Oxytocin, a nonapeptide hormone, has a key role in female reproductive functions as well as in social memory in the brain. In our recent Communications Biology article, we reported that oxytocin is transported from the peripheral blood into the brain by the receptor for advanced glycation end-products (RAGE) in endothelial cells at the blood−brain barrier. Additionally, we found that oral oxytocin is absorbed by RAGE on intestinal epithelial cells at the blood−intestinal barrier. From a physiological perspective, we herein outline the continuing research regarding oxytocin and social behaviour.
Yamamoto and Higashido discuss the possible routes of the hormone oxytocin in the body, and highlight their recent study in Communications Biology where they showed that the RAGE receptor is a transporter for oxytocin across the blood−brain barrier.
Oxytocin in female reproduction and beyond
Oxytocin is primarily synthesized in the oxytocinergic neurons in the hypothalamus within the brain1,2. Oxytocin in brain neurons is secreted somato-axono-dendritically into the brain3,4 and released from the nerve terminals in the posterior pituitary into the circulation1–4. Within the past decade, oxytocin in the brain has attracted increasing attention because of the importance of oxytocin in species-specific social memory functioning5–7. Oxytocin in the blood circulation has a primary hormonal role in female reproduction but was not thought to re-enter the brain. Recently, however, based on the practical nasal administration of large doses of oxytocin to humans with and without social deficit-related psychiatric disorders, such as autism spectrum disorders and schizophrenia8,9, oxytocin has been thought to cross the blood−brain barrier (BBB)3,10. However, there is little or no direct evidence for this transport process, with the exception of two reports that examined human cerebrospinal fluid (CSF) and microdialysates from the mouse and rat hippocampus and amygdala11,12. They found that peak levels of oxytocin were observed 30–60 min after the nasal administration of oxytocin in three species12,13, which is confirmed in our study as well14. Furthermore, if oxytocin is indeed transferred from the blood to the brain, the underlying molecular mechanism is unclear.
How oxytocin crosses the blood−brain barrier
The transport of peptides across the BBB requires specific or nonspecific interactions with proteins or receptors that are expressed on the luminal and/or abluminal surfaces of brain capillary cells15. Endocytosis and transcytosis play important roles in uni- and bidirectional transport across the BBB. Therefore, it was necessary to hypothesize that oxytocin transfer across the BBB is mediated by a binding protein15. We considered that such a protein should meet the following criteria: it should exist on brain microvessel endothelial cells; it should have multiligand binding capacity (non-oxytocin-specific receptors exist, for example in the uterus); and its binding to oxytocin should not be popularly known.
Regarding possible candidates, we suspected the receptor for advanced glycation end-products (receptor for AGEs, RAGE), which belongs to the immunoglobulin superfamily and is involved in a variety of diseases related to inflammation and blood vessel impediments caused by diabetes16–22. Membrane-bound full-length RAGE (mRAGE) is expressed on the cell surfaces of the endothelial cells of various organs, including the endothelial cells of neurovascular units23. Additionally, plasma contains the soluble form of RAGE (sRAGE), which is composed of endogenous soluble RAGE (esRAGE), an alternative splicing product of the AGER gene and an ectodomain-shedded form of mRAGE20. These different forms of RAGE possess identical multiple ligand binding properties for AGE, high mobility group box 1 (HMGB1), S100 proteins, lipopolysaccharides, and soluble amyloid-beta peptide18,19 but have never been demonstrated to bind to physiological ligands, such as oxytocin. The interaction of β-amyloid and RAGE results in its transport across the BBB23. Thus, RAGE seems to fulfill the aforementioned criteria, and we hypothesized that RAGE is a candidate protein for the mediation of oxytocin transfer from the blood to the brain.
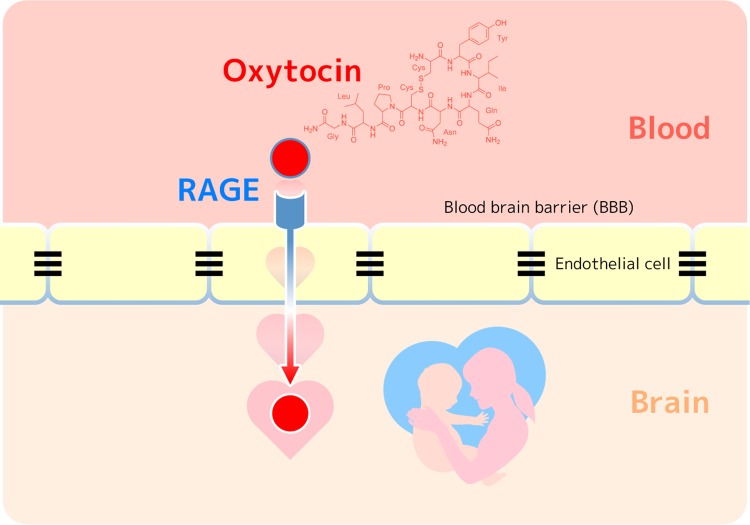
Indeed, we demonstrated that RAGE is the oxytocin’s binding protein using the plasmon resonance method and that it is a transporter of oxytocin primarily from the blood to the brain using reconstituted BBB in culture14. Interestingly, the transfer is rather unidirectional; the blood to brain direction is 5–10-fold more efficient than transfer in the reverse direction (brain to blood). It has only recently been proposed that blood oxytocin re-enters the brain (Fig. 1)14.
Fig. 1. RAGE-dependent oxytocin transport from the blood into the brain via the blood−brain barrier (BBB).
Oxytocin is a peptide hormone released by the posterior pituitary and plays important roles in maternal behaviors and social bonding. Endothelial RAGE is an essential molecule existing at the BBB for the oxytocin transfer and the functions.
Possible routes and functions of oxytocin
Although oxytocin receptors have been identified in numerous brain regions24 and occasionally in remote brain areas far from the oxytocin synthesizing neurons in the hypothalamus2,24, oxytocin transmission is believed to occur either via release from collateral axons into the amygdala or nucleus accumbens or via diffusion processes through extracellular spaces in a process known as volume transmission2. Interestingly, our finding regarding RAGE suggests a third possibility, in that oxytocin is able to function in brain after transport from the blood (Fig. 1)14. This system might allow the transfer of oxytocin’s peripheral information to the central nervous system during labor, lactation, or social interactions with or without stress.
More characteristically, the binding of oxytocin as a ligand to RAGE did not induce the activation of intracellular signaling, which contrasts with the binding of AGEs, S100, HMGB1, and amyloid β14. The lack of activation of cellular responses by oxytocin strongly supports the role of RAGE over other cell remodeling activities in pathological conditions such as diabetes, renal or retinal vessel dysfunction, and Alzheimer’s disease16–23.
The presence of RAGE in the caveolae of the hippocampus and choroid plexus suggests that RAGE might be a carrier of oxytocin in vesicles (endocytosis) or for transcytosis in a manner similar to that suggested for Mfsd2a and omega-3 fatty acids25. However, it is not clear at the cellular level how RAGE transports oxytocin into the brain.
RAGE seems to play a role not only in the BBB, but also in the blood−CSF barrier because RAGE is found in the choroid plexus14. The transport of oxytocin to the CSF might thus be mediated via the choroid plexus, which could be one of the reasons that the intranasal administration of oxytocin is effective in the central delivery of oxytocin and results in beneficial effects on impaired social interaction behavior6,8. We suggest that the circulation route for the rapid transfer of oxytocin following intranasal administration is via a specific nasal structure and that it passes through intercellular clefts in the olfactory epithelium to diffuse into the subarachnoid space thus reaching the choroid plexus26. However, even if this is the case, RAGE might play an important role.
In human neurovascular units, RAGE is confirmed to be present in vascular endothelial cells in the human brain27. Therefore, since the physiological properties of RAGE do not differ between human and mice, it is reasonable to hypothesize that RAGE contributes to the transport of plasma oxytocin to the brain in humans. Further, oxytocin transfer to the brain is increased when RAGE expression is enhanced by transient brain ischemia14. This suggests that oxytocin plays an important role in the wound-healing process and/or stress after brain ischemic attack
Human plasma oxytocin has an affinity to esRAGE in the affinity column14. This suggests that esRAGE may be a binding protein in the circulation. When human plasma is pretreated to remove plasma proteins, the remaining concentration of free oxytocin in the plasma is estimated to be extremely low (<10 pg/ml, below the detectable level), suggesting that oxytocin is present in a protein-binding form. Although such binding proteins have not yet been identified in human plasma, it is highly possible that this protein could be esRAGE. This point should be further examined. Interestingly, it is also found that circulating esRAGE is transported into the brain through the BBB by mRAGE on endothelial cells, potentially contributing to the oxytocin transfer into the brain28.
RAGE-mediated oxytocin transport from the intestinal tract into the blood circulation across the intestinal barrier was also found to occur in mice29. This RAGE-dependent intestinal permeability was demonstrated following the oral administration of oxytocin in mouse pups at the very narrow window of postnatal days 4–6, during which the barrier will be formed but still digestion is not active. The blood oxytocin levels of the mice were high at approximately 1–3 days after birth, likely due to nonspecific bulk absorption, and no uptake of oxytocin was observed after 7 days when the same concentration of oxytocin was orally applied29. Although it was previously reported that oxytocin is absorbed in neonates and infants, our results show that oxytocin transport across the intestinal barrier is RAGE-dependent29.
Oxytocin absorption via the intestine was more difficult to detect in adults. However, when we used a tenfold higher concentration for gavage, the uptake was visible29. Oxytocin seems to be somewhat resistant to digestion. This result suggests that novel delivery methods for oral oxytocin supplementation may be advantageous for the treatment of a range of conditions, such as impaired social development in preterm infants who are at risk of autism spectrum disorder, and obesity or diabetes in adults19.
Outlook
Future directions for currently unsolved questions include answering questions about whether oxytocin transport is increased in the excess of sRAGE or esRAGE via mRAGE. We would also like to know what extent does RAGE-dependent oxytocin transport rely on other transport means, such as the direct intranasal pathway or trigeminal nerve transport26. It will be important to follow up results on additional oxytocin binding proteins in plasma. The molecular weight of sRAGE/esRAGE is approximately 50 kDa. However, it is known that there is an oxytocin binding protein with a molecular weight of 10 kDa30. It has frequently been demonstrated that no oxytocin transport to the brain occurs in rats and it would be interesting to know if this could be due to lack of RAGE function. Another question is whether oxytocin recruited in cortical regions that are far from the hypothalamus with no axon collaterals from oxytocinergic neurons2. With regards to behavior, there is one hypothesis that personal differences in social behavior in humans depends on plasma oxytocin levels. Given our data, it is reasonable to ask whether molecular variants or single nucleotide polymorphisms of the AGER gene coding RAGE contribute to this phenomenon. Further, can orally administered oxytocin reach the brain? This is particularly relevant for oxytocin in milk. We also need to determine the precise bioavailability of oxytocin from the intestine and the blood. Several questions arise about oxytocin movement. Can oxytocin enter the lymph and intraperitoneal space, and tight junctions? Can oxytocin be absorbed from mouse epithelial cells and esophageal epithelial cells in a RAGE-dependent fashion across epithelial barriers? Overall, it will be important to determine how oxytocin is transported intracellularly, transcellularly and intercellularly25.
To summarize, although we need to determine the pharmacokinetics of oxytocin, our data present a better understanding of how exogenous oxytocin from the intestine or blood can influence the brain targets of oxytocin, which results in social behavioral improvement in humans with or without social impairments.
Acknowledgements
We acknowledge financial support from Kanazawa University SAKIGAKE project 2018 and the Strategic Research Program for Brain Sciences from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Grants-in-Aid for Scientific Research (24590375, 25461335 and 18K06889) and the Program for Fostering Globally Talented Researchers from Japan Society for Promotion of Sciences.
Author contributions
Y.Y. and H.H. wrote the draft. All authors approved the final submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Castel M, Gainer H, Dellmann HD. Neuronal secretory systems. Int. Rev. Cytol. 1984;88:303–459. doi: 10.1016/S0074-7696(08)62760-6. [DOI] [PubMed] [Google Scholar]
- 2.Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatry. 2016;79:155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Jin D, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 4.Higashida H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J. Physiol. Sci. 2016;66:275–282. doi: 10.1007/s12576-015-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashida H, et al. CD38, CD157, and RAGE as molecular determinants for social behavior. Cells. 2020;9:62. doi: 10.3390/cells9010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashida H, et al. Social interaction improved by oxytocin in the subclass of autism with comorbid intellectual disabilities. Diseases. 2019;7:24. doi: 10.3390/diseases7010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horta M, Kaylor K, Feifel D, Ebner NC. Chronic oxytocin administration as a tool for investigation and treatment: a cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 2020;108:1–23. doi: 10.1016/j.neubiorev.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ermisch A, et al. On the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol. Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- 11.Lefevre A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci. Rep. 2017;7:17222. doi: 10.1038/s41598-017-17674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019;2:76. doi: 10.1038/s42003-019-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks WA. The blood-brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 2019;15:444–455. doi: 10.1038/s41574-019-0213-7. [DOI] [PubMed] [Google Scholar]
- 16.Rabbani N, Thornalley PJ. Hexokinase-2 glycolytic overload in diabetes and ischemia-reperfusion injury. Trends Endocrinol. Metab. 2019;30:419–431. doi: 10.1016/j.tem.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Senatus LM, Schmidt AM. The AGE-RAGE axis: implications for age-associated arterial diseases. Front. Genet. 2017;8:187. doi: 10.3389/fgene.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLean M, et al. The receptor for advanced glycation end products (RAGE) and DIAPH1: implications for vascular and neuroinflammatory dysfunction in disorders of the central nervous system. Neurochem. Int. 2019;126:154–164. doi: 10.1016/j.neuint.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Yamamoto H. RAGE-mediated inflammation, type 2 diabetes, and diabetic vascular complication. Front. Endocrinol. 2013;4:105. doi: 10.3389/fendo.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonekura H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003;370:1097–1109. doi: 10.1042/bj20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto Y, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J. Clin. Invest. 2001;108:261–268. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myint KM, et al. RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes. 2006;55:2510–2522. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- 23.Candela P, et al. Apical-to-basolateral transport of amyloid-β peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J. Alzheimers Dis. 2010;22:849–859. doi: 10.3233/JAD-2010-100462. [DOI] [PubMed] [Google Scholar]
- 24.Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98:1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 25.Andreone BJ, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94:581–594. doi: 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintana DS, Alvares GA, Hickie IB, Guastella AJ. Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neurosci. Biobehav. Rev. 2015;49:182–192. doi: 10.1016/j.neubiorev.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C, et al. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Mod. Pathol. 2005;18:1385–1396. doi: 10.1038/modpathol.3800450. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu, Y. et al. Neuroprotective effects of endogenous secretory receptor for advanced glycation end-products in brain ischemia. Aging Dis. in press, 10.14336/AD.2019.0715 (2020). [DOI] [PMC free article] [PubMed]
- 29.Higashida H, et al. Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice. Sci. Rep. 2017;7:7883. doi: 10.1038/s41598-017-07949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szeto A, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]