Abstract
Acute kidney injury (AKI) is defined as a rapid decline in renal function and is characterized by excessive renal inflammation and programmed death of resident cells. AKI shows high morbidity and mortality, and severe or repeated AKI can transition to chronic kidney disease (CKD) or even end-stage renal disease (ESRD); however, very few effective and specific therapies are available, except for supportive treatment. Growth factors, such as epidermal growth factor (EGF), insulin-like growth factor (IGF), and transforming growth factor-β (TGF-β), are significantly altered in AKI models and have been suggested to play critical roles in the repair process of AKI because of their roles in cell regeneration and renal repair. In recent years, a series of studies have shown evidence that growth factors, receptors, and downstream effectors may be highly involved in the mechanism of AKI and may function in the early stage of AKI in response to stimuli by regulating inflammation and programmed cell death. Moreover, certain growth factors or correlated proteins act as biomarkers for AKI due to their sensitivity and specificity. Furthermore, growth factors originating from mesenchymal stem cells (MSCs) via paracrine signaling or extracellular vesicles recruit leukocytes or repair intrinsic cells and may participate in AKI repair or the AKI-CKD transition. In addition, growth factor-modified MSCs show superior therapeutic potential compared to that of unmodified controls. In this review, we summarized the current therapeutic and diagnostic strategies targeting growth factors to treat AKI in clinical trials. We also evaluated the possibilities of other growth factor-correlated molecules as therapeutic targets in the treatment of AKI and the AKI-CKD transition.
Subject terms: Predictive markers, Prognostic markers
Introduction
Acute kidney injury (AKI) is a clinical syndrome with acute renal dysfunction. The major causes of AKI include ischemic reperfusion, drug toxicity, and sepsis.1 The common pathological feature of AKI is damage to tubular epithelial cells (TECs), accompanied by endothelial damage and accumulation of inflammatory cells.2–4 AKI shows high morbidity and mortality, and severe or repeated AKI may progress to chronic kidney disease (CKD) or even end-stage renal disease (ESRD).5 Unfortunately, effective and specific therapies are unavailable, except for supportive management.1,6,7
In the last century, growth factors such as epidermal growth factor (EGF), insulin-like growth factor (IGF), and fibroblast growth factor (FGF) have been widely investigated as an interesting research area since they are significantly dysregulated and dysfunctional in different AKI models8 (Table 1). Evidence has shown that the administration of these growth factors promotes renal repair and restores renal function in animals; however, treatment with growth factors has not been used clinically.9 With the rapid progress in research technology, growth factors, receptors, and downstream effectors have been found to be highly involved in the mechanism of AKI, including the regulation of inflammation, programmed cell death, necrosis, cell proliferation, and dedifferentiation.10 Moreover, certain growth factors or correlated proteins, such as IGF binding protein (IGFBP)-7 and FGF-23, can serve as biomarkers for AKI due to their sensitivity and specificity.11 Paracrine or extracellular vesicle-delivered growth factors, such as hepatocyte growth factor (HGF) or vascular endothelial growth factor (VEGF), are major mechanisms by which mesenchymal stem cells (MSCs) exert therapeutic effects on renal injury. Growth factor-modified MSCs show superior therapeutic effects in AKI treatment.12 Therefore, the current review focused on summarizing the use of various growth factors as biomarkers for predicting AKI and interpreting their functions and the mechanisms underlying their roles in both renal injury and renal repair in AKI. We also evaluated the current growth factor-targeted therapy or diagnosis in clinical trials and analyzed the limitations of growth factors in clinical treatment. These findings may add new information to the search for a target and prediction of AKI and AKI-CKD progression.
Table 1.
Growth factors may contribute to different types of AKI.
| Model of AKI | Growth factor |
|---|---|
| Ischemia-reperfusion Injury |
BMP-7/EGF/FGF-2/HGF/ IGF-1/TGF-β1/VEGF/PDGF |
| Folic acid-induced AKI |
BMP-7/EGF/FGF-23/HGF/ TGF-β1/VEGF/IGF-1 |
| Cisplatin-induced AKI |
BMP-7/TGF-β1/VEGF/ FGF-21/FGF-10/EGF/IGF-1/HGF |
| Lipopolysaccharide-induced AKI | BMP-7/FGF-2/TGF-β1/HGF/EGF |
| Mercuric chloride-induced AKI | EGF/IGF-1 |
| Glycerol-induced AKI | HGF/TGF-β1 |
| Colistin-induced AKI | TGF-β1/EGF |
| Gentamicin-induced AKI | IGF-1/TGF-β1/EGF/PDGF/VEGF |
Growth factors in AKI
Bone morphogenetic proteins in AKI
Bone morphogenetic proteins (BMPs) are conserved signaling molecules that belong to the transforming growth factor-β1 (TGF-β) superfamily. Structurally, BMPs and some TGF-β family members act as monomeric prepro-forms, including signal sequences, long latency-associated peptides (LAPs), and mature cytokines. These precursor dimers are cleaved by an enzyme at R-X-X-R proteolytic processing sites, which release the biologically active domain. There is the highest degree of similarity (~40–70%) at the carboxy-terminal regions among mature peptides that are the biologically active form of BMP-7 and TGF-β1.13 To date, no less than fifteen BMPs have been identified.14 Recently, more attention has been focused on BMP-7, which is also known as osteogenic protein-1 (OP-1), for its protective role in acute and chronic kidney diseases. In the adult kidney, BMP-7 was detected specifically in the collecting tubule, the thick ascending limb, and podocytes15 (Fig. 1); however, BMP-7 expression is significantly reduced in different kidney diseases, including AKI.16
Fig. 1. Localization of growth factor expression in the kidney.
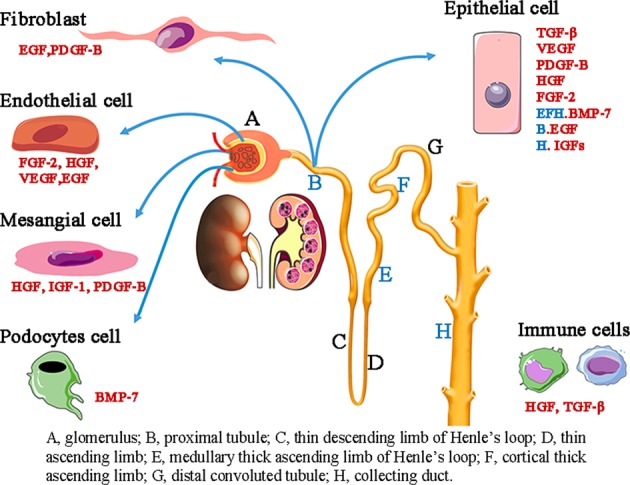
In the glomerulus, endothelial cells mainly secrete FGF-2, HGF, and VEGF, mesangial cells secrete HGF, IGF-1, and PDGF-B, and podocytes are the major source of BMP-7. In renal tubules, growth factors are primarily expressed in fibroblasts and epithelial cells. Fibroblasts express and secrete EGF and PDGF-B, and epithelial cells secrete TGF-β, VEGF, PDGF-B, HGF, and FGF-2. Specifically, BMP-7 is only detected in thick ascending limb and collecting duct epithelial cells. EGF is expressed in the proximal tubule, and IGFs are secreted in the collecting duct. Infiltrating inflammatory cells, such as macrophages, are the key source of HGF, TGF-β, and PDGF-B.
The first evidence regarding the protective role of BMP-7 in AKI was found in a study that indicated that OP-1 injection preserved kidney function and increased the survival rate after ischemic AKI through several mechanisms. These mechanisms included reducing apoptosis and necrosis of tubular epithelial cells, suppressing inflammation by limiting neutrophil infiltration and the level of intercellular adhesive molecules, and maintaining the vascular smooth muscle cell phenotype in pericellular capillaries.17 The anti-inflammatory effect of BMP-7 was also highlighted in another study that indicated that BMP-7 regulated the expression of chemokines, cytokines, and hemodynamic genes (vasoactive genes) in proximal tubule cells.15 By generating tubular-specific BMP receptor 1A knockout mice, a recent study showed that BMP-7/Smad1/5/8 signaling accelerated tubular regeneration by targeting the inhibition of DNA-binding (ID) proteins (Id1, Id2, and Id4), thereby mediating recovery after AKI and preventing fibrosis.18
Evidence shows that BMP-7 acts as a key target in the pathological process of AKI. By modifying ligand-receptor interactions to enhance BMP-7 and suppress TGF-β signaling, Kielin/chordin-like protein (KCP) is capable of halting folic acid-induced AKI by decreasing mortality while enhancing the recovery of renal function.19 Signal peptide-CUB epithelial growth factor domain-containing protein 1 (SCUBE1) directly binds to the BMP-7 ligand and stimulates Smad1/5/8 phosphorylation, thereby accelerating tubular cell proliferation and re-epithelization after renal ischemia-reperfusion injury (IRI).20 Knockout of uterine sensitization-associated gene-1 (USAG)-1, the most abundant BMP antagonist in the kidney, significantly prolonged survival, and preserved renal function in the AKI model, whereas the administration of neutralizing antibodies against BMP-7 abrogated the renoprotective effect of USAG-1 deficiency, further indicating that BMPs are promising therapeutic targets in AKI treatment.21 In addition, MyoR inhibits cisplatin-induced apoptosis and deterioration of renal function by targeting BMP-7.22 Propofol, a sedative, suppresses oxidative stress in sepsis-induced AKI by upregulating BMP-7.23 Additionally, epigenetic modification of BMP-7 plays a critical role in AKI progression. In AKI models induced by both ischemic reperfusion and sepsis, dexmedetomidine (DEX), an α (2)-adrenoceptor (α(2)-AR) agonist, protects against renal injury by restoring BMP-7 levels via a histone deacetylase 5 (HDAC5)-dependent mechanism.24,25 Furthermore, we recently showed that the HDAC inhibitors trichostatin A (TSA) or valproic acid (VPA) attenuated cisplatin-induced renal tubular epithelial cell apoptosis by restoring BMP-7 expression via targeting HDAC2.26
The protective effect of BMPs in AKI is also attributed to their protective role in endothelial cells. A study found that BMP-5 promoted the migration and survival of early endothelial outgrowth cells (eEOCs), thereby improving renal function in the short term.27 Collectively, the therapeutic effect of BMP-7 may be due to its anti-inflammatory, antiapoptotic, and proliferative effects. However, the function of other members of the BMP family in AKI is still unknown and needs to be further determined.
EGF and the EGF receptor in AKI
The EGF-related peptide growth factor family consists of groups of ligands. The first group includes EGF, transforming growth factor-α (TGF-α), and amphiregulin. These factors work by specifically binding to EGF receptor (EGFR). Members of the second group, including heparin-binding EGF (HB-EGF) and betacellulin, bind to both EGFR and ErbB4.28 Activation of EGF/EGFR triggers downstream intracellular pathways, including MAP kinase, JAK/STAT, and PI3K/AKT, to control cell apoptosis, proliferation, and differentiation.
In the kidney, EGF is highly expressed in renal proximal tubule epithelial cells (RPTCs) and transiently decreases after IRI29 (Fig. 1). Clinical evidence shows that urinary human EGF (hEGF) levels are largely downregulated in patients with AKI compared to those of control subjects.30 A study showed that the administration of exogenous EGF increased the DNA replication and recovery of renal function in IRI.31 EGF also attenuates mercuric chloride (HgCl2)-induced tubular necrosis by stimulating the regeneration of resident cells rather than bone marrow-derived cells.32
EGFR function has attracted more attention in recent years. EGFR is widely expressed in mammalian kidneys, with high levels of expression in RPTCs and interstitial fibroblasts. It is a transmembrane protein with intrinsic tyrosine kinase activity and can be activated by several ligands, such as EGF, TGF-β1 and IGF.28,33 Activation of EGFR can be detected 5–30 min after reperfusion, accompanied by generation of superoxide anion/hydrogen peroxide and a reduction in EGF. This finding indicates that early activation of EGFR may not be EGF-dependent.29 Functional studies showed that conditional deletion of EGFR from RPTCs or treatment with an EGFR tyrosine kinase inhibitor (erlotinib) delayed renal function recovery on day 6 after IRI, but activation of EGFR with exogenous EGF or HB-EGF accelerated renal repair.34 Furthermore, a recent study identified that EGFR promoted the dedifferentiation and proliferation of surviving RPTCs by activating Yes-associated protein (YAP) and transcriptional coactivator with PDZ binding motif (TAZ).35 Strikingly, deletion of ErbB4, a type I transmembrane receptor tyrosine kinase of the EGFR superfamily, accelerated cell proliferation and unbalanced cell apoptosis, which was related to the activation of YAP, resulting in renal function deterioration and fibrosis following ischemic injury. This finding was further confirmed in other renal fibrosis models, such as polycystic kidney disease and UUO nephropathy.36,37 As mentioned previously, activating EGF/EGFR signaling appears to be a promising strategy for treating AKI and recovery after AKI.38 However, it is noteworthy that sustained activation of EGFR is associated with cell cycle arrest at the G2/M phase, leading to renal fibrogenesis after AKI.39,40 Therefore, exogenous EGF or HB-EGF may not be suitable for long-term treatment. Consistently, functional inactivation of EGFR by overexpression of dominant-negative EGFR in RPTCs decreases tubulointerstitial lesions after renal injury.41 These findings indicate that EGFR may function as a double-edged sword by regulating both repair and fibrosis, which may be determined by the degree and duration of EGFR activation in response to renal injury.39
FGF in AKI
Mammalian fibroblast growth factor signaling involves interactions between 18 FGF ligands and 4 FGF receptors (FGFR1–4).42 Some FGFs, such as FGF-2 and FGF-23, play specific roles in mediating or predicting AKI.
FGF-2, also called basic fibroblast growth factor (bFGF), is a well-characterized survival factor for both endothelial cells and epithelial cells43 (Fig. 1). Administration of bFGF induces an early repair process after ischemic AKI by inducing various morphogens that are involved in renal repair, such as FGF-2 itself, HGF, BMP-7 and VEGF.44,45 This observation was further confirmed by a recent study that found that FGF-2 protected against mitochondrial damage and the HMGB1-mediated inflammatory response induced by IRI.46 However, the function of FGF-2 is still controversial. Other studies have shown that increased circulating FGF-2 levels fails to improve the outcome of lipopolysaccharide (LPS)-induced AKI but leads to further renal damage because circulating FGF-2 may predispose endothelial cells to undergo apoptosis in response to LPS or induce inflammatory changes.43 This was further confirmed by high serum FGF-2 levels in children with sepsis who were at a high risk of developing AKI. This discrepancy may be explained by the difference in AKI insults.
As a novel predictive and prognostic biomarker for AKI, FGF-23 has recently been widely investigated in different types of animal models and AKI patients.11 FGF-23, induced by multiple factors such as IRI, folic acid, and rhabdomyolysis, is significantly increased in AKI patients and murine models.11,47,48 Clinical evidence confirmed that FGF-23 levels significantly increase in infants, children, adults, and older individuals suffering AKI.49–53 FGF-23 is not only an early prognostic marker for cardiac surgery-associated AKI and intensive care unit (ICU)-associated AKI but also serves as a prognostic marker for adverse outcomes in patients with established AKI.11 Several studies have revealed the mechanisms underlying the upregulation of FGF-23 in AKI. A recent study showed that hyper-IL-6 (HIL-6) activates the FGF-23 promoter by STAT3 phosphorylation and increases circulating FGF-23 in both AKI and CKD.54 Activation of FGF receptor 1 (FGFR1) further increases FGF-23 synthesis in folic acid-induced AKI.55 Moreover, decreased FGF-23 clearance in AKI also contributes to high circulating levels of FGF-23.11 However, whether FGF-23 plays a functional role in mediating AKI is an important topic and remains to be explored.
The participation of other FGF ligands or receptors has also been determined. Evidence shows that inhibiting nitric oxide synthase with Nw-nitro-l-arginine (L-NNA) abolishes the suppressive effects of FGF-1 on neutrophil infiltration, indicating that nitric oxide may be involved in the anti-inflammatory effects of FGF-1.56 FGF-10 works by binding to the high-affinity receptor FGFR2-IIIb splicing isoform and protects against kidney IRI by inhibiting excessive autophagy and the inflammatory response.57 FGF-21, a key regulator of the energy metabolic balance and cell stress responses, is induced in cisplatin nephropathy. FGF-21 knockdown accelerates cisplatin-induced tubular cell injury via p53-dependent mechanisms, but this effect is attenuated by supplementation with recombinant FGF-21.58 Additionally, a study showed that bFGFR2 knockdown prevented the repair process and induced a fibrotic response after ischemic injury, indicating the therapeutic potential of bFGFR2 in AKI.59
Hepatocyte growth factor and c-met in AKI
HGF was originally isolated as a potent mitogen for hepatocytes that binds to the c-met receptor and stimulates its transactivation. HGF exerts multiple effects on tubular repair and regeneration in the kidney.60–62 Epithelial cells, mesangial cells, endothelial cells, and macrophages are the major origins of renal HGF62 (Fig. 1). In the early phase of AKI, HGF, and c-met mRNA significantly increase; however, the total protein level of HGF in the kidney is downregulated 24 h post injury.60 Evidence shows that previous partial hepatectomy-induced HGF overexpression attenuates tubular apoptosis and necrosis.63 Furthermore, HGF gene therapy reduces renal failure and mortality by attenuating tubulointerstitial damage, proinflammatory cytokine production, necrosis, and hemodynamic deterioration.64,65 Previous studies showed that human umbilical cord-derived MSC (hucMSC) transplantation improved renal function in ischemia/reperfusion-induced AKI rats, and HGF-modified hucMSCs showed high efficiency in treating AKI via antiapoptotic and anti-inflammatory mechanisms.66 Additionally, evidence showed that HGF-transgenic mesothelial cell sheet transplantation supports renal recovery and attenuates fibrosis in AKI murine models.67,68 It is noteworthy that the balance between HGF and TGF-β signaling at the initial stage of IRI facilitates the acute repair response, but the balance switches to TGF-β signaling during abnormal repair and fibrogenesis.69 In addition, HGF is highly correlated with active β-catenin in fibroblasts. β-catenin deficiency in renal fibroblasts induces HGF expression and activates tyrosine phosphorylation of the c-met receptor after IRI, thereby promoting cell proliferation and renal repair.70 In injured kidneys, proHGF is processed and cleaved to form mature HGF that binds to the c-met receptor.62 Conditional knockout of c-met in renal tubules exacerbates renal injury and inhibits renal regeneration after AKI. This indicates that tubule-specific c-met signaling plays an essential role in renal protection due to its proliferative, antiapoptotic, and anti-inflammatory properties.71–73 Consistently, HGF/c-met attenuates renal injury and inflammation while accelerating repair after glycerol-induced AKI.74
IGF and IGFBPs in AKI
IGF, a peptide growth factor that is secreted by the collecting duct of the adult kidney, binds with IGF1R and phosphorylates insulin receptor substrate proteins, thereby initiating downstream pathways, including PI3K-Akt-mTOR, to participate in the regulation of cell proliferation and apoptosis75,76 (Fig. 1). Infusion of IGF-1 improves hemodynamic parameters, such as renal plasma flow (RPF), inulin clearances (GFR), and renal vascular resistance (RVR), in fasted rats.77 Previous studies have shown that IGF signaling is highly involved in kidney development and different types of kidney diseases, including AKI.78,79 However, the function of IGF in AKI is still controversial. IGF-1 decreases following ischemic injury, and treatment with exogenous IGF-1 accelerates recovery by limiting cell apoptosis and promoting cell proliferation.80,81 These findings were further confirmed by a study indicating that administration of rhIGF-1 2 h post injury suppresses the renal inflammatory response and upregulates EGF levels.82 IGF-1 also promotes tubular regeneration after AKI by transactivating EGFR.83 In contrast, it is unfortunate that data from a clinical trial showed less salutary results for IGF-1 treatment,84,85 because administration of IGF-1 induced an inflammatory response, especially neutrophil accumulation, in rats with AKI, and this may lead to a higher mortality risk in patients.86,87 In addition, induction of the fibrotic response in mesangial cells may be another reason for the failure of IGF-1 treatment.88 Although IGF-1-based AKI therapy is disappointing, serum IGF-1 appears to be a potential biomarker because a reduced level of serum IGF is clearly correlated with increased mortality and the nutritional status of patients. The serum stability and short half-life of IGF-1 make it a suitable candidate as an early and sensitive biomarker for AKI mortality in intensive care units.89 In addition to IGF ligands, receptors, and insulin, a family of high-affinity IGFBPs has been identified in the IGF system and has gained more attention. These factors primarily antagonize IGF actions and may serve as biomarkers for AKI.90 Among these IGFBPs, IGFBP-7 is well studied, and emerging evidence shows that urinary IGFBP-7 and tissue inhibitor of metalloproteinase-2 (TIMP-2) can be applied as early diagnostic biomarkers for AKI following cardiac surgery,91 sepsis,92 and other renal insults of varied etiology.93–96 These factors appear to be ideal biomarkers for moderate and severe AKI, and the US Food and Drug Administration already permitted marketing of NephroCheck® (Astute Medical) to detect urinary [TIMP-2]*[IGFBP-7] in critically ill patients in 2014.90,95,97 However, it is noteworthy that the kinetics of urinary TIMP-2 and IGFBP-7 do not match the exposure of radiocontrast in patients suffering from stage 2–3 AKI.98
TGF-β in AKI
TGF-β exerts multiple biological functions in renal diseases by binding to its receptors and activating downstream Smad and non-Smad pathways, and renal TGF-β mainly originates from epithelial cells, leukocytes, or the circulation99,100 (Fig. 1). TGF-β1 is a well-recognized profibrotic factor.101–103 Activation of TGF-β/Smad signaling is detected in AKI models induced by different types of insults, such as IRI.104 In the IRI model, the level of TGF-β1 is increased by 1.5-fold at 12 h and more than 3-fold at 24 h and is sustained at a high level until 14 days,105 which was confirmed by our recent study showing that the production of TGF-β1 was significantly induced in cisplatin nephropathy.106 However, the exact role of TGF-β in AKI is not fully understood.
Several studies have provided evidence that TGF-β1 may be protective in AKI. It has been reported that a deficiency in TGF-β1 in mice increases renal damage and deteriorates renal function,107 and this was further confirmed by another study showing that sevoflurane protects against IRI-induced renal injury.108 Additionally, a recent study showed that TGF-β-induced CD4 + Foxp3 + Tregs prevented antibody-mediated acute renal allograft injury by targeting multiple effectors.109 However, other studies have shown controversial data that TGF-β1 is possibly detrimental in AKI. In the IRI rat model, blockade of TGF-β1 signaling with anti-TGF-β antibodies attenuates renal hypertrophy and interstitial cellularity and has a beneficial effect on microvascular structure but fails to accelerate the recovery of renal function.110 This finding was further confirmed by a recent study showing that SB4315432, a TGF-β1 receptor I inhibitor, decreased Nox4 levels and cell injury following colistin exposure.111 In addition, overexpression of type I TGF-β receptors specifically in tubular epithelial cells is sufficient to induce acute tubular injury and renal inflammation, which partly depends on mitochondrial-derived ROS.112 Consistently, conditional knockout of type II receptors from tubular epithelial cells blocks hydrogen peroxide–induced apoptosis, at least partly, through a Smad-dependent mechanism.113 Some studies revealed the potential function of downstream Smads in AKI. Global knockout of Smad3 protects against ischemic AKI by reducing IL-6 production.114 Moreover, Smad3 binds directly to p27 and inhibits the CDK2/cyclin E complex, thereby promoting AKI.115 As an inhibitory Smad, Smad7 protects against AKI by rescuing tubular epithelial cells from Smad3-mediated G1 cell cycle arrest.116 The function of Smad2 in AKI has drawn attention. A recent study showed that the activation of Smad2 is highly correlated with AKI progression.109 Our group further identified that conditional knockout of Smad2 protects against AKI by alleviating cell necroptosis, apoptosis and inflammation via the Smad/p53 interaction.106 Interestingly, we previously reported that Smad2 protects against renal fibrosis by suppressing Smad3 signaling;117 however, Smad2 and Smad3 are both detrimental in the progression of AKI, which indicates that the functional interaction between Smad2 and Smad3 might be distinct in different conditions; this needs to be further determined in future studies.106
It is noteworthy that TGF-β/Smads play a predominant role in the progression of AKI to CKD.118 In the tubular injury phase, proximal tubular cells dedifferentiate and proliferate to replace lost epithelial cells. However, when the insult is severe and unresolvable, some cells fail to redifferentiate and continue to produce growth factors such as TGF-β, finally leading to renal fibrosis.119 Additionally, a recent study showed that TGF-βRII deletion in macrophages prevents tubulointerstitial fibrosis following severe ischemic renal injury by abrogating TGF-β-dependent chemoattraction of macrophages.118 Collectively, the functions of TGF-β/Smads may vary according to their activation level, disease stages, and types of AKI models, which need to be further validated. Exploring the detailed function of TGF-β and downstream Smads may help us to better understand the pathological mechanisms of AKI and its progression to CKD.
VEGF in AKI
In the kidney, VEGF is mainly expressed in epithelial and endothelial cells (Fig. 1). Five isoforms of amino acids 121, 145, 165, 189, and 206 are produced through alternative splicing of VEGF mRNA. These amino acids bind to VEGFR-1 (flt-1), VEGFR-2 (flk-1), or VEGFR-3 to perform biological functions. In response to ischemic AKI insults, VEGFR-2 is upregulated in kidney tissues, although VEGF mRNA and protein levels are not increased, suggesting the possibility for exogenous VEGF treatment.120–122 A study showed that treatment with VEGF-121 protects against renal microvessel structure and prevents the AKI-CKD transition in response to increased sodium intake.123 Mechanistically, VEGF promotes renal repair following AKI by directly mediating mitogenic and antiapoptotic effects on TECs.124 In addition, VEGF expression stabilizes microvascular density, diminishes capillary rarefaction, and improves renal perfusion, which decreases chronic hypoxia and hemodynamics in ischemic AKI.125,126 Of note, the transcriptional regulation of VEGF has drawn increasing attention. As a key transcription factor, hypoxia inducible factor-1 (HIF-1) induces VEGF production to protect against hypoxic renal injury in the acute hypoxia phase of the ischemic AKI model.127 Preischemic targeting of HIF prolyl hydroxylation attenuates AKI and prevents AKI-CKD progression.128 However, HIF-1-induced overproduction of several growth factors (such as VEGF and connective tissue growth factor (CTGF)) contribute to renal fibrosis in chronic hypoxia conditions.129,130 Thus, the disease condition might be critical when HIF-1/VEGF-targeted therapy is applied.
Platelet-derived growth factor in AKI
Platelet-derived growth factors (PDGFs) consist of five dimers termed PDGF-AA, -AB, -BB, -CC, and -DD, and they bind and activate PDGF receptors (PDGFR-αα, -αβ, and -ββ) with distinct binding affinities.131 PDGFs are secreted by injured epithelial cells after AKI, and other cells involved in the progression of CKD also secrete PDGFs, including mesangial cells, fibroblasts, and pericytes131 (Fig. 1). Similarly, PDGF receptors are predominantly expressed on mesenchymal cells.132 In the early phase of IRI, PDGF-B/PDGFR is expressed in the S3 segments of the proximal tubule. This is related to proliferation activated by Src kinase, which induces tubular epithelial cell self-renewal.133,134 Concurrently, PDGF-B signaling is highly involved in fibroblast transformation, capillary damage, and rarefaction that result in alterations in renal hemodynamics. This indicates that PDGF contributes to the development of the AKI-CKD transition.10 However, the function of PDGF and PDGFR in the AKI-CKD transition, especially in the early stage, should be verified with conditional knockout models.
Growth factors and the AKI-CKD transition
Pathophysiology of the AKI-CKD transition
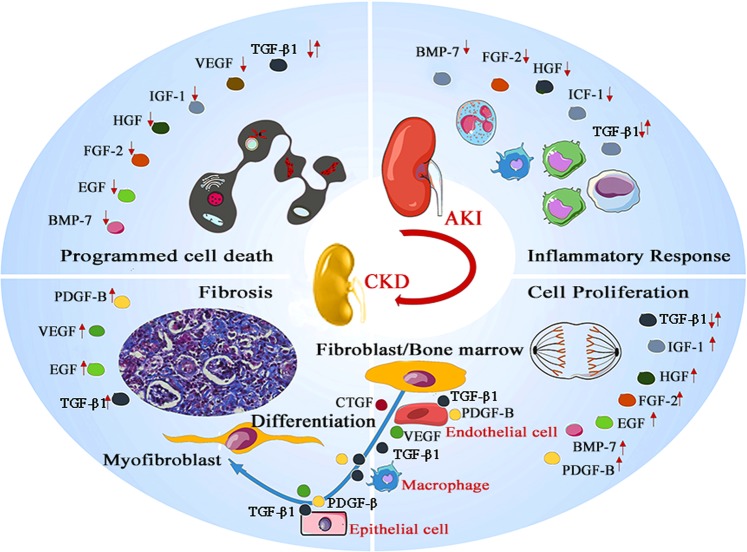
Accumulating evidence indicates that the severity of AKI and the number of AKI episodes are positively correlated with the subsequent development of CKD.135 When renal ischemia, toxic exposure, or obstruction occurs, TECs initiate renal self-renewal, including redifferentiation and proliferation, to replace the injured cells.136 Moreover, G2/M phase cell cycle arrest of some TECs results in a failure to regenerate and acquire a profibrotic phenotype, mediating the secretion of fibrotic cytokines such as TGF-β and CTGF, which accelerate the course of interstitial fibrosis, including fibroblast/bone marrow (major precursors of fibroblasts) differentiation or proliferation.136,137 Additionally, ischemia and oxidative stress induce endothelial cell apoptosis, which mediates microvasculature rarefaction, causing leakage of large macromolecules that are responsible for inflammatory and profibrotic responses in the interstitium137 (Fig. 2). An epidemiological study showed that the incidence of AKI-CKD transition occurs in ~15–20% of 1.5 million AKI survivors per year.138 Therefore, the molecular mechanisms underlying the AKI-CKD transition attract much attention. Possible mechanisms contributing to AKI-CKD progression include unresolved renal inflammation, tubular epithelial cell G2/M phase cell cycle arrest, hypoxia, microvascular rarefaction, transdifferentiation, and senescence of resident renal cells, myofibroblast activation, and interstitial fibrosis.139
Fig. 2. Effect of growth factors on AKI and AKI-CKD progression.
Many growth factors, such as BMP-7, EGF, FGF-2, HGF, IGF-1, VEGF, and TGF-β1, are involved in the programmed cell death of endothelial or epithelial cells in the acute injury phase. BMP-7, FGF-2, HGF, TGF-β1, and IGF-1 participate in the regulation of the inflammatory microenvironment that is responsible for cytokine production and immune cell recruitment. TGF-β1 is a double-edged growth factor. In addition, TGF-β1 exerts anti-inflammatory effects, and TGF-β1 overproduction leads to acute tubular injury. After injured epithelial cells fail to regenerate through differentiation, fibrosis is induced as a self-limiting repair process to limit damage. In this stage, overproduction of growth factors such as TGF-β1, PDGF, and FGF induces fibroblast/pericyte proliferation, transdifferentiation of tubular epithelial cells, endothelial cells, and macrophages, and extracellular matrix production, leading to CKD. Concurrently, abnormal synthesis of PDGF-B, VEGF, EGF, and TGF-β1 has a negative impact on endothelial integrity and causes capillary rarefaction, accelerating renal fibrosis.
Growth factors in the AKI-CKD transition
Emerging evidence shows that growth factors are highly involved in the progression of AKI to CKD.10,140 EGFR signaling is closely correlated with CKD progression. In a vancomycin-induced AKI mouse model, mutations in EGFR or inactivation of EGFR with gefitinib prevents the AKI-CKD transition via the STAT3/homeodomain interacting protein kinase 2 (HIPK2) axis.141 TGF-β is another key mediator that links AKI to CKD, although it has anti-inflammatory effects in certain conditions. TGF-β has multiple effects on renal cells in the AKI stage. For instance, TGF-β induces macrophage chemotaxis to accelerate inflammation and increase apoptosis of tubular epithelial and endothelial cells by promoting cell cycle arrest in renal tubular epithelial cells, which leads to abnormal repair, activation of myofibroblasts, and production of extracellular matrix. In addition, TGF-β signaling promotes endothelial injury and myofibroblast differentiation after AKI. TGF-β, PDGF-β, and CTGF mediate fibroblast/bone marrow transformation and reinforce the endothelial-fibroblast interface that is involved in fibroblast proliferation and capillary rarefaction in the pathological process of CKD. Furthermore, TGF-β and PDGF-β are secreted by epithelial cells that fail to recover after AKI, which accelerates fibrogenesis.10 Future studies on the inhibition of TGF-β signaling after cessation of AKI are needed to better define the role of TGF-β in the progression of acute to chronic renal injury.102,142 As previously mentioned, dysregulation of VEGF signaling is a key factor in promoting renal injury in CKD, since endothelial dysfunction and failure to maintain endothelial integrity lead to renal fibrosis.123,143 Furthermore, many growth factors, including TGF-β, BMP-7, VEGF, and HGF, are highly involved in the course of the AKI-CKD transition through regulating inflammation and immune reactions. In this setting, targeting abnormal activation of these signals may prevent AKI progression to CKD.
Growth factors may serve as biomarkers
To date, a series of studies have evaluated growth factors and correlated molecules as biomarkers for the early diagnosis and prediction of renal recovery from AKI. AKI diagnosis is currently dependent on increased serum creatinine (sCr) or other biomarkers. Considering that these factors are indirect biomarkers of kidney function, direct markers of tissue damage may be better candidates for predicting AKI (Table 2). As critical cell arrest modulators, the urine biomarkers IGFBP-7 and TIMP-2 are involved in the early phase of cellular stress and are used to predict AKI, especially moderate and severe AKI.95,144 In 2019, Kellum et al. published a guide for the clinical use of the [TIMP-2]* [IGFBP-7] biomarker to assess the risk of AKI in critical care.145 Recent evidence shows that these biomarkers may also predict adverse outcomes of AKI patients in the ICU.146,147 There are other potential biomarkers for AKI. The Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury (TRIBE-AKI) cohort study on adults undergoing cardiac surgery found that proangiogenic markers, such as VEGF, correlated with a reduced risk of AKI and mortality, but antiangiogenic VEGFR-1 was associated with an increased risk of AKI and mortality.148 Moreover, VEGF-C and VEGF-D, which are the main ligands for lymphangiogenesis, are abundantly expressed in tubules and increased in the serum and urine after injury. They are involved in renal inflammation and possibly serve as novel urinary biomarkers for AKI and the progression of kidney disease.149 In addition, increased urine or plasma FGF-23 levels may be promising novel biomarkers for AKI and other adverse outcomes in critically ill patients.148,150–152 A previous study also showed that low IGF-1 levels might serve as mortality predictors in AKI patients in the ICU.89
Table 2.
Diagnosis and treatment in patient with AKI.
| Biomarkers/novel therapy | Patients | |
|---|---|---|
| Diagnosis |
[TIMP-2]•[IGFBP7] ≤0.3 Low risk of AKI; >0.3 >92% of stage2/3 AKI; (FDA approved)145 |
Postoperative cardiovascular surgery |
| Shock/hemodynamically unstable | ||
| Sepsis | ||
| Postoperative major non-cardiovascular surgery | ||
| Cardiac arrest | ||
| Oliguria after acute resuscitation | ||
| FGF-23 (Clinical trial) | Severe sepsis/septic shock | |
| IGF-189 | In the intensive care unit | |
|
VEGF VEGFR-1148 |
Cardiac surgery | |
| Treatment |
HGF mimetic (ANG-3777, clinical trial) |
Kidney transplantation Cardiac surgery |
Growth factors and stem cell-based AKI therapy
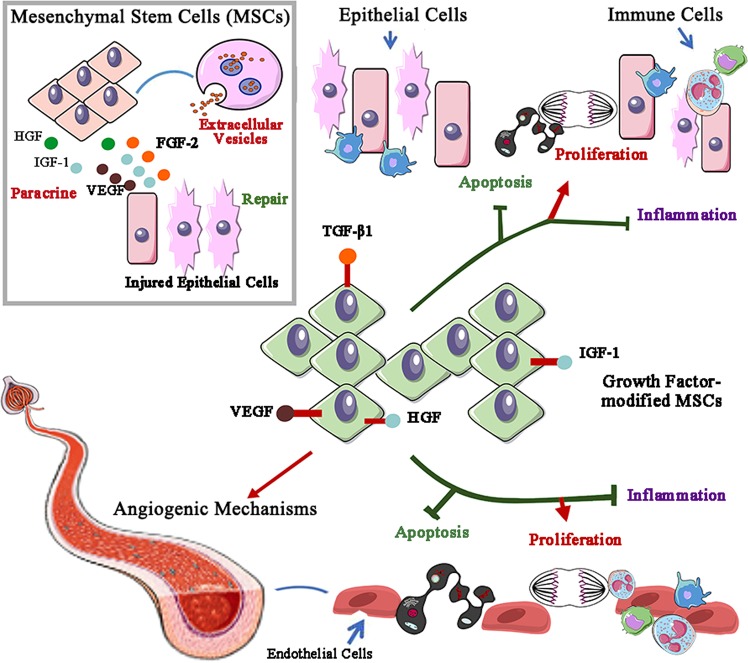
The therapeutic effect of stem cells, especially MSCs, in AKI has been widely investigated in the last decade. MSCs can be isolated from bone marrow, umbilical cord, placenta, or adipose tissue, and they show potent anti-inflammatory and immunosuppressive properties.153 Previous studies found that MSC transplantation prolonged mouse survival and promoted renal repair in AKI models induced by toxic drugs and ischemic/reperfusion.154 Several mechanisms have been proposed regarding the effect of stem cells on renal repair, including paracrine growth factors or extracellular vesicles.155 Stem cells accelerate renal repair by paracrine signaling through multiple types of growth factors, such as VEGF, FGF-2, IGF, and HGF.156,157 However, recent studies have indicated that extracellular vesicles (EVs), particularly microvesicles and exosomes, are responsible for the therapeutic effect of MSCs in many types of disease.158 A previous study on the biodistribution of MSC-derived extracellular vesicles in an AKI model showed that exosomes appear to be able to move to the injury site.159,160 Further evidence also indicated that horizontal transfer of IGF-1 receptor mRNA to tubular cells through MSC-derived exosomes accelerates renal repair post AKI.161 In addition, MSC-derived extracellular vesicles directly secrete bFGF, VEGF, IGF-1, and other proangiogenic factors,162 which have therapeutic effects on AKI.12
Moreover, growth factor-modified stem cells show more therapeutic potential than untreated controls. For example, IGF-1-incubated umbilical cord-derived MSCs had an enhanced renoprotective effect in the treatment of gentamicin-induced AKI.163 Consistently, a compound containing the C domain peptide of IGF-1 and chitosan hydrogel imitated the microenvironment of adipose-derived MSCs and had therapeutic effects on AKI.164 In addition, the VEGF165 gene conferred MSCs with protection against cisplatin-induced AKI by exerting beneficial effects on cell apoptosis, proliferation, and peritubular capillaries.165 In contrast, knockdown of VEGF in MSCs largely reduced the therapeutic potential of these cells and decreased the microvessel density in an AKI model.166 Consistently, a recent study identified that VEGF overexpression in amniotic fluid stem cells attenuated renal ischemia-reperfusion injury via mitogenic, anti-inflammatory, and angiogenic mechanisms.167 As a key immunomodulatory growth factor, TGF-β1-modified MSCs produce a local immunosuppressive effect and prevent IRI.168 Additionally, other studies indicated that HGF gene therapy or HGF-modified MSCs play a more effective role in AKI via antiapoptotic and anti-inflammatory mechanisms.66 A brief summary of stem cell-based AKI therapy is provided in Fig. 3.
Fig. 3. Growth factors and stem cell-based AKI therapy.
Extracellular vesicle (EV)-delivered and paracrine factors such as HGF, IGF-1, VEGF, and FGF-2 from mesenchymal stem cells contribute to repair after renal injury. More importantly, stem cells modified by growth factors, including VEGF, TGF-β1, and IGF-1, efficiently protect against AKI by decreasing apoptosis and the inflammatory response and promoting tubular epithelial and endothelial cell proliferation. VEGF-modified stem cells change capillary density via angiogenic mechanisms to attenuate renal ischemia-reperfusion injury.
Anti-AKI therapy targeting growth factors
Potential growth factor-targeted therapy for AKI
As previously mentioned, therapeutic strategies targeting growth factors and downstream effectors have been tested in animal models of AKI. BMP-7 seems to be a potential therapeutic target, since treatment with recombinant BMP-7 preserved kidney function and increased the survival rate post ischemic AKI,17 and restoration of BMP-7 by Dex or TSA attenuated renal injury by inhibiting HDAC5 or HDAC2-mediated suppression of BMP-7, respectively.24 In addition, members of the FGF family, such as FGF-10 and FGF-21, protect against AKI induced by cisplatin and IRI.57,58 HGF and c-met are also ideal targets because activation of HGF/c-met signaling attenuates tubular injury and renal inflammation in murine models of multiple types of AKI.74 Moreover, TGF-β/Smad signaling may also be a good target in the treatment of AKI because recent studies showed that the restoration of Smad7 or knockdown of Smad3 prevents both AKI and CKD progression.118 Although administration of VEGF effectively alleviated renal injury, we should be cautious because overactivation of VEGF in the late stage of AKI may promote the AKI-CKD transition.123 These strategies should be further evaluated in more animal model studies before clinical trials.
Clinical trials of growth factors for treating AKI
Effective and specific therapies for AKI in the clinic are still unavailable, and only a small number of agents targeting growth factors have been tested in clinical trials (Table 2). A small molecule hepatocyte growth factor/scatter factor (HGF/SF) mimetic, termed ANG-3777 or BB3, is undergoing clinical trial in patients who are susceptible to kidney injury.169 Investigators from Angion Biomedica Corp have demonstrated that ANG-3777 improves renal function in patients after kidney transplantation.170 Furthermore, research by this company is assessing whether ANG-3777 can reduce the severity of delayed graft function in recipients of a deceased donor kidney.171 Other similar clinical trials are underway. A phase 2 study to assess the safety and efficacy of ANG-3777 in patients who develop AKI after cardiac surgery is ongoing.172 Unfortunately, some clinical trials have already failed; for example, exogenous IGF-1 is beneficial in the recovery after kidney injury in mouse models, but a therapeutic trial in patients with acute renal failure (ARF) failed to demonstrate the efficacy of IGF-1 in humans because it induced a fibrotic response in mesangial cells and extensive neutrophil infiltration that reduced patient survival.56 This may be due to different renal lesions in ARF. Patients with ARF always have other severe illnesses, unlike experimental models with isolated disorders. Taken together, more precise dosing and targeted drug delivery systems need to be used and further studied.
Concluding remarks
In conclusion, growth factors function in the entire process of AKI, including initiation, renal repair, and the AKI-CKD transition. Considering the multiple roles of growth factors in kidney injury, directly targeting them may result in unexpected side effects such as renal fibrosis, which may impede their clinical application. Therefore, their downstream effectors should be characterized and evaluated as new targets in future studies. In addition, growth factors and correlated proteins, such as IGFBP-7, could serve as biomarkers for the prediction of AKI. MSCs modified by certain growth factors have great merit and may contribute to AKI treatment in the future.
Acknowledgements
We apologize to all colleagues whose important findings could not be cited owing to space limitations. This study was supported by the National Natural Science Foundation of China (No. 81570623 and No. 81970584), Science and Technological Fund of Anhui Province for Outstanding Youth of China (Grant number: 1608085J07), the Innovation and Entrepreneurship Support Program for Overseas Returnees in Anhui Province, the Key Projects of Outstanding Youth Foundation in Colleges of Anhui Province of China (No. gxyqZD2017021).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Li Gao, Xiang Zhong, Juan Jin
References
- 1.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang JN, et al. RIPK1 inhibitor Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via attenuating necroptosis, inflammation and oxidative stress. Clin. Sci. 2019;133:1609–1627. doi: 10.1042/CS20190599. [DOI] [PubMed] [Google Scholar]
- 3.Meng XM, et al. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab Invest. 2018;98:63–78. doi: 10.1038/labinvest.2017.120. [DOI] [PubMed] [Google Scholar]
- 4.Gao L, et al. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Front. Pharmacol. 2016;7:479. doi: 10.3389/fphar.2016.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CY. Yes, AKI truly leads to CKD. J. Am. Soc. Nephrol. 2012;23:967–969. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- 6.Forni LG, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickkers P, et al. Effect of human recombinant alkaline phosphatase on 7-Day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320:1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsurkan MV, et al. Growth factor delivery from hydrogel particle aggregates to promote tubular regeneration after acute kidney injury. J. Control Release. 2013;167:248–255. doi: 10.1016/j.jconrel.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Hammerman MR, Miller SB. Therapeutic use of growth factors in renal failure. J. Am. Soc. Nephrol. 1994;5:1–11. doi: 10.1681/ASN.V511. [DOI] [PubMed] [Google Scholar]
- 10.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 2015;26:1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christov M, Neyra JA, Gupta S, Leaf DE. Fibroblast growth factor 23 and klotho in AKI. Semin. Nephrol. 2019;39:57–75. doi: 10.1016/j.semnephrol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res. Ther. 2017;8:273. doi: 10.1186/s13287-017-0727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiskirchen R, et al. BMP-7 as antagonist of organ fibrosis. Front Biosci. 2009;14:4992–5012. doi: 10.2741/3583. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Choe S. BMPs and their clinical potentials. BMB Rep. 2011;44:619–634. doi: 10.5483/BMBRep.2011.44.10.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51–60. doi: 10.1046/j.1523-1755.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 16.Almanzar MM, et al. Osteogenic protein-1 mRNA expression is selectively modulated after acute ischemic renal injury. J. Am. Soc. Nephrol. 1998;9:1456–1463. doi: 10.1681/ASN.V981456. [DOI] [PubMed] [Google Scholar]
- 17.Vukicevic S, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Investig. 1998;102:202–214. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigolo E, et al. Canonical BMP signaling in tubular cells mediates recovery after acute kidney injury. Kidney Int. 2019;95:108–122. doi: 10.1016/j.kint.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Soofi A, Zhang P, Dressler GR. Kielin/chordin-like protein attenuates both acute and chronic renal injury. J. Am. Soc. Nephrol. 2013;24:897–905. doi: 10.1681/ASN.2012070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao WJ, et al. SCUBE1-enhanced bone morphogenetic protein signaling protects against renal ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:329–338. doi: 10.1016/j.bbadis.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Yanagita M, et al. Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J. Clin. Investig. 2006;116:70–79. doi: 10.1172/JCI25445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiura N, et al. Basic helix-loop-helix transcriptional factor MyoR regulates BMP-7 in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2013;304:F1159–F1166. doi: 10.1152/ajprenal.00510.2012. [DOI] [PubMed] [Google Scholar]
- 23.Hsing CH, et al. Propofol increases bone morphogenetic protein-7 and decreases oxidative stress in sepsis-induced acute kidney injury. Nephrol. Dial. Transplant. 2011;26:1162–1172. doi: 10.1093/ndt/gfq572. [DOI] [PubMed] [Google Scholar]
- 24.Hsing CH, et al. Alpha2-Adrenoceptor agonist dexmedetomidine protects septic acute kidney injury through increasing BMP-7 and inhibiting HDAC2 and HDAC5. Am. J. Physiol. Renal Physiol. 2012;303:F1443–F1453. doi: 10.1152/ajprenal.00143.2012. [DOI] [PubMed] [Google Scholar]
- 25.Marumo T, Hishikawa K, Yoshikawa M, Fujita T. Epigenetic regulation of BMP7 in the regenerative response to ischemia. J. Am. Soc. Nephrol. 2008;19:1311–1320. doi: 10.1681/ASN.2007091040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma T, et al. Suppression of BMP-7 by histone deacetylase 2 promoted apoptosis of renal tubular epithelial cells in acute kidney injury. Cell Death Dis. 2017;8:e3139. doi: 10.1038/cddis.2017.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patschan D, et al. Bone morphogenetic protein-5 and early endothelial outgrowth cells (eEOCs) in acute ischemic kidney injury (AKI) and 5/6-chronic kidney disease. Am. J. Physiol. Renal Physiol. 2013;305:F314–F322. doi: 10.1152/ajprenal.00677.2012. [DOI] [PubMed] [Google Scholar]
- 28.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009;315:602–610. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yano T, et al. Activation of epidermal growth factor receptor in the early phase after renal ischemia-reperfusion in rat. Nephron. 1999;81:230–233. doi: 10.1159/000045281. [DOI] [PubMed] [Google Scholar]
- 30.Taira T, et al. Urinary epidermal growth factor levels in patients with acute renal failure. Am. J. Kidney Dis. 1993;22:656–661. doi: 10.1016/S0272-6386(12)80427-6. [DOI] [PubMed] [Google Scholar]
- 31.Humes HD, et al. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J. Clin. Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen TH, et al. Epidermal growth factor attenuates tubular necrosis following mercuric chloride damage by regeneration of indigenous, not bone marrow-derived cells. J. Cell. Mol. Med. 2015;19:463–473. doi: 10.1111/jcmm.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo CK, et al. Ligand release-independent transactivation of epidermal growth factor receptor by transforming growth factor-beta involves multiple signaling pathways. Oncogene. 2008;27:614–628. doi: 10.1038/sj.onc.1210649. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82:45–52. doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, et al. EGF Receptor-Dependent YAP Activation Is Important for Renal Recovery from AKI. J. Am. Soc. Nephrol. 2018;29:2372–2385. doi: 10.1681/ASN.2017121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice. Kidney Int. 2014;86:538–547. doi: 10.1038/ki.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. ErbB4 deletion accelerates renal fibrosis following renal injury. Am. J. Physiol. Renal Physiol. 2018;314:F773–F787. doi: 10.1152/ajprenal.00260.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 2013;304:F356–F366. doi: 10.1152/ajprenal.00553.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, et al. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am. J. Pathol. 2013;183:160–172. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013;83:804–810. doi: 10.1038/ki.2012.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terzi F, et al. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Investig. 2000;106:225–234. doi: 10.1172/JCI8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinkle A, Mohammadi M. Structural Biology of the FGF7 Subfamily. Front. Genet. 2019;10:102. doi: 10.3389/fgene.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattison PC, et al. Role of circulating fibroblast growth factor-2 in lipopolysaccharide-induced acute kidney injury in mice. Pediatr. Nephrol. 2012;27:469–483. doi: 10.1007/s00467-011-2001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 45.Villanueva S, Cespedes C, Gonzalez A, Vio CP. bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1677–R1687. doi: 10.1152/ajpregu.00023.2006. [DOI] [PubMed] [Google Scholar]
- 46.Tan XH, et al. Fibroblast growth factor 2 protects against renal ischaemia/reperfusion injury by attenuating mitochondrial damage and proinflammatory signalling. J. Cell. Mol. Med. 2017;21:2909–2925. doi: 10.1111/jcmm.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egli-Spichtig D, Zhang MYH, Perwad F. Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid-induced acute kidney injury. Front. Physiol. 2018;9:1494. doi: 10.3389/fphys.2018.01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leaf DE, et al. Fibroblast growth factor 23 levels associate with AKI and death in critical illness. J. Am. Soc. Nephrol. 2017;28:1877–1885. doi: 10.1681/ASN.2016080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leaf DE, et al. FGF-23 levels in patients with AKI and risk of adverse outcomes. Clin. J. Am. Soc. Nephrol. 2012;7:1217–1223. doi: 10.2215/CJN.00550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leaf DE, et al. Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int. 2016;89:939–948. doi: 10.1016/j.kint.2015.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volovelsky O, et al. Early postoperative measurement of fibroblast growth factor 23 predicts severe acute kidney injury in infants after cardiac surgery. Clin. Nephrol. 2018;90:165–171. doi: 10.5414/CN109359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanudel MR, et al. Effects of acute kidney injury and chronic hypoxemia on fibroblast growth factor 23 levels in pediatric cardiac surgery patients. Pediatr. Nephrol. 2016;31:661–669. doi: 10.1007/s00467-015-3257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown JR, et al. Fibroblast growth factor-23 and the long-term risk of hospital-associated AKI among community-dwelling older individuals. Clin. J. Am. Soc. Nephrol. 2014;9:239–246. doi: 10.2215/CJN.05830513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durlacher-Betzer K, et al. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94:315–325. doi: 10.1016/j.kint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Hassan A, et al. The fibroblast growth factor receptor mediates the increased FGF23 expression in acute and chronic uremia. Am. J. Physiol. Renal Physiol. 2016;310:F217–F221. doi: 10.1152/ajprenal.00332.2015. [DOI] [PubMed] [Google Scholar]
- 56.Cuevas P, et al. Fibroblast growth factor protects the kidney against ischemia-reperfusion injury. Eur. J. Med. Res. 1999;4:403–410. [PubMed] [Google Scholar]
- 57.Tan X, et al. FGF10 protects against renal ischemia/reperfusion injury by regulating autophagy and inflammatory signaling. Front. Genet. 2018;9:556. doi: 10.3389/fgene.2018.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han W, et al. A nanomedicine approach enables co-delivery of cyclosporin A and gefitinib to potentiate the therapeutic efficacy in drug-resistant lung cancer. Signal Transduct. Target. Ther. 2018;3:16. doi: 10.1038/s41392-018-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villanueva S, et al. Inhibition of bFGF-receptor type 2 increases kidney damage and suppresses nephrogenic protein expression after ischemic acute renal failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R819–R828. doi: 10.1152/ajpregu.00273.2007. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, et al. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999;55:442–453. doi: 10.1046/j.1523-1755.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 61.Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J. Am. Soc. Nephrol. 1994;4:1387–1395. doi: 10.1681/ASN.V471387. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–2038. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- 63.Homsi E, et al. Attenuation of glycerol-induced acute kidney injury by previous partial hepatectomy: role of hepatocyte growth factor/c-met axis in tubular protection. Nephron Exp. Nephrol. 2007;107:e95–e106. doi: 10.1159/000109828. [DOI] [PubMed] [Google Scholar]
- 64.Herrero-Fresneda I, et al. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int. 2006;70:265–274. doi: 10.1038/sj.ki.5001510. [DOI] [PubMed] [Google Scholar]
- 65.Nakatani T, et al. Hepatocyte growth factor ameliorates renal hemodynamic disorder after ischemia/reperfusion. Int. J. Mol. Med. 2002;10:217–219. [PubMed] [Google Scholar]
- 66.Chen Y, et al. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 67.Miyabe Yoei, Sekiya Sachiko, Sugiura Naoko, Oka Masatoshi, Karasawa Kazunori, Moriyama Takahito, Nitta Kosaku, Shimizu Tatsuya. Renal subcapsular transplantation of hepatocyte growth factor-producing mesothelial cell sheets improves ischemia-reperfusion injury. American Journal of Physiology-Renal Physiology. 2019;317(2):F229–F239. doi: 10.1152/ajprenal.00601.2018. [DOI] [PubMed] [Google Scholar]
- 68.Oka M, et al. Hepatocyte growth factor-secreting mesothelial cell sheets suppress progressive fibrosis in a rat model of CKD. J. Am. Soc. Nephrol. 2019;30:261–276. doi: 10.1681/ASN.2018050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du T, et al. Human Wharton’s jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res. Ther. 2013;4:59. doi: 10.1186/scrt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou D, et al. Fibroblast-specific beta-catenin signaling dictates the outcome of AKI. J. Am. Soc. Nephrol. 2018;29:1257–1271. doi: 10.1681/ASN.2017080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou D, et al. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84:509–520. doi: 10.1038/ki.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pu T, et al. Augmenter of liver regeneration regulates autophagy in renal ischemia-reperfusion injury via the AMPK/mTOR pathway. Apoptosis. 2017;22:955–969. doi: 10.1007/s10495-017-1370-6. [DOI] [PubMed] [Google Scholar]
- 73.Gui Yuan, Lu Qingmiao, Gu Mengru, Wang Mingjie, Liang Yan, Zhu Xingwen, Xue Xian, Sun Xiaoli, He Weichun, Yang Junwei, Zhao Allan Zijian, Xiao Bo, Dai Chunsun. Fibroblast mTOR/PPARγ/HGF axis protects against tubular cell death and acute kidney injury. Cell Death & Differentiation. 2019;26(12):2774–2789. doi: 10.1038/s41418-019-0336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Homsi E, Janino P, Amano M, Saraiva Camara NO. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am. J. Nephrol. 2009;29:283–291. doi: 10.1159/000159275. [DOI] [PubMed] [Google Scholar]
- 75.Solarek Wojciech, Koper Michal, Lewicki Slawomir, Szczylik Cezary, Czarnecka Anna M. Insulin and insulin-like growth factors act as renal cell cancer intratumoral regulators. Journal of Cell Communication and Signaling. 2019;13(3):381–394. doi: 10.1007/s12079-019-00512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bridgewater DJ, Ho J, Sauro V, Matsell DG. Insulin-like growth factors inhibit podocyte apoptosis through the PI3 kinase pathway. Kidney Int. 2005;67:1308–1314. doi: 10.1111/j.1523-1755.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 77.Hirschberg R, Kopple JD. Evidence that insulin-like growth factor I increases renal plasma flow and glomerular filtration rate in fasted rats. J. Clin. Invest. 1989;83:326–330. doi: 10.1172/JCI113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bach LA, Hale LJ. Insulin-like growth factors and kidney disease. Am. J. Kidney Dis. 2015;65:327–336. doi: 10.1053/j.ajkd.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 79.Friedlaender M, et al. Insulin-like growth factor-1 (IGF-1) enhances recovery from HgCl2-induced acute renal failure: the effects on renal IGF-1, IGF-1 receptor, and IGF-binding protein-1 mRNA. J. Am. Soc. Nephrol. 1995;5:1782–1791. doi: 10.1681/ASN.V5101782. [DOI] [PubMed] [Google Scholar]
- 80.Ding H, Kopple JD, Cohen A, Hirschberg R. Recombinant human insulin-like growth factor-I accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J. Clin. Invest. 1993;91:2281–2287. doi: 10.1172/JCI116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Z, et al. IGF-1 protects tubular epithelial cells during injury via activation of ERK/MAPK signaling pathway. Sci. Rep. 2016;6:28066. doi: 10.1038/srep28066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goes N, et al. Effect of recombinant human insulin-like growth factor-1 on the inflammatory response to acute renal injury. J. Am. Soc. Nephrol. 1996;7:710–720. doi: 10.1681/ASN.V75710. [DOI] [PubMed] [Google Scholar]
- 83.Lin JJ, et al. Insulin-like growth factor-1 enhances epidermal growth factor receptor activation and renal tubular cell regeneration in postischemic acute renal failure. J. Lab. Clin. Med. 1995;125:724–733. [PubMed] [Google Scholar]
- 84.Hladunewich MA, et al. A randomized, placebo-controlled trial of IGF-1 for delayed graft function: a human model to study postischemic ARF. Kidney Int. 2003;64:593–602. doi: 10.1046/j.1523-1755.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 85.Hirschberg R, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423–2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez M, et al. Exacerbated inflammatory response induced by insulin-like growth factor I treatment in rats with ischemic acute renal failure. J. Am. Soc. Nephrol. 2001;12:1900–1907. doi: 10.1681/ASN.V1291900. [DOI] [PubMed] [Google Scholar]
- 87.Osterziel KJ, Dietz R, Ranke MB. Increased mortality associated with growth hormone treatment in critically ill adults. N. Engl. J. Med. 2000;342:134–135. doi: 10.1056/NEJM200001133420214. [DOI] [PubMed] [Google Scholar]
- 88.Schreiber BD, Hughes ML, Groggel GC. Insulin-like growth factor-1 stimulates production of mesangial cell matrix components. Clin. Nephrol. 1995;43:368–374. [PubMed] [Google Scholar]
- 89.Guimaraes SM, et al. Low insulin-like growth factor-1 and hypocholesterolemia as mortality predictors in acute kidney injury in the intensive care unit. Crit. Care Med. 2008;36:3165–3170. doi: 10.1097/CCM.0b013e318186ab70. [DOI] [PubMed] [Google Scholar]
- 90.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin. Chim. Acta. 2015;438:350–357. doi: 10.1016/j.cca.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, et al. The molecular determinants governing the immunogenicity of Japanese encephalitis live attenuated vaccines. Signal Transduct. Target. Ther. 2017;2:17005. doi: 10.1038/sigtrans.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Honore PM, et al. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit. Care Med. 2016;44:1851–1860. doi: 10.1097/CCM.0000000000001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aregger F, et al. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014;85:909–919. doi: 10.1038/ki.2013.363. [DOI] [PubMed] [Google Scholar]
- 94.Jia HM, Huang LF, Zheng Y, Li WX. Diagnostic value of urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7 for acute kidney injury: a meta-analysis. Crit. Care. 2017;21:77. doi: 10.1186/s13054-017-1660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vijayan A, et al. Clinical Use of the Urine Biomarker [TIMP-2] x [IGFBP7] for Acute Kidney Injury Risk Assessment. Am. J. Kidney Dis. 2016;68:19–28. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen W, et al. TIFA suppresses hepatocellular carcinoma progression via MALT1-dependent and -independent signaling pathways. Signal Transduct. Target Ther. 2016;1:16013. doi: 10.1038/sigtrans.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koyner JL, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J. Am. Soc. Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ostermann M, et al. Kinetics of urinary cell cycle arrest markers for acute kidney injury following exposure to potential renal insults. Crit. Care Med. 2018;46:375–383. doi: 10.1097/CCM.0000000000002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology. 2005;10:48–56. doi: 10.1111/j.1440-1797.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 100.Meng XM. Inflammatory mediators and renal fibrosis. Adv. Exp. Med. Biol. 2019;1165:381–406. doi: 10.1007/978-981-13-8871-2_18. [DOI] [PubMed] [Google Scholar]
- 101.Meng XM, Tang PM, Li J, Lan HY. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015;6:82. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat. Rev. Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Meng XM, Huang XR, Lan HY. The preventive and therapeutic implication for renal fibrosis by targetting TGF-beta/Smad3 signaling. Clin. Sci. 2018;132:1403–1415. doi: 10.1042/CS20180243. [DOI] [PubMed] [Google Scholar]
- 104.Kilari S, et al. Increased transforming growth factor beta (TGF-beta) and pSMAD3 signaling in a Murine Model for Contrast Induced Kidney Injury. Sci. Rep. 2018;8:6630. doi: 10.1038/s41598-018-24340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Basile DP, Rovak JM, Martin DR, Hammerman MR. Increased transforming growth factor-beta 1 expression in regenerating rat renal tubules following ischemic injury. Am. J. Physiol. 1996;270:F500–F509. doi: 10.1152/ajpcell.1996.270.2.C500. [DOI] [PubMed] [Google Scholar]
- 106.Yang Q, et al. Conditional knockout of TGF-betaRII /Smad2 signals protects against acute renal injury by alleviating cell necroptosis, apoptosis and inflammation. Theranostics. 2019;9:8277–8293. doi: 10.7150/thno.35686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guan Q, Nguan CY, Du C. Expression of transforming growth factor-beta1 limits renal ischemia-reperfusion injury. Transplantation. 2010;89:1320–1327. doi: 10.1097/TP.0b013e3181d8e9dc. [DOI] [PubMed] [Google Scholar]
- 108.Lee HT, et al. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am. J. Physiol. Ren. Physiol. 2008;295:F128–F136. doi: 10.1152/ajprenal.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liao T, et al. In vivo attenuation of antibody-mediated acute renal allograft rejection by ex vivo TGF-beta-induced CD4(+)Foxp3(+) regulatory T cells. Front. Immunol. 2017;8:1334. doi: 10.3389/fimmu.2017.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spurgeon KR, Donohoe DL, Basile DP. Transforming growth factor-beta in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am. J. Physiol. Ren. Physiol. 2005;288:F568–F577. doi: 10.1152/ajprenal.00330.2004. [DOI] [PubMed] [Google Scholar]
- 111.Jeong BY, et al. TGF-beta-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J. Antimicrob. Chemother. 2018;73:962–972. doi: 10.1093/jac/dkx479. [DOI] [PubMed] [Google Scholar]
- 112.Gentle ME, et al. Epithelial cell TGFbeta signaling induces acute tubular injury and interstitial inflammation. J. Am. Soc. Nephrol. 2013;24:787–799. doi: 10.1681/ASN.2012101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gewin L, et al. Deleting the TGF-beta receptor attenuates acute proximal tubule injury. J. Am. Soc. Nephrol. 2012;23:2001–2011. doi: 10.1681/ASN.2012020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 2011;301:F436–F442. doi: 10.1152/ajprenal.00162.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lai W, et al. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int. 2016;90:610–626. doi: 10.1016/j.kint.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fu S, et al. Smad7 protects against acute kidney injury by rescuing tubular epithelial cells from the G1 cell cycle arrest. Clin. Sci. 2017;131:1955–1969. doi: 10.1042/CS20170127. [DOI] [PubMed] [Google Scholar]
- 117.Meng XM, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J. Am. Soc. Nephrol. 2010;21:1477–1487. doi: 10.1681/ASN.2009121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chung, S. et al. TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 3, 123563 (2018). [DOI] [PMC free article] [PubMed]
- 119.Fattah H, Vallon V. Tubular recovery after acute kidney injury. Nephron. 2018;140:140–143. doi: 10.1159/000490007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanellis J, et al. Renal ischemia-reperfusion increases endothelial VEGFR-2 without increasing VEGF or VEGFR-1 expression. Kidney Int. 2002;61:1696–1706. doi: 10.1046/j.1523-1755.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 121.Basile DP, et al. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am. J. Physiol. Ren. Physiol. 2008;294:F928–F936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 122.Kanellis J, et al. Redistribution of cytoplasmic VEGF to the basolateral aspect of renal tubular cells in ischemia-reperfusion injury. Kidney Int. 2000;57:2445–2456. doi: 10.1046/j.1523-1755.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- 123.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008;295:F1648–F1657. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu KD, Brakeman PR. Renal repair and recovery. Crit. Care Med. 2008;36:S187–S192. doi: 10.1097/CCM.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- 125.Lee SY, et al. MMP-9 gene deletion mitigates microvascular loss in a model of ischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 2011;301:F101–F109. doi: 10.1152/ajprenal.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 127.Smith SF, Hosgood SA, Nicholson ML. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int. 2019;95:50–56. doi: 10.1016/j.kint.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 128.Kapitsinou PP, et al. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am. J. Physiol. Ren. Physiol. 2012;302:F1172–F1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haase VH. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Ren. Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andringa KK, Agarwal A. Role of hypoxia-inducible factors in acute kidney injury. Nephron Clin. Pract. 2014;127:70–74. doi: 10.1159/000363669. [DOI] [PubMed] [Google Scholar]
- 131.Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol. Dial. Transplant. 2014;29:i45–i54. doi: 10.1093/ndt/gft273. [DOI] [PubMed] [Google Scholar]
- 132.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat. Rev. Nephrol. 2014;10:700–711. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- 133.Takikita-Suzuki M, et al. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am. J. Pathol. 2003;163:277–286. doi: 10.1016/S0002-9440(10)63651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakagawa T, et al. Role of PDGF B-chain and PDGF receptors in rat tubular regeneration after acute injury. Am. J. Pathol. 1999;155:1689–1699. doi: 10.1016/S0002-9440(10)65484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2014;307:F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 136.Yang L. How acute kidney injury contributes to renal fibrosis. Adv. Exp. Med. Biol. 2019;1165:117–142. doi: 10.1007/978-981-13-8871-2_7. [DOI] [PubMed] [Google Scholar]
- 137.Lovisa S, Zeisberg M, Kalluri R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol. Metab. 2016;27:681–695. doi: 10.1016/j.tem.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 138.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 139.Strausser SA, Nakano D, Souma T. Acute kidney injury to chronic kidney disease transition: insufficient cellular stress response. Curr. Opin. Nephrol. Hypertens. 2018;27:314–322. doi: 10.1097/MNH.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 140.Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014;10:493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 141.Xu L, et al. EGFR drives the progression of AKI to CKD through HIPK2 overexpression. Theranostics. 2019;9:2712–2726. doi: 10.7150/thno.31424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gewin Leslie S. Transforming Growth Factor-β in the Acute Kidney Injury to Chronic Kidney Disease Transition. Nephron. 2019;143(3):154–157. doi: 10.1159/000500093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin SL, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bihorac A, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am. J. Respir. Crit. Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 145.Guzzi LM, et al. Clinical use of [TIMP-2]*[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care. 2019;23:225. doi: 10.1186/s13054-019-2504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kashani K, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xie Y, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)* IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int. 2019;95:1486–1493. doi: 10.1016/j.kint.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 148.Mansour SG, et al. The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am. J. Kidney Dis. 2019;74:36–46. doi: 10.1053/j.ajkd.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zarjou Abolfazl, Black Laurence M., Bolisetty Subhashini, Traylor Amie M., Bowhay Sarah A., Zhang Ming-Zhi, Harris Raymond C., Agarwal Anupam. Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Laboratory Investigation. 2019;99(9):1376–1388. doi: 10.1038/s41374-019-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Role of FGF-23 as a Prognosis Biomarker in Intensive Care Patients. ClinicalTrials.gov., https://clinicaltrials.gov/ct2/show/NCT01801501, (2019).
- 151.Spaich S, et al. Fibroblast growth factor 23 (FGF-23) is an early predictor of mortality in patients with cardiac arrest. Resuscitation. 2016;98:91–96. doi: 10.1016/j.resuscitation.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 152.Volovelsky O, et al. Pre-operative level of FGF23 predicts severe acute kidney injury after heart surgery in children. Pediatr. Nephrol. 2018;33:2363–2370. doi: 10.1007/s00467-018-4024-1. [DOI] [PubMed] [Google Scholar]
- 153.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morigi M, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 155.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Imberti B, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J. Am. Soc. Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 157.Zhao L, et al. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J. Cell. Mol. Med. 2019;23:720–730. doi: 10.1111/jcmm.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qiu G, et al. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018;9:320. doi: 10.1186/s13287-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark. Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Grange C, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014;33:1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bruno S, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng. A. 2017;23:1262–1273. doi: 10.1089/ten.tea.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsuji K, Kitamura S, Wada J. Secretomes from mesenchymal stem cells against acute kidney injury: possible heterogeneity. Stem Cells Int. 2018;2018:8693137. doi: 10.1155/2018/8693137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu P, et al. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. Sci. Rep. 2016;6:20287. doi: 10.1038/srep20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Feng G, et al. IGF-1 C domain-modified hydrogel enhances cell therapy for AKI. J. Am. Soc. Nephrol. 2016;27:2357–2369. doi: 10.1681/ASN.2015050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yuan L, et al. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2011;300:F207–F218. doi: 10.1152/ajprenal.00073.2010. [DOI] [PubMed] [Google Scholar]
- 166.Togel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J. Cell. Mol. Med. 2009;13:2109–2114. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mori da Cunha MG, et al. Vascular endothelial growth factor up-regulation in human amniotic fluid stem cell enhances nephroprotection after ischemia-reperfusion injury in the rat. Crit. Care Med. 2017;45:e86–e96. doi: 10.1097/CCM.0000000000002020. [DOI] [PubMed] [Google Scholar]
- 168.Cai J, et al. Transforming growth factor-beta1-overexpressing mesenchymal stromal cells induced local tolerance in rat renal ischemia/reperfusion injury. Cytotherapy. 2019;21:535–545. doi: 10.1016/j.jcyt.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 169.Hulse M, Rosner MH. Drugs in development for acute kidney injury. Drugs. 2019;79:811–821. doi: 10.1007/s40265-019-01119-8. [DOI] [PubMed] [Google Scholar]
- 170.Study to improve renal function after kidney transplantation. ClinicalTrials.gov., https://clinicaltrials.gov/ct2/show/NCT01286727, (2015).
- 171.Reduce the severity of DGF in recipients of a deceased donor kidney. ClinicalTrials.gov., https://clinicaltrials.gov/ct2/show/NCT02474667, (2019).
- 172.Study to prevent acute kidney injury after cardiac surgery involving cardiopulmonary bypass. ClinicalTrials.gov., https://clinicaltrials.gov/ct2/show/NCT02771509, (2019).