Abstract
Objective
Pap tests hold promise as a molecular diagnostic for serous ovarian cancer, but previous studies reported limited sensitivity. Furthermore, the presence of somatic mutations in normal tissue is increasingly recognized as a challenge to the specificity of mutation-based cancer diagnostics. We applied an ultra-deep sequencing method with the goal of improving sensitivity and characterizing the landscape of low-frequency somatic TP53 mutations in Pap tests.
Methods
We used CRISPR-DS to deeply sequence (mean Duplex depth ~3,000x) the TP53 gene in 30 Pap tests from 21 women without cancer and 9 women with serous ovarian carcinoma with known TP53 driver mutations. Mutations were annotated and compared to those in the TP53 cancer database.
Results
The tumor-derived mutation was identified in 3 of 8 Pap tests from women with ovarian cancer and intact tubes. In addition, 221 low-frequency (≲0.001) exonic TP53 mutations were identified in Pap tests from women with ovarian cancer (94 mutations) and without ovarian cancer (127 mutations). Many of these mutations resembled TP53 mutations found in cancer: they impaired protein activity, were predicted to be pathogenic, and clustered in exons 5 to 8 and hotspot codons. Cancer-like mutations were identified in all women but at higher frequency in women with ovarian cancer.
Conclusions
Pap tests have low sensitivity for ovarian cancer detection and carry abundant low-frequency TP53 mutations. These mutations are more frequently pathogenic in women with ovarian cancer. Determining whether low-frequency TP53 mutations in normal gynecologic tissues are associated with an increased cancer risk warrants further study.
Keywords: Epithelial ovarian cancer, deep sequencing, Papanicolaou test, biomarker, TP53 gene
INTRODUCTION
High-grade serous ovarian, fallopian tube, and peritoneal carcinoma (HGSC) is the most common subtype of ovarian cancer [1]. Most women with HGSC present in advanced stage, which renders a dismal overall 5-year survival of approximately 30% in contrast to 90% for early stage ovarian cancers [2]. The development of screening methods to detect ovarian cancer has been a principal but elusive research goal for decades. Since the advent of Next Generation Sequencing (NGS), the “liquid biopsy” approach (the detection of tumor-derived DNA in minimally-invasive clinical samples) has emerged with promise to revolutionize cancer diagnostics [3].
Pap tests are particularly attractive as a potential tool for ovarian cancer screening because they are minimally-invasive and already an established component of women’s preventive healthcare [4]. Pap tests consist primarily of cervical epithelial cells, but are known to contain small populations of cells from proximal components of the female reproductive tract including endometrial, fallopian tube, and ovarian epithelium [5]. In two promising initial studies, Kinde et al. [6] and Wang et al. [7] detected ovarian tumor-derived DNA in 41% and 29% of associated Pap tests, respectively, using Safe-SeqS, a high-accuracy deep sequencing method [8]. While Safe-SeqS improves the mutation detection threshold of NGS by two orders of magnitude [9], it may still be insufficient for this clinical application. We hypothesized that the use of a more accurate sequencing technique could improve sensitivity and better define the utility of Pap tests to detect ovarian cancer.
Duplex Sequencing (DS) is an ultra-accurate NGS method estimated to have an error rate two orders of magnitude less than Safe-SeqS [9–11]. We previously demonstrated that DS can identify a single tumor mutation among ~24,000 normal genomes [12]. Here we propose to utilize the high sensitivity of DS for the detection of tumor-derived DNA in Pap tests. We targeted TP53 exclusively because mutations in this gene are highly prevalent (>96%) in both HGSC [13–15] and high grade intraepithelial tubal neoplasia [16].
In addition to determining the sensitivity of Pap tests for HGSC detection, we also aimed to better define the specificity of this approach by analyzing Pap tests from women without malignant disease. Somatic mutations in cancer “driver” genes (such as TP53) were long presumed to be specific for malignancy, but mounting evidence indicates that these mutations are common in non-cancerous tissues, where they accumulate with age [17–19]. These findings challenge the concept of the “liquid biopsy”, which must be interpreted in context of the mutational background of the non-malignant tissue that is sampled [20]. Here we characterize the landscape of somatic TP53 mutations in Pap tests from women without cancer and compare with that of women with HGSC in order to better understand the potential role of Pap tests as a diagnostic tool for HGSC.
METHODS
Patients and samples
The study included a cohort of 30 women with or without HGSC who underwent gynecological surgery at the University of Washington due to pelvic masses suspicious for malignancy or prophylactic salpingo-oophorectomy due to inherited mutations in BRCA1 or BRCA2 (BRCA mutation carriers). Women were enrolled at diagnosis under an IRB-approved protocol at the University of Washington and consented to tissue collection including blood, tumor, and a pre-operative Pap test. Pap tests were collected in the OR prior to the start of surgery with an endocervical cytobrush (Thinprep, Hologic, MA, USA) according to manufacturer’s protocol. Samples were centrifuged at 2,700 rpm for 15 min and cell pellets were stored in 1.7 mL of media at −80°C at the University of Washington Gynecologic Oncology Tissue Bank. Final patient selection was based on availability of sufficient DNA samples given the pilot study design. Clinico-pathological information was recorded for each patient (Table 1, Supplementary Table S1). None of the patients had prior salpingectomy, but four patients had prior tubal ligations. One of the patients was in the HGSC group and thus was excluded from sensitivity analyses. Tumors were surgically staged according to the International Federation of Obstetrics and Gynecology (FIGO) criteria. Sectioning and extensively examining the fimbriated end (SEE-FIM) was performed for all cases of cancer and all germline BRCA mutation carriers (in patients without cancer no gross or microscopic disease was detected). The BROCA sequencing panel was used to determine germline DNA mutations in BRCA1, BRCA2 and other known ovarian cancer genes in all cancer patients [21]. In the benign group, only the patients with high-risk family history and/or known inherited BRCA mutations undergoing risk-reducing salpingo-oophorectomy were tested for germline mutations.
Table 1.
Clinico-pathological characteristics of patients
| No. (%) |
|||
|---|---|---|---|
| Total | Benign (N=21) | High grade serous cancer (N=9) | |
| Median age (range) | 54.5 (19–82) | 51 (19–82) | 65 (48–72) |
| Menopausal status | |||
| Pre | 10 (33.3%) | 9 (42.9%) | 1 (11.1%) |
| Post | 20 (66.7%) | 12 (57.1%) | 8 (88.9%) |
| Mutation status | |||
| None | 18 (60.0%) | 12 (57.1%) | 6 (66.7%) |
| BRCA1 | 6 (20.0%) | 3 (14.3%) | 3 (33.3%) |
| BRCA2 | 6 (20.0%) | 6 (28.6%) | |
| Histology | |||
| No neoplasm | 12 (40%) | 12 (57.1%) | |
| Benign neoplasm* | 9 (30%) | 9 (42.9%) | |
| High grade serous cancer | 9 (30%) | 9 (100%) | |
| Stage | |||
| III | 8 (88.9%) | ||
| IV | 1 (11.1%) | ||
| Prior cancer diagnosis | |||
| No | 29 (96.7%) | 21 (100%) | 8 (88.9%) |
| Yes | 1 (3.3%) | 1 (11.1%) | |
| Prior chemotherapy | |||
| No | 28 (93.3%) | 21 (100%) | 7 (77.8%) |
| Yes | 2 (6.7%)† | 2 (22.2%)† | |
Benign neoplasms included serous cystadenoma, mucinous cystadenoma, serous cystadenofibroma, endometriosis, salpingitis, fibroma, cellular angiofibroma, and struma ovarii
Received neoadjuvant chemotherapy for ovarian cancer
Pap test DNA extraction
Pap tests were centrifuged at 1,000g for 3 minutes and DNA was extracted from the cell pellet using the QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA). The yield of Pap test DNA averaged 6.46 μg (range: 650 ng – 14.5 μg). DNA was also extracted from fresh-frozen tumors (from areas of ≥60% neoplastic cellularity).
TP53 mutation detection in cancers
TP53 mutations in primary tumors were determined by NGS as previously described [22].
CRISPR-DS
We used CRISPR-DS to sequence TP53 as previously described [23]. CRISPR-DS incorporates CRISPR/Cas9 target enrichment prior to library preparation, which reduces the need for two rounds of hybridization capture while preserving the high accuracy of DS. 250 ng of genomic Pap test DNA was digested with CRISPR/Cas9 using guide RNAs designed to excise the coding region of TP53 (exons 2–11) in fragments of ~500bp (Supplementary Fig. S1). Then the excised fragments were recovered by size selection using AMPure XP Beads (Beckman Coulter, Brea, CA, USA) and processed for library preparation (Supplementary Methods). Sequencing data was analyzed using the CRISPR-DS pipeline available at https://github.com/risqueslab/CRISPR-DS [23]. Raw reads were grouped to produce single-strand consensus sequences for each DNA strand. Then complementary strands of the same DNA molecule were compared to produce Duplex reads (Supplementary Fig. S1), which were aligned to the human genome reference hg19 (GRCH37) and processed to extract mutational information.
Mutational analysis
For each individual mutation, mutant allele frequency (MAF) was calculated as number of reads with the mutation divided by the total Duplex reads sequenced at that position. For each sample, TP53 mutation frequency was calculated as the number of unique coding or splicing mutations in TP53 (excluding the tumor mutation) divided by the total number of Duplex nucleotides sequenced in exons (Supplementary Table S2). For each sample, Duplex depth was calculated as the number of Duplex nucleotides sequenced in exons divided by the size of the TP53 coding region. The mean Duplex depth for the 30 Pap tests was 3,068x (range: 811x – 7,440x) (Supplementary Table S2).
Characterization of TP53 mutations using Seshat
All TP53 variants identified in exons and splice-sites in Pap tests from women with and without HGSC (n=224) were submitted to Seshat (https://p53.fr/TP53-database/seshat), a web service that performs TP53 mutation annotation and characterization using data derived from the UMD TP53 database [24] (Supplementary Methods, Supplementary Table S3). Mutations were analyzed based on mutation type (missense, nonsense, splice, insertion or deletion, synonymous), mutation spectrum (6 possible nucleotide substitutions), location in exons 5 to 8 (which encode the DNA binding domain), location in mutational hotspot codons (9 most commonly mutated codons in the UMD TP53 database for all cancers: 175, 179, 213, 220, 245, 248, 249, 273, 282), reported frequency of the mutation in the cancer database, functional activity, and predicted pathogenicity. For each patient, we calculated the mutation frequency in each category by dividing the number of mutations by the total number of Duplex nucleotides sequenced in exons in order to compensate for differences in sequencing depth between patients.
Comparison with TP53 mutations without selection
A list of all possible single nucleotide substitutions in the TP53 coding region (n=3,546) was generated in silico and submitted to Seshat to determine the distribution of mutations in the following categories: frequency in the cancer database, protein activity, predicted pathogenicity, location in exons 5–8, and location in hotspots [18]. The values obtained represent the distribution of all possible TP53 mutations in the absence of selection and were used for comparison with TP53 Pap test mutations identified in women with and without HGSC.
Comparison with TP53 cancer database mutations
From the UMD TP53 database (April 2017 version), we identified 71,051 mutations reported within human tumors (mutations from cell lines, normal and premalignant tissue were excluded). Then we determined the distribution of mutations in the following categories: frequency in the cancer database, protein activity, predicted pathogenicity, location in exons 5–8, and location in hotspots [18]. These values were used to compare TP53 cancer mutations with TP53 Pap test mutations identified in women with and without HGSC.
Statistical analysis
Correlations were tested with Spearman’s rank test or with quasibinomial tests in order to explicitly account for the different sequencing depth across individuals (lower depth results in a lower probability of finding mutations). Comparison of means across categories of mutations was performed by t-test. Comparison of the distribution of mutational features in Pap tests vs. all possible TP53 mutations was performed with chi-square. All tests were two-sided at an alpha level (type 1 error rate) of 0.05. Statistical analyses were performed with STATA and R.
RESULTS
Low-frequency somatic TP53 mutations are detected in Pap tests from all patients with and without HGSC
The cohort included nine patients with HGSC (30%) and 21 patients with benign pathology (70%). Clinico-pathological information is summarized in Table 1. All patients with HGSC had stage III or IV disease. Three patients with HGSC (33%) and nine patients with benign pathology (43%) carried a germline BRCA1 or BRCA2 mutation. A single clonal TP53 mutation was found in all tumors (Supplementary Table S1). No additional clonal or subclonal TP53 mutations were found in any of the primary tumors using conventional NGS.
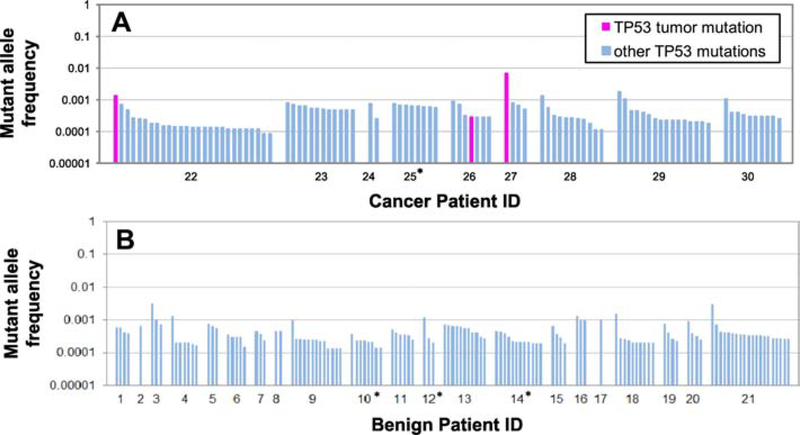
We performed ultra-deep sequencing of TP53 (mean depth = 3,068x) in Pap tests from all 30 women. A total of 571 TP53 variants were identified, 256 of which were located in exons or splicing sites and 315 were located in introns. 137 variants were germline polymorphisms (32 exonic and 105 intronic) as demonstrated by MAF around 0.5 and 1.0 and Seshat database confirmation. After removing polymorphisms and intronic mutations, 224 exonic mutations (including coding and splicing, Supplementary Table S3) remained for analysis. All these mutations were present at very low-frequency (MAF<0.01) and were identified in patients with and without HGSC (Fig. 1).
Figure 1: TP53 mutations identified in Pap tests. A. Women with HGSC. B. Women without cancer.
Each bar represents a TP53 mutation. For each patient, mutations are sorted by descending Mutant Allele Frequency (MAF). Note that all mutations have MAF<0.01 and most mutations have MAF<0.001. The three mutations that correspond to matching primary tumor mutations are indicated in fuchsia. Patient ID is indicated in the X-axis. The four patients with prior tubal ligations are indicated with an asterisk.
Of the 224 exonic mutations, only 3 were tumor-derived mutations (i.e. matched the clonal driver TP53 mutation present in the tumor). This resulted in a sensitivity of 37.8% for the detection of HGSC mutations in Pap test DNA (3 positive patients out of 8 patients with HGSC and intact fallopian tubes, Fig. 1). The MAF of the tumor-derived mutations were 0.00135, 0.00029, and 0.00671 for patients 22, 26, and 27, respectively. All 3 Pap tests with tumor-derived mutations contained additional low-frequency TP53 mutations, although in 2 of these 3 cases (patients 22 and 27), the tumor-derived mutant allele was the most abundant mutation. All patients carried low-frequency TP53 mutations, including the four patients that had undergone prior tubal ligations. In total, we identified 94 TP53 mutations in ovarian cancer patients and 127 TP53 mutations in benign patients. The mean number of mutations per sample was 7.4 (range: 1 – 26, Supplementary Table S2).
The number of mutations was correlated with the Duplex depth for each sample (p=0.0007 by Spearman’s rank correlation test, Supplementary Fig. S2). To account for that association, samples were compared based on mutation frequency, which is calculated as the number of mutations divided by the total number of nucleotides sequenced. TP53 mutation frequency was highly variable across patients and it was not significantly associated with age in benign or cancer patients when tested using Spearman’s rank test (Supplementary Fig. S3, p=0.51 and p=0.41 respectively). However, because lower sequencing depth results in a lower probability to find mutations, we also used a quasibinomial model that considered different sequencing depth across individuals. The quasibinomial model revealed near-significant association between TP53 mutation frequency and age in patients without cancer but not in patients with cancer (Supplementary Fig. S3, p=0.06 and p=0.42 respectively).
Cancer-like low-frequency TP53 mutations are more frequent in women with ovarian cancer than in women without cancer
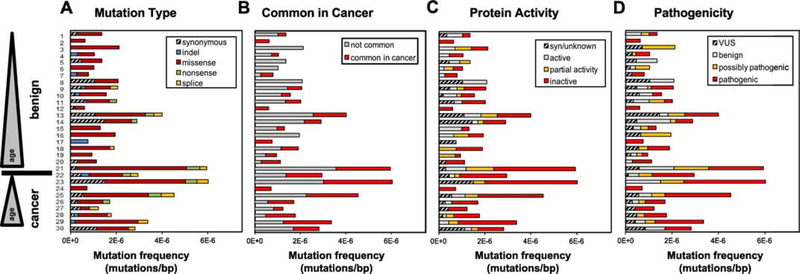
We next performed a detailed characterization of the TP53 mutations identified in Pap tests. Pap tests from all patients both with and without cancer contained non-synonymous mutations (Fig. 2A) and mutations with functional consequences (Fig. 2B-D). Overall, most mutations observed in the Pap tests were likely to have functional effects: 81.5% were non-synonymous, 68.3% resulted in an inactive or partially active protein in vitro, and 62.4% are predicted to be “possibly” or “likely” pathogenic. Consistent with this pathogenic role, 43% of all low-frequency TP53 mutations identified in Pap tests were reported to be very frequent or frequent in the UMD cancer database, supporting the cancer-like nature of these mutations.
Figure 2. Distribution of TP53 mutations identified in Pap tests from patients with and without HGSC based on (A) mutation type, (B) common appearance in the UMD cancer database, (C) impact on in vitro protein activity, and (D) predicted pathogenicity.
Patient ID is indicated in the Y-axis and patients are sorted by ascending age within each group. For each patient, mutation frequency was calculated as the number of TP53 mutations divided by the total number of Duplex nucleotides sequenced in the TP53 coding region. Syn: synonymous. VUS: variant of unknown significance.
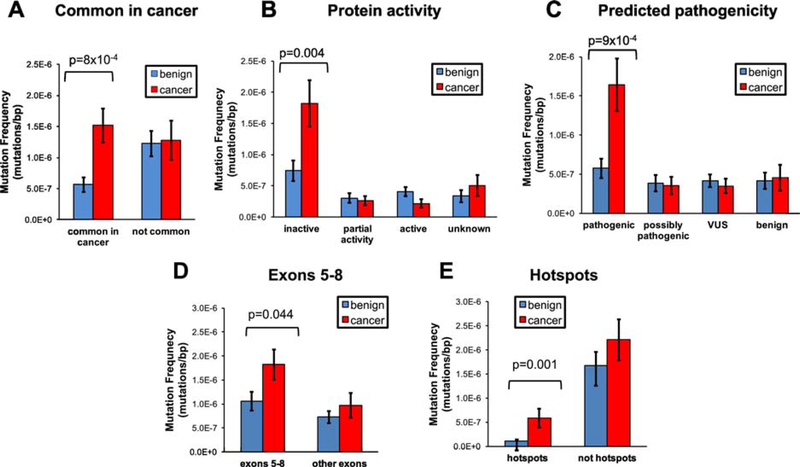
While cancer-like mutations were present in Pap tests from all patients, they appeared to be more abundant in cancer patients. When mutations were grouped according to their presence in the UMD TP53 cancer database, we observed that mutations commonly found in cancers were approximately 3x more abundant in Pap tests from women with HGSC than in Pap tests from women without cancer (Fig. 3A, p=8×10−4). Remarkably, the frequency of mutations infrequent in cancer was similar in both groups. A similar pattern emerged with mutation effect on protein activity and predicted pathogenicity (Fig. 3B and 3C). TP53 mutations that inactivate the protein and are predicted to be pathogenic were approximately 2.7x and 2.9x more frequent, respectively, in Pap tests from women with HGSC than in women without cancer (Fig. 3B, p=0.004 and Fig. 3C, p=9×10−4). Conversely, there was no difference in the mutation frequency of any of the other categories of activity or pathogenicity between both groups of women. In summary, Pap tests from patients with ovarian cancer bear a higher abundance of low-frequency mutations with the most cancer-like characteristics: commonly reported in cancers, inactivating the protein, and likely pathogenic by computational prediction. A sensitivity analysis was performed for the purpose of excluding that the difference in mutation pattern observed could have been a product of the different proportion of germline BRCA mutation carriers in each group. When BRCA mutation carriers were excluded from both HGSC and benign groups and the analysis was repeated, Pap tests from patients with ovarian cancer were still significantly more likely to carry TP53 mutations that were commonly reported in tumors (p=0.011) and likely pathogenic (p=0.018) (Supplementary Fig. S4A and S4C).
Figure 3. Comparison of TP53 mutation frequency in Pap tests from women with and without HGSC based on (A) common appearance in the UMD cancer database, (B) inactivation of protein activity, (C) predicted pathogenicity, (D) location in exons 5–8, and (E) location in hotspot codons.
For each mutation category, the mean of mutation frequency for patients with and without HGSC was compared using t-tests (benign n=21, cancer n=9). Only significant p-values are displayed. Error bars represent standard error of the mean.
Given the functional differences observed between TP53 mutations in Pap tests from women with and without ovarian cancer, we sought to determine whether there was a difference in whether mutations affected gene locations with biological impact. Indeed, we observed that the frequency of TP53 mutations located in exons 5–8 (which encode the protein DNA binding domain) and in hotspot codons (the 9 most commonly mutated codons in the UMD TP53 database) was significantly higher in Pap tests from women with cancer than in those from women cancer-free (p=0.044 and 0.001, respectively, Fig. 3D and Fig. 3E). The association with hotspot codons remained statistically significant when BRCA mutations carriers were excluded (Supplementary Fig. S4E, p=0.027).
The mutational spectrum of TP53 mutations identified in Pap tests from women with and without HGSC was similar, with a predominance of C:G>T:A transitions consistent with mutational signatures in cancer associated with age [25] (Supplementary Fig. S5). These results indicate that while the underlying mechanism causing mutations might be similar in both groups, functional TP53 mutations in women with cancer were more common, as reflected by an enrichment of cancer-like mutations and predominant location in exons 5–8 and hotspot codons.
TP53 mutations in Pap tests are not random and resemble TP53 mutations found in cancers
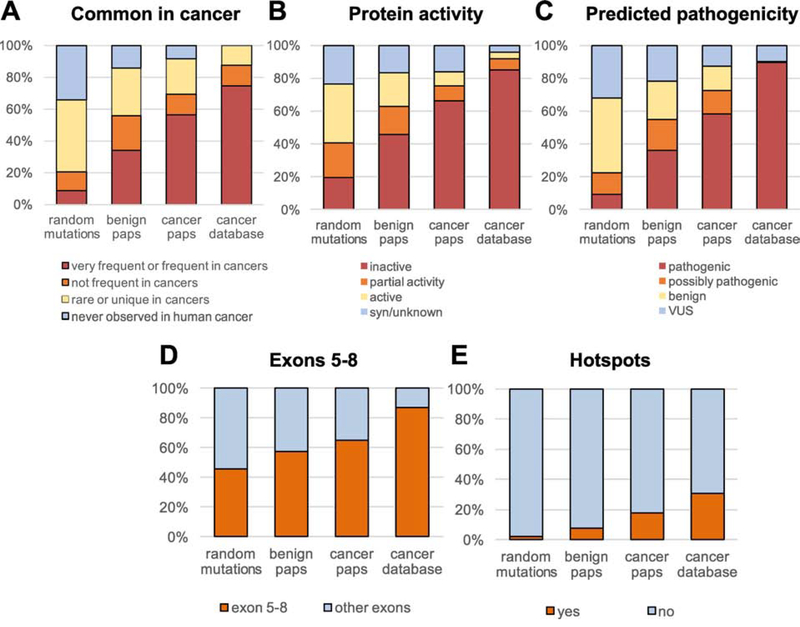
We next compared the features of TP53 mutations in Paps with the features of random TP53 mutations and with TP53 mutations identified in human cancer (Fig. 4). To determine the features of random TP53 mutations, we generated the full set of all possible TP53 mutations by changing in silico each coding nucleotide to the other 3 possible bases. We then cataloged this list of mutations (n=3,546) according to 5 features extracted from Seshat analysis: common in cancer (Fig. 4A), protein activity (Fig. 4B), predicted pathogenicity (Fig. 4C), exons 5–8 location (Fig. 4D), and hotspot location (Fig. 4E). The same features were extracted for the full set of TP53 mutations reported in the UMD cancer database (April 2017, n=71,051). We observed that, for these 5 distinct features, TP53 mutations in Pap tests differed from random mutations and gradually shifted towards the distribution seen with cancer mutations. This shift indicates that TP53 mutations found in Pap DNA are enriched for pathogenic features, especially in women with HGSC. Accordingly, for these 5 features, the percentage of Pap mutations positive for the feature was statistically significantly higher than the expectation from random mutations both in women with and without HGSC, but with highest significance in the former (Supplementary Fig. S6).
Figure 4. Comparison of TP53 mutations identified in Pap tests from women with and without HGSC vs. random TP53 mutations and TP53 mutations in the cancer database.
Mutations were compared based on (A) common appearance in cancers, (B) inactivation of protein activity, (C) predicted pathogenicity, (D) location in exons 5–8, and (E) location in hotspot codons. ‘Random mutations’ includes all possible mutations in the TP53 coding region (n=3,546), benign Paps include 127 mutations, cancer Paps include 94 mutations, and cancer database includes all TP53 mutations identified in human cancers in the April 2017 version of the UMD cancer database (n=71,051).
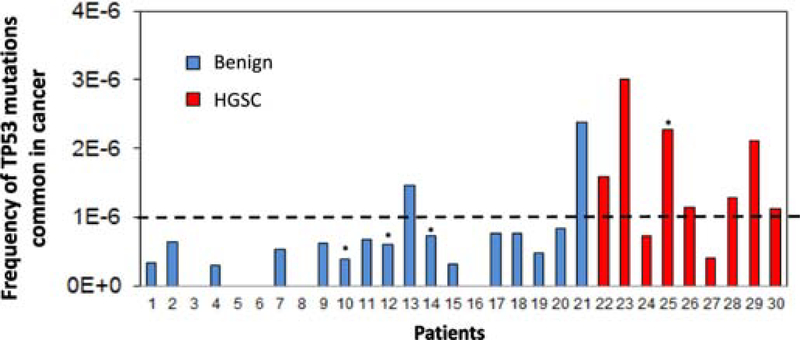
The Pap test frequency of TP53 mutations commonly reported in the UMD cancer database discriminates patients with and without ovarian cancer
As seen in Fig. 3A, patients with HGSC have a higher frequency of mutations that have been commonly reported in the UMD TP53 database. The frequency of common-in-cancer TP53 mutations identified in Pap tests could thus potentially discriminate patients with and without HGSC. In this small study, a threshold of >1×10−6 mutations/bp yield a sensitivity of 78% and specificity of 90% (Fig. 5). Of note, the woman with HGSC and tubal ligation had a high frequency of TP53 mutations common in cancer, indicating that these mutations are not necessarily related to exfoliation from distal fallopian tube epithelium. Because the age range was wider in the group of women without cancer than in the group of women with cancer, we also plotted the data including the regression by age, which was significant for women without cancer (Supplementary Fig. S7, Spearman’s rank correlation p=0.008). We then calculated the sensitivity and specificity for the subset of patients matched by age (range 45 to 75). Sensitivity remained at 78% and specificity increased to 92% because one woman without cancer but with high frequency of mutation was older than 75 years. Upon exclusion of BRCA mutation carriers, sensitivity was 71% and specificity was 100%.
Figure 5. The frequency of Pap test TP53 mutations commonly reported in cancer distinguishes women with and without HGSC.
TP53 mutation frequency included only mutations identified by Seshat as very frequent or frequent in the UMD cancer database. The four patients with prior tubal ligations are indicated with an asterisk.
DISCUSSION
The objective of this study was to use ultra-deep sequencing to improve the sensitivity of the Pap test to detect HGSC and to characterize the landscape of low-frequency somatic TP53 mutations and their potential impact on specificity. We detected the tumor-derived TP53 mutation in 3 of 8 cancer patients with intact fallopian tubes (38%), a sensitivity similar to prior reports [6, 7] despite the fact that we used a sequencing method with higher resolution [9]. This result suggests that the limited sensitivity of the Pap test to detect HGSC mutations is likely due to the absence of neoplastic DNA in the sample and not technical resolution. The resolution of Duplex Sequencing, however, enabled the novel identification of abundant low-frequency TP53 mutations in Pap tests from all women, with and without HGSC. A substantial proportion of these mutations were common in cancer, inactivated protein activity, and were predicted to be pathogenic by computational models. Interestingly, common TP53 cancer mutations were more frequent in Pap tests from women with ovarian cancer than in Pap tests from women without cancer, suggesting an increased presence of potentially pathogenic TP53 mutated clones in association with HGSC. Thus, it is possible that the mutational burden of pathogenic TP53 mutations could serve as a tool in risk assessment, but such an application would require further study with longitudinal follow-up.
The finding of functional low-frequency mutations in Pap tests is consistent with a growing body of literature demonstrating the presence of cancer-like somatic mutations in normal tissues [17]. These mutations were initially studied in blood, where clones with leukemia-associated driver gene mutations (including TP53) were found in otherwise healthy individuals [26–29]. Similar findings of abundant, positively-selected, age-associated, cancer gene mutations have also been reported in normal skin [30], esophagus [31, 32], and several other human tissues [19]. Relevant to gynecology, our group has demonstrated pervasive, positively selected, low-frequency TP53 mutations in peritoneal fluid [12], uterine lavage [18], and normal gynecological tissues [18], and others have demonstrated cancer driver mutations in both normal endometrium and endometriosis [33–35]. Collectively, these findings support the concept of somatic evolution within normal tissue [17], which has important implications for the development of cancer detection tests based on mutational analysis of liquid biopsies, including Pap tests [20]. Because even archetypal cancer “driver” mutations (e.g. in TP53) are not specific to cancer, caution must be taken when interpreting the results from such tests. We and others have targeted TP53, because mutations in this gene are highly prevalent even in intraepithelial tubal neoplasia [16]. However, our results suggest that the simple presence or absence of a TP53 mutation at any mutant allele frequency threshold in a Pap test is unlikely to be a robust biomarker to distinguish women with and without HGSC or precursor lesions. Our study highlights that future “liquid biopsy” studies should approach this challenge of specificity through careful analysis of patients without cancer from the target population for screening.
We have demonstrated, in agreement with prior studies [6, 7], that the Pap test has limited sensitivity for ovarian cancer detection when relying on NGS identification of tumor-derived TP53 mutations. Because we used an extremely sensitive sequencing technology [9] and detected mutations at frequencies <0.001, we have demonstrated this limitation is probably not technical. Most likely, the limited sensitivity of Pap tests is due to the distance between the site of collection (cervical canal) and the fallopian tubes, which are the presumed site of origin for most serous ovarian cancers [16, 36]. This hypothesis is supported by the fact that Tao brushes and uterine lavages, which sample the uterine cavity, both have higher sensitivity for the detection of ovarian cancer cells [7, 37]. Uterine lavage has the highest sensitivity reported so far (80%) [18, 37], which makes it the most promising option as a minimally invasive method for ovarian cancer detection
The most novel and clinically relevant finding from this study is the increased frequency of TP53 cancer-like mutations in Pap tests from women with ovarian cancer compared to cancer-free women. This finding is supported by five mutational features analyzed in the study: frequency in cancer, protein activity, predicted pathogenicity, location in exons 5–8, and location in hotspot codons. The excess of pathogenic mutations in women with ovarian cancer is unlikely to be due to ovarian cancer subclones since no subclonal mutations were identified in any of the primary cancers. In addition, the finding of abundant pathogenic mutations in a woman with HGSC but prior tubal ligation indicates that, in this case, mutated cells could have not derived from the shedding of synchronous pathogenic clones located in the distal fimbria. However, one possibility is for these clones to have disseminated early in carcinogenesis, seeding the lower gynecological tract prior to the ligation of the tubes. An alternative hypothesis is that TP53 mutant clones present in Pap DNA are simply derived from cervical or endometrial epithelium and reflect more advanced somatic evolution in individuals prone to developing cancer. If the latter hypothesis is true, then other non-malignant tissues (especially those that may be sampled non-invasively such as blood or buccal mucosa) may also exhibit an excess of TP53 cancer-like mutations in individuals with or at risk of cancer, enabling new venues for cancer biomarker development. Our analysis suggests that significant differences in TP53 cancer-like mutations still exist while excluding BRCA mutation carriers, but large, properly designed longitudinal studies are needed to validate these results and to determine their clinical utility.
In summary, our study adds further evidence that the sensitivity of the Pap test is likely insufficient for ovarian cancer detection even using highly-accurate and ultra-deep sequencing methods. In addition, the finding of low-frequency TP53 mutations in Pap tests is yet another example of prevalent deleterious mutations in a non-cancerous biopsy. The fact that cancer-like TP53 mutations are more common in women with ovarian cancer is of clinical interest because Pap tests might be utilized in a two-stage approach to ovarian cancer diagnosis [38] (e.g. in which Pap tests with high burden of cancer-like mutations could be an indication to follow up with other diagnostic assays). Beyond the clinical applications, our findings raise questions regarding the cell of origin of these ubiquitous TP53 mutated subclones and the mechanisms that prevent them from malignant transformation. The answers to these questions will provide important insight into refining our understanding of carcinogenesis and will reveal targets for future diagnostics and therapeutics.
Supplementary Material
Highlights.
Tumor-derived TP53 mutations were detected in Pap tests in about one-third of women with ovarian cancer
Low-frequency TP53 mutations were present in Pap tests in all women with and without ovarian cancer
Pap test TP53 mutations resemble TP53 mutations found in cancer
Cancer-like Pap test mutations are more common in women with ovarian cancer
Acknowledgements
We thank Brendan Kohrn and Scott Kennedy for data analysis and computational support. We deeply thank the patients who provided clinical samples, without which this research would not have been possible.
Funding
NIH grant R01CA181308 to RAR; Mary Kay Foundation grant 045–15 to RAR; Rivkin Center for Ovarian Cancer grant 567612 to RAR.
Footnotes
Conflict of Interest Statement
RAR shares equity in NanoString Technologies Inc. and is the principal investigator on a NIH SBIR R44CA221426 subcontract research agreement with TwinStrand Biosciences Inc.
Data and materials availability
Data deposition: Sequencing data from this Paper have been deposited online at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA529688 (BioProject ID: PRJNA529688).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowtell DD et al. (2015) Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 15 (11), 668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL et al. (2019) Cancer statistics, 2019. CA Cancer J Clin 69 (1), 7–34. [DOI] [PubMed] [Google Scholar]
- 3.Diaz LA Jr. and Bardelli A (2014) Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 32 (6), 579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakkum-Gamez J and Dowdy S (2014) Retooling the pap smear for ovarian and endometrial cancer detection. Clin Chem 60 (1), 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSimone CP et al. (2006) Rate of pathology from atypical glandular cell Pap tests classified by the Bethesda 2001 nomenclature. Obstet Gynecol 107 (6), 1285–91. [DOI] [PubMed] [Google Scholar]
- 6.Kinde I et al. (2013) Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med 5 (167), 167ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y et al. (2018) Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med 10 (433). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinde I et al. (2011) Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A 108 (23), 9530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salk JJ et al. (2018) Enhancing the accuracy of next-generation sequencing for detecting rare and subclonal mutations. Nat Rev Genet 19 (5), 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy SR et al. (2014) Detecting ultralow-frequency mutations by Duplex Sequencing. Nat Protoc 9 (11), 2586–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt MW et al. (2012) Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci U S A 109 (36), 14508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krimmel JD et al. (2016) Ultra-deep sequencing detects ovarian cancer cells in peritoneal fluid and reveals somatic TP53 mutations in noncancerous tissues. Proc Natl Acad Sci U S A 113 (21), 6005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed AA et al. (2010) Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol 221 (1), 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.TCGA (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474 (7353), 609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vang R et al. (2016) Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma: A Rereview of Cases Lacking TP53 Mutations in The Cancer Genome Atlas Ovarian Study. Int J Gynecol Pathol 35 (1), 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn E et al. (2012) TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol 226 (3), 421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risques RA and Kennedy SR (2018) Aging and the rise of somatic cancer-associated mutations in normal tissues. PLoS Genet 14 (1), e1007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salk JJ et al. (2019) Ultra-Sensitive TP53 Sequencing for Cancer Detection Reveals Progressive Clonal Selection in Normal Tissue over a Century of Human Lifespan. Cell Rep 28 (1), 132–144 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yizhak K et al. (2019) RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 364 (6444). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy SR et al. , Cancer-associated mutations but no cancer: insights into the early steps of carcinogenesis and implications for early cancer detection, Trends in Cancer 5 (9) (2019) 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh T et al. (2011) Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 108 (44), 18032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennington KP et al. (2014) Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20 (3), 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nachmanson D et al. (2018) Targeted genome fragmentation with CRISPR/Cas9 enables fast and efficient enrichment of small genomic regions and ultra-accurate sequencing with low DNA input (CRISPR-DS). Genome Res 28 (10), 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tikkanen T et al. (2018) Seshat: A Web service for accurate annotation, validation, and analysis of TP53 variants generated by conventional and next-generation sequencing. Hum Mutat. 39 (7), 925–933. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov LB et al. (2013) Signatures of mutational processes in human cancer. Nature 500 (7463), 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genovese G et al. (2014) Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371 (26), 2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaiswal S et al. (2014) Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371 (26), 2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie M et al. (2014) Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 20 (12), 1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young AL et al. (2016) Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun 7, 12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martincorena I et al. (2015) Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348 (6237), 880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martincorena I et al. (2018) Somatic mutant clones colonize the human esophagus with age. Science 362 (6417), 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama A et al. (2019) Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565 (7739), 312–317. [DOI] [PubMed] [Google Scholar]
- 33.Anglesio MS et al. , Cancer-associated mutations in endometriosis without cancer, N Engl J Med. 376 (19) (2017) 1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair N et al. (2016) Genomic Analysis of Uterine Lavage Fluid Detects Early Endometrial Cancers and Reveals a Prevalent Landscape of Driver Mutations in Women without Histopathologic Evidence of Cancer: A Prospective Cross-Sectional Study. PLoS Med 13 (12), e1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suda K et al. (2018) Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep 24 (7), 1777–1789. [DOI] [PubMed] [Google Scholar]
- 36.Kurman RJ and Shih Ie M (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34 (3), 433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maritschnegg E et al. (2015) Lavage of the Uterine Cavity for Molecular Detection of Mullerian Duct Carcinomas: A Proof-of-Concept Study. J Clin Oncol. 33 (36), 4293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu KH (2018) Screening for Ovarian Cancer in Asymptomatic Women. JAMA 319 (6), 557–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.