with these gynecologic cancers and the subsequent onset of lymphedema were also assessed. Because of the small numbers of cervical and vulvar cancer patients, only the endometrial cohort underwent regression analysis and the monitored risk factor must have preceded the onset of an LVC ≥10% in a patient who had at least 5 assessed measurements. There have been many risk factors previously associated with lymphedema that were not validated in this large prospective trial. For instance, pelvic radiation has frequently been reported to be associated with an increased risk of lymphedema [9, 32, 34–37]. But in this prospective trial, radiation was not found to be a risk factor through 2 years of follow-up. The presence of metastatic disease in lymph nodes or advanced stage were other risk factors not validated in this study [35, 38]. The lack of validation in these areas may be reflective of the true differences of a large prospective trial or of issues with the definitions and measurements used in this trial. A descriptive analysis was performed to further evaluate several other risk factors. That analysis was limited to the endometrial population as any results would be dominated by that population. In that analysis, this study did identify that a lymph node dissection that exceeded 8 nodes was associated with a significant increased risk of lymphedema development (p=0.0333).
These findings would corroborate the reported reduced risk of lymphedema associated with a robotically assisted sentinel lymph node evaluation, in which fewer lymph nodes were removed [39]. As 23.2% of those with ≤8 nodes removed still experienced some LVC, an effort to manage these patients through treatment pathways that would further reduce the number of lymph nodes removed without affecting overall survival warrants further investigation. This study also identified a lower incidence of lymphedema associated with advancing age, a finding that could not be corroborated in the literature. For this analysis, there were a significant number of patients in their fifth and sixth decade of life. This clustering did not allow a specific age risk cutoff to be defined during exploration. The significance of age was identified when age was used as a continuous variable in the logistic regression model and was the only significant variable for the whole population
The study had several strengths. It was the largest cohort of gynecologic cancer patients to undergo a baseline assessment followed by sequential evaluation for lymphedema using objective measurements over a 2-year interval. It standardized the assessment process and increased the number of measurements in an attempt to improve the quality of the data. Other strengths of this study include the 2 years of follow-up and its multi-institutional nature, which give the findings broad applicability.
The study also had significant limitations. The large cohort size and multi-institutional nature of the study were also weaknesses, as a large number of research associates were involved in the assessments used to evaluate for LVC, the primary surrogate for diagnosing lymphedema in this trial. Sequential measurements were tedious and labor-intensive. Measurement accuracy was paramount to confident conclusions. Despite the training, however, discrepancies were identified. The measurements were to be taken twice and verified to be within 1 cm of each other. But 11.3% of the paired measurements reported had a discrepancy greater than 1 cm. In addition, 32% of the patients had some reported variance in leg length. If there was variance in leg length, then one could surmise that there may have been variance in the location of the leg measurement. A hypothetical proximal movement of just 1.5 cm that was then propagated along the length of the leg may have skewed the measurements enough to generate a 10% increase in LVC. The protocol analysis required the completion of at least 5 measurements to be eligible for risk factor analysis. The risk factor analysis was weakened by the large number of patients who were lost to follow-up, with only half of the patients completing the 24 months of follow-up. Patients with a complete data set were compared to those who were lost to follow-up. The additional analysis could not identify any significant data inconsistencies or trends that would suggest that those with lymphedema were more or less likely to complete the scheduled follow-up. Another weakness was that the protocol did not require notification of the clinician when there was an ≥10% LVC identified by the research staff. While it is not typical to immediately communicate research results to the clinician, the failure to do so meant that there was not an immediate concurrent clinical collaboration in those who manifested an LVC by measurements or reported a new lymphedema diagnosis on the GCLQ. Therefore, the lymphedema diagnosis was only assessed and documented through the patient-reported questionnaire. This made it more difficult to draw conclusions when there were discrepancies between an increase in LVC measurement that was not corroborated by a PRO or a change in the GCLQ score (See Carter et al. in the accompanying manuscript).
Lymphedema is a complicated, dynamic process for which a “static” volumetric measurement was applied in this study. Using volumetric measurements for LVC as a surrogate potentially overestimated the true incidence of lymphedema while using PROs likely underreported the true incidence. This opinion is not unique [7]. Perhaps the ideal assessment strategy to assess LLE would include a less labor-intensive form of objective limb measurement (bioimpedance) in conjunction with PROs of LLE symptoms (i.e., GCLQ). Since the presentation of the results of this study, an international panel of experts met at the NCI to discuss controversies around circumferential measurements with the intent to publish lymphedema assessment recommendations for future studies.
In conclusion, this is the largest prospective study to comprehensively assess lymphedema and its associated risk factors in a cohort of gynecologic cancer patients. The findings confirm that lymphedema is a common problem for these patients. The study also hightlights some of the challenges with the diagnosis of this potentially debilitating problem.
Supplementary Material
RESEARCH HIGHLIGHTS.
Lymphedema as defined by volume change ≥10% was found in 34% of endometrial, 35% of cervical, and 43% of vulvar patients.
Regression analysis showed risk decreased with advanced age (p=0.0467) and increased with a node count >8 (p=0.033).
Increase risk of lymphedema was not associated with radiation, advanced stage or other commonly reported risk factors.
Final conclusions were weakened by 50% lost to follow-up and discrepancies in measurements identified in 32% of patients.
Acknowledgments
This study was supported by NCI grant R01CA162139 and grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1 U10 CA180822), and NRG Operations (U10CA180868). Drs. Carter, Zivanovic, and Barakat are also supported in part by the NIH/NCI Memorial Sloan Kettering Cancer Center support grant P30 CA008748.
The following Gynecologic Oncology institutions participated in this study: University of Oklahoma, Women’s Cancer Center of Nevada, Cancer Research for the Ozarks NCORP, University of North Carolina at Chapel Hill, Women and Infants Hospital, Memorial Sloan Kettering Cancer Center, Georgia Center for Oncology Research and Education (CORE), Metro-Minnesota CCOP, Abington Memorial Hospital, University of California at Los Angeles Health System, Ohio State University Comprehensive Cancer Center, Froedhert and the Medical College of Wisconsin, University of New Mexico, Roswell Park Comprehensive Cancer Center, University of New Mexico, Mayo Clinic, The Hospital of Central Connecticut, University of Minnesota Medical Center-Fairview, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Delaware/Christiana Care CCOP, Virginia Commonwealth University, University of Alabama at Birmingham, Hartford Hospital, Emory University School of Medicine, Washington University School of Medicine, Gynecologic Oncology of West Michigan PLLC, Lewis Cancer and Research Pavilion at St. Joseph’s/Candler, Stony Brook University Medical Center, Saint Joseph’s Hospital and Medical Center, University of Arkansas for Medical Sciences, Cancer Research Consortium of West Michigan NCORP, Michigan Cancer Research Consortium Community Clinical Oncology Program, Main Medical Center - Scarborough Campus, William Beaumont Hospital, University of Iowa Hospitals and Clinics, University of California Medical Center at Irvine-Orange Campus, Case Western Reserve University, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Baystate Medical Center, Carle Cancer Center, Mainline Health CCOP, Southeast Cancer Control Consortium CCOP, University of Texas Southwestern Medical Center, MD Anderson Cancer Center, City of Hope, Wichita CCOP, Northside Hospital, Avera Cancer Institute, Upstate Carolina CCOP, Tulane University MBCCOP, New Hanover Regional Medical Center/Zimmer Cancer Center and Cleveland Clinic Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Dr. Alan Hutson received grant funding from NRG SDMC.
Dr. Jeanne Carter received grant funding from grant RO1 as a co-investigator.
Dr. Jane Armer received grant funding from NCI LEG study.
Dr. Suzy Lockwood received grant funding from NIH ROICA 162139.
Dr. Susan Nolte grant funding from NCI ROICA162139, salary support to institution, payments for patient accrual from GOG and NRG Oncology to institution.
Dr. Robert Stewart received grant funding from NCI LEG study.
Dr. Lari Wenzel received grant funding from NRG Oncology.
All other co-authors have no conflicts of interest to declare.
REFERENCES
- [1].Koh WJ, Abu-Rustum NR, Bean S, Bradely K, Campos SM, Cho KR, et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:64–84. [DOI] [PubMed] [Google Scholar]
- [2].Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:170–99. [DOI] [PubMed] [Google Scholar]
- [3].Koh WJ, Greer BE, Abu-Rustum NR, Campos SM, Cho KR, Chon HS, et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:92–120. [DOI] [PubMed] [Google Scholar]
- [4].Halaska MJ, Novackova M, Mala I, Pluta M, Chmel R, Stankusova H, et al. A prospective study of postoperative lymphedema after surgery for cervical cancer. Int J Gynecol Cancer. 2010;20:900–4. [DOI] [PubMed] [Google Scholar]
- [5].Kim JH, Choi JH, Ki EY, Lee SJ, Yoon JH, Lee KH, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer. 2012;22:686–91. [DOI] [PubMed] [Google Scholar]
- [6].Salani R, Preston MM, Hade EM, Johns J, Fowler JM, Paskett EP, et al. Swelling among women who need education about leg lymphedema: a descriptive study of lymphedema in women undergoing surgery for endometrial cancer. Int J Gynecol Cancer. 2014;24:1507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lindqvist E, Wedin M, Fredrikson M, Kjolhede P. Lymphedema after treatment for endometrial cancer - A review of prevalence and risk factors. Eur J Obstet Gynecol Reprod Biol. 2017;211:112–21. [DOI] [PubMed] [Google Scholar]
- [8].Rowlands IJ, Beesley VL, Janda M, Hayes SC, Obermair A, Quinn MA, et al. Quality of life of women with lower limb swelling or lymphedema 3–5 years following endometrial cancer. Gynecol Oncol. 2014;133:314–8. [DOI] [PubMed] [Google Scholar]
- [9].Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Do JH, Choi KH, Ahn JS, Jeon JY. Effects of a complex rehabilitation program on edema status, physical function, and quality of life in lower-limb lymphedema after gynecological cancer surgery. Gynecol Oncol. 2017;147:450–5. [DOI] [PubMed] [Google Scholar]
- [11].Hayes SC, Janda M, Ward LC, Reul-Hirche H, Steele ML, Carter J, et al. Lymphedema following gynecological cancer: Results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol Oncol. 2017;146:623–9. [DOI] [PubMed] [Google Scholar]
- [12].Carlson JW, Kauderer J, Walker JL, Gold MA, O’Malley D, Tuller E, et al. A randomized phase III trial of VH fibrin sealant to reduce lymphedema after inguinal lymph node dissection: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;110:76–82. [DOI] [PubMed] [Google Scholar]
- [13].Langbecker D, Hayes SC, Newman B, Janda M. Treatment for upper limb and lower limb lymphedema by professionals specializing in lymphedema care. Eur J Cancer Care. 2008;17:557–64. [DOI] [PubMed] [Google Scholar]
- [14].Petersen EJ, Irish SM, Lyons CL, Miklaski SF, Bryan JM, Henderson NE, et al. Reliability of water volumetry and the figure of eight method on subjects with ankle joint swelling. J Orthop Sports Phys Ther. 1999;29:609–15. [DOI] [PubMed] [Google Scholar]
- [15].Meijer RS, Rietman JS, Geertzen JH, Bosmans JC, Dijkstra PU. Validity and intra- and interobserver reliability of an indirect volume measurements in patients with upper extremity lymphedema. Lymphology. 2004;37:127–33. [PubMed] [Google Scholar]
- [16].Pani SP, Vanamail P, Yuvaraj J. Limb circumference measurement for recording edema volume in patients with filarial lymphedema. Lymphology. 1995;28:57–63. [PubMed] [Google Scholar]
- [17].Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86:205–14. [PubMed] [Google Scholar]
- [18].Karges JR, Mark BE, Stikeleather SJ, Worrell TW. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther. 2003;83:134–45. [PubMed] [Google Scholar]
- [19].Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–9. [DOI] [PubMed] [Google Scholar]
- [20].Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–17. [DOI] [PubMed] [Google Scholar]
- [21].Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical care tool to identify lower extremity lymphedema in gynecologic cancer survivors. Gynecol Oncol. 2010;117:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cemal Y, Jewell S, Albornoz CR, Pusic A, Mehrara BJ. Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity lymphedema. Lymphat Res Biol. 2013;11:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, et al. Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015;13:395–404; quiz [DOI] [PubMed] [Google Scholar]
- [24].Klein MJ, Alexander MA, Wright JM, Redmond CK, LeGasse AA. Treatment of adult lower extremity lymphedema with the Wright linear pump: statistical analysis of a clinical trial. Arch Phys Med Rehabil. 1988;69:202–6. [PubMed] [Google Scholar]
- [25].McNeely ML, Magee DJ, Lees AW, Bagnall KM, Haykowsky M, Hanson J. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: a randomized controlled trial. Breast Cancer Res Treat. 2004;86:95–106. [DOI] [PubMed] [Google Scholar]
- [26].Stanton AW, Badger C, Sitzia J. Non-invasive assessment of the lymphedematous limb. Lymphology. 2000;33:122–35. [PubMed] [Google Scholar]
- [27].Framework TL. Best Practice for the Management of Lymphoedema. London, UK: Medical Education Partnership (MEP) Ltd; 2006. [Google Scholar]
- [28].Schmielau J, Rick O, Reuss-Borst M, Kalusche-Bontemps EM, Steimann M. Rehabilitation of Cancer Survivors with Long-Term Toxicities. Oncol Res Treat. 2017;40:764–71. [DOI] [PubMed] [Google Scholar]
- [29].Beesley VL, Rowlands IJ, Hayes SC, Janda M, O’Rourke P, Marquart L, et al. Incidence, risk factors and estimates of a woman’s risk of developing secondary lower limb lymphedema and lymphedema-specific supportive care needs in women treated for endometrial cancer. Gynecol Oncol. 2015;136:87–93. [DOI] [PubMed] [Google Scholar]
- [30].Kuroda K, Yamamoto Y, Yanagisawa M, Kawata A, Akiba N, Suzuki K, et al. Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: a hospital-based retrospective cohort study. BMC Womens Health. 2017;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moss HA, Havrilesky LJ. The use of patient-reported outcome tools in Gynecologic Oncology research, clinical practice, and value-based care. Gynecol Oncol. 2018;148:12–8. [DOI] [PubMed] [Google Scholar]
- [32].Karabuga H, Gultekin M, Tulunay G, Yuce K, Ayhan A, Yuce D, et al. Assessing the Quality of Life in Patients With Endometrial Cancer Treated With Adjuvant Radiotherapy. Int J Gynecol Cancer. 2015;25:1526–33. [DOI] [PubMed] [Google Scholar]
- [33].Yost KJ, Cheville AL, Weaver AL, Al Hilli M, Dowdy SC. Development and validation of a self-report lower-extremity lymphedema screening questionnaire in women. Phys Ther. 2013;93:694–703. [DOI] [PubMed] [Google Scholar]
- [34].Bae HS, Lim MC, Lee JS, Lee Y, Nam BH, Seo SS, et al. Postoperative Lower Extremity Edema in Patients with Primary Endometrial Cancer. Ann Surg Oncol. 2016;23:186–95. [DOI] [PubMed] [Google Scholar]
- [35].Mitra D, Catalano PJ, Cimbak N, Damato AL, Muto MG, Viswanathan AN. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J Gynecol Oncol. 2016;27:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ryan M, Stainton MC, Slaytor EK, Jaconelli C, Watts S, Mackenzie P. Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust N Z J Obstet Gynaecol. 2003;43:148–51. [DOI] [PubMed] [Google Scholar]
- [37].Tada H, Teramukai S, Fukushima M, Sasaki H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer. 2009;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev.. 2017;10:Cd007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Geppert B, Lonnerfors C, Bollino M, Persson J. Sentinel lymph node biopsy in endometrial cancer-Feasibility, safety and lymphatic complications. Gynecol Oncol. 2018;148:491–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.